Abstract
Currently, large numbers of clinical trials are performed to investigate different forms of experimental therapy for patients suffering from chronic spinal cord injury (SCI). However, for the enrollment process, there are different views on how the time period between injury and interventions should be determined. Herein, we sought to evaluate the impact of time-to-enrollment in chronic SCI clinical trials. A data set comprising 957 clinical studies from clinicalTrials.gov was downloaded and analyzed focusing on the eligibility criteria for post-injury time-to-enrollment. We also aggregated individual patient data from nine clinical trials of regenerative interventions for chronic SCI selected by a systematic literature search from 1990 to 2018. Characteristics of the studies were assessed and compared by dividing into three groups based on time-to-enrollment (group 1 ≤ 12 months, group 2 = 12–23 months and group 3 ≥ 24 months). In ClinicalTrials.gov registry, 445 trials were identified for chronic SCI where 87% (385) were unrestricted in the maximum post-injury time for trial eligibility. From systematic literature search, nine studies and 156 patients (group 1 = 30, group 2 = 55 and group 3 = 71) were included. The range of time-to-enrollment was 0.5 to 321 months in those studies. We also observed various degrees of motor and sensory improvement in between three time-to-enrollment groups. Our results indicate that enrolling wide ranges of time-to-enrollment in a group may present imprecise outcomes. Clinical trial designs should consider appropriate post-injury time frames to evaluate therapeutic benefit.
Key Words: chronic, clinical trial, spinal cord injury, systematic review, time-to-enrollment
Introduction
Spinal cord injury (SCI) is a chronic disability with neurological impairment that is currently being managed symptomatically with no effective treatment available. According to pathophysiological response to trauma, SCI can be divided into three phases: acute, subacute, and chronic (Kim et al, 2017; Dalamagkas et al., 2018). Among them, the chronic phase is defined when inflammation has subsided and any kind of neural plasticity and spontaneous regeneration has already failed (Dalamagkas et al., 2018). Regenerative medicine is an exciting and promising approach for the treatment of chronic spinal cord injury. Currently, there are several clinical trials worldwide that attempt to deliver feasibility/proof of concept for regenerative therapies (Kim et al., 2017). A common underlying premise among previous studies suggests that the interval of time between injury and initiation of the experimental treatment has been thought to play a crucial role in recovery. This idea stems from multiple mechanisms of injury occurring at different time points after the initial insult (Ahuja et al., 2017). However, the chronic phase encompasses a broad range of months and years, and it is common among physician practice to encounter non-static injury profiles at multiple time points in a given patent's follow-up visits (Gomes-Osman et al., 2016). In this point, researchers consider paucity of neurological improvement for a certain period to enroll chronic SCI patients for a trial. However, it is unlikely to expect equivalent responses in all patients with a broad range of post injury time (PIT). We believe that including patients with wide-ranging injury durations undergoing an experimental therapy may not allow for adequate generalizability of the results.
In this review, we initially examined the characteristics of clinical trials in the spinal cord injury field contained in the ClinicalTrial.gov registry with a focus on the eligibility of patients based on post-injury time-to-enrollment for enrollment in a trial. Then, we evaluated the effect of time-to-enrollment on outcomes in patients with chronic SCI by performing a comprehensive literature search including clinical trials using regenerative intervention for chronic SCI from 1990 to 2018. Only studies with reported individual patient data were included and subsequently analyzed by different time-to-enrollment groups to observe the characteristics of outcomes of their experimental therapies.
Data and Methods
ClinicalTrials.gov registry data
Our analysis was restricted to “Spinal cord injury” clinical trials registered with ClinicalTrials.gov until April 2020 (Gomes-Osman et al., 2016). A data set of 957 clinical studies with a status of “Not yet recruiting”, “Recruiting”, “Enrolling by invitation”, “Active, not recruiting”, or “Completed” registered with ClinicalTrials.gov was downloaded and locked from the website on April 4th, 2020. Trial data was reported by the trial sponsors or investigators as required by the ClinicalTrials.gov registry (Zarin et al., 2011). Each record contained a set of data elements describing the study's condition, enrolment, study design, eligibility criteria, and other protocol information. Two investigators identified the post-injury enrollment time frame from the eligibility criteria. All trials were divided into “Acute”, defined as 2 weeks or below, “Chronic”, defined as above 2 weeks, and “Others”, defined as no specified PIT frame. For chronic SCI trials, we subdivided groups into “3 months and longer”, “6 months and longer”, “12 months and longer” and “18 months and longer” depending on each trial's eligibility criteria. Further, trials were subdivided by restricted and unrestricted maximum PIT. The characteristics of the trials were assessed overall and presented as percentages.
Literature search strategy and selection criteria
A comprehensive search of PubMed, Ovid MEDLINE and Ovid EMBASE databases was conducted for all clinical trials treating SCI using keywords ‘spinal cord injury’, ‘treatment’, ‘regenerative’, ‘cellular’, ‘biomaterial’, ‘scaffold’, ‘stem cell’ and ‘stimulation’. A manual search was also conducted to identify all other eligible studies that may have been missed during the original query. Among all identified studies, screening for eligibility according to inclusion and exclusion criteria was conducted by two investigators (YUY and WW), and disagreements were resolved by a third investigator (FMM). The following inclusion criteria were applied to all search results: 1) clinical trials using regenerative treatments including cellular or biomaterial therapy in purpose to restore neurological function; 2) for chronic spinal cord injury patients who received treatment not less than 0.5 months after injury, 3) report individual patient data on outcomes of interest; 4) have at least 6 months of follow-up, and 5) are published in English language. Full electronic search strategy is presented in Additional file 1.
Outcomes of interest
The following variables were extracted: first author, year of publication, age, gender, level of injury, time-to-enrollment (in months), type of intervention, baseline International Standards for Neurological Classification of Spinal Cord Injury (ISNCSCI) motor score, baseline ISNCSCI sensory (or pinprick + light touch) score, baseline ASIA impairment scale, ISNCSCI motor score at last follow-up, ISNCSCI sensory score at last follow-up, and ASIA impairment scale (AIS) at last follow-up. Primary outcomes were: change in motor and sensory ISNCSCI scores and improvement of AIS grade.
International Standards for Neurological Classification of Spinal Cord Injury score
ISNCSCI score is a mathematical representation of injury severity in patients with spinal cord injury. The scoring is based on 2 major components (motor and sensory). Motor scores assess the muscle strength in 10 specified muscle groups on a 5-point scale (with a maximum unilateral total of 50 points). Sensory scores are assessed using pinprick and light touch sensations separately and use a 2-point scale for each dermatome (bilateral total of 112 points for each sensory evaluation). The evaluations are made for both sides of the body and all scores are added for a total score out of 324 (Kirshblum et al., 2011). The revised version of the scoring worksheet is presented as Additional file 2 (361.2KB, pdf) .
ASIA Impairment Scale
ASIA Impairment Scale (AIS) grading stratifies the severity of impairment for spinal cord injury patients in five grades: A through E (Kirshblum et al., 2011). The scale is adopted from a previously used neurological assessment scale called “Frankel Classification”(Cantu et al., 2013). Similar to Frankel Classification, AIS categorizes various levels of injury with Grade A being the most severe and “complete” impairment while Grade E representing normal neurological function.
Statistical analysis
All patients were divided into three groups based on their time-to-enrollment interval (Group 1: < 12 months, Group 2: 12–23 months and Group 3: ≥ 24 months). Mean and standard deviations of motor and sensory ISNCSCI scores were calculated of the three groups with regards to primary outcomes were conducted.
Results
Characteristics of SCI trials in ClinicalTrials.gov registry
In order to observe how researchers are defining the enrollment criteria for chronic SCI trials, we analyzed 957 trials registered in ClinicalTrials.gov registry. Of these trials, 46% (445) of trials were for chronic SCI, 3% (28) for acute SCI and the remaining 51% of trials were missing or did not restrict the PIT in the eligibility criteria (Figure 1A). Among the chronic SCI trials, we observed 87% (385) that did not limit the maximum PIT while only 13% (60) of trials restricted maximum PIT for their eligibility. The range of maximum PIT was 1 month to 10 years. We also observed higher trends of unrestricted trials in each subcategory (Figure 1B). It means that there is a high probability of participants enrolled with a wide range of PITs.
Figure 1.
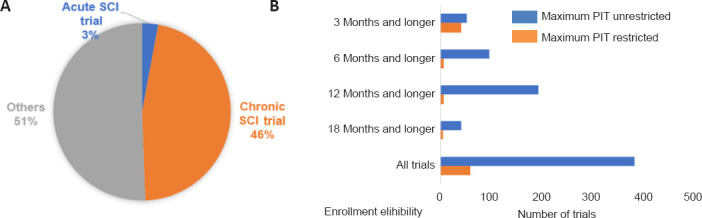
Characteristics of studies in ClinicalTrials.gov.
(A) Distribution of trials according to eligibility criteria status by “Acute” as 2 weeks or below, “Chronic” as above 2 weeks and “Others” as nothing available information about PIT frame. (B) Characteristics of eligibility of PIT frame of chronic SCI trials. Trials were subdivided by restricted and unrestricted maximum PIT. PIT: Post-injury time; SCI: spinal cord injury.
Search results, studies and patient characteristics
To understand the range of time-to-enrollment in clinical trials, we performed a literature search which revealed a total of 508 studies (Figure 2). Following inclusion and exclusion criteria evaluation, 19 studies remained and were evaluated for individual patient data after a primary screening of article title and abstracts. Of the 19 studies, nine clinical trials which presented individual patient data were included in the final analysis (Table 1; Grijalva et al., 2010; Lima et al., 2010; Rao et al., 2013; Chen et al., 2014; El-Kheir et al., 2014; Mendonça et al., 2014; Shin et al., 2015; Hur et al., 2016; Vaquero et al., 2016). Median (range) time-to-enrollment was 18.5 months (0.5–321). Specifically, five studies (Grijalva et al., 2010; Lima et al., 2010; Chen et al., 2014; Mendonca et al., 2014; Vaquero et al., 2016) included participants who had a wide range of time-to-enrollment (Figure 3). This wide variability could potentially affect the outcome of chronic SCI.
Figure 2.
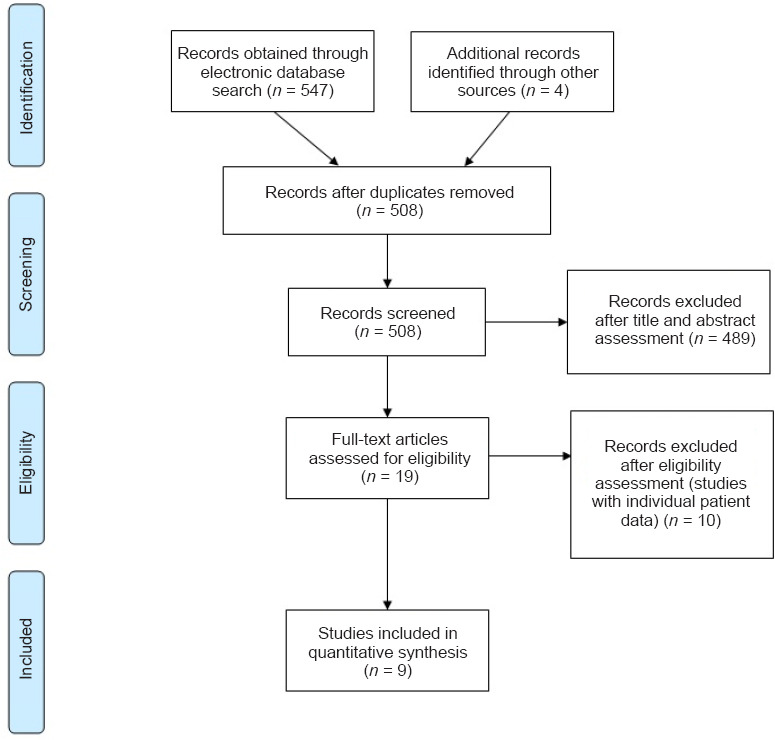
PRISMA flow diagram of search strategy and study selection.
PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1.
Characteristics of included studies
| Study | Country | Sample size | Type of treatment | Range time-to-enrollment (mon) |
|---|---|---|---|---|
| Grijalva et al., 2010 | Mexico | 8 Cervical | 4-Aminopyridine | 24–132 |
| 4 Thoracic | ||||
| Lima et al., 2010 | Portugal | 13 Cervical | Olfactory mucosal cells + rehabilitation | 18–189 |
| 7 Thoracic | ||||
| Rao et al., 2013 | China | 8 Cervical | Olfactory ensheathing cells | 8–15 |
| Chen et al., 2014 | China | 5 Cervical | 3 Olfactory ensheathing cells | 62–165 |
| 1 Schwann cells | ||||
| 1 Olfactory ensheathing cells + Schwann cells | ||||
| Control arm: 2 Cervical | Placebo | 16–186 | ||
| El-Kheir et al., 2014 | Egypt | 10 Cervical | Autologous bone marrow-derived cells + rehabilitation | 12–36 |
| 40 Thoracic | ||||
| Control arm: 5 Cervical | Placebo rehabilitation | N/A | ||
| 10 Thoracic | ||||
| Mendonca et al., 2014 | Brazil | 13 Thoracic | Bone marrow-derived mesenchymal stem cells + rehabilitation | 18–180 |
| Shin et al., 2015 | South Korea | 19 Cervical | Human neural stem/progenitor cells | 0.5–7.1 |
| Control arm: 15 Cervical | Placebo | 0.25–6 | ||
| Hur et al., 2016 | South Korea | 7 Cervical | Adipose-derived mesenchymal stem cells | 3–28 |
| 6 Thoracic | ||||
| Vaquero et al., 2016 | Spain | 12 Thoracic | Bone marrow-derived mesenchymal stem cells | 38–321 |
N/A: Not available.
Figure 3.
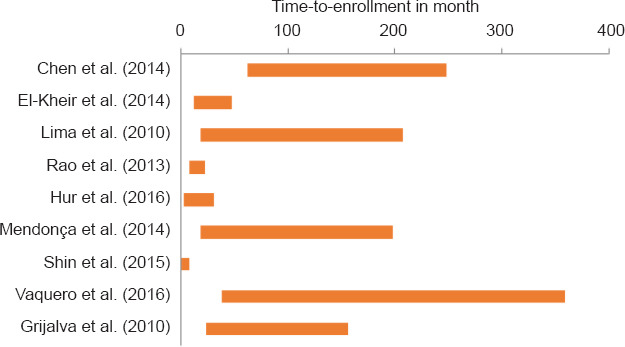
Range of time-to-enrollment of participants in each study.
Neurological recovery after SCI
To independently evaluate the effect of time-to-enrollment on chronic SCI outcomes, we separated the participants based on their time-to treatment into three groups in each trial. Several degrees of improvement were reported in AIS grade in a select group of subjects across all studies. Particularly, we observed total percentages of AIS grade improvement were varied between groups of time-to-enrollment within each study (Table 2). Although the majority of patients remained unchanged at last follow-up compared to baseline with regards to AIS including 67% (20/30) in the Group 1, 64% (35/55) in the Group 2 and 68% (48/71) in the Group 3 (Figure 4).
Table 2.
Change of AIS grade by time-to-enrollment groups
| Study | Total No. of patients | AIS grade change | Percent age of change | Group 1 | Group 2 | Group 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| No. of patients | AIS grade change | Percentage of change | No. of patients | AIS grade change | Percentage of change | No. of patients | AIS grade change | Percentage of change | ||||
| Grijalva et al., 2010 | 14 | 1 | 7 | 14 | 1 | 7 | ||||||
| Lima et al., 2010 | 20 | 6 | 30 | 3 | 0 | 0 | 17 | 8 | 47 | |||
| Rao et al., 2013 | 8 | 8 | 100 | 5 | 5 | 100% | 3 | 3 | 100 | |||
| Chen et al., 2014 | 5 | 0 | 0 | 5 | 0 | 0 | ||||||
| El-Kheir et al., 2014 | 50 | 17 | 34 | 41 | 15 | 37 | 9 | 2 | 22 | |||
| Mendonca et al., 2014 | 14 | 8 | 57 | 2 | 0 | 0 | 12 | 8 | 67 | |||
| Shin et al., 2015 | 19 | 5 | 16 | 19 | 5 | 26% | ||||||
| Hur et al., 2016 | 14 | 2 | 14 | 6 | 0 | 0% | 6 | 2 | 33 | 2 | 0 | 0 |
| Vaquero et al., 2016 | 12 | 4 | 33 | 12 | 4 | 33 | ||||||
Figure 4.
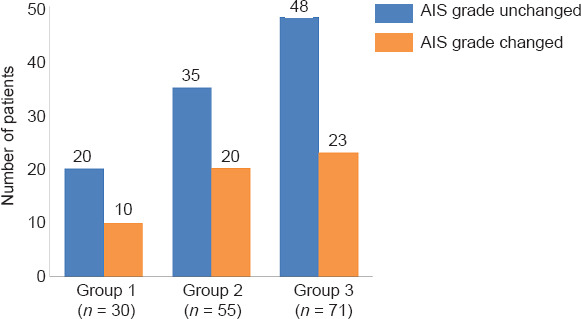
Distribution of number of patients with AIS grade change from baseline to last follow-up after therapeutic intervention in Group 1: < 12 months, Group 2: 12–23 months, and Group 3: ≥ 24 months.
AIS: ASIA impairment scale.
The average improvements in motor and sensory ISNCSCI scores in each study are displayed in Table 3. We observed that studies with available data had different degrees of improvement between the three time-to-enrollment groups. For example, results from a study by Hur et al. (2016) showed an average improvement in sensory ISNCSCI score of 11.0 ± 15.9, which included 17 ± 22.7, 7.3 ± 7.8 and 4 ± 5.7 in the Groups 1, 2 and 3, respectively (Table 3). It is important to mention that time-to-enrollment in chronic SCI may have a significant impact on outcomes.
Table 3.
Change in ASIA score by time-to-enrollment group
| Study | Motor score | Sensory score | Group 1 | Group 2 | Group 3 | |||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||
| Motor score | Sensory score | Motor score | Sensory score | Motor score | Sensory score | |||
| Grijalva et al., 2010 | N/A | N/A | ||||||
| Lima et al., 2010 | 3.2±3.8 | 11.9±29.6 | 1.3±1.2 | –2.3±3.2 | 3.5±4.0 | 14.4±31.5 | ||
| Rao et al., 2013 | N/A | N/A | ||||||
| Chen et al., 2014 | 0.4±0.5 | 3.8±4.3 | 0.4±0.5 | 3.8±4.3 | ||||
| El-Kheir et al., 2014 | 9.0±4.6 | 57.7±23.0 | 9.0±4.3 | 57.2±23.0 | 9.3±5.8 | 60±24.3 | ||
| Mendonça et al., 2014 | N/A | N/A | ||||||
| Shin et al., 2015 | 11.1±11.1 | 13.8±18 | 11.1±11.1 | 13.8±18 | ||||
| Hur et al., 2016 | 1.4±2.1 | 11.0±15.9 | 1.8±2.6 | 17.0±22.7 | 0.7±1.6 | 7.3±7.8 | 2.0±2.8 | 4.0±5.7 |
| Vaquero et al., 2016 | N/A | N/A | ||||||
Group 1: < 12 months, Group 2: 12–23 months and Group 3: ≥ 24 months. Data are expressed as the mean ± SD. N/A: Not available.
The average improvements in motor and sensory ISNCSCI scores in the three groups based on their initial AIS grades are presented in Table 4. Regardless of the type of therapy, the average improvement in motor scores for baseline AIS A was 6.59 ± 5.14 in the Group 1, 6.85 ± 5.98 in the Group 2 and 2.46 ± 4.14 in the Group 3. However, for baseline AIS B patients, the average motor score improvement was 25.3 ± 24.1 in the Group 1, 8.24 ± 4.15 in the Group 2 and 6.82 ± 5.62 in the Group 3. Interestingly, the average sensory score improvement was more prominent in the Group 2 (33.5 ± 32.2 and 58.8 ± 22.0 for both baseline AIS A and AIS B, respectively) (Table 4).
Table 4.
Change in ISNCSCI score by time-to-enrollment group
| ASIA score | Group 1 | Group 2 | Group 3 | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| AIS A | AIS B | AIS A | AIS B | AIS A | AIS B | |
| Motor | 6.59±5.14 (22) | 25.3±24.1 (3) | 6.85±5.98 (20) | 8.24±4.15 (29) | 2.46±4.14 (35) | 6.82±5.62 (11) |
| Sensory | 14.0±15.7 (22) | 19.0±40.0 (3) | 33.5±32.2 (20) | 58.8±22.0 (29) | 11.8±22.4 (35) | 37.4±46.0 (11) |
| Total | 24.2±18.7 (25) | 48.5±47.2 (4) | 40.3±35.7 (20) | 64.4±26.2 (31) | 14.3±24.6 (35) | 44.2±49.7 (11) |
Group 1: < 12 months, Group 2: 12–23 months and Group 3: ≥ 24 months. Data are expressed as the mean ± SD (N). AIS: ASIA impairment scale; ISNCSCI: International Standards for Neurological Classification of Spinal Cord Injury.
Discussion
Study design, specifically time-to-enrollment, has historically been inconsistent across SCI clinical trials particularly in studies evaluating chronic stage SCI where baseline functional status is most static and minimal spontaneous neurological improvement occurs. Here, our study provides important data that may shape and optimize future clinical trials. This method favors particular time-to-enrollment ranges depending on the targeted pathophysiology and mechanism of the therapeutic intervention being investigated. However, we acknowledge that the chronic injury period is potentially the most difficult time point to elicit biological influence over the injured spinal cord in a beneficial manner (Fawcett et al., 2007).
In our initial observation, we found that the trials related to chronic SCI in ClinicalTrials.gov registry mainly consisted of inclusion criteria with no upper limit on post-injury times for enrollment. Such a criterion leads to inclusion of participants in a trial with a wide range of post-injury time. These findings raise questions about the capacity of the trials to supply sufficient amounts of evidence of optimal time-to-enrollment for intervention. Hence, we included nine clinical trials for the treatment of chronic SCI patients using regenerative therapies where time-to-enrollment after SCI varied from 0.5 to 321 months. It will, therefore, be important to understand the relationship between treatment efficacy and how soon after injury the treatment should be initiated. A previous study by Kirshblum et al. (2011) examined the degree of spontaneous improvement with regards to improvement in AIS scale between 1 and 5 years after SCI in 987 subjects (Kirshblum et al., 2004). The authors noted that 5.6% of people with injuries classified as complete (AIS A) 1 year after SCI still converted to an incomplete injury by year 5, with 3.5% converting to AIS B and about 1% to either AIS C or AIS D (Kirshblum et al., 2004). This result suggests that, to some extent, spontaneous recovery overlaps with a therapeutic benefit. Therefore, well powered studies with robust control arms are necessary to differentiate the impact of treatment from spontaneous recovery. It is also unclear how best to determine individual therapeutic efficacy thresholds at different time points in the chronic stages of SCI. In other words, it is difficult to determine the therapeutic benefit for an individual on the basis of average outcome from a wide range of time-to-enrollment. Figure 4 demonstrates the distribution of patients and their improvement in AIS grade as a function of their respective time-to-enrollment. These results may serve as a suggestion for future investigators in designing clinical trials with the goal of maximizing therapeutic benefit. However, we strongly encourage adequate power in future trials to avoid statistical limitations resulting from small sample sizes across groups. In the present study, we acknowledge that this very issue, small sample sizes across groups, is a limitation but provides further justification for larger scale future studies.
In chronic SCI studies, the total ISNCSCI score may not be representative of the most important aspect of functional changes after SCI (Steeves et al., 2007). In many respects, the ISNCSCI motor score is considered more reliable than the ISNCSCI sensory score in predicting functional outcome after SCI (Marino and Graves, 2004). In clinical trials, it is necessary to establish a functionally meaningful ISNCSCI motor score threshold in order to report the benefit of a therapeutic intervention. In general, this threshold depends on both the level and severity of the SCI, as well as the degree of spontaneous recovery after SCI with conventional treatments. Previous studies have reported that a low cervical, ASIA A-injured patient is likely to spontaneously improve about 10 points in ISNCSCI motor score 1 year following SCI (Waters et al., 1993; Marino et al., 1999; Geisler et al., 2001). It was proposed that a response to treatment of an additional 10-point improvement in the ISNCSCI motor score (efficacy threshold now being 20 points) might be considered a valid primary outcome end point to demonstrate the efficacy of a therapeutic intervention (Fawcett et al., 2007). Our analysis with baseline AIS A presented 2.4 to 6.8 point improvement depending on their enrollment period. It might suggest that in chronic SCI where motor recovery has been relatively sieged the therapeutic efficacy threshold of ISNCSCI motor score should optimize according to the enrollment period.
The ISNCSCI sensory score has been recognized as a valid outcome measurement (Steeves et al., 2007). The lack of sophistication and highly variable light touch at different assessment times of sensory score does not seem useful. However, the ISNCSCI pin-prick score describes more accurately preserved spinal sensory function (e.g. sacral sparing in people with an ASIA B classification) (Crozier et al., 1991; Katoh and el Masry, 1995). It is still valuable for classifying and stratifying ISNCSCI sensory scores of participants for clinical trial to predict the future recovery. We indicated that time-to-enrollment had significant association with sensory and motor improvement.
The presented study has certain limitations. First, there are many more trials investigating experimental treatment modalities for spinal cord injury. However, the usual way of reporting includes a mean/median time period for study group(s). Therefore, only a portion of the clinical trials could be analyzed with regards to time-to-enrollment. Second, heterogeneity among studies by age, sex, level of injury, severity of injury is also accountable. Although the main purpose of the presented study is to evaluate the impact of time-to-enrollment, we speculate that more clear views can be obtained with a similar set of interventions.
Conclusion
Future studies are required to validate our findings with more precise understanding of optimal time-to-enrollment protocol therapies for chronic SCI patients. Results from this study may be used as supportive evidence to compare the therapeutic benefit of different interventions at particular time points following a SCI. Depending on the mechanism of proposed regenerative treatment, clinical trials should consider the appropriate timing after injury to start intervention.
Additional files:
Additional file 1: Search strategy.
Additional file 1.
Search Strategy
| Ovid | ||
|---|---|---|
| Database(s): Embase 1988 to 2018 Week 33, EBM Reviews -Cochrane Central Register of Controlled Trials July 2018, EBM Reviews -Cochrane Database of Systematic Reviews 2005 to August 8, 2018, Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations and Daily 1946 to August 14, 2018 Search Strategy: | ||
| # | Searches | Results |
| 1 | exp Spinal Cord Injuries/ | 104014 |
| 2 | exp Paraplegia/ | 29007 |
| 3 | exp Quadriplegia/ | 20703 |
| 4 | (“brown presentation” or “brown sequard disease” or “Brown Sequard syndrome” or “Brown-Sequards syndrome” or “central cord syndrome” or “central spinal cord syndrome” or “medullary transverse lesion*” or Paraplegia* or “Post Traumatic Myelopath*” or Quadriplegia* or “Spinal Cord Contusion*” or “Spinal Cord Injur*” or “Spinal Cord Laceration*” or “Spinal Cord Transection*” or “spinal cord transsection*” or “spinal cord transverse lesion*” or “Spinal Cord Trauma*” or “transverse cord lesion*” or “transverse lesion*” or “transverse spinal cord lesion*” or “Traumatic Myelopath*”).ti,ab,hw,kw. |
150827 |
| 5 | 1 or 2 or 3 or 4 | 168989 |
| 6 | exp Bone Marrow Transplantation/ | 98496 |
| 7 | exp Stem Cells/ | 483689 |
| 8 | exp Tissue Scaffolds/ | 29389 |
| 9 | exp Biocompatible Materials/ | 140685 |
| 10 | (((“bone marrow” or hematopoietic or haematopoietic) adj4 (transplant* or graft* or transfer* or transfus*)) or (embryonic adj2 cell*) or “Biocompatible Material*” or Biomaterial* or Cellular or “colony forming unit*” or “embryoid bodies” or “embryoid body” or haemangioblast or haemangioblasts or hemangioblast or hemangioblasts or “megakaryocyte erythroid progenitor*” or “mother cell*” or myoblast or myoblasts or “precursor cell*” or “progenitor cell*” or scaffold* or “side population cell*” or “stem cell*”).ti,ab,hw,kw. |
2763364 |
| 11 | 6 or 7 or 8 or 9 or 10 | 2805329 |
| 12 | 5 and 11 | 11539 |
| 13 | exp Time Factors/ | 1210355 |
| 14 | ("best time*" or earlier or earliest or early or late or later or latest or "time factor*" or timeliness or timing).ti,ab,hw,kw. | 5989767 |
| 15 | 13 or 14 | 5989767 |
| 16 | 12 and 15 | 1981 |
| 17 | exp controlled study/ | 5778758 |
| 18 | exp Randomized Controlled Trial/ | 937436 |
| 19 | exp triple blind procedure/ | 156 |
| 20 | exp Double-Blind Method/ | 410168 |
| 21 | exp Single-Blind Method/ | 73906 |
| 22 | exp latin square design/ | 338 |
| 23 | exp Placebos/ | 327932 |
| 24 | exp Placebo Effect/ | 10288 |
| 25 | ((control* adj3 study) or (control* adj3 trial) or (randomized adj3 study) or (randomized adj3 trial) or (randomised adj3 study) or (randomised adj3 trial) or "pragmatic clinical trial" or (doubl* adj blind*) or (doubl* adj mask*) or (singl* adj blind*) or (singl* adj mask*) or (tripl* adj blind*) or (tripl* adj mask*) or (trebl* adj blind*) or (trebl* adj mask*) or "latin square" or placebo* or nocebo* or random*).mp,pt. |
9059302 |
| 26 | or/17-25 | 9059442 |
| 27 | 16 and 26 | 550 |
| 28 | limit 27 to (editorial or erratum or note or addresses or autobiography or bibliography or biography or blogs or comment or dictionary or directory or interactive tutorial or interview or lectures or legal cases or legislation or news or newspaper article or overall or patient education handout or periodical index or portraits or published erratum or video-audio media or webcasts) [Limit not valid in Embase,CCTR,CDSR,Ovid MEDLINE(R),Ovid MEDLINE(R) Daily Update,Ovid MEDLINE(R) In-Process,Ovid MEDLINE(R) Publisher; records were retained] | 3 |
| 29 | 27 not 28 | 547 |
| 30 | remove duplicates from 29 | 504 |
Additional file 2 (361.2KB, pdf) : International Standards for Neurological Classification of Spinal Cord Injury (ISNSCI) scoring sheet.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest to disclose.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: John Houle, Drexel University, USA.
P-Reviewer: Houle J; C-Editors: Zhao M, Song LP; T-Editor: Jia Y
References
- 1.Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, Choi D, Fehlings MG. Traumatic spinal cord injury-repair and regeneration. Neurosurgery. 2017;80:S9–22. doi: 10.1093/neuros/nyw080. [DOI] [PubMed] [Google Scholar]
- 2.Cantu RC, Li YM, Abdulhamid M, Chin LS. Return to play after cervical spine injury in sports. Curr Sports Med Rep. 2013;12:14–17. doi: 10.1249/JSR.0b013e31827dc1fb. [DOI] [PubMed] [Google Scholar]
- 3.Chen L, Huang H, Xi H, Zhang F, Liu Y, Chen D, Xiao J. A prospective randomized double-blind clinical trial using a combination of olfactory ensheathing cells and Schwann cells for the treatment of chronic complete spinal cord injuries. Cell Transplant. 2014;23(Suppl 1):S35–44. doi: 10.3727/096368914X685014. [DOI] [PubMed] [Google Scholar]
- 4.Crozier KS, Graziani V, Ditunno JF, Jr, Herbison GJ. Spinal cord injury: prognosis for ambulation based on sensory examination in patients who are initially motor complete. Arch Phys Med Rehabil. 1991;72:119–121. [PubMed] [Google Scholar]
- 5.Dalamagkas K, Tsintou M, Seifalian A, Seifalian AM. Translational regenerative therapies for chronic spinal cord injury. Int J Mol Sci. 2018;19:1776. doi: 10.3390/ijms19061776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El-Kheir WA, Gabr H, Awad MR, Ghannam O, Barakat Y, Farghali HA, El Maadawi ZM, Ewes I, Sabaawy HE. Autologous bone marrow-derived cell therapy combined with physical therapy induces functional improvement in chronic spinal cord injury patients. Cell Transplant. 2014;23:729–745. doi: 10.3727/096368913X664540. [DOI] [PubMed] [Google Scholar]
- 7.Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, Bartlett PF, Blight AR, Dietz V, Ditunno J, Dobkin BH, Havton LA, Ellaway PH, Fehlings MG, Privat A, Grossman R, Guest JD, Kleitman N, Nakamura M, Gaviria M, Short D. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. doi: 10.1038/sj.sc.3102007. [DOI] [PubMed] [Google Scholar]
- 8.Geisler FH, Coleman WP, Grieco G, Poonian D Sygen Study Group. Measurements and recovery patterns in a multicenter study of acute spinal cord injury. Spine (Phila Pa 1976) 2001;26(24 Suppl):S68–86. doi: 10.1097/00007632-200112151-00014. [DOI] [PubMed] [Google Scholar]
- 9.Gomes-Osman J, Cortes M, Guest J, Pascual-Leone A. A systematic review of experimental strategies aimed at improving motor function after acute and chronic spinal cord injury. J Neurotrauma. 2016;33:425–438. doi: 10.1089/neu.2014.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grijalva I, García-Pérez A, Díaz J, Aguilar S, Mino D, Santiago-Rodríguez E, Guizar-Sahagún G, Castañeda-Hernández G, Maldonado-Julián H, Madrazo I. High doses of 4-aminopyridine improve functionality in chronic complete spinal cord injury patients with MRI evidence of cord continuity. Arch Med Res. 2010;41:567–575. doi: 10.1016/j.arcmed.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 11.Hur JW, Cho TH, Park DH, Lee JB, Park JY, Chung YG. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: a human trial. J Spinal Cord Med. 2016;39:655–664. doi: 10.1179/2045772315Y.0000000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katoh S, el Masry WS. Motor recovery of patients presenting with motor paralysis and sensory sparing following cervical spinal cord injuries. Paraplegia. 1995;33:506–509. doi: 10.1038/sc.1995.110. [DOI] [PubMed] [Google Scholar]
- 13.Kim YH, Ha KY, Kim SI. Spinal cord injury and related clinical trials. Clin Orthop Surg. 2017;9:1–9. doi: 10.4055/cios.2017.9.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirshblum S, Millis S, McKinley W, Tulsky D. Late neurologic recovery after traumatic spinal cord injury. Arch Phys Med Rehabil. 2004;85:1811–1817. doi: 10.1016/j.apmr.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, Johansen M, Jones L, Krassioukov A, Mulcahey MJ, Schmidt-Read M, Waring W. International standards for neurological classification of spinal cord injury (revised 2011) J Spinal Cord Med. 2011;34:535–546. doi: 10.1179/204577211X13207446293695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lima C, Escada P, Pratas-Vital J, Branco C, Arcangeli CA, Lazzeri G, Maia CA, Capucho C, Hasse-Ferreira A, Peduzzi JD. Olfactory mucosal autografts and rehabilitation for chronic traumatic spinal cord injury. Neurorehabil Neural Repair. 2010;24:10–22. doi: 10.1177/1545968309347685. [DOI] [PubMed] [Google Scholar]
- 17.Marino RJ, Ditunno JF, Jr, Donovan WH, Maynard F., Jr Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch Phys Med Rehabil. 1999;80:1391–1396. doi: 10.1016/s0003-9993(99)90249-6. [DOI] [PubMed] [Google Scholar]
- 18.Marino RJ, Graves DE. Metric properties of the ASIA motor score: subscales improve correlation with functional activities. Arch Phys Med Rehabil. 2004;85:1804–1810. doi: 10.1016/j.apmr.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 19.Mendonça MV, Larocca TF, de Freitas Souza BS, Villarreal CF, Silva LF, Matos AC, Novaes MA, Bahia CM, de Oliveira Melo Martinez AC, Kaneto CM, Furtado SB, Sampaio GP, Soares MB, dos Santos RR. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res Ther. 2014;5:126. doi: 10.1186/scrt516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rao Y, Zhu W, Liu H, Jia C, Zhao Q, Wang Y. Clinical application of olfactory ensheathing cells in the treatment of spinal cord injury. J Int Med Res. 2013;41:473–481. doi: 10.1177/0300060513476426. [DOI] [PubMed] [Google Scholar]
- 21.Shin JC, Kim KN, Yoo J, Kim IS, Yun S, Lee H, Jung K, Hwang K, Kim M, Lee IS, Shin JE, Park KI. Clinical trial of human fetal brain-derived neural stem/progenitor cell transplantation in patients with traumatic cervical spinal cord injury. Neural Plast 2015. 2015 doi: 10.1155/2015/630932. 630932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steeves JD, Lammertse D, Curt A, Fawcett JW, Tuszynski MH, Ditunno JF, Ellaway PH, Fehlings MG, Guest JD, Kleitman N, Bartlett PF, Blight AR, Dietz V, Dobkin BH, Grossman R, Short D, Nakamura M, Coleman WP, Gaviria M, Privat A. International Campaign for Cures of Spinal Cord Injury Paralysis (2007) Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord. 45:206–221. doi: 10.1038/sj.sc.3102008. [DOI] [PubMed] [Google Scholar]
- 23.Vaquero J, Zurita M, Rico MA, Bonilla C, Aguayo C, Montilla J, Bustamante S, Carballido J, Marin E, Martinez F, Parajon A, Fernandez C, Reina L. Neurological Cell Therapy Group (2016) An approach to personalized cell therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II clinical trial. Cytotherapy. 18:1025–1036. doi: 10.1016/j.jcyt.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Waters RL, Adkins RH, Yakura JS, Sie I. Motor and sensory recovery following complete tetraplegia. Arch Phys Med Rehabil. 1993;74:242–247. [PubMed] [Google Scholar]
- 25.Zarin DA, Tse T, Williams RJ, Califf RM, Ide NC. The ClinicalTrials.gov results database--update and key issues. N Engl J Med. 2011;364:852–860. doi: 10.1056/NEJMsa1012065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


