ABSTRACT
The exploitation of microorganisms for the fabrication of nanoparticles (NPs) has garnered considerable research interest globally. The microbiological transformation of metals and metal salts into respective NPs can be achieved under environmentally benign conditions, offering a more sustainable alternative to chemical synthesis methods. Species of the metal-reducing bacterial genus Shewanella are able to couple the oxidation of various electron donors, including lactate, pyruvate, and hydrogen, to the reduction of a wide range of metal species, resulting in biomineralization of a multitude of metal NPs. Single-metal-based NPs as well as composite materials with properties equivalent or even superior to physically and chemically produced NPs have been synthesized by a number of Shewanella species. A mechanistic understanding of electron transfer-mediated bioreduction of metals into respective NPs by Shewanella is crucial in maximizing NP yields and directing the synthesis to produce fine-tuned NPs with tailored properties. In addition, thorough investigations into the influence of process parameters controlling the biosynthesis is another focal point for optimizing the process of NP generation. Synthesis of metal-based NPs using Shewanella species offers a low-cost, eco-friendly alternative to current physiochemical methods. This article aims to shed light on the contribution of Shewanella as a model organism in the biosynthesis of a variety of NPs and critically reviews the current state of knowledge on factors controlling their synthesis, characterization, potential applications in different sectors, and future prospects.
KEYWORDS: biosynthesis, microbial, anaerobic, biofabrication, catalysis, green synthesis, metal nanoparticles, Shewanella
INTRODUCTION
Nanotechnology has become increasingly popular with the development of a variety of nanoparticles (NPs) with unique properties for wide-ranging applications across various sectors of science and technology (1, 2). The physical and chemical properties of NPs differ substantially from bulk materials due to their small size (particle size of ≤100 nm), high surface-area-to-volume ratio, and increased presence of defect sites and edges. Owing to these unique properties, the application of NPs is a rapidly growing field of research with diverse applications in a wide range of industries, including farming (3, 4), consumer products (5), coatings (6), cosmetics (7), catalysis (8), chemicals (9), electronics and optics (10), environmental remediation (11, 12), food packaging (13), fuel additives (14), energy (15), textile and paints (16), and next-generation medicine (17).
Worldwide, the area of NP synthesis is growing rapidly, and it is expected that the global market of NPs could reach $25.26 billion by 2022 (18, 19). The physicochemical approaches currently being employed are capital exhaustive and often require the use of aggressive reagents and processing conditions (20, 21). Thus, there is a need for cost-effective, clean, biocompatible, and eco-friendly methods for the facile synthesis of NPs. Biological resources, and in particular microorganisms, offer a potential solution as nano-factories for NP synthesis (22, 23). Numerous publications can be found in the literature dealing with the biosynthesis of metal-based NPs using different organisms, including bacteria (24, 25), algae (26), yeasts (27), fungi (28), and plant extracts (29, 30).
Biosynthesis of NPs has several advantages, including high purity, low cost, and sustainable environmentally benign methods. However, consideration must be given to eventual scale-up for mass production via optimization of culture conditions such as pH, incubation time, temperature, and the concentration of the metal ions. Developing an environment-friendly, low-risk approach that effectively modulates the size, morphology, stability, and properties of NPs is an important focus of current research concerned with biogenic NP synthesis. In this minireview, an in-depth critical synthesis pertaining to the application of Shewanella as a model microorganism for the green synthesis of NPs is provided, including factors influencing the synthesis and properties of NPs and mechanistic details of electron transfer routes culminating in metal reduction and NPs synthesis. Finally, concluding discussions on potential future opportunities and challenges of microbial synthesis of NPs are summarized.
SYNTHESIS OF NANOPARTICLES USING BACTERIA
Bacteria have attracted significant attention in the green synthesis of NPs due to their ease of cultivation in simple growth media, the large diversity of organisms, and rapid adaptation under changing environmental conditions (31, 32). Extensive studies are available in the literature focusing on the bacterial synthesis of a wide variety of NPs. However, the identification of key bacterial enzymes, metabolic products, and factors affecting the synthesis of NPs is required to develop a systematic understanding of these processes. Among different bacterial taxa, the metal- and sulfur-reducing Shewanella spp. have shown promising potential in the fabrication of a variety of NPs for a wide range of applications (33, 34) and are discussed in this minireview. The biosynthesis of NPs using Shewanella are generalized in Fig. 1.
FIG 1.
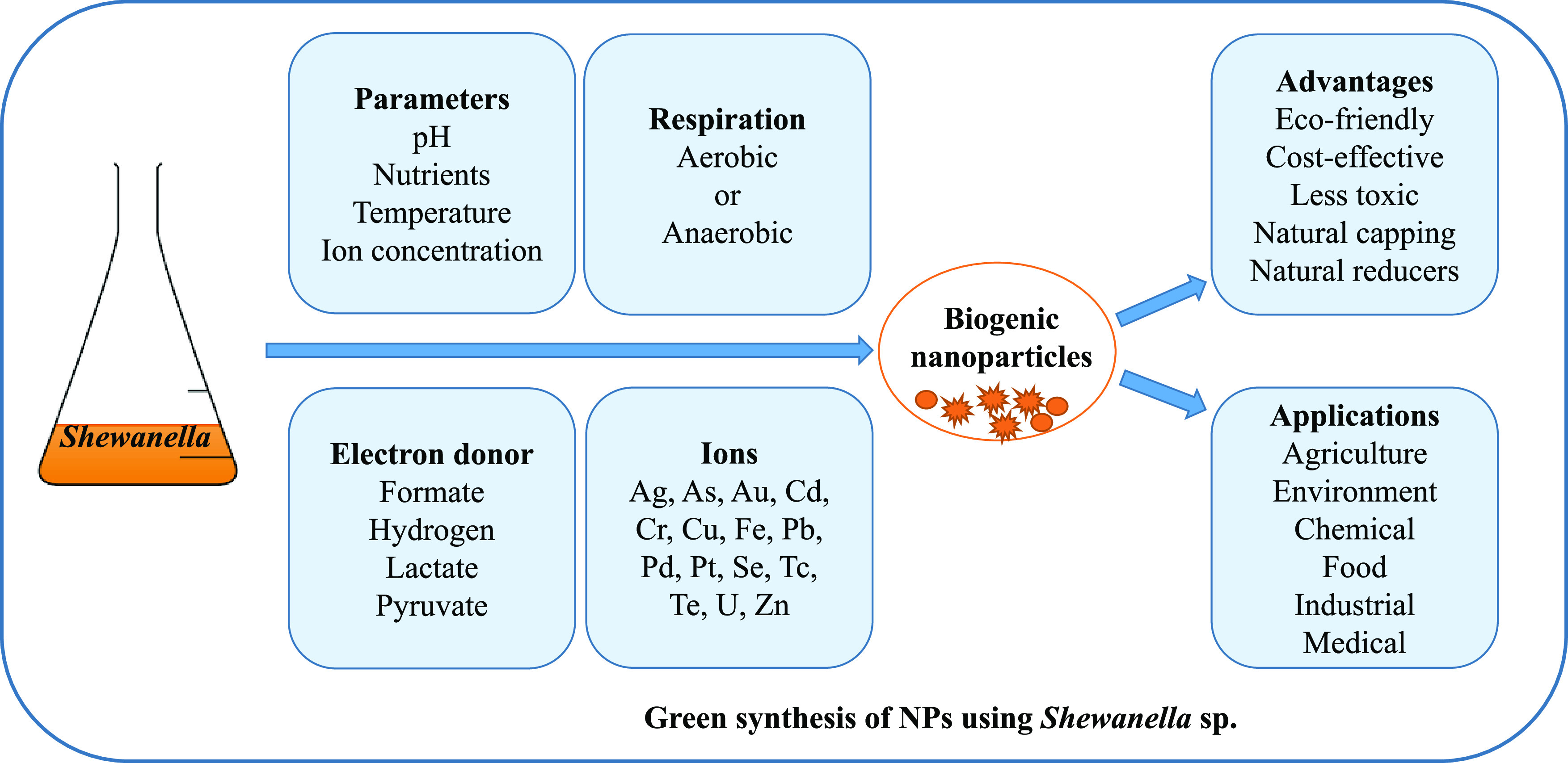
Green synthesis of nanoparticles using Shewanella species.
Role of Shewanella species in the facile synthesis of nanoparticles.
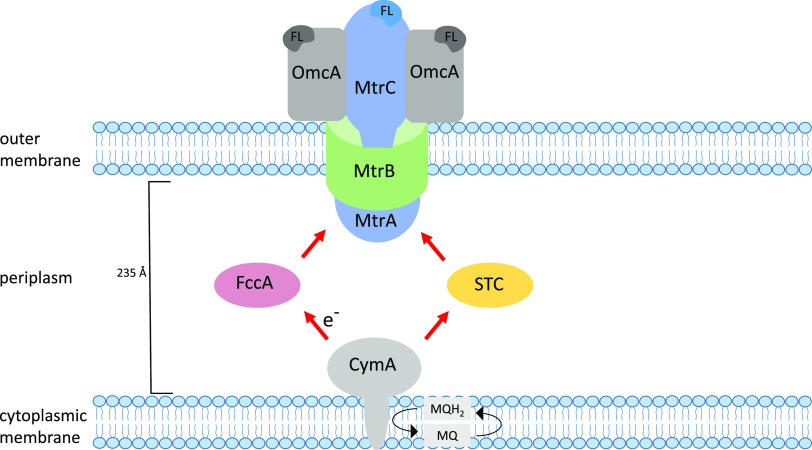
Shewanella is the sole genus within the family Shewanellaceae, belonging to the Gammaproteobacteria, a class of Gram-negative bacteria. Among Shewanella, S. oneidensis MR-1 is often used as a model organism to study the anaerobic reduction of metals. S. oneidensis MR-1 (formally Alteromonas putrefaciens), was isolated from Lake Oneida, New York, and described with the capability of utilizing Fe and Mn as terminal electron acceptors for growth (35, 36). Since then, a variety of other Shewanella strains with the capacity for metal reduction have been identified from a range of soil, sediment, and aquatic environments, including, S. xiamenensis BC01 (SXM), S. algae, S. putrefaciens, S. loihica PV-4, S. piezotolerans, and S. decolorationis S12. The versatility of Shewanella in reducing metals extends beyond Fe(III) and Mn(IV) to “nonstandard” metals, including U(VI) (37), Cr(VI) (38), Np(V) (39), Tc(VII) (40), Pu(IV) (41), V(V) (42), Se(IV) (43), Te(IV) (43), Au(III) (44), Ag(I) (45), Pd(II) (46), and Cu(II) (47). The reduction of these diverse terminal electron acceptors is facilitated by the complex multicomponent branched electron transport system (ETS) in Shewanella which includes inner membrane-localized dehydrogenases, menaquinone, and a multitude of cytochromes (48). Of these electron transport systems, the best characterized is the Mtr pathway of S. oneidensis, which is required for the reduction of insoluble metal oxides and electrodes and also plays an important role in the reduction of various soluble electron acceptors (49). Five primary protein components have been identified in the Mtr pathway, the inner membrane-bound CymA, the periplasmic c-type cytochrome MtrA, the outer membrane (OM) β-barrel protein MtrB, and the OM-anchored c-type cytochromes MtrC and OmcA (Fig. 2). CymA, a tetraheme c-type cytochrome, has been described as an “electron transport hub,” transferring electrons from menaquinol to a variety of electron transport pathways with terminal electron acceptors that are reduced either within the periplasm or at the cell surface. Electrons from CymA are transferred to MtrA via perisplasmic carriers, including the fumarate reductase (FccA) and the small tetraheme cytochrome (STC) (50).
FIG 2.
Illustration of the Mtr electron transport pathway in Shewanella oneidensis MR-1. Electrons from the menaquinone (MQ) pool are passed to CymA, a cytoplasmic membrane-associated tetraheme cytochrome. The electrons from CymA can be distributed to a range of electron transport pathways with terminal electron acceptors that can be reduced in the periplasm or at the cell surface. In the case of extracellular metal reduction, electrons are transferred from CymA to MtrA via periplasmic c-type cytochromes, FccA and STC. MtrA, a decaheme c-type cytochrome is located on the periplasmic side of the outer membrane, deeply embedded within the β-barrel protein MtrB. The MtrAB complex connects to MtrC, another decaheme c-type cytochrome, located on the external side of the outer membrane, allowing transfer of electron to extracellular electron acceptors. A further decaheme c-type cytochrome, OmcA, completes the structure, forming a 2:1 complex with MtrC and facilitating reduction. FL, flavin cofactor of OM cyctochromes. Adapted from Beblawy et al. (128).
MtrA is one of three proteins, along with MtrB and MtrC, that form the porin-cytochrome complex, MtrCAB. MtrA is embedded within MtrB, which together span the OM and bind to MtrC on the extracellular side of the membrane (51). Electrons can be transferred directly from MtrA to MtrC and then distributed to various extracellular terminal electron acceptors. OmcA has also been suggested to accept electrons from the MtrAB complex and can facilitate extracellular electron transfer (52). This pathway plays a central role in the bioreduction of many metals and the subsequent formation of metal NPs and is discussed in more detail in the relevant sections.
The current information pertaining to the contribution of different Shewanella spp. in the biosynthesis of various metal-based NPs is presented in the following section.
Iron-based nanoparticles.
A range of biogenic Fe minerals were produced using S. putrefaciens (strain CN32), via reduction of an amorphous hydrous ferric oxide (ferrihydrite) (53). The nature of the resulting biominerals was heavily dependent upon the composition of the aqueous media. In the absence of P in the media, nanoscale magnetite was formed. When an electron shuttle, anthraquinone-2,6-disulfonate (AQDS), was present, the magnetite NPs were highly crystalline, with crystallinity lower in the absence of the electron shuttle (52). S. oneidensis MR-1 has also been shown to produce magnetite NPs from a ferrihydrite precursor. These biogenic magnetite NPs were less crystalline than abiogenic magnetite, a feature that could potentially serve as a biosignature for natural magnetites (54). Magnetite NPs have also been produced by a range of other Shewanella spp. Ferrihydrite was partially converted to superparamagnetic magnetite nanocrystals by S. algae (55). The synthesis of magnetite NPs (>35 nm) by S. loihica, a marine psychrotolerant species, resulted in well-formed single-domain magnetite, in contrast to the mostly superparamagnetic magnetite NPs produced by mesophilic species (56), highlighting that strain choice could be important for directing NP properties.
The biosynthesis of doped extracellular magnetite using Fe(III)-reducing bacteria, including Shewanella, has also attracted great attention in order to control and exploit the magnetic properties of these biominerals for applications, including remediation, catalysis, drug delivery, and data storage (57). S. oneidensis was used to produce Co and Ni doped magnetite NPs (58). The produced NiFe2O4 contained Ni2+, with at least 80% in Oh coordination, whereas the CoFe2O4 contained Co2+ but with a significant proportion (up to 45%) in Td coordination. The formation of Ni2+-substituted magnetite NPs from a Ni-doped ferrihydrite precursor was also shown to be promoted by S. putrefaciens (59). S. loihica was able to facilitate the production of transition metal- and lanthanide-substituted magnetite nanocrystals from a doped iron oxyhydroxide precursor (60, 61). The Mn- and Zn-substituted magnetites had a higher magnetic susceptibility (κRT) and saturation magnetization (MS) than pure biogenic magnetite with a maximum κRT at 0.2 cationic mole fraction, while lanthanide substitution was found to generally decrease MS, with Gd- and Ho-substituted magnetites having the highest magnetization (61).
Biomineralization of spherical FeS-NPs by S. loihica and S. oneidensis following bioreduction of iron citrate and thiosulfate has also been demonstrated (62, 63). These extracellular NPs were not directly associated with the cells but were linked to cells via biogenic FeS nanowires which could facilitate long-distance extracellular electron transport.
Copper-based nanoparticles.
The role of S. loihica in facilitating the synthesis of Cu-NPs possessing antimicrobial activity was reported (63). The authors concluded that the NPs were primarily extracellular, leading them to suggest that extracellular bioreduction could be responsible for their formation (64). However, in a study using S. oneidensis, it was demonstrated that key OM cytochromes (OmcA, MtrC, and MtrF) and periplasmic cytochromes (including MtrABCDEF) did not play a role in the bioreduction of Cu(II) by this organism (47). In addition, electron microscopy work clearly demonstrated that Cu-NPs were not restricted to the extracellular environment but were also present in the periplasm and cytoplasm of S. oneidensis (47). Although this could indicate that electron transport pathways and characteristics of synthesized Cu-NPs are strain dependent, there was little evidence to support either the involvement of OM cytochromes in S. loihica or even that the NPs were indeed extracellular. Furthermore, it was claimed that X-ray photoelectron spectroscopy (XPS) analysis supports the bioreduction of Cu(II) to Cu(0) by S. loihica; however, the XPS peaks are more reflective of Cu(II) than Cu(0) (65). The high Cu(II) concentration (1 mM) used in combination with additional salts in the S. loihica medium, may have contributed to the abiotic precipitation of oxidized Cu. In contrast, the Cu-NPs produced by S. onediensis were confirmed by X-ray absorption spectroscopy to comprise Cu(0). The efficiency of these Cu(0)-NPs as catalysts for “click chemistry” reactions, an important synthesis pathway for new pharmaceuticals (66), was also demonstrated.
S. oneidensis has also been shown to facilitate the synthesis of CuS-NPs when sodium thiosulfate was used as an electron acceptor (66). The CuS-NPs displayed a high photothermal conversion efficiency, demonstrating the potential for cost-effective and environment-friendly biosynthesis of CuS-NPs by Shewanella for possible applications in photothermal therapy for cancer treatment (66). A similar study showed the formation of hollow CuS nanospheres following bioreduction of thiosulfate by S. oneidensis and subsequent addition of Cu(II) (67). The CuS shells, located on the cell surface, enhanced the adsorption capacity of S. oneidensis toward toxic Cr(VI). These studies demonstrate that the use of an additional electron acceptor, thiosulfate, can lead to the formation of CuS-NPs by S. oneidensis, in contrast to the Cu(0)-NPs synthesized where no additional electron acceptor (other than Cu(II)) was used. This highlights the potential for controlling Cu-based NP properties, which could be tailored for specific applications, using a single species of Shewanella (S. oneidensis).
Gold-based nanoparticles.
Synthesis of Au-NPs by S. oneidensis from a tetracholoraurate solution was investigated and revealed a synthesis process involving an initial, fast biosorption step followed by a slower reduction step which required the presence of an electron donor (68). While using H2 as an electron donor, it was found that the location and size of the Au-NPs could be directed. However, as the reduction was also observed in a heat-killed cell control, the synthesis process was considered to be of nonenzymatic nature. The Au-NPs synthesized by S. oneidensis were hydrophilic and resisted aggregation even after several months and were not toxic or inhibitory to strains of Gram-negative and Gram-positive bacteria (69). The synthesis of Au-NPs by a biofilm of S. oneidensis on the anode of a microbial fuel cell (MFC) and their impact on energy generation were also investigated (70). An anode decorated with the Au-NPs produced a 62.5% higher power density than the MFC containing a plain carbon foam electrode and allowed rapid detection of H2O2 at a very low concentration (20 μM) (70).
Photo-induced biosynthesis of Au-NPs by S. oneidensis has also been reported (71). The light-induced activation of certain functional groups and extracellular polymer molecules, as revealed by the excision of selected genes, was suggested as the possible mechanism underlying the synthesis of the Au-NPs. Interestingly, light intensity and wavelength were found to influence the rate of Au-NP synthesis. These studies demonstrate several pathways that could induce Au-NP formation in the presence of Shewanella spp.; however, the impact of these different pathways on the properties of the resulting Au-NPs has not been studied and should be further investigated so as to identify methods that could produce tailored Au-NPs for specific applications.
Recent work has investigated the synthesis of Au-NPs by S. oneidensis and S. xiamenensis under various conditions such as pH, initial metal concentration, and cellular biomass of selected anaerobic bacteria (72). S. xiamenensis was found to produce a higher yield of Au-NPs, but differences in the properties or characteristics of the NPs produced by the different strains were not reported. The study deciphered the binding of Au ions onto the bacterial OM followed by the precipitation of Au-NPs and presented a viable strategy for metal extraction from large volumes of industrially produced wastes. In a similar study, synthesis of Au-, Pd- and Pt-NPs by an electrochemically active biofilm of S. loihica was described (73). Interestingly, cell extracts from S. algae were found to produce Au-NPs and nanoplates with H2 as an electron donor at room temperature and pH 2.8 (33), demonstrating the potential use of Shewanella spp. for Au-NP synthesis under harsh conditions, such as those found in metal-rich waste streams.
Palladium-based nanoparticles.
The majority of work on Shewanella-directed Pd-NP synthesis has focused on S. oneidensis and demonstrated the bioreduction of Pd(II) to Pd(0) in the presence of a variety of electron donors (46). Biogenic Pd-NPs have the ability to reductively dehalogenate the contaminant, polychlorinated biphenyl (PCB), with comparable activity to commercial Pd(0) powder (74). The size and reactivity of Pd-NPs synthesized by S. oneidensis could be fine-tuned by controlling the Pd/cell weight (dry weight) to tailor catalytic activity toward either PCB or perchlorate (74).
Several studies have sought to elucidate key electron transport pathways involved in Pd(II) reduction and Pd-NP formation, focusing on S. oneidensis, with contrasting reports on the roles of hydrogenases and cytochromes. Initial work by Ng et al. (75) suggested that hydrogenases were key enzymes in the reduction of Pd(II), with mutants lacking either the [NiFe]-hydrogenase, HyaB, or [FeFe]-hydrogenase, HydA, displaying decreased Pd(II) reduction rates relative to the wild type when using formate as an electron donor. On the other hand, a mutant lacking both the OM cytochromes, MtrC and OmcA, did not show any decrease in Pd(II)-reducing capability when either lactate or formate was used as an electron donor (75). It should be noted that the concentration of formate used as an electron donor in this study has been shown to result in abiotic reduction of Pd(II) and so may have contributed to the reduction rates, potentially contributing to differences between the mutant strains (76, 77). More recently, Dundas et al. presented contrasting results showing that the loss of these two OM cytochromes (MtrC and OmcA) did attenuate Pd(II) reduction and were also important in controlling particle size and deposition (78). A minimal decrease in Pd(II) reduction using hydrogenase deletion mutants was also observed (78). In the latter study, transmission electron microscopy (TEM) images revealed clear differences in the cellular location of NPs produced by the different mutant strains. MtrC and OmcA were required for the precipitation of extracellular NPs, with their removal resulting in Pd precipitation in the periplasm, further supporting the role of these OM cytochromes in facilitating Pd(II) reduction. This demonstrated use of mutants to control NP size and localization (78) could prove a valuable tool for the biosynthesis of bespoke Pd catalysts. A difference in buffering capacity used in the two studies could potentially explain the discrepancy in results. Ng et al. (75) used 30 mM HEPES, whereas Dundas et al. (78) used a more heavily buffered medium containing 100 mM HEPES. Dundas et al. (78) suggested that their more strongly buffered system may have better countered pH changes caused by cell metabolism which could, in turn, have affected the results of the study by Ng et al. (75). However, it should also be pointed out that Good’s buffers, including HEPES, have been shown to have an effect on aqueous Pd chemistry and at high concentrations (100 mM), and can cause abiotic Pd precipitation, potentially complicating the interpretation in these studies (79). Indeed, Dundas et al. (78) highlighted that they observed decreased reduction rates of Pd(II) with increasing age of the HEPES buffer, further highlighting the importance of buffer chemistry in controlling Pd reduction. A recent study by Yang et al. (77) sought to address these conflicting results and elucidate the role of dehydrogenases and hydrogenases in Pd(II) reduction and NP synthesis. In the presence of formate as an electron donor, the inhibition of NADH dehydrogenases (NADH-DH) resulted in a strong decrease in Pd(II)-reducing capability relative to the wild type with almost no Pd-NPs observed on the OM (77). A smaller decrease in Pd(II) reduction was observed in mutants lacking both the hydrogenases, HydA and HydB. Based on these data, two pathways resulting in Pd(II) reduction in S. oneidensis using formate as the electron donor were proposed by the authors (77). Pathway I involves electron transfer from NADH produced by formate dehydrogenases, which couple formate oxidation to NAD+ reduction (80), via NADH-DH to CymA and the Mtr pathway. This pathway is blocked when NADH-DH is inhibited, resulting in the decrease in Pd(II) reduction and lack of OM-associated NPs observed by Yang et al. (77). Pathway II involves Pd(II) reduction by hydrogen, produced from formate by hydrogenases in combination with formate dehydrogenase. Pathway I, which appeared to be the more dominant mechanism in the study by Yang et al. (77) supports the observation from Dundas et al. (78) that the Mtr pathway, and likely the OM cytochromes, play an important role in Pd(II) reduction. Pathway II supports the earlier observations from Ng et al. (75) that hydrogenases may also be involved in Pd(II) reduction, albeit in a smaller capacity than the cytochromes. It is clear that despite detailed mechanistic work with deletion mutants, the complex electron transport pathways of Shewanella require further study focusing on the formation of Pd-NPs to achieve directed control and optimization of NP synthesis. As discussed above, this may also require a deeper understanding of the role of solution chemistry in Pd(II) bioreduction and NP formation (see “Factors Affecting Biosynthesis of Nanoparticles” below).
Recently, in situ biosynthesis of Pd-NPs on cells of S. oneidensis coated with reduced graphene oxide (rGO), forming a bio-nano hybrid material has been reported (81). The integration of Pd-NPs with rGO and Shewanella cells promoted Cr(VI) removal 10 times that of native cells and 5 times that of cells and Pd-NPs without rGO (81). S. oneidensis has also been exploited for Pd-NP synthesis in microbial electrolysis cells via bioelectrochemical reduction of the metal on the cathode (82). The size of NPs synthesized via the bioelectrochemical route was in the range of 10 to 100 nm, in contrast to 200- to 250-nm Pd-NPs generated through an electrochemical reduction process lacking cells. Furthermore, in the same experiment, the bioelectrochemical Pd-NPs also displayed significantly enhanced activity toward hydrogen production by 60% compared to Pd catalysts in the absence of microbial reduction, highlighting the substantial potential of biological routes in producing NPs with smaller size and better catalytic efficiency (82).
Platinum-based nanoparticles.
So far, limited investigations have been made regarding the synthesis of Pt-NPs using Shewanella spp. A biofilm of S. loihica successfully directed the biosynthesis of ultralow-sized Pt-NPs when supplied with acetate as an electron donor (73). The particle size was observed to be influenced by pH, with no Pt-NPs produced at pH 4.0, 2- to 6-nm Pt-NPs produced at pH 7.0, and 2- to 10-nm Pt-NPs produced at pH 9.0 (73). Metal precursor concentration was also found to influence particle size, with higher initial metal concentration producing larger NPs. However, no information on the potential electron transport pathway(s) involved or localization of the Pt-NPs was provided. The bacterium S. algae was also found to produce Pt-NPs, with an average diameter of 5 nm, using lactate as an electron donor at ambient temperature and pH 7.0 (83). Resting cells of S. algae catalyzed the fabrication of Pt-NPs via bioreduction within 1 h. TEM analysis of thin sections indicated the Pt-NPs were located in the cell periplasm suggesting that periplasmic reductases, such as hydrogenases or periplasmic cytochromes, may be important for Pt reduction. However, further work is required to elucidate the mechanism of Pt reduction in Shewanella. The use of deletion mutants, such as those studied in Pd(II) reduction, could prove useful in identifying key Pt reductases. In addition, the catalytic activity of Pt-NPs synthesized by Shewanella has not been tested and remains an avenue for further research.
Cadmium-based nanoparticles.
Anaerobic synthesis of spherical CdS-NPs by S. oneidensis, possessing high activity in the degradation of the diazo dye, trypan blue, was reported (84). When the biogenic CdS-NPs were excited by visible light, the electronic energy transfer (EET) of S. oneidensis could deliver electrons to photogenerated holes in the valance band and maintain the ongoing photoexcitation to produce photogenerated electrons and catalyze the photoreduction of trypan blue (84). Participation of S. oneidensis has been reported in the synthesis of metastable spherical CdS-NPs in a culture medium containing Na2S and CdCl2 salts as precursors (32). In abiotic controls, dense precipitates of agglomerated CdS minerals were observed, suggesting that the Shewanella cells acted as nucleation sites for CdS-NP precipitation and prevented their agglomeration.
The toxicity of CdS-NPs, synthesized in the presence of S. oneidensis, against brain cancer cell lines was demonstrated, thereby offering an environmentally benign biosynthesis method for these important NPs (31). However, the CdS-NPs were largely aggregated which may limit their surface area and potential efficiency for biotechnological applications. A possible solution to problems associated with Cd-NP aggregation was investigated using cells of Shewanella to catalyze the synthesis of Cd-based NPs immobilized on polymer supports, resulting in stable and well-dispersed NPs (85).
Silver-based nanoparticles.
AgS-NPs synthesized in the presence of S. oneidensis displayed enhanced toxicity toward both Gram-negative and Gram-positive bacteria compared to chemically synthesized Ag-NPs (86). Biosynthesis was found to proceed most efficiently in the presence of metabolically active cells, and an increase in the initial concentration of AgNO3 and Na2S2O3 from 1 mM to 10 mM was reported to improve the final mass of NPs synthesized (87). Nevertheless, the yield with respect to the concentration of Ag in the medium was observed to decline. The in situ synthesis of well-dispersed Ag-NPs on carbon nanotubes assisted by S. oneidensis bioreduction was demonstrated, with the resulting NPs displaying satisfactory catalytic activity in the degradation of 4-nitrophenol (88). Studies on Ag-based NP biosynthesis using Shewanella spp. were also demonstrated by several other researchers (85, 89, 90).
Composite nanoparticles.
Recently, the cost-effective and eco-friendly generation of composite NPs (CNPs) consisting of Cu-NPs and carbon nanotubes (CNTs) assisted by S. oneidensis, possessing efficient catalytic activity for the conversion of the environmental pollutant, 4-nitrophenol, was demonstrated (91). Highly crystalline Cu-NPs were formed over the exterior of the CNTs with a size range of 4 to 10 nm. A weight ratio of 3% (Cu-NPs/CNTs) was found to be the optimal loading before higher Cu-NP loadings started to decrease the catalytic activity, potentially due to increasing NP aggregation. The use of S. oneidensis as a bioreducing agent leading to in situ fabrication of CNPs consisting of Ag2S-NPs on the surfaces of TiO2 nanotubes with a potential application in contaminant degradation was also reported (92). Characterization of the Ag2S-NPs suggested a relatively uniform distribution on the TiO2 surface with a particle size of <8 nm. The composite materials synthesized by S. oneidensis displayed excellent catalytic activity toward the reduction of 4-nitrophenol, and again, the molar ratio of the components was important in maximizing the catalytic efficiency (92).
The biological synthesis of Pt and Pd bimetallic NPs by S. oneidensis as catalysts for the reduction of environmental pollutants has been investigated by several authors (93–95). The addition of an electron shuttle was found to decrease the size of bimetallic PdPt-NPs synthesized by S. oneidensis and resulted in the increased catalytic activity of these smaller bimetallic NPs relative to their larger counterparts and monometallic Pd- or Pt-NPs (94). The PdPt-NPs could be reused for up to six cycles in the reduction of 4-nitrophenol. Differences in X-ray diffraction (XRD) peaks between the bimetallic PdPt-NPs and monometallic NPs of Pd and Pt were used to infer an alloyed structure (94). Localization of the bimetallic NPs could not be determined in this study. However, a similar study imaging thin-section TEM samples suggested that bimetallic PdPt-NPs synthesized by S. oneidensis were present both intracellularly and extracellularly (93), potentially pointing to the involvement of both OM cytochromes and periplasmic enzymes (e.g., cytochromes or hydrogenases) in the metal reduction and NP precipitation process.
S. oneidensis has also been used in the synthesis of PdAg-NPs supported on reduced graphene oxide (rGO). Characterization of the nanocomposites suggested that individual Pd- and Ag-NPs were separated by the rGO. The nanocomposites were catalytically active toward the reduction of 4-nitrophenol with the highest efficiency demonstrated for a 1:1 ratio of Pd to Ag (96). Several authors have also reported the biosynthesis of bimetallic PdAu-NPs using S. oneidensis for promising applications in environmental remediation and catalysis. The bimetallic NPs delivered more reproducible results with a broader reaction scope as catalysts for Suzuki coupling compared to monometallic Pd-NPs (97, 98). However, these PdAu-NPs were synthesized under an H2-containing atmosphere which is known to abiotically reduce Pd and thus may offer less control on NP structure than purely enzymatic synthesis routes (97, 98). This is highlighted in a recent study where the enzymatic synthesis of PdAu-NPs by S. oneidensis, in the absence of H2, resulted in bimetallic NPs with a smaller average size and smaller size range than those seen when H2 is present as a potential electron donor (99). In the same study, the catalytic activity of these PdAu-NPs was compared against PdAg-NPs and monometallic Pd-NPs in Suzuki-Miyaura cross coupling reactions. The catalytic activity of the NPs was found to follow in the order PdAg>PdAu≫Pd, demonstrating the potential application of CNPs in key commercial reactions (99).
The complexes of NPs synthesized by a single Shewanella species could foster the development of composite NPs with novel characteristics (82, 92). However, the complexity of multicomponent NP synthesis offers both challenges and opportunities for developing such novel materials.
Other nanoparticles.
Apart from the above discussed NPs, Shewanella is credited to contribute to the synthesis of a suite of metallic NPs, including Cr, Zn, T, Pb, Se, Te, Tc, U, As, and Mn by numerous researchers (Table 1).
TABLE 1.
Application of Shewanella to synthesize various nanoparticlesa
| Produced NPs | Organism | Chemical(s) used | Aerobic or anaerobic |
Electron donor |
NP size (nm) |
Significance | Reference(s) |
|---|---|---|---|---|---|---|---|
| Arsenic-Fe | Shewanella spp. | Na3AsO4 | Anaerobic | Pyruvate | 129 | ||
| As4S4 nanotubes | Shewanella sp. | As5+ and S2O32− | Anaerobic | Lactate | Photoactive | 130 | |
| As4S4-rGO | Strain HN-41 | As5+, S2O32−, GO | Anaerobic | Lactate | Li ion storage | 131 | |
| Cadmium sulfide | S. oneidensis | CdCl2·2.5H2O | Aerobic | 5 ± 1 | 85 | ||
| Chromium | S. oneidensis | K2CrO4 | Anaerobic | Lactate | nd | 132 | |
| Chromium | S. oneidensis | K2CrO4 | Anaerobic | Lactate | nd | 133 | |
| Cr-Te | S. oneidensis | K2Cr2O7 | Anaerobic | Lactate | 10−16 | Precipitation of Te(IV) | 134 |
| Copper | S. oneidensis | CuSO4 | Anaerobic | Lactate | 20−40 | Catalysis | 47 |
| Copper | S. loihica | CuCl2·2H2O | Anaerobic | 10−16 | Antibacterial activity | 64 | |
| Copper | S. oneidensis | CuCl2 | Anaerobic | Lactate | 5 | Photothermal agent | 66 |
| Gold | S. algae | HAuCl4 | Anaerobic | H2 | 10−20 | Intracellular recovery of gold | 135 |
| Gold | S. oneidensis | HAuCl4·3H2O | Anaerobic | H2 | 5−10 | 68 | |
| Aerobic | Formate | ||||||
| Gold | EPS of S. oneidensis | HAuCl4 | 1−8 | 136 | |||
| Gold | S. oneidensis | HauCl4·3H2O | Anaerobic | Lactate | 15 | 71 | |
| Gold | S. oneidensis | HauCl4 | Aerobic | 12 ± 5 | 69 | ||
| Gold | S. haliotis | HauCl4·3H2O | Aerobic | Lactate | 10−30 | Degradation of p-nitrophenol | 137 |
| Iron | S. oneidensis | Fe(III) oxide | Anaerobic | Lactate | 27−31 | 138 | |
| Lead | S. putrefaciens | Pb-jarosite | Anaerobic | 113.2 | Biogeochemical cycling of Pb | 139 | |
| Palladium | S. oneidensis | Na2PdCl4 | Aerobic | Formate | nd | Dechlorination of PCB | 46 |
| Palladium | S. oneidensis | Na2PdCl4 | Anaerobic | Formate | nd | Dechlorination and degradation of PCBs | 74 |
| Palladium | S. oneidensis | Na2PdCl4 | Aerobic | Formate | nd | Dechlorination of lindane | 140 |
| Palladium | S. oneidensis | Na2PdCl4 | Aerobic | Formate | nd | Dechlorination of trichloroethylene | 122 |
| Palladium | S. oneidensis | Na2PdCl4 | Aerobic | Formate | nd | Remediation of trichloroethylene | 141 |
| Palladium | S. oneidensis | Na2PdCl4 | Anaerobic | Formic acid | nd | Pd recovery from waste | 76 |
| Palladium | S. oneidensis | Na2PdCl4 | Aerobic | Formate | 20−100 | Reduction of p-nitrophenol | 142 |
| Pd-Au | S. oneidensis | Na2PdCl4 + HauCl4·3H2O | Anaerobic | 6.88 ± 5.16 | Dechlorination of diclofenac, trichlorethylene | 97 | |
| Pd-cells-rGO | S. oneidensis | Na2PdCl4 | Anaerobic | Formate | 6.8 ± 2.9 | Electrocatalyst | 143 |
| Platinum | S. algae | PtCl62− | Anaerobic | Lactate | 5 | 83 | |
| Selenium | Shewanella spp. | Na2SeO3·5H2O | Anaerobic | Lactate | 164 ± 24 | 144 | |
| Selenium | Shewanella spp. | SeO32− | Anaerobic | Lactate | 1−20 | 145 | |
| Selenium | S. putrefaciens | Na2SeO3 | Aerobic | nd | Coreduction of Hg and Se | 146 | |
| Selenium | S. oneidensis | SeO32− | Anaerobic | Lactate | 20−100 | 147 | |
| Silver | S. oneidensis | AgNO3 | Anaerobic | Lactate | 24, 41 | Reduction of methylviologen | 148 |
| Silver | S. oneidensis | AgNO3 | Aerobic | 4 ± 1.5 | Antibacterial activity | 149 | |
| Silver on carbon nanotubes | S. oneidensis | AgNO3 | Anaerobic | 20 | Nitrophenol catalysis | 88 | |
| Silver | EPS | AgNO3 | Anaerobic | 5−35 | 150 | ||
| Silver | S. oneidensis | AgNO3 | Anaerobic | 20−50 | 45 | ||
| Technetium | S. oneidensis | NH499Tc(VII)O4 | Anaerobic | H2 or lactate | 151 | ||
| Tellurium nanorods | S. oneidensis | Na2TeO3 | Anaerobic | Lactate | 100−200 | 152 | |
| Tellurium nanorods | S. oneidensis | Te(IV) | Anaerobic | Lactate | 25−240 | Li ion storage material | 153, 154 |
| Uranium | S. oneidensis | Uranyl acetate | Anaerobic | Lactate | 1−5 | 155 | |
| Uranium | S. oneidensis | UO2Cl2·3H2O | Anaerobic | Lactate | 3−5 | 156 | |
| Uranium | S. oneidensis | Uranyl acetate | Anaerobic | H2 | 157 | ||
| Uranium | S. putrefaciens | UO2(NO3)2·6H2O | Anaerobic | Lactate | ∼3 | 158 | |
| U nanowires | S. oneidensis | Uranyl acetate | Anaerobic | Lactate | 2−5 | 159 | |
| U nanowires | S. oneidensis | Uranyl acetate | Anaerobic | Lactate | 1−4 | 160 | |
| Arsenic sulfide nanotube |
Shewanella spp. | Thiosulfate, As(V) | Anaerobic | Lactate | 20–100 | 130 | |
| Iron sulfide | S. oneidensis | Thiosulfate, iron citrate | Anaerobic | Lactate | ∼100 | 62 | |
| Silver sulfide | S. oneidensis | AgNO3, Na2S2O3 | Aerobic | 9 ± 3.5 | 86 | ||
| Silver sulfide | S. oneidensis | AgNO3, Na2S2O3 | Anaerobic | Lactate | 27, 53 | Reduction of methylviologen | 148 |
| Silver sulfide | S. oneidensis | AgNO3 Na2S2O3 | Aerobic | 4−10 | 87 | ||
| Zinc sulfide | S. oneidensis | ZnSO4 | Anaerobic | Lactate | 5 | Photocatalytic | 161 |
| Mn-doped zinc sulfide |
S. oneidensis | Mn(IV), Na2S, ZnSO4 | Anaerobic | Lactate | 2−70 | 162 | |
| Magnetic |
S. algae, Shewanella spp. |
Ferrihydrite | Anaerobic | Lactate | 6−26 | Superparamagnetic magnetite particles | 55 |
| Magnetic | S. piezotolerans | Ferrihydrite | Anaerobic | Lactate | 4−8 | Superparamagnetic magnetite particles | 163 |
| Pd/Fe3O4 | S. oneidensis | Na2PdCl4 | Anaerobic | Lactate | 5.5 ± 2.2 | Reduction of nitroaromatic compounds | 95 |
| Au/Fe3O4 | S. oneidensis | HAuCl4 | Anaerobic | Lactate | 15.4 ± 6.8 | Reduction of nitroaromatic compounds | 95 |
| PdAu/Fe3O4 | S. oneidensis | Na2PdCl4 and HAuCl4 | Anaerobic | Lactate | 8.3 ± 3.2 | Reduction of nitroaromatic compounds | 95 |
EPS, exopolysaccharide; nd, not determined.
FACTORS AFFECTING BIOSYNTHESIS OF NANOPARTICLES
There are several factors that affect the synthesis and properties of NPs, including solution pH, pressure, temperature, time, types of microorganisms, the concentration of the raw materials and extracts, ionic substances, extracellular materials secreted in the medium, carbon source, precursor salts, composition of growth media, microbial growth phase, and presence of other chemical substances employed in microscopic techniques (100–103).
One of the most important factors that influence the size and morphology of NPs synthesized by microorganisms is solution chemistry. For example, pH is known to affect the surface properties of Shewanella, controlling metal adsorption and reduction kinetics (104–106). Controlling reaction kinetics through careful selection of pH could offer increased control over NP synthesis by Shewanella, as has been well documented for chemical synthesis of NPs (107, 108). The effect of pH on the properties and crucially, the catalytic activity, of NPs produced by Shewanella spp. should be explored to optimize reactivity and identify the potential for controlling pH to produce bespoke NPs. As highlighted above (“Palladium-based nanoparticles”), the impact of buffer chemistry can influence the kinetics of metal reduction and must be considered when designing and reporting experiments. Buffering capacity should be carefully considered to minimize potential pH changes from microbial metabolism but also to limit any possible abiotic reduction or precipitation that could complicate interpretation of the results. As reported by Dundas et al. (78) aging of the buffer can also influence reduction kinetics, and so freshly prepared buffer solutions should be used (and reported in methods) to limit this effect and simplify comparisons between studies. Detailed studies on the effects of different buffers on metal precursor chemistry and the subsequent impact on microbial reduction and NP synthesis would greatly enhance our understanding of the role of solution chemistry in NP biosynthesis and enable better comparisons between studies. Additional details, such as the metal precursor used, aging of metal or buffer solutions, and the order of metal and biomass addition to solutions may all influence the reduction kinetics and properties of biosynthesized NPs. Indeed, studies on the abiotic synthesis of NPs highlight how such factors can affect metal ion hydrolysis, formation of polymeric or colloidal species, and precipitation which in turn control the properties of the final NP precipitates (109–111). These factors could also be expected to influence the biosynthesis of NPs by Shewanella and other microbes but remain largely unexplored. A wide range of studies has shown that temperature changes over even a relatively narrow window can still influence biological NP synthesis (112–114). The temperature has been shown to affect the kinetics of metal reduction by S. oneidensis (106), which could in turn affect the properties of resulting NPs. For example, magnetic NPs produced by the psychrotolerant species S. loihica were found to vary in size and shape at temperatures ranging from 0 to 37°C (56).
Variations in the synthesis and storage time may result in aggregation or dissolution of any NPs, potentially affecting their properties (115). However, to our knowledge, there have been limited studies on the effect of prolonged storage on the efficacy of NPs synthesized by any microbe, which may have implications for their contribution in a range of commercial applications.
It was reported that the melting point of NPs is reduced with decreasing NP size (116). The particle shape and size play a vital role in determining the properties of NPs. Although it is often controlled by the synthesis conditions, several postsynthesis processes can also impact these characteristics. The choice of methods used in the purification and separation of the synthesized NPs can also influence their properties. In a number of cases, centrifugation is performed for the separation of NPs based on their gravitational force (100). In other cases, chromatography techniques are described to be used (117).
In the case of Shewanella-synthesized NPs, careful consideration must be given to the extraction, separation, as well as purification of the desired NPs to minimize alterations that could negatively affect their performance. This may be particularly important for the separation and extraction of intracellularly precipitated NPs. Physiochemical methods to separate NPs from cells include freeze-thawing, heating processes, osmotic shock, ultrasound treatment, centrifugation, and the use of organic solvents or surfactants (118). However, the impact of these different processing methods on the stability, agglomeration, and oxidation of Shewanella-synthesized NPs requires further study to ensure that their functional and structural attributes are conserved.
FUTURE PERSPECTIVES AND CONCLUDING REMARKS
This minireview highlights the versatility of Shewanella for the biosynthesis of metal-based NPs. However, several challenges remain before Shewanella-based NP synthesis routes could be considered viable sustainable alternatives to more traditional chemical routes. We believe these challenges center on two primary topics: (i) control of NP physiochemical properties and (ii) scale-up of biological NP synthesis.
Despite the many advantages of microbial synthesis of NPs, traditional chemical synthesis still often provides greater control over the properties of the final NP product. However, recent investigations into controlling NP properties via directing the electron transport pathway involved in metal reduction provide an exciting opportunity to produce tailored biosynthesized NPs. Controlling the electron transfer pathway, for example, using cytochrome or hydrogenase deletion mutants, can preferentially precipitate NPs of a desired size in a desired cellular location (78). Recent studies continue to highlight the complex nature of the branching electron transfer pathways of Shewanella involved in metal reduction and NP synthesis and occasionally provide conflicting results on the primary enzymes involved. Strain selection may also prove important in controlling NP properties; however, as highlighted in this minireview, there are only limited comparisons that can be made currently between NPs produced by different Shewanella strains. This is perhaps primarily due to a focus of the majority of studies on the model species, S. oneidensis. In addition, when other Shewanella strains have been investigated, a lack of reported detail on the characteristics and properties of the synthesized NPs makes a comparison between these strains difficult. In our view, this is an important aspect to be rectified in future research. We suggest that in addition to simply expanding the range of Shewanella strains studied in NP synthesis, a focus on the electron transport pathways involved and greater characterization of NP properties and reactivity should also be applied to identify optimal pathways for bespoke NP production.
Additionally, the issue of scale-up must also be considered if Shewanella-directed biosynthesis of NPs is to replace traditional chemical and physical synthesis processes. While Shewanella present an attractive method for the production of biogenic NPs due to their versatility as metal reducers and their potential to tune the physiochemical properties of NPs by carefully controlling reaction conditions (pH, electron donor, etc.), balancing this delicate biological process could also present problems for the biosynthesis of NPs at scale. There is little literature exploring whether these carefully controlled biogeochemical conditions required for NP synthesis can be replicated at scale using Shewanella, where heterogeneities in bioreactors could reduce the NP yield and lead to increased heterogeneity in the properties of the biosynthesized NPs (119). However, existing studies have demonstrated the potential for scale-up of Fe-NP (magnetite) production using another model metal-reducing bacterium, Geobacter sulfurreducens (120). Like Shewanella, Geobacter species also contain complex electron transport chains capable of reducing a wide range of metals and producing NPs (121). Cells of Geobacter were successfully grown in 50-liter bioreactors, and magnetite was produced in a second stage using biomass at 10-ml to 10-liter scales, producing a maximum of 120 g in under 24 h (120). Crucially, the Fe-NPs retained similar physical properties and catalytic activity at both scales. The successful synthesis of NPs at scale using Geobacter could be potentially replicated by Shewanella but is yet to be tested. As well as their synthesis at scale, assessing their performance or reactivity at scale is also critical if these NPs are to move from benchtop reactions to industrial or commercial applications. Pd-NPs synthesized by S. oneidensis have been demonstrated to successfully treat the contaminant, trichloroethylene (TCE), in a pilot-scale membrane reactor. Under the conditions investigated, a continuous flow reactor containing 20 liters of 50 mg liter−1 of Pd-NPs, could remove up to 2,515 mg TCE day−1 g−1 Pd (122). These studies highlight the potential for scaling up the following: (i) growth of bacterial cells, (ii) synthesis of metal NPs at scale, and (iii) application of these biosynthesized NPs in pilot plant reactors. However, only limited studies have been performed, and significant work is required to be done in this area. Such studies would benefit greatly from industrial collaboration so that engineering and logistical challenges can be addressed in the early designs of pilot-scale reactors.
When considering scale-up, the life cycle analysis of NP synthesis and application will also be crucial to assess the economic viability and environmental sustainability of biosynthesized NPs. A promising direction toward sustainability involves the potential for microbial processes, including the use of Shewanella spp., to produce valuable NPs from metals present in waste streams. This offers potential cost savings by revalorizing existing waste streams and utilizing cheap, readily available feedstocks. In addition, the ability to recover and recycle metals from existing waste offers a possible solution to the security of the supply of critical metals required for industry (123). Recent studies have started to explore the sustainable biosynthesis of Fe-NPs (magnetite) using naturally abundant raw materials or waste products to replace analytical-grade or lab-synthesized precursor materials (124, 125). Interestingly, Sadhukhan et al. (124) reported that when using analytical-grade raw materials, the “state-of-the-art” biosynthesis of magnetite has an increased environmental cost relative to industrial magnetite production. However, when biosynthesis was optimized to reduce raw material use, environmental benefits were generated. These potential environmental benefits were even greater when the analytical raw materials were replaced with naturally abundant or waste materials. These environmental benefits, reported as “fossil resource savings” in the study by Sadhukhan et al. (124), may also translate to economic savings through decreased energy and carbon usage. However, direct comparisons exploring the economic viability of Shewanella (or other microbially)-synthesized NPs in relation to established synthesis methods are lacking and must be explored. The potential to produce catalytically active Pd-NPs and Pt-NPs from waste streams has been demonstrated using the bacteria, Escherichia coli and Desulfovibrio desulfuricans (126, 127). Whether the diverse electron transport pathways of Shewanella can be harnessed for the sustainable production of functional NPs from waste or natural sources remains to be investigated. Significant challenges in this area could include the following: (i) difficulty in controlling NP properties under harsh conditions in industrial waste streams, (ii) selectivity of metal recovery and NP precipitation in complex waste streams, and (iii) slow reaction kinetics or reduced NP yield relative to synthetic solutions. However, the possibility of utilizing the metabolic diversity of Shewanella toward achieving the goal of a sustainable, circular economy for valuable metals offers great potential rewards.
ACKNOWLEDGMENTS
The research was financially supported by the Russian Science Foundation, project no. 21-77-20089. J.R.L. acknowledges funding from the BBSRC from grant BB/R010412/1.
We have no conflicts of interest to declare.
Contributor Information
Vishnu D. Rajput, Email: rajput.vishnu@gmail.com.
Jeremy D. Semrau, University of Michigan—Ann Arbor
REFERENCES
- 1.Singh VK, Das S, Dwivedy AK, Rathore R, Dubey NK. 2019. Assessment of chemically characterized nanoencapuslated Ocimum sanctum essential oil against aflatoxigenic fungi contaminating herbal raw materials and its novel mode of action as methyglyoxal inhibitor. Postharvest Biol Technol 153:87–95. 10.1016/j.postharvbio.2019.03.022. [DOI] [Google Scholar]
- 2.Renuka R, Devi KR, Sivakami M, Thilagavathi T, Uthrakumar R, Kaviyarasu K. 2020. Biosynthesis of silver nanoparticles using phyllanthus emblica fruit extract for antimicrobial application. Biocatal Agric Biotechnol 24:101567. 10.1016/j.bcab.2020.101567. [DOI] [Google Scholar]
- 3.do Espirito Santo Pereira A, Caixeta Oliveira H, Fernandes Fraceto L, Santaella C. 2021. Nanotechnology potential in seed priming for sustainable agriculture. Nanomaterials 11:267. 10.3390/nano11020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salem SS, Fouda A. 2021. Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol Trace Elem Res 199:344–370. 10.1007/s12011-020-02138-3. [DOI] [PubMed] [Google Scholar]
- 5.Verma SK, Das AK, Gantait S, Kumar V, Gurel E. 2019. Applications of carbon nanomaterials in the plant system: a perspective view on the pros and cons. Sci Total Environ 667:485–499. 10.1016/j.scitotenv.2019.02.409. [DOI] [PubMed] [Google Scholar]
- 6.Arroyo BJ, Bezerra AC, Oliveira LL, Arroyo SJ, Melo EA, Santos AMP. 2020. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem 309:125566. 10.1016/j.foodchem.2019.125566. [DOI] [PubMed] [Google Scholar]
- 7.Carrouel F, Viennot S, Ottolenghi L, Gaillard C, Bourgeois D. 2020. Nanoparticles as anti-microbial, anti-inflammatory, and remineralizing agents in oral care cosmetics: a review of the current situation. Nanomaterials 10:140. 10.3390/nano10010140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kimber RL, Parmeggiani F, Joshi N, Rakowski AM, Haigh SJ, Turner NJ, Lloyd JR. 2019. Synthesis of copper catalysts for click chemistry from distillery wastewater using magnetically recoverable bionanoparticles. Green Chem 21:4020–4024. 10.1039/C9GC00270G. [DOI] [Google Scholar]
- 9.Lu Y, Yan Q, Han J, Cao B, Street J, Yu F. 2017. Fischer–Tropsch synthesis of olefin-rich liquid hydrocarbons from biomass-derived syngas over carbon-encapsulated iron carbide/iron nanoparticles catalyst. Fuel 193:369–384. 10.1016/j.fuel.2016.12.061. [DOI] [Google Scholar]
- 10.Kefeni KK, Msagati TAM, Mamba BB. 2017. Ferrite nanoparticles: synthesis, characterisation and applications in electronic device. Mater Sci Eng B 215:37–55. 10.1016/j.mseb.2016.11.002. [DOI] [Google Scholar]
- 11.Singh J, Dutta T, Kim K-H, Rawat M, Samddar P, Kumar P. 2018. ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol 16:84. 10.1186/s12951-018-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baig MM, Zulfiqar S, Yousuf MA, Shakir I, Aboud MFA, Warsi MF. 2021. Dy(x)MnFe(2-)(x)O(4) nanoparticles decorated over mesoporous silica for environmental remediation applications. J Hazard Mater 402:123526. 10.1016/j.jhazmat.2020.123526. [DOI] [PubMed] [Google Scholar]
- 13.Basavegowda N, Mandal TK, Baek K-H. 2020. Bimetallic and trimetallic nanoparticles for active food packaging applications: a review. Food Bioprocess Technol 13:30–44. 10.1007/s11947-019-02370-3. [DOI] [Google Scholar]
- 14.Ahmed A, Shah AN, Azam A, Uddin GM, Ali MS, Hassan S, Ahmed H, Aslam T. 2020. Environment-friendly novel fuel additives: investigation of the effects of graphite nanoparticles on performance and regulated gaseous emissions of CI engine. Energy Convers Manag 211:112748. 10.1016/j.enconman.2020.112748. [DOI] [Google Scholar]
- 15.Li Z, Xu Q. 2017. Metal-nanoparticle-catalyzed hydrogen generation from formic acid. Acc Chem Res 50:1449–1458. 10.1021/acs.accounts.7b00132. [DOI] [PubMed] [Google Scholar]
- 16.Mishra R, Militky J, Baheti V, Huang J, Kale B, Venkataraman M, Bele V, Arumugam V, Zhu G, Wang Y. 2014. The production, characterization and applications of nanoparticles in the textile industry. Text Prog 46:133–226. 10.1080/00405167.2014.964474. [DOI] [Google Scholar]
- 17.Anselmo AC, Mitragotri S. 2019. Nanoparticles in the clinic: an update. Bioeng Transl Med 4:e10143. 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keller AA, Adeleye AS, Conway JR, Garner KL, Zhao L, Cherr GN, Hong J, Gardea-Torresdey JL, Godwin HA, Hanna S, Ji Z, Kaweeteerawat C, Lin S, Lenihan HS, Miller RJ, Nel AE, Peralta-Videa JR, Walker SL, Taylor AA, Torres-Duarte C, Zink JI, Zuverza-Mena N. 2017. Comparative environmental fate and toxicity of copper nanomaterials. NanoImpact 7:28–40. 10.1016/j.impact.2017.05.003. [DOI] [Google Scholar]
- 19.Rajput V, Minkina T, Ahmed B, Sushkova S, Singh R, Soldatov M, Laratte B, Fedorenko A, Mandzhieva S, Blicharska E. 2019. Interaction of copper-based nanoparticles to soil, terrestrial, and aquatic systems: critical review of the state of the science and future perspectives. Rev Environ Contam Toxicol 252:51–96. 10.1007/398_2019_34. [DOI] [PubMed] [Google Scholar]
- 20.Pedone D, Moglianetti M, De Luca E, Bardi G, Pompa PP. 2017. Platinum nanoparticles in nanobiomedicine. Chem Soc Rev 46:4951–4975. 10.1039/c7cs00152e. [DOI] [PubMed] [Google Scholar]
- 21.Priya K, Vijayakumar M, Janani B. 2020. Chitosan-mediated synthesis of biogenic silver nanoparticles (AgNPs), nanoparticle characterisation and in vitro assessment of anticancer activity in human hepatocellular carcinoma HepG2 cells. Int J Biol Macromol 149:844–852. 10.1016/j.ijbiomac.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Plaza DO, Gallardo C, Straub YD, Bravo D, Perez-Donoso JM. 2016. Biological synthesis of fluorescent nanoparticles by cadmium and tellurite resistant Antarctic bacteria: exploring novel natural nanofactories. Microb Cell Fact 15:76. 10.1186/s12934-016-0477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owaid MN, Ibraheem IJ. 2017. Mycosynthesis of nanoparticles using edible and medicinal mushrooms. Eur J Nanomed 9:5–23. [Google Scholar]
- 24.Dahm H, Brzezińska J, Wrótniak-Drzewiecka W, Golińska P, Różycki H, Rai M. 2015. Myxobacteria as a potential biocontrol agent effective against pathogenic fungi of economically important forest trees. Dendrobiology 74:13–24. 10.12657/denbio.074.002. [DOI] [Google Scholar]
- 25.Bhople S, Gaikwad S, Deshmukh S, Bonde S, Gade A, Sen S, Brezinska A, Dahm H, Rai M. 2016. Myxobacteria-mediated synthesis of silver nanoparticles and their impregnation in wrapping paper used for enhancing shelf life of apples. IET Nanobiotechnol 10:389–394. 10.1049/iet-nbt.2015.0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rajeshkumar S, Malarkodi C, Paulkumar K, Vanaja M, Gnanajobitha G, Annadurai G. 2014. Algae mediated green fabrication of silver nanoparticles and examination of its antifungal activity against clinical pathogens. Int J Met 2014:692643. 10.1155/2014/692643. [DOI] [Google Scholar]
- 27.Apte M, Girme G, Bankar A, RaviKumar A, Zinjarde S. 2013. 3, 4-Dihydroxy-L-phenylalanine-derived melanin from Yarrowia lipolytica mediates the synthesis of silver and gold nanostructures. J Nanobiotechnol 11:2. 10.1186/1477-3155-11-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gade A, Gaikwad S, Duran N, Rai M. 2014. Green synthesis of silver nanoparticles by Phoma glomerata. Micron 59:52–59. 10.1016/j.micron.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Saha J, Begum A, Mukherjee A, Kumar S. 2017. A novel green synthesis of silver nanoparticles and their catalytic action in reduction of Methylene Blue dye. Sustain Environ Res 27:245–250. 10.1016/j.serj.2017.04.003. [DOI] [Google Scholar]
- 30.Santhoshkumar J, Kumar SV, Rajeshkumar S. 2017. Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resource-Efficient Technol 3:459–465. 10.1016/j.reffit.2017.05.001. [DOI] [Google Scholar]
- 31.Wang L, Chen S, Ding Y, Zhu Q, Zhang N, Yu S. 2018. Biofabrication of morphology improved cadmium sulfide nanoparticles using Shewanella oneidensis bacterial cells and ionic liquid: for toxicity against brain cancer cell lines. J Photochem Photobiol B 178:424–427. 10.1016/j.jphotobiol.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Zhuravliova OA, Voeikova TA, Khaddazh MK, Bulushova NV, Ismagulova TT, Bakhtina AV, Gusev SA, Gritskova IA, Lupanova TN, Shaitan KV, Debabov VG. 2018. Bacterial synthesis of cadmium and zinc sulfide nanoparticles: characteristics and prospects of application. Mol Genet Microbiol Virol 33:233–240. 10.3103/S0891416818040092. [DOI] [Google Scholar]
- 33.Ogi T, Saitoh N, Nomura T, Konishi Y. 2010. Room-temperature synthesis of gold nanoparticles and nanoplates using Shewanella algae cell extract. J Nanopart Res 12:2531–2539. 10.1007/s11051-009-9822-8. [DOI] [Google Scholar]
- 34.Jeong M, Kim Y, Roh Y. 2019. Biogenesis of magnetite nanoparticles using Shewanella species isolated from diverse regions. J Nanosci Nanotechnol 19:963–966. 10.1166/jnn.2019.15907. [DOI] [PubMed] [Google Scholar]
- 35.Myers CR, Nealson KH. 1988. Bacterial manganese reduction and growth with manganese oxide as the sole electron acceptor. Science 240:1319–1321. 10.1126/science.240.4857.1319. [DOI] [PubMed] [Google Scholar]
- 36.Myers CR, Nealson KH. 1988. Microbial reduction of manganese oxides: interactions with iron and sulfur. Geochim Cosmochim Acta 52:2727–2732. 10.1016/0016-7037(88)90041-5. [DOI] [Google Scholar]
- 37.Caccavo F, Blakemore RP, Lovley DR. 1992. A hydrogen-oxidizing, Fe(III)-reducing microorganism from the Great Bay Estuary, New Hampshire. Appl Environ Microbiol 58:3211–3216. 10.1128/aem.58.10.3211-3216.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wielinga B, Mizuba MM, Hansel CM, Fendorf S. 2001. Iron promoted reduction of chromate by dissimilatory iron-reducing bacteria. Environ Sci Technol 35:522–527. 10.1021/es001457b. [DOI] [PubMed] [Google Scholar]
- 39.Lloyd JR, Yong P, Macaskie LE. 2000. Biological reduction and removal of Np(V) by two microorganisms. Environ Sci Technol 34:1297–1301. 10.1021/es990394y. [DOI] [Google Scholar]
- 40.Wildung RE, Gorby YA, Krupka KM, Hess NJ, Li SW, Plymale AE, McKinley JP, Fredrickson JK. 2000. Effect of electron donor and solution chemistry on products of dissimilatory reduction of technetium by Shewanella putrefaciens. Appl Environ Microbiol 66:2451–2460. 10.1128/AEM.66.6.2451-2460.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boukhalfa H, Icopini GA, Reilly SD, Neu MP. 2007. Plutonium(IV) reduction by the metal-reducing bacteria Geobacter metallireducens GS15 and Shewanella oneidensis MR1. Appl Environ Microbiol 73:5897–5903. 10.1128/AEM.00747-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Carpentier W, De Smet L, Van Beeumen J, Brigé A. 2005. Respiration and growth of Shewanella oneidensis MR-1 using vanadate as the sole electron acceptor. J Bacteriol 187:3293–3301. 10.1128/JB.187.10.3293-3301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klonowska A, Heulin T, Vermeglio A. 2005. Selenite and tellurite reduction by Shewanella oneidensis. Appl Environ Microbiol 71:5607–5609. 10.1128/AEM.71.9.5607-5609.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konishi Y, Tsukiyama T, Saitoh N, Nomura T, Nagamine S, Takahashi Y, Uruga T. 2007. Direct determination of oxidation state of gold deposits in metal-reducing bacterium Shewanella algae using X-ray absorption near-edge structure spectroscopy (XANES). J Biosci Bioeng 103:568–571. 10.1263/jbb.103.568. [DOI] [PubMed] [Google Scholar]
- 45.Wang H, Law N, Pearson G, van Dongen BE, Jarvis RM, Goodacre R, Lloyd JR. 2010. Impact of silver(I) on the metabolism of Shewanella oneidensis. J Bacteriol 192:1143–1150. 10.1128/JB.01277-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Windt WD, Aelterman P, Verstraete W. 2005. Bioreductive deposition of palladium (0) nanoparticles on Shewanella oneidensis with catalytic activity towards reductive dechlorination of polychlorinated biphenyls. Environ Microbiol 7:314–325. 10.1111/j.1462-2920.2005.00696.x. [DOI] [PubMed] [Google Scholar]
- 47.Kimber RL, Lewis EA, Parmeggiani F, Smith K, Bagshaw H, Starborg T, Joshi N, Figueroa AI, van der Laan G, Cibin G, Gianolio D, Haigh SJ, Pattrick RAD, Turner NJ, Lloyd JR. 2018. Biosynthesis and characterization of copper nanoparticles using Shewanella oneidensis: application for click chemistry. Small 10.1002/smll.201703145. [DOI] [PubMed] [Google Scholar]
- 48.Myers CR, Myers JM. 1997. Cloning and sequence of cymA, a gene encoding a tetraheme cytochrome c required for reduction of iron(III), fumarate, and nitrate by Shewanella putrefaciens MR-1. J Bacteriol 179:1143–1152. 10.1128/jb.179.4.1143-1152.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shi L, Squier TC, Zachara JM, Fredrickson JK. 2007. Respiration of metal (hydr)oxides by Shewanella and Geobacter: a key role for multihaem c-type cytochromes. Mol Microbiol 65:12–20. 10.1111/j.1365-2958.2007.05783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sturm G, Richter K, Doetsch A, Heide H, Louro RO, Gescher J. 2015. A dynamic periplasmic electron transfer network enables respiratory flexibility beyond a thermodynamic regulatory regime. ISME J 9:1802–1811. 10.1038/ismej.2014.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edwards MJ, White GF, Butt JN, Richardson DJ, Clarke TA. 2020. The crystal structure of a biological insulated transmembrane molecular wire. Cell 181:665–673.e10. 10.1016/j.cell.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Coursolle D, Gralnick JA. 2010. Modularity of the Mtr respiratory pathway of Shewanella oneidensis strain MR-1. Mol Microbiol 77:995–1008. 10.1111/j.1365-2958.2010.07266.x. [DOI] [PubMed] [Google Scholar]
- 53.Fredrickson JK, Zachara JM, Kennedy DW, Dong H, Onstott TC, Hinman NW, Li S. 1998. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim Cosmochim Acta 62:3239–3257. 10.1016/S0016-7037(98)00243-9. [DOI] [Google Scholar]
- 54.Perez-Gonzalez T, Jimenez-Lopez C, Neal AL, Rull-Perez F, Rodriguez-Navarro A, Fernandez-Vivas A, Iañez-Pareja E. 2010. Magnetite biomineralization induced by Shewanella oneidensis. Geochim Cosmochim Acta 74:967–979. 10.1016/j.gca.2009.10.035. [DOI] [Google Scholar]
- 55.Li YL, Pfiffner SM, Dyar MD, Vali H, Konhauser K, Cole DR, Rondinone AJ, Phelps TJ. 2009. Degeneration of biogenic superparamagnetic magnetite. Geobiology 7:25–34. 10.1111/j.1472-4669.2008.00186.x. [DOI] [PubMed] [Google Scholar]
- 56.Roh Y, Gao H, Vali H, Kennedy DW, Yang ZK, Gao W, Dohnalkova AC, Stapleton RD, Moon JW, Phelps TJ, Fredrickson JK, Zhou J. 2006. Metal reduction and iron biomineralization by a psychrotolerant Fe(III)-reducing bacterium, Shewanella sp. strain PV-4. Appl Environ Microbiol 72:3236–3244. 10.1128/AEM.72.5.3236-3244.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Byrne JM, Coker VS, Moise S, Wincott PL, Vaughan DJ, Tuna F, Arenholz E, van der Laan G, Pattrick RAD, Lloyd JR, Telling ND. 2013. Controlled cobalt doping in biogenic magnetite nanoparticles. J R Soc Interface 10:20130134. 10.1098/rsif.2013.0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coker VS, Pearce CI, Pattrick RAD, van der Laan G, Telling ND, Charnock JM, Arenholz E, Lloyd JR. 2008. Probing the site occupancies of Co-, Ni-, and Mn-substituted biogenic magnetite using XAS and XMCD. Am Mineral 93:1119–1132. 10.2138/am.2008.2681. [DOI] [Google Scholar]
- 59.Fredrickson JK, Zachara JM, Kukkadapu RK, Gorby YA, Smith SC, Brown CF. 2001. Biotransformation of Ni-substituted hydrous ferric oxide by an Fe(III)-reducing bacterium. Environ Sci Technol 35:703–712. 10.1021/es001500v. [DOI] [PubMed] [Google Scholar]
- 60.Moon JW, Roh Y, Lauf RJ, Vali H, Yeary LW, Phelps TJ. 2007. Microbial preparation of metal-substituted magnetite nanoparticles. J Microbiol Methods 70:150–158. 10.1016/j.mimet.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 61.Moon J-W, Yeary LW, Rondinone AJ, Rawn CJ, Kirkham MJ, Roh Y, Love LJ, Phelps TJ. 2007. Magnetic response of microbially synthesized transition metal- and lanthanide-substituted nano-sized magnetites. J Magn Magn Mater 313:283–292. 10.1016/j.jmmm.2007.01.011. [DOI] [Google Scholar]
- 62.Kondo K, Okamoto A, Hashimoto K, Nakamura R. 2015. Sulfur-mediated electron shuttling sustains microbial long-distance extracellular electron transfer with the aid of metallic iron sulfides. Langmuir 31:7427–7434. 10.1021/acs.langmuir.5b01033. [DOI] [PubMed] [Google Scholar]
- 63.Nakamura R, Okamoto A, Tajima N, Newton GJ, Kai F, Takashima T, Hashimoto K. 2010. Biological iron-monosulfide production for efficient electricity harvesting from a deep-sea metal-reducing bacterium. Chembiochem 11:643–645. 10.1002/cbic.200900775. [DOI] [PubMed] [Google Scholar]
- 64.Lv Q, Zhang B, Xing X, Zhao Y, Cai R, Wang W, Gu Q. 2018. Biosynthesis of copper nanoparticles using Shewanella loihica PV-4 with antibacterial activity: novel approach and mechanisms investigation. J Hazard Mater 347:141–149. 10.1016/j.jhazmat.2017.12.070. [DOI] [PubMed] [Google Scholar]
- 65.Biesinger MC, Lau LWM, Gerson AR, Smart RSC. 2010. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl Surf Sci 257:887–898. 10.1016/j.apsusc.2010.07.086. [DOI] [Google Scholar]
- 66.Zhou NQ, Tian LJ, Wang YC, Li DB, Li PP, Zhang X, Yu HQ. 2016. Extracellular biosynthesis of copper sulfide nanoparticles by Shewanella oneidensis MR-1 as a photothermal agent. Enzyme Microb Technol 95:230–235. 10.1016/j.enzmictec.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 67.Xiao X, Liu Q-Y, Lu X-R, Li T-T, Feng X-L, Li Q, Liu Z-Y, Feng Y-J. 2017. Self-assembly of complex hollow CuS nano/micro shell by an electrochemically active bacterium Shewanella oneidensis MR-1. Int Biodeterior Biodegrad 116:10–16. 10.1016/j.ibiod.2016.09.021. [DOI] [Google Scholar]
- 68.De Corte S, Hennebel T, Verschuere S, Cuvelier C, Verstraete W, Boon N. 2011. Gold nanoparticle formation using Shewanella oneidensis: a fast biosorption and slow reduction process. J Chem Technol Biotechnol 86:547–553. 10.1002/jctb.2549. [DOI] [Google Scholar]
- 69.Suresh AK, Pelletier DA, Wang W, Broich ML, Moon J-W, Gu B, Allison DP, Joy DC, Phelps TJ, Doktycz MJ. 2011. Biofabrication of discrete spherical gold nanoparticles using the metal-reducing bacterium Shewanella oneidensis. Acta Biomater 7:2148–2152. 10.1016/j.actbio.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 70.Karim MR, Han TH, Sawant SY, Shim J, Lee MY, Kim WK, Kim JS, Cho MH. 2020. Microbial fuel cell-assisted biogenic synthesis of gold nanoparticles and its application to energy production and hydrogen peroxide detection. Korean J Chem Eng 37:1241–1250. 10.1007/s11814-020-0539-9. [DOI] [Google Scholar]
- 71.Huang BC, Yi YC, Chang JS, Ng IS. 2019. Mechanism study of photo-induced gold nanoparticles formation by Shewanella oneidensis MR-1. Sci Rep 9:7589. 10.1038/s41598-019-44088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu J-W, Ng IS. 2017. Biofabrication of gold nanoparticles by Shewanella species. Bioresour Bioprocess 4:50. 10.1186/s40643-017-0181-5. [DOI] [Google Scholar]
- 73.Ahmed E, Kalathil S, Shi L, Alharbi O, Wang P. 2018. Synthesis of ultra-small platinum, palladium and gold nanoparticles by Shewanella loihica PV-4 electrochemically active biofilms and their enhanced catalytic activities. J Saudi Chem Soc 22:919–929. 10.1016/j.jscs.2018.02.002. [DOI] [Google Scholar]
- 74.De Windt W, Boon N, Van den Bulcke J, Rubberecht L, Prata F, Mast J, Hennebel T, Verstraete W. 2006. Biological control of the size and reactivity of catalytic Pd(0) produced by Shewanella oneidensis. Antonie Van Leeuwenhoek 90:377–389. 10.1007/s10482-006-9088-4. [DOI] [PubMed] [Google Scholar]
- 75.Ng CK, Cai Tan TK, Song H, Cao B. 2013. Reductive formation of palladium nanoparticles by Shewanella oneidensis: role of outer membrane cytochromes and hydrogenases. RSC Adv 3:22498. 10.1039/c3ra44143a. [DOI] [Google Scholar]
- 76.Rotaru AE, Jiang W, Finster K, Skrydstrup T, Meyer RL. 2012. Non-enzymatic palladium recovery on microbial and synthetic surfaces. Biotechnol Bioeng 109:1889–1897. 10.1002/bit.24500. [DOI] [PubMed] [Google Scholar]
- 77.Yang Z-N, Hou Y-N, Zhang B, Cheng H-Y, Yong Y-C, Liu W-Z, Han J-L, Liu S-J, Wang A-J. 2020. Insights into palladium nanoparticles produced by Shewanella oneidensis MR-1: roles of NADH dehydrogenases and hydrogenases. Environ Res 191:110196. 10.1016/j.envres.2020.110196. [DOI] [PubMed] [Google Scholar]
- 78.Dundas CM, Graham AJ, Romanovicz DK, Keitz BK. 2018. Extracellular electron transfer by Shewanella oneidensis controls palladium nanoparticle phenotype. ACS Synth Biol 7:2726–2736. 10.1021/acssynbio.8b00218. [DOI] [PubMed] [Google Scholar]
- 79.Torgeman E. 2017. Biosynthesis of gold and palladium nanoparticles via bacteria. MSc thesis. University of Oslo, Olso, Norway.
- 80.Tishkov VI, Popov VO. 2004. Catalytic mechanism and application of formate dehydrogenase. Biochemistry (Mosc) 69:1252–1267. 10.1007/s10541-005-0071-x. [DOI] [PubMed] [Google Scholar]
- 81.Chen YX, Liu X, Fang Z, Zhang CL, Abbas SZ, Yu YY, Yong YC. 2020. Self-assembling of Shewanella@rGO@Pd bionanohybrid for synergistic bio-abiotic removal of Cr(VI). J Chem Technol Biotechnol 95:2222–2228. 10.1002/jctb.6409. [DOI] [Google Scholar]
- 82.Wang W, Zhang B, He Z. 2019. Bioelectrochemical deposition of palladium nanoparticles as catalysts by Shewanella oneidensis MR-1 towards enhanced hydrogen production in microbial electrolysis cells. Electrochim Acta 318:794–800. 10.1016/j.electacta.2019.06.038. [DOI] [Google Scholar]
- 83.Konishi Y, Ohno K, Saitoh N, Nomura T, Nagamine S, Hishida H, Takahashi Y, Uruga T. 2007. Bioreductive deposition of platinum nanoparticles on the bacterium Shewanella algae. J Biotechnol 128:648–653. 10.1016/j.jbiotec.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 84.Xiao X, Han X, Wang L-G, Long F, Ma X-L, Xu C-C, Ma X-B, Wang C-X, Liu Z-Y. 2020. Anaerobically photoreductive degradation by CdS nanocrystal: biofabrication process and bioelectron-driven reaction coupled with Shewanella oneidensis MR-1. Biochem Eng J 154:107466. 10.1016/j.bej.2019.107466. [DOI] [Google Scholar]
- 85.Zhuravliova OA, Voeikova TA, Bulushova NV, Veiko VP, Ismagulova TT, Lupanova TN, Lobastov SL, Retivov VM, Debabov VG. 2019. Biotechnological method of obtaining nanoparticles of silver, cadmium, and zinc sulfides. Physico-chemical characteristics. creation of polymeric nanocomposites. Inorg Mater Appl Res 10:1394–1400. 10.1134/S2075113319060303. [DOI] [Google Scholar]
- 86.Suresh AK, Doktycz MJ, Wang W, Moon JW, Gu B, Meyer HM, III, Hensley DK, Allison DP, Phelps TJ, Pelletier DA. 2011. Monodispersed biocompatible silver sulfide nanoparticles: facile extracellular biosynthesis using the gamma-proteobacterium, Shewanella oneidensis. Acta Biomater 7:4253–4258. 10.1016/j.actbio.2011.07.007. [DOI] [PubMed] [Google Scholar]
- 87.Voeikova TA, Zhuravliova OA, Gracheva TS, Bulushova NV, Ismagulova TT, Shaitan KV, Debabov VG. 2018. Optimization of microbial synthesis of silver sulfide nanoparticles. Appl Biochem Microbiol 54:800–807. 10.1134/S0003683818080070. [DOI] [Google Scholar]
- 88.Song X, Shi X. 2017. Bioreductive deposition of highly dispersed Ag nanoparticles on carbon nanotubes with enhanced catalytic degradation for 4-nitrophenol assisted by Shewanella oneidensis MR-1. Environ Sci Pollut Res Int 24:3038–3044. 10.1007/s11356-016-8076-0. [DOI] [PubMed] [Google Scholar]
- 89.Voeikova TA, Shebanova AS, Ivanov YD, Kaysheva AL, Novikova LM, Zhuravliova OA, Shumyantseva VV, Shaitan KV, Kirpichnikov MP, Debabov VG. 2016. The role of proteins of the outer membrane of Shewanella oneidensis MR-1 in the formation and stabilization of silver sulfide nanoparticles. Appl Biochem Microbiol 52:769–775. 10.1134/S0003683816080081. [DOI] [Google Scholar]
- 90.Voeikova TA, Zhuravliova OA, Bulushova NV, Veiko VP, Ismagulova TT, Lupanova TN, Shaitan KV, Debabov VG. 2017. The “protein corona” of silver-sulfide nanoparticles obtained using Gram-negative and -positive bacteria. Mol Genet Microbiol Virol 32:204–211. 10.3103/S0891416817040103. [DOI] [Google Scholar]
- 91.Li C, Huang R, Shi X. 2020. Biosynthesis of Cu nanoparticles supported on carbon nanotubes and its catalytic performance under different test conditions. J Chem Technol Biotechnol 95:1511–1518. 10.1002/jctb.6344. [DOI] [Google Scholar]
- 92.Yang M, Shi X. 2019. Biosynthesis of Ag2S/TiO2 nanotubes nanocomposites by Shewanella oneidensis MR-1 for the catalytic degradation of 4-nitrophenol. Environ Sci Pollut Res Int 26:12237–12246. 10.1007/s11356-019-04462-1. [DOI] [PubMed] [Google Scholar]
- 93.Xu H, Xiao Y, Xu M, Cui H, Tan L, Feng N, Liu X, Qiu G, Dong H, Xie J. 2019. Microbial synthesis of Pd-Pt alloy nanoparticles using Shewanella oneidensis MR-1 with enhanced catalytic activity for nitrophenol and azo dyes reduction. Nanotechnology 30:e065607. 10.1088/1361-6528/aaf2a6. [DOI] [PubMed] [Google Scholar]
- 94.Tuo Y, Liu G, Dong B, Yu H, Zhou J, Wang J, Jin R. 2017. Microbial synthesis of bimetallic PdPt nanoparticles for catalytic reduction of 4-nitrophenol. Environ Sci Pollut Res Int 24:5249–5258. 10.1007/s11356-016-8276-7. [DOI] [PubMed] [Google Scholar]
- 95.Tuo Y, Liu G, Dong B, Zhou J, Wang A, Wang J, Jin R, Lv H, Dou Z, Huang W. 2015. Microbial synthesis of Pd/Fe3O4, Au/Fe3O4 and PdAu/Fe3O4 nanocomposites for catalytic reduction of nitroaromatic compounds. Sci Rep 5:13515. 10.1038/srep13515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Han R, Song X, Wang Q, Qi Y, Deng G, Zhang A, Wang Q, Chang F, Wu C, Cheng Y. 2019. Microbial synthesis of graphene-supported highly-dispersed Pd-Ag bimetallic nanoparticles and its catalytic activity. J Chem Technol Biotechnol 94:3375–3383. 10.1002/jctb.6150. [DOI] [Google Scholar]
- 97.De Corte S, Hennebel T, Fitts JP, Sabbe T, Bliznuk V, Verschuere S, van der Lelie D, Verstraete W, Boon N. 2011. Biosupported bimetallic Pd-Au nanocatalysts for dechlorination of environmental contaminants. Environ Sci Technol 45:8506–8513. 10.1021/es2019324. [DOI] [PubMed] [Google Scholar]
- 98.Heugebaert TSA, De Corte S, Sabbe T, Hennebel T, Verstraete W, Boon N, Stevens CV. 2012. Biodeposited Pd/Au bimetallic nanoparticles as novel Suzuki catalysts. Tetrahedron Lett 53:1410–1412. 10.1016/j.tetlet.2012.01.030. [DOI] [Google Scholar]
- 99.Kimber RL, Parmeggiani F, Neill TS, Merroun ML, Goodlet G, Powell NA, Turner NJ, Lloyd JR. 2021. Biotechnological synthesis of Pd/Ag and Pd/Au nanoparticles for enhanced Suzuki–Miyaura cross-coupling activity. Microb Biotechnol 10.1111/1751-7915.13762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Baker S, Rakshith D, Kavitha KS, Santosh P, Kavitha HU, Rao Y, Satish S. 2013. Plants: emerging as nanofactories towards facile route in synthesis of nanoparticles. Bioimpacts 3:111–117. 10.5681/bi.2013.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Patra JK, Baek K-H. 2014. Green nanobiotechnology: factors affecting synthesis and characterization techniques. J Nanomater 2014:417305. 10.1155/2014/417305. [DOI] [Google Scholar]
- 102.Raman G, Park SJ, Sakthivel N, Suresh AK. 2017. Physico-cultural parameters during AgNPs biotransformation with bactericidal activity against human pathogens. Enzyme Microb Technol 100:45–51. 10.1016/j.enzmictec.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 103.Tan L, Ray Jones T, Poitras J, Xie J, Liu X, Southam G. 2020. Biochemical synthesis of palladium nanoparticles: the influence of chemical fixatives used in electron microscopy on nanoparticle formation and catalytic performance. J Hazard Mater 398:122945. 10.1016/j.jhazmat.2020.122945. [DOI] [PubMed] [Google Scholar]
- 104.Mishra B, Boyanov M, Bunker BA, Kelly SD, Kemner KM, Fein JB. 2010. High- and low-affinity binding sites for Cd on the bacterial cell walls of Bacillus subtilis and Shewanella oneidensis. Geochim Cosmochim Acta 74:4219–4233. 10.1016/j.gca.2010.02.019. [DOI] [Google Scholar]
- 105.Furukawa Y, Dale JR. 2013. The surface properties of Shewanella putrefaciens 200 and S. oneidensis MR-1: the effect of pH and terminal electron acceptors. Geochem Trans 14:3. 10.1186/1467-4866-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han R, Li F, Liu T, Li X, Wu Y, Wang Y, Chen D. 2016. Effects of incubation conditions on Cr(VI) reduction by c-type cytochromes in intact Shewanella oneidensis MR-1 cells. Front Microbiol 7:746. 10.3389/fmicb.2016.00746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mozaffari S, Li W, Thompson C, Ivanov S, Seifert S, Lee B, Kovarik L, Karim AM. 2017. Colloidal nanoparticle size control: experimental and kinetic modeling investigation of the ligand–metal binding role in controlling the nucleation and growth kinetics. Nanoscale 9:13772–13785. 10.1039/c7nr04101b. [DOI] [PubMed] [Google Scholar]
- 108.Özkar S, Finke RG. 2019. Nanoparticle formation kinetics and mechanistic studies important to mechanism-based particle-size control: evidence for ligand-based slowing of the autocatalytic surface growth step plus postulated mechanisms. J Phys Chem C 123:14047–14057. 10.1021/acs.jpcc.9b03220. [DOI] [Google Scholar]
- 109.Andrieux-Ledier A, Tremblay B, Courty A. 2013. Synthesis of silver nanoparticles using different silver phosphine precursors: formation mechanism and size control. J Phys Chem C 117:14850–14857. 10.1021/jp4040248. [DOI] [Google Scholar]
- 110.Briñas RP, Hu M, Qian L, Lymar ES, Hainfeld JF. 2008. Gold nanoparticle size controlled by polymeric Au(I) thiolate precursor size. J Am Chem Soc 130:975–982. 10.1021/ja076333e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kettemann F, Wuithschick M, Caputo G, Kraehnert R, Pinna N, Rademann K, Polte J. 2015. Reliable palladium nanoparticle syntheses in aqueous solution: the importance of understanding precursor chemistry and growth mechanism. CrystEngComm 17:1865–1870. 10.1039/C4CE01025F. [DOI] [Google Scholar]
- 112.Gericke M, Pinches A. 2006. Biological synthesis of metal nanoparticles. Hydrometallurgy 83:132–140. 10.1016/j.hydromet.2006.03.019. [DOI] [Google Scholar]
- 113.Gurunathan S, Kalishwaralal K, Vaidyanathan R, Venkataraman D, Pandian SR, Muniyandi J, Hariharan N, Eom SH. 2009. Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf B Biointerfaces 74:328–335. 10.1016/j.colsurfb.2009.07.048. [DOI] [PubMed] [Google Scholar]
- 114.Kathiresan K, Manivannan S, Nabeel MA, Dhivya B. 2009. Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf B Biointerfaces 71:133–137. 10.1016/j.colsurfb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 115.Baer D. 2011. Surface characterization of nanoparticles: critical needs and significant challenges. J Surf Anal 17:163–169. 10.1384/jsa.17.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Akbari B, Tavandashti MP, Zandrahimi M. 2011. Particle size characterization of nanoparticles – a practical approach. Iran J Mater Sci Eng 8:48–56. [Google Scholar]
- 117.Jimenez VL, Leopold MC, Mazzitelli C, Jorgenson JW, Murray RW. 2003. HPLC of monolayer-protected gold nanoclusters. Anal Chem 75:199–206. 10.1021/ac0260589. [DOI] [PubMed] [Google Scholar]
- 118.Iravani S. 2014. Bacteria in nanoparticle synthesis: current status and future prospects. Int Scholar Res Notices 2014:359316. 10.1155/2014/359316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lara AR, Galindo E, Ramírez OT, Palomares LA. 2006. Living with heterogeneities in bioreactors: understanding the effects of environmental gradients on cells. Mol Biotechnol 34:355–381. 10.1385/MB:34:3:355. [DOI] [PubMed] [Google Scholar]
- 120.Byrne JM, Muhamadali H, Coker VS, Cooper J, Lloyd JR. 2015. Scale-up of the production of highly reactive biogenic magnetite nanoparticles using Geobacter sulfurreducens. J R Soc Interface 12:20150240. 10.1098/rsif.2015.0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zou L, Zhu F, Long Z, Huang Y. 2021. Bacterial extracellular electron transfer: a powerful route to the green biosynthesis of inorganic nanomaterials for multifunctional applications. J Nanobiotechnol 19:120. 10.1186/s12951-021-00868-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hennebel T, Simoen H, De Windt W, Verloo M, Boon N, Verstraete W. 2009. Biocatalytic dechlorination of trichloroethylene with bio-palladium in a pilot-scale membrane reactor. Biotechnol Bioeng 102:995–1002. 10.1002/bit.22138. [DOI] [PubMed] [Google Scholar]
- 123.Løvik AN, Hagelüken C, Wäger P. 2018. Improving supply security of critical metals: current developments and research in the EU. Sustain Mater Technol 15:9–18. 10.1016/j.susmat.2018.01.003. [DOI] [Google Scholar]
- 124.Sadhukhan J, Joshi N, Shemfe M, Lloyd JR. 2017. Life cycle assessment of sustainable raw material acquisition for functional magnetite bionanoparticle production. J Environ Manag 199:116–125. 10.1016/j.jenvman.2017.05.048. [DOI] [PubMed] [Google Scholar]
- 125.Joshi N, Filip J, Coker VS, Sadhukhan J, Safarik I, Bagshaw H, Lloyd JR. 2018. Microbial reduction of natural Fe(III) minerals; toward the sustainable production of functional magnetic nanoparticles. Front Environ Sci 6:127. 10.3389/fenvs.2018.00127. [DOI] [Google Scholar]
- 126.Mabbett AN, Sanyahumbi D, Yong P, Macaskie LE. 2006. Biorecovered precious metals from industrial wastes: single-step conversion of a mixed metal liquid waste to a bioinorganic catalyst with environmental application. Environ Sci Technol 40:1015–1021. 10.1021/es0509836. [DOI] [PubMed] [Google Scholar]
- 127.Murray AJ, Zhu J, Wood J, Macaskie LE. 2017. A novel biorefinery: biorecovery of precious metals from spent automotive catalyst leachates into new catalysts effective in metal reduction and in the hydrogenation of 2-pentyne. Miner Eng 113:102–108. 10.1016/j.mineng.2017.08.011. [DOI] [Google Scholar]
- 128.Beblawy S, Bursac T, Paquete C, Louro R, Clarke TA, Gescher J. 2018. Extracellular reduction of solid electron acceptors by Shewanella oneidensis. Mol Microbiol 109:571–583. 10.1111/mmi.14067. [DOI] [PubMed] [Google Scholar]
- 129.Jiang S, Lee JH, Kim D, Kanaly RA, Kim MG, Hur HG. 2013. Differential arsenic mobilization from As-bearing ferrihydrite by iron-respiring Shewanella strains with different arsenic-reducing activities. Environ Sci Technol 47:8616–8623. 10.1021/es400534z. [DOI] [PubMed] [Google Scholar]
- 130.Lee JH, Kim MG, Yoo B, Myung NV, Maeng J, Lee T, Dohnalkova AC, Fredrickson JK, Sadowsky MJ, Hur HG. 2007. Biogenic formation of photoactive arsenic-sulfide nanotubes by Shewanella sp. strain HN-41. Proc Natl Acad Sci USA 104:20410–20415. 10.1073/pnas.0707595104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kim T-Y, Ahn H, Jeon J, Kim MS, Kim MG, Hur H-G. 2017. Biogenic realgar As4S4 molecular clusters formed by a one-pot microbial-driven process as a Li-ion storage material. Adv Sustain Syst 1:1700056. 10.1002/adsu.201700056. [DOI] [Google Scholar]
- 132.Belchik SM, Kennedy DW, Dohnalkova AC, Wang Y, Sevinc PC, Wu H, Lin Y, Lu HP, Fredrickson JK, Shi L. 2011. Extracellular reduction of hexavalent chromium by cytochromes MtrC and OmcA of Shewanella oneidensis MR-1. Appl Environ Microbiol 77:4035–4041. 10.1128/AEM.02463-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang Y, Sevinc PC, Belchik SM, Fredrickson J, Shi L, Lu HP. 2013. Single-cell imaging and spectroscopic analyses of Cr(VI) reduction on the surface of bacterial cells. Langmuir 29:950–956. 10.1021/la303779y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kim D-H, Park S, Kim M-G, Hur H-G. 2014. Accumulation of amorphous Cr(III)–Te(IV) nanoparticles on the surface of Shewanella oneidensis MR-1 through reduction of Cr(VI). Environ Sci Technol 48:14599–14606. 10.1021/es504587s. [DOI] [PubMed] [Google Scholar]
- 135.Konishi Y, Tsukiyama T, Ohno K, Saitoh N, Nomura T, Nagamine S. 2006. Intracellular recovery of gold by microbial reduction of AuCl4− ions using the anaerobic bacterium Shewanella algae. Hydrometallurgy 81:24–29. 10.1016/j.hydromet.2005.09.006. [DOI] [Google Scholar]
- 136.Dong B, Liu G, Zhou J, Cai L, Wang J, Jin R. 2020. Roles of molecular weight-fractionated extracellular polymeric substance in transformation of Au(III) to Au nanoparticles in aqueous environments. Sci Total Environ 728:138889. 10.1016/j.scitotenv.2020.138889. [DOI] [PubMed] [Google Scholar]
- 137.Zhu N, Cao Y, Shi C, Wu P, Ma H. 2016. Biorecovery of gold as nanoparticles and its catalytic activities for p-nitrophenol degradation. Environ Sci Pollut Res Int 23:7627–7638. 10.1007/s11356-015-6033-y. [DOI] [PubMed] [Google Scholar]
- 138.Coker VS, Byrne JM, Telling ND, van der Laan G, Lloyd JR, Hitchcock AP, Wang J, Pattrick RAD. 2012. Characterisation of the dissimilatory reduction of Fe(III)-oxyhydroxide at the microbe-mineral interface: the application of STXM-XMCD. Geobiology 10:347–354. 10.1111/j.1472-4669.2012.00329.x. [DOI] [PubMed] [Google Scholar]
- 139.Smeaton CM, Fryer BJ, Weisener CG. 2009. Intracellular precipitation of Pb by Shewanella putrefaciens CN32 during the reductive dissolution of Pb-jarosite. Environ Sci Technol 43:8086–8091. 10.1021/es901629c. [DOI] [PubMed] [Google Scholar]
- 140.Mertens B, Blothe C, Windey K, De Windt W, Verstraete W. 2007. Biocatalytic dechlorination of lindane by nano-scale particles of Pd(0) deposited on Shewanella oneidensis. Chemosphere 66:99–105. 10.1016/j.chemosphere.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 141.Hennebel T, Verhagen P, Simoen H, De Gusseme B, Vlaeminck SE, Boon N, Verstraete W. 2009. Remediation of trichloroethylene by bio-precipitated and encapsulated palladium nanoparticles in a fixed bed reactor. Chemosphere 76:1221–1225. 10.1016/j.chemosphere.2009.05.046. [DOI] [PubMed] [Google Scholar]
- 142.De Corte S, Bechstein S, Lokanathan AR, Kjems J, Boon N, Meyer RL. 2013. Comparison of bacterial cells and amine-functionalized abiotic surfaces as support for Pd nanoparticle synthesis. Colloids Surf B Biointerfaces 102:898–904. 10.1016/j.colsurfb.2012.08.045. [DOI] [PubMed] [Google Scholar]
- 143.Hou YN, Sun SY, Yang ZN, Yun H, Zhu TT, Ma JF, Han JL, Wang AJ, Cheng HY. 2020. Shewanella oneidensis MR-1 self-assembled Pd-cells-rGO conductive composite for enhancing electrocatalysis. Environ Res 184:109317. 10.1016/j.envres.2020.109317. [DOI] [PubMed] [Google Scholar]
- 144.Lee JH, Han J, Choi H, Hur HG. 2007. Effects of temperature and dissolved oxygen on Se(IV) removal and Se(0) precipitation by Shewanella sp. HN-41. Chemosphere 68:1898–1905. 10.1016/j.chemosphere.2007.02.062. [DOI] [PubMed] [Google Scholar]
- 145.Tam K, Ho CT, Lee JH, Lai M, Chang CH, Rheem Y, Chen W, Hur HG, Myung NV. 2010. Growth mechanism of amorphous selenium nanoparticles synthesized by Shewanella sp. HN-41. Biosci Biotechnol Biochem 74:696–700. 10.1271/bbb.90454. [DOI] [PubMed] [Google Scholar]
- 146.Jiang S, Ho CT, Lee JH, Duong HV, Han S, Hur HG. 2012. Mercury capture into biogenic amorphous selenium nanospheres produced by mercury resistant Shewanella putrefaciens 200. Chemosphere 87:621–624. 10.1016/j.chemosphere.2011.12.083. [DOI] [PubMed] [Google Scholar]
- 147.Li DB, Cheng YY, Wu C, Li WW, Li N, Yang ZC, Tong ZH, Yu HQ. 2014. Selenite reduction by Shewanella oneidensis MR-1 is mediated by fumarate reductase in periplasm. Sci Rep 4:3735. 10.1038/srep03735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Ng CK, Sivakumar K, Liu X, Madhaiyan M, Ji L, Yang L, Tang C, Song H, Kjelleberg S, Cao B. 2013. Influence of outer membrane c‐type cytochromes on particle size and activity of extracellular nanoparticles produced by Shewanella oneidensis. Biotechnol Bioeng 110:1831–1837. 10.1002/bit.24856. [DOI] [PubMed] [Google Scholar]
- 149.Suresh AK, Pelletier DA, Wang W, Moon J-W, Gu B, Mortensen NP, Allison DP, Joy DC, Phelps TJ, Doktycz MJ. 2010. Silver nanocrystallites: biofabrication using Shewanella oneidensis, and an evaluation of their comparative toxicity on Gram-negative and Gram-positive bacteria. Environ Sci Technol 44:5210–5215. 10.1021/es903684r. [DOI] [PubMed] [Google Scholar]
- 150.Li SW, Zhang X, Sheng GP. 2016. Silver nanoparticles formation by extracellular polymeric substances (EPS) from electroactive bacteria. Environ Sci Pollut Res Int 23:8627–8633. 10.1007/s11356-016-6105-7. [DOI] [PubMed] [Google Scholar]
- 151.Marshall MJ, Plymale AE, Kennedy DW, Shi L, Wang Z, Reed SB, Dohnalkova AC, Simonson CJ, Liu C, Saffarini DA, Romine MF, Zachara JM, Beliaev AS, Fredrickson JK. 2008. Hydrogenase- and outer membrane c-type cytochrome-facilitated reduction of technetium(VII) by Shewanella oneidensis MR-1. Environ Microbiol 10:125–136. 10.1111/j.1462-2920.2007.01438.x. [DOI] [PubMed] [Google Scholar]
- 152.Kim DH, Kanaly RA, Hur HG. 2012. Biological accumulation of tellurium nanorod structures via reduction of tellurite by Shewanella oneidensis MR-1. Bioresour Technol 125:127–131. 10.1016/j.biortech.2012.08.129. [DOI] [PubMed] [Google Scholar]
- 153.Kim DH, Kim MG, Jiang S, Lee JH, Hur HG. 2013. Promoted reduction of tellurite and formation of extracellular tellurium nanorods by concerted reaction between iron and Shewanella oneidensis MR-1. Environ Sci Technol 47:8709–8715. 10.1021/es401302w. [DOI] [PubMed] [Google Scholar]
- 154.Kim MG, Kim D-H, Kim T, Park S, Kwon G, Kim MS, Shin TJ, Ahn H, Hur H-G. 2015. Unusual Li-ion storage through anionic redox processes of bacteria-driven tellurium nanorods. J Mater Chem A 3:16978–16987. 10.1039/C5TA04038H. [DOI] [Google Scholar]
- 155.Marshall MJ, Beliaev AS, Dohnalkova AC, Kennedy DW, Shi L, Wang Z, Boyanov MI, Lai B, Kemner KM, McLean JS, Reed SB, Culley DE, Bailey VL, Simonson CJ, Saffarini DA, Romine MF, Zachara JM, Fredrickson JK. 2006. c-Type cytochrome-dependent formation of U(IV) nanoparticles by Shewanella oneidensis. PLoS Biol 4:e268. 10.1371/journal.pbio.0040268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sani RK, Peyton BM, Dohnalkova A. 2008. Comparison of uranium(VI) removal by Shewanella oneidensis MR-1 in flow and batch reactors. Water Res 42:2993–3002. 10.1016/j.watres.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 157.Bernier-Latmani R, Veeramani H, Vecchia ED, Junier P, Lezama-Pacheco JS, Suvorova EI, Sharp JO, Wigginton NS, Bargar JR. 2010. Non-uraninite products of microbial U(VI) reduction. Environ Sci Technol 44:9456–9462. 10.1021/es101675a. [DOI] [PubMed] [Google Scholar]
- 158.Lee SY, Baik MH, Choi JW. 2010. Biogenic formation and growth of uraninite (UO2). Environ Sci Technol 44:8409–8414. 10.1021/es101905m. [DOI] [PubMed] [Google Scholar]
- 159.Jiang S, Kim MG, Kim SJ, Jung HS, Lee SW, Noh DY, Sadowsky MJ, Hur HG. 2011. Bacterial formation of extracellular U(VI) nanowires. Chem Commun (Camb) 47:8076–8078. 10.1039/c1cc12554k. [DOI] [PubMed] [Google Scholar]
- 160.Jiang S, Hur HG. 2013. Effects of the anaerobic respiration of Shewanella oneidensis MR-1 on the stability of extracellular U(VI) nanofibers. Microbes Environ 28:312–315. 10.1264/jsme2.me12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xiao X, Ma XB, Yuan H, Liu PC, Lei YB, Xu H, Du DL, Sun JF, Feng YJ. 2015. Photocatalytic properties of zinc sulfide nanocrystals biofabricated by metal-reducing bacterium Shewanella oneidensis MR-1. J Hazard Mater 288:134–139. 10.1016/j.jhazmat.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 162.Chellamuthu P, Naughton K, Pirbadian S, Silva KPT, Chavez MS, El-Naggar MY, Boedicker J. 2019. Biogenic control of manganese doping in zinc sulfide nanomaterial using Shewanella oneidensis MR-1. Front Microbiol 10:938. 10.3389/fmicb.2019.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wu WF, Wang FP, Li JH, Yang XW, Xiao X, Pan YX. 2013. Iron reduction and mineralization of deep-sea iron reducing bacterium Shewanella piezotolerans WP3 at elevated hydrostatic pressures. Geobiology 11:593–601. 10.1111/gbi.12061. [DOI] [PubMed] [Google Scholar]