Abstract
Leptomeningeal carcinomatosis (LC) is a rare but challenging manifestation of advanced breast cancer with a severe impact on morbidity and mortality. We performed a systematic review of the evidence published over the last two decades, focusing on recent advances in the diagnostic and therapeutic options of LC. Lobular histology and a triple-negative intrinsic subtype are well-known risk factors for LC. Clinical manifestations are diverse and often aspecific. There is no gold standard for LC diagnosis: MRI and cerebrospinal fluid cytology are the most frequently used modalities despite the low accuracy. Current standard of care involves a multimodal strategy including systemic and intrathecal chemotherapy in combination with brain radiotherapy. Intrathecal chemotherapy has been widely used through the years despite the lack of data from randomized controlled trials and conflicting evidence on patient outcomes. No specific chemotherapeutic agent has shown superiority over others for both intrathecal and systemic treatment. Although endocrine therapy was heuristically considered unable to exert significant control on central nervous system metastatic disease, retrospective data suggest a favourable toxicity profile and even a possible positive impact on survival. In recent years, encouraging data on the use of targeted agents has emerged but further research in this field is required. Palliative treatment in the form of whole brain or stereotactic radiotherapy is associated with improvement in clinical manifestations and quality of life, with no proven impact on survival. The most investigated prognostic factors include performance status, non-triple-negative disease and multimodal treatment. Validation of prognostic scores is necessary to aid clinicians in the identification of patient subgroups that are most likely to benefit from an intensive therapeutic approach.
Keywords: breast cancer, leptomeningeal carcinomatosis, leptomeningeal metastasis, systematic review
Introduction
Leptomeningeal carcinomatosis (LC) is defined as the infiltration by cancer cells of the pia mater, the arachnoid mater and the subarachnoid space.1 Although breast cancer (BC) is the tumour most commonly associated with leptomeningeal disease due to its high prevalence worldwide, LC remains a rare manifestation, occurring in up to 5% of patients with BC.2 However, it is characterized by a severe impact on morbidity and mortality and retains a dismal prognosis even in patients treated with multimodal, aggressive treatment.1
The primary aim of this review was to conduct a complete revision of the literature on the topic from the past two decades focusing on recent advances in the diagnostic approach and management of the disease.
Methods
Literature review
This review was designed to collect and evaluate evidence in the literature about (1) the clinical management and (2) current therapeutic options of leptomeningeal carcinomatosis in breast cancer. We performed a systematic review in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Eligible articles were identified through a search in the MEDLINE database using the following string: “breast cancer” AND “leptomeningeal carcinomatosis” OR “leptomeningeal metastasis”. We initially included all studies published between January 2000 and June 4, 2021. This work was not eligible for PROSPERO database registration as it did not meet their inclusion criteria.
Results
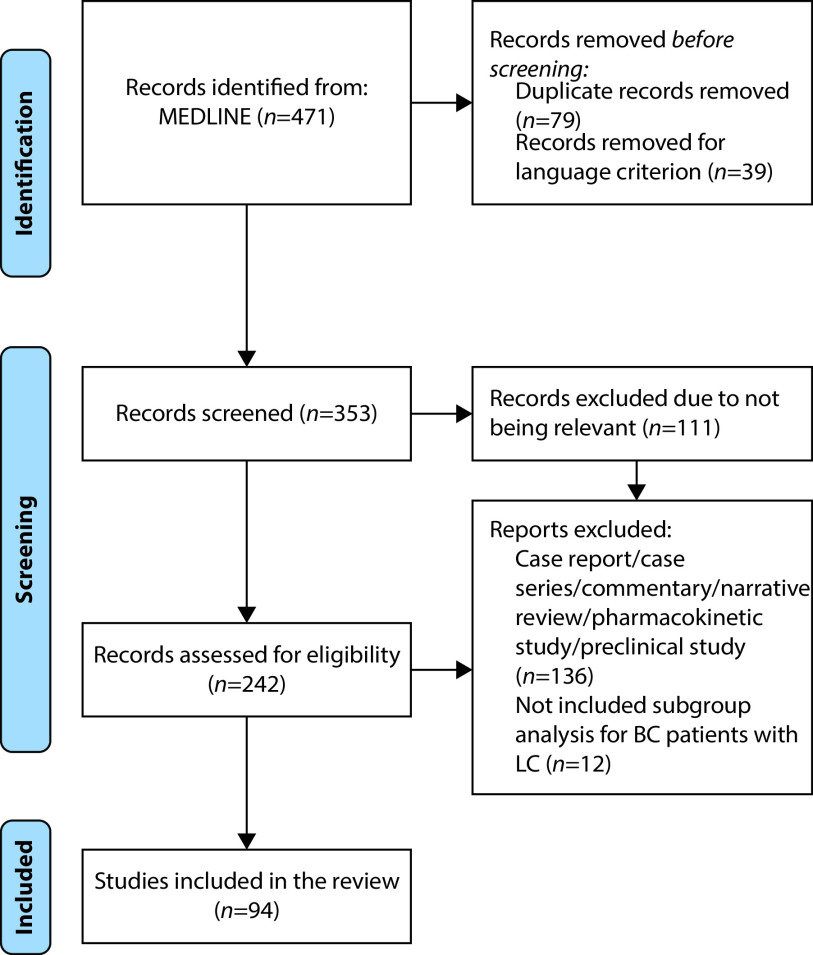
Our search was performed on June 4, 2021, and we retrieved 471 articles. After the exclusion of duplicates and articles not meeting prespecified criteria (absence of English translation), a total of 353 articles were selected for the next screening step. We excluded 111 articles considered not relevant for the objectives of this review. The remaining 242 articles were evaluated for eligibility. We decided to include only clinical trials, prospective studies, retrospective studies, other systematic reviews, and survey studies to increase the quality of the reported evidence. We excluded 136 articles that were case series, case reports, commentaries, studies on pharmacokinetics and preclinical studies. Finally, we excluded all the articles about LC that enrolled patients with different solid tumours and did not provide specific data for the BC subgroup (Figure 1).
Figure 1.
PRISMA flow diagram.105
Epidemiology
LC is a rare complication of BC, with historical rates of occurrence averaging at 5% of total BC patients.1 In more recent times, the incidence of LC appears to be higher than in the past possibly due to advances in diagnostic methods, increased awareness of clinicians and therapeutic improvements, with a positive impact on BC survival. A retrospective study analysing a large cohort of patients surgically treated for BC (n=1915) described a risk of developing leptomeningeal metastasis of 0.3% at 5 years and of 0.6% at 10 years, suggesting a much lower incidence of LC in the subgroup of patients initially diagnosed with early BC.3 LC seems to be a late event in the natural course of the disease, with a reported median time from initial BC diagnosis of 7.4 years (range 0–23.4 years) compared to a median time from the onset of metastatic disease of 21 months (range 0–230 months).4
Several studies addressed a possible correlation between BC histology and the development of LC. Sacco et al.2 investigated the association between LC incidence and histological and biological subtypes of BC: whereas the prevalence of invasive lobular carcinoma ranges from 17% to 28% of total BC cases, this histological subtype is more represented in the subpopulation with leptomeningeal metastasis (up to 35%). Niwińska et al.5 also detected a propensity of the lobular subtype to metastasize to the leptomeninges, with a prevalence two times higher amongst patients with leptomeningeal disease than amongst the whole BC population.
The intrinsic subtype most commonly associated with LC is triple-negative breast cancer (TNBC): it is estimated that up to 40% of the totality of patients who develop metastases at this site have triple-negative primary tumours1–3,6 and TNBC is 3.5 times more represented amongst patients with LC than in the whole BC population.5 Although human epidermal growth factor receptor 2-positive (HER2+) BC shows central nervous system (CNS) tropism in the form of metastasis to the brain parenchyma, LC is not a common manifestation of this intrinsic subtype.1,2 As most HER2-targeted agents do not cross the blood–brain barrier (BBB) and increased rates of CNS metastases had been reported in patients receiving trastuzumab,7 Lai et al. retrospectively investigated the incidence of brain and leptomeningeal metastasis in a cohort of patients receiving trastuzumab for their metastatic disease but found no significant evidence of a correlation between anti-HER2 treatment and the development of LC.8
Data from several studies included in our review suggest that hormone receptor status may influence the time to development of LC. In fact, TNBC-associated LC occurs earlier in the history of metastatic disease (in 9–25% of patients as the first presentation), whereas it is a late event in hormone receptor-positive (HR+) BC.2,3,9–12 In a retrospective study by Griguolo et al.,13 median time from initial BC diagnosis to LC was 55.3 months and, at multivariate analysis, varied significantly according to HR status and tumour stage at diagnosis. Data on the incidence of histological and intrinsic subtypes in the cohorts of selected studies are shown in Table 1.
Table 1.
Histological and molecular subtypes of the primary tumour in breast cancer patients with leptomeningeal metastases (selected literature).
| First author, year | Number of patients | Histiotype | Molecular subtype | |||||
|---|---|---|---|---|---|---|---|---|
| IDC (%) | ILC (%) | ER+ (%) | PR+ (%) | ER+ and/or PR+ (%) | HER2+ (%) | TN (%) | ||
| Gauthier et al., 20104 | 91 | 63 | 28 | 70 | 44 | 74 | 10 | 21 |
| De Azevedo et al., 201197 | 60 | 78,3 | 21,6 | 51,7 | 43,3 | NA | 15 | 30 |
| Lee et al., 201111 | 68 | 69.1 | 4.4 | NA | NA | 35.3 | 27.9 | 36.8 |
| Lara-Medina et al., 201294 | 49 | 76 | 14 | 20 | 27 | NA | 20 | 39 |
| Meattini et al., 201298 | 33 | 63.6 | 36.4 | NA | NA | 60.6 | NA | NA |
| Jo et al., 201323 | 95 | NA | NA | NA | NA | 25.3 | 15.8 | 53.7 |
| Yust-Katz et al., 20139 | 154 | 78.2 | 21.8 | 50.5 | 37.9 | 55.3 | 47.4 | 22.8 |
| Niwińska et al., 20135 | 118 | 59 | 35 | 42 | NA | NA | 19 | 40.5 |
| Torrejón et al., 201310 | 38 | NA | NA | 34.2 | 26.3 | NA | 26.3 | 23.7 |
| Kingston et al., 201799 | 182 | 84.1 | 9.9 | NA | NA | 49.5 | 26.4 | 14.8 |
| Morikawa et al., 201793 | 318 | NA | NA | NA | NA | 62 | 26 | 25.5 |
| Griguolo et al., 201813 | 153 | 64.7 | 25.5 | 71.2 | 54.9 | 74.5 | 20.9 | 15.0 |
| Le Rhun et al., 2020102 | 104 | 64 | 22 | 10 | 10 | NA | 25 | 24 |
ER, estrogen receptor; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; NA, not available; PR, progesterone receptor; TN, triple negative.
LC occurs more frequently in patients undergoing surgical treatment for brain metastasis, especially if surgery involves the ventricular system.14,15 Several studies have assessed the risk of developing LC after stereotactic radiosurgery (SRS) for brain metastasis. A retrospective study by Jung et al. showed an increased incidence of LC in BC patients treated with radiosurgery compared to those who received whole-brain radiotherapy (WBRT); additional risk factors associated with the increased risk of LC at multivariate analysis were young age (<40 years) and progressing systemic disease.16 A retrospective review by Trifletti et al.17 identified only one risk factor for the development of LC after SRS, namely the presence of active disease in the chest at the time of SRS; oestrogen receptor (ER) status, progesterone receptor (PR) status, HER2 status, tumour size and location, previous craniotomy, number of intra-axial tumours, cystic tumour morphology, prior WBRT, and active bone and liver metastases were not associated with LC onset. Notably, BC displays a higher predisposition for leptomeningeal dissemination after SRS compared to other tumours18–20 as suggested by a recent meta-analysis by Brown et al. (HR of 2.22, p<0.001).21
A prospective study by Kosmas et al. compared the incidence of LC in BC patients who progressed after having obtained a major response with first-line taxane chemotherapy to a controlled group treated with a non-taxane regimen. They reported a non-significant increased rate of leptomeningeal dissemination in the taxane group; however, this was not associated with a significant difference in time to progression and in survival between the two treatment arms.22
Pathophysiology
Metastatic dissemination to the leptomeninges can occur by haematogenous spread (either through the arterial or the venous circulation), endoneural/perineural/perivascular spread, lymphatic spread, or direct invasion. The presence of parenchymal brain metastases or bone metastases (especially vertebral) is the most common risk factors for the development of LC by direct invasion or perivascular infiltration; most patients present with secondary lesions at these sites at the time of LC onset (38% and 48%, respectively).2,23
As mentioned earlier in this review, lobular BC shows a particular predisposition for leptomeningeal involvement. A proposed explanation for this phenomenon involves changes in cell adhesion molecules: specifically, the vast majority of lobular BC is characterized by complete loss of expression of E-cadherin mostly due to inactivating CDH1 mutations.24,25 Because of E-cadherin deregulation, intercellular adherence junctions lose their function and the tumour acquires a phenotype prone to disruption of epithelial integrity, promotion of angiogenesis and tissue invasion.26,27 Tumours with disrupted E-cadherin expression are prone to early invasion and metastatization and are associated with unfavourable prognosis. In BC, this molecular alteration clinically manifests with an increased risk of mesothelial and leptomeningeal invasion.1
Another putative mechanism involved in meningeal dissemination is BBB dysregulation. A preclinical study conducted by Boire et al. in mouse models of LC showed that interaction between the complement molecule C3 and its receptor on choroid plexus cells (C3aR) led to disruption of the BBB and leakage of plasma components into the cerebrospinal fluid (CSF), where they acted as cancer cell growth factors.28
A molecular alteration detected in CSF floating cancer cells is the aberrant expression of MUC1 and Syndecan 1 products, a finding associated with cancer dissemination and aggressive tumour phenotypes. CD15, CD24, CD44 and CD113 are cancer stem cell markers with proven roles in non-CNS metastatic disease and their increased expression seems to also be associated with meningeal disease.29
Clinical presentation
LC can manifest with a variety of signs and symptoms, reflecting multifocal involvement of the CNS. Findings may be subtle and aspecific, especially in the initial phases of disease, and this may often lead to a delay in diagnosis. Neurological deficits in patients with known metastatic disease, especially if rapidly progressive, should always arise suspicion for LC.1,30
The most frequent manifestations include headache (present in >80% of patients at diagnosis), nausea, vomiting, neurocognitive deficits, gait abnormalities, cranial nerve palsy, hearing loss, visual disturbances, seizures, dizziness and radicular signs (including pain, weakness and cauda equina syndrome).1,30,31 An uncommon presentation of LC is a newly diagnosed psychiatric disorder.32 Rare clinical pictures such as central fever and diabetes insipidus have been reported in the literature.31 In a minority of cases, LC may be diagnosed incidentally in asymptomatic patients.1
Diagnostic workup
LC can be difficult to diagnose, presenting with elusive clinical and radiological findings. Currently, there is no consensus in the diagnosis of LC, which often requires a combination of neurological assessment, imaging and CSF analysis. The lack of a gold standard for LC diagnosis also contributes to the high heterogeneity of the patients enrolled in dedicated studies.33 A recent survey highlighted the major differences in diagnostic and therapeutic approaches across Europe. Only a minority of physicians (16%) declared the routine use of a standardized score for neurological examination, 23% of physicians did not always use MRI to evaluate the presence of LC, 56% reported that CSF analysis was always performed when leptomeningeal dissemination from solid tumours was suspected, and 35% of clinicians requested CSF analysis only after non-conclusive clinical and MRI evaluation. These findings may be justified by the commonly poor performance status of LC patients, which often limits the intensity of the diagnostic process.34
MRI role in leptomeningeal disease diagnosis from breast cancer
Imaging is crucial for initial LC assessment. MRI of the entire neuroaxis is required to evaluate whether both the brain and spine are involved and has an estimated sensitivity of 66–98%.30 Typical findings are meningeal and/or cranial and spinal nerve root enhancement, sulcal enhancement or obliteration, and linear or nodular lesions into the ependymal space and/or vertebral canal. Other suggestive but non-specific signs include nodular enhancement in cerebral cortex, dural enhancement in the intracalvarium and communicating hydrocephalus.30,34
Bier et al. retrospectively analysed a cohort of 78 patients with advanced solid tumours receiving systemic therapy (23 with BC) to assess the prevalence of false-positive meningeal contrast enhancement suggestive of neoplastic meningitis, a generally rare phenomenon (<1%). However, the recent introduction of modern cancer therapies (e.g. immunotherapy) increased the rate of false-positive results (a phenomenon called ‘pseudomeningeosis’). This was a clinically relevant finding due to its impact on subsequent therapeutic decisions. Unfortunately, a clear imaging pattern allowing discrimination between treatment-induced meningeal enhancement and LC was not identified. Even considering the retrospective nature of these results, cytological confirmation may be warranted in patients with suspicious meningeal enhancement, especially if they are receiving immunotherapy.35
Mayinger et al.36 compared volumetric differences in brain substructures amongst BC patients with oligo/multiple parenchymal brain metastases and/or LC. Enlargement of the fourth ventricle, a complication probably caused by LC cells invading the base of the brain and preventing CSF outflow, was more frequently associated with LC. Tumour-associated inflammatory response can also reduce CSF absorption. The corpus callosum was found to be smaller in LC patients, a finding correlating with cognitive decline.
CSF analysis
CSF analysis in LC patients is frequently associated with aspecific pathological results, including increased opening pressure (>200 mmHg), increased leukocyte count (>4/mm3), increased proteinorrachia (>50 mg/dL) and decreased glycorrhachia (<2.3 mmol/L).30 The gold standard for LC diagnosis is the identification of malignant cells in the CSF or in a leptomeningeal biopsy; the latter is a highly invasive procedure rarely performed due to the frailty of patients. ESMO guidelines suggest that CSF cytology can be considered ‘positive’ if malignant cells are found in the CSF, ‘equivocal’ if there are atypical cells in the sample or ‘negative’ if they are totally absent.30
Cytomorphological features of metastatic BC in the CSF were evaluated in a retrospective study;37 23 positive CSF samples obtained from a cohort of 15 BC patients were processed as Cytospin preparations and stained by Papanicolaou and Diff-Quik techniques, with both methods achieving the same accuracy. Cytological evaluation demonstrated, in most cases, high variation in tumour cell size, hyperchromatic nuclei, prominent nucleoli and diffuse cytosol vacuolization.37
Although the low invasiveness of MRIs makes it the preferred diagnostic technique, radiological findings may be often aspecific or misleading. CSF cytology is widely performed to confirm LC but its sensitivity remains low (approximately 50% and 85% for the first and the second lumbar puncture, respectively).38
There were many attempts to improve the diagnostic power of CSF analysis. Rare cell capture technology is approved for the identification of circulating tumour cells (CTCs) in the blood of patients with epithelial tumours. This method uses an immunomagnetic-enriched platform covered with epithelial cell adhesion molecule (EpCAM) antibodies that bind EpCAM-positive CTCs in the blood.39 The same technique was evaluated for CTC detection in the CSF in the below-mentioned studies.
In 2012, Le Rhun et al.40 published the results of the phase III DEPOSEIN trial: they had adapted the Veridex CellSearchTM technology to detect malignant cells in the CSF of a subgroup of breast cancer patients with LC. This technique was initially designed to detect CTCs in peripheral blood and was used in an exploratory analysis to detect malignant cells using only 5 mL of CSF. Tumour cells were observed in 14 out of 16 samples with the CellSearchTM technology (tumour cell numbers ranging between 1 and 10,500/5 mL).40
A prospective study conducted on a cohort of 95 patients with epithelial tumours and clinical suspicion of LC (36 of which had BC as the primary tumour) aimed to establish the diagnostic accuracy of the CSF-CTC count obtained with the EpCAM rare cell capture technology immunomagnetic platform. Based on ROC analysis, concentrations of 1 CTC per millilitre of CSF guaranteed the best threshold for LC diagnosis (sensitivity 93%, specificity 95%, positive predictive value 90% and negative predictive value 97%).41
A previous prospective study had already shown that CTC-based techniques used to study CTCs in the peripheral blood were feasible also for CSF-CTC analysis in patients with BC and LC. Two different techniques were used (the CellSearchTM assay and an EpCAM-based method composed of immunomagnetic enrichment and flow cytometry), and they both showed higher sensitivity than CSF cytology alone (80.95% versus 66.67%). Specificity was lower as the detection of malignant cells at cytological examination is the current accepted golden standard (84.67% versus 100%). These findings suggested that CSF-CTC detection with the CellSearchTM assay can improve the early diagnosis of LC in patients with BC.42 These results have been further confirmed by another study by Torre et al.,43 where the CellSearch immunomagnetic assay was used to identify CSF-CTCs in 20 patients with LC from solid tumours (9 of which with BC). The assay reached a sensitivity of 88.9% and specificity of 100% using a threshold of 1 CTC per millilitre of CSF. These data are consistent with the previously mentioned studies and can support the role of CSF-CTC testing in selected cases with negative CSF cytology and high clinical suspicion of LC.
Another retrospective study assessed the role of SE-i•FISH, a subtraction enrichment and immunostaining fluorescence in situ assay previously used for CTCs isolation in the peripheral blood, in the identification and quantification of CTCs in the CSF of eight patients with BC and LC. According to these results, SE-i•FISH can accurately and feasibly detect CSF-CTCs. There was significant concordance between CSF-CTC count and typical clinical-cytological markers of LC (increased intracranial pressure, CSF cytology and clinical presentation). Interestingly, CSF-CTCs were also isolated, cultured and subjected to genomic sequencing. Shared non-synonymous single-nucleotide variants were observed in both CSF-CTCs and matched primary tumours, confirming their common origin. Heterogeneous patterns of genetic mutations were also identified, a finding consistent with the clonal changes expected during tumour progression. The authors suggested that this method could be used to increase diagnostic sensitivity and, more importantly, to monitor tumour dynamics, drug sensitivity and treatment response.44 A similar study showed high concordance between the HER2 status of CSF-CTCs and of the primary tumour.45 This finding, together with older data showing high concordance rates between baseline HER2 status and FISH on CSF cytology, suggests that patients with HER2+ BC may be treated with anti-HER2 drugs even without confirming HER2 positivity on CSF samples.46
On this trial, a retrospective analysis by Yu et al.47 investigated the dynamic changes of CSF-CTC features following intrathecal (IT) CT. In particular, the SE-i•FISH was applied for surveillance and function analysis of CSF-CTCs in five patients with BC and LC. The study focused on the status of CK18 as the upregulation of this cytokeratin is frequently correlated to cell migration, metastasis and tumour progression. Baseline CTC immunofluorescence for CK18 was intensively positive, whereas CTCs from patients receiving IT treatment displayed a gradual decrease in fluorescence intensity. At least six IT cycles were necessary to induce a reduction in CSF-CTCs. These data confirmed the negative correlation of CK18 expression on CSF-CTCs with clinical improvement, CSF-CTC count and treatment response.
Recently, mutation and aneuploidy status in CSF-derived circulating tumour DNA was investigated in patients with BC with a clinical suspicion of LC using next-generation sequencing and the modified fast aneuploidy screening test-sequencing system. Although low DNA availability caused insufficient material in most samples analysed with next-generation sequencing, the modified fast aneuploidy screening test-sequencing system method proved successful in 93% of samples, detecting aneuploidy in 24 out of 121 patients. Aneuploidy was also demonstrated in patients with negative cytology and imaging who later developed clinically relevant metastases. Even though more data are needed to confirm this hypothesis, aneuploidy in the CSF-derived circulating tumour DNA showed the potential to become an early marker of LC associated with worse overall survival (OS).48
Role of tumour markers in LC diagnosis
Considering the diagnostic challenges and poor outcomes in patients with BC and LC, great efforts have been undertaken to find novel tumour biomarkers and facilitate the early diagnosis of leptomeningeal involvement.
The role of tumour marker elevation in patients developing intracranial metastasis (including LC) has been investigated throughout the years. Ishibashi et al.49 tried to prove a correlation between an increase in serum tumour markers (such as NSE, Pro-GRP, CEA and CA15-3) and the development of CNS metastasis in a population of 53 patients affected by solid tumours, 26.4% of whom had BC. In 42.9% of patients with BC, CA15-3 levels did not increase upon CNS metastasization. Amongst patients who developed LC, there was a statistically non-significant trend towards tumour marker elevation. This may be due to the increased permeability of the BBB allowing tumour markers to diffuse through the CSF to the bloodstream. The presence of an obstruction (i.e. hydrocephalus) can cause CSF congestion and facilitate tumour marker backflow.50 A single retrospective study compared the use of CSF CA15-3 levels to CSF cytology for the diagnosis of BC-associated LC. CSF CA15-3 measurement was not associated with a superior sensitivity nor did it show concordance with cytology. Its use in clinical practice should be discouraged.
TGFβ1, urokinase, tissue plasminogen activator and VEGF are all marker proteins that play a role in metastasization. Elevated CSF VEGF levels have been reported in patients with breast carcinoma. A comparative study measured biomarker concentration in the serum and CSF of patients with proven or suspected LC, and then compared them with cases of infective meningitis and other neurological disorders. Intrathecal production of VEGF (calculated through the VEGF index) was increased in LC patients, whilst the tissue plasminogen activator index was decreased. The combination of these two parameters reached a sensitivity close to 100% for the diagnosis of LC. Urokinase and TGFβ1 levels did not show specificity for LC.51,52
The role of proteomic profiles was investigated by Dekker et al.,53 who used a mass spectrum-based method to evaluate protein expression patterns in the CSF of patients with and without LC. The authors proposed a predictive model for LC diagnosis based on the differential expression of a set of proteins. Its high sensitivity (79%) and specificity (76%) suggest a possible use of proteomic analysis in support of standard CSF cytology for LC diagnosis.
Treatment
Our search retrieved 30 articles concerning current evidence on the use of chemotherapy (both systemic and intrathecal), target therapy, immunotherapy and novel target molecules in patients with LC from BC. The aim of treatment for LC is to stabilize neurological symptoms, improve quality of life and prolong survival.
Prospective observational trials
We included three prospective trials whose main characteristics are summarized in Table 2. These studies were conducted over a very long time span, between 1993 and 2019, and analysed the outcome of patients with LC from BC or other malignancies treated with IT CT alone54 or in association with other systemic agents. The studies suggest the feasibility of both these approaches despite dismal results in terms of clinical benefit and survival.55,56 These data should be considered carefully because of the limited number of patients enrolled.
Table 2.
Detailed characteristics of prospective studies.
| Author (year) | Study duration | N (total) | Intrinsic subtypes | Treatment | Other therapies (ITT, ET, ChT, RT) | Safety (G3–G4 AEs) | CNS-specific response rate | Survival (OS) |
|---|---|---|---|---|---|---|---|---|
| Yoshida et al. (2005)54 | 1993–2002 | 58 (9 patients with BC) | – | IT MTX (15 mg/mq) and PSL (10 mg) 6 times in 2 weeks via Ommaya reservoir plus ARA-C (10 mg/mq) for 4 doses of MTX | Yes | – | CSF cytology and clinical improvement in 3 pts (duration 10.8 months ± 3.7 and 13.4 months ± 5.8, respectively) | mOS: 18.4 months ± 7.4 in BC patients |
| Wu et al. (2015)56 | 2010–2013 | 8 | 3 Luminal 2 HER2+ 3 TNBC |
BEEP every 3 weeks for a maximum of 6 cycles | 6 patients received IT MTX; 4 patients undergone RT |
Neutropenia (23.1%) Leukopenia (23.1%) Hyponatraemia (23.1%) |
CNS response was evaluated in 5 pts CSF clearance from tumour cells = 3 pts Clinical improvement = 3 pts Neurological PFS = 4.7 months (95% CI 0.0–10.5 months) |
mOS: 4.7 months (95% CI 0.3–9.0) |
| Niwìnska et al. (2015)55 | 1999–2011 | 149 | 51 Luminal A/B (38%) 12 HER2+ Luminal like (9%) 17 HER2+ non-luminal (13%) 53 TNBC (40%) |
IT MTX (10 mg) and dexamethasone (4 mg) twice a week for the first 2 weeks, then total dose of 150 mg weekly until PD or IT liposomal cytarabine (50 mg) every 2 weeks for 5 cycles, then once every 4 weeks until PD |
Yes 77 pts received systemic ChT |
AEs ≥3 in the IT MTX group 67% AEs≥3 in the IT liposomal cytarabine group 77% |
Clinical response: 88 pts of ITT (63%) Complete response: 21 pts of ITT (15%) |
mOS in ITT 4.2 months (range 1–37) mOS with IT MTX vs IT liposomal cytarabine: 4.2 vs 4.6 months (p=0.717) mOS with systemic therapy vs none: 5.9 vs 2.3 months (p<0.001) |
AEs, adverse events; ARA-C, cytosine arabinoside; BEEP, bevacizumab combined with etoposide and cisplatin; BC, breast cancer; ChT, chemotherapy; CNS, central nervous system; CSF, cerebrospinal fluid; ET, endocrine therapy; HER2, human epidermal growth factor receptor 2; IT, intrathecal; ITT, intrathecal therapy; mOS, median overall survival; MTX, methotrexate; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PSL, prednisolone; pts, patients; RT, radiotherapy; TNBC, triple-negative breast cancer; TT, target therapy.
Clinical trials
Our MEDLINE search included 11 clinical trials, 3 of which were phase I dose-escalation studies, 6 phase II trials and 2 randomized phase III trials. Principal data are summarized in Tables 3–5.
Table 3.
Detailed characteristics of phase I trials.
| Author, year, type of study | Time of study | N (total) | Intrinsic subtypes | TLDM | Treatment | Other therapies (ITT, ET, ChT, RT) | Safety | CNS-specific response rate | Pharmacokinetics and toxicity |
|---|---|---|---|---|---|---|---|---|---|
| Tetef et al. (2000)57 Phase I |
…–1999 | 16 pts: 13 treated with MTX (9 with BC) and 3 controls | – | – | Dose escalating i.v. MTX at 1h loading dose of 200–1500 mg/mq, followed by a 23h infusion of 800–6000 mg/mq, every 1–2 weeks | Yes | G3 neutropenia and thrombocytopenia at dose level 2: 1 pt | No CNS cytology response. 5 out of 9 pts with BC were refractory to MTX |
Potentially cytotoxic CSF MTX concentration (>1 μM) can be safely delivered by i.v. infusion; Recommended dose: loading dose 700 mg/mq and 23-hour infusion 2800 mg/mq |
| Bonneau et al. (2018)58 Phase I |
2011–2018 | 16 pts with HER2+ BC | 6 HER2+ luminal like 10 HER2+ non-luminal | 7.4 years | Dose escalation IT trastuzumab (30 mg, 60 mg, 100 mg, 150 mg) once a week for at least 4 weeks | Yes (except lapatinib); Prior systemic trastuzumab allowed |
G1–G2 headache, nausea, vomiting, cervical, pain, and peripheral neuropathy were reporter for 60 mg dose or higher | Clinical response: 3 pts clinically improved, 7 pts remained stable, 4 pts had PD Radiological response (RECIST criteria): 9 pts SD, 5 pts PD Mean trastuzumab CSF concentrations: 1.23 mg/L, 0.79 mg/L, 7.08 mg/L and 27.88 mg/L for the 30, 60, 100 and 150 mg dose levels, respectively (target concentration being 30 mg/L) |
Recommended weekly dose of IT trastuzumab for phase II studies: 150 mg mOS: 7.3 months |
| Morikawa et al. (2020)59 Phase I |
2016–2017 | 11 HER2+ BC (4 patients with leptomeningeal involvement) | 5 HER2+ luminal-like 6 HER2+ non-luminal | – | Escalating dose of Lapatinib (ranging from 500 mg BID to 2500 mg BID) day 1–3, q14 – alternating with intermittent Capecitabine 1500 mg os BID for 7 days on – 7 days off | Yes | At the 2000 mg BID, there were two DLT: G3 nausea (1 pt), G3 vomiting (1 pt) | Radiological response of the 4 LMD pts: 1 pt had a partial response and 1 had stable disease | MTD 1500 mg BID lapatinib 3 days on 11 days off alternating with capecitabine 1500 mg oral BID on 7 days on 7 days off |
BC, breast cancer; ChT, chemotherapy; CNS, central nervous system; CSF, cerebrospinal fluid; DLT, dose-limiting toxicity; ET, endocrine therapy; HER2, human epidermal growth factor receptor 2; IT, intrathecal; ITT, intrathecal therapy; LMD, leptomeningeal disease; mOS, median overall survival; MTD, maximum tolerated toxicity; MTX, methotrexate; PD, progressive disease; pts, patients; RT, radiotherapy; SD, stable disease.
Table 4.
Detailed characteristics of phase II trials.
| Author, year, type of study | Time of study | N (total) | Intrinsic subtypes | TLDM | Treatment | Other therapies (ITT, ET, ChT, RT) | Safety | Efficacy |
|---|---|---|---|---|---|---|---|---|
| Orlando et al. (2002)60 Phase II |
– | 13 | – | – | Weekly IT schedule: Day 1 Thiotepa 10 mg plus MTX 15 mg plus hydrocortisone 30 mg: Day 5 ARA-C 70 mg, MTX 15 mg, hydrocortisone 30 mg | Yes (concomitant systemic therapy in 5 pts and WBRT in 7 pts) | Minimal AEs | ITC failed to provide OR |
| Pan et al. (2016)61 Phase II |
2010–2014 | 59 patients with solid tumours (11 with BC) | – | – | IT MTX 12.5–15 plus dexamethasone 5 mg, weekly and IF-RT (40 Gy/20f) | Yes | Acute cerebral meningitis G5 (2%) Encephalopathy G2–G3 (15%) Leukodystrophy G3 (27%) Radiculitis G3–G4 (12%) Bone marrow depression G3–G4 (14%) Mucositis G3–G4 (3%) |
Overall clinical RR 86.4%, including CR (23.7%), OR (49.1%), PR (13.6%), SD (8.5%), PD (5.1%) No differences amongst different primitivities (p=0.568) |
| Mrugala et al. (2019)62 Phase II |
2011–2014 | 3 patients (22 planned accruals, study was closed prematurely from poor accrual) | 2 Luminal B 1 HER2+ luminal like) |
– | High dose i.v. MTX 8 mg/mq plus IT liposomal cytarabine (IT Depocyt) 50 mg divided in induction, consolidation and maintenance phase | – | G4 patient had lymphopenia (1 pt) G3 transient transaminitis (all 3 pts) No signs of neurotoxicity were recorded |
mPFS 14 months mOS 8.2 months 2 patients showed PD after the end of induction phase 1 patient showed PD after the end of consolidation phase |
| Brastianos et al. (2020)63 Phase II |
2016–2018 | 20 patients (of which 17 BC) | 3 TNBC pts; 3 HER2+ non luminal pts; 3 HER2+ luminal like pts 7 luminal A/B 7 pts; 1 pt with unknown receptor status |
39.5 months | Pembrolizumab 200 mg i.v. every 2 weeks – single arm | Yes (systemic trastuzumab 2 pts, endocrine therapy 2 pts) | G3 headache (15%) G3 nausea (10%), G3 immune-related AST increasing (10%) G3 vomiting (5%) No G4 AEs were recorded G1 vomiting and hyperglycaemia were the most common AEs |
OS at 3 months: 60%. mOS = 3.6 months CNS radiological response (iRANO): 55% pts had stable disease as the best response |
| Kumthekar et al. (2020)64 Phase II |
2014– | 72 BC pts (28 with LMD) | In the LMD subgroup: 4 TNBC pts, 16 HER2+ pts, 8 luminal A/B pts | 4.4 years | ANG1005 (3 paclitaxel molecules covalently linker to Angiopep-2) 600 mg/m2 i.v. every 3 weeks | Yes | G ≥ 3 myelosuppression: reduced leucocytes (62%), reduced neutrophil (64%), reduced lymphocyte (43%), reduced platelets (15%) and anaemia (13%) G≥3 febrile neutropenia (17%) G3 fatigue (11%) G3 nausea (6%) G3 peripheral neuropathy (8%) |
iORR 8% in ITT and 17% in LMD pts iCBR 77% in ITT and 79% in LMD pts 3 months iPFS 67% in ITT and 83% in LMD pts OS 7.8 and 9.9 months in ITT and HER2+ pts, respectively OS 8.0 and 9.0 months in the LMD subgroup and HER2+ LMD, respectively |
AEs, adverse events; ARA-C, cytosine arabinoside; BC, breast cancer; ChT, chemotherapy; CNS, central nervous system; CR, complete response; CSF, cerebrospinal fluid; ET, endocrine therapy; HER2, human epidermal growth factor receptor 2; iCBR, intracranial clinical benefit rate; IF-RT, involved field-radiotherapy; iORR, intracranial objective response rate; IT, intrathecal; ITC: intrathecal chemotherapy; ITT, intrathecal therapy; LMD, leptomeningeal disease; mOS, median overall survival; mPFS, media progression-free survival; MTX, methotrexate; OR, overall response; OS, overall survival; PD, progressive disease; PR, partial response; pts, patients; RR, response rate; RT, radiation therapy; SD, stable disease; TNBC, triple-negative breast cancer; WBRT, whole-body radiotherapy.
Table 5.
Detailed characteristics of phase III trials.
| Author, year, type of study | Time of study | N (total) | Intrinsic subtypes | TLDM | Treatment | Other therapies (ITT, ET, ChT, RT) | Safety | CNS-specific response | Survival |
|---|---|---|---|---|---|---|---|---|---|
| Boogerd et al. (2004)65 Phase III |
1991–1998 | 35 BC pts with LMD (study closed prematurely because of poor accrual in the IT arm) | – | – | IT arm: systemic therapy plus IF/RT (30 Gy/10 fr) plus IT MTX Non-IT arm: systemic therapy (CAF, FEC and CMF) plus IF/RT (30 Gy/10 fr) |
Yes | Delayed leukoencephalopathy 18% (IT) vs 6% (non-IT) Serious gait disturbance 65% (IT) vs 28% (non-IT) Moderate headache 41% (IT) vs 22% (non-IT) Moderate cognitive impairment 53% (IT) vs 22% (non-IT) Neurological complications 47% (IT) vs 6% (non-IT) (p=0.0072) 1 death in the IT group after Ommaya reservoir insertion and before MTX infusion |
Neurological improvement: 41% (IT) vs 39% (non-IT) Neurological stabilization = 18% vs 28% Mean time to neurological progression in responders = 43 weeks (IT) vs 64 weeks (non-IT) |
mOS 18.3 weeks in the IT arm vs 30.33 weeks in the non-IT arm (p=0.32) mOS of 6 IT patients who achieved cytological response 52 weeks |
| Le Rhun et al. (2020)66 Phase III DEPOSEIN trial |
2011–2018 | 73 pts | In the control arm: 7 pts TNBC (19%), 9 pts HER2+ (24%) – 24 patients ER+ (65%) – 18 PR+ (29%) In the experimental arm: 5 TNBC (14%) – 2 HER2+ (6%) 27 ER+ (75%) – 24 PR+ (67%) |
4.5 years in the control arm 7.3 years in the experimental arm 4.7 years in all patients |
Control arm: systemic therapy Experimental arm: systemic therapy plus intrathecal liposomal cytarabine 50 mg every 14 days for 2 months (5 total injections) followed by monthly injection of 50 mg until PD |
Yes | No significant differences in the incidence of severe (G3 or higher) AEs between the two groups | Median LM PFS in the ITT: 2.2 (control) vs 3.8 (experimental) (HR 0.61; p=0.04) Clinical LM response in the ITT: improvement 3% vs 17%; stabilization 81% vs 72%, deterioration 11% vs 11%, missing 5% vs 0% Radiological LM response in the ITT: PR 8% vs 19%, SD 32% vs 56%, PD 30% vs 14%, missing 30% vs 11% |
Overall PFS in the ITT: 2.0 months (control) vs 2.4 months (experimental) (HR 0.66; p=0.09) OS in the ITT: 4.0 months (control) vs 7.3 months (experimental) (HR 0.85; p=0.051) |
BC, breast cancer; CAF, cyclophosphamide, doxorubicin, 5-fluorouracil; CMF, cyclophosphamide, methotrexate, 5-fluorouracil; FEC, 5-fluorouracil, epidoxorubicin, cyclophosphamide; IF/RT, involved field radiotherapy; IT, intrathecal; MTX, methotrexate.
Phase I dose-escalation studies
In 2000, Tetef et al.57 published a dose-escalation trial to evaluate the tolerability of high dose i.v. methotrexate (MTX) in patients with confirmed LC from solid tumours (13 patients enrolled, of whom 9 had BC). The theoretical advantage of this schedule was the prolonged exposure to cytotoxic levels of MTX in all parts of the CNS. No severe neurological toxicities related to protocol were reported, which confirmed MTX as a well-tolerated drug even at high doses. In the subgroup of patients with LC from BC, 55% of patients were refractory to standard MTX therapy, meaning a high prevalence of resistance to the antimetabolite in this population. According to the authors, a high dose of leucovorin can also have a role in antagonizing the efficacy of MTX. Nevertheless, this study proves that high dose i.v. MTX guarantees a more uniform and less invasive drug exposure to the drug than IT MTX. A dose of 700 mg/mq over 1 hour followed by 2800 mg/mq over 23 hours showed induction of a potentially cytotoxic concentration in the CSF with a good safety profile.
The other two dose-escalation studies included in our review focused on the tolerability of anti-HER2 agents in a population of patients with HER2+ BC and with confirmed LC. In 2018, Bonneau et al.58 evaluated the maximum tolerated dose of intrathecal/intraventricular weekly trastuzumab. The recommended dose was 150 mg/week until disease progression as no dose-limiting toxicity was seen. Antileptomeningeal activity – defined as clinical or radiological stabilization – was shown in this cohort of heavily pretreated patients (including systemic anti-HER2 drugs). There was no statistical difference in the survival of patients with stable or responsive disease compared to patients with progressive disease defined by CSF cytology, which may reflect the inadequate role of cytology as a treatment response marker.58
Morikawa et al. studied the CNS penetration of oral lapatinib in patients with HER2+ BC with isolated CNS progression (both brain metastases and LC) and concomitant systemic disease control. The maximum tolerated dose was 1500 mg BID lapatinib 3 days on/11 days off, alternating with 1500 mg BID capecitabine 7 days on/7 days off. An intermittent schedule was chosen to mitigate gastrointestinal toxicity. Three of 11 patients were on study for at least 6 months before disease progression and 2 of these had LC. In this study, an interesting exploratory analysis was performed on CTCs in the LC cohort. The dynamic changes in CTC enumeration appeared to correlate with the clinical course of the disease, suggesting that CTC count may be used for treatment response assessment. Because of the paucity of patients evaluated (only 3), these data need to be further confirmed. In summary, this study supports the evaluation of a new treatment schedule with drugs that have already shown efficacy against CNS disease (such as tucatinib, neratinib and TDM-1) with the aim of improving safety and tolerability without affecting efficacy.59
Phase II trials
In 2002, Orlando et al. evaluated the efficacy of IT CT in patients with LC from BC. The weekly schedule consisted of day 1 thiotepa 10 mg plus MTX 15 mg plus hydrocortisone 30 mg and day 5 cytarabine (ARA-C) 70 mg plus MTX 15 mg plus hydrocortisone 30 mg. A total of 12 patients were evaluated and none of them had clinical response or improvement of symptoms. This study emphasized the controversial role of IT CT in this cohort of patients.60
In a prospective single-arm phase II study, Pan et al.61 evaluated the efficacy and safety of concurrent IT MTX and involved-field radiotherapy (RT) in patients with a poor prognosis affected by LC from different solid tumours. BC represented 18% of the intention-to-treat (ITT) population. In this subgroup, the treatment was effective in 72.7% of cases, with a median OS of 5.4 months. No differences were observed in response and survival of patients with different primitivities, whilst statistically significant OS prolongation was observed in patients with clinical response (p=0.009). IT CT plus RT can improve the outcome of patients with LC from solid tumours with adverse prognostic factors. MTX and RT act at different moments of the cellular cycle; moreover, MTX has a radiosensitizing effect and RT can relieve CSF block. This multimodal strategy can help to improve neurological symptoms, allowing the physician to continue with systemic therapy in those patients with the active extra-CNS disease. This study also showed that CSF cytological clearance does not correlate with a relative risk (p=0.423) or OS (p=0.988).
In 2019, Mrugala et al.62 evaluated the efficacy of i.v. high dose MTX and IT liposomal cytarabine in patients with metastatic BC with brain and leptomeninges involvement. Even if these data must be considered preliminary due to poor accruals and premature study closure, the combination of systemic therapy and IT approach appears to be feasible and potentially effective as the longest survival was observed in the only patient who completed the treatment schedule until the consolidation phase.
A recent phase II study by Brastianos et al.63 examined the role of pembrolizumab in patients with LC from solid tumours. The background of the study refers to previous preclinical data suggesting a potential role of immune-checkpoint inhibitors in overcoming the anatomic BBB in patients with LC. All patients enrolled were heavily pretreated and most (85%) had breast primitivities. The primary endpoint of 3-months OS was achieved by 12 of 20 patients; median OS in the ITT was 3.6 months. In the subgroup of BC patients, no significant correlations were found between survival outcomes and receptor status. Moreover, PD-L1 expression was measured from archival intracranial or extracranial tissue and it was shown that it did not have any impact on median OS. These relevant evaluations can be underpowered as the size of the study was very small. Nonetheless, there is a rationale in raising the hypothesis that prior lines of therapy (including RT) can sensitize tumour cells allowing them to be responsive to immunotherapy.
Kumthekar et al.64 studied the efficacy of an innovative peptide-drug conjugate, ANG1005 (paclitaxel trevatide), given intravenously at the dose of 600 mg/mq every 3 weeks in a population of patients with CNS involvement from metastatic BC. The novel drug was designed properly to cross the BBB barriers via LDL receptor-related protein 1 (LRP1)-mediated transcytosis. Even if the primary endpoint of intracranial overall response rate (ORR) was not met in the ITT, a clinically relevant CNS and systemic response was shown as well as survival prolongation compared to historical control. Of note, in the LC subset, best response and survival rates were observed in HER2+ patients, whilst worst outcomes were seen in TNBCs. In conclusion, ANG1005 showed significant benefit both in CNS and systemic lesions in a heavily pretreated population (including taxane-based therapies).
Phase III randomized trials
In 2004, Boogerd et al.65 designed a multicentre, randomized study to evaluate the benefits of IT therapy (MTX or ARA-C) compared to physician-chosen systemic therapy, which included standard regimens of the time such as cyclophosphamide + adriamycin + 5-fluorouracil (CAF), 5-fluorouracil + epirubicina + cyclophosphamide (FEC), and cyclophosphamide + methotrexate + 5-fluorouracil (CMF). This study corroborated the growing evidence in those years that adding IT to systemic therapy does not lead to significant survival improvement and yet certainly increases treatment-related neurotoxicity. The hypothesis is that the lack of response may be related to CSF obstruction as reported in previous studies, which may also explain why systemic therapy shows efficacy in this setting. Moreover, this study offers further evidence of leukoencephalopathy after IT MTX in patients who did not receive WBRT. According to these results, IT therapy can be omitted safely as it did not improve patient outcomes and it increased treatment-related toxicity.
More recently, Le Rhun et al.66 published a second randomized trial after Boogerd’s study comparing IT therapy and systemic therapy. In this trial, the primary endpoint was leptomeningeal progression-free survival (PFS) in patients who received IT liposomal cytarabine in addition to conventional systemic therapy. IT liposomal cytarabine at a dose of 50 mg every 2 weeks was chosen for its longer half-life, allowing fewer administrations. In the ITT population, leptomeningeal PFS was 2.2 months in the control arm versus 3.8 months in the experimental arm (HR 0.61, 95% CI: 0.38–0.98; p=0.04). Median OS was 4.0 months in the control arm versus 7.3 months in the experimental arm (HR 0.85, 95% CI: 0.53–1.36; p=0.05) in the unadjusted analysis. In an adjusted analysis of the per-protocol population, the differences between overall PFS and OS became significant. No significant difference was shown in the quality of life or severe adverse events between the two groups, even if infections and chemical meningitis were more frequent in the experimental arm. Even if a clinically significant benefit was demonstrated in the experimental arm, the data from this trial are not adequate to confirm the superiority of the addition of IT therapy to systemic treatment. In the control arm, more heavily pretreated patients and tumours with a higher prevalence of HER2+ were included. Moreover, systemic therapy was not standardized. Liposomal cytarabine is currently unavailable in clinical practice in contrast to regular cytarabine, which needs twice weekly administration.
Retrospective studies
Our research included 11 retrospective studies.
Kim et al.67 retrospectively compared the efficacy of IT single therapy MTX versus a three-drug combination therapy (MHA group: MTX, hydrocortisone, ARA-C) in patients with LC from BC. They found that the cytological response rate (defined as total absence of malignant cells at lumbar puncture) was higher in the MHA treatment arm (38.5% versus 13.8%; p=0.036). This difference was more accentuated in patients who did not receive CNS irradiation (58.3% versus 12.5%; p=0.017). Amongst patients with BC, the survival was higher in the MHA arm than in the MTX-alone arm, although it was not statistically significant (23.7 versus 10.1 weeks; p=0.445). Moreover, ARA-C is no longer considered active against LC, even though, in 2003, it was considered a reasonable therapeutic approach due to the absence of valid alternatives.
Lassman et al.68 observed that, amongst patients with LC (from different tumours, including BC), there was a good response to high-dose intravenous MTX (3.5 g/m2) with an estimated ORR of 26% and a median survival of 12.6 weeks. According to the authors, in this subgroup of patients, systemic treatment has many advantages: it is not invasive, it does not require an Ommaya reservoir, it is effective also in patients with parenchymal metastases (which co-existed in 32% of patients) and its administration does not depend on the presence of obstructed CSF flow.
In a small retrospective study by Ekenel et al.,69 it was shown that oral capecitabine may contribute to disease control as all seven patients with CNS involvement from BC experienced a clinical benefit. Data from this study are limited by poor accrual (only three patients with LC) and the concomitant administration of other anticancer drugs.
Rudnicka et al.70 studied the efficacy of multimodality treatment in LC from BC aiming to identify which strategy had the greatest impact on patient outcomes. Results underline how systemic chemotherapy (drugs used: vinorelbine, fluorouracil, anthracyclines, platinum, paclitaxel, etoposide, carmustine, temozolomide) plays a crucial role in prolonging survival. Performance status was the most relevant predictive factor in patients with leptomeningeal disease. In this study, RT had a controversial impact on survival although it improved quality of life due to the alleviation of neurological signs and symptoms.
In another small study by Kiewe et al.,71 the efficacy of topotecan/ifosfamide (TOPO/IFO) in patients with CNS involvement from different solid tumours was evaluated. Only seven patients with LC from BC were enrolled. Response was seen in two patients (both received intrathecal MTX in addition) and progression disease in two, whilst three patients were not evaluated for response. The TOPO/IFO combination resulted in important haematological toxicity and moderate efficacy in this subgroup of heavily pretreated patients with CNS lesions of solid tumours.
In 2013, Le Rhun et al.72 described survival outcomes in a cohort of 103 patients with LC from BC evaluated retrospectively and treated with IT-CT (first-line liposomal cytarabine, second-line thiotepa and third-line MTX). Median PFS after first-line treatment was 2.1 months and median OS was 3.8 months. In the univariate analysis, some prognostic factors associated with significant better OS were initial systemic therapy, initial clinical response, second-line treatment with IT thiotepa, initial ECOG performance status (PS) of 0–2, combination therapy with WBRT, non-TNBC, initial treatment including endocrine therapy, a CSF protein level of 2 g/L at diagnosis, and absence of intracranial hypertension at LC diagnosis. According to this retrospective analysis, intra-CSF liposomal cytarabine seems to be the most appropriate treatment in the context of multimodal therapy.
In the same year, first-line or second-line IT thiotepa was further investigated in a large cohort of patients with BC and LC. The aim of the study was to assess survival and to describe prognostic factors in this subgroup of patients. The regimen proposed consisted of weekly IT thiotepa (10 mg) and methylprednisolone (40 mg). Median OS was 4.5 months (range 0.1–5.0), and no statistical difference was observed between patients receiving thiotepa as first-line or second-line IT-CT. According to these results, a subgroup of patients may benefit from IT thiotepa even as the second line. At the multivariate analysis performed at the time of LC diagnosis, adverse prognostic factors were performance status and more than three previous chemotherapy lines. At the start of treatment, high tumour grade, elevated Cyfra21-1 in CSF and clinical symptoms failure were also identified as negative prognostic factors.73
In 2014, Scott et al.74 evaluated the tolerability of a combination of concurrent IT MTX and liposomal ARA-C in patients with LC from solid tumours. Of 30 patients enrolled, 15 had BC. Clinical improvement was more common amongst patients with BC. Amongst these patients, the median time to neurological progression was 13.4 weeks and the median OS was 33.2 weeks. According to these results, combination therapy with two IT drugs did not aggravate toxicity. Meningitis was the most common grade III adverse event, but its incidence was lower than in other reported studies with single-agent IT liposomal ARA-C. Three patients interrupted treatment because of toxicity due to meningitis, herpes zoster and mucositis, respectively. The retrospective nature of the study and the limited number of patients enrolled did not allow conclusions to be drawn about the impact on survival of different histology, HR status and other tumour features. However, according to these results, other novel IT-CT combinations may be feasible and could be explored in future randomized trials.
In a large single-cohort study conducted by Berger et al.75 in a population of women with CNS involvement from hormone HR+ BC, the impact of endocrine therapy (ET) after the diagnosis of brain metastasis was evaluated. In patients with concomitant leptomeningeal disease, ET had a positive impact on OS. No significant differences were demonstrated between fulvestrant, tamoxifen and aromatase inhibitors. Moreover, ET appeared to have activity even if patients had previously received ET before CNS involvement and after developing ‘secondary’ endocrine resistance.
Chen et al.76 retrospectively evaluated 34 patients with leptomeningeal disease confirmed by positive CSF cytology receiving modern systemic therapy, especially the BEEP (bevacizumab, cisplatin and etoposide) regimen. Intrinsic subtypes were predominantly ER+ (21/34), followed by TNBC (10/34) and HER2+ (7/34). Eight patients had lobular histology (23.5%). Most of these patients received at least one dose of MTX; 23 of 34 patients received systemic therapy after LC diagnosis. From multivariate analyses, the BEEP regimen and IT trastuzumab were identified as significant prognostic factors. In this cohort, the BEEP regimen not only showed activity but also demonstrated improved OS. This can be explained by the fact that VEGF plays a role in LC pathophysiology. It is not possible to quantify the benefit deriving from the BEEP regimen and from intrathecal MTX as these two treatments were administered concurrently in most patients. In this study, IT trastuzumab was also administered in three of seven HER2+ patients and was associated with improved survival.
More recently, triple IT-CT (MTX, cytosine arabinoside and hydrocortisone) was evaluated retrospectively in a cohort of 20 patients with solid tumours (45% of whom had BC). Amongst patients with BC, the median OS was 20 months. The cytological response rate (defined as negative cytological analyses on two consecutive cytological CSF tests after 4-weekly administration) was 65%. The presence of concomitant parenchymal metastases was the only predictor of poorer outcomes at univariate analysis.77
In a recent large real-life study, Carausu et al.78 studied survival outcomes in a population of 312 patients with LC and BC treated with IT-CT between 2008 and 2016. Consistent with previous studies, the median OS was 4.5 months and the 1-year survival rate was 25.6%. At the multivariate analysis TNBC, more than three treatment lines and more than three metastatic sites excluding CNS and IT cytarabine or thiotepa (versus IT-MTX) were associated with poor prognosis. Concomitant systemic therapy was instead associated with better OS (HR 0.47, 95% CI: 0.35–0.62; p<0.001). No differences in terms of better outcomes were found in patients treated more recently (2012–2016). This study suggests that concomitant systemic therapy and IT MTX (instead of cytarabine or thiotepa) may improve patient survival.
Systematic reviews
Five systematic reviews addressing the role of IT-CT, systemic CT, ET and anti-HER2 agents in LC were published in the last two decades. The lack of high-quality, prospective evidence was widely reported and represented a strong limitation for the accuracy of conclusions.79–83
Two reviews highlighted the scarcity of high-quality limited data regarding the benefit of IT-CT compared to systemic treatment despite its widespread use in clinical practice.80,81 Lee et al.80 reported a median OS of 15, 13 and 10 months in patients receiving ET, systemic therapy and IT-CT, respectively.
As LC is often a late event in HR+ BC, thus occurring in a context of endocrine resistance, ET is often avoided in favour of more aggressive systemic treatment. However, data collected by Fernandes et al.12 suggest a survival benefit in a subgroup of patients with HR+ disease. Although the use of newer targeted agents has not been studied in LC patients, CDK4/6 inhibitors and PI3K/AKT/mTOR inhibitors have shown activity against CNS disease and represent an important future line of research.
Three systematic reviews evaluated the use of IT trastuzumab alone or in combination with other therapies in patients with LC from HER2+ BC. IT trastuzumab showed good tolerability when administered intrathecally, even in association with systemic treatment. Median OS ranged from 10.7 to 13.5 months, an impressive result when compared with historical controls from the same population (5.9 months).79,82,83
Ongoing trials on novel treatment options in LC from advanced BC
A further search was conducted to identify current ongoing trials aiming to assess novel therapeutic options for LC from BC. Most of the ongoing studies are phase I/II, designed to evaluate the efficacy and tolerability of anti-HER2 drugs in LC from HER2+ BC. After the promising results of the phase I study published by Bonneau et al.,58 a phase II study has been developed to assess the antitumour activity of IT trastuzumab; however, preliminary results are not yet available (NCT01373710). Another phase II study will evaluate the safety of RT followed by IT trastuzumab/pertuzumab in patients with LC from HER2+ BC (NCT04588545). Of note, some new anti-HER2 drugs are gaining major interest. The DEBBRAH study (NCT04420598) is a phase II study currently recruiting patients with HER2+ BC with brain metastases and/or LC who will be treated with trastuzumab deruxtecan with or without concomitant ET. Similarly, in a non-randomized phase II study, the efficacy of triplet tucatinib/trastuzumab/capecitabine will be assessed in LC from HER2+ BC (NCT03501979).
The only phase III study currently ongoing (NCT03613181) has been designed to further evaluate the survival outcome of patients treated with ANG1005 following the promising preliminary results of the phase II study published by Kumthekar et al.64
On the translation side, two phase I studies (NCT03661424, NCT03696030) are currently recruiting patients with LC from solid tumours to evaluate the best and safest dose of bispecific antibody armed activated T cells and HER2+ CAR T cells given intraventricularly, respectively.
Finally, a prospective study aiming to evaluate exosome CSF concentration in patients with BC with suspected LC has been designed to evaluate the possible role of proteomic profiling issued from CSF exosomes for LC diagnosis (NCT03974204).
Radiotherapy
WBRT and focal RT are therapeutic options for patients with LC, including those considered unsuitable for systemic therapy, helping to delay neurological deterioration and to improve the symptom burden. A direct favourable impact on survival is less clear as indicated by some retrospective analysis.84,85 The literature also reports case series regarding the feasibility of SRS for the treatment of focal leptomeningeal disease.86 The most frequently reported toxicities are myelosuppression and other mild non-haematological toxicities (dysphagia, mucositis, enteritis, and nausea). Concomitant treatment with IT-CT is feasible and could be superior to irradiation alone.87 Good Karnofsky performance status, moderate neurological deficits, clinical response to RT and systemic therapy are associated with a better prognosis, whilst high-grade myelosuppression and high serum LDH levels (>500 U/I) were associated with limited survival.88,89
Patients undergoing surgical brain metastasis resection can receive WBRT to reduce the risk of intracranial failure. Because of the long-term cognitive decline associated with WBRT, partial RT has been evaluated as a substitution. There are controversial data on the associated risks of leptomeningeal recurrence following WBRT and partial RT on the surgical cavity.
A retrospective review aiming to evaluate the use of SRS on the cavity bed of resected brain metastasis found out that SRS can spare or at least delay the need for WBRT in patients with small-volume resected brain metastases.15 On the contrary, Ha et al.14 compared the incidence of LC or dural metastasis in patients treated with WBRT, partial RT and no RT after the resection of brain metastases from BC. In this population, relapse-free survival was longer in patients treated with WBRT compared to partial RT and to systemic therapy alone (p<0.001 at the multivariate analysis).
Palliative ventriculoperitoneal shunt for LC-associated intracranial hypertension
Leptomeningeal disease from solid tumours can be complicated by the occurrence of intracranial hypertension (ICH) and hydrocephalus. Clinical presentation includes headache, nausea/vomiting, impaired awareness and walking, papilledema, and sixth nerve palsy. Hydrocephalus is generally communicating but a clinical picture of non-communicating hydrocephalus can be associated with bulky brain disease or metastasis causing CSF flow obstructions. An accurate identification of such a scenario is essential not only for the correct management of the symptoms but also to avoid IT-CT, which is absolutely contraindicated in patients with ICH because an impaired CSF dynamic can increase acute and chronic neurotoxicity. Corticosteroids and other palliative pharmacological solutions may not be sufficient to obtain a prompt relief of the symptoms and sometimes a ventriculoperitoneal shunt (VPS) may be required. We included in our review four retrospective studies about the clinical outcome of patients with ICH from BC-LC treated with VPS.
In a retrospective study, Omuro et al.90 evaluated 37 patients (23 of whom had BC) with leptomeningeal disease treated with a ventriculoperitoneal insertion for ICG or recurrent CSF leak after brain surgery. Improvements in ICH symptoms were observed in 77% of patients (decreased nausea/vomiting, improved level of awareness) and none of them had evidence of peritoneal carcinomatosis and infections. No deaths were directly correlated to the shunt. The median OS was 2 months.
Hydrocephalus usually precludes the possibility of using IT-CT. To overcome this complication, a combined approach that includes a subcutaneous reservoir connected in series to an on/off valve and a VPS for both diversion of CSF and injection of IT-CT was proposed (RO-VPS construct). In a retrospective case–control study, Lin et al.91 reviewed their experience with this dispositive in patients with leptomeningeal disease and symptomatic hydrocephalus from different primitivities (of 48 patients enrolled, 19 had BC, 9 in the case group and 10 in the control group). Patients who underwent placement of the RO-VPS dispositive experienced a symptomatic improvement and performance status improvement in 83.3% of cases; they also received intraventricular CT in 75% of cases with a cytological response noted in 61.1% of patients. Median PFS and OS were 14 and 31 weeks, respectively. The procedure-associated complication rate was confirmed to be low (8.3%), in agreement with previous results. Of note, BC patients treated with RO-VPS had significantly better survival outcomes when compared to a matched population treated with CSF reservoir only (35 versus 21 weeks; p=0.04). This difference was not observed in patients with other primitivities. This benefit may be attributable to the possibility of continued Depocyt (cytarabine liposomal injection) administration intrathecally. In 2014, Jung et al.92 evaluated the prognosis of surgically treated hydrocephalus in patients with leptomeningeal disease from solid tumours (71 patients enrolled, of whom 14 had BC). According to their results, RTOG-RPA class II and systemic and local treatment (both RT and IT-CT) were independent factors associated with improved OS, whilst surgically untreated hydrocephalus was associated with poor outcome compared to surgically treated patients (1.7 versus 5.7 months).
Prognosis
The prognosis of patients with BC affected by LC remains dismal, even in patients receiving aggressive, multimodal treatment. Based on data reported in retrospective studies, median OS from the time of LC diagnosis ranges from 7 to 21 weeks, with similar data obtained from cohorts of patients diagnosed across the past two decades.5,6,9–12,93–99
Many retrospective studies have aimed to identify prognostic factors that could be used in clinical practice to stratify patients and recognize those who gain the highest benefit from intensified, multimodal treatment. Performance status is the single most investigated factor, with several studies showing the negative impact of poor performance on survival.4,5,13,93,96,98,100 Available prognostic scores are addressed in Table 6.
Table 6.
Summary of prognostic scores available in the literature for breast cancer patients with leptomeningeal carcinomatosis.
| References | Type of study | Population | Prognostic variables | Classification | Median OS | |
|---|---|---|---|---|---|---|
|
Curie scorea Gauthier et al. (2010)4 |
Retrospective | 91 consecutive patients with BC and CM treated with intrathecal MTX |
|
Group 1 (score 0–1; score 0 for simplified version) | 12 months | |
| Group 2 (score 2; score 1 for simplified version) | 4 months | |||||
| Group 3 (score 3–4; score 2–3 for simplified version) | 2 months | |||||
|
| ||||||
| Lara-Medina et al. (2012)94 | Retrospective | 49 consecutive patients with BC and CM |
|
Favourable prognostic group (≤1 RF) | 14 weeks | |
| Poor prognostic group (≥2 RF) | 2 weeks | |||||
|
| ||||||
| INDEX scoreb Niwińska et al. (2018)96 |
Prospective | 187 consecutive patients with BC and CM |
|
Group 1 (score = 1) | 9.6 months | |
| Group 2 (score = 2) | 6.9 months | |||||
| Group 3 (score = 3–4) | 3.9 months | |||||
| Group 4 (score = 5–8) | 1.5 months | |||||
|
| ||||||
| EANO-ESMO classificationc Le Rhun et al. (2020)102 |
Retrospective | 254 patients with histologically confirmed solid tumour and LM (104 patients with BC) |
|
Type I | Type A | 2.2 months |
| Type B | 3.1 months | |||||
| Type C | 4.7 months | |||||
| Type D | 2.1 months | |||||
| Type II | Type A | 11.4 months | ||||
| Type B | 2.7 months | |||||
| Type C | 5.0 months | |||||
| Type D | 3.5 months | |||||
The simplified Curie score omits baseline Cyfra21-1 levels, with no difference in median OS within the same prognostic groups.
The INDEX score is calculated with the following formula: INDEX = 7 + (age < 53) – (KPS ≥ 70) – (luminal) – (2×RT) – (intrathecal) – (2×systemic).
The aim of EANO-ESMO Classification is to guide treatment decisions in patients with LM and solid tumours. Le Rhun et al.102 validated its prognostic use in a cohort of patients with different solid tumours; the data inserted in this table pertain to the subgroup of breast cancer patients.
BC, breast cancer; CM, carcinomatous meningitis; CSF, cerebrospinal fluid; CT, chemotherapy; KPS, Karnofsky performance status; LM, leptomeningeal metastases; MTX, methotrexate; OS, overall survival; RF, risk factor; TN, triple negative.
As discussed previously, the triple-negative subtype shows a predisposition for LC. A further association between receptor expression profiles and prognosis in patients with leptomeningeal metastases was investigated in several retrospective studies that observed a correlation between an HR− status4,9,95,96 or the HR−/HER2− subtype93,94,99 and shorter survival after diagnosis of meningeal involvement. In 2011, Lee et al.11 retrospectively analysed a cohort of 68 patients with BC with LC: whereas the median survival duration from LC onset to death (LM-OS) was similar amongst intrinsic subtypes, the median survival time from the diagnosis of distant metastasis was significantly shorter in TNBC (11.8 months versus 28.3 months in HR+ and 29.1 months in HER2+; p<0.0001), a finding at least partially explained by the tendency of TNBC to metastasize to the CNS earlier in the course of the disease.
Other important factors influencing survival in this patient population are systemic treatment and the multimodal therapeutic approach.4,5,9–11,30,95,96,100,101 In 2013, Yust-Katz et al.9 conducted a retrospective analysis of 103 patients with BC and LC, the majority of whom (80.8%) had received treatment for their leptomeningeal disease. Therapeutic approaches included WBRT (52%), spinal RT (19%), systemic CT (36%) and IT-CT (55%). Patients who did not undergo any type of treatment had a significantly shorter OS. At multivariate analysis, systemic CT (HR 0.38; p<0.0001), IT-CT (HR 0.43; p=0.0002) and WBRT (HR 0.57; p=0.0081) were all independent prognostic factors for patient survival, with the caveat that subjects in these subgroups tended to have a better performance status.9 In 2013, Niwińska et al.5 conducted a single-centre retrospective cohort study on 318 patients with LC from BC: Cox multivariate analysis revealed that systemic treatment (including intravenous/oral CT, ET and targeted therapy) significantly influenced survival in this patient population, with an absolute difference in median OS of 4 months (HR 0.477; p=0.012). The same group authored a similar study in 2017 on a cohort of 187 patients consecutively referred to the same centre between 1999 and 2015. Multivariate analysis was used to identify factors associated with prolonged survival from LM: six potential positive prognostic factors were proposed, namely Karnofsky Performance Status ≥70, older age (>53 years), luminal biological subtype, systemic intravenous/oral treatment, IT treatment and RT. Amongst the various therapeutic options, systemic therapy was the one with the strongest impact on OS (HR 0.418; p<0.001). Of note, a comparison between treatment outcomes before and after 2005 showed no difference in terms of survival, a finding coherent with the lack of therapeutic advances in the last two decades.96
In 2016, the largest known cohort of BC patients with LC (318 subjects) was retrospectively analysed to assess the presence of previously underestimated prognostic factors. Recent diagnosis (after 2006), triple-negative subtype, performance status and no evidence of metastatic disease outside the CNS were independently associated with improved survival at multivariate analysis. Notably, in an exploratory univariate analysis, a more recent diagnosis of HER2+ BC with LC (after 2005 and thus coinciding with the increased use of anti-HER2-targeted agents in this setting) was associated with better survival.93
In 2017, Kingston et al.99 conducted a retrospective trial including 182 patients with BC and LC diagnosed by MRI in a 10-year period (2004–2014). Initial management of LC consisted of WBRT or partial-brain RT (34.1%), systemic therapy (standard BC therapy, most commonly capecitabine; 24.7%), supportive care alone (20.3%) or IT-CT/trastuzumab (7.7%). Patients treated with systemic therapy had the longest OS (median 8.8 months versus 6.1 with RT, 2.9 with IT therapy and 1.7 with supportive care). On multivariate analysis, older age at diagnosis of LC, a triple-negative subtype, prior or concomitant brain metastases, presence of brain and spinal LC, and low albumin levels at LC diagnosis were predictive of poorer prognosis.
A study published by Griguolo et al.13 in 2018 described patient characteristics, treatment patterns and prognostic factors in a contemporary multicentric cohort of 153 consecutive patients with BC diagnosed with LC at two European institutions, divided into two subgroups based on HER2 expression. In the multivariate analysis for the HER2− BC patients, ECOG PS, histological grade and having received treatment (either systemic or, to a lesser extent, intrathecal) were confirmed as independent prognostic factors. In the HER2+ subgroup, the only factor maintaining independent prognostication at multivariate analysis was treatment with HER2-targeted therapy (HR 0.12).
In 2017, the EANO-ESMO Clinical Practice Guidelines proposed a classification of LC in solid tumours based on the presence of clinical manifestations, the type of meningeal alteration detected by MRI, CSF analysis and histology of biopsy specimens. Type I (confirmed) LM is verified cytologically or histologically, whereas the diagnosis of type II (probable or possible) LM is based on the presence of typical clinical signs and neuroimaging findings. Moreover, LM metastasis was subdivided into four subgroups based on their MRI appearance: linear leptomeningeal disease (type A), nodular leptomeningeal disease (type B), both (type C) or neither (type D, might include indirect signs like hydrocephalus).30 The prognostic value of this classification was assessed in a retrospective study conducted in 2020 that included 254 patients with LM from solid tumours, 41% of whom had a diagnosis of BC. In this subgroup, median OS was 3.4 months (IQR 1.3–7.5), with a better prognosis for patients with probable/possible LM compared to definitive LM (median OS 4.5 months versus 2.4 months, respectively).102
Recent literature includes few papers addressing factors predictive of treatment response and markers to assess treatment efficacy in patients with BC and LC.
A German study published in 2008 retrospectively analysed all patients with BC diagnosed with LM between 1998 and 2005 from a single centre (n=27). Detection of a contrast-enhancing lesion at MRI was associated with longer median OS (33 weeks versus 8 weeks, p=0.0407). Moreover, a contrast-enhancing lesion appeared to be an effect modifier for systemic therapy (multivariate p=0.03): patients with enhancing lesions benefited the most from systemic therapy (they were the subgroup with the longest survival), whereas in the subgroup with no enhancing lesions, patients who received systemic treatment tended to fare even worse than those who did.95
In 2009, Clatot et al.103 retrospectively analysed the CSF of 24 patients treated with high-dose IT MTX for BC LM. Cytologic response (i.e. CSF cytology without neoplastic cells after treatment) was observed in 46% of patients: there was an association between cytologic response and symptom improvement, and a statistically significant correlation with survival was detected at univariate analysis (p<0.005). This finding was later detected by subgroup analysis of a retrospective study from Lee et al.,11 who reported a statistically significant interaction between cytological negative conversion to IT and survival after LC diagnosis at multivariate analysis.
Conclusion
LC is a rare complication of BC and can be considered a terminal event in its natural history, leading to severe morbidity, decreased quality of life and poor prognosis. BC is the type of tumour most commonly associated with LC.21 This can be especially observed in triple-negative subtypes and lobular histology because of the increased CNS tropism in the former and the loss of E-cadherin in the latter – two factors that facilitate early invasion. Different timing in clinical presentation has been reported, with patients with triple-negative disease developing LC as an earlier event than in HR+ BC, where it generally arises as a late phenomenon.
Great efforts have been undertaken through the years to identify risk factors for the development of leptomeningeal colonization, including histology, intrinsic subtypes and the presence of parenchymal metastases. Previous neurosurgery on the ventricular system and SRS for focal brain metastases have also shown a correlation with an increased risk of leptomeningeal involvement. This finding can be explained by surgical spillover or drop metastasis of malignant cells into the leptomeninges induced by iatrogenic disruption of the BBB.
LC diagnosis can be challenging for several reasons: the varied and aspecific clinical presentation, the lack of a standardized diagnostic approach and the low sensitivity of commonly used tests (MRI and CSF cytology).26 According to our review of the literature, CSF-CTCs analysis significantly increases diagnostic accuracy and can be proposed to selected subgroups of patients with good PS and symptomatic disease, which are those that may benefit the most from further treatment for their leptomeningeal disease. Future prospects include the potential role of CSF samples as a substrate for liquid biopsy in order to help physicians achieve a deeper understanding of the tumour, follow tumour dynamics and detect molecular changes induced by treatment pressure.
Leptomeningeal disease treatment is exceedingly challenging. The therapeutic strategy cannot ignore a prognostic evaluation and multidisciplinary discussion. We believe that setting realistic goals, such as reducing the burden of symptoms and increasing survival whilst preserving the quality of life, should be the key objective of LC treatment. A multimodal approach appears to be the best therapeutic option available, especially in patients with good prognosis. There is an urgent need to validate a prognostic score to identify the subgroup of patients that can benefit from a multimodality approach, possibly tailored on intrinsic subtypes. Our current knowledge suggests that the most important independent prognostic factors are PS, non-triple-negative receptor status, extra-CNS involvement and the presence of encephalopathy at LC onset.93
IT therapy has been historically widely used in LC patients. The studies evaluated in our systematic review highlighted the controversial role of IT therapy as it was not possible to describe a certain survival benefit or improvement in quality of life when IT-CT was administered.49,60,80,81 The concomitant use of a multimodality approach made it harder to quantify the benefits deriving individually from IT-CT, systemic therapy and, eventually, RT. In the phase II study published by Pan et al.,61 IT-CT in combination with RT improved the outcomes of a subgroup of patients with poor prognosis whereas it is difficult to understand how much RT alone contributed to symptoms control compared to IT-MTX.61 In a large real-life retrospective study, IT cytarabine and thiotepa were associated with poor prognosis when compared to IT MTX, whilst concomitant systemic therapy led to improved survival, independently from the drugs administered. All the patients received concomitant systemic and IT therapy, which prevents us from understanding the benefit deriving from systemic therapy alone. The most relevant negative evidence on IT therapy derives from a large phase III randomized study, where the addition of IT therapy (MTX, ARA-C or liposomal cytarabine) to standard systemic therapy did not lead to an improvement in survival; however, these results must be interpreted carefully as the study was closed prematurely for poor accrual.65 On the other hand, the phase III randomized trial (DEPOSEIN study) suggested a small clinical benefit, especially in terms of LM-PFS66 and other retrospective studies reported similar results.72–74
We hypothesize that a subgroup of LC patients may benefit from concomitant IT treatment and systemic therapy. As we highlighted in this review, there is a lack of optimized selection criteria that can guide clinical decisions. It is therefore important to consider the consequences of proposing an invasive treatment with proven toxicity to a population of extremely fragile patients.
Active systemic drugs with proven efficacy in the setting of LC include etoposide, cisplatin, bevacizumab, methotrexate, capecitabine, vinorelbine, gemcitabine and 5-FU. Scarce evidence is available on the role of immunotherapy. The rationale of the potential role of immune-checkpoint inhibitors is that BBB disruption allows immune cells to access the subarachnoid space. Previous treatment lines can modify tumour cells and microenvironment, induce sensitization to tumour antigens, and trigger an immune response against them. Moreover, there is proof of the high frequency of PD-L1 and PD-L2 expression in BC brain metastases, regardless the receptor status.104 Pembrolizumab proved effective in a population of heavily pretreated patients with LC from solid tumours (mostly BC) evaluated in a phase II study63 but further data are needed to confirm these preliminary positive results.
In patients with HER2+ BC with LC, IT trastuzumab combined with systemic therapy and other additional IT drugs (MTX, cytarabine) showed promising efficacy and tolerable toxicities.79,83,84 Consistently with the positive results obtained from phase I dose-escalating studies,58,59 most of the ongoing phase II studies are exploring the antitumoural activity of IT anti-HER2 drugs and probably, in the next years, the interest for these agents will overcome the interest for IT-CT. Research should also focus on the efficacy of novel anti-HER2 drugs (TDM-1, tucatinib, neratinib) in leptomeningeal disease as these drugs have already proven activity against brain metastases.
Endocrine therapy (alone or in association with other drugs) has been historically neglected in retrospective studies in favour of CT, even in populations with HR+ BC. This can be explained by the fact that LC mostly occurs in the setting of endocrine-resistant disease or because ET was considered unable to control advanced disease. Contrary to expectations, a recent systematic review suggested that ET was associated with prolonged survival and an acceptable toxicity profile, leading the authors to elect ET as the preferred first-line therapy for LC in HR+ BC.12 In the future, the efficacy of novel targeted agents, such as CDK4/6 inhibitors and AKT-mTOR-PI3K inhibitors, all of which are able to cross the BBB, should be investigated in the setting of leptomeningeal disease.
We decided to extend our investigation also to the current evidence on palliative RT. We found that both WBRT and focal RT can delay the onset of cognitive decline and the worsening of neurological symptoms. Scant evidence is available about its effect on survival and most of it is indirect but an association between shortened OS and WBRT discontinuation seems plausible.88 The multimodality approach showed superiority to other strategies across all studies included in our review.
The treatment considered in different studies showed great heterogeneity according to the characteristics of the patients enrolled, receptor status, combination of intrathecal and systemic therapy, and dosage of the investigated regimens. We hope that, in the future, patients with similar features (histology, intrinsic subtype, disease burden, presence of symptoms, PS) will be treated with greater uniformity to obtain more solid evidence in support of the definition of a standard of care.
Acknowledgements
None.
Footnotes
Contributions: FS and LM conceived the content of the article. LM, CL, SP and SS performed the initial searches and wrote the first draft. F.S. provided input about the contents and reviewed the final draft. All authors reviewed and approved the final manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole and have given their approval for this version to be published.
Disclosure and potential conflicts of interest: The authors declare that they have no conflicts of interest relevant to this manuscript. The International Committee of Medical Journal Editors (ICMJE) Potential Conflicts of Interests form for the authors is available for download at: https://www.drugsincontext.com/wp-content/uploads/2021/09/dic.2021-6-6-COI.pdf
Funding declaration: There was no funding associated with the preparation of this article.
Correct attribution: Copyright © 2021 Mollica L, Leli C, Puglisi S, Sardi S, Sottotetti F. https://doi.org/10.7573/dic.2021-6-6. Published by Drugs in Context under Creative Commons License Deed CC BY NC ND 4.0.
Provenance: Invited; externally peer reviewed.
Drugs in Context is published by BioExcel Publishing Ltd. Registered office: Plaza Building, Lee High Road, London, England, SE13 5PT.
BioExcel Publishing Limited is registered in England Number 10038393. VAT GB 252 7720 07.
For all manuscript and submissions enquiries, contact the Editorial office editorial@drugsincontext.com
For all permissions, rights and reprints, contact David Hughes david.hughes@bioexcelpublishing.com
References
- 1.Franzoi MA, Hortobagyi GN. Leptomeningeal carcinomatosis in patients with breast cancer. Crit Rev Oncol Hematol. 2019;135:85–94. doi: 10.1016/j.critrevonc.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 2.Sacco K, Muhammad A, Saleem W, et al. Leptomeningeal carcinomatosis as primary presentation of relapse in breast cancer. Oncol Lett. 2016;12(2):779–782. doi: 10.3892/ol.2016.4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mittica G, Senetta R, Richiardi L, et al. Meningeal carcinomatosis underdiagnosis and overestimation: incidence in a large consecutive and unselected population of breast cancer patients. BMC Cancer. 2015;15:1021. doi: 10.1186/s12885-015-2042-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauthier H, Guilhaumem MN, Bidard FC, et al. Survival of breast cancer patients with meningeal carcinomatosis. Ann Oncol. 2010;21:2183–2187. doi: 10.1093/annonc/mdq232. [DOI] [PubMed] [Google Scholar]
- 5.Niwińska A, Rudnicka H, Murawska M. Breast cancer leptomeningeal metastasis: propensity of breast cancer subtypes for leptomeninges and the analysis of factors influencing survival. Med Oncol. 2013;30(1):408. doi: 10.1007/s12032-012-0408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alnajar H, Rosen L, Javidiparsijani S, et al. Prognostic markers and histologic subtypes in patients with meningeal carcinomatosis in breast cancer. Acta Cytol. 2017;61(2):140–144. doi: 10.1159/000455115. [DOI] [PubMed] [Google Scholar]
- 7.Bendell JC, Domchek SM, Burstein HJ, et al. Central nervous system metastases in women who receive trastuzumab-based therapy for metastatic breast carcinoma. Cancer. 2003;97(12):2972–2977. doi: 10.1002/cncr.11436. [DOI] [PubMed] [Google Scholar]
- 8.Lai R, Dang CT, Malkin MG, et al. The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer. 2004;101(4):810–816. doi: 10.1002/cncr.20418. [DOI] [PubMed] [Google Scholar]
- 9.Yust-Katz S, Garciarena P, Liu D, et al. Breast cancer and leptomeningeal disease (LMD): hormone receptor status influences time to development of LMD and survival from LMD diagnosis. J Neurooncol. 2013;114(2):229–235. doi: 10.1007/s11060-013-1175-6. [DOI] [PubMed] [Google Scholar]
- 10.Torrejón D, Oliveira M, Cortes J, et al. Implication of breast cancer phenotype for patients with leptomeningeal carcinomatosis. Breast. 2013;22(1):19–23. doi: 10.1016/j.breast.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Lee S, Ahn HK, Park YH, et al. Leptomeningeal metastases from breast cancer: intrinsic subtypes may affect unique clinical manifestations. Breast Cancer Res Treat. 2011;129(3):809–817. doi: 10.1007/s10549-011-1682-0. [DOI] [PubMed] [Google Scholar]
- 12.Fernandes L, de Matos LV, Cardoso D, et al. Endocrine therapy for the treatment of leptomeningeal carcinomatosis in luminal breast cancer: a comprehensive review. CNS Oncol. 2020;9(4):CNS65. doi: 10.2217/cns-2020-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Griguolo G, Pouderoux S, Dieci MV, et al. Clinicopathological and treatment-associated prognostic factors in patients with breast cancer leptomeningeal metastases in relation to tumor biology. Oncologist. 2018;23(11):1289–1299. doi: 10.1634/theoncologist.2018-0200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ha B, Chung SY, Kim YJ, et al. Effects of postoperative radiotherapy on leptomeningeal carcinomatosis or dural metastasis after resection of brain metastases in breast cancer patients. Cancer Res Treat. 2017;49(3):748–758. doi: 10.4143/crt.2016.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ojerholm E, Lee JYK, Thawani JP, et al. Stereotactic radiosurgery to the resection bed for intracranial metastases and risk of leptomeningeal carcinomatosis. J Neurosurg. 2014;121:75–83. doi: 10.3171/2014.6.GKS14708. [DOI] [PubMed] [Google Scholar]
- 16.Jung JM, Kim S, Joo J, et al. Incidence and risk factors for leptomeningeal carcinomatosis in breast cancer patients with parenchymal metastases. J Korean Neurosurg Soc. 2012;52(3):193–199. doi: 10.3340/jkns.2012.52.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trifiletti DM, Romano KD, Xu Z, et al. Leptomeningeal disease following stereotactic radiosurgery for brain metastases from breast cancer. J Neuro Oncol. 2015;124(3):421–427. doi: 10.1007/s11060-015-1854-6. [DOI] [PubMed] [Google Scholar]
- 18.Atalar B, Modlin LA, Choi CYH, et al. Risk of leptomeningeal disease in patients treated with stereotactic radiosurgery targeting the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2013;87(4):713–718. doi: 10.1016/j.ijrobp.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 19.Foreman PM, Jackson BE, Singh KP, et al. Postoperative radiosurgery for the treatment of metastatic brain tumor: evaluation of local failure and leptomeningeal disease. J Clin Neurosci. 2018;49:48–55. doi: 10.1016/j.jocn.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang AJ, Huang KE, Page BR, et al. Risk factors for leptomeningeal carcinomatosis in patients with brain metastases who have previously undergone stereotactic radiosurgery. J Neuro Oncol. 2014;120(1):163–169. doi: 10.1007/s11060-014-1539-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown DA, Lu VM, Himes BT, et al. Breast brain metastases are associated with increased risk of leptomeningeal disease after stereotactic radiosurgery: a systematic review and meta-analysis. Clin Exp Metastasis. 2020;37(2):341–352. doi: 10.1007/s10585-020-10019-1. [DOI] [PubMed] [Google Scholar]
- 22.Kosmas C, Malamos NA, Tsavaris NB, et al. Isolated leptomeningeal carcinomatosis (carcinomatous meningitis) after taxane-induced major remission in patients with advanced breast cancer. Oncology. 2020;63(1):6–15. doi: 10.1159/000065714. [DOI] [PubMed] [Google Scholar]
- 23.Jo JC, Kang MJ, Kim JE, et al. Clinical features and outcome of leptomeningeal metastasis in patients with breast cancer: a single center experience. Cancer Chemother Pharmacol. 2013;72(1):201–207. doi: 10.1007/s00280-013-2185-y. [DOI] [PubMed] [Google Scholar]
- 24.Berx G, Cleton-Jansen AN, Nollet F, et al. E-cadherin is a tumour/invasion suppressor gene mutated in human lobular breast cancers. EMBO J. 1995;14:6107–6115. doi: 10.1002/j.1460-2075.1995.tb00301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vos C, Cleton-Jansen AN, Berx G, et al. E-cadherin inactivation in lobular carcinoma in situ of the breast: an early event in tumorigenesis. Br J Cancer. 1997;76:1131–1133. doi: 10.1038/bjc.1997.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teo K, Gómez-Cuadrado L, Tenhagen M, et al. E-cadherin loss induces targetable autocrine activation of growth factor signalling in lobular breast cancer. Sci Rep. 2018;8(1):15454. doi: 10.1038/s41598-018-33525-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corso G, Pravettoni G, Galimberti V, et al. Clinical implication of E-cadherin deficiency in lobular breast cancer. Breast Cancer Res Treat. 2019;173(3):751–752. doi: 10.1007/s10549-018-5051-0. [DOI] [PubMed] [Google Scholar]
- 28.Boire A, Zou Y, Shieh J, et al. Complement component 3 adapts the cerebrospinal fluid for leptomeningeal metastasis. Cell. 2017;168(6):1101–1113.e13. doi: 10.1016/j.cell.2017.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cordone I, Masi S, Summa V, et al. Overexpression of syndecan-1, MUC-1, and putative stem cell markers in breast cancer leptomeningeal metastasis: a cerebrospinal fluid flow. Breast Cancer Res. 2017;19(1):46. doi: 10.1186/s13058-017-0827-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le Rhun E, Weller M, Brandsma D, et al. EANO–ESMO Clinical Practice Guidelines for diagnosis, treatment, and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol. 2017;28:iv84–iv99. doi: 10.1093/annonc/mdx221. [DOI] [PubMed] [Google Scholar]
- 31.Pan Z, Yang G, He H, et al. Leptomeningeal metastasis from solid tumors: clinical features and its diagnostic implication. Sci Rep. 2018;8(1):10445. doi: 10.1038/s41598-018-28662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Rhun E, Galanis E. Leptomeningeal metastases of solid cancer. Curr Opin Neurol. 2016;29(6):797–805. doi: 10.1097/WCO.0000000000000393. [DOI] [PubMed] [Google Scholar]
- 33.Figura N, Victoria VT, Armaghani AJ, et al. Breast leptomeningeal disease: a review of current practices and updates on management. Breast Cancer Res Treat. 2019;177(2):277–294. doi: 10.1007/s10549-019-05317-6. [DOI] [PubMed] [Google Scholar]
- 34.Le Rhun E, Rudà R, Devos P, et al. Diagnosis and treatment patterns for patients with leptomeningeal metastasis from solid tumors across Europe. J NeuroOncol. 2017;133(2):419–427. doi: 10.1007/s11060-017-2452-6. [DOI] [PubMed] [Google Scholar]
- 35.Bier G, Klumpp B, Roder C, et al. Meningeal enhancement depicted by magnetic resonance imaging in tumor patients: neoplastic meningitis or therapy-related enhancement? Neuroradiology. 2019;61(7):775–782. doi: 10.1007/s00234-019-02215-y. [DOI] [PubMed] [Google Scholar]
- 36.Mayinger M, Reibelt A, Borm KJ, et al. MRI based neuroanatomical segmentation in breast cancer patients: leptomeningeal carcinomatosis vs. oligometastatic brain disease vs. multimetastastic brain disease. Radiat Oncol. 2019;14(1):170. doi: 10.1186/s13014-019-1380-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao R, Hoda SA, Marcus A, et al. Metastatic breast carcinoma in cerebrospinal fluid: a cytopathological review of 15 cases. Breast J. 2017;23(4):456–460. doi: 10.1111/tbj.12766. [DOI] [PubMed] [Google Scholar]
- 38.Mack F, Baumert BG, Schäfer N, et al. Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat Rev. 2016;3:83–91. doi: 10.1016/j.ctrv.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Went PT, Lugli A, Meier S, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35(1):122–128. doi: 10.1016/j.humpath.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 40.Le Rhun E, Massin F, Tu Q, et al. Development of a new method for identification and quantification in cerebrospinal fluid of malignant cells from breast carcinoma leptomeningeal metastasis. BMC Clin Pathol. 2012;12:21. doi: 10.1186/1472-6890-12-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin X, Fleisher M, Rosenblum M, et al. Cerebrospinal fluid circulating tumor cells: a novel tool to diagnose leptomeningeal metastases from epithelial tumors. Neuro Oncol. 2017;19(9):1248–1254. doi: 10.1093/neuonc/nox066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee JS, Melisko ME, Magbanua MJM, et al. Detection of cerebrospinal fluid tumor cells and its clinical relevance in leptomeningeal metastasis of breast cancer. Breast Cancer Res Treat. 2015;154(2):339–349. doi: 10.1007/s10549-015-3610-1. [DOI] [PubMed] [Google Scholar]
- 43.Torre M, Lee EQ, Chukwueke UN, et al. Integration of rare cell capture technology into cytologic evaluation of cerebrospinal fluid specimens from patients with solid tumors and suspected leptomeningeal metastasis. J Am Soc Cytopathol. 2020;9(1):45–54. doi: 10.1016/j.jasc.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Zhang Y, Ding J, et al. Clinical significance of detecting CSF-derived tumor cells in breast cancer patients with leptomeningeal metastasis. Oncotarget. 2018;9(2):2705–2714. doi: 10.18632/oncotarget.23597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Magbanua MJ, Melisko M, Roy R, et al. Molecular profiling of tumor cells in cerebrospinal fluid and matched primary tumors from metastatic breast cancer patients with leptomeningeal carcinomatosis. Cancer Res. 2013;73(23):7134–7143. doi: 10.1158/0008-5472.CAN-13-2051. [DOI] [PubMed] [Google Scholar]
- 46.Park IH, Kwon Y, Ro JY, et al. Concordant HER2 status between metastatic breast cancer cells in CSF and primary breast cancer tissue. Breast Cancer Res Treat. 2010;123(1):125–128. doi: 10.1007/s10549-009-0627-3. [DOI] [PubMed] [Google Scholar]
- 47.Yu J, Dong Y, Yu J, et al. Longitudinal monitoring of cerebrospinal fluid-derived circulating tumor cells: a pilot study. Int J Clin Exp Pathol. 2018;11(10):5002–5007. [PMC free article] [PubMed] [Google Scholar]
- 48.Angus L, Deger T, Jager A, et al. Detection of aneuploidy in cerebrospinal fluid from patients with breast cancer can improve diagnosis of leptomeningeal metastases. Clin Cancer Res. 2021;27(10):2798–2806. doi: 10.1158/1078-0432.CCR-20-3954. [DOI] [PubMed] [Google Scholar]
- 49.Kenyon SM, Flieth TL, Algeciras-Schimnich A, et al. Comparing the performance of CA 15–3 CSF to cytology in a cohort of patients with breast cancer leptomeningeal metastasis. Clin Biochem. 2018;58:122–124. doi: 10.1016/j.clinbiochem.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 50.Ishibashi N, Maebayashi T, Aizawa T, et al. Serum tumor marker levels at the development of intracranial metastasis in patients with lung or breast cancer. J Thorac Dis. 2019;11(5):1765–1771. doi: 10.21037/jtd.2019.05.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Herrlinger U, Wiendl H, Renninger M, et al. Vascular endothelial growth factor (VEGF) in leptomeningeal metastasis: diagnostic and prognostic value. Br J Cancer. 2004;91(2):219–224. doi: 10.1038/sj.bjc.6601953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Van de Langerijt B, Gijtenbeek JM, De Reus HPM, et al. CSF levels of growth factors and plasminogen activators in leptomeningeal metastases. Neurology. 2006;67(1):114–119. doi: 10.1212/01.wnl.0000223348.42106.97. [DOI] [PubMed] [Google Scholar]
- 53.Dekker LJ, Boogerd W, Stockhammer G, et al. MALDI-TOF mass spectrometry analysis of cerebrospinal fluid tryptic peptide profiles to diagnose leptomeningeal metastases in patients with breast cancer. Moll Cell Proteomics. 2005;4(9):1341–1349. doi: 10.1074/mcp.M500081-MCP200. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida S, Morii K. Intratechal chemotherapy for patients with meningeal carcinomatosis. Surg Neurol. 2005;63(1):52–55. doi: 10.1016/j.surneu.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 55.Niwińska A, Rudnicka H, Murawska M. Breast cancer leptomeningeal metastasis: the results of combined treatment and the comparison of methotrexate and liposomal cytarabine as intra-cerebrospinal fluid. Clin Breast Cancer. 2015;15(1):66–72. doi: 10.1016/j.clbc.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 56.Wu PF, Lin CH, Kuo CH, et al. A pilot study of bevacizumab combined with etoposide and cisplatin in breast cancer patients with leptomeningeal carcinomatosis. BMC Cancer. 2015;15:299. doi: 10.1186/s12885-015-1290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tetef M, Margolin K, Doroshow J, et al. Pharmacokinetics and toxicity of high-dose intravenous methotrexate in the treatment of leptomeningeal carcinomatosis. Cancer Chemother Pharmacol. 2000;46(1):19–26. doi: 10.1007/s002800000118. [DOI] [PubMed] [Google Scholar]
- 58.Bonneau C, Paintaud G, Trédan O, et al. Phase I feasibility study for intrathecal administration of trastuzumab in patients with HER2 positive breast carcinomatous meningitis. Eur J Cancer. 2018;95:75–84. doi: 10.1016/j.ejca.2018.02.032. [DOI] [PubMed] [Google Scholar]
- 59.Morikawa A, de Stanchina E, Pentsova E, et al. Phase I study of intermittent high-dose lapatinib alternating with capecitabine for HER2-positive breast cancer patients with central nervous system metastases. Clin Cancer Res. 2019;25(13):3784–3792. doi: 10.1158/1078-0432.CCR-18-3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Orlando L, Curigliano G, Colleoni M, et al. Intrathecal chemotherapy in carcinomatous meningitis from breast cancer. Anticancer Res. 2002;22(5):3057–3059. [PubMed] [Google Scholar]
- 61.Pan Z, Yang G, He H, et al. Concurrent radiotherapy and intrathecal methotrexate for treating leptomeningeal metastasis from solid tumors with adverse prognostic factors: a prospective and single-arm study. Int J Cancer. 2016;139(8):1864–1872. doi: 10.1002/ijc.30214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mrugala M, Kim B, Sharma A, et al. Phase II study of systemic high-dose methotrexate and intrathecal liposomal cytarabine for treatment of leptomeningeal carcinomatosis from breast cancer. Clin Breast Cancer. 2019;19(5):311–316. doi: 10.1016/j.clbc.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 63.Brastianos PK, Lee EQ, Cohen JV, et al. Single-arm, open-label phase 2 trial of pembrolizumab in patients with leptomeningeal carcinomatosis. Nat Med. 2020;26(8):1280–1284. doi: 10.1038/s41591-020-0918-0. [DOI] [PubMed] [Google Scholar]
- 64.Kumthekar P, Tang SC, Brenner AJ, et al. ANG1005, a brain-penetrating peptide-drug conjugate, shows activity in patients with breast cancer with leptomeningeal carcinomatosis and recurrent brain metastases. Clin Cancer Res. 2020;26(12):2789–2799. doi: 10.1158/1078-0432.CCR-19-3258. [DOI] [PubMed] [Google Scholar]
- 65.Boogerd W, van den Bent MJ, Koehler PJ, et al. The relevance of intraventricular chemotherapy for leptomeningeal metastasis in breast cancer: a randomised study. Eur J Cancer. 2004;40(18):2726–2733. doi: 10.1016/j.ejca.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 66.Le Rhun E, Wallet J, Mailliez A, et al. Intrathecal liposomal cytarabine plus systemic therapy versus systemic chemotherapy alone for newly diagnosed leptomeningeal metastasis from breast cancer. Neuro Oncol. 2020;22(4):524–538. doi: 10.1093/neuonc/noz201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kim DY, Lee KW, Yun T, et al. Comparison of intrathecal chemotherapy for leptomeningeal carcinomatosis of a solid tumor: methotrexate alone versus methotrexate in combination with cytosine arabinoside and hydrocortisone. Jpn J Clin Oncol. 2003;33(12):608–612. doi: 10.1093/jjco/hyg118. [DOI] [PubMed] [Google Scholar]
- 68.Lassman AB, Abrey LE, Shah GG, et al. Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol. 2006;78(3):255–260. doi: 10.1007/s11060-005-9044-6. [DOI] [PubMed] [Google Scholar]
- 69.Ekenel M, Hormigo AM, Peak S, et al. Capecitabine therapy of central nervous system metastases from breast cancer. J Neurooncol. 2007;85(2):223–227. doi: 10.1007/s11060-007-9409-0. [DOI] [PubMed] [Google Scholar]
- 70.Rudnicka H, Niwińska A, Murawska M. Breast cancer leptomeningeal metastasis—the role of multimodality treatment. J Neurooncol. 2007;84(1):57–62. doi: 10.1007/s11060-007-9340-4. [DOI] [PubMed] [Google Scholar]
- 71.Kiewe P, Thiel E, Reinwald M, et al. Topotecan and ifosfamide systemic chemotherapy for CNS involvement of solid tumors. J Neurooncol. 2011;103(3):629–634. doi: 10.1007/s11060-010-0434-z. [DOI] [PubMed] [Google Scholar]
- 72.Le Rhun E, Taillibert S, Zairi F, et al. A retrospective case series of 103 consecutive patients with leptomeningeal metastasis and breast cancer. J Neurooncol. 2013;113(1):83–92. doi: 10.1007/s11060-013-1092-8. [DOI] [PubMed] [Google Scholar]
- 73.Comte A, Jdid W, Guilhaume MN, et al. Survival of breast cancer patients with meningeal carcinomatosis treated by intrathecal thiotepa. J Neurooncol. 2013;115(3):445–452. doi: 10.1007/s11060-013-1244-x. [DOI] [PubMed] [Google Scholar]
- 74.Scott BJ, van Vugt VA, Rush T, et al. Concurrent intrathecal methotrexate and liposomal cytarabine for leptomeningeal metastasis from solid tumors: a retrospective cohort study. J Neurooncol. 2014;119(2):361–368. doi: 10.1007/s11060-014-1486-2. [DOI] [PubMed] [Google Scholar]
- 75.Bergen ES, Berghoff A, Medjedovic M, et al. Continued endocrine therapy is associated with improved survival in patients with breast cancer brain metastases. Clin Cancer Res. 2019;25(9):2737–2744. doi: 10.1158/1078-0432.CCR-18-1968. [DOI] [PubMed] [Google Scholar]
- 76.Chen TWW, Jan Is, Chang DY, et al. Systemic treatment of breast cancer with leptomeningeal metastases using bevacizumab, etoposide and cisplatin (BEEP regimen) significantly improves overall survival. J Neurooncol. 2020;148(1):165–172. doi: 10.1007/s11060-020-03510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Srinivasalu VK, Subramaniam N, Philip A, et al. Triple intrathecal chemotherapy for leptomeningeal carcinomatosis in solid tumors: treatment outcomes, response and their determinants. Indian J Cancer. 2021;58(1):84–90. doi: 10.4103/ijc.IJC_730_18. [DOI] [PubMed] [Google Scholar]
- 78.Carausu M, Carton M, Darlix A, et al. Breast cancer patients treated with intrathecal therapy for leptomeningeal metastases in a large real-life database. ESMO Open. 2021;6(3):100150. doi: 10.1016/j.esmoop.2021.100150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zagouri F, Sergentanis TN, Bartsch R, et al. Intrathecal administration of trastuzumab for the treatment of meningeal carcinomatosis in HER2-positive metastatic breast cancer: a systematic review and pooled analysis. Breast Cancer Res Treat. 2013;139(1):13–22. doi: 10.1007/s10549-013-2525-y. [DOI] [PubMed] [Google Scholar]
- 80.Dudani S, Mazzarello S, Hilton J, et al. Optimal management of leptomeningeal carcinomatosis in breast cancer patients – a systematic review. Clin Breast Cancer. 2016;16(6):456–470. doi: 10.1016/j.clbc.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 81.Lee YC, Hsiech CC, Chuang JP, et al. The necessity of intrathecal chemotherapy for the treatment of breast cancer patients with leptomeningeal metastasis: a systematic review and pooled analysis. Curr Probl Cancer. 2017;41(5):355–370. doi: 10.1016/j.currproblcancer.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 82.Thomas KH, Ramirez RA. Leptomeningeal disease and the evolving role of molecular targeted therapy and immunotherapy. Ochsner J. 2017;17(4):362–378. [PMC free article] [PubMed] [Google Scholar]
- 83.Zagouri F, Zoumpourlis P, Le Rhun E, et al. Intrathecal administration of anti-HER2 treatment for the treatment of meningeal carcinomatosis in breast cancer: a metanalysis with meta-regression. Cancer Treat Rev. 2020;88:102046. doi: 10.1016/j.ctrv.2020.102046. [DOI] [PubMed] [Google Scholar]
- 84.Gani C, Müller A, Eckert F, et al. Outcome after whole brain radiotherapy alone in intracranial leptomeningeal carcinomatosis from solid tumors. Strahlenther Onkol. 2012;188(2):148–153. doi: 10.1007/s00066-011-0025-8. [DOI] [PubMed] [Google Scholar]
- 85.El Shafie R, Bohm K, Weber D, et al. Palliative radiotherapy for leptomeningeal carcinomatosis-analysis of outcome, prognostic factors, and symptom response. Front Oncol. 2019;8:641. doi: 10.3389/fonc.2018.00641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wolf A, Donahue B, Silverman JS, et al. Stereotactic radiosurgery for focal leptomeningeal disease in patients with brain metastases. J Neurooncol. 2017;134(1):139–143. doi: 10.1007/s11060-017-2497-6. [DOI] [PubMed] [Google Scholar]
- 87.Hermann B, Hültenschmidt B, Sautter-Bihl ML. Radiotherapy of the neuroaxis for palliative treatment of leptomeningeal carcinomatosis. Strahlenther Onkol. 2001;177(4):195–199. doi: 10.1007/PL00002398. [DOI] [PubMed] [Google Scholar]
- 88.Okada Y, Aba T, Shinozaki M, et al. Evaluation of imaging findings and prognostic factors after whole-brain radiotherapy for carcinomatous meningitis from breast cancer: a retrospective analysis. Medicine. 2020;99(31):e21333. doi: 10.1097/MD.0000000000021333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Devecka M, Duma MN, Wilkens JJ, et al. Craniospinal irradiation (CSI) in patients with leptomeningeal metastases: risk-benefit-profile and development of a prognostic score for decision making in the palliative setting. BMC Cancer. 2020;20(1):501. doi: 10.1186/s12885-020-06984-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Omuro AMP, Lallana E, Bilsky MH, et al. Ventriculoperitoneal shunt in patients with leptomeningeal metastasis. Neurology. 2005;64(9):1625–1627. doi: 10.1212/01.WNL.0000160396.69050.DC. [DOI] [PubMed] [Google Scholar]
- 91.Lin N, Dunn IF, Glantz M, et al. Benefit of ventriculoperitoneal cerebrospinal fluid shunting and intrathecal chemotherapy in neoplastic meningitis: a retrospective, case-controlled study. J Neurosurg. 2011;115(4):730–736. doi: 10.3171/2011.5.JNS101768. [DOI] [PubMed] [Google Scholar]
- 92.Jung TY, Chung WK, Oh IJ. The prognostic significance of surgically treated hydrocephalus inleptomeningeal metastases. Clin Neurol Neurosurg. 2014;119:80–83. doi: 10.1016/j.clineuro.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 93.Morikawa A, Jordan L, Rozner R, et al. Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin Breast Cancer. 2017;17(1):23–28. doi: 10.1016/j.clbc.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lara-Medina F, Crismatt A, Villarreal-Garza C, et al. Clinical features and prognostic factors in patients with carcinomatous meningitis secondary to breast cancer. Breast J. 2012;18(3):233–241. doi: 10.1111/j.1524-4741.2012.01228.x. [DOI] [PubMed] [Google Scholar]
- 95.Regierer AC, Stroux A, Kühnhardt D, et al. Contrast-enhancing meningeal lesions are associated with longer survival in breast cancer-related leptomeningeal metastasis. Breast Care. 2008;3(2):118–123. doi: 10.1159/000121688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Niwińska A, Pogoda K, Michalski W, et al. Determinants of prolonged survival for breast cancer patient groups with leptomeningeal metastasis. J Neurooncol. 2018;138(1):191–198. doi: 10.1007/s11060-018-2790-z. [DOI] [PubMed] [Google Scholar]
- 97.De Azevedo CRAS, Cruz MRS, Chinen LTD, et al. Meningeal carcinomatosis in breast cancer: prognostic factors and outcome. J Neurooncol. 2011;104(2):565–572. doi: 10.1007/s11060-010-0524-y. [DOI] [PubMed] [Google Scholar]
- 98.Meattini I, Livi L, Saieva C, et al. Prognostic factors and clinical features in patients with leptominengeal metastases from breast cancer: a single center experience. J Chemother. 2012;24(5):279–284. doi: 10.1179/1973947812Y.0000000034. [DOI] [PubMed] [Google Scholar]
- 99.Kingston B, Kayhanian H, Brooks C, et al. Treatment and prognosis of leptomeningeal disease secondary to metastatic breast cancer: a single-centre experience. Breast. 2017;36:54–59. doi: 10.1016/j.breast.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 100.Scott BJ, Kesari S. Leptomeningeal metastases in breast cancer. Am J Cancer Res. 2013;3(2):117–126. [PMC free article] [PubMed] [Google Scholar]
- 101.Watanabe J, Mitsuya K, Nakamoto S, et al. Leptomeningeal metastasis in ER+ HER2− advanced breast cancer patients: a review of the cases in a single institute over a 15-year period. Breast Cancer Res Treat. 2021;189(1):225–236. doi: 10.1007/s10549-021-06246-z. [DOI] [PubMed] [Google Scholar]
- 102.Le Rhun E, Devos P, Weller J, et al. Prognostic validation and clinical implications of the EANO ESMO classification of leptomeningeal metastasis from solid tumors. Neuro Oncol. 2021;23(7):1100–1112. doi: 10.1093/neuonc/noaa298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Clatot F, Philippin-Lauridant G, Ouvrier MJ, et al. Clinical improvement and survival in breast cancer leptomeningeal metastasis correlate with the cytologic response to intrathecal chemotherapy. J Neurooncol. 2009;95(3):421–426. doi: 10.1007/s11060-009-9940-2. [DOI] [PubMed] [Google Scholar]
- 104.Duchnowska R, Pęksa R, Radecka B, et al. Immune response in breast cancer brain metastases and their microenvironment: the role of the PD-1/PD-L axis. Breast Cancer Res. 2016;18(1):43. doi: 10.1186/s13058-016-0702-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]