Key Words: cognitive impairment, diffusion tensor imaging, fractional anisotropy, functional connectivity, functional magnetic resonance imaging, hippocampus, local consistency, low frequency oscillation amplitude, mean diffusivity, multiple sclerosis, neurodegeneration
Abstract
Multiple sclerosis is associated with structural and functional brain alterations leading to cognitive impairments across multiple domains including attention, memory, and the speed of information processing. The hippocampus, which is a brain important structure involved in memory, undergoes microstructural changes in the early stage of multiple sclerosis. In this study, we analyzed hippocampal function and structure in patients with relapsing-remitting multiple sclerosis and explored correlations between the functional connectivity of the hippocampus to the whole brain, changes in local brain function and microstructure, and cognitive function at rest. We retrospectively analyzed data from 20 relapsing-remitting multiple sclerosis patients admitted to the Department of Neurology at the China-Japan Union Hospital of Jilin University, China, from April 2015 to November 2019. Sixteen healthy volunteers were recruited as the healthy control group. All participants were evaluated using a scale of extended disability status and the Montreal cognitive assessment within 1 week before and after head diffusion tensor imaging and functional magnetic resonance imaging. Compared with the healthy control group, the patients with relapsing-remitting multiple sclerosis had lower Montreal cognitive assessment scores and regions of simultaneously enhanced and attenuated whole-brain functional connectivity and local functional connectivity in the bilateral hippocampus. Hippocampal diffusion tensor imaging data showed that, compared with the healthy control group, patients with relapsing-remitting multiple sclerosis had lower hippocampal fractional anisotropy values and higher mean diffusivity values, suggesting abnormal hippocampal structure. The left hippocampus whole-brain functional connectivity was negatively correlated with the Montreal cognitive assessment score (r = −0.698, P = 0.025), and whole-brain functional connectivity of the right hippocampus was negatively correlated with extended disability status scale score (r = −0.649, P = 0.042). The mean diffusivity value of the left hippocampus was negatively correlated with the Montreal cognitive assessment score (r = −0.729, P = 0.017) and positively correlated with the extended disability status scale score (r = 0.653, P = 0.041). The right hippocampal mean diffusivity value was positively correlated with the extended disability status scale score (r = 0.684, P = 0.029). These data suggest that the functional connectivity and presence of structural abnormalities in the hippocampus in patients with relapse-remission multiple sclerosis are correlated with the degree of cognitive function and extent of disability. This study was approved by the Ethics Committee of China-Japan Union Hospital of Jilin University, China (approval No. 201702202) on February 22, 2017.
Chinese Library Classification No. R452; R363; R445
Introduction
Cognitive deficits adversely affect the daily function of multiple sclerosis (MS) patients, especially in terms of quality of life of indices such as independence, social inclusion, and emotional stability (Chan et al., 2017; Oset et al., 2020; Gaetani et al., 2021). Changes in cognitive function may be related to alterations in brain structure and function (Ontaneda and Fox, 2017; Karavasilis et al., 2019; Lashkari et al., 2021). Functional magnetic resonance imaging (fMRI) can be used to generate dynamic physiological information via blood oxygen level-dependent (BOLD) signals (Filippi and Rocca, 2013). In regard to cognition, MS patients initially exhibit increased connectivity as a manifestation of compensation mechanisms. However, brain connectivity declines as the disease progresses (Penner et al., 2003). fMRI studies have reported on the re-organization of neural functional activity during cognitive tasks in MS patients (Mainero et al., 2004; Margulies et al., 2010). In studies of spontaneous brain activity, voxel-based regional homogeneity (ReHo) analysis and amplitude of low-frequency fluctuation (ALFF) analysis reflect the temporal synchrony of neuronal activities (Chen et al., 2017) and the measurement of local resting-state fMRI time course cortical low-frequency oscillation amplitude values (Duff et al., 2008; Zuo et al., 2010), respectively. Diffusion tensor imaging (DTI), where water molecule motion is characterized by applying multiple diffusion gradients (Sbardella et al., 2013; Bagnato et al., 2019; Stock et al., 2020), is ideal for identifying pathological changes in cognition in MS patients (Vrenken et al., 2006; Yu et al., 2012). Microscopic features have been used to distinguish MS lesions with more axonal damage from lesions that are hyperintense in T2-weighted sequences (Bagnato et al., 2019).
The increasing evidence for a pure amnestic-like profile in MS supports the role of hippocampal formation in MS episodic memory function (Karavasilis et al., 2019). The hippocampus, as an important brain structure for memory, undergoes microstructural changes in the early stage of MS. Microstructural damage measured by DTI has been observed in patients with clinically isolated syndrome and RRMS, and the MRI findings were strongly correlated with long-term recall (Planche et al., 2017). Moreover, in patients examined two to 6 months after clinically isolated syndrome, performance on learning trials was correlated with hippocampal volume (Planche et al., 2018). Here, we hypothesized that there would be a correlation between changes in hippocampal structure and function in patients with RRMS. We used DTI voxel-based analysis and multimodal fMRI to investigate the relationship between structural and functional changes in the hippocampus and cognitive deficits and other symptoms in patients with RRMS.
Participants and Methods
Participants
This was a retrospective case control study. We retrospectively collected data from 20 patients with RRMS who were admitted to the Department of Neurology at the China-Japan Union Hospital of Jilin University, China from April 2015 to November 2019. The patients included 5 men and 15 women with an average age of 43.00 ± 12.25 (27–64) years old. The mean disease duration was 6.50 ± 2.27 (4–12) years, and the average education level was 10.70 ± 3.09 (5–15) years. A total of 16 physically healthy volunteers from the China-Japan Union Hospital were retrospectively recruited for the healthy control group (HC), including 8 men and 8 women with an average age of 41.37 ± 11.27 (27–56) years old. The average educational level of the HC group was 10.88 ± 3.52 (5–16) years.
All subjects were evaluated using the extended disability status scale (EDSS) (Wingerchuk et al., 2006) and the Montreal cognitive assessment (MoCA) (Nasreddine et al., 2005) within 1 week before and after the fMRI. The clinical data, including patient disease duration, EDSS scores, and MoCA scores were recorded by two experienced neurologists (YL and JBG). The EDSS quantifies disability by assigning a functional system score for the pyramidal, cerebellar, brainstem, sensory, bowel and bladder, visual, cerebral, and other functional systems. The following cognitive assessments were performed for all subjects using the MoCA: visuospatial/exclusion, naming, memory, attention, language, abstraction, and delayed recall. This study was approved by the Ethics Committee of the China-Japan Union Hospital (approval No. 2017022202) on February 22, 2017, and all participants signed informed consent forms. This study was performed in strict accordance with the Declaration of Helsinki formulated by the World Medical Association.
Inclusion criteria for RRMS patients
The patient inclusion criteria were: (1) McDonald's 2010 revised diagnostic criteria for MS (Polman et al., 2011) and the 2016 European MS Magnetic Resonance Imaging Multicenter Collaborative Group (MAGNIMS) MS MRI diagnostic criteria (Filippi et al., 2016). (2) 22–65 years of age (to avoid the influence of physiological atrophy); (3) no serious mental illness or neurological disease history other than MS; (4) Chinese as a primary language; (5) right-handedness; (6) a MRI head scan conducted using the same scanner at our institution; (7) relapse-free status without steroid treatment for at least 6 weeks and no current disease modifying treatments.
Exclusion criteria for RRMS patients
The patient exclusion criteria were: (1) clear intracranial infarction, intracranial space-occupying lesion, or other lesions that could affect the outcome of the study; (2) severe cardiopulmonary diseases; (3) age over 65 years.
Inclusion criteria for healthy volunteer controls
The inclusion criteria for healthy volunteers were: (1) right-handedness, matched with the patients in terms of age, sex, and education level; (2) good physical health status, with no neurological diseases, underlying systematic diseases affecting the nervous system, neurologically positive symptoms, or abnormalities observed on a routine MRI scan.
Exclusion criteria for healthy volunteer controls
The exclusion criteria for the healthy volunteers were: (1) clear intracranial infarction, intracranial space-occupying lesion, or other lesions that could affect the outcome of the study; (2) severe cardiopulmonary disease; (3) age over 65 years.
The RRMS patients were relapse-free and without steroid treatment for at least 6 weeks prior to scanning and were not undergoing any disease modifying treatment. Volunteers in the HC group were evaluated using the EDSS (Wingerchuk et al., 2006) and the MoCA (Nasreddine et al., 2005) within 1 week before and after the fMRI.
Image acquisition
All MRI scans were performed on the same Siemens Trio Tim 3.0T MR scanner (Siemens Medical Systems, Erlangen, Germany) from April 2015 to November 2019. The standard protocol consisted of three sequences: blood oxygen level-dependent functional magnetic resonance imaging (BOLD-fMRI) (echo time = 30 ms, repetition time = 2500 ms, flip angle = 90°, slice thickness = 3.0, slice number = 43, field of view = 210 mm), DTI (echo time = 95.0 ms, repetition time = 3700 ms, slice thickness = 4.0, slice number = 25, and field of view = 220 mm), and 3DT1 weighted imaging (echo time/repetition time = 2.98/5000 ms, slice thickness/gap = 1.0/0 mm, field of view = 256 mm). The participants were instructed to close their eyes while staying awake and to try not to think about anything.
Data processing and analysis
We used the REST_V1.8 resting-state fMRI data analysis toolkit (http://www.restfmri.net) in MATLAB7.14 (R2012a) (Math Works, Inc. Natick, MA, USA, http://www.mathworks.com/products/matlab) to divide the bilateral hippocampus into regions. The hippocampal regions were identified using an automated anatomical labeling template (Tzourio-Mazoyer et al., 2002). The brain regions and hippocampal data were confirmed in Montreal Neurological Institute (MNI) space using MRIcron (http://www.sph.sc.edu/comd/rorden/mricron/) (automated anatomical labeling 37 and 38). MNI space is a coordinate system based on a series of MRI images of healthy participants (Fjalldal et al., 2018). The left and right hippocampi were sampled at 3 × 3 × 3 mm3. The voxels of the re-sampled left and right hippocampi were 273 and 288, respectively. The collected image data were classified and analyzed using RadiAnt DICOM Viewer (http://www.radiantviewer.com) software, and the BOLD-fMRI, 3DT1 weighted imaging, and DTI sequence data were extracted from the image data of each subject. We used a data processing assistant for resting-state fMRI (DPARSM, http://www.restfmri.net) in MATLAB to pre-process the BOLD-fMRI data, and REST software for functional connectivity (FC) analysis, ReHo analysis, and analysis. We used Functional MRI Software Library software based on the Pipeline for Analyzing Brain Diffusion Images (PANDA, http://www.nitrc.org/projects/panda/) to process the DTI data and obtain bilateral hippocampal FA and MD Values. The voxel-based analysis method in Statistical Parametric Mapping (SPM8, http://www.fil.ion.ucl.ac.uk/spm/software/Spm8/) was used to analyze the data.
Quality control
The patient clinical assessment data, including disease duration, EDSS score, and MoCA score, were recorded by experienced neurologists (YL and JBG).
Outcomes
All subjects underwent the EDSS and MoCA within 1 week before and after the MRI.
MoCA: Cognitive functions were examined using the MoCA (Nasreddine et al., 2005). The following cognitive assessments were performed for all subjects: (1) visuospatial, (2) naming, (3) memory, (4) attention, (5) language, (6) abstraction, (7) delayed recall. A score of 26 or greater is considered normal.
EDSS: Disability status was examined using the EDSS. The EDSS quantifies disability by assigning a functional system score to each of eight functional systems: pyramidal, cerebellar, brainstem, sensory, bowel and bladder, visual, cerebral, other.
Statistical analysis
The demographic variables and clinical scores of the RRMS patients and HC subjects were statistically analyzed using SPSS 20.0 software (IBM, Armonk, NY, USA). Two-sample t-tests were used to evaluate differences in age, duration of disease, years of education, and clinical assessment scores between the MS patients and HC volunteers. The chi-square test was used to compare the sex distribution of the two groups. The data are expressed as the mean ± standard deviation. For non-normally distributed data (EDSS score), the median (interquartile range) is provided. Independent two-sample t-tests were performed for the standardized FC, ReHo, and ALFF maps using REST software with age, sex, and years of education as co-variates. Multiple corrections were carried out using AlphaSim (https://afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf), for voxels > 5. P-values less than 0.05 indicated statistical significance. The DTI parameters were analyzed using SPM8 software (https://www.fil.ion.ucl.ac.uk/spm/) for the two-sample t-tests between groups. FDR was used for multiple correction, for voxels > 10.
We initially evaluated the distribution of the values for each parameter. After testing, all of the values had a normal distribution, so the Pearson correlation coefficient was adopted. The resting-state fMRI measurements (FC, ReHo, and ALFF values) and DTI measurements (FA and MD values) in the RRMS group were evaluated to assess correlations with the clinical scores.
Results
Participant demographics and clinical data
We assessed data from a total of 20 RRMS patients. All patients underwent clinical assessments and structural MRI. After statistical analysis, the sex, age, and education level were matched between the two groups. The median EDSS score (range) was 2.50 (1.0–4.50). Compared with the HC group, the MoCA scores in the RRMS group were significantly lower (P < 0.001), while there were no between-group differences in the other variables (P > 0.05; Figure 1 and Table 1).
Figure 1.

Flow chart of study procedure.
DTI: Diffusion tensor imaging; EDSS: expanded disability status scale; FA: fractional anisotropy; fMRI: functional magnetic resonance imaging; HC: healthy control; MD: values and higher mean diffusivity; MoCA: Montreal congnitive assessment; RRMS: relapsing-remitting multiple sclerosis.
Table 1.
Demographics and clinical data from the RRMS and healthy control groups
| Variables | RRMS group (n = 20) | Healthy control group (n = 16) | P-value | t/χ2 value |
|---|---|---|---|---|
| Age (yr) | 43.00 ± 12.25 | 41.37 ± 11.27 | 0.08 | 1.80 |
| Sex (male/female, n) | 5/15 | 8/8 | 0.83# | 0.79 |
| Disease history (yr) | 3.50 ± 1.27 | / | / | |
| Education level (yr) | 10.70 ± 3.09 | 10.88 ± 3.52 | 0.71 | –1.89 |
| Median EDSS (score) | 2.50(1.00–4.50) | / | / | |
| MoCA (score) | 25.20 ± 0.92 | 27.75 ± 1.28 | 0.001* | –4.28 |
Data are expressed as the mean ± standard deviation. For non-normally distributed data, the median (interquartile range) is provided. *Two-sample t-tests; #Chi-squared test. EDSS: Expanded disability status scale; MoCA: Montreal cognitive assessment; RRMS: relapsing-remitting multiple sclerosis.
Differences in FC between the groups
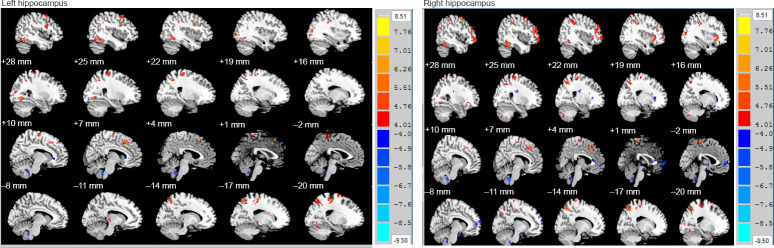
Compared with the HC group, the whole-brain FC values of the bilateral hippocampi in the RRMS group indicated that there were both enhanced and attenuated regions. The left hippocampal regions with attenuated FC on the left and right sides of the brain were the cingulate gyrus, limbic lobe, and right brain stem; while the regions with enhanced whole-brain FC were the right cerebellum (posterior cerebellum), bilateral temporal lobe, suboccipital lobules, frontal lobe (prefrontal lobe, frontal gyrus), amygdala, lateral globus pallidus, and lentiform nucleus (Table 2 and Figure 2). The right hippocampal regions with attenuated FC on the left and right sides of the brain were the right brain stem, limbic lobe, middle temporal gyrus, para-hippocampal gyrus, and left frontal lobe; while the regions with enhanced whole-brain FC were the right cerebellum (posterior cerebellum), left occipital lobe, left frontal lobe, insula, and parietal lobe (P < 0.001, corrected by AlphaSim, voxel > 5; Table 3 and Figure 2).
Table 2.
Brain regions in which the FC with the left and right side of the brain in the left hippocampus differed between the RRMS and healthy control groups
| Brain region (automated anatomical labeling template) | Voxel (mm3) | MNI coordinates | T value | ||
|---|---|---|---|---|---|
|
| |||||
| X | Y | Z | |||
| Left side | |||||
| Temporal lobe (middle temporal gyrus, superior temporal gyrus) | 5 | −42 | 6 | −30 | −5.166 |
| Temporal lobe (inferior temporal gyrus), fusiform gyrus | 5 | 54 | −6 | −30 | −4.451 |
| left cerebellum (posterior cerebellar lobe), fusiform gyrus, anterior cerebellar lobe, medial occipital gyrus | 48 | −33 | −78 | −21 | 6.178 |
| Amygdala, lateral globus pallidus, lentiform nucleus | 9 | −21 | −6 | −9 | 7.043 |
| Frontal lobe, precentral gyrus | 161 | −48 | 39 | 3 | 7.672 |
| Temporal lobe (middle temporal gyrus) | 44 | −57 | −51 | −3 | 5.526 |
| Insula | 7 | −42 | −3 | 0 | 6.489 |
| Frontal lobe, insula | 15 | −30 | 18 | −3 | 4.953 |
| Occipital lobe | 5 | −39 | −84 | 6 | 4.300 |
| Frontal lobe, precentral gyrus | 6 | −51 | −6 | 6 | 5.158 |
| Occipital lobe, occipital lobe | 9 | −24 | −90 | 9 | 5.312 |
| Occipital lobe | 21 | 33 | −84 | 12 | 5.686 |
| Parietal lobe, postcentral gyrus, temporal lobe (transverse temporal gyrus), frontal lobe | 8 | −60 | −12 | 15 | 5.311 |
| Frontal lobe, caudate nucleus | 5 | −24 | −24 | 27 | −5.812 |
| Precentral gyrus, postcentral gyrus, frontal lobe, parietal lobe | 21 | 57 | −3 | 24 | 5.366 |
| Occipital lobe, occipital lobe | 7 | −9 | −90 | 18 | 4.607 |
| Superior parietal lobe, precuneus | 63 | −18 | −69 | 60 | 7.266 |
| Frontal lobe, precentral gyrus, parietal lobe, postcentral gyrus, paracentral lobule | 43 | −24 | −27 | 60 | 6.429 |
| Hypothalamus, midbrain, left brain stem, limbic lobe, hippocampus, mammillary body | 13 | −21 | −27 | −6 | 6.678 |
| Right side | |||||
| Posterior cerebellar lobe, cerebellar tonsil, right brain stem | 34 | 6 | −42 | −45 | −9.504 |
| Limbic lobe, para-hippocampal gyrus | 5 | −18 | 3 | −36 | 4.998 |
| Temporal lobe (inferior temporal gyrus), fusiform gyrus | 5 | 54 | −6 | −30 | −4.451 |
| Temporal lobe (middle temporal gyrus, inferior temporal gyrus), fusiform gyrus, right cerebellum (anterior cerebellar lobe) | 42 | 45 | −54 | −21 | 7.193 |
| Occipital (medial occipital gyrus), temporal lobe, posterior cerebellar lobe | 17 | 45 | −66 | −15 | 5.224 |
| Parietal lobe, postcentral gyrus, frontal lobe, precentral gyrus | 6 | 60 | −12 | 15 | 4.342 |
| Frontal lobe (inferior frontal gyrus) | 34 | 54 | 21 | 27 | 5.330 |
| Limbic lobe, posterior cingulate gyrus | 7 | 6 | −57 | 24 | −5.215 |
| Frontal lobe | 47 | −39 | 12 | 30 | 8.507 |
| Occipital lobe, temporal lobe, angular gyrus | 7 | 33 | −78 | 27 | 5.274 |
| Limbic gyrus, cingulate gyrus, medial frontal lobe | 33 | 0 | 18 | 45 | 6.895 |
RRMS patients (corrected P < 0.001 using AlphaSim voxel > 5). T value < 0, brain regions with less FC than in the healthy control group; T value > 0, brain regions with more FC than in the healthy control group. FC: Functional connectivity; MNI: Montreal Neurological Institute; RRMS: relapsing-remitting multiple sclerosis.
Figure 2.
Whole-brain FC analysis of the bilateral hippocampus in relapsing-remitting multiple sclerosis patients and healthy control subjects.
The left hippocampal regions with attenuated FC on the left and right side of the brain were the cingulate gyrus, limbic lobe, and right brain stem; while the regions with enhanced whole brain FC were the right cerebellum (posterior cerebellum), bilateral temporal lobe, suboccipital lobules, frontal lobe (prefrontal lobe, frontal gyrus), amygdala, lateral globus pallidus, and lentiform nucleus. The right hippocampal regions with attenuated FC on the left and right side of the brain were the right brain stem, limbic lobe, middle temporal gyrus, para-hippocampal gyrus, and left frontal lobe; while the regions with enhanced whole-brain FC were the right cerebellum (posterior cerebellum), left occipital lobe, left frontal lobe, insula, and parietal lobe. Red represents FC-enhanced regions and blue represents FC-attenuated regions. Independent two-sample t-tests were performed for the standardized FC (P < 0.001, corrected by AlphaSim, voxel > 5). Ordinate unit: T-value. FC: Functional connectivity.
Table 3.
Brain regions in which the FC with the left and right side of the brain in the right hippocampus differed between the RRMS and healthy control groups
| Brain region (automated anatomical labeling template) | Voxel (mm3) | MNI coordination | T value | ||
|---|---|---|---|---|---|
|
| |||||
| X | Y | Z | |||
| Left side | |||||
| Temporal lobe (inferior temporal gyrus, middle temporal gyrus), hippocampal uncus, limbic lobe | 7 | −21 | −3 | −42 | 4.930 |
| Middle temporal gyrus, superior temporal gyrus | 12 | −45 | 0 | −33 | −5.117 |
| Frontal lobe | 9 | 0 | 33 | −27 | −5.281 |
| Temporal lobe, fusiform gyrus | 5 | −48 | −18 | −27 | 5.785 |
| Limbic lobe, hippocampal uncus, para-hippocampal gyrus | 11 | −9 | −3 | −21 | −9.253 |
| Occipital lobe (inferior occipital lobe, medial occipital lobe), fusiform gyrus, posterior cerebellar lobe | 76 | −27 | −75 | −18 | 7.879 |
| Insula, frontal lobe | 29 | −42 | −3 | 0 | 8.138 |
| Medial frontal lobe, limbic lobe, anterior cingulate gyrus | 56 | −3 | 60 | 3 | −6.545 |
| Frontal lobe, temporal lobe (superior temporal gyrus), precentral gyrus | 12 | −54 | 3 | 9 | 5.274 |
| Occipital lobe, occipital lobe, middle temporal gyrus | 15 | −24 | −87 | 9 | 5.451 |
| Parietal lobe, precuneus | 98 | −15 | −60 | 48 | 8.056 |
| Parietal lobe (inferior parietal lobule), postcentral gyrus, frontal lobe | 147 | −39 | −48 | 39 | 7.619 |
| Frontal lobe, paracentral lobule | 32 | 0 | −33 | 69 | 7.176 |
| Right side | |||||
| Posterior cerebellar lobe | 21 | 18 | −72 | −42 | 6.304 |
| Posterior cerebellar lobe, cerebellar tonsil, right brain stem | 60 | 6 | −45 | −45 | −7.962 |
| Fusiform gyrus, temporal lobe, occipital lobe, anterior cerebellar lobe | 38 | 42 | −48 | −27 | 7.231 |
| Temporal lobe (superior temporal gyrus) | 5 | 45 | 18 | −27 | −6.073 |
| Temporal lobe (middle temporal gyrus) | 27 | 63 | −45 | −6 | 6.119 |
| Midbrain | 10 | 3 | −15 | −15 | −7.379 |
| Frontal lobe, insula | 58 | 30 | 24 | −6 | 6.433 |
| Frontal lobe (anterior medial frontal gyrus, anterior inferior frontal gyrus) | 184 | −39 | 39 | 0 | 7.437 |
| Occipital lobe | 17 | 33 | −81 | 12 | 5.657 |
| Frontal lobe (anterior medial frontal gyrus) | 130 | 42 | 39 | 15 | 6.985 |
| Insula, parietal lobe | 6 | 42 | −21 | 21 | 5.066 |
| Caudate nucleus, frontal lobe | 12 | 18 | 15 | 21 | −6.595 |
| Temporal lobe (middle temporal gyrus), angular gyrus, parietal lobe | 22 | 48 | −66 | 24 | 6.248 |
| Prefrontal lobe, precentral gyrus | 6 | 60 | −6 | 24 | 4.479 |
| Precuneus, parietal lobe | 48 | 21 | −57 | 48 | 6.948 |
| Frontal lobe (medial frontal lobe), cingulate gyrus, limbic lobe | 44 | 6 | 27 | 45 | 8.231 |
| Frontal lobe, precentral gyrus, postcentral gyrus | 37 | 24 | −27 | 69 | 4.955 |
RRMS patients (corrected P < 0.001 using AlphaSim voxel > 5). T value < 0, brain regions with less FC thanin the healthy control group; T value > 0, brain regions with more FC than in the healthy control group. FC: Functional connectivity; MNI: Montreal Neurological Institute; RRMS: relapsing-remitting multiple sclerosis.
Differences in ReHo between the groups
Compared with the HC group, the ReHo values were decreased in the left hippocampus, limbic lobe, and para-hippocampal gyrus of the RRMS patients, whereas the ReHo values were increased in the partial hippocampus, temporal lobe, limbic lobe, and para-hippocampal gyrus (Table 4 and Figure 3). Compared with the HC group, ReHo values were significantly higher in the right para-hippocampal gyrus, limbic lobe, hippocampus, and temporal lobe in the RRMS group (P < 0.05, corrected by AlphaSim, voxel > 5; Table 4 and Figure 3).
Table 4.
Brain regions in which the ReHo values in the left and right hippocampus differed between the patients in the RRMS group and the healthy control group
| Brain region (automated anatomical labeling template) | Voxel (mm3) | MNI coordination | T value | ||
|---|---|---|---|---|---|
|
| |||||
| X | Y | Z | |||
| Left side | |||||
| Hippocampus, limbic lobe, para-hippocampal gyrus | 6 | −21 | −15 | −21 | −3.604 |
| Hippocampus, temporal lobe, limbic lobe, para-hippocampal gyrus | 13 | −33 | −27 | −15 | 3.705 |
| Right side | |||||
| Para-hippocampal gyrus, limbic lobe, hippocampus, temporal lobe | 9 | 33 | −15 | −21 | 3.219 |
RRMS patients (corrected P < 0.05 using AlphaSim voxel > 5). T value < 0, brain regions with less ReHo than in the healthy control group; T value > 0, brain regions with more ReHo than in the healthy control group. MNI: Montreal Neurological Institute; ReHo: regional homogeneity; RRMS: relapsingremitting multiple sclerosis.
Figure 3.
ReHo analysis of the bilateral hippocampus in relapsing-remitting multiple sclerosis patients and healthy control subjects.
The ReHo values of the left hippocampus, limbic lobe, and para-hippocampal gyrus were decreased in the patients compared with the control subjects, whereas the ReHo values of the partial hippocampus, temporal lobe, limbic lobe, and para-hippocampal gyrus were increased in the relapsing-remitting multiple sclerosis patient group compared with the healthy control group. The right para-hippocampal gyrus, limbic lobe, hippocampus, and temporal lobe had significantly higher ReHo values in the relapsing-remitting multiple sclerosis group compared with the healthy control group. Red represents an ReHo increase and blue represents an ReHo decrease. Independent two-sample t-tests were performed for the standardized ReHo maps (P < 0.05, corrected by AlphaSim, voxel > 5). Ordinate unit: T-value. ReHo: Regional homogeneity.
Differences in ALFF between the groups
Compared with the HC group, when the left hippocampus was used as the template, the ALFF values of patients in the RRMS group were lower in the left hippocampus, limbic lobe, para-hippocampal gyrus, temporal lobe, and caudate nucleus. The ALFF values of the following brain regions were higher in the RRMS group than in the HC group: the limbic lobe, para-hippocampal gyrus, hippocampus, and amygdala (Table 5 and Figure 4). When the right hippocampus was used as the template, the ALFF values of patients in the RRMS group were lower than those in the HC group in the hippocampus, limbic lobe, temporal lobe, para-hippocampal gyrus, and amygdala; and the ALFF values were higher in the hippocampus, para-hippocampal gyrus, and limbic lobe (Table 5 and Figure 4).
Table 5.
Brain regions in which the ALFF values in the left and right hippocampus differed between the patients in the RRMS and healthy control groups
| Brain region (automated anatomical labeling template) | Voxel (mm3) | MNI coordination | T value | ||
|---|---|---|---|---|---|
|
| |||||
| X | Y | Z | |||
| Left side | |||||
| Limbic lobe, para-hippocampal gyrus, hippocampus, amygdala | 17 | −24 | −21 | −15 | 7.384 |
| Hippocampus, limbic lobe, para-hippocampal gyrus, temporal lobe, caudate nucleus | 49 | −36 | −27 | −9 | −5.329 |
| Right side | |||||
| Hippocampus, limbic lobe, temporal lobe, para-hippocampal gyrus, amygdala | 36 | 36 | −12 | −24 | −7.822 |
| Hippocampus, para-hippocampal gyrus, limbic lobe | 10 | 24 | −21 | −15 | 4.154 |
RRMS patients (corrected P < 0.05 using AlphaSim voxel > 5). T value < 0, brain regions with less ALFF than in the healthy control group; T value > 0, brain regions with more ALFF than in the healthy control group. ALFF: Amplitude of low-frequency fluctuation; MNI: Montreal Neurological Institute; RRMS: relapsing-remitting multiple sclerosis.
Figure 4.
ALFF analysis of the bilateral hippocampus of relapsing-remitting multiple sclerosis patients and healthy control subjects.
Compared with the healthy control group, ALFF values in the relapsing-remitting multiple sclerosis group were lower in the left hippocampus, limbic lobe, para-hippocampal gyrus, temporal lobe, and caudate nucleus. The ALFF values of the following brain regions were higher in the relapsing-remitting multiple sclerosis group than in the healthy control group: limbic lobe, para-hippocampal gyrus, hippocampus, and amygdala. Red represents the ALFF increase; blue represents ALFF decrease. Independent two-sample t-tests were performed for the standardized ALFF maps (P < 0.05, corrected by AlphaSim, voxel > 5). Ordinate unit: T-value. ALFF: Amplitude of low-frequency fluctuation.
Differences in hippocampal and whole-brain FA and MD between the groups
Compared with the HC group, the FA values were lower in the RRMS group in the bilateral hippocampus (P < 0.05, corrected by FDR, voxel > 10; Table 6). The MD values in the left hippocampus were significantly higher in the RRMS group than in the HC group (P < 0.05, corrected by FDR, voxel > 10; Table 6).
Table 6.
Comparison of FA and MD values in the bilateral hippocampi between the RRMS and healthy control groups
| DTI parameter | RRMS group (n = 20) | Healthy control group (n = 16) | T value | P value |
|---|---|---|---|---|
| FA value of left hippocampus | 0.373±0.016 | 0.397±0.015 | −2.374 | 0.008* |
| FA value of right hippocampus | 0.336±0.021 | 0.379±0.025 | −4.635 | 0.023* |
| MD value of left hippocampus | 0.167±0.052 | 0.034±0.012 | 3.430 | 0.003* |
| MD value of right hippocampus | 0.079±0.025 | 0.041±0.015 | −1.416 | 0.200 |
Data are expressed as the mean ± standard deviation, *P < 0.05, vs. healthy control group. Corrected by FDR, voxel > 10. DTI: Diffusion tensor imaging; FA: fractional anisotropy; MD: mean diffusivity; RRMS: relapsing-remitting multiple sclerosis.
Correlation between clinical characteristics and functional and structural changes in RRMS patients
We conducted correlation analyses to examine the relationships between the left and right resting-state fMRI measurements (including the FC, ReHo, and ALFF values) and the MoCA and EDSS scores in the RRMS group. The results indicated that the whole-brain FC was negatively correlated with the MoCA score in the left hippocampus (r = −0.698, P = 0.025), and that whole-brain FC was negatively correlated with EDSS score in the right hippocampus (r = −0.649, P = 0.042). The MD value was negatively correlated with the MoCA score (r = −0.729, P = 0.017) and positively correlated with the EDSS score (r = 0.653, P = 0.041) in the left hippocampus. Furthermore, the MD value was positively correlated with the EDSS score in the right hippocampus (r = 0.684, P = 0.029). The ReHO value, ALFF value, and FA value were not statistically significantly correlated with the EDSS or MoCA scores (P > 0.05). The ALFF value in the right hippocampus was positively correlated with the right FA value (r = 0.693, P = 0.026), and the ReHo value in the right hippocampus was negatively correlated with the right FA value (r = −0.709, P = 0.022; Table 7 and Figure 5).
Table 7.
Correlation analysis between hippocampal function/structure and MoCA and EDSS scores in RRMS patients
| DTI parameter | MoCA score | EDSS score | ||
|---|---|---|---|---|
|
|
|
|||
| r | P | r | P | |
| zFCmap of left hippocampus | −0.698 | 0.025 | / | / |
| zFCmap of right hippocampus | / | / | −0.649 | 0.042 |
| MD value of left hippocampus | −0.729 | 0.017 | 0.653 | 0.041 |
| MD value of right hippocampus | / | / | 0.684 | 0.029 |
We used the Pearson correlation coefficient. The left hippocampus wholebrain FC was negatively correlated with the MoCA score. The whole-brain FC of the right hippocampus was negatively correlated with the EDSS score. The MD value of the left hippocampus was negatively correlated with the MoCA score and positively correlated with the EDSS score. The right hippocampal MD value was positively correlated with the EDSS score. DTI: Diffusion tensor imaging; EDSS: extended disability status scale; FC: functional connectivity; MD: mean diffusivity; MoCA: Montreal cognitive assessment; RRMS: relapsing-remitting multiple sclerosis.
Figure 5.

Correlation between the hippocampal FC or MD value and clinical scores (Pearson correlation coefficient).
(A) The correlation between the FC of the left hippocampus and the MoCA score (r = −0.698, P = 0.025). (B) The correlation between the FC of the right hippocampus and the EDSS score (r = −0.649, P = 0.042). (C) The correlation between the MD value of the left hippocampus and the MoCA score (r = −0.729, P = 0.017). (D) The correlation between the MD value of the left hippocampus and the EDSS score (r = 0.653, P = 0.041). (E) The correlation between the MD value of the right hippocampus and the EDSS score (r = 0.684, P = 0.029). EDSS: Extended disability status scale; FC: functional connectivity; MD: mean diffusivity; MoCA: Montreal cognitive assessment.
Discussion
In this study, we used structural and functional MRI to investigate how cognitive impairment was associated with hippocampal structure and connectivity in RRMS patients. Our data indicated that the whole brain and local FC of the hippocampus in patients with RRMS were both enhanced and weakened, such that abnormal FC of the left hippocampus was related to cognitive function, while abnormal FC of the right hippocampus was related to the extent of disability.
Although MS is defined as the presence of white matter lesions in the central nervous system, it can also involve changes in gray matter, including cortical atrophy and damage observed via MRI and histology (Kuceyeski et al., 2018; Yamout and Alroughani, 2018). Subcortical atrophy in the thalamus (Amin and Ontaneda, 2020; Zhao et al., 2020) and hippocampal atrophy (van Geest et al., 2018; Lashkari et al., 2021) are also associated with cognitive deficits (Karavasilis et al., 2019). fMRI can be used to effectively assess neural connectivity and damage in various brain regions (Filippi and Rocca, 2013). DTI is a non-invasive imaging technique, and the only method capable of illustrating the structure and orientation of white matter fibrous structures in vivo. Thus, it allows for the evaluation of changes in the brain microstructure (Pokryszko-Dragan et al., 2018; Schneider et al., 2019; Martínez-Heras et al., 2020). Martínez-Heras et al. (2020) demonstrated that MS lesions could be classified into two types based on the severity of changes in macroscopic DTI parameters and microscopic diffusion properties.
Based on the distribution of resting-state FC patterns of the left and right hippocampi in patients in the RRMS and HC groups, the FC patterns of the bilateral hippocampi, cerebral cortex, and subcortical regions showed enhanced and attenuated regions in RRMS patients. The abnormal regions were mainly distributed in the prefrontal lobe, parietal lobe, temporal lobe, limbic lobe, and cerebellum regions. The correlation between cognitive dysfunction and the left hippocampus was more significant than that for the right hippocampus (r = –0.698), which was consistent with previous studies (Sicotte et al., 2008; Hulst et al., 2012). Attenuated connectivity indicates impaired FC. The enhanced connectivity was due to cerebral cortical reorganization in adjacent areas, which is a compensatory mechanism for maintaining neurological stability that is highly common in RRMS patients (Filippi et al., 2013; Sacco et al., 2013; Sampath et al., 2017; Laura et al., 2018). In this study, the amygdala, lateral globus pallidus, and lentiform nucleus exhibited enhanced FC patterns. Given the presence of compensatory mechanisms, we predicted that those brain regions would be abnormal in patients with cognitive impairment. fMRI of cognitively impaired patients revealed a functional relationship between the posterior cingulate gyrus and the hippocampus. The activation of the posterior cingulate gyrus during episodic memory coding was related to the activation of the right hippocampus. The identification of memory was related to the activation of the left hippocampus, indicating the presence of hemispherical characteristics (Klawiter et al., 2011).
The ReHo value represents brain functional activity based on the BOLD signal, which can be interpreted as a network-centric indicator. This underscores the importance of connectivity nodes in the human brain via local functional interactions (Jiang and Zuo, 2016). In the present study, we found that RRMS patients exhibited abnormal ReHo values in the limbic lobe, para-hippocampal gyrus, and temporal lobe. These brain regions are mainly involved in cognitive-related activities. Indeed, the changes in ReHo values in these regions were related to cognitive impairment. In the left hippocampus, the ALFF values of the hippocampus, limbic lobe, para-hippocampal gyrus, temporal lobe, and caudate nucleus were decreased in the RRMS patient group compared with the HCs. The ALFF values of the hippocampus, limbic lobe, temporal lobe, para-hippocampal gyrus, and amygdala were also lower in the right hippocampus in the RRMS group. These findings suggest that these brain regions may be highly susceptible to cognitive impairment caused by immunological white matter lesions. The spontaneous changes in ALFF values in these brain regions are a potential indicator of MS nerve damage. In behavioral studies, MS patients exhibited significant cognitive, memory, and executive function deficits, with abnormal information processing speed (Defrancesco et al., 2013; Li et al., 2017; Koenig et al., 2019; Toko et al., 2021). The hippocampus is a key structure in multiple memory functions, including memory coding and retrieval, and is also strongly associated with learning and emotional control. Previous studies have reported that the hippocampus is particularly sensitive to ischemia and hypovolemia (Fein et al., 2000; Chai et al., 2010; Yang et al., 2010). Thus, a decrease in ALFF values in the hippocampus may indicate memory impairment in subjects with MS. The association between altered caudate nucleus activity and reduced cognitive function has not been reported in previous fMRI studies.
We identified an interesting phenomenon in the current study: the FC of the hippocampus was both enhanced and attenuated, in different regions. This could be related to changes in connectivity caused by the spontaneous activities of neurons in other brain regions. We postulated that this was the result of compensatory mechanisms in these brain regions. Indeed, the hippocampus is not a monolithic structure (Heine et al., 2020). Recently, a study on hippocampal connectivity precisely defined the pre- and post-differentiation regions of the hippocampus. The phenomenon observed in the present study could have been caused by variation between the distinctive histological regions of the hippocampus. This topic merits in-depth examination in future research.
In this study, our voxel-based analysis revealed that bilateral hippocampal myelination (MD value) and the loss of white matter integrity (FA value) in patients in the RRMS group were significantly different from those in the HC group. Because of the presence of a small amount of fibrous bundles in the gray matter, axonal injury may occur in the hippocampus of patients with RRMS, and neuronal cells may be retrogradely damaged, resulting in cell degeneration, apoptosis, necrosis, increased cell junction, and reduced diffusion of water molecules. These form the pathological basis for FA and MD abnormalities (Feinstein et al., 2010), and lead to shrinkage in the corresponding gray matter volume. Previous studies have used voxel-based morphological analyses to identify reduced hippocampal volume in RRMS patients (Han et al., 2017). Hippocampal atrophy in MS is caused by neuronal damage, and fibrous bundle demyelination is also evident (Papadopoulos et al., 2009). Recent studies have reported abnormal changes in FA values or MD values in cortical or subcortical lesions in RRMS patients (Ciccarelli et al., 2001; Ceccarelli et al., 2007; Preziosa et al., 2011). Although MD values are usually increased, FA values may either increase (Ciccarelli et al., 2001; Tovar-Moll et al., 2009) or decrease (Ceccarelli et al., 2007). These inconsistent changes could be explained by grey matter inflammation, where the resting state or activation of microglia during grey matter inflammation could lead to an increase or decrease in FA values, respectively (Calabrese et al., 2011). In this study, FA values in both hippocampal regions decreased, indicating that this brain region was inflamed. The FA value of the left hippocampus was greater than that of the right hippocampus because the FA value was positively correlated with the voxel size (Han et al., 2017). We postulate that the volume shrinkage of the right hippocampus was more obvious than that of the left in this study, which is consistent with previous results (Roosendaal et al., 2010).
We assessed structural and functional changes in hippocampal memory networks and their association with cognitive dysfunction in RRMS patients. Our study demonstrates that whole-brain FC was abnormal in the bilateral hippocampi, but only abnormal left hippocampal connectivity was associated with cognitive function. The lateralization and asynchrony of hippocampal damage observed in RRMS patients may indicate that bilateral hippocampal damage occurs in a different time frame, although more evidence is needed on this topic (Roosendaal et al., 2010). The results from this study reveal that the whole-brain FC of the right hippocampus was negatively correlated with the EDSS score. Previous studies have suggested that gray matter atrophy is associated with white matter fiber damage (Koubiyr et al., 2018). We postulate that the hippocampus directly or indirectly affects the degree of disability in patients with RRMS through white matter fiber damage. A reduction in hippocampal volume is associated with impairment of general knowledge and contextual visual memory (Resmini et al., 2012). Fjalldal et al. (2018) reported that changes in white matter integrity in the right hippocampus were associated with decreased visual spatial capacity, whereas decreased white matter integrity and demyelination/edema in the left hippocampus were associated with impaired general knowledge and delayed recall in episodic memory. Here, we report a correlation between functional and structural changes in the hippocampus. The changes in the ALFF value, ReHo value, and ipsilateral FA value in the right hippocampus were particularly robust. We thus conclude that local hippocampal functional changes are associated with fibrous bundle damage. Previous studies have reported that gray matter atrophy was strongly correlated with the FA values in white matter fibrous bundles (Han et al., 2017). We postulate that local hippocampal functional changes are associated with hippocampal volume shrinkage and white matter fiber damage. There are numerous causes for the decreases in cognitive ability associated with changes in hippocampal function and volume. Previous studies have reported that a slight decrease in FC could occur in patients with normal hippocampal volume, suggesting that FC changes can occur before hippocampal volume atrophy takes place (Roosendaal et al., 2010). Longitudinal studies are needed to clarify how FC changes are related to atrophy in hippocampal memory systems.
Improvements in the reliability and repeatability of functional connectivity models will enhance the utility of this approach (Penner and Aktas, 2017). Currently, it is difficult to determine whether individual changes are normal compensatory phenomena (Rocca and Filippi, 2017) or pathological changes. Therefore, to examine differences in FC in the brain, the underlying disease mechanisms must be combined with the characteristics of the disease (Abrol et al., 2017). Alterations in brain FC is expected to provide potential biomarkers (Du et al., 2010) for classifying or predicting brain disorders, enabling diagnoses at the early stage of disease.
There are some limitations to this study. First, this study was a preliminary cross-sectional study. Future longitudinal studies are warranted to investigate the reproducibility of the FC and DTI abnormalities in the hippocampus of MS patients, and longitudinal studies are necessary to clarify how FC changes are related to hippocampal memory system atrophy. Second, this study had a small sample size (20 subjects). Further studies with a larger sample size and comprehensive clinical data are warranted to explore the correlations between FC, structural abnormalities in the hippocampus, and clinical metrics.
In conclusion, the FC and structural abnormalities of the hippocampus are correlated with the degree of cognitive function and extent of disability in RRMS patients.
Acknowledgments: Thanks to Yingyu Zhang and Xinying Wang for their great help about the Image processing and format of the references.
Additional file (87.3KB, pdf) : Open peer review report 1.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This study was supported by the Project of International Cooperation of Jilin Province in China, No. 20180414062GH (to XMH) and Health research talents Project of Jilin Province in China, No. 2019sc2 018 (to XMH). The funding sources had no role in study conception and design, data analysis or interpretation, paper writing or deciding to submit this paper for publication.
Institutional review board statement: The study was approved by the Ethics Committee of China-Japan Union Hospital of Jilin University, China (approval No. 201702202) on February 22, 2017.
Declaration of patient consent: The authors certify that they will obtain all appropriate patient consent forms. In the form, the patients will give their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity.
Reporting statement: The writing and editing of the article was performed in accordance with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) Statement statement.
Biostatistics statement: The statistical methods of this study were reviewed by China-Japan Union hospital of Jilin University, China.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: Tetsuya Akaishi, Tohoku University, Japan.
Funding: This study was supported by the Project of International Cooperation of Jilin Province in China, No. 20180414062GH (to XMH) and Health research talents Project of Jilin Province in China, No. 2019sc2018 (to XMH).
P-Reviewer: Akaishi T; C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Koke S, Norman C, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Abrol A, Damaraju E, Miller RL, Stephen JM, Claus ED, Mayer AR, Calhoun VD. Replicability of time-varying connectivity patterns in large resting state fMRI samples. NeuroImage. 2017;163:160–176. doi: 10.1016/j.neuroimage.2017.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amin M, Ontaneda D. Thalamic injury and cognition in multiple sclerosis. Front Neurol. 2020;11:623914. doi: 10.3389/fneur.2020.623914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagnato F, Franco G, Li H, Kaden E, Ye F, Fan R, Chen A, Alexander DC, Smith SA, Dortch R, Xu J. Probing axons using multi-compartmental diffusion in multiple sclerosis. Ann Clin Transl Neurol. 2019;6:1595–1605. doi: 10.1002/acn3.50836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Calabrese M, Rinaldi F, Seppi D, Favaretto A, Squarcina L, Mattisi I, Perini P, Bertoldo A, Gallo P. Cortical diffusion-tensor imaging abnormalities in multiple sclerosis: a 3-year longitudinal study. Radiology. 2011;261:891–898. doi: 10.1148/radiol.11110195. [DOI] [PubMed] [Google Scholar]
- 5.Ceccarelli A, Rocca MA, Falini A, Tortorella P, Pagani E, Rodegher M, Comi G, Scotti G, Filippi M. Normal-appearing white and grey matter damage in MS. A volumetric and diffusion tensor MRI study at 3.0 Tesla. J Neurol. 2007;254:513–518. doi: 10.1007/s00415-006-0408-4. [DOI] [PubMed] [Google Scholar]
- 6.Chai XJ, Ofen N, Jacobs LF, Gabrieli JD. Scene complexity: influence on perception, memory, and development in the medial temporal lobe. Front Human Neurosci. 2010;4:21. doi: 10.3389/fnhum.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan D, Binks S, Nicholas JM, Frost C, Cardoso MJ, Ourselin S, Wilkie D, Nicholas R, Chataway J. Effect of high-dose simvastatin on cognitive, neuropsychiatric, and health-related quality-of-life measures in secondary progressive multiple sclerosis: secondary analyses from the MS-STAT randomised, placebo-controlled trial. Lancet Neurol. 2017;16:591–600. doi: 10.1016/S1474-4422(17)30113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Z, An Y, Zhao B, Yang W, Yu Q, Cai L, Ni H, Yin J. The value of resting-state functional magnetic resonance imaging for detecting epileptogenic zones in patients with focal epilepsy. PLoS One. 2017;12:e0172094. doi: 10.1371/journal.pone.0172094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ciccarelli O, Werring DJ, Wheeler-Kingshott CA, Barker GJ, Parker GJ, Thompson AJ, Miller DH. Investigation of MS normal-appearing brain using diffusion tensor MRI with clinical correlations. Neurology. 2001;56:926–933. doi: 10.1212/wnl.56.7.926. [DOI] [PubMed] [Google Scholar]
- 10.Defrancesco M, Marksteiner J, Deisenhammer E, Kemmler G, Djurdjevic T, Schocke M. Impact of white matter lesions and cognitive deficits on conversion from mild cognitive impairment to Alzheimer's disease. J Alzheimer's Dis. 2013;34:665–672. doi: 10.3233/JAD-122095. [DOI] [PubMed] [Google Scholar]
- 11.Du Y, Fu Z, Calhoun VD. Classification and prediction of brain disorders using functional connectivity: promising but challenging. Front Neurosci. 2018;12:525. doi: 10.3389/fnins.2018.00525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duff EP, Johnston LA, Xiong J, Fox PT, Mareels I, Egan GF. The power of spectral density analysis for mapping endogenous BOLD signal fluctuations. Human Brain Mapp. 2008;29:778–790. doi: 10.1002/hbm.20601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fein G, Di Sclafani V, Tanabe J, Cardenas V, Weiner MW, Jagust WJ, Reed BR, Norman D, Schuff N, Kusdra L, Greenfield T, Chui H. Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology. 2000;55:1626–1635. doi: 10.1212/wnl.55.11.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feinstein A, O’Connor P, Akbar N, Moradzadeh L, Scott CJ, Lobaugh NJ. Diffusion tensor imaging abnormalities in depressed multiple sclerosis patients. Multi Scler. 2010;16:189–196. doi: 10.1177/1352458509355461. [DOI] [PubMed] [Google Scholar]
- 15.Filippi M, Agosta F, Spinelli EG, Rocca MA. Imaging resting state brain function in multiple sclerosis. J Neurol. 2013;260:1709–1713. doi: 10.1007/s00415-012-6695-z. [DOI] [PubMed] [Google Scholar]
- 16.Filippi M, Rocca MA. Present and future of fMRI in multiple sclerosis. Expert Rev Neurother. 2013;13:27–31. doi: 10.1586/14737175.2013.865871. [DOI] [PubMed] [Google Scholar]
- 17.Filippi M, Rocca MA, Ciccarelli O, De Stefano N, Evangelou N, Kappos L, Rovira A, Sastre-Garriga J, Tintorè M, Frederiksen JL, Gasperini C, Palace J, Reich DS, Banwell B, Montalban X, Barkhof F. MAGNIMS Study Group (2016) MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. Lancet Neurol. 15:292–303. doi: 10.1016/S1474-4422(15)00393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fjalldal S, Follin C, Svärd D, Rylander L, Gabery S, Petersén Å, van Westen D, Sundgren PC, Björkman-Burtscher IM, Lätt J, Ekman B, Johanson A, Erfurth EM. Microstructural white matter alterations and hippocampal volumes are associated with cognitive deficits in craniopharyngioma. Eur J Endocrinol. 2018;178:577–587. doi: 10.1530/EJE-18-0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaetani L, Salvadori N, Chipi E, Gentili L, Borrelli A, Parnetti L, Di Filippo M. Cognitive impairment in multiple sclerosis: lessons from cerebrospinal fluid biomarkers. Neural Regen Res. 2021;16:36–42. doi: 10.4103/1673-5374.286949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han XM, Tian HJ, Han Z, Zhang C, Liu Y, Gu JB, Bakshi R, Cao X. Correlation between white matter damage and gray matter lesions in multiple sclerosis patients. Neural Regen Res. 2017;12:787–794. doi: 10.4103/1673-5374.206650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heine J, Prüß H, Scheel M, Brandt AU, Gold SM, Bartsch T, Paul F, Finke C. Transdiagnostic hippocampal damage patterns in neuroimmunological disorders. NeuroImage Clin. 2020;28:102515. doi: 10.1016/j.nicl.2020.102515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hulst HE, Schoonheim MM, Roosendaal SD, Popescu V, Schweren LJ, van der Werf YD, Visser LH, Polman CH, Barkhof F, Geurts JJ. Functional adaptive changes within the hippocampal memory system of patients with multiple sclerosis. Human Brain Mapp. 2012;33:2268–2280. doi: 10.1002/hbm.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang L, Zuo XN. Regional homogeneity: a multimodal, multiscale neuroimaging marker of the human connectome. Neuroscientist. 2016;22:486–505. doi: 10.1177/1073858415595004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karavasilis E, Christidi F, Velonakis G, Tzanetakos D, Zalonis I, Potagas C, Andreadou E, Efstathopoulos E, Kilidireas C, Kelekis N, Evdokimidis I. Hippocampal structural and functional integrity in multiple sclerosis patients with or without memory impairment: a multimodal neuroimaging study. Brain Imaging Behav. 2019;13:1049–1059. doi: 10.1007/s11682-018-9924-y. [DOI] [PubMed] [Google Scholar]
- 25.Klawiter EC, Schmidt RE, Trinkaus K, Liang HF, Budde MD, Naismith RT, Song SK, Cross AH, Benzinger TL. Radial diffusivity predicts demyelination in ex vivo multiple sclerosis spinal cords. NeuroImage. 2011;55:1454–1460. doi: 10.1016/j.neuroimage.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koenig KA, Rao SM, Lowe MJ, Lin J, Sakaie KE, Stone L, Bermel RA, Trapp BD, Phillips MD. The role of the thalamus and hippocampus in episodic memory performance in patients with multiple sclerosis. Mult Scler. 2019;25:574–584. doi: 10.1177/1352458518760716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koubiyr I, Deloire M, Coupé P, Dulau C, Besson P, Moroso A, Planche V, Tourdias T, Brochet B, Ruet A. Differential gray matter vulnerability in the 1 year following a clinically isolated syndrome. Front Neurol. 2018;9:824. doi: 10.3389/fneur.2018.00824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuceyeski A, Monohan E, Morris E, Fujimoto K, Vargas W, Gauthier SA. Baseline biomarkers of connectome disruption and atrophy predict future processing speed in early multiple sclerosis. NeuroImage Clin. 2018;19:417–424. doi: 10.1016/j.nicl.2018.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lashkari A, Davoodi-Bojd E, Fahmy L, Li L, Nejad-Davarani SP, Chopp M, Jiang Q, Cerghet M. Impairments of white matter tracts and connectivity alterations in five cognitive networks of patients with multiple sclerosis. Clin Neurol Neurosurg. 2021;201:106424. doi: 10.1016/j.clineuro.2020.106424. [DOI] [PubMed] [Google Scholar]
- 30.Laura G, Silvia T, Nikolaos P, Patrizia P. The role of fMRI in the assessment of neuroplasticity in ms: a systematic review. Neural Plast 2018. 2018 doi: 10.1155/2018/3419871. 3419871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li Q, Zhao LQ, Hu FY. Characteristics of cognitive impairment and the resting state functional MRI in patients with leukoaraiosis. Zhonghua Yi Xue Za Zhi. 2017;97:3529–3533. doi: 10.3760/cma.j.issn.0376-2491.2017.45.003. [DOI] [PubMed] [Google Scholar]
- 32.Mainero C, Caramia F, Pozzilli C, Pisani A, Pestalozza I, Borriello G, Bozzao L, Pantano P. fMRI evidence of brain reorganization during attention and memory tasks in multiple sclerosis. NeuroImage. 2004;21:858–867. doi: 10.1016/j.neuroimage.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Margulies DS, Böttger J, Long X, Lv Y, Kelly C, Schäfer A, Goldhahn D, Abbushi A, Milham MP, Lohmann G, Villringer A. Resting developments: a review of fMRI post-processing methodologies for spontaneous brain activity. MAGMA. 2010;23:289–307. doi: 10.1007/s10334-010-0228-5. [DOI] [PubMed] [Google Scholar]
- 34.Martínez-Heras E, Solana E, Prados F, Andorrà M, Solanes A, López-Soley E, Montejo C, Pulido-Valdeolivas I, Alba-Arbalat S, Sola-Valls N, Sepúlveda M, Blanco Y, Saiz A, Radua J, Llufriu S. Characterization of multiple sclerosis lesions with distinct clinical correlates through quantitative diffusion MRI. NeuroImage Clin. 2020;28:102411. doi: 10.1016/j.nicl.2020.102411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 36.Ontaneda D, Fox RJ. Imaging as an outcome measure in multiple sclerosis. Neurotherapeutics. 2017;14:24–34. doi: 10.1007/s13311-016-0479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oset M, Stasiolek M, Matysiak M. Cognitive dysfunction in the early stages of multiple sclerosis-how much and how important? Curr Neurol Neurosci Rep. 2020;20:22. doi: 10.1007/s11910-020-01045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Papadopoulos D, Dukes S, Patel R, Nicholas R, Vora A, Reynolds R. Substantial archaeocortical atrophy and neuronal loss in multiple sclerosis. Brain Pathol. 2009;19:238–253. doi: 10.1111/j.1750-3639.2008.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Penner IK, Aktas O. Functional reorganization is a maladaptive response to injury - NO. Mult Scler. 2017;23:193–194. doi: 10.1177/1352458516679895. [DOI] [PubMed] [Google Scholar]
- 40.Penner IK, Rausch M, Kappos L, Opwis K, Radü EW. Analysis of impairment related functional architecture in MS patients during performance of different attention tasks. J Neurol. 2003;250:461–472. doi: 10.1007/s00415-003-1025-0. [DOI] [PubMed] [Google Scholar]
- 41.Planche V, Koubiyr I, Romero JE, Manjon JV, Coupé P, Deloire M, Dousset V, Brochet B, Ruet A, Tourdias T. Regional hippocampal vulnerability in early multiple sclerosis: dynamic pathological spreading from dentate gyrus to CA1. Human Brain Mapp. 2018;39:1814–1824. doi: 10.1002/hbm.23970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Planche V, Ruet A, Coupé P, Lamargue-Hamel D, Deloire M, Pereira B, Manjon JV, Munsch F, Moscufo N, Meier DS, Guttmann CR, Dousset V, Brochet B, Tourdias T. Hippocampal microstructural damage correlates with memory impairment in clinically isolated syndrome suggestive of multiple sclerosis. Multi Scler. 2017;23:1214–1224. doi: 10.1177/1352458516675750. [DOI] [PubMed] [Google Scholar]
- 43.Pokryszko-Dragan A, Banaszek A, Nowakowska-Kotas M, Jeżowska-Jurczyk K, Dziadkowiak E, Gruszka E, Zagrajek M, Bilińska M, Budrewicz S, Sąsiadek M, Bladowska J. Diffusion tensor imaging findings in the multiple sclerosis patients and their relationships to various aspects of disability. J Neurol Sci. 2018;391:127–133. doi: 10.1016/j.jns.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Preziosa P, Rocca MA, Mesaros S, Pagani E, Stosic-Opincal T, Kacar K, Absinta M, Caputo D, Drulovic J, Comi G, Filippi M. Intrinsic damage to the major white matter tracts in patients with different clinical phenotypes of multiple sclerosis: a voxelwise diffusion-tensor MR study. Radiology. 2011;260:541–550. doi: 10.1148/radiol.11110315. [DOI] [PubMed] [Google Scholar]
- 46.Resmini E, Santos A, Gómez-Anson B, Vives Y, Pires P, Crespo I, Portella MJ, de Juan-Delago M, Barahona MJ, Webb SM. Verbal and visual memory performance and hippocampal volumes, measured by 3-Tesla magnetic resonance imaging, in patients with Cushing's syndrome. J Clin Endocrinol Metab. 2012;97:663–671. doi: 10.1210/jc.2011-2231. [DOI] [PubMed] [Google Scholar]
- 47.Rocca MA, Barkhof F, De Luca J, Frisén J, Geurts JJG, Hulst HE, Sastre-Garriga J, Filippi M. The hippocampus in multiple sclerosis. Lancet Neurol. 2018;17:918–926. doi: 10.1016/S1474-4422(18)30309-0. [DOI] [PubMed] [Google Scholar]
- 48.Rocca MA, Filippi M. Functional reorganization is a maladaptive response to injury - YES. Mult Scler. 2017;23:191–193. doi: 10.1177/1352458516667242. [DOI] [PubMed] [Google Scholar]
- 49.Roosendaal SD, Hulst HE, Vrenken H, Feenstra HE, Castelijns JA, Pouwels PJ, Barkhof F, Geurts JJ. Structural and functional hippocampal changes in multiple sclerosis patients with intact memory function. Radiology. 2010;255:595–604. doi: 10.1148/radiol.10091433. [DOI] [PubMed] [Google Scholar]
- 50.Sacco R, Bonavita S, Esposito F, Tedeschi G, Gallo A. The contribution of resting state networks to the study of cortical reorganization in MS. Multi Scler Int 2013. 2013 doi: 10.1155/2013/857807. 857807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sampath D, Sathyanesan M, Newton SS. Cognitive dysfunction in major depression and Alzheimer's disease is associated with hippocampal-prefrontal cortex dysconnectivity. Neuropsychiatr Dis Treat. 2017;13:1509–1519. doi: 10.2147/NDT.S136122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sbardella E, Tona F, Petsas N, Pantano P. DTI measurements in multiple sclerosis: evaluation of brain damage and clinical implications. Multi Scler Int 2013. 2013 doi: 10.1155/2013/671730. 671730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schneider R, Genç E, Ahlborn C, Gold R, Lukas C, Bellenberg B. Temporal dynamics of diffusion metrics in early multiple sclerosis and clinically isolated syndrome: a 2-year follow-up tract-based spatial statistics study. Front Neurol. 2019;10:1165. doi: 10.3389/fneur.2019.01165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sicotte NL, Kern KC, Giesser BS, Arshanapalli A, Schultz A, Montag M, Wang H, Bookheimer SY. Regional hippocampal atrophy in multiple sclerosis. Brain. 2008;131:1134–1141. doi: 10.1093/brain/awn030. [DOI] [PubMed] [Google Scholar]
- 55.Stock B, Shrestha M, Seiler A, Foerch C, Hattingen E, Steinmetz H, Deichmann R, Wagner M, Gracien RM. Distribution of cortical diffusion tensor imaging changes in multiple sclerosis. Front Physiol. 2020;11:116. doi: 10.3389/fphys.2020.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Toko M, Kitamura J, Ueno H, Ohshita T, Nemoto K, Ochi K, Higaki T, Akiyama Y, Awai K, Maruyama H. Prospective memory deficits in multiple sclerosis: voxel-based morphometry and double inversion recovery analysis. Int Med. 2021;60:39–46. doi: 10.2169/internalmedicine.5058-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tovar-Moll F, Evangelou IE, Chiu AW, Richert ND, Ostuni JL, Ohayon JM, Auh S, Ehrmantraut M, Talagala SL, McFarland HF, Bagnato F. Thalamic involvement and its impact on clinical disability in patients with multiple sclerosis: a diffusion tensor imaging study at 3T. AJNR Am J Neuroradiol. 2009;30:1380–1386. doi: 10.3174/ajnr.A1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 59.van Geest Q, Hulst HE, Meijer KA, Hoyng L, Geurts JJG, Douw L. The importance of hippocampal dynamic connectivity in explaining memory function in multiple sclerosis. Brain Behav. 2018;8:e00954. doi: 10.1002/brb3.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vrenken H, Pouwels PJ, Geurts JJ, Knol DL, Polman CH, Barkhof F, Castelijns JA. Altered diffusion tensor in multiple sclerosis normal-appearing brain tissue: cortical diffusion changes seem related to clinical deterioration. J Magn Reson Imaging. 2006;23:628–636. doi: 10.1002/jmri.20564. [DOI] [PubMed] [Google Scholar]
- 61.Wingerchuk DM, Lennon VA, Pittock SJ, Lucchinetti CF, Weinshenker BG. Revised diagnostic criteria for neuromyelitis optica. Neurology. 2006;66:1485–1489. doi: 10.1212/01.wnl.0000216139.44259.74. [DOI] [PubMed] [Google Scholar]
- 62.Yamout BI, Alroughani R. Multiple sclerosis. Semin Neurol. 2018;38:212–225. doi: 10.1055/s-0038-1649502. [DOI] [PubMed] [Google Scholar]
- 63.Yang Y, Raine A, Han CB, Schug RA, Toga AW, Narr KL. Reduced hippocampal and parahippocampal volumes in murderers with schizophrenia. Psychiatry Res. 2010;182:9–13. doi: 10.1016/j.pscychresns.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu HJ, Christodoulou C, Bhise V, Greenblatt D, Patel Y, Serafin D, Maletic-Savatic M, Krupp LB, Wagshul ME. Multiple white matter tract abnormalities underlie cognitive impairment in RRMS. NeuroImage. 2012;59:3713–3722. doi: 10.1016/j.neuroimage.2011.10.053. [DOI] [PubMed] [Google Scholar]
- 65.Zhao L, Ng A, Chen Q, Lam B, Abrigo J, Au C, Mok VCT, Wong A, Lau AY. Impaired cognition is related to microstructural integrity in relapsing remitting multiple sclerosis. Ann Clin Transl Neurol. 2020;7:1193–1203. doi: 10.1002/acn3.51100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, Castellanos FX, Biswal BB, Milham MP. The oscillating brain: complex and reliable. NeuroImage. 2010;49:1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.