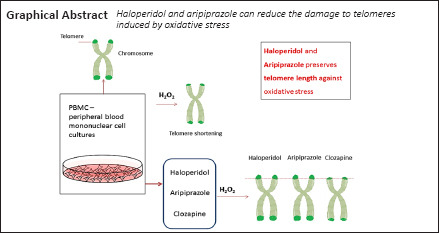
Key Words: antipsychotic, aripiprazole, cell culture, clozapine, haloperidol, oxidative stress, peripheral blood mononuclear cell, telomere length
Abstract
Antipsychotics may prolong or retain telomere length, affect mitochondrial function, and then affect the metabolism of nerve cells. To validate the hypothesis that antipsychotics can prolong telomere length after oxidative stress injury, leukocytes from healthy volunteers were extracted using Ficoll-Histopaque density gradient. The mononuclear cells layer was resuspended in cell culture medium. Oxidative stress was induced with hydrogen peroxide in cultured leukocytes. Four days later, leukocytes were treated with aripiprazole, haloperidol or clozapine for 7 days. Real-time PCR revealed that treatments with aripiprazole and haloperidol increased the telomere length by 23% and 20% in peripheral blood mononuclear cells after acute oxidative stress injury. These results suggest that haloperidol and aripiprazole can reduce the damage to telomeres induced by oxidative stress. The experiment procedure was approved by the Ethics Committee of Faculty of Medicine of the University of São Paulo (FMUSP/CAAE approval No. 52622616.8.0000.0065).
Chinese Library Classification No. R453; R741; Q2
Introduction
Telomeres are tandem repeats of the non-coding sequence TTAGGG at the end of each chromosome. Human telomeres protect the DNA from end-to-end fusion and cellular erosion, but they diminish progressively with cellular division (Manchia et al., 2020). In counterpart, telomerase is the enzyme responsible for adding new TTAGGG sequences at the end of telomeres, so that telomere erosion does not occur prematurely. Some people consider telomeres are “biological clocks”, since the cell becomes senescent when they reach a critical limit (Savage, 2018). Interestingly, several conditions have been related to decreased telomere length, including cardiovascular (Yeh and Wang, 2016), neurologic (Eitan et al., 2014), and some psychiatric diseases (Lindqvist et al., 2015), probably due to chronic inflammation and oxidative stress.
Antipsychotics consist of a class of drugs used for the treatment of several neuropsychiatric conditions, including schizophrenia. The exact mechanism of action of antipsychotics is still debated and each antipsychotic has its own pharmacological features, largely represented by dopamine (DA) and serotonin (5-HT) receptor antagonism at different degrees of affinity and specificity (Farlow and Shamliyan, 2017).
Recently, we demonstrated a mechanism through which serotonin or dopamine antagonism would upregulate telomerase expression and therefore telomere lengthening (Polho et al., 2015). The blockage of D2 or 5-HT2A receptors causes a different regulation of protein kinase B (Akt)/glycogen synthase kinase 3 beta (GSK3β)/β-catenin pathway (Beaulieu and Gainetdinov, 2011; Bersani et al., 2015; Polho et al., 2015). Antipsychotics were able to alter Akt-GSK3β and Wnt signaling pathways (Kang et al., 2004; Alimohamad et al., 2005; Emamian et al., 2004; Freyberg et al., 2010). These pathways regulate β-catenin expression, which binds to transcriptional factors, such as transcriptional factor 4 and regulates human telomerase reverse transcriptase expression (catalytic subunit of telomerase) (Zhang et al., 2012), suggesting that antipsychotics can modulate telomere length by this mechanism. Bersani et al. (2015) suggested other potential mechanisms through which antipsychotics may influence telomerase activity, such as antioxidant properties, brain-derived neurotrophic factors upregulation and serotoninergic modulation.
There is limited evidence that antipsychotic drugs may influence telomere dynamics. Aoyama et al. (2014) showed that atypical antipsychotics elongated telomere length in the hippocampus of mice. Conversely, Monroy-Jaramillo et al. (2017) showed decreased telomere length in leukocytes of schizophrenic patients treated with olanzapine. Yu et al. (2008) found longer telomeres in schizophrenic patients who had a good response to pharmacological treatment, but Li et al. (2015) showed that patients who responded to antipsychotic treatment had longer telomeres at baseline, in which case telomere length was a predictor of good response, not directly affected by the antipsychotic treatment itself. In an ex vivo study using cultures of peripheral blood mononuclear cells (PBMC) drawn from schizophrenic patients, haloperidol and clozapine administered for 5 days at therapeutic concentrations did not modify telomerase expression (Porton et al., 2008). In view of the conflicting reports in the literature regarding the possible influence of antipsychotics on telomere length, we designed the present study to determine whether the effect of antipsychotic drugs on telomere length may be related to the occurrence of oxidative stress. We hypothesize that the deleterious effect of oxidative stress on telomeres may be attenuated by treatment with clozapine, aripiprazole and haloperidol.
Materials and Methods
Peripheral blood mononuclear cell culture
The experiment procedure was approved by the Ethics Committee of Faculty of Medicine of University of São Paulo (FMUSP/CAAE approval No. 52622616.8.0000.0065) and conformed to the guidelines of the Declaration of Helsinki. All participants filled in the informed consent. Peripheral blood was collected from healthy volunteers (without previous psychiatric disorder and previous blood disorders and non-smokers) for the isolation of peripheral blood mononuclear cells (PBMCs). Blood was collected (14 mL) in heparinized tubes and diluted 1:1 in sterile phosphate buffered saline (PBS). The diluted blood was carefully transferred to a conic tube containing 6 mL of Ficoll (Sigma, Sigma, St. Louis, MO, USA). The mixture was centrifuged at 700 × g for 30 minutes at room temperature. The mononuclear cell layer was removed and centrifuged with 15 mL of sterile PBS at 700 × g for 30 minutes at room temperature, for removal of contaminant platelets. The supernatant was discarded and the cell pellet was suspended with 1 mL of RPMI 1640 medium (Thermo Fisher Scientific, Waltham, MA, USA). Cell count was performed with a Neubauer chamber. For each well, we diluted the cells to a final concentration of 5 × 105 cells/mL.
Antipsychotic preparation
The antipsychotics were diluted in the following way: haloperidol (Sigma): 2.5 mg in 1.2 mL of DMSO (Sigma); clozapine (Biogen, Cambridge, MA, USA): 25 mg in 1 mL DMSO; aripiprazole (Sigma): 2.5 mg in 500 μL PBS. After solubilization, the clozapine and aripiprazole samples were diluted to 100 μg/mL and the haloperidol sample to 10 μg/mL in RPMI 1640 culture medium (Thermo fisher, USA) and stored at –20°C. The final concentration of dimethyl sulfoxide (DMSO, as vehicle) was ≤ 0.02% (de Abreu Costa et al., 2017).
PBMC culture maintenance, hydrogen peroxide and antipsychotic treatment
The PBMCs were maintained in a 6-well dish, in RPMI 1640 medium supplemented with 10% bovine fetal serum (Sigma) and antibiotics (penicillin/streptomycin 1% v/v) at 37°C and an atmosphere with 5% of CO2. To cause initial telomeric damage, we tested different hydrogen peroxide (H2 O2) (Sigma) concentrations: 100, 200, 400, and 600 μM. The cultures with H2O2 were maintained for 3 days in the same conditions described.
For each individual culture of PBMCs, we induced oxidative stress with 200 μM of H2O2 for 3 days, after which the distinct antipsychotics were added to the culture medium for additional 4 days. We used working concentrations of antipsychotic drugs within the range of plasma therapeutic levels in three experimental (intervention) groups: (a) haloperidol 7 ng/mL (Van Putten et al., 1992); (b) clozapine 1000 ng/mL (Ulrich et al., 2003); (c) aripiprazole 125 ng/mL (McEvoy et al., 2014). Two comparison groups [controls and vehicle (DMSO ≤ 0.02%)] were established: (d) PBMCs maintained for 7 days in culture media only (no H2O2 and no antipsychotics), presumably accounting for baseline in vitro effect (no/minimal undisturbed change in telomere length); and (e) PBMCs treated with H2O2 for 3 days but no antipsychotics added between days 4 and 7 (negative control), indicative of the effect of oxidative stress on telomeres).
Cell viability
The PBMC viability was assessed according to MTT ((3-(4, 5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide) (Sigma) protocol (De-Paula et al., 2016). MTT was reduced by active cells, generating purple formazan, which can be quantified by spectrophotometry.
For each sample, a 100 μL aliquot was transferred to a 96-well dish, in triplicate and added 10 μL of MTT reagent was added. The cells were incubated for 6 hours and 100 μL of sodium dodecyl sulfate (SDS) 20% was added to each well. Then, the absorbance at 570 nm was recorded using a microplate reader (BioLab Inc., Lawrenceville, GA, USA).
DNA extraction and quality evaluation
The PBMCs were harvested and stored after the experiment in a refrigerator at –20°C. DNA was extracted using QIAamp DNA Mini Blood kit (QIAGEN, CA, USA) with QIAcube protocol. After extraction, DNA was diluted to 4 ng/mL and kept at –80°C. DNA quality was assessed with spectrophotometry (Nanodrop - Thermo Fisher Scientific, CA, USA). We also performed capillary electrophoresis (Fragment Analyzer – Advanced Analytical Technologies, CA, USA) to verify DNA integrity.
Telomere length measurement – real-time polymerase chain reaction
Telomere length in PBMCs was measured using the methods described previously (Cawthon, 2009), with some modifications. We used 36B4 for reference as a single copy gene. The primer sequences (5′–3′) were: telomere forward: GGT TTT TGA GGG TGA GGG TGA GGG TGA GGG TGA GGG T; telomere reverse: TCC CGA CTA TCC CTA TCC CTA TCC CTA TCC CTA TCC CTA; 36B4 forward/reverse CAG CAA GTG GGA AGG TGT AAT CC; 36B4 forward/reverse CCC ATT CTA TCA TCA ACG GGT ACA A. The 36B4 reaction included 7.5 μL of SyBr Green PCR MasterMix (Promega, UK), 0.45 μL of primer forward, 0.75 μL of primer reverse and 3.3 μL of miliQ water. For telomere reaction: 7.5 μL of SyBr Green PCR MasterMix (Promega), 0.45 μL of primer forward, 0.45 μL of primer reverse and 3 μL of miliQ water.
The real-time polymerase chain reaction (PCR) was run with 7500 Fast Real Time PCR System (Promega, CA, USA) at 50°C for 2 minutes, then 40 cycles of 95°C for 2 minutes, 55°C for 15 seconds and 60°C for 1 minute.
Statistical analysis
Statistical analysis was conducted using software R (version 3.2.0). For telomere length quantification, we used the ratio (average Ct telomere/average Ct 36B4) (T/S ratio). Differences in TL between control and vehicle within each treatment were investigated with one-way analysis of variance (ANOVA) followed by Tukey's post hoc test. The Turkey's package (post hoc analysis) was used in the R software. Statistical differences were considered significant if adjusted P < 0.05.
Results
After 3 days of treatment with different H2O2 concentrations, we harvested the cells to perform cell viability analysis and telomere mensuration. ANOVA test showed there was no difference among groups (P = 0.051). As observed, we found the tendency of diminished telomeres after H2O2 treatment, more pronounced after 200 μM H2O2 treatment. In the light of these results, we opted for the use of concentration 200 μM (Figure 1).
Figure 1.
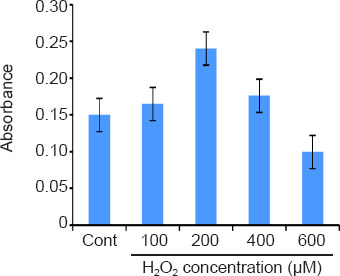
Average cell viability (MTT absorbance at 570 nm) after treatment with different concentrations of hydrogen peroxide (H2 O2).
Cont: Control; MTT: (3-(4, 5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide.
We recruited six individuals with no history of psychiatric disease or use of psychotropic medication and no smokers. Four were female and two were male subjects and the average age was 28 ± 6.2 years.
We observed significant differences in actin reactivity for only one treatment. The MTT results indicated increases at aripiprazole (13%) in comparison with vehicle (P = 0.05). Although not statistically significant, H2O2 treatment reduced cell viability by 11% compared to the control.
The telomere length ratio was changed to percentage to facilitate the visualization of the treatments compared with control, vehicle and H2O2. The vehicle condition showed a reduction of 4% compared to the control (culture with medium only), showing that the vehicles did not cause telomere length alteration. Cells treated with H2O2 showed 46% reduction in telomere length, compared to the control condition, with no statistical significance (Figure 2).
Figure 2.
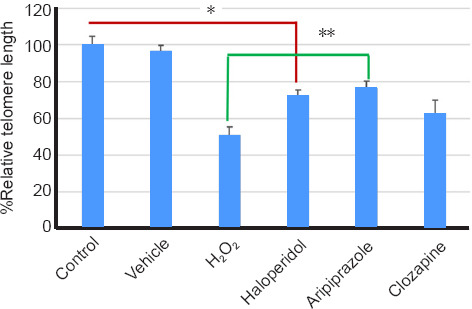
Telomere length ratio in the samples studied.
The average telomere length was increased by 19% and 23% after treatment with Haloperidol and Aripiprazole, respectively compared to H2O2 treatment. *P < 0.05, **P < 0.01 (one-way analysis of variance followed by Tukey's post hoc test).
The PBMCs treated with haloperidol showed longer telomeres compared to cells with H2O2 toxicity. However, they showed a decrease in telomeric length when compared to the control group (P < 0.05). Telomere length in cells was significantly longer after aripiprazole treatment compared with after H2O2 treatment (P < 0.01).
Discussion
Currently, in psychiatry, there is a difficulty in acquiring proper cell models for a disease study, since the physiopathology of most diseases has not been completely understood. Some effort has been made to model induced pluripotent stem cells, for example, to study schizophrenia, but characteristic cell phenotypes (perhaps based on ‘omics’) need yet to be studied and validated (Falk et al., 2016). This is the first work in the literature to show that PBMCs subjected to oxidative damage to DNA have telomeric shortening. However, this shortening of telomeres can be avoided, since antipsychotic treatment before the event of oxidative stress protects and prevents telomere length.Here we showed that aripiprazole increased telomere length by 23% and haloperidol tended to increase telomere length in PBMC after H2O2 treatment. Besides enlightening novel mechanisms of action of antipsychotics, results from this study provide the basis for the use of antipsychotics in ageing-related diseases (Aviv and Shay, 2018). It has been suggested that treatment with antipsychotics in patients with schizophrenia may lengthen, or preserve the length of, the telomere and also affect mitochondrial function and consequently cell metabolism. Several cross-sectional studies have shown that psychiatric patients with longer telomeres tend to respond better to antipsychotic treatment, thus telomere length has been become a candidate for a biological marker (Polho et al., 2015; Brand et al., 2020).
The aripiprazole has demonstrated neuroprotective effects through increasing transcripts and protein level of brain-derived neurotrophic factor, phosphorylation and GSK-3β in SH-SY5Y cells (Gibson et al., 1999; Xu et al., 2015), and decreasing presynaptic-like glutamate release in synaptosomes (Yu et al, 2008). Although the mechanism through which aripiprazole exhibits neuroprotective effects is unknown, aripiprazole is distinct from all other antipsychotics in that it has partial agonist activity at the dopamine D2 receptor and serotonin 5-HT1A receptor (Cardenas et al., 2019).
Atypical antipsychotics are associated with a lower risk of many reasons for mortality and extrapyramidal symptoms compared with conventional antipsychotics. However, atypical antipsychotics have been associated with a higher risk of stroke compared with conventional antipsychotics (Farlow and Shamliyan, 2017).
A significant decrease was observed in mitochondrial complex I activity in PBMCs obtained from patients with chronic and stabilized schizophrenia when compared with healthy controls. An increase in plasma level of TBARS suggests lipid peroxidation in schizophrenia patients (Gubert et al., 2013). Considering that PBMC results should reflect the status of the brain, and then increased neuronal oxidative stress could produce impairments in signal transduction and neuronal plasticity (Lindqvist et al., 2015). Biomarkers of oxidative stress allow for the identification of cellular impairment via measurable parameters. Arvindakshan et al. (2003) found that the level of lipid peroxidation was certainly correlated with symptom severity in drug-free schizophrenic patients. Casademont et al. (2007) described a stronger effect of haloperidol, on decreasing complex I activity and increasing lipid peroxidation when associated with atypical agents, such as clozapine and risperidone, in PBMCs from schizophrenia. Some studies showed the relationship between telomere length and treatment response to psychiatric medications (Honig et al., 2006; Khan et al., 2017; Polho et al., 2015). It has been shown that shortened telomere length in schizophrenia is an indicator of oxidative stress, and the subsequent cellular dysfunction may be a factor contributing to the progressive deterioration in treatment resistant schizophrenia (Yu et al, 2008). These results indicate that telomere length is shortened largely in a subgroup of chronic schizophrenia patients who respond poorly to treatment.
Interestingly, it has been demonstrated that haloperidol decreases the viability of neuronal cells in vitro (Brinholi et al., 2016), which increases the activity of apoptosis proteins. On the other hand, studies in vitro have shown that while haloperidol is apparently neurotoxic, second-generation antipsychotics offer protection against serum withdrawal-induced apoptosis in SK-N-SH cells (Gassó et al., 2012), or against cytotoxin-induced apoptosis in the pheochromocytoma cell line (Lindqvist et al., 2015). In vitro experiments using haloperidol separately and above the therapeutic dosage, showed decreased telomerase expression in leukocytes of healthy individuals (Porton et al., 2008). Perhaps the tendency of haloperidol increasing telomere length observed in our experiments may only represent a selection of the leukocyte population with long telomeres.
Our study has some limitations. The number of subjects was small, so the lack of statistical significance may be explained by underpowered sample. These results demonstrate the effects of antipsychotics on oxidative stress and only three antipsychotics were tested and on a single dose. Since different antipsychotics have different pharmacodynamics, new studies must be made to demonstrate other effects on telomere length. Oxidative stress is a well described phenomenon in schizophrenia and some authors suggest it may contribute to the pathology. Although schizophrenia may be better represented by chronic oxidative stress, our purpose was to verify only DNA damage (more specifically, telomere attrition) (Bitanihirwe and Woo, 2011). As expected, H2O2 treatment reduced telomere length in PBMCs of healthy subjects.
In conclusion, our results showed that aripiprazole can reduce the damage caused by oxidative stress and preserve telomere length and cell viability.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest related to the contests of the present work.
Financial support: This study was supported by grants from FAPESP (Fundação de Amparo à Pesquisa de São Paulo, Grant nº 2016/01302-9 and 2014/27129-6) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) 88887.463672/2019-00.
Institutional review board statement: The experiment procedure was approved by the Ethics Committee (FMUSP/CAAE approval number 52622616.8.0000.0065) and conformed to the guidelines of the Declaration of Helsinki.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This study was supported by grants from FAPESP (Fundação de Amparo à Pesquisa de São Paulo, Grant nº 2016/01302-9 and 2014/27129-6) and CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior) 88887.463672/2019-00.
C-Editors: Zhao M, Liu WJ; S-Editor: Li CH; L-Editor: Song LP; T-Editor: Jia Y
References
- 1.Alimohamad H, Rajakumar N, Seah YH, Rushlow W. Antipsychotics alter the protein expression levels of β-catenin and GSK-3 in the rat medial prefrontal cortex and striatum. Biol Psychiatry. 2005;57:533–542. doi: 10.1016/j.biopsych.2004.11.036. [DOI] [PubMed] [Google Scholar]
- 2.Aoyama Y, Mouri A, Toriumi K, Koseki T, Narusawa S, Ikawa N, Mamiya T, Nagai T, Yamada K, Nabeshima T. Clozapine ameliorates epigenetic and behavioral abnormalities induced by phencyclidine through activation of dopamine D1 receptor. Int J Neuropsychopharmacol. 2014;17:723–737. doi: 10.1017/S1461145713001466. [DOI] [PubMed] [Google Scholar]
- 3.Aviv A, Shay JW. Reflections on telomere dynamics and ageing-related diseases in humans. Philos Trans R Soc Lond B Biol Sci. 2018;373:20160436. doi: 10.1098/rstb.2016.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 5.Bersani FS, Lindqvist D, Mellon SH, Penninx BW, Verhoeven JE, Révész D, Reus VI, Wolkowitz OM. Telomerase activation as a possible mechanism of action for psychopharmacological interventions. Drug Discov Today. 2015;20:1305–1309. doi: 10.1016/j.drudis.2015.06.016. [DOI] [PubMed] [Google Scholar]
- 6.Bitanihirwe BK, Woo TU. Oxidative stress in schizophrenia: an integrated approach. Neurosci Biobehav Rev. 2011;35:878–893. doi: 10.1016/j.neubiorev.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brand BA, de Boer JN, Oude Ophuis SBJ, Slot MIE, De Wilde B, Catthoor KCEER, Goverde AJ, Bakker PR, Marcelis MC, Grootens KP, Luykx JJ, Heringa SM, Weickert CS, Sommer IEC, Weickert TW. Raloxifene augmentation in men and women with a schizophrenia spectrum disorder: A study protocol. Contemp Clin Trials Commun. 2020;20:100681. doi: 10.1016/j.conctc.2020.100681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brinholi FF, Farias CC, Bonifácio KL, Higachi L, Casagrande R, Moreira EG, Barbosa DS. Clozapine and olanzapine are better antioxidants than haloperidol, quetiapine, risperidone and ziprasidone in in vitro models. Biomed Pharmacother. 2016;81:411–415. doi: 10.1016/j.biopha.2016.02.047. [DOI] [PubMed] [Google Scholar]
- 9.Cardenas A, Faleschini S, Cortes Hidalgo A, Rifas-Shiman SL, Baccarelli AA, DeMeo DL, Litonjua AA, Neumann A, Felix JF, Jaddoe VWV, El Marroun H, Tiemeier H, Oken E, Hivert MF, Burris HH. Prenatal maternal antidepressants, anxiety, and depression and offspring DNA methylation: epigenome-wide associations at birth and persistence into early childhood. Clin Epigenetics. 2019;11:56. doi: 10.1186/s13148-019-0653-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cawthon RM. Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res. 2009;37:e21. doi: 10.1093/nar/gkn1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De-Paula VJ, Kerr DS, Scola G, Gattaz WF, Forlenza OV. Lithium distinctly modulates the secretion of pro- and anti-inflammatory interleukins in co-cultures of neurons and glial cells at therapeutic and sub-therapeutic concentrations. Curr Alzheimer Res. 2016;13:848–852. doi: 10.2174/1567205013666160219112612. [DOI] [PubMed] [Google Scholar]
- 12.de Abreu Costa L, Henrique Fernandes Ottoni M, Dos Santos MG, Meireles AB, Gomes de Almeida V, de Fátima Pereira W, Alves de Avelar-Freitas B, Eustáquio Alvim Brito-Melo G. Dimethyl sulfoxide (DMSO) decreases cell proliferation and TNF-α, IFN-γ, and IL-2 cytokines production in cultures of peripheral blood lymphocytes. Molecules. 2017;22:1789. doi: 10.3390/molecules22111789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eitan E, Hutchison ER, Mattson MP. Telomere shortening in neurological disorders: An abundance of unanswered questions. Trends Neurosci. 2014;37:256–263. doi: 10.1016/j.tins.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Emamian ES, Hall D, Birnbaum MJ, Karayiorgou M, Gogos JA. Convergent evidence for impaired AKT1-GSK3beta signaling in schizophrenia. Nat Genet. 2004;36:131–137. doi: 10.1038/ng1296. [DOI] [PubMed] [Google Scholar]
- 15.Falk A, Heine VM, Harwood AJ, Sullivan PF, Peitz M, Brüstle O, Shen S, Sun YM, Glover JC, Posthuma D, Djurovic S. Modeling psychiatric disorders: from genomic findings to cellular phenotypes. Mol Psychiatry. 2016;21:1167–1179. doi: 10.1038/mp.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Farlow MR, Shamliyan TA. Benefits and harms of atypical antipsychotics for agitation in adults with dementia. Eur Neuropsychopharmacol. 2017;27:217–231. doi: 10.1016/j.euroneuro.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 17.Freyberg Z, Ferrando SJ, Javitch JA. Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry. 2010;167:388–396. doi: 10.1176/appi.ajp.2009.08121873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gassó P, Mas S, Molina O, Bernardo M, Lafuente A, Parellada E. Neurotoxic/neuroprotective activity of haloperidol, risperidone and paliperidone in neuroblastoma cells. Prog Neuropsychopharmacol Biol Psychiatry. 2012;36:71–77. doi: 10.1016/j.pnpbp.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Gibson GE, Park LC, Zhang H, Sorbi S, Calingasan NY. Oxidative stress and a key metabolic enzyme in Alzheimer brains, cultured cells, and an animal model of chronic oxidative deficits. Ann N Y Acad Sci. 1999;893:79–94. doi: 10.1111/j.1749-6632.1999.tb07819.x. [DOI] [PubMed] [Google Scholar]
- 20.Gubert P, Aguiar GC, Mourão T, Bridi JC, Barros AG, Soares FA, Romano-Silva MA. Behavioral and metabolic effects of the atypical antipsychotic ziprasidone on the nematode Caenorhabditis elegans. PLoS One. 2013;8:e74780. doi: 10.1371/journal.pone.0074780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Honig LS, Schupf N, Lee JH, Tang MX, Mayeux R. Shorter telomeres are associated with mortality in those with APOE epsilon4 and dementia. Ann Neurol. 2006;60:181–187. doi: 10.1002/ana.20894. [DOI] [PubMed] [Google Scholar]
- 22.Kang UG, Seo MS, Roh MS, Kim Y, Yoon SC, Kim YS. The effects of clozapine on the GSK-3-mediated signaling pathway. FEBS Lett. 2004;560(1-3):115–119. doi: 10.1016/S0014-5793(04)00082-1. [DOI] [PubMed] [Google Scholar]
- 23.Khan SS, Singer BD, Vaughan DE. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell. 2017;16:624–633. doi: 10.1111/acel.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z, Hu M, Zong X, He Y, Wang D, Dai L, Dong M, Zhou J, Cao H, Lv L, Chen X, Tang J. Association of telomere length and mitochondrial DNA copy number with risperidone treatment response in first-episode antipsychotic-naïve schizophrenia. Sci Rep. 2015;5:18553. doi: 10.1038/srep18553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindqvist D, Epel ES, Mellon SH, Penninx BW, Révész D, Verhoeven JE, Reus VI, Lin J, Mahan L, Hough CM, Rosser R, Bersani FS, Blackburn EH, Wolkowitz OM. Psychiatric disorders and leukocyte telomere length: Underlying mechanisms linking mental illness with cellular aging. Neurosci Biobehav Rev. 2015;55:333–364. doi: 10.1016/j.neubiorev.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manchia M, Paribello P, Arzedi C, Bocchetta A, Caria P, Cocco C, Congiu D, Cossu E, Dettori T, Frau DV, Garzilli M, Manca E, Meloni A, Montis MA, Mura A, Nieddu M, Noli B, Pinna F, Pisanu C, Robledo R. A multidisciplinary approach to mental illness: do inflammation, telomere length and microbiota form a loop? A protocol for a cross-sectional study on the complex relationship between inflammation, telomere length, gut microbiota and psychiatric disorders. BMJ Open. 2020;10:e032513. doi: 10.1136/bmjopen-2019-032513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McEvoy J, Millet RA, Dretchen K, Morris AA, Corwin MJ, Buckley P. Quantitative levels of aripiprazole parent drug and metabolites in urine. Psychopharmacology (Berl) 2014;231:4421–4428. doi: 10.1007/s00213-014-3781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monroy-Jaramillo N, Rodríguez-Agudelo Y, Aviña-Cervantes LC, Roberts DL, Velligan DI, Walss-Bass C. Leukocyte telomere length in Hispanic schizophrenia patients under treatment with olanzapine. J Psychiatr Res. 2017;90:26–30. doi: 10.1016/j.jpsychires.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 29.Polho GB, De-Paula VJ, Cardillo G, dos Santos B, Kerr DS. Leukocyte telomere length in patients with schizophrenia: a meta-analysis. Schizophr Res. 2015;165(2-3):195–200. doi: 10.1016/j.schres.2015.04.025. [DOI] [PubMed] [Google Scholar]
- 30.Porton B, Delisi LE, Bertisch HC, Ji F, Gordon D, Li P, Benedict MM, Greenberg WM, Kao HT. Telomerase levels in schizophrenia: a preliminary study. Schizophr Res. 2008;106(2-3):242–247. doi: 10.1016/j.schres.2008.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savage SA. Beginning at the ends: telomeres and human disease. F1000Research. 2018;7:524. doi: 10.12688/f1000research.14068.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ulrich S, Baumann B, Wolf R, Lehmann D, Peters B, Bogerts B, Meyer FP. Therapeutic drug monitoring of clozapine and relapse--a retrospective study of routine clinical data. Int J Clin Pharmacol Ther. 2003;41:3–13. doi: 10.5414/cpp41003. [DOI] [PubMed] [Google Scholar]
- 33.Van Putten T, Marder SR, Mintz J, Poland RE. Haloperidol plasma levels and clinical response: a therapeutic window relationship. Am J Psychiatry. 1992;149:500–505. doi: 10.1176/ajp.149.4.500. [DOI] [PubMed] [Google Scholar]
- 34.Xu T, Park SK, Venable JD, Wohlschlegel JA, Diedrich JK, Cociorva D, Lu B, Liao L, Hewel J, Han X, Wong CCL, Fonslow B, Delahunty C, Gao Y, Shah H, Yates JR., 3rd ProLuCID: an improved SEQUEST-like algorithm with enhanced sensitivity and specificity. J Proteomics. 2015;129:16–24. doi: 10.1016/j.jprot.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yeh JK, Wang CY. Telomeres and telomerase in cardiovascular diseases. Genes (Basel) 2016;7:58. doi: 10.3390/genes7090058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu WY, Chang HW, Lin CH, Cho CL. Short telomeres in patients with chronic schizophrenia who show a poor response to treatment. J Psychiatry Neurosci. 2008;33:244–247. [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Toh L, Lau P, Wang X. Human telomerase reverse transcriptase (hTERT) is a novel target of the Wnt/β-catenin pathway in human cancer. J Biol Chem. 2012;287:32494–32511. doi: 10.1074/jbc.M112.368282. [DOI] [PMC free article] [PubMed] [Google Scholar]


