Abstract
Parkinson's disease is the second most prevalent neurodegenerative disorder worldwide. Clinically, it is characterized by severe motor complications caused by progressive degeneration of dopaminergic neurons. Current treatment is focused on mitigating the symptoms through the administration of levodopa, rather than on preventing dopaminergic neuronal damage. Therefore, the use and development of neuroprotective/disease-modifying strategies is an absolute need that can lead to promising gains on translational research of Parkinson's disease. For instance, N-acetylcysteine, a natural compound with strong antioxidant effects, has been shown to modulate oxidative stress, preventing dopamine-induced cell death. Despite the evidence of neuroprotective and modulatory effects of this drug, as far as we know, it does not induce per se any regenerative process. Therefore, it would be of interest to combine the latter with innovative therapies that induce dopaminergic neurons repair or even differentiation, as stem cell-based strategies. Stem cells secretome has been proposed as a promising therapeutic approach for Parkinson's disease, given its ability to modulate cell viability/preservation of dopaminergic neurons. Such approach represents a shift in the paradigm, showing that cell-transplantation free therapies based on the use of stem cells secretome may represent a potential alternative for regenerative medicine of Parkinson's disease. Thus, in this review, we address the current understanding of the potential combination of stem cell free-based strategies and neuroprotective/disease-modifying strategies as a new paradigm for the treatment of central nervous system neurodegenerative diseases, like Parkinson's disease.
Key Words: disease-modifying strategies, mesenchymal stem cells, N-acetylcysteine, neuroprotection, Parkinson's disease, stem cells secretome
Introduction
If one could imagine a youth elixir capable of preventing age-related neurodegenerative diseases, then it would be envisaged that the affected cells could be re-educated with such treatment by reprograming their cell fate, making them go backwards in their developmental program. Ageing plays an unquestionable role in Parkinson's disease (PD) pathophysiology, being the most relevant risk factor. With the increase of life expectancy, one of the major challenges for neuroscience will, definitely, be able to delay or reverse the ageing process of the nervous system. How can this be attained specifically in PD? In this perspective article, we challenge the idea that symptomatic and disease-modifying therapies for PD could be approached globally with a rejuvenating combinatorial tactic able to tackle simultaneously different disease mechanisms and cell types. Specifically, we highlight the potential regenerative and youthful effects of stem cells secretome (SCS) and the promising disease-modifying effects of N-acetylcysteine (NAC) supplementation, a powerful and well-known antioxidant.
Search Strategy and Selection Criteria
The studies cited in the current review, published from 2000 to 2020, were searched on PubMed and Web of Science databases using the following keywords/terms: neurodegeneration; Parkinson's disease; Stem Cells secretome (SCS); N-Acetylcysteine (NAC); neuroprotection and repair. Furthermore, we also used variations of the above main search terms to meticulously reach the largest number of studies in the literature.
Parkinson's Disease
PD affects about 1–5 % of the population over the age of 60. The Global Burden of Diseases Study in 2016 estimated that PD affected 6.1 million individuals worldwide and its prevalence is expected to increase by more than 50% in the next decade (GBD 2016 Parkinson's Disease Collaborators, 2018).
PD is clinically complex, characterized by a broad spectrum of central motor features comprising bradykinesia, uncontrollable resting tremor, muscular rigidity and impaired postural reflexes. Non-motor symptoms are also present in PD patients, including sleep disturbances, humor alterations and depression behavioral and/or cognitive dysfunction. The pathological common denominator of PD is the predominant dopaminergic neuronal loss and a deficient dopamine production in the substantia nigra and striatal projections. Nevertheless, it is becoming clear that dysfunction of microglia, astrocytes, oligodendrocytes and other neuron cell types also contribute to PD pathogenesis and progression. Some of the underlying pathogenic mechanisms that are associated with the disease progression include α-synuclein aggregation, mitochondrial dysfunction, endoplasmic reticulum stress, impaired autophagy and the loss of calcium homeostasis (Teixeira et al., 2018).
Additionally, a significant amount of evidence in the past years has suggested that oxidative stress is a crucial element of dopaminergic neurodegeneration, marked by glutathione depletion, increased generation of reactive oxygen species and inhibition of the mitochondrial complex, thus contributing to heterogeneous symptom manifestations. The present available pharmacological strategies to treat PD put an emphasis on the symptomatic dimension as an attempt to provide transient symptom relief to the patients and improve their functional capacity. The most common therapies consist on dopamine replacement, being the administration of levodopa the most potent and widely used, alone or combined with dopamine receptor agonists and monoamine oxidase-B or catechol-O-methyltransferase inhibitors (Teixeira et al., 2018). At first stage, these strategies are effective in controlling primary motor symptoms but as the disease progresses there is a decline in responsiveness, eliciting undesirable side effects, such as dyskinesias, motor fluctuations, and behavior changes. Though there is a clear increase in the levels of dopamine, they are not able to replace or counteract dopaminergic neuronal death (Maiti et al., 2017).
Here, we challenge the “one-disease-one-target” viewpoint that has been followed in the past decades and that may constitute the rationale behind the current failure in effective disease modifying treatments for PD. Now recognized as a multifaced disorder, a PD-focused multimodal treatment perspective may open different avenues in the endorsement of nigro-striatal dopaminergic cells survival and regeneration.
Stem Cells Secretome
In recent years, the use of SCS is looking as a promising therapeutic tool for central nervous system neurodegenerative diseases, like PD. Within such concept, the secretome of mesenchymal stem cells (MSCs) has been the focus of intense research on potential therapeutic strategies for CNS repair and regeneration (Teixeira and Salgado, 2020). In the past years, we have shown that the therapeutic properties of MSCs are closely related to their secretome - a mixture of released soluble and vesicular fractions of proteins and nucleic acids – with proven encouraging data in pre-clinical models for PD (Mendes-Pinheiro et al., 2019). In vitro, we have demonstrated positive effects of MSCs secretome in neuronal and glial survival and differentiation and axonal growth (Martins et al., 2017; Teixeira et al., 2017). We also showed that the treatment with the secretome of MSCs from bone marrow have promising effects in a rat pre-clinical model of PD, where sole administration lead to significant motor improvements and rescue of dopaminergic neurons (Teixeira et al., 2017, 2020; Mendes-Pinheiro et al., 2019). Using unbiased proteomics analysis, we identified in MSCs secretome the presence of important protective molecules such as DJ-1, TRXR1, PRDX1, brain-derived neurotrophic factor (BDNF), glial cell line-derived neurotrophic factor (GDNF), PEDF, Gal-1 and Cystatin C, which may explain the multi-target beneficial effects observed (Teixeira and Salgado, 2020).
In addition to MSCs, encouraging evidence have also demonstrated that the use of SCS from other stem cells sources such as neural progenitor cells (NPCs), also confer beneficial properties to Parkinson's treatment (Willis et al., 2020b). Indeed, we have revealed for the first time that the sole injection of human NPCs secretome in a 6-OHDA rat model of PD, provided support to promote dopaminergic neuronal survival, which consequently lead to an improvement on animal motor deficits (Mendes-Pinheiro et al., 2018). Actually, NPCs are believed to be neurotrophic factors secreting cells, capable of releasing neurotrophic components such as GDNF and BDNF that can sustain the growth and differentiation of dopaminergic (im)mature neurons in PD models, and regulate many biological processes, comprising cell survival, proliferation and differentiation, as well as immunomodulation, and regulation of the apoptotic process (Mendes-Pinheiro et al., 2018; Willis et al., 2020a). Although promising, the studies involving the action of NPCs secretome in PD are still limited, being too early to take sustainable conclusions (Willis et al., 2020a).
Such findings have driven SCS to be considered a promising and valuable therapeutic purpose for PD. With it, we expect to overcome the disadvantages of cell transplantation itself (high numbers of cells for transplantation; low rates of cell survival and engraftment upon transplantation), as well as the preceding differentiation processes. By overcoming these disadvantages with SCS-based approaches, it should be possible to develop a faster and reliable therapy, using off-the-shelf ready to use products.
N-Acetylcysteine
Both at the cellular and physiological levels, oxidative stress is considered to be a potential driver of PD progression. NAC is an old antioxidant nutraceutical with an ample spectrum of use, but with potential new tricks in nervous system regeneration. In fact, recent controlled and randomized clinical trials have demonstrated that intravenous administration of NAC increased blood redox rations of glutathione (Holmay et al., 2013) and may positively affect the dopaminergic system in patients with PD (Monti et al., 2019, 2016). These findings were supported by previous in vitro and rodent pre-clinical in vivo models of PD where NAC supplementation decreased the levels of brain aggregated alpha-synuclein, modulated the localization of NFkappaB and protected dopaminergic cells (Bagh et al., 2008; Clark et al., 2010; Berman et al., 2011). Additionally, NAC administration substantially increased brain synaptic and non-synaptic connections, diminished oxidative damage, raised brain synaptic mitochondrial complex I activity and anti-inflammatory protection and prevented reactive oxygen species accumulation (Mart??nez Banaclocha, 2000; Chen et al., 2007). The recent observed similar protective effects of NAC on oligodendrocytes and astrocytes (Shieh et al., 2019; Zhou et al., 2020) make NAC a promising multi-target prodrug in PD treatment.
Future Perspectives
To date, no regenerative, neuroprotective or disease-modifying strategy has been approved as a PD therapy, a fact that can be partially attributed to the “one-disease-one-target” view that has been followed in PD treatment pipeline. Although SCS presents promising PD therapeutical effects, due to the complex nature of PD pathophysiology it is unlikely that SCS per se can completely repair it. Thus, combining SCS with strategies that can tackle oxidative stress, excitotoxicity, inflammation, and dopamine (DA) will be essential. A combination of SCS with potential neuroprotective/disease-modifying PD drugs may represent an efficient way to handle PD in a multi-dimensional way. As so, could SCS be supplemented with NAC represent a novel strategy to PD modeling and repair?
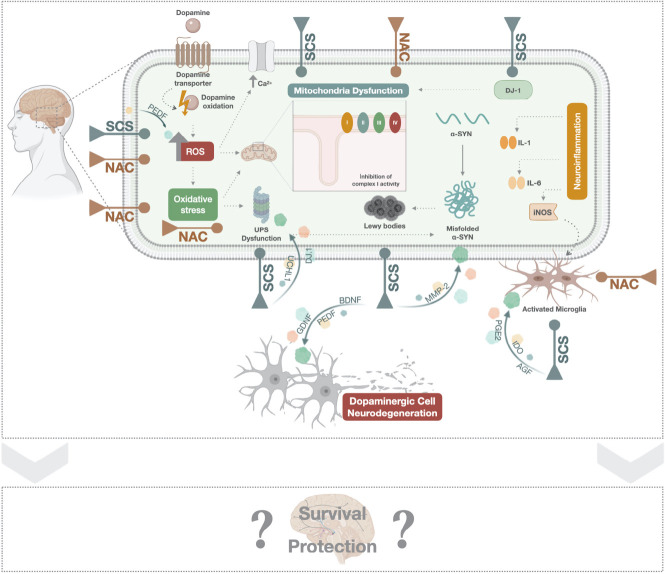
In the last decades, research has been devoted to investigate the neurodegenerative processes and symptomology of PD instead of the loss of the dopaminergic system in the nigrostriatal pathway. Nowadays it is recognized that Parkinson's comprises different cell types, mechanisms and circuitries in the brain (and in the periphery), and the erstwhile obsession with dopaminergic neurons and mainly with DA levels reposition has been a limiting factor in advancing new approaches towards modifying the trajectory of the disease. Indeed, single treatment approaches (or even with adjuvant strategies) have failed, by showing quite variable outcomes, either displaying benefits (during the first years after disease diagnosis) or leading to adverse effects (due to higher doses in medium-late stages of the disease), thereby being insufficient to tackle the dissimilar strands of PD. Therefore, synergistic approaches seem greater than isolated perspectives when employing them in this pathology, as we can hold distinct impacts on different cell, mechanisms, and brain areas. In fact, a logical design of combinatorial strategies that can overcome the limitations of single drug/surgical approach could be the path to pursue to help clinicians successfully manage patients’ symptomatology by lengthening the period of response to medication with enhanced tolerance, and so, modify the progression of the disease. Therefore, hypothesize the combination of NAC neuroprotective/disease-modifying properties with the SCS regenerative potentialities may hold a multimodal and promising approach for treating PD (Figure 1).
Figure 1.
SCS and NAC mechanistic effects.
Administration of SCS (from different sources) was found to significantly reduce ROS production, alpha-synuclein aggregation and as consequence Lewy bodies formation. Still, potential properties in the attenuation of UPS alterations, and mitochondrial dysfunction have also been assigned to SCS, thereby leading to decreased microglia activation and dopaminergic cell death. Trophic factors including PEDF, DJ-1, UCHL-1, GDNF, BDNF, MMP-2, PGE2, and IDO, are responsible for this secretome effects. Similarly, NAC has also revealed the capability to tackle identical mechanisms, such as neuroinflammation, oxidative stress, mitochondria impairments and UPS dysfunction. Despite the multifactorial profile of PD, the multi-targeted approach of SCS and NAC per se or (eventually) in combination opens a promising and reliable therapeutic route to the protection/survival of the preexisting dopaminergic neurons in PD brain lesioned areas, which may lead to the improvement of cellular and molecular mechanisms/circuitries, as well as to functional amelioration. BDNF: Brain-derived neurotrophic factor; DJ-1: protein deglycase; GDNF: glial cell line-derived neurotrophic factor; IDO: indoleamine 2,3-dioxygenase; MMP-2: metalloproteinase 2; NAC: N-acetylcysteine; PEDF: pigment epithelium-derived factor; PGE2: prostaglandin E2; ROS: reactive oxygen species; SCS: stem cells secretome; UCHL-1: ubiquitin carboxyl-terminal hydrolase L1; UPS: ubiquitin-proteosome system.
With such possibility, we could tackle major hallmarks of PD, relying on the rationale of SCS and NAC capacity to potentiate the number of dopaminergic positive cells in the affected areas and tackle most of the molecular issues responsible for the failure of dopaminergic cell survival and DA degradation after PD, thereby slowing its progression.
Thus, even being a promising approach, future pre-clinical studies are warranted to support this ground-breaking hypothesis, to understand how the combinatorial content modulates the molecular and cellular PD pathophysiological mechanisms. Likewise, routes of administration, dosage regimens, extent of therapies, monitoring plans and randomized procedures should be considered and explored as a way to support the hypothetical outcomes. Still, quite challenging will also be to understand the impact of these combinatorial formulations and how different pre-conditional SCS conditions might be a tool to enhance the feasibility, safety and disease-specific therapeutical potential of SCS as an adjuvant strategy to PD clinical use.
Footnotes
Conflicts of interest: The authors declare no conflict of interest.
Financial support: The authors would like to acknowledge the financial support from Prémios Santa Casa Neurociências Prize Mantero Belard for Neurodegenerative Diseases Research (MB-28-2019). This work was supported by the European Regional Development Fund (FEDER), through the Competitiveness Internationalization Operational Programme (POCI), and by National funds, through the Foundation for Science and Technology (FCT), under the scope of projects UIDB/50026/2020; UIDP/50026/2020 and POCI-01-0145-FEDER-029751. This article has also been developed under the scope of the project NORTE-01-0145-FEDER-000023, supported by the Northern Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER). This work has been funded by ICVS Scientific Microscopy Platform, member of the national infrastructure PPBI - Portuguese Platform of Bioimaging (PPBI-POCI-01-0145-FEDER-022122) (to FGT).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: The authors would like to acknowledge the financial support from Prémios Santa Casa Neurociências Prize Mantero Belard for Neurodegenerative Diseases Research (MB-28-2019). This work was supported by the European Regional Development Fund (FEDER), through the Competitiveness Internationalization Operational Programme (POCI), and by National funds, through the Foundation for Science and Technology (FCT), under the scope of projects UIDB/50026/2020; UIDP/50026/2020 and POCI-01-0145-FEDER-029751. This article has also been developed under the scope of the project NORTE-01-0145-FEDER-000023, supported by the Northern Portugal Regional Operational Programme (NORTE 2020), under the Portugal 2020 Partnership Agreement, through the European Regional Development Fund (FEDER). This work has been funded by ICVS Scientific Microscopy Platform, member of the national infrastructure PPBI - Portuguese Platform of Bioimaging (PPBI-POCI-01-0145-FEDER-022122) (to FGT).
C-Editors: Zhao M, Liu WJ, Qiu Y; T-Editor: Jia Y
References
- 1.Ashpole NM, Hudmon A. Excitotoxic neuroprotection and vulnerability with CaMKII inhibition. Mol Cell Neurosci. 2011;46:720–730. doi: 10.1016/j.mcn.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 2.Berman AE, Chan WY, Brennan AM, Reyes RC, Adler BL, Suh SW, Kauppinen TM, Edling Y, Swanson RA. N-acetylcysteine prevents loss of dopaminergic neurons in the EAAC1−/− mouse. Ann Neurol. 2011;69:509–520. doi: 10.1002/ana.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen CM, Yin MC, Hsu CC, Liu TC. Antioxidative and anti-inflammatory effects of four cysteine-containing agents in striatum of MPTP-treated mice. Nutrition. 2007;23:589–597. doi: 10.1016/j.nut.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Clark J, Clore EL, Zheng K, Adame A, Masliah E, Simon DK. Oral N-acetyl-cysteine attenuates loss of dopaminergic terminals in α-synuclein overexpressing mice. PLoS One. 2010;5:e12333. doi: 10.1371/journal.pone.0012333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.GBD 2016 Parkinson's Disease Collaborators (2018) Global, regional, and national burden of Parkinson's disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 17:939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmay MJ, Terpstra M, Coles LD, Mishra U, Ahlskog M, Öz G, Cloyd JC, Tuite PJ. N-acetylcysteine boosts brain and blood glutathione in Gaucher and Parkinson diseases. Clin Neuropharmacol. 2013;36:103–106. doi: 10.1097/WNF.0b013e31829ae713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maiti P, Manna J, Dunbar GL. Current understanding of the molecular mechanisms in Parkinson's disease: targets for potential treatments. Transl Neurodegener. 2017;6:28. doi: 10.1186/s40035-017-0099-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martínez Banaclocha M. N-acetylcysteine elicited increase in complex i activity in synaptic mitochondria from aged mice: implications for treatment of Parkinson's disease. Brain Res. 2000;859:173–175. doi: 10.1016/s0006-8993(00)02005-9. [DOI] [PubMed] [Google Scholar]
- 9.Martins LF, Costa RO, Pedro JR, Aguiar P, Serra SC, Teixeira FG, Sousa N, Salgado AJ, Almeida RD. Mesenchymal stem cells secretome-induced axonal outgrowth is mediated by BDNF. Sci Rep. 2017;7:4153. doi: 10.1038/s41598-017-03592-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mendes-Pinheiro B, Anjo SI, Manadas B, Da Silva JD, Marote A, Behie LA, Teixeira FG, Salgado AJ. Bone marrow mesenchymal stem cells’ secretome exerts neuroprotective effects in a Parkinson's disease rat model. Front Bioeng Biotechnol. 2019;7:294. doi: 10.3389/fbioe.2019.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mendes-Pinheiro B, Teixeira FG, Anjo SI, Manadas B, Behie LA, Salgado AJ. Secretome of undifferentiated neural progenitor cells induces histological and motor improvements in a rat model of Parkinson's disease. Stem Cells Transl Med. 2018;7:829–838. doi: 10.1002/sctm.18-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monti DA, Zabrecky G, Kremens D, Liang TW, Wintering NA, Cai J, Wei X, Bazzan AJ, Zhong L, Bowen B, Intenzo CM, Iacovitti L, Newberg AB. N-acetyl cysteine may support dopamine neurons in Parkinson's disease: preliminary clinical and cell line data. PLoS One. 2016;11:e0157602. doi: 10.1371/journal.pone.0157602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monti DA, Zabrecky G, Kremens D, Liang TW, Wintering NA, Bazzan AJ, Zhong L, Bowens BK, Chervoneva I, Intenzo C, Newberg AB. N-acetyl cysteine is associated with dopaminergic improvement in Parkinson's disease. Clin Pharmacol Ther. 2019;106:884–890. doi: 10.1002/cpt.1548. [DOI] [PubMed] [Google Scholar]
- 14.Shieh P, Jan CR, Liang WZ. The protective effects of the antioxidant N-acetylcysteine (NAC) against oxidative stress-associated apoptosis evoked by the organophosphorus insecticide malathion in normal human astrocytes. Toxicology. 2019;417:1–14. doi: 10.1016/j.tox.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Teixeira FG, Gago MF, Marques P, Moreira PS, Magalhães R, Sousa N, Salgado AJ. Safinamide: a new hope for Parkinson's disease? Drug Discov Today. 2018;23:736–744. doi: 10.1016/j.drudis.2018.01.033. [DOI] [PubMed] [Google Scholar]
- 16.Teixeira FG, Carvalho MM, Panchalingam KM, Rodrigues AJ, Mendes-Pinheiro B, Anjo S, Manadas B, Behie LA, Sousa N, Salgado AJ. Impact of the secretome of human mesenchymal stem cells on brain structure and animal behavior in a rat model of Parkinson's disease. Stem Cells Transl Med. 2017;6:634–646. doi: 10.5966/sctm.2016-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teixeira FG, Vilaça-Faria H, Domingues AV, Campos J, Salgado AJ. Preclinical comparison of stem cells secretome and levodopa application in a 6-hydroxydopamine rat model of Parkinson's disease. Cells. 2020;9:315. doi: 10.3390/cells9020315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Teixeira FG, Salgado AJ. Mesenchymal stem cells secretome: current trends and future challenges. Neural Regen Res. 2020;15:75. doi: 10.4103/1673-5374.264455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willis CM, Nicaise AM, Peruzzotti-Jametti L, Pluchino S. The neural stem cell secretome and its role in brain repair. Brain Res. 2020a;1729:146615. doi: 10.1016/j.brainres.2019.146615. [DOI] [PubMed] [Google Scholar]
- 20.Willis CM, Nicaise AM, Hamel R, Pappa V, Peruzzotti-Jametti L, Pluchino S. Harnessing the neural stem cell secretome for regenerative neuroimmunology. Front Cell Neurosci. 2020b;14:590960. doi: 10.3389/fncel.2020.590960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou J, Terluk MR, Basso L, Mishra UR, Orchard PJ, Cloyd JC, Schröder H, Kartha RV. N-acetylcysteine provides cytoprotection in murine oligodendrocytes through heme oxygenase-1 activity. Biomedicines. 2020;8:240. doi: 10.3390/biomedicines8080240. [DOI] [PMC free article] [PubMed] [Google Scholar]