Abstract
The main advantage of diffusion tensor tractography is that it allows the entire neural tract to be evaluated. In addition, configurational analysis of reconstructed neural tracts can indicate abnormalities such as tearing, narrowing, or discontinuations, which have been used to identify axonal injury of neural tracts in concussion patients. This review focuses on the characteristic features of axonal injury in concussion or mild traumatic brain injury (mTBI) patients through the use of diffusion tensor tractography. Axonal injury in concussion (mTBI) patients is characterized by their occurrence in long neural tracts and multiple injuries, and these characteristics are common in patients with diffuse axonal injury and in concussion (mTBI) patients with axonal injury. However, the discontinuation of the corticospinal tract is mostly observed in diffuse axonal injury, and partial tearing and narrowing in the subcortical white matter are frequently observed in concussion (mTBI) patients with axonal injury. This difference appears to be attributed to the observation that axonal injury in concussion (mTBI) patients is the result of weaker forces than those producing diffuse axonal injuries. In addition, regarding the fornix, in diffuse axonal injury, discontinuation of the fornical crus has been frequently reported, but in concussion (mTBI) patients, many collateral branches form in the fornix in addition to these findings in many case studies. It is presumed that the impact on the brain in TBI is relatively weaker than that in diffuse axonal injury, and that the formation of collateral branches occurs during the fornix recovery process. Although the occurrence of axonal injury in multiple areas of the brain is an important feature of diffuse axonal injury, case studies in concussion (mTBI) have shown that axonal injury occurs in multiple neural tracts. Because axonal injury lesions in mTBI patients may persist for approximately 10 years after injury onset, the characteristics of axonal injury in concussion (mTBI) patients, which are reviewed and categorized in this review, are expected to serve as useful supplementary information in the diagnosis of axonal injury in concussion (mTBI) patients.
Key Words: axonal injury, brain injury, concussion, diffusion tensor imaging, diffusion tensor tractography, mild traumatic brain injury, neural tract
Introduction
Concussion is defined as a transient reversible neurologic dysfunction resulting from external mechanical force transmitted to the brain through a physical impact to the head (Hill et al., 2016; Jang, 2018) (Table 1). In other words, it refers to the transient changes in the neurological function of the brain resulting from an acute head trauma that does not involve any organic brain syndromes and is not accompanied by any signs of lesions on conventional brain magnetic resonance imaging (MRI) (Anderson et al., 2006). Head trauma is classified as a concussion if loss of consciousness following the trauma lasts less than 6 hours, and it is classified as diffuse axonal injury if loss of consciousness lasts 6 hours or more (Gennarelli, 1993). A mild traumatic brain injury (mTBI) refers to a traumatic brain injury with loss of consciousness for less than 30 minutes, and because of their similar definitions, the mTBI and concussion terms are often used interchangeably (Barth, 2006). However, there is a lack of clear evidence supporting the use of the 6-hour loss of consciousness criterion used to distinguish between concussion and diffuse axonal injury, leading to the problem that lesions indicative of diffuse axonal injury are detected in concussion cases (Gennarelli et al., 1982; Mittl et al., 1994; Topal et al., 2008). Axonal injury should not be detected in concussion cases, but in an experiment using monkeys, nearly half (46.7%) of the animals that lost consciousness for less than 6 hours after concussion were shown, upon pathologic examination, to have lesions indicative of axonal injury (Gennarelli et al., 1982). Axonal injury has been shown to be detected by conventional MRI in 12.5% to 30% of concussion patients (Mittl et al., 1994; Topal et al., 2008).
Table 1.
Classification of traumatic brain injuries
| Patho-anatomy | |||
|---|---|---|---|
|
| |||
| Diffuse | Focal | ||
| Concussion (< 6 h) | Contusion (≥ 6 h) | ||
| Traumatic axonal injury/diffuse axonal injury | Penetrating | ||
| Explosion | Hematoma | ||
| Abusive head trauma | - Epidural | ||
| - Subarachnoid | |||
| - Subdural | |||
| - Intraventricular | |||
| - Intracerebral | |||
|
| |||
| Severity of head trauma | |||
|
| |||
| LOC | PTA | GCS | |
|
| |||
| Mild | ≤ 30 min | ≤ 24 h | 13–15 |
| Moderate | > 30 min, ≤ 24 h | > 24 h, ≤ 7 d | 9–12 |
| Severe | > 24 h | > 7 d | 3–8 |
The data are sourced from Hill et al. (2016) and Jang (2018). GCS: Glasgow Coma Scale; LOC: loss of consciousness; PTA: post-traumatic amnesia.
In concussion cases, the absence of neural injury has been determined mainly based on observing no abnormal results on conventional brain MRI. There are at least about 10 billion brain cells in the human brain, and conventional brain MRI consists of about 300,000 voxels. A voxel is a term referring to a point on a three-dimensional plane. Based on the above-mentioned values, one brain MRI voxel represents the state of about 30,000 neurons. At that resolution, it is difficult to accurately reflect the state of any brain cell damage; thus, normal findings on brain MRI may not indicate that the brain has a normal state (Jang, 2020). Due to the resolution limitation of conventional MRI, and because 80% of diffuse axonal injury lesions are non-hemorrhagic lesions or microscopic lesions that can be identified only by pathological examination, it is sometimes suggested that the lesions in diffuse axonal injury that can be detected by conventional brain MRI in patients with diffuse axonal injury are only the tip of the iceberg (Adams et al., 1982; Gentry et al., 1988). It is also known that brain lesions associated with many brain diseases cannot be detected by conventional MRI (Wang et al., 2008; Shenton et al., 2012; Jang, 2018; Yu et al., 2019).
In contrast, diffusion tensor imaging (DTI), which was developed in the 1990s, provides valuable information on the subcortical white matter that cannot be obtained from conventional MRI (Basser et al., 1994). DTI has a unique advantage in the identification of microstructural white matter abnormalities, such as axonal injuries, in traumatic brain injury, anomalies that are not usually detectable on conventional brain MRI (Basser et al., 1994). In this respect, the development of DTI opened a new era in the research on the subcortical white matter of the live human brain (Basser et al., 1994). In addition, diffusion tensor tractography (DTT), which reconstructs the neural tracts of the brain three-dimensionally from the DTI data, enables the visualization and evaluation of neural tracts in the live human brain for the first time in human history, thereby leading to breakthroughs in neuroscience (Mori et al., 1999). Thus, diffuse tensor imaging enables the diagnosis of lesions of various neurological diseases, such as axonal injury in concussion (mTBI), stroke, hypoxic-ischemic brain injury, cerebral palsy, and multiple sclerosis, lesions that cannot be detected by conventional MRI (Wang et al., 2008; Shenton et al., 2012; Jang, 2018; Yu et al., 2018).
Since the 1960s, axonal injuries have been reported in patients with concussion (mTBI) through autopsy studies conducted after the patients died from other diseases without any lesions observed on conventional brain computed tomography or MRI. Organic brain injuries have also been demonstrated by a variety of other tests, such as the nuclear medicine and neurophysiologic tests and electroencephalography (Blumbergs et al., 1994; Major et al., 2015; Bonfante et al., 2018; Raji and Henderson, 2018). With respect to DTI, cerebral white matter injury and axonal injury in concussion (mTBI) have been reported in hundreds of articles published in international journals since 2002, and dozens of studies have demonstrated axon injuries in concussion (mTBI) patients by applying DTT (Shenton et al., 2012; Yallampalli et al., 2013; D’Souza et al., 2015; Kim et al., 2015a; Lee and Jang, 2015; Jang and Kim, 2016; Jang and Lee, 2017a, b; Jang, 2018). In the above studies, two DTI-based analytical methods have been used to detect axonal injury in concussion (mTBI): 1) measurement of DTI parameters such as fractional anisotropy and mean diffusivity in specific regions of interest of the brain, and 2) DTT assessment of neural tracts, assessment of DTT parameters such as fractional anisotropy, mean diffusivity, and tract volume, and configurational analysis of reconstructed tracts (Jang, 2018). The main advantage of DTT over DTI is that it allows the entire neural tract to be evaluated based on a variety of DTT parameter measurements. In addition, configurational analysis of reconstructed neural tracts can indicate abnormalities such as tearing, narrowing, or discontinuations, which have been used to identify axonal injury of neural tracts in concussion (mTBI) patients (Jang, 2018). Although many of the characteristics of axonal injury in concussion (mTBI) observed on DTT have not yet been fully elucidated, and much research is still needed, several characteristic features have been reported. In this review, we selected relevant studies according to the process presented in the flow diagram in Figure 1.
Figure 1.
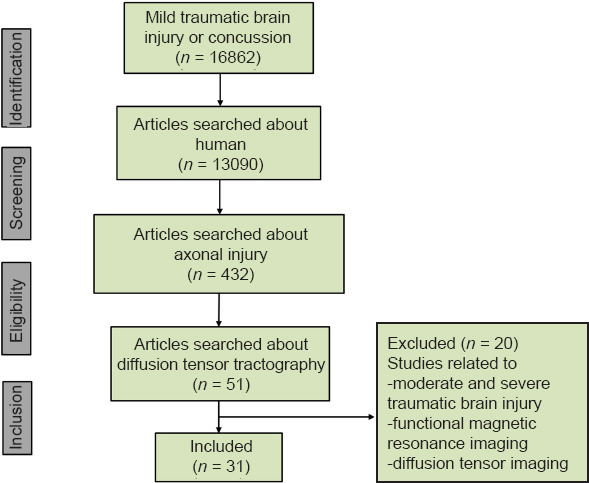
Flow diagram of the process of selecting studies for review.
In this review, we reviewed the characteristic features of axonal injury in concussion (mTBI) from previous studies that have used DTT to demonstrate axonal injury in concussion (mTBI) patients.
Database Search Strategy
In this review, DTI studies that have demonstrated characteristics of axonal injury in concussion and mild traumatic brain injury are reviewed. Relevant studies published during 2009 to 2020 were identified by searching the following electronic databases: PubMed, Google Scholar, and MEDLINE. The following key words and abbreviations were used to search the databases: DTI, DTT, mTBI, concussion, axonal injury. This review was limited to studies of humans. We selected relevant studies according to the flow diagram shown in Figure 2.
Figure 2.
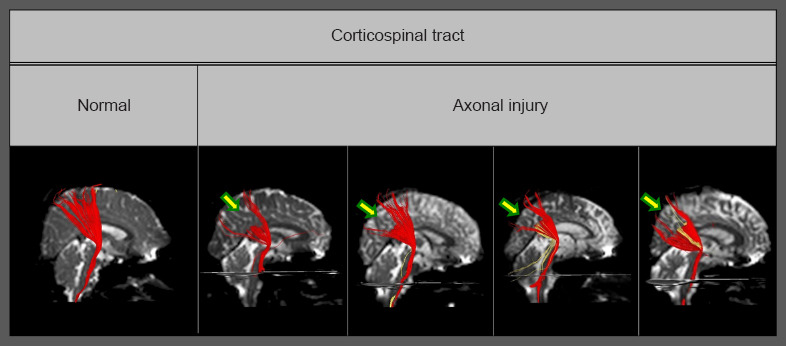
Diffusion tensor tractography-reconstructed corticospinal tracts.
Compared with the normal corticospinal tract, partial tears (arrows) are observed in the subcortical white matter of corticospinal tracts with traumatic axonal injury. Reprinted with permission from Jang and Kim (2016).
Classification of the Characteristic Features of Axonal Injury in Concussion or Mild Traumatic Brain Injury
In this review, the patterns of axonal injury in concussion (mTBI) identified by using DTT (Shenton et al., 2012; Yallampalli et al., 2013; D’Souza et al., 2015; Kim et al., 2015a; Lee and Jang, 2015; Jang and Kim, 2016; Jang and Lee, 2017a, b) were classified into three categories (Table 2): (1) injury of long neural tracts, such as the corticospinal tract, spinothalamic tract, corticoreticulospinal tract, corticofugal pathway, corticobulbar tract, corticopontocerebellar tract, dentatorubrothalamic tract, and neural tracts that run along an anterior-posterior pathway, such as the cingulum and arcuate fasciculus; (2) collateral branches of the fornix; and (3) multiple injuries of various neural tracts, including the corticospinal tract, spinothalamic tract, corticoreticulospinal tract, cingulum, dentatorubrothalamic tract, prefrontothalamic tract, prefrontocaudate tract, and fornix. The parameters used for DTI scanning and the analysis methods for DTT differ among different study institutions. However, because each study provided control data obtained using study-consistent scanning parameters and analytic methods, the importance of these differences is reduced.
Table 2.
Classification of the characteristic features of axonal injuries in concussion (mild traumatic brain injury)
| Classification | Neural tracts |
|---|---|
| Injury of long neural tracts | Corticospinal tract |
| Medial lemniscus | |
| Spinothalamic tract | |
| Corticoreticulospinal tract | |
| Corticofugal tract | |
| Corticobulbar tract | |
| Corticopontocerebellar tract | |
| Dentatorubrothalamic tract | |
| Collateral branches of the fornix | Discontinuation of the fornical crus |
| Discontinuation of the fornical column | |
| Collateral branches of the fornix | |
| Multiple injuries of various neural tracts | Spinothalamic tract |
| Corticoreticulospinal tract | |
| Corticobulbar tract | |
| Dentatorubrothalamic tract | |
| Fornix | |
| Cingulum | |
| Prefrontothalamic tract | |
| Prefrontocaudate tract |
Injury of long neural tracts
Previous studies have reported that diffuse axonal injury frequently occurs in long neural tracts such as the corticospinal tract and the medial lemniscus (Maxwell et al., 1997; Meythaler et al., 2001). In concussion (mTBI), axonal injury has been shown to occur in the long neural tracts running vertically in the brain, such as the corticospinal tract, spinothalamic tract, corticoreticulospinal tract, corticofugal pathway, corticobulbar tract, corticopontocerebellar tract, and dentatorubrothalamic tract (Lee and Jang, 2015; Jang and Kim, 2016). In addition, axonal injury has also been reported in the neural tracts running along the anterior-posterior pathways such as the cingulum and arcuate fasciculus (D’Souza M et al., 2015; Kim et al., 2015a).
In patients with diffuse axonal injury, previous studies on corticospinal tract injuries have reported that discontinuation is a characteristic feature frequently observed in various parts of the brain (Jang et al., 2009b). However, a study of mTBI patients published in 2016 examined the degree of damage for each type of corticospinal tract injury and reported that partial tearing in the subcortical white matter was observed in 80% of the 35 patients with corticospinal tract lesions out of 98 mTBI patients, and narrowing was detected in 20% of the patients (Figure 2) (Jang and Kim, 2016). Other case studies have also reported partial tearing and narrowing in the subcortical white matter in the corticospinal tract of mTBI patients (Seo and Jang, 2015). With regard to axonal injury in the spinothalamic tract, observations of partial tearing, narrowing, and discontinuation in the subcortical white matter in the spinothalamic tract have been reported. Studies of the corticoreticulospinal tract also reported detection of partial tearing, narrowing, and discontinuation in the subcortical white matter. In this regard, according to a report by Lee and Jang (2015) on patients with proximal muscle weakness, the rate of discontinuation in the subcortical white matter was the highest and the rate of partial tearing was lowest among the injury patterns of the corticoreticulospinal tract: discontinuation in 51.7% of the patients, narrowing in 22.4%, and partial tearing in the subcortical white matter in 15.5% of the patients. In addition, case studies have shown the presence of axonal injury in the corticofugal pathway, corticobulbar tract, corticopontocerebellar tract, and dentatorubrothalamic tract, and although partial tearing and narrowing were observed in each of those cases, discontinuation was characteristically observed in the neural tracts that run along right-left pathways in the brain, such as the corticopontocerebellar tract and the dentatorubrothalamic tract.
In concussion (mTBI) patients, it has been reported that neural structures show more damage in the anterior portion than in the posterior part (Cubon et al., 2018). Regarding the cingulum, Jang et al. (2013) conducted a study of the injury of the cingulum in 21 diffuse axonal injury patients with cognitive impairment and reported that the most severe nerve damage occurred in the anterior cingulum located in the frontal lobe. Likewise, the major common injury feature appearing in other case reports was discontinuation in the anterior portion of the cingulum in concussion (mTBI) patients (Figure 3) (Kim et al., 2015a). Damage to the arcuate fasciculus of the dominant hemisphere was observed in 39% of patients, with the identified characteristic damage patterns being narrowing and discontinuation, and all of the cases of discontinuation involved injury of the anterior portion of the arcuate fasciculus that is connected to Broca's area.
Figure 3.
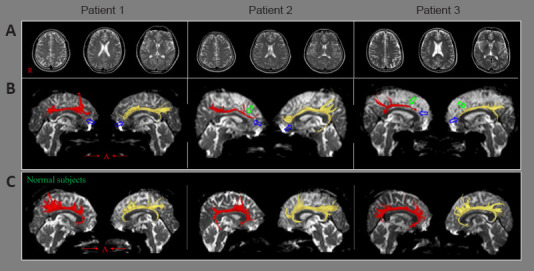
Results of brain magnetic resonance imaging and diffusion tensor tractography in patients injured by a motor vehicle accident.
(A) No lesion was detected by conventional brain magnetic resonance imaging. (B) Results of diffusion tensor tractography of the cingulum in patients with mild traumatic brain injury. Bilateral discontinuations are detected between the anterior cingulum and the basal forebrain in all three patients (blue arrows), and tract narrowing was observed in three of the cingula (green arrows). (C) Diffusion tensor tractography for the cingulum in three normal subjects. Reprinted with permission from Kim et al. (2015a).
Collateral branches of the fornix
A previous study of the fornix in diffuse axonal injury has reported that the fornix injury pattern is characterized by discontinuation of the fornical crus (Jang et al., 2009a). In mTBI cases, Jang and Lee (2017b) reported that discontinuation of the fornical crus or fornical column was observed in 86 patients with memory loss following mTBI, and an additional distinctive injury pattern observed only in the fornix was the formation of collateral branches of the fornix following injury (Figure 4). These collateral branches form a recovery or compensation mechanism following fornix injury, providing a connection to the medial temporal lobe, which is the normal fornix pathway, by extending nerve branches along various pathways from the damaged fornical crus, fornical body, and fornical column (Figure 5). In TBI, the impact on the brain might be relatively weaker than that in diffuse axonal injury, and it is suggested that, while the partially damaged fornix is recovering, collateral branches going toward the ipsi-lesional medial temporal lobe or contra-lesional medial temporal lobe may be formed (Jang and Lee, 2017a).
Figure 4.
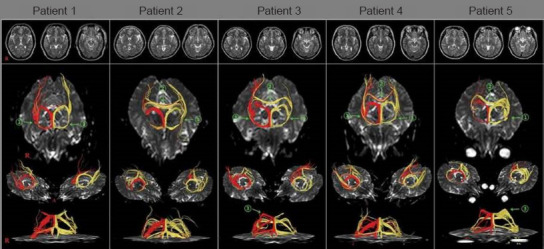
Results of diffusion tensor tractography of the fornix (right – red, left – yellow) of patients with mild traumatic brain injury.
Abundant unusual neural tracts (green arrows) are observed. These unusual neural tracts can be classified as follows: (1) the neural branch originating from the fornical column to the ipsilateral medial temporal lobe, (2) the neural branch originating from the fornical column to the contralateral medial temporal lobe via the splenium of the corpus callosum and (3) the neural branch originating from the fornical body to the ipsilateral medial temporal lobe via the splenium of the corpus callosum. Reprinted with permission from Jang and Lee (2017b).
Figure 5.
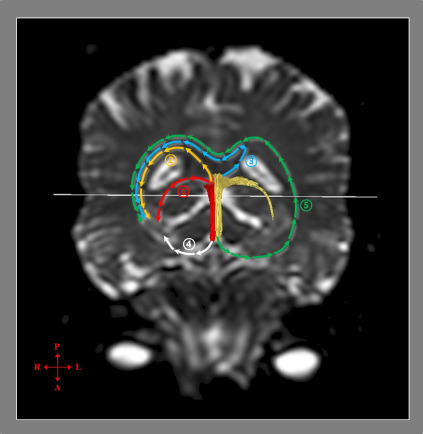
Mechanisms of recovery of injured fornical crus as determined by diffusion tensor tractography.
Mechanism 1: Recovery through the neural tract from the injured fornical crus to the medial temporal lobe via the normal pathway of the fornical crus. Mechanism 2: Recovery through the neural tract originating from an ipsilesional fornical body connected to the ipsilesional medial temporal lobe via the splenium of the corpus callosum. Mechanism 3: Recovery through the neural tract from the ipsilesional fornical body extending to the contralesional medial temporal lobe via the splenium of the corpus callosum. Mechanism 4: Recovery through the neural tract originating from the ipsi-lesional fornical column connected to the ipsi-lesional medial temporal lobe. Mechanism 5: Recovery through the nerve tract originating from the contra-lesional fornical column connected to the ipsi-lesional medial temporal lobe via the contra-lesional medial temporal lobe and the splenium of the corpus callosum. Reprinted with permission from Jang and Lee (2017a).
Multiple injuries of various neural tracts
Diffuse axonal injury is characterized by multiple injuries in various parts of the brain. The subcortical white matter, corpus callosum, and brainstem are most frequently affected, but the thalamus, hypothalamus, basal ganglia, cerebellum, and inner temporal lobe including the hippocampus and parahippocampal gyrus can also be damaged (Meythaler et al., 2001). Analysis of the whole brain by applying a tract-based spatial statistics approach has shown that multiple injuries can occur in overall areas of the brain following mTBI, but since only a few studies have compared the axonal injuries of concussion (mTBI) patients with those of diffuse axonal injury patients, it is difficult to make a straightforward comparison (Xiong et al., 2014). However, several case studies of concussion (mTBI) have reported that multiple injuries can be observed in various neutral tracts. To date, the neural tract where multiple injuries were observed most frequently is the corticospinal tract, followed in descending order by the fornix, spinothalamic tract, corticoreticulospinal tract, cingulum, dentatorubrothalamic tract, prefrontothalamic tract, prefrontocaudate tract, and corticobulbar tract. Many of the above neural tracts are also long neural tracts, which are frequently involved in concussion (mTBI) axonal injury.
Conclusion
In this study, characteristic features of axonal injury in concussion (mTBI) patients were investigated by reviewing previous studies that demonstrated axonal injury in concussion (mTBI) patients through the application of DTT. That review revealed that axonal injury lesions in concussion (mTBI) patients are characterized by their occurrence in long neural tracts and the appearance of multiple injuries, and these characteristics are common to both the axonal injuries of concussion (mTBI) patients and diffuse axonal injury patients. However, concussion (mTBI) patients had less severe injury results than those of diffuse axonal injury patients. In addition, regarding the fornix, in diffuse axonal injury, findings of discontinuation of the fornical crus were frequently reported, but in concussion (mTBI) patients, many collateral branches formed in the fornix following the injury. The above differences appear to be attributed to the reality that axonal injuries in concussion (mTBI) patients are caused by weaker forces than those producing diffuse axonal injuries. Many studies have reported that axonal injury is a consistent feature of all traumatic brain injuries and have shown that both the distribution and number of damaged axons increase with trauma severity from mild to moderate and finally severe TBI, and from concussion to diffuse axonal injury (Povlishock et al., 1992; Wallace et al., 2018). However, the majority of the reviewed studies were case reports. Thus, further studies with large numbers of subjects that compare the characteristics of concussion (mTBI) injuries with those of diffuse axonal injuries need to be conducted. Because axonal injury lesions in mTBI patients are known to persist for about 10 years after the injury, the characteristic features of axonal injury in concussion (mTBI) patients reviewed and categorized in this review are expected to serve as supplementary information useful in the diagnosis of axonal injury in concussion (mTBI) subjects (Eierud et al., 2014; Rajesh et al., 2017). However, DTT abnormalities related to previous head trauma, concurrent neurological disease, aging, or artifacts associated with the DTT procedure should be ruled out. Furthermore, several limitations of DTT should be considered (Parker and Alexander, 2005; Wedeen et al., 2008; Yamada et al., 2009). First, the fiber tracking technique is operator-dependent, although the DTT method is reported to have excellent reliability compared to that of DTI (Seo et al., 2019). Second, DTT may underestimate the finer features of neural fiber tracts. DTT is a powerful anatomic imaging tool that can demonstrate the gross fiber architecture, but it cannot precisely define functional or synaptic connections. Third, regions of fiber complexity and fiber crossing can prevent DTT from fully reflecting the underlying fiber architecture. Therefore, further studies to overcome the above limitations and that employ advanced magnetic resonance-based techniques such as diffusion kurtosis imaging should be undertaken (Gatto et al., 2019a, b; Zheng et al., 2020).
Footnotes
Conflicts of interest: The authors declare that they have no competing interests.
Financial support: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Goverment (No.2018R1A6A3A11050913).
Copyright license agreement: The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean Goverment, No. 2018R1A6A3A11050913 (to YSS).
C-Editor: Zhao M; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Jia Y
References
- 1.Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury in humans: An analysis of 45 cases. Ann Neurol. 1982;12:557–563. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- 2.Anderson T, Heitger M, Macleod AD. Concussion and mild head injury. Pract Neurol. 2006;6:342–357. [Google Scholar]
- 3.Barth JT, Ruff R, Espe-Pfeifer P. Mild Traumatic Brain Injury: Definitions. In: Young G, Nicholson K, Kane AW, editors. Psychological Knowledge in Court. Boston, MA: Springer; 2006. [Google Scholar]
- 4.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344:1055–1056. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- 6.Bonfante E, Riascos R, Arevalo O. Imaging of chronic concussion. Neuroimaging Clin N Am. 2018;28:127–135. doi: 10.1016/j.nic.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 7.Cubon VA, Murugavel M, Holmes KW, Dettwiler A. Preliminary evidence from a prospective DTI study suggests a posterior-to-anterior pattern of recovery in college athletes with sports-related concussion. Brain Behav. 2018;8:e01165. doi: 10.1002/brb3.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Souza M M, Trivedi R, Singh K, Grover H, Choudhury A, Kaur P, Kumar P, Tripathi RP. Traumatic brain injury and the post-concussion syndrome: A diffusion tensor tractography study. Indian J Radiol Imaging. 2015;25:404–414. doi: 10.4103/0971-3026.169445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eierud C, Craddock RC, Fletcher S, Aulakh M, King-Casas B, Kuehl D, LaConte SM. Neuroimaging after mild traumatic brain injury: review and meta-analysis. Neuroimage Clin. 2014;4:283–294. doi: 10.1016/j.nicl.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gatto RG, Ye AQ, Colon-Perez L, Mareci TH, Lysakowski A, Price SD, Brady ST, Karaman M, Morfini G, Magin RL. Detection of axonal degeneration in a mouse model of Huntington's disease: comparison between diffusion tensor imaging and anomalous diffusion metrics. MAGMA. 2019a;32:461–471. doi: 10.1007/s10334-019-00742-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gatto RG, Amin M, Finkielsztein A, Weissmann C, Barrett T, Lamoutte C, Uchitel O, Sumagin R, Mareci TH, Magin RL. Unveiling early cortical and subcortical neuronal degeneration in ALS mice by ultra-high field diffusion MRI. Amyotroph Lateral Scler Frontotemporal Degener. 2019b;20:549–561. doi: 10.1080/21678421.2019.1620285. [DOI] [PubMed] [Google Scholar]
- 12.Gennarelli TA. Cerebral concussion and diffuse brain injuries. Head injury, 3rd Edition: Cooper. 1993 [Google Scholar]
- 13.Gennarelli TA, Thibault LE, Adams JH, Graham DI, Thompson CJ, Marcincin RP. Diffuse axonal injury and traumatic coma in the primate. Ann Neurol. 1982;12:564–574. doi: 10.1002/ana.410120611. [DOI] [PubMed] [Google Scholar]
- 14.Gentry LR, Godersky JC, Thompson B. MR imaging of head trauma: review of the distribution and radiopathologic features of traumatic lesions. AJR Am J Roentgenol. 1988;150:663–672. doi: 10.2214/ajr.150.3.663. [DOI] [PubMed] [Google Scholar]
- 15.Hill CS, Coleman MP, Menon DK. Traumatic axonal injury: mechanisms and translational opportunities. Trends Neurosci. 2016;39:311–324. doi: 10.1016/j.tins.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jang SH. Traumatic axonal injury in mild traumatic brain injury. In: Gorbunoy N, editor. Traumatic brain injury. London: Intech; 2018. pp. 137–154. [Google Scholar]
- 17.Jang SH. Diagnostic problems in diffuse axonal injury. Dignostics. 2020;10:117. doi: 10.3390/diagnostics10020117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jang SH, Kim SY. Injury of the corticospinal tract in patients with mild traumatic brain injury: a diffusion tensor tractography study. J Neurotrauma. 2016;33:1790–1795. doi: 10.1089/neu.2015.4298. [DOI] [PubMed] [Google Scholar]
- 19.Jang SH, Lee HD. Diffusion tensor tractography studies on mechanisms of recovery of injured fornix. Neural Regen Res. 2017a;12:1742–1744. doi: 10.4103/1673-5374.217355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang SH, Lee HD. Abundant unusual neural branches from the fornix in patients with mild traumatic brain injury: a diffusion tensor tractography study. Brain Inj. 2017b;31:1530–1533. doi: 10.1080/02699052.2017.1350997. [DOI] [PubMed] [Google Scholar]
- 21.Jang SH, Kim SH, Kim OL. Fornix injury in a patient with diffuse axonal injury. Arch Neurol. 2009a;66:1424–1425. doi: 10.1001/archneurol.2009.242. [DOI] [PubMed] [Google Scholar]
- 22.Jang SH, Kim SH, Kim OL, Byun WM, Ahn SH. Corticospinal tract injury in patients with diffuse axonal injury: a diffusion tensor imaging study. NeuroRehabilitation. 2009b;25:229–233. doi: 10.3233/NRE-2009-0519. [DOI] [PubMed] [Google Scholar]
- 23.Jang SH, Kim SH, Kim OR, Byun WM, Kim MS, Seo JP, Chang MC. Cingulum injury in patients with diffuse axonal injury: a diffusion tensor imaging study. Neurosci Lett. 2013;543:47–51. doi: 10.1016/j.neulet.2013.02.058. [DOI] [PubMed] [Google Scholar]
- 24.Kim JW, Lee HD, Jang SH. Severe bilateral anterior cingulum injury in patients with mild traumatic brain injury. Neural Regen Res. 2015a;10:1876–1878. doi: 10.4103/1673-5374.170321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee HD, Jang SH. Injury of the corticoreticular pathway in patients with mild traumatic brain injury: a diffusion tensor tractography study. Brain Inj. 2015;29:1219–1222. doi: 10.3109/02699052.2015.1045028. [DOI] [PubMed] [Google Scholar]
- 26.Major BP, Rogers MA, Pearce AJ. Using transcranial magnetic stimulation to quantify electrophysiological changes following concussive brain injury: a systematic review. Clin Exp Pharmacol Physiol. 2015;42:394–405. doi: 10.1111/1440-1681.12363. [DOI] [PubMed] [Google Scholar]
- 27.Maxwell WL, Povlishock JT, Graham DL. A mechanistic analysis of nondisruptive axonal injury: a review. J Neurotrauma. 1997;14:419–440. doi: 10.1089/neu.1997.14.419. [DOI] [PubMed] [Google Scholar]
- 28.Meythaler JM, Peduzzi JD, Eleftheriou E, Novack TA. Current concepts: diffuse axonal injury-associated traumatic brain injury. Arc Phys Med Rehabil. 2001;82:1461–1471. doi: 10.1053/apmr.2001.25137. [DOI] [PubMed] [Google Scholar]
- 29.Mittl RL, Grossman RI, Hiehle JF, Hurst RW, Kauder DR, Gennarelli TA, Alburger GW. Prevalence of MR evidence of diffuse axonal injury in patients with mild head injury and normal head CT findings. AJNR Am J Neuroradiol. 1994;15:1583–1589. [PMC free article] [PubMed] [Google Scholar]
- 30.Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Parker GJ, Alexander DC. Probabilistic anatomical connectivity derived from the microscopic persistent angular structure of cerebral tissue. Philos Trans R Soc Lond B Biol Sci. 2005;360:893–902. doi: 10.1098/rstb.2005.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Povlishock JT, Erb DE, Astruc J. Axonal response to traumatic brain injury: reactive axonal change, deafferentation, and neuroplasticity. J Neurotrauma. 1992;9(Suppl1):S189–200. [PubMed] [Google Scholar]
- 33.Rajesh A, Cooke GE, Monti JM, Jahn A, Daugherty AM, Cohen NJ, Kramer AF. Differences in brain architecture in remote mild traumatic brain injury. J Neurotrauma. 2017;34:3280–3287. doi: 10.1089/neu.2017.5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Raji CA, Henderson TA. PET and single-photon emission computed tomography in brain concussion. Neuroimaging Clin N Am. 2018;28:67–82. doi: 10.1016/j.nic.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Seo JP, Kwon YH, Jang SH. Mini-review of studies reporting the repeatability and reproducibility of diffusion tensor imaging. Invstig Magn Reson Imaging. 2019;1:26–33. [Google Scholar]
- 36.Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, Vu MA, Purohit MP, Helmer K, Koerte I, Lin AP, Westin CF, Kikinis R, Kubicki M, Stern RA, Zafonte R. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:137–192. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topal NB, Hakyemez B, Erdogan C, Bulut M, Koksal O, Akkose S, Dogan S, Parlak M, Ozguc H, Korfali E. MR imaging in the detection of diffuse axonal injury with mild traumatic brain injury. Neurol Res. 2008;30:974–978. doi: 10.1179/016164108X323799. [DOI] [PubMed] [Google Scholar]
- 38.Wallace EJ, Mathias JL, Ward L. Diffusion tensor imaging changes following mild, moderate and severe adult traumatic brain injury: a meta-analysis. Brain Imaging Behav. 2018;12:1607–1621. doi: 10.1007/s11682-018-9823-2. [DOI] [PubMed] [Google Scholar]
- 39.Wang JY, Bakhadirov K, Devous MD, Sr, Abdi H, McColl R, Moore C, Marquez de la Plata CD, Ding K, Whittemore A, Babcock E, Rickbeil T, Dobervich J, Kroll D, Dao B, Mohindra N, Madden CJ, Diaz-Arrastia R. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch Neurol. 2008;65:619–626. doi: 10.1001/archneur.65.5.619. [DOI] [PubMed] [Google Scholar]
- 40.Wedeen VJ, Wang RP, Schmahmann JD, Benner T, Tseng WYI, Dai G, Pandya DN, Hagmann P, D’Arceuil H, de Crespignya AJ. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. Neuroimage. 2008;41:1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 41.Xiong K, Zhu Y, Zhang Y, Yin Z, Zhang J, Qiu M, Zhang W. White matter integrity and cognition in mild traumatic brain injury following motor vehicle accident. Brain Res. 2014;1591:86–92. doi: 10.1016/j.brainres.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 42.Yallampalli R, Wilde EA, Bigler ED, McCauley SR, Hanten G, Troyanskaya M, Hunter JV, Chu ZL, Li XQ, Levin HS. Acute white matter differences in the fornix following mild traumatic brain injury using diffusion tensor imaging. J Neuroimaging. 2013;23:224–227. doi: 10.1111/j.1552-6569.2010.00537.x. [DOI] [PubMed] [Google Scholar]
- 43.Yamada K, Sakai K, Akazawa K, Yuen S, Nishimura T. MR tractography: a review of its clinical applications. Magn Reson Med Sci. 2009;8:165–174. doi: 10.2463/mrms.8.165. [DOI] [PubMed] [Google Scholar]
- 44.Yu FF, Chiang FL, Stephens N, Huang SY, Bilgic B, Tantiwongkosi B, Romero R. Characterization of normal-appearing white matter in multiple sclerosis using quantitative susceptibility mapping in conjunction with diffusion tensor imaging. Neuroradiology. 2019;61:71–79. doi: 10.1007/s00234-018-2137-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zheng T, Yuan Y, Yang H, Du J, Wu S, Jin Y, Wang Z, Liu D, Shi Q, Wang X, Liu L. Evaluating the therapeutic effect of low-intensity transcranial ultrasound on traumatic brain injury with diffusion kurtosis imaging. J Magn Reson Imaging. 2020;52:520–531. doi: 10.1002/jmri.27063. [DOI] [PubMed] [Google Scholar]


