The COVID-19 pandemic, with all its tragedy, has also ushered in a new era of biological medicine. This global crisis has brought along with it an opportunity that had not been foreseen by many – it brought the therapeutic use of subcellular biological material to the forefront of medicine. Though clinical trials for certain biological therapeutics began more than 15 years ago, in many nations there has been public hesitation to these approaches. With this shift of public thought, there will likely be many more similar therapeutic efforts in days ahead. In the case of the pioneering COVID-19 vaccines, mRNA was used as medicine for this inflammatory pathology. Further, the use of lipid delivery systems for these mRNA vaccines has opened the eyes of many regarding new possibilities for biological therapeutics and delivery systems. Within the body during normal physiology, such endogenous delivery systems are continuously operating. These extracellular vesicles (EVs) are lipid-enclosed transport systems that allow continuous communication between cells and organ systems. EVs transport diverse biological cargo such mRNAs, miRNAs, DNA, histones, long non-coding RNAs, proteins, bio-active lipids, and more. Previously considered to be inactive, not physiological, EVs have now emerged as critical mediators in a variety of biological processes and disease states (Jeppesen et al., 2019). As such, much interest has emerged for using EVs not only as disease biomarkers, but also as biological drug delivery systems or even as direct treatments themselves. Disease states with inflammatory components in particular have been studied for the involvement of EVs. Like the COVID-19 mRNA vaccines, EVs represent a new dimension of subcellular biological medicine.
EVs have been classified into categories based upon their size and origin, though significant overlap can occur across their sizes. Exosomes are ~30–150 nm in diameter and are released from the endosomal system after fusion of multivesicular endosome bodies with the cell membrane. Microvesicles (MVs, also known as ectosomes or microparticles) are approximately 50 nm to 1 μm in diameter and are thought to form primarily from budding from the cell membrane. However, other similarly sized EVs can also be released by secretory autophagy. Since many of the cell surface molecules are shared and there is often overlap in size, it can be difficult to discriminate between these different subtypes, though efforts are being made to identify specific markers (Jeppesen et al., 2019; Mathieu et al., 2019). Apoptotic bodies are a typically larger class (≥ 1 μm, though smaller apoptotic bodies can be seen as well) and are released as cells undergo apoptosis. In general, exosomes and MVs are considered the most common and relevant biological mediators in the setting of inflammatory conditions. EVs can be considered subcellular communication vehicles. Given their diverse cargo, EVs likely communicate many facets of cellular and tissue state between cells locally and across great distances between organ systems. EVs are released from nearly all cell types and are found in all body fluid and tissue matrices. The circulating vesicle pool in blood and other fluids is a conglomerate of all the ongoing processes from cells across the body. This intercellular- and interorgan-level communication could have profound influences on normal physiology as well as functional adaptations across tissues and organs during normal and disease states. Inflammatory diseases in particular have been found to involve EV signaling in their pathology. Several studies have identified EV signaling as critical during both acute and chronic inflammatory pathologies. This includes but is not limited to sepsis, arthritis, bacterial infection, lupus, various cancers, alcohol-related diseases, Alzheimer's disease, burn injuries, acute lung injury, and alcohol-induced neuroinflammation (Mukherjee et al., 2020; Crews et al., 2021). Essentially, when it comes to inflammatory conditions, wherever people have looked, a role for EV signaling has been found. This is likely because EVs seem to represent a snapshot in time of the dynamic intercellular states of the organism. In fact, circulating EVs could be viewed as a pool of real-time thermometers which, as we learn to read them more clearly, will likely give us great insight into the status of cells throughout the body. Therefore, they are perturbed across a broad range of conditions. One class of diseases that involves multiples organ systems wherein EV signaling has been implicated are alcohol-related disorders.
It has recently been identified that alcohol-related disorders are also inflammatory conditions that involve EV signaling. This includes a variety of disorders such as alcohol use disorder (AUD), fetal alcohol spectrum disorders, alcoholic liver disease, and others. In AUD neuropathology, a role for both exosomes and MVs has been reported. Astrocytes treated with ethanol secrete toxic exosomes that damage neurons (Ibanez et al., 2019), while microglia secrete the miRNA let-7b in MVs to promote neuronal death through an immune-mediated mechanism (Coleman et al., 2017). In the setting of fetal alcohol spectrum disorders, microglia promote neuronal death in the hypothalamus through secretion of C1q-containing exosomes (Mukherjee et al., 2020). In addition to promoting alcohol-induced neuronal death, EVs may also drive the underlying neuroinflammation seen in AUD. MVs secreted from organotypic brain slice culture in response to ethanol reproduced neuroinflammatory responses in naïve cultures, and blockade of MV secretion prevented ethanol induction of inflammatory signaling (Crews et al., 2021). Moreover, alcohol causes systemic alterations in EV composition that contribute to alcoholic liver disease, pancreatitis, oral cancer, and other alcohol-related diseases (Rahman et al., 2020). These studies suggest perturbations in EV composition or uptake could underlie many alcohol-related disorders and may represent novel therapeutic targets or biomarkers for determining an individual's risk for an alcohol-related disorder. This may be true not only for alcohol-related disorders, but many other diseases. Their involvement in pathological processes will likely make EVs targets for therapeutic intervention. Though EVs are involved in many different pathologies, one example of a peripheral immune pathology that may benefit from EV-targeted therapeutics is sepsis. Sepsis is a complex setting of immune and metabolic dysfunction that results in organ failure and, quite often, death. Unfortunately, clinical trials targeting promising individual circulating immune molecules have not been successful. This may be due to the complex extracellular milieu which involves many different types of mediators and biological signals that are attempting to both respond to the acute insult and normalize the aberrant physiology. Individual EVs contain multiple types of mediators with diverse signaling molecules. Therefore, neutralizing certain classes of EVs marked by specific molecules may be beneficial. Consistent with this idea, a recent study found that chelation of a class of EVs that are enriched with C-reactive protein in septic patients reduced their proinflammatory potential (Fendl et al., 2021). This proof of principle supports that certain EV subtypes may be removed in order to normalize physiology in different patient populations. EV dialysis and plasmapheresis techniques will likely be emerging as novel therapeutic approaches in the near future.
On the other hand, in addition to promoting pathology, EVs from certain cell types may also afford protection against inflammatory conditions such as sepsis and AUD. In fact, the promotion of proinflammatory signaling itself is often protective in certain cases such as microbial infection. Further, transfer of trophic/anti-inflammatory EVs from mesenchymal or adipose tissue-derived stem cells protect against both sepsis and alcohol-induced neuropathology (Ezquer et al., 2019; Bai et al., 2020) (discussed further below). Protective EVs may provide trophic factors and miRNA cargo that promote cell vitality. Understanding the balance between these two – protective versus pathologic EVs – will likely help to guide future treatment approaches.
Circulating EVs seem to offer a real-time picture of the functional status of cells throughout the body as well as the intercellular dynamics that are occurring. Since EVs are membrane-enclosed, they protect cargo such as nucleic acids that would otherwise be degraded in the extracellular environment. These qualities make EVs strong candidates for identifying biomarkers for early detection or monitoring of disease states. One condition in which EVs are being investigated as biomarkers is Alzheimer's disease. Increasing evidence supports a role for neuroinflammation as a driving contributor to Alzheimer's disease pathology. The natural history of Alzheimer's progresses over many years prior to the development of clinical symptoms. Postmortem diagnostic markers include accumulation of amyloid beta plaques and tau fibrils in brain. Recent studies have found several proteins and miRNAs in MVs from cerebrospinal fluid and serum that reached criteria for preclinical markers or prognostic indicators (Vandendriessche et al., 2020). EVs may also be useful for early detection of tumors that historically have been difficult to detect before late stages of disease. For instance, exosomes containing the proteoglycan glypican-1 and a characteristic miRNA signature show high sensitivity and specificity for diagnosis of pancreatic ductal adenocarcinoma, even during early stages (Hou et al., 2021). Similar efforts at identifying early-stage EV biomarkers are underway for other late-presenting cancers such as esophageal adenocarcinoma, glioblastoma, and other hepatobiliary malignancies.
Beyond using them for diagnosis and directly removing them as a form of treatment, perhaps an even more exciting possibility for EV therapeutics is using EVs themselves as treatments. This can occur either through selective modification of EV cargo or the biological production of protective EVs. EVs have the potential for delivery by non-invasive over-the-counter methods such as aerosol inhalers or intranasal sprays. These lipid bilayer vesicles can be stored at either 4°C or –20°C for long periods of time and can protect their nucleic acid or protein cargo from unwanted degradation by circulating enzymes. Once in circulation, EVs can deliver their cargo to the desired location through multiple strategies. Components in the EV membrane such as proteins or bioactive lipids can directly activate cell surface receptors on target cells. EVs can also cross into the cell through fusion with the cell membrane or through endocytosis mechanisms to deliver protein, lipid, or nucleic acid cargo to intracellular organelles within the cytoplasm or even the nucleus. Thus, EVs could potentially be bioengineered to deliver cargo to the necessary intracellular location to affect cell biological processes. Further, cell surface protein modification will likely allow for organ- and cell-specific targeting, as cell-specific uptake markers for different cell types are currently being identified. Therefore, the potential for selective targeting of therapeutic agents using modified EVs is significant. Also, since EVs can carry diverse cargo, one could imagine a time when multiple therapeutics are packaged into a single EV preparation. Polypharmacy is a real concern with current small-molecule pharmaceutics, as many patients are on several drugs for various chronic medical conditions. Treatments for conditions that commonly occur together (such as high blood pressure and hypercholesterolemia) could be packaged together in EVs, possibly reducing the number of medications a person would need to take. However, care must be taken when engineering EVs, as the presence of unwanted mediators could produce unintended side effects. Thorough screening of the contents of carrier EVs must be performed to know their full cargo. Further, beyond the possible benefits associated with the bioengineering of EVs, EVs from healthy, protective cells also possess many beneficial effects. This has been clearly demonstrated by the benefits of EVs harvested from mesenchymal stem cells.
Mesenchymal stem cells (MSCs) have shown great promise as a biological medical treatment approach for a variety of conditions, at least in part due to their immunomodulatory actions. MSCs have shown therapeutic improvements in wound healing, ischemic cardiomyocyte injury, acute kidney injury, liver damage, graft vs host disease, various cancers, alcohol abuse, and others (Qiu et al., 2018). These benefits are often observed even in the absence of incorporation of the MSCs into the tissue of interest. Strikingly, several studies have found that EVs that are derived from MSCs are often able to reproduce many of the therapeutic benefits seen with MSC transplants themselves. These beneficial effects are likely due to protective EV cargo which includes trophic factors, miRNAs, lipid species, mRNAs, and other contents. This follows the idea that EVs from healthy cells promote health in other cells, with MSCs being some of the most protective cells in the body. Similar to the idea of packaging treatments for multiple conditions into the same EV preparations, MSC-EVs could be beneficial for multiple chronic diseases, reducing the number of medications an individual might need and lowering the risks associated with polypharmacy, as MSC-EVs might be beneficial for multiple chronic diseases. Returning to alcohol use disorder, MSC-EVs have shown promise in recent preclinical studies. In chronic alcohol drinking rats, intranasal delivery of MSC-EVs profoundly reduced alcohol intake and astrocyte morphological activation (Ezquer et al., 2019). This underscores the fact that EVs can be administered in an over-the-counter method, can cross the blood-brain barrier, and can modulate behavior.
There is much to be said about the work that has been done surrounding EVs and their potential in the future. This commentary is by no means an exhaustive description of the role of EVs in normal physiology, pathology, and their utility as therapeutics. Rather, we have hoped to emphasize some of the unique aspects of EVs and their exciting potential future uses in medicine as biomarkers, biologics, and delivery vehicles (Figure 1). The flood gates for subcellular biological medicine have been opened by the recent COVID-19 vaccination approaches. EVs have great potential as they are biological and carry a vast array of mediators. It will be exciting to see how this new world of biological medicine takes form.
Figure 1.
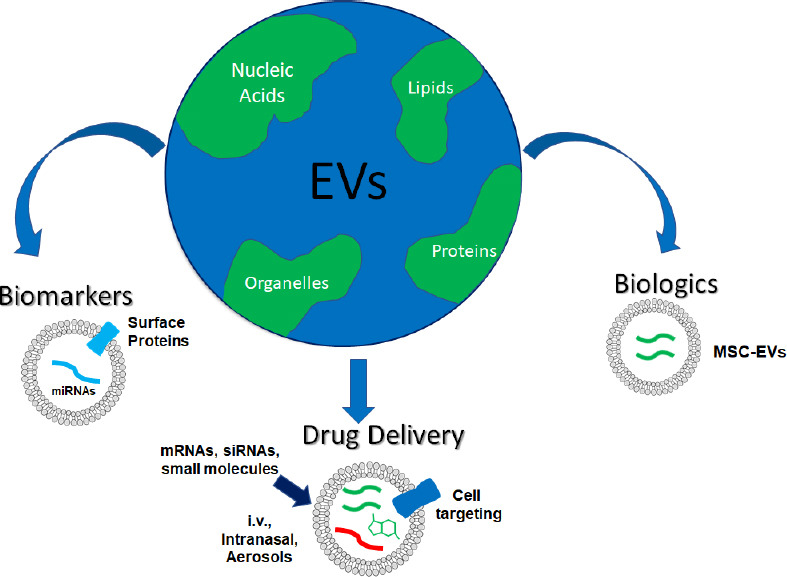
The emerging world EVs.
Circulating EVs contain nucleic acid, protein, lipid and cellular organelle cargo such as mitochondria. The future of EVs will likely involve their use as biomarkers, biologic agents, and drug delivery vehicles. EV surface markers and cargo may be used to predict disease risk or progression. EV biologics such as mesenchymal stem cell EVs (MSC-EVs) show efficacy across a range of immune disorders. EVs can be modified or loaded with mRNAs, siRNAs, or small molecules to allow for targeted delivery to cells of interest. EVs may be given intravenously (i.v.) or intramuscularly, as well as in over the counter or home prescription formulations as intranasal sprays or aerosol inhalers. EVs: Extracellular vesicles.
This work was supported by National Institute on Alcohol Abuse and Alcoholism AA024829, AA028924, AA028599 and the Bowles Center for Alcohol Studies (all to LGC).
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by the author before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Liu WJ, Li JY; T-Editor: Jia Y
References
- 1.Bai X, Li J, Li L, Liu M, Liu Y, Cao M, Tao K, Xie S, Hu D. Extracellular vesicles from adipose tissue-derived stem cells affect Notch-miR148a-3p axis to regulate polarization of macrophages and alleviate sepsis in mice. Front Immunol. 2020;11:1391. doi: 10.3389/fimmu.2020.01391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coleman LG, Jr, Zou J, Crews FT. Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J Neuroinflammation. 2017;14:22. doi: 10.1186/s12974-017-0799-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crews F, Zou J, Coleman L., Jr Extracellular microvesicles promote microglia-mediated proinflammatory responses to ethanol. J Neurosci Res. 2021 doi: 10.1002/jnr.24813. doi: 10.1002/jnr.24813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ezquer F, Quintanilla ME, Morales P, Santapau D, Ezquer M, Kogan MJ, Salas-Huenuleo E, Herrera-Marschitz M, Israel Y. Intranasal delivery of mesenchymal stem cell-derived exosomes reduces oxidative stress and markedly inhibits ethanol consumption and post-deprivation relapse drinking. Addict Biol. 2019;24:994–1007. doi: 10.1111/adb.12675. [DOI] [PubMed] [Google Scholar]
- 5.Fendl B, Weiss R, Eichhorn T, Linsberger I, Afonyushkin T, Puhm F, Binder CJ, Fischer MB, Weber V. Extracellular vesicles are associated with C-reactive protein in sepsis. Sci Rep. 2021;11:6996. doi: 10.1038/s41598-021-86489-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hou J, Li X, Xie KP. Coupled liquid biopsy and bioinformatics for pancreatic cancer early detection and precision prognostication. Mol Cancer. 2021;20:34. doi: 10.1186/s12943-021-01309-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibanez F, Montesinos J, Urena-Peralta JR, Guerri C, Pascual M. TLR4 participates in the transmission of ethanol-induced neuroinflammation via astrocyte-derived extracellular vesicles. J Neuroinflammation. 2019;16:136. doi: 10.1186/s12974-019-1529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jeppesen DK, Fenix AM, Franklin JL, Higginbotham JN, Zhang Q, Zimmerman LJ, Liebler DC, Ping J, Liu Q, Evans R, Fissell WH, Patton JG, Rome LH, Burnette DT, Coffey RJ. Reassessment of exosome composition. Cell. 2019;177:428–445. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathieu M, Martin-Jaular L, Lavieu G, Thery C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. 2019;21:9–17. doi: 10.1038/s41556-018-0250-9. [DOI] [PubMed] [Google Scholar]
- 10.Mukherjee S, Cabrera MA, Boyadjieva NI, Berger G, Rousseau B, Sarkar DK. Alcohol increases exosome release from microglia to promote complement C1q induced cellular death of proopiomelanocortin neurons in the hypothalamus in a rat model of fetal alcohol spectrum disorders. J Neurosci. 2020;40:7965–7979. doi: 10.1523/JNEUROSCI.0284-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, Xu J. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther. 2018;9:320. doi: 10.1186/s13287-018-1069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahman MA, Patters BJ, Kodidela S, Kumar S. Extracellular vesicles: intercellular mediators in alcohol-induced pathologies. J Neuroimmune Pharmacol. 2020;15:409–421. doi: 10.1007/s11481-019-09848-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vandendriessche C, Bruggeman A, Van Cauwenberghe C, Vandenbroucke RE. Extracellular vesicles in Alzheimer's and Parkinson's disease: small entities with large consequences. Cells. 2020;9:2485. doi: 10.3390/cells9112485. [DOI] [PMC free article] [PubMed] [Google Scholar]


