Abstract
While Fibro-Adipogenic Progenitors (FAPs) have been originally identified as muscle-interstitial mesenchymal cells activated in response to muscle injury and endowed with inducible fibrogenic and adipogenic potential, subsequent studies have expanded their phenotypic and functional repertoire and revealed their contribution to skeletal muscle response to a vast range of perturbations. Here we review the emerging contribution of FAPs to skeletal muscle responses to motor neuron injuries and to systemic physiological (e.g., exercise) or pathological metabolic (e.g., diabetes) perturbations. We also provide an initial blueprint of discrete sub-clusters of FAPs that are activated by specific perturbations and discuss their role in muscle adaptation to these conditions.
Keywords: FAPs, Skeletal Muscle, Denervation, Neuromuscular Junctions, Diabetes, Exercise
Muscle-resident cell activation by homeostatic perturbations of skeletal muscles
In vertebrate animals, skeletal muscles support essential functions, such as locomotion, maintenance of posture, breathing, control of body temperature and metabolism. Both contractile units (myofibers) and non-contractile cellular (muscle-resident cells) and extra-cellular (the extra-cellular matrix – ECM) components of skeletal muscles contribute to these functions. Thus, maintenance of anatomical and functional integrity of skeletal muscles is required for long-term health of vertebrates. In turn, skeletal muscles are subjected to continuous homeostatic perturbations, ranging from systemic signals released by other tissues and organs to local injuries of structural and cellular components. Skeletal muscle responses to homeostatic perturbations typically entail well-coordinated processes that require functional interactions between cellular and non-cellular components. Repair of physical injuries to myofibers has been the most studied process, whereby successful regeneration of damaged myofibers is capable of restoring the original tissue architecture and contractile activity. Although a successful skeletal muscle regeneration is ultimately mediated by muscle stem cells (MuSCs, also known as muscle satellite cells) [1], it also requires the concerted action of other muscle-resident cells, such as Fibro-Adipogenic Progenitors (FAPs), fibroblasts, resident macrophages, pericytes and endothelial cells, as well as the inflammatory infiltrate [2–8]. The recent advent of single cell transcriptomics has revealed additional potential sub-populations of FAPs [9] and identified other muscle resident cell types, such as tenocytes/myotenocytes, glial/Schwann cells, and smooth muscle mesenchymal cells (SMMCs) [10–17]. Reciprocal interactions between muscle-resident cells control timing and magnitude of their activation for optimal execution of the regeneration process. This is well illustrated in the typical experimental models of acute muscle damage by physical injury (myotrauma), whereby the sequential and overlapping waves of activation of muscle resident cells is followed by their clearance, upon successful myofiber regeneration, in order to restore the original size and proportion of these cells. However, upon repeated cycles of injuries, such as contraction-mediated degeneration/regeneration of dystrophic muscles, loss of coordination between muscle-resident cell interactions leads to an aberrant accumulation of pathogenic cell types that promote compensatory repair of damaged muscle by deposition of non-contractile fibrotic and adipose tissues, instead of new contractile myofibers [2,18–20].
Among muscle resident cells, FAPs have emerged as central component of the cellular network that maintains muscle homeostasis. FAPs have been initially identified as muscle interstitial mesenchymal cells that express Sca1 and Platelet-Derived Growth Factor (PDGF) receptor alpha (PDGFRα) and are endowed with the potential to adopt either a fibrogenic or adipogenic potential, when exposed to specific culture conditions, and to enhance the myogenic potential of MuSCs [21,22]. Further works demonstrated that, in response to acute myotrauma, FAPs activation is regulated by functional interactions with cells of the inflammatory infiltrate and possibly other muscle-resident cells, in order to achieve a transient expansion. This is followed by their clearance to return to the original size of quiescent FAPs [23]. Failure to clear injury-activated FAPs in regenerating muscles leads to the formation of ectopic fat and fibrosis [24] – an outcome observed in most muscular dystrophies and other chronic muscular diseases. Indeed, FAPs have been shown to aberrantly accumulate in muscles of patients or mouse models of Duchenne Muscular Dystrophy (DMD), as well as other forms of muscular dystrophies, and targeting pathogenic populations of FAPs is a current strategy for pharmacological treatment of muscular dystrophies [9,25–28]. Conversely, inducible depletion of FAPs using a genetically modified mouse model – PdgfraCreER; R26RDTA demonstrated the essential role of FAPs in maintaining MuSCs pool and in facilitating injury-induced muscle regeneration in healthy (wild type) mice [29]. Overall, these data are consistent with a model whereby timely activation of FAPs warrants transient deposition of collagens and possibly other ECM components for optimal regeneration of injured muscles. Because FAPs-mediated fibrosis is regulated by reciprocal signals exchanged between FAPs and other cell types [30–32], control of spatial and temporal interplay between muscle-resident cells appears a major determinant of whether FAPs adopt physiological and transient dynamics of cellular states versus constitutive pathological phenotypes [9].
The ability to fully repair injured muscle emphasizes the great regenerative potential of skeletal muscles and indicates that, in addition to MuSCs-mediated formation of new myofibers, other individual components of skeletal muscles – such as, vasculature, neuro-muscular junctions (NMJ), the myotendinous junctions (MTJ), and the interstitial ECM – are also recovered. Conceivably, full repair of these structures requires specific cell progenitors and types of interactions among muscle-resident cells, different from those that are required for MuSCs-mediated regeneration of myofibers [33–38]. Selective damage of some of these structures can occur without a concomitant myotrauma in specific conditions, such as motor neuron damage (ranging from complete denervation to reversible nerve crush or various forms of progressive neuromuscular diseases) and systemic perturbations (including physical exercise and pathogenic metabolic conditions, such as diabetes).
Here we will review the emerging role of FAPs, as sensors of systemic and local perturbations of skeletal muscle homeostasis that affect the integrity of NMJ and ECM, without a primary myofiber lesion, and their contribution to the physiological or pathological recovery of these anatomical structures of skeletal muscles. Interestingly, the mild inflammatory infiltrate and modest activation of MuSCs observed in muscle perturbations that do not include acute myotrauma, predict that FAPs might establish different types of functional interactions with other muscle-resident cells, in order to promote responses specific to the type of perturbation – i.e., ECM remodeling for repair/maintenance of NMJ and MTJ.
Role of FAPs in skeletal muscle adaptation to motor neuron injury
As an integral component of skeletal muscles, the NMJ is invariably affected by the large majority of skeletal muscle perturbations, including acute injury induced by toxins/chemicals (e.g., cardiotoxin – CTX, notexin – NTX, barium chloride – BaCl2) or by physical damage (e.g., freeze, laceration, denervation), and chronic injury (e.g., muscular dystrophy, aging, diabetes). The identity, extent and magnitude of activation of muscle-resident cell types in response to these different types of perturbations might vary accordingly, in order to restore the integrity and functionality of neuromuscular activity [34,39–41]. While loss of integrity of NMJ can be completely restored when it occurs within the context of reversible injuries, NMJ repair cannot be accomplished in case of acute denervation, such as complete nerve transection by spinal cord injury, or during progression of neuromuscular disorders, such as ALS, possibly because of lack of survival signals and growth factors from permanently damaged motor neurons [42]. Skeletal muscle adaptation to conditions of irreversible repair of NMJ includes myofiber atrophy and changes of ECM components leading to deposition of ectopic fibrotic and adipose tissues. However, the relationship between NMJ, activation of FAPs and other muscle-resident cells in the final outcome of repair of different types of muscle nerve lesions has only begun to be investigated in the last few years.
Structure and function of the NMJ
Control of skeletal muscle by the nervous system relies on a synaptic structure – the NMJ – which consists of a presynaptic nerve terminal and a postsynaptic endplate on the myofiber plasma membrane. The NMJ formation and maturation is finely regulated by motor neurons and myofibers during the embryonic and postnatal development, with the ultimate outcome that each myofiber is innervated by one motor neuron [42,43]. A number of molecular players implicated in NMJ formation and maintenance have been identified. For instance, Neuregulin 1 (Nrg1)/ErbB3/ErbB2, the agrin/low-density lipoprotein receptor-related protein 4 (Lrp4)/muscle specific kinase (MuSK) signaling, and the muscle expressed Yes-associated protein (Yap)/β-catenin signaling, all play essential roles in regulating the formation, maturation, regeneration, and/or function of the NMJ [42,44,45]. Neurotransmitters released from the presynaptic nerve terminal across the synaptic cleft, a ~50nm space between pre- and post-synaptic structures, bind their receptors on the post-synaptic side to trigger the skeletal muscle contraction [44]. A major component of the NMJ on the myofiber membrane is indeed the family of acetylcholine receptors (AChRs), whose morphology can be easily detected due to its strong binding affinity to bungarotoxin (BTX) and which is widely used as an indicator of NMJ post-synaptic integrity [46]. Loss of NMJ occurs via Wallerian degeneration of the nerve (i.e., degeneration of the axon distal to the nerve injury), gradually resulting in muscle atrophy and further body paralysis, which are common consequences of major nerve injuries and neurodegenerative diseases such as Amyotrophic Lateral Sclerosis (ALS) and Spinal Muscular Atrophy (SMA) [34,44]. When the nerve injury is not severe, such as nerve crush, degenerated motor neuron’s axons have the potential to grow back to innervate again the denervated myofibers [34,47]. Typically, in response to sciatic nerve crush, disrupted NMJ are reestablished through an initial partial reinnervation (at 2 weeks post-injury), followed by hyperinnervation at 3 and 4 weeks, and restoration of a 1:1 axon to motor endplate relationship at 6 weeks post-injury [48]. This process is regarded as reversible muscle nerve injury, while severe nerve injuries, such as complete nerve transection or repeated nerve crush, cause irreversible impairment of functional recovery of NMJ [49]. In the latter case, long-term loss of NMJ leads to the functional impairment of myofiber contractile activity and progressive muscle atrophy, accompanied by ectopic formation of fat tissue and fibrotic scars [50,51].
Schwann cells are fundamental cellular components of NMJ. About 1~3 Schwann cells that uniquely locate at the peri-synaptic area of NMJ are named Peri-synaptic Schwann Cells (PSCs, also known as terminal Schwann Cells). Interestingly, PSCs not only participate in the maturation of the NMJ during development, but also sustain the growth of degenerated nerve axons, via trophic factors, and guide them to re-establish contacts with the denervated myofibers [34,44,47]. Although it is known that Schwann cells participate in NMJ repair post-injury, and recent works have revealed potential interplays between muscle glial cells, MuSCs and other muscle-resident cell types in NMJ recovery following motor neuron injury [16,17,52], it is currently unclear the precise origin, identity and functions of muscle-resident cells implicated in the process of muscle nerve repair post-injury.
FAPs’ activation by loss of integrity of NMJ upon motor neuron injury
Initial evidence that FAPs are activated by motor neuron injury and could contribute to myofiber atrophy and fibrosis was provided by Gonzalez and colleagues, using a mouse model of ALS – SODG93A, in which progressive motor neuron degeneration and atrophy leads to loss of structural and functional integrity of NMJ [53]. The authors reported on the association between symptomatic stages of ALS, higher abundance in fibrotic composition of ECM (e.g., increased amounts of fibronectin and collagen III) and increased frequency of interstitial mesenchymal cells exhibiting identity markers of FAPs (PDGFRα and TCF4) [53]. Further studies performed in a model of irreversible denervation by complete sciatic nerve transection showed that FAPs undergo activation following denervation [50]. Compared to FAPs activated by acute injury (INJ-FAPs), FAPs from denervated muscle (DEN-FAPs) exhibited a lower proliferation rate that was however sustained even after 30 days post denervation. Consistently, increased number of FAPs was also observed in additional models of skeletal muscle denervation, either by acute spinal cord injury (SCI) or by progressive motor neuron degeneration and atrophy, such as the SODG93A mice, as well as in skeletal muscle sections of ALS patients [50]. Of note, DEN-FAPs exhibited a persistent activation of the Interleukin-6 (IL-6)-STAT3 signaling, which promoted myofiber atrophy and interstitial fibrosis [50]. It is worth noting that DEN-FAPs are activated in the absence of an intense inflammatory infiltrate as well as without concomitant MuSCs activation – two events that are typically observed following acute myotrauma. In response to acute injury, INJ-FAPs are activated, massively expand at 2~4 days post injury (dpi) and return to the basal level at around 7 dpi, possibly through immune-mediated clearance [24]. Within this context, INJ-FAPs exert a transient pro-regenerative role by promoting myogenesis, via secreted factors, including IL-6 [50], WNT family members as well as a variety of cytokines and growth factors [4,9,21]. Clearance of activated FAPs by anti-inflammatory macrophages has been proposed as a key mechanism to prevent aberrant accumulation of pathogenic FAPs [24]. Thus, it is possible that increased amount of DEN-FAPs in denervated muscles is caused by lack of clearance by anti-inflammatory infiltrate. In contrast, it is still unclear how nerve injury activates FAPs and what cues are responsible for FAPs proliferation and differentiation over the course of muscle denervation. Conceivably, signals derived from disrupted NMJ and/or injured motor neurons might activate DEN-FAPs. Likewise, acute lack of inhibitory signals released by intact NMJ could also contribute to the activation of DEN-FAPs. In this regard, it is interesting that, different from the other aforementioned models of denervation, the gradual loss of integrity of NMJ typically associated with aging did not trigger DEN-FAPs activation in geriatric mice [50], thereby suggesting that the type, extent and timing of NMJ loss could also be a key determinant for DEN-FAPs activation and aberrant accumulation.
Interactions with other muscle-resident cell types activated by denervation could also contribute to the activation of DEN-FAPs. Consistently, a recent scRNA-seq analysis of mouse skeletal muscle post-denervation by complete transection of sciatic nerve revealed a selective activation of two specific muscle resident cells – the muscle glial cells and activated fibroblasts [17]. It should be noted that mouse activated fibroblasts partly share, at least to a certain degree, some identity markers with FAPs, including PDGFRα, Sca1, and CD90, and are in close contiguity to FAPs in the UMAP-derived representation of muscle-resident cells in denervated muscles [17]. While these data suggest that activated fibroblasts can potentially derive from FAPs, the relationship between DEN-FAPs and activated fibroblasts post-denervation remains to be elucidated. Interestingly, denervation-activated fibroblasts express fibrogenic factors, but also neurotrophic signals that could regulate muscle glial cells to promote NMJ repair [17]. This is also reminiscent of the dual role of INJ-FAPs as pro-fibrogenic and supportive cells for MuSCs to promote myofiber regeneration. Recent work has established that different sub-populations of FAPs could mediate these distinct effects upon muscle injury [9]. Similarly, following denervation different sub-clusters of FAPs appear to express discrete subsets of genes belonging to functional networks potentially implicated in distinct biological outcomes. Among them, one fraction of FAPs within the cluster of activated FAPs exhibited a persistent increase of Il6 expression (Fig. 1) [17]. These cells also show increased activation of STAT3 signaling components, including Apod and Lif [17]. The persistent activation of IL-6 expression in DEN-FAPs is of particular interest, as it substantially differs from the typical induction of IL-6 post-injury or post-exercise, which is milder and reversible [54]. Future studies should establish whether this difference occurs at epigenetic level – i.e., denervation-activated enhancers different from injury- or exercise-activated enhancers of Il6 gene.
Fig. 1. Versatile responses of FAPs and Activated Fibroblasts to skeletal muscle perturbations.
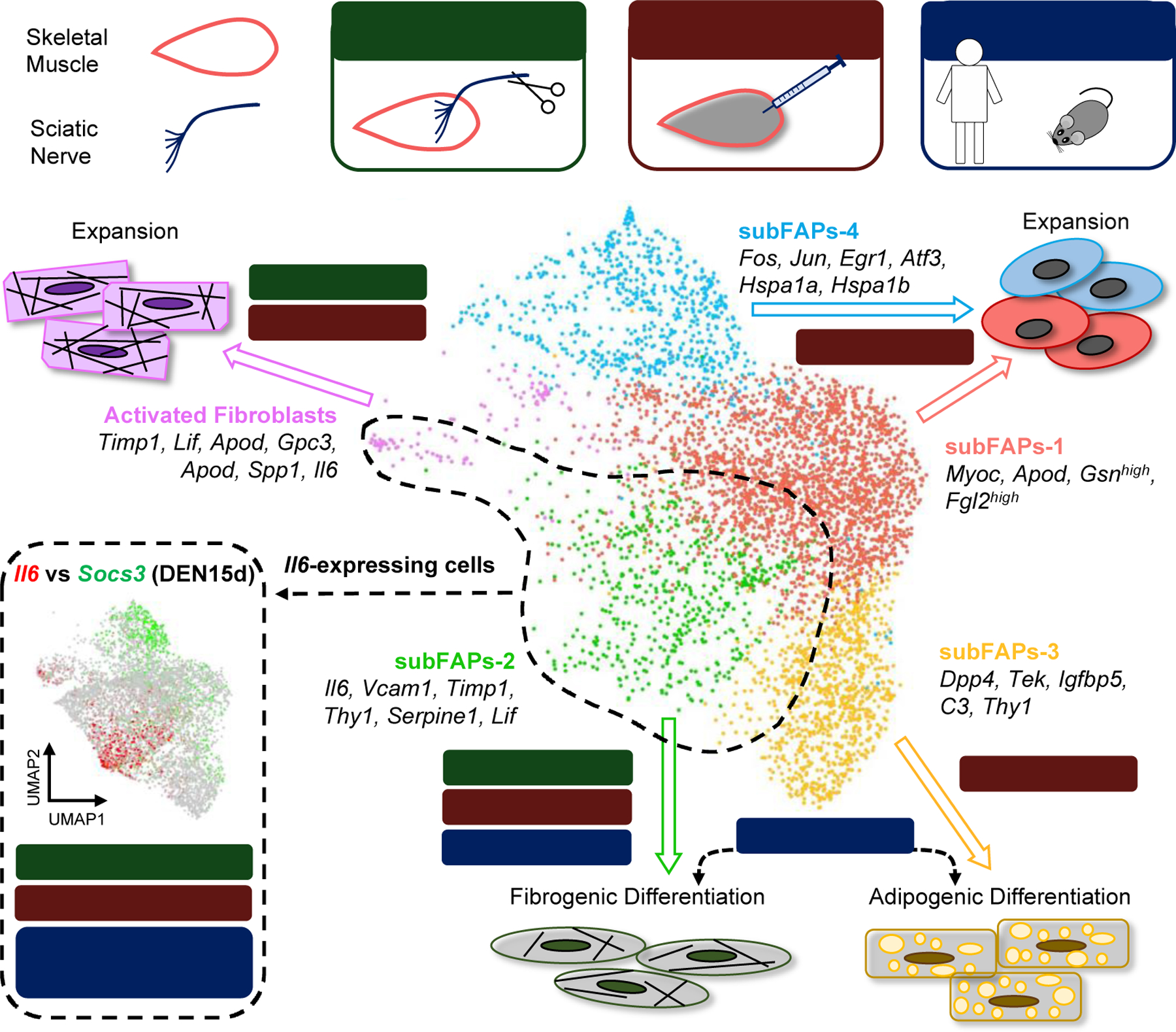
UMAP plot of four FAPs sub-clusters (subFAPs) and Activated Fibroblasts, as identified in unperturbed mouse muscles (tibialis anterior and gastrocnemius) was adapted from Nicoletti et al. [17]. SubFAPs-1, coral; subFAPs-2, green; subFAPs-3, yellow; subFAPs-4, blue; Activated Fibroblasts, violet. Selected marker genes are listed next to each cell cluster. The figure illustrates how different types of perturbations of muscle homeostasis – including acute muscle injury (myotrauma), denervation, neuromuscular diseases, and systemic perturbations (T2DM) - can activate specific subFAPs and Activated Fibroblasts, which expand and adopt different fates, e.g., adipogenic or fibrogenic differentiation. A discrete cluster of IL-6-expressing cells, which anti-correlates with SOCS3 expressing FAPs, is shown at the bottom left corner. IL-6 expressing FAPs belong to a cluster of FAPs (subFAPs-2) that undergoes activation, albeit with distinctive pattern of expansion, in response to different types of muscle perturbation. VCAM1 expressing subFAPs-2 are transiently activated upon myotrauma, while subFAPs-2, exhibiting constitutive activation of IL6-STAT3 signaling (partly overlapping with VCAM1 expression), gradually expands in response to irreversible denervation (e.g., post sciatic nerve transection and SCI or in conditions of progressive degeneration of motor neurons and loss of NMJ integrity – ALS, SMA). SCI: Spinal cord injury; T2DM: Type 2 Diabetes Mellitus; ALS: Amyotrophic Lateral Sclerosis; SMA: Spinal Muscular Atrophy; DEN15d: 15 days post irreversible denervation.
Reciprocal interactions between FAPs, denervated myofibers, other muscle resident cells, ECM and NMJ
The constitutive activation of STAT3 signaling and IL-6 expression detected in DEN-FAPs is in principle at odds with the negative feedback that typically regulates the well-known IL-6-mediated activation of STAT3 signaling, whereby downstream STAT3 targets inhibit IL-6 expression and limit magnitude and duration of the IL-6-STAT3 signaling [55]. In this regard it is interesting to note that the expression of one major component of this feedback – STAT3-activated Suppressor of Cytokine Signaling 3 (SOCS3) [56] – anti-correlated with IL-6 expression in DEN-FAPs [17] (see Fig. 1). This suggests that constitutive activation of IL6-STAT3 signaling might arise in a sub-population of FAPs that escapes a negative feedback that limits IL6-STAT3 activation. Regardless the mechanism that sustains persistent activation of IL6-STAT3 signaling in DEN-FAPs, functional evidence indicates that this signaling is implicated in both denervation-induced myofiber atrophy and fibrosis. Indeed, genetic ablation of STAT3, pharmacological inhibition of STAT3 or antibody-mediated neutralization of IL-6 all invariably reduce myofiber atrophy and muscle fibrosis in denervated muscles [50]. Further studies have shown that denervation-mediated myofiber atrophy requires the integrity of p38 pathway in myofibers [57], suggesting that DEN-FAPs-derived pro-atrophic IL-6 signaling to myofibers could be mediated by p38 kinases. However, whether IL-6-activated p38 signaling is also implicated in denervation-induced pro-fibrotic changes of ECM composition remains unknown. It is likely that DEN-FAPs-derived pro-fibrotic factors bias the ECM composition, but the role of STAT3-IL-6 signaling in the activation of pro-fibrotic genes in DEN-FAPs is currently unclear. In this regard, the interplay between FAPs, activated fibroblasts and other muscle-resident cells in promoting transient vs persistent ECM reconfiguration toward fibrosis for NMJ repair or fibro-degeneration of denervated muscles, respectively, appears of particular interest.
As a fundamental component of many tissues and organs, the highly dynamic ECM and its homeostasis are essential for the basic functions of multicellular organisms [58,59]. Finely regulated ECM remodeling is required for the perturbation-induced tissue repair as the ECM was shown to interact with tissue-resident cells and regulate their adhesion, migration, proliferation and differentiation [60]. Under homeostatic condition, the ECM turnover through proteolysis and synthesis is primarily regulated by fibroblasts, which inhabit most soft connective tissues [58,59]. As mentioned above, in response to perturbations, tissue resident mesenchymal stromal cells, such as FAPs in skeletal muscle, also participate in the ECM remodeling usually by adopting a fibrogenic phenotype [4,5]. However, the aberrant accumulation of fibrogenic FAPs is associated with dysregulated ECM remodeling, resulting in muscle fibrosis, which is not permissive to muscle regeneration and mostly identifies chronic muscle injury conditions and disease progression stages [9,50,61]. The use of Col1a1-GFP transgenic mouse allowed the identification of at least three Collagen 1 (COL1)-expressing cell types, including Integrin-α7-positive myogenic cells and SCA1-positive FAPs, hinting at their ability to remodel the ECM directly [62]. Based on the scRNA-seq datasets from skeletal muscle under homeostatic and injury conditions, FAPs invariably account for a relatively large proportion of muscle-resident cells, which suggests a more robust contribution of FAPs to the remodeling of the ECM compared to other cell types [9–15].
Using the PdgfraCreER; R26RDTA mouse model of inducible depletion of FAPs, Uezumi and colleagues observed defects of the NMJ in skeletal muscle 17 days after FAPs depletion, indicating that FAPs are also important for maintenance of myofiber innervation and NMJ integrity. Within this context, Bone Morphogenetic Protein 3 (Bmp3b) was identified as a potential mediator of functional interactions between NMJ and FAPs. Indeed, Bmp3b knockout mouse muscles displayed a phenotype similar to that of FAPs-depleted muscles. Moreover, addition of BMP3b to cultures of Schwann cells could stabilize their phenotype [63], suggesting that FAP-derived Bmp3b is required for maintenance of NMJ integrity. A close localization of neuronal cells and Interleukin-33 (IL-33)-expressing mesenchymal stromal cells (mSCs) has also been observed in both skeletal muscle and visceral adipose tissue (VAT), further pointing to a potential crosstalk between these two types of cells under homeostasis [64,65]. Moreover, Wang and colleagues showed that a sub-cluster of FAPs in skeletal muscle expresses IL-33 as well as neuropeptides, neuropeptide receptors and nerve-related genes, supporting again a direct interaction potential between FAPs and nerve-associated cells, such as glial/Schwann cells [64]. To verify this possibility, a rigorous characterization of glial/Schwann cells behavior after FAPs depletion in vivo in the same mouse model needs to be performed. Meanwhile, discovering the effect of FAPs on the NMJ regeneration after reversible nerve injury is of great interest. In this regard, it should be noted that ECM composition in NMJ is different than in the interstitial spaces between myofibers [36,66,67], suggesting that FAPs-mediated reconfiguration of ECM could change depending on the type of injury (i.e., nerve lesion vs myotrauma). Thus, it is likely that ECM can adopt an optimal composition to promote the expansion and migration of progenitors of different muscle structures – e.g., muscle-glial cells at NMJ or MuSCs at myofibers. This is also consistent with the specificity of expression pattern of discrete collagen types at NMJ vs interstitial spaces between myofibers [68]. Interestingly, scRNA-seq analysis of denervated muscles revealed that denervation-activated FAPs and fibroblasts express several ECM components and signaling molecules toward ECM reconfiguration [17]. However, further studies are necessary to establish the functional relevance of these signals and their comparative analysis with FAPs- and fibroblast-mediated regulation of ECM composition during myotrauma. Loss of NMJ integrity has been observed also in other conditions. Age-related sarcopenia, as defined by loss of skeletal muscle mass, strength and function, is one of the most representative conditions [69,70]. Consistently, the expression of muscle atrophy-related genes, such as Murf1, is significantly upregulated in the muscles of aging mice [71,72]. Transcriptome analysis of young and aged muscle by microarray assay showed an elevated expression of genes associated with protein catabolism/degradation, ECM and NMJ degeneration in aged muscle [72]. Moreover, energy metabolism pathways (e.g., oxidative phosphorylation in mitochondria) are highly repressed in muscles of aged rats and tightly correlated with sarcopenia [71]. Autophagy is the cellular process by which abnormal proteins and dysregulated organelles (e.g., mitochondria) are degraded through their inclusion in an autophagosome that is later fused to a lysosome. Impaired autophagy results in accumulation of damaged mitochondria and elevated production of reactive oxygen species (ROS) [73]. It was shown that defective autophagy in aging mice leads to altered NMJ morphology and function, and eventually to muscle atrophy [74,75]. In addition, chronic low-grade inflammatory profile (CLIP), characterized by an increase of pro-inflammatory factors in the bloodstream, including TNFα and IL-6, is also a common feature of aging [76,77]. Through the detection of the denervation-specific sodium channel – Nav1.5 - Rowan and colleagues suggested that aging-associated muscle atrophy is primarily due to muscle denervation, as Nav1.5-positive myofibers are smaller than Nav1.5-negative myofibers [78]. Although many trophic factors, for instance brain derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), and fibroblast growth factor (FGF), have been shown to play essential roles in modifying NMJ integrity or function in the course of aging, it remains unclear how aging causes the partial loss of muscle innervation. Hence, whether FAPs functional changes during aging, such as the decreased proliferation ability and reduced adipogenic differentiation coupled with increased fibrogenic potential [79], is a direct or indirect consequence of defective nerve-muscle communication requires additional investigation. Of note, unlike ALS and SMA, FAPs from aged muscles do not exhibit constitutive activation of IL6-STAT3 signaling [50], further emphasizing differences in the dynamics of FAP activation by physiological (aging) vs pathological (ALS, SMA) loss of NMJ integrity.
Role of FAPs in skeletal muscle adaptation to systemic perturbations
While primarily known for their role in the response to local traumatic events of skeletal muscle, FAPs have recently reported to also respond to systemic perturbations of skeletal muscle homeostasis, such as metabolic stress (e.g., high fat diet – HFD, diabetes) and endurance exercise. In turn, FAP-derived intermuscular adipose tissue (IMAT) beneath the deep fascia between groups of muscle fibers [80] and deposition of ECM components can impair skeletal muscle contractile and metabolic properties [81]. Thus, FAPs appear candidate cellular mediators of reciprocal, functional interactions between skeletal muscles and systemic homeostasis.
Earlier studies revealed the adipogenic potential of FAPs, upon exposure to glycerol-induced muscle injury. Furthermore, when cultured in vitro FAPs also exhibited both spontaneous and inducible abilities to differentiate into adipogenic cells [21,22]. Transcriptomic analysis at single cell level revealed that FAP-derived adipogenic progenies originate from a specific sub-cluster marked by the expression of Tek (i.e., subFAPs-3 shown in the UMAP of Figure 1) [9]. Adipocyte formation in skeletal muscle post glycerol injection was completely blocked upon depletion of the transcriptional activator peroxisome proliferator-activated receptor (PPARγ). Moreover, FAPs isolated from PPARγ knockout mouse were unable to differentiate into adipocytes in vitro [82]. As the master regulator of adipogenesis, PPARγ activates and/or enhances the expression of adipocytes-specific genes, like those involved in lipid storage [83]. Simultaneous muscle injury and dexamethasone treatment in wild type (WT) mice induced an increase in both the transcript and protein levels of PPARγ, resulting in the muscle adiposity, while such an increase was abolished in interleukin-4 (IL-4) knockout mouse model [84]. Furthermore, it was shown that IL-4 expressed by eosinophils inhibits FAPs adipogenic differentiation while promoting FAPs proliferation to facilitate muscle regeneration after acute muscle injury [23]. Likewise, Hedgehog (Hg) signaling through FAPs cilia blocks adipogenesis via TIMP3, a secreted inhibitor of matrix metalloproteinase 14 (MMP14) [85]. Apart from Hg and IL-4 signaling, NOTCH pathway also exerts a repressive role on FAPs adipogenic potential. This negative regulation was shown to be fundamental in the inhibitory effect of myotubes on FAPs adipogenesis [22,86]. Marinkovic and colleagues proposed that the increased adipogenesis of FAPs in mdx mouse (mdx-FAPs) was due to the insensitivity of mdx-FAPs to NOTCH signaling, which could be rescued with the supplement of TNFα that is secreted by macrophages in acute muscle injury [86]. Additionally, there is evidence that the intramuscular fat deposition in mdx muscles is, at least in part, due to the reduced activation of WNT5a/β-catenin signaling, which causes the upregulation of PPARγ in mdx-FAPs. A further reduction of WNT5a/β-catenin signaling activity in old mdx-FAPs is consistent with the higher fat accumulation in muscle, as compared with young mdx mice [87]. Conversely, in aged skeletal muscle of WT mice, FAPs adipogenic potential is greatly reduced [79]. However, muscles of both aged WT or mdx mice display functional and regenerative defects as well as pronounced fibrosis [79,87]. It remains currently unclear whether alterations in FAPs adipogenic differentiation occur as a primary, cell intrinsic events, or are secondary to changes in muscle microenvironment. Recently, short-term HFD-induced metabolic reprogramming of FAPs was found to modulate FAPs fibrogenic and adipogenic abilities, thus emerging as a potential method to recover mdx muscle atrophy in a FAPs-dependent manner [88]. Interestingly, the adipogenic potential of FAPs is repressed by a microRNA (miR-206), which was originally thought to be expressed only in myogenic cells (and thereby defined as myomiR), but could be upregulated in FAPs of mdx mice, upon exposure to HDAC inhibitors (HDACi) – a treatment that inhibits the fibro-adipogenic potential of FAPs from dystrophic mice) [27].
The IMAT has been linked to reduced insulin sensitivity and muscle performance [89], as well as to insulin resistance in obese individuals [90]. In contrast, a different type of fat deposits found in skeletal muscle, the intramyocellular lipids (IMCLs) [91], can be either associated to muscle metabolic impairment in obesity or linked to increased muscle performance in endurance-trained athletes, depending on their cellular localization [92]. This topic is progressively gaining interest, as conditions that increase muscle adiposity and fibrosis might eventually lead to muscle atrophy and loss of contractility [90,93]. At the same time, muscle enrichment in fibro/fatty tissue reduces insulin sensitivity and glucose uptake in muscle, overall exacerbating systemic insulin resistance [90]. While further studies are needed to understand how FAPs balance their adipogenic and fibrogenic abilities and reprogram their metabolic states in order to benefit muscle homeostatic and regenerative functions, we review here current evidence implicating FAPs as potential cellular mediators of the interplay between skeletal muscle and metabolic stress (e.g., type 2 diabetes) or endurance exercise.
Diabetes-induced activation of FAPs
Emerging evidence links the expansion of the IMAT to FAP-mediated adipogenesis, both in mouse [94] and human [95], in chronic conditions such as obesity and diabetes. In obese mice, FAPs expansion is promoted by an increase in circulating adipokines such as Thrombospondin 1 (THBS1) and transforming growth factor-β1 (TGF-β1), released by the hypertrophic adipose tissue [81,96]. In particular, Buras and colleagues found that long-term HFD is able to trigger FAPs expansion as well as their differentiation into adipocytes and COL1-depositing fibroblasts. Farup and colleagues have recently identified FAPs as key players in the muscle fibrotic degeneration observed in Type 2 Diabetes Mellitus (T2DM) [97]. Single-cell transcriptome analysis of biopsies from healthy subjects and T2DM patients revealed enrichment of a FAPs sub-population in the latter, characterized by THY1 (CD90) expression that was more prone to proliferate in vitro and exhibited higher glycolytic activity, as compared to their CD90-negative (CD90−) counterparts [97]. Glycolytic flux has been correlated to a higher propensity of cells differentiating toward the fibrogenic fate [98], which is consistent with the evidence that CD90-positive (CD90+) FAPs from T2DM patients express different types of collagen [97]. Treatment with a common anti-diabetic drug, Metformin, could reduce the pathogenic phenotype of CD90+ FAPs, mainly through inhibition of their proliferation and fibrogenic potential [98]. Importantly, this study identified the PDGF signaling as key regulator of FAP phenotype, as it promoted proliferation and collagen production at the expense of adipogenesis, an effect accompanied with a metabolic shift towards glycolysis [98]. This evidence is also supported by previous works linking PDGF to increased reliance on glycolysis in other biological contexts [99]. Although all FAPs express PDGFRα, data identify PDGF-driven conversion of a sub-population of FAPs as a key event in the pathogenic accumulation of extracellular matrix in T2DM muscles.
Exercise-induced activation of FAPs
Endurance training proves particularly beneficial in T2DM, leading to improved insulin sensitivity and glycemic control, mainly due to the action of myokines such as IL-6, released from skeletal muscle in response to contraction [100]. IL-6 function is still controversial, because when expressed at moderate levels it acts as a “myokine”, exerting beneficial effects at the systemic and local levels on different tissues, e.g., promoting muscle hypertrophy and enhancing glucose uptake. However, when expressed at constitutively higher levels, as in the context of chronic pathological inflammatory conditions (e.g. cancer cachexia, sepsis, etc.) or irreversible muscle denervation, IL6 turns into a key effector of skeletal muscle atrophy and fibrosis [50].
Interestingly, adipose tissue is also responsible for the release of another adipokine, TGF-β2, which improves muscle glucose tolerance in mice in response to exercise, due to the cross-talk between the two tissues mediated by muscle lactate production [101]. Additionally, exercise-induced production of Nitric Oxide (NO) by myofibers acts as a suppressor of intramuscular adipogenesis, through the inhibition of the adipogenesis master regulator PPARγ in FAPs [102]. Another study has shown that exercise-induced muscle damage promotes a transient expression of inflammatory cytokines that resembles the senescence-associated secretory pathway (SASP) in mouse FAPs, possibly associated to the release of signals implicated in the regulation of muscle regeneration [103]. Muscle ECM composition and homeostasis, commonly disrupted in obese and diabetic individuals, are also ameliorated by exercise, which contributes to a higher rate of ECM turnover and to an increased expression of specific collagen types [104]. A similar conclusion on the inhibitory effect of exercise on FAPs proliferation stems from the observation that male mice on a HFD show lower FAPs accumulation under exercise training, compared to their sedentary counterparts [105]. In contrast to this evidence, Farup and colleagues show that, in humans, resistance training is responsible for an increase in FAPs, coupled to a decline in pericytes, which favors the activation and expansion of MuSCs post-training [106]. Although preliminary evidence links eccentric force and stimulation of muscle-resident mesenchymal populations to release pro-myogenic factors favorable to MuSCs expansion [107], further evidence is needed to determine FAPs contribution to this phenomenon and to identify the molecular players involved in the release of trophic factors in response to exercise. In this respect, it is worth noting that FAPs are endowed with a secretory potential ranging from soluble factors to Extracellular Vesicles (EVs) that predict reciprocal exchange with myofibers and other muscle resident cells, including MuSCs [103,108,109].
Concluding Remarks
The contribution of FAPs to the response of skeletal muscle to homeostatic perturbations different from the typical myotrauma caused by physical injury or muscular dystrophies expands our knowledge on FAP biology and provides new potential avenues for pharmacological control of condition- or disease-specific activation of discrete sub-populations of FAPs. In particular, the identification of denervation-activated FAPs as source of pathogenic signals (e.g., IL6-STAT3 signaling) is a finding of special biomedical interest, as it provides new insights into the contribution of the muscle component of NMJ to the pathogenesis of neuromuscular diseases, such as ALS [110]. Likewise, the contribution of FAPs to skeletal muscle adaptation to systemic perturbations (i.e., exercise, T2DM and possibly other physiological or pathological metabolic changes) might provide novel biological insights into the molecular mechanism that governs the phenotype of discrete subFAPs in response to specific stimuli. Conceivably, this knowledge might inspire novel pharmacological interventions toward limiting the adoption of FAP phenotypes that promotes pathogenic systemic loops – e.g., fatty degeneration of skeletal muscles that impairs their metabolic performance in chronic systemic diseases, such as T2DM. In this regard, earlier works have reported that expression of miR-206 in FAPs antagonizes their adipogenic potential [27]. Of note, miR-206 expression can be induced in FAPs by pharmacological interventions (i.e., HDAC inhibitors) that also promote FAPs’ release of miR-206-containing EVs, up-taken by neighboring cells [109]. Given that miR-206 expression and function are implicated in many skeletal muscle-related disorders [111,112] and increased expression of miR-206 has been associated to positive outcomes in mouse models of ALS and DMD [27,113], pharmacological modulation of miR-206 (and possibly other miRs) could be exploited to prevent development of pathogenic events in skeletal muscle during progression of these diseases.
Acknowledgements
We thank all Puri’s lab members and Dr Alessandra Sacco for insightful comments and discussions during the manuscript preparation. This work has been supported by the funding from R01AR076247–01 NIH/NIAMS to PLP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing Interests
The authors declare no competing interests.
References
- [1].Sambasivan R, Yao R, Kissenpfennig A, Wittenberghe LV, Paldi A, Gayraud-Morel B, Guenou H, Malissen B, Tajbakhsh S, Galy A., Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration, Development. (2011) 18. [DOI] [PubMed]
- [2].Farup J, Madaro L, Puri PL, Mikkelsen UR, Interactions between muscle stem cells, mesenchymal-derived cells and immune cells in muscle homeostasis, regeneration and disease, Cell Death & Disease. 6 (2015) e1830–e1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Evano B, Tajbakhsh S., Skeletal muscle stem cells in comfort and stress, Npj Regenerative Medicine. 3 (2018) 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Wosczyna MN, Rando TA, A Muscle Stem Cell Support Group: Coordinated Cellular Responses in Muscle Regeneration, Developmental Cell. 46 (2018) 135–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Biferali B, Proietti D, Mozzetta C, Madaro L., Fibro–Adipogenic Progenitors Cross-Talk in Skeletal Muscle: The Social Network, Front. Physiol. 10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].V. M, A. Y, M. Bsr, P. T, L. C, C. B, M. Lk, A. A, Muscle Satellite Cell Cross-Talk With a Vascular Niche Maintains Quiescence via VEGF and Notch Signaling, Cell Stem Cell. 23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tidball JG, Regulation of muscle growth and regeneration by the immune system, Nature Reviews Immunology. 17 (2017) 165–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA, Cellular dynamics in the muscle satellite cell niche, EMBO Reports. 14 (2013) 1062–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Malecova B, Gatto S, Etxaniz U, Passafaro M, Cortez A, Nicoletti C, Giordani L, Torcinaro A, Bardi MD, Bicciato S, Santa FD, Madaro L, Puri PL, Dynamics of cellular states of fibro-adipogenic progenitors during myogenesis and muscular dystrophy, Nature Communications. 9 (2018) 3670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].De Micheli AJ, Laurilliard EJ, Heinke CL, Ravichandran H, Fraczek P, Soueid-Baumgarten S, De Vlaminck I, Elemento O, Cosgrove BD, Single-Cell Analysis of the Muscle Stem Cell Hierarchy Identifies Heterotypic Communication Signals Involved in Skeletal Muscle Regeneration, Cell Reports. 30 (2020) 3583–3595.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Dell’Orso S, Juan AH, Ko K-D, Naz F, Perovanovic J, Gutierrez-Cruz G, Feng X, Sartorelli V., Single cell analysis of adult mouse skeletal muscle stem cells in homeostatic and regenerative conditions, Development. 146 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Giordani L, He GJ, Negroni E, Sakai H, Law JYC, Siu MM, Wan R, Corneau A, Tajbakhsh S, Cheung TH, Le Grand F., High-Dimensional Single-Cell Cartography Reveals Novel Skeletal Muscle-Resident Cell Populations, Molecular Cell. 74 (2019) 609–621.e6. [DOI] [PubMed] [Google Scholar]
- [13].Oprescu SN, Yue F, Qiu J, Brito LF, Kuang S., Temporal Dynamics and Heterogeneity of Cell Populations during Skeletal Muscle Regeneration, IScience. 23 (2020) 100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pawlikowski B, Betta ND, Elston T, O’Rourke R, Jones K, Olwin BB, A cellular atlas of skeletal muscle regeneration and aging, BioRxiv. (2019) 635805.
- [15].Petrany MJ, Swoboda CO, Sun C, Chetal K, Chen X, Weirauch MT, Salomonis N, Millay DP, Single-nucleus RNA-seq identifies transcriptional heterogeneity in multinucleated skeletal myofibers, Nature Communications. 11 (2020) 6374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Proietti D, Giordani L, Bardi MD, D’Ercole C, Lozanoska-Ochser B, Amadio S, Volontè C, Marinelli S, Muchir A, Bouchè M, Borsellino G, Sacco A, Puri PL, Madaro L., Activation of skeletal muscle-resident glial cells upon nerve injury, JCI Insight. (2021). [DOI] [PMC free article] [PubMed]
- [17].Nicoletti C, Wei X, Etxaniz U, Proietti D, Madaro L, Puri PL, scRNA-seq-based analysis of skeletal muscle response to denervation reveals selective activation of muscle-resident glial cells and fibroblasts, BioRxiv. (2020) 2020.12.29.424762.
- [18].Morgan J, Partridge T., Skeletal muscle in health and disease, Disease Models & Mechanisms. 13 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Judson RN, Zhang R-H, Rossi FMA, Tissue-resident mesenchymal stem/progenitor cells in skeletal muscle: collaborators or saboteurs?, FEBS J. 280 (2013) 4100–4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Serrano AL, Muñoz-Cánoves P., Fibrosis development in early-onset muscular dystrophies: Mechanisms and translational implications, Seminars in Cell & Developmental Biology. 64 (2017) 181–190. [DOI] [PubMed] [Google Scholar]
- [21].Joe AWB, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FMV, Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis, Nature Cell Biology. 12 (2010) 153–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K., Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle, Nature Cell Biology. 12 (2010) 143–152. [DOI] [PubMed] [Google Scholar]
- [23].Heredia JE, Mukundan L, Chen FM, Mueller AA, Deo RC, Locksley RM, Rando TA, Chawla A., Type 2 Innate Signals Stimulate Fibro/Adipogenic Progenitors to Facilitate Muscle Regeneration, Cell. 153 (2013) 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Lemos DR, Babaeijandaghi F, Low M, Chang C-K, Lee ST, Fiore D, Zhang R-H, Natarajan A, Nedospasov SA, Rossi FMV, Nilotinib reduces muscle fibrosis in chronic muscle injury by promoting TNF-mediated apoptosis of fibro/adipogenic progenitors, Nature Medicine. 21 (2015) 786–794. [DOI] [PubMed] [Google Scholar]
- [25].Mozzetta C, Consalvi S, Saccone V, Tierney M, Diamantini A, Mitchell KJ, Marazzi G, Borsellino G, Battistini L, Sassoon D, Sacco A, Puri PL, Fibroadipogenic progenitors mediate the ability of HDAC inhibitors to promote regeneration in dystrophic muscles of young, but not old Mdx mice, EMBO Molecular Medicine. 5 (2013) 626–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Uezumi A, Fukada S, Yamamoto N, Ikemoto-Uezumi M, Nakatani M, Morita M, Yamaguchi A, Yamada H, Nishino I, Hamada Y, Tsuchida K., Identification and characterization of PDGFR α + mesenchymal progenitors in human skeletal muscle, Cell Death & Disease. 5 (2014) e1186–e1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Saccone V, Consalvi S, Giordani L, Mozzetta C, Barozzi I, Sandoná M, Ryan T, Rojas-Muñoz A, Madaro L, Fasanaro P, Borsellino G, Bardi MD, Frigè G, Termanini A, Sun X, Rossant J, Bruneau BG, Mercola M, Minucci S, Puri PL, HDAC-regulated myomiRs control BAF60 variant exchange and direct the functional phenotype of fibro-adipogenic progenitors in dystrophic muscles, Genes Dev. 28 (2014) 841–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hogarth MW, Defour A, Lazarski C, Gallardo E, Diaz Manera J, Partridge TA, Nagaraju K, Jaiswal JK, Fibroadipogenic progenitors are responsible for muscle loss in limb girdle muscular dystrophy 2B, Nature Communications. 10 (2019) 2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Wosczyna MN, Konishi CT, Perez Carbajal EE, Wang TT, Walsh RA, Gan Q, Wagner MW, Rando TA, Mesenchymal Stromal Cells Are Required for Regeneration and Homeostatic Maintenance of Skeletal Muscle, Cell Reports. 27 (2019) 2029–2035.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Juban G, Saclier M, Yacoub-Youssef H, Kernou A, Arnold L, Boisson C, Ben Larbi S, Magnan M, Cuvellier S, Théret M, Petrof BJ, Desguerre I, Gondin J, Mounier R, Chazaud B., AMPK Activation Regulates LTBP4-Dependent TGF-β1 Secretion by Pro-inflammatory Macrophages and Controls Fibrosis in Duchenne Muscular Dystrophy, Cell Reports. 25 (2018) 2163–2176.e6. [DOI] [PubMed] [Google Scholar]
- [31].Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G., Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration, Development. 138 (2011) 3625–3637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fry CS, Kirby TJ, Kosmac K, McCarthy JJ, Peterson CA, Myogenic Progenitor Cells Control Extracellular Matrix Production by Fibroblasts during Skeletal Muscle Hypertrophy, Cell Stem Cell. 20 (2017) 56–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hic1 Defines Quiescent Mesenchymal Progenitor Subpopulations with Distinct Functions and Fates in Skeletal Muscle Regeneration, Cell Stem Cell. 25 (2019) 797–813.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Liu W, Chakkalakal JV, The Composition, Development, and Regeneration of Neuromuscular Junctions, Curr. Top. Dev. Biol. 126 (2018) 99–124. [DOI] [PubMed] [Google Scholar]
- [35].Gordon T., Peripheral Nerve Regeneration and Muscle Reinnervation, International Journal of Molecular Sciences. 21 (2020) 8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Csapo R, Gumpenberger M, Wessner B., Skeletal Muscle Extracellular Matrix – What Do We Know About Its Composition, Regulation, and Physiological Roles? A Narrative Review, Front. Physiol. 11 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ciciliot S, Schiaffino S., Regeneration of mammalian skeletal muscle. Basic mechanisms and clinical implications, Curr Pharm Des. 16 (2010) 906–914. [DOI] [PubMed] [Google Scholar]
- [38].Strenzke M, Alberton P, Aszodi A, Docheva D, Haas E, Kammerlander C, Böcker W, Saller MM, Tenogenic Contribution to Skeletal Muscle Regeneration: The Secretome of Scleraxis Overexpressing Mesenchymal Stem Cells Enhances Myogenic Differentiation In Vitro, International Journal of Molecular Sciences. 21 (2020) 1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Hardy D, Besnard A, Latil M, Jouvion G, Briand D, Thépenier C, Pascal Q, Guguin A, Gayraud-Morel B, Cavaillon J-M, Tajbakhsh S, Rocheteau P, Chrétien F., Comparative Study of Injury Models for Studying Muscle Regeneration in Mice, PLOS ONE. 11 (2016) e0147198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Feige P, Brun CE, Ritso M, Rudnicki MA, Orienting Muscle Stem Cells for Regeneration in Homeostasis, Aging, and Disease, Cell Stem Cell. 23 (2018) 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Collao N, Farup J, De Lisio M., Role of Metabolic Stress and Exercise in Regulating Fibro/Adipogenic Progenitors, Front. Cell Dev. Biol. 8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Li L, Xiong W-C, Mei L., Neuromuscular Junction Formation, Aging, and Disorders, Annual Review of Physiology. 80 (2018) 159–188. [DOI] [PubMed] [Google Scholar]
- [43].Baudet C, Pozas E, Adameyko I, Andersson E, Ericson J, Ernfors P., Retrograde Signaling onto Ret during Motor Nerve Terminal Maturation, J. Neurosci. 28 (2008) 963–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Darabid H, Perez-Gonzalez AP, Robitaille R., Neuromuscular synaptogenesis: coordinating partners with multiple functions, Nature Reviews Neuroscience. 15 (2014) 703–718. [PubMed] [Google Scholar]
- [45].Zhao K, Shen C, Lu Y, Huang Z, Li L, Rand CD, Pan J, Sun X-D, Tan Z, Wang H, Xing G, Cao Y, Hu G, Zhou J, Xiong W-C, Mei L., Muscle Yap Is a Regulator of Neuromuscular Junction Formation and Regeneration, J. Neurosci. 37 (2017) 3465–3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Zuo Y, Lubischer JL, Kang H, Tian L, Mikesh M, Marks A, Scofield VL, Maika S, Newman C, Krieg P, Thompson WJ, Fluorescent Proteins Expressed in Mouse Transgenic Lines Mark Subsets of Glia, Neurons, Macrophages, and Dendritic Cells for Vital Examination, J. Neurosci. 24 (2004) 10999–11009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kang H, Tian L, Mikesh M, Lichtman JW, Thompson WJ, Terminal Schwann Cells Participate in Neuromuscular Synapse Remodeling during Reinnervation following Nerve Injury, J. Neurosci. 34 (2014) 6323–6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Magill CK, Tong A, Kawamura D, Hayashi A, Hunter DA, Parsadanian A, Mackinnon SE, Myckatyn TM, Reinnervation of the tibialis anterior following sciatic nerve crush injury: A confocal microscopic study in transgenic mice, Experimental Neurology. 207 (2007) 64–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sakuma M, Gorski G, Sheu S-H, Lee S, Barrett LB, Singh B, Omura T, Latremoliere A, Woolf CJ, Lack of motor recovery after prolonged denervation of the neuromuscular junction is not due to regenerative failure, Eur J Neurosci. 43 (2016) 451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Madaro L, Passafaro M, Sala D, Etxaniz U, Lugarini F, Proietti D, Alfonsi MV, Nicoletti C, Gatto S, Bardi MD, Rojas-García R, Giordani L, Marinelli S, Pagliarini V, Sette C, Sacco A, Puri PL, Denervation-activated STAT3–IL-6 signalling in fibro-adipogenic progenitors promotes myofibres atrophy and fibrosis, Nat Cell Biol. 20 (2018) 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Carlson BM, The biology of long-term denervated skeletal muscle, European Journal of Translational Myology. 24 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Liu W, Wei-LaPierre L, Klose A, Dirksen RT, Chakkalakal JV, Inducible depletion of adult skeletal muscle stem cells impairs the regeneration of neuromuscular junctions, ELife. 4 (2015) e09221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Gonzalez D, Contreras O, Rebolledo DL, Espinoza JP, van Zundert B, Brandan E., ALS skeletal muscle shows enhanced TGF-β signaling, fibrosis and induction of fibro/adipogenic progenitor markers, PLoS One. 12 (2017) e0177649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Muñoz-Cánoves P, Scheele C, Pedersen BK, Serrano AL, Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword?, The FEBS Journal. 280 (2013) 4131–4148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Johnson DE, O’Keefe RA, Grandis JR, Targeting the IL-6/JAK/STAT3 signalling axis in cancer, Nature Reviews Clinical Oncology. 15 (2018) 234–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Sims NA, The JAK1/STAT3/SOCS3 axis in bone development, physiology, and pathology, Experimental & Molecular Medicine. 52 (2020) 1185–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Odeh M, Tamir-Livne Y, Haas T, Bengal E., P38α MAPK coordinates the activities of several metabolic pathways that together induce atrophy of denervated muscles, The FEBS Journal. 287 (2020) 73–93. [DOI] [PubMed] [Google Scholar]
- [58].Grzelkowska-Kowalczyk K., The Importance of Extracellular Matrix in Skeletal Muscle Development and Function, Composition and Function of the Extracellular Matrix in the Human Body. (2016).
- [59].Humphrey JD, Dufresne ER, Schwartz MA, Mechanotransduction and extracellular matrix homeostasis, Nature Reviews Molecular Cell Biology. 15 (2014) 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Bonnans C, Chou J, Werb Z., Remodelling the extracellular matrix in development and disease, Nature Reviews Molecular Cell Biology. 15 (2014) 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fiore D, Judson RN, Low M, Lee S, Zhang E, Hopkins C, Xu P, Lenzi A, Rossi FMV, Lemos DR, Pharmacological blockage of fibro/adipogenic progenitor expansion and suppression of regenerative fibrogenesis is associated with impaired skeletal muscle regeneration, Stem Cell Research. 17 (2016) 161–169. [DOI] [PubMed] [Google Scholar]
- [62].Chapman MA, Mukund K, Subramaniam S, Brenner D, Lieber RL, Three distinct cell populations express extracellular matrix proteins and increase in number during skeletal muscle fibrosis, American Journal of Physiology-Cell Physiology. 312 (2016) C131–C143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Uezumi A, Ikemoto-Uezumi M, Zhou H, Kurosawa T, Yoshimoto Y, Nakatani M, Hitachi K, Yamaguchi H, Wakatsuki S, Araki T, Morita M, Yamada H, Toyoda M, Kanazawa N, Nakazawa T, Hino J, Fukada S, Tsuchida K., Mesenchymal Bmp3b expression maintains skeletal muscle integrity and decreases in age-related sarcopenia, J Clin Invest. 131 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wang K, Yaghi OK, Spallanzani RG, Chen X, Zemmour D, Lai N, Chiu IM, Benoist C, Mathis D., Neuronal, stromal, and T-regulatory cell crosstalk in murine skeletal muscle, PNAS. 117 (2020) 5402–5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Spallanzani RG, Zemmour D, Xiao T, Jayewickreme T, Li C, Bryce PJ, Benoist C, Mathis D., Distinct immunocyte-promoting and adipocyte-generating stromal components coordinate adipose tissue immune and metabolic tenors, Science Immunology. 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Singhal N, Martin PT, Role of extracellular matrix proteins and their receptors in the development of the vertebrate neuromuscular junction, Developmental Neurobiology. 71 (2011) 982–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Dityatev A, Schachner M, Sonderegger P., The dual role of the extracellular matrix in synaptic plasticity and homeostasis, Nature Reviews Neuroscience. 11 (2010) 735–746. [DOI] [PubMed] [Google Scholar]
- [68].Legay C, Dobbertin A., Collagens at the vertebrate neuromuscular junction, from structure to pathologies, Neuroscience Letters. 735 (2020) 135155. [DOI] [PubMed] [Google Scholar]
- [69].Beyer I, Mets T, Bautmans I , Chronic low-grade inflammation and age-related sarcopenia, Current Opinion in Clinical Nutrition & Metabolic Care. 15 (2012) 12–22. [DOI] [PubMed] [Google Scholar]
- [70].Jang YC, Van Remmen H., Age-associated alterations of the neuromuscular junction, Experimental Gerontology. 46 (2011) 193–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ibebunjo C, Chick JM, Kendall T, Eash JK, Li C, Zhang Y, Vickers C, Wu Z, Clarke BA, Shi J, Cruz J, Fournier B, Brachat S, Gutzwiller S, Ma Q, Markovits J, Broome M, Steinkrauss M, Skuba E, Galarneau J-R, Gygi SP, Glass DJ, Genomic and Proteomic Profiling Reveals Reduced Mitochondrial Function and Disruption of the Neuromuscular Junction Driving Rat Sarcopenia, Molecular and Cellular Biology. 33 (2013) 194–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Barns M, Gondro C, Tellam RL, Radley-Crabb HG, Grounds MD, Shavlakadze T., Molecular analyses provide insight into mechanisms underlying sarcopenia and myofibre denervation in old skeletal muscles of mice, The International Journal of Biochemistry & Cell Biology. 53 (2014) 174–185. [DOI] [PubMed] [Google Scholar]
- [73].Hansen M, Rubinsztein DC, Walker DW, Autophagy as a promoter of longevity: insights from model organisms, Nature Reviews Molecular Cell Biology. 19 (2018) 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M., Autophagy Is Required to Maintain Muscle Mass, Cell Metabolism. 10 (2009) 507–515. [DOI] [PubMed] [Google Scholar]
- [75].Carnio S, LoVerso F, Baraibar MA, Longa E, Khan MM, Maffei M, Reischl M, Canepari M, Loefler S, Kern H, Blaauw B, Friguet B, Bottinelli R, Rudolf R, Sandri M., Autophagy Impairment in Muscle Induces Neuromuscular Junction Degeneration and Precocious Aging, Cell Reports. 8 (2014) 1509–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Dalle S, Rossmeislova L, Koppo K., The Role of Inflammation in Age-Related Sarcopenia, Front. Physiol. 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Gomarasca M, Banfi G, Lombardi G, Chapter Four - Myokines: The endocrine coupling of skeletal muscle and bone, in: Makowski GS (Ed.), Advances in Clinical Chemistry, Elsevier, 2020: pp. 155–218. [DOI] [PubMed] [Google Scholar]
- [78].Rowan SL, Rygiel K, Purves-Smith FM, Solbak NM, Turnbull DM, Hepple RT, Denervation Causes Fiber Atrophy and Myosin Heavy Chain Co-Expression in Senescent Skeletal Muscle, PLOS ONE. 7 (2012) e29082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Lukjanenko L, Karaz S, Stuelsatz P, Gurriaran-Rodriguez U, Michaud J, Dammone G, Sizzano F, Mashinchian O, Ancel S, Migliavacca E, Liot S, Jacot G, Metairon S, Raymond F, Descombes P, Palini A, Chazaud B, Rudnicki MA, Bentzinger CF, Feige JN, Aging Disrupts Muscle Stem Cell Function by Impairing Matricellular WISP1 Secretion from Fibro-Adipogenic Progenitors, Cell Stem Cell. 24 (2019) 433–446.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Addison O, Marcus RL, LaStayo PC, Ryan AS, Intermuscular Fat: A Review of the Consequences and Causes, International Journal of Endocrinology. 2014 (2014) e309570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Buras ED, Converso-Baran K, Davis CS, Akama T, Hikage F, Michele DE, Brooks SV, Chun T-H, Fibro-Adipogenic Remodeling of the Diaphragm in Obesity-Associated Respiratory Dysfunction, Diabetes. 68 (2019) 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Dammone G, Karaz S, Lukjanenko L, Winkler C, Sizzano F, Jacot G, Migliavacca E, Palini A, Desvergne B, Gilardi F, Feige JN, PPARγ Controls Ectopic Adipogenesis and Cross-Talks with Myogenesis During Skeletal Muscle Regeneration, International Journal of Molecular Sciences. 19 (2018) 2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Tontonoz P, Spiegelman BM, Fat and Beyond: The Diverse Biology of PPARγ, Annu. Rev. Biochem. 77 (2008) 289–312. [DOI] [PubMed] [Google Scholar]
- [84].Dong Y, Silva KAS, Dong Y, Zhang L., Glucocorticoids increase adipocytes in muscle by affecting IL-4 regulated FAP activity, The FASEB Journal. 28 (2014) 4123–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kopinke D, Roberson EC, Reiter JF, Ciliary Hedgehog Signaling Restricts Injury-Induced Adipogenesis, Cell. 170 (2017) 340–351.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Marinkovic M, Fuoco C, Sacco F, Perpetuini AC, Giuliani G, Micarelli E, Pavlidou T, Petrilli LL, Reggio A, Riccio F, Spada F, Vumbaca S, Zuccotti A, Castagnoli L, Mann M, Gargioli C, Cesareni G., Fibro-adipogenic progenitors of dystrophic mice are insensitive to NOTCH regulation of adipogenesis, Life Science Alliance. 2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Reggio A, Rosina M, Palma A, Cerquone Perpetuini A, Petrilli LL, Gargioli C, Fuoco C, Micarelli E, Giuliani G, Cerretani M, Bresciani A, Sacco F, Castagnoli L, Cesareni G., Adipogenesis of skeletal muscle fibro/adipogenic progenitors is affected by the WNT5a/GSK3/β-catenin axis, Cell Death & Differentiation. 27 (2020) 2921–2941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Reggio A, Rosina M, Krahmer N, Palma A, Petrilli LL, Maiolatesi G, Massacci G, Salvatori I, Valle C, Testa S, Gargioli C, Fuoco C, Castagnoli L, Cesareni G, Sacco F., Metabolic reprogramming of fibro/adipogenic progenitors facilitates muscle regeneration, Life Science Alliance. 3 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Delmonico MJ, Harris TB, Visser M, Park SW, Conroy MB, Velasquez-Mieyer P, Boudreau R, Manini TM, Nevitt M, Newman AB, Goodpaster BH, Health, Aging, and Body, Longitudinal study of muscle strength, quality, and adipose tissue infiltration, Am J Clin Nutr. 90 (2009) 1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Goodpaster BH, Thaete FL, Kelley DE, Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus, The American Journal of Clinical Nutrition. 71 (2000) 885–892. [DOI] [PubMed] [Google Scholar]
- [91].Gemmink A, Goodpaster BH, Schrauwen P, Hesselink MKC, Intramyocellular lipid droplets and insulin sensitivity, the human perspective, Biochimica et Biophysica Acta (BBA) - Molecular and Cell Biology of Lipids. 1862 (2017) 1242–1249. [DOI] [PubMed] [Google Scholar]
- [92].Samjoo IA, Safdar A, Hamadeh MJ, Glover AW, Mocellin NJ, Santana J, Little JP, Steinberg GR, Raha S, Tarnopolsky MA, Markers of Skeletal Muscle Mitochondrial Function and Lipid Accumulation Are Moderately Associated with the Homeostasis Model Assessment Index of Insulin Resistance in Obese Men, PLOS ONE. 8 (2013) e66322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Kao T-W, Peng T-C, Chen W-L, Han D-S, Chen C-L, Yang W-S, Impact of adiposity on muscle function and clinical events among elders with dynapenia, presarcopenia and sarcopenia: a community-based cross-sectional study, Aging (Albany NY). 13 (2021) 7247–7258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Khan IM, Perrard X-Y, Brunner G, Lui H, Sparks LM, Smith SR, Wang X, Shi Z-Z, Lewis DE, Wu H, Ballantyne CM, Intermuscular and perimuscular fat expansion in obesity correlates with skeletal muscle T cell and macrophage infiltration and insulin resistance, International Journal of Obesity. 39 (2015) 1607–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Sachs S, Zarini S, Kahn DE, Harrison KA, Perreault L, Phang T, Newsom SA, Strauss A, Kerege A, Schoen JA, Bessesen DH, Schwarzmayr T, Graf E, Lutter D, Krumsiek J, Hofmann SM, Bergman BC, Intermuscular adipose tissue directly modulates skeletal muscle insulin sensitivity in humans, American Journal of Physiology-Endocrinology and Metabolism. 316 (2019) E866–E879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Kong P, Gonzalez-Quesada C, Li N, Cavalera M, Lee D-W, Frangogiannis NG, Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation, American Journal of Physiology-Endocrinology and Metabolism. 305 (2013) E439–E450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Farup J, Just J, de Paoli F, Lin L, Jensen JB, Billeskov T, Roman IS, Cömert C, Møller AB, Madaro L, Groppa E, Fred RG, Kampmann U, Pedersen SB, Bross P, Stevnsner T, Eldrup N, Pers TH, Rossi FMV, Puri PL, Jessen N., Human skeletal muscle CD90+ fibro-adipogenic progenitors are associated with muscle degeneration in type 2 diabetic patients, BioRxiv. (2020) 2020.08.25.243907. [DOI] [PMC free article] [PubMed]
- [98].Zhao X, Kwan JYY, Yip K, Liu PP, Liu F-F, Targeting metabolic dysregulation for fibrosis therapy, Nature Reviews Drug Discovery. 19 (2020) 57–75. [DOI] [PubMed] [Google Scholar]
- [99].Ran C, Liu H, Hitoshi Y, Israel MA, Proliferation-Independent Control of Tumor Glycolysis by PDGFR-Mediated AKT Activation, Cancer Res. 73 (2013) 1831–1843. [DOI] [PubMed] [Google Scholar]
- [100].Karstoft K, Pedersen BK, Exercise and type 2 diabetes: focus on metabolism and inflammation, Immunology & Cell Biology. 94 (2016) 146–150. [DOI] [PubMed] [Google Scholar]
- [101].Takahashi H, Alves CRR, Stanford KI, Middelbeek RJW, Nigro P, Ryan RE, Xue R, Sakaguchi M, Lynes MD, So K, Mul JD, Lee M-Y, Balan E, Pan H, Dreyfuss JM, Hirshman MF, Azhar M, Hannukainen JC, Nuutila P, Kalliokoski KK, Nielsen S, Pedersen BK, Kahn CR, Tseng Y-H, Goodyear LJ, TGF-β2 is an exercise-induced adipokine that regulates glucose and fatty acid metabolism, Nature Metabolism. 1 (2019) 291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Cordani N, Pisa V, Pozzi L, Sciorati C, Clementi E., Nitric Oxide Controls Fat Deposition in Dystrophic Skeletal Muscle by Regulating Fibro-Adipogenic Precursor Differentiation, STEM CELLS. 32 (2014) 874–885. [DOI] [PubMed] [Google Scholar]
- [103].Saito Y, Chikenji TS, Matsumura T, Nakano M, Fujimiya M., Exercise enhances skeletal muscle regeneration by promoting senescence in fibro-adipogenic progenitors, Nature Communications. 11 (2020) 889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Martinez-Huenchullan S, McLennan SV, Verhoeven A, Twigg SM, Tam CS, The emerging role of skeletal muscle extracellular matrix remodelling in obesity and exercise, Obesity Reviews. 18 (2017) 776–790. [DOI] [PubMed] [Google Scholar]
- [105].D’Souza D, Roubos S, Larkin J, Lloyd J, Emmons R, Chen H, De Lisio M., The Late Effects of Radiation Therapy on Skeletal Muscle Morphology and Progenitor Cell Content are Influenced by Diet-Induced Obesity and Exercise Training in Male Mice, Scientific Reports. 9 (2019) 6691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Farup J, De Lisio M, Rahbek SK, Bjerre J, Vendelbo MH, Boppart MD, Vissing K., Pericyte response to contraction mode-specific resistance exercise training in human skeletal muscle, Journal of Applied Physiology. 119 (2015) 1053–1063. [DOI] [PubMed] [Google Scholar]
- [107].De Lisio M, Jensen T, Sukiennik RA, Huntsman HD, Boppart MD, Substrate and strain alter the muscle-derived mesenchymal stem cell secretome to promote myogenesis, Stem Cell Research & Therapy. 5 (2014) 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Consalvi S, Tucciarone L, Macrì E, Bardi MD, Picozza M, Salvatori I, Renzini A, Valente S, Mai A, Moresi V, Puri PL, Partial resistance to HDAC inhibitors in FAPs of dystrophic muscles at late stages of disease is associated to epigenetic and transcriptional features of cellular senescence, BioRxiv. (2021) 2021.04.26.441412.
- [109].Sandonà M, Consalvi S, Tucciarone L, De Bardi M, Scimeca M, Angelini DF, Buffa V, D’Amico A, Bertini ES, Cazzaniga S, Bettica P, Bouché M, Bongiovanni A, Puri PL, Saccone V., HDAC inhibitors tune miRNAs in extracellular vesicles of dystrophic muscle-resident mesenchymal cells, EMBO Reports. 21 (2020) e50863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Loeffler J-P, Picchiarelli G, Dupuis L, Aguilar J-LGD, The Role of Skeletal Muscle in Amyotrophic Lateral Sclerosis, Brain Pathology. 26 (2016) 227–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ma G, Wang Y, Li Y, Cui L, Zhao Y, Zhao B, Li K., MiR-206, a key modulator of skeletal muscle development and disease, Int J Biol Sci. 11 (2015) 345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Vienberg S, Geiger J, Madsen S, Dalgaard LT, MicroRNAs in metabolism, Acta Physiologica. 219 (2017) 346–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Williams AH, Valdez G, Moresi V, Qi X, McAnally J, Elliott JL, Bassel-Duby R, Sanes JR, Olson EN, MicroRNA-206 Delays ALS Progression and Promotes Regeneration of Neuromuscular Synapses in Mice, Science. 326 (2009) 1549–1554. [DOI] [PMC free article] [PubMed] [Google Scholar]


