Abstract
Background
Atopic eczema (AE), also known as atopic dermatitis, is a chronic inflammatory skin condition that causes significant burden. Phototherapy is sometimes used to treat AE when topical treatments, such as corticosteroids, are insufficient or poorly tolerated.
Objectives
To assess the effects of phototherapy for treating AE.
Search methods
We searched the Cochrane Skin Specialised Register, CENTRAL, MEDLINE, Embase, and ClinicalTrials.gov to January 2021.
Selection criteria
We included randomised controlled trials in adults or children with any subtype or severity of clinically diagnosed AE. Eligible comparisons were any type of phototherapy versus other forms of phototherapy or any other treatment, including placebo or no treatment.
Data collection and analysis
We used standard Cochrane methodology. For key findings, we used RoB 2.0 to assess bias, and GRADE to assess certainty of the evidence. Primary outcomes were physician‐assessed signs and patient‐reported symptoms. Secondary outcomes were Investigator Global Assessment (IGA), health‐related quality of life (HRQoL), safety (measured as withdrawals due to adverse events), and long‐term control.
Main results
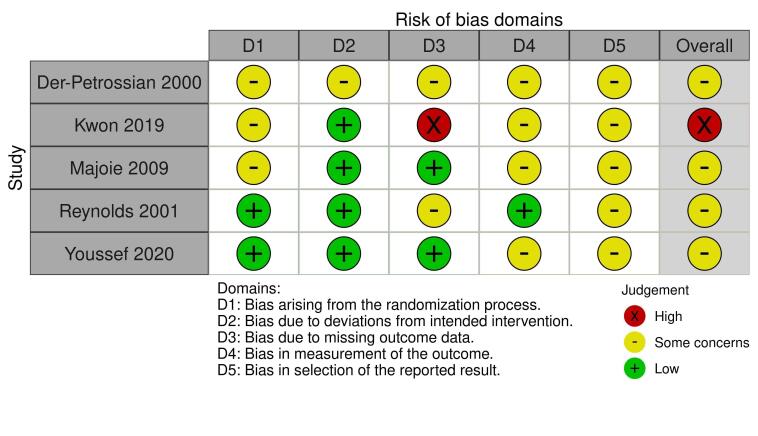
We included 32 trials with 1219 randomised participants, aged 5 to 83 years (mean: 28 years), with an equal number of males and females. Participants were recruited mainly from secondary care dermatology clinics, and study duration was, on average, 13 weeks (range: 10 days to one year). We assessed risk of bias for all key outcomes as having some concerns or high risk, due to missing data, inappropriate analysis, or insufficient information to assess selective reporting.
Assessed interventions included: narrowband ultraviolet B (NB‐UVB; 13 trials), ultraviolet A1 (UVA1; 6 trials), broadband ultraviolet B (BB‐UVB; 5 trials), ultraviolet AB (UVAB; 2 trials), psoralen plus ultraviolet A (PUVA; 2 trials), ultraviolet A (UVA; 1 trial), unspecified ultraviolet B (UVB; 1 trial), full spectrum light (1 trial), Saalmann selective ultraviolet phototherapy (SUP) cabin (1 trial), saltwater bath plus UVB (balneophototherapy; 1 trial), and excimer laser (1 trial). Comparators included placebo, no treatment, another phototherapy, topical treatment, or alternative doses of the same treatment.
Results for key comparisons are summarised (for scales, lower scores are better):
NB‐UVB versus placebo/no treatment
There may be a larger reduction in physician‐assessed signs with NB‐UVB compared to placebo after 12 weeks of treatment (mean difference (MD) ‐9.4, 95% confidence interval (CI) ‐3.62 to ‐15.18; 1 trial, 41 participants; scale: 0 to 90). Two trials reported little difference between NB‐UVB and no treatment (37 participants, four to six weeks of treatment); another reported improved signs with NB‐UVB versus no treatment (11 participants, nine weeks of treatment).
NB‐UVB may increase the number of people reporting reduced itch after 12 weeks of treatment compared to placebo (risk ratio (RR) 1.72, 95% CI 1.10 to 2.69; 1 trial, 40 participants). Another trial reported very little difference in itch severity with NB‐UVB (25 participants, four weeks of treatment).
The number of participants with moderate to greater global improvement may be higher with NB‐UVB than placebo after 12 weeks of treatment (RR 2.81, 95% CI 1.10 to 7.17; 1 trial, 41 participants).
NB‐UVB may not affect rates of withdrawal due to adverse events. No withdrawals were reported in one trial of NB‐UVB versus placebo (18 participants, nine weeks of treatment). In two trials of NB‐UVB versus no treatment, each reported one withdrawal per group (71 participants, 8 to 12 weeks of treatment).
We judged that all reported outcomes were supported with low‐certainty evidence, due to risk of bias and imprecision. No trials reported HRQoL.
NB‐UVB versus UVA1
We judged the evidence for NB‐UVB compared to UVA1 to be very low certainty for all outcomes, due to risk of bias and imprecision. There was no evidence of a difference in physician‐assessed signs after six weeks (MD ‐2.00, 95% CI ‐8.41 to 4.41; 1 trial, 46 participants; scale: 0 to 108), or patient‐reported itch after six weeks (MD 0.3, 95% CI ‐1.07 to 1.67; 1 trial, 46 participants; scale: 0 to 10). Two split‐body trials (20 participants, 40 sides) also measured these outcomes, using different scales at seven to eight weeks; they reported lower scores with NB‐UVB. One trial reported HRQoL at six weeks (MD 2.9, 95% CI ‐9.57 to 15.37; 1 trial, 46 participants; scale: 30 to 150). One split‐body trial reported no withdrawals due to adverse events over 12 weeks (13 participants). No trials reported IGA.
NB‐UVB versus PUVA
We judged the evidence for NB‐UVB compared to PUVA (8‐methoxypsoralen in bath plus UVA) to be very low certainty for all reported outcomes, due to risk of bias and imprecision. There was no evidence of a difference in physician‐assessed signs after six weeks (64.1% reduction with NB‐UVB versus 65.7% reduction with PUVA; 1 trial, 10 participants, 20 sides). There was no evidence of a difference in marked improvement or complete remission after six weeks (odds ratio (OR) 1.00, 95% CI 0.13 to 7.89; 1 trial, 9/10 participants with both treatments). One split‐body trial reported no withdrawals due to adverse events in 10 participants over six weeks. The trials did not report patient‐reported symptoms or HRQoL.
UVA1 versus PUVA
There was very low‐certainty evidence, due to serious risk of bias and imprecision, that PUVA (oral 5‐methoxypsoralen plus UVA) reduced physician‐assessed signs more than UVA1 after three weeks (MD 11.3, 95% CI ‐0.21 to 22.81; 1 trial, 40 participants; scale: 0 to 103). The trial did not report patient‐reported symptoms, IGA, HRQoL, or withdrawals due to adverse events.
There were no eligible trials for the key comparisons of UVA1 or PUVA compared with no treatment.
Adverse events
Reported adverse events included low rates of phototoxic reaction, severe irritation, UV burn, bacterial superinfection, disease exacerbation, and eczema herpeticum.
Authors' conclusions
Compared to placebo or no treatment, NB‐UVB may improve physician‐rated signs, patient‐reported symptoms, and IGA after 12 weeks, without a difference in withdrawal due to adverse events. Evidence for UVA1 compared to NB‐UVB or PUVA, and NB‐UVB compared to PUVA was very low certainty. More information is needed on the safety and effectiveness of all aspects of phototherapy for treating AE.
Keywords: Adult; Child; Female; Humans; Male; Dermatitis, Atopic; Dermatitis, Atopic/drug therapy; Eczema; Phototherapy; Quality of Life; Ultraviolet Therapy
Plain language summary
What are the benefits and risks of light therapy for treating atopic eczema (also known as eczema or atopic dermatitis)?
Key messages
Narrowband (NB) ultraviolet B (UVB), compared to placebo (a sham treatment), may improve eczema severity (including itch) and may not affect the number of people leaving a study because of unwanted effects.
We were unable to confidently draw conclusions for other phototherapy (light therapy) treatments.
Future research needs to assess longer term effectiveness and safety of NB‐UVB and other forms of phototherapy for eczema.
What is eczema?
Eczema is a condition that results in dry, itchy patches of inflamed skin. Eczema typically starts in childhood, but can improve with age. Eczema is caused by a combination of genetics and environmental factors, which lead to skin barrier dysfunction. Eczema can negatively impact quality of life, and the societal cost is significant.
How is eczema treated?
Eczema treatments are often creams or ointments that reduce itch and redness, applied directly to the skin. If these are unsuccessful, systemic medicines that affect the whole body, or phototherapy are options. Phototherapy can be UVB, ultraviolet A (UVA), or photochemotherapy (PUVA), where phototherapy is given alongside substances that increase sensitivity to UV light.
What did we want to find out?
We wanted to find out whether phototherapy was better than no treatment or other types of treatment for treating eczema, and whether it caused unwanted effects.
What did we do?
We searched for studies that investigated phototherapy compared with no treatment, placebo, other forms of phototherapy, or another type of eczema treatment. Studies could include people of all ages, who had eczema diagnosed by a healthcare professional.
We compared and summarised the results of the studies, and rated our confidence in the evidence.
What did we find?
We found 32 studies, involving 1219 people with eczema (average age: 28 years), who were recruited from dermatology clinics. Most studies assessed people with skin type II to III (which is classed as white to medium skin colour), and moderate to severe eczema, with which they had lived for many years. Studies included similar numbers of males and females.
The studies were conducted in Europe, Asia, and Egypt (setting was not reported by seven studies), and lasted, on average, for 13 weeks. Almost half of the studies reported their source of funding; two were linked to commercial sponsors.
Our included studies mostly assessed NB‐UVB, followed by UVA1, then broadband ultraviolet B; fewer studies investigated other types of phototherapy. The studies compared these treatments to placebo, or no treatment, another type of phototherapy, different doses of the same sort of phototherapy, or other eczema treatments applied to the skin or taken by tablet.
None of the studies investigated excimer lamp (a source of UV radiation) or heliotherapy (the use of natural sunlight), that were other light therapies in which we were interested.
What are the main results of our review?
When compared to placebo, NB‐UVB may:
‐ improve signs of eczema assessed by a healthcare professional (1 study, 41 people);
‐ increase the number of people reporting less severe itching (1 study, 41 people);
‐ increase the number of people reporting moderate or greater improvement of eczema, measured by the Investigator Global Assessment scale (IGA), a 5‐point scale that measures improvement in eczema symptoms (1 study, 40 people); and
‐ have no effect on the rate of people withdrawing from treatment due to unwanted effects (3 studies, 89 people).
None of the studies assessing NB‐UVB against placebo measured health‐related quality of life.
We do not know if NB‐UVB (compared with UVA1 or PUVA) or UVA1 (compared with PUVA) has an effect on the following:
‐ signs of eczema assessed by a healthcare professional;
‐ patient‐reported eczema symptoms;
‐ IGA;
‐ health‐related quality of life; and
‐ withdrawals due to unwanted effects.
This is because either we are not confident in the evidence, or they were not reported.
We did not identify any studies that investigated UVA1 or PUVA compared with no treatment.
Some studies reported that phototherapy caused some unwanted effects, including skin reactions or irritation, UV burn, worsening of eczema, and skin infections. However, these did not occur in most people.
What are the limitations of the evidence?
Our confidence in the evidence is limited, mainly because only a few studies could be included in each comparison, and the studies generally involved only small numbers of people.
How up to date is this evidence?
The evidence is up to date to January 2021.
Summary of findings
Summary of findings 1. Summary of findings table ‐ NB‐UVB compared to placebo for atopic eczema.
| NB‐UVB compared to placebo for atopic eczema | ||||||
| Patient or population:atopic eczema Setting:outpatient or not stated (Egypt; Korea; Taiwan; UK) Intervention:NB‐UVB Comparison:placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with NB‐UVB | |||||
| Physician‐assessed changes in clinical signs assessed with: mean reduction in total disease activity score: lower score is better Scale from: 0 to 90 follow‐up: mean 12 weeks | The mean physician‐assessed changes in clinical signs was ‐0.4 | MD 9.4 lower (15.18 lower to 3.62 lower) | ‐ | 41 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | This result is from Reynolds 2001. Three other studies reported this outcome but did not report any dispersion data. In Kwon 2019, EASI score was 2.1 (n=6) with NB‐UVB versus 3.6 (n=5) with no treatment (after 9 weeks). In Tzung 2005 (split‐body study, 6 weeks, n=12), the side treated with NB‐UVB had a mean reduction of 56% in EASI versus 54% with no treatment. In Youssef 2020 (n=25), SCORAD reduced by 50.8% with NB‐UVB versus 48.6% with no treatment (4 weeks of treatment). |
| Patient‐reported changes in symptoms assessed with: number of participants reporting a reduction in itch on VAS follow‐up: mean 12 weeks | 526 per 1000 | 905 per 1000 (579 to 1000) | RR 1.72 (1.10 to 2.69) | 40 (1 RCT) | ⊕⊕⊝⊝ Lowb,c | This result is from Reynolds 2001 (19 of 21 participants with NB‐UVB versus 10 of 19 with placebo). One other study reported this outcome but did not report any dispersion data. Youssef 2020 reported a ‐55.7% change in VAS itch after 4 weeks of treatment with NB‐UVB (n=13), compared to a ‐53.6% change in VAS itch in patients with no treatment (n=12). |
| Investigator Global Assessment (short‐term) assessed with: number of participants with moderate or greater improvement follow‐up: mean 12 weeks | 211 per 1000 | 592 per 1000 (232 to 1000) | RR 2.81 (1.10 to 7.17) | 41 (1 RCT) | ⊕⊕⊝⊝ Lowb,d | This result is from Reynolds 2001 (13 of 22 participants with NB‐UVB versus 4 of 19 with placebo). Long‐term data (measured at 6 months, 3 months post‐treatment) showed a similar result (RR 1.89, 95% CI 0.92 to 3.89, n=35). |
| Health‐related quality of life ‐ not measured | ‐ | ‐ | ‐ | None of the studies measured this outcome | ||
| Safety: withdrawals due to adverse events (short‐term) assessed with: number of participants follow‐up: range 8 weeks to 12 weeks | See comments box for narrative description. | 89 (3 RCTs) | ⊕⊕⊝⊝ Lowb,e | In Reynolds 2001, one patient in each group withdrew because of burning (measured up to week 12, n=41). In Youssef 2020, two patients withdrew because of adverse events: one patient in the NB‐UVB group (phototoxic reaction) and one patient in the glycerol 85% group (severe irritation) (measured up to week 8, n=30). Kwon 2019 reported no withdrawals in both groups (measured up to week 9, n=18). | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_425206404975446768. | ||||||
a Downgraded one level due to risk of bias. Overall risk of bias was 'some concerns' in Reynolds 2001 due to concerns with missing outcome data (13% of participants withdrew but numbers were similar in both groups) and selection of the reported results (no protocol available). Kwon 2019 was considered high risk overall (deviations from intended interventions, missing outcome data). In Tzung 2005, overall risk of bias was 'some concerns' (concerns in all domains apart from measurement of outcome). In Youssef 2020, overall risk of bias was 'some concerns' (deviations from intended interventions and selection of reported result). b Downgraded one level due to imprecision ‐ small sample sizes. c Downgraded one level due to risk of bias. Overall risk of bias was 'some concerns' in Reynolds 2001 due to concerns with missing outcome data (13% of participants withdrew but numbers were similar in both groups) and selection of the reported results (no protocol available). There were 'some concerns' with Youssef 2020 due to deviations from intended interventions, measurement of the outcome and selection of reported result. d Downgraded one level due to risk of bias. Overall risk of bias was 'some concerns' in Reynolds 2001 due to concerns with missing outcome data (13% of participants withdrew but numbers were similar in both groups) and selection of the reported results (no protocol available). e Downgraded one level due to risk of bias. Overall risk of bias was 'some concerns' in Reynolds 2001 due to concerns with missing outcome data (13% of participants withdrew but numbers were similar in both groups) and selection of the reported results (no protocol available). Kwon 2019 was considered 'some concerns' overall (deviations from intended interventions, missing outcome data). In Tzung 2005, overall risk of bias was 'some concerns' (concerns in all domains). In Youssef 2020, overall risk of bias was 'some concerns' (Measurement of outcome and selection of reported result).
Summary of findings 2. Summary of findings table ‐ NB‐UVB compared to UVA1 for atopic eczema.
| NB‐UVB compared to UVA1 for atopic eczema | ||||||
| Patient or population:atopic eczema Setting:not stated (Germany; the Netherlands) Intervention:NB‐UVB Comparison:UVA1 | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with UVA1 | Risk with NB‐UVB | |||||
| Physician‐assessed changes in clinical signs (short‐term) assessed with: SASSAD: lower score is better Scale from: 0 to 108 follow‐up: mean 12 weeks | The mean physician‐assessed changes in clinical signs (short‐term) was 22 | MD 2 lower (8.41 lower to 4.41 higher) | ‐ | 46 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | This result is from Gambichler 2009. Two split‐body studies could not be included due to insufficient data. Legat 2003 (n=7) reported median Costa (scale 0‐123) score of 40 (range 26 to 89) and 58 (27 to 89) and median Leicester score (maximum 162) of 23 (12 to 56) and 52 (14 to 69) after 7 weeks with NB‐UVB and UVA1, respectively. Majoie 2009 reported mean Leicester sign score (scale 0‐108) of 9.2 and 11.6 in 26 body‐halves (13 participants) treated with NB‐UVB and UVA1, respectively (8 weeks). |
| Patient‐reported changes in symptoms assessed with: VAS for itch Scale from: 0 to 10 follow‐up: mean 6 weeks | The mean patient‐reported changes in symptoms was 4.2 | MD 0.3 higher (1.07 lower to 1.67 higher) | ‐ | 46 (1 RCT) | ⊕⊝⊝⊝ Very lowb,c | This result is from Gambichler 2009. Two split‐body studies could not be included due to insufficient data. After 7 weeks of treatment, seven participants in Legat 2003 reported a median VAS itch (scale 0‐10) of 2 (0.1 to 8.5) for their body‐half that was treated with NB‐UVB, compared to 3.9 (0.2 to 8.4) for the UVA1 treated body‐half. At week 8, Majoie 2009 showed a mean itch VAS of 2.9 and 3.6 for NB‐UVB and UVA1 in 13 participants, respectively. |
| Investigator Global Assessment ‐ not measured | ‐ | ‐ | ‐ | |||
| Health‐related quality of life assessed with: German Skindex‐29: lower score is better Scale from: 30 to 150 follow‐up: mean 6 weeks | The mean health‐related quality of life was 69.8 | MD 2.9 higher (9.57 lower to 15.37 higher) | ‐ | 46 (1 RCT) | ⊕⊝⊝⊝ Very lowb,d | This result is from Gambichler 2009. |
| Safety: withdrawal due to adverse events assessed with: number of participants follow‐up: mean 12 weeks | See comments box for narrative description (right). | 13 (1 RCT) | ⊕⊝⊝⊝ Very lowe,f | Majoie 2009 was the only study that reported the number of withdrawals due to adverse events. There were no withdrawals due to adverse events in this split‐body trial (13 participants, 26 sides). | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_425498097763422661. | ||||||
a Downgraded by two levels due to very serious risk of bias. High risk of bias overall as there was high risk of bias due to deviations from intended interventions (did not follow intention to treat analysis, excluded participants due to inefficiency), missing outcome data (45% missing) and selection of reported results (retrospective clinical trial register entry which specified SCORAD been used instead). Legat 2003 was also rated high risk of bias overall (measurement of the outcome). Majoie 2009 had some concerns (randomisation process, selection of the reported result). b Downgraded by one level due to serious imprecision ‐ small sample sizes. c Downgraded by two levels due to very serious risk of bias. High risk of bias overall as there was high risk of bias due to deviations from intended interventions (did not follow intention to treat analysis, excluded participants due to inefficiency) and missing outcome data (45% missing). Legat 2003 was also rated high risk of bias overall (measurement of the outcome). Majoie 2009 had some concerns (randomisation process, measurement of the outcome, selection of the reported result). d Downgraded by two levels due to very serious risk of bias. High risk of bias overall as there was high risk of bias due to deviations from intended interventions (did not follow intention to treat analysis, excluded participants due to inefficiency) and missing outcome data (40% missing). e Downgraded one level due to serious risk of bias. Majoie 2009 had some concerns (randomisation process, selection of the reported result). f Downgraded by two levels due to very serious imprecision ‐ single study with very small sample size.
Summary of findings 3. Summary of findings table ‐ NB‐UVB compared to PUVA for atopic eczema.
| NB‐UVB compared to PUVA for atopic eczema | ||||||
| Patient or population:atopic eczema Setting:not stated Intervention:NB‐UVB Comparison:PUVA | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with PUVA | Risk with NB‐UVB | |||||
| Physician‐assessed changes in clinical signs assessed with: percentage reduction in modified SCORAD follow‐up: mean 6 weeks | See comments box for narrative description. | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | Data was only presented on a graph and it wasn't clear if standard deviations were shown. At week 6, a 64.10% percentage reduction in SCORAD was seen in the NB‐UVB treated body‐half, compared to a similar percentage reduction of 65.7% in the body‐half treated with PUVA. This is a split‐body study where the number of participants in the study was 10 ‐ but there were 20 'sides' analysed. | ||
| Patient‐reported changes in symptoms ‐ not measured | ‐ | ‐ | ‐ | |||
| Investigator Global Assessment assessed with: number of participants with marked improvement or complete remission follow‐up: mean 6 weeks | 900 per 1000 | 900 per 1000 (539 to 986) | OR 1.00 (0.13 to 7.89) | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowa,c | This is a split‐body study where the number of participants in the study was 10 ‐ but there were 20 'sides' analysed (which has been adjusted for in the analysis). |
| Health‐related quality of life ‐ not measured | ‐ | ‐ | ‐ | |||
| Safety: withdrawal due to adverse events assessed with: number of participants follow‐up: mean 6 weeks | See comments box for narrative description (right). | 20 (1 RCT) | ⊕⊝⊝⊝ Very lowb,d | There were no severe adverse events and no withdrawals due to adverse events in this split‐body study (10 participants, 20 sides). | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_425498309567386282. | ||||||
a Downgraded one level due to serious risk of bias. Some concerns in all domains apart from measurement of the outcome which was considered low risk of bias. b Downgraded two levels due to very serious imprecision (small sample size; n=10 participants, 20 sides). c Downgraded two levels due to very serious imprecision. Small sample size (n=10 participants, 20 sides) and a wide 95% CI. d Downgraded one level due to serious risk of bias. Some concerns in all domains.
Summary of findings 4. Summary of findings table ‐ UVA1 compared to PUVA for atopic eczema.
| UVA1 compared to PUVA for atopic eczema | ||||||
| Patient or population:atopic eczema Setting:outpatient (Austria) Intervention:UVA1 Comparison:PUVA | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with PUVA | Risk with UVA1 | |||||
| Physician‐assessed changes in clinical signs assessed with: SCORAD: lower score is better Scale from: 0 to 103 follow‐up: mean 3 weeks | The mean physician‐assessed changes in clinical signs was 28.8 | MD 11.3 higher (0.21 lower to 22.81 higher) | ‐ | 40 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | |
| Patient‐reported changes in symptoms ‐ not measured | ‐ | ‐ | ‐ | |||
| Investigator Global Assessment ‐ not measured | ‐ | ‐ | ‐ | |||
| Health‐related quality of life ‐ not measured | ‐ | ‐ | ‐ | |||
| Safety: withdrawals due to adverse events ‐ not measured | ‐ | ‐ | ‐ | |||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
| See interactive version of this table: https://gdt.gradepro.org/presentations/#/isof/isof_question_revman_web_425498095256539586. | ||||||
a Downgraded one level due to serious risk of bias. Some concerns overall in randomisation, missing outcome data, and selection of the reported result. b Downgraded by two levels due to very serious imprecision ‐ small sample size and wide 95% CI.
Background
Description of the condition
Atopic eczema, also known as atopic dermatitis, is a chronic inflammatory skin condition that causes a significant burden to people with the condition and society. Atopic eczema can have a relapsing‐remitting or a continuous disease course. The clinical presentation is characterised by xerosis (dry skin), pruritus, and flaky, excoriated "eczematous" lesions (Weidinger 2016). Atopic eczema is diagnosed clinically by its signs and symptoms, and its distribution, which varies in different age groups (Spergel 2003). Diagnosis is based on the presence of other atopic diseases, like asthma. In research settings, the most commonly used diagnostic criteria are the Hanifin and Rajka criteria (Hanifin 1980), and the UK Working Party Diagnostic Criteria for Atopic Dermatitis (Williams 1994).
The prevalence of atopic eczema is reported to be up to 20% in children, and between 7% and 10% in adults, and may be increasing (De Lusignan 2020; Flohr 2014). Often, atopic eczema manifests at infancy, but it can start at any age. A cross‐sectional survey of 1760 children with atopic eczema found that 84% suffered from mild disease; 14% from moderate, and 2% from severe atopic eczema (Emerson 1998). Typically, the condition improves during childhood, with more than 50% of childhood atopic eczema resolving by adolescence (Williams 2005). However, some aspects of skin barrier and immune dysfunction may persist into adulthood (Abuabara 2018).
The International Study of Asthma and Allergies in Childhood (ISAAC) uses consistent measurement tools to study the prevalence of atopic eczema in children 6 to 7 years old, and 13 to 14 years old. One study within this research programme, examining time trends in the prevalence of atopic eczema, found a decreased prevalence of atopic eczema in developed countries, especially in Northwest Europe, between 2001 and 2003, compared to results from an earlier study that was conducted between 1994 and 1995. On the other hand, they found an increased prevalence, particularly for the younger age group, in many formerly low‐prevalence, low‐income countries in Latin America and Southeast Asia (Odhiambo 2009; Williams 2008). This variation in reported prevalence over time, and between different regions, suggests that disease prevalence is influenced by environmental factors. A large epidemiological study, using a UK primary care research database of 3.85 million children and adults, showed that the incidence of atopic eczema was higher in people with Black and Asian ethnicity than in white ethnic groups (De Lusignan 2020). A greater incidence of atopic eczema was seen in children younger than two years old with higher socioeconomic status, but for all other age groups, higher socioeconomic status was associated with a lower incidence of the condition. Both incidence and prevalence of atopic eczema are higher in urban areas (De Lusignan 2020; Schram 2010). It seems that environmental factors play a role during early life, as a relatively higher atopic eczema prevalence is seen in children from immigrants who moved from a low‐prevalence country to a country with higher prevalence (Martin 2013). The strongest determinant of atopic eczema is a positive family history (i.e. genetics (Apfelbacher 2011)).
The pathophysiology of atopic eczema is complex, and includes multiple interactions between genetic, immune, and external factors (Stefanovic 2020). It involves defects in epidermal structure and barrier dysfunction, alterations in cell‐mediated immune responses and immunoglobulin E‐mediated hypersensitivity (Weidinger 2016). An underlying genetic predisposition is identified with the discovery of mutations in the gene coding for the skin barrier protein, filaggrin (Palmer 2006). However, filaggrin mutations do not occur in all people with atopic eczema, so other genes and environmental factors seem to play an important role in its pathophysiology. The exposome is the total amount of external factors that an individual is exposed to throughout their lifetime (Stefanovic 2020). Exposomal influences play an important role in atopic eczema pathogenesis, and can be categorised into nonspecific exposures (e.g. human and natural factors), specific exposures (environmental factors, e.g. diet, allergens, humidity, ultraviolet radiation, pollution, and water hardness), and internal exposures (e.g. microbiota of the skin and gut, and host cell interaction (Stefanovic 2020)).
Atopic eczema causes a significant burden to both the person with the condition and their families, and it has been found that an increase in the condition's severity can result in lower quality of life, anxiety, and depression (Maksimović 2012). In addition, atopic eczema has important effects on society due to high medical costs, psychosocial effects, and co‐morbidities (Mancini 2008). The Global Burden of Disease Study, providing annually updated numbers on disease‐related morbidity and mortality worldwide, showed that atopic eczema disease burden, as measured by disability‐adjusted life years (DALYs), ranks fifteenth among all nonfatal diseases, and has the highest disease burden of all skin diseases (Laughter 2020). The worldwide DALY rate was 123.31 per 100,000 (95% uncertainty interval 66.79 to 205.17) in 2017 (Laughter 2020). The outcomes of the Cochrane Skin Prioritisation Exercise 2020 showed that the total number of DALYs for atopic eczema in 2017 was 9,003,374 (Cochrane Skin 2020a).
The main physician‐assessed outcome measures are the EASI (Eczema Area and Severity Index) score (Ricci 2009); the SCORAD (severity SCORing of Atopic Dermatitis) Index, which also includes a self‐assessment component, the Subjective SCORAD (Kunz 1997); the SASSAD (Six Area Six Sign Atopic Dermatitis Severity) score (Charman 2002); and Costa's Simple Scoring System (Costa (Costa 1989)). Subjective tools used for self‐assessment are the POEM (Patient‐Oriented Eczema Measure) Scale (Charman 2004); the PO‐SCORAD (Patient‐Oriented SCORAD (Stalder 2011)); and the SA‐EASI (Self‐Administered Eczema Area and Severity Index) Rating Scale (Housman 2002). The Harmonising Outcome Measures for Eczema (HOME) initiative reached consensus that the EASI score should be the core instrument used for clinician‐reported signs; POEM and NRS‐11 (Numeric Rating Scale, 11‐point scale for peak itch over past 24 hours) should be used for self‐reported symptoms; RECAP (Recap of Atopic Eczema (Howells 2020)) or ADCT (Atopic Dermatitis Control Test (Simpson 2019)) should be used for long‐term control; and the DLQI (Dermatology Life Quality Index (Finlay 1994)), should be used for quality of life assessment (Schmitt 2014; Spuls 2017).
The severity of atopic eczema is variable, with symptoms ranging from mild disease with localised redness and localised involvement, to moderate to severe disease characterised by more generalised involvement of the whole body, with widespread redness, oozing, crusting, and secondarily infected lesions. Assessment of clinical severity is based on both objective clinical signs and subjective symptoms, such as itch and loss of sleep (Schmitt 2014). The EASI score corresponds to disease severity as follows: 0 = clear; 0.1 to 1.0 = almost clear; 1.1 to 7.0 = mild; 7.1 to 21.0 = moderate; 21.1 to 50.0 = severe; 50.1 to 72.0 = very severe (Barbarot 2016).
Description of the intervention
For people with moderate to severe atopic eczema, for whom topical treatments, including corticosteroids and emollients, are insufficient, systemic immunomodulating medication, phototherapy, or photochemotherapy are therapeutic options. Photochemotherapy is a subtype of phototherapy, which is defined as the use of phototherapy combined with adjuvant ultraviolet light‐activated drug photosensitisers. Several types of phototherapy are beneficial for disease control in people with atopic eczema. These include: broadband ultraviolet B (BB‐UVB; wavelength 280 nm to 315 nm); narrowband ultraviolet B (NB‐UVB; wavelength 311 nm to 313 nm); ultraviolet A (UVA; wavelength 315 nm to 400 nm); ultraviolet A1 (UVA1; wavelength 340 nm to 400 nm); cold‐light UVA1 (containing a cooling system eliminating wavelengths greater than 530 nm, decreasing the heat load); ultraviolet AB (UVAB; wavelength 280 nm to 400 nm); full‐spectrum light (wavelength 320 nm to 500 nm, including UVA, visible, and infrared light); saltwater bath plus UVB (balneophototherapy); coal tar plus UVB (Goeckerman therapy); and excimer laser and excimer lamp (generating radiation in the ultraviolet B range (Garritsen 2014)). Photochemotherapy includes treatment with psoralen plus UVA (PUVA) and khellin plus UV. Phototherapy is usually administered in institutional settings, but for certain types of phototherapy, home phototherapy is also available.
Ultraviolet B (UVB)
UVB phototherapy can be administered using different wavelengths of emission. BB‐UVB lamps deliver ultraviolet radiation in the range of 280 nm to 315 nm, while NB‐UVB lamps deliver radiation of a much narrower spectrum, between 311 nm and 313 nm. UVB absorption occurs mainly through chromophores in the epidermis and superficial dermis (Weichenthal 2005). In order to increase the effectiveness of UVB therapy, and thereby, reduce UV exposure and risks, UVB treatment is often combined with topical agents (Mahrle 1987).
For psoriasis, it was shown that wavelengths around 311 nm were more effective than broad‐spectrum UVB, which led to the development of NB‐UVB lamps, which emit selective UVB spectra in the range of 311 nm to 313 nm (Fischer 1976; Parrish 1981). While the equivalent action spectra studies are not available for atopic eczema, NB‐UVB is now the most established and widely used form of phototherapy for the treatment of a wide range of other skin diseases, including atopic eczema (Herzinger 2016; Honig 1994; Van Weelden 1988; Vermeulen 2020). NB‐UVB devices contain fluorescent lamps emitting UVB in the 311 nm to 313 nm range (Van Weelden 1988). Although much less widely available in current times, devices used for BB‐UVB emit wavelengths in both the UVB range (280 nm to 315 nm, approximately two‐thirds of the output) and the UVA range (320 nm to 400 nm, approximately one‐third of the output (Jaleel 2019)).
The starting dose of UVB phototherapy is established by determining the person’s minimal erythema dose (MED), and basing treatment on that (e.g. 70% MED as first dose), or it is based on the person’s Fitzpatrick skin phototype (a system that classifies skin type by its reaction to exposure to sunlight). After treatment initiation, doses are gradually increased to 2000 mJ/cm2 to 5000 mJ/cm2, or to the maximum tolerated dose (Ibbotson 2004). Dose increments usually vary between 5% and 40% of the last dose used, most often in 10% to 20% increments. Treatment frequency varies from two to five times per week. Each treatment lasts from seconds at the onset of treatment, to minutes, depending on the type of device used. Guidelines on the dosimetry of NB‐UVB have mainly been published for psoriasis, but the same dosing protocols are often used for atopic eczema (Beani 2010; Ibbotson 2004; Menter 2010; Sidbury 2014; Spuls 2004). UVB phototherapy can also be administered in the person's home, described as home phototherapy.
BB‐UVB is sometimes combined with topical crude coal tar, in a regimen called Goeckerman therapy. This therapy was first reported by Goeckerman in 1925 for the treatment of psoriasis, but can also be used for the treatment of severe atopic eczema (Dennis 2013).
Balneotherapy (saltwater immersion) can also be combined with UVB (balneophototherapy). The addition of UVB phototherapy to balneotherapy may enhance the anti‐inflammatory effect of thermal spring water. UVB can be administered simultaneously, or after saltwater immersion (Huang 2018).
Ultraviolet A (UVA)
The different types of UVA phototherapies can be sub‐categorised into conventional UVA (315 nm to 400 nm) and UVA1 (340 nm to 400 nm). Conventional UVA requires longer exposure times for effective doses. However, as UVA1 equipment is relatively expensive to buy and maintain, conventional UVA lamps can still be used as a less costly alternative to UVA1, as 90% of their emission is in the UVA1 range (Darsow 2010; Legat 2003; Zandi 2012).
UVA1 lamps that eliminate ultraviolet A2 (UVA2; 320 nm to 340 nm) wavelengths from their emission spectrum have enabled higher doses to be delivered, while minimising risk of adverse effects, notably erythema. In practice, metal halide sources are required to achieve such high doses, as fluorescent sources at much lower irradiance are unable to achieve this. UVA1 can be administrated at a high dose (HD; 80 J/cm2 to 130 J/cm2), medium dose (MD; 40 J/cm2 to 80 J/cm2), or low dose (LD; less than 40 J/cm2), with sessions lasting from 10 minutes to one hour (Darsow 2010; Legat 2003). Dosimetry has not yet been standardised internationally, but based on reports of the approximate dose needed to produce minimum erythema and treatment durations, it can be assumed that low, medium, and high doses are approximately equivalent between centres; although quoted dosages are unlikely to be precisely equivalent (Dawe 2003). Efficacy of high dose UVA1 has been reported in acute flares of severe atopic eczema, although the specific phenotype of atopic eczema that responds most effectively has not been evaluated, and is a matter for further study (Krutmann 1998).
For people receiving high dose UVA1, UVA1 cold light lamps that filter infrared radiation with a cooling ventilation machine, enable treatment to be delivered more comfortably, without the high levels of heat produced during high dose UVA1 exposure (Von Kobyletzki 1999b).
UVAB radiation includes wavelengths of both UVA and ultraviolet B (UVB), given either simultaneously by a single device (such as Metec Helarium©), or in subsequent emissions. Its use for atopic eczema was initiated by Jekler and Larkö, but it is rarely used today, as it has largely been replaced with other UV‐based phototherapies (Grundmann 2012; Jekler 1990).
Full spectrum light (FSL) is an alternative modality of phototherapy, generating the full spectrum of light with a continuous wavelength ranging from 320 nm to 5000 nm, usually in combination with emollients (Byun 2011).
Photochemotherapy
Photochemotherapy uses ultraviolet light‐activated drug photosensitisers combined with phototherapy. It typically uses a systemic drug photosensitiser combined with phototherapy. In photochemotherapy, the anti‐inflammatory, anti‐proliferative, and immunosuppressive effects only occur in the skin on irradiation, when the drug absorbs ultraviolet light. The most common form of photochemotherapy is psoralen‐UVA (PUVA); during which the administration of UVA is combined with psoralen as the photosensitiser. Psoralen can be administered orally or topically, either by immersing in a bath, or applying it as soaks, creams, or gels. The main psoralens used for oral PUVA are 8‐methoxypsoralen (8‐MOP) and 5‐methoxypsoralen (5‐MOP). 8‐MOP is most commonly used for bath PUVA, although this is not useful in atopic eczema if the face requires treatment. Usually, the dose and treatment schedule of PUVA is based on the minimum phototoxic dose (MPD) to ensure adequate drug bioavailability, or on people's sensitivity to sunlight, corresponding to the Fitzpatrick sun‐reactive skin phototype (Sachdeva 2009; Sidbury 2014). The treatment schedule of PUVA is usually twice weekly for atopic eczema; the UVA radiation dose is gradually increased during the course of treatment by increments, often in the order of 20% to 40%. The total number of PUVA treatments per course will depend on disease response and tolerance. Cumulative treatment numbers will depend on individual factors.
Another form of photochemotherapy is khellin, combined with UV (natural sunlight or UVA). Khellin is a photosensitiser that can be administered topically or orally.
Excimer lamp and excimer laser
Excimer is a complex of excited gases, which upon decomposition, give off excess energy in the form of UV radiation. The excimer exists both as a lamp and a laser. The lamp is a polychromatic (wavelengths 306 nm to 310 nm), non‐targeted (incoherent) light used to treat a range of body surface areas. On the other hand, the laser is a monochromatic (308 nm), targeted (coherent), intermittent (pulsing) light (Brenninkmeijer 2010; Park 2012).
Safety and adverse events
The various forms of phototherapy available for people with atopic eczema have different risk profiles that must be taken into account by the physician (Goldsmith 2012; Menter 2010; Morison 1998; Stern 1997). Common adverse events for any type of UV‐based phototherapy are erythema, pruritus, and a sense of burning or stinging, although it is important to be aware that erythema from PUVA may not be apparent until 48 hours to 96 hours after exposure. Other less common consequences of phototherapy are induction of polymorphous light eruption, folliculitis, herpes simplex virus reactivation, and photo‐onycholysis (with PUVA). The most common side effect of oral psoralen is nausea. Uncommonly, pain may occur, and seems specific to PUVA rather than other UV‐based phototherapies. It is likely that this is neuropathic in nature, and is important to recognise, as PUVA should be discontinued in that instance. The risk of squamous cell carcinoma is increased if people are exposed to high cumulative numbers of PUVA treatments (more than 150 to 200 (Stern 1998)). While a delayed risk of melanoma was reported, it has not been replicated, nor has a causal role been proven (Stern 1997). A larger Swedish study, including people with atopic eczema, did not show this association (Lindelöf 1991; Lindelöf 1999).
The incidence of adverse events of phototherapy is considered to be low, although the true incidence is unknown. Most publications on the safety and adverse events of phototherapy concern the treatment of people with psoriasis, and it is unclear how the outcomes of these studies relate to outcomes for people with atopic eczema. However, noncompliance rates secondary to side effects are very low in the available studies for atopic eczema (Clayton 2007; Grundmann‐Kollmann 1999; Jekler 1988; Meduri 2007; Tay 1996).
Prescribing practices
A recent survey was conducted by the European TREatment of ATopic eczema (TREAT) Registry Taskforce. Invited via a mailing list of the European Academy of Dermatology and Venereology and national societies, 238 dermatologists from 30 European countries participated (Vermeulen 2020). The most common first‐line non‐topical therapy for people with moderate to severe atopic eczema was phototherapy, prescribed by 41.5% of survey participants, followed by day‐care therapy (39.3%), and systemic therapy (26.6%). NB‐UVB and PUVA were the most frequently prescribed first‐ and second‐line choices of phototherapy for atopic eczema. Only a small minority of participants prescribed UVA1. The most important reason participants stated for using phototherapy was personal experience with the treatment (58.8%).
There is an absence of published data on phototherapy practice patterns for the treatment of atopic eczema for regions outside Europe. The guidelines of care for the management of atopic eczema by the American Academy of Dermatology (AAD) state that phototherapy is a second‐line treatment, and that choice of phototherapy modality should be guided by factors, such as availability, cost, skin phototype, skin cancer history, and the use of photosensitising medications (Sidbury 2014). Anecdotally, different types of UVB (NB and BB) may be the most commonly used form of phototherapy for atopic eczema in North America. In general, NB‐UVB is often recommended, taking into account its relative efficacy, low adverse effects profile, and availability (Sidbury 2014). A study on phototherapy utilisation and costs in the USA found that the total invoice of phototherapy services for all diseases increased 5% annually from 2000 to 2015. UVB comprised 77% of phototherapy volume, and 92% of phototherapy was prescribed by dermatologists (Tan 2018).
Previous evidence
A previous systematic review, using GRADE methodology, showed that phototherapy can be a valid therapeutic option for people with atopic eczema (Garritsen 2014). Garritsen and colleagues highlighted that the best evidence on efficacy is available for the use of NB‐UVB and UVA1 (Garritsen 2014). These findings are in line with the recommendations in the Atopic Eczema treatment guideline from the European Dermatology Forum (Wollenberg 2018). The review further showed that there was little information available on the duration of remission, long‐term safety, efficacy in children, and in acute versus chronic atopic eczema. The review authors also identified some shortcomings in the quality of the included studies. They argued that studies should adequately measure the use of concomitant topical corticosteroids, and use validated diagnostic atopic eczema criteria and outcome measurements.
Another systematic review supported the findings of Garritsen 2014 regarding the evidence for the use of NB‐UVB and UVA1 phototherapy in moderate to severe atopic eczema (Pérez‐Ferriols 2015). These review authors found that there was scarce evidence supporting the use of PUVA, and little information on phototherapy for atopic eczema in children. The authors recommended standardisation of radiation methods, and the use of comparable criteria, scales, and minimum length of follow‐up in future studies (Pérez‐Ferriols 2015).
A randomised controlled trial (RCT) on high versus medium UVA1 phototherapy reported that UVA1 phototherapy should be considered among the first approaches in people with severe atopic eczema, and stated that high dose was more effective than medium dose UVA1 for dark skin types (Pacifico 2019).
In an observational multicentre study, researchers observed 207 people with psoriasis, and 144 people with atopic eczema, in eight centres (Väkevä 2019). For the people with atopic eczema, scores from the Patient‐Oriented SCORing Atopic Dermatitis (PO‐SCORAD) index and Dermatology Life Quality Index (DLQI) improved significantly during and after treatment (measured at three months or more). Alleviation of pruritus correlated with better quality of life. The study authors indicated that further studies in atopic eczema were necessary to determine the best treatment dose.
How the intervention might work
Several factors are believed to contribute to the effectiveness of phototherapy (Gambichler 2009). First, suppression of the antigen‐presenting function of Langerhans cells is believed to be the mechanism of the immune‐suppressing effect, together with induction of apoptosis of infiltrating T‐cells (Majoie 2009). Second, phototherapy is found to thicken the stratum corneum. This causes the skin to be less susceptible to pathogens and antigens, resulting in smaller eczematous reactions (Jekler 1990). And last, there seems to be suppression of the colonisation of the skin with Staphylococcus aureus and Pityrosporum orbiculare (the yeast form of Malassezia furfur), which is helpful for people with atopic eczema, as their skin often shows superabundance of these organisms. S. aureus secretes toxins that drive atopic eczema (Alexander 2020; Faergemann 1987; Weidinger 2016), while P. orbiculare can trigger the development and persistence of atopic eczema through the generation of autoantigens (Nowicka 2019).
The mechanisms of action of different phototherapeutic options differ, but include anti‐inflammatory, antiproliferative, and immunosuppressive effects, which will be of differing importance in contributing to the effects seen in different diseases. Anti‐inflammatory and immunosuppressive effects are of importance in atopic eczema.
UVB exerts its effects mainly at the level of the epidermis and superficial dermis, while UVA‐based phototherapies affect mid‐ and deep‐dermal components, including blood vessels. UVB radiation is absorbed by endogenous chromophores, such as nuclear DNA, initiating a cascade of events. Absorption of UV light by nucleotides leads to the formation of DNA photoproducts and suppresses DNA synthesis. UV light stimulates the synthesis of prostaglandins and cytokines that play important roles in immune suppression. It can reduce the number of Langerhans cells, cutaneous T‐lymphocytes, and mast cells in the dermis. UV radiation can also affect extranuclear molecular targets located in the cytoplasm and cell membrane. The combination of immune suppression, alteration in cytokine expression, and cell cycle arrest contributes to the suppression of disease activity (Bulat 2011).
With PUVA, the conjunction of psoralens with epidermal DNA inhibits DNA replication, and causes cell cycle arrest. Psoralen photosensitisation also causes an alteration in the expression of cytokines and cytokine receptors. Psoralens interact with RNA, proteins, and other cellular components, and indirectly modify proteins and lipids via single oxygen‐mediated reactions, or by generating free radicals. Infiltrating lymphocytes are strongly suppressed by PUVA, with variable effects on different T‐cell subsets (Bulat 2011).
Studies in Asian populations have suggested that both NB‐UVB, and a combination of UVA plus NB‐UVB, are effective in the treatment of moderate to severe atopic eczema (Mok 2014). NB‐UVB, which is usually the preferred modality for treating atopic eczema, requires higher doses in more pigmented skin types (Meduri 2007; Syed 2011a; Syed 2011b).
UVA1 is thought to be faster and more efficacious for treating acute atopic eczema, and is equally effective in skin types I to V, without requiring dose adjustments (Jacobe 2008; Mok 2014). However, it is not clear how atopic eczema disease phenotype (e.g. predominantly flexural versus discoid, or follicular) impacts on the responsiveness to the different types of phototherapy; this area requires further study.
Why it is important to do this review
A good summary of the evidence of the different types of phototherapy will be useful to detect the gaps of evidence and to determine the future research agenda. The knowledge gap and varying prescribing practices have led to limited reimbursement of phototherapy for atopic eczema by healthcare insurance companies in some countries, making a promising treatment modality unattainable for some people for whom topical corticosteroids are insufficient. The costs of atopic eczema per person are expected to rise in the coming years, when dupilumab, a fully human monoclonal antibody that inhibits IL‐4 and IL‐13, and baricitinib, a janus kinase (JAK) inhibitor are approved for the treatment of atopic dermatitis, and most importantly, because of the arrival of other new systemic treatments, such as new JAK inhibitors. Thus, high‐quality research into therapeutic alternatives, which have longstanding track records for efficacy, safety, and cost‐effectiveness, is very important.
Limitations on the reimbursement of phototherapy and other off‐label treatments in the future, may lead to a shift to new on‐label, and much more expensive systemic treatments that have been proven effective in RCTs. The question is whether this is desirable, as not all new treatments are widely available globally. Therefore, our aim is to investigate the effectiveness and safety of phototherapy in the treatment of atopic eczema. With the results, we aim to strengthen existing and evolving guidelines for atopic eczema, and provide meaningful evidence to support treatment decisions. We will also highlight the gaps in evidence in relation to this topic.
Cochrane Skin undertook an extensive prioritisation exercise in 2020 to identify a core portfolio of the most clinically important questions. The topic of phototherapy for eczema was identified as one of the top three titles (Cochrane Skin 2020b). This review is also directly applicable to, and is being conducted to inform the update of the European and American guidelines on the use of phototherapy for atopic eczema.
Objectives
To assess the effects of phototherapy regimens (e.g. narrowband ultraviolet B (NB‐UVB), broadband ultraviolet B (BB‐UVB), psoralen plus ultraviolet A (PUVA), ultraviolet A1 (UVA1)) for people with atopic eczema.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs), including cross‐over trials, and randomised within‐participant trials.
Types of participants
We included studies conducted in participants with atopic eczema of any phenotype and severity. We included participants of all ages with a clinical diagnosis of atopic eczema. The diagnostic criteria could include the Hanifin and Rajka definition (Hanifin 1980), or the UK modification (Williams 1994), or they could have been diagnosed clinically by a healthcare professional, using the terms 'atopic eczema' or 'atopic dermatitis', for example. Studies in children who were described as having ‘eczema’, as opposed to ‘atopic eczema’, were also eligible.
We assessed the distribution of relevant participant characteristics, including severity of atopic eczema, age, and concomitant medications.
We imposed no restrictions on age, sex, or ethnicity of participants.
We excluded studies that included participants with other types of eczema, such as contact dermatitis, seborrhoeic eczema (seborrhoeic dermatitis), varicose eczema, discoid eczema, irritant dermatitis, and hand eczema.
We only included participants with diagnoses, such as 'Besnier's prurigo' or 'neurodermatitis' if there was additional descriptive evidence of atopic eczema in the flexures. We only included studies in which not all participants had atopic eczema if separate results were reported for the participants with atopic eczema.
Types of interventions
Any kind of phototherapy, including the following.
Broadband ultraviolet B (BB‐UVB; 280 nm to 315 nm)
Narrowband UVB (NB‐UVB; 311 nm to 313 nm; i.e. TL‐01)
UVA (315 nm to 400 nm)
UVA1 (340 nm to 400 nm)
Cold‐light UVA1 (containing a cooling system eliminating wavelengths greater than 530 nm)
UVAB (280 nm to 400 nm)
Full‐spectrum light (320 nm to 5000 nm, including UVA, visible, and infrared light)
Saltwater bath plus UVB (balneophototherapy)
Coal tar plus UVB radiation (Goeckerman therapy)
Psoralen plus UVA (PUVA) with oral 8‐methoxypsoralen (8‐MOP)
Psoralen plus UVA (PUVA) with 5‐methoxypsoralen (5‐MOP)
Oral trimethylpsoralen with UVA (PUVA)
Oral khellin plus UV
Topical khellin plus UV
Heliotherapy
Excimer laser
Excimer lamp
For the comparators, we accepted any other type of treatment regimen, namely: any type of phototherapy; systemic treatment (e.g. prednisolone, cyclosporin, methotrexate, azathioprine, biologics); topical treatment (e.g. topical corticosteroids, topical tacrolimus, coal tar); placebo; or no treatment. We included studies in which concomitant medications or co‐interventions were given, as long as the medication regimen was the same in each treatment arm. We included treatment given in any setting, for example clinic‐based or home phototherapy.
In studies where two treatment intervention groups from different categories were compared against a single comparator group, the relevant treatment group and the same comparator group were included in two separate pair‐wise meta‐analyses.
Types of outcome measures
We defined treatment outcomes as short‐term (up to and including 16 weeks after initiating treatment, taking the measurement closest to 12 weeks if outcomes were measured at multiple time points), and long‐term (more than 16 weeks after initiating treatment, taking the longest time point if outcomes were measured at multiple time points). Long‐term control was defined as the closest time point to six months after the end of the course of phototherapy, assessed in the same way as the primary outcome for physician‐assessed and participant‐reported changes in signs and symptoms of atopic eczema. Outcomes of interest in this review were in accordance with the core outcomes (including core outcome instruments) of the Harmonising Outcome Measures for Eczema (HOME) initiative (Schmitt 2014).
We included studies in this review regardless of whether our primary and secondary outcomes were measured.
Primary outcomes
-
Physician‐assessed changes in clinical signs of atopic eczema
Using the following measurement instruments (in hierarchy, starting with the most preferred instrument): EASI (Ricci 2009), Objective SCORAD (or compound SCORAD if objective SCORAD was not reported (Kunz 1997), Costa (Costa 1989), SASSAD (Charman 2002)
-
Patient‐reported changes in symptoms of atopic eczema, including itch
Using the following multi‐item measurement instruments for atopic eczema symptoms (in hierarchy, starting with the most preferred instrument): POEM (Charman 2004), subjective SCORAD; and the following single‐item measurement instruments for itch (in hierarchy, starting with the most preferred instrument): peak numerical rating scale (NRS (Yosipovitch 2019)), average NRS, visual analogue scale (VAS (Reich 2012)), verbal rating scale (VRS(Phan 2012))
Secondary outcomes
Investigator Global Assessment (IGA)
Health‐related quality of life, measured with the (Skindex‐29 (Chren 1996), Dermatology Life Quality Index (DLQI (Finlay 1994)), Children's DLQI (CDLQI (Lewis‐Jones 1995))
Safety (adverse events and tolerability (i.e. withdrawals due to adverse events))
Long‐term control, at the closest time point to six months after the end of the course of phototherapy, assessed in the same way as the primary outcome (e.g. EASI or POEM)
Search methods for identification of studies
We aimed to identify all relevant RCTs, regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
The Cochrane Skin Information Specialist (Liz Doney) searched the following databases, using strategies based on the draft strategy for MEDLINE in our published protocol (Musters 2021).
The Cochrane Skin Specialised Register (searched 13 January 2021, using the search strategy in Appendix 1);
The Cochrane Central Register of Controlled Trials (CENTRAL; 2021, Issue 1) in the Cochrane Library (searched 13 January 2021, using the strategy in Appendix 2);
MEDLINE Ovid (from 1946 to 13 January 2021), using the strategy in Appendix 3;
Embase Ovid (from 1974 to 13 January 2021), using the strategy in Appendix 4.
Trials registers
The Cochrane Skin Information Specialist searched the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 19 January 2021, using the search strategy in Appendix 5). The World Health Organization International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch/) was not available at this time, due to technical issues.
Searching other resources
Searching reference lists
We checked the bibliographies of included studies and any relevant systematic reviews for further references to relevant trials.
Searching by contacting relevant individuals or organisations
We contacted experts and organisations in the field to request additional information on relevant trials (Table 5).
1. Correspondence with investigators.
| Study ID | Correspondence | Response |
| Agrawal 2018 | Email sent 26 May 2021 to purbi1@yahoo.com to request raw dataset (original data). | No reply received |
| Byun 2011 | Email sent 26 May 2021 to entdoctor@cau.ac.kr to request raw dataset (original data). | No reply received |
| Der‐Petrossian 2000 | Email sent 26 May 2021 to manon.der‐petrossian@akh‐wien.ac.at to request raw dataset (original data). | No reply received |
| Dittmar 2001 | Email sent 26 May 2021 to dittmar@haut.ukl.uni‐freiburg.de to request raw dataset (original data). | No reply received |
| NCT02915146 | Email sent 02 March 2021 to r.s.dawe@dundee.ac.uk to confirm study is ongoing. | Reply received 02 March 2021 |
| Gambichler 2009 | Email sent 26 May 2021 to thilo.gambichler@klinikum‐bochum.de to request raw dataset (original data). | Reply received on 26 May 2021: authors are unable to share the raw data of this trial. |
| Granlund 2001 | Email sent 26 May 2021 to hakan.granlund@hus.fi to request raw dataset (original data). | No reply received |
| Heinlin 2011 | Email sent 26 May 2021 to sigrid.karrer@klinik.uni‐regensburg.de to request raw dataset (original data). | No reply received |
| Hoey 2006 | Email sent 26 May 2021 to hoeysusannah@hotmail.com to request raw dataset (original data). | No reply received |
| Keemss 2016 | Email sent 17 Feb 2021 to vvonfelbert@ukaachen.de to clarify inclusion criteria for this study (whether any participants were included with conditions excluded from this systematic review). | No reply received |
| Kromer 2019 | Emails sent 24 Feb 2021 to timo.buhl@meduni‐goettingen.de to clarify if linked to Keemss 2016. | Reply received 24 Feb 2021 |
| Krutmann 1992 | Email sent 26 May 2021 to krutmann@uni‐duesseldorf.de to request raw dataset (original data). | No reply received |
| Krutmann 1998 | Email sent 26 May 2021 to krutmann@uni‐duesseldorf.de to request raw dataset (original data). | No reply received |
| Legat 2003 | Email sent 26 May 2021 to peter.wolf@uni‐graz.at to request raw dataset (original data). | No reply received |
| Leone 1998 | Email sent 26 May 2021 to gleone@ifo.it to request raw dataset (original data). | No reply received |
| Majoie 2009 | Email sent 26 May 2021 to iml.majoie@meandermc.nl to request raw dataset (original data). | No reply received |
| Maul 2017 | Email sent 02 March 2021 to alexander.navarini@usz.ch to request information on atopic dermatitis patients separately. | No reply received |
| Maul 2017 | Email sent 26 May 2021 to alexander.navarini@usz.ch to request raw dataset (original data). | No reply received |
| NCT01402414 | Emails sent 26 January 2021 to s.terras@klinikum‐bochum.de and t.gambichler@klinikum‐bochum.de to clarify if study is eligible for inclusion. | Reply received on 27 Jan 2021 to confirm recruitment was terminated |
| Pacifico 2019 | Email sent 26 May 2021 to alessia.pacifico@gmail.com to request raw dataset (original data). | No reply received |
| Qayyum 2016 | Email sent 19 April 2021 to drsadiaqayyum@hotmail.com to clarify the type of UVB lamps used in the study. | No reply received |
| Qayyum 2016 | Email sent 26 May 2021 to drsadiaqayyum@hotmail.com to request raw dataset (original data). | No reply received |
| Reynolds 2001 | Email sent 26 May 2021 to nick.reynolds@ncl.ac.uk to request raw dataset (original data). | No reply received |
| Tzaneva 2001 | Email sent 26 May 2021 to anislava.tzaneva@meduniwien.ac.at to request raw dataset (original data). | No reply received |
| Tzaneva 2010 | Email sent 26 May 2021 to anislava.tzaneva@meduniwien.ac.at to request raw dataset (original data). | No reply received |
| Tzung 2006 | Email sent 26 May 2021 to tytzung@isca.vghks.gov.tw to request raw dataset (original data). | No reply received |
| Youssef 2020 | Email sent 02 March 2021 to randayoussef@kasralainy.edu.eg and ahmedhm@gmail.com to request further information. | No reply received |
| Youssef 2020 | Email sent 26 May 2021 to vanessahafez@kasralainy.edu.eg to request raw dataset (original data). | Reply received on 27 May 2021: authors are happy to share the raw data of this trial; however, we did not receive it after our reply on 27 May 2021 |
Unpublished literature
We sought information about unpublished or incomplete trials by corresponding with investigators or organisations, or both, known to be involved in previous relevant studies (Table 5).
Correspondence with trialists, experts, and organisations
We contacted original authors for clarification and further data if trial reports were unclear (Table 5).
Adverse effects
We did not perform a separate search for adverse effects of phototherapy interventions used for the treatment of eczema. We only considered adverse effects described in included studies.
Errata and retractions
The Cochrane Skin Information Specialist ran a specific search to identify errata or retractions related to our included studies on 13 July 2021. No relevant retraction statements or errata were retrieved.
Data collection and analysis
We used the software, Covidence, to manage the study selection and Microsoft Excel for the data extraction process (Covidence).
Selection of studies
Two pairs of review authors (SM and AM, and SL and JH) independently screened all identified titles and abstracts using Covidence. We examined the full texts of studies that potentially met the criteria, as well as studies for which abstracts did not provide sufficient information. We resolved disagreements through discussion with a senior review author (PS).
Data extraction and management
Two pairs of review authors (SM and AM, and SL and JH) independently extracted outcome data from the included studies. One review author (JH) entered the characteristics of each study into Review Manager Web, and another reviewer (JH) checked these data for accuracy (RevMan Web 2020). For studies that met the inclusion criteria, we extracted relevant information into evidence tables, using an a priori defined proforma, piloting data extraction on a subset of studies before final extraction. We resolved disagreements through discussion with a senior review author (PS).
We extracted data on methodological quality, participants, interventions, and outcomes of interest, according to the Harmonising Outcome Measures for Eczema (HOME) consensus, from the included studies, using the following data extraction fields.
Author and year of publication
Year and country
Sample size
Study design
Age
Setting (hospital or population‐based)
Type of phototherapy
Length and frequency of treatment
Cumulative doses of UV radiation
Duration of follow‐up
-
Primary outcomes:
Physician‐assessed changes in the clinical signs of atopic eczema
Patient‐reported changes in symptoms of atopic eczema, including itch
-
Secondary outcomes:
Investigator Global Assessment (IGA)
Health‐related quality of life
Safety (adverse events and tolerability (i.e. withdrawals due to adverse events))
Long‐term control, at the closest time point to six months after the end of the course of phototherapy, assessed in the same way as the primary outcome
Translation (yes/no)
Assessment of risk of bias in included studies
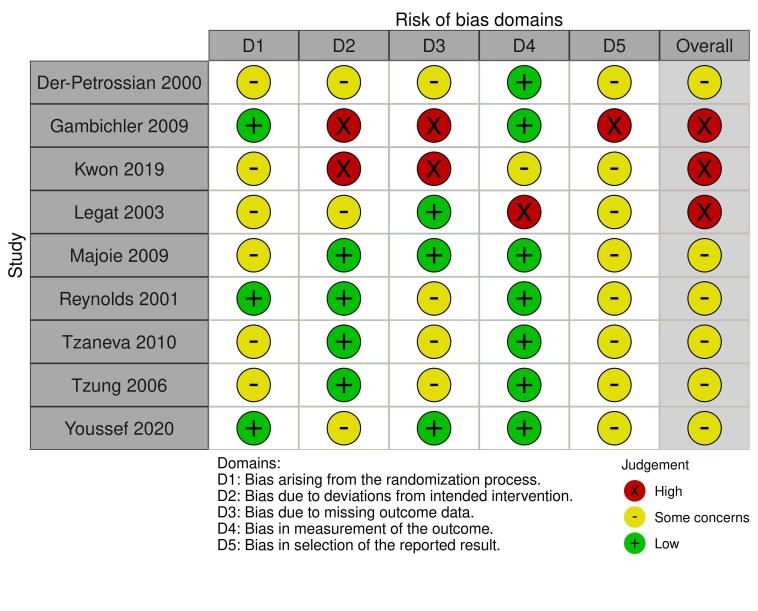
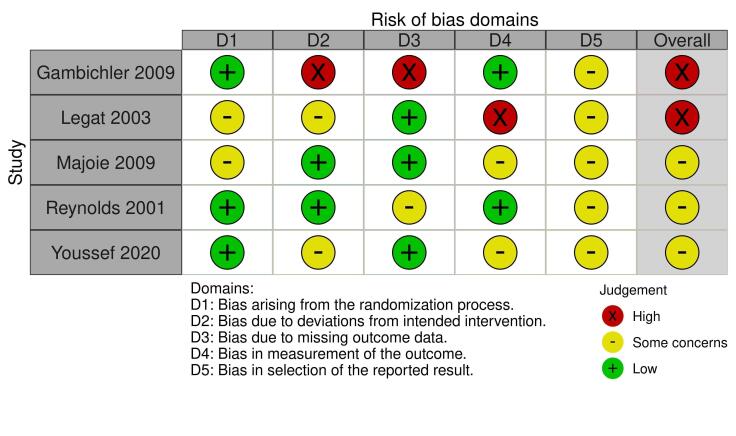
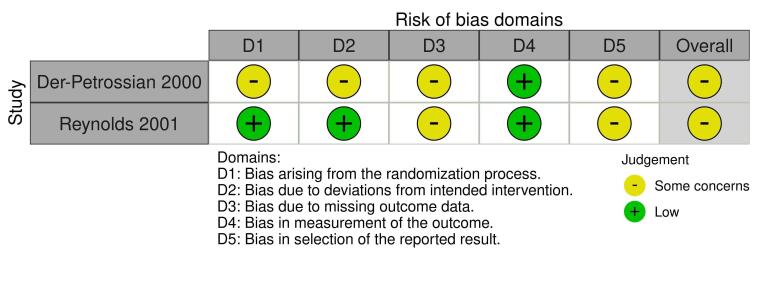
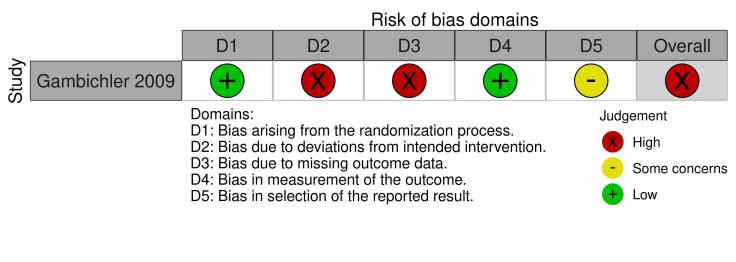
Two review authors (EA and RB) independently assessed the risk of bias for the effect of assignment to the intervention, using the Cochrane RoB 2 tool (Higgins 2020b; Sterne 2019). We only assessed the outcomes in the summary of findings tables (see Summary of findings and assessment of the certainty of the evidence section). We resolved disagreements through discussion. The RoB 2 tool addresses the following domains.
Bias arising from the randomisation process
Bias due to deviations from intended interventions
Bias due to missing outcome data
Bias in measurement of the outcome
Bias in selection of the reported result
We answered a number of signalling questions, which led to the tool algorithm assessing each domain as high risk, low risk, or some concerns. The tool algorithm also calculates an overall risk of bias, as high risk, low risk, or some concerns. To undertake these assessments, we used the RoB 2 Excel Tool. The answers to these signalling questions are available on an online repository.
We did not use the cross‐over variant of the RoB 2 tool, because we only included data from the first phase of cross‐over trials.
Measures of treatment effect
We presented continuous outcomes, where possible, on the original scale reported in each individual study, with a mean change from baseline and its associated standard deviation (SD). We used the standardised mean difference (SMD) as a measure of effect for continuous outcomes that used different scales (e.g. EASI and SCORAD). We calculated risk ratios (RR) for dichotomous outcomes, and presented either the number needed to treat for one additional beneficial outcome (NNTB), or the number needed to treat for one additional harmful outcome (NNTH), when the results, including their measure of variance, fell on the same side of the line of no effect. We calculated odds ratios (OR) for within‐participant studies, and in meta‐analyses in which we combined parallel and within‐participant studies.
If outcome data were reported as 'physician‐assessments of the time needed until skin improvement', we presented these narratively, highlighting the general trend within the groups at the first time point at which an improvement was seen.
We reported all outcome data with their associated 95% confidence intervals (CIs), where possible.
Unit of analysis issues
Cross‐over studies
Unit of analysis issues can arise in studies where participants have been randomised to multiple treatments in multiple periods, or when there has been an inadequate wash‐out period. For cross‐over trials, we used data from the first treatment period, due to concerns with carry‐over effects, unless otherwise stated.
Within‐participant studies
For paired data from studies with no suspicion of contamination across intervention sites, we planned to analyse using the generic inverse‐variance method in Review Manager Web, after accounting for the within‐participant variability (Higgins 2020a). In studies that reported paired data, but did not adjust for the within‐participant variability, we planned to use a McNemar's test with the corresponding P value. However, no such data were available. When paired data were not reported, we performed variance corrections for the within‐participant studies using the Becker‐Balagtas method (Elbourne 2002). We assumed an intra‐class correlation coefficient (ICC) of 0.5 in our calculations.
For dichotomous outcomes, we calculated OR for both study designs (number of participants with the event receiving the intervention, multiplied by the number of participants without the event in the control group, divided by the number of participants with the event receiving the control, multiplied by the number of participants without the event in the intervention group (Higgins 2020a)). A continuity correction of 0.5 was used in the case of zero events (Sweeting 2004). We pooled data from within‐participant studies with data from parallel‐group studies in meta‐analyses using the generic inverse‐variance method, inputting the natural log of the OR.
More than two treatment comparisons
We included multi‐arm trials in the review if at least one arm examined a type of phototherapy for atopic eczema, and completed a separate data extraction for each pair‐wise comparison. We included these studies as pair‐wise comparisons. For future updates, to prevent double‐counts of participants if treatment arms from multi‐arm studies are pooled in more than one meta‐analysis, we will partition them according to the number of comparisons carried out, and analyse them following the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020a).
Dealing with missing data
If data were missing from trials that were carried out less than 10 years ago, we attempted to contact the investigators or sponsors of these studies. We re‐analysed data according to the intention‐to‐treat (ITT) principle whenever possible. For dichotomous outcomes, if study authors had conducted a per‐protocol analysis, and we had concerns about the level of missing data, we attempted to carry out an ITT analysis with imputation, using baseline values for the missing data, after checking the degree of imbalance in the dropouts between the arms, to determine the potential impact of bias (Higgins 2020a). We planned to carry out a per‐protocol analysis instead of an ITT analysis for continuous outcomes.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining the study characteristics, the similarity between the types of participants, interventions, comparisons, and outcomes, as specified in the criteria for included studies. Although a degree of heterogeneity between the studies included in a review is inevitable, we entered them into a meta‐analysis if we could explain the heterogeneity by clinical reasoning, and make a coherent argument for combining the studies. We assessed statistical heterogeneity using the Chi2 test and the I2 statistic. We interpreted the I2 as follows.
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
70% to 100%: considerable heterogeneity
We acknowledge that I2 depends on magnitude and direction of effects, and the strength of the evidence for heterogeneity (e.g. the P values from the Chi2 test). We explored heterogeneity through subgroup and sensitivity analysis. If we could not explain it through these methods, we downgraded the evidence for inconsistency in the GRADE assessments.
Assessment of reporting biases
Had we included a sufficient number of trials (10 or more) that assessed similar effects, we planned to assess publication bias according to the recommendations on testing for funnel plot asymmetry, described in the Cochrane Handbook (Higgins 2020a). If we did identify asymmetry, we planned to assess possible causes and explore these in the discussion section, if appropriate.
Data synthesis
One review author (EA) analysed the data in Review Manager Web, and reported them as specified in the Cochrane Handbook (Higgins 2020a). We carried out data synthesis only if we were able to identify two or more studies that investigated similar treatments, and reported data that could be pooled. We used a random‐effects model to combine the results of individual studies. For comparisons where data synthesis was not feasible, we reported data separately in tables as 'Incomplete data on which further analysis is not possible', and presented them in a narrative summary, where appropriate. If applicable, for synthesis of data and reporting of analyses from multiple studies evaluating similar interventions, we took into consideration individual studies we had categorised at high risk of bias. When results were estimated for individual parallel RCTs with low numbers of events (less than 10 in total), or when the total sample size was less than 30 participants, and we calculated a risk ratio, we reported the proportion of events in each group, together with a P value from a Fisher’s Exact test.
Subgroup analysis and investigation of heterogeneity
Adults versus children
Different Fitzpatrick skin types
participants with HIV/AIDS and atopic eczema
We planned to use the formal Chi2 test for subgroup differences to test for subgroup interactions. We planned to compare subgroups using the analysis option of the 'Test for subgroup differences' in Review Manager Web (RevMan Web 2020).
Sensitivity analysis
We planned to explore reasons for heterogeneity in studies, and if necessary, we planned to perform sensitivity analyses, examining the effects of excluding study subgroups, e.g. those studies for which we had judged the results at high risk of bias, or we had some concerns.
Summary of findings and assessment of the certainty of the evidence
We generated summary of findings (SoF) tables for the most clinically important comparisons of this review:
NB‐UVB versus placebo/no treatment;
NB‐UVB versus UVA1;
NB‐UVB versus PUVA;
UVA1 versus PUVA
UVA1 versus no treatment; and
PUVA versus no treatment
The outcomes selected for inclusion in the SoF tables were:
Physician‐assessed changes in the clinical signs of atopic eczema (AE)
Patient‐reported changes in symptoms of AE including itch
Investigator Global Assessment (IGA);
Health‐related quality of life and
Safety (adverse events and tolerability i.e. withdrawals due to adverse events).
For each outcome result in the summary of findings tables, we assessed the certainty of the body of evidence using the GRADE approach (Schünemann 2013), and GRADEpro GDT software, which identify four levels of certainty (high, moderate, low, and very low). As all studies included in the review were RCTs, the starting level for all assessments was high certainty. We downgraded the level of certainty according to the presence of the following factors: study limitations (risk of bias); indirectness of evidence; unexplained heterogeneity; imprecision of results; and likelihood of publication bias. Two review authors (AM and PS) independently assessed the certainty of the evidence, with any disagreement resolved by discussion, or input from a senior review author (RB).
Results
Description of studies
Results of the search
The database searches (see Electronic searches) retrieved a total of 616 records. We identified an additional three records through other sources (see Searching other resources), giving a total of 619 records. After removing duplicates, we had 613 records to screen. We excluded 514 records based on titles and abstracts. We obtained the full text of the remaining 99 records. We excluded 32 studies, reported in 33 references. We classified four studies (in seven references) as ongoing, and four studies as awaiting classification. We included 32 studies, reported in 55 references. For a further description of our screening process, see the study flow diagram (Figure 1).
1.
Included studies
Please see the Characteristics of included studies tables for or a full description of the studies.
Design
All studies were prospective, randomised controlled trials. Seventeen of the studies were parallel group trials (Agrawal 2018; Byun 2011; Dittmar 2001; Granlund 2001; Heinlin 2011; Hoey 2006; Krutmann 1998; Krutmann 1992; Kwon 2019; Leone 1998; Maul 2017; Pacifico 2019; Qayyum 2016; Reynolds 2001; Von Kobyletzki 1999a; Youssef 2020; Zimmerman 1994). Thirteen of the studies were within‐participant studies (Brenninkmeijer 2010; Der‐Petrossian 2000; Jekler 1988a; Jekler 1988b; Jekler 1990; Jekler 1991; Jekler 1991b Study 1; Jekler 1991b Study 2; Legat 2003; Majoie 2009; Selvaag 2005; Tzaneva 2001; Tzung 2006). There were also two cross‐over trials (Gambichler 2009; Tzaneva 2010).
The trial duration, including active treatment and follow‐up, ranged from 10 days to 1 year; two trials did not mention the total length of follow‐up (Hoey 2006; Maul 2017). The average trial duration was 13 weeks.
Five trials were multicentre (Granlund 2001; Heinlin 2011; Qayyum 2016; Tzaneva 2010; Zimmerman 1994). The rest of the studies were either single centre, or did not mention whether they were single‐ or multicentre.
Setting
All included studies recruited participants from secondary care clinics, the vast majority of which were dermatology outpatient clinics. The studies were conducted in many parts of the world. Nineteen studies were conducted in Europe (UK, Germany, the Netherlands, Finland, Norway, Sweden, Denmark, Switzerland, Austria, and Italy), five in Asia (Pakistan, Taiwan, and Korea), and one in Egypt. Seven studies did not report in which country they were conducted (Der‐Petrossian 2000; Dittmar 2001; Krutmann 1992; Krutmann 1998; Legat 2003; Leone 1998; Von Kobyletzki 1999a).
Participants
Studies recruited participants ranging in age from 5 to 83 years, with a mean age of 28. Nine studies included paediatric < 18‐year‐old participants (Agrawal 2018; Jekler 1988b; Kwon 2019; Leone 1998; Qayyum 2016; Selvaag 2005; Tzung 2006; Youssef 2020; Zimmerman 1994). Five studies did not report the mean age; three of them focused on children, and two had a mix of adults and children of at least 16 years.
Five studies did not provide data on the gender of participants. Based on the studies that did provide data on gender, the number of male and female participants was almost equal (ratio 0.99 males:1 female).
Fitzpatrick skin type was reported by 21 studies. The majority of studies included participants with Fitzpatrick skin type II to IV. Only four studies included participants with skin type I, and only two studies included participants with skin type V or VI. One study evaluated physician‐assessed changes in clinical signs separately for participants with skin type II versus skin type III or IV, and compared a medium dose of UVA1 with a high dose UVA1 (Pacifico 2019).
The duration of atopic eczema was reported by 15/32 included studies; mean or median total disease duration ranged from 1 to 30.3 years.
Baseline atopic eczema severity was reported by all but two of the included studies. Studies used a variety of measurement outcomes to report the disease severity of their included participants. Thirteen studies used the SCORAD; however, only one study reported the use of the compound SCORAD. We assumed that most studies used either the compound or objective SCORAD. The range of (compound) SCORAD scores lies between 0 and 83. Mean or median baseline compound SCORAD of the participants included in these 13 trials ranged from 35 (moderate) to 67 (severe). Other outcome measures that were used to report baseline atopic eczema severity were the SASSAD (one study), Costa (three studies), Leicester sign score (one study), EASI (one study), Investigator Global Assessment (IGA; two studies), visual analogue scale (VAS) for itch (one study), or other (self‐developed) measurement instruments to assess disease severity (nine studies).
None of the included studies reported HIV or AIDs comorbidity.
Sample sizes
A total of 1219 participants were randomised across the 32 RCTs included in this review, sample sizes ranged from 8 to 180 participants, with a mean of 38 participants.
Funding
Overall, 11 studies were funded by research grants, one study was sponsored by the pharmaceutical industry, one was sponsored by primary health insurance companies, two had no funding, and the rest of the studies did not report their source of funding.
Correspondence
We contacted 27 corresponding authors to obtain further information about their studies. For further details, see Table 5.
Interventions
The included studies fell into the following 10 broad phototherapeutic categories: narrowband ultraviolet B (NB‐UVB; 14 RCTS), broadband ultraviolet B (BB‐UVB; 5 RCTS), psoralen plus UVA (PUVA; 2 RCTS), UVA1 (11 RCTS), UVA (3 RCTS), UVB (unspecified; 1 RCT), UVAB (9 RCTS), full spectrum light (1 RCT), excimer laser (1 RCT), and other (Saalmann selective ultraviolet phototherapy lamp (SUP) cabin; 1 RCT). This list includes all types of phototherapy, included in both the intervention and comparator groups.
Comparisons
Sixteen studies included comparisons of phototherapy with other types of phototherapy, seven studies compared different dosages of the same phototherapy, six studies compared phototherapy with no treatment or placebo, three studies compared phototherapy with topical corticosteroid (betamethasone valerate 0.1%, clobetasol proprionate 0.05% fluocortolone 0.5%), one study compared phototherapy with systemic treatments (ciclosporin), two studies compared the same phototherapy in both arms with the addition of a co‐intervention in one arm (balneotherapy one study and pimecrolimus one study). Finally, one study used the same phototherapy in both arms with the addition of different concentrations of salt bath in the two comparator groups.
* We included multi‐arm trials if at least one arm examined a type of phototherapy for atopic eczema, and separate data extraction was carried out for each pair‐wise comparison. We included these multi‐arm studies as pair‐wise comparisons.
1. NB‐UVB
Thirteen RCTs.
NB‐UVB versus no treatment or placebo (Kwon 2019; Reynolds 2001*; Tzung 2006*; Youssef 2020)
Youssef 2020 compared NB‐UVB with 85% glycerol. NB‐UVB was administered three times a week for four weeks in a UV cabin (Waldmann GmbH, Germany), with 16 TL‐01/100W fluorescent lamps producing NB‐UVB with a peak emission at 311 nm. The starting dose was 70% minimal erythema dose (MED), with increments according to erythema response. The 85% glycerol was applied daily to the affected sites for four weeks.
Kwon 2019 compared NB‐UVB against no treatment. Participants were treated with NB‐UVB, administered two to three times a week for six weeks (12 to 18 treatments). The initial dose was 350 mJ/cm2 to 400 mJ/cm2, which was gradually increased to 1,100 mJ/cm2. There was a follow‐up period of three weeks.
Tzung 2006 compared NB‐UVB combined with 1% pimecrolimus cream with 1% pimecrolimus cream alone. One half of the body was randomly selected to also be treated with NB‐UVB twice a day for six weeks. NB‐UVB was delivered using 24 Waldmann TL‐01/100 fluorescent tubes, mounted in a UV 5001 BL cabinet (Waldmann, Villingen‐Schwenningen, Germany). The starting dose was 70% MED with percentage‐based increments every week (to a maximum of 1.5J/cm2). After the six‐week treatment phase, there was a post‐treatment follow‐up of four weeks.
Reynolds 2001 compared NB‐UVB with visible fluorescent light. The NB‐UVB unit contained 40 TL‐100 W/01 lamps (Philips) and participants received a starting dose of 0.4 J/cm2. Percentage‐based increments were made weekly (maximum 1.5 J/cm2, if tolerated). The cumulative dose was 24.8 J/cm2 (range 2.8 to 32.2). The other group received visible fluorescent light through Philips’ 75 to 85 W/96 Northlight fluorescent lamps (fitted into a Sovereign 8‐tube vertical sunbed canopy). The exposure time was increased from 5 to 15 minutes, and participants were turned by 180º halfway through the treatment period. The median cumulative exposure time was 320 min (5 to 345). Participants in both groups were treated twice weekly for 12 weeks.
NB‐UVB versus topical corticosteroid (betamethasone valerate 0.1%) (Agrawal 2018)
Agrawal 2018 compared NB‐UVB, administered three times a week for eight weeks (closed chamber Philips TL‐01), with betamethasone valerate 0.1%, applied twice a day for four weeks. The dose used for the NB‐UVB started at 75% MED, and increased incrementally by 20% each visit, if well tolerated.
NB‐UVB versus UVA1 (Gambichler 2009; Legat 2003; Majoie 2009)
Gambichler 2009 compared NB‐UVB (delivered via a stand‐up cubicle Cosmedico GP‐42 (Cosmedico Medizintechnik GmbH, VS‐Schwenningen, Germany) cabin fitted with ARIMED 311 fluorescent lamps; wavelength 310 nm to 315 nm (peak 311 nm)) with UVA1 (delivered via an air‐conditioned UVA1 bed Sellamed 24000 (Sellamed, Gevelsberg, Germany), wavelength 340 nm to 400 nm). Both were delivered three times a week for six weeks. The initial dose of the NB‐UVB therapy was 70% of MED, determined by a TL‐01 ⁄12W lamp (Philips, Eindhoven, the Netherlands), with 10% to 20% increments, for a maximum dose of 1.2 J/cm2 for skin phototype II, and 1.5 J/cm2 for skin phototypes III and IV. The dose delivered for the UVA treatment was 50 J/cm2.
Majoie 2009 compared NB‐UVB, delivered using a light cabin (Waldmann, Schwenningen, Germany) with 20 311‐nm lamps (TL‐01, Philips, Eindhoven, the Netherlands), with UVA1, delivered using a light cabin (Waldmann, Schwenningen, Germany) with 40 lamps (TL‐10R, Philips), emitting wavelengths of 350 nm to 400 nm only, with a maximum of ± 370 nm. The treatments were given three times a week for up to eight weeks. UVB treatment was started with an initial dose of 70% of the minimal erythemal dose. Subsequent dose increments were given on the basis of erythemic reactions of the skin. The intention was for each exposure to induce slight erythema. If the previous exposure failed to induce any reaction, the dose was increased by 20%. If the resulting erythema was slight, the dose was increased by 10%. Participants received median cumulative doses of 10.5 J/cm2 of NB‐UVB (range 9.9 to 11.5, average increment 10%/exposure) to one body side. The initial dose for the UVA1 treatment was 30 J/cm2. In two steps, the dose was increased to 45 J/cm2. The average dose of UVA1 was more than 40 J/cm2. Participants received median cumulative doses of 930.6 J/cm2 of MD UVA1 (range 717.1 to 1067.4) to the body side treated with UVA1.
Legat 2003 compared NB‐UVB (delivered using a UV 7001 light box (Waldmann Medizinische Technik, Villingen‐Schwenningen, Germany)) with UVA1 (delivered using a Sellas UV‐A1 bench system (Sellamed 24000A; Sellas Medizinische Gerate GmbH, Gevelsberg, Germany)). Both were administered three times a week for up to eight weeks. The starting dose for the NB‐UVB was 70% of the participant's minimal erythema dose, and dose increases were usually 10% to 20%, depending on the erythema response induced by the previous exposure. The NB‐UVB median MED was 0.77 J/cm2, (range 0.55‐1.56 J/cm2). The starting dose for UVA1 irradiation was 10 J/cm2, with 20 J/cm2 applied at the second,30 J/cm2 at the third, and 40 J/cm2 applied at the fourth treatment. At the fifth, and each subsequent treatment, 50 J/cm2 was administered. Participants received a median of 23 treatments (range 12 to 24 treatments), with a mean cumulative dose of 26.7 J/cm2 NB‐UVB (range 15.7 to 59.2 J/cm2), and 1000 J/cm2 UVA1 irradiation (range 500 J/cm2 to 1150 J/cm2).
NB‐UVB versus UVA (Reynolds 2001*)
Reynolds 2001 compared NB‐UVB (using 40 TL‐100 W/01 lamps (Philips)) against UVA (40 fluorescent lamps (Performance 100 W, Philips)). Both treatments were given twice a week. The dosing schedule of NB‐UVB was 0.4 J/cm2, with percentage‐based increments weekly (maximum 1.5 J/cm2, if tolerated). Cumulative dose was 24.8 J/cm2 (range 2.8 to 32.2). The dosing schedule of UVA was a starting dose of 5 J/cm2, increasing to 10 J/cm2, if tolerated, then to a maximum of 15 J/cm2. Cumulative dose of 315 J/cm2 (range 15 to 345). Participants were treated for 12 weeks.
NB‐UVB versus UVAB (Leone 1998; Maul 2017)
Maul 2017 compared NB‐UVB with UVAB. The treatment regimen for NB‐UVB alone, performed with a NB‐UVB light cabin (model UV7001, Waldmann (Waldmann Lichttechnik GmbH, Kuttingen, Switzerland), 310 nm to 315 nm), was NB‐UVB, started at a dose of 0.1 J/cm2, with increments of 20% per session, if there were no side effects, to a maximum of 2.0 J/cm2; three treatment sessions per week for 16 weeks. In the UVAB group, in addition to standard NB‐UVB treatment, UVA was given at a starting dose of 0.5 J/cm2, and increased incrementally by 20%, to a maximum of 5.0 J/cm2. The treatment was performed with a UVA/NB‐UVB light cabin (model UV7002, Waldmann, UVA 320 nm to 410 nm, to a peak of 351 nm; UVB output 310 nm to 315 nm, to a peak of 311 nm).
In two arms of a three‐arm trial, Leone 1998 compared NB‐UVB (using an irradiation bed equipped with 14 TL01/100w tubes) versus UVAB (phototherapy booth with F85/100W UV21 tubes emitting in the UVB, and F85/100W PUVA tubes emitting in the UVA). Participants were treated three times a week. The UVB irradiation protocol (for both narrowband and broadband UVB) was based on the MED: starting at 70% MED, with 40% dose increments after every third treatment, if tolerated, for a total of 10 to 15 treatments in both groups. In the UVAB group, the participants also received the UVA irradiation protocol; the initial dose was 3 J to 4 J (based on skin type) with a 1 J increment after every third treatment, up to a maximum of 10 J.
NB‐UVB versus NB‐UVB with a different dosing regimen (Hoey 2006; Selvaag 2005)
Hoey 2006 compared a standard increasing dose of UVB‐TL01 treatment, with a fixed dose of UVB‐TL01; the length of the study was unclear. In the standard increasing dose group, the first treatment was 70% of MED; subsequent treatments were increased by 20% increments. In the fixed‐dose group, the first treatment was 70% of MED, followed by two subsequent increments; the maximum dose was then used for the remaining treatments. The number of treatments and the maximum dose was not reported in either case.
Selvaag 2005 compared a fixed dose of NB‐UVB with an optimised regimen of UVB, with the dose based on skin reflectance measures. UVB was delivered using a bank of Philips TL‐01 UVB tubes. One standard erythema dose (SED) is 10 mJ/cm2 at 298 nm, using the International Commission on Illumination (CIE) erythema action spectrum, and is equivalent to 1.6 kJ/m2 of the UVB lamp. Skin reflectance measurement was performed on non‐lesional skin on the chest or between shoulder blades, with UV‐Optimize 555 (MaticH, Copenhagen, Denmark). Participants were treated for up to six weeks, three to five times a week.
In the fixed regimen, a starting dose of 1.6 SED was used, with 25% incremental increases with each treatment session. The mean cumulative dose was 124 SED (range 29 to 186). In the optimised regimen group, UVB was administered according to skin reflectance measurements of skin pigmentation and erythema. The mean cumulative dose was 39 SED (16 to 88).
NB‐UVB versus NB‐UVB + pimecrolimus (Tzung 2006*)
Tzung 2006 compared NB‐UVB (delivered using 24 Waldmann TL‐01/100 fluorescent tubes mounted in a UV 5001BL cabinet (Waldmann, Villingen‐Schwenningen, Germany)) with NB‐UVB (delivered in the same way) plus topical pimecrolimus. The whole body was irradiated with NB‐UVB twice a week for six weeks. Only lesions on one side of the body (randomly selected) received a thin film of pimecrolimus 1% cream (Elidel®, Novartis Pharma GmbH, Nuremberg, Germany), twice a day (1 hour after irradiation on days when phototherapy was received). The starting dose of NB‐UVB was 70% MED, with percentage‐based increments every week (to maximum of 1.5J/cm2).
NB‐UVB versus NB‐UVB + synchronous balneotherapy (Heinlin 2011)
Heinlin 2011 compared NB‐UVB (using a Phillips and Okkaido‐Vario‐System Tomesa® Alteglofsheim, Germany; wavelength 311 nm) with NB‐UVB (delivered in the same way) plus synchronous balneotherapy. Both groups received treatments three to five times a week, for up to 35 sessions (approximately 7 to 12 weeks). The starting dose of NB‐UVB was determined according to the individual skin type. All trial physicians were provided with a dose‐escalation schedule for each skin type. The dose per treatment unit was increased by simultaneously prolonging the bathing time. Incremental steps to reach the final dose depended on the participant's skin type and individual acceptance (erythema threshold). Sessions lasted from 15 minutes to 30 minutes, including a bathing time of at least four minutes, before the UV light was started. In the group treated with synchronous balneotherapy, a 10% Dead Sea salt solution (Tomesa®) was delivered in an anatomically shaped bath tub with a computer‐controlled purification system. Turning over every four minutes guaranteed a constant and total covering of the irradiated skin with the solution. In addition, participants moistened their face regularly with salt solution. Mean total light dose received was 34.9 J/cm2. For the group that did not receive balneotherapy, participants lay on a couch placed in the tub instead of bathing. In this group, the mean total light dose received was 34.6 J/cm2.
2. BB‐UVB
Five RCTs.
BB‐UVB versus placebo (Jekler 1988a)
In a split‐body study, Jekler 1988a compared BB‐UVB (delivered using 14 Philips TL 12 40 W and 14 Philips TL 12 20 W tubes arranged in a cubicle; wavelength 280 nm to 315 nm) with visible light (placebo tubes; ordinary daylight tubes — Osram L 36 W/30 — with no measurable UV content). Treatments were given three times a week, for up to eight weeks. For the side that received BB‐UVB, each participant's MED of UVB was determined before the commencement of the phototherapy. The participants were randomised into two treatment groups — one starting with 0.5 MED, and one with 1 MED UVB, randomised to the right or left side of the body. In the 0.5 MED group, the dose was increased by 20% each time, until erythema appeared, at which point, the dose was decreased to half of the last dose given. Thereafter, the 20% increase schedule was resumed. In the 1 MED group, the doses were increased similarly. However, in this group, no dose reduction was made at the appearance of erythema. Instead, the dose was kept unchanged until erythema was no longer seen; after which, the 20% dose increase schedule was resumed. The initial doses were in the range of 20 mJ/cm2 to 153 mJ/cm2; the final doses in the range of 63 mJ/cm2 to 816 mJ/cm2; and the mean total dose was 3.18 J/cm2.
BB‐UVB versus UVA (Jekler 1991)
Jekler 1991 compared BB‐UVB (14 Philips TL 12 40 W and 14 Philips TL 12 20 W tubes arranged in a cubicle) with UVA (delivered using a cubicle containing 24 Philips TL 85/100 W/09 (TL09) fluorescent tubes (Philips, Roosendaal, the Netherlands)). Both arms were treated three times a week for up to eight weeks, or until healing occurred. For the UVB, each participant was phototested before the start of treatment, and the initial dose was set at approximately 80% of the MED. Subsequently, dose increments of 10% to 25% were made at each treatment session. With the appearance of erythema, there was a reduction in the dose of about 10% to 30%. The mean initial dose was 20.8 mJ/cm2 (SD 3.4; the mean final dose was 131 mJ/cm2 (SD 49); and the mean total dose was 1589 mJ/cm2 (SD 534). For the UVA, the initial dose was set at 7, 9, or 11 J/cm2, depending on the participant's skin type and previous experience with solaria. At each subsequent treatment session, the dose was increased in steps of 2 J/cm2, up to a maximum of 15 J/cm2. The mean initial dose was 7.9 J/cm2 (SD 1.4); the mean final dose was 14.3 J/cm2 (SD 1.5); and the mean total dose was 255 J/cm2 (SD 51).
BB‐UVB versus UVAB (Jekler 1990, Jekler 1991b Study 1 )
Jekler 1990 compared BB‐UVB (14 Philips TL 12 40W and 14 Philips TL 12 20 W tubes arranged in a cubicle (Philips, Roosendaal, the Netherlands)) with UVAB (24 Wolff Helarium System tubes B1‐12 100W (Cosmedico, Stuttgart, Germany) in an arrangement similar to that used for UVB therapy). Participants in both arms were treated three times a week for up to eight weeks, or until one body half was deemed to be healed. For the BB‐UVB treatment, the initial dose of UVB was set at 80% of the MED. It was then increased each treatment session by 20%. With the appearance of erythema, the dose was reduced by 50%, and thereafter, the 20% increase schedule was resumed. For the UVAB treatment, a dose increment schedule was set at 5, 7, 10, 12, 15, 17.5, 20, 22.5, and 25 minutes. The dose that preceded the MED was set as the initial dose. The dose was incremented at every other treatment until a maximum of 25 minutes was reached (corresponding to 30 mJ/cm2 UVB, and 8.3 J/cm2 UVA). When erythema appeared, the dose was reduced to the preceding dose. In the treatment of participants with insensitive skin (MED ≥ 15 minutes; 18 mJ/cm2 UVB, 5 J/cm2 UVA), the steps at 17.5 and 22.5 minutes were omitted. With UVB, the mean initial dose was 37 mJ/cm2. The mean final dose was 204 mJ/cm2. The mean total dose was 2.47 J/cm2. With UVAB, the mean initial dose was 13 mJ/cm2 (range 6 mJ/cm2 to 18 mJ/cm2) UVB, and 3.7 J/cm2 (1.7 mJ/cm2 to 5 J/cm2) UVA. The mean final doses were 29 mJ/cm2 (range 18 mJ/cm2 to 30mJ/cm2) UVB, and 8 J/cm2 (range 5 mJ/cm2 to 8.3 J/cm2) UVA. The mean total dose was 0.47 J/cm2 UVB, and 130 J/cm2 UVA.
Jekler 1991b Study 1 compared low dose BB‐UVB with UVAB. The BB‐UVB was administered using 14 Philips TL 12 40W and 14 Philips TL 12 20 W tubes arranged in a cubicle (Philips, Roosendaal, the Netherlands). The UVAB was administered using a cubicle containing 24 Wolff Helarium System tubes B1‐12 100W (Cosmedico, Stuttgart, Germany), or a sunbed containing 20 tubes of the same kind. The wavelengths of the UVA irradiation were 315 nm to 400 nm and the UVB 280 nm to 315 nm. Both treatments were given three times a week for up to eight weeks, or the healing of one body side. A mean of 18.5 (SD 4.4) treatments were given in 7.5 (SD 1.0) weeks. For the low dose UVB, each participant's minimal erythema dose of UVB was determined before the study, and thereafter, every other week. The aim was to give treatment with 20% of the MED. Dose increments were made stepwise, every other week, each time maintaining a dose of 0.2 MED. For the UVAB treatment, a dose increment schedule, depending on the participant's skin type was set up. The initial exposure time of 7 to 10 minutes was subject to an increment every, or every other treatment session of 2 to 5 minutes, to a maximum of 25 minutes (corresponding to 45 mJ/cm2 UVB, and 10.5 J/cm2 UVA). The mean initial BB‐UVB dose was 10mJ/cm2 (SD 3.6), the final dose was 18 mJ/cm2 (SD 7.8), and total (cumulative) dose was 282 mJ/cm2 (SD 152). For the UVAB arm, the mean initial dose was 14 mJ/cm2 (SD 2.2) BB‐UVB, and 3.2 J/cm2 (SD 0.5) UVA: the mean final dose was 41 mJ/cm2 (SD 6.8) BB‐UVB, and 9.5 J/cm2 (SD 1.6) UVA; and the mean total dose was 558 mJ/cm2 (SD 193) BB‐UVB, and 130 J/cm2 (SD 45) UVA.
BB‐UVB versus BB‐UVB with a different dosing regimen (Jekler 1988b)
Jekler 1988b compared two different doses of BB‐UVB, administered using 14 Philips TL 12 40W and 14 Philips TL 12 20 W tubes arranged in a cubicle (wavelength 280 nm to 315 nm) in a split‐body study. Participants were treated three times a week for up to eight weeks, or until one half of the body was healed. The MED was determined every other week on the right and left body halves separately. One side of the body was treated with 0.4 MED, while the other was treated with 0.8 MED. Dose increments were made stepwise, every other week, on the basis of the MED. The initial doses on the 0.4 MED sides were in the range of 7 mJ/cm2 to 36 mJ/cm2; on the 0.8 MED sides, they were 14 mJ/cm2 to 72 mJ/cm2. The final doses were in the range of 20 mJ/cm2 to 77 mJ/cm2 on the 0.4 MED sides, and 51 mJ/cm2 to 173 mJ/cm2, on the 0.8 MED side. The mean total dose for the UVB 0.4 MED group was 0.44 J/cm2, and 1.08 J/cm2 for the 0.8 group
3. PUVA
Two RCTs.
PUVA (8‐methoxypsoralen plus UVA) versus NB‐UVB (Der‐Petrossian 2000)
Der‐Petrossian 2000 compared PUVA (8‐methoxypsoralen plus UVA) with NB‐UVB. This was a within‐participant study; first the participant received narrowband UVB treatment on one side of the body (according to a prior randomisation), then the participant bathed in the 8‐methoxypsoralen (8‐MOP) bath, then the participant received the UVA treatment on the previously unirradiated body half. The treatment was delivered three times a week for up to a maximum of six weeks. The NB‐UVB treatment was delivered using a Waldmann UV 3003 lay‐down irradiation unit (H. Waldmann, Werk fűr Lichttechnik, Schwenningen, Germany) equipped with 15 Philips TL 100W/01 fluorescent tubes. The initial dosage was one MED of NB‐UVB. Subsequent dose increments in both regimens were set to elicit or maintain a slight erythematous reaction. In the absence of erythema, the UV dose was increased by 30% in participants with skin type III, and 15% in participant with skin types I or II. In the presence of erythema, the last dose was maintained. After irradiation with NB‐UVB, the participant bathed in the 8‐MOP (1 mg/L) solution. The participant bathed for 15 minutes in 100 L of tap water at 38 °C. After the bath, the skin was gently dried, and the previously unirradiated body half exposed to UVA (Waldmann PUVA 4000 lay‐down unit equipped with 40 Sylvania FR 90 T 12/PUVA fluorescent tubes). The initial dosage was 0.5 minimum phototoxic dose (MPD) for bath‐PUVA. Subsequent dose increments in both regimens were set to elicit, or maintain a slight erythematous reaction. Owing to delayed erythema formation, the UVA dose was never increased before 96 hours after the last bath‐PUVA exposure. The initial mean doses were: NB‐UVB 235 mJ/cm2, SD ± 55 mJ/cm2; bath‐PUVA 1.0 J/cm2, SD ± 0.7 J/cm2. The final mean single doses were: NB‐UVB 922 mJ/cm2, SD ± 138 mJ/cm2; bath‐PUVA 3.3 J/cm2, SD ± 1.7 J/cm2. The mean cumulative UV doses were: NB‐UVB 14.0 J/cm2, SD ± 3.5 J/cm2; bath‐PUVA 48.3 J/cm2, SD ± 8.7 J/cm2. The mean number of total treatments was 17, SD ± 1.4.
PUVA (5‐methoxypsoralen plus UVA) versus UVA1 (Tzaneva 2010)
Tzaneva 2010 compared PUVA (5‐methoxypsoralen (5‐MOP) plus UVA) administered three times a week over five weeks, with UVA1 treatment administered five times a week over three weeks. The PUVA arm used 5‐MOP treatment in the form of liquid capsules (Geralen®), at a dose of 1.2 mg/kg two hours prior to each irradiation with UVA. The MPD was determined before treatment for all participants in this group. The first dose was 70% of MPD, with no increments in week one. The UVA was increased by 20% in the second week, if there was no erythematous response (by 10% if there was a light reaction), but no fewer than 96 hours after the last increment. UVA treatment was delivered using Waldmann PUVA 7001 units equipped with Waldmann F15 T8 ⁄PUVA tubes (Waldmann, Schwenningen, Germany). The cumulative PUVA dose was 48.1 J/cm2, SD ± 21.8 J/cm2.
UVA1 phototherapy was delivered with a 24 kW Dermalight ultrA1 lay‐down unit (Systems Dr Sellmeier, Gevelsberg Vogelsang, Germany). Prior to UVA1 treatment, the MED was determined. The participants in the UVA1 arm alone were treated with single exposure doses of 70 J/cm2. If this was higher than the erythema threshold dose, treatment was initiated at one MED. The dose in this group was increased (if no erythema) by 10 J/cm2, to a maximum of 70 J/cm2. The cumulative UVA1 dose was 1138.8 J/cm2, SD± 350 J/cm2.
4. UVA1
Seven RCTs.
UVA1 versus topical corticosteroid (fluocortolone 0.5% (Krutmann 1998*))
Krutmann 1998 compared UVA1 (delivered with the UVASUN 30,000 Biomed (Mutzhas, Munich, Germany), filtered to give wavelengths of > 340 nm) with topical corticosteroid. Both treatments were given daily for ten days. The dose of the UVA1 treatment was 130 J/cm2 per body half, with a maximum dose of 1300 J/cm2. To rule out hypersensitivity to UVA1R, all participants in the high‐dose UVA1 group were phototested before receiving phototherapy with increasing doses (0 to 130 J/cm2 UVA1), with a UVASUN 5000 (Mutzhas) irradiation device, which emitted 100% wavelengths greater than 340 nm. Participants in the topical steroid arm applied fluocortolone 0.5% cream or ointment; the participant's entire body was treated with cream or ointment once a day.
UVA1 versus UVAB (Jekler 1991b Study 2; Krutmann 1992; Krutmann 1998*; Von Kobyletzki 1999a*)
Jekler 1991b Study 2 compared UVA1 (delivered using UVASUN 3000 lamp (Mutzhas, Munic, Germany) with a UVA filter eliminating wavelengths shorter than 340 nm) with UVAB (delivered via a cubicle containing 24 Wolff Helarium System tubes B1‐12 100W (Cosmedico, Stuttgart, Germany) or a sunbed containing 20 tubes of the same kind, with wavelengths 315 nm to 400 nm, UVB 280 nm to 315 nm). Both treatments were given five times a week for three weeks, or until clearing of at least one body side (the study was a split‐body study). A mean of 13.0 (SD 2.5) treatments were given in 2.9 (SD 0.42) weeks. For the UVA1 treatment, an initial dose of 10 J/cm2 or 20 J/cm2 UVA was increased by 10 J/cm2 each treatment session, to a final dose of 30 J/cm2. The mean initial dose of UVA was 11 J/cm2 (SD 2.8), mean final dose was 30 J/cm2 (SD 0), and total dose was 361 J/cm2 (SD 75). For the UVAB treatment, depending on the participant's skin type, an initial exposure time of 8 to 14 minutes was determined for UVAB therapy. Dose increments of 2 to 4 minutes were made at each treatment session, to a maximum of 25 minutes. The mean initial dose was 16 mJ/cm2 (SD 3.1) UVB, 3.8 J/cm2 (SD 0.7) UVA; final doses were 43 mJ/cm2 (SD 5.0) UVB, 10.1 J/cm2 (SD 1.2) UVA; and the mean total dosages were 466 mJ/cm2 (SD 119) UVB, and 109 J/cm2 (SD 27.7) UVA.
Krutmann 1992 compared UVA1 with UVAB. The treatment in both groups was administered daily; total number of treatments was 15. The device used to deliver the UVA1 treatment was the UVASUN 30,000 BIOMED (Mutzhas, Munich, F.R.G.) irradiation device. The emission was filtered with UVACRYL (Mutzhas) and UG 1 (Schott Glasswerke, Munich) and consisted exclusively of wavelengths greater than 340 nm. The device used to deliver the UVAB treatment was the Metec Helarium, model 1480 (Metec Helarium, Munich) radiation device, equipped with 20 Wolff Helarium System tubes B1‐12 100W (Cosmedico, Stuttgart, F.R.G.). This delivered wavelengths of 300 nm to 400 nm. The dose for the UVA1 treatment was 130 J/cm2 UVA1 per body half. The total dose for each participant was 1950 J/cm2. To rule out hypersensitivity to UVA light, all participants in the high‐dose UVA1 group were phototested before phototherapy with increasing doses (0 to 130 J/cm2) of UVA1 with a UVASUN 5000 (Mutzhas) irradiation device, which emitted 100% UVA1 light. For the UVAB therapy, the dose preceding the MED for UVB was used as the initial dose. Subsequently, the doses were successively increased, up to a maximum of 30 mJ/cm2 UVB, and 7.5 J/cm2 UVA. If erythema was induced, the preceding dose was used for the next treatment. The mean final dose in the UVAB group was 28 mJ/cm2 UVB, and 7 J/cm2 UVA.
Krutmann 1998 compared UVA1 (delivered with a UVASUN 30,000 Biomed (Mutzhas, Munich, Germany), filtered to give wavelengths of > 340 nm) with UVAB (machine not specified). The total number of treatments in both cases was 10. The UVA1 treatment was administered daily; it was not clear how frequently the UVAB treatment was used. The dose of UVA1 treatments was 130 J/cm2 per body half, with a maximum dosage of 1300 J/cm2. To rule out hypersensitivity to UVA1R, all participants in the high‐dose UVA1 group were phototested before phototherapy with increasing doses (0 to 130 J/cm2 UVA1) with a UVASUN 5000 (Mutzhas) irradiation device, which emitted 100% wavelengths greater than 340 nm. For the UVAB arm, the dose preceding the MED for UVB was used as the initial dose. Doses increased by a maximum of 40 mJ/cm2 UVB, and 7.5 J/cm2 UVA. If erythema occurred, the preceding dose was used for the next treatment. The mean final doses in the UVAB treatment group were 33 mJ/cm2 UVB, and 6.8 J/cm2 UVA.
Von Kobyletzki 1999a compared two forms of UVA1 (one being cold‐light therapy) versus UVAB. The UVA1 was delivered using the Sellas WL 20,000 bed (Systems Dr Sellmeier, Ennepetal, Germany), which produced wavelengths of 340 nm to 400 nm (also scattered radiation higher than 530 nm, including infrared radiation, 780 nm to 3000 nm). The UVA1 cold‐light therapy was delivered with the Photomed CL 300,000 liquid (Photomed, Hamburg, Germany) device. This produced wavelengths of 340 nm to 530 nm. The UVA1 treatments were both administered five times a week for three weeks. The dosing regimen for the UVA1 treatment was 2.3 J/cm2 per minute; the average time to apply 50 J/cm2 was 44 minutes (22 minutes on each side). The dosing regimen for the UVA1 cold light therapy was 1.9 J/cm2 per minute; the average time to apply 50 J/cm2 was 52 minutes (26 minutes each side). With 50 J/cm2 applied 15 times, the participant should receive a cumulative dose of 750 J/cm2.
For the UVAB treatment, 40 fluorescent tubes (UVA – Waldmann F85/100‐PUVA, UVB – Waldmann F85/UV6) arranged in a cubicle (Waldmann, Villingen‐Schwenningen, Germany) were used. UVB treatment was started at 80% of the MED. After each session, the UVB dosage was increased by 20% of the MED, to a maximum of 0.3 J/cm2. UVA was introduced at 2.0 J/cm2, and then increased daily by 1.0 J/cm2, to a maximum single dose of 8.0 J/cm2. When erythema appeared, the UVA and UVB doses were reduced to the preceding dose. Successive dose increments were performed daily for 15 days, under close participant control. The mean final doses were 0.29 J/cm2, SD ± 0.03 for UVB; and 7.9 J/cm2, SD ± 0.4 for UVA.
UVA1 versus UVA1 with a different dosing regimen (Dittmar 2001*; Pacifico 2019; Tzaneva 2001; Von Kobyletzki 1999a*)
Dittmar 2001 compared UVA1 (delivered using the UVA1 24 kW, Sellas/Dr. Honle, Medizintechnik GmbH, Munchen, Germany device) across three different doses (wavelength 340 nm to 430 nm). Participants were treated five times a week for three weeks, and were scheduled to receive 15 treatments. The low‐dose group received a maximum single dose of 20 J/cm2,with a maximum cumulative dose of 300 J/cm2. The medium‐dose group received a maximum single dose of 65 J/cm2, with a maximum cumulative dose of 975 J/cm2.The high‐dose group received one dose of a maximum of 60 J/cm2, one dose of a maximum of 90 J/cm2, and then received a maximum single dose of 130J/cm2 at the remaining 13 sessions. The maximum cumulative dose for the high‐dose group was 1840 J/cm2. The mean cumulative doses were 276 J/cm2 (SD ± 43)in the low‐, 866 J/cm2 (SD ± 152) in the medium‐, and 1759 J/cm2 (SD ± 104)in the high‐dose group.
Pacifico 2019 compared a medium and low dose of UVA1 (administered using a Sellamed 24,000 lay‐down unit (Systems Dr Sellmeier; Gevelsberg‐Vogelsang, Germany)). The high‐dose group received 130 J/cm2 UVA1, while the medium‐dose received 60 J/cm2. The cumulative dose was 1950 J/cm2 in the high‐dose group, and 750 J/cm2 in the medium‐dose group. Both groups were treated five times a week for three weeks.
Tzaneva 2001 also compared high and medium dose UVA1 using the 24 kW Dermalight UltrA1 lay‐down unit (Systems Dr Sellmeier, Gevelsberg‐Vogelsang, Germany) device, which emitted UVA1 light (96.9% 340 nm to 400 nm). The high‐dose group starting dose was the MED, with increments of 10 J/cm2, providing there was no erythemal response (maximum of 130 J/cm2). The medium‐dose group received 50% of the high‐dose regimen. Both treatments were delivered five times a week for three weeks. For the high‐dose UVA1 group, the median final single exposure dose was 120 J/cm2 (range 80 J/cm2 to130 J/cm2), and the median cumulative dose was 1710 J/cm2 (range 1020 J/cm2 to 1950 J/cm2). For the medium‐dose group, the median final single exposure dose was 60 J/cm2 (range 40 J/cm2 to 65 J/cm2), and median cumulative dose was 855 J/cm2 (range 510 J/cm2 to 975 J/cm2; two participants received only 10 exposures).
Von Kobyletzki 1999b compared two forms of UVA1 (one of which was cold‐light therapy). The UVA1 was delivered using the Sellas WL 20,000 bed (Systems Dr Sellmeier, Ennepetal, Germany), which produced wavelengths of 340 nm to 400 nm (also scattered radiation higher than 530 nm, including infrared radiation, 780 nm to 3000 nm). The UVA1 cold‐light therapy was delivered using the Photomed CL 300,000 liquid device (Photomed, Hamburg, Germany). This produced wavelengths of 340 nm to 530 nm. The UVA1 treatments were both administered five times a week for three weeks. The dosing regimen for the UVA1 treatment was 2.3 J/cm2 per minute; the average time to apply 50 J/cm2 was 44 minutes (22 minutes on each side). The dosing regimen for the UVA1 cold light therapy was 1.9 J/cm2 per minute; the average time to apply 50 J/cm2 was 52 minutes (26 minutes each side). With 50 J/cm2 applied 15 times, the participant received a cumulative dose of 750 J/cm2.
5. UVA
One RCT
UVA versus visible fluorescent light (placebo (Reynolds 2001*))
Reynolds 2001 compared UVA (40 fluorescent lamps (Performance 100 W, Philips)) against visible fluorescent light (Philips 75 W to 85 W/96 Northlight fluorescent lamps, fitted into a Sovereign 8‐tube vertical sunbed canopy (Sun Health Services, Crowborough, UK)). Both treatments were given twice a week. The dosing schedule of UVA started at 5 J/cm2, increasing to 10 J/cm2 if tolerated, then to a maximum of 15 J/cm2. The cumulative dose was 315 J/cm2 (range 15 J/cm2 to 345 J/cm2). For the fluorescent light group (placebo), the exposure time was increased from 5 to 15 minutes, and participants turned 180º halfway through the treatment period. The median cumulative exposure time was 320 minutes (5 minutes to 345 minutes). Participants were treated for 12 weeks.
6. UVB (unspecified)
One RCT.
UVB versus UVA (Qayyum 2016)
Qayyum 2016 compared whole body UVB (1.25 mW/cm2, Waldmann 1000) with whole body UVA (4 mW/cm2, Waldmann 1000). The treatments were delivered three times a week until skin cleared, or a maximum of 12 weeks. For the UVB, the starting dose was 75% of MED for the skin type, with 20% increments each visit according to the participant's tolerance. For the UVB, the starting dose 1 J/cm2, with 0.5 J/cm2 increments until response. Mean cumulative dose for UVA was 121 J/cm2; for UVB, it was 8151 mJ/cm2.
7. UVAB
Two RCTs
UVAB versus topical corticosteroid (fluocortolone 0.5% (Krutmann 1998*))
Krutmann 1998 compared UVAB therapy with topical corticosteroid. Participants received topical corticosteroid treatment for ten days, or a total of ten UVA‐UVB exposures. The dose preceding the MED for UVB was used as the initial dose. Doses increased by a maximum of 40 mJ/cm2 UVB, and 7.5 J/cm2 UVA. If erythema occurred, the preceding dose was used for the next treatment. The mean final doses were 33 mJ/cm2 UVB, and 6.8 J/cm2 UVA. Participants in the topical steroid group applied fluocortolone 0.5% cream or ointment; participants' entire bodies were treated with cream or ointment once daily.
UVAB versus ciclosporin (Granlund 2001)
Granlund 2001 compared UVAB (delivered with a Waldmann UV 8001 K phototherapy cabin) with oral ciclosporin. In both groups, treatment was administered intermittently, with a treatment period of eight weeks (treatment phase), followed by a period of only topical treatment (remission phase). Participants received at least 16 treatments per cycle, and could receive multiple cycles over the year during which the study took place. The phototherapy was received two to three times a week. The initial dose depended on the participant's skin type and previous experience with UVAB therapy. Successive dose increments were delivered at every other treatment visit, according to a standard treatment schedule, up to maximum doses of 15 J/cm2 of UVA, and 0.26 J/cm2 of UVB. If remission occurred before the maximum dose was achieved, there were no further dose increments. If erythema appeared, the dose was reduced to the preceding dose. Participants in the ciclosporin group received initial doses of 4 mg/kg/day. During the first two treatment cycles, the dose was either increased or decreased at each scheduled visit, in increments of 1 mg/kg/day, according to response. The lowest dose was 1 mg/kg/day; the maximum dose was 4 mg/kg/day. The second treatment phase was initiated using the lowest effective dose from the first treatment phase. The lowest effective dose in the second cycle was chosen as a constant maintenance dose in subsequent cycles.
8. Full spectrum light
One RCT
Full spectrum light versus no treatment (Byun 2011)
Byun 2011 compared full‐spectrum light (delivered using FSL®, BMC Co. LTD, Anyang‐si, South Korea), which included wavelengths of 320 nm to 5000 nm, with no treatment. Phototherapy was administered twice a week for four weeks (total of eight treatments). The anterior side of the body was irradiated for 20 minutes, then the posterior side of the body for 20 minutes. The fluence of each irradiation was 530 J/cm2, including 121 J/cm2 of UVA, and 409 J/cm2 of visible and infrared light. Participants in the control group applied emollient twice a day, without any other treatment (emollient was also used in the FSL arm).
9. Excimer laser
One RCT
Excimer laser versus topical corticosteroid (clobetasol proprionate 0.05% (Brenninkmeijer 2010))
Brenninkmeijer 2010 compared excimer laser (308 nm xenon chloride excimer laser) with topical corticosteroid (clobetasol proprionate 0.05% ointment (Dermovate, GlaxoSmithKline)). Both treatments were used for 10 weeks. The laser treatment was administered twice a week (20 treatments), while the tropical corticosteroid was used once a day.
10. Other
One RCT
Saalmann SUP cabin (295 nm to 335 nm) + 15% salt solution versus Saalmann SUP cabin (295 nm to 335 nm) + 3% saline solution (Zimmerman 1994)
Zimmerman 1994 compared two strengths of salt solution before irradiation. The intervention group bathed in a 15% salt solution of 35 kg synthetic Dead Sea salt in 220 L water. The control group bathed in a 3% saline solution for 20 minutes prior to irradiation. For both groups, irradiation was carried out in a Saalmann SUP cabin, 295 nm to 335 nm, in increasing time intervals and doses, according to the photosensitivity of the skin and manufacturer's recommendations, over four weeks.
Outcomes
Thirty out of 32 included trials (94%) measured our primary outcome of physician‐assessed changes in clinical signs of atopic eczema, and 15 trials (47%) measured our primary outcome of patient‐reported changes in symptoms of atopic eczema, including itch. Of the secondary outcomes, eight trials (25%) measured Investigator Global Assessment (IGA), and three trials (9%) measured health‐related quality of life. Eighteen trials (56%) reported data on safety (adverse events and tolerability (i.e. withdrawals due to adverse events)). Long‐term control, measured at the closest time point to six months after the end of the course of phototherapy was reported (assessed in the same way as the primary outcome) in four trials (13%).
Excluded studies
We excluded 32 studies due to: wrong study design (25), wrong population (4), trial terminated with no data available (1), wrong indication (1), and wrong comparator (1). More details about the excluded studies are listed in the Characteristics of excluded studies tables.
Studies awaiting classification
Four trials are still waiting for classification. For these studies, only the study title or abstract was available, and we were unable to get access to the full papers. Hannuksela 1985 involved ultraviolet light therapy; however, there was insufficient information to confirm whether the study followed a randomised controlled trial design. Kim 2012 compared the StoneTouch® far‐infrared device to a sham device in a randomised controlled trial; however, there was insufficient information in the abstract alone to judge if the study was appropriate for inclusion. Potapenko 2000 looked at photo‐oxidised psoralen; however, no other information was available, and it was unclear if it followed a randomised controlled trial design. Pullman 1985 compared two UVA regimens; however, it was unclear if it followed a randomised controlled design. Limited further details can be found in the Characteristics of studies awaiting classification tables.
Ongoing studies
We identified four ongoing studies. These studies had no available data to include in this review. ACTRN12620000546954 is comparing NB‐UVB therapy to natural sunlight with an amino acid lecithin cream, and appears to be a randomised controlled trial; however, this must be confirmed. Droitcourt 2019 is a randomised, controlled cross‐over trial of phototherapy combined with vitamin D supplementation. Kromer 2019 is a randomised controlled three‐arm trial of 415 nm versus 450 nm blue light compared to a non‐therapeutically active dose of 450 nm blue light. NCT02915146 is a randomised controlled trial of NB‐UVB combined with UVA1 versus NB‐UVB monotherapy. Please see the Characteristics of ongoing studies tables for more details.
Risk of bias in included studies
EA and RB independently assessed the risk of bias, using the Cochrane RoB 2 tool (Higgins 2020b; Sterne 2019; ). The results‐level RoB 2 tables are located in the risk of bias section of the characteristics of studies section and in Table 6; Table 7; and Table 8, which also include domain judgements and support for judgement. Figure 2; Figure 3; Figure 4; Figure 5; and Figure 6 show graphical summaries for each outcome.
2. RoB 2 assessments of narrative data (not included in a forest plot) — NB‐UVB versus placebo/no treatment.
| Study | Outcome | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | ||
| Kwon 2019 | Physician‐assessed changes in clinical signs (EASI, short‐term, week 6 and 9) | Some concerns | Quote. "Randomization was performed using random
tables."
Comment: not clear if allocation was
concealed. Quote. "No significant difference was observed in age, TISS, and EASI score between the 2 groups." ... "Of the 18 subjects, 13 and 5 subjects were randomly allocated to the NBUVB + TCS and TCS groups," Comment. no differences between groups, but it's not clear why the groups were so uneven in numbers ‐ only five participants in TCS group, which seems odd. |
High | Comment. There was no mention of blinding, but it's
unlikely they were, as one group received NB‐UVB while
the other group did not, and there was no mention of any
kind of dummy treatment. Assume people delivering the
intervention were also not blinded to treatment
allocation. No mention of any deviations from intended
intervention. Quote. "The other 2 subjects dropped out due to aggravation of symptoms and consequent treatment change. No significant side effects occurred in either group. The subjects who dropped out were excluded from data analysis." Comment. the two participants who changed treatments were excluded from the analysis. This equals more than 10% so likely to have impact on the results |
High | Quote. "All 5 subjects in the TCS group completed the study. However, only 6 out of 13 subjects in the NBUVB + TCS group finished the study. The 5 subjects in the NBUVB + TCS group were lost to follow‐up. The other 2 subjects dropped out due to aggravation of symptoms and consequent treatment change." Comment: a large number of dropouts/ exclusions (54%) in NB‐UVB group. No sensitivity analysis to explore impact of missing data. Two participants were excluded due to treatment failure — the other 5 participants were lost to follow‐up, and potentially could be for similar reasons. | Some concerns | Quote. "Overall eczema severity was evaluated using Eczema Area and Severity Index (EASI) at week 0 (baseline), week 3, week 6 (end of treatment), and week 9 (3 weeks after discontinuation of treatment) by 2 dermatologists" Comment: the outcome measured is the recommended instrument by HOME (core outcome set) Comment: unlikely to differ across groups. No mention of outcome assessment being blinded, and only one group received phototherapy. No differences seen between groups in this outcome so unlikely that knowledge of the intervention influenced assessment. | Some concerns | Comment: no protocol or analysis plan available | High | Comment: high risk in two domains, some concerns in the other domains |
| Tzung 2006 | Physician‐assessed changes in clinical signs (EASI, short‐term, week 6) | Some concerns | Quote. "Patients were randomized to treatment with a thin film of 1% pimecrolimus cream (Elidel®, Novartis Pharma GmbH, Nuremberg, Germany) twice daily on all skin lesions and one half of the body was chosen at random to be treated with nUVB twice weekly for 6 weeks. The other half of the body was shielded from irradiation with tailored UV‐filtering clothing" Comment: sides of the body were randomised but no details of sequence and if allocation was concealed. No details regarding baseline differences. | Some concerns | Quote. "This was a single‐centre, prospective,
randomized, investigator‐blind, bilateral comparison
study approved by the local ethics and pharmacy
committee."
Comment: no mention of blinding, but
one side of body received NB‐UVB while the other side
didn't, and there is no mention of a dummy treatment.
Investigators were blinded, but carers (parents of the
children) would likely know which side of the body
received each treatment. No mention of any deviations
from the intended intervention. Quote. "We compared the clinical efficacy of monotherapy with either twice daily topical 1% pimecrolimus cream or twice weekly narrowband UVB, and combination therapy in 26 children and adolescents with moderate to severe atopic dermatitis in a half‐side manner for 6 weeks." Comment: there isn't a clear description of the number of participants included in the analysis. However, it is likely the two dropouts were from group B of the study (which is not included for this comparison); hence, all participants were probably analysed. |
Some concerns | Comment: there isn't a clear description of how many participants were included in the analysis. No sensitivity analysis or reasons given for dropout/exclusion. However, it is likely the two dropouts were from group B of the study (which is not included for this comparison); hence, all participants were probably analysed. | Low | Quote. "The primary outcome measure was the change of
EASI scores."
Comment: EASI used to assess
outcome, and this is the recommended instrument from
HOME (core outcome set). Measurement unlikely to differ
across groups. Quote. "The evaluation was performed by the same blinded investigator at week 0 (baseline), 1, 2, 4, 6, and post‐treatment week 2 and 4 with the aid of a set of reference photographs whose severity had been agreed among the investigators." Comment: outcome assessment was blinded |
Some concerns | Comment: no protocol or analysis plan available | Some concerns | Comment: some concerns in three domains, low risk in the other domains |
| Youssef 2020 | Physician‐assessed changes in clinical signs (SCORAD, short‐term, week 4) | Low | Quote. "Patients were randomized into one of two
interventional arms
(A or B) based on a
computer‐generated list in blocks of five". "Sealed
opaque envelopes" (from clinical trial
register)
Comment: randomisation via computer and
allocation concealed Quote. "As shown in Table 5, comparative BL characteristics of patients in both interventional groups were homogenous as regards clinical and laboratory parameters." Comment: baseline characteristics shown in table 1; nothing to suggest problems with randomisation. |
Some concerns | Quote. "This study was designed as a randomized,
controlled, parallel group, single‐blinded clinical
trial with two interventional arms." "For determination
of clinical efficacy, the SCORAD score was calculated at
BL and EOT by one non‐blinded and two
blinded
investigators, and the mean was
calculated."
Comment: participants were not
blinded to treatment allocation. Not all investigators
were blinded. There doesn't seem to be any deviations
from intended intervention. Quote. "Data for analysis of treatment success were analyzed on intention‐to‐treat basis." Comment. However, SCORAD was only actually reported for 13/15 and 12/15, and 1 participant from each group was missing due to an adverse event, and they should have been included. Less than 10% were excluded so unlikely to have a substantial impact. |
Low | Comment: according to figure 1, 2 participants were not available for follow‐up in NB‐UVB group and 3 participants in glycerol group. No sensitivity analysis used to explore missing data. One participant in each group discontinued due to adverse events. Two lost to follow‐up due to non‐compliance in glycerol group and 1 lost to follow‐up due to not being able to attend clinic. Small number of dropouts and similar across groups, so unlikely to have impact on results. | Low | Quote. "Primary outcomes were defined as: (i) clinical
effectiveness as
assessed by reduction of
SCORAD"
Comment: SCORAD is commonly used to assess
this outcome. Measurement unlikely to differ across
groups. Quote. "For determination of clinical efficacy, the SCORAD score was calculated at BL and EOT by one non‐blinded and two blinded investigators, and the mean was calculated." Comment: one investigator knew treatment allocation, but the other two did not. |
Some concerns | Comment: the trial was registered on Pan African Clinical Trials Registry (PACTR201810815694251), but there are no details about analysis plan. Clinical improvement is stated as an outcome on the registry, and SCORAD is mentioned for inclusion criterion, but the outcomes to be evaluated, time points, etc. are not stated in the registration. | Some concerns | Comment: some concerns in two domains, low risk of bias in other domains |
| Youssef 2020 | Patient‐reported changes in symptoms (itch measured on VAS, short‐term, week 4) |
Low | Quote. "Patients were randomized into one of two
interventional arms
(A or B) based on a
computer‐generated list in blocks of five."... "Sealed
opaque envelopes" (from clinical trial
register)
Comment: randomisation via computer and
allocation concealed. Quote. "As shown in Table 5, comparative BL characteristics of patients in both interventional groups were homogenous as regards clinical and laboratory parameters." Comment: baseline characteristics shown in table 1 and nothing to suggest problems with randomisation. |
Some concerns | Quote. "This study was designed as a randomized,
controlled, parallel group,
single‐blinded
clinical trial with two interventional arms."... "For
determination of clinical efficacy, the SCORAD score
was
calculated at BL and EOT by one non‐blinded
and two blinded investigators, and the mean was
calculated."
Comment: participants were not
blinded to treatment allocation. Not all investigators
were blinded. There doesn't seem to be any deviation
from intended intervention. Quote. "Data for analysis of treatment success were analyzed on intention‐to‐treat basis." Comment. However, itch score was only actually reported for 13/15 and 12/15, and 1 participant from each group was missing due to an adverse event, and they should have been included. Less than 10% were excluded so unlikely to have a substantial impact. |
Low | Comment: according to figure 1, 2 participants were not available for follow‐up in NB‐UVB group and 3 participants in glycerol group. No sensitivity analysis used to explore missing data. One participant in each group discontinued due to adverse event. Two lost to follow‐up due to non‐compliance in glycerol group and 1 lost to follow‐up due to not being able to attend clinic. Small number of dropouts and similar across groups so unlikely to have impact on results. | Some concerns | Comment: assume VAS itch is part of SCORAD, which is
commonly used to assess this outcome. Measurement
unlikely to differ across groups. Quote. "For determination of clinical efficacy, the SCORAD score was calculated at BL and EOT by one non‐blinded and two blinded investigators, and the mean was calculated." Comment: one investigator knew treatment allocation but the other two did not. However, itch would be assessed by participants, and they knew treatment allocation. Reduction in itch scores was similar across groups, so unlikely to be influence by knowledge of intervention. |
Some concerns | Comment: the trial was registered on Pan African Clinical Trials Registry (PACTR201810815694251) but there are no details about analysis plan. Instrument (SCORAD) and reference to measuring itch, and time points are given in trial register and correspond with report. | Some concerns | Comment: some concerns in three domains, low risk in other domains |
| Kwon 2019 | Safety: withdrawal due to adverse events (short‐term, up to week 9) |
Some concerns | Quote."Randomization was performed using random
tables."
Comment: not clear if allocation was
concealed. Quote."No significant difference was observed in age, TISS, and EASI score between the 2 groups." ... "Of the 18 subjects, 13 and 5 subjects were randomly allocated to the NBUVB + TCS and TCS groups," Comment: no differences between groups, but it's not clear why the groups were so uneven in numbers ‐ only five participants in TCS group seems odd. |
Low | Comment: there was no mention of blinding, but it's
unlikely there was, as one group received NB‐UVB while
the other group did not, and there was no mention of any
kind of dummy treatment. Assume people delivering the
intervention were also not blinded to treatment
allocation. No mention of any deviations from intended
interventions. Quote. "The other 2 subjects dropped out due to aggravation of symptoms and consequent treatment change. No significant side effects occurred in either group. The subjects who dropped out were excluded from data analysis." Comment: they were still included in the analysis of adverse events |
High | Quote. "All 5 subjects in the TCS group completed the study. However, only 6 out of 13 subjects in the NBUVB + TCS group finished the study. The 5 subjects in the NBUVB + TCS group were lost to follow‐up. The other 2 subjects dropped out due to aggravation of symptoms and consequent treatment change." Comment: large number of dropout/exclusions in NB‐UVB group. No sensitivity analysis to explore impact of missing data. Two participants were excluded due to treatment failure — the other 5 participants were lost to follow‐up. Large proportion of dropouts and asymmetrical dropout suggest a serious issue with attrition, and they may well have dropped out due to adverse effects of treatment, without this being recorded. | Some concerns | Comment: no mention of how adverse events were monitored. It's very likely participants knew which treatment they were receiving. However, no significant adverse events were reported, so it's unlikely that knowledge of intervention influenced this outcome. | Some concerns | Comment: no protocol or analysis plan available, therefore, no information available to make a judgement | High | Comment: high risk in one domain, some concerns in three domains, and low risk in one domain |
| Reynolds 2001 | Safety: withdrawal due to adverse events (short‐term, up to week 12) |
Low | Quote. "Individuals were randomly assigned narrowband UVB, broadband UVA, or visible fluorescent light by means of the Minim computer program (version 1.5), by one investigator (VF) who was not involved with assessment of patients" Comment: randomisation method described and allocation was likely concealed. Baseline characteristics presented in table 1 and look similar across groups. | Low | Quote. "We designed a randomised, controlled,
double‐blind trial to assess efficacy of narrowband UVB
and broadband UVA (as used, for example,
in
psoralen photochemotherapy) as second‐line, adjunctive
treatment in adult patients with moderate to severe
atopic eczema." ... "Some patients might also have
worked out which treatment they were receiving because
of differences between exposure units or markings on
lamps, although the markings were technical in
nature"
Comment: says double‐blind but doesn't
specify who is blinded. However, the comment in the
discussion suggests participants were blinded to
treatment group but may have guessed due to units or
markings on lamps. Although, they acknowledge this is
technical in nature so perhaps unlikely. Assume the
people delivering the intervention weren't blinded, as
they would know what the units and markings on the lamps
meant. There is nothing to suggest there were any
deviations from the intended intervention. Quote. "Of the 69 patients who began phototherapy, nine were excluded from analysis because of insufficient follow‐up data. Thus, 60 patients were analysed on an intention‐to‐treat basis." Comment: they used a modified ITT approach, as there were exclusions due to insufficient follow‐up, but these were similar between groups. Participants were analysed in the group to which they were randomised. |
Some concerns | Quote. "Of the 69 patients who began phototherapy, nine were excluded from analysis because of insufficient follow‐up data. Thus, 60 patients were analysed on an intention‐to treat basis." Comment: a further 5 from UVB group and 4 from light group withdrew (no reasons given) but were included in the ITT analysis. No sensitivity or other analyses done to investigate risk of bias. Withdrawal reasons not given (other than adverse events), but rates were similar across groups. | Low | Quote. "We recorded adverse events."
Comment:
limited details, but assume adverse events were reported
by participants. We did not include exacerbation of
eczema in this outcome, as it's considered more related
to lack of efficacy or non‐adherence to treatment.
Measurement unlikely to differ between groups. Quote. "Some patients might also have worked out which treatment they were receiving because of differences between exposure units or markings on lamps, although the markings were technical in nature." Comment: participants did not know which treatment they were receiving, however they might have guessed. |
Some concerns | Comment: no protocol available but withdrawal due to adverse events were reported during study; no analysis was performed | Some concerns | Some concerns in two domains, low risk in the other domains |
| Youssef 2020 | Safety: withdrawal due to adverse events (short‐term, up to week 8) |
Low | Quote. "Patients were randomized into one of two
interventional arms (A or B) based on a
computer‐generated list in blocks of five" ... "Sealed
opaque envelopes" (from clinical trial
register)
Comment: randomisation via computer and
allocation concealed. Quote: "As shown in Table 5, comparative BL characteristics of patients in both interventional groups were homogenous as regards clinical and laboratory parameters." Comment: baseline characteristics in table 1 and nothing to suggest problems with randomisation. |
Low | Quote. "This study was designed as a randomized,
controlled, parallel group,
single‐blinded
clinical trial with two interventional arms." ... "For
determination of clinical efficacy, the SCORAD score
was
calculated at BL and EOT by one non‐blinded
and two blinded
investigators, and the mean was
calculated."
Comment: participants were not
blinded to treatment allocation. Not all investigators
were blinded. There doesn't seem to be any deviation
from intended intervention. Quote. "Data for analysis of treatment success were analyzed on intention‐to‐treat basis." Comment: participants were analysed in the groups to which they were assigned, and participants who withdrew due to adverse events were obviously included here. |
Low | Comment: according to figure 1, 2 participants were not available for follow‐up in NB‐UVB group and 3 participants in glycerol group. No sensitivity analysis used to explore missing data. One participant in each group discontinued due to adverse events. Two lost to follow‐up due to non‐compliance in glycerol group and 1 lost to follow‐up due to not being able to attend clinic. Small number of dropouts and similar across groups, so unlikely to have impact on results. | Some concerns | Quote. "Patients were followed up for side effects and flares. Patients were excluded from the study if they developed phototoxic reactions to NB‐UVB, irritant contact dermatitis to glycerol, or uncontrollable flare of AD. Other adverse events and skin infections were monitored and recorded." Comment: common adverse events monitored and recorded. Measurement unlikely to differ across groups. Participants were not blinded to treatment allocation. Withdrawal rates (1 in each group) the same across groups, so unlikely that this outcome was influenced by knowledge of treatment. | Some concerns | Comment: the trial was registered on Pan African Clinical Trials Registry (PACTR201810815694251) but there are no details about analysis plan. Adverse events not mentioned in trial register. | Some concerns | Some concerns in two domains, low risk in other domains |
BL: baseline; EASI: Eczema Area and Severity Index; EOT: end of treatment; HOME: Harmonising Outcome Measures for Eczema; ITT: intention‐to‐treat; nUVB/NB‐UVB: narrowband UVB; SCORAD: SCORing Atopic Dermatitis; TISS: Three Item Severity Score; TCS: topical corticosteroids; UV: ultraviolet; UVA: ultraviolet A; UVB: ultraviolet B; VAS: Visual Analogue Scale.
3. RoB 2 assessments of narrative data (not included in a forest plot) — NB‐UVB versus UVA1.
| Study | Outcome | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | ||
| Legat 2003 | Physician‐assessed changes in clinical signs: Costa and Leicester scales at weeks 4 to 8 (median 7 weeks) | Some concerns | Quote. "The NB‐UVB and UVA1 treatments were randomly assigned to the body halves of each patient" Comment: the word randomly is used, but no further information about whether allocation sequence was concealed. No information about whether there were baseline differences | Some concerns | Comment: no mention of blinding; there is nothing to
suggest there were deviations from the intended
protocol, but limited information given in trial
report. Quote: "Moreover, in 2 patients, the half‐side treatment had to be terminated after 4 and 6 weeks, respectively, because in these patients, the score values obtained from the NB‐UVB treatment body halves were more than 30% lower than those obtained from UVA1 body halves" Comment: appears everyone was analysed according to the treatment they received. Two patients were terminated, and it seems the reasons was appropriate, but not sure if they were included in final analysis (assume they were, as treatment was up to 8 weeks, and the table indicates all 9 were included in the results at the end of therapy). |
Low | Comment: 2 participants had treatment terminated at 4 and 6 weeks, but assume they were included in analysis (as 9 patients referred to in table, and results given at end of therapy, which could be up to 8 weeks). | High | Comment: the Leicester score and Costa score were used, which assess diagnostic features of atopic dermatis and likely to be appropriate for this outcome. Measurements unlikely to differ across groups, but there is no mention of blinding outcome assessment. There is no evidence to suggest the outcome was heavily influenced by knowledge of the intervention, but there is not enough information given to make a judgement. | Some concerns | Comment: no protocol available | High | High risk in one domain, some concerns in three domains, and low risk in one domain |
| Majoie 2009 | Physician‐assessed changes in clinical signs: Leicester sign score weeks 8 and 12 |
Some concerns | Quote: "The study was done in a randomized,
investigator‐
blinded, and half‐sided comparison
design"
Comment: randomised study, but no
information on sequence and whether allocation was
likely concealed. Quote: "Baseline characteristics were same for both body sides before half‐sided phototherapy" Comment: nothing to suggest differences in baseline characteristics due to inadequate randomisation |
Low | Quote: "The study was done in a randomized, investigator‐blinded, and half‐sided comparison design." Comment: they don't explicitly state whether participants were blinded. No deviations from intended intervention identified. It appears that everyone was analysed according to treatment received. | Low | Quote. "All patients completed the trial." Comment: no missing data | Low | Quote. "Severity of the eczema was evaluated by the Leicester sign score (LSS; range 0 to 108) by a blinded investigator. Severity is scored by 6 clinical features (erythema, purulence, excoriation or crusting, dryness or scaling, cracking or fissuring, and lichenification), graded at 6 defined body sites on a scale of 0 (none) to 3 (severe)." Comment: it is likely to be an appropriate measure and unlikely to differ between groups. Outcome assessment was blinded. | Some concerns | Comment: no protocol available | Some concerns | Some concerns in two domains, low risk in the other domains |
| Legat 2003 | Patient‐reported changes in symptoms: VAS measures of skin lesions, pruritus, and overall therapeutic effect. Weeks 4 to 8 (median of 7 weeks) | Some concerns | Quote. "The NB‐UVB and UVA1 treatments were randomly assigned to the body halves of each patient" Comment: the word randomly is used, but no further information about whether allocation sequence was concealed. No information about whether there were baseline differences | Some concerns | Comment: no mention of blinding; there is nothing to
suggest there were deviations from the intended
protocol, but limited information given in trial
report Quote. "Moreover, in 2 patients, the half‐side treatment had to be terminated after 4 and 6 weeks, respectively, because in these patients, the score values obtained from the NB‐UVB treatment body halves were more than 30% lower than those obtained from UV‐1 body halves" Comment: it appears that everyone was analysed according to the treatment they received. Two patients were terminated, and it seems the reason was appropriate, but not sure if they were included in final analysis (assume they were as treatment was up to 8 weeks, and the table indicates all 9 were included in the results at the end of therapy). |
Low | Comment: 2 participants had treatment terminated at 4 and 6 weeks, but assume they were included in analysis (as 9 patients referred to in table and results given at end of therapy, which could be up to 8 weeks). | High | Comment: self‐reported VAS of pruritus (itch) was used to assess participant's report of itch, and likely to be appropriate for this outcome. Measurement unlikely to differ across groups. However, there is no mention of blinding. There is no evidence to suggest the outcome was heavily influenced by knowledge of the intervention. However, there is not enough information given to make a judgement. | Some concerns | Comment: no protocol available | High | High risk in one domain, some concerns in three domains and low risk in one domain |
| Majoie 2009 | Patient‐reported changes in symptoms: itch/pruritis measured on VAS at weeks 8 and 12 | Some concerns | Quote: "The study was done in a randomized,
investigator‐
blinded, and half‐sided comparison
design"
Comment: randomised study but no
information on sequence and whether allocation was
likely concealed. Quote: "Baseline characteristics were same for both body sides before half‐sided phototherapy" Comment: nothing to suggest differences in baseline characteristics due to inadequate randomisation. |
Low | Quote: "The study was done in a randomized, investigator‐blinded, and half‐sided comparison design." Comment: only investigators were blinded. No deviations from intended intervention identified. It appears that everyone was analysed according to treatment received. | Low | Quote. "All patients completed the trial." Comment: no missing data | Some concerns | Quote. "Patients were asked to complete a visual analog scale (VAS) for pruritus, where the level of their itch is reflected on a scale of 0 to 10 (0 = no itch and 10 = most intense itch imaginable). Comment: likely to be an appropriate measure and unlikely to differ between groups. It's not explicitly stated whether participants were blinded to treatment. If participants were not blinded, then they could potentially have favoured one intervention over the other. But since there are 2 active interventions, then it's perhaps unlikely knowledge of intervention influenced the outcome by much | Some concerns | Comment: no protocol available | Some concerns | Some concerns in three domains and low risk in other domains |
| Majoie 2009 | Safety: withdrawal due to adverse events | Some concerns | Quote: "The study was done in a randomized,
investigator‐
blinded, and half‐sided comparison
design"
Comment: randomised study but no
information on sequence and whether allocation was
likely concealed. Quote: "Baseline characteristics were same for both body sides before half‐sided phototherapy" Comment: nothing to suggest differences in baseline characteristics due to inadequate randomisation |
Low | Quote: "The study was done in a randomized, investigator‐blinded, and half‐sided comparison design." Comment: only investigators were blinded. No deviations from intended intervention identified. It appears everyone was analysed according to treatment received. | Low | Quote. "All patients completed the trial." Comment: no missing data | Some concerns | Quote. "Patients were asked to complete a visual analog scale (VAS) for pruritus, where the level of their itch is reflected on a scale of 0 to 10 (0 = no itch and 10 = most intense itch imaginable). Comment: likely to be an appropriate measure. Unlikely to differ between groups. Not explicitly stated whether participants were blinded to treatment. If participants were not blinded, then they could potentially have favoured one intervention over the other. But since there are 2 active interventions, then it's perhaps unlikely knowledge of intervention influenced the outcome very much. | Some concerns | Comment: no protocol available | Some concerns | Some concerns in three domains and low risk in two domains |
LSS: Leicester sign score; NB‐UVB: narrowband UVB; UVA1: ultraviolet A1; VAS: Visual Analogue Scale.
4. RoB 2 assessments of narrative data (not included in a forest plot) — NB‐UVB versus PUVA.
| Study | Outcome | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | ||||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | ||
| Der‐Petrossian 2000 | Physician‐assessed changes in clinical signs Modified SCORAD Week 6 |
Some concerns | Quote. "We have investigated this issue by means of a randomized investigator‐blinded half‐side comparison study." Comment: study referred to as randomised but no details of sequence or whether allocation was concealed. No information on baseline differences | Some concerns | Quote. "We have investigated this issue by means of a
randomized investigator‐blinded half‐side comparison
study."
Comment: only investigators were blinded.
There don't seem to be any deviations from intended
intervention. Quote. "Of the 12 patients who had entered the study, two were excluded from evaluation. One patient experienced an exacerbation of AD after 3 weeks of treatment and had started to take oral corticosteroids. In the other patient, considerably fewer erythema reactions were recorded in response to bath‐PUVA as compared with narrowband UVB, and thus the criterion of equi‐erythemogenic dosages was not fulfilled." Comment: two patients were excluded from analyses, which is not appropriate. Only 2 patients excluded out of 12, so unlikely to have a large impact on the results |
Some concerns | Quote. "Of the 12 patients who had entered the study, two were excluded from evaluation." Comment: two patients were not included in the analysis (83% were analysed). Only two patients not included (excluded by study authors) and unlikely to affect overall results. Missing data from two participants unlikely to affect overall results | Low | Quote. "A modified SCORAD score was used to assess the
half‐side severity of AD before and after 2, 4, and 6
weeks of bilateral treatment. In the modified SCORAD
score, the involvement of the face was not included, as
this part of the patient was irradiated with narrowband
UVB only. In addition, erythema was discarded as one of
the six intensity items as the delivery of erythemogenic
UV doses interfered with the assessment of AD‐related
erythema. Finally, sleep loss, which cannot be evaluated
in a half‐side fashion, was also excluded from the
modified SCORAD score"
Comment: a modified version
of SCORAD was used, but it appears the reasons for
modification were appropriate. Measurement unlikely to
differ between groups. Quote. "The half‐side evaluation was always performed by the same blinded investigator (A.T.)." Comment: the outcome assessment was blinded |
Some concerns | Comment: no protocol or analysis plan provided; no information available to make a judgement | Some concerns | Some concerns in four domains, low risk in one domain |
| Der‐Petrossian 2000 | Safety: withdrawals due to adverse events Week 6 |
Some concerns | Quote. "We have investigated this issue by means of a randomized investigator‐blinded half‐side comparison study." Comment: study referred to as randomised but no details of sequence or whether allocation was concealed. No information on baseline differences | Some concerns | Quote. "We have investigated this issue by means of a
randomized investigator‐blinded half‐side comparison
study."
Comment: only investigators were blinded.
Doesn't seem to be any deviation from intended
intervention. Quote: "Of the 12 patients who had entered the study, two were excluded from evaluation. One patient experienced an exacerbation of AD after 3 weeks of treatment and had started to take oral corticosteroids. In the other patient, considerably fewer erythema reactions were recorded in response to bath‐PUVA as compared with narrowband UVB, and thus the criterion of equi‐erythemogenic dosages was not fulfilled." Comment: two patients were excluded from analyses, which is not appropriate. However, it's not clear whether these patients were included in the analysis of adverse events. Only 2 patients excluded out of 12, so unlikely to have a large impact on the results. |
Some concerns | Quote. "Of the 12 patients who had entered the study, two were excluded from evaluation." Comment: two patients were not included in the analysis (83% were analysed). Only two patients not included (excluded by study authors) and unlikely to affect overall results. Missing data from two participants unlikely to affect overall results | Some concerns | Comment: adverse events are only mentioned in the abstract, no details of how they were recorded in methods; no information available. Not explicitly stated if participants were blinded. No serious adverse events were recorded, so assume knowledge of intervention had no effect on this outcome. | Some concerns | Comment: no protocol or analysis plan provided, so no information available to make a judgement | Some concerns | Some concerns in all domains |
AD: atopic dermatitis; NB‐UVB: narrowband UVB; PUVA: psoralen ultraviolet; SCORAD: SCORing Atopic Dermatitis; UV: ultraviolet; UVB: ultraviolet B.
2.
RoB 2 summary ‐ Physician‐assessed changes in clinical signs
3.
RoB 2 summary ‐ patient‐reported symptoms
4.
RoB 2 summary ‐ Investigator Global Assessment (IGA)
5.
RoB 2 summary ‐ HR QoL
6.
RoB 2 summary ‐ withdrawals due to adverse events
For the outcome Physician‐assessed changes in clinical signs, we assessed results from nine studies for risk of bias (Der‐Petrossian 2000; Gambichler 2009; Kwon 2019; Legat 2003; Majoie 2009; Reynolds 2001; Tzaneva 2010; Tzung 2006; Youssef 2020). We considered three of them at high risk (Gambichler 2009; Kwon 2019; Legat 2003); we had some concerns about the rest. The high risk of bias assessments were in the following domains: deviations from intended interventions (Gambichler 2009; Kwon 2019); missing outcome data (Gambichler 2009; Kwon 2019); and bias in the measurement of the outcome (Legat 2003).
For the outcome Patient‐reported changes in symptoms, we assessed results from five studies for risk of bias (Gambichler 2009; Legat 2003; Majoie 2009; Reynolds 2001; Youssef 2020). We considered two to be at high risk (Gambichler 2009; Legat 2003); we had some concerns about the rest. The high risk of bias assessments were in the following domains: deviations from intended interventions (Gambichler 2009); missing outcome data (Gambichler 2009); and bias in the measurement of the outcome (Legat 2003).
For the outcome Investigator Global Assessment (IGA), we assessed results from two studies for risk of bias (Der‐Petrossian 2000; Reynolds 2001). We had some concerns for both: Reynolds 2001 in missing outcome data, and selection of reported results; and Der‐Petrossian 2000 in all domains apart from measurement of the outcome.
For the outcome Health‐related quality of life, there was the result from one study available to assess for risk of bias (Gambichler 2009). We considered the overall risk to be high, because we assessed two domains at high risk of bias: deviations from intended interventions, and missing outcome data.
For the outcome Safety: withdrawals due to adverse events, we assessed results from five studies for risk of bias (Der‐Petrossian 2000; Kwon 2019; Majoie 2009; Reynolds 2001; Youssef 2020). We considered one at high risk due to high levels of missing data (Kwon 2019). We had some concerns for the results from the other four studies, mainly with the measurement of outcome data (the studies did not specify how they monitored adverse events), and selection of reported result (no protocol available).
Across domains, we assessed risk of bias from randomisation as either low risk, or we had some concerns (none were at high risk). For Deviations from intended intervention, we assessed four studies at high risk of bias, we had some concerns for seven, and we assessed 11 at low risk of bias. For Missing outcome data, we assessed five studies at high risk of bias, we had some concerns for nine, and we assessed eight at low risk of bias. For Measurement of outcome, we assessed two studies at high risk of bias, had some concerns for seven, and assessed 13 at low risk of bias. For Selection of reported result, we assessed none at low risk of bias, one study at high risk, and had some concerns for the remaining studies (mainly due to no pre‐registered protocols).
The full answers to the signalling questions are available here.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Throughout this section lower scores for continuous outcome scales are better.
1.NB‐UVB versus no treatment or placebo
Four studies compared NB‐UVB with no treatment (Kwon 2019; Tzung 2006; Youssef 2020), or placebo (Reynolds 2001). See Table 1.
Kwon 2019 compared NB‐UVB against no treatment in 18 participants with moderate disease. Thirteen participants were treated with NB‐UVB, administered two to three times a week for six weeks (12 to 18 treatments). Five participants were enrolled in the no treatment group. Participants in both groups received topical corticosteroids (methylprednisolone cream), applied to lesional skin only, plus an oral antihistamine. The participants received six weeks of active treatment, and were followed up for three weeks. Reynolds 2001, comparing NB‐UVB with placebo, was a parallel‐group study with three arms. The arm comparing NB‐UVB with visible fluorescent light included 46 participants, 24 of whom were treated with NB‐UVB, and 22 who were treated with visible fluorescent light; both groups received treatment twice a week for 12 weeks. Tzung 2006 was a multi‐arm, split‐body study. One arm of this trial, which compared NB‐UVB combined with 1% pimecrolimus cream with 1% pimecrolimus cream alone, included 12 children with moderate to severe atopic eczema. One half of the body was treated with NB‐UVB twice a day for six weeks. On the contralateral side, pimecrolimus cream was applied twice a day on all skin lesions; this side of the body was shielded from UV transmission, using tailored UV‐filtering clothing. The study consisted of a six‐week treatment phase, and four‐week post‐treatment follow‐up. Youssef 2020 compared NB‐UVB with 85% glycerol in 30 participants with mild to moderate disease, aged six years and older. Fifteen participants received NB‐UVB three times a week for four weeks. The other 15 participants were treated with 85% glycerol, applied daily to affected sites, for four weeks.
Primary outcomes
Physician‐assessed changes in clinical signs
Reynolds 2001 used their own disease activity score to measure physician‐assessed changes in clinical signs. The disease activity score instrument assessed erythema, papulovesicles, excoriation, scaling or dryness, and lichenification, and graded these signs from 0 to 3 (a higher score indicates more severe disease) at six sites. NB‐UVB reduced the total disease activity score more than placebo, measured at 12 weeks (mean difference (MD) ‐9.40, 95% confidence interval (CI) ‐15.18 to ‐3.62; 1 study, 41 participants; low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: NB‐UVB versus placebo/no treatment, Outcome 1: Physician‐assessed changes in clinical signs (mean reduction in total disease activity score)
After 9 weeks of treatment, participants who received NB‐UVB (N = 6) had an EASI score of 2.1; and those who received no treatment (N = 5) had a score of 3.6 (low‐certainty evidence; Analysis 1.2). At baseline, those in the NB‐UVB group (N = 13) had a mean EASI score of 13; and those who received no treatment (N = 5) had a mean EASI score of 11.6. A higher EASI score is associated with more severe disease, so it appears that the participants in the NB‐UVB‐treated group had better outcomes; however, without any measures of dispersion available, we could not determine whether the results were conclusive (Kwon 2019).
1.2. Analysis.
Comparison 1: NB‐UVB versus placebo/no treatment, Outcome 2: Physician‐assessed changes in clinical signs – incomplete data on which further analysis is not possible (short‐term)
| Physician‐assessed changes in clinical signs – incomplete data on which further analysis is not possible (short‐term) | |||||
| Study | Measure of effect and time point | NB‐UVB | Placebo/no treatment | Comments | Risk of bias 2 |
| Kwon 2019 | EASI (unclear if it is the mean that is reported) |
3.2 (N = 6) Week 6 |
3 (N = 5) | Unable to include in analysis as no dispersion data and unclear if means are presented. Data extracted by WebPlotDigitizer (automeris.io/WebPlotDigitizer/). | High |
| Kwon 2019 | EASI (unclear if it is the mean that is reported) Week 9 (3 weeks after end of treatment) |
2.1 (N = 6) | 3.6 (N = 5) | Unable to include in analysis as no dispersion data, and unclear if means are presented. Data extracted by WebPlotDigitizer. | High |
| Kwon 2019 | Mean EASI (unclear dispersion data) |
13 ± 6.0 (N = 13) Baseline |
11.6 ± 4.1 (N = 5) | Does not mention what type of dispersion data are presented. | High |
| Tzung 2006 | Percentage mean reduction in EASI Week 6 |
56% (N = 12) | 54% (N = 12) | Unable to include in analysis, as no dispersion data. Split‐body study. | Some concerns |
| Youssef 2020 | Percentage change in SCORAD Week 4 |
‐50.8% (N = 13) | ‐48.6% (N = 12) | Unable to include in analysis, as no dispersion data. | Some concerns |
After six weeks of treatment, Tzung 2006 (N = 24)reported a mean reduction in EASI of 56% for the body half that was treated with NB‐UVB combined with pimecrolimus cream, versus a mean reduction in EASI of 54% in the body half treated with pimecroliumus cream alone (low‐certainty evidence; Analysis 1.2).
After four weeks of treatment, Youssef 2020 (N = 25) reported a ‐50.8% change in SCORAD in participants treated with NB‐UVB, compared to a ‐48.6% change in SCORAD in participants treated with 85% glycerol (low‐certainty evidence; Analysis 1.2). Higher SCORAD and EASI scores are associated with poorer outcomes; however, in the case of the later two studies there was very little difference between the treatment arms.
Patient‐reported changes in symptoms
In Reynolds 2001, participants who received NB‐UVB were more likely to report less severe itch than those who received placebo after 12 weeks (risk ratio (RR) 1.72, 95% CI 1.10 to 2.69; 1 study, 40 participants; low‐certainty evidence; Analysis 1.3; number needed to treat for an additional beneficial outcome (NNTB) = 3).
1.3. Analysis.

Comparison 1: NB‐UVB versus placebo/no treatment, Outcome 3: Patient‐reported changes in symptoms (number of participants reporting a reduction in VAS for itch; short‐term)
Youssef 2020 (N = 25) reported a 55.7% reduction in itch, measured on VAS, after four weeks of treatment with NB‐UVB, compared to a 53.6% reduction in itch in participants treated with 85% glycerol; therefore, very little difference was seen between the two treatment arms; (low‐quality evidence; Analysis 1.4).
1.4. Analysis.
Comparison 1: NB‐UVB versus placebo/no treatment, Outcome 4: Patient‐reported changes in symptoms – incomplete data on which further analysis is not possible (short‐term)
| Patient‐reported changes in symptoms – incomplete data on which further analysis is not possible (short‐term) | |||||
| Study | Measure of effect and time point | NB‐UVB | Placebo/no treatment | Comments | RoB 2 |
| Youssef 2020 | % change on VAS for itch Week 4 |
‐55.7 (N = 13) |
‐53.6 (N = 12) |
Unable to include in analysis as no dispersion data. | Some concerns |
Secondary outcomes
Investigator Global Assessment (IGA)
Measured at 12 weeks, 13 out of 22 participants treated with NB‐UVB compared to 4 out of 19 participants treated with placebo in the study by Reynolds 2001 showed a moderate or greater improvement in IGA (RR 2.81, 95% CI 1.10 to 7.17, NNT = 3). This result is in favour of NB‐UVB. The IGA scale was a 6‐point investigator global assessment (exacerbation of disease, no change, slight improvement, moderate improvement, marked improvement, or complete resolution). Three months post‐treatment a moderate or greater improvement in IGA was seen in 12 out of 18 participants treated with NB‐UVB and 6 out of 17 participants treated with placebo (RR 1.89, 95% CI 0.92 to 3.89). See Analysis 1.5. We rated the certainty of evidence (GRADE) for these outcomes as low.
1.5. Analysis.

Comparison 1: NB‐UVB versus placebo/no treatment, Outcome 5: Investigator Global Assessment (number of participants with moderate or greater improvement)
Health‐related quality of life
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
In general, the trials reported few adverse events. In one study of 41 participants (Reynolds 2001), one participant withdrew from each group because of burning. In another study (Youssef 2020) of 15 participants, one participant withdrew from the NB‐UVB group because of a phototoxic reaction, and one withdrew from the glycerol 85% group because of severe irritation (low‐certainty evidence; Analysis 1.6).
1.6. Analysis.
Comparison 1: NB‐UVB versus placebo/no treatment, Outcome 6: Safety: withdrawal due to adverse events (short‐term)
| Safety: withdrawal due to adverse events (short‐term) | |||||
| Study | Time point | NB‐UVB | Placebo/no treatment | Comments | RoB 2 |
| Kwon 2019 | Up to week 9 | 0 (N = 13) |
0 (N = 5) |
High | |
| Reynolds 2001 | Up to week 12 | 1 (burning) (N = 22) |
1 (burning) (N = 19) |
Some concerns | |
| Youssef 2020 | Up to week 8 | 1 (phototoxic reaction) (N = 15) |
1 (severe irritation) (N = 15) |
Some concerns | |
Long‐term control
Analysis 1.7 shows long‐term control in Reynolds 2001, measured 3 months post‐treatment (6 months from baseline). The number of participants with a total disease activity score improved relative from baseline was 15 out of 18 participants compared to 8 out of 17 participants treated with NB‐UVB and placebo, respectively (RR 1.77, 95% CI 1.03 to 3.05, NNT=3). This result is in favour of NB‐UVB. For itch VAS, 14 out of 18 and 11 out of 17 participants treated with NB‐UVB and placebo, respectively, reported improvement relative from baseline (RR 1.20, 95% CI 0.78 to 1.85).
1.7. Analysis.

Comparison 1: NB‐UVB versus placebo/no treatment, Outcome 7: Long‐term control
2. NB‐UVB versus UVA1
Three small studies compared NB‐UVB with UVA1 (Gambichler 2009; Legat 2003; Majoie 2009). See Table 2.
The two‐treatment, two‐period cross‐over trial by Gambichler 2009 included 47 participants, 22 of whom were randomised to NB‐UVB, and 25 to UVA1 in the first period. There were two six‐week treatment periods, separated by at least eight weeks. Legat 2003 compared NB‐UVB with UVA1 in a split‐body study of nine adults with atopic eczema. Another split‐body study compared NB‐UVB with UVA1 (Majoie 2009). Clinical effectiveness of both treatment modalities was assessed in 13 adult participants with moderate to severe atopic eczema. There was an eight‐week, eight treatment period, followed by a four‐week follow‐up period.
Primary outcomes
Physician‐assessed changes in clinical signs
The SASSAD severity score was used by Gambichler 2009 for physician‐assesed changes in the clinical signs of AE. After 6 weeks of treatment, participants treated with NB‐UVB had a mean SASSAD score (from 0 to 108) of 20 (SD 9.6) compared to a mean SASSAD score of 22 (SD 12.14) in the UVA1 group (mean difference (MD) ‐2.00, 95% CI ‐8.4 to 4.41). See Analysis 2.1.
2.1. Analysis.

Comparison 2: NB‐UVB versus UVA1, Outcome 1: Physician‐assessed changes in clinical signs (SASSAD; short‐term)
Legat 2003 reported a median Costa (scale 0‐123) score of 40 (26 to 89) and 58 (27 to 89) over 7 weeks of treatment with NB‐UVB and UVA1, respectively. The participants had a median Leicester sign score (maximum score 162) over 7 weeks of treatment of 23 (12 to 56) in the NB‐UVB treated body‐half and a much higher median Leicester sign score of 52 (14 to 69) in the UVA1 treated body‐half. A higher score indicates more severe disease when AE is assessed using both of these instruments. Therefore, it appears from these results that NB‐UVB provided better outcomes; however, as the studies did not report any measures of dispersion, we cannot determine whether this result is statistically significant. Majoie 2009 did not show such a difference between the two treatment modalities at week 8: a mean Leicester sign score (scale 0‐108) of 9.2 was seen in the NB‐UVB group compared to a score of 11.6 in UVA1. Four weeks after end of treatment (week 12), a mean Leicester sign score of 9 and 10.1 was seen for NB‐UVB and UVA1, respectively. This study found lower scores with NB‐UVB; however, again no measures of dispersion were reported therefore we were unable to determine whether this result was statistically significant. See Analysis 2.2.
2.2. Analysis.
Comparison 2: NB‐UVB versus UVA1, Outcome 2: Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short‐term)
| Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | |||||
| Study | Measure of effect and time point | NB‐UVB | UVA1 | Comments | RoB 2 |
| Legat 2003 | Costa scale (0 to 123) Median and range Weeks 4 to 8 (median 7 weeks) |
40 (26 to 89) (N = 7) |
58 (27 to 89) (N = 7) |
Median and ranges given; unable to add to analysis. Split‐body study. | High |
| Legat 2003 | Leicester (maximum 162); median and range Median 7 weeks |
23 (12 to 56) (N = 7) |
52 (14 to 69) (N = 7) |
Median and ranges given; unable to add to analysis. Split‐body study | High |
| Majoie 2009 | Mean Leicester score (0 to 108) Week 8 |
9.2 (N = 13) |
11.6 (N = 13) |
Data extracted by WebPlotDigitizer, but error bars are not shown for both treatments, so unable to add to analysis Split‐body study | Some concerns |
| Majoie 2009 | Mean Leicester score (0 to 108) Week 12 (4 weeks after end of treatment) |
9 (N = 13) |
10.1 (N = 13) |
Split‐body study. Data extracted by WebPlotDigitizer, but error bars are not shown for both treatments, so unable to add to analysis | Some concerns |
We rated the certainty of evidence (GRADE) for these outcomes as very low.
Patient‐reported changes in symptoms
Participants in the trial by Gambichler 2009 reported a mean itch VAS of 4.5 (SD 2.3) after 6 weeks of treatment with NB‐UVB, compared to a mean itch VAS of 4.2 (SD 2.42); Analysis 2.3.
2.3. Analysis.

Comparison 2: NB‐UVB versus UVA1, Outcome 3: Patient‐reported changes in symptoms ( VAS for pruritus; short‐term)
Legat 2003 measured the VAS for skin lesions, overall effect, and itch. Over seven weeks of treatment, participants reported a median VAS for itch of 2 (range 0.1 to 8.5) for their body half that was treated with NB‐UVB, compared to 3.9 (range 0.2 to 8.4) for the UVA1 treated body half. At week eight, Majoie 2009 reported a mean VAS for itch of 2.9 for the NB‐UVB group and 3.6 for the UVA1 group. After four weeks of follow‐up, participants reported a mean VAS for itch of 2.2 for the NB‐UVB group, compared to 2.6 for the UVA group.
As higher itch scores are associated with more severe disease, these results appears to favour NB‐UVB; however, as the studies did not report any measures of dispersion, we could not determine whether these results were conclusive (very low‐certainty evidence; Analysis 2.4).
2.4. Analysis.
Comparison 2: NB‐UVB versus UVA1, Outcome 4: Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short‐term)
| Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short‐term) | |||||
| Study | Measure of effect and time point | NB‐UVB | UVA1 | Comments | RoB 2 |
| Legat 2003 | VAS of overall therapeutic effect (0 to 10); median
and range Weeks 4 to 8 (median 7 weeks) |
6.4 (1.2 to 9.2) (N = 7?) |
4.5 (0.5 to 9.1) (N = 9) |
Only median and range given, so unable to add to analysis. Split body study | High |
| Legat 2003 | VAS of pruritis (0 to 10) Median and range Weeks 4 to 8 (median 7 weeks) |
2 (0.1 to 8.5) (N = 7?) |
3.9 (0.2 to 8.4) (N = 7?) |
Only median and range given, so unable to add to analysis. Split body study | High |
| Legat 2003 | VAS of skin lesions (0 to 10); median and range Median 7 weeks |
1.5 (0.1 to 8.5) (N = 9) |
1.9 (0.1 to 8.5) (N = 9) |
Only median and range given, so unable to add to analysis. Split body study | High |
| Majoie 2009 | Mean VAS for itch Week 8 |
2.9 (N = 13) |
3.6 (N = 13) |
Data extracted by WebPlotDigitizer, but error bars are not shown for both treatments, so unable to add to analysis. Split‐body study | Some concerns |
| Majoie 2009 | Median VAS for pruritis Week 8 |
1.8 (N = 13) |
4.1 (N = 13) |
Only medians given, no dispersion data, so unable to add to analysis. Split‐body study | Some concerns |
| Majoie 2009 | Mean VAS for itch Week 12 (4 weeks after end of treatment) |
2.2 (N = 13) |
2.6 (N = 13) |
Data extracted by WebPlotDigitizer, but error bars are not shown for both treatments, so unable to add to analysis. Split‐body study | Some concerns |
We rated the certainty of evifor itchdence (GRADE) for these outcomes as very low.
Secondary outcomes
Investigator Global Assessment (IGA)
None of the trials measured this outcome.
Health‐related quality of life
To measure health‐related quality of life, participants filled in a German version of the Skindex‐29 questionnaire (range 30‐150) in the study of Gambichler 2009. A mean score of 72.7 (SD 23.2) was reported by participants after 6 weeks of treatment with NB‐UVB, compared to a slightly lower score of 68.8 (SD 19.94) when treated with UVA1 (MD 2.90, 95% CI ‐9.57 to 15.37). A lower score is more favourable. See Analysis 2.5.
2.5. Analysis.

Comparison 2: NB‐UVB versus UVA1, Outcome 5: Health‐related quality of life (German Skindex‐29)
There were baseline differences identified for this outcome (80.47 versus 69.8), meaning the end values may be unreliable. The percentage reduction given in the paper was 23.8% (SD 16.1) for NB‐UVB group versus 13.56% (SD 12) for UVA1 group, favouring stated that those receiving NB‐UVB therapy reported better health‐related quality of life than those receiving UVA1 (MD ‐10.24%, 95% CI ‐18.37 to ‐2.11; Gambichler 2009).
Safety: withdrawals due to adverse events
Only one study measured the number of withdrawals due to adverse events: there were none (1 study, 26 participants; very low‐certainty evidence).
Long‐term control
None of the trials measured this outcome.
3. NB‐UVB versus PUVA
One split‐body study investigated the clinical effectiveness of NB‐UVB compared to bath‐PUVA. Der‐Petrossian 2000 included 10 adults with chronic, severe atopic eczema. Each participant was treated with NB‐UVB on one side of the body, then they bathed in an 8‐MOP bath solution, then received UVA on the previously unirradiated body half (PUVA). Treatment was provided until there was complete remission on at least one‐half of the body. Treatment was provided for a maximum of six weeks. See Table 3.
Primary outcomes
Physician‐assessed changes in clinical signs
At week six, a 64.1% percentage reduction in SCORAD was seen in the NB‐UVB treated body‐half, compared to a similar percentage reduction of 65.7% in the body‐half treated with PUVA. See Analysis 3.1. We rated the certainty of evidence (GRADE) for this outcomes as very low.
3.1. Analysis.
Comparison 3: NB‐UVB versus PUVA, Outcome 1: Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term)
| Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | |||||
| Study | Measure of effect and time point | NB‐UVB | PUVA | Comments | RoB 2 |
| Der‐Petrossian 2000 | Percentage reduction in modified SCORAD Week 6 |
64.10% (N = 10) |
65.7% (N = 10) |
Dispersion data provided on the graph, but not clear if they are SDs. Split‐body study | Some concerns |
Patient‐reported changes in symptoms
None of the trials measured this outcome.
Secondary outcomes
Investigator Global Assessment (IGA)
Marked improvement or complete remission (IGA 0, 1 or 2: moderate improvement, marked improvement or complete remission) measured at a maximum of 6 weeks was seen in 9 of 10 sides treated with NB‐UVB and 9 of 10 sides treated with PUVA (OR 1.00, 95% CI 0.13 to 7.89). See Analysis 3.2. We rated the certainty of evidence (GRADE) for this outcome as very low.
3.2. Analysis.

Comparison 3: NB‐UVB versus PUVA, Outcome 2: Investigator Global Assessment (number of participants with marked improvement or complete remission; short‐term)
Safety: withdrawals due to adverse events
There were no severe adverse events and no withdrawals due to adverse events reported in the Der‐Petrossian 2000 study (20 participants). See Analysis 3.3. We rated the certainty of evidence (GRADE) for this outcome as very low.
3.3. Analysis.
Comparison 3: NB‐UVB versus PUVA, Outcome 3: Safety: withdrawal due to adverse events
| Safety: withdrawal due to adverse events | |||||
| Study | Time point | NB‐UVB | PUVA | Comments | RoB 2 |
| Der‐Petrossian 2000 | Week 6 | 0 (N = 10) |
0 (N = 10) |
No severe adverse events; split‐body study | Some concerns |
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
4. UVA1 versus PUVA
One cross‐over study compared UVA1 with 5‐MOP in 40 participants aged 18 years or older. Twenty‐three participants were allocated to medium dose UVA1, and 17 participants were allocated to 5‐MOP PUVA. UVA1 was administered five times a week over three weeks, and PUVA was given three times a week over five weeks (Tzaneva 2010). See Table 4.
Primary outcomes
Physician‐assessed changes in clinical signs
Tzaneva 2010 shows a better response in participants treated with 5‐MOP PUVA compared to UVA1. After 3 weeks of treatment, a mean SCORAD of 40.1 (SD 19.1) was seen in the UVA1 group, compared to a much lower mean SCORAD of 28.8 (SD 17.8) in the PUVA group (MD 11.30, 95% CI ‐0.21 to 22.81, 40 participants, Analysis 4.1). As higher SCORAD scores are associated with more severe disease, this result is in favour of PUVA. We rated the certainty of evidence (GRADE) for these outcomes as very low (see Table 4).
4.1. Analysis.

Comparison 4: UVA1 versus PUVA, Outcome 1: Physician‐assessed changes in clinical signs (SCORAD)
Patient‐reported changes in symptoms
None of the trials measured this outcome.
Secondary outcomes
Investigator Global Assessment
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
None of the trials measured this outcome.
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
5. NB‐UVB versus UVA
Two arms of the three‐arm parallel‐group study by Reynolds 2001 compared NB‐UVB with UVA in participants aged 16 to 65 years old. Twenty‐six participants were randomised to be treated with NB‐UVB and 24 participants were randomised to be administered UVA. Approximately half of the participants had a Fitzpatrick skin type of I/II. Participants were excluded if they had mild disease. Treatment was given twice weekly for 12 weeks and participants were followed up at 3 months post‐treatment end.
Primary outcomes
Physician‐assessed changes in clinical signs
Reynolds 2001 used their own disease activity score as an instrument for measuring physician‐assessed changes in clinical signs. The mean difference between groups was ‐5.00 (95% CI ‐10.60 to 0.60] in favour of NB‐UVB measured at 12 weeks (n=41). However, the confidence interval included zero, so there is uncertainty around this result. See Analysis 5.1.
5.1. Analysis.

Comparison 5: NB‐UVB versus UVA, Outcome 1: Physician‐assessed changes in the clinical signs (mean reduction in total disease activity score)
Patient‐reported changes in symptoms
Patient‐reported changes in symptoms were reported by Reynolds 2001. The number of participants reporting a reduction in itch measured using VAS (10cm; none at the left, severe at the right, a higher score is associated with more severe itch) after 12 weeks of treatment is shown in Analysis 5.2. Nineteen out of 21 participants in the NB‐UVB group reported a reduction in itch VAS, versus 12 out of 19 participants in the UVA group (RR 1.43, 95% CI 0.99 to 2.07). This was measured at 12 weeks.
5.2. Analysis.

Comparison 5: NB‐UVB versus UVA, Outcome 2: Patient‐reported changes in symptoms (number of participants reporting a reduction in VAS for itch (short‐term)
Secondary outcomes
Investigator Global Assessment
At 12 weeks (Reynolds 2001), 13 out of 22 participants treated with NB‐UVB compared to 7 out of 19 participants treated with UVA showed a moderate or greater improvement in IGA (RR 1.60, 95% CI 0.81 to 3.18). At 6 months (3 months post‐treatment) 12 of 18 participants treated with NB‐UVB showed a moderate or greater improvement in IGA compared to 6 of 19 treated with UVA (RR 2.11, 95% CI 1.01 to 4.42). This result is in favour of NB‐UVB. See Analysis 5.3.
5.3. Analysis.

Comparison 5: NB‐UVB versus UVA, Outcome 3: Investigator Global Assessments (number of participants with moderate or greater improvement)
Safety: withdrawals due to adverse events
In Reynolds 2001, one participant in the NB‐UVB arm (n=22) withdrew because of burning, no participants withdrew due to adverse events in the UVA arm (n=19). See Analysis 5.4.
5.4. Analysis.
Comparison 5: NB‐UVB versus UVA, Outcome 4: Safety: withdrawal due to adverse events
| Safety: withdrawal due to adverse events | ||||
| Study | Time point | NB‐UVB | UVA | Comments |
| Reynolds 2001 | Up to week 12 | 1 (burning) (N = 22) |
0 (N = 19) |
|
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
Analysis 5.5 shows long‐term control in Reynolds 2001, measured 3 months post‐treatment (6 months from baseline). The number of participants with a total disease activity score improved relative to baseline was 15 out of 18 participants in the NB‐UVB group compared to 9 out of 19 in the UVA group (RR 1.76, 95% CI 1.05 to 2.95, NNT = 3). This result is in favour of NB‐UVB. For itch VAS 14 out of 18 participants in the NB‐UVB group showed an improvement relative to baseline in comparison to 14 out of 19 in the UVA group (RR 1.06, 95% CI 0.73 to 1.52).
5.5. Analysis.

Comparison 5: NB‐UVB versus UVA, Outcome 5: Long‐term control
6. NB‐UVB versus UVAB
Two parallel studies (Leone 1998; Maul 2017), both including adults, compared NB‐UVB with UVAB. One study was in participants with severe AE (n=12) (Leone 1998), one study in participants with eczema severity unspecified (n=24) (Maul 2017). In the study by Leone 1998 participants received treatments thrice weekly for approximately 5 weeks (10‐15 treatments). In the study by Maul 2017, participants also received treatment three times a week for up to 16 weeks. The skin type of participants was not reported in either study.
Primary outcomes
Physician‐assessed changes in clinical signs
Leone 1998 reported that they measured physician‐assessed clinical signs using the SCORAD score. They reported NB‐UVB was significantly better than UVAB with a P value less than 0.05 (around week 5); however, no further data were provided per group to support this statement (6 participants were in each group). See Analysis 6.1.
6.1. Analysis.
Comparison 6: NB‐UVB versus UVAB, Outcome 1: Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term)
| Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | ||||
| Study | Measure of effect and timepoint | NB‐UVB | UVAB | Comments |
| Leone 1998 | SCORAD Around week 5 |
See comments | See comments | No raw data given per group; narrowband UVB better
than UVAB; 6 participants in each group Quote. "However, a difference in the clinical efficacy among the groups was noted using the Kruskall‐Wallis test and Mann and Withney test: UVBTL01 > UVA‐B (P < 0.05)." |
Patient‐reported changes in symptoms
None of the trials measured this outcome.
Secondary outcomes
Investigator Global Assessment
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
There were no withdrawals due to adverse events in the trial by Maul 2017 (Analysis 6.2).
6.2. Analysis.
Comparison 6: NB‐UVB versus UVAB, Outcome 2: Safety: withdrawal due to adverse events (short‐term)
| Safety: withdrawal due to adverse events (short‐term) | ||||
| Study | Time point | NB‐UVB | UVAB | Comments |
| Maul 2017 | Up to 16 weeks | 0 | 0 | |
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
7. NB‐UVB versus topical corticosteroids
Agrawal 2018 compared NB‐UVB (n=30) with topical corticosteroids (n=30), specifically betamethasone valerate 0.1%, in a parallel study in adults and children (aged 5‐60 years). Participants were included in the study if they had a SCORAD between 15 and 60 and a skin type of III or IV. Participants in the phototherapy group received treatment thrice weekly for 8 weeks, whilst those in the topical corticosteroid group received treatment twice daily for 4 weeks.
Primary outcomes
Physician‐assessed changes in clinical signs
Mean SCORAD in the NB‐UVB group (n=30) was higher than in the topical corticosteroid group (n=30) at week 4 (Agrawal 2018). The mean SCORAD was 25.93 (range 16.5 to 49) in the NB‐UVB group and 15.07 (range 10.0 to 34.0) in the TCS group. A higher SCORAD score indicates a greater severity of AE. However, the absence of the standard deviation or similar measures of dispersion limited the interpretation of this result. See Analysis 7.1.
7.1. Analysis.
Comparison 7: NB‐UVB versus topical corticosteroids, Outcome 1: Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term)
| Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | ||||
| Study | Measure of effect and time point | NB‐UVB | Topical corticosteroids | Comments |
| Agrawal 2018 | Mean SCORAD and range Week 4 |
25.93 (16.5 to 49) (N = 30) |
15.07 (10.0 to 34.0) (N = 30) |
Only range given, so can’t include in analysis. |
Patient‐reported changes in symptoms
None of the trials measured this outcome.
Secondary outcomes
Investigator Global Assessment
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
None of the trials measured this outcome.
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
8. Standard increasing NB‐UVB versus fixed‐dose NB‐UVB
Hoey 2006 conducted a parallel group study (n=10) which compared a standard increasing dose of NB‐UVB (UVB‐TL01) against a fixed‐dose dose NB‐UVB (UVB‐TL01). The age, severity and skin type of the participants was not reported. The length of the study was also unclear.
Primary outcomes
Physician‐assessed changes in clinical signs
Hoey 2006 measured SCORAD; however, results were only reported narratively. It was also unclear how many participants were randomised to each group, as was the length of treatment. The authors noted that a significant difference was only noted between the two groups for the 18th session SCORAD though there is no information as to what this difference was. Three participants were reported to have a mild flare but it is unclear what proportion of the original groups this related to. See Analysis 9.1.
9.1. Analysis.
Comparison 9: Standard increasing NBUVB versus fixed dose NBUVB, Outcome 1: Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term)
| Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | ||||
| Study | Measure of effect and time point | Standard increasing UVB‐TL01 | Fixed dose UVB‐TL01 | Comments |
| Hoey 2006 | SCORAD Unclear time point |
See comments | See comments | Narrative only; quote. "A significant difference was only noted between the two groups for the 18th session SCORAD." ... "Three patients had a mild….(flare)” |
Patient‐reported changes in symptoms
None of the trials measured this outcome.
Secondary outcomes
Investigator Global Assessment
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
None of the trials measured this outcome.
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
9. NB‐UVB with optimised dose by skin reflectance measurements versus NB‐UVB with fixed‐dose increments
Selvaag 2005 compared different dosing regimens of NB‐UVB in a split‐body study of 20 participants. The participants were adults with mild to moderate AE, skin type was not reported. Participants were treated for up to 6 weeks, 3‐5 times per week. In the fixed‐dose regimen, half of the body was treated with a starting dose of 1.6 SED with 25% increments with each treatment session. One SED is 10 mJ/cm2 at 298 nm using the International Commission on Illumination (CIE) erythema action spectrum and is equivalent to 1.6 kJ/m2 of the UVB lamp. In the optimised regimen group UVB was administered according to skin reflectance measurements of skin pigmentation and erythema.
Primary outcomes
Physician‐assessed changes in clinical signs
Selvaag 2005 measured the number of weeks to a SCORAD measurement of <10 in both groups. The median time to SCORAD <10 was 3.0 weeks (5‐95 percentile 2.0 to 5.5) in the optimised dose NB‐UVB group (n=20) compared with 3.5 weeks (5‐95 percentile 1.5 to 6.0) in the fixed‐dose group (n=20). See Analysis 8.1.
8.1. Analysis.
Comparison 8: NB‐UVB with optimised dose by skin reflectance measurements versus NB‐UVB with fixed dose increments , Outcome 1: Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term)
| Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | ||||
| Study | Measure of effect and time point | NB‐UVB with optimised dose by skin reflectance measurements | NB‐UVB with fixed dose increments | Comments |
| Selvaag 2005 | Number of weeks to reach a SCORAD<10 Result given as median (5 to 95 percentiles) Week 6 |
3.0 (2.0 to 5.5) (N = 20) |
3.5 (1.5 to 6.0) (N = 20) |
Split body study. |
Patient‐reported changes in symptoms
None of the trials measured this outcome.
Secondary outcomes
Investigator Global Assessment
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
None of the trials measured this outcome.
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
10. UVB 0.8 MED versus UVB 0.4 MED
Only one study, Jekler 1988b compared different dosages of UVB (0.8 MED vs 0.4 MED) in a split‐body study that included 31 participants aged 16 years and over. In this split‐body study, 31 participant were treated on both sides of the body for up to 8 weeks or until healing of at least one body part. The eczema was of unknown severity and the participant had the following skin types: 8 were type II, 15 type III and 2 type IV. Participants received treatment three times a week for up to 8 weeks or until the body half was healed.
Primary outcomes
Physician‐assessed changes in clinical signs
Jekler 1988b used their own scale to assess clinical signs which assessed 8 variables; pruritus, lichenification, scaling, xerosis, vesiculation, excoriations and erythema and an overall evaluation, rated on a 4 point scale of 0=none to 3=severe, with a maximum score of 24. The mean severity score was 7 in the group treated with UVB 0.8 MED group (n=25 sides) and 6.6 in the group treated with UVB 0.4 MED (n=25 sides) at the final time point which was either 8 weeks or the time taken for healing of at least one body half. No dispersion data were reported, so this study could not be included in a forest plot. See Analysis 10.1.
10.1. Analysis.
Comparison 10: UVB 0.8 MED versus UVB 0.4 MED , Outcome 1: Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term)
| Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | ||||
| Study | Measure of effect and time point | UVB 0.8 MED | UVB 0.4 MED | Comments |
| Jekler 1988b | Mean severity score (total) up to 8 weeks or healing of one side |
7 (N = 25) |
6.6 (N = 25) |
No dispersion data given, so cannot include in analysis. Split‐body study |
Patient‐reported changes in symptoms
The mean pruritus score (rated on a 4 point scale as above, Jekler 1988b) was 1.2 in the group treated with UVB 0.8 MED group (n=25 sides) and 1.2 in the group treated with UVB 0.4 MED (n=25 sides) at the final time point which was either 8 weeks or the time taken for healing of at least one body half. No dispersion data were reported, so this study could not be included in a forest plot. See Analysis 10.2.
10.2. Analysis.
Comparison 10: UVB 0.8 MED versus UVB 0.4 MED , Outcome 2: Patient‐reported changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term)
| Patient‐reported changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | ||||
| Study | Measure of effect and time point | UVB 0.8 MED | UVB 0.4 MED | Comments |
| Jekler 1988b | Mean pruritis score up to 8 weeks or healing of one side |
1.2 (N = 25) |
1.2 (N = 25) |
No dispersion data given so cannot include in analysis. Split‐body study |
Secondary outcomes
Investigator Global Assessment
Jekler 1988b reported that 15 out of 25 sides of the body were healed or considerably improved by treatment in the 0.8 UVB MED group in comparison with 16 out of 25 sides in the group treated with 0.4 MED (OR 0.84, 95% CI 0.38 to 1.89) measured at 8 weeks or the time taken for healing of at least one body half. This result is uncertain as the confidence intervals are wide and cross the line of no effect. See Analysis 10.3.
10.3. Analysis.

Comparison 10: UVB 0.8 MED versus UVB 0.4 MED , Outcome 3: Investigator Global Assessment (short‐term)
Safety: withdrawals due to adverse events
In Jekler 1988b, one participant in the group that received UVB 0.8 MED withdrew due to experiencing UVB burn. See Analysis 10.4.
10.4. Analysis.
Comparison 10: UVB 0.8 MED versus UVB 0.4 MED , Outcome 4: Safety: withdrawals due to adverse events
| Safety: withdrawals due to adverse events | ||||
| Study | Time point | UVB 0.8 MED | UVB 0.4 MED | Comments |
| Jekler 1988b | Up to week 8 | 1 (UVB burn) (N = 31) |
0 (N = 31) |
Split‐body study |
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
11. UVB (unspecified) versus UVA
One parallel‐design study (Qayyum 2016) compared UVB with UVA. The type of UVB that was used in this trial was not specified. The study included 60 participants, adults and children, with moderate to severe AE. Participants were treated three times weekly, up to 12 weeks.
Primary outcomes
Physician‐assessed changes in clinical signs
The mean difference in SCORAD values in the study by Qayyum 2016 between UVA and UVB groups was 3 (95% CI ‐1.09 to 7.08), with the point estimate slightly in favour of UVA at week 12. However, the confidence interval crosses the line of no effect, so this result is uncertain. See Analysis 11.1.
11.1. Analysis.

Comparison 11: UVB versus UVA, Outcome 1: Physician‐assessed changes in clinical signs (SCORAD; short‐term)
Patient‐reported changes in symptoms
None of the trials measured this outcome.
Secondary outcomes
Investigator Global Assessment
The number of participants achieving excellent improvement was 12 out of 30 in the UVB group compared to 17 out of 30 in the UVA group. The risk ratio calculated from this study was 0.71 (in favour of UVA treatment); however, this result crossed the line of no effect, so the result was uncertain (95% CI 0.41 to 1.21), see Analysis 11.2. This was measured at 12 weeks.
11.2. Analysis.

Comparison 11: UVB versus UVA, Outcome 2: Investigator Global Assessment (number of participants with excellent improvement; short‐term)
Safety: withdrawals due to adverse events
No participants withdrew from the UVA group (n=30) and two participants withdrew due to adverse events from the UVB group (n=30) in the study by Qayyum 2016, this study had up to 12 weeks of active treatment. See Analysis 11.3.
11.3. Analysis.
Comparison 11: UVB versus UVA, Outcome 3: Safety: withdrawals due to adverse events
| Safety: withdrawals due to adverse events | ||||
| Study | Time point | UVB | UVA | Comments |
| Qayyum 2016 | 3‐month post‐treatment follow‐up (active treatment 12 weeks) | 2 (N = 30) |
0 (N = 30) |
|
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
12. BB‐UVB versus placebo
Jekler 1988a was a within‐participant trial comparing BB‐UVB with placebo (ordinary daylight tubes) in 17 participants. The participants were randomized into two treatment groups—one starting with 0.5 MED and one with 1 MED BB‐UVB, randomized to the right or left side of the body. Treatment was given three times a week for a maximum of 8 weeks or until the healing of at least one body half. Participants were assessed for 8 variables scored 0 to 3 (0=none, 1=light, 2= moderate and 3= severe) on the following variables; pruritus, lichenification, scaling, xerosis, vesiculation, excoriations, erythema and an overall evaluation.
Primary outcomes
Physician‐assessed changes in clinical signs
After 8 weeks of treatment, Jekler 1988a reported a modified severity score of 5 (n=17) in the body half that was treated with BB‐UVB compared to a severity score of 8 (n=17) in the body half that received placebo. No dispersion data were reported, so this study could not be included in a forest plot. See Analysis 12.1.
12.1. Analysis.
Comparison 12: BB‐UVB versus placebo, Outcome 1: Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term)
| Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | ||||
| Study | Measure of effect and time point | BB‐UVB | Placebo | Comments |
| Jekler 1988a | Severity score (total) Week 8 |
5 (N = 17) |
8 (N = 17) |
Unable to include in analysis as no dispersion data; split‐body study |
Patient‐reported changes in symptoms
Jekler 1988a showed a mean pruritis score of 0.8 (n=17) and 1.8 (n=17) on the sides treated with BB‐UVB and placebo, respectively. No dispersion data were reported, so this study could not be included in a forest plot. See Analysis 12.2.
12.2. Analysis.
Comparison 12: BB‐UVB versus placebo, Outcome 2: Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short‐term)
| Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short‐term) | ||||
| Study | Measure of effect and time point | BB‐UVB | Placebo | Comments |
| Jekler 1988a | Mean pruritis score Week 8 |
0.8 (N = 17) |
1.8 (N = 17) |
Unable to include in analysis as no dispersion data; split‐body study |
Secondary outcomes
Investigator Global Assessment
Jekler 1988a reported that 13 of 17 participants were healed or considerably improved on the side treated with BB‐UVB compared to 1 of 17 on the side treated with placebo at 8 weeks. This result favours BB‐UVB with OR=52.00 (95% CI 9.01 to 300.17). See Analysis 12.3.
12.3. Analysis.

Comparison 12: BB‐UVB versus placebo, Outcome 3: Investigator Global Assessment (number of participants healed or considerably improved; short‐term)
Safety: withdrawals due to adverse events
Jekler 1988a reported that one participant withdrew from the study because of a UVB burn experienced on the side treated with BB‐UVB (n=28). No withdrawals were due to adverse events on the side treated with placebo. See Analysis 12.4.
12.4. Analysis.
Comparison 12: BB‐UVB versus placebo, Outcome 4: Safety: withdrawal due to adverse events (short‐term)
| Safety: withdrawal due to adverse events (short‐term) | ||||
| Study | Time point | BB‐UVB | Placebo | Comments |
| Jekler 1988a | Up to week 8 | 1 (UVB burn) (N = 28) |
0 (N = 28) |
Split body study. |
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
13. BB‐UVB versus UVA
One study compared BB‐UVB with UVA, Jekler 1991 (n=33 (though results and characteristics only reported for 21 participants)) was a split‐body study and included those aged 15 years and over. Disease severity was not specified. Participants were treated three times weekly for up to 8 weeks. All but 2 participants had a skin type of III (the remaining had a skin type of II).
Primary outcomes
Physician‐assessed changes in clinical signs
In the study by Jekler 1991 (which used a scale that measured the severity of clinical signs defined by the authors) no dispersion data were provided; therefore, it was not possible to include the data in a forest plot. However, the mean severity score was 6.4 on the sides treated with UVB (n=21 sides, split‐body study) and 5.5 on the sides treated with UVA (n=21 sides, split‐body study) at week 8. See Analysis 13.1.
13.1. Analysis.
Comparison 13: BB‐UVB versus UVA, Outcome 1: Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term)
| Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | ||||
| Study | Measure of effect and time point | BB‐UVB | UVA | Comments |
| Jekler 1991 | Mean severity score (total) Week 8 |
6.4 (N = 21) |
5.5 (N = 21) |
No dispersion data given, so cannot include in analysis; split‐body study |
Patient‐reported changes in symptoms
Jekler 1991 reported a mean pruritus score (measured on a 4 point scale 0=none to 3=severe) for both treatments; however, again there were no dispersion data provided. The mean values for the sides treated with UVB was 1.3 (n=21) compared to 1 on the sides treated with UVA (n=21). See Analysis 13.2.
13.2. Analysis.
Comparison 13: BB‐UVB versus UVA, Outcome 2: Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short‐term)
| Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short‐term) | ||||
| Study | Measure of effect and time point | BB‐UVB | UVA | Comments |
| Jekler 1991 | Mean pruritis score Week 8 |
1.3 (N = 21) |
1 (N = 21) |
No dispersion data given so cannot include in analysis; split‐body study |
Secondary outcomes
Investigator Global Assessment
The number considerably improved or healed was 13 of 21 sides treated with UVB compared to 15 of 21 sides treated with UVA. The odds ratio calculated was 0.65 in favour of UVA treatment; however, this result crossed the line of no effect, so the result was uncertain (95% CI 0.26 to 1.62), see Analysis 13.3. This was measured at 8 weeks.
13.3. Analysis.

Comparison 13: BB‐UVB versus UVA, Outcome 3: Investigator Global Assessment (number of participants considerably improved or healed; short‐term)
Safety: withdrawals due to adverse events
No participants withdrew due to adverse events from Jekler 1991. See Analysis 13.4.
13.4. Analysis.
Comparison 13: BB‐UVB versus UVA, Outcome 4: Safety: withdrawals due to adverse events
| Safety: withdrawals due to adverse events | ||||
| Study | Time point | BB‐UVB | UVA | Comments |
| Jekler 1991 | up to 8 weeks or healing of one side of the body | 0 (N = 33) |
0 (N = 33) |
Split‐body study |
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
14. BB‐UVB versus UVAB
Two studies compared BB‐UVB versus UVAB (Jekler 1990; Jekler 1991b Study 1), both split body studies, in participants aged 15 years and over with unspecified eczema severity. In both studies, the majority of participants had skin type of III. Participants were treated three times a week for up to 8 weeks or healing of one body side. In Jekler 1991b Study 1 a lower dose of UVB was used.
Primary outcomes
Physician‐assessed changes in clinical signs
Both studies assessed physician‐assessed clinical signs using an instrument defined by the authors, which assessed 8 variables; pruritus, lichenification, scaling, xerosis, vesiculation, excoriations and erythema and an overall evaluation, rated on a 4 point scale of 0 = none to 3 = severe. As the numerical data were incomplete (no usable measures of dispersion) it was not possible to include these studies in a meta‐analysis. In Jekler 1990 (n=30 participants, 60 sides treated overall in both groups) the BB‐UVB group scored a mean 6.1 with range of 0‐17 whilst in the UVAB group the mean was 5.2 with a range of 0‐15. In Jekler 1991b Study 1 (n=18 participants, 36 sides treated overall in both groups) the mean in the BB‐UVB group was 8.8 with a range of 4.5 to 14, whilst in the UVAB group the mean was 5.3 with a range of 1.5 to 11. See Analysis 14.1.
14.1. Analysis.
Comparison 14: BB‐UVB versus UVAB, Outcome 1: Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term)
| Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | ||||
| Study | Measure of effect and time point | BB‐UVB | UVAB | Comments |
| Jekler 1990 | Score for lichenification, scaling, xerosis,
vesiculation, excoriations, erythema Mean and range Week 8 |
6.1 (0 to 17) (N = 30) |
5.2 (0 to 15) (N = 30) |
Cannot include in analysis as only range given as dispersion data; split‐body study |
| Jekler 1991b Study 1 | Disease severity total score Mean and range Week 8 (or at healing) |
8.8 (4.5 to 14) (N = 18) |
5.3 (1.5 to 11) (N = 18) |
Cannot include in analysis as only range given as dispersion data; split‐body study |
Patient‐reported changes in symptoms
Both studies (Jekler 1990; Jekler 1991b Study 1) reported itch measured on a 4 point scale (0=none, 1=light, 2=moderate and 3=severe). As the numerical data were incomplete (no usable measures of dispersion) it was not possible to include these studies in a meta‐analysis. In Jekler 1990 (n=30 participants, 60 sides treated overall in both groups) the BB‐UVB score was 1.2, whilst in the UVAB group the mean score was 1. The range was 0 to 3 in both arms. In Jekler 1991b Study 1, which used the same itch measurement scale (n=18 participants, 36 sides treated overall in both groups), the mean in the BB‐UVB group was 1.5 whilst in the UVAB group the mean was 0.8. The range in both groups was 0 to 2. In both cases, the timepoint at which the outcome was measured was 8 weeks or upon healing of one body side. See Analysis 14.2.
14.2. Analysis.
Comparison 14: BB‐UVB versus UVAB, Outcome 2: Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short‐term)
| Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short‐term) | ||||
| Study | Measure of effect and time point | BB‐UVB | UVAB | Comments |
| Jekler 1990 | Itch ‐ participants were assessed for 8 variables
scored 0 to 3 (0 = none, 1 = light, 2 = moderate,
and 3 = severe) Mean and range Week 8 (or at healing) |
1.2 (0 to 3) (N = 30) |
1 (0 to 3) (N = 30) |
Cannot include in analysis as only range given as dispersion data; split‐body study |
| Jekler 1991b Study 1 | Itch (unspecified) Mean and range Week 8 (or at healing) |
1.5 (0 to 2) (N = 18) |
0.8 (0 to 2) (N = 18) |
Cannot include in analysis as only range given as dispersion data; split‐body study |
Secondary outcomes
Investigator Global Assessment
Both studies (Jekler 1990; Jekler 1991b Study 1) measured IGA. Both studies were split‐body studies in which 48 participants were treated on both half of the body. On treatment with BB‐UVB 30 out of 48 body sides were healed or showed considerable improvement whilst on treatment with UVAB, 45 out of 48 body sides were healed or showed considerable improvement (odds ratio 0.14, 95% CI 0.00 to 4.49). In both cases, the timepoint at which the outcome was measured was 8 weeks or upon healing of one body side. See Analysis 14.3.
14.3. Analysis.

Comparison 14: BB‐UVB versus UVAB, Outcome 3: Investigator Global Assessment (number of participants healed or considerably improved; short‐term)
Safety: withdrawals due to adverse events
No participants in either study (Jekler 1990; Jekler 1991b Study 1) withdrew due to adverse events. See Analysis 14.4.
14.4. Analysis.
Comparison 14: BB‐UVB versus UVAB, Outcome 4: Safety: withdrawals due to adverse events
| Safety: withdrawals due to adverse events | ||||
| Study | Time point | BB‐UVB | UVAB | Comments |
| Jekler 1990 | Up to week 8. | 0 (N = 30) |
0 (N = 30) |
Split‐body study. |
| Jekler 1991b Study 1 | Up to week 8. | 0 (N = 18) |
0 (N = 18) |
Split‐body study. |
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
15. UVA1 versus UVAB
Four studies compared UVA1 to UVAB (Jekler 1991b Study 2, Krutmann 1992; Krutmann 1998; Von Kobyletzki 1999a).
Jekler 1991b Study 2 compared UVA1 and UVAB. Jekler 1991b Study 2 was a within‐participant, randomised controlled trial and had 28 participants. Phototherapy in both arms was delivered five times a week for up to three weeks.
Krutmann 1992 was a parallel randomised controlled trial with 25 participants, with up to 15 treatments given daily over approximately two to three weeks.
Krutmann 1998 was a randomised, multi‐centre, three‐armed, parallel study with 53 participants, daily treatments conducted over a 10‐day period.
Von Kobyletzki 1999a 1999 was a parallel, three‐armed, randomised, active‐control trial with 120 participants. Participants received treatment 5 times per week for 3 weeks with 4 weeks of follow‐up post treatment.
Primary outcomes
Physician‐assessed changes in clinical signs
Data on physician‐assessed changes in clinical signs from these 3 studies were added to the meta‐analyses. The pooled standardised mean difference was ‐2.10 (95% CI ‐2.84 to ‐1.35) in favour of UVA1. See Analysis 15.1.
15.1. Analysis.

Comparison 15: UVA1 versus UVAB, Outcome 1: Physician‐assessed changes in clinical signs (short‐term)
In Analysis 15.1, values are given at end of treatment for Krutmann 1992 and Krutmann 1998. However, for Von Kobyletzki 1999a the values are given at 7 weeks (4 weeks after completing active treatment) as this is the closest timepoint to 12 weeks, as per our protocol. The end of treatment (at 3 weeks) mean SCORAD values (plus SD) for Von Kobyletzki 1999a were: UVA medium dose: 28.8 (6.9), UVA medium dose cold light: 23.3 (10.6) and UVAB: 41.4 (9.9), also showing lower values in the UVA groups compared to UVAB.
Jekler 1991b Study 2 could not be added to this meta‐analysis as only the range was given (rather than another measure of dispersion such as SD). Disease severity was graded using a scale defined by Jekler 1991b Study 2 that comprised of 8 variables (pruritus, lichenification, scaling, xerosis, vesiculation, excoriations, erythema and an overall evaluation), scored 0 to 3 (0=none, 1=slight, 2= moderate and 3= severe). The mean disease severity total score at week 3 in the UVA1 arm was 7.2 (range 3 to 14) compared to 6 (range 1 to 12) in the UVAB arm. Results were reported for 25 participants treated on both sides of the body, therefore "50 sides". See Analysis 15.2.
15.2. Analysis.
Comparison 15: UVA1 versus UVAB, Outcome 2: Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short term)
| Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short term) | ||||
| Study | Measure of effect and timepoint | UVA1 | UVAB | Comments |
| Jekler 1991b Study 2 | Disease severity total score Mean and range Up to week 3 (or healing) |
7.2 (3 to 14) (n=25) |
6 (1 to 12) (n=25) |
Split‐body. Can’t add to analysis as only range given. |
Patient‐reported changes in symptoms
Only Jekler 1991b Study 2 reported patient‐reported changes in symptoms. The mean itch score (0=none, 1=slight, 2= moderate and 3= severe) at week 3 in the UVA arm was 1.3 (range 0 to 2) compared to 1.1 (range 0 to 2) in the UVAB arm. No dispersion data were reported, so this study could not be included in a forest plot. See Analysis 15.3. This was measured at week 3 or upon healing. Results were reported for 25 participants treated on both sides of the body, therefore "50 sides".
15.3. Analysis.
Comparison 15: UVA1 versus UVAB, Outcome 3: Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short term)
| Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short term) | ||||
| Study | Measure of effect and timepoint | UVA | UVAB | Comments |
| Jekler 1991b Study 2 | Itch (unspecified) Mean and range Up to week 3 (or healing) |
1.3 (0 to 2) (n=25) |
1.1 (0 to 2) (n=25) |
Split‐body. Can’t add to analysis as only range given. |
Secondary outcomes
Investigator Global Assessment
In Jekler 1991b Study 2, 17 of 25 sides treated with UVA achieved healing or considerable improvement compared to 23 of 25 receiving UVAB at 3 weeks. The odds ratio was 0.18 (CI 0.05 to 0.65). See Analysis 15.4. Results were reported for 25 participants treated on both sides of the body, therefore "50 sides".
15.4. Analysis.

Comparison 15: UVA1 versus UVAB, Outcome 4: Investigator Global Assessment (IGA) ‐ number of participants who healed or considerably improved (short term)
Safety: withdrawals due to adverse events
Jekler 1991b Study 2 reported one withdrawal due to bilateral polymorphic light eruption. Results were reported for 25 participants treated on both sides of the body, therefore "50 sides". Krutmann 1998 reported no withdrawals due to adverse events. Von Kobyletzki 1999b reported a total of 6 withdrawals in the UVA1 arm (1 for bacterial superinfection treated with antibiotics; 5 due to exacerbation of disease) compared to 1 withdrawal in the UVAB arm (due to bacterial superinfection). See Analysis 15.5.
15.5. Analysis.
Comparison 15: UVA1 versus UVAB, Outcome 5: Withdrawals due to adverse events
| Withdrawals due to adverse events | ||||
| Study | Timepoint | UVA1 | UVAB | Comments |
| Jekler 1991b Study 2 | Up to week 3 | See comments | See comment | Split‐body study (n=25). One patient withdrew due to bilateral polymorphic light eruption (not clear which treatment they were receiving). |
| Krutmann 1998 | Up to day 10 | 0 | 0 | |
| Von Kobyletzki 1999a | Up to week 3 | 6 (1 for bacterial superinfection, treated with
antibiotics; 5 due to discomfort and intense
sweating combined with progressive pruritis, leading
to exacerbation of disease) (n=50) |
1 (due to bacterial superinfection) (n=20) |
|
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
16. High dose UVA1 versus medium dose UVA1
Three studies compared high dose UVA1 and medium dose UVA1 in adults; Dittmar 2001, Pacifico 2019 and Tzaneva 2001.
Dittmar 2001 was a randomised, controlled, parallel, prospective study conducted with 15 treatments (5 a week) over 3 weeks for a total of 34 participants.
Pacifico 2019 was a randomised, controlled, open, parallel‐group study with 27 participants with a total of 15 treatments over 3 weeks.
Tzaneva 2001 was an investigator‐blinded, within‐participant study with 10 participants receiving treatment 5 times per week for 3 weeks.
Primary outcomes
Physician‐assessed changes in clinical signs
Both Dittmar 2001 and Pacifico 2019 assessed the SCORAD score, and the mean difference between groups was ‐8.24 (95% CI ‐14.14 to ‐2.34), favouring high dose. See Analysis 16.1.
16.1. Analysis.

Comparison 16: High dose UVA1 versus medium dose UVA1, Outcome 1: Physician‐assessed changes in the clinical signs (short term) ‐ SCORAD
Tzaneva 2001 reported a mean modified SCORAD reduction of 34.7% (range 0 to 46.9%) at week 3 in the high dose UVA1 arm compared to 28.2% (range 0 to 46.9%) in the medium dose UVA1 group. No dispersion data were reported, so this study could not be included in a forest plot. See Analysis 16.2.
16.2. Analysis.
Comparison 16: High dose UVA1 versus medium dose UVA1, Outcome 2: Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short term)
| Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short term) | ||||
| Study | Measure of effect and timepoint | High dose UVA1 | Medium dose UVA1 | Comments |
| Tzaneva 2001 | Modified SCORAD Percentage median reduction and range Week 3 |
34.70% (0 to 46.9%) (n=10) |
28.20% (0 to 46.9%) (n=10) |
Split‐body study. Can't include in analysis as only median and range given. |
Subgroup analysis (Skin type): Physician‐assessed changes in the clinical signs
Pacifico 2019 reported subgroup analysis for the SCORAD at week 3 of two different skin type groups: skin type II and skin type II/IV. In skin type II group they reported a mean difference of 2.30 (CI ‐1.85 to 6.45) at week 3. In skin type II/IV they reported a mean difference of ‐20.92 (CI ‐28.68 to ‐13.15) at week 3. See Analysis 16.3.
16.3. Analysis.

Comparison 16: High dose UVA1 versus medium dose UVA1, Outcome 3: Subgroup analysis (Skin type): Physician‐assessed changes in the clinical signs (short term) ‐ SCORAD
Patient‐reported changes in symptoms
None of the trials measured this outcome.
Secondary outcomes
Investigator Global Assessment
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
Dittmar 2001 had no withdrawals due to adverse events (n=23) during 3 weeks of treatment. See Analysis 16.4.
16.4. Analysis.
Comparison 16: High dose UVA1 versus medium dose UVA1, Outcome 4: Withdrawals due to adverse events
| Withdrawals due to adverse events | ||||
| Study | Timepoint | High dose UVA1 | Medium dose UVA1 | Comments |
| Dittmar 2001 | Up to week 3 | 0 (n=11) |
0 (n=12) |
|
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
17. High dose UVA1 versus low dose UVA1
Only Dittmar 2001 compared high dose UVA1 to low dose UVA1. Dittmar 2001 was a randomised, controlled, parallel, prospective study conducted with 15 treatments (5 a week) over 3 weeks for a total of 34 adult participants.
Primary outcomes
Physician‐assessed changes in clinical signs
The mean difference of SCORAD at week 3 in high dose UVA1 versus low dose UVA1 was ‐12.97 (CI ‐35.16 to 9.22). See Analysis 17.1.
17.1. Analysis.

Comparison 17: High dose UVA1 versus low dose UVA1, Outcome 1: Physician‐assessed changes in the clinical signs (short term) ‐ SCORAD
Patient‐reported changes in symptoms
None of the trials measured this outcome.
Secondary outcomes
Investigator Global Assessment
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
Dittmar 2001 had no withdrawals due to adverse events (n=22) after 3 weeks of treatment. See analysis Analysis 17.2.
17.2. Analysis.
Comparison 17: High dose UVA1 versus low dose UVA1, Outcome 2: Withdrawals due to adverse events
| Withdrawals due to adverse events | ||||
| Study | Timepoint | High dose UVA1 | Low dose UVA1 | Comments |
| Dittmar 2001 | Up to week 3 | 0 (n=11) |
0 (n=11) |
|
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
18. Medium dose UVA1 versus low dose UVA1
Only Dittmar 2001 compared medium dose UVA1 to low dose UVA1. Dittmar 2001 was a randomised, controlled, parallel, prospective study conducted with 15 treatments (5 a week) over 3 weeks. Eleven s were treated with low dose UVA1 and 12 participants received medium dose UVA1.
Primary outcomes
Physician‐assessed changes in clinical signs
Dittmar 2001 showed a mean difference in SCORAD at week 3 of ‐6.75 (CI ‐31.80 to 18.30) for medium dose UVA1 versus low dose UVA1. See Analysis 18.1.
18.1. Analysis.

Comparison 18: Medium dose UVA1 versus low dose UVA1, Outcome 1: Physician‐assessed changes in the clinical signs (short term) ‐ SCORAD
Patient‐reported changes in symptoms
None of the trials measured this outcome.
Secondary outcomes
Investigator Global Assessment
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
Dittmar 2001 had no withdrawals due to adverse events (n=23). See Analysis 18.2.
18.2. Analysis.
Comparison 18: Medium dose UVA1 versus low dose UVA1, Outcome 2: Withdrawals due to adverse events
| Withdrawals due to adverse events | ||||
| Study | Timepoint | Medium dose UVA1 | Low dose UVA1 | Comments |
| Dittmar 2001 | Up to week 3 | 0 (n=12) |
0 (n=11) |
|
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
19. UVA1 medium dose versus UVA1 medium dose cold‐light
Von Kobyletzki 1999b compared medium dose UVA1 with cold light medium dose UVA1. This was a parallel, three‐armed, randomised, active‐control trial with 120 adult participants. Participants received treatment 5 times per week for 3 weeks with 4 weeks of follow‐up post treatment.
Primary outcomes
Physician‐assessed changes in clinical signs
Von Kobyletzki 1999b showed a mean difference in SCORAD of medium dose UVA1 versus cold light medium dose UVA1 at 3 weeks of 5.90 (CI 1.94 to 9.86) in favour of cold light treatment. See Analysis 19.1.
19.1. Analysis.

Comparison 19: UVA1 medium dose versus UVA1 medium dose cold‐light, Outcome 1: Physician‐assessed changes in the clinical signs (short term) ‐ SCORAD
Patient‐reported changes in symptoms
None of the trials measured this outcome.
Secondary outcomes
Investigator Global Assessment
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
There were 6 withdrawals due to adverse events in Von Kobyletzki 1999b in the medium dose UVA1 arm (1 for bacterial superinfection, 5 due to exacerbation of disease); this is compared to 2 withdrawals in the cold light medium dose UVA1 (1 due to eczema herpeticum; 1 due to bacterial superinfection). See Analysis 19.2.
19.2. Analysis.
Comparison 19: UVA1 medium dose versus UVA1 medium dose cold‐light, Outcome 2: Withdrawals due to adverse events
| Withdrawals due to adverse events | ||||
| Study | Timepoint | Medium dose UVA1 | Medium dose UVA1 cold‐light | Comments |
| Von Kobyletzki 1999a | Up to week 3 | 6 (1 for bacterial superinfection, treated with antibiotics; 5 due to discomfort and intense sweating combined with progressive pruritis, leading to exacerbation of disease) (n=50) |
2 (1 due to eczema herpeticum; 1 due to bacterial superinfection requiring additional antiseptic therapy) (n=50) |
|
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
20. UVA1 versus topical corticosteroids
Only Krutmann 1998 compared UVA1 to topical steroids. Krutmann 1998 was a randomised, multi‐centre, three‐armed, parallel study with 53 adult participants, daily treatments conducted over a 10‐day period. They compared UVA1 (daily for 10 days) with topical steroids (fluocortolone 0.5% cream or ointment), applied to the entire body once daily for 10 consecutive days.
Primary outcomes
Physician‐assessed changes in the clinical signs
Krutmann 1998 showed a ‐8.00 (CI ‐16.01 to 0.01) mean difference in Costa score between UVA1 versus topical steroids at 10 days. See Analysis 20.1.
20.1. Analysis.

Comparison 20: UVA1 versus topical steroids, Outcome 1: Physician‐assessed changes in the clinical signs (short term) ‐ Costa
Patient‐reported changes in symptoms
None of the trials measured this outcome.
Secondary outcomes
Investigator Global Assessment
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
No participants in Krutmann 1998 withdrew due to adverse events.
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
21. UVA versus placebo
Reynolds 2001 compared UVA to placebo. This was a 3‐arm randomised, controlled, double‐blind, parallel‐group study with 73 adult participants. 24 participant were randomised to receive UVA1 and 23 to visible fluorescent light (placebo). Phototherapy was administered to the whole body twice weekly for 12 weeks. After this, participants were followed up for a further 3 months. Disease severity was scored based on Sowden and colleagues (Sowden 1991) with the following parameters: erythema, papulovesicles, excoriation, scaling or dryness, and lichenification graded from 0 to 3 at six sites (maximum=90). Assessed at baseline, after 6, 12, 18, and 24 treatments, and 3 months after the final treatment.
Primary outcomes
Physician‐assessed changes in the clinical signs (short‐term)
Reynolds 2001 also reported a mean reduction in total disease activity score of ‐4.40 (CI ‐9.80 to 1.00) for UVA vs placebo at 12 weeks. See Analysis 21.1.
21.1. Analysis.

Comparison 21: UVA versus placebo, Outcome 1: Physician‐assessed changes in the clinical signs ‐ mean reduction in total disease activity score
Patient‐reported changes in symptoms (short‐term)
Reynolds 2001 reported that 12 of 19 participants achieved a reduction in itch VAS on treatment with UVA compared to 10 of 19 treated with placebo at 12 weeks. This gave a risk ratio of 1.20 (CI 0.69 to 2.07). See Analysis 21.2.
21.2. Analysis.

Comparison 21: UVA versus placebo, Outcome 2: Patient‐reported changes in symptoms ‐ number of participants reporting a reduction in itch VAS (short term)
Secondary outcomes
Investigator Global Assessment (short‐term)
Reynolds 2001 reported that 7 of 19 participants achieved moderate or greater improvement in IGA on treatment with UVA compared to 4 of 19 treated with placebo at 12 weeks. This gave a risk ratio of 1.75 (CI 0.61 to 5.01). See Analysis 21.3.
21.3. Analysis.

Comparison 21: UVA versus placebo, Outcome 3: Investigator Global Assessment (IGA) ‐ number of participants with moderate or greater improvement
Investigator Global Assessment (long‐term)
Reynolds 2001 reported that 6 of 19 participants achieved moderate or greater improvement in IGA following treatment with UVA compared to 6 of 17 treated with placebo at 3 months after the 12‐week treatment course. This gave a risk ratio of 0.89 (CI 0.36 to 2.25). See Analysis 21.3.
Safety: withdrawals due to adverse events
In Reynolds 2001 there were no withdrawals due to adverse events in the UVA arm compared to one withdrawal in the placebo arm (secondary to burning). See Analysis 21.4.
21.4. Analysis.
Comparison 21: UVA versus placebo, Outcome 4: Withdrawals due to adverse events
| Withdrawals due to adverse events | ||||
| Study | Timepoint | UVA1 | Placebo | Comments |
| Reynolds 2001 | Up to week 12 | 0 (n‐=10) |
1 (burning) (n=19) |
|
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
Reynolds 2001 reported that 9 of 19 participants improved in total disease activity score following treatment with UVA compared to 8 of 17 treated with placebo at 3 months after the 12‐week treatment course. This gave a risk ratio of 1.01 (CI 0.50 to 2.01). See Analysis 21.5.
21.5. Analysis.

Comparison 21: UVA versus placebo, Outcome 5: Long‐term control
Reynolds 2001 reported that 14 of 19 participants achieved a reduction in itch VAS following treatment with UVA compared to 11 of 17 treated with placebo at 3 months after the 12‐week treatment course. This gave a risk ratio of 1.14 (CI 0.73 to 1.77). See Analysis 21.5.
22. UVAB versus topical corticosteroids
Krutmann 1998 was the only study that compared UVAB with topical steroids. It was a randomised, multi‐centre, three‐armed, parallel study with 53 adult participants with daily treatments (UVAB or Fluocortolone) conducted over a 10‐day period.
Primary outcomes
Physician‐assessed changes in the clinical signs
Krutmann 1998 showed a mean difference in Costa score of 7.00 (CI ‐1.59 to 15.59) at day 10. See Analysis 22.1.
22.1. Analysis.

Comparison 22: UVAB versus topical steroid, Outcome 1: Physician‐assessed changes in the clinical signs (short term) ‐ Costa
Patient‐reported changes in symptoms
None of the trials measured this outcome.
Secondary outcomes
Investigator Global Assessment
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
No participants in Krutmann 1998 withdrew due to adverse events. See Analysis 22.2.
22.2. Analysis.
Comparison 22: UVAB versus topical steroid, Outcome 2: Withdrawals due to adverse events
| Withdrawals due to adverse events | ||||
| Study | Timepoint | UVAB | Topical corticosteroids | Comments |
| Krutmann 1998 | Up to day 10. | 0 | 0 | |
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
23. UVAB versus ciclosporin
Granlund 2001 was the only study that compared UVAB to ciclosporin. This was a randomised, controlled, parallel group, multi‐centre study with 72 adult participants with 1 year follow‐up, during which the participants received different cycles of treatment. UVAB was given 2‐3 times a week with the intention that participants received at least 16 visits per cycle. Ciclosporin was given with initial doses of 4 mg/kg/day.
Primary outcomes
Physician‐assessed changes in the clinical signs
The mean change in SCORAD from baseline in Granlund 2001 was ‐7.00 (CI ‐14.09 to 0.09] at week 10 (2 weeks after completion of round 1).
Patient‐reported changes in symptoms
Granlund 2001 reported that 18 of 30 participants achieved very good or good effectiveness when treated with UVAB compared to 30 of 35 treated with ciclosporin at 8 weeks. The risk ratio was 0.70 (CI 0.51 to 0.97) in favour of ciclosporin. See Analysis 23.2.
23.2. Analysis.

Comparison 23: UVAB versus cyclosporin, Outcome 2: Patient‐reported changes in symptoms ‐ number of participants reporting very good or good efficacy (short term)
Secondary outcomes
Investigator Global Assessment
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
None of the trials measured this outcome.
Health‐related quality of life
The mean difference in the eczema disability index score (range 0‐6) (Salek 1993) for Granlund 2001 was 5.00 (CI ‐1.21 to 11.21) at 8 weeks and 1.00 (CI ‐4.56 to 6.56) at 1 year after up to 5 cycles of treatment. See Analysis 23.3.
23.3. Analysis.

Comparison 23: UVAB versus cyclosporin, Outcome 3: Health‐related quality of life ‐ Eczema disability index score
Long‐term control
For physician‐assessed changes in clinical signs, the mean change in SCORAD from baseline in Granlund 2001 was ‐ 2.00 (CI confidence interval‐5.73 to 9.73] at 1 year (after up to 5 cycles of treatment). See Analysis 23.4.
23.4. Analysis.

Comparison 23: UVAB versus cyclosporin, Outcome 4: Long‐term control
24. Excimer laser versus topical steroid
Brenninkmeijer 2010 was the only study that compared excimer laser to topical steroids. This was a prospective, randomised, within‐participant, controlled study with 13 adult participants conducted over 34 weeks. Participants were either allocated to receive excimer laser twice weekly laser for 10 weeks or clobetasol proprionate 0.05% ointment topically once daily for 10 weeks.
Primary outcomes
Physician‐assessed changes in the clinical signs
The clinical signs in Brenninkmeijer 2010 were assessed using an unnamed scale incorporating number of nodules, excoriation, erythema, induration and pruritus (VAS). The mean difference for excimer laser versus topical steroid was ‐0.50 (CI ‐2.40 to 1.40) at 10 weeks. See Analysis 24.1.
24.1. Analysis.

Comparison 24: Excimer laser versus topical steroid, Outcome 1: Physician‐assessed changes in the clinical signs ‐ unnamed scale: number of nodules, excoriation, erythema, induration and pruritus (VAS) (short term)
Patient‐assessed clinical symptoms
The mean itch VAS reported by participants in Brenninkmeijer 2010 was 3.5 when treated with excimer laser compared to 4.5 when treated with topical steroids at week 10. No dispersion data were reported, so this study could not be included in a forest plot. See Analysis 24.2.
24.2. Analysis.
Comparison 24: Excimer laser versus topical steroid, Outcome 2: Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible
| Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible | ||||
| Study | Measure of effect and timepoint | Excimer laser | Topical corticosteroids | Comments |
| Brenninkmeijer 2010 | Mean itch VAS Week 10 (short term) |
3.5 (n=10) |
4.5 (n=10) |
Split‐body. Data extracted using WebPlotDigitizer. |
Secondary outcomes
Investigator Global Assessment (IGA)
Brenninkmeijer 2010 reported that 1 of 10 participants achieved cleared or almost clear on IGA on the side treated with excimer laser compared to 0 of 10 on the side treated with topical steroid at 10 weeks: odds ratio of 3.32 (CI 0.28 to 39.42). At 34 weeks, 2 of 10 achieved cleared or almost clear on the side treated with excimer laser compared to 0 of 10 on the side treated with topical steroid. This gave an odds ratio of 6.18 (CI 0.53 to 72.07). See Analysis 24.3.
24.3. Analysis.

Comparison 24: Excimer laser versus topical steroid, Outcome 3: Investigator Global Assessment (IGA) ‐ number of participants cleared or almost clear
Safety: withdrawals due to adverse events
There were no withdrawals due to adverse events in either arm of Brenninkmeijer 2010. See Analysis 24.4.
24.4. Analysis.
Comparison 24: Excimer laser versus topical steroid, Outcome 4: Withdrawals due to adverse events
| Withdrawals due to adverse events | ||||
| Study | Timepoint | Excimer laser | Topical corticosteroids | Comments |
| Brenninkmeijer 2010 | Up to week 34. | 0 | 0 | Split‐body study. |
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
For physician‐assessed clinical signs, the mean difference between excimer laser versus topical steroid was ‐2.00 (CI confidence interval‐3.92 to ‐0.08), favouring laser treatment at 34 weeks. See Analysis 24.5.
24.5. Analysis.

Comparison 24: Excimer laser versus topical steroid, Outcome 5: Long‐term control ‐ physician‐assessed changes in clinical signs
For patient‐assessed symptoms, the mean itch VAS in Brenninkmeijer 2010 was 3 in the excimer laser group compared to 4 in the topical steroid group at week 34. No dispersion data were reported, so this study could not be included in a forest plot. See Analysis 24.6.
24.6. Analysis.
Comparison 24: Excimer laser versus topical steroid, Outcome 6: Long‐term control ‐ patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible.
| Long‐term control ‐ patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible. | ||||
| Study | Measure of effect and timepoint | Excimer laser | Topical corticosteroids | Comments |
| Brenninkmeijer 2010 | Mean itch VAS Week 34 |
3 (n=10) |
4 (n=10) |
Split‐body. |
25. Full spectrum light versus no treatment
Byun 2011 was the only study comparing full spectrum light to no treatment. This was an open, randomised, controlled, parallel, prospective study with 38 adult participants receiving treatment for 8 weeks. Phototherapy was administered twice per week for 4 consecutive weeks. The control arm received only emollients twice a day.
Primary outcomes
Physician‐assessed changes in clinical signs
The mean SCORAD in Byun 2011 was 36.81 (11.6 SD) in the full spectrum light arm compared to 35.39 (8.9 SD) in the no treatment arm at week 4.
The mean SCORAD was 30.76 (12.25 SD) in the full spectrum light arm compared to 33.85 (12.15 SD) in the no treatment arm at week 8 (4 weeks after completion of treatment). See Analysis 25.1.
25.1. Analysis.
Comparison 25: Full spectrum light versus no treatment, Outcome 1: Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short term)
| Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short term) | ||||
| Study | Measure of effect and timepoint | Full spectrum light | No treatment | Comments |
| Byun 2011 | Mean (SD) SCORAD Week 8 (4 weeks after end of treatment) |
30.76 (12.25) (n=20) |
33.85 (12.15) (n=18) |
SDs extracted using webplotdigitizer. Not included in a forest plot as comparison considered not clinically relevant. |
| Byun 2011 | Mean (SD) SCORAD Week 4 (end of treatment) |
36.81 (11.6) (n=20) |
35.39 (8.9) (n=18) |
SDs extracted using webplotdigitizer. Not included in a forest plot as comparison considered not clinically relevant. |
Patient‐reported changes in symptoms
The number of participants self‐reporting an excellent improvement (76% to 100%) at week 8 in Byun 2011was 6/20 in the full spectrum light group compared to 2/18 in the no treatment group at week 8. See Analysis 25.2.
25.2. Analysis.
Comparison 25: Full spectrum light versus no treatment, Outcome 2: Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short term)
| Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short term) | ||||
| Study | Measure of effect and timepoint | Full spectrum light | No treatment | Comments |
| Byun 2011 | Patients’ subjective assessments of clinical
improvement Number of participants with excellent improvement (76–100%) Week 8 (4 weeks after end of treatment) |
6/20 | 2/18 | Not included in a forest plot as comparison considered not clinically relevant. |
Secondary outcomes
Investigator Global Assessment (IGA)
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
No participants withdrew due to adverse events in either arm of the Byun 2011 study. See Analysis 25.3.
25.3. Analysis.
Comparison 25: Full spectrum light versus no treatment, Outcome 3: Withdrawals due to adverse events
| Withdrawals due to adverse events | ||||
| Study | Timepoint | Full spectrum light | No treatment | Comments |
| Byun 2011 | 8 weeks | 0 (n=20) |
0 (n=18) |
|
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
26. NB‐UVB versus NB‐UVB + pimecrolimus
Only Tzung 2006 compared NB‐UVB to NB‐UVB + pimecrolimus. This was a single centre, prospective, randomised, investigator‐blind, within‐participant study. There were 26 participants receiving NB‐UVB twice weekly for 6 weeks with or without pimecrolimus cream twice daily.
Primary outcomes
Physician assessed changes in the clinical signs
The mean reduction in EASI from baseline at 6 weeks in Tzung 2006 was 59% in NB‐UVB + pimecrolimus compared to 55% in NB‐UVB alone. See Analysis 26.1.
26.1. Analysis.
Comparison 26: NB‐UVB + pimecrolimus versus NB‐UVB, Outcome 1: Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short term)
| Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short term) | ||||
| Study | Measure of effect and timepoint | NB‐UVB + pimecrolimus | NB‐UVB | Comments |
| Tzung 2006 | Mean reduction in EASI from baseline 6 weeks |
59% (n=14) |
55% (n=14) |
Split‐body study. Not included in a forest plot as comparison considered not clinically relevant. No dispersion data. |
Patient‐reported changes in symptoms
None of the trials measured this outcome.
Secondary outcomes
Investigator Global Assessment (IGA)
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
None of the trials measured this outcome.
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
27. NB‐UVB versus NB‐UVB + synchronous balneotherapy
Only Heinlin 2011 compared NB‐UVB to NB‐UVB + synchronous balneotherapy. This was a parallel, randomised, controlled trial with 180 participants over 24 weeks. Participants received 3 to 5 sessions a week of either NB‐UVB alone or combined with balneotherapy for up to 35 sessions.
Primary outcomes
Physician‐assessed changes in the clinical signs
The mean SCORAD of Heinlin 2011 at 7 to 12 weeks was 34.6 (22.3 SD) in NB‐UVB alone compared to 25.6 (22 SD) combined with balneotherapy. See Analysis 27.1.
27.1. Analysis.
Comparison 27: NB‐UVB versus NB‐UVB + synchronous balneotherapy, Outcome 1: Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short term)
| Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short term) | ||||
| Study | Measure of effect and timepoint | NB‐UVB | NB‐UVB + synchronous balneotherapy | Comments |
| Heinlin 2011 | Mean (SD) SCORAD 7 to 12 weeks |
34.6 (22.3) (n=54) |
25.6 (22) (n=60) |
Not included in a forest plot as comparison considered not clinically relevant. |
Patient‐reported changes in symptoms
Using the Patient Global Assessment 6‐point Likert scale (improvement from very good to very bad), 55.4% of participants judged their treatment to be very good or good at 7 to 12 weeks in the NB‐UVB alone group compared to 76.3% in the combined group. See Analysis 27.2.
27.2. Analysis.
Comparison 27: NB‐UVB versus NB‐UVB + synchronous balneotherapy, Outcome 2: Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short term)
| Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short term) | ||||
| Study | Measure of effect and timepoint | NB‐UVB | NB‐UVB + synchronous balneotherapy | Comments |
| Heinlin 2011 | Patient global assessment: 6 step likert scale
(improvement from very good to very bad) Percentage of participants who judged treatment to be very good or good 7‐12 weeks |
55.4 (n=54) |
76.3 (n=60) |
Not included in a forest plot as comparison considered not clinically relevant. |
Secondary outcomes
Investigator Global Assessment (IGA)
None of the trials measured this outcome.
Safety: withdrawals due to adverse events
There were six withdrawals due to adverse events in the NB‐UVB group compared to 2 in the combined with balneotherapy group. See Analysis 27.4,
27.4. Analysis.
Comparison 27: NB‐UVB versus NB‐UVB + synchronous balneotherapy, Outcome 4: Withdrawals due to adverse events
| Withdrawals due to adverse events | ||||
| Study | Timepoint | NB‐UVB | NB‐UVB + synchronous balneotherapy | Comments |
| Heinlin 2011 | Up to week 12 | 6 (n=89) |
2 (n=88) |
|
Health‐related quality of life
The mean Sickness Impact Profile summary score (Finlay 1990) at 6 months after end of treatment was 3.3 (5.7 SD) in the NB‐UVB arm compared to 4.3 (7.4 SD) in the combined arm. The mean Sickness Impact Profile summary score at 7 to 12 weeks was 4 (5.5 SD) in the NB‐UVB arm compared to 4.6 (6.8 SD) in the combined arm. See Analysis 27.3.
27.3. Analysis.
Comparison 27: NB‐UVB versus NB‐UVB + synchronous balneotherapy, Outcome 3: Health‐related Quality of Life
| Health‐related Quality of Life | ||||
| Study | Measure of effect and timepoint | NB‐UVB | NB‐UVB + synchronous balneotherapy | Comments |
| Heinlin 2011 | Sickness Impact Profile, summary score Mean (SD) 7‐12 weeks |
4 (5.5) (n=54?) |
4.6 (6.8) (n=60?) |
Not included in a forest plot as comparison considered not clinically relevant. |
| Heinlin 2011 | Sickness Impact Profile, summary score Mean (SD) 6 months after end of treatment |
3.3 (5.7) (n=60) |
4.3 (7.4) (n=52) |
Not included in a forest plot as comparison considered not clinically relevant. |
Long‐term control
For physician‐assessed changes in clinical signs, the mean SCORAD of Heinlin 2011 at 6 months after completing treatment was 25.3 (21.9 SD) in NB‐UVB alone compared to 18 (16.4 SD) combined with balneotherapy.
For patient‐reported symptoms, 49% of participants judged their treatment to be very good or good, 6 months after end of treatment in the NB‐UVB alone group compared to 77.5% in the combined group. See Analysis 27.5.
27.5. Analysis.
Comparison 27: NB‐UVB versus NB‐UVB + synchronous balneotherapy, Outcome 5: Long‐term control
| Long‐term control | ||||
| Study | Measure of effect and timepoint | NB‐UVB | NB‐UVB + synchronous balneotherapy | Comments |
| Heinlin 2011 |
Patient‐reported changes in symptoms Patient global assessment: 6 step likert scale (improvement from very good to very bad) Percentage of participants who judged treatment to be very good or good 6 months after end of treatment |
49 (n=60) |
77.5 (n=52) |
Not included in a forest plot as comparison considered not clinically relevant. |
| Heinlin 2011 |
Physician‐assessed changes in the clinical
signs Mean (SD) SCORAD 6 months after end of treatment |
25.3 (21.9) (n=60) |
18 (16.4) (n=52) |
Not included in a forest plot as comparison considered not clinically relevant. |
28. Saalmann SUP cabin (295 nm to 335 nm) + 15% salt solution versus Saalmann SUP cabin (295 nm to 335 nm) + 3% saline solution
Zimmerman 1994 was the only study to compare Saalmann SUP cabin with 15% salt versus 3% salt. This was a prospective, randomised, parallel‐group study with 8 participants. For both groups, irradiation was carried out in a Saalmann SUP cabin, 295 to 335 nm, in increasing time intervals and doses according to photosensitivity of the skin and manufacturer's recommendations over 4 weeks.
Primary outcomes
Physician‐assessed changes in the clinical signs
None of the trials measured this outcome.
Patient‐reported changes in symptoms
None of the trials measured this outcome.
Secondary outcomes
Investigator Global Assessment (IGA)
Both arms (Saalmann SUP cabin (295 to 335 nm) + 15% salt solution and Saalmann SUP cabin (295 to 335 nm) + 3% saline solution) of Zimmerman 1994 showed 3 participants with very good (complete healing) or good response at week 4. See Analysis 28.1.
28.1. Analysis.
Comparison 28: Saalmann SUP cabin (295 to 335 nm) + 15% salt solution versus Saalmann SUP cabin (295 to 335 nm) + 3% saline solution, Outcome 1: Investigator Global Assessment (IGA) (short term)
| Investigator Global Assessment (IGA) (short term) | ||||
| Study | Measure of effect and timepoint | 15% Dead Sea salt bath | 3% saline bath | Comments |
| Zimmerman 1994 | Number of participants with very good (complete
healing) or good response (>80% healing) Week 4. |
3 (n=4) |
3 (n=4) |
Not included in a forest plot as comparison considered not clinically relevant. |
Subgroup analyses
We were unable to perform subgroup analyses for ‘adults versus children’ or ‘HIV/AIDS participants with atopic eczema’ due to the small number of studies included in each comparison. In addition, these data were not presented separately in any of the studies. One study (Pacifico 2019) reported a subgroup analysis for different Fitzpatrick skin types (see Analysis 16.3).
Safety: withdrawals due to adverse events
None of the trials measured this outcome.
Health‐related quality of life
None of the trials measured this outcome.
Long‐term control
None of the trials measured this outcome.
Discussion
Summary of main results
Atopic eczema is a common chronic inflammatory skin condition with several treatment options available. Therapeutic options for moderate to severe atopic eczema include phototherapy and photochemotherapy. We aimed to give a complete summary of the evidence on clinical effectiveness and safety of the different types of phototherapy, to detect the gaps in evidence, and to determine the future research agenda. We included 32 randomised controlled trials in this review that randomised a total of 1219 participants. Thirteen studies assessed narrowband ultraviolet B (NB‐UVB), so most of the evidence was for this type of phototherapy. Data from the included studies were synthesised into 28 comparisons. We considered NB‐UVB versus no treatment or placebo, NB‐UVB versus UVA1, NB‐UVB versus psoralen plus UVA (PUVA), UVA1 versus PUVA, UVA1 versus no treatment or placebo, and PUVA versus no treatment or placebo as the main comparisons in this review. We found studies assessing four of our six proposed key comparisons, which we reported in summary of findings tables.
NB‐UVB versus placebo or no treatment
We included four studies (89 participants) that compared NB‐UVB with no treatment or placebo. We rated the certainty of evidence for outcomes from these studies as low.
Physician‐assessed changes in clinical signs (assessed using a total disease activity score) may improve more with NB‐UVB than with placebo after 12 weeks of treatment.
For patient‐reported changes in symptoms (number of participants reporting a reduction in itch), itch may be reduced more with NB‐UVB than with placebo after 12 weeks of treatment. After four weeks of treatment, there seems to be very little difference reported between NB‐UVB and no treatment.
NB‐UVB may provide moderate or greater improvement (measured by Investigator Global Assessment (IGA)) than placebo after 12 weeks of treatment.
NB‐UVB may not affect the rate of withdrawal due to adverse events compared to placebo or no treatment. In total, only 4 out of 89 participants withdrew due to adverse events, none of which were serious in nature (reasons for withdrawal included burning, severe irritation, or phototoxic reaction).
None of the studies measured health‐related quality of life (HRQoL).
For further details, see Table 1.
NB‐UVB versus UVA1
We included three studies (66 participants) that compared NB‐UVB with UVA1. These three studies provided very low‐certainty evidence for each of the outcomes.
We are uncertain if there is a difference between groups in clinical signs measured by clinicians (using SASSAD), self‐reported itch, or HRQoL, after six weeks of treatment.
One split‐body trial (13 participants) reported no withdrawals over 12 weeks.
None of the studies measured IGA.
For further details, see Table 2.
NB‐UVB versus PUVA
One study (10 participants, 20 sides) compared NB‐UVB and PUVA (8‐methoxypsoralen (8‐MOP) bath plus UVA).
There was no evidence of a difference between treatment groups in physician‐assessed (modified SCORAD) after six weeks (very low‐certainty evidence). Patient‐reported symptoms were not reported.
We are uncertain whether there is a difference between groups in marked improvement or complete remission (IGA; very low‐certainty evidence). There were no withdrawals due to adverse events over six weeks (very low‐certainty evidence).
The study did not report HRQoL.
For further details, see Table 3.
UVA1 versus PUVA
One study compared UVA1 with PUVA (oral 5‐MOP) in 40 participants.
We are uncertain if there was a difference between groups in physician‐assessed signs (SCORAD) after three weeks of treatment (very low‐certainty evidence). The study did not measure any other outcomes.
For further details, see Table 4.
We did not identify any eligible trials for our other key comparisons of UVA1 or PUVA compared with no treatment or placebo.
Adverse events
Reported adverse events from phototherapy included low rates of phototoxic reaction, severe irritation, UV burn, bacterial superinfection, disease exacerbation, and eczema herpeticum.
Overall completeness and applicability of evidence
This review gives a complete overview of the evidence that is available on phototherapy for atopic eczema. The 32 included studies assessed 12 different phototherapeutic interventions for atopic eczema. Our primary and secondary outcomes were addressed to varying degrees by the evidence we identified.
Although atopic eczema is common in children, the mean age of the study participants was 28 years (range: 5 to 83 years old; five studies did not report the mean age). Most studies recruited either adults or a mixture of adults and young people under the age of 18 years. In nine studies, paediatric participants younger than 18 years of age were eligible for inclusion. Most studies reported the gender of the participants; the number of males and females were similar.
Participants had different Fitzpatrick skin types and severity of disease. Thirteen studies did not report the skin type of their participants, which is limiting, as skin type is a factor that should be taken into account when determining dosage. However, in the studies that did report, almost 90% of participants had skin type II or III, and around twice as many participants had skin type III than II.
All studies, except two, reported baseline severity of atopic eczema. Most studies assessed moderate to severe disease, and mean or median total disease duration of the participants ranged from 1 to 30.3 years; many participants had eczema for over 10 years (only around half of the studies reported duration of the eczema).
Only one small study analysed data according to Fitzpatrick skin type. We were unable to conduct our planned subgroup analyses on either people with HIV or AIDS and atopic eczema, or adults versus children: HIV/AIDS status was not reported, and no studies exclusively investigated the age‐related subgroups. In addition, there was a very small number of studies included in each comparison, and data were not presented separately in any of the studies. No studies made specific distinctions between atopic eczema phenotypes, so we are unable to draw conclusions on which of the phototherapies may be best used, for example for acute versus chronic atopic eczema disease. The effect of seasonal differences on the symptoms and severity of atopic eczema was not mentioned by the majority of the included studies; most trials did not report if they were conducted in summer or winter.
UVB was the most prevalent intervention type assessed by our included studies: approximately 40% of the studies assessed NB‐UVB, which reflects its status as the most recognised and widespread form of phototherapy treatment for atopic eczema. A further five studies assessed BB‐UVB, and one study assessed UVB, but did not specify the type. A quarter of the studies assessed ultraviolet A (UVA), with six studies investigating UVA1 (not including the studies where UVA1 was used as a comparator) given in various doses (low to high dose, including cold‐light therapy).
According to a recent survey among 238 dermatologists in Europe, psoralen‐UVA (PUVA) is the second most frequently prescribed second‐line phototherapeutic treatment for atopic eczema (Vermeulen 2020); however, it was assessed by only two studies. Only single studies assessed full‐spectrum light, balneotherapy, and excimer laser, which are infrequently used phototherapy types.
The following categories of phototherapy were not assessed by any of our included studies:
coal tar plus UVB radiation (Goeckerman therapy);
oral trimethylpsoralen with UVA;
oral khellin in combination with UV;
topical khellin in combination with UV;
heliotherapy; and
excimer lamp.
The trial duration, including active treatment and follow‐up, ranged from 10 days to 1 year; two trials did not mention the total length of follow‐up. The average trial duration was 13 weeks, which we defined as short‐term. Whether longer‐term UV treatment or intermittent courses would be helpful for atopic eczema needs further exploration. Only four studies measured outcomes at six months or more; it would have been more helpful to know how long the treatment lasted, rather than the follow‐up period from start of treatment.
We were able to include 28 comparisons, 21 of which were active comparisons. We selected six comparisons as main comparisons for this review: NB‐UVB, UVA1, or PUVA compared to placebo, no treatment, or to each other. However, only four of these comparisons were assessed by nine of the included studies, which provided low to very low‐certainty evidence. We were only able to pool data for a very small number of outcomes, and only from a maximum of three studies each. NB‐UVB versus PUVA was assessed by one study; PUVA versus UVA1 by one study; and NB‐UVB versus UVA1 by three studies. Meta‐analysis was often not feasible because many comparisons were assessed by only one study, or there were insufficient data (e.g. no dispersion data reported).
Half of the included studies compared one type of phototherapy or photochemotherapy to another type of phototherapy (10 comparisons assessed by 16 studies). Six studies compared phototherapy versus placebo or no treatment. Different dosing regimens of a certain phototherapy type, for example high‐dose UVA1 versus medium‐dose UVA1, were assessed by seven studies. NB‐UVB was compared to NB‐UVB combination therapy in two studies (pimecrolimus and synchronous balneotherapy). Three studies compared phototherapy with topical corticosteroids, one study compared UVAB with ciclosporin, and one study compared Saalmann selective ultraviolet phototherapy (SUP) cabin (295 nm to 335 nm) + 15% salt solution versus Saalmann SUP cabin (295 nm to 335 nm) + 3% saline solution. No studies reported that they provided phototherapy at home.
Most of the included studies (94%) reported our primary outcome, physician‐assessed changes in clinical signs, and 47% assessed patient‐reported changes in symptoms of atopic eczema. SCORAD (objective or compound) was the most commonly used tool for measuring physician‐assessed changes (used by approximately half of the studies). EASI, which is the HOME (Harmonising Outcome Measures for Eczema) initiative approved core instrument for physician‐reported clinical signs, was only used by 2 of the 30 studies assessing physician‐assessed changes. Eight studies assessed the outcome using an unnamed total severity score. Other measurement tools used were Costa, SASSAD, and a modified version of the SCORAD. For patient‐reported symptoms of AE, the POEM, which HOME recommends as the core instrument for measuring this outcome, was not assessed by any included study. Eighty per cent of the studies that assessed this outcome used a single‐item measurement instrument for itch e.g. VAS itch. Other measurement tools used were PGA and Patients’ overall assessment of efficacy. A reason why the HOME core outcomes for trials were not used by most of the included studies is that the majority was published before the core outcome set was developed.
Regarding our secondary outcomes, 18 studies (56%) reported data on safety (i.e. withdrawals due to adverse events), and 10 studies assessed Investigator Global Assessment (IGA). Long‐term control (physician‐assessed or patient‐reported outcomes measured at the closest time point to six months after the end of the course of phototherapy) was evaluated by only four studies (13%). HRQoL was only evaluated by three studies, but again, no study used the HOME initiative’s recommended tools (Dermatology Life Quality Index (DLQI), the Children’s Dermatology Life Quality Index (CDLQI), the Infants' Dermatitis Quality of Life Index (IDQOL)). The measurement tools used were Skindex‐29, Eczema disability index score, and the Sickness Impact Profile.
Almost half of the studies reported their source of funding, with two linked to potential commercial sponsors (Granlund 2001; Heinlin 2011).
Quality of the evidence
We completed GRADE assessments for the results included in all four summary of findings tables. We did not rate the evidence for any of the results at moderate or high certainty. We considered the evidence to be of either low or very low certainty. We downgraded for serious or very serious risk of bias and imprecision.
In the comparison NB‐UVB versus placebo, we rated the evidence for all outcomes as low certainty. We downgraded by one level due to serious imprecision (small sample sizes), and one level due to serious risk of bias. We either had some concerns or considered the studies at high risk of bias. This was usually due to missing outcome data, or concerns with the selection of reported results (e.g. no protocol available to make an assessment).
In the comparison NB‐UVB versus UVA1, we judged the evidence for all outcomes as very low certainty. We downgraded all results by two levels due to very serious risk of bias, as we judged two out of the three included studies at high risk of bias overall. We also downgraded by one or two levels for serious or very serious imprecision (small sample size or wide 95% CI).
In the comparison NB‐UVB versus PUVA, we downgraded physician‐assessed changes in clinical signs, Investigator Global Assessment, and safety (withdrawals due to adverse events) by one level due to serious risk of bias (some concerns in all domains, apart from measurement of the outcome). We downgraded them all by a further two levels due to very serious imprecision (small sample size); Investigator Global Assessment also had a very wide 95% CI.
In the comparison UVA1 versus PUVA, evidence was only available for physician‐assessed changes in clinical signs. We considered it to be very low certainty due to a serious risk of bias (some concerns in three domains), and very serious imprecision (small sample size and wide 95% CI).
The decision whether to downgrade by one or two levels for imprecision was influenced by the width of the confidence interval; the effect of different results within the confidence intervals on the clinical interpretation of effectiveness or safety; the absolute effect size and number of events, participants, and studies contributing to both the reported effect measure and to other relevant outcome data, which could not be combined in meta‐analyses with the reported effect measure.
Potential biases in the review process
We attempted to conduct a comprehensive search for studies, but the four Studies awaiting classification may be a potential source of bias. Review authors independently assessed eligibility of studies to minimise bias in the study selection process. There were some minor deviations from the original protocol, as we became aware of certain factors within the studies as the review progressed, such as the use of the Leicester sign score as an outcome measurement instrument in one of the included studies. Bias may have been introduced by the time points chosen for some of our outcomes. For example, when faced with outcome data with a range of time points, we had to make a decision on which time point to include for the different comparisons. We attempted to minimise this bias by coming to a consensus among all the review authors as to what should be the best time point to include. The decision was made to select a time point (one short‐term and one long‐term outcome measure) based on what was most commonly reported in trials.
The interventions used in included trials varied in their details. This led to difficulty in classification of the intervention for the purpose of subgroup analysis. For example, the studies described as UVA had to be reclassified as broadband UVA, others reclassified to UVA1 based on the frequency of light given. The regimens used also varied, as well as the machines used. We took advice from the phototherapy experts in our group (JF, SI, RD). We acknowledge that other groups may have classified the interventions differently.
While there was a set list of pre‐defined outcomes outlined in the protocol, due to the nature of the trials, we had to deviate from the protocol and include other outcome measures not specified, such as Leicester sign score and disease severity scores that did not fit into one of the validated scores. We discussed these scoring criteria with the lead authors, and decided on the validity of these outcome measures depending on the parameters they included. We decided to include these, as an exclusion would lead to a significant amount of missing data, using the risk of bias tool to mitigate this as far as possible.
We estimated that the potential bias introduced by small deviations from the protocol was not of considerable impact.
Agreements and disagreements with other studies or reviews
Three previously published systematic reviews have evaluated the evidence on phototherapy for atopic eczema. The first systematic review evaluating phototherapy in the treatment of atopic eczema was published in 2007, and did not include PUVA (Meduri 2007). The authors of this review included nine studies, and concluded that UVA1 should be used for acute flares of atopic eczema, and chronic forms of atopic eczema should be treated with NB‐UVB. Meduri 2007 found most of their evidence for UVA1 in trials including participants with acute atopic eczema flares, compared to UVAB. As for chronic atopic eczema, they found more evidence on UVAB and NB‐UVB compared to UVA or UVA1. Eight out of nine trials included in Meduri 2007 are also included in our systematic review. We excluded one trial from our review because it was a non‐randomised controlled trial (non‐RCT) study design. We did not focus on the same investigational theme addressed by Meduri 2007. Many of our included studies did not specify whether their studied population had acute or chronic atopic eczema, and did not report baseline atopic eczema duration and severity, so little data were available to affirm these conclusions. In general, our findings are in line with the findings of Meduri 2007, i.e. we found that most evidence on efficacy was available for NB‐UVB and UVA1, compared to other types of phototherapy in the treatment of atopic eczema.
Two other systematic reviews evaluating the efficacy of phototherapy for atopic eczema, published in 2014 and 2015, also highlighted that the best‐quality evidence on effectiveness was available for the use of NB‐UVB and UVA1 (Garritsen 2014; Pérez‐Ferriols 2015). Garritsen 2014 used GRADE methodology, and developed a treatment algorithm for the use of phototherapy for atopic eczema, based on their findings. They suggested that both medium dose UVA1 and NB‐UVB should be considered first‐choice phototherapeutic treatments.
Regarding the dosing regimen of UVA1, Garritsen 2014 noted that they found little to no difference in efficacy between medium dose UVA1 and high dose UVA1. When we compared medium dose versus high dose UVA1, our analysis showed that physician‐assessed clinical signs were slightly more reduced with high dose UVA1 (short‐term). Evidence from our included studies found that low dose UVA1 was less effective than medium dose and high dose UVA1. However, it should be taken into account that higher doses of UVA1 are associated with photodamage and carcinogeneses.
Unlike Meduri 2007, Garritsen 2014 and Pérez‐Ferriols 2015 did include PUVA; and they found that evidence evaluating the use of PUVA in atopic eczema was scarce. Our findings confirmed this. We only identified and included two trials comparing bath and oral PUVA to either NB‐UVB or UVA1, and we are uncertain if there is a difference between treatments, because the evidence was very low certainty. Interestingly, a recent survey among 238 dermatologists from 30 European countries found that PUVA was the most frequently prescribed choice of phototherapy for atopic eczema after NB‐UVB, despite that fact there is only scant evidence for PUVA.
Garritsen 2014 found that UVAB was more effective at reducing clinical signs than BB‐UVB and UVA, but less effective than UVA1, when assessed by physicians. Another study showed that ciclosporin was more effective than UVAB at reducing clinical signs (Granlund 2001). Garritsen 2014 stated that they would not recommend BB‐UVB, UVA, and full‐spectrum light for the treatment of atopic eczema, due to the small size and low quality of these studies.
Recommendations about other phototherapy modalities included in our review, including balneophototherapy, excimer laser, and Saalmann SUP cabin, were not made by any of these previous reviews. As we identified only single studies assessing each of these phototherapy types, we could not give more than a summary of the results of these studies either.
Our findings are in line with the recommendations in the atopic eczema guidelines from the European Dermatology Forum (EDF), which are currently being updated (Wollenberg 2018). The guidelines' preliminary recommendations state that NB‐UVB and medium dose UVA1 are first‐line treatment options in adults with atopic eczema who do not respond to topical therapy. The EDF guidelines also made recommendations about treatment cycles and maintenance regimens; stating that prolonged or repeated treatment cycles and maintenance regimens should be avoided in all phototherapy modalities.
Studies included in our review used various treatment schedules, but phototherapy was administered two to three times a week in most trials. Dose increments were generally made using a fixed percentage, and an erythema threshold was used by the majority of included studies. No previous reviews made recommendations about dose increments during phototherapy treatment. We included two studies that assessed a dosing regimen of NB‐UVB; they compared a standard increasing dose with a fixed dose, and a fixed dose regimen of NB‐UVB with an optimised regimen (Hoey 2006; Selvaag 2005). However, these studies reported incomplete data, on which further analysis was not possible.
Both Garritsen 2014 and Pérez‐Ferriols 2015 recognised that little information was available on duration of remission, long‐term safety, efficacy in children, or in acute versus chronic atopic eczema. Unfortunately, we were unable to include new data from RCTs that tackled these shortcomings in the evidence. We could only analyse data on long‐term control from four studies, and none of the included studies mentioned a separate evaluation of paediatric participants.
Authors' conclusions
Implications for practice.
We found little evidence for our key comparisons, each of which were assessed by a range of only one to four studies that we were often unable to pool. Furthermore, our key results were based on very low‐ to low‐certainty evidence. This means we cannot draw firm conclusions about the effectiveness and safety of phototherapy for atopic eczema.
Reported adverse events associated with phototherapy included phototoxic reaction, severe irritation, ultraviolet‐induced erythema, bacterial superinfection, exacerbation of disease, and eczema herpeticum. However, rates of occurrence were low, and did not differ between different phototherapy modalities.
However, lack of high quality RCT evidence does not mean lack of effectiveness of these treatments. Besides, the included studies did not provide the data needed to determine how the interventions differ according to age, Fitzpatrick skin type, AE phenotype, or HIV/AIDS co‐morbidity, which limits external validity. The studies assessed our outcomes in the short‐term (less than 16 weeks), which does not align with AE as a long‐term condition. The vast majority of studies did not report long‐term control or duration on remission after the phototherapy treatment course has ended.
We found no studies assessing coal tar plus UVB radiation (Goeckerman therapy), oral trimethylpsoralen with UVA, oral or topical khellin in combination with UV, heliotherapy and excimer lamp. Only two trials investigated PUVA, so there is a lack of evidence to assess this treatment, while it's frequently prescribed in Europe (Vermeulen 2020). Studies in psoriasis showed that there are indications for an increased incidence of actinic keratoses and skin malignancies after systemic PUVA treatment and a positive correlation is seen with the cumulative UVA dose/number of PUVA exposures (Archier 2012; Stern 1998; Henseler 1987; Stern 1994). A Swedish study assessing the risk of skin malignancies in people with AE treated with PUVA did not find any increased risk for melanoma, but confirmed previous reports of an increased incidence of cutaneous squamous cell carcinoma (Lindelöf 1991; Lindelöf 1999). This information should be taken into account when prescribing PUVA.
Our primary outcome physician‐assessed changes in clinical signs was reported by almost all studies (compared to patient‐reported changes in symptoms, which was assessed by just less than half); however, the tools used to measure these outcomes were not HOME core instruments and were very heterogeneous. Safety data related to withdrawals were limited.
Implications for research.
Currently, only very low‐ to low‐certainty evidence is available on the efficacy of narrowband ultraviolet B (NB‐UVB) versus no treatment or placebo, NB‐UVB versus UVA1, and PUVA versus UVA1 or NB‐UVB. We found no studies evaluating the other main comparisons of our review (UVA1 versus no treatment or placebo and psoralenUVA (PUVA) versus no treatment or placebo), so future studies are needed to assess these and our other main comparisons, focusing on NB‐UVB, UVA1, and PUVA. Information on duration of remission and long‐term efficacy and safety (especially skin cancer risk) of phototherapy for atopic eczema is scarce, and more research is needed to investigate these outcomes. Collecting data on (long‐term) safety of combinations of phototherapy with other systemic or topical treatments (e.g. tacrolimus) or certain treatment sequences (e.g. phototherapy after systemic immunomodulating treatment) would also be of interest, as people with moderate to severe atopic eczema receive numerous treatment modalities and sequences.
Studies evaluating the efficacy of phototherapy for atopic eczema use a wide variation of outcome measurements and study parameters. Future studies should use outcome measures that reflect the core outcomes (including core outcome instruments) of the Harmonising Outcome Measures for Eczema (HOME) initiative in order to compare and pool data. As we found that previous studies evaluating the efficacy of phototherapy in atopic eczema reported very little data on (skin specific) quality of life and other self‐reported outcomes, these outcomes should be assessed in future studies.
Trials used different methods for participant selection (including atopic eczema diagnosis), phototherapy dosing regimens, and administration. Future studies should include participants who were diagnosed with atopic eczema using validated criteria, and longer follow‐up periods (≥ six months). More homogeneous study designs, with standardised treatment procedures and cumulative doses should also be used, so that they can be pooled in future systematic reviews. Researchers investigating the effectiveness of phototherapy in trials in which participants are treated with concomitant topical corticosteroids are advised to keep track of the amount of topicals that are used.
Correctly designed randomised controlled trials (RCT) should be used to evaluate the effectiveness and safety of phototherapy for atopic eczema in the future, as insufficient reporting of study methodology may lead to biased assessment of treatment effects (Schulz 1995). Future RCTs should include power calculations to establish that adequate participant numbers are included. We recommend that investigators of future (parallel‐group) RCTs assessing the effectiveness and safety of phototherapy for atopic eczema consult the CONSORT statement (Schulz 2010).
Data on the effectiveness and safety of phototherapy in certain populations, such as children or people with particular skin types are lacking, and should be considered for future research. We emphasise the need of future studies to investigate the effectiveness and safety of phototherapy in people with skin of colour. Phototherapy for acute versus chronic atopic eczema and other phenotypes should be further investigated. Home phototherapy should also be considered in future studies.
In addition to the results of this systematic review evaluating the existing evidence on phototherapy assessed through RCTs, cohort data of clinical daily practice could be useful. Real‐world data on the (long‐term) efficacy of phototherapy, for example from the European TREatment of ATopic eczema (TREAT) Registry Taskforce, could be beneficial to develop recommendations and inform clinical guidelines.
As the costs of atopic eczema per person are rising, due to the introduction of new systemic treatments, such as monoclonal antibodies and Janus kinase (JAK) inhibitors, high‐quality research into the effectiveness, safety, and cost‐effectiveness of skin‐directed alternatives, like phototherapy, is of great importance.
What's new
| Date | Event | Description |
|---|---|---|
| 10 November 2021 | Amended | Clarification made to the PLS regarding the type of phototherapy included |
History
Protocol first published: Issue 2, 2021 Review first published: Issue 10, 2021
Risk of bias
Risk of bias for analysis 1.1 Physician‐assessed changes in clinical signs (mean reduction in total disease activity score) .
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Reynolds 2001 | Low risk of bias | Quote: "Individuals were randomly assigned narrow‐band UVB, broad‐band UVA, or visible fluorescent light by means of the Minim computer program (version 1.5), by one investigator (VF) who was not involved with assessment of patients" Comment: randomisation method described and allocation likely concealed. Baseline characteristics presented in table 1 and look similar across groups. | Low risk of bias | Quote: "We designed a randomised, controlled,
double‐blind trial to assess efficacy of narrowband UVB
and broad‐band UVA (as used, for example, in psoralen
photochemotherapy) as second‐line, adjunctive treatment
in adult patients with moderate to severe atopic
eczema." "Some patients might also have worked out which
treatment they were receiving because of differences
between exposure units or markings on lamps, although
the markings were technical in nature."
Comment:
says 'double blind' but doesn't state who is blinded.
However, the comment in the discussion suggests
participants were blinded to treatment group but may
have guessed due to units or markings on lamps. However,
they acknowledge this is technical in nature so perhaps
unlikely. Assume the people delivering the intervention
weren't blinded as they would know what the units and
markings on the lamps mean. There is nothing to suggest
there were any deviations from the intended
intervention. Quote: "Of the 69 patients who began phototherapy, nine were excluded from analysis because of insufficient follow‐up data. Thus, 60 patients were analysed on an intention‐totreat basis." Comment: they used a 'modified ITT' approach as there were exclusions due to insufficient follow up but these were similar between groups. Participants were analysed in the group they were randomised to. |
Some concerns | Quote: "Of the 69 patients who began phototherapy, nine were excluded from analysis because of insufficient follow‐up data. Thus, 60 patients were analysed on an intention‐totreat basis." Comment: a further 5 from UVB group and 4 from light group withdrew (no reasons given) but were included in the ITT analysis. No sensitivity or other analyses done to investigate risk of bias. Withdrawal reasons not given (other than adverse events) but rates were similar across groups. | Low risk of bias | Quote: "We graded erythema, papulovesicles, excoriation, scaling or dryness, and lichenification on a 0–3 scale at six body sites to provide a maximum score of 90." Comment: not a familiar tool but uses a method similar to other well known tools. Measurements unlikely to differ between groups. Quote: "Investigators unaware of treatment assignment assessed patients." Comment: outcome assessors did not know which group the patients were allocated to. | Some concerns | Comment: no protocol available. Data provided after 6 treatments (every 3 weeks) up to 24 treatments (12 weeks) then at 3 months post‐treatment. Analysis method given seems sensible, but no protocol available to assess if this is what they planned. | Some concerns | Comment: some concerns in two domains, low risk of bias in other domains. |
Risk of bias for analysis 1.3 Patient‐reported changes in symptoms (number of participants reporting a reduction in VAS for itch; short‐term).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Reynolds 2001 | Low risk of bias | Quote: "Individuals were randomly assigned narrow‐band UVB, broad‐band UVA, or visible fluorescent light by means of the Minim computer program (version 1.5), by one investigator (VF) who was not involved with assessment of patients" Comment: randomisation method described and allocation likely concealed. Baseline characteristics presented in table 1 and look similar across groups. | Low risk of bias | Quote: "We designed a randomised, controlled,
double‐blind trial to assess efficacy of narrowband UVB
and broad‐band UVA (as used, for example, in psoralen
photochemotherapy) as second‐line, adjunctive treatment
in adult patients with moderate to severe atopic
eczema." "Some patients might also have worked out which
treatment they were receiving because of differences
between exposure units or markings on lamps, although
the markings were technical in nature"
Comment:
says 'double blind' but doesn't state who is blinded.
However, the comment in the discussion suggests
participants were blinded to treatment group but may
have guessed due to units or markings on lamps. However,
they acknowledge this is technical in nature so perhaps
unlikely. Assume the people delivering the intervention
weren't blinded as they would know what the units and
markings on the lamps mean. There is nothing to suggest
there were any deviations from the intended
intervention. Quote: "Of the 69 patients who began phototherapy, nine were excluded from analysis because of insufficient follow‐up data. Thus, 60 patients were analysed on an intention‐to treat basis." Comment: they used a 'modified ITT' approach as there were exclusions due to insufficient follow up but these were similar between groups. Participants were analysed in the group they were randomised to. |
Some concerns | Quote: "Of the 69 patients who began phototherapy, nine were excluded from analysis because of insufficient follow‐up data. Thus, 60 patients were analysed on an intention‐to treat basis." Comment: a further 5 from UVB group and 4 from light group withdrew (no reasons given) but were included in the ITT analysis. No sensitivity or other analyses done to investigate risk of bias. Withdrawal reasons not given (other than adverse events) but rates were similar across groups. | Low risk of bias | Quote: "Patients graded itch and loss of sleep (as a result of eczema) on a 10 cm visual analogue scale, with none at the left and severe on the right." Comment: a VAS itch scale was used, unlikely to be inappropriate. Measurements unlikely to differ between groups. Quote: "Some patients might also have workedout which treatment they were receiving because of differences between exposure units or markings on lamps, although the markings were technical in nature." Comment: participants did not know which treatment they were receiving, however they might have guessed. | Some concerns | Comment: no protocol available to assess if this is what they planned. | Some concerns | Comment: Some concerns in two domains, low risk of bias in other domains. |
Risk of bias for analysis 1.5 Investigator Global Assessment (number of participants with moderate or greater improvement).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Subgroup 1.5.1 Short‐term | ||||||||||||
| Reynolds 2001 | Low risk of bias | Quote: "Individuals were randomly assigned narrow‐band UVB, broad‐band UVA, or visible fluorescent light by means of the Minim computer program (version 1.5), by one investigator (VF) who was not involved with assessment of patients" Comment: randomisation method described and allocation likely concealed. Baseline characteristics presented in table 1 and look similar across groups. | Low risk of bias | Quote: "We designed a randomised, controlled,
double‐blind trial to assess efficacy of narrowband UVB
and broad‐band UVA (as used, for example, in psoralen
photochemotherapy) as second‐line, adjunctive treatment
in adult patients with moderate to severe atopic
eczema." "Some patients might also have worked out which
treatment they were receiving because of differences
between exposure units or markings on lamps, although
the markings were technical in nature"
Comment:
says 'double blind' and doesn't specifiy state who is
blinded. However, the comment in the discussion suggests
participants were blinded to treatment group but may
have guessed due to units or markings on lamps. However,
they acknowledge this is technical in nature so perhaps
unlikely. Assume the people delivering the intervention
weren't blinded as they would know what the units and
markings on the lamps mean. There is nothing to suggest
there were any deviations from the intended
intervention. Quote: "Of the 69 patients who began phototherapy, nine were excluded from analysis because of insufficient follow‐up data. Thus, 60 patients were analysed on an intention‐to treat basis." Comment: they used a 'modified ITT' approach as there were exclusions due to insufficient follow up but these were similar between groups. Participants were analysed in the group they were randomised to. |
Some concerns | Quote: "Of the 69 patients who began phototherapy, nine were excluded from analysis because of insufficient follow‐up data. Thus, 60 patients were analysed on an intention‐to treat basis." Comment: a further 5 from UVB group and 4 from light group withdrew (no reasons given) but were included in the ITT analysis. No sensitivity or other analyses done to investigate risk of bias. Withdrawal reasons not given (other than adverse events) but rates were similar across groups. | Low risk of bias | Quote: "Global dermatological assessment compared with baseline was graded as: exacerbation of disease, no change, slight improvement, moderate improvement, marked improvement, or complete resolution" Comment: IGA based on a disease activity score ‐ not a familiar tool but uses a method similar to other well known tools. Measurements unlikely to differ between groups. Quote: "Investigators unaware of treatment assignment assessed patients." Comment: outcome assessors did not know which group the patients were allocated to. | Some concerns | Comment: no protocol available. Data provided after treatment period (12 weeks) then at 3 months post‐treatment. Analysis method given seems sensible, but no protocol available to assess if this is what they planned. | Some concerns | Comment: Some concerns in two domains, low risk in other domains. |
| Subgroup 1.5.2 Long‐term | ||||||||||||
| Reynolds 2001 | Low risk of bias | Quote: "Individuals were randomly assigned narrow‐band UVB, broad‐band UVA, or visible fluorescent light by means of the Minim computer program (version 1.5), by one investigator (VF) who was not involved with assessment of patients" Comment: randomisation method described and allocation likely concealed. Baseline characteristics presented in table 1 and look similar across groups. | Low risk of bias | Quote: "We designed a randomised, controlled,
double‐blind trial to assess efficacy of narrowband UVB
and broad‐band UVA (as used, for example, in psoralen
photochemotherapy) as second‐line, adjunctive treatment
in adult patients with moderate to severe atopic
eczema." "Some patients might also have worked out which
treatment they were receiving because of differences
between exposure units or markings on lamps, although
the markings were technical in nature"
Comment:
says 'double blind' and doesn't specifiy state who is
blinded. However, the comment in the discussion suggests
participants were blinded to treatment group but may
have guessed due to units or markings on lamps. However,
they acknowledge this is technical in nature so perhaps
unlikely. Assume the people delivering the intervention
weren't blinded as they would know what the units and
markings on the lamps mean. There is nothing to suggest
there were any deviations from the intended
intervention. Quote: "Of the 69 patients who began phototherapy, nine were excluded from analysis because of insufficient follow‐up data. Thus, 60 patients were analysed on an intention‐to treat basis." Comment: they used a 'modified ITT' approach as there were exclusions due to insufficient follow up but these were similar between groups. Participants were analysed in the group they were randomised to. |
Some concerns | Quote: "Of the 69 patients who began phototherapy, nine were excluded from analysis because of insufficient follow‐up data. Thus, 60 patients were analysed on an intention‐to treat basis." Comment: a further 5 from UVB group and 4 from light group withdrew (no reasons given) but were included in the ITT analysis. No sensitivity or other analyses done to investigate risk of bias. Withdrawal reasons not given (other than adverse events) but rates were similar across groups. | Low risk of bias | Quote: "Global dermatological assessment compared with baseline was graded as: exacerbation of disease, no change, slight improvement, moderate improvement, marked improvement, or complete resolution" Comment: IGA based on a disease activity score ‐ not a familiar tool but uses a method similar to other well known tools. Measurements unlikely to differ between groups. Quote: "Investigators unaware of treatment assignment assessed patients." Comment: outcome assessors did not know which group the patients were allocated to. | Some concerns | Comment: no protocol available. Data provided after treatment period (12 weeks) then at 3 months post‐treatment. Analysis method given seems sensible, but no protocol available to assess if this is what they planned. | Some concerns | Comment: Some concerns in two domains, low risk in other domains. |
Risk of bias for analysis 2.1 Physician‐assessed changes in clinical signs (SASSAD; short‐term).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Gambichler 2009 | Low risk of bias | Quote: "Using the software for parallel group randomized trials recently introduced by Saghaei, all patients were randomly allocated to start treatment with either NB‐UVB or UVA1." Comment: used computer software to randomly assign treatments, it's likely the allocation was concealed. Quote: "Mean ± SD baseline SASSAD of therapy period A (P = 0.66) andB (P = 0.56) did not differ significantly between the UVA1 and NB‐UVB treatment arms (Table 5)." Comment: no differences in SASSAD scores at baseline. Other measures seem similar in table 1. | High risk of bias | Quote: "Study staff and participants were blinded to
treatment assignment. Only the study statistician and
the data monitoring assistant saw unblinded data, but
neither had any contact with study
participants."
Comment: participants and people
delivering intervention were blinded to treatment
allocation Quote: "Twenty‐eight patients who completed both UVA1 and NB‐UVB phototherapy courses on an ITT basis were analysed according to the two by two crossover design as follows." Comment: they refer to the analysis at 'intention to treat' but according to table 1 only 28 participants were included in the analysis (including the 1st phase), so it seems participants were excluded if they didn't complete the therapy. A higher percentage of people received full therapy and were included in the analysis in the UVA1 ‐ reasons for not continuing treatment were due to inefficency. |
High risk of bias | Comment: results given in table 1 are only for participants who completed both treatments. Data are only presented for 28 of 47 randomised participants = 40% missing outcome data. No sensitivity analyses investigating impact of missing data. Only 28 participants analysed, and slightly more participants receiving NB‐UVB were excluded/dropped out due to inefficancy or requiring systemic therapy. Some reasons for missing data are likely to be related to the outcome e.g. switching treatment due to poor treatment response. | Low risk of bias | Quote: "The clinical outcome was measured using the validated SASSAD score." Comment: well known validated score used to assess this outcome. Measurements likely to be similar across treatments. Quote: "For each patient, the SASSAD scoring was carried out by the same physician who was blinded to the treatment allocation." Comment: outcome assessment was blinded. | High risk of bias | Comment: a clinical trial register entry is avaiable but this was registered in January 2007 ‐ the study started in March 2005. The clinical trial register says SCORAD index was used but in the report SASSAD was chosen instead with no explanation of why. | High risk of bias | Comment: High risk of bias in three domains, low risk of bias in the other domains. |
Risk of bias for analysis 2.3 Patient‐reported changes in symptoms ( VAS for pruritus; short‐term).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Gambichler 2009 | Low risk of bias | Quote: "Using the software for parallel group randomized trials recently introduced by Saghaei, all patients were randomly allocated to start treatment with either NB‐UVB or UVA1." Comment: used computer software to randomly assign treatments, it's likely the allocation was concealed. Quote: "Mean ± SD baseline SASSAD of therapy period A (P = 0.66) andB (P = 0.56) did not differ significantly between the UVA1 and NB‐UVB treatment arms (Table 5)." Comment: no differences in SASSAD scores at baseline. Other measures seem similar in table 1. | High risk of bias | Quote: "Study staff and participants were blinded to
treatment assignment. Only the study statistician and
the data monitoring assistant saw unblinded data, but
neither had any contact with study
participants."
Comment: participants and people
delivering intervention were blinded to treatment
allocation Quote: "Twenty‐eight patients who completed both UVA1 and NB‐UVB phototherapy courses on an ITT basis were analysed according to the two by two crossover design as follows." Comment: they refer to the analysis at 'intention to treat' but according to table 1 only 28 participants were included in the analysis (including the 1st phase), so it seems participants were excluded if they didn't complete the therapy. There appear to be inappropriate exclusions, for refusal to continue treatment or switching to alternative treatment. A higher percentage of people received full therapy and were included in the analysis in the UVA1 ‐ reasons for not continuing treatment were due to inefficency. |
High risk of bias | Comment: results given in table 1 are only for participants who completed both treatments. Data are only presented for 28 of 47 randomised participants = 40% missing outcome data. No sensitivity analyses investigating impact of missing data. Only 28 participants analysed, and slightly more participants receiving NB‐UVB were excluded/dropped out due to inefficancy or requiring systemic therapy. Some reasons for missing data are likely to be related to the outcome e.g. switching treatment due to poor treatment response. | Low risk of bias | Quote: "At these times, pruritus was also estimated by each patient using a visual analogue scale (VAS; range: 0, no itch; 10, maximum itch)." Comment: a common way to assess itch and unlikely to differ between groups. Participant‐assessed outcome and they were blinded to treatment allocation. | Some concerns | Comment: a clinical trial register entry is available but this was registered in January 2007 ‐ the study started in March 2005. No information about outcome or analysis plan on clinical trial register. | High risk of bias | High risk of bias in two domains, some concerns in one domain and low risk of bias in the other domains. |
Risk of bias for analysis 2.5 Health‐related quality of life (German Skindex‐29).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Gambichler 2009 | Low risk of bias | Quote: "Using the software for parallel group randomized trials recently introduced by Saghaei, all patients were randomly allocated to start treatment with either NB‐UVB or UVA1." Comment: used computer software to randomly assign treatments, it's likely the allocation was concealed. Quote: "Mean ± SD baseline SASSAD of therapy period A (P = 0.66) andB (P = 0.56) did not differ significantly between the UVA1 and NB‐UVB treatment arms (Table 5)." Comment: no differences in SASSAD scores at baseline. Other measures seem similar in table 1. | High risk of bias | Quote: "Study staff and participants were blinded to
treatment assignment. Only the study statistician and
the data monitoring assistant saw unblinded data, but
neither had any contact with study
participants."
Comment: participants and people
delivering intervention were blinded to treatment
allocation. Quote: "Twenty‐eight patients who completed both UVA1 and NB‐UVB phototherapy courses on an ITT basis were analysed according to the two by two crossover design as follows." Comment: they refer to the analysis at 'intention to treat' but according to table 1 only 28 participants were included in the analysis (including the 1st phase), so it seems participants were excluded if they didn't complete the therapy. There appear to be inappropriate exclusions, for refusal to continue treatment or switching to alternative treatment. A higher percentage of people received full therapy and were included in the analysis in the UVA1 ‐ reasons for not continuing treatment were due to inefficency. |
High risk of bias | Comment: results given in table 1 are only for participants who completed both treatments. Data are only presented for 28 of 47 randomised participants = 40% missing outcome data. No sensitivity analyses investigating impact of missing data. Only 28 participants analysed, and slightly more participants receiving NB‐UVB were excluded/dropped out due to inefficancy or requiring systemic therapy. Some reasons for missing data are likely to be related to the outcome e.g. switching treatment due to poor treatment response. | Low risk of bias | Quote: "Skin disease‐specific health‐related quality of life was measured using the German adaptation of Skindex‐29, a validated, self‐administered, 30‐item questionnaire." Comment: unlikely to differ between groups. Outcome was participant‐assessed, and they were blinded to study group. | Some concerns | Comment: a clinical trial register entry is available but this was registered in January 2007 ‐ the study started in March 2005. No information about outcome or analysis plan on clinical trial register. | High risk of bias | Comment: High risk of bias in two domains, some concerns in one domain and low risk of bias in the other domains. |
Risk of bias for analysis 3.2 Investigator Global Assessment (number of participants with marked improvement or complete remission; short‐term).
| Study | Bias | |||||||||||
| Randomisation process | Deviations from intended interventions | Missing outcome data | Measurement of the outcome | Selection of the reported results | Overall | |||||||
| Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | Authors' judgement | Support for judgement | |
| Der‐Petrossian 2000 | Some concerns | Quote: "We have investigated this issue by means of a randomized investigator‐blinded half‐side comparison study." Comment: study refered to as randomised but no details of sequence or whether allocation was concealed. No information on baseline differences. | Some concerns | Quote: "We have investigated this issue by means of a
randomized investigator‐blinded half‐side comparison
study."
Comment: only investigators were blinded.
There doesn't seem to be any deviations from intended
intervention. Quote: "Of the 12 patients who had entered the study, two were excluded from evaluation. One patient experienced an exacerbation of AD after 3 weeks of treatment and had started to take oral corticosteroids. In the other patient considerably fewer erythema reactions were recorded in response to bath‐PUVA as compared with narrowband UVB, and thus the criterion of equi‐erythemogenic dosages was not fulfilled." Comment: two patients were excluded from analyses which is not appropriate. Only 2 patients excluded out of 12 so unlikely to have a large impact on the results. |
Some concerns | Quote: "Of the 12 patients who had entered the study, two were excluded from evaluation." Comment: two patients were not included in the analysis (83% were analysed). Only two patients not included (excluded by study authors) and unlikely to affect overall results. | Low risk of bias | Comment: based on a modified version of SCORAD which seems appropriate and unlikely to differ between groups. Quote: "The half‐side evaluation was always performed by the same blinded investigator (A.T.)." Comment: the outcome assessment was blinded. | Some concerns | Comment: no protocol or analysis plan provided. No information available to make a judgement. | Some concerns | Comment: Some concerns in four domains and low risk of bias in the fifth domain. |
Acknowledgements
The Cochrane Skin editorial base wishes to thank the American Academy of Dermatology (AAD) Guidelines Working Group for commenting on the review, as well as the clinical referee, Professor Sara Brown, and the consumer referee, Amanda Roberts. We would also like to thank Cochrane Dermatology Editor Luigi Naldi, Statistical Editor Matthew Grainge, Network Associate Editor Jen Hilgart for reviewing methods, Iris Gordon, who reviewed the search methods at protocol stage, and Vicki Pennick, who copy‐edited the review.
Appendices
Appendix 1. CRSW online search strategy
1. eczema* or dermatit* or neuro dermatit* or neurodermatit* AND INREGISTER 2. ultraviolet or ultra‐violet or UV or UVA or UVA1 or UVB or UVAB or NBUVB or NUVB or BUVB or BBUVB or PUVA or PUVA1 or PUVB AND INREGISTER 3. narrowband* or NB or broadband* or narrow band* or broad band* AND INREGISTER 4. photother* or photo‐ther* or photoradi* or photo‐radi* or photochemo* or photo‐chemo* or chemophotothera* or photodynam* or photo‐dynam* or photopheres* or chromotherap* or chromo‐ther* or PDT or IPL AND INREGISTER 5. psoralen* or furocoumarin* or furanocoumarin* or ficusin* or khellin* or visammin* or deltasoralen* or ammoidin* or meladinin* or meloxin* or methoxa* or methoxsa* or oxsoralen or ultramop or ultra‐MOP or xanthotoxin* or dermox or puvalen* or methoxypsoralen* or geroxalen* or 8‐MOP or 8MOP or 5‐MOP or 5MOP or trioxsale* or trioxysale* or nsc‐71047 or nsc71047 or trimethylpsoral* or trisoralen AND INREGISTER 6. heliother* or helio‐ther* or heliothalasso* or helio‐thalas* AND INREGISTER 7. excimer* or 308 nm or 308nm or MEL or xenon chloride or XTRAC AND INREGISTER 8. balneophoto* or balneo‐photo* or balneology AND INREGISTER 9. coal tar AND INREGISTER 10. low‐level light therap* AND INREGISTER 11. photosensitizing agents or 5 methoxypsoralen or furocoumarins or methoxsalen or trioxsalen AND INREGISTER 12. goeckerman* AND INREGISTER 13. (light and (therap* or treatment*)) AND INREGISTER 14. ((full spectrum or blue or intense pulsed or cold) and light) AND INREGISTER 15. #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 16. #1 AND #15
Appendix 2. CENTRAL search strategy, in the Cochrane Library
#1 MeSH descriptor: [Eczema] explode all trees #2 MeSH descriptor: [Dermatitis, Atopic] explode all trees #3 (atopic dermatit*):ti,ab #4 (atopic and (neuro dermatit* or neurodermatit*)):ti,ab #5 MeSH descriptor: [Neurodermatitis] this term only #6 eczema*:ti,ab #7 {OR #1‐#6} #8 MeSH descriptor: [Phototherapy] this term only #9 MeSH descriptor: [Heliotherapy] this term only #10 MeSH descriptor: [Intense Pulsed Light Therapy] this term only #11 MeSH descriptor: [Low‐Level Light Therapy] this term only #12 MeSH descriptor: [Photochemotherapy] this term only #13 MeSH descriptor: [Ultraviolet Therapy] this term only #14 MeSH descriptor: [PUVA Therapy] explode all trees #15 MeSH descriptor: [Ultraviolet Rays] this term only #16 MeSH descriptor: [Photosensitizing Agents] this term only #17 MeSH descriptor: [5‐Methoxypsoralen] this term only #18 MeSH descriptor: [Furocoumarins] explode all trees #19 MeSH descriptor: [Methoxsalen] this term only #20 MeSH descriptor: [Trioxsalen] this term only #21 MeSH descriptor: [Lasers, Excimer] this term only #22 photo*:so #23 (ultraviolet or ultra violet or UV or UVA or UVA1 or UVB or UVAB or NBUVB or NUVB or BUVB or BBUVB or PUVA or PUVA1 or PUVB):ti,ab #24 (narrowband* or NB or broadband* or narrow band* or broad band*):ti,ab #25 ((full spectrum or blue or intense pulsed or cold) and light):ti,ab #26 (light and (therap* or treatment*)):ti,ab #27 (photother* or photo ther* or photoradi* or photo radi* or photochemo* or photo chemo* or chemophotothera* or photodynam* or photo dynam* or photopheres* or chromotherap* or chromo ther* or PDT or IPL):ti,ab #28 (psoralen* or furocoumarin* or furanocoumarin* or ficusin* or khellin* or visammin* or deltasoralen* or ammoidin* or meladinin* or meloxin* or methoxa* or methoxsa* or oxsoralen or ultramop or ultra MOP or xanthotoxin* or dermox or puvalen* or methoxypsoralen* or geroxalen* or 8 MOP or 8MOP or 5 MOP or 5MOP or trioxsale* or trioxysale* or nsc 71047 or nsc71047 or trimethylpsoral* or trisoralen):ti,ab #29 goe?kerman*:ti,ab #30 (heliother* or helio ther* or heliothalasso* or helio thalas*):ti,ab #31 (excimer* or 308 nm or 308nm or MEL or xenon chloride or XTRAC):ti,ab #32 MeSH descriptor: [Balneology] this term only #33 (balneophoto* or balneo photo*):ti,ab #34 MeSH descriptor: [Coal Tar] this term only #35 {OR #8‐#34} #36 #7 and #35
Appendix 3. MEDLINE Ovid search strategy
1. Eczema/
2. Dermatitis, Atopic/
3. (atopic adj6 (dermatit* or neurodermati*)).tw,kf,ot.
4. (disseminated adj4 (neurodermatit* or neuro‐dermatit*)).tw,kf,ot.
5. eczema.tw,kf,ot.
6. or/1‐5
7. phototherapy/ or heliotherapy/ or intense pulsed light therapy/ or low‐level light therapy/ or photochemotherapy/ or ultraviolet therapy/
8. exp PUVA Therapy/
9. Ultraviolet Rays/
10. Photosensitizing Agents/
11. 5‐Methoxypsoralen/
12. exp Furocoumarins/
13. Methoxsalen/
14. Trioxsalen/
15. Lasers, Excimer/
16. photo*.jw.
17. (ultraviolet or ultra‐violet or UV or UVA or UVA1 or UVB or UVAB or NBUVB or NUVB or BUVB or BBUVB or PUVA or PUVA1 or PUVB).tw,ot,kf.
18. (narrowband* or NB or broadband* or narrow band* or broad band*).tw,kf.
19. ((full spectrum or blue or intense pulsed or cold) adj light).tw,kf.
20. (light adj2 (therap* or treatment*)).tw.
21. (photother* or photo‐ther* or photoradi* or photo‐radi* or photochemo* or photo‐chemo* or chemophotothera* or photodynam* or photo‐dynam* or photopheres* or chromotherap* or chromo‐ther* or PDT or IPL).tw,kf.
22. (psoralen* or furocoumarin* or furanocoumarin* or ficusin* or khellin* or visammin* or deltasoralen* or ammoidin* or meladinin* or meloxin* or methoxa* or methoxsa* or oxsoralen or ultramop or ultra‐MOP or xanthotoxin* or dermox or puvalen* or methoxypsoralen* or geroxalen* or 8‐MOP or 8MOP or 5‐MOP or 5MOP or trioxsale* or trioxysale* or nsc‐71047 or nsc71047 or trimethylpsoral* or trisoralen).tw,kf,ot.
23. goe?kerman*.tw,kf.
24. (heliother* or helio‐ther* or heliothalasso* or helio‐thalas*).tw,kf.
25. (excimer* or 308 nm or 308nm or MEL or xenon chloride or XTRAC).tw,kf.
26. Balneology/
27. (balneophoto* or balneo‐photo*).tw,kf.
28. Coal Tar/
29. or/7‐28
30. randomized controlled trial.pt.
31. controlled clinical trial.pt.
32. randomized.ab.
33. placebo.ab.
34. clinical trials as topic.sh.
35. randomly.ab.
36. trial.ti.
37. 30 or 31 or 32 or 33 or 34 or 35 or 36
38. exp animals/ not humans.sh.
39. 37 not 38
40. 6 and 29 and 39
[Lines 30‐39: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision); Ovid format, from section 3.6.1 in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, Noel‐Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook]
Appendix 4. Embase Ovid search strategy
1. eczema/ 2. atopic dermatitis/ 3. (atopic adj6 (dermatit* or neurodermatit* or neuro‐dermatit*)).tw,kw,ot. 4. (disseminated adj4 (neurodermatit* or neuro‐dermatit*)).tw,kw,ot. 5. eczema*.tw,kw,ot. 6. 1 or 2 or 3 or 4 or 5 7. phototherapy/ or heliotherapy/ or intense pulsed light therapy/ or low level laser therapy/ or ultraviolet phototherapy/ 8. photochemotherapy/ 9. exp PUVA/ 10. ultraviolet radiation/ 11. photosensitizing agent/ 12. bergapten/ 13. exp furocoumarin derivative/ 14. methoxsalen/ 15. trioxysalen/ 16. excimer laser/ 17. photo*.jn. 18. (ultraviolet or ultra‐violet or UV or UVA or UVA1 or UVB or UVAB or NBUVB or NUVB or BUVB or BBUVB or PUVA or PUVA1 or PUVB).tw,ot,kw. 19. (narrowband* or NB or broadband* or narrow band* or broad band*).tw,kw,ot. 20. ((full spectrum or blue or intense pulsed or cold) adj light).tw,kw,ot. 21. (light adj2 (therap* or treatment*)).tw,kw,ot. 22. (photother* or photo‐ther* or photoradi* or photo‐radi* or photochemo* or photo‐chemo* or chemophotothera* or photodynam* or photo‐dynam* or photopheres* or chromotherap* or chromo‐ther* or PDT or IPL).tw,kw,ot. 23. (psoralen* or furocoumarin* or furanocoumarin* or ficusin* or khellin* or visammin* or deltasoralen* or ammoidin* or meladinin* or meloxin* or methoxa* or methoxsa* or oxsoralen or ultramop or ultra‐MOP or xanthotoxin* or dermox or puvalen* or methoxypsoralen* or geroxalen* or 8‐MOP or 8MOP or 5‐MOP or 5MOP or trioxsale* or trioxysale* or nsc‐71047 or nsc71047 or trimethylpsoral* or trisoralen).tw,kw,ot. 24. goe?kerman*.tw,kw,ot. 25. (heliother* or helio‐ther* or heliothalasso* or helio‐thalas*).tw,kw,ot. 26. (excimer* or 308 nm or 308nm or MEL or xenon chloride or XTRAC).tw,kw,ot. 27. balneotherapy/ 28. (balneophoto* or balneo‐photo*).tw,kw,ot. 29. coal tar/ 30. or/7‐29 31. Randomized controlled trial/ 32. Controlled clinical study/ 33. random$.ti,ab. 34. randomization/ 35. intermethod comparison/ 36. placebo.ti,ab. 37. (open adj label).ti,ab. 38. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 39. double blind procedure/ 40. parallel group$1.ti,ab. 41. (crossover or cross over).ti,ab. 42. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 43. (controlled adj7 (study or design or trial)).ti,ab. 44. trial.ti. 45. or/31‐44 46. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 47. human/ or normal human/ 48. 46 and 47 49. 46 not 48 50. 45 not 49 51. 6 and 30 and 50
[Lines 31‐45: Based on terms suggested for identifying RCTs in Embase (section 3.6.2) in Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M‐I, Noel‐Storr A, Rader T, Shokraneh F, Thomas J, Wieland LS. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from: www.training.cochrane.org/handbook]
Appendix 5. ClinicalTrials.gov
Condition or disease: eczema OR "atopic dermatitis" OR neurodermatitis
Intervention/treatment ‐ 3 searches run due to limits on number of terms you can search in one string
phototherapy OR heliotherapy OR photochemotherapy OR ultraviolet OR light OR PUVA OR PUVAB OR balneophototherapy OR balneology OR “helio‐thalassotherapy” OR “coal tar” OR UVA OR UVB OR BUVB OR BBUVB OR narrowband OR broadband OR NBUVB OR NUVB
photoradiation OR chemophototherapy OR PDT OR IPL OR excimer OR XTRAC OR psoralen OR furocoumarin OR furanocoumarin OR ficusin OR khellin OR visammin OR deltasoralen OR ammoidin OR meladinin OR methoxsalen OR methoxypsoralen OR oxsoralen OR ultramop
“ultra‐MOP” OR xanthotoxin OR dermox OR puvalen OR methoxypsoralen OR geroxalen OR “8‐MOP” OR 8MOP OR “5‐MOP” OR 5MOP OR trioxsalen OR trimethylpsoralen OR trisoralen OR photodynamic OR chromotherapy OR “narrow band” OR “broad band”
Applied filters: interventional (trials)
Data and analyses
Comparison 1. NB‐UVB versus placebo/no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Physician‐assessed changes in clinical signs (mean reduction in total disease activity score) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.2 Physician‐assessed changes in clinical signs – incomplete data on which further analysis is not possible (short‐term) | 3 | Other data | No numeric data | |
| 1.3 Patient‐reported changes in symptoms (number of participants reporting a reduction in VAS for itch; short‐term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.4 Patient‐reported changes in symptoms – incomplete data on which further analysis is not possible (short‐term) | 1 | Other data | No numeric data | |
| 1.5 Investigator Global Assessment (number of participants with moderate or greater improvement) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5.1 Short‐term | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5.2 Long‐term | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6 Safety: withdrawal due to adverse events (short‐term) | 3 | Other data | No numeric data | |
| 1.7 Long‐term control | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.1 Physician‐assessed changes in clinical signs (total disease activity score: number of participants improved relative to baseline) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.7.2 Patient‐reported changes in symptoms ‐ itch VAS: number of participants improved relative to baseline | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. NB‐UVB versus UVA1.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Physician‐assessed changes in clinical signs (SASSAD; short‐term) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.2 Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | 2 | Other data | No numeric data | |
| 2.3 Patient‐reported changes in symptoms ( VAS for pruritus; short‐term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.4 Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short‐term) | 2 | Other data | No numeric data | |
| 2.5 Health‐related quality of life (German Skindex‐29) | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.6 Safety: withdrawal due to adverse events | 1 | Other data | No numeric data |
2.6. Analysis.
Comparison 2: NB‐UVB versus UVA1, Outcome 6: Safety: withdrawal due to adverse events
| Safety: withdrawal due to adverse events | |||||
| Study | Time point | NB‐UVB | UVA1 | Comments | RoB 2 |
| Majoie 2009 | Up to 12 weeks (8 weeks treatment, 4 weeks follow‐up) | 0 (N = 13) |
0 (N = 13) |
Split‐body study | Some concerns |
Comparison 3. NB‐UVB versus PUVA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | 1 | Other data | No numeric data | |
| 3.2 Investigator Global Assessment (number of participants with marked improvement or complete remission; short‐term) | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.3 Safety: withdrawal due to adverse events | 1 | Other data | No numeric data |
Comparison 4. UVA1 versus PUVA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Physician‐assessed changes in clinical signs (SCORAD) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 5. NB‐UVB versus UVA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Physician‐assessed changes in the clinical signs (mean reduction in total disease activity score) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5.2 Patient‐reported changes in symptoms (number of participants reporting a reduction in VAS for itch (short‐term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.3 Investigator Global Assessments (number of participants with moderate or greater improvement) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.3.1 Investigator Global Assessments (number of participants with moderate or greater improvement; short‐ term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.3.2 Investigator Global Assessments (number of participants with moderate or greater improvement; long‐term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.4 Safety: withdrawal due to adverse events | 1 | Other data | No numeric data | |
| 5.5 Long‐term control | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.5.1 Physician‐assessed changes in clinical signs (total disease activity score: number of participants improved relative to baseline) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.5.2 Patient‐reported changes in symptoms (VAS for itch: number of participants improved relative to baseline) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. NB‐UVB versus UVAB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | 1 | Other data | No numeric data | |
| 6.2 Safety: withdrawal due to adverse events (short‐term) | 1 | Other data | No numeric data |
Comparison 7. NB‐UVB versus topical corticosteroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | 1 | Other data | No numeric data |
Comparison 8. NB‐UVB with optimised dose by skin reflectance measurements versus NB‐UVB with fixed dose increments .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | 1 | Other data | No numeric data |
Comparison 9. Standard increasing NBUVB versus fixed dose NBUVB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | 1 | Other data | No numeric data |
Comparison 10. UVB 0.8 MED versus UVB 0.4 MED .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | 1 | Other data | No numeric data | |
| 10.2 Patient‐reported changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | 1 | Other data | No numeric data | |
| 10.3 Investigator Global Assessment (short‐term) | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 10.3.1 Number of participants healed or considerably improved | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 10.4 Safety: withdrawals due to adverse events | 1 | Other data | No numeric data |
Comparison 11. UVB versus UVA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Physician‐assessed changes in clinical signs (SCORAD; short‐term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11.2 Investigator Global Assessment (number of participants with excellent improvement; short‐term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 11.3 Safety: withdrawals due to adverse events | 1 | Other data | No numeric data |
Comparison 12. BB‐UVB versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | 1 | Other data | No numeric data | |
| 12.2 Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short‐term) | 1 | Other data | No numeric data | |
| 12.3 Investigator Global Assessment (number of participants healed or considerably improved; short‐term) | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 12.4 Safety: withdrawal due to adverse events (short‐term) | 1 | Other data | No numeric data |
Comparison 13. BB‐UVB versus UVA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | 1 | Other data | No numeric data | |
| 13.2 Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short‐term) | 1 | Other data | No numeric data | |
| 13.3 Investigator Global Assessment (number of participants considerably improved or healed; short‐term) | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 13.4 Safety: withdrawals due to adverse events | 1 | Other data | No numeric data |
Comparison 14. BB‐UVB versus UVAB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Physician‐assessed changes in clinical signs ‐ incomplete data on which further analysis is not possible (short‐term) | 2 | Other data | No numeric data | |
| 14.2 Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short‐term) | 2 | Other data | No numeric data | |
| 14.3 Investigator Global Assessment (number of participants healed or considerably improved; short‐term) | 2 | 96 | Odds Ratio (IV, Random, 95% CI) | 0.14 [0.00, 4.49] |
| 14.4 Safety: withdrawals due to adverse events | 2 | Other data | No numeric data |
Comparison 15. UVA1 versus UVAB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Physician‐assessed changes in clinical signs (short‐term) | 3 | 170 | Std. Mean Difference (IV, Random, 95% CI) | ‐2.10 [‐2.84, ‐1.35] |
| 15.2 Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short term) | 1 | Other data | No numeric data | |
| 15.3 Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short term) | 1 | Other data | No numeric data | |
| 15.4 Investigator Global Assessment (IGA) ‐ number of participants who healed or considerably improved (short term) | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 15.5 Withdrawals due to adverse events | 3 | Other data | No numeric data |
Comparison 16. High dose UVA1 versus medium dose UVA1.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Physician‐assessed changes in the clinical signs (short term) ‐ SCORAD | 2 | 46 | Mean Difference (IV, Random, 95% CI) | ‐8.24 [‐14.14, ‐2.34] |
| 16.2 Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short term) | 1 | Other data | No numeric data | |
| 16.3 Subgroup analysis (Skin type): Physician‐assessed changes in the clinical signs (short term) ‐ SCORAD | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐9.07 [‐31.81, 13.68] |
| 16.3.1 Skin type II | 1 | 13 | Mean Difference (IV, Random, 95% CI) | 2.30 [‐1.85, 6.45] |
| 16.3.2 Skin type II/IV | 1 | 14 | Mean Difference (IV, Random, 95% CI) | ‐20.92 [‐28.68, ‐13.15] |
| 16.4 Withdrawals due to adverse events | 1 | Other data | No numeric data |
Comparison 17. High dose UVA1 versus low dose UVA1.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Physician‐assessed changes in the clinical signs (short term) ‐ SCORAD | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 17.2 Withdrawals due to adverse events | 1 | Other data | No numeric data |
Comparison 18. Medium dose UVA1 versus low dose UVA1.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 18.1 Physician‐assessed changes in the clinical signs (short term) ‐ SCORAD | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 18.2 Withdrawals due to adverse events | 1 | Other data | No numeric data |
Comparison 19. UVA1 medium dose versus UVA1 medium dose cold‐light.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 19.1 Physician‐assessed changes in the clinical signs (short term) ‐ SCORAD | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 19.2 Withdrawals due to adverse events | 1 | Other data | No numeric data |
Comparison 20. UVA1 versus topical steroids.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 20.1 Physician‐assessed changes in the clinical signs (short term) ‐ Costa | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 20.2 Withdrawals due to adverse events | 1 | Other data | No numeric data |
20.2. Analysis.
Comparison 20: UVA1 versus topical steroids, Outcome 2: Withdrawals due to adverse events
| Withdrawals due to adverse events | ||||
| Study | Timepoint | UVA1 | Topical corticosteroids | Comments |
| Krutmann 1998 | Up to day 10. | 0 | 0 | |
Comparison 21. UVA versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 21.1 Physician‐assessed changes in the clinical signs ‐ mean reduction in total disease activity score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 21.2 Patient‐reported changes in symptoms ‐ number of participants reporting a reduction in itch VAS (short term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 21.3 Investigator Global Assessment (IGA) ‐ number of participants with moderate or greater improvement | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 21.3.1 Short term | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 21.3.2 Long term | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 21.4 Withdrawals due to adverse events | 1 | Other data | No numeric data | |
| 21.5 Long‐term control | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 21.5.1 Physician‐assessed changes in the clinical signs ‐ total disease activity score: number of participants improved relative to baseline | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 21.5.2 Patient‐reported changes in symptoms ‐ itch VAS: number of participants improved relative to baseline | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 22. UVAB versus topical steroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 22.1 Physician‐assessed changes in the clinical signs (short term) ‐ Costa | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 22.2 Withdrawals due to adverse events | 1 | Other data | No numeric data |
Comparison 23. UVAB versus cyclosporin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 23.1 Physician‐assessed changes in the clinical signs ‐ mean change SCORAD from baseline (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23.2 Patient‐reported changes in symptoms ‐ number of participants reporting very good or good efficacy (short term) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 23.3 Health‐related quality of life ‐ Eczema disability index score | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23.3.1 Short term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23.3.2 Long term | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23.4 Long‐term control | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 23.4.1 Physician‐assessed changes in the clinical signs ‐ mean change SCORAD from baseline | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
23.1. Analysis.

Comparison 23: UVAB versus cyclosporin, Outcome 1: Physician‐assessed changes in the clinical signs ‐ mean change SCORAD from baseline (short term)
Comparison 24. Excimer laser versus topical steroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 24.1 Physician‐assessed changes in the clinical signs ‐ unnamed scale: number of nodules, excoriation, erythema, induration and pruritus (VAS) (short term) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 24.2 Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible | 1 | Other data | No numeric data | |
| 24.3 Investigator Global Assessment (IGA) ‐ number of participants cleared or almost clear | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 24.3.1 Short term | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 24.3.2 Long term | 1 | Odds Ratio (IV, Random, 95% CI) | Totals not selected | |
| 24.4 Withdrawals due to adverse events | 1 | Other data | No numeric data | |
| 24.5 Long‐term control ‐ physician‐assessed changes in clinical signs | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 24.5.1 Physician‐assessed changes in the clinical signs ‐ unnamed scale: number of nodules, excoriation, erythema, induration and pruritus (VAS) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 24.6 Long‐term control ‐ patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible. | 1 | Other data | No numeric data |
Comparison 25. Full spectrum light versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 25.1 Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short term) | 1 | Other data | No numeric data | |
| 25.2 Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short term) | 1 | Other data | No numeric data | |
| 25.3 Withdrawals due to adverse events | 1 | Other data | No numeric data |
Comparison 26. NB‐UVB + pimecrolimus versus NB‐UVB.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 26.1 Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short term) | 1 | Other data | No numeric data |
Comparison 27. NB‐UVB versus NB‐UVB + synchronous balneotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 27.1 Physician‐assessed changes in the clinical signs ‐ incomplete data on which further analysis is not possible (short term) | 1 | Other data | No numeric data | |
| 27.2 Patient‐reported changes in symptoms ‐ incomplete data on which further analysis is not possible (short term) | 1 | Other data | No numeric data | |
| 27.3 Health‐related Quality of Life | 1 | Other data | No numeric data | |
| 27.4 Withdrawals due to adverse events | 1 | Other data | No numeric data | |
| 27.5 Long‐term control | 1 | Other data | No numeric data |
Comparison 28. Saalmann SUP cabin (295 to 335 nm) + 15% salt solution versus Saalmann SUP cabin (295 to 335 nm) + 3% saline solution.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 28.1 Investigator Global Assessment (IGA) (short term) | 1 | Other data | No numeric data |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Agrawal 2018.
| Study characteristics | ||
| Methods |
Trial design Randomised, parallel‐group, quasi‐experimental study Trial registration number Not reported Country Pakistan Outpatient or hospital Dermatology Department Unit II, King Edward Medical University, Mayo Hospital, Lahore Date trial conducted Not reported Duration of trial participation 8 weeks Additional design details None Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 60 Age Topical corticosteroid group: mean 11 years (range 5 to 40) NB‐UVB group: mean 22 years (range 5 to 53) Sex Male:female was 1.3:1 overall Race/ethnicity/Fitzpatrick skin type Not reported Duration of eczema Not reported Severity of eczema Topical corticosteroid group: mean baseline SCORAD was 35 (range 20 to 50) NB‐UVB group: mean baseline SCORAD was 39 (range 23 to 60) HIV/AIDs comorbidity Not reported Number of withdrawals None Notes None |
|
| Interventions |
Run‐in details Not reported Groups A: betamethasone valerate 0.1% applied twice daily for 4 weeks B: NB‐UVB three times weekly for 8 weeks. Starting dose 75% MED, incremented by 20% each visit if well tolerated. Closed chamber (Philips TL‐01®) Cumulative dose of NB‐UVB not reported Weaning regimen not reported Co‐interventions Both groups were permitted to use emollients Notes None |
|
| Outcomes |
*denotes relevance to this review |
|
| Notes |
Funding source Not reported Declarations of interest Not declared Notes None |
|
Brenninkmeijer 2010.
| Study characteristics | ||
| Methods |
Trial design Prospective, randomised, within‐person, controlled study Trial registration number NTR797; ISRCTN38773821; EUCTR2006‐005602‐31‐NL Country The Netherlands Outpatient or hospital In‐ and outpatient clinic (Netherlands Institute for Pigment Disorders, Department of Dermatology of the AMC in Amsterdam) Date trial conducted November 2006 to August 2007 Duration of trial participation 34 weeks Additional design details None Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 13 Age Mean age 50 years (range 31 to 69) Sex 7 males; 6 females Race/ethnicity/Fitzpatrick skin type 7 skin type II; 3 skin type III; 3 skin type IV Duration of eczema 3 participants had eczema 1 to 5 years; 3 had eczema 5 to 10 years; 7 had eczema greater than 10 years Severity of eczema Physician global assessment was severe for all cases; mean physician assessment of individual signs (0 to maximum 15) was 12.5 (range 9 to 15) HIV/AIDs comorbidity Not reported Number of withdrawals 3; 2 owing to eczema exacerbation and another owing to non‐compliance Notes None |
|
| Interventions |
Run‐in details None Groups Excimer laser: 308 nm xenon chloride excimer laser treatment twice weekly laser for 10 weeks (total = 20) Topical corticosteroid: clobetasol proprionate 0.05% ointment (Dermovate, GlaxoSmithKline) applied by the participant topically once daily for 10 weeks. Corticosteroids and immunomodulators could be applied to other affected areas, but not the target sites for the trial interventions. Emollient could be applied to all lesions throughout. Non‐sedating antihistamines were also permitted. Cumulative dose: not reported Weaning regimen: not reported Co‐interventions It is unclear if the concurrent treatment described for the TCS control group was also the case for the excimer laser group. Notes None |
|
| Outcomes |
*denotes relevance to this review |
|
| Notes |
Funding source Academic medical centre (The Netherlands) Declarations of interest None declared |
|
Byun 2011.
| Study characteristics | ||
| Methods |
Trial design Open, randomized, controlled, parallel, prospective study Trial registration number Not reported Country South Korea (authors addresses and supported by research grant from South Korean university) Outpatient or hospital Not reported Date trial conducted August 2007 to July 2008 Duration of trial participation Active treatment ‐ 4 weeks, follow‐up after cessation of therapy ‐ 4 weeks Additional design details None Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 38 (FSL group N = 20, control group N = 18) Age FSL group 25.68 ± 7.69 years, control group 25.63 ± 8.41 years; range 25 to 48 years Sex FSL group 8 male 12 female, control group 9 male 9 female. Race/ethnicity/Fitzpatrick skin type All participants were Korean with skin of phototypes III or IV Duration of eczema FSL group 11.09 years, control group 10.95 years Severity of eczema SCORAD mean (SD) FSL group 47.87 ± 15.45, control group 39.79 ± 9.76 HIV/AIDs comorbidity Not reported Number of withdrawals None reported Notes There were no statistically significant differences in baseline SCORAD values between the two groups. (P = 0.167 Mann‐Whitney U‐test). |
|
| Interventions |
Run‐in details None Groups Full‐spectrum light Delivered using the FSL®, (BMC Co. LTD, Anyang‐si, South Korea) device (320 nm to 5000 nm). Further details on the Full Spectrum Light device are in the article. Twice per week for 4 consecutive weeks. The anterior side of the body was irradiated for 20 minutes then the posterior side of the body for 20 minutes. Total treatments: 8. Dosage: fluence of each irradiation was 530 J/cm2 including 121 J/cm2 of UVA and 409 J/cm2 of visible and infrared light Pilot study showed exposures of 20 minutes were effective and safe. Weaning regimen: not reported Cumulative dose: not reported Control Emollient applied twice daily without any other treatment Co‐interventions Emollient only, physiogel was permitted in both groups, topical or systemic agents were not permitted in either group Notes None |
|
| Outcomes |
*denotes relevance to this review Funding source Supported by research grant from Chung‐Ang University awarded in 2010 Declarations of interest None reported |
|
| Notes | No mention of how the control group were followed up for adverse events | |
Der‐Petrossian 2000.
| Study characteristics | ||
| Methods |
Trial design Within‐participant, randomised, investigator blinded trial Trial registration number Not reported Country Not reported (author affiliation is the University of Vienna) Outpatient or hospital Not reported Date trial conducted Not reported Duration of trial participation Maximum of 6 weeks. Mean duration ± SD; 40 days ± 2.8 Additional design details None Inclusion criteria
Exclusion criteria None reported Notes None |
|
| Participants |
Total number randomised 12 Age Mean ± SD; 27 ± 11.3 years Sex Not reported Race/ethnicity/Fitzpatrick skin type Skin type I n = 1 Skin type II n = 5 Skin type III n = 4 Duration of eczema Mean ± SD; 17 ± 18.4 years Severity of eczema Inclusion criteria stated chronic severe AD Mean pretreatment SCORAD score ± SD; 67.9 ± 15.6 HIV/AIDs comorbidity Not reported Number of withdrawals 2; one participant experienced an exacerbation after 3 weeks and started to take oral corticosteroids, while the other participant had considerably fewer erythema reactions recorded in response to bath‐PUVA as compared with narrowband UVA, and thus the criteria for equi‐erythemogenic dosages was not fulfilled Notes The remaining 10 participants had a comparable number of erythema responses to both treatments throughout the whole study period. |
|
| Interventions |
Run‐in details Not reported Groups This was a within‐participant study, first the participant received narrowband UVB treatment on one side of the body (according to a prior randomisation), then the participant bathed in the 8‐MOP PUVA bath, then the participant received the UVA treatment on the previously unirradiated body half. Narrowband UVB Three times weekly, the treatment was delivered using a Waldmann UV 3003 lay down irradiation unit (H. Waldmann, Werk fűr Lichttechnik, Schwenningen, Germany) equipped with 15 Philips TL 100W/01 fluorescent tubes. On the treatment day, one‐half of the participant's body, including the whole face, was first exposed to narrowband UVB according to prior randomisation. The other half of the body was shielded with 4 layers of white, tightly woven cotton, which completely prevented the transmission of UV radiation. The initial dosage was 1 minimal erythema dose of narrowband UVB. Subsequent dose increments in both regimens were set to elicit or maintain a slight erythematous reaction. In the absence of erythema, the UV dose was increased by 30% in participants with skin type III and 15% in participant with skin type I/II. In the presence of erythema, the last dose was maintained. Maximum dose: not reported Weaning regimen: not reported Total number of treatments: mean (SD) 17 ± 1.4 Initial dose mean (± SD); NB UVB (mJ/cm2) 235 ± 55 Final single dose mean (± SD); NB UVB (mJ/cm2) 922 ± 138 Cumulative UV dose mean (± SD); NB UVB (J/cm2) 14.0 ± 3.5 8‐MOP bath PUVA After irradiation with narrowband UVB, the participant bathed in the 8‐MOP solution. The bath contained 8‐methoxypsoralen (8‐MOP) 1mg/L. The participant bathed for 15 minutes in 100 L of tap water at 38 °C. After the bath, the skin was gently dried and the previously unirradiated body half exposed to UVA (Waldmann PUVA 4000 lay down unit equipped with 40 Sylvania FR 90 T 12/PUVA fluorescent tubes) The initial dosage was 0.5 minimal phototoxic dose for bath‐PUVA. Subsequent dose increments in both regimens were set to elicit or maintain a slight erythematous reaction. In the absence of erythema, the UV dose was increased by 30% in participants with skin type III and 15% in participants with skin type I/II. In the presence of erythema, the last dose was maintained. Owing to delayed erythema formation, the UVA dose was never increased before 96 hours after the last bath‐PUVA exposure. Maximum dose: not reported Weaning regimen: not reported Total number of treatments: mean (SD) 17 ± 1.4 Initial dose mean (± SD); bath‐PUVA (J/cm2) 1.0 ± 0.7 Final single dose mean (± SD); bath‐PUVA (J/cm2) 3.3 ± 1.7 Cumulative UV dose mean (± SD); bath‐PUVA (J/cm2) 48.3 ± 8.7 Co‐interventions No additional topical or systemic treatments were allowed, except emollients, which were always applied after irradiation. Notes None |
|
| Outcomes |
*Denotes relevance to this study Funding source Not reported Declarations of interest Not reported |
|
| Notes | None | |
Dittmar 2001.
| Study characteristics | ||
| Methods |
Trial design Randomised, controlled, parallel, prospective study Trial registration number Not reported Country Not reported (author affiliated to University of Freiburg, Germany) Outpatient or hospital Not reported Date trial conducted Between 1998 and 1999 Duration of trial participation 3 weeks (15 treatments at 5 per week) Additional design details None Inclusion criteria
Exclusion criteria
Notes Exclusion criteria in German translation also included in the following exclusion criteria:
|
|
| Participants |
Total number randomised 34 (low dose N = 11, medium dose N = 12, high dose N = 11) Age Low dose; average age (years) 31, range (years) 19 to 50 Medium dose; average age (years) 30, range (years) 18 to 57 High dose: average age (years) 29, range (years) 21 to 40 Sex Low dose; female 7 males 4 Medium dose; female 10 males 2 High dose; female 6 males 5 Race/ethnicity/Fitzpatrick skin type Not reported Duration of eczema Not reported Severity of eczema Baseline SCORAD of the three groups as follows: Low dose; 55.22 ± 18.43 Medium dose; 56.29 ± 14.74 High dose; 70.81 ± 9.03 HIV/AIDs comorbidity No participants had HIV (exclusion criteria) Number of withdrawals Low dose; 5 (2 participants lost to follow‐up, 3 participants showed exacerbation of AD and received steroids) Medium dose; 2 (2 participants showed exacerbation of AD and received steroids) High dose; 2 (2 participants lost to follow‐up) Notes Table 5 states 3 participants in the medium dose group withdrew, while the text in both articles states 2 withdrew. |
|
| Interventions |
Run‐in details Not reported Groups
Five times per week for three weeks using the UVA1 24 kW, Sellas/Dr. Honle, Medizintechnik GmbH, Munchen, Germany (340 nm to 430 nm) device MED/MPDs conducted: yes tested for immediate pigmentation dose before randomisation Schedule says participants should receive 15 treatments, but the mean number of treatments received in the low‐ and medium‐dose groups was 14 (15 in high). Weaning regimen: not reported Actual cumulative doses received:
Co‐interventions Only the use of emollient was permitted in addition to 'external nursing care'. No other local or systemic therapies were used. Notes none |
|
| Outcomes |
*denotes relevance to this review Funding source Not reported Declarations of interest Not reported |
|
| Notes | None | |
Gambichler 2009.
| Study characteristics | ||
| Methods |
Trial design Randomised, double‐blind, controlled, two‐treatment two‐period crossover Trial registration number NCT00419406 Country Germany Outpatient or hospital Not reported Date trial conducted March 2005 to December 2007 Duration of trial participation The study included a two‐week initial wash‐out followed by two six‐week treatment periods separated by at least 8 weeks. Participants were followed up for two months post‐treatment. Additional design details None Inclusion criteria Participants with extrinsic atopic eczema (standard criteria including that of Hanifin and Rajka); SASSAD score > 20 (protocol stated > 30) Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 47 Age Mean 37.5 years (range 18 to 83) Sex 23 males; 24 females Race/ethnicity/Fitzpatrick skin type Not reported Duration of eczema Not reported Severity of eczema SASSAD 41.92 ± 12.7 in those receiving NB‐UVB first; 42.87 ± 9.97 in those receiving UVA1 first HIV/AIDs comorbidity Not reported Number of withdrawals Of 22 randomised to UVA1 in the first period:
Of 25 randomised to NB‐UVB in the first period:
Notes None |
|
| Interventions |
Run‐in details There was a two‐week initial wash‐out Groups UVA1: air‐conditioned UVA1 bed Sellamed 24000 (Sellamed, Gevelsberg, Germany), 340 nm to 400 nm, 50J/cm2, three times weekly for 6 weeks (N = 18) NB‐UVB: stand‐up cubicle Cosmedico GP‐42 (Cosmedico Medizintechnik GmbH, VS‐Schwenningen, Germany) cabin fitted with ARIMED 311 fluorescent lamps; 310 nm to 315 nm (peak 311 nm), three times weekly for 6 weeks (N = 18); initial dose 70% of MED, determined by TL‐01 ⁄12W lamp (Philips, Einthoven, the Netherlands), 10% to 20% increments, maximum dose 1.2 J/cm2 for skin phototype II and 1.5 J/cm2 for skin phototypes III and IV Cumulative dose: not reported Weaning regimen: not reported Co‐interventions Not reported Notes None |
|
| Outcomes |
*denotes relevance to this review |
|
| Notes |
Funding source Not reported Declarations of interest Not reported Notes None |
|
Granlund 2001.
| Study characteristics | ||
| Methods |
Trial design Randomised, controlled, parallel group, multi‐centre study Trial registration number Not reported Country Finland and Norway Outpatient or hospital Not reported Date trial conducted Not reported Duration of trial participation Up to one year Additional design details In both arms, treatment was administered intermittently with a treatment period of 8 weeks (treatment phase) followed by a period of only topical treatment (remission phase). The remission phase continued until relapse or at least 2 weeks. The total treatment time was 12 months and contained as many treatment cycles as needed to keep the participant in remission. Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 72 (36 per group) Age UVAB; mean age (SD) years, 33.2 ± 10.6 Cyclosporin; mean age (SD) years, 33.3 ± 12.2 Sex UVAB; 14 males, 21 females Cyclosporin; 21 males, 15 females Race/ethnicity/Fitzpatrick skin type Not reported; people with skin type I were excluded (see exclusion criteria) Duration of eczema UVAB; mean duration (SD) years, 30.0 ± 10.9 Cyclosporin; mean duration (SD) years, 30.3 ± 11.8 Severity of eczema UVAB: Rajka and Langeland baseline mean severity 1989 (SD) 7.7 ± 1.0 Cyclosporin: Rajka and Langeland baseline mean severity 1989 (SD) 7.8 ± 0.8 UVAB: mean SCORAD baseline severity (SD) 46.8 ± 15.3 Cyclosporin: mean SCORAD baseline severity (SD) 48.5 ± 12.7 HIV/AIDs comorbidity Not reported Number of withdrawals One participant who was randomised never appeared for treatment and so was excluded. A further 24 participants discontinued treatment prematurely:
* due to lack of adherence to the treatment schedule or other practical difficulties with treatment Notes Major protocol deviations not resulting in premature withdrawal occurred in 13 participants. Except for concomitant asthma, which was more common in the cyclosporin group, no significant differences in demographics, previous therapy or severity grading were noted at baseline. |
|
| Interventions |
Run‐in details Not reported Groups In both arms, treatment was administered intermittently with a treatment period of 8 weeks (treatment phase) followed by a period of only topical treatment (remission phase). The remission phase continued until relapse or at least 2 weeks. The total treatment time was 12 months and contained as many treatment cycles as needed to keep the participant in remission. UVAB Treatment was received 2 to 3 times per week using Waldmann UV 8001 K phototherapy cabin. It was intended that participants received at least 16 visits per cycle and no more than one cycle was allowed to be incomplete. The initial dose depended on the participant's skin type and on previous experience with UVAB therapy. Successive dose increments were performed at every other treatment visit according to a standard treatment schedule, up to maximal doses of 15 J/cm2 of UVA and 0.26 J/cm2 of UVB. If remission occurred before the maximal dose was achieved, no further dose increments were performed. If erythema appeared, the dose was reduced to the preceding dose. UVAB treatment was stopped in cases of inefficacy, if relevant side effects were observed, at the wish of the participant, in cases of lack of compliance, and if the investigator believed that continuation was detrimental to the participant's health. Total UVA dose at the end of the first cycle was mean (SD) 116 ± 64 J/cm2, UVB 1.5 ± 0.9 J/cm2 Total UVA dose at the end of the fifth cycle was mean (SD) 176 ± 54 J/cm2, UVB 2.3 ± 0.8 J/cm2 MED/MPD conducted: not reported Weaning regimen: not reported Cyclosporin Cyclosporin initial doses 4 mg/kg/day (Microemulsion form). During the first two treatment cycles, the dose was either increased or decreased at each scheduled visit in increments of 1 mg/kg/day, according to response. The lowest dose used was 1 mg/kg/day, and the maximum dose used was 4 mg/kg/day. The second treatment phase was initiated using the lowest effective dose from the first treatment phase. The lowest effective dose in the second cycle was chosen as a constant maintenance dose in subsequent cycles. In case of significant adverse effects, the dose of cyclosporin was decreased or treatment discontinued as per the protocol. Mean dose of cyclosporin at end of cycle 1, 2.7 ± 1.0 mg/kg/day Mean dose of cyclosporin at end of cycle 3, 2.3 ± 1.2 mg/kg/day Co‐interventions Topical non‐halogenated corticosteroids not stronger than hydrocortisone‐17‐butyrate were allowed in order to keep participants in remission. The participants were encouraged to use emollients as needed. Notes None |
|
| Outcomes |
*denotes relevance to this review Funding source “Supported by Novartis Finland and by grants from Finska Lakaresallskapet” Declarations of interest Not reported |
|
| Notes | None | |
Heinlin 2011.
| Study characteristics | ||
| Methods |
Trial design Parallel, randomised, controlled trial Trial registration number Not reported Country Germany Outpatient or hospital Dermatological outpatient practice Date trial conducted Not reported Duration of trial participation Up to 35 treatments (approx 7 to 12 weeks) or early cure, the follow‐up phase was 6 months Additional design details This was a multicentre trial. After completion of the treatment period, no limitation was put on the type or duration of additional active treatments until the end of follow‐up. Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 180 Synchronous balneotherapy (sBPT) N = 90 Narrowband UVB monotherapy (PT) N = 90 Age sBPT mean (SD) 42.5 (16.5); PT 39.5 (16.5) Sex sBPT 61 females (71.8%), PT 50 females (59.5%) Race/ethnicity/Fitzpatrick skin type sBPT skin type I: 3 (3.5%), II: 34 (40.0%), III: 37 (43.5%), IV: 10 (11.8%), V: 1 (1.2%). PT I: 9 (10.7%), II: 41 (48.8%), III: 26 (31.0%), IV: 8 (9.5%), V: 0 Duration of eczema Duration of current attack mean (SD) months, sBPT 5.2 (1.2), PT 5.5 (1.6) Severity of eczema Baseline mean (SD) SCORAD of the sBPT group 61.8 (14.1) Baseline mean (SD) SCORAD of the PT group 61.5 (12.4) HIV/AIDs comorbidity Not reported Number of withdrawals 2 sBPT and 1 PT did not start treatment 4 sBPT and 5 PT withdrew early, before the second evaluation of SCORAD at session 10 (excluded from efficacy but included in the safety analysis) 25 sBPT and 30 PT withdrew before the end of treatment (5 sBPT with clearance and 3 PT with clearance) From the trial participant flow chart, it appears that more participants were lost between the end of treatment and the end of follow‐up phase, although the number of participants lost at this stage is not clear. Notes No significant differences were identified between groups in terms of demographics, SCORAD, or skin type. |
|
| Interventions |
Run‐in details UV therapy and specific systemic therapy for AD had to be stopped 4 weeks before, topical treatment 1 week before the study and were disallowed during the treatment period. Groups Balneophototherapy including UVB (sBPT) Phillips and Okkaido‐Vario‐System Tomesa® Alteglofsheim, Germany. Wavelength: 311 nm 3 to 5 sessions a week, up to 35 sessions in total with increasing treatment duration. Sessions lasted from 15 minutes to 30 minutes, including a bathing time of at least 4 minutes before UV light started. The starting dose was determined according to the individual skin type. All trial physicians were provided with a dose‐escalation schedule for each skin type. The dose per treatment unit was increased by simultaneously prolonging the bathing time. Incremental steps to reach this final dose again depended on the skin type of the participants and a participant’s individual acceptance (erythema threshold). Total treatments: up to 35 treatments Maximum dose: not reported MED/MPD conducted: not reported Weaning regimen: not reported Concurrent treatment: 10% Dead Sea salt (Tomesa®) solution delivered in an anatomically shaped bath tub with a computer‐controlled purification system. Turning over every 4 minutes guaranteed a constant and all over covering of the irradiated skin with the solution. In addition, participants had to moisten their face regularly with salt solution. Mean total light dose received was 34.9 J/cm2. Mean starting UVB dose 0.35 J⁄ cm2 (ranging from 0.09 to 0.56 J⁄ cm2 depending on the skin type) Mean UVB dose after the 35th session was 2.53 J⁄ cm2 (ranging from 0.72 to 3.38 J⁄ cm2 depending on skin type) Participants received an average of 27.3 sessions. Narrowband UVB alone (PT) As above, however, participants lay on a couch placed in the tub instead of bathing. Mean total light dose received was 34.6 J/cm2. Mean starting UVB dose 0.35 J⁄ cm2 (ranging from 0.09 to 0.56 J⁄ cm2 depending on the skin type) Participants received an average of 26.3 sessions Mean UVB dose after the 35th session was 2.85 J⁄ cm2 (1.13 to 3.38 J⁄ cm2) Co‐interventions A proportion of 22.7% of sBPT participants and 24.7% of PT participants used additional topical corticosteroids during therapy, and 46.4% of sBPT participants and 46.6% of PT participants used corticosteroids during the follow‐up period. Notes None |
|
| Outcomes |
Funding source This study was sponsored by the primary health insurance companies in Bavaria, Germany and is completely independent of the producer of the medical devices used. Declarations of interest None declared |
|
| Notes | None | |
Hoey 2006.
| Study characteristics | ||
| Methods |
Trial design Randomised, single‐blinded, parallel‐group Trial registration number Not reported Country Belfast, UK (assumed from author affiliations) Outpatient or hospital Not reported Date trial conducted Not reported Duration of trial participation Unclear; includes 2 months of post‐treatment follow‐up; 18th session SCORAD is also mentioned, but the time between sessions is not reported Additional design details None Inclusion criteria Not reported Exclusion criteria Not reported Notes None |
|
| Participants |
Total number randomised 10 Age Not reported Sex Not reported Race/ethnicity/Fitzpatrick skin type Not reported Duration of eczema Not reported Severity of eczema Not reported HIV/AIDs comorbidity Not reported Number of withdrawals Not reported Notes None |
|
| Interventions |
Run‐in details None Groups UVB‐TL01 standard increasing dose: first treatment was 70% of MED; subsequent treatments were 20% increments; number of treatments and maximum dose not reported UVB‐TL01 fixed dose: first treatment was 70% of MED, there were two subsequent increments, and then this dose was used for the remaining treatments; number of treatments and maximum dose not reported Cumulative dose: not reported Weaning regimen: not reported Co‐interventions Not reported Notes None |
|
| Outcomes | SCORAD at baseline and regular intervals
(unspecified)* Number of participants with a flare* *denotes relevance to this review |
|
| Notes |
Funding source Not reported Declarations of interest Not declared |
|
Jekler 1988a.
| Study characteristics | ||
| Methods |
Trial design Within‐participant, randomised, placebo controlled trial Trial registration number Not reported Country Sweden Outpatient or hospital Daycare centre where people can receive phototherapy without making an appointment Date trial conducted Not reported Duration of trial participation Up to 8 weeks; participants were treated for 8 weeks or until healing of at least one body half Additional design details The participants receiving the UVB treatment appeared to be further randomised into two groups receiving different dosage regimens. Inclusion criteria
Exclusion criteria
Notes Though not explicitly stated in the exclusion criteria, the linked thesis states "Patients with severe disease were excluded as it was considered unethical to withhold potent corticosteroids from these patients (Jekler 1992)." No phototherapy was performed during summer months. |
|
| Participants |
Total number randomised 28 (characteristics and results are only reported for the 17 participants who did not drop out) Age Mean age 24.9 years, range 20 to 42 years Sex 10 men, 7 women Race/ethnicity/Fitzpatrick skin type Skin type 1, 2 participants Skin type 2, 2 participants Skin type 3, 11 participants Skin type 4, 2 participants Duration of eczema 2 to 31 years; mean 20.1 years Severity of eczema Baseline severity score: the participants were assessed for 8 variables, scored 0 to 3 (0 = none, 1 = light, 2 = moderate, and 3 = severe) on the following variables; pruritus, lichenification, scaling, xerosis, vesiculation, excoriations, erythema, and an overall evaluation. Both groups, mean total score 9.9, range 6.5 to 19 Both groups, mean overall evaluation score 1.5, range 1 to 3 HIV/AIDs comorbidity Not reported Number of withdrawals 11. One was excluded because of side effects, namely UVB burn. The remaining 10 stopped treatment on other grounds, primarily intercurrent disease or lack of time for treatment. The linked thesis states, "Even though no patients stated the reason for withdrawal had been lack of efficacy, this may have been a factor" (Jekler 1992). Notes None |
|
| Interventions |
Run‐in details Only mild corticosteroid preparations (hydrocortisone 0.5% to 1%) and emollient creams were allowed as topical treatment during, and 2 weeks prior to the start of phototherapy. Groups UVB 14 Philips TL 12 40W and 14 Philips TL 12 20 W tubes arranged in a cubicle. 280 nm to 315 nm Treatment was given three times a week for a maximum of 8 weeks, or until the healing of at least one body half Each participant's minimal erythema dose (MED) of UVB was determined before the commencement of the phototherapy. The participants were randomized into two treatment groups—one starting with 0.5 MED and one with 1 MED UVB, randomized to the right or left side of the body. In the 0.5 MED group, the dose was increased by 20% each time until erythema appeared, when the dose was decreased to half of the last dose given. Thereafter, the 20% increase schedule was resumed. In the 1 MED group, the doses were similarly increased. However, in this group, no dose reduction was made at the appearance of erythema. Instead, the dose was kept unchanged until erythema was no longer seen. The 20% dose increase schedule was then resumed. The participants were given the same exposure time in the UVB cabinet as in the visible light cabinet; in both cases, one side of the body was shielded with two layers of thick dark cotton sheeting. No treatments were given during the summer months. Weaning regimen: not reported The initial doses were in the range of 20 to 153 mJ/cm2, and the final doses in the range of 63 to 816 mJ/cm2; mean total dose 3.18 J/cm2 Visible light (placebo) The placebo tubes used in this study were ordinary daylight tubes—Osram L 36 W/30—with no measurable UV content. Co‐interventions Only mild corticosteroid preparations (hydrocortisone 0.5% to 1%) and emollient creams were allowed as topical treatment during, and 2 weeks prior to the start of phototherapy. Ten of the 17 participants stated they had used more corticosteroids on the placebo side, while only one had used more on the UVB side. The remaining 6 participants had used equal amounts on both sides, or no topical corticosteroids at all. Two participants used more emollients on the placebo side, 4 used more on the UVB side and 11 used equal amounts on both sides. Notes None |
|
| Outcomes |
*denotes relevance to this review Funding source Supported by a grant from the Edvard Welander Foundation. Declarations of interest Not reported |
|
| Notes | None | |
Jekler 1988b.
| Study characteristics | ||
| Methods |
Trial design Within‐participant, randomised, controlled trial Trial registration number Not reported Country Sweden Outpatient or hospital Daycare centre where people can receive phototherapy without making an appointment Date trial conducted Not reported Duration of trial participation 8 weeks; participants were treated for 8 weeks, or healing of at least one body half Additional design details None Inclusion criteria
Exclusion criteria
Notes No phototherapy was performed during summer months |
|
| Participants |
Total number randomised 31 (characteristics and results only reported for 25 participants, 6 participants were excluded) Age Mean age 25.9 years, range 16 to 59 years Sex 5 men 20 women Race/ethnicity/Fitzpatrick skin type Skin type 1, 0 participants Skin type 2, 8 participants Skin type 3, 15 participants Skin type 4, 2 participants Duration of eczema 4 to 54 years, mean 21.4 years Severity of eczema Baseline severity score: the participants were assessed for 8 variables, scored 0 to 3 (0 = none, 1 = light, 2 = moderate and 3 = severe) on the following variables; pruritus, lichenification, scaling, xerosis, vesiculation, excoriations, erythema, and an overall evaluation Both groups, mean total score 10.7, range 6 to 19 Both groups, mean overall evaluation score 1.6, range 1 to 3 HIV/AIDs comorbidity Not reported Number of withdrawals 6; 1 experienced troublesome UVB burn, 1 experienced no benefit from treatment, 1 had severe AD and could not manage without more potent steroids, the remaining three stopped treatment owing to lack of time Notes None |
|
| Interventions |
Run‐in details Only mild corticosteroid preparations (hydrocortisone 0.5% to 1%) and emollient creams were allowed as topical treatment during, and 2 weeks prior to the start of phototherapy. Groups 14 Philips TL 12 40W and 14 Philips TL 12 20 W tubes arranged in a cubicle 280 nm to 315 nm The MED was determined every other week on the right and left body halves separately. Dose increments were made stepwise every other week on the basis of the MED. One side of the body was shielded with two layers of thick dark cotton sheeting. Participants were treated three times a week for up to 8 weeks, or until one half of the body was healed. UVB 0.8 minimal erythema dose One side of the body was treated with 0.8 MED. The initial doses on the 0.8 MED sides were in the range 14 to 72 mJ/cm2. Final doses were in the range 51 to 173 mJ/cm2. The mean total dose of the UVB 0.8 MED group was 1.08 J/cm2 UVB 0.4 minimal erythema dose One side of the body was treated with 0.4 MED. The initial doses on the 0.4 MED sides were in the range 7 to 36 mJ/cm2. Final doses were in the range 20 to 77 mJ/cm2. The mean total dose of the UVB 0.4 MED group was 0.44 J/cm2. Maximum dose: not reported Weaning regimen: not reported Co‐interventions Only mild corticosteroid preparations (hydrocortisone 0.5% to 1%) and emollient creams were allowed as topical treatment during, and 2 weeks prior to the start of phototherapy. Notes None |
|
| Outcomes |
*denotes relevance to this review Funding source Supported by a grant from the Edvard Welander Foundation. Declarations of interest Not reported |
|
| Notes | None | |
Jekler 1990.
| Study characteristics | ||
| Methods |
Trial design Within‐participant, randomised trial Trial registration number Not reported Country Sweden Outpatient or hospital Not reported Date trial conducted Not reported Duration of trial participation Up to 8 weeks; participants were treated for 8 weeks, or healing of at least one body half Additional design details None Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 39 (characteristics and results are only reported for the 17 participants who completed the study) Age Mean age 24.8 years, range 15 to 40 years Sex 11 men, 19 women Race/ethnicity/Fitzpatrick skin type Skin type 1, 0 participants Skin type 2, 5 participants Skin type 3, 22 participants Skin type 4, 2 participants Skin type 5, 1 participant Duration of eczema Mean disease duration 20.5 years, range 4 to 40 years Severity of eczema Baseline severity score: participants were assessed for 8 variables scored 0 to 3 (0 = none, 1 = light, 2 = moderate, and 3 = severe) on the following variables; pruritus, lichenification, scaling, xerosis, vesiculation, excoriations, erythema, and an overall evaluation Both groups, mean total score 10.8, range 7 to 19 Both groups, mean overall evaluation score 1.7, range 1 to 3 HIV/AIDs comorbidity Not reported Number of withdrawals Nine participants withdrew:
Notes None |
|
| Interventions |
Run‐in details Only mild corticosteroid preparations (hydrocortisone 0.5% to 1%) and emollient creams were allowed as topical treatment during, and 2 weeks prior to the start of phototherapy Groups Treatments were given 3 times per week for 8 weeks or until healing. The side not irradiated was shielded with two layers of thick dark cotton sheeting. UVB 14 Philips TL 12 40W and 14 Philips TL 12 20 W tubes arranged in a cubicle (Philips, Roosendaal, the Netherlands); wavelength: 280 nm to 315 nm Participants were irradiated with UVB on one side of the body The initial dose of the UVB was set at 80% of the MED. It was then increased at each treatment session by 20%. With the appearance of erythema, the dose was reduced by 50% and thereafter, the 20% increase schedule was resumed. UVB: mean initial dose was 37 mJ/cm2; mean final dose was 204 mJ/cm2; mean total dose was 2.47 J/cm2 Maximum dose: not reported Weaning regimen: not reported UVAB 24 Wolff Helarium System tubes B1 to12/100W (Cosmedico, Stuttgart, Germany) in an arrangement similar to that used for UVB therapy; wavelength: 280 nm to 400 nm Participants were irradiated with UVAB on one side of the body For UVAB therapy, a dose increment schedule was set at 5, 7, 10, 12, 15, 17.5, 20, 22.5, and 25 minutes. The dose that preceded the MED was set as the initial dose. Successive dose increments were performed at every other treatment until a maximum of 25 minutes (corresponding to 30 mJ/cm2 UVB and 8.3 J/cm2 UVA). When erythema appeared, the dose was reduced to the preceding dose. In the treatment of participants with insensitive skin (MED ≥ 15 minutes: 18 mJ/cm2 UVB, 5 J/cm2 UVA), the steps at 17.5 and 22.5 minutes were omitted. UVAB: mean initial dose 13 mJ/cm2 (range 6 to 18 mJ/cm2) UVB, and 3.7 J/cm2 (1.7 to 5 J/cm2) UVA. The mean final doses were 29 mJ/cm2 (range 18 to 30 mJ/cm2) UVB, and 8 J/cm2 (range 5 to 8.3 J/cm2) UVA. The mean total dose was 0.47 J/cm2 UVB, and 130 J/cm2 UVA. Weaning regimen: not reported Co‐interventions Only mild corticosteroid preparations (hydrocortisone 0.5% to 1%) and emollient creams were allowed as topical treatment during, and 2 weeks prior to the start of phototherapy. Of 20 participants who were using hydrocortisone at the termination of therapy, 3 stated they had been using more preparation on the UVB‐ treated body half; the reverse was true for one participant. The other 16 participants were using the same amounts bilaterally. Notes Treatment was terminated after 6 weeks for one participant, and after 7 weeks for six participants |
|
| Outcomes | Physician's assessment of signs; participants scored
0 to 3 (0 = none, 1 = slight, 2 = moderate, and 3 =
severe) on the following variables; pruritus,
lichenification, scaling, xerosis, vesiculation,
excoriations, erythema, and an overall evaluation at
week 0, 2, 4, 6, and 8 (or on completion)* Results for total, overall, and pruritus scores were reported. Assessment of healing on a 5‐point scale: 1 = worsened, 0 = unchanged, 1 = somewhat improved, 2 = considerably improved, 3 = healed. A body half was considered healed if no erythema, papules, excoriations, vesicles, lichenification, or scaling remained. The designation 'considerably improved' was used when a body half was almost healed, while 'somewhat improved' designated slight to moderate improvement. Treatment of the face and the hands was not evaluated. Assessed at week 0, 2, 4, 6, and 8 (or on completion), we assumed.* Percentage of skin involved using the rule of nine. Evaluation of the face and hands not included. Assessed at week 0, 2, 4, 6, and 8 (or on completion), we assumed. Participants reported amount of emollient and hydrocortisone applied to each body half. Participant preference based on pruritus, assessed on completion Participant preference based on xerosis, assessed on completion Participant preference overall, assessed on completion Participant‐reported side effects, assessed on completion* *denotes relevance to this review Funding source Supported by a grant from the Edvard Welander Foundation. Declarations of interest Not reported |
|
| Notes | None | |
Jekler 1991.
| Study characteristics | ||
| Methods |
Trial design Within‐participant, randomised, controlled trial Trial registration number Not reported Country Sweden Outpatient or hospital Daycare centre where people can receive phototherapy without making an appointment Date trial conducted Not reported Duration of trial participation Up to 8 weeks; participants were treated for 8 weeks, or until healing occurred Additional design details Inclusion criteria
Exclusion criteria
Notes The study was not performed during the summer months |
|
| Participants |
Total number randomised 33 (characteristics and results are only reported for the 21 participants who did not drop out) Age Mean age 23.3 years (SD 5.2 years) Sex 12 men and 9 women Race/ethnicity/Fitzpatrick skin type Skin type II n = 2 Skin type III n = 19 Duration of eczema Mean duration 19.6 years (SD 6.9 years) Severity of eczema Baseline severity score: participants were assessed for 8 variables scored 0 to 3 (0 = none, 1 = light, 2 = moderate, and 3 = severe) on the following variables; pruritus, lichenification, scaling, xerosis, vesiculation, excoriations, erythema, and an overall evaluation. Both groups, mean total score 10.3, range 6 to 18 Both groups, mean overall evaluation score 1.8, range 1 to 3 HIV/AIDs comorbidity Not reported Number of withdrawals 12: no details provided regarding reason participants withdrew Notes None |
|
| Interventions |
Run‐in details Only mild corticosteroid preparations (hydrocortisone 0.5% to 1%) and emollient creams were allowed as topical treatment during, and 2 weeks prior to the start of phototherapy. Groups Generally, phototherapy was given three times a week for 8 weeks, or until healing occurred. Shielding of the contralateral side was accomplished with two layers of thick dark cotton sheeting. A mean of 18.9 (SD 3.5) treatments were given over 7.9 (SD 1.1) weeks BB‐UVB 14 Philips TL 12 40W and 14 Philips TL 12 20 W tubes arranged in a cubicle; wavelength: 280 nm to 315 nm Before the start of treatment, each participant was phototested, and the initial dose was set at approximately 80% of the MED. Subsequently, dose increments of 10% to 25% were made at each treatment session. With the appearance of erythema, there was a reduction in the dose of about 10% to 30%. UVB: The mean initial dose was 20.8 mJ/cm2 (SD 3.4 ); mean final dose was 131 mJ/cm2 (SD 49); and mean total dose was 1589 mJ/cm2 (SD 534). Weaning regimen: not reported UVA A cubicle containing 24 Philips TL 85/100W/09 (TL09) fluorescent tubes (Philips, Roosendaal, the Netherlands) was used; wavelength: 315nm to 400 nm The initial dose was set at 7, 9, or 11 J/cm2, depending on the participant's skin type and previous experience with solaria. At each subsequent treatment session, the dose was increased in steps of 2 J/cm2, up to a maximum of 15 J/cm2 UVA: The mean initial dose was 7.9 J/cm2 (SD 1.4); mean final dose was 14.3 J/cm2 (SD 1.5); and mean total dose was 255 J/cm2 (SD 51). Weaning regimen: not reported Co‐interventions Only mild corticosteroid preparations (hydrocortisone 0.5% to 1%) and emollient creams were allowed as topical treatment during, and 2 weeks prior to the start of phototherapy. Of the 15 participants using hydrocortisone during the study, five used more on the UVB‐treated side, while the remainder used equal amounts bilaterally. All the participants used emollients, and four used more on the UVB treated side, while the remainder used equal amounts on both sides. Notes None |
|
| Outcomes | Physician's assessment of signs; participants scored
0 to 3 (0 = none, 1 = slight, 2 = moderate, and 3 =
severe) on the following variables; pruritus,
lichenification, scaling, xerosis, vesiculation,
excoriations, erythema, and an overall evaluation at
week 0, 2, 4, 6, and 8 (or on completion)* Assessment of healing on a 5‐point scale: 1 = worsened, 0 = unchanged, 1 = somewhat improved, 2 = considerably improved, 3 = healed. A body half was considered healed if no erythema, papules, excoriations, vesicles, lichenification, or scaling remained. The designation 'considerably improved' was used when a body half was almost healed, while 'somewhat improved' designated slight to moderate improvement. Assessed at week 0, 2, 4, 6, and 8 (or on completion), we assumed* Percentage of skin involved using the rule of nine. Evaluation of the face and hands not included. Assessed at week 0, 2, 4, 6, and 8 (or on completion), we assumed Physician's judgement as to which treatment gave a better result, assessed on completion Participant preference based on pruritus, assessed on completion Participant preference based on xerosis, assessed on completion Participant preference overall, assessed on completion Participant‐reported side effects, assessed on completion* Amount of topical corticosteroid and emollient used on one side of the body compared to the other *denotes relevance to this review Funding source Supported by a grant from the Edvard Welander Foundation Declarations of interest Not reported |
|
| Notes | None | |
Jekler 1991b Study 1.
| Study characteristics | ||
| Methods |
Trial design Within‐participant, randomised trial Trial registration number Not reported Country Sweden Outpatient or hospital Not reported Date trial conducted Not reported (however, the study was not conducted during the summer months) Duration of trial participation For 8 weeks, or until healing of at least one body half (in some cases, 7 weeks) Additional design details None Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 20 participants "entered the study"; however, characteristics and results reported for 18 participants only Age Mean age, years (SD) 28.3 (11.7) Sex 8 men, 10 women Race/ethnicity/Fitzpatrick skin type Skin type 1, 0 participants Skin type 2, 5 participants Skin type 3, 12 participants Skin type 4, 1 participant Duration of eczema Mean total disease duration, years (SD), 24.8 (9.8) Severity of eczema The participants were assessed for 8 variables, scored 0 to 3 (0 = none, 1 = slight, 2 = moderate, and 3 = severe) on the following variables: pruritus, lichenification, scaling, xerosis, vesiculation, excoriations, erythema, and an overall evaluation. The total baseline score; mean 10.8, range 7 to 15.5. The score for the overall evaluation component; mean 1.9, range 1 to 2.5 HIV/AIDs comorbidity Not reported Number of withdrawals 2 participants: one who failed to improve within the first 2.5 weeks in the study and had to be treated with potent topical corticosteroids, and one who had been using a moderately potent topical steroid believing it was identical to hydrocortisone Notes None |
|
| Interventions |
Run‐in details Only mild corticosteroid preparations (hydrocortisone 0.5% to 1%) and emollient creams were allowed as topical treatment during, and 2 weeks prior to the start of phototherapy Groups Three times a week for up to 8 weeks (or healing of one body half) A mean of 18.5 (SD 4.4) treatments were given in 7.5 (SD 1.0 weeks) Low dose UVB 14 Philips TL 12 40W and 14 Philips TL 12 20 W tubes arranged in a cubicle (Philips, Roosendaal, the Netherlands) One half of the body was treated with low‐dose UVB, left or right according to randomisation (except for the face which was treated with UVAB). The side not irradiated was shielded with 2 layers of thick dark cotton sheeting. Each participant's minimal erythema dose of UVB was determined before the study, and thereafter, every other week. The aim was to give treatment with 20% of the MED. Dose increments were made stepwise every other week, each time maintaining a dose of 0.2 MED. UVB doses: mean initial 10mJ/cm2 (SD 3.6), final 18 mJ/cm2 (SD 7.8), and total (cumulative doses) 282 mJ/cm2 (SD 152) Maximum dose: not reported Weaning regimen: not reported UVAB Cubicle containing 24 Wolff Helarium System tubes B1 to 12/100 W (Cosmedico, Stuttgart, Germany) or a sunbed containing 20 tubes of the same kind; wavelength: UVA 315 nm to 400 nm, UVB 280 nm to 315 nm One half of the body was treated with UVAB, left or right according to randomisation (the face was treated with UVAB). The side not irradiated was shielded with 2 layers of thick dark cotton sheeting. A dose increment schedule, depending on the participant's skin type was set up. The initial exposure time of 7 to 10 minutes was subject to incremental increase every, or every other treatment session by 2 to 5 minutes, to a maximum of 25 min (corresponding to 45 mJ/cm2 UVB and 10.5 J/cm2 UVA). The mean initial dose was 14 mJ/cm2 (SD 2.2) UVB and 3.2 J/cm2 (SD 0.5) UVA; the mean final dose was 41 mJ/cm2 (SD 6.8) UVB and 9.5 J/cm2 (SD 1.6) UVA; and the mean total dose was 558 mJ/cm2 (SD 193) UVB and 130 J/cm2 (SD 45) UVA Weaning regimen: not reported Co‐interventions Only mild corticosteroid preparations (hydrocortisone 0.5% to 1%) and emollient creams were allowed as topical treatment during, and 2 weeks prior to the start of phototherapy. Notes Of the 15 participants who were using topical hydrocortisone during the study, 9 stated that at some point, they used more on the UVB‐treated body half, whereas no participant had used more on the UVAB‐treated one. |
|
| Outcomes |
*denotes relevance to this review Funding source Supported by a grant from the Edvard Welander Foundation. Declarations of interest Not reported |
|
| Notes | None | |
Jekler 1991b Study 2.
| Study characteristics | ||
| Methods |
Trial design Within‐participant, randomised trial Trial registration number Not reported Country Sweden Outpatient or hospital Not reported Date trial conducted Not reported (however, the study was not conducted during the summer months) Duration of trial participation For 3 weeks, or until clearing of at least one side Additional design details Results are also provided in the study for a control patch of untreated skin, however, the results for this area were not extracted, as it's unlikely this was allocated at random. Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 28 participants "entered the study" however, characteristics and results reported for 25 participants only Age Mean age, years (SD) 24.0 (4.8) Sex 8 men, 17 women Race/ethnicity/Fitzpatrick skin type Skin type 1, 0 participants Skin type 2, 6 participants Skin type 3, 17 participants Skin type 4, 2 participants Duration of eczema Mean total disease duration, years (SD), 20.4 (8.3) Severity of eczema The participants were assessed for 8 variables, scored 0 to 3 (0 = none, 1 = slight, 2 = moderate, and 3 = severe) on the following variables; pruritus, lichenification, scaling, xerosis, vesiculation, excoriations, erythema, and an overall evaluation. The total baseline score; mean 12.3, range 7 to 21.5; score for the overall evaluation component; mean 2.1, range 1 to 3 HIV/AIDs comorbidity Not reported Number of withdrawals 3 participants; one due to lack of time for treatment, one had severe AD and could not manage without corticosteroids, and one with type I skin was diagnosed as having polymorphic light eruption bilaterally after 2 weeks of treatment Notes None |
|
| Interventions |
Run‐in details Only mild corticosteroid preparations (hydrocortisone 0.5% to 1%) and emollient creams were allowed as topical treatment during, and 2 weeks prior to the start of phototherapy Groups Dermatitis areas of equal clinical status were selected on participant (arms or legs). The treatment with each modality was given to right side or left side of the body (though due to the size of the UVA lamp, not the whole body) according to randomisation. The rest of the body was shielded with two‐layer thick dark cotton sheeting, except for the face, which was unshielded in the UVAB cabinet. UVA1 UVA1 treatment 5 times a week (Monday to Friday) for 3 weeks, or until clearing of at least one side UVASUN 3000 lamp (Mutzhas, Munic, Germany) with a UVA filter eliminating wavelengths shorter than 340 nm, yields mainly UVA1 (340 nm to 400 nm) An initial dose of 10 or 20 J/cm2 UVA was increased by 10 J/cm2 each treatment session to a final dose of 30 J/cm2; MED/MPD was measured UVA doses: mean initial 11 J/cm2 (SD 2.8), final 30 J/cm2 (SD 0), and total doses 361 J/cm2 (SD 75) A mean of 13.0 (SD 2.5) treatments were given in 2.9 (SD 0.42) weeks Weaning regimen: not reported MED/MPD conducted: Yes UVAB UVAB treatment 5 times a week (Monday to Friday) for 3 weeks, or until clearing of at least one side Cubicle containing 24 Wolff Helarium System tubes B1 to 12/100 W (Cosmedico, Stuttgart, Germany) or a sunbed containing 20 tubes of the same kind. UVA 315 nm to 400 nm, UVB 280 nm to 315 nm Depending on the participant's skin type, an initial exposure time of 8 to 14 minutes was determined for UVAB therapy. Dose increments were made at each treatment session with 2 to 4 minutes added, to a maximum of 25 minutes A mean of 13.0 (SD 2.5) treatments were given in 2.9 (SD 0.42) weeks The mean initial doses were 16 mJ/cm2 (SD 3.1) UVB, 3.8 J/cm2 (SD 0.7) UVA; mean final doses were 43 mJ/cm2 (SD 5.0) UVB and 10.1 J/cm2 (SD 1.2) UVA; and the mean total doses were 466 mJ/cm2 (SD 119) UVB and 109 J/cm2 (SD 27.7) UVA Maximum dose: not reported MED/MPD conducted: Yes Weaning regimen: not reported Co‐interventions Only mild corticosteroid preparations (hydrocortisone 0.5% to 1%) and emollient creams were allowed as topical treatment during, and 2 weeks prior to the start of phototherapy Of the 10 participants who used hydrocortisone,1 stated they used more on the UVAB treated side |
|
| Outcomes |
*denotes relevance to this review Funding source Supported by a grant from the Edvard Welander Foundation. Declarations of interest Not reported |
|
| Notes | None | |
Krutmann 1992.
| Study characteristics | ||
| Methods |
Trial design Parallel, randomised, controlled trial Trial registration number Not reported Country Not reported (author affiliated to the University of Freiburg, Germany) Outpatient or hospital Not reported Date trial conducted Not reported Duration of trial participation Up to 15 treatments (daily, approximately two/three weeks) Additional design details None Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 25 participants (15 high‐dose UVA1, 10 UVA‐UVB) Age High dose UVA1 25 (20 to 33) years (statistic type not reported) UVA‐UVB 25 (19 to 35) years (statistic type not reported) Sex High dose UVA1 male 10 female 5 UVA‐UVB male 5 female 5 Race/ethnicity/Fitzpatrick skin type All participants were white High dose UVA1 Skin type III n = 12 Skin type IV n = 3 UVA‐UVB Skin type III n = 6 Skin type IV n = 4 Duration of eczema Not reported Severity of eczema Baseline COSTA 1989 score mean ± SE (range): high‐dose UVA1 52 (though this appears to be 55 on the graph) ± 2.6 (36) Baseline COSTA 1989 score mean ± SE (range), UVA‐UVB 53 ± 1.9 (17) HIV/AIDs comorbidity Not reported, however, high risk for HIV an exclusion criterion Number of withdrawals One participant due to dissatisfaction with the therapeutic result (UVA‐UVB group) withdrew after the third exposure. Notes The two groups did not differ significantly in terms of sex, age, clinical severity |
|
| Interventions |
Run‐in details Not reported Groups High dose UVA1 UVASUN 30,000 BIOMED (Mutzhas, Munich, F.R.G.) irradiation device. The emission was filtered with UVACRYL (Mutzhas) and UG 1 (Schott Glasswerke, Munich), and consisted exclusively of wavelengths greater than 340 nm; wavelength: 340 nm to 400 nm High‐dose UVA1 exposures were given daily. The total number of exposures were limited to 15. Participants had to turn from back to front every 10 minutes during the irradiation (as the device only allows exposure from the top). The dosage was 130 J/cm2 UVA1 per body half; total dose for each participant was 1950 J/cm2. To rule out hypersensitivity to UVA light, all participants in the high‐dose UVA1 group were phototested before phototherapy with increasing doses (0 to 130 J/cm2) of UVA I with a UVASUN 5000 (Mutzhas) irradiation device emitting 100% UVA I light Weaning regimen: not reported Maximum dose: not reported UVA‐UVB Metec Helarium model 1480 (Metec Helarium, Munich) radiation device equipped with 20 Wolff Helarium System tubes B1 to 12/100 W (Cosmedico, Stuttgart, F.R.G.); wavelength: 300 nm to 400nm The total number of exposures was limited to 15 The dose preceding the minimal erythema dose for UVB was used as the initial dose. Subsequently, the doses were successively increased up to a maximum of 30 mJ/cm2 UVB and 7.5 J/cm2 UVA. If erythema was induced, the preceding dose was used for the next treatment. Treatments were given daily. The mean final doses were 28 mJ/cm2 UVB and 7 J/cm2 UVA Weaning regimen: not reported Co‐interventions Unlimited use of emollients only. Each participant was allowed one bath per day, preferably immediately after phototherapy. Notes None |
|
| Outcomes | Clinical severity according to the COSTA 1989 scoring
system; severity criteria (erythema, edema,
vesicles, exudation, crusts, excoriations, scales,
lichenification, pruritus, and loss of sleep) scored
from 0 (no lesion) to 6 (extremely severe),
topographic score following areas assessed for
extent of involvement (face, neck, anterior and
posterior aspects of the trunk, buttocks, arms,
hands, legs, knees, and feet) and scored 0 to 3.
Severity, topographic, and total score reported at
baseline, after 6 treatments, and after 15
treatments (approximately two /three weeks)* Adverse events at two/three weeks* Serum Eosinophil cationic protein (ECP) level measured at baseline and at two/three weeks *denotes relevance to this review Funding source Not reported Declarations of interest Not reported |
|
| Notes | None | |
Krutmann 1998.
| Study characteristics | ||
| Methods |
Trial design Randomised, multi‐centre, three armed, parallel study Trial registration number Not reported Country Not reported (author affiliations are all in Germany) Outpatient or hospital Inpatients Date trial conducted Not reported Duration of trial participation 10 days Additional design details None Inclusion criteria
Exclusion criteria
Notes Entry into the study required the following:
|
|
| Participants |
Total number randomised 53 High dose UVA1 N = 20 Fluocortolone N = 17 UVA‐UVB N = 16 Age Assumed mean age in years: High dose UVA1 26 Fluocortolone 27 UVA‐UVB 28 Sex High dose UVA1 (M/F) 8/12 Fluocortolone (M/F) 8/9 UVA‐UVB (M/F) 8/8 Race/ethnicity/Fitzpatrick skin type All participants included in the study were white High dose UVA; skin type III 15, skin type IV 5 Fluocortolone; not reported UVA‐UVB; skin type III 11, skin type IV 5 Duration of eczema Not reported Severity of eczema Inclusion criteria was clinical score greater than 40 (Costa 1989) and person had to have had an acute severe flare Total clinical score (Costa 1989). Mean ± SE (Range) High dose UVA1; 56 ± 11 Fluocortolone; 60 ± 7 UVA‐UVB; 60 ± 13 HIV/AIDs comorbidity Not reported, though high‐risk groups for HIV excluded Number of withdrawals None Notes The three treatment groups did not differ significantly in terms of age, sex, clinical severity, and serum ECP |
|
| Interventions |
Run‐in details NA Groups High‐dose UVA1 High dose UVA1 exposures were given daily for 10 days UVASUN 30,000 Biomed (Mutzhas, Munich, Germany). The emission was filtered with UVACRYL (Mutzhaus, Munich, Germany) and UG1 (Schott Glasswerke, Munich, Germany) and consisted exclusively of wavelengths > 340 nm The dosage was 130 J/cm2 per body half; maximum dose: 1300 J/cm2, participants turned from back to front every ten minutes The total number of treatments was 10 To rule out hypersensitivity to UVA1R, all participants in the high‐dose UVA1 group were phototested before phototherapy with increasing doses (0 to 130 J/cm2 UVA1) with a UVASUN 5000 (Mutzhas) irradiation device emitting 100% wavelengths > 340 nm Topical Corticosteroid fluocortolone 0.5% cream or ointment; the participant's entire body was treated with cream or ointment once daily for 10 consecutive days UVA‐UVB The dose preceding the minimal erythema dose for UVB was used as the initial dose. Doses increased by a maximum of 40mJ/cm2 UVB and 7.5 J/cm2 UVA. If erythema occurred, the preceding dose was used for the next treatment. The total number of treatments was 10 The paper references Krutmann 1992 and Jekler 1990 for details of this intervention, though it is not clear to what extent the interventions were similar. The mean final doses were 33 mJ/cm2 UVB and 6.8 J/cm2 UVA (in the UVA‐UVB treatment group). Co‐interventions Unlimited use of emollients was permitted. Each participant was allowed one bath per day. |
|
| Outcomes |
*Denotes relevance to this review Funding source Not reported Declarations of interest Not reported |
|
| Notes | None | |
Kwon 2019.
| Study characteristics | ||
| Methods |
Trial design Parallel, randomised, controlled trial Trial registration number Not reported Country Korea Outpatient or hospital Not reported Date trial conducted November 2014 to August 2015 Duration of trial participation 6 weeks of active treatment 3 weeks of follow‐up Additional design details None Inclusion criteria
Exclusion criteria
Notes The number of participants enrolled to each group was kept similar by season to minimise seasonal differences |
|
| Participants |
Total number randomised 18 TCS plus NBUVB N = 13 TCS alone N = 5 Age NBUVB + TCS mean ages (assumed SD) 14.8 ± 2.4 years TCS mean ages (assumed SD) 14.8 ± 4.9 Sex Not reported Race/ethnicity/Fitzpatrick skin type Not reported Duration of eczema Not reported Severity of eczema NBUVB + TCS mean EASI score (assumed SD) 13.0 ± 6.0 TCS mean EASI score (assumed SD) 11.6 ± 4.1 HIV/AIDs comorbidity Not reported Number of withdrawals 7 participants withdrew from the TCS plus NBUVB group: 5 were lost to follow‐up, 2 dropped out due to aggravation of symptoms and consequent treatment change All participants in the TCS group alone completed the study. Notes Participants who required systemic corticosteroids, immunosuppressants, or oral or systemic antibiotics due to aggravation were excluded from analysis. |
|
| Interventions |
Run‐in details NA Groups Narrowband UVB plus topical corticosteroid; narrowband UVB administered 2 to 3 times a week for 6 weeks (12 to 18 treatments). The initial dose was 350 to 400 mJ/cm2, which was gradually increased to 1100 mJ/cm2. Methylprednisolone cream was applied to lesional skin only plus an oral antihistamine Topical corticosteroid alone: methylprednisolone cream applied to lesional skin only plus an oral antihistamine Co‐interventions Daily bathing and twice a day moisturiser use Notes Any substance containing antibiotics or antiseptics was not allowed |
|
| Outcomes |
*Denotes relevance to this review Funding source This study was supported by grant 12‐2013 from the SNUBH Research Fund. Declarations of interest "The authors have no conflicts of interest to declare." |
|
| Notes | None | |
Legat 2003.
| Study characteristics | ||
| Methods |
Trial design Within‐participant, randomised, controlled trial Trial registration number Not reported Country Not reported (authors affiliated with Graz University Austria) Outpatient or hospital Not reported Date trial conducted Not reported Duration of trial participation Up to 8 weeks Median duration of 7 weeks, range 4 to 8 weeks Additional design details None Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 9 Age Median 27 years, range 23 to 41 years Sex 6 women, 3 men Race/ethnicity/Fitzpatrick skin type Not reported Duration of eczema Median disease duration 22 years, range 2 to 33 years Severity of eczema NB‐UVB Costa score at baseline, median (range), 74 (46 to 93) Medium dose UV‐A1 Costa score at baseline, median (range), 74 (46 to 95) HIV/AIDs comorbidity Not reported Number of withdrawals 2 participants: half‐sided treatment had to be terminated at 4 and 6 weeks because score values for the NB‐UVB treated body halves were more than 30% lower than those obtained from the UV‐A1 body halves. Notes None |
|
| Interventions |
Run‐in details Not reported Groups Treatment was administered 3 times weekly for up to 8 weeks. Light‐shielding half body overalls were used to deliver the treatment to one half of the body only. Participants received a median of 23 treatments, (range 12 to 24 treatments), with a mean cumulative dose of 26.7 J/cm2 NBUV B (range 15.7 to 59.2 J/cm2) and 1000 J/cm2 UVA1 irradiation (range 500 to 1150 J/cm2) Narrow Band UVB UV 7001 light box (Waldmann Medizinische Technik, Villingen‐Schwenningen, Germany) The starting dose was 70% of the participant's minimum erythema dose, and dose increases were usually 10% to 20%, depending on the erythema response induced by the previous exposure. NB‐UVB median MED 0.77 J/cm2, (range 0.55 to 1.56 J/cm2) Maximum dose: not reported Weaning regimen: not reported UV‐A1 Sellas UV‐A1 bench system (Sellamed 24000A; Sellas Medizinische Gerate GmbH, Gevelsberg, Germany) The starting dose for UVA1 irradiation was 10 J/cm2, with 20 J/cm2 applied at the second, 30 J/cm2 at the third, and 40 J/cm2 at the fourth treatment; 50 J/cm2 was administered at the fifth and each subsequent treatment Maximum dose: not reported Weaning regimen: not reported Co‐interventions Topical therapy restricted to emollients when needed Notes None |
|
| Outcomes |
*Denotes relevance to this review Funding source Not reported Declarations of interest Not reported |
|
| Notes | None | |
Leone 1998.
| Study characteristics | ||
| Methods |
Trial design Parallel, randomised, controlled, three‐arm trial Trial registration number Not reported Country Not reported (author affiliations are Italian) Outpatient or hospital Not reported Date trial conducted Not reported Duration of trial participation Approximately 5 weeks (10 to 15 treatments, three times a week) Additional design details None Inclusion criteria Adults with severe atopic eczema Exclusion criteria Not reported Notes None |
|
| Participants |
Total number randomised 18 (6 per group) Age Mean age 28 years, range 16 to 54 Sex 11 males, 7 females Race/ethnicity/Fitzpatrick skin type Not reported Duration of eczema Not reported Severity of eczema Not reported (though see inclusion criteria) HIV/AIDs comorbidity Not reported Number of withdrawals There was no information about participants withdrawing from the study Notes None |
|
| Interventions |
Run‐in details Not reported Groups Narrowband UVB Participants were treated three times a week; irradiation bed equipped with 14 TL01/100 w tubes was used for narrowband UVB treatment The UVB irradiation protocol (for both narrowband and broadband UVB) was based on the MED: start at 70% MED, with 40% dose increments after every third treatment, if tolerated Total treatments: 10 to 15 treatments Maximum dose: not reported Cumulative dose: not reported Weaning regimen: not reported UVAB Participants were treated 3 times a week using a phototherapy booth with F85/100W UV21 tubes emitting UVB and F85/100 W PUVA tubes emitting UVA The UVB irradiation protocol (for both narrowband and broadband UVB) was based on the MED: start at 70% MED, with 40% dose increments after every third treatment, if tolerated. In the UVA irradiation protocol, the initial dose was 3 to 4 J (based on skin type), with a 1 J increment after every third treatment, up to a maximum of 10 J. Total treatments: 10 to 15 treatments Maximum dose: not reported Cumulative dose: not reported Weaning regimen: not reported Narrowband UVB plus UVA Participants were treated 3 times a week, using a combination of both devices described in the two groups above to deliver the treatment. The UVB irradiation protocol (for both narrowband and broadband UVB) was based on the MED: start at 70% MED, with 40% dose increments after every third treatment, if tolerated. In the UVA irradiation protocol, the initial dose was 3 to 4 J (based on skin type) with a 1 J increment after every third treatment, up to a maximum of 10 J. Total treatments: 10 to 15 treatments Maximum dose: not reported Cumulative dose: not reported Weaning regimen: not reported Co‐interventions Not reported Notes None |
|
| Outcomes | SCORAD index before treatment and after 10 to 15
treatments Funding source Not reported Declarations of interest Not reported |
|
| Notes | None | |
Majoie 2009.
| Study characteristics | ||
| Methods |
Trial design Randomised, investigator‐blinded, within‐participant study Trial registration number Not reported Country The Netherlands Outpatient or hospital Not reported Date trial conducted Not reported Duration of trial participation 4 weeks wash‐out period 8 weeks treatment period 4 weeks follow‐up period Additional design details None Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 13 (within‐participant) Age Median age 25 years, range 20 to 56 years Sex 5 males, 8 females Race/ethnicity/Fitzpatrick skin type Not reported Duration of eczema Not reported Severity of eczema Baseline median Leicester symptom score for NB‐UVB group: 19, range (9 to 29); baseline median VAS for pruritus: 7.5, range (3.5 to 10) Baseline median Leicester symptom score for medium‐dose UVA1 group: 20, range (8 to 31); baseline median VAS for pruritus: 7.5, range (3.5 to 10) HIV/AIDs comorbidity Not reported Number of withdrawals All participants completed the study Notes None |
|
| Interventions |
Run‐in details No specific details were provided regarding the wash‐out period in the paper, however, the exclusion criteria state that participants had to have 2 weeks without topical corticosteroids before starting the study, and 4 weeks without systemic antibiotics, corticosteroids, or oral immunosuppressants. This is likely to have been during the run‐in period. Groups Narrowband UVB A light cabin (Waldmann, Schwenningen, Germany) with 20 311 nm lamps (TL‐01, Philips, Eindhoven, the Netherlands); wavelength: 311 nm Three times weekly during a period of 8 weeks. Half of the body was exposed to each treatment, with the non‐exposed body sides covered with a half‐sided overall. UVB treatment was started with an initial dose of 70% of the minimal erythemal dose (MED). Subsequent dose increments were given on the basis of erythemic reactions of the skin. The intention was for each exposure to induce slight erythema. If the previous exposure failed to induce any reaction, the dose was increased by 20%. If the resulting erythema was slight, the dose was increased by 10%. Total treatments: not reported Maximum dose: not reported Weaning regimen: not reported Participants received median cumulative dose of 10.5 J/cm2 of NB‐UVB (range 9.9 to 11.5, average increment 10%/exposure) Medium dose UVA1 A light cabin (Waldmann, Schwenningen, Germany) with 40 lamps (TL‐10R, Philips) emitting wavelengths of 350 nm to 400 nm only, with a maximum of ± 370 nm. Three times weekly during a period of 8 weeks. Half of the body was exposed to each treatment, with the non‐exposed body sides covered with a half‐sided overall. The first dose was 30 J/cm2. In two steps the dose was increased to 45 J/cm2. In 3 of the participants, the dose of UVA1 had to be decreased because the reaction (erythema/papules) was too strong. The average dose of UVA1 was more than 40 J/cm2. Participants received median cumulative dose of 930.6 J/cm2 of MD UVA1 (range 717.1 to 1067.4) to the other body side Total treatments: not reported Maximum dose: not reported Weaning regimen: not reported Co‐interventions During the treatment period, no other topical treatments, other than emollients were allowed. During the follow‐up period topical, corticosteroids were allowed, if needed (most participants used topical corticosteroids during this period). |
|
| Outcomes |
*Denotes relevance to this review Funding source None Declarations of interest None declared |
|
| Notes | Each scoring of outcomes was done just before the next phototherapy session, so the erythema caused by phototherapy could not influence scoring. The face was excluded from half‐sided comparison and analysis. It was only treated with medium dose UVA1, and if necessary, mild topical corticosteroids (European Class I or II). | |
Maul 2017.
| Study characteristics | ||
| Methods |
Trial design Double‐blind, randomised, parallel‐group trial Trial registration number NCT01254240 Country Switzerland Outpatient or hospital Outpatient clinic, Department of Dermatology, University Hospital of Zürich Date trial conducted 2010 to 2015 Duration of trial participation 16 weeks Additional design details Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 24 participants with atopic dermatitis; 10 randomised to NB‐UVB alone and 14 to NB‐UVB with UVA Age Not reported for participants with only AD Sex Not reported for participants with only AD Race/ethnicity/Fitzpatrick skin type Not reported for participants with only AD Duration of eczema Not reported Severity of eczema Not reported for participants with only AD HIV/AIDs comorbidity Not reported Number of withdrawals Of 53 participants enrolled, 45 completed the trial, however it was not clear how many withdrawals were participants with AD. Notes None |
|
| Interventions |
Run‐in details Not reported Groups NB‐UVB alone: NB‐UVB started at a dosage of 0.1 J/cm2 with increments of 20% per session if no side effects were observed, to maximum 2.0 J/cm2; three treatment sessions per week for 16 weeks. Performed with a NB‐UVB light cabin (Model UV7001, Waldmann (Waldmann Lichttechnik GmbH, Kuttingen, Switzerland), 310 nm to 315 nm) UVA/NB‐UVB: in addition to standard NB‐UVB treatment, UVA was also given at a starting dose of 0.5 J/cm2 and increased by increments of 20%, to a maximum of 5.0 J/cm2. Performed with a UVA/NB‐UVB light cabin (Model UV7002, Waldmann, UVA 320 nm to 410 nm, peak 351 nm; UVB output 310 nm to 315 nm, peak 311 nm). Cumulative dose: not reported Weaning regimen: not reported Co‐interventions Not reported Notes None |
|
| Outcomes |
*denotes relevance to this review |
|
| Notes |
Funding source Department of Dermatology, University Hospital of Zürich Declarations of interest None declared Notes None |
|
Pacifico 2019.
| Study characteristics | ||
| Methods |
Trial design Randomised, controlled, open, parallel‐group study Trial registration number Not reported Country Italy Outpatient or hospital Phototherapy Unit of S Gallicano Institute; unclear if participants were treated on an out‐ or inpatient basis Date trial conducted October 2008 to February 2010 Duration of trial participation 3 weeks Additional design details None Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 27; 13 randomised to receive high dose UVA1 and 14 to medium dose Age Mean 34.7 years (range 19 to 47) Sex 14 females; 13 males Race/ethnicity/Fitzpatrick skin type 13 were Fitzpatrick skin type II; 6 type III; 8 type IV Duration of eczema Not reported Severity of eczema Median SCORAD was 53 (range 45 to 60) in the high dose group, and 53.5 (range 45 to 65) in the medium dose group HIV/AIDs comorbidity Not reported Number of withdrawals None Notes None |
|
| Interventions |
Run‐in details None Groups High dose: 130 J/cm2 UVA1 administered five times weekly for 3 weeks (total = 15; cumulative dose 1950 J/cm2) Medium dose: 60 J/cm2 UVA1 administered five times weekly for 3 weeks (total = 15; cumulative dose 750 J/cm2) Weaning regimen: not reported Almost exclusively UVA1 light was delivered using a Sellamed 24000 lay down unit (Systems Dr Sellmeier; Gevelsberg‐Vogelsang, Germany) Co‐interventions Emollients were used to treat skin dryness associated with mild pruritus immediately after therapy; no other co‐interventions were reported Notes None |
|
| Outcomes |
*denotes relevance to this review |
|
| Notes |
Funding source Grants from National Institute of Health and National Institute of Arthritis and Musculoskeletal and Skin Diseases Declarations of interest None declared Notes None |
|
Qayyum 2016.
| Study characteristics | ||
| Methods |
Trial design Randomised, parallel‐group study Trial registration number Not reported Country Pakistan Outpatient or hospital Dermatology Department Unit‐II, Outpatient Department of King Edward Medical University Mayo Hospital, Lahore Date trial conducted January 2011 to June 2012 Duration of trial participation Up to 12 weeks of treatment with 3 months post‐treatment follow‐up Additional design details None Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 60; 30 in each group Age UVA group: mean 21 ± 18 years (range 5 to 62) UVB group: mean 22 ± 21 years (range 5 to 70) Sex UVA group: 21 males and 9 females UVB group: 17 males and 13 females Race/ethnicity/Fitzpatrick skin type Not reported Duration of eczema Not reported Severity of eczema UVA group: mean baseline SCORAD 45 (range 34 to 58) UVB group: mean baseline SCORAD 51 (range 30 to 70) HIV/AIDs comorbidity Not reported Number of withdrawals UVA group: no withdrawals UVB group: 4 withdrawals; 2 because of side effects and 2 lost to follow‐up Notes None |
|
| Interventions |
Run‐in details Not reported Groups UVA: whole body UVA (4 mW/cm2, Waldmann 1000) three times weekly until clearance (maximum 12 weeks). Starting dose 1 J/cm2 with 0.5 J/cm2 increment until response UVB: whole body UVB (1.25 mW/cm2, Waldmann 1000) three times weekly until clearance (maximum 12 weeks). Starting dose 75% of MED for the skin type with 20% increments each visit according to participant tolerance Mean cumulative dose for UVA was 121 J/cm2 and for UVB, it was 8151 mJ/cm2 Weaning regimen not reported Co‐interventions Emollients were permitted Notes None |
|
| Outcomes |
*denotes relevance to this review |
|
| Notes |
Funding source Not reported Declarations of interest None declared Notes None |
|
Reynolds 2001.
| Study characteristics | ||
| Methods |
Trial design Randomised, controlled, double‐blind, parallel‐group study (3 arms) Trial registration number ISRCTN10725589 (retrospectively registered) Country United Kingdom Outpatient or hospital Not reported Date trial conducted April 1995 to November 1997 Duration of trial participation 12 weeks treatment period and 3 months post‐treatment follow‐up Additional design details None Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 73 were randomised (26 to NB‐UVB group; 24 to UVA; 23 to visible fluorescent light), however 4 withdrew before treatment (2 from NB‐UVB group; 1 from UVA; 1 from visible fluorescent light), and baseline data were only presented for the treated participants Age Mean (SD) was 29 years (11) in the NB‐UVB group, 25 (8) in the UVA group, and 25 (8) in the visible fluorescent light group Sex 15 males:14 females in the NB‐UVB group; 11 males:12 females in the UVA group; 10 males:12 females in the visible fluorescent light group Race/ethnicity/Fitzpatrick skin type 12 Fitzpatrick skin type I/II in the NB‐UVB group; 13 in the UVA group; 12 in the visible fluorescent light group Duration of eczema Not reported Severity of eczema There were 19 participants with moderate/severe disease in the NB‐UVB group, mean total disease score (SD) 32.3 (9.2), median (range) participant‐assessed itch on 10 cm VAS 59 (0 to 95); 20 in the UVA group, mean total disease score (SD) 29.8 (9.3), median (range) participant‐assessed itch on 10 cm VAS 60 (3 to 94); and 19 in the visible fluorescent light group, mean total disease score (SD) 30.8 (9.5), median (range) participant‐assessed itch on a 10 cm VAS 35 (0 to 88) HIV/AIDs comorbidity Not reported Number of withdrawals In addition to the above, 9 were excluded because of insufficient follow‐up (2 from NB‐UVB group; 4 from UVA; 3 from visible fluorescent light); and so data from 60 participants were included in the intention‐to‐treat analysis. A further 13 participants subsequently withdrew; combined reasons were burning (1 from NB‐UVB, 0 from UVA, and 1 from visible fluorescent light); exacerbation of eczema (1 from NB‐UVB, 2 from UVA, and 1 from visible fluorescent light); dislike of treatment (0 from NB‐UVB, 2 from UVA, and 1 from visible fluorescent light); moved away (1 from NB‐UVB, 0 from UVA, and 1 from visible fluorescent light); unable to attend owing to work or family commitments (1 from NB‐UVB, 3 from UVA, and 1 from visible fluorescent light); and failure to attend (3 from NB‐UVB, 1 from UVA, and 2 from visible fluorescent light). Notes None |
|
| Interventions |
Run‐in details None Groups Phototherapy was administered to the whole body twice weekly for 12 weeks (total = 24). Participants were monitored after treatment for an erythemal response. NB‐UVB: exposure unit containing 40 TL‐100 W/01 lamps (Philips). Starting dose 0.4 J/cm2, percentage‐based increments weekly (maximum 1.5 J/cm2 if tolerated); cumulative dose 24.8 J/cm2 (range 2.8 to 32.2) UVA: exposure unit containing 40 fluorescent lamps (Performance 100 W, Philips). Starting dose 5 J/cm2, increasing to 10 J/cm2 if tolerated; then to a maximum 15 J/cm2; cumulative dose 315 J/cm2 (range 15 to 345) Visible fluorescent light: Philips’ 75 to 85 W/96 Northlight fluorescent lamps, fitted into a Sovereign 8‐tube vertical sunbed canopy (Sun Health Services, Crowborough, UK). Exposure time was increased from 5 to 15 minutes and participants turned by 180º halfway through the treatment period. Median cumulative exposure time was 320 min (5 to 345). Weaning regimen: not reported Co‐interventions Emollients and mild to potent topical steroids were permitted as required. Only betamethasone valerate 0.1%, clobetasone butyrate 0.05%, and hydrocortisone 1% were prescribed. Participants were advised to use emulsifying ointment or aqueous cream as emollients. Notes None |
|
| Outcomes |
*denotes relevance to this review |
|
| Notes |
Funding source An NHS Research and Development grant partly funded this study; no other funding sources reported. Declarations of interest None declared Notes None |
|
Selvaag 2005.
| Study characteristics | ||
| Methods |
Trial design Randomised, open, controlled, within‐participant study Trial registration number Not reported Country Denmark Outpatient or hospital Not reported Date trial conducted Not reported Duration of trial participation Up to 6 weeks Additional design details None Inclusion criteria People with mild to moderate atopic dermatitis Exclusion criteria Not reported Notes None |
|
| Participants |
Total number randomised 20 Age Median 24 years (range 16 to 38) Sex 9 males; 11 females Race/ethnicity/Fitzpatrick skin type Not reported Duration of eczema Not reported Severity of eczema Mean SCORAD at baseline was 32 (range 15 to 53) HIV/AIDs comorbidity Not reported Number of withdrawals Not reported Notes None |
|
| Interventions |
Run‐in details Not reported Groups UVB was delivered using a bank of Philips TL 01 UVB tubes. One SED is 10 mJ/cm2 at 298 nm using the CIE erythema action spectrum and is equivalent to 1.6 kJ/m2 of the UVB lamp. Skin reflectance measurement was performed on non‐lesional skin on the chest or between shoulder blades with UV‐Optimize 555 (MaticH, Copenhagen, Denmark). Fixed regimen: UVB administered 3 to 5 times weekly to one half of the body. Starting dose 1.6 SED with 25% increments with each treatment session. Cumulative dose was mean 124 SED (range 29 to 186). Optimised regimen: UVB administered according to skin reflectance measurements of skin pigmentation and erythema. Cumulative dose was mean 39 SED (range 16 to 88). Weaning regimen: not reported The whole face was always given the standard treatment. Co‐interventions Topical corticosteroids and emollients were permitted if used symmetrically, except during UV treatment. Notes None |
|
| Outcomes |
*denotes relevance to this review |
|
| Notes |
Funding source Not reported Declarations of interest None declared Notes None |
|
Tzaneva 2001.
| Study characteristics | ||
| Methods |
Trial design Investigator‐blinded, within‐participant study Trial registration number Not reported Country Austria Outpatient or hospital Outpatient Date trial conducted Not reported Duration of trial participation 3 weeks treatment phase followed by 6 months post‐treatment follow‐up Additional design details None Inclusion criteria Severe, generalised atopic dermatitis; diagnosis according to Hanifin and Rajka criteria Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 10 Age Median age 30 years (range 22 to 58) Sex 5 males, 5 females Race/ethnicity/Fitzpatrick skin type 3 had Fitzpatrick skin type II; 5 had type III; 2 had type IV Duration of eczema Median duration 22.5 years (range 3 to 55) Severity of eczema Severe; median baseline SCORAD score of 67 (range 45 to 90) HIV/AIDs comorbidity Not reported Number of withdrawals Two participants received 10 of 15 treatments, as they were unable to attend for the remainder. Notes None |
|
| Interventions |
Run‐in details None Groups Both sides were treated using a 24 kW Dermalight UltrA1 lay down unit (Systems Dr Sellmeier, Gevelsberg‐Vogelsang, Germany) emitting UVA1 light (96.9% 340 nm to 400 nm). High dose UVA1: starting dose was MED with increments of 10 J/cm2 provided there was no erythema response (maximum of 130 J/cm2) 5 times per week for 3 weeks (total = 15) Medium dose UVA1: 50% of the high‐dose regimen 5 times per week for 3 weeks (total = 15) Doses received:
Weaning regimen: not reported Co‐interventions Medium‐dose treatment was used on the face. In 9 participants, one half of the buttocks was shielded with 4 layers of tightly woven cotton sheets to prevent transmission of UVA1 as a negative control, in order to exclude a systemic effect of the treatments. Only emollients were permitted as additional treatments. Notes At the end of treatment, three participants asked for continuation of treatment, and were switched to NB‐UVB; the other 7 continued with emollients alone. |
|
| Outcomes |
*denotes relevance to this review |
|
| Notes |
Funding source The authors stated "no outside funding of this study" Declarations of interest None declared Notes None |
|
Tzaneva 2010.
| Study characteristics | ||
| Methods |
Trial design Randomised, observer blinded, crossover study Trial registration number NCT00533195; EudraCT 2006‐00698217 Country Austria Outpatient or hospital Outpatient clinic; Medical University of Vienna; University Clinic of Dermatology; Division of Special and Environmental Dermatology, Vienna Date trial conducted October 2007 to January 2009 Duration of trial participation Up to 5 weeks for each treatment period, a minimum of 4 weeks wash‐out, and 12 months follow‐up following the last treatment Additional design details None Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 40; 17 allocated to PUVA and 23 allocated to UVA1 Age Mean 32.9 years (SD 14.6) Sex 15 males; 25 females Race/ethnicity/Fitzpatrick skin type 18 skin type II; 22 skin type III Duration of eczema Mean 21.5 years (SD 13.7) Severity of eczema Severe; mean SCORAD in the PUVA group was 62.5 (SD 13.1); UVA1 group was 63.7 (SD 15.6) HIV/AIDs comorbidity Not reported Number of withdrawals All 23 participants receiving UVA1 in the first period completed the treatment. 5 were not available for the follow‐up period for unknown reasons. 5 did not proceed to period B; 1 moved, 1 for lack of efficacy, 1 more minimal disease, 2 for unknown reasons. All 10 participants allocated to UVA1 in the second period completed the treatment. One was unavailable for the follow‐up period having missed two visits. One participant receiving PUVA in the first period (of 17) withdrew for lack of efficacy. 2 were not available for the follow‐up period for unknown reasons. 4 did not proceed to period B; 2 because they did not relapse, and 2 for unknown reasons. All 13 participants allocated to PUVA in the second period completed the treatment. 6 were unavailable for the follow‐up period; 1 because of stable disease, 1 missed two visits, 3 requested new treatment, and 1 for an unknown reason. Notes None |
|
| Interventions |
Run‐in details No run‐in, however, there was a minimum wash‐out interval of 4 weeks between treatment periods A and B. Groups PUVA: 5‐Methoxypsoralen plus ultraviolet A (UVA) three times weekly over 5 weeks on an outpatient basis, with no maintenance therapy (total = 15). 1.2 mg/kg Geralen 2 hours prior to each irradiation. First dose 70% of MPD with no increments in week 1. Increase UVA by 20% in the second week if no erythematous response (10% if light reaction), but no fewer than 96 hours after the last increment. UVA treatment was delivered using Waldmann PUVA 7001 units equipped with Waldmann F15 T8 ⁄PUVA tubes (Waldmann, Schwenningen, Germany). UVA1: medium dose UVA1 five times weekly over 3 weeks on an outpatient basis, with no maintenance therapy (total = 15). First dose MED if < 70 J/cm2, with 20% increments if no erythematous reaction and good tolerability, to a maximum of 70 J/cm2. UVA1 phototherapy was delivered with a 24 kW Dermalight ultrA1 lay down unit (Systems Dr Sellmeier, GevelsbergVogelsang, Germany) Cumulative dose: 48.1 ± 21.8 J/cm2 with PUVA; 1138.8 ± 350 J/cm2 with UVA1 Weaning regimen: not reported Co‐interventions No additional treatment was permitted except for emollients, as required. Notes None |
|
| Outcomes |
*denotes relevance to this review |
|
| Notes |
Funding source Not reported Declarations of interest None declared Notes None |
|
Tzung 2006.
| Study characteristics | ||
| Methods |
Trial design Single centre, prospective, randomised, investigator‐blind, within‐participant study Trial registration number Not reported Country Taiwan (assumed from authors' affiliations) Outpatient or hospital Not reported Date trial conducted Not reported Duration of trial participation 6‐week treatment phase and 4 weeks post‐treatment follow‐up Additional design details None Inclusion criteria Children with moderate to severe AD of symmetrical distribution Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 26 Age Range 5 to 17 years Sex 12 males; 14 females Race/ethnicity/Fitzpatrick skin type Not reported Duration of eczema Not reported Severity of eczema Investigator’s Global Assessment ≥ 3, mean 4.2; mean whole body EASI 30.5 (SD = 11.7, range 12.2 to 52.5); mean involved body surface 48.5% (range 15% to 95%); bilateral EASI scores were similar at baseline (P = 0.477) HIV/AIDs comorbidity Not reported Number of withdrawals Twenty‐four patients completed the study. It is not clear if the dropouts were from group A or B. No reasons given. Notes None |
|
| Interventions |
Run‐in details Not reported Groups A1 and A2 were used on bilateral sites on participants randomised to group A (N = 12).
A thin film of 1% pimecrolimus cream (Elidel®, Novartis Pharma GmbH, Nuremberg, Germany) was applied twice daily on all skin lesions. One half of the body was randomly selected to also be treated with NB‐UVB twice daily for 6 weeks. The contralateral side was shielded from UV transmission completely using tailored UV‐filtering clothing. B1 and B2 were used on bilateral sites on individuals randomised to group B (N = 14).
The whole body was irradiated with NB‐UVB twice weekly for 6 weeks. Only lesions on one side of the body received pimecrolimus cream, twice daily (1 hour after irradiation on days when phototherapy was received). NB‐UVB was delivered using 24 Waldmann TL‐01/100 fluorescent tubes mounted in a UV 5001BL cabinet (Waldmann, Villingen‐Schwenningen, Germany). The starting dose was 70% MED with percentage‐based increments every week (to maximum 1.5 J/cm2). Cumulative dose: not reported Weaning regimen: not reported Co‐interventions No other treatments were permitted, including emollients. Petrolatum was permitted for liberal use in the post‐treatment follow‐up period. Notes None |
|
| Outcomes |
*denotes relevance to this review |
|
| Notes |
Funding source Not reported Declarations of interest None declared Notes None |
|
Von Kobyletzki 1999a.
| Study characteristics | ||
| Methods |
Trial Design Parallel, three‐armed, randomised, active‐control trial Trial Registration Number Not reported Country Not reported Outpatient or hospital Not reported Date trial conducted Not reported Duration of trial participation 3 weeks active treatment 4 weeks of follow‐up post treatment Additional design details None Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 120 (UVA1 n=50, UVA1 cold‐light n=50, UVA‐UVB n=20) Age UVA1 (unspecified measure assumed mean) 36 years, range 18 to 61 UVA1 cold‐light (unspecified measure assumed mean) 38 years, range 19 to 59 UVA‐UVB (unspecified measure assumed mean) 32 years, range 18 to 52 Sex UVA1 M/F 23/2 UVA1 cold‐light M/F 28/22 UVA‐UVB M/F 12/8 Race/Ethnicity/Fitzpatrick skin type UVA1 skin type II n=6, type III n=41, type VI n=3 UVA1 cold‐light skin type II n=9, type III n=36, type VI n=5 UVA‐UVB skin type II n=4, type III n=14, type VI n=2 Duration of eczema Not reporte Severity of eczema Inclusion criteria SCORAD greater than 45 UVA1 baseline SCORAD mean (SD) 69.8 ± 10.2 UVA1 cold‐light SCORAD mean (SD) 71.7 ± 12.6 UVA‐UVB SCORAD mean (SD) 71.0 ± 9.4 HIV/AIDs comorbidity Not reported Number of withdrawals UVA1 n=6 (12.0%) due to adverse effects UVA1 cold‐light n=2 (4.0%) due to adverse effects UVA‐UVB n=4 (20.0%) due to the fact no effect was seen and in some cases the skin status deteriorated Notes Pre‐treatment disease severity did not differ significantly between the 3 study groups (P greater than 0.2). The treatment protocol was allowed to be discontinued prematurely when skin status, as assessed by means of the SCORAD score, had improved by less than 5% or even deteriorated after 2 weeks of therapy or when bacterial superinfection or herpes simplex infection occurred, thus requiring additional external or internal treatment. |
|
| Interventions |
Run‐in details NA Groups UVA1 Machine type: Sellas WL 20.000 bed (Systems Dr Sellmeier, Ennepetal, Germany). Wavelength: 340‐400nm (also scattered radiation higher than 530nm including infrared radiation, 780‐3000nm). Treatment regimen: 5 times per week for 3 weeks. Total treatments: 15. Dosage: 2.3 J/cm2 per minute. The average time to apply 50 J/cm2 was 44 minutes (22 minutes on each side) Cumulative dose: 750 J/cm2 UVA1 cold‐light Machine type: Photomed CL 300,000 liquid (Photomed, Hamburg, Germany). Wavelength: 340‐530 nm. Regimen: 5 times per week for 3 weeks. Total number of treatments: 15. Dosage: 1.9 J/cm2 per minute. Average time to apply 50 J/cm2 was 52 minutes (26 minutes each side). Cumulative dose: 750 J/cm2. UVA‐UVB Machine type: 40 fluorescent tubes (UVA ‐ Waldmann F85/100‐PUVA, UVB ‐ Waldmann F85/UV6) arranged in a cubicle (Waldmann, Villingen‐Schwenningen, Germany). Treatment regimen: "successive dose increments were performed daily under close meshed patient control for 15 days." Size of increments: UVB treatment was started at 80%of the minimal erythema dose. After each session the UVB dosage was increased by 20% of the minimal erythema dose to a maximum of 0.3 J/cm2. UVA was introduced at 2.0 J/cm2 and then increased daily by 1.0 J/cm2 to a maximum single dose of 8.0 J/cm2. When erythema appeared, the UVA and UVB dose was reduced to the preceding dose. Total treatments: 15 Mean final dosages actually received by participants was 0.29 ±0.03 J/cm2 for UVB and 7.9 ± 0.4 J/cm2 for UVA. Co‐interventions Use of emollients Notes None |
|
| Outcomes |
*denotes relevance to this review. Funding source Not reported Declarations of interest Not reported |
|
| Notes | None. | |
Youssef 2020.
| Study characteristics | ||
| Methods |
Trial design Randomised, controlled, parallel‐group, single‐blinded clinical trial Trial registration number PACTR201810815694251 Country Egypt Outpatient or hospital Outpatient clinic of Kasr Al‐Ainy Hospital, Faculty of Medicine, Cairo University Date trial conducted Not reported Duration of trial participation 4‐week treatment period and 4 weeks of post‐treatment follow‐up Additional design details None Inclusion criteria
Exclusion criteria
Notes None |
|
| Participants |
Total number randomised 30; 15 to each group Age Mean age 9.9 years ± SD 4.1 years in the glycerol group and 13.7 years ± 8.7 years in the NB‐UVB group Sex 8 males and 7 females in the glycerol group; 4 males and 11 females in the NB‐UVB group Race/ethnicity/Fitzpatrick skin type Not reported Duration of eczema Not reported Severity of eczema Mean SCORAD 34.32 ± 10.95 in the glycerol group and 37.24 ± 9.06 in the NB‐UVB group HIV/AIDs comorbidity Not reported Number of withdrawals 3 participants withdrew from the glycerol group; 1 owing to severe irritation, and 2 were lost to follow‐up. 2 withdrew from the NB‐UVB group; 1 owing to phototoxicity, and 1 was lost to follow‐up. Notes None |
|
| Interventions |
Run‐in details None Groups Glycerol: 85% glycerol, without additives and preservatives, applied daily to affected sites for 4 weeks NB‐UVB: NB‐UVB administered 3 times weekly for 4 weeks (total = 12 sessions) in a UV cabin (Waldmann GmbH, Germany) with 16 TL‐01/ 100 W fluorescent lamps producing NB‐UVB with a peak emission of 311 nm. Starting dose 70% MED, with increments according to erythemal response. If faint erythema, dose was fixed; if mild erythema, dose reverted to previous dose; if moderate erythema, sessions were halted, then resumed at 50% of previous dose. If localised moderate erythema, patient was instructed to cover it with a cloth during the following session, then gradually expose for half the time subsequently. Participants who miss 1 or 2 sessions resumed their last dose and continued until they completed all 12 sessions. Cumulative dose: not reported Weaning regimen: not reported Co‐interventions Participants received pure petroleum jelly to apply to all dry skin at bedtime and after bathing. Those in the phototherapy group were asked to clean it off with a moist towel or bath prior to sessions. No other topical or systemic treatments were permitted. Notes None |
|
| Outcomes |
*denotes relevance to this review |
|
| Notes |
Funding source There was no specific public, commercial, or not‐for‐profit grant funding. Declarations of interest None declared Notes None |
|
Zimmerman 1994.
| Study characteristics | ||
| Methods |
Trial design Prospective, randomised, parallel‐group study Trial registration number Not reported Country Germany (assumed from authors' affiliation) Outpatient or hospital Both Date trial conducted February 1992 to August 1993 Duration of trial participation 4‐week treatment period. Follow‐up treatment was mentioned, however it was not clear if this was between phototherapy treatments or outside the 4‐week period. Additional design details None Inclusion criteria Participants with psoriasis vulgaris or atopic eczema; we only extracted data for the participants with atopic eczema Exclusion criteria Requirement for systemic treatment with retinoids, immunosuppressants, corticosteroids, and antihistamines Notes None |
|
| Participants |
Total number randomised 8 people with atopic eczema Age Not reported for participants with atopic eczema alone. Range across all included participants was 15 to 66 years. Sex Not reported for participants with atopic eczema alone Race/ethnicity/Fitzpatrick skin type Not reported Duration of eczema Not reported Severity of eczema Not reported HIV/AIDs comorbidity Not reported Number of withdrawals Not reported Notes None |
|
| Interventions |
Run‐in details None Groups The intervention group bathed in 15% salt solution: 220 L water to 35 kg synthetic Dead Sea salt The control group bathed in 3% saline solution for 20 minutes prior to irradiation. For both groups, irradiation was carried out in a Saalmann SUP cabin, 295 nm to 335 nm, in increasing time intervals and doses according to photosensitivity of the skin and manufacturer's recommendations. Cumulative dose: not reported Weaning regimen: not reported Co‐interventions Topical dithranol or corticoids were not permitted during the study. Nourishing topicals were stated to be used as follow‐up treatment to prevent drying. Notes None |
|
| Outcomes |
*denotes relevance to this review |
|
| Notes |
Funding source Not reported Declarations of interest Not declared Notes None |
|
Clinical trial protocols on the WHO platform were inaccessible February 2021
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anonymous 2016 | Wrong population |
| Biella 1993 | Wrong study design |
| Breuckmann 2003 | Wrong study design |
| Collins 1995 | Wrong study design |
| Dittmar 1999 | Wrong study design |
| Edstrom 2010 | Wrong study design |
| Falk 1985 | Wrong study design |
| Gambichler 2000 | Wrong study design |
| Grabbe 1996 | Wrong study design |
| Hjerppe 2001 | Wrong study design |
| Jekler 1990 | Wrong study design |
| Jekler 1990a | Wrong study design |
| JPRN‐UMIN000018462 | Wrong study design |
| Keemss 2016 | Wrong population |
| Kowalzick 1994 | Wrong study design |
| Kowalzick 1995 | Wrong study design |
| Krutmann 1991 | Wrong study design |
| Lajevardl 2015 | Wrong study design |
| Legat 2017 | Wrong population |
| Midelfart 1985 | Wrong study design |
| Morison 1978 | Wrong study design |
| NCT00129415 | Wrong study design |
| NCT01402414 | Trial terminated with no data available |
| NCT03083730 | Wrong study design |
| NCT03402412 | Wrong study design |
| NCT04444726 | Wrong population |
| Pasic 1996 | Wrong study design |
| Salo 1983 | Wrong study design |
| Schiffner 2002 | Wrong study design |
| Shephard 1996 | Wrong population |
| Snellman 2000 | Wrong study design |
| Valkova 2004 | Wrong comparator |
Characteristics of studies awaiting classification [ordered by study ID]
Hannuksela 1985.
| Methods | No information given; does not appear to be a randomised controlled trial, but we were unable to rule it out during full text screening. |
| Participants | 196 participants with atopic dermatitis |
| Interventions | Psorilux 9050 (1.24 mW/cm2 at 280 nm to 315 nm and
7.33 mW/cm2 at 315 nm to 400 nm). Some participants
received one treatment course, some 2 to 3 courses,
and some more than 3 treatment courses; there is an
imbalance of group sizes, which may be a consequence
of this not being a randomised controlled trial. From 1982 onwards, participants were treated with Metec Helarium model 1480 (UVB and UVA; 310 nm to 340 nm, with a peak at 320 nm to 330 nm). Some received one treatment course (mean 19 weeks), and some received two treatment courses. Again, there was an imbalance in the groups. |
| Outcomes | Effectiveness and requirement for topical
corticosteroids Burning and erythema were reported |
| Notes | No full text or contact information for the study authors available; information extracted from the abstract. |
Kim 2012.
| Methods | Randomised single‐blind, placebo‐controlled, parallel‐group study |
| Participants | 92 participants with mild to moderate atopic dermatitis |
| Interventions | StoneTouch® far‐infrared versus sham device three times daily for 14 days |
| Outcomes | Efficacy including pruritus visual analogue scale and
physician assessment; reported improvement in the
StoneTouch® group. Safety, including transient erythema and mild irritation reported "in a few patients"; diminished after 1 to 2 days of treatment. |
| Notes | No full text or contact information for the study authors available. Information extracted from the abstract. |
Potapenko 2000.
| Methods | No information given |
| Participants | People with eczema; otherwise no information |
| Interventions | Photo‐oxidized psoralen; otherwise no information |
| Outcomes | No information given |
| Notes | No abstract, full text, or contact information available for the study authors. |
Pullman 1985.
| Methods | No information given; unclear if this is a randomised controlled trial. |
| Participants | People with endogenous eczema |
| Interventions | UVA for five treatments a week for 3 weeks versus UVA twice weekly for 6 to 8 weeks; otherwise no information |
| Outcomes | No information given |
| Notes | No abstract, full text, or contact information available for the study authors. Information extracted from Jekler 1991b. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12620000546954.
| Study name | Comparing the effect of narrowband ultraviolet B (UVB) therapy to therapy with natural sunlight and an amino acid lecithin cream on dermatologic symptoms |
| Methods |
Trial design Unclear; presumed to be a randomised controlled trial, however, this is not explicitly stated. Country Queensland, Australia Outpatient or hospital Outpatient Duration of trial participation 12 weeks Inclusion criteria
Exclusion criteria Not reported |
| Participants | Total number randomised not reported |
| Interventions | NB‐UVB versus amino acid lecithin cream with natural
sunlight.
|
| Outcomes |
|
| Starting date | Not yet recruiting; anticipated to start 01 June 2020. |
| Contact information | Not reported |
| Notes | Extracted from Key Trial Information and Cochrane Central listing: www.cochranelibrary.com/central/doi/10.1002/central/CN‐02165320/full |
Droitcourt 2019.
| Study name | PRADA |
| Methods |
Trial design Randomised, multi‐centre, double‐blind (except phototherapy), parallel‐group, cross‐over, pragmatic trial Country France; recruitment in primary care with study conducted in hospital settings Duration of trial participation 2 years (in addition to up to 9 months of pre‐screening period prior to randomisation) Inclusion criteria
Exclusion criteria
|
| Participants | The study aims to enrol 200 participants |
| Interventions |
Phototherapy: NB‐UVB; period of escalation with three sessions per week for 3 weeks, followed by every 2 weeks for 6 months in total (winter: October to March). Dose initiated at 0.2 J/cm2 (phototype II to III) or 0.3 J/cm2 (phototype IV to V) with increments of 0.1 up to the ninth session. Then, the dosage of the ninth session (1.0 or 1.1 J/cm2 for type II to III; 1.1 or 1.2 J/cm2 for type IV to V) will be used for maintenance. The exposure can be altered according to clinical tolerance. All participants will continue to receive standard care, i.e. topical anti‐inflammatory treatments, strictly as usual, and without recommendations to change lifestyle. |
| Outcomes |
|
| Starting date | 27 October 2015; currently recruiting |
| Contact information | Catherine Droitcourt: +33 2 99 28 43 49; catherine.droitcourt@chu‐rennes.fr |
| Notes |
Kromer 2019.
| Study name | AD‐Blue; NCT03085303 |
| Methods |
Trial design Multi‐centre, placebo‐controlled, double‐blinded, three‐armed, prospective, randomised controlled trial Country Germany and Switzerland Duration of trial participation 13 weeks; 1‐week enrolment, 8‐week treatment phase and 4‐week post‐treatment follow‐up Inclusion criteria
Exclusion criteria
|
| Participants | 87 participants were randomised |
| Interventions | Full body irradiation given three times weekly for 8
weeks with FBB‐CT01 devices (Philips; Aachen,
Germany; not Conformité Européene, CE, marked). LEDs
emitting blue light for 30 minutes (15 minutes each
body side) with the following settings:
Unguentum leniens cream was also permitted If EASI increased by ≥ 50% from baseline after ≥ 4 weeks, rescue therapy with topical steroids or antihistamines was prescribed and documented. |
| Outcomes |
|
| Starting date | 16 March 2017 |
| Contact information | Timo Buhl (timo.buhl@med.uni‐goettingen.de), Department of Dermatology, Venereology, and Allergology, University Medical Center Göttingen |
| Notes |
NCT02915146.
| Study name | Narrowband ultraviolet B versus narrowband ultraviolet B plus ultraviolet A1 for atopic eczema |
| Methods |
Trial design Randomised, controlled, single‐blind, parallel‐group trial Country Scotland, UK Duration of trial participation 51 weeks (25‐week treatment and 26‐week post‐treatment follow‐up) Inclusion criteria
Exclusion criteria
|
| Participants | 39 participants were enrolled |
| Interventions | NB‐UVB combined with UVA1 versus NB‐UVB monotherapy |
| Outcomes |
|
| Starting date | August 2016 |
| Contact information | Robert S Dawe, Ninewells Hospital, Dundee, United Kingdom, DD1 9SY |
| Notes |
Differences between protocol and review
Assessment of risk of bias in included studies: We did not use the cross‐over variant of the RoB 2 tool because we only extracted data during the first period of the studies, due to concerns with carry‐over effects (Higgins 2016).
Unit of analysis issues: in split‐body studies, paired data were not reported. Therefore, to be able to include such data in a meta‐analysis and combine with parallel studies, we performed variance corrections using the Becker‐Balagtas method (Elbourne 2002). We assumed an intra‐class correlation coefficient (ICC) of 0.5 in our calculations. A continuity correction of 0.5 was used in the case of zero events (Sweeting 2004). We combined data from within‐participant studies with data from between‐participant studies into a meta‐analysis using the generic inverse‐variance method, and calculated odds ratios (OR).
Contributions of authors
AM was the contact person with the editorial base. AM co‐ordinated contributions from the co‐authors and wrote the final draft of the review. AM, SM, SL and JH screened papers against eligibility criteria. AM, SM, SL and JH obtained data on ongoing and unpublished studies. AM, RB and PS appraised the quality of papers. AM, SM, SL and JH extracted data for the review and sought additional information about papers. AM, SM, SL and JH entered data into RevMan. AM, SM, SL, JH and EA analysed and interpreted data. AM, SM, SL, JH, CF, AD, LG, JF, SI, RD, FG, MB, JL, RB, and PS worked on the methods sections. AM, SM and LG drafted the clinical sections of the background and responded to the clinical comments of the referees. EA responded to the methodology and statistics comments of the referees. EA and RB undertook GRADE certainty of evidence assessments and completed the summary of findings tables and abstract results and conclusions sections. EA and LP drafted other summary sections of the review based on the abstract conclusions. RB oversaw the project progress, delivery and quality. PS was the consumer co‐author and checked the review for readability and clarity, as well as ensuring outcomes are relevant to consumers.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Skin. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Sources of support
Internal sources
-
Department of Dermatology, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands
Departmental funding
External sources
-
The National Institute for Health Research (NIHR), UK
The NIHR, UK, is the largest single funder of Cochrane Skin
-
American Academy of Dermatology (AAD), USA
This project was supported by a grant from the AAD (4783981)
Declarations of interest
Emma Axon: has declared that they have no conflict of interest.
Robert J Boyle: reports receiving personal income from several private paediatric allergy practice clinics that include eczema management.
Marijke Brouwer: has declared that they have no conflict of interest.
Robert S Dawe: is a member of the steering group for the Scottish National Managed Clinical Network for Phototherapy (Photonet).
Aaron Drucker: reports receiving compensation from the British Journal of Dermatology (Section Editor and Reviewer; honorarium paid to institution) and the American Academy of Dermatology (guidelines writer; paid to institution). AD reports being interviewed for the Eczema Society of Canada’s educational resource ‘Ask the doctor about...Phototherapy’ (eczemahelp.ca/wp-content/uploads/hcp-resources/ESC_Ask-the-Doctor_Phototherapy_2020.pdf) (no payment received), and he has been a grant reviewer for the National Eczema Association (no payment received).
John Ferguson: reports paid consultancy (personal payment) with Genesis Care, a personal healthcare company with an interest in providing radiotherapy for benign skin disease. This could include the treatment of eczema, particularly in its more chronic forms. The relevance of this work with respect to this Cochrane Review is limited, but Dr Ferguson wishes readers to be aware of the potential conflict. Dr Ferguson reports that a charitable trust in the UK (Photobiology Trust) has given money to Guy's and St Thomas' Hospital Trust (GSTT) towards the purchase of an excimer lamp for people with GSTT. Excimer lamp technology can be used for treating eczema, particularly chronic forms. Dr Ferguson wishes readers to be aware of this potential conflict. JF is a member of the British Photo‐dermatology Group.
Carsten Flohr: has declared that they have no conflict of interest.
Floor Garritsen: reports payment from AbbVie for a presentation about treatment of atopic dermatitis (personal payment); payment from AbbVie for participation on an atopic dermatitis advisory board (personal payment); and payment from the Dutch Society of Dermatology (NVDV) for an atopic dermatitis guideline panel (personal payment).
Louise Gerbens: has declared that they have no conflict of interest.
Jane Harvey: has declared that they have no conflict of interest.
Sally Ibbotson: reports payment from La Roche‐Posay as an invited speaker at a masterclass November 2019 (paid to institution). SI reports personal payment from UCB Pharma for registration fees for the British Association of Dermatologists annual meeting September 2020 (invited speaker), the American Academy of Dermatology VMX virtual meeting April 2021 (invited speaker), and the British Association of Dermatologists annual meeting July 2021. SI reports personal payment from Galderma (UK) for registration, accommodation, and travel expenses to support attendance at the World Congress of Dermatology June 2019 (invited speaker), and registration fees for the European Academy of Dermatology and Venereology virtual congress in October 2020 (invited speaker).
Stephanie J Lax: has declared that they have no conflict of interest.
Jacqueline Limpens: has declared that they have no conflict of interest.
Soudeh Mashayekhi: has declared that they have no conflict of interest.
Annelie H Musters: has declared that they have no conflict of interest.
Laura E Prescott: has declared that they have no conflict of interest.
Phyllis I Spuls: reports consultancies in the past for Sanofi (2017) and AbbVie (2017) (unpaid). PIS received a departmental independent research grant (paid to institution) for her role as Chief Investigator of the systemic and phototherapy atopic eczema registry (TREAT NL) for adults and children; this grant was from a governmental grant office (ZonMW in 2017), LEO Pharma (in 2019), and Novartis (in 2020); other companies have already agreed to sponsor in order to have multi‐sponsoring. PIS reports involvement in performing clinical trials with many pharmaceutical industries that manufacture drugs used for the treatment of psoriasis and atopic dermatitis. Financial compensation for this work is paid to the department or hospital. PIS is one of the main investigators of the SECURE‐AD registry. PIS is currently trying to get funding for a study that could be included in a future update of this review. The funding resource is ZonMW, governmental funding body, Netherlands. Our cohort study TREAT NL registry is not a randomised controlled trial, thus, not eligible.
Clinical referee, Sara Brown: Wellcome Trust Senior Fellow and Professor of Dermatology, University of Edinburgh and NHS Lothian: I trained in dermatology with Nick Reynolds and colleagues from 2000 to 2008. My research is focussed on genetic mechanisms in atopic eczema; I receive grant funding from the Wellcome Trust, British Skin Foundation, EU‐IMI (including multiple pharmaceutical partners), and philanthropic donors. I received a grant from Pfizer for an investigator‐initiated research study 3 years ago. I am a consultant for Sosei Heptares and AbbVie (reimbursement paid to the University of Edinburgh – no personal financial reward). I have received honoraria for speaking about my research at academic conferences and symposia, including the British Association of Dermatologists, British Society for Paediatric Dermatology, Harvard Grand Rounds, and Wellcome Trust Advanced Course.
Edited (no change to conclusions)
References
References to studies included in this review
Agrawal 2018 {published data only}
- Agrawal R, Bashir B, Qayuum S, Inayat S, Khurshid K, Pal SS, et al. Comparison of efficacy and safety of topical betamethasone valerate 0.1% with narrowband-UVB in atopic dermatitis. Journal of Pakistan Association of Dermatologists 2018;28(2):239‐44. [Google Scholar]
Brenninkmeijer 2010 {published data only}
- Brenninkmeijer E, Spuls P, Bos J, Wolkerstorfer A. Excimer 308 nm laser vs clobetasol propionate 0.05% ointment in the prurigo form of atopic dermatitis: a randomized controlled trial. Lasers in Surgery and Medicine 2009;41:107. [DOI] [PubMed] [Google Scholar]
- Brenninkmeijer EE, Spuls PI, Lindeboom R, Wal AC, Bos JD, Wolkerstorfer A. Excimer laser vs. clobetasol propionate 0.05% ointment in prurigo form of atopic dermatitis: a randomized controlled trial, a pilot. British Journal of Dermatology 2010;163(4):823-31. [DOI] [PubMed] [Google Scholar]
- EUCTR2006-005602-31-NL. Excimer laser versus clobetason propionaat in prurigo form of atopic dermatitis. www.clinicaltrialsregister.eu/ctr-search/trial/2006-005602-31/NL (first received 24 October 2006).
- ISRCTN38773821. Excimer laser versus clobetason propionate in prurigo form of atopic dermatitis. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN38773821 (accessed before 13 July 2021).
- NTR797. Excimer laser versus clobetason propionaat in prurigo form of atopic dermatitis. www.who.int/trialsearch/Trial2.aspx?TrialID=NTR797 (accessed before 13 July 2021).
Byun 2011 {published data only}
- Byun HJ, Lee HI, Kim B, Kim MN, Hong H, Choi Y, et al. Full-spectrum light phototherapy for atopic dermatitis. International Journal of Dermatology 2011;50(1):94‐101. [DOI] [PubMed] [Google Scholar]
Der‐Petrossian 2000 {published data only}
- Der-Petrossian M, Honigsman H, Tanew A. Halfside comparison study on the efficacy of 8-MOP bath PUVA versus narrow band UVB phototherapy in patients with severe atopic dermatitis. Journal of Investigative Dermatology 1998;110(4):682. [ABSTRACT NUMBER: 1262] [DOI] [PubMed] [Google Scholar]
- Der-Petrossian M, Seeber A, Hönigsmann H, Tanew A. Half-side comparison study on the efficacy of 8-methoxypsoralen bath-PUVA versus narrow-band ultraviolet B phototherapy in patients with severe chronic atopic dermatitis. British Journal of Dermatology 2000;142(1):39‐43. [DOI] [PubMed] [Google Scholar]
Dittmar 2001 {published data only}
- Dittmar HC, Pflieger D, Schöpf E, Simon JC. UVA1 phototherapy. Pilot study of dose finding in acute exacerbated atopic dermatitis. Der Hautarzt 2001;52(5):423‐7. [DOI] [PubMed] [Google Scholar]
- Dittmar HC, Pflieger D, Schopf E, Simon JC. UVA1 therapy dose-finding study in patients with acute exacerbated atopic dermatitis. International Archives of Allergy and Immunology 2001;124(1‐3):386‐8. [Google Scholar]
Gambichler 2009 {published data only}
- Gambichler T, Othlinghaus N, Tomi NS, Holland-Letz T, Boms S, Skrygan M, et al. Medium-dose ultraviolet (UV) A1 vs. narrowband UVB phototherapy in atopic eczema: a randomized crossover study. British Journal of Dermatology 2009;160(3):652‐8. [DOI] [PubMed] [Google Scholar]
- NCT00419406. Medium-dose UVA1 versus narrow-band UVB in atopic dermatitis. clinicaltrials.gov/ct2/show/NCT00419406 (first received 8 January 2007).
Granlund 2001 {published data only}
- Granlund H, Erkko P, Remitz A, Langeland T, Helsing P, Nuutinen M, et al. Comparison of cyclosporin and UVAB phototherapy for intermittent one-year treatment of atopic dermatitis. Acta Dermato-Venereologica 2001;81(1):22‐7. [DOI] [PubMed] [Google Scholar]
- Granlund H. Comparison of cyclosporine and UVA/B phototherapy in long-term intermittent treatment of atopic dermatitis. Australasian Journal of Dermatology 1997;38(Suppl 2):236. [ABSTRACT NUMBER: 4144] [Google Scholar]
- Salo H, Pekurinen M, Granlund H, Nuutinen M, Erkko P, Reitamo S. An economic evaluation of intermittent cyclosporin A therapy versus UVAB phototherapy in the treatment of patients with severe atopic dermatitis. Acta Dermato-Venereologica 2004;84(2):138‐41. [DOI] [PubMed] [Google Scholar]
Heinlin 2011 {published data only}
- Heinlin J, Schiffner-Rohe J, Schiffner R, Einsele-Krämer B, Landthaler M, Klein A, et al. A first prospective randomized controlled trial on the efficacy and safety of synchronous balneophototherapy vs. narrow-band UVB monotherapy for atopic dermatitis. Journal of the European Academy of Dermatology and Venereology 2011;25(7):765‐73. [DOI] [PubMed] [Google Scholar]
Hoey 2006 {published data only}
- Hoey SEH, Catney D, Maguire S, McKenna K. Fixed low dose versus increasing dose of ultraviolet B-TL01 in the treatment of atopic eczema. British Journal of Dermatology 2006;155(Suppl 1):121‐2. [ABSTRACT NUMBER: PD-4] [Google Scholar]
Jekler 1988a {published data only}
- Jekler J, Larkö O. UVB phototherapy of atopic dermatitis. British Journal of Dermatology 1988;119(6):697‐705. [DOI] [PubMed] [Google Scholar]
- Jekler J. Phototherapy of atopic dermatitis with ultraviolet radiation. Acta Dermato-Venereologica. Supplementum 1992;171:1‐37. [PubMed] [Google Scholar]
Jekler 1988b {published data only}
- Jekler J, Larkö O. UVB phototherapy of atopic dermatitis. British Journal of Dermatology 1988;119(6):697‐705. [DOI] [PubMed] [Google Scholar]
- Jekler J. Phototherapy of atopic dermatitis with ultraviolet radiation. Acta Dermato-Venereologica. Supplementum 1992;171:1‐37. [PubMed] [Google Scholar]
Jekler 1990 {published data only}
- Jekler J, Larko O. Combined UVA-UVB versus UVB phototherapy for atopic dermatitis: a paired-comparison study. Journal of the American Academy of Dermatology 1990;22(1):49-53. [DOI] [PubMed] [Google Scholar]
- Jekler J. Phototherapy of atopic dermatitis with ultraviolet radiation. Acta Dermato-Venereologica. Supplementum 1992;171:1‐37. [PubMed] [Google Scholar]
Jekler 1991 {published data only}
- Jekler J, Larkö O. UVA solarium versus UVB phototherapy of atopic dermatitis: a paired-comparison study. British Journal of Dermatology 1991;125(6):569‐72. [DOI] [PubMed] [Google Scholar]
- Jekler J. Phototherapy of atopic dermatitis with ultraviolet radiation. Acta Dermato-Venereologica. Supplementum 1992;171:1‐37. [PubMed] [Google Scholar]
Jekler 1991b Study 1 {published data only}
- Jekler J, Larkö O. Phototherapy for atopic dermatitis with ultraviolet A (UVA), low-dose UVB and combined UVA and UVB: two paired comparison studies. Photodermatology, Photoimmunology & Photomedicine 1991;8(4):151-6. [PubMed] [Google Scholar]
- Jekler J. Phototherapy of atopic dermatitis with ultraviolet radiation. Acta Dermato-Venereologica. Supplementum 1992;171:1‐37. [PubMed] [Google Scholar]
Jekler 1991b Study 2 {published data only}
- Jekler J, Larkö O. Phototherapy for atopic dermatitis with ultraviolet A (UVA), low-dose UVB and combined UVA and UVB: two paired comparison studies. Photodermatology, Photoimmunology & Photomedicine 1991;8(4):151-6. [PubMed] [Google Scholar]
- Jekler J. Phototherapy of atopic dermatitis with ultraviolet radiation. Acta Dermato-Venereologica. Supplementum 1992;171:1‐37. [PubMed] [Google Scholar]
Krutmann 1992 {published data only}
- Krutmann J, Czech W, Diepgen T, Niedner R, Kapp A, Schöpf E. High-dose UVA1 therapy in the treatment of patients with atopic dermatitis. Journal of the American Academy of Dermatology 1992;2(26):225-30. [DOI] [PubMed] [Google Scholar]
Krutmann 1998 {published data only}
- Krutmann J, Diepgen TL, Luger TA, Grabbe S, Meffert H, Sönnichsen N, et al. High-dose UVA1 therapy for atopic dermatitis: results of a multicenter trial. Journal of the American Academy of Dermatology 1998;38(4):589‐93. [DOI] [PubMed] [Google Scholar]
- Krutmann J. High dose UVA1 therapy for the atopic dermatitis (AD): a multicentrer trial. Journal of Investigative Dermatology 1995;105(3):458. [ABSTRACT NUMBER: 65] [Google Scholar]
Kwon 2019 {published data only}
- Kwon S, Choi JY, Shin JW, Huh CH, Park KC, Du MH, et al. Changes in lesional and non-lesional skin microbiome during treatment of atopic dermatitis. Acta Dermato-Venereologica 2019;99(3):284‐90. [DOI] [PubMed] [Google Scholar]
Legat 2003 {published data only}
- Legat FJ, Hofer A, Brabek E, Quehenberger F, Kerl H, Wolf P. Narrowband UV-B vs medium-dose UV-A1 phototherapy in chronic atopic dermatitis. Archives of Dermatology 2003;139(2):223‐4. [DOI] [PubMed] [Google Scholar]
Leone 1998 {published data only}
- Leone G, Cristaudo A, Ferraro C, Capitanio B, Morrone A, Fazio M. Evaluation of different phototherapies in severe atopic dermatitis: preliminary results . Journal of the European Academy of Dermatology and Venereology 1998;11(Suppl 2):S319. [ABSTRACT NUMBER: P567] [Google Scholar]
Majoie 2009 {published data only}
- Majoie IM, Oldhoff JM, Weelden H, Knol EF, Bousema MT, Bruijnzeel-Koomen CA, et al. Phototherapy in atopic dermatitis. narrow-band UVB versus medium-dose UVA1. Journal of the European Academy of Dermatology and Venereology 2005;19(Suppl 2):2‐3. [ABSTRACT NUMBER: FC01.7] [Google Scholar]
- Majoie IM, Oldhoff JM, Weelden H, Laaper-Ertmann M, Bousema MT, Sigurdsson V, et al. Narrowband ultraviolet B and medium-dose ultraviolet A1 are equally effective in the treatment of moderate to severe atopic dermatitis. Journal of the American Academy of Dermatology 2009;60(1):77‐84. [DOI] [PubMed] [Google Scholar]
Maul 2017 {published data only}
- Maul JT, Kretschmer L, Anzengruber F, Pink A, Murer C, French LE, et al. Impact of UVA on pruritus during UVA/B phototherapy of inflammatory skin diseases: a randomized double-blind study. Journal of the European Academy of Dermatology and Venereology 2017;31(7):1208-13. [DOI] [PubMed] [Google Scholar]
- NCT01254240. Efficacy study of two choices of phototherapy on itching skin diseases. clinicaltrials.gov/ct2/show/NCT01254240 (first received 6 December 2010).
Pacifico 2019 {published data only}
- Pacifico A, Iacovelli P, Damiani G, Ferraro C, Cazzaniga S, Conic RZ, et al. 'High dose' vs. 'medium dose' UVA1 phototherapy in Italian patients with severe atopic dermatitis. Journal of the European Academy of Dermatology and Venereology 2019;33(4):718‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Qayyum 2016 {published data only}
- Qayyum S, Asad F, Agrawal R, Khurshid K, Rani Z, Pal SS. Comparison of efficacy and safety of ultraviolet A radiation versus ultraviolet B radiation in atopic dermatitis. Journal of Pakistan Association of Dermatologists 2016;26(3):223‐8. [Google Scholar]
Reynolds 2001 {published data only}
- ISRCTN10725589. Ultraviolet light (UV) therapy for atopic dermatitis: double blind, randomised trial of narrow band (TLO1) versus UVA versus placebo. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN10725589 (accessed before 13 July 2021).
- Reynolds N, Franklin V, Gray J, Diffey B, Farr P. Effectiveness of narrow-band UVB (TL01) compared to UVA in adult atopic eczema: a randomized controlled trial. British Journal of Dermatology 1999;141(Suppl 55):20-1. [Google Scholar]
- Reynolds N, Franklin V, Gray J, Diffey B, Farr P. Randomized, controlled trial of narrow-band UVB (TL01) and UVA in adult atopic dermatitis. Journal of Investigative Dermatology 1999;112(4):655. [ABSTRACT NUMBER: 794] [Google Scholar]
- Reynolds NJ, Franklin V, Gray JC, Diffey BL, Farr PM. Narrow-band ultraviolet B and broad-band ultraviolet A phototherapy in adult atopic eczema: a randomised controlled trial. Lancet 2001;357(9273):2012‐6. [DOI] [PubMed] [Google Scholar]
Selvaag 2005 {published data only}
- Selvaag E, Caspersen L, Bech-Thomsen N, Wulf HC. Optimized UVB treatment of atopic dermatitis. A controlled, left-right comparison trial. Annales de Dermatologie et de Venereologie 2002;129(Suppl 1 Pt 2):1S734. [Google Scholar]
- Selvaag E, Caspersen L, Bech-Thomsen N, Wulf HC. Optimized UVB treatment of atopic dermatitis using skin reflectance measurements. A controlled, left-right comparison trial. Acta Dermato-Venereologica 2005;85(2):144‐6. [DOI] [PubMed] [Google Scholar]
Tzaneva 2001 {published data only}
- Tzaneva S, Seeber A, Schwaiger M, Hönigsmann H, Tanew A. High-dose versus medium-dose UVA1 phototherapy for patients with severe generalized atopic dermatitis. Journal of the American Academy of Dermatology 2001;45(4):503‐7. [DOI] [PubMed] [Google Scholar]
Tzaneva 2010 {published data only}
- EUCTR2006-006982-17-AT. UVA 1 therapy versus 5-MOP UVA photochemotherapy for patients with severe generalized atopic dermatitis. www.clinicaltrialsregister.eu/ctr-search/trial/2006-006982-17/AT (first received 14 March 2007).
- NCT00533195. Comparison of UVA1 phototherapy versus photochemotherapy for patients with severe generalized atopic dermatitis. clinicaltrials.gov/show/NCT00533195 (first received 21 September 2007).
- Tzaneva S, Kittler H, Holzer G, Reljic D, Weber M, Hönigsmann H, et al. 5-methoxypsoralen plus ultraviolet (UV) A is superior to medium-dose UVA1 in the treatment of severe atopic dermatitis: a randomized crossover trial. British Journal of Dermatology 2010;162(3):655‐60. [DOI] [PubMed] [Google Scholar]
Tzung 2006 {published data only}
- Tzung TY, Lin CB, Chen YH, Yang CY. Pimecrolimus and narrowband UVB as monotherapy or combination therapy in children and adolescents with atopic dermatitis. Acta Dermato-Venereologica 2006;86(1):34‐8. [DOI] [PubMed] [Google Scholar]
Von Kobyletzki 1999a {published data only}
- Frietag M, Von Kobyletzki G, Pieck F, Breuckmann F, Hoffmann K, Altmeyer P. Medium-dose UVA1 cold light phototherapy in the treatment of severe atopic dermatitis. Journal of the European Academy of Dermatology and Venereology 1999;12(Suppl 2):S122. [DOI] [PubMed] [Google Scholar]
- Von Kobyletzki G, Freitag M, Herde M, Höxtermann S, Stücker M, Hoffmann K, et al. Phototherapy in severe atopic dermatitis. Comparison between current UVA1 therapy, UVA1 cold light and combined UVA-UVB therapy. Der Hautarzt 1999;50(1):27-33. [DOI] [PubMed] [Google Scholar]
- Von Kobyletzki G, Freitag M, Herde M, Stücker M, Reuther T, Hoffmann K, et al. Comparison between conventional UVA1 phototherapy, UVA1 cold light phototherapy and combined UVA-UVB phototherapy in atopic dermatitis. Journal of the European Academy of Dermatology and Venereology 1998;11(Suppl 2):S318. [Google Scholar]
- Von Kobyletzki G, Pieck C, Hoffmann K, Freitag M, Altmeyer P. Medium dose UVA1 cold light phototherapy in the treatment of severe atopic dermatitis. Dermatology 1999;199(1):83. [DOI] [PubMed] [Google Scholar]
- Von Kobyletzki G, Pieck C, Hoffmann K, Freitag M, Altmeyer P. Medium-dose UVA1 cold-light phototherapy in the treatment of severe atopic dermatitis. Journal of the American Academy of Dermatology 1999;41(6):931‐7. [DOI] [PubMed] [Google Scholar]
Youssef 2020 {published data only}
- PACTR201810815694251. A randomised controlled trial comparing topical glycerol versus narrowband ultraviolet light B in atopic dermatitis: a clinical and bacteriological evaluation. www.who.int/trialsearch/Trial2.aspx?TrialID=PACTR201810815694251 (accessed before 13 July 2021).
- Youssef R, Hafez V, Elkholy Y, Mourad A. Glycerol 85% efficacy on atopic skin and its microbiome: a randomized controlled trial with clinical and bacteriological evaluation. Journal of Dermatological Treatment 2020 Jan 6 [Epub ahead of print]. [DOI: 10.1080/09546634.2019.1708246] [DOI] [PubMed]
Zimmerman 1994 {published data only}
- Zimmermann J, Utermann S. Photo-brine therapy in patients with psoriasis and neurodermatitis atopica. Der Hautarzt 1994;45(12):849-53. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Anonymous 2016 {published data only}
- Anonymous. Erratum: Prospective, randomized study on the efficacy and safety of local UV-free blue light treatment of eczema (Dermatology (2016) 232 (496-502) DOI: 10.1159/000448000). Dermatology 2016;232(4):522. [DOI] [PubMed] [Google Scholar]
Biella 1993 {published data only}
- Biella U, Biella B, Huse C. Visible light and infrared versus low-dose UVA in the treatment of atopic eczema in childhood. Aktuelle Dermatologie 1993;19(7):185‐6. [Google Scholar]
Breuckmann 2003 {published data only}
- Breuckmann F, Kobyletzki G, Avermaete A, Kreuter A, Altmeyer P, Gambichler T. Mast cells in atopic dermatitis: resistance against medium-dose UVA1 phototherapy? Dermatology 2003;207(3):334-6. [DOI] [PubMed] [Google Scholar]
Collins 1995 {published data only}
- Collins P, Ferguson J. Narrowband (TL-01) UVB air-conditioned phototherapy for atopic eczema in children . British Journal of Dermatology 1995;133(4):653-67. [DOI] [PubMed] [Google Scholar]
Dittmar 1999 {published data only}
- Dittmar HC, Pflieger D, Schempp CM, Schöpf E, Simon JC. Comparison of balneophototherapy and UVA/B mono-phototherapy in patients with subacute atopic dermatitis. Der Hautarzt 1999;50(9):649‐53. [DOI] [PubMed] [Google Scholar]
Edstrom 2010 {published data only}
- Edstrom DW, Linder J, Wennersten G, Brismar K, Ros AM. Phototherapy with ultraviolet radiation: a study of hormone parameters and psychological effects. Journal of the European Academy of Dermatology and Venereology 2010;24(4):403-9. [DOI] [PubMed] [Google Scholar]
Falk 1985 {published data only}
- Falk ES. UV-light therapies in atopic dermatitis. Photo-Dermatology 1985;2(4):241‐6. [PubMed] [Google Scholar]
Gambichler 2000 {published data only}
- Gambichler T, Kuster W, Kreuter A, Altmeyer P, Hoffmann K. Balneophototherapy — combined treatment of psoriasis vulgaris and atopic dermatitis with salt water baths and artificial ultraviolet radiation . Journal of the European Academy of Dermatology and Venereology 2000;14(5):425-8. [DOI] [PubMed] [Google Scholar]
Grabbe 1996 {published data only}
- Grabbe J, Welker P, Humke S, Grewe M, Schopf E, Henz BM, et al. High-dose ultraviolet A1 (UVA1), but not UVA/UVB therapy, decreases IgE-binding cells in lesional skin of patients with atopic eczema. Journal of Investigative Dermatology 1996;107(3):419-22. [DOI] [PubMed] [Google Scholar]
Hjerppe 2001 {published data only}
- Hjerppe M, Hasan T, Saksala I, Reunala T. Narrow-band UVB treatment in atopic dermatitis. Acta Dermato-Venereologica 2001;81(6):439‐40. [DOI] [PubMed] [Google Scholar]
Jekler 1990 {published data only}
- Jekler J, Diffey B, Larkö O. Ultraviolet radiation dosimetry in phototherapy for atopic dermatitis. Journal of the American Academy of Dermatology 1990;23(1):49‐51. [DOI] [PubMed] [Google Scholar]
Jekler 1990a {published data only}
- Jekler J, Larko O. The effect of ultraviolet radiation with peaks at 300 nm and 350 nm in the treatment of atopic dermatitis. Photodermatology, Photoimmunology & Photomedicine 1990;7(4):169‐72. [PubMed] [Google Scholar]
JPRN‐UMIN000018462 {published data only}
- JPRN-UMIN000018462. Efficacy study of long-wave ultraviolet light therapy with LED for the skin disease. www.who.int/trialsearch/Trial2.aspx?TrialID=JPRN-UMIN000018462 (accessed before 13 July 2021).
Keemss 2016 {published data only}
- Keemss K, Pfaff SC, Born M, Liebmann J, Merk HF, Felbert V. Prospective, randomized study on the efficacy and safety of local UV-free blue light treatment of eczema. Dermatology 2016;232(4):496‐502. [DOI] [PubMed] [Google Scholar]
- NCT02002871. Blue light for treating eczema. clinicaltrials.gov/show/NCT02002871 (first received 6 December 2013).
Kowalzick 1994 {published data only}
- Kowalzick L, Kleinheinz A, Weichenthal M, Neuber K, Kohler I, Grosch J, et al. Effects of medium-dose UV-A1 on clinical course and serum levels of sICAM-1,sELAM-1 and ECP in severe atopic eczema. Journal of Investigative Dermatology 1994;103(3):410. [ABSTRACT NUMBER: 82] [Google Scholar]
Kowalzick 1995 {published data only}
- Kowalzick L, Kleinheinz A, Weichenthal M, Neuber K, Kohler I, Grosch J, et al. Low dose versus medium dose UV-A1 treatment in severe atopic eczema. Acta Dermato-Venereologica 1995;75(1):43‐5. [DOI] [PubMed] [Google Scholar]
Krutmann 1991 {published data only}
- Krutmann J, Czech W, Diepgen T, Niedner R, Kapp A, Schopf E. High dose UVA1 therapy in the treatment of patients with atopic dermatitis. Journal of Investigative Dermatology 1991;96(4):568. [ABSTRACT NUMBER: 223] [DOI] [PubMed] [Google Scholar]
Lajevardl 2015 {published data only}
- Lajevardl V, Ghiasl M, Hejazl P, Ansarl M, Akbarl Z, Shakibl H, et al. The effect of narrow band UVB on serum levels of folate: trial on patients with dermatologic disorders. Iranian Journal of Dermatology 2015;18(71):36-7. [Google Scholar]
Legat 2017 {published data only}
- Legat FJ, Hofer A, Gruber-Wackernagel A, Quehenberger F, Waltner K, Wolf P. Both narrowband-UVB and broadband UVB are equally effective in reducing itch in chronic pruritus patients. Acta Dermato-Venereologica 2017;97(8):1056‐7. [Google Scholar]
Midelfart 1985 {published data only}
- Midelfart K, Stenvold SE, Volden G. Combined UVB and UVA phototherapy of atopic eczema. Dermatologica 1985;171(2):95‐8. [DOI] [PubMed] [Google Scholar]
Morison 1978 {published data only}
- Morison WL, Parrish J, Fitzpatrick TB. Oral psoralen photochemotherapy of atopic eczema. British Journal of Dermatology 1978;98(1):25‐30. [DOI] [PubMed] [Google Scholar]
NCT00129415 {published data only}
- NCT00129415. Ultraviolet (UVA and UVB) light therapy in the treatment of inflammatory skin conditions. ClinicalTrials.gov/show/NCT00129415 (first received 11 August 2005).
NCT01402414 {published data only}
- NCT01402414. Narrow-band (NB)-UVB vs. Bath-PUVA and NB-UVB plus salt water baths in atopic dermatitis. ClinicalTrials.gov/show/NCT01402414 (first received 26 July 2011).
NCT03083730 {published data only}
- NCT03083730. Impact of narrowband UVB phototherapy on systemic inflammation in patients with atopic dermatitis. ClinicalTrials.gov/show/NCT03083730 (first received 20 March 2017).
NCT03402412 {published data only}
- NCT03402412. Atopic dermatitis: early gene expression changes as predictors of therapeutic response to narrow-band UVB treatment. ClinicalTrials.gov/show/NCT03402412 (first received 18 January 2018).
NCT04444726 {published data only}
- NCT04444726. Phototherpy versus tapwater iontophoresis for management of atopic dermatitis in children, randomized clinical trial. clinicaltrials.gov/show/NCT04444726 (first received 23 June 2020).
Pasic 1996 {published data only}
- Pasic A, Lipozencic J, Milavec-Puretic V, Murat-Susic S. Ultraviolet light in the treatment of atopic dermatitis. Acta Dermatovenerologica Croatica 1996;4(2):59-64. [Google Scholar]
Salo 1983 {published data only}
- Salo O, Lassus A, Juvakoski T, Kanerva L, Lauharanta J. Treatment of atopic dermatitis and seborrheic dermatitis with selective UV-phototherapy and PUVA. A comparative study. Dermatologische Monatsschrift 1983;169(6):371‐5. [PubMed] [Google Scholar]
Schiffner 2002 {published data only}
- Schiffner R, Schiffner-Rohe J, Landthaler M, Stolz W. How large is the loss of effectiveness of a treatment procedure between "theory" and "practice"? Evaluating health economics basic data within the scope of a trial model of ambulatory synchronous balenophototherapy of atopic eczema. Der Hautarzt 2002;53(1):22-9. [DOI] [PubMed] [Google Scholar]
Shephard 1996 {published data only}
- Shephard SE, Schregenberger N, Dummer R, Panizzon R. Comparison of two forms of local PUVA therapy: bath-PUVA versus topical meladinine lotion. Dermatology 1996;193(2):162. [DOI] [PubMed] [Google Scholar]
Snellman 2000 {published data only}
- Snellman E, Rantanen T, Sundell J. Cumulative UV radiation dose and outcome in clinical practice: effectiveness of trioxsalen bath PUVA with minimal UVA exposure. Photodermatology Photoimmunology & Photomedicine 2000;16(5):207-10. [DOI] [PubMed] [Google Scholar]
Valkova 2004 {published data only}
- Valkova S, Velkova A. UVA/UVB phototherapy for atopic dermatitis revisited. Journal of Dermatological Treatment 2004;15(4):239‐44. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Hannuksela 1985 {published data only}
- Hannuksela M, Karvonen J, Husa M, Jokela R, Katajamäki L, Leppisaari M. Ultraviolet light therapy in atopic dermatitis. Acta Dermato-Venereologica 1985;114:137-9. [DOI] [PubMed] [Google Scholar]
Kim 2012 {published data only}
- Kim HK, Park MK, Park KY, Kim MN, Oh G, Seo SH. Clinical Study of StoneTouch® far-infrared device on atopic dermatitis. Korean Journal of Dermatology 2012;50(10):874‐9. [Google Scholar]
Potapenko 2000 {published data only}
- Potapenko A, Butov Y, Levinzon E, Mamedov I, Kyagova A, Nikonenko B, et al. Treatment of eczema with photooxidized psoralen. British Journal of Dermatology 2000;143(Suppl 57):35. [Google Scholar]
Pullman 1985 {published data only}
- Pullmann H, Möres E, Reinbach S. Infrared and UVA rays on human skin and their effectiveness in treating endogenous eczema [Infrarot- und UVA-Strahlen auf die menschliche Haut und ihre Wirksamkeit bei der Behandlung des endogenen Ekzems]. Zeitschrift fur Hautkrankheiten 1985;60:171-7. [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12620000546954 {published data only}
- ACTRN12620000546954. Comparing the effect of narrowband ultraviolet B (UVB) therapy to therapy with natural sunlight and an amino acid lecithin cream on dermatologic symptoms. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12620000546954 (accessed before 13 July 2021).
Droitcourt 2019 {published data only}
- Droitcourt C, Barbarot S, Maruani A, Darrieux L, Misery L, Brenaut E, et al, Groupe de Recherche sur l'Eczema Atopique de la Societe Francaise de Dermatologie. A new phototherapy regimen during winter as an add-on therapy, coupled with oral vitamin D supplementation, for the long-term control of atopic dermatitis: study protocol for a multicentre, randomized, crossover, pragmatic trial — the PRADA trial. Trials 2019;20(1):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUCTR2015-000881-73-FR. PRAgmatic trial, Multicentre, cross-over, evaluating in patients with atopic dermatitis the long-term control effectiveness of new phototherapy regimen during winter, in supplement of standard topical treatments, with oral vitamin D supplementation or not. www.clinicaltrialsregister.eu/ctr-search/trial/2015-000881-73/FR (first received 29 July 2015).
- NCT02537509. PRAgmatic Trial in atopic dermatitis testing long-term control effectiveness of new phototherapy regimen during winter coupled with oral vitamin D supplementation vs. placebo. ClinicalTrials.gov/show/NCT02537509 (first received 1 September 2015).
Kromer 2019 {published data only}
- Kromer C, Nuhnen VP, Pfutzner W, Pfeiffer S, Laubach HJ, Boehncke WH, et al. Treatment of atopic dermatitis using a full-body blue light device (AD-Blue): protocol of a randomized controlled trial. JMIR Research Protocols 2019;8(1):e11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03085303. Treatment of atopic dermatitis by a full-body blue light device (AD-Blue). ClinicalTrials.gov/show/NCT03085303 (first received 21 March 2017).
NCT02915146 {published data only}
- NCT02915146. Narrowband ultraviolet B versus narrowband ultraviolet B plus ultraviolet A1 for atopic eczema. ClinicalTrials.gov/show/NCT02915146 (first received 26 September 2016).
Additional references
Abuabara 2018
- Abuabara K, Yu AM, Okhovat JP, Allen IE, Langan SM. The prevalence of atopic dermatitis beyond childhood: a systematic review and meta-analysis of longitudinal studies. Allergy 2018;73(3):696-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
Alexander 2020
- Alexander H, Paller AS, Traidl‐Hoffmann C, Beck LA, De Benedetto A, Dhar S, et al. The role of bacterial skin infections in atopic dermatitis: expert statement and review from the International Eczema Council Skin Infection Group. British Journal of Dermatology 2020 Jun;182(6):1331-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Apfelbacher 2011
- Apfelbacher CJ, Diepgen TL, Schmitt J. Determinants of eczema: population-based cross-sectional study in Germany. Allergy 2011;66(2):206-13. [DOI] [PubMed] [Google Scholar]
Archier 2012
- E Archier, S Devaux, E Castela, A Gallini, F Aubin, M Le Maître, et al. Carcinogenic risks of psoralen UV-A therapy and narrowband UV-B therapy in chronic plaque psoriasis: a systematic literature review. Journal of the European Academy of Dermatology and Venereology 2012;26(3):22-31. [DOI] [PubMed] [Google Scholar]
Barbarot 2016
- Barbarot S, Rogers NK, Abuabara K, Aubert H, Chalmers J, Flohr C, et al. Strategies used for measuring long-term control in atopic dermatitis trials: a systematic review. Journal of the American Academy of Dermatology 2016;75(5):1038-44. [DOI] [PubMed] [Google Scholar]
Beani 2010
- Beani JC, Jeanmougin M. Narrow-band UVB therapy in psoriasis vulgaris: good practice guideline and recommendations of the French Society of Photodermatology. Annales de Dermatologie et de Venereologie 2010;137(1):21-31. [DOI] [PubMed] [Google Scholar]
Brenninkmeijer 2010
- Brenninkmeijer EEA, Spuls PI, Lindeboom R, Van Der Wal AC, Bos JD, Wolkerstorfer A. Excimer laser vs. clobetasol propionate 0·05% ointment in prurigo form of atopic dermatitis: a randomized controlled trial, a pilot. British Journal of Dermatology 2010;163(4):823-31. [DOI] [PubMed] [Google Scholar]
Bulat 2011
- Bulat V, Situm M, Dediol I, Ljubicić I, Bradić L. The mechanisms of action of phototherapy in the treatment of the most common dermatoses. Collegium Antropologicum 2011;35(2):147-51. [PubMed] [Google Scholar]
Charman 2002
- Charman CR, Venn AJ, Williams HC. Reliability testing of the Six Area, Six Sign Atopic Dermatitis severity score. British Journal of Dermatology 2002;146(6):1057-60. [DOI] [PubMed] [Google Scholar]
Charman 2004
- Charman CR, Venn AJ, Williams HC. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients' perspective. Archives of Dermatology 2004;140(12):1513–9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Chren 1996
- Chren MM, Lasek RJ, Quinn LM, Mostow EN, Zyzanski SJ. Skindex, a quality-of-life measure for patients with skin disease: reliability, validity, and responsiveness. Journal of Investigative Dermatology 1996;107(5):707-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Clayton 2007
- Clayton TH, Clark SM, Turner D, Goulden V. The treatment of severe atopic dermatitis in childhood with narrowband ultraviolet B phototherapy. Clinical and Experimental Dermatology: Clinical Dermatology 2007;32(1):28-33. [DOI] [PubMed] [Google Scholar]
Cochrane Skin 2020a
- Scott H, Doney E, on behalf of Cochrane Skin. Outcomes of the Cochrane Skin Prioritisation Exercise 2020. Available at www.skin.cochrane.org/sites/skin.cochrane.org/files/public/uploads/outcomes_of_the_cochrane_skin_prioritisation_exercise_2020_final.docx.
Cochrane Skin 2020b
- Cochrane Skin Group. Cochrane Skin Prioritisation results 2020. www.skin.cochrane.org/prioritisation-results-2020 (accessed prior to 14 December 2020).
Costa 1989
- Costa C, Rilliet A, Nicolet M, Saurat JH. Scoring atopic dermatitis: the simpler the better? Acta Dermato-venereologica 1989;69(1):41-5. [PMID: ] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Melbourne, Australia: Veritas Health Innovation, accessed prior to 4 November 2020. Available at www.covidence.org.
Darsow 2010
- Darsow U, Wollenberg A, Simon D, Taïeb A, Werfel T, Oranje A, et al. ETFAD/EADV Eczema Task Force 2009 position paper on diagnosis and treatment of atopic dermatitis. Journal of the European Academy of Dermatology and Venereology 2010;24(3):317–28. [DOI] [PubMed] [Google Scholar]
Dawe 2003
- Dawe RS. Ultraviolet A1 phototherapy. British Journal of Dermatology 2003;148(4):626-37. [DOI] [PubMed] [Google Scholar]
De Lusignan 2020
- De Lusignan S, Alexander H, Broderick C, Dennis J, McGovern A, Feeney C, et al. The epidemiology of eczema in children and adults in England: a population-based study using primary care data. Clinical & Experimental Allergy 2020 Nov 11 [Epub ahead of print]. [DOI: 10.1111/cea.13784] [PMID: ] [DOI] [PMC free article] [PubMed]
Dennis 2013
- Dennis M, Bhutani T, Koo J, Liao W. Goeckerman therapy for the treatment of eczema: a practical guide and review of efficacy. Journal of Dermatological Treatment 2013;24(1):2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. International Journal of Epidemiology 2002;31:140-9. [DOI] [PubMed] [Google Scholar]
Emerson 1998
- Emerson RM, Williams HC, Allen BR. Severity distribution of atopic dermatitis in the community and its relationship to secondary referral. British Journal of Dermatology 1998;139(1):73-6. [DOI] [PubMed] [Google Scholar]
Faergemann 1987
- Faergemann J, Larkö O. The effect of UV-light on human skin microorganisms. Acta Dermato-venereologica 1987;67(1):69–72. [PMID: ] [PubMed] [Google Scholar]
Finlay 1990
- Finlay AY, Khan GK, Luscombe DK, Salek MS. Validation of sickness impact profile and psoriasis disability index in psoriasis. British Journal of Dermatology 1990;123:751–6. [DOI] [PubMed] [Google Scholar]
Finlay 1994
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI) — a simple practical measure for routine clinical use. Clinical and Experimental Dermatology 1994;19(3):210-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fischer 1976
- Fischer T. UV-light treatment of psoriasis. Acta Dermato-Venereologica 1976;56:473. [PubMed] [Google Scholar]
Flohr 2014
- Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy 2014;69(1):3–16. [DOI] [PubMed] [Google Scholar]
Gambichler 2009
- Gambichler T. Management of atopic dermatitis using photo(chemo)therapy. Archives of Dermatological Research 2009;301(3):197–203. [PMID: ] [DOI] [PubMed] [Google Scholar]
Garritsen 2014
- Garritsen FM, Brouwer MW, Limpens J, Spuls PI. Photo(chemo)therapy in the management of atopic dermatitis: an updated systematic review with implications for practice and research. British Journal of Dermatology 2014;170(3):501-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Goldsmith 2012
- Goldsmith LK, Katz SI, Gilchrest B, Paller A, Lefell D, Wolff K. Fitzpatrick’s Dermatology in General Medicine. New York: McGraw-Hill, 2012. [Google Scholar]
Grundmann 2012
- Grundmann SA, Beissert S. Modern aspects of phototherapy for atopic dermatitis. Journal of Allergy (Cairo) 2011 Dec 15 [Epub ahead of print]. [DOI: 10.1155/2012/121797] [DOI] [PMC free article] [PubMed]
Grundmann‐Kollmann 1999
- Grundmann-Kollmann M, Behrens S, Podda M, Peter RU, Kaufmann R, Kerscher M. Phototherapy for atopic eczema with narrow-band UVB. Journal of the American Academy of Dermatology 1999;40:995-7. [DOI] [PubMed] [Google Scholar]
Hanifin 1980
- Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Dermato-venereologica. Supplementum 1980;60(92):44-7. [DOI: 10.2340/00015555924447] [DOI] [Google Scholar]
Henseler 1987
- Henseler T, Christophers E, Hönigsmann H, Wolff K. Skin tumors in the European PUVA Study. Eight-year follow-up of 1,643 patients treated with PUVA for psoriasis. Journal of the American Academy of Dermatology 1987;16:108–16. [PubMed] [Google Scholar]
Herzinger 2016
- Herzinger T, Berneburg M, Ghoreschi K, Gollnick H, Hölzle E, Hönigsmann H, et al. Guidelines on UV phototherapy and photochemotherapy. Journal der Deutschen Dermatologischen Gesellschaft 2016;14(8):853-76. [DOI] [PubMed] [Google Scholar]
Higgins 2016
- Higgins JP, on behalf of the RoB 20 Working Group. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0). Additional considerations for cross-over trials. Available at www.riskofbias.info/welcome/rob-2-0-tool/archive-rob-2-0-cross-over-trials-2016.
Higgins 2020a
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available at www.training.cochrane.org/handbook.
Higgins 2020b
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available at www.training.cochrane.org/handbook.
Honig 1994
- Honig B, Morison WL, Karp D. Photochemotherapy beyond psoriasis. Journal of the American Academy of Dermatology 1994;31:775. [DOI] [PubMed] [Google Scholar]
Housman 2002
- Housman TS, Patel MJ, Camacho F, Feldman SR, Fleischer AB Jr, Balkrishnan R. Use of the Self-Administered Eczema Area and Severity Index by parent caregivers: results of a validation study. British Journal of Dermatology 2002;147(6):1192-8. [DOI] [PubMed] [Google Scholar]
Howells 2020
- Howells LM, Chalmers JR, Gran S, Ahmed A, Apfelbacher C, Burton T, et al. Development and initial testing of a new instrument to measure the experience of eczema control in adults and children: Recap of atopic eczema (RECAP). British Journal of Dermatology 2020;183(3):524-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2018
- Huang A, Seité S, Adar T. The use of balneotherapy in dermatology. Clinical Dermatology 2018;36(3):363-8. [DOI] [PubMed] [Google Scholar]
Ibbotson 2004
- Ibbotson SH, Bilsland D, Cox NH, Dawe RS, Diffey B, Edwards C, et al. An update and guidance on narrowband ultraviolet B phototherapy: a British Photodermatology Group workshop report. British Journal of Dermatology 2004;151(2):283-97. [DOI] [PubMed] [Google Scholar]
Jacobe 2008
- Jacobe HT, Cayce R, Nguyen J. UVA1 phototherapy is effective in darker skin: a review of 101 patients of Fitzpatrick skin types I-V. British Journal of Dermatology 2008;159(3):691-6. [DOI] [PubMed] [Google Scholar]
Jaleel 2019
- Jaleel T, Pollack BP, Elmets CA. Phototherapy. In: Kang S, Amagai M, Bruckner AL, Enk AH, Margolis DJ, McMichael AJ, et al, editors(s). Fitzpatrick's Dermatology. 9 edition. Vol. 2. New York: McGraw-Hill Education, 2019:3635. [Google Scholar]
Jekler 1988
- Jekler J, Larkö O. UVB phototherapy of atopic dermatitis. British Journal of Dermatology 1988;119(6):697-705. [DOI] [PubMed] [Google Scholar]
Kunz 1997
- Kunz B, Oranje AP, Labrèze L, Stalder JF, Ring J, Taïeb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology (Basel, Switzerland) 1997;195(1):10-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Laughter 2020
Lewis‐Jones 1995
- Lewis-Jones MS, Finlay AY. The Children’s Dermatology Life Quality Index (CDLQI): initial validation and practical use. British Journal of Dermatology 1995;132(6):942-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lindelöf 1991
- Lindelöf B, Sigurgeirsson B, Tegner E, Larkö O, Johannesson A, Berne B, et al. PUVA and cancer: a large-scale epidemiological study. Lancet 1991;338(8759):91-3. [DOI] [PubMed] [Google Scholar]
Lindelöf 1999
- Lindelöf B, Sigurgeirsson B, Tegner E, Larkö O, Johannesson A, Berne B, et al. PUVA and cancer risk: the Swedish follow-up study. British Journal of Dermatology 1999;141(1):108-12. [DOI] [PubMed] [Google Scholar]
Mahrle 1987
- Mahrle G. Phototherapie in Kombination mit Cignolin, Teer und Retinoiden. In: Braun-Falco O, Schill WB, editors(s). Fortschritte der praktischen Dermatologie und Venerologie. Berlin, Heidelberg: Springer, 1987. [Google Scholar]
Maksimović 2012
- Maksimović N, Janković S, Marinković J, Sekulović LK, Zivković Z, Spirić VT. Health-related quality of life in patients with atopic dermatitis. Journal of Dermatology 2012;39(1):42–7. [DOI] [PubMed] [Google Scholar]
Mancini 2008
- Mancini AJ, Kaulback K, Chamlin SL. The socioeconomic impact of atopic dermatitis in the United States: a systematic review. Pediatric Dermatology 2008;25(1):1-6. [DOI] [PubMed] [Google Scholar]
Martin 2013
- Martin PE, Koplin JJ, Eckert JK, Lowe AJ, Ponsonby AL, Osborne NJ, et al. The prevalence and socio-demographic risk factors of clinical eczema in infancy: a population-based observational study. Clinical & Experimental Allergy 2013;43(6):642-51. [DOI] [PubMed] [Google Scholar]
Meduri 2007
- Meduri NB, Vandergriff T, Rasmussen H, Jacobe H. Phototherapy in the management of atopic dermatitis: a systematic review. Photodermatology, Photoimmunology & Photomedicine 2007;23(4):106-12. [DOI] [PubMed] [Google Scholar]
Menter 2010
- Menter A, Korman NJ, Elmets CA, Feldman SR, Gelfand JM, Gordon KB, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis, Section 5: guidelines of care for the treatment of psoriasis with phototherapy and photochemotherapy. Journal of the American Academy of Dermatology 2010;62:114-35. [DOI] [PubMed] [Google Scholar]
Mok 2014
- Mok ZR, Koh MJ, Chong WS. Is phototherapy useful in the treatment of atopic dermatitis in Asian children? A 5-year report from Singapore. Pediatric Dermatology 2014;31(6):698-702. [DOI] [PubMed] [Google Scholar]
Morison 1998
- Morison WL, Baughman RD, Day RM, Forbes PD, Hoenigsmann H, Krueger GG, et al. Consensus workshop on the toxic effects of long-term PUVA therapy. Archives of Dermatology 1998;134:595-8. [DOI] [PubMed] [Google Scholar]
Nowicka 2019
- Nowicka D, Nawrot U. Contribution of Malassezia spp. to the development of atopic dermatitis. Mycoses 2019;62(7):588-96. [DOI] [PubMed] [Google Scholar]
Odhiambo 2009
- Odhiambo J, Williams H, Clayton T, Robertson CF, Asher MI, ISAAC Phase Three Study Group. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. Journal of Allergy and Clinical Immunology 2009;124(6):1251-8. [DOI] [PubMed] [Google Scholar]
Palmer 2006
- Palmer CN, Irvine AD, Terron-Kwiatkowski A, Zhao Y, Liao H, Lee SP, et al. Common loss-of-function variants of the epidermal barrier protein filaggrin are a major predisposing factor for atopic dermatitis. Nature Genetics 2006;38(4):441-6. [DOI] [PubMed] [Google Scholar]
Park 2012
- Park KK, Swan J, Koo J. Effective treatment of etanercept and phototherapy-resistant psoriasis using excimer laser. Dermatology Online Journal 2012;18(3):2. [PubMed] [Google Scholar]
Parrish 1981
- Parrish JA, Jaenicke KF. Action spectrum for phototherapy of psoriasis. Journal of Investigative Dermatology 1981;76(5):359-62. [DOI] [PubMed] [Google Scholar]
Pérez‐Ferriols 2015
- Pérez-Ferriols A, Aranegui B, Pujol-Montcusí JA, Martín-Gorgojo A, Campos-Domínguez M, Feltes RA, et al. Phototherapy in atopic dermatitis: a systematic review of the literature. Actas Dermo-sifiliograficas 2015;106(5):387-401. [PMID: ] [DOI] [PubMed] [Google Scholar]
Phan 2012
- Phan NQ, Blome C, Fritz F, Gerss J, Reich A, Ebata T, et al. Assessment of pruritus intensity: prospective study on validity and reliability of the visual analogue scale, numerical rating scale and verbal rating scale in 471 patients with chronic pruritus. Acta Dermato-Venereologica 2012;92(5):502-7. [DOI] [PubMed] [Google Scholar]
Reich 2012
- Reich A, Heisig M, Phan NQ, Taneda K, Takamori K, Takeuchi S, et al. Visual analogue scale: evaluation of the instrument for the assessment of pruritus. Acta Dermato-venereologica 2012;92(5):497-501. [PMID: ] [DOI] [PubMed] [Google Scholar]
RevMan Web 2020 [Computer program]
- RevMan Web. Version 3.8.0. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Ricci 2009
- Ricci G, Dondi A, Patrizi A. Useful tools for the management of atopic dermatitis. American Journal of Clinical Dermatology 2009;10(5):287-300. [DOI] [PubMed] [Google Scholar]
RoB 2 Excel Tool
- RoB 2 Excel Tool. Available from www.bristol.ac.uk/population-health-sciences/centres/cresyda/barr/riskofbias/rob2-0/ 2019.
Sachdeva 2009
- Sachdeva S. Fitzpatrick skin typing: applications in dermatology. Indian Journal of Dermatology, Venereology and Leprology 2009;75(1):93-6. [DOI] [PubMed] [Google Scholar]
Salek 1993
- Salek MS, Finlay AY, Luscombe DK, Allen BR, Berth-Jones J, Camp RDR, et al. Cyclosporin greatly improves the quality of life of adults with severe atopic dermatitis. A randomized, double- blind, placebo-controlled trial. British Journal of Dermatology 1993;129:422-30. [DOI] [PubMed] [Google Scholar]
Schmitt 2014
- Schmitt J, Spuls PI, Thomas KS, Simpson E, Furue M, Deckert S, et al. The Harmonising Outcome Measures for Eczema (HOME) statement to assess clinical signs of atopic eczema in trials. Journal of Allergy and Clinical Immunology 2014;134(4):800-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schram 2010
- Schram ME, Tedja AM, Spijker R, Bos JD, Williams HC, Spuls PI. Is there a rural/urban gradient in the prevalence of eczema? A systematic review. British Journal of Dermatology 2010;162(5):964-73. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. JAMA 1995;273(5):408-12. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. Updated October 2013. Available from guidelinedevelopment.org/handbook (accessed prior to 4 November 2020).
Sidbury 2014
- Sidbury R, Davis DM, Cohen DE, Cordoro KM, Berger TG, Bergman JN, et al. Guidelines of care for the management of atopic dermatitis. Section 3. Management and treatment with phototherapy and systemic agents. Journal of the American Academy of Dermatology 2014;71(2):327-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 2019
- Simpson E, Eckert L, Gadkari A, Mallya UG, Yang M, Nelson L, et al. Validation of the Atopic Dermatitis Control Tool (ADCT©) using a longitudinal survey of biologic-treated patients with atopic dermatitis. BMC Dermatology 2019;19(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sowden 1991
- Sowden JM, Berth-Jones J, Ross JS, Motley RJ, R Marks, Finlay AY, et al. Double-blind, controlled, crossover study of cyclosporin in adults with severe refractory atopic dermatitis. Lancet 1991;338: 137–40. [DOI] [PubMed] [Google Scholar]
Spergel 2003
- Spergel JM, Paller AS. Atopic dermatitis and the atopic march. Journal of Allergy & Clinical Immunology 2003 Dec;112(6 Suppl):118-27. [DOI] [PubMed] [Google Scholar]
Spuls 2004
- Spuls PI, Tuut MK, Van Everdingen JJ, De Rie MA, Werkgroep Psoriasis van de Nederlandse Vereniging voor Dermatologie en Venereologie. The practice guideline 'Photo(chemo)therapy and systemic therapy in severe chronic plaque-psoriasis'. Nederlands Tijdschrift voor Geneeskunde 2004;148(43):2121-5. [PubMed] [Google Scholar]
Spuls 2017
- Spuls PI, Gerbens LAA, Simpson E, Apfelbacher CJ, Chalmers JR, Thomas KS, et al. Patient-Oriented Eczema Measure (POEM), a core instrument to measure symptoms in clinical trials: a Harmonising Outcome Measures for Eczema (HOME) statement. British Journal of Dermatology 2017;176(4):979-84. [DOI] [PubMed] [Google Scholar]
Stalder 2011
- Stalder JF, Barbarot S, Wollenberg A, Holm EA, De Raeve L, Seidenari S, et al. Patient‐Oriented SCORAD (PO‐SCORAD): a new self‐assessment scale in atopic dermatitis validated in Europe. Allergy 2011;66(8):1114-21. [DOI] [PubMed] [Google Scholar]
Stefanovic 2020
- Stefanovic N, Flohr C, Irvine AD. The exposome in atopic dermatitis. Allergy 2020;75(1):63-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stern 1994
- Stern RS, Laird N. The carcinogenic risk of treatments for severe psoriasis. Photochemotherapy follow-up study. Cancer 1994;73(11):2759–64. [DOI] [PubMed] [Google Scholar]
Stern 1997
- Stern RS, Nichols KT, Vakeva LH. Malignant melanoma in patients treated for psoriasis with methoxsalen (psoralen) and ultraviolet A radiation (PUVA). The PUVA follow-up study. New England Journal of Medicine 1997;336:1041-5. [DOI] [PubMed] [Google Scholar]
Stern 1998
- Stern RS, Liebman EJ, Vakeva L. Oral psoralen and ultraviolet-A light (PUVA) treatment of psoriasis and persistent risk of nonmelanoma skin cancer. PUVA follow-up study. Journal of the National Cancer Institute 1998;90(17):1278-84. [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
Sweeting 2004
- Sweeting, MJ, Sutton AJ, Lambert PC. What to add to nothing? Use and avoidance of continuity corrections in meta-analysis of sparse data. Statistics in Medicine 2004;23:1351-75. [DOI] [PubMed] [Google Scholar]
Syed 2011a
- Syed ZU, Hamzavi IH. Role of phototherapy in patients with skin of color. Seminars in Cutaneous Medicine and Surgery 2011;30(4):184-9. [DOI] [PubMed] [Google Scholar]
Syed 2011b
- Syed ZU, Hamzavi IH. Photomedicine and phototherapy considerations for patients with skin of color. Photodermatology, Photoimmunology & Photomedicine 2011;27(1):10-6. [DOI] [PubMed] [Google Scholar]
Tan 2018
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. Journal of the American Academy of Dermatology 2018;79(4):672-9. [DOI] [PubMed] [Google Scholar]
Tay 1996
- Tay YK, Morelli JG, Weston WL. Experience with UVB phototherapy in children. Pediatric Dermatology 1996;13:406-9. [DOI] [PubMed] [Google Scholar]
Väkevä 2019
- Väkevä L, Niemelä S, Lauha M, Pasternack R, Hannuksela-Svahn A, Hjerppe A, et al. Narrowband ultraviolet B phototherapy improves quality of life of psoriasis and atopic dermatitis patients up to 3 months: Results from an observational multicenter study. Photodermatology, Photoimmunology & Photomedicine 2019;35(5):332-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Van Weelden 1988
- Van Weelden H, De La Faille HB, Young E, Van der Leun JC. A new development in UVB phototherapy of psoriasis. British Journal of Dermatology 1988;119(1):11-9. [DOI] [PubMed] [Google Scholar]
Vermeulen 2020
- Vermeulen FM, Gerbens LA, Schmitt J, Deleuran M, Irvine AD, Logan K, et al. The European TREatment of ATopic eczema (TREAT) Registry Taskforce survey: prescribing practices in Europe for phototherapy and systemic therapy in adult patients with moderate-to-severe atopic eczema. British Journal of Dermatology 2020;183(6):1073-82. [DOI: 10.1111/bjd.18959] [DOI] [PMC free article] [PubMed] [Google Scholar]
Von Kobyletzki 1999b
- Von Kobyletzki G, Pieck C, Höxtermann S, Freitag M, Altmeyer P. Circulating activation markers of severe atopic dermatitis following ultraviolet A1 cold light phototherapy: eosinophil cationic protein, soluble interleukin-2 receptor and soluble interleukin-4 receptor. British Journal of Dermatology 1999;140(5):966–8. [DOI] [PubMed] [Google Scholar]
Weichenthal 2005
- Weichenthal M, Schwarz T. Phototherapy: how does UV work? Photodermatology, Photoimmunology & Photomedicine 2005;21(5):260-6. [DOI] [PubMed] [Google Scholar]
Weidinger 2016
- Weidinger S, Novak N. Atopic dermatitis. Lancet 2016;387(10023):1109-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Williams 1994
- Williams HC, Burney PG, Hay RJ, Archer CB, Shipley MJ, Hunter JJ, et al. The UK Working Party's Diagnostic Criteria for Atopic Dermatitis. I. Derivation of a minimum set of discriminators for atopic dermatitis. British Journal of Dermatology 1994;131(3):383–96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Williams 2005
- Williams HC. Clinical practice. Atopic dermatitis. New England Journal of Medicine 2005;352(22):2314-24. [DOI] [PubMed] [Google Scholar]
Williams 2008
- Williams H, Stewart A, Mutius E, Cookson W, Anderson HR, International Study of Asthma and Allergies in Childhood (ISAAC) phase one and three study groups. Is eczema really on the increase worldwide? Journal of Allergy and Clinical Immunology 2008;121(4):947–54.e15. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wollenberg 2018
- Wollenberg A, Barbarot S, Bieber T, Christen-Zaech S, Deleuran M, Fink-Wagner A, et al. EDF guidelines for treatment of atopic eczema (atopic dermatitis). Consensus based European guidelines for treatment of atopic eczema (atopic dermatitis) in adults and children. www.edf.one/dam/jcr:5abe6f7c-b653-4259-b03a-f5b35258a926/EDF%20guideline%20AE%202018_modified_190508.pdf (accessed 4 November 2020).
Yosipovitch 2019
- Yosipovitch G, Reaney M, Mastey V, Eckert L, Abbé A, Nelson L, et al. Peak pruritus numerical rating scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. British Journal of Dermatology 2019;181(4):761–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zandi 2012
- Zandi S, Kalia S, Lui H. UVA1 phototherapy: a concise and practical review. Skin Therapy Letters 2012;17(1):1-4. [PubMed] [Google Scholar]
References to other published versions of this review
Musters 2021
- Musters AH, Mashayekhi S, Flohr C, Drucker AM, Gerbens L, Ferguson J, et al. Phototherapy for atopic eczema. Cochrane Database of Systematic Reviews 2021, Issue 2. Art. No: CD013870. [DOI: 10.1002/14651858.CD013870] [DOI] [PMC free article] [PubMed] [Google Scholar]