Neurons of the mature central nervous system (CNS, mainly the brain and spinal cord) are unable to regenerate spontaneously after a lesion, in contrast to neurons of the peripheral nervous system (PNS). While the extraneuronal environment was long thought to be limiting, evidence was given less than 15 years ago that neurons themselves are critical players of their own regeneration (Park et al., 2008). Indeed, CNS neurons show a decline of axon growth capacity as they mature and after an injury. Today, the role of axonal translation is actively explored in the paradigm of embryonic neuronal growth and in peripheral nerve injury and regeneration, but less is known about the role of local protein synthesis in regrowth of adult CNS axons. Here we discuss how the current understanding of axonal translation in the CNS may contribute to the development of novel strategies to enhance axon regeneration in the injured CNS.
Evidence for local translation in adult CNS axons: In mammals, new protein synthesis events have been robustly detected in embryonic axons and in adult axons of the PNS (Jung et al., 2012). In addition, the remarkable regeneration potential of injured PNS neurons is partly dependent on local protein synthesis events. Indeed, inhibiting new protein synthesis in the axon impairs the structure of the growth cone, necessary for axon regeneration (Verma et al., 2005). On the other hand, most results linking axonal translation and axon regeneration in the injured adult CNS are inferred from in vitro studies or extrapolation from elegant demonstrations in embryonic neurons. The limited regeneration of lesioned CNS axons renders technically difficult to detect mRNA transcripts, the translational machinery and the new protein synthesis events in this compartment. In addition, due to the limited sensitivity of proteomics techniques, the collection of sufficient starting amount of material remains challenging to determine the protein content of the axonal compartment.
Impaired regeneration is linked to decreased translation detected at the soma level. In addition, robust axon regeneration in the mature CNS has been obtained by activation of mTOR signaling pathway, an important regulator of protein synthesis (Park et al., 2008). Enhancement of global translation in the soma may have a repercussion on local translation in the axon, but this remains to be demonstrated. Alternatively, local translation of specific mRNA in the axon may be regulated independently of global enhancement of translation. Therefore, decoding the spatio-temporal regulation of the local proteome in injured CNS axons may be key to obtain axon regeneration.
In intact CNS axons, compartmentalized translation occurs at synapses, which accounts for a rapid turnover of neurotransmitters and receptors necessary for efficient signal conduction. Although the existence of local translation in mature axons has long been debated, multiple studies have recently brought robust evidence for such events in the adult CNS. In particular, the detection and characterization of the adult local translatome–i.e., ribosome-bound mRNA–has been achieved through translating ribosome affinity purification in the superior colliculus, a distal primary target of retina ganglion cells (Shigeoka et al., 2016). This study highlighted that the axonal translatome is dynamically regulated during development, whereby specific subsets of mRNA are primed for ribosome-binding and translation at key steps of circuit establishment, e.g., axon elongation, synaptogenesis, circuit refinement and eventually synaptic activity and axon survival in adulthood (Shigeoka et al., 2016). Furthermore, compelling evidence for the presence of the translation machinery in axons of the mature brain has been provided by the use of electron microscopy and super-resolution fluorescence microscopy. Ribosomes and translation initiation factors, such as eIF4E, eIF4G1 and eIF2α, are found in cortical axons of the rat lateral amygdala (Ostroff et al., 2019). Components of the translation machinery (rRNA) as well as mRNA are robustly found in both pre- and post-synaptic comparments of neurons of several adult brain regions (Hafner et al., 2019). Importantly, metabolic labeling conjugated with high-resolution microscopy led to the extensive detection of new protein synthesis events in pre- and post-synaptic compartments (Hafner et al., 2019).
Are local translation events absent in lesioned adult CNS axons, and if not are they efficient to trigger a regeneration response? After injury, adult CNS axons may fail to initiate a regenerative response due to decreased dynamics of local translation, e.g., smaller number of axonal transcripts, absence of functional ribosomes in the axon, or absence of critical co-factors and efficient translational machinery in the axon. How the presence and functionality of ribosomes in CNS axons are regulated remains an unanswered question in the field. Once assembled in the nucleolus, ribosomes may be actively transported to the tip of the axon and may hold there until required. High-resolution microscopy techniques allow the detection of ribosomal proteins, rRNA and assembled ribosomes in individual presynaptic compartments of mature forebrain, cerebellum and hippocampus (Younts et al., 2016; Hafner et al., 2019). Furthermore, the presence of mRNA coding for ribosomal proteins as well as recent evidence for their local translation and assembly into pre-existing ribosomes brings up the possibility for a local remodeling of functional ribosomes (Shigeoka et al., 2019). In the context of CNS axon injury, axonal localization of the translational machinery–whether by efficient transport or local remodeling – may be absent and account for defective axon regeneration.
Another idea is that the local translation response in injured CNS axons is present but ineffective to trigger a regrowth programme, for example because of the absence of pro-regenerative or pro-survival mRNA species in the axon. Which steps of local translation could actually be responsible for impaired regeneration of adult CNS axons? Efficient sorting of mRNA to the axon shaft/growth cone is done by active anterograde transport of RNA granules addressing selected messengers to the axon thanks to specific 3’-UTR localization motifs. One can hypothesize either impaired mRNA transport or incorrect addressing of regeneration-associated mRNA to the axon. Consistent with these hypotheses, low levels of the RNA-binding protein zip-code binding protein 1 affects axonal transport and local translation of mRNA normally required for regeneration in dorsal root ganglion axons (Donnelly et al., 2011). In addition, local translation in CNS neurons may be impaired due to mRNA retention in stress granules. In axotomized dorsal root ganglion axons, axon regrowth is triggered by the disassembly of the stress granule protein G3BP1 that normally blocks translation of axonally localized mRNA (Sahoo et al., 2018). If and how these features are integrated in the lesioned CNS axons remains to be determined. Altogether, the elucidation of intra-axonal mRNA trafficking and sorting, as well as local presence of a functional translation machinery is critical to understand the role of local translation in inducing a regrowth programme in adult CNS neurons.
Why would local translation be important in the context of axon regeneration?
Initiation of a regenerative programme: Several limiting steps are critical to achieve regeneration of adult CNS neurons, including retrograde signaling of injury signals, integration of these signals, growth cone formation and axon elongation. Interestingly, these steps rely on effective and dynamic local translation events in the axon (Jung et al., 2012; Figure 1). During circuit formation, axon elongation is sustained by anterograde transport of biomaterial (proteins, lipids) and organelles. In addition, embryonic axons possess the capacity to synthesize this material, providing an immediate, local source of material for cue-induced axon elongation. It is conceivable that producing biomaterial locally requires less energy than transporting it from the soma to the tip of the axon. Two possibilities arise here: defective material sourcing that prevents adult CNS axon elongation is due (i) to decreased axonal transport observed after injury; and/or (ii) to impaired local production of the material necessary to form a new axon. While the former has been demonstrated for organelles such as mitochondria, the latter remains to be tested in an injured CNS system.
Figure 1.
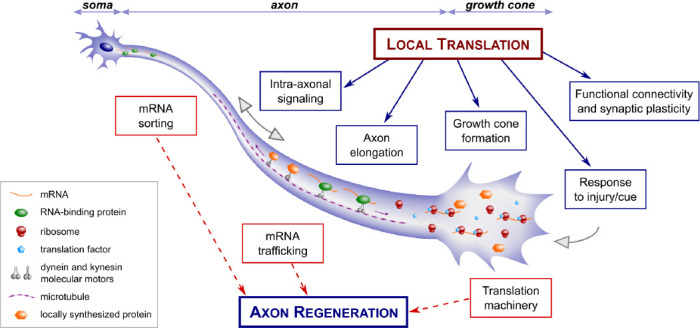
Role of local protein synthesis on the initiation of a regenerative programme in adult axons.
The initiation of a regenerative programme requires local protein synthesis in the axon and growth cone, as does axon elongation, axonal transport and growth cone steering. Future studies on central nervous system axon regeneration could focus on determining: the presence and identity of mRNA transcripts in the axon; the presence of the translation machinery in the axon, including ribosomes and co-factors; whether amino acids are incorporated locally via techniques such as puromycin incorporation and labeling; whether newly synthesized proteins undergo post-translational modifications locally.
In the injured PNS, axon regeneration relies on local synthesis of injury signals retrogradely transported and integrated at the soma level, and on local translation of pro-growth mRNA transported in the axon such as Gap-43 and β-actin (Donnelly et al., 2011). Interestingly, several regeneration-associated mRNA, including Gap-43 and β-actin, are detected in injured CNS spinal axons regenerating into a peripheral nerve graft, in a proportionally higher number of axons than in naive (uninjured) PNS axons (Kalinski et al., 2015). Moreover, several elements of the translation machinery are present in axons regenerating in the peripheral nerve graft. This suggests that, when presented with a growth-favorable environment, CNS axons have the tools to integrate injury signals, which in turn induces mRNA axonal transport and translation in order to initiate/sustain a regenerative programme.
Circuit formation: Despite the successful development of long-distance CNS axon regeneration models via modulation of pro-growth pathways, regenerating axons display strong guidance defects, suggesting that they are unable to respond to the environment or that the environment does not pave the correct path to appropriate targets. Axon guidance is a growth cone-autonomous mechanism largely dependent on efficient local protein synthesis. In particular, spatial polarization of mRNA translation induces steering (Jung et al., 2012). Importantly, integration of guidance signals has been shown to directly regulate translation dynamics at the local level. In this context, adaptation of local translation mechanisms may result in proper guidance of these axons, e.g., by sorting correct mRNA cargoes or by translating specific mRNA subsets involved in the response to guidance cues.
Synapse formation and synaptic activity critically rely on fine spatio-temporal regulation of the local proteome. Indeed, the local translatome is regulated during all phases of circuit formation during development, including synapse formation and strengthening (Shigeoka et al., 2016). In the intact adult CNS, synapse strengthening requires dynamic local translation in both pre- and post-synaptic compartments, with different forms of plasticity driven by specific local translation events (Hafner et al., 2019). In addition, presynaptic translation is critical for long-term plasticity in the hippocampus (Younts et al., 2016), and local translation of specific subsets of mRNA is differentially regulated during the learning process in cortical axons projecting to the amygdala (Ostroff et al., 2019). Interestingly, in vivo and in vitro observations show that synapse formation during development is accompanied by a local decrease of axonally-localized ribosomes (Costa et al., 2019). This decrease may correlate with a reduction of the axonal translatome during maturation (Shigeoka et al., 2016). Yet, notably, the axonal localization of both ribosomes and mRNA is not totally suppressed and a local translation activity is maintained during adulthood. This supports a need for adaptation of the local proteome at diverse stages of maturation and functioning. As CNS axons mature, the regulation of axonally-localized ribosomes correlates with loss of regrowth capacity, and may possibly underlie the changes in mRNA translation specificity at different stages. Therefore, as functional connectivity is the endpoint of axon regeneration, it is necessary to understand the dynamics of the translational machinery and the translated mRNA repertoire to reform a functional circuit.
Conclusions: Following injury, mature CNS axons fail to regenerate spontaneously, particularly because of the growth-inhibitory nature of the environment and decreased intrinsic growth capabilities. Lessons from the development reveal that cue-induced modifications of the local proteome are necessary for the rapid growth and steering response of the axon. In addition, the differential regenerative potential of PNS versus CNS neurons in adult correlates with the dynamics in local protein synthesis. Therefore, integration of an active local translation programme may be required to counterbalance the energetic cost of axonal transport of biomaterial in order to obtain axon regeneration.
Studies in the PNS have revealed that several key steps of axon regeneration critically depend on efficient local translation. Moreover, axonal protein synthesis occurs in the intact mature CNS and is regulated spatio-temporally for circuit connectivity and plasticity. Today, increasing sensitivity of high-throughput omics technologies, combined with increasing imaging resolution and powerful ex vivo and in vivo models of CNS regeneration, renders possible the characterization of the local translation response in injured CNS axons. Future therapeutic strategies will have to focus on local regeneration reservoirs, i.e., efficient sorting of pro-growth mRNA cargoes in the axon, and presence and functionality of the translation machinery, including ribosomes.
We apologize to authors whose work we did not reference in this perspective due to space limitations. We thank Homaira Nawabi, Noemie Vilallongue and Charlotte Decourt Université Grenoble Alpes, Grenoble Institut Neurosciences, Grenoble, France for useful comments on our manuscript.
JS was supported by a post-doctoral fellowship from Fondation pour la Recherche Médicale (FRM) (SPF201909009106). SB was supported by Agence Nationale pour la Recherche (ANR) (ANR-18-CE16-0007).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Zhao LJ, Li JY; T-Editor: Jia Y
References
- 1.Costa RO, Martins H, Martins LF, Cwetsch AW, Mele M, Pedro JR, Tomé D, Jeon NL, Cancedda L, Jaffrey SR, Almeida RD. Synaptogenesis stimulates a proteasome-mediated ribosome reduction in axons. Cell Rep. 2019;28:864–876. doi: 10.1016/j.celrep.2019.06.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donnelly CJ, Willis DE, Xu M, Tep C, Jiang C, Yoo S, Schanen NC, Kirn-Safran CB, van Minnen J, English A, Yoon SO, Bassell GJ, Twiss JL. Limited availability of ZBP1 restricts axonal mRNA localization and nerve regeneration capacity. EMBO J. 2011;30:4665–4677. doi: 10.1038/emboj.2011.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hafner AS, Donlin-Asp PG, Leitch B, Herzog E, Schuman EM. Local protein synthesis is a ubiquitous feature of neuronal pre- and postsynaptic compartments. Science. 2019;364:eaau3644. doi: 10.1126/science.aau3644. [DOI] [PubMed] [Google Scholar]
- 4.Jung H, Yoon BC, Holt CE. Axonal mRNA localization and local protein synthesis in nervous system assembly, maintenance and repair. Nat Rev Neurosci. 2012;13:308–324. doi: 10.1038/nrn3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalinski AL, Sachdeva R, Gomes C, Lee SJ, Shah Z, Houle JD, Twiss JL. mRNAs and protein synthetic machinery localize into regenerating spinal cord axons when they are provided a substrate that supports growth. J Neurosci. 2015;35:10357–10370. doi: 10.1523/JNEUROSCI.1249-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostroff LE, Santini E, Sears R, Deane Z, Kanadia RN, LeDoux JE, Lhakhang T, Tsirigos A, Heguy A, Klann E. Axon TRAP reveals learning-associated alterations in cortical axonal mRNAs in the lateral amygdala. ELife. 2019;8:e51607. doi: 10.7554/eLife.51607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science. 2008;322:963–966. doi: 10.1126/science.1161566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahoo PK, Lee SJ, Jaiswal PB, Alber S, Kar AN, Miller-Randolph S, Taylor EE, Smith T, Singh B, Ho TS, Urisman A, Chand S, Pena EA, Burlingame AL, Woolf CJ, Fainzilber M, English AW, Twiss JL. Axonal G3BP1 stress granule protein limits axonal mRNA translation and nerve regeneration. Nat Commun. 2018;9:3358. doi: 10.1038/s41467-018-05647-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shigeoka T, Jung H, Jung J, Turner-Bridger B, Ohk J, Lin JQ, Amieux PS, Holt CE. Dynamic axonal translation in developing and mature visual circuits. Cell. 2016;166:181–192. doi: 10.1016/j.cell.2016.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shigeoka T, Koppers M, Wong HH, Lin JQ, Cagnetta R, Dwivedy A, de Freitas Nascimento J, van Tartwijk FW, Ströhl F, Cioni JM, Schaeffer J, Carrington M, Kaminski CF, Jung H, Harris WA, Holt CE. On-site ribosome remodeling by locally synthesized ribosomal proteins in axons. Cell Rep. 2019;29:3605–3619. doi: 10.1016/j.celrep.2019.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Verma P, Chierzi S, Codd AM, Campbell DS, Meyer RL, Holt CE, Fawcett JW. Axonal protein synthesis and degradation are necessary for efficient growth cone regeneration. J Neurosci. 2005;25:331–342. doi: 10.1523/JNEUROSCI.3073-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Younts TJ, Monday HR, Dudok B, Klein ME, Jordan BA, Katona I, Castillo PE. Presynaptic protein synthesis is required for long-term plasticity of GABA release. Neuron. 2016;92:479–492. doi: 10.1016/j.neuron.2016.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]


