Abstract
Introduction
The clinical effect of pharmacotherapy on prostate morphometric parameters is largely unknown. The sole exception is 5α-reductase inhibitors (5-ARI) that reduce prostate volume and prostate-specific antigen (PSA). This review assesses the effect of pharmacotherapy on prostate parameters effect on prostate parameters, namely total prostate volume (TPV), transitional zone volume (TZV), PSA and prostate perfusion.
Material and methods
We conducted a systematic review and meta-analysis of randomized controlled trials (RCTs) reporting on morphometric parameters’ changes after pharmacotherapy, as primary or secondary outcomes. The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. RCTs’ quality was assessed by the Cochrane tool and the criteria of the Agency for Healthcare Research and Quality. The effect magnitude was expressed as standard mean difference (SMD). The study protocol was published on PROSPERO (CRD42020170172).
Results
Sixty-seven RCTs were included in the review and 18 in the meta-analysis. The changes after alpha-blockers are comparable to placebo. Long-term studies reporting significant changes from baseline, result from physiologic growth. Finasteride and dutasteride demonstrated large effect sizes in TPV reduction ([SMD]: -1.15 (95% CI: -1.26 to -1.04, p <0.001, and [SMD]:-0.66 (95% CI: -0.83 to -0.49, p <0.001, respectively), and similar PSA reductions. Dutasteride’s effect appears earlier (1st vs 3rd month), the changes reach a maximum at month 12 and are sustained thereafter. Phosphodiesterase-5 (PDE-5) inhibitors have no effect on morphometric parameters. Phytotherapy’s effect on TPV is non-significant [SMD]: 0.12 (95% CI: -0.03 to 0.27, p = 0.13). Atorvastatin reduces TPV as compared to placebo (-11.7% vs +2.5%, p <0.01). Co-administration of testosterone with dutasteride spares the prostate from the androgenic stimulation as both TPV and PSA are reduced significantly.
Conclusions
The 5-ARIs show large effect size in reducing TPV and PSA. Tamsulosin improves perfusion but no other effect is evident. PDE-5 inhibitors and phytotherapy do not affect morphometric parameters. Atorvastatin reduces TPV and PSA as opposed to testosterone supplementation.
Keywords: prostate volume changes, prostate perfusion, lower urinary tract pharmacotherapy, morphometric parameters
INTRODUCTION
Benign prostatic obstruction (BPO) is a common cause of lower urinary tract symptoms (LUTS) in men older than 50 years [1]. Benign prostatic enlargement (BPE) is defined as prostatic enlargement due to histologic benign prostatic hyperplasia [2]. BPO involves the static component or the physical mass of the prostate and the dynamic component or smooth muscle tone of the prostate stroma and the bladder neck [1, 2]. It is reasonable to assume a potential relation between prostate size, degree of obstruction and LUTS severity, but population-based studies failed to demonstrate a direct link [3]. Prostate morphometric parameters are prognostic indicators of BPE progression. Data analysis from the placebo arm of Medical Therapy of Prostatic Symptoms (MTOPS) trial showed that men with baseline total prostate volume (TPV) 31 ml and prostate-specific antigen (PSA) of 1.6 ng/dl or greater are at significantly higher risk of BPE progression, defined as a 4-point or more increase in AUA-SS, acute urinary retention, urinary incontinence, renal insufficiency or recurrent urinary tract infections [4]. Baseline flow rate, post-void residual and age were the additional predictors. TPV and PSA are among the baseline factors which could predict conservative treatment failure and/or the need for combination therapy [5]. Baseline PSA is higher in men with larger prostates and is associated with higher annual volume increase (2.2%) compared to smaller prostates (1.7%) [6]. However, a multivariate analysis of the Baltimore Longitudinal Study of Aging in 242 men without prostate cancer, reported no correlation between PSA or PSA changes and annual prostate growth rate during 4.2 years of follow-up [6]. The median rate of TPV and PSA change per year was 0.6 ml and 0.03 ng/ml respectively.
Existing data supports the hypothesis that ischemia of the lower urinary tract may cause BPE and LUTS. Azadzoi et al. were first to document bladder dysfunction and increased prostate contractility in an animal model of pelvic atherosclerosis [7]. The underlying mechanism of ischemic injury involves oxidative stress, free radical injury to smooth muscle cells, epithelium, mitochondria, endoplasmic reticulum and nerve fibers, impairment of the nitric oxide (NO/cGMP) pathway, activation of degenerative processes and deposition of collagen [7]. Chronic ischemia induces prostate stromal fibrosis, decreases cGMP and increases prostate tissue sensitivity to contractile stimuli [7].
The clinical effect of pharmacotherapy on prostate morphometric parameters is largely unknown. The sole exception is 5α-reductase inhibitors (5-ARI) which reduce TPV, transitional zone volume (TZV) and PSA. There is preclinical evidence that all medications influence prostate volume or perfusion. Experiments have shown the anti-apoptotic effect of sympathomimetics, and the potent apoptotic effect on human prostate cancer cell cultures of quinazoline-based α-blockers [8].
Phosphodiesterase-5 (PDE-5) inhibitors influence prostate cell proliferation via upregulation of NO/cGMP and Rho-kinase activity [9, 10]. Evidence supports that finasteride reduces prostate blood flow via downregulation of vascular endothelial growth factor (VEGF) [11]. Tamsulosin antagonizes vesical arteries adrenoceptors, thus improving LUT perfusion [12]. PDE5 inhibitors improve perfusion via the reduction of endothelin-1 levels and regulation of vascular smooth muscle cells proliferation [10].
This review aims to investigate the effect of both urological and non-urological medication on prostate morphometric parameters, namely TPV, TZV, PSA and prostate perfusion.
MATERIAL AND METHODS
Literature search
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [13]. The Embase, MEDLINE, Cochrane Database of Systematic Reviews, Cochrane Central (Cochrane Health Technology Assessment, Database of Abstracts of Reviews of Effects, Health Economics Evaluations Database) and Google Scholar were searched with no restriction on publication date. Additional sources for articles were the reference lists of included studies and relevant review articles.
Study selection
We included randomized-controlled trials (RCTs) of adult men with LUTS due to BPE, who received pharmacotherapy, and reported post-intervention changes of prostate parameters as primary or secondary outcome. The included studies had 10 participants minimum, were written in English language and used ultrasound or MRI to assess morphometric parameters. There was no restriction in study duration. In the event of open extension of double-blind studies, only data from the double-blind period were included. If data were not reported separately, studies were excluded.
Two reviewers (AG and DC) screened the titles and abstracts of identified records, and the full text of potentially eligible records was evaluated using a standardized form. Disagreement was resolved by discussion. If there was no agreement, a third independent party acted as an arbitrator (VS).
Data extraction
Data from eligible studies were extracted in duplicate. Discrepancies were resolved by a third reviewer. The variables assessed included the year of publication, number of randomized subjects, number of subjects who completed the follow up, baseline values and post treatment changes in morphometric parameters presented as mean (±standard deviation) and percentage changes from baseline.
Risk of bias and study quality assessment
Risk of bias (RoB) was assessed using the revised version of Cochrane Collaboration’s RoB Assessment tool [14]. Two reviewers (AG and DC) independently assessed RoB in each study, while a third reviewer (VS) acted as an arbitrator. The RoB was considered high if the confounder had not been considered by the individual study. The RoB tables were developed in Review Manager 5.3 (RevMan-Informatics and Knowledge Management Department, Cochrane, London, UK).
To ensure reliability and validity of measures and reported measurements, each included RCT had an overall rating based on the criteria developed by Agency for Healthcare Research and Quality (AHRQ). The ratings were ‘Low-risk’, ‘Moderate-risk’ or ‘High-risk’ [15, 16]. The RCTs should have been characterized as low risk in measurement bias (points 3d & 3e) based on the criteria developed by AHRQ.
Statistical analysis
The primary outcome was the post-intervention changes in TPV. The secondary outcomes were the changes in TZV, PSA and prostate perfusion as defined by the trialist. Owing to the expected heterogeneity, a narrative synthesis of all included studies was planned [17]. Data are presented as post-treatment absolute mean changes (±SD) and percentage changes.
Statistical heterogeneity was tested using chi-square test. A value of p <0.10 or I2 >50% was used to define heterogeneity. A list of potential confounders was developed a priori: use of LUTS-related medications, follow-up duration, LUTS not related to BPE, previous catheter use, previous LUT surgery and history of LUT malignancy.
A meta-analysis was considered for each endpoint if two or more RCTs had similar study design, dosing scheme and follow-up duration. Meta-analysis was conducted using RevMan. The effect magnitude was expressed as standard mean difference (SMD) with 95% confidence interval (CI) for continuous outcomes. The treatment effect size was considered small for SMD values of 0–0.2, moderate for SMD range 0.2–0.8 and large if SMD was >0.8.
RESULTS
Evidence acquisition: Study selection
Sixty-seven RCTs were eligible for inclusion (Figure 1). Eighteen were eligible for quantitative synthesis. The search was updated in October 2020.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.
Study characteristics
We identified 28 placebo-controlled RCTs and 39 non-placebo RCTs. Since the included RCTs had 2 or more study arms, we studied 36 active medications versus placebo comparisons and 48 active medications versus active medication comparisons. Phytotherapy’s effect on morphometric parameters was assessed in 18 comparisons, α-blockers’ effect in 18 comparisons, 5-ARI’s effect in 23 comparisons, PDE5’s effect in 6 comparisons, combination treatments in 10 comparisons, while 9 comparisons assessed the effect of non-urological medications. Among them, only 10 trials were powered to assess changes in morphometric parameters, while 57 reported a morphometric parameter change as secondary outcome. The characteristics of included RCTs are presented in Table 1.
Table 1.
The characteristics of included trials
| Study, [reference] | Comparator 1, Daily dosage | Comparator 2, Daily dosage | Comparator 3, Daily dosage | Comparator 4, Daily dosage | No. subjects randomized | Duration of Follow up | Reported parameters | Primary or Secondary endpoints | Study rating based on AHRQ criteria |
|---|---|---|---|---|---|---|---|---|---|
| Lepor 1996, [18] | Terazosin, 10 mg OD | Finasteride, 5 mg OD | Terazosin 10 mg OD plus Finasteride, 5 mg OD | Placebo | 1230 | 12 months | TPV, PSA | Secondary | Low Risk |
| McConnell 2003, [19] | Doxazosin, 4 or 8 mg OD | Finasteride, 5 mg OD | Doxazosin, 4 or 8 mg OD plus Finasteride, 5 mg OD | Placebo | 3047 | 4.5 years | TPV, PSA | Secondary | Low Risk |
| Yokoyama 2012, [20] | Tadalafil. 2.5 mg OD | Tadalafil 5 mg OD | Tamsulosin, 0.2 mg OD | Placebo | 612 | 3 months | PSA | Secondary | Low Risk |
| Roehrborn 2006, [21] | Alfuzosin, 10 mg OD | Placebo | n/a | n/a | 1522 | 24 months | PSA | Secondary | Low Risk |
| Roehrborn 2006, [22] | Alfuzosin, 10 mg OD | Placebo | n/a | n/a | 528 | 3 months | TPV, TZV | Primary | Moderate Risk |
| Turkeri 2001, [23] | Doxazosin, 4 mg OD | Placebo | n/a | n/a | 29 | 4 weeks | TPV, PSA | Secondary | High Risk |
| Debruyne 2002, [24] | Tamsulosin, 0.4 mg OD | Serenoa repens, 320 mg OD | n/a | n/a | 704 | 12 months | TPV, PSA | Primary | Low Risk |
| Sengupta 2011, [25] | Tamsulosin, 0.4 mg OD | Phytotherapy (Non-Sr), OD | n/a | n/a | 46 | 3 months | TPV | Secondary | Moderate Risk |
| Latil 2015, [26] | Tamsulosin, 0.4 mg OD | Hexanic Extract Serenoa repens, 320 mg OD | n/a | n/a | 203 | 3 months | TPV | Secondary | High Risk |
| Pande 2014, [27] | Tamsulosin, 0.4 mg OD | Silodosin, 8 mg OD | n/a | n/a | 61 | 3 months | TPV | Secondary | Moderate Risk |
| Karami 2016, [28] | Tamsulosin, 0.4 mg OD | Tadalafil, 20 mg OD | n/a | n/a | 119 | 3 months | PSA | Primary | High Risk |
| Griwan 2014, [99] | Tamsulosin, 0.4 mg OD | Naftopidil, 75 mg OD | n/a | n/a | 60 | 3 months | TPV | Secondary | Moderate Risk |
| HIzli 2007, [29] | Tamsulosin, 0.4 mg OD | Serenoa repens, 320 mg OD | n/a | n/a | 40 | 6 months | TPV, PSA | Secondary | High Risk |
| Odysanya 2017, [30] | Tamsulosin, 0.4 mg OD | Finasteride, 5 mg OD | Tamsulosin, 0.4 mg OD plus Finasteride, 5 mg OD | n/a | 60 | 6 months | TPV | Secondary | High Risk |
| Morgia 2014, [31] | Tamsulosin, 0.4 mg OD | Phytotherapy (Non-Sr) | Tamsulosin, 0.4 mg OD plus Phytotherapy (Non-Sr) |
n/a | 150 | 12 months | TPV, PSA | Secondary | Low Risk |
| Roehrborn 2010, [32] | Tamsulosin, 0.4 mg OD | Dutasteride, 0.5 mg OD | Tamsulosin, 0.4 mg OD plus Dutasteride, 0.5 mg OD | n/a | 3221 | 4 years | TPV, PSA | Secondary | Low Risk |
| Debruyne 1998, [33] | Alfuzosin SR, OD | Finasteride, 5 mg OD | Alfuzosin SR, OD plus Finasteride, 5 mg OD |
n/a | 707 | 6 months | TPV, PSA | Secondary | Low Risk |
| Sakalis 2018, [34] | Tamsulosin, 0.4 mg OD | Solifenacin, 5 or 10 mg OD | n/a | n/a | 69 | 6 months | TPV, TZV, PSA, Perfusion parameters | Primary | Moderate Risk |
| Andersen 1995, [35] | Finasteride, 5 mg OD | Placebo | n/a | n/a | 707 | 24 months | TPV, PSA | Secondary | Moderate Risk |
| Nickel 1996, [36] | Finasteride, 5 mg OD | Placebo | n/a | n/a | 613 | 24 months | TPV, PSA | Primary | Low Risk |
| McConnell 1998, [37] | Finasteride, 5 mg OD | Placebo | n/a | n/a | 312 | 48 months | TPV | Secondary | Low Risk |
| Marberger 1998, [38] | Finasteride, 5 mg OD | Placebo | n/a | n/a | 2902 | 24 months | TPV | Secondary | Moderate Risk |
| Kirby 1992, [39] | Finasteride, 5 mg OD | Finasteride, 10 mg OD | Placebo | n/a | 66 | 3 months | TPV, PSA | Secondary | High Risk |
| Finasteride group 1993, [40] | Finasteride, 1 mg OD | Finasteride, 5 mg OD | Placebo | n/a | 750 | 12 months | TPV, PSA | Secondary | Moderate Risk |
| Tammela 1995, [41] | Finasteride, 5 mg OD | Placebo | n/a | n/a | 36 | 6 months | TPV | Secondary | High Risk |
| Pannek 1998, [42] | Finasteride, 5 mg OD | Placebo | n/a | n/a | 34 | 6 months | TPV, PSA | Secondary | High Risk |
| Marks 1997, [43] | Finasteride, 5 mg OD | Placebo | n/a | n/a | 41 | 6 months | TPV, PSA | Secondary | Moderate Risk |
| Gormley 1992, [44] | Finasteride, 5 mg OD | Placebo | n/a | n/a | 597 | 12 months | TPV, PSA | Secondary | Moderate Risk |
| Roehrborn 2002, [45] | Dutasteride, 0.5 mg OD | Placebo | n/a | n/a | 4325 | 24 months | TPV, TZV, PSA | Secondary | Low Risk |
| Na 2012, [46] | Dutasteride, 0.5 mg OD | Placebo | n/a | n/a | 253 | 6 months | TPV, PSA | Primary | Moderate Risk |
| Tsukamoto 2009, [47] | Dutasteride, 0.5 mg OD | Placebo | n/a | n/a | 378 | 6 months | TPV, PSA | Secondary | Moderate Risk |
| Andriole 2010, [48] | Dutasteride, 0.5 mg OD | Placebo | n/a | n/a | 8231 | 48 months | TPV | Secondary | Moderate Risk |
| Nickel 2011, [49] | Finasteride, 5 mg OD | Dutasteride, 0.5 mg OD | n/a | n/a | 1630 | 12 months | TPV, PSA | Primary | Moderate Risk |
| Carraro 1996, [50] | Finasteride, 5 mg OD | Serenoa repens, 320 mg OD | n/a | n/a | 1098 | 6 months | TPV, PSA | Secondary | Low Risk |
| Kuo 1998, [51] | Dibenyline, 10 mg BD | Finasteride, 5 mg OD | n/a | n/a | 125 | 6 months | TPV | Secondary | High Risk |
| Jeong 2009, [52] | a blocker OD plus Finasteride, 5 mg OD |
a blocker OD plus Dutasteride, 0.5 mg OD |
n/a | n/a | 120 | 24 months | TPV, PSA | Secondary | Moderate Risk |
| Pinggera 2014, [53] | Tadalafil, 5 mg OD | Placebo | n/a | n/a | 97 | 8 weeks | Perfusion parameters | Primary | Moderate Risk |
| Morgia 2018, [54] | Serenoa repens plus Selenium, OD | Tadalafil, 5 mg OD | n/a | n/a | 427 | 6 months | TPV, PSA | Secondary | Moderate Risk |
| Kosilov 2019, [55] | Tadalafil, 5 mg OD | Tadalafil, 5 mg OD plus Solifenacin, 10 mg OD | n/a | n/a | 214 | 12 months | TPV | Secondary | High Risk |
| Oztrurk 2011, [56] | Alfuzosin XL OD | Alfuzosin XL OD plus Sildenafil, 50 mg OD | n/a | n/a | 100 | 3 months | TPV, PSA | Secondary | High Risk |
| Joo 2012, [57] | Tamsulosin, 0.2 mg OD | Tamsulosin, 0.2 mg OD plus Dutasteride, 0.5 mg OD |
n/a | n/a | 216 | 12 months | TPV, TZV, PSA | Secondary | High Risk |
| Choi 2016, [58] | Tamsulosin, 0.2 mg OD | Tamsulosin, 0.2 mg OD plus Dutasteride, 0.5 mg OD |
n/a | n/a | 118 | 12 months | TPV, TZV, PSA | Secondary | Low Risk |
| Mohanty 2006, [59] | Tamsulosin, 0.4 mg OD plus Finasteride, 5 mg OD |
Tamsulosin, 0.4 mg OD plus Dutasteride, 0.5 mg OD |
n/a | n/a | 106 | 6 months | TPV, PSA | Secondary | High Risk |
| Yamanishi 2017, [60] | Tamsulosin, 0.2 mg OD plus Dutasteride, 0.5 mg OD |
Tamsulosin, 0.2 mg OD plus Dutasteride, 0.5 mg OD plus imidafenacin, 0.2 mg OD |
n/a | n/a | 163 | 24 weeks | TPV, PSA | Secondary | Moderate Risk |
| Ryu 2014, [61] | Tamsulosin, 0.2 mg OD | Tamsulosin, 0.2 mg OD plus Serenoa repens, 320 mg OD |
n/a | n/a | 120 | 12 months | TPV, PSA | Secondary | Moderate Risk |
| Argirovic 2013, [62] | Tamsulosin, 0.4 mg OD | Serenoa repens, 320 mg OD | Tamsulosin, 0.4 mg OD plus Serenoa repens, 320 mg OD |
n/a | 184 | 6 months | TPV, PSA | Secondary | High Risk |
| Beiraghdar 2017, [63] | Phytotherapy (Non-Sr) | Placebo | n/a | n/a | 86 | 2 weeks | TPV | Secondary | Moderate Risk |
| Berges 1995, [64] | Phytotherapy (Non-Sr) | Placebo | n/a | n/a | 163 | 6 months | TPV | Secondary | Moderate Risk |
| Safarinejad 2005, [65] | Phytotherapy (Non-Sr) | Placebo | n/a | n/a | 620 | 6 months | TPV, PSA | Secondary | High Risk |
| Bent 2006, [66] | Serenoa repens, 160 mg BD | Placebo | n/a | n/a | 225 | 12 months | TPV, TZV, PSA | Secondary | Low Risk |
| Marks 2000, [67] | Serenoa repens | Placebo | n/a | n/a | 44 | 24 weeks | TPV, TZV, PSA | Secondary | Moderate Risk |
| Ye 2019, [68] | Serenoa repens, 320 mg OD | Placebo | n/a | n/a | 325 | 24 weeks | TPV, PSA | Secondary | Low Risk |
| Zhang 2008, [69] | Phytotherapy (Non-Sr) | Placebo | n/a | n/a | 49 | 4 months | TPV | Secondary | High Risk |
| Shi 2008, [70] | Serenoa repens | Placebo | n/a | n/a | 94 | 12 weeks | TPV, PSA | Secondary | Moderate Risk |
| Guzman 2019, [71] | Phytotherapy (Non-Sr), OD | Terazosin, 5 mg OD | n/a | n/a | 100 | 6 months | TPV | Secondary | Moderate Risk |
| Braeckman 1997, [72] | Serenoa repens, 320 mg OD | Serenoa repens, 160 mg OD | n/a | n/a | 84 | 12 months | TPV | Secondary | High Risk |
| Allott 2019, [73] | Statin users | Non- Statin users | n/a | n/a | 4106 | 48 months | TPV | Primary | Moderate Risk |
| Mills 2007, [74] | Atorvastatin, 80 mg OD | Placebo | n/a | n/a | 350 | 26 weeks | TPV, TZV, PSA | Secondary | Low Risk |
| Zhang 2015, [75] | Atorvastatin, 20 mg OD | Placebo | n/a | n/a | 81 | 12 months | TPV, PSA | Secondary | Moderate Risk |
| Safwat 2018, [76] | Tamsulosin, 0.4 mg OD | Tamsulosin, 0.4 mg OD plus Cholecalciferol 600IU OD |
n/a | n/a | 389 | 24 months | TPV, PSA | Secondary | Moderate Risk |
| Ghadian 2017, [77] | Ω3 300 mg plus Tamsulosin 0.4 mg plus Finasteride 5 mg | Tamsulosin 0.4 mg plus Finasteride 5 mg | n/a | n/a | 100 | 6 months | TPV | Secondary | High Risk |
| Di Silverio 2005, [78] | Finasteride, 5 mg OD | Finasteride, 5 mg OD plus Rofecoxib, 25 mg OD | n/a | n/a | 46 | 6 months | TPV, PSA | Secondary | Moderate Risk |
| Goodarzt 2011, [79] | Terazosin, 2 mg OD | Terazosin, 2 mg OD plus Celecoxib, 200 mg OD | n/a | n/a | 160 | 12 weeks | TPV, PSA | Secondary | High Risk |
| Jhang 2013, [80] | Doxazosin, 4 mg OD | Doxazosin, 4 mg OD plus Celecoxib, 200 mg OD | n/a | n/a | 122 | 3 months | TPV, PSA | Secondary | High Risk |
| Page 2011, [81] | Testosterone 1% 7.5 mg OD plus placebo | Testosterone 1% 7.5 mg OD plus Dutasteride, 0.5 mg OD |
n/a | n/a | 53 | 6 months | TPV, PSA | Secondary | Moderate Risk |
| Kacker 2014, [82] | Testosterone plus placebo | Testosterone plus Dutasteride, 0.5 mg OD | n/a | n/a | 23 | 12 months | TPV, PSA | Primary | Moderate Risk |
| Chung 2011, [83] | a blocker OD plus 5ARI | a blocker OD plus 5ARI plus Tolterodine |
n/a | n/a | 137 | 12 months | TPV, TZI, PSA | Secondary | Moderate Risk |
AHRQ – Agency for Healthcare Research and Quality; BD – Twice Daily; n/a – not applicable; Non-Sr – other than Serenoa repens; OD – once daily; PSA – prostate-specific antigen; Sr – Serenoa repens; TPV – total prostate volume; TZI – transitional zone index; TZV – transitional zone volume
Assessment of study quality
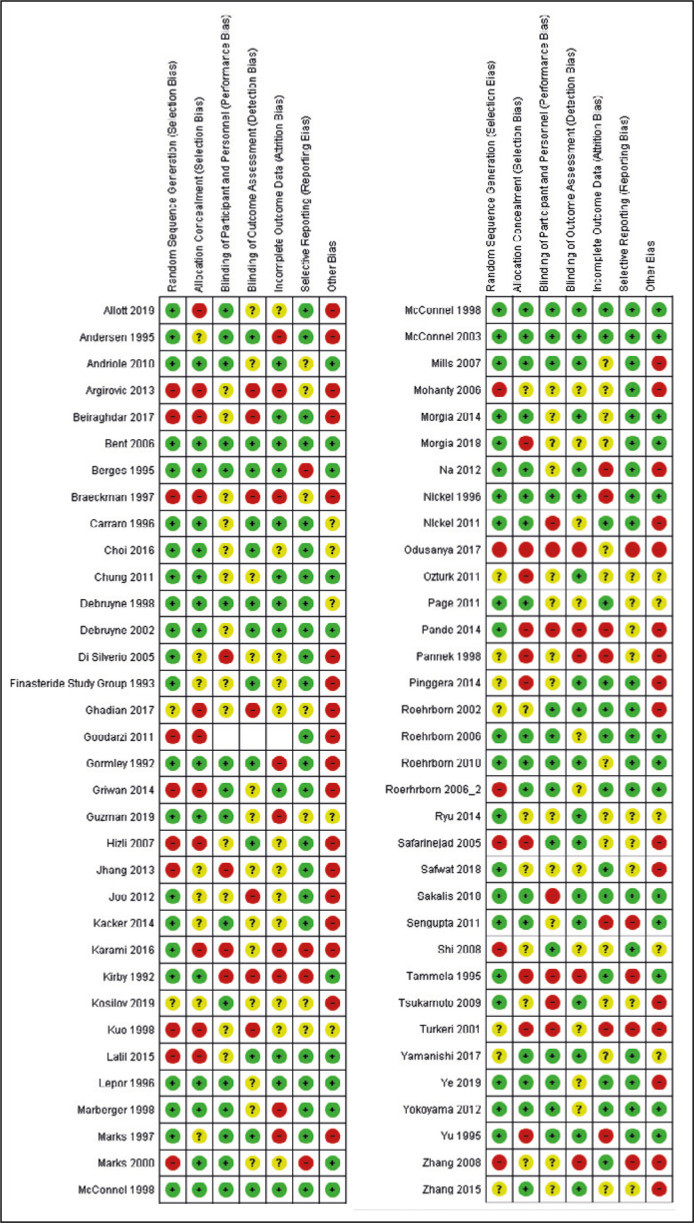
The summary of RoB assessment is presented in Figure 2 and Figure 3. Based on AHRQ criteria, 16 RCTs were graded as low-risk, 31 as moderate-risk and 20 as high-risk (Table 2).
Figure 2.
The risk of bias summary.
Figure 3.
The risk of bias graph.
Table 2.
Detailed rating for included trials based on criteria developed by the Agency for Healthcare Research and Quality (AHRQ). The ratings were ‘Low-risk’, ‘Moderate-risk’ or ‘High-risk’
| Study | Individual Quality Assessment Criteria Ratings | Overall Rating | COI Absent? | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1a | 1b | 1c | 2a | 2b | 3a | 3b | 3c | 3d | 3e | 4 | 5 | |||
| Lepor 1996, [18] | LR | LR | LR | UR | LR | LR | UR | LR | LR | LR | LR | LR | Low Risk | No |
| McConnell 2003, [19] | LR | LR | LR | UR | LR | LR | UR | LR | LR | LR | LR | LR | Low Risk | No |
| Yokoyama 2012, [20] | LR | UR | LR | UR | LR | HR | UR | LR | LR | LR | LR | LR | Low Risk | No |
| Roerhborn 2006, [21] | LR | UR | LR | LR | LR | UR | UR | LR | LR | LR | LR | LR | Low Risk | No |
| Roerhborn 2006, [22] | HR | HR | LR | LR | LR | UR | LR | LR | LR | LR | LR | LR | Moderate Risk | No |
| Turkeri 2001, [23] | UR | UR | HR | HR | LR | UR | UR | UR | LR | LR | HR | HR | High Risk | Unclear |
| Debruyne 2002, [24] | LR | LR | LR | UR | LR | LR | LR | UR | LR | LR | LR | LR | Low Risk | No |
| Sengupta 2011, [25] | LR | LR | LR | UR | UR | LR | UR | UR | LR | LR | HR | HR | Moderate Risk | No |
| Latil 2015, [26] | HR | HR | LR | UR | LR | UR | UR | UR | LR | LR | LR | LR | High Risk | Unclear |
| Pande 2014, [27] | LR | HR | LR | LR | HR | HR | UR | LR | LR | LR | HR | UR | Moderate Risk | Unclear |
| Karami 2016, [28] | LR | HR | HR | UR | HR | UR | HR | HR | LR | LR | HR | HR | High Risk | Unclear |
| Hizli 2007, [29] | HR | HR | HR | UR | LR | HR | HR | UR | LR | LR | UR | LR | High Risk | Unclear |
| Odusanya 2017, [30] | HR | HR | LR | HR | UR | HR | HR | HR | LR | LR | UR | HR | High Risk | Unclear |
| Morgia 2014, [31] | LR | LR | LR | UR | LR | LR | UR | LR | LR | LR | UR | LR | Low Risk | Unclear |
| Roehrborn 2010, [32] | LR | LR | LR | LR | UR | UR | UR | LR | LR | LR | LR | LR | Low Risk | Unclear |
| Debruyne 1998, [33] | LR | LR | LR | UR | LR | LR | UR | LR | LR | LR | LR | LR | Low Risk | Unclear |
| Sakalis 2018, [34] | LR | LR | LR | HR | HR | LR | LR | HR | LR | LR | LR | LR | Moderate Risk | Yes |
| Andersen 1995, [35] | LR | UR | LR | LR | LR | UR | UR | LR | LR | LR | HR | LR | Moderate Risk | No |
| Nickel 1996, [36] | LR | LR | UR | LR | LR | LR | LR | LR | LR | LR | HR | LR | Low Risk | No |
| McConnel 1998, [37] | LR | LR | LR | LR | LR | LR | LR | LR | LR | LR | LR | LR | Low Risk | No |
| Marberger 1998, [38] | LR | UR | LR | UR | LR | LR | LR | LR | LR | LR | HR | LR | Moderate Risk | No |
| Kirby 1992, [39] | LR | UR | UR | HR | HR | HR | HR | UR | LR | LR | LR | HR | High Risk | Unclear |
| Finasteride group 1993, [40] | LR | UR | UR | UR | UR | UR | HR | HR | LR | LR | UR | LR | Moderate Risk | No |
| Tammela 1995, [41] | LR | HR | UR | HR | HR | UR | HR | HR | LR | LR | LR | HR | High Risk | Unclear |
| Pannek 1998, [42] | UR | HR | HR | UR | UR | UR | UR | UR | LR | LR | HR | UR | High Risk | Unclear |
| Marks 1997, [43] | LR | UR | UR | LR | UR | UR | UR | UR | LR | LR | HR | LR | Moderate Risk | Unclear |
| Gormley 1992, [44] | LR | LR | UR | UR | UR | LR | LR | LR | LR | LR | HR | LR | Moderate Risk | Unclear |
| Roehrborn 2002, [45] | UR | UR | UR | LR | LR | LR | LR | LR | LR | LR | LR | LR | Low Risk | Unclear |
| Na 2012, [46] | LR | LR | UR | UR | UR | LR | LR | LR | LR | LR | LR | LR | Moderate Risk | Unclear |
| Tsukamoto 2009, [47] | LR | UR | UR | HR | HR | UR | UR | UR | LR | LR | UR | UR | Moderate Risk | No |
| Andriole 2010, [48] | LR | LR | UR | UR | UR | UR | LR | UR | LR | LR | LR | UR | Moderate Risk | No |
| Nickel 2011, [49] | LR | LR | UR | HR | UR | UR | LR | UR | LR | LR | LR | LR | Moderate Risk | No |
| Carraro 1996, [50] | LR | LR | LR | UR | LR | LR | UR | UR | LR | LR | LR | LR | Low Risk | Unclear |
| Kuo 1998, [51] | HR | UR | UR | HR | UR | UR | LR | LR | LR | LR | UR | HR | High Risk | Unclear |
| Jeong 2009, [52] | UR | UR | LR | LR | UR | UR | UR | UR | LR | LR | LR | UR | Moderate risk | Unclear |
| Jeong 2009, [52] | UR | UR | LR | LR | UR | UR | UR | UR | LR | LR | LR | UR | Moderate risk | Unclear |
| Pinggera 2014, [53] | UR | UR | LR | LR | LR | UR | UR | UR | LR | LR | LR | LR | Moderate Risk | No |
| Morgia 2018, [54] | LR | HR | LR | UR | LR | UR | UR | UR | LR | LR | UR | LR | Moderate Risk | No |
| Kosilov 2019, [55] | UR | UR | HR | LR | UR | UR | UR | UR | LR | LR | UR | UR | High Risk | Unclear |
| Ozturk 2011, [56] | UR | HR | LR | UR | UR | LR | HR | UR | LR | LR | UR | UR | High Risk | Unclear |
| Joo 2012, [57] | LR | UR | UR | UR | HR | UR | UR | LR | LR | LR | UR | UR | High risk | Unclear |
| Choi 2016, [58] | LR | LR | UR | LR | UR | UR | UR | LR | LR | LR | LR | UR | Low Risk | Yes |
| Mohanty 2006, [59] | HR | UR | UR | UR | UR | UR | UR | UR | LR | LR | UR | UR | High Risk | Unclear |
| Yamanishi 2017, [60] | UR | LR | LR | LR | LR | LR | LR | UR | LR | LR | UR | LR | Moderate Risk | No |
| Ryu 2014, [61] | LR | UR | UR | UR | UR | UR | UR | UR | LR | LR | UR | UR | Moderate Risk | Unclear |
| Argirovic 2013, [62] | HG | HG | HR | UR | UR | HR | UR | HR | LR | LR | HR | UR | High Risk | Unclear |
| Beiraghdar, 2017 [63] | HR | HR | LR | LR | UR | HR | UR | LR | LR | LR | LR | LR | Moderate risk | Yes |
| Berges, 1995 [64] | LR | LR | LR | LR | LR | UR | UR | LR | LR | LR | UR | LR | Moderate risk | No |
| Safarinejad, 2005 [65] | HR | HR | LR | UR | LR | LR | LR | UR | LR | LR | UR | UR | High Risk | Yes |
| Bent, 1995 [66] | LR | LR | LR | LR | UR | LR | UR | LR | LR | LR | LR | UR | Low Risk | Unclear |
| Marks, 2000 [67] | LR | LR | HR | LR | LR | LR | UR | HR | LR | LR | UR | HR | Moderate risk | Unclear |
| Ye, 2019 [68] | LR | LR | LR | UR | LR | LR | UR | LR | LR | LR | LR | LR | Low Risk | No |
| Zhang 2008, [69] | HR | UR | LR | UR | UR | LR | HR | HR | LR | LR | LR | HR | High Risk | Unclear |
| Shi, 2008, [70] | LR | LR | HR | LR | LR | LR | UR | UR | LR | LR | UR | LR | Moderate risk | Unclear |
| Guzman 2019, [71] | LR | LR | LR | LR | HR | LR | UR | HR | LR | LR | HR | UR | Moderate Risk | No |
| Braeckman 1997, [72] | HR | HR | HR | UC | UC | HR | UC | HR | LR | LR | HR | HR | High Risk | Unclear |
| Allott 2019, [73] | LR | HR | UR | UR | UR | UR | LR | UR | LR | LR | UR | LR | Moderate Risk | Unclear |
| Mills 2007, [74] | LR | LR | UR | UR | LR | LR | LR | LR | LR | LR | UR | LR | Low Risk | No |
| Zhang 2015, [75] | UR | LR | UR | LR | UR | UR | UR | LR | LR | LR | UR | LR | Moderate Risk | Yes |
| Safwat 2018, [76] | LR | UR | UR | LR | LR | UR | UR | HR | LR | LR | UR | UR | Moderate Risk | Yes |
| Ghadian 2017, [77] | UR | HR | UR | LR | UR | UR | UR | UR | LR | LR | UR | UR | High Risk | Unclear |
| Di Silverio 2005, [78] | LR | UR | UR | UR | UR | UR | UR | LR | LR | LR | HR | UR | Moderate Risk | Unclear |
| Goodarzt 2011, [79] | HR | HR | UR | UR | UR | UR | UR | UR | LR | LR | LR | UR | High Risk | Unclear |
| Jhang 2013, [80] | HR | UR | UR | UR | UR | HR | UR | UR | LR | LR | UR | UR | High Risk | Unclear |
| Page 2011, [81] | LR | LR | UR | UR | LR | UR | UR | UR | LR | LR | LR | UR | Moderate Risk | Unclear |
| Kacker 2014, [82] | LR | UR | LR | UR | UR | LR | UR | UR | LR | LR | UR | UR | Moderate Risk | Unclear |
| Chung 2011, [83] | LR | LR | UR | UR | UR | LR | LR | HR | LR | LR | LR | LR | Moderate Risk | Unclear |
| Griwan 2014, [99] | LR | HR | UR | LR | UR | UR | UR | LR | UR | UR | LR | LR | Moderate Risk | Unclear |
Data Synthesis: α1-blockers
Six trials randomized men (n = 4525) to α-blocker versus placebo (Table 1) [18–23]. The MTOPS randomized men to receive doxazosin, finasteride, combination or placebo and reported +24% (+10.1 ml) change in TPV of patients receiving doxazosin at 4 years, similar to placebo (+24% or +8.8 ml) [19]. The Veteran Affairs Cooperative Study (VA-COOP Study) reported similar changes in terazosin and placebo arms at 12 months (+2.0% or +0.5 ml vs +2.3% or +0.5 ml) [18]. The ALFUS trial reported non-significant changes from baseline at 3 months in men who received alfuzosin or placebo in both TPV (-2% or 0.25 ml vs +3% or +0.46 ml) and TZV (-2% vs -5% or -0.8 ml vs -0.39 ml) [22]. Five RCTs reported on post treatment PSA changes, which were similar to placebo [18–21, 23]. There was no information on prostate perfusion parameters.
Ten RCTs randomized men (n = 5479) to an α-blocker versus an active comparator with a follow-up to 24 weeks [24–33]. All studies reported non-significant TPV changes from baseline (-3.4% to +9.5% or -1.4 ml to +6.32 ml). CombAT randomized men to receive tamsulosin, dutasteride or combination and followed them up for 4.5 years [32]. Men in the tamsulosin arm increased TPV by +4.6% (+2.57 ml) and TZV by +18.2% (+5.5 ml). A single trial compared tamsulosin to silodosin and reported a reduction of TPV after 6 months, which was greater in the silodosin arm (-2.8% vs -8.6% or -1.0 ml vs -3.6 ml, p = 0.594) [27]. TPV changes after 3-months of Naftopidil treatment were negligible and comparable to tamsulosin [99]. A trial with high RoB reported +9.5% (+6.32 ml) increase in TPV after 6 months tamsulosin monotherapy, which was neither significantly different from baseline (p = 0.17) nor from the comparator [30]. The Alfin study reported no significant change in TPV (-1% or -0.2 ml) or PSA value (+3.3% or +0.1 ng/dl) after 6 months of alfuzosin treatment [33]. PSA was reported unchanged in four tamsulosin studies (-5.0% to +7.4%) [24, 28, 29, 31]. Tamsulosin monotherapy enhanced prostate perfusion (+146%) as opposed to tamsulosin and solifenacin combination treatment (-41%) in a male overactive bladder (OAB) cohort [34].
5-ARIs
Sixteen trials randomized men (n = 21109) to 5-ARI versus placebo (Table 1) [18, 19, 35–48]. Twelve finasteride trials reported significant changes in TPV as compared to baseline and to placebo [18, 19, 35–44]. The quantitative synthesis revealed a large effect size in favor of finasteride [SMD]: -1.15 (95%CI: -1.26 to -1.04, p <0.001) (Figure 2). The effect on TPV varies between studies with different follow-ups. Trials with 3-6 months’ follow-up report changes between -4.8% and -26.1%, while trials with follow-up of 12 months or longer report higher TPV changes (-15.3% to -22.4% or -8.1 ml to -10.53 ml). The finasteride study group randomized men to finasteride 1 mg versus finasteride 5 mg versus placebo, and reported similar TPV changes at 12 months (-23.6% vs -22.4% vs -5%), but the later was superior in improvement of clinical parameters such as maximum flow rate and relevant questionnaires scores [40].
Four dutasteride trials reported significant changes in TPV both from baseline or as compared to placebo [45-48]. The quantitative analysis revealed a large size effect [SMD]: -0.66 (95%CI: -0.83 to -0.49, p <0.001) (Figure 4). The effect on TPV appears homogenous among studies with different follow-up and ranges between -17.5% and- 27.0% (-7.2 ml to -13.6 ml). Dutasteride also significantly reduces TZV (-20.1% or -7.1 ml, p <0.001), an effect which is evident from the first month of treatment.
Figure 4.
Meta-analysis of 5-ARI effect on prostate morphometric parameters in placebo-controlled trials. A) Forrest plot of the effect of finasteride versus placebo on total prostate volume (TPV). B) Forrest plot of the effect of dutasteride versus placebo on total prostate volume (TPV). C) Forrest plot of the effect of finasteride versus placebo on prostate-specific antigen (PSA). D) Forrest plot of the effect of finasteride on total prostate volume in placebo-controlled trials with 6 months follow-up.
CI – confidence interval; SD – standard deviation
Nine finasteride RCTs report significant changes in PSA as compared to baseline or to placebo [18, 19, 35, 36, 39, 40, 42, 43, 44]. The quantitative analysis revealed a moderate size effect in favor of finasteride ([SMD]:-0.63, 95%CI:-0.76 to 0.51), p <0.001) (Figure 4). Trials with 12 months follow-up or more report a PSA change of -46.0% to -52%. Three dutasteride RCTs, report significant reduction in PSA (-42.2% to -52.4%), compared to both baseline (p <0.05) and placebo (p <0.05) [45, 46, 47].
Five RCTs randomized men (n = 3615) to finasteride versus an active comparator. All studies report significant TPV changes from baseline (-10.5% to -24.3% or -4.3 ml to -7.5 ml) and significant difference from the active comparator [30, 33, 49, 50, 51]. The dutasteride arm of CombAT reported -28.0% (-15.3 ml) and -26.5% (-8.03 ml) reduction of TPV and TZV, respectively [32]. The EPICS study randomized men to finasteride or dutasteride for 12 months and found significant change from baseline in both arms (-26.7% vs -26.3% or -13.99 ml vs -14.2 ml) without intergroup difference (p = 0.65) [49]. Another trial reported similar changes after 12 months’ treatment with finasteride or dutasteride (-24.5% vs -26.1% or -9.76 ml vs -10.2 ml) but a significant increase of TPV (+11.2% vs 8.66%) 12 months after discontinuation of 5-ARI therapy [52]. PSA changes were different from baseline (-47.7% vs 49.5%, p <0.01), without difference between groups (p = 0.776). The ALFIN study reported a -50% change (-1.7 ±1.9, p <0.05) in PSA from baseline [33]. There was no information on prostate perfusion parameters.
PDE-5 inhibitors
Yokoyama et al., randomized 612 men to receive tadalafil 2.5 mg, tadalafil 5 mg, tamsulosin 0.2 mg or placebo for 3 months (Table 3) [20]. Authors reported non-significant changes in PSA from baseline in either tadalafil group (-7% vs -2%) that were similar to placebo. Pinggera et al., reported that tadalafil does not affect prostate perfusion as evaluated by 3 basic perfusion parameters [53]. There was no information on TPV and TZV changes.
Table 3.
Baseline and outcome measures of included studies
| Study Description | Results | Outcome | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Active medication | Placebo | ||||||||||
| Author (yr), [ref] (RoB overall rating) | Comparison | Main inclusion criteria | Study duration | Randomized patients (N) in each arm | Baseline mean (mls), ±SD | Total No. of patients (N) Analysed | Mean change from baseline, ±SD) (p value) (% mean change ±SD, p value) | Baseline mean (mls), ±SD | Total No. of patients (N) Analysed | Mean change from baseline, ±SD) (p value) (% mean change ±SD, p value) | |
| Beiraghdar 2017, [63] Moderate Risk |
Viola odorata, Echiumamoneum and Physalis Alkekengi vs Placebo | Men 40–75 yo, with LUTS due to BPH, Prostate volume >30 ml, IPSS ≥13 | 2 weeks | 57 vs 29 | TPV: 37.25 ±2.2 | 57 | TPV: not given absolute values (-16.92% ±0.89) (p <0.001) |
TPV: 42.67 ±4.3 | 29 | TPV: not given absolute values (+2.91% ±0.81) NS |
Significant reduction in TPV in phytotherapy vs placebo (p <0.001) |
| Berges 1995, [64] Moderate Risk |
β-sitosterol vs Placebo | Men <75 yo, Qmax <15 ml/s and residual volume 20–150 ml | 6 months | 83 vs 80 | TPV: 44.6 ±19.4 | 83 | TPV: -3.1 ±8.8 (-6.95%) NS |
TPV: 48.0 ±27.9 | 80 | TPV: -0.3 ±9.0 (-0.6%) NS |
Non-significant change in TPV compared to baseline or between groups |
| Safarinejad 2005, [65] High Risk |
Urtica Diopa vs Placebo | Men 55–72 yo, with LUTS due to BPH | 6 months | 305 vs 315 | TPV: 40.1 ±6.8 PSA: 2.4 ±1.4 |
287 | TPV: -3.8 ±5.94 (-9.47%) (p <0.01) PSA: -0.2 ±1.31 (-8.34%) NS |
TPV: 40.8 ±6.2 PSA: 1.8 ±1.4 |
271 | TPV:-0.2 ±5.73 (-0.5%) NS PSA: +0.01 ±1.0 (+1.0%) NS |
Significant reduction in TPV in phytotherapy group from baseline (p <0.01) |
| Bent 2006, [66] Low Risk |
Saw Palmetto vs Placebo | Men >49 yo, with moderate to severe LUTS due to BPH, Qmax 8–15 ml/s, PVR <250 | 52 weeks | 112 vs 113 | TPV: 34.7 ±13.9 TZV: 13.2 ±10.4 PSA: 1.8 ±1.4 |
112 | TPV: +3.76 ±10.4 (+10.8%) (NP) TZV: +3.26 ±10.9 (+25.3%) (NP) PSA: -0.005 ±0.74 (-0.3%) (NP) |
TPV: 33.9 ±15.2 TZV: 12.5 ±11.0 PSA: 1.6 ±1.4 |
113 | TPV: +4.98 ±10.2 (+14.7%) (NP) TZV: +2.01 ±10.7 (+15.1%) (NP) PSA: +0.15 ±0.74 (+8.8%) (NP) |
Non-significant changes in prostate size and PSA between groups |
| Marks 2000, [67] Moderate Risk |
Saw Palmetto vs Placebo | Men 45–80 yo, with IPSS >9, PSA <15 ng/dl, Prostate volume >30 ml | 24 weeks | 21 vs 23 | TPV: 58.5 ±6.5 TZV: 32.2 ±6.3 PSA: 2.67 ±0.4 |
21 | TPV: +3.42 ±6.9 (+5.8%) (NS) TZV: -0.92 ±6.3 (-2.9%) (NS) PSA: +0.13 ±0.46 (+4.9%) (NS) |
TPV: 55.5 ±5.6 TZV: 27.4 ±4.6 PSA: 4.06 ±0.7 |
23 | TPV: +0.22 ±5.7 (+0.5%) (NS) TZV: +0.42 ±4.7 (+1.5%) (NS) PSA: -0.17 ±0.79 (-4.2%) (NS) |
Non-significant changes in prostate size and PSA between groups. Saw palmetto - epithelial contraction in the transition zone (p <0.01) |
| Ye 2019, [68] Low Risk |
Saw Palmetto vs Placebo | Men 50–70 yo, with LUTS due to BPH, IPSS ≤19, Stable sexual life, 2 week BPH medication withdrawal | 24 weeks | 159 vs 166 | TPV: 34.3 ±18.3 PSA: 2.41 ±4.6 |
150 | TPV: +0.77 ±9.4 (+2.25%) (NS) PSA: -0.24 ±1.36 (-9.96%) (NS) |
TPV: 34.4 ±22.1 PSA: 1.99 ±2.5 |
154 | TPV:+0.31 ±11.4 (+0.9%) (NS) PSA: -0.01 ±2.3 (-1.0%) (NS) |
Non significant change in TPV (p = 0.74) and in PSA (p = 0.289) compared to baseline. No difference between groups |
| Zhang 2008, [69] High Risk |
Flaxseed LIgnan Extractvs Placebo | Men 55–80 yo, IPSS ≥7, Prostate volume ≥30 ml, Qmax 5–15 ml/s, normal kidney function | 4 months | 25 vs 24 | TPV: 46.7 ±3.7 | 25 | TPV: -5.39 ±4.5 (-11.5%) (p<0.01) |
TPV: 41.01 ±2.4 | 24 | TPV: -6.6 ±6.1 (-16.1%) (p<0.01) |
Significant reduction in TPV from baseline). Non-significant difference between groups |
| Shi 2008, [70] Moderate Risk |
Saw Palmetto vs Placebo | Men 49–75 yo, treatment naïve, LUTS due to BOH, clinical BPH on DRE, PSA ≤4 ng/dl | 12 weeks | 46 vs 48 | TPV: 47.72 ±8.1 PSA: 1.84 ±0.88 |
46 | TPV: -2.08 ±6.12 (-4.4%) NS PSA: -0.05 ±0.78 (-2.7%) (NS) |
TPV: 48.38 ±7.4 PSA: 1.9 5 ±1.03 |
46 | TPV: -2.48 ±6.4 (-5.1%) NS PSA: -0.26 ±0.65 (-13.8%) (NS) |
Non significant difference between groups in TPV (p = 0.826) and PSA (p = 0.305). |
| Andersen 1995, [35] Moderate Risk |
Finasteride 5 mg vs Placebo | Men age ≤80yo, Qmax 5–15 ml/s, LUTS (2 moderate symptoms), enlarged prostate on DRE, PSA ≤10 ng/dl, PVR ≤150 mls | 24 months | 354 vs 353 | TPV: 40.6 mls PSA: NR |
197 | TPV: -19.2 ±23.27 (-17.9%) (p <0.01) PSA: -52% (p <0.001) |
TPV: 41.7 mls PSA: NR |
197 | TPV: +11.5 ±23.8 (+11.5%) (p <0.05) PSA: +6% (NS) |
Significant difference between groups in TPV (p <0.01) and PSA (p <0.001) |
| Nickel 1996, [36] PROSPECT Study Low Risk |
Finasteride 5 mg vs Placebo | Men age ≤80 yo, Qmax 5–15 ml/s, LUTS (2 moderate symptoms), enlarged prostate on DRE, PSA ≤10 ng/dl, PV R ≤150 mls | 24 months | 310 vs 303 | TPV: 44.1 ±23.5 PSA: not reported |
246 | TPV: -8.63 ±9.04 (-21.0%) (p <0.05) PSA: -50% (p <0.01) |
TPV: 45.8 ±22.4 PSA: not reported |
226 | TPV: +3.84 ±11.4 (+8.4%) NS PSA: +13.3% (p <0.01) |
Siignificant difference between groups (P <0.01) in both TPV and PSA |
| McConnel 1998, [37] Low Risk |
Finasteride 5 mg vs Placebo | Treatment naïve men, Qmax <15 ml/s, BPH on DRE, PSA <10 ng/dl | 48 months | 157 vs 155 *TVP measurement only in 10% of study population |
TPV: 54.1 ±26 | 130 | TPV: -9.72 ± n/a (-18.0%) (p <0.01) |
TPV: 55 ±26 | 119 | TPV: +5.5 ±n/a (+14.0%) (p <0.05) |
Difference between groups, 32% P <0.001) |
| Marberger 1998, [38] Moderate Risk |
Finasteride 5 mg vs Placebo | Men 50–75 yo, BPH, Qmax 5-15 ml/s, VV >150 ml, LUTS (2 at least symptoms), enlarged prostate on DRE, PSA <10 ng/dl, PVR <150 ml | 24 months | 1450 vs 1452 | TPV: 38.7 ±20.1 | 890 | TPV: -8.1 ±25.6 (-15.3%) (p <0.01) |
TPV: 39.2 ±20.2 | 906 | TPV: +1.5 ±19.9 (+8.9%) (p <0.05) |
Significant reduction in TPV (p <0.01) from 12th months. Statistical significant difference between groups (p <0.001) |
| Kirby 1992, [39] High Risk |
Finasteride 5 mg vs Finasteride 10 mg vs Placebo | Men 48–87 yo, BPH, Urodynamically proven obstruction | 3 months | 29 vs 16 vs 21 | TPV: 49.7 ±NR PSA: 4.1 ±NR |
25 | TPV: -2.5 ±27.0 (-4.8%) NS PSA: -1.1 ± n/a (-20.5%) (p <0.05) At 12 months TPV -14.1% and PSA -28% |
TPV: 54.3 ±NR PSA: 5.0 ±NR |
10 | TPV: -1.8 ± 14.4 (-4.22%) NS PSA: -1.0 ±n/a (-6.2%) NS |
Statistical significant reduction of PSA (p <0.05) in finasteride arm. No dose related effect at 3 months 10 mg: TPV -3.7&, PSA NS |
| Finasteride group 1993, [40] Moderate Risk |
Finasteride 1 mg vs Finasteride 5 mg vs Placebo | Men 40–80 yo, Qmax <15 ml/s, TPV >30 ml, clinical BPO, No infection or neurogenic bladder |
12 months | 249 vs 246 vs 255 | TPV: 47.0 ±20.8 PSA: 5.8 ±6.7 |
246 | TPV: -10.53 ±n/a (-22.4%) (p <0.001) PSA: -2.67 ±n/a (-46.0%) (p <0.001) |
TPV: 46.3 ±23.4 PSA: 5.7 ±7.2 |
TPV: -2.31 ±n/a (-5.0%) NS PSA: -0.11 ±n/a (-2.0%) NS |
Significant reduction of TPV and PSA from 3rd month. No change in placebo arm. The effect of 1 mg were similar of 5 mg (TPV -23.6%, PSA -43%). Statistical significant difference between groups (p <0.001). There was great difference on clinical improvement with 5 mg |
|
| Tammela 1995, [41] High Risk |
Finasteride 5 mg vs Placebo | Ambulatory men, with LUTS due to BPO. Qmax <15 ml/s, Negative history for Prostate cancer | 6 months | 18 vs 18 | TPV: 56.0 ±25.0 | 18 | TPV: -15.0 ±22.6 (-26.1%) (p <0.05) |
TPV: 47.0 ±17.0 | 18 | TPV: -2.0 ±18.0 (-4.3%) NS |
Statistical significant reduction of TPV (p <0.05) as compared to placebo |
| Pannek 1998, [42] High Risk |
Finasteride 5 mg vs Placebo | Treatment naïve Men 45–78 yo, IPSS ≥9, PSA <10 ng/dl | 6 months | 24 vs 10 | TPV: 36.7 ±17.0 PSA: 3.02 ±2.9 |
24 | TPV: -7.1 ±15.2 (-21.4%) (p <0.01) PSA: -1.53 ±2.52 (-50.7%) (p = 0.005) |
TPV: 37.2 ±11.4 PSA: 3.74 ±3.3 |
10 | TPV: -1.0 ±11.9 (-2.7%) NS PSA: -1.03 ±2.96 (-27.3%) (p <0.05) |
Statistical significant reduction of PSA from baseline but no difference between groups |
| Marks 1997, [43] Moderate Risk |
Finasteride 5 mg vs Placebo | Treatment naïve Men 45–78 yo, IPSS ≥9, PSA <10 ng/dl | 6 months | 26 vs 15 | TPV: 37.0 ±17.0 PSA: 2.7 ±2.5 |
26 | TPV: -8.0 ±15.1 (-21.0%) (p <0.01) PSA: -1.3 ±2.16 (-49.0%) (p <0.01) | TPV: 37.0 ±10.0 PSA: 3.3 ±3.1 |
13 | TPV: -0.4 ±10.0 (-3.0%) NS PSA: -0.2 ±2.0 (-1.0%) NS |
Statistical significant reduction of TPV and PSA in finasteride arm (p <0.01) as compared to placebo. 6% reduction of transitional zone epithelium (p <0.01) |
| Lepor 1996, [18] Prostate Hyperplasia Study Group Low Risk |
Finasteride 5 mg vs Placebo | Treatment naïve men, AUASI score ≥8, Qmax 4–15 ml/s, PVR <300 ml, Clinical BPH, no other obvious cause of LUTS | 12 months | 306 vs 310 vs 309 vs 305 | TPV: 36.2 ± 1.0 PSA: 2.2 ±1.8 |
252 | TPV: -6.1 ±NR (-18.4%) (p <0.001) PSA: -0.9 ±NR (-29.0%) (p <0.001) |
TPV: 38.4 ±1.3 PSA: 2.4 ±2.1 |
264 | TPV: +0.5 ±NR (+2.3%) NS PSA: -0.1 ±NR (-4.0%) NS |
Statistical significant reduction of TPV and PSA in finasteride arm (p <0.001) from baseline. Statistical significant difference between groups (p <0.001) |
| Gormley 1992, [44] Finasteride study group Moderate Risk |
Finasteride 5 mg vs Placebo | Treatment naïve men 40–83 yo, enlarged prostate on DRE, Qmax <15 ml/s, PSA <40 ng/dl, No other cause of LUTS | 12 months | 297 vs 300 | TPV: 58.6 ±30.5 PSA: 3.6 ±4.2 |
257 | TPV: -11.1 ±27.6 (-19.0%) (p <0.01) PSA: n/a (-50%) (p <0.001) |
TPV: 61.0 ±36.5 PSA: 4.1 ±4.8 |
263 | TPV: -1.2 ±38.0 (-3.0%) NS PSA: non-significant changes |
Statistical significant reduction of TPV and PSA in finasteride as compared to placebo (p <0.001). Drop of PSA from month 3 and then stable |
| McConnell 2003, [19] MTOPS research group Low Risk |
Finasteride 5 mg vs Placebo | Treatment naïve men 50 yo and older, AUASI 8–35, Qmax 4–15 ml/s, No other cause of LUTS | 4.5 years | 756 vs 768 vs 786 vs 737 | TPV: 36.9 ±20.6 PSA: 2.4 ±2.1 |
551 | TPV: -12.0 ±26.6 (-19.0%) (p <0.05) PSA: NR (-50%) (p <0.001) |
TPV: 35.2 ±18.8 PSA: 2.3 ±2.0 |
519 | TPV: +8.8 ±36.0 (+24.0%) (p <0.001) PSA: NR (+15%) (p <0.001) |
4 years results. Statistical significant reduction of TPV and PSA in finasteride |
| Roehrborn 2002, [45] pooled analyses 3 different trials Low Risk |
Dutasteride 0.5 mg vs Placebo | Treatment naïve men, AUASI score ≥12, Qmax <15 ml/s, PSA 1.5–10 ng/dl, Prostate volume ≥30 mls | 24 months | 2167 vs 2158 | TPV: 54.9 ±23.9 TZV: 26.8 ±17.1 PSA: 4.0 ±2.1 |
1510 | TPV: -14.6 ±13.5 (-25.7%) (p <0.001) TZV: -7.1 ±9.7 (-20.4%) (p <0.001) PSA: -2.2 ±2.0 (-52.4%) (p <0.001) |
TPV: 54.0 ±21.9 TZV: 26.8 ±17.4 PSA: 4.0 ±2.1 |
1441 | TPV: +0.8 ±14.3 (+2.0%) p = 0.04 TZV: +1.8 ±11.2 (+5.9%) (p <0.01) PSA: +0.5 ±2.1 (+15.8%) (p <0.001) |
Significant difference between groups (p <0.001) TPV and TZV decreased significantly from month 1 and continuing through 24 months |
| Na 2012, [46] Moderate Risk |
Dutasteride 0.5 mg vs Placebo | Men ≥50 yo, clinical BPH, TPV ≥30 ml, AUASI ≥12, Qmax 5–15 ml/s, VV ≥125 ml | 6 months | 126 vs 127 | TPV: 48.2 ±27.7 PSA: 3.33 ±1.9 |
113 | TPV: -7.2 ±11.1 (-17.1%) (p <0.05) PSA: -1.44 ±NR (-43.3%) (p <0.05) |
TPV: 42.3 ±16.5 PSA: 3.14 ±1.9 |
116 | TPV: -1.6 ±12.8 (-3.7%) PSA: -0.12 ±NR (-4.0%) |
Significant improvements in PSA and TPV in dutasteride group |
| Tsukamoto 2009, [47] Moderate Risk |
Dutasteride 0.5 mg vs Placebo | Men ≥50 yo, clinical BPH, TPV ≥30 ml, IPSS ≥8 m qmax <15 ml/s, VV ≥150 mls, PSA <4 ng/dl | 6 months | 193 vs 185 | TPV: 50.2 ±19.8 PSA: 3.5 ±n/a |
184 | TPV: -13.6 ±12.8 (-27.0%) (p <0.05) PSA: -42.2% (p <0.05) |
TPV: 49.4 ±17.2 PSA: 3.5 ±n/a |
181 | TPV: -4.94 ±8.7 (-10.0%) (p <0.05) PSA: +12.0 % |
Significant improvements in PSA and TPV in dutasteride group |
| Andriole 2010, [48] REDUCE Study group Moderate Risk |
Dutasteride 0.5 mg vs Placebo | Men 50–75 yo, PSA 2.5-10 ng/dl, and had TRUSg prostate biopsy 6 months before enrollemnt | 48 months | 4105 vs 4126 | TPV: 45.7 ±18.2 | 3299 | TPV: -6.7 ±18.3 (-17.5%) (p = NR) |
TPV: 45.7 ±18.8 | 3407 | TPV: +3.9 ±18.5 (+19.7%) (p = NR) |
Significant change between groups in TPV (P <0.001) |
| Yokoyama 2012, [20] Low Risk |
Tadalafil 5 mg vs Placebo | Asian men ≥45 yo, BPH-LUTS, Total IPSS ≥13, Qmax 4–12 ml/s, volume >20 ml, PS | 3 months | 155 vs 154 | PSA: 1.71 ±1.14 | 153 | PSA: +0.13 ±0.59 (p = 0.083) (+7%) | PSA: 1.74 ±1.35 | 152 | PSA: -0.03 ±0.55 (-1%) | Non-significant changefrom baseline. No difference between groups |
| Roerhborn 2006 [21] ALTESS Study group Low Risk |
Alfuzosin vs Placebo | Men ≥55 yo, history of LUTS due to BPH, IPSS ≥13, Qmax 5–12 ml/s, VV ≥150 ml, PVR <350 mls, Prostate volume ≥30 mls, PSA 1.4–10 ng/dl | 24 months | 759 vs 763 | PSA: 3.4 ±2.0 | 754 | PSA: -0.1 ±N/a (-0.6%) NS | PSA: 3.6 ±2.1 | 761 | PSA: +0.2 ±N/a (-3.6%) NS | No significant changes from baseline or between group (p >0.05) |
| Roerhborn 2006 [22] ALFUS Trial Moderate Risk |
Alfuzosin vs Placebo | Men ≥55 yo, history of LUTS due to BPH, IPSS ≥13, Qmax 5–12 ml/s, VV ≥150 ml, PVR <350 mls, Prostate volume ≥30 mls, PSA 1.4–10 ng/dl | 3 months | 353 vs 175 | TPV: 39.3 ±17.9 TZV: 18.0 ±11.7 |
307 | TPV: -0.25 ±8.3 (-2%) NS TZV: -0.8 ±6.8 (-2%) NS |
TPV: 36.0 ±18.3 TZV: 16.3 ±12.7 |
157 | TPV: +0.46 ±8.5 (+3%) NS TZV: -0.39 ±8.2 (-5%) NS |
None of thedifferences between placebo and alfuzosin was statistically significant |
| McConnell 2003, [19] MTOPS research group Low Risk |
Doxazosin vs Placebo | Treatment naïve men 50 yo and older, AUASI 8–35, Qmax 4–15 ml/s, No other cause of LUTS | 4.5 years | 756 vs 768 vs 786 vs 737 | TPV: 36.9 ±21.6 PSA: 2.4 ±2.1 |
582 | TPV: +10.1 ±36 (+24.0%) (p <0.001) PSA: NR (+13%) (p <0.001) |
TPV: 35.2 ±18.8 PSA: 2.3 ±2.0 |
519 | TPV: +8.8 ±36.0 (+24.0%) (p <0.01) PSA: NR (+15%) (p <0.00) |
4 years results. Non-significant differences between doxazosin and placebo groups |
| Turkeri 2001 [23] High Risk |
Doxazosin 4 mg vs Placebo | Men with LUTS due to BPH | 4 weeks | 15 vs 14 | TPV: 53.7 ±22.8 PSA: 3.6 ±0.6 |
15 | TPV: -3.3 ±n/a (-6.2%) (p = NR) PSA: -0.47 ±N/a (-13.9%) (p = NR) |
TPV: 56.7 ±17.6 PSA: 3.5 ±0.7 |
14 | TPV: -5.7 ±n/a (-10.4%) (p = NR) PSA: +0.4 ±N/a (+10%) (p = NR) |
Non-significant differences between groups PSA, but small sample size |
| Lepor 1996, [18] Prostate Hyperplasia Study Group Low Risk |
Terazosin vs Placebo | Treatment naïve men, AUASI score ≥8, Qmax 4–15 ml/s, PVR <300 ml, Clinical BPH, no other obvious cause of LUTS | 12 months | 306 vs 310 vs 309 vs 305 | TPV: 37.5 ±1.1 PSA: 2.2 ±1.9 |
275 | TPV: +0.5 ±NR (+2.0%) NS PSA: -0.4 ±NR (-20.0%) NS |
TPV: 38.4 ±1.3 PSA: 2.4 ±2.1 |
264 | TPV: +0.5 ±NR (+2.3%) NS PSA: -0.1 ±NR (-4.0%) NS |
No statistical significant difference between groups in TPV and PSA |
| Yokoyama 2012, [20] Low Risk |
Tamsulosin 0.2 mg vs Placebo | Asian men ≥45 yo, >6 months history of BPH-LUTS, Total IPSS ≥13, Qmax 4–12 ml/s, Prostate volume >20 ml, PSA <4 or else negative biopsy | 3 months | 152 vs 154 | PSA: 1.75 ±1.60 | 150 | PSA: -0.06 ±0.61 (-4%) NS | PSA: 1.74 ±1.35 | 152 | PSA: -0.03 ±0.55 (-1%) NS | Non significant changes between groups |
| Lepor 1996, [18] Prostate Hyperplasia Study Group Low Risk |
Terazosin plus Finasteride combination vs Placebo | Treatment naïve men, AUASI score ≥8, Qmax 4–15 ml/s, PVR <300 ml, Clinical BPH, no other obvious cause of LUTS | 12 months | 309 vs 305 | TPV: 37.2 ±1.1 PSA: 2.3 ±2.0 |
277 | TPV: -7.0 ±NR (-18.8%) (p <0.001) PSA: +0.9 ±NR (+39.1%) (p <0.001) |
TPV: 38.4 ±1.3 PSA: 2.4 ±2.1 |
264 | TPV: +0.5 ±NR (+2.3%) NS PSA: -0.1 ±NR (-4.0%) NS |
Statistical significant difference between groups in TPV and PSA. Max TPV and PSA reduction at 26th week, as in finasteride group |
| McConnell 2003, [19] MTOPS research group Low Risk |
Doxazosin plus finasteride vs Placebo |
Treatment naïve men 50 yo and older, AUASI 8–35, Qmax 4–15 ml/s, No other cause of LUTS | 4.5 years | 786 vs 737 | TPV: 36.4 ±19.2 PSA: 2.3 ±1.9 |
574 | TPV: -12.1 ±30 (-19.0%) (p <0.001) PSA: NR (-50%) (p <0.001) |
TPV: 35.2 ±18.8 PSA: 2.3 ±2.0 |
519 | TPV: +8.8 ±36.0 (+24.0%) (p <0.01) PSA: NR (+15%) (p <0.01) |
4 years results. Significant differences between combination and placebo groups in TPV and PSA (p <0.001) |
| Joo 2012, [57] High Risk |
Tamsulosin 0.2 mg vs Tamsulosin 0.2 mg and Dutasteride |
Treatment naïve men ≥40 yo, IPSS ≥13, Qmax 4–15 ml/s, VV ≥150 ml, PVR <200 ml, Clinical BPH, no other obvious cause of LUTS | 12 months | 108 vs 108 | TPV: 36.63 ±13.2 TZV: 14.94 ±7.16 PSA: 1.7 ±1.23 |
95 | TPV: +0.38 ±2.1 (+1.0%) NS TZV: +0.24±0.66 (+1.6%) NS PSA: -0.06 ±0.22 (-3.5%) NS |
TPV: 37.26 ±13.2 TZV: 15.36 ±7.56 PSA: 1.77 ±1.4 |
98 | TPV: -10.04 ±6.14 (-26.9%) (p <0.05) TZV: -3.03 ±2.32 (-19.7%) (p <0.05) PSA: -0.73 ±0.68 (-41.2%) (p <0.05) |
Statistical significant change from baseline (<0.05) in combination group. Integroup comparison p <0.05 inTPV, TZV and PSA |
| Choi 2016, [58] Low Risk |
Tamsulosin 0.2 mg vs Tamsulosin 0.2 mg and Dutasteride |
Treatment naïve men ≥40 yo, Prostate volume >30 ml, IPSS ≥13, Qmax 4–15 ml/s, VV ≥150 ml, PVR <200 ml, Clinical BPH, no other obvious cause of LUTS | 12 months | 59 vs 59 | TPV: 40.34 ±1.4 TZV: 16.0 ±1.26 PSA: 1.35 ±0.12 |
55 | TPV: 0.0 ±NR (0%) NS TZV: 0.0 ±NR (0%) NS PSA: +0.17 ±NR (+12.6%) (p <0.05) |
TPV: 41.05 ±2.7 TZV: 16.95 ±2.33 PSA: 1.31 ±0.15 |
46 | TPV: -8.0 ±NR (-19.5%), p <0.001 TZV: -3.0 ±NR (-17.7%), p <0.001 PSA: -0.24 ±NR (-18.3%), p <0.001 |
Statistical significant differences between groups in TPV (p = 0028) and TZV (p <0.001). PSA didn’t differ (p = 0.108) |
| Ryu 2014, [61] Moderate Risk |
Tamsulosin 0.2 mg vs Tamsulosin 0.2 mg and Serenoa repens 320 mg |
Treatment naïve men 50–70 yo, IPSS >10, Qmax 5–15, VV >150 ml, Prostate volume ≥25 ml, PSA <4 ng/dl | 12 months | 60 vs 60 | TPV: 30.2 ±0.67 PSA: 1.1 ±0.16 |
53 | TPV: +0.1 ±0.15 (+1.0%) NS PSA: +0.2 ±0.12 (+18.0%) (p = NR) |
TPV: 30.1 ±0.93 PSA: 1.2 ±0.11 |
50 | TPV: -0.7 ±0.27 (-2.0%) NS PSA: +0.2 ±0.12 (+8.0%) (p = NR) |
No significant changes between groups in prostate volume (p = 0.096) or PSA (p = 0.521) |
| Debruyne 2002, [24] PERMAL Study Group Low Risk |
Tamsulosin 0.4 mg vs Serenoa repens 320 mg |
Treatment naïve men 50–85 yo, IPSS >10, Qmax 5–15, VV >150 ml, Prostate volume ≥25 ml, PSA< 4 ng/dl or negative biopsy if PSA ≥4 ng/dl | 12 months | 354 vs 350 | TPV: 48.0 ±19.0 PSA: 2.7 ±2.2 |
TPV N: 270 PSA N: 268 |
TPV: +0.2 ±12.8 (+1.0%) NS (p = 0.75) PSA: +0.2 ±1.6 (+7.4%) NS (p = 0.09) |
TPV: 48.2 ±18.0 PSA: 2.5 ±1.9 |
TPV N: 269 PSA N: 266 |
TPV: -0.9 ±13.4 (-2.0%) NS (p = 0.75) PSA: +0.2 ±1.4 (+10.0%) NS (p = 0.09) |
No significant changes between groups in TPV (p = 0.27) or PSA (p = 0.5) |
| Sengupta 2011, [25] Moderate Risk |
Tamsulosin 0.4 mg vs phytotherapy (MurrayaKoenigii and tribulusterrestris) |
Treatment naïve men >50 yo, Clinical BPH, no other obvious cause of LUTS, IPSS >7, enlarged prostate | 12 weeks | 23 vs 23 | TPV: 41.3 ±26.8 | 21 | TPV: -1.4 ±23.1 (-3.4%) NS (p = 0.099) |
TPV: 33.5 ±24.1 | 23 | TPV: -1.9 ±13.9 (-5.6%) (p = 0.04 from baseline) |
Significant difference TPV between groups (p = 0.037) |
| Latil 2015, [26] High Risk |
Tamsulosin 0.4 mg vs hexamic extract Serenoa repens 320 mg |
Treatment naïve men 45–85 yo, BPH related LUTS >12 months, IPSS ≥12, prostate volume 30 ml, Qmax 5–15 ml/s, VV 150-500 ml, PSA ≤4 or negative biopsy | 12 weeks | 101 vs 102 | TPV: 46. 3 ±13.8 | 86 | TPV: -0.53 ±10.5 (-1.0%) NS |
TPV: 48.8 ±20.8 | 83 | TPV: -0.99 ±10.9 (-2.0%) NS |
No significant changes between groups in prostate volume NS |
| Pande 2014, [27] Moderate Risk |
Tamsulosin 0.4 mg vs Silodosin 8 mg |
Treatment naïve men >50 yo, LUTS due to BPH, IPSS >7, low PSA | 12 weeks | 29 vs 32 | TPV: 35.6 ±9.6 | 27 | TPV: -1.0 ±13.5 (-2.8%) NS (p = 0.677) |
TPV: 42.0 ±20 | 26 | TPV: -3.6 ±19.6 (-8.6%) NS (p = 0.594) |
No significant changes between groups in prostate volume (p = 0.996) |
| Sakalis 2018, [34] Moderate Risk |
Tamsulosin 0.4 mg vs Tamsulosin 0.4 mg and Solifenacin | Treatment naïve men >50 yo, storage LUTS due to BPH, IPSS >7, Q3 IPSS ≥, Qmax ≥10, PSA <4 or negative biopsy | 6 months | 34 vs 35 | TPV: 48.9 ±13.6 TZV: 24.4 ±10.2 PSA: 1.36 ±1.0 |
31 | TPV: +3.88 ±14.6 (+9.2%) (p <0.001) TZV: +3.74 ±10.7 (+17.4%) (p <0.001) PSA: +0.26 ±1.0 (+19.1%) (p <0.051) |
TPV: 52.6 ±13.0 TZV: 28.4 ±21.4 PSA: 1.9 ±1.6 |
32 | TPV: -5.49 ±16.1 (-9.5%) (p <0.001) TZV: -2.48 ±21.1 (-12.5%) (p <0.001) PSA: +0.2 ±1.5 (+10.5%) (p <0.549) |
Significant changes in TPV and TZV in both groups from baselines and in intergroup comparison (p <0.001). Non-significant PSA changes |
| Safwat 2018, [76] Moderate Risk |
Tamsulosin 0.4 mg vs Tamsulosin plus Cholecalciferol 600IU/day | Men with AUA-SI score >7 | 24 months | 193 vs 196 | TPV: 55.4 ±13.1 PSA: 0.26 ±0.09 |
TPV: +3.3 ±3.5 (+5.9%) NS PSA: +0.01 ±0.0009 (+3.8%) NS |
TPV: 60.2 ±10.8 PSA: 0.19 ±0.05 |
TPV: +4.9 ±2.2 (+8.1%) NS PSA: +0.032 ±0.0022 (+16.8%) NS |
Non significant changes in TPV (p = 0.098) between groups. Significant difference between groups in PSA (p = 0.044) |
||
| Griwan 2014, [99] Moderate Risk |
Tamsulosin 0.4 mg vs Naftopidil 75 mg | Men >45 yo, symptomatic BPH, Frequency >8, Nocturia >2, Qmax 5–15 ml/s, IPSS >13 | 3 months | 30 vs 30 | TPV: 57.73 ±7.33 | 30 | TPV: -0.04 ±7.37 (-1.0%) NS (p = 0.15) |
TPV: 56.81 ±6.45 | 30 | TPV: +0.01 ±6.52 (-1.0%) NS (p = 0.18) |
No significant changes between groups TPV or from baseline |
| Nickel 2011, [49] EPICS Study Moderate Risk |
Finasteride 5 mg vs Dutasteride 0.5 mg | Men ≥50 yo, with clinical BPH, AUASI score ≥12, Vol Prostate ≥30 ml, Qmax <15 mls/s, VV ≥125 ml, PVR <250 ml | 12 months | 817 vs 813 | TPV: 52.4 ±19.4 PSA: 4.3 ±2.2 |
735 | TPV: -13.99 ±n/a (-26.7%) (p <0.05) PSA: -2.05 ±n/a (+47.7%) (p <0.05) |
TPV: 54.2 ±21.9 PSA: 4.3 ±2.3 |
719 | TPV: -14.2 ±n/a (-26.3%) (p <0.05) PSA: -2.12 ±n/a (+49.5%) (p <0.05) |
Non-significant changes between groups (p = 0.776) Greater reductions in men with prostates >40 grs |
| Jeong 2009, [52] Moderate Risk |
Finasteride 5 mg plus a-blocker versus Dutasteride 0.5 mg plus a-blocker | Men ≥50 yo, with moderate to severe LUTS (determined by IPSS), without previous 5ARI treatment but on a blocker, with prostate volume ≥25 ml | 12 months Plus 12 months of 5ARI discontinuation |
60 vs 60 | TPV: 39.78 ±9.3 PSA: 1.83 ±1.19 |
37 | TPV: -9.76 ±8.24 (-24.51%) (p <0.001) PSA: -0.89 ±0.49 (-48.9%) (p <0.001) |
TPV: 39.22 ±12.3 PSA: 1.85 ±1.31 |
40 | TPV: -10.25 ±9.98 (-26.11%) (p <0.001) PSA: -0.94 ±0.79 (-50.9%) (p <0.001) |
Non significant difference between arms inTPV change (p = 0.568) and PSA changes (p = 0.352). Significant increase of TPV (+11.2% and +8.66%) and PSA (+46.2% and +43.1%) at 12 months after 5ARI discontinuation |
| Carraro 1996, [50] Low Risk |
Finasteride 5 mg vs Serenoa repens 320 mg | Clinical BPH, IPSS >6, Qmax 4-5 mls/s, Prostate volume >25 mls, PSA according to predefined prostate volume limits | 6 months | 545 vs 553 | TPV: 44.0 ±20.6 PSA: 3.23 ±3.34 |
484 | TPV: -7.3 ±19.12 (-18.0%) (p <0.001) PSA: -1.23 ±2.9 (-41.0%) (p <0.001) |
TPV: 43.0 ±19.6 PSA: 3.26 ±3.41 |
467 | TPV: -1.5 ±20.0 (-7.0%) (p = NR) PSA: -0.04 ±3.7 (-3.0%)(p = NR) |
Both treatments reduced prostate size, but the reduction was significantly greater in finasteride arm (p <0.001) |
| Di Silverio 2005, [78] Moderate Risk |
Finasteride 5 mg vs Finasteride 5 mg and Rofecoxib 25 mg | Men 50–80 yo, IPSS >12, Qmax 5–15 ml/s, VV >150 mls, Prostate volume >40 mls and PSA <10 ng/dl | 6 months | 23 vs 23 | TPV: 51.65 ±9.1 PSA: 2.68 ±1.18 |
23 | TPV: -8.83 ±8.35 (-20.2%) PSA: -0.98 ±1.1 (-36.4%) (p <0.001) |
TPV: 49.65 ±9.5 PSA: 2.62 ±1.16 |
23 | TPV: -8.79 ±8.93 (-20.1%) (p = NR) PSA: -0.93 ±1.02 (-35.4%) (p <0.001) |
Significant changes from baseline in both groups (p <0.001) but insignificant changes between groups |
| Guzman 2019, [71] Moderate Risk |
Phytotherapy (Roystonearegia lipid exctract D-004) 320 mg vs Terazosin 5 mg | Men ≥50 yo, Clinical BPH on DRE and, IPSS 7–19, without prior LUT surgery, PSA <5 ng/dl | 6 months | 50 vs 50 | TPV: 31.4 ±23.2 | 50 | TPV: -3.4 ±21.8 (-10.8%) (p <0.01) |
TPV: 29.7 ±19.4 | 50 | TPV: -1.4 ±18.7 (-4.7%) (p <0.01) |
Statistical significant reduction in TPV both groups. Non-significant difference between groups |
| Morgia 2018, [54] SPRITE Study Moderate Risk |
Phytotherapy (Serenoa repens + selenium + lycopene) vs Tadalafil 5 mg | Men 50–80 yo, negative DRE for PCa, PSA <4 ng/dl, IPSS ≥12, Qmax ≤15 ml/s, PVR <100 ml | 6 months | 291 vs 136 Randomization 2:1 |
TPV: 45.0 ±13.1 PSA: 1.8 ±1.0 median value |
276 | TPV: -2.0 ±n/a (-4.5%) (NS) PSA: -0.1 ±1.65 (-5.5%) (NS) |
TPV: 45.0 ±13.0 PSA: 1.9 ±1.1 median value |
128 | TPV: 0.0 ±n/a (0.0%) (NS) PSA: -0.06 ±1.1 (-3.1%)(NS) |
Non-significant changes from baseline or between groups in TPV and PSA |
| Ozturk 2011, [56] High Risk |
Alfuzosin XL vs AlfuzosinXL + Sildenafil 50 mg | Men >45 yo, with moderate to severe LUTS and ED, IPSS ≥12, QoL ≥3 | 3 months | 50 vs 50 | TPV: 47.6 ±30.0 PSA: 1.83 ±1.6 |
50 | TPV: +0.7 ±29.3 (+1.5%) (NS) PSA: -0.04 ±1.5 (-2.2%) (NS) |
TPV: 44.8 ±22.2 PSA: 1.4 ±1.4 |
50 | TPV: -1.6 ±22.6 (-3.6%) (NS) PSA: -0.12 ±1.3 (-8.6%) (NS) |
No significant differences from baseline or between group comparison in TPV and PSA |
| Mohanty 2006, [59] High Risk |
Tamsulosin 0.4 mg plus Finasteride vs Tamsulosin 0.4 mg plus Dutasteride |
Men 40–80 yo, with BPH | 6 months | 53 vs 53 | TPV: 45.4 ±22.5 PSA: 2.3 ±2.2 |
50 | TPV: -8.9 ±20.0 (-19.6%) (p <0.001) PSA: -0.2 ±2.1 (-8.7%) (p <0.001) |
TPV: 41.1 ±15.1 PSA: 2.0 ±2.2 |
50 | TPV: -6.0 ±14.0 (-14.6%) (p <0.01) PSA: -0.5 ±1.3 (-25.0%) (p <0.001) |
Significant differences from baseline but no difference in intergroup comparison in TPV and PSA |
| Ghadian 2017, [77] High Risk |
Ω3 300 m g plus Tamsulosin 0.4 mg plus Finasteride 5 mg versus Tamsulosin 0.4 mg plus Finasteride 5 mg |
Men 50–70 yo, with LUTS due to BPH, prostate volume >40 ml, IPSS 8–19 | 6 months | 50 vs 50 | TPV: 62.1 ±5.2 | 50 | TPV: -17.1 ±6.0 (-27.5%), (p <0.001) |
TPV: 61.4 ±5.6 | 50 | TPV: -9.62 ±5.7 (-15.6%), (p <0.001) |
Significant differences from baseline but no difference in intergroup comparison in TPV (p <0.001) |
| Page 2011, [81] Moderate Risk |
Testosterone gel 1% 7.5 gr plus placebo versus Testosterone gel 1% 7.5 gr plus dutasteride 0.5 mg | Men ≥50 yo, at least one symptom of androgen deficiency syndrome, Total testosterone <280 nng/dl, Prostate >30 ml, PSA 1.5–10 ng/dl, PVR <200 ml | 6 months | 27 vs 26 | TPV: 54.2 ±38.1 PSA: 2.9 ±2.9 |
27 | TPV: +4.1 ±38.4 (+7.6%) (p <0.05) PSA: +0.3 ±2.9 (10.7%) (p <0.05) |
TPV: 44.4 ±19.8 PSA: 2.1 ±1.3 |
26 | TPV: -5.8 ±19.1 (-13.1%) (p <0.05) PSA: -0.7 ±1.3 (33.3%) (p <0.05) |
Significant differences from baseline both TPV and PSA in testosterone plus dutasteride group. Significant difference in intergroup comparison in TPV and PSA (p <0.05) |
| Kacker 2014, [82] Moderate Risk |
Testosterone plus placebo vs testosterone plus dutasteride |
Men 40–85 yo, who already receive testosterone therapy, ±LUTS | 12 months | 11 vs 12 | TPV: 57.4 ±29.3 PSA: 2.58 ±1.2 |
11 | TPV: +3.4 ±14.6 (+5.9%) (NS p = 0.530) PSA: +0.21 ±1.1 (+8.2%) (NS p = 0.458) |
TPV: 45.0 ±25.4 PSA: 1.98 ±0.8 |
11 | TPV: -6.65±11.0 (-14.7%) (p = 0.018) PSA: -0.46 ±0.81 (42.6%)(p = 0.04) |
No significant difference between dutasteride and placebo groups in TPV (p = 0.085) and PSA (p = 0.113) |
| Yamanishi 2017, [60] DIrecT Study Moderate Risk |
Tamsulosin plus dutasteride versus Tamsulosin plus Dutasteride plus imidafenacin | Men 40–89 yo, OAB symptoms (OABS S ≥3), prostate volume ≥30 ml | 24 weeks | 81 vs 82 | TPV: 43.7 ±15.2 PSA: 4.1 ±4.2 |
72 (TPV) 68 (PSA) |
TPV: -9.48 ±n/a (-21.7%) (p <0.05) PSA: -1.88 ±n/a (-47.2%) (p <0.001) |
TPV: 44.6 ±18.7 PSA: 3.3 ±2.7 |
69 (TPV) 64 (PSA) |
TPV: -10.07 ±n/a (-22.6%) (p <0.05) PSA: -1.28 ±n/a (-38.8%) (p <0.01) |
Significant changes in TPV and PSA from baseline in both groups. Non significant difference between groups in TPV (p = 0.78), PSA (p = 0.113) |
| Goodarzt 2011, [79] High Risk |
Terazosin 2 mg vs Terazosin 2 mg plus Celecoxib 200 mg | Men ≥50 yo, LUTS due to BPH, AUA Symptom scale 7–25, benign DRE | 12 weeks | 80 vs 80 | TPV: 43.4 ±18.9 PSA: 3.54 ±3.6 |
80 | TPV: -0.4 ±4.8 (-1.0%) (NS p = 0.454) PSA: -0.37 2.9 (-10.5%) (NS p = 0.238) |
TPV: 44.0 ±19.3 PSA: 3.36 ±2.4 |
80 | TPV: -5.7 ±7.0 (-12.9%) (p <0.001) PSA: -0.59 ±2.1 (-17.6%) (p = 0.013) |
Significant changes in Celecoxib group from baseline inTPV and PSA. Significant difference between groups in TPV (p < 0.001) only |
| Jhang 2013, [80] High Risk |
Doxazosin 4 mg vs Doxazosin 4 mg plus Celecoxib 200 mg | Men ≥40 yo, LUTS due to BPH, PSA ≥4 ng/dl, IPSS ≥8, Benign DRE | 3 months | 58 vs 64 | TPV: 67.0 ±34.0 PSA: 16.2 ±16.8 |
37 | TPV: +3.7 ±34.8 (+5.5%) NS PSA: -0.2 ±22.4 (-2.0%) NS |
TPV: 68.3 ±33.5 PSA: 10.7 ±16.8 |
45 | TPV: -1.0 ±33.0 (-2.0%) NS PSA: -1.82 ±6.1 (-17.0% p < 0.05) |
Significant changes in Celecoxib group from baseline in PSA. 22 patients diagnosed with PCa. Non significant difference between group in TPV (p = 0.122), PSA (p = 0.545) |
| Karami 2016, [28] High Risk |
Tamsulosin 0.4 mg vs Tadalafil 20 mg | Men ≥45 yo, IPS S≥12, LUTS due to BPH and ED, PVR <200 ml | 3 months | 59 vs 60 | PSA: 2.3 ±1.9 | 59 | PSA: 0.0 ±0.3 (0%) NS |
PSA: 2.5 ±1.8 | 60 | PSA: 0.0 ± 0.1 (0%) NS |
No significant changes from baseline or between groups in PSA |
| Hizli 2007, [29] High Risk |
Tamsulosin 0.4 mg vs Serenoa repens 320 mg | Men 43–73yo, LUTS due to BPH, IPSS ≥10, Qmax 5–15 ml/s, PVR ≤150 ml, Prostate volume ≥25 ml, PSA ≤4 ng/ml | 6 months | 20 vs 20 | TPV: 28.6 ±11.6 PSA: 2.1 ±0.9 |
20 | TPV: -1.0 ±2.2 (-3.5%) NS PSA: -0.1 ±0.2 (-5.0%) NS |
TPV: 35.2 ±10.3 PSA: 1.9 ±0.9 |
20 | TPV: -0.7 ±2.6 (-2.0%) NS PSA: -0.1 ±0.3 (-1.0%) NS |
No significant changes between groups in prostate volume (p = 0.61) or PSA (p = 0.07). |
| Hizli 2007, [29] High Risk |
Tamsulosin 0.4 mg vs Tamsulosin 0.4 mg plus Serenoa repens 320 mg |
Men 43–73 yo, LUTS due to BPH, IPSS ≥10, Qmax 5–15 ml/s, PVR ≤150 ml, Prostate volume ≥25 ml, PSA ≤4 ng/ml | 6 months | 20 vs 20 | TPV: 28.6 ±11.6 PSA: 2.1 ±0.9 |
20 | TPV: -1.0 ±2.2 (-3.5%) NS PSA: -0.1 ±0.2 (-5.0%) NS |
TPV: 31.2 ±4.2 PSA: 1.7 ±0.7 |
20 | TPV: -0.8± 2.0 (-2.5%) NS PSA: -0.2 ±0.3 (-1.0%) N |
No significant changes between groups in prostate volume (p = 0.55) or PSA (p = 0.07) |
| Lepor 1996, [18] Prostate Hyperplasia Study Group |
Terazosin vs Finasteride 5 mg | Treatment naïve men, AUASI score ≥ 8, Qmax 4–15 ml/s, PVR <300 ml, Clinical BPH, no other obvious cause of LUTS | 12 months | 305 vs 310 | TPV: 37.5 ± 1.1 PSA: 2.2 ±1.9 |
277 | TPV: +0.5 ±NR (-13.4%) (p <0.001) PSA: -0.4 ±NR (-18.2%) (p <0.01) |
TPV: 36.2 ±1.0 PSA: 2.2 ±1.8 |
264 | TPV: -6.1 ± NR (-16.8%) PSA: +0.9 ±NR (+40.1%) (p <0.01) |
Statistical significant difference between groups in TPV and PSA. Significant difference from baseline in finasteride group |
| Lepor 1996, [18] Prostate Hyperplasia Study Group Low Risk |
Terazosin vs Finasteride 5 mg plus Terazosin | Treatment naïve men, AUASI score ≥8, Qmax 4–15 ml/s, PVR <300 ml, Clinical BPH, no other obvious cause of LUTS | 12 months | 305 vs 309 | TPV: 37.5 ± 1.1 PSA: 2.2 ±1.9 |
277 | TPV: +0.5 ±NR (-13.4%) (p <0.001) PSA: -0.4 ±NR (-18.2%) (p <0.01) |
TPV: 38.4 ±1.3 PSA: 2.4 ±2.1 |
264 | TPV: +0.5 ±NR (+2.3%) NS PSA: -0.1 ±NR (-4.0%) NS |
Statistical significant difference between groups in TPV and PSA. Significant difference from baseline in finasteride group |
| Odusanya 2017, [30] High Risk |
Tamsulosin 0.4 mg versus Finasteride 5 mg | Men with LUTS due to BPH and enlarged prostate on DRE | 6 months | 30 vs 30 | TPV: 66.2 ±NR | 21 | TPV: +6.32 ±NR (+9.5%) (p = 0.17) | TPV: 66.57 ±NR | 20 | TPV: -6.8 ±NR (-10.2%), (p = 0.49) |
Non-significant change from baseline, no significant difference between groups |
| Odusanya 2017, [30] High Risk |
Tamsulosin 0.4 mg versus Tamsulosin 0.4 mg plus Finasteride 5 mg | Men with LUTS due to BPH and enlarged prostate on DRE | 6 months | 30 vs 30 | TPV: 66.2 ±n/a | 21 | TPV: +6.32 ±n/a (+9.5%) (p = 0.17) |
TPV: 55.43 n/a | 24 | TPV: -8.19 n/a (-11.8%), (p = 0.13) |
Non significant change from baseline. Significant difference between groups (p = 0.006) |
| Morgia 2014, [31] PROCOMB Trial Low Risk |
Tamsulosin vs Phytotherapy | Men 55–80 yo, benign DRE, PSA ≤4 ng/ml, IPSS ≥12, prostate volume ≤60 ml, PVR <150 ml | 12 months | 79 vs 71 | TPV: 45.0 ±n/a PSA: 2.1 ±n/a |
78 | TPV: -1.0 ±NR (-2.2%) NS PSA: -0.09 ±NR (-4.3%) NS |
TPV: 43.0 ±NR PSA: 1.94 ±NR |
67 | TPV: -1.5 ±NR (-3.5%) NS PSA: -0.0 ±NR (0%) NS |
No significant changes between groups in TPV and PSA. No significant changes form baseline |
| Morgia 2014, [31] Low Risk |
Tamsulosin vs Tamsulosin plus Phytotherapy | Men 55–80 yo, benign DRE, PSA ≤4 ng/ml, IPSS≥12, prostate volume ≤60 ml, PVR <150 ml | 12 months | 79 vs 75 | TPV: 45.0 ±n/a PSA: 2.1 ±n/a |
78 | TPV: -1.0 ±NR (-2.2%) NS PSA: -0.09 ±NR (-4.3%) NS |
TPV: 45.0 ±NR PSA: 2.11 ±NR |
74 | TPV: -2.5 ±NR (-5.5%) NS PSA: -0.16 ±NR (7.6%) NS |
No significant changes between groups in prostate volume and PSA. No significant changes form baseline |
| McConnell 2003, [19] MTOPS Low Risk |
Doxazosin vs Finasteride 5 mg | Treatment naïve men 50 yo and older, AUASI 8–35, Qmax 4–15 ml/s, No other cause of LUTS | 4.5 years | 756 vs 768 | TPV: 36.9 ±21.6 PSA: 2.4 ±2.1 |
N = TPV 582 N = PSA 655 |
TPV: +29.0 ±36 (+24.0%) (p <0.05) PSA: NR (+13%) (p <0.05) |
TPV: 36.9 ±20.6 PSA: 2.4 ±2.1 |
N=TPV 519 N=PSA 631 |
TPV: -12.0 ±30.0 (-19.0%) (p <0.05) PSA: n/a (-50%) p <0.001 |
4 years results. Significant differences between groups |
| McConnell 2003, [19] MTOPS Low Risk |
Doxazosin vs Doxazosin plus Finasteride 5 mg | Treatment naïve men 50 yo and older, AUASI 8–35, Qmax 4–15 ml/s, No other cause of LUTS | 4.5 years | 756 vs 786 | TPV: 36.9 ±21.6 PSA: 2.4 ±2.1 |
N = TPV 582 N = PSA 655 |
TPV: +29.0 ±36 (+24.0%) (p <0.05) PSA: NR (+13%) (p <0.05) |
TPV: 36.5 ±19.2 PSA: 2.3 ±1.9 |
N=TPV 574 N=PSA 673 |
TPV: -12.0 ±30.0 (-19.0%) (p <0.05) PSA: NR (-50%) p <0.001 |
4 years results. Significant differences between groupn and from baseline |
| Kuo 1998, [51] High Risk |
Dibenyline vs Finasteride | NP | 6 months | 71 vs 54 | TPV: 27.5 ±16.9 | 53 | TPV: -0.1 ±23.1 (-3.6%) |
TPV: 30.9 ±12.9 | 47 | TPV: -7.5 ± 11.5 (-24.3%), (p <0.05) |
Significant changes in finasteride group |
| Roehrborn 2010, [32] Low Risk |
Tamsulosin vs Dutasteride | Men ≥50 yo, with LUTS due to BPH, IPSS ≥12, prostate volume ≥30 ml, PSA 1.5–10 ng/dl, Qmax 5–15 ml/s | 4 years | 1611 vs 1623 | TPV: 55.8 ±24.2 TZV: 30.5 ±24.5 |
989 | TPV: +2.57 ±NR (+4.6%) TZV: +5.55 ±NR (+18.2%) |
TPV: 54.6 ±23.0 TZV: 30.3 ±21.0 |
1093 | TPV: -15.29 ±NR (-28%) TZV: -8.03 ±R (-26.5%) |
Significant change from baseline in dutasteride group. Significant difference between groups in TPV (p <0.001) and TZV (p <0.001) |
| Roehrborn 2010, [32] Low Risk |
Tamsulosin vs Tamsulosin plus Dutasteride | Men ≥50 yo, with LUTS due to BPH, IPSS ≥12, prostate volume ≥30 ml, PSA 1.5–10 ng/dl, Qmax 5–15 ml/s | 4 years | 1611 vs 1610 | TPV: 55.8 ±24.2 TZV: 30.5 ±24.5 |
989 | TPV: +2.57 ±NR (+4.6%) TZV: +5.55 ±NR (+18.2%) |
TPV: 54.7 ±23.5 TZV: 27.7 ±20.2 |
1113 | TPV: -14.93 ±NR (-27.3%) TZV: -4.96 ±NR (-17.9%) |
Significant difference between groups in TPV (p <0.001) and TZV (p <0.001) |
| Yokoyama 2012, [20] Low Risk |
Tamsulosin 0.2 mg vs Tadalafil 5 mg | Asian men ≥45 yo, >6 months history of BPH-LUTS, Total IPSS ≥13, Qmax 4–12 ml/s, Prostate volume >20 ml, PSA <4 or else negative biopsy | 3 months | 152 vs 155 | PSA: 1.75 ±1.6 | 143 | PSA: -0.06 ±0.61 (-3.5%) NS |
PSA: 1.71 ±1.14 | 137 | PSA: +0.13 ±0.59 (8.0%) p = 0.083 NS | Non-significant changes from baseline Small tendency in tadalafil arm without significance. Non-significant changes between groups |
| Debruyne 1998, [33] Low Risk |
Alfuzosin SR vs Finasteride 5 mg | Men 50–75 yo, LUTS due to BPH, IPSS >7, Qmax 5–15 ml/s for VV >150 mls | 6 months | 358 vs 344 | TPV: 41.4 ±25.7 PSA: 3.0 ±2.5 |
318 | TPV: -0.2 ±14.3 (+1.0%) NS PSA: +0. 1 ±2.7 (+3.3%) NS |
TPV: 40.9 ±23.5 PSA: 3.4 ±2.5 |
305 | TPV: -4.3 ±15.0 (-10.5%) (p = 0.05) PSA: -1.7 ±1.9 (-50.0%) (p = 0.05) |
Significant changes in finasteride group from baseline and in between group comparison for TPV (p <0.001) and PSA (p <0.001) |
| Debruyne 1998, [33] Low Risk |
Alfuzosin SR vs Alfuzosin SR plus Finasteride 5 mg | Men 50-75 yo, LUTS due to BPH, IPSS >7, Qmax 5–15 ml/s for VV >150 mls | 6 months | 358 vs 349 | TPV: 41.4 ±25.7 PSA: 3.0 ±2.5 |
318 | TPV: -0.2 ±14.3 (+1.0%) NS PSA: +0.1 ±2.7 (+3.3%) NS |
TPV: 41.1 ±22.6 PSA: 3.1 ±2.7 |
295 | TPV: -4.9 ±12.4 (-11.9%) (p <0.01) PSA: -1.4±1.7 (-45.2%) (p <0.01) |
Significant changes in combination group from baseline and in between group comparison for TPV (p <0.001) and PSA (p <0.001) |
| Argirovic 2013, [62] High Risk |
Tamsulosin 0.4 mg vs Serenoa repens 320 mg | Men with LUTS due to BPH, Prostate volume <50 ml, IPSS 7–18, QoL >3, Qmax 5–15 ml/s, PVR <150 ml, PSA 1.5–4 ng/ml | 6 months | 87 vs 97 | TPV: 38.6 ±11.6 PSA: 2.1 ±0.9 |
87 | TPV: -1.0 ±0.6 (-2.6%) PSA: -0.1 ±0. 2 (-4.8%) |
TPV: 35.2 ±10.3 PSA: 1.9 ±0.9 |
97 | TPV: -0.7± 0.1 (-2.0%) PSA: -0.3 ±1.4 (-15.0%) |
No significant changes between groups in prostate volume or PSA |
| Argirovic 2013, [62] High Risk |
Tamsulosin 0.4 mg vs Tamsulosin 0.4 mg plus Serenoa repens 320 mg | Men with LUTS due to BPH, Prostate volume <50 ml, IPSS 7–18, QoL >3, Qmax 5–15 ml/s, PVR <150 ml, PSA 1.5–4 ng/ml | 6 months | 87 vs 81 | TPV: 38.6 ±11.6 PSA: 2.1 ±0.9 |
87 | TPV: -1.0 ±0.6 (-2.6%) NS PSA: -0.1 ±0. 2 (-4.8%) |
TPV: 31.2 ±4.2 PSA: 1.97 ±0.7 |
81 | TPV: -0.8 ±0.3 (-2.6%) NS PSA: -0.25 ±0.2 (-14.7%) (NS p = 0.25) |
No significant changes between groups in prostate volume or PSA |
| Braeckman 1997, [72] High Risk |
Serenoa repens 320 OD vs Serenoa repens 160 BD | Men <75 yo, LUTS due to BPH, BPE from DRE and TRUS, Qmax 5–15 ml/s, IPSS 12–24, PVR <100 ml, PSA <10 ng/dl | 12 months | 42 vs 42 | TPV: 46.4 ±44.1 | 33 | TPV: -6.7 ±40.5 (-14.5%) (p <0.001) |
TPV: 37.6 ±17.6 | 34 | TPV: -3.63± 23.7 (-9.6%) (p <0.001) |
Significant difference from baseline in both groups, non-significant difference between groups |
| Chung 2011, [83] Moderate Risk |
Tolterodine plus a blocker plus 5ARI vs a blocker plus 5ARI |
Men <70 yo, IPSS >8, IPSS storage Subscore >5, QoL >3, TPV >20 ml, Qmax <15 ml/s, urodynamically confirmed BPH/BOO | 12 months | 50 vs 87 | TPV: 49.2 ±26.3 TZI: 0.46 ±0.13 PSA: 3.44 ±1.55 |
50 | TPV: -9.5 ±22.9 (-19.3%) (p <0.001) TZI: -0.02 ±0.12 (-4.5%) (p <0.039) PSA: -1.44 ±1.61 (-41.8%) (p <0.001) |
TPV: 53.3 ±22.1 TZI: 0.47 ±0.15 PSA: 3.9 ±2.06 |
87 | TPV: -9.1 ± 21.8 (-17.1%) (p <0.001) TZI: -0.04 ±0.13 (-12.8%) (p <0.001) PSA: -0.97 ±3.1 (-24..8%) (p <0.013) |
Significant difference from baseline in both groups, non significant difference between groups in TPV (p = 0.877), TZI (p = 0.671) and PSA (p = 0.434) |
| Kosilov 2019, [55] HighRisk |
Tadalafil 5 mg versus Tadalafil 5 mg plus Solifenacin 10 mg | ED, LUTS due to BPH, IPSS 8–19, TPV <45 ml, PSA <10 ng/dl | 12 weeks | 107 vs 107 | TPV: 37.4 ±4.8 | 107 | TPV: -2.2 ±4.1 (-5.9%) (NS) |
TPV: 42.4 ±6.4 | 107 | TPV: -1.4 ±5.6 (-3.3%) (NS) |
Non significant difference from baseline or between groups |
| Allott 2019, [73] post hoc analysis of REDUCE trial Moderate Risk |
Subgroup analysis statinsusers vs non statinusers |
Men 50–75 yo, PSA 2.5-10 ng/dl, and had TRUSg prostate biopsy 6 months before enrollment | 48 months | 692 vs 3414 | Dutasteride arm Statin users TPV: 45.3 ±18.2 Non-statin users TPV: 45.7 ±22.4 |
NR | Dutasteride arm Statin users TPV: -6.8 ±18.5 (NR%) (p <0.033) Non-statin users TPV: -5.6 ±23.2 (NR%) (p = NR) |
Placebo arm Statin users TPV: 45.2 ±18.8 Non-statin users TPV: 45.7 ±10.7 |
NR | Placebo arm Statin users TPV: +11.4 ±19.2 (-NR%) (p <0.32) Non-statin users TPV: +12.6 ±24.3 (-NR%) (p = NR) |
Statistical significant difference (p = 0.032) in dutasteride group in patients receiving statins over the non-statin users (4.5% smaller prostate). Similan differences between statin and non-statin users in placebo arm (3–3.3%) without statistical significance (p ≥0.18) |
| Mills 2007, [74] Low Risk |
Atorvastatin 80 mg vs Placebo | Men ≥50 yo, IPSS score ≥13, Vol prostate ≥30 ml, Qmax 5–15 ml/s, LDL100–190 mg/dl | 26 weeks | 176 vs 174 | TPV: 48.7 ±19.0 TZV: 21.4 ±15.3 PSA: 2.73 ±2.2 |
160 | TPV: -2.0 ±0.83 (-4.1%) TZV: -0.3 ±0.64 (-12.5%) PSA: -0.1 ±0.08 (-3.6%) |
TPV: 50.7 ±19.0 TZV: 22.4 ±13.6 PSA: 2.81 ±2.3 |
159 | TPV: -2.4 ±0.85 (-4.7%) TZV: -0.3 ±0.66 (-13.4%) PSA: 0 ±0.08 (0%) |
No significant change from baseline I both groups. non-significant difference between groups in TPV (p = 0.654), in TZV (p = 0.421) and PSA (p = 0.235) |
| Zhang 2015, [75] Moderate Risk |
Atorvastatin 20 mg vs Placebo | Men ≥60 yo, with LUTS due to BPH, TPV >30 ml, IPSS score >7, PSA <4 ng/dl, MetS as defined by NCEP ATPIII criteria | 12 months | 40 vs 41 | TPV: 50.69 ±17.7 PSA: 1.93 ±1.8 |
40 | TPV: -5.91 ±19.5 (-11.7%), (p <0.001) PSA: -0.06 ±1.77 (-3. 1%), (p = NR) |
TPV: 47.14 ±16.3 PSA: 2.0 ±1.9 |
41 | TPV: +1.17 ±17.4 (+2.5%), (p = NR) PSA: +0.02 ±1.8 (+1.0%), (p = NR) |
Significant changes in TPV in favor of Atorvastatin group as compared to placebo (p <0.01). Non significant changes in PSA between groups or from baseline |
| Pinggera 2014, [53] Moderate Risk |
Tadalafil 5 mg vs Placebo | Men ≥45 yo, with LUTS due to BPH ≥6 months history, Qmax ≥4 to ≤15 ml/s, IPSS ≥13 | 8 weeks | 47 vs 50 | mRI: 0.65 ±0.7 mCPI: 77.88 ±28.4 mCPD: 11.73 ±7.4 |
39 | mRI: 0.01 ±0.01 (1.5%) (NS) mCPI: 4.3 ±2.6 (5.5%) (NS) mCPD: 0.36 ±1.3 (3.0%) (NS) |
mRI: 0.63 ±0.7 mCPI: 79.16 ±22.9 mCPD: 12.81 ±9.9 |
45 | mRI:-0.01 ±0.01 (-1.6%) NS mCPI: 1.67 ±2.5 (2.1%) (NS) mCPD: 0.39 ±1.2 (3.0%) (NS) |
Non-significant changes from baseline in either parameters. Non-significant difference from placebo |
AUA-SI score – American Urology Association Symptom Index score; BD – twice daily; BPH – benign prostate hyperplasia; DRE – digital rectal examination; IPSS – international prostate symptom score; LUTS – lower urinary tract symptoms; Non-Sr – other than Serenoa repens; MetS – metabolic syndrome; NR – not reported, NCEP ATPIII – National Cholesterol Education Program Adult Treatment Panel III; NS – non sinificant; OD – once daily; PVR – postvoid residual; Qmax – maximum flow rate; PCa – prostate cancer; PSA – prostate specific antigen; Qmax – maximum flow rate; TPV – total prostate volume, TZI – transitional zone index; TZV – transitional zone volume; Vol – volume; TRUS – transrectal ultrasonography; VV – voided volume; yo – years old
The SPRITE study randomized men to tadalafil 5 mg or phytotherapy [54]. No change of TPV was observed in the tadalafil arm at 6 months. Two trials with high RoB reported a non-significant reduction in TPV from baseline after tadalafil (-5.9%) and sildenafil (-3.9%) treatment [55, 56]. PSA changes as reported in three trials were not significantly different from baseline (-8.6% -0%) [28, 54, 56]. There was no information on TZV or prostate perfusion parameters.
Combination treatment
The 12-month VA-COOP study reported a significant reduction in TPV from baseline (-18.8% or -7.0 ml, p <0.001) after terazosin and finasteride combination compared to non-significant changes in the placebo arm (+2.3% or +0.5 ml) (Table 3) [18]. MTOPS reported a similar reduction in the combination arm (-19% or -12.1 ml, p <0.001), while TPV increased significantly in the placebo arm (+24% or +8.8 ml, p <0.01) [19]. MTOPS reported a 50% PSA reduction, while VA-COOP reported an unexplained 39.1% increase from baseline. There was no information on TZV or prostate perfusion parameters.
Two 12-month studies randomized men to tamsulosin 0.2 mg versus tamsulosin plus dutasteride combination and reported a significant reduction of TPV (-18.8% to -26.9%), TZV (-17.7% to -19.7%) and PSA (-18.3% to -41.2%) in combination arms and no changes in tamsulosin arms [57, 58]. Mohanty et al., compared tamsulosin plus finasteride versus tamsulosin plus dutasteride combination, and found similar TPV changes after 6 months of treatment [59].
Two 6-month RCTs assessed the influence of anticholinergics in prostate morphometric parameters in men with OAB and BPE [34, 60]. A moderate risk trial randomized men to tamsulosin versus tamsulosin plus solifenacin combination [34]. Authors reported a significant reduction of TPV (-9.5% or 5.5 ml, p <0.001), TZV (-12.5% or -2.5 ml, p <0.001) and prostate perfusion (-41%) in the combination arm. Yamahishi et al., randomized men to tamsulosin plus dutasteride alone or with imidafenacin. Both arms significantly improved TPV (-21.7% vs -22.6%) and PSA (-47.2% vs -38.8%), without significant differences between them. Three trials randomized men to tamsulosin monotherapy versus tamsulosin plus Serenoa repens (Sr). No significant differences in TPV or PSA were reported [29, 61, 62].
Phytotherapy
Eight trials randomized men (n = 1608) to phytotherapy versus placebo (Table 3) [63–70]. Four Sr trials reported non-significant changes in TPV as compared to placebo [SMD:0.12(95%CI:-0.03 to 0.27, p = 0.13) (Figure 5) [66, 67, 68, 70]. Non-Sr trials reported significant TPV reduction from baseline up to -16.9% [63, 64, 69]. Two trials reported similar TZV changes to placebo at 52 and 24 weeks respectively [SMD:0.06 (95%CI:-0.18 to 0.30, p = 0.64) (Figure 3) [66, 67]. A small trial reported pronounced epithelial component involution in the transitional zone as compared to baseline (17.8% to 10.7%, p <0.01) in the Sr group [67]. Four trials reported non-significant small effects of phytotherapy on PSA as compared to placebo [SMD: -0.06 (95%CI: -0.21 to 0.10, p = 0.46) (Figure 5) [66, 67, 68, 70]. There was no information regarding prostate perfusion parameters.
Figure 5.
Meta-analysis of Serenoa repens (Sr) and testosterone effect on prostate morphometric parameters effect in placebo-controlled trials. A) Forrest plot of the effect of Sr versus placebo on total prostate volume (TPV). B) Forrest plot of the effect of Sr versus placebo on transitional zone volume (TZV). C) Forrest plot of the effect of Sr versus placebo on prostate-specific antigen (PSA). D) Forrest plot of the effect of testosterone plus dutasteride combination versus testosterone monotherapy on total prostate volume (TPV). E) Forrest plot of the effect of testosterone plus dutasteride combination versus testosterone monotherapy on PSA.
CI – confidence interval; SD – standard deviation
Ten trials randomized men (n = 2972) to phytotherapy versus active component [24, 25, 26, 29, 31, 50, 54, 62, 71, 72]. Six RCTs compared Sr to an active comparator and reported non-significant changes in TPV from baseline (-7% to –2% or -2.0 ml to -0.7 ml) [24, 26, 29, 31, 50, 62]. A single trial reported significant TPV change from baseline after Sr 320 mg once daily (-14.5%, p <0.001) or Sr 160 mg twice daily for 12 months (-9.6%, p <0.001) [72]. Two non-Sr trials reported significant reduction in TPV as compared to baseline (-5.6% and -10.8%) [25, 71]. Six trials reported non-significant changes in PSA as compared to baseline or to comparator (-15% to +10% or -0.3 ng/dl to +0.2 ng/dl) [24, 29, 31, 50, 62]. There was no information regarding TZV and prostate perfusion parameters.
Other medications
A post hoc analysis of the REDUCE trial classified men on dutasteride as statin and non-statin users (Table 1) [73, 48]. Authors reported a significant TPV change from baseline (-15.8% or -6.8 ml, p = 0.033) in the statin users’ subgroup as compared to the non-statin users. The effect of dutasteride on lowering TPV was roughly 10-fold greater than the statin-associated effect at year 2 (p <0.001) and year 4 (p <0.001) [73]. A 26-week RCT compared atorvastatin 80 mg versus placebo and reported no difference from baseline or between groups in TPV (-4.1% vs -4.7%, p = 0.654), in TZV (-12.5% vs -13.4%, p = 0.421) and in PSA (-3.6% vs 0%, p = 0.235) [74]. In contrast, a 12-month trial reported a significant difference in TPV in favor of atorvastatin group as compared to placebo (-11.7% vs +2.5%, p <0.01), changes which were more pronounced in obese patients compared to normoweight individuals [75].
A single study compared cholecalciferol 600 IU plus tamsulosin versus tamsulosin monotherapy and reported non-significant TPV changes between groups (+8.1% vs +5.9%, p = 0.098) at 24 months [76]. Ω3-fatty acids in combination with tamsulosin and finasteride were non-superior to tamsulosin plus finasteride combination in reducing TPV [77]. Di Silverio studied the effect of rofecoxib 25 mg with finasteride versus finasteride monotherapy and reported comparable reductions in TPV (-20.1% vs -20.2%) and PSA (-35.4% vs -36.4%) at 6 months [78]. They reported an accelerated effect in rofecoxib group. The effect of celecoxib was tested in two studies against terazosin and doxazosin [79, 80]. The first reported that celecoxib reduces significantly TPV (-12.9%) and PSA (-17.6%) and the latter reported a significant PSA change (-17.0%) only. Two RCTs studied the effect of testosterone replacement in TPV and PSA of men with androgen deficiency syndrome [81, 82]. Both studies reported an increase in TPV (+7.6% and +5.9%) and PSA (10.7% and +8.2%) after testosterone supplementation. The co-administration of dutasteride spares prostate from androgenic stimulation since both TPV (-13.1% and -14.7%) and PSA (-33.3% and -42.6%) were reduced significantly. The treatment effect was considered moderate in favor of combination regarding TPV [SMD: -0.44 (95%CI: -0.90 to 0.02, p = 0.06) (Figure 3) and PSA change [SMD: -0.50 (95%CI: -0.96 to 0.04, p = 0.03).
Placebo arm
In phytotherapy trials, the changes in TPV were from -5.1% to +2.91% and in PSA from -4.2% to -1.0% [63–70]. A trial with 12-month follow-up reported +14.7% increase in TPV and +8.8% increase in PSA [66]. In short-term RCTs with α-1 blockers, the changes in TPV and PSA were not significant, ranging from 2.3% to 3% and -4% to +10% respectively. In 5-ARI trials TPV change was reported between -10.0% and -2.7% in 6-month studies, -5.0% to -2.3% in 12-month studies, +2.0% to +14.0% in 24-month studies [18, 19, 35–48]. REDUCE and MTOPS trials, both with long follow-up, reported TPV change +19.7% and +24.0% respectively [48, 19]. The changes in PSA were -6.0% to -1.0%, -5.0% to -2.0% and +6% to +15.8% respectively.
DISCUSSION
Herein, we systematically reviewed the effect of pharmacotherapy on prostate morphometric parameters, namely TPV, TZV, PSA and prostate perfusion. The strengths of this review include the systematic and transparent approach to analyze the evidence base, including the Cochrane review methodology, the adherence to PRISMA guidelines and a-priori written protocol. We also used a comprehensive approach to determine RoB and to include studies with well-defined protocol assessing morphometric parameters.
The weaknesses relate to the limitations of the body of evidence that we analyzed. Based on AHRQ standards, 16 RCTs were considered as low-risk, 31 RCT as moderate-risk and 20 as high-risk. Thirteen out of 28 placebo-controlled trials were considered of moderate-risk. Most trials were powered to assess post-treatment changes in clinical parameters such as relevant questionnaires or flow test. Only 10 RCTs were powered to assess changes in morphometric parameters as a primary outcome. An additional methodological issue relates to the technique used to evaluate prostate parameters. To overcome measurement bias, we included studies that describe in detail the method of volume calculation. Concerning PSA and perfusion parameters, we relied on data provided by each group.
α-blockers do not affect TPV, TZV or PSA. Studies with long-term follow-up report changes similar to placebo, while the observed significant differences from baseline result from physiologic growth. Animal experiments demonstrated that sympathomimetics induce prostate hyperplasia, whereas quinazoline-based α-blockers exert apoptotic effect on human prostate cancer cell cultures [8]. This in vitro effect is not evident in clinical setting [83]. There is evidence that tamsulosin improves prostate perfusion, possibly by the antagonistic action on α1A- and α1D-adrenoceptors of vesical arteries [12, 84]. A single RCT in OAB population reported increased perfusion up to +149%, which was similar to previous findings (+132.8%), hence the beneficial effect of tamsulosin on LUTS [85].
Robust evidence supports the effect of 5-ARIs on TPV, TZV and PSA. Dihydrotestosterone (DHT) induces prostate growth via enhanced proteinosynthesis and reduced apoptotic rates [86]. 5-ARIs reduce TPV, TZV and PSA in at least 85.3% of patients after six to twelve months of treatment [87]. A head-to-head comparison of finasteride and dutasteride showed similar efficacy, but dutasteride effect appears sooner [49, 52]. A pooled analysis of dutasteride trials reports significant changes of TPV starting at 1st month of treatment, as a result of the faster DHT suppression [45, 47]. These changes reach the maximum effect at 12 months and this change is sustained thereafter [18, 19, 35–38, 40, 44]. DHT increases prostatic blood flow via increased expression of VEGF [86]. Finasteride downregulates VEGF and reduces prostate blood flow as early as 7 days after administration [11, 86]. Preliminary literature search on single-arm studies revealed two dutasteride single-arm trials reporting a reduction in perfusion parameters [88, 89].
Even though there is little evidence, PDE5 inhibitors do not affect TPV, TZV and PSA. Studies on human prostatic tissue strips, suggested that upregulation of intracellular cGMP by PDE5 inhibition decreases smooth muscle tone and might attenuate prostate cells proliferation [3, 10, 90, 91]. Animal models of chronic pelvic ischemia demonstrated that PDE5 inhibitors increase cGMP levels and improve lower urinary tract perfusion [10]. Using contrast-enhanced ultrasound, an observational study demonstrated improvements in prostate perfusion after tadalafil administration [10, 92]. In men at high-risk for endothelial dysfunction, tadalafil significantly improves flow-mediated dilation of brachial artery as compared to controls [21, 93]. However, these vasoactive effects of PDE5 inhibitors were not evident at clinical level [9, 10, 92, 94, 95]. A single RCT did not report any significant effect on prostate perfusion parameters [53].
The overall effect of phytotherapy on prostate morphometric parameters is ambiguous. Both placebo-controlled and active medication-controlled trials on Sr reported no significant difference from comparators, while non-Sr trial reported a significant reduction in TPV. These trials are characterized by high heterogeneity and poor quality. Conclusions from phytotherapy trials are difficult due to differences in consistency, concentration or extraction techniques. As a result, the biological activity might differ even among studies with same extracts. A recent meta-analysis reported significant reduction in TPV and non-significant increase of PSA after administration of hexanic extract of Sr [96].
Combination treatment is indicated when monotherapy fails to control symptoms. According to European Association of Urology (EAU) guidelines, α-blockers are combined with 5-ARIs to improve residual voiding LUTS or with an anticholinergic for residual storage symptoms [1]. CombAT reported similar TPV changes between combination and dutasteride monotherapy arm (-27.3% vs -28.0%) [32]. TZV changes differ, almost statistically significantly (-17.9% vs -26.5%, p = 0.052). In the case of α-blocker with anticholinergic combination the data is limited. A single RCT reported significant reduction in TPV, TZV and perfusion parameters with combination of solifenacin and tamsulosin as opposed to tamsulosin monotherapy [34]. There is no data on the effect of β3-agonists on TPV. Evidence from basic science shows that mirabegron improves bladder wall blood flow and bladder dysfunction through amelioration of pelvic blood flow [97].
Statins reduce TPV, albeit ten times less than dutasteride [73]. Recent evidence shows that atorvastatin has pro-cell apoptotic action, a pro-cellular adhesion effect, a pro-proliferation effect and an anti-inflammatory action via reduction of Interleukin-6 and IGF-1 [75]. Cholecalciferol and rofecoxib did not differ from their comparators. By contrast, celecoxib reduces both TPV and PSA [79, 80]. Testosterone replacement therapy restores DHT levels, thus TPV and PSA do not change further from a saturation point [82]. Dutasteride reduces TPV, PSA in men who receive testosterone replacement therapy, an effect that validates the influence of intraprostatic DHT on morphometric parameters.
A single summary for the effect of medications on prostate morphometric parameters is not possible. The degree of heterogeneity renders inappropriate any formal data pooling. The reasons of heterogeneity were the differences in study design, in follow-up duration, in sample size, in drop-out rates, in the inadequacy of reporting standards and in the forced unilateral regression to the mean (due to inclusion/exclusion criteria other than volume such as uroflowmetry). In addition, a small number of trials were powered enough to detect changes in morphometric parameters while others were characterized as low-quality due to high risk of bias [100]. The placebo response differs surprisingly among trials. A similar effect has been previously described [98]. The relevant mechanisms of this effect are poorly understood.
CONCLUSIONS
A detailed review of the effect of medical therapy on prostate morphometric parameters has been presented. The 5-ARIs show large effect size in reducing TPV as compared to placebo. There is no difference between finasteride and dutasteride but data support an earlier influence of dutasteride on TPV. Quinazolin-based α-blockers are associated with significant TPV changes in 4-year trials which are similar to placebo and represent the natural growth of prostate. Non-Sr phytotherapy appears to reduce TPV in contrast to a non-effect of Sr, but relevant studies suffer from moderate or high risk of bias. PDE5-inhibitors’ trials reported non-significant TPV changes. Among other medications, atorvastatin and celecoxib were found to significantly reduce TPV. A large effect on TZV is observed after either 5-ARI monotherapy or after combination treatment with an α-blocker, but the reduction in the latter group is less. PSA changes are significant in patients receiving 5ARI monotherapy or in combination. No other treatment class appears to affect PSA. There is less robust evidence to suggest that tamsulosin improves prostate perfusion while tadalafil has no effect on clinical perfusion parameters.
CONFLICTS OF INTEREST
The authors report no conflicts of interest.
DEPARTMENT AND INSTITUTION WHERE THE WORK WAS CARRIED OUT
Agios Pavlos General Hospital of Thessaloniki, Greece
2nd Department of Urology, Aristotele University of Thessaloniki, Greece
References
- 1.Gravas S, Cornu JN, Gacci M, Gratzke C, Herrmann TRW, Mamoulakis C, et al. Edn. Presented at the EAU Annual Congress Barcelona. Arnhem: EAU Guideline Office; EAU guidelines on management of non-neurogenic male lower urinary tract symptoms (LUTS), incl. benign prostatic obstruction (BPO) [Google Scholar]
- 2.Abrams P, Cardozo L, Fall M, et al. the standardization of terminology in lower urinary tract function: report from the standardization sub-committee of the International Continence Society. Neurourol Urodyn. 2002;61:37–49. doi: 10.1002/nau.10052. [DOI] [PubMed] [Google Scholar]
- 3.Jacobsen SJ, Girman CJ, Lieber MM. Natural history of benign prostatic hyperplasia. Urology. 2001;58:5–16. doi: 10.1016/s0090-4295(01)01298-5. [DOI] [PubMed] [Google Scholar]
- 4.Crawford ED, Wolson SS, McConnell JD, et al. for the MTOPS Research group Baseline factors as predictors of clinical progression of benign prostatic hyperplasia in men treated with placebo. J Urol. 2006;175:1422–1427. doi: 10.1016/S0022-5347(05)00708-1. [DOI] [PubMed] [Google Scholar]
- 5.Kozminski MA, Wei JT, Nelson J, Kent DM. Baseline characteristics predict risk of progression and response to combined medical therapy for benign prostatic hyperplasia (BPH) BJU Int. 2015;115:308–318. doi: 10.1111/bju.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Loeb S, Kettermann A, Carter HB, Ferrucci L, Metter EJ, Walsh PC. Does prostate growth confound prostate specific antigen velocity? Data from the Baltimore Longitudinal Study of Aging. J Urol. 2008;180:1314–1317. doi: 10.1016/j.juro.2008.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azadzoi KM, Babayan RK, Kozlowski R, Siroky MB. Chronic ischemia increases prostatic smooth muscle contraction in the rabbit. J Urol. 2003;170:659–663. doi: 10.1097/01.ju.0000064923.29954.7e. [DOI] [PubMed] [Google Scholar]
- 8.Kyprianou N, Benning CM. Suppression of human prostate cancer cell growth by alpha 1-adrenoreceptor antagonists doxazosin and terazosin via induction of apoptosis. Cancer Res. 2000;60:4550–4555. [PubMed] [Google Scholar]
- 9.Morelli A, Sarchielli E, Comegiol P, et al. Phosphodiesterase type 5 expression in human and rat lower urinary tract tissues and the effect of tadalafil on prostate gland oxygenation in spontaneously hypertensive rats. J Sex Med. 2011;8:2746–2760. doi: 10.1111/j.1743-6109.2011.02416.x. [DOI] [PubMed] [Google Scholar]
- 10.Andesson KE, de Groat WC, McVary KT, et al. Tadalafil for the treatment of lower urinary tract symptoms secondary to benign prostatic hyperplasia: Pathophysiology and mechanism of action. Neurourol Urodyn. 2011;30:292–301. doi: 10.1002/nau.20999. [DOI] [PubMed] [Google Scholar]
- 11.Lekas E, Bergh A, Damber JE. Effects of finasteride and bicalutamide on prostatic blood flow in the rat. BJU Int. 2000;85:962–965. doi: 10.1046/j.1464-410x.2000.00671.x. [DOI] [PubMed] [Google Scholar]
- 12.Mine S, Yamamoto T, Mizuno H, et al. Effect of tamsulosin on bladder microcirculation in rat model of bladder outlet obstruction using pencil lens charge-coupled device microscopy system. Urology. 2013;81:155–159. doi: 10.1016/j.urology.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Sterne JAC, Savovic J, et al. A revised tool for assessing risk of bias in randomized trials. In: Chandler J, McKenzie J, Boutron I, Welch V, editors. Cochrane Methods. ISA: Cochrane Database of Systematic Reviews; 2016. [Google Scholar]
- 15.Viswanathan M, Ansari MT, Berkman ND, et al. Methods guide for effectiveness and comparative effectiveness reviews. AHRQ methods for effective health care. Rockville, MD: Agency for Healthcare Research and Quality (US); 2008. Assessing the risk of bias on individual studies in systematic reviews of health care interventions. Available at: http://www.ncbi.nlm.nih.gov/books/NBK91433/ accessed: June 12, 2020. [PubMed] [Google Scholar]
- 16.Berkman ND, Lohr KN, Ansari M, et al. Grading the strength of a body of evidence when assessing health care interventions for the effective health care program of the Agency for Healthcare Research and Quality: an update. AHRQ Publication No. 13(14) - EHC130-EF. Rockville, MD: Agency for Healthcare Research and Quality; 2013. Nov, www.effectivehealthcare.ahrq.gov/reports/final.cfm. [PubMed] [Google Scholar]
- 17.Popay J, Roberts H, Sowden A, Petticrew M, Arai L, Rodgers M, Britten N. Guidance on the conduct of narrative synthesis in systematic reviews. ESRC Research Methods Programme; 2006. [Google Scholar]
- 18.Lepor H, Williford WO, Barry MJ, et al. The efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. N Eng J Med. 1996;335:533–539. doi: 10.1056/NEJM199608223350801. [DOI] [PubMed] [Google Scholar]
- 19.McConnell JD, Roehrborn CG, Bautista OM, et al. The long-term effects of doxazoin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Eng J Med. 2003;349:2387–2398. doi: 10.1056/NEJMoa030656. [DOI] [PubMed] [Google Scholar]
- 20.Yokoyama O, Yoshida M, Kim SC, Wang CJ, Imaoka T, Morisaki Y. Tadalafil once daily for lower urinary tract symptoms suggestive of benign prostatic hyperplasia: A randomize placebo- and tamsulosin-controlled 12-week study in Asian men. Int J Urol. 2013;20:193–201. doi: 10.1111/j.1442-2042.2012.03130.x. [DOI] [PubMed] [Google Scholar]
- 21.Roehrborn CG, for the ALTESS Study Group Alfuzosin 10 mg once daily prevents overall clinical progression of benign prostatic hyperplasia but not acute urinary retention: results of a 2-year placebo-controlled study. BJU Int. 2006;97:734–741. doi: 10.1111/j.1464-410X.2006.06110.x. [DOI] [PubMed] [Google Scholar]
- 22.Roehrborn CG. Three months’ treatment with the α1-blocker alfuzosin does not affect total of transition zone volume of the prostate. Prostate Cancer Prostatic Dis. 2006;9:121–125. doi: 10.1038/sj.pcan.4500849. [DOI] [PubMed] [Google Scholar]
- 23.Turkeri LN, Ozyurek M, Ersev D, Akdas A. Apoptotic regression of prostatic tissue induced by short-term doxazosin treatment in benign prostatic hyperplasia. Arch Esp de Urol. 2001;54:191–196. [PubMed] [Google Scholar]
- 24.Debruyne F, Koch G, Boyle P, et al. Comparison of a phytotherapeutic agent (Permixon) with an α-blocker (tamsulosin) in the treatment of benign prostatic hyperplasia: A 1-year randomized international study. Eur Urol. 2002;41:497–507. [PubMed] [Google Scholar]
- 25.Sengupta G, Hazra A, Kundu A, Ghosh A. Comparison of Murraya koenigii- and Tribulus terrestris-based oral formulation versus tamsulosin in the treatment of benign prostatic hyperplasia in men aged >50 years: a double-blind, double-dummy, randomized controlled trial. Clin Ther. 2011;33:1943–1952. doi: 10.1016/j.clinthera.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Latil A, Petrissans MT, Rouquet J, Robert G, de la Taille A. Effects of hexanic extract of Serenoa repens (Permixon® 160 mg) on inflammation biomarkers in the treatment of lower urinary tract symptoms related to benign prostatic hyperplasia. Prostate. 2015;75:1857–1867. doi: 10.1002/pros.23059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pande S, Hazra A, Kundu AK. Evaluation of silodosin in comparison to tamsulosin in benign prostatic hyperplasia. Indian J Pharmacol. 2014;46:601–607. doi: 10.4103/0253-7613.144912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karami H, Hassanzadeh-Hadad A, Fallah-Karkan M. Comparing monotherapy with tadalafil or tamsulosin and their combination therapy in men with benign prostatic hyperplasia: A randomized clinical trial. Urol J. 2016;13:2920–2926. [PubMed] [Google Scholar]
- 29.Hizli F, Uygur MC. A prospective study of the efficacy of Serenoa repens, tamsulosin, and Serenoa repens plus tamsulosin treatment for patients with benign prostate hyperplasia. Int Urol Nephrol. 2007;39:879–886. doi: 10.1007/s11255-006-9106-5. [DOI] [PubMed] [Google Scholar]
- 30.Odusanya BO, Tijani KH, Jeje EA, Ogunjimi MA, Ojewola RW. Short-term effect of tamsulosin and finasteride monotherapy and their combination on Nigerian men with benign prostatic hyperplasia. Nig J Surg. 2017;23:5–10. doi: 10.4103/1117-6806.199963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgia G, Russo GI, Voce S, et al. Serenoa repens, Lycopene and Selenium versus tamsulosin for the treatment of LUTS/BPH. An Italian multicentre double-blinded randomized study between single or combination therapy (PROCOMB Trial) Prostate. 2014;74:1471–1480. doi: 10.1002/pros.22866. [DOI] [PubMed] [Google Scholar]
- 32.Roerhborn CG, Siami P, Barkin J, et al. The effects of combination therapy with dutasteride and tamsulosin on clinical outcomes in men with symptomatic benign prostatic hyperplasia: 4-year results from the CombAT Study. Eur Urol. 2010;57:123–131. doi: 10.1016/j.eururo.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 33.Debruyne FMJ, Jardin A, Colloi D, et al. Sustained-release alfuzosin, finasteride and the combination of both in the treatment of benign prostatic hyperplasia. Eur Urol. 1998;34:169–175. doi: 10.1159/000019706. [DOI] [PubMed] [Google Scholar]
- 34.Sakalis V, Sfiggas V, Vouros I, Salpiggidis G, Papathanasiou A, Apostolidis A. Combination of solifenacin with tamsulosin reduces prostate volume and vascularity as opposed to tamsulosin monotherapy in patients with benign prostate enlargement and overactive bladder symptoms: Results from a randomized pilot study. Int J Urol. 2018;25:737–745. doi: 10.1111/iju.13721. [DOI] [PubMed] [Google Scholar]
- 35.Andersen JT, Ekman P, Wokf H, et al. Can finasteride reverse the progress of benign prostatic hyperplasia: A two-year placebo controlled study. Urology. 1995;46:631–637. doi: 10.1016/s0090-4295(99)80291-x. [DOI] [PubMed] [Google Scholar]
- 36.Nickel JC, Fradet Y, Boake R, et al. Efficacy and safety of finasteride therapy for benign prostatic hyperplasia: Result of a 2-year randomized controlled trial (the PROSPECT Study) Can Med Assoc J. 1996;155:1251–1259. [PMC free article] [PubMed] [Google Scholar]
- 37.McConnell JD, Bruskewitz R, Walsh P, et al. The effect of finasteride on the risk on the risk of acute urinary retention and the need for surgical treatment among men with benign prostatic hyperplasia. N Eng J Med. 1998;338:557–563. doi: 10.1056/NEJM199802263380901. [DOI] [PubMed] [Google Scholar]
- 38.Marberger MJ, on behalf of the PROWESS Study Group Long-term effects of finasteride in patients with benign prostatic hyperplasia: A double-blind, placebo-controlled, multicentre study. Urology. 1998;51:677–686. doi: 10.1016/s0090-4295(98)00094-6. [DOI] [PubMed] [Google Scholar]
- 39.Kirby RS, Bryan J, Eardley I, Christmas TJ, Liu S, Holmes SAV, Vale JA, Shanmuganathan K, Webb JA. Finasteride in the treatment on benign prostatic hyperplasia. A urodynamic evaluation. Br J Urol. 1992;70:65–72. doi: 10.1111/j.1464-410x.1992.tb15666.x. [DOI] [PubMed] [Google Scholar]
- 40.Stoner E, on behalf of The finasteride Study group The clinical effects of a 5α-reductase inhibitor, finasteride, on benign prostatic hyperplasia. J Urol. 1992;147:1298–1302. doi: 10.1016/s0022-5347(17)37547-x. [DOI] [PubMed] [Google Scholar]
- 41.Tammela TLJ, Kontturi MJ. Long-term effects of finasteride on invasive urodynamics and symptoms in the treatment of patients with bladder outflow obstruction due to benign prostatic hyperplasia. J Urol. 1995;154:1466–1469. [PubMed] [Google Scholar]
- 42.Pannek J, Marks LS, Pearson JD, et al. Influence of finasteride on free and total serum prostate specific antigen levels in men with benign prostatic hyperplasia. J Urol. 1998;159:449–453. doi: 10.1016/s0022-5347(01)63946-6. [DOI] [PubMed] [Google Scholar]
- 43.Marks LS, Partin AW, Gormley GL, et al. Prostate tissue composition and response to finasteride in men with symptomatic benign prostatic hyperplasia. J Urol. 1997;157:2171–2178. [PubMed] [Google Scholar]
- 44.Gormley GJ, Stoner E, Bruskewitz RC, et al. the effect of finasteride in men with benign prostatic hyperplasia. N Eng J Med. 1992;327:1185–1191. doi: 10.1056/NEJM199210223271701. [DOI] [PubMed] [Google Scholar]
- 45.Roerhborn CG, Boyle P, Nickel JC, Hoefner K, Andriole G. Efficacy and safety of a dual inhibitor of 5-alpha-reductase types 1 and 1 (Dutasteride) in men with benign prostatic hyperplasia. Urology. 2002;60:434–441. doi: 10.1016/s0090-4295(02)01905-2. [DOI] [PubMed] [Google Scholar]
- 46.Na Y, Ye Z, Zhang S, on behalf of the Chinese Dutasteride Phase III trial (ARIA108898) Study group Efficacy and safety of dutasteride in Chinese men with benign prostatic hyperplasia. Clin Drug Investig. 2012;32:29–39. doi: 10.2165/11593750-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 47.Tsukamoto T, Endo Y, Narita M. Efficacy and safety of dutasteride in Japanese men with benign prostatic hyperplasia. Int J Urol. 2009;16:747–750. doi: 10.1111/j.1442-2042.2009.02357.x. [DOI] [PubMed] [Google Scholar]
- 48.Andriole G, Bostwick DG, Brawley OW, et al. Effect of dustasteride on the risk of prostate cancer. N Eng J Med. 2010;362:1192–1202. doi: 10.1056/NEJMoa0908127. [DOI] [PubMed] [Google Scholar]
- 49.Nickel JC, Gilling P, Tammela TL, Morrill B, Wilson TH, Rittmaster RS. Comparison of dutasteride and finasteride for treating benign protatic hyperplasia: the Enlarged Prostate International Comparator Study (EPICS) BJU Int. 2011;108:388–394. doi: 10.1111/j.1464-410X.2011.10195.x. [DOI] [PubMed] [Google Scholar]
- 50.Carraro JC, Raynaud JP, Koch G, et al. Comparison of phytotherapy (Permixon®) with finasteride in the treatment of benign prostate hyperplasia: A randomized international study of 1098 patients. Prostate. 1996;29:231–240. doi: 10.1002/(SICI)1097-0045(199610)29:4<231::AID-PROS4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 51.Kuo HC. Comparative study of therapeutic effect of dibenyline, finasteride, and combination drugs for symptomatic benign prostatic hyperplasia. Urol Int. 1998;60:85–91. doi: 10.1159/000030217. [DOI] [PubMed] [Google Scholar]
- 52.Jeong YB, Kwon KS, Kim SD, Kim HJ. Effect of discontinuation of 5α-reductase inhibitors on prostate volume and symptoms in men with BPH: A prospective study. Urology. 2009;73:802–806. doi: 10.1016/j.urology.2008.10.046. [DOI] [PubMed] [Google Scholar]
- 53.Pinggera GM, Frauscher F, Paduch DA, et al. Effect of tadalafil once daily on prostate blood flow and perfusion in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia: A randomized, double-blind, multicenter, placebo-controlled trial. Urology. 2014;84:412–420. doi: 10.1016/j.urology.2014.02.063. [DOI] [PubMed] [Google Scholar]
- 54.Morgia G, Vespasiani G, Pareo RM, et al. Serenoa repens + selenium + lycopene vs tadalafil 5 mg for the treatment of lower urinary tract symptoms secondary to benign prostatic obstruction: a Phase IV, non-inferiority, open-label, clinical study (SPRITE study) BJU Int. 2018;122:317–325. doi: 10.1111/bju.14209. [DOI] [PubMed] [Google Scholar]
- 55.Kosilov KV, Kuzina IG, Kuznetsov V, Kosilova EK. Improvement of the symptoms of lower urinary tract and sexual function with tadalafil and solifenacin after the treatment of bening prostatic hyperplasia with dutasteride. Prostate Int. 2020;8:78–84. doi: 10.1016/j.prnil.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozturk MI, Kalkan S, Koca O, Gunes M, Akyuz M, Karaman MI. Efficacy of alfuzosin and sildenafil combination in male patients with lower urinary tract symptoms. Andrologia. 2012;44:791–795. doi: 10.1111/j.1439-0272.2011.01268.x. [DOI] [PubMed] [Google Scholar]
- 57.Joo KJ, Sung WS, Park SH, Yang WJ, Kim TH. Comparison of α-blocker monotherapy and α-blocker plus 5α-reductase inhibitor combination therapy based on prostate volume for treatment f benign prostatic hyperplasia. J Int Med Res. 2012;40:899–908. doi: 10.1177/147323001204000308. [DOI] [PubMed] [Google Scholar]
- 58.Choi JD, Kim JH, Ahn SH. Transitional zone index as a preditor of the efficacy of α-blocker and 5α-reductase inhibitor combination therapy in Korean patients with benign prostatic hyperplasia. Urol Int. 2016;96:406–412. doi: 10.1159/000442995. [DOI] [PubMed] [Google Scholar]
- 59.Mohanty NK, Singh UP, Sharma NK, Arora RP, Amtabh V. A comparative study of fixed dose of tamsulosin with finasteride vs tamsulosin with dutasteride in the management of benign prostatic hyperplasia. Indian J Urol. 2006;22:130–134. [Google Scholar]
- 60.Yamanishi T, Asakura H, Seki N, Tokunaga S. Efficacy and safety of combination therapy with tamsulosin, dutasteride and imidafenacin for the management of overactive bladder symptoms associated with benign prostatic hyperplasia: A multicenter, randomized, open-label, controlled trial (DIrect Study) Int J Urol. 2017;24:525–531. doi: 10.1111/iju.13359. [DOI] [PubMed] [Google Scholar]
- 61.Ryu YW, Lim SW, Kim JH, Ahn SH, Choi JD. Comparison of tamsulosin plus serenoa repens with tamsulosin in the treatment of benign prostatic hyperplasia in Korean men: 1-year randomized open label study. Urol Int. 2015;94:187–193. doi: 10.1159/000366521. [DOI] [PubMed] [Google Scholar]
- 62.Argirovic A, Argirovic D. Does the addition of Serenoa repens to tamsulosin improve its therapeutical efficacy in benign prostatic hyperplasia? Vojnosanit Pregl. 2013;70:1091–1096. doi: 10.2298/vsp110620029a. [DOI] [PubMed] [Google Scholar]
- 63.Beiraghdar F, Einollahi B, Panahi Y, Hadjiakhoondi A, Vazirian M, Salarytabar A, Darvishi B. A two-week, double-blind, placebo controlled trial of viola odorata, Echiumamoneum and Physalis alkekengi mixture in symptomatic benign prostate hyperplasia (BPH) in men. Pharm Biol. 2017;55:1800–1805. doi: 10.1080/13880209.2017.1328445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berges RR, Windeler J, Trampisch HJ, Senge Th, the β-sitosterol study group Randomised, placebo-controlled, double-blind clinical trial of β-sitosterol in patients with benign prostatic hyperplasia. Lancet. 1995;345:1529–1532. doi: 10.1016/s0140-6736(95)91085-9. [DOI] [PubMed] [Google Scholar]
- 65.Safarinejad MR. Urtica dioca for treatment of benign prostatic hyperplasia: A prospective, randomized, double-blind, placebo-controlled, crossover study. J Herb Pharmacother. 2005;5:1–11. [PubMed] [Google Scholar]
- 66.Bent S, Kane C, Shinohara K, Neuhaus J, Hudes ES, Goldberg H, Avins AL. Saw Palmetto for benign prostatic hyperplasia. N Eng J Med. 2006;354:557–566. doi: 10.1056/NEJMoa053085. [DOI] [PubMed] [Google Scholar]
- 67.Marks LS, Partin AW, Epstein JI, et al. Effects of a saw palmetto herbal blend in men with symptomatic benign prostatic hyperplasia. J Urol. 2000;163:1451–1456. [PubMed] [Google Scholar]
- 68.Ye Z, Huang J, Zhou L, et al. Efficacy and safety of Serenoa repens extract among patients with benign prostatic hyperplasia in China: A multicenter, randomized, double-blind, placebo-controlled trial. Urology. 2019;129:172–179. doi: 10.1016/j.urology.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 69.Zhang W, Wang X, Liu Y, et al. Effects of dietary flaxseed lignin extract on symptoms of benign prostatic hyperplasia. J Med Food. 2008;11:207–214. doi: 10.1089/jmf.2007.602. [DOI] [PubMed] [Google Scholar]
- 70.Shi R, Xie Q, Gang X, et al. Effect of saw palmetto soft gel capsule on lower urinary tract symptoms associated with benign prostatic hyperplasia: a randomized trial in Shanghai, China. J Urol. 2008;179:610–615. doi: 10.1016/j.juro.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 71.Guzman R, Fernandez JC, Perdoso M, et al. Efficacy and tolerability of Roystonea regia lipid extract (D-004) and terazosin in men with symptomatic benign prostatic hyperplasia: a 6-month study. Ther Adv Urol. 2019;11:1–12. doi: 10.1177/1756287219854923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Braeckman J, Bruhwyler J, Vandekerckhove K, Geczy J. Efficacy and safety of the extract of serenoa repens in the treatment of benign prostatic hyperplasia: Therapeutic equivalence between twice and once daily dosage forms. Phytother Res. 1997;11:558–563. [Google Scholar]
- 73.Allott EH, Csizmadi I, Howard LE, et al. Statin use and longitudinal changes in prostate volume;results from the REduction by DUtasteride of prostate Cancer Events (REDUCE) trial. BJU Int. 2020;125:226–233. doi: 10.1111/bju.14905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mills IW, Crossland A, Patel A, Ramonas H. Atorvastatin treatment for men with lower urinary tract symptoms and benign prostatic enlargement. Eur Urol. 2007;52:503–509. doi: 10.1016/j.eururo.2007.02.032. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X, Zeng X, Dong L, Zhao X, Qu X. The effects of statins on benign prostatic hyperplasia in elderly patients with metabolic syndrome. World J Urol. 2015;33:2071–2077. doi: 10.1007/s00345-015-1550-3. [DOI] [PubMed] [Google Scholar]
- 76.Safwat AS, Hasanain A, Shahat A, et al. Cholecalciferol for the prophylaxis against recurrent urinary tract infection among patients with benign prostatic hyperplasia: A randomized, comparative study. World J Urol. 2019;37:1347–1352. doi: 10.1007/s00345-018-2536-8. [DOI] [PubMed] [Google Scholar]
- 77.Ghadian A, Rezaei M. Combination therapy with omega-3 fatty acids plus tamsulosin and finasteride in the treatment of men with lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH) Inflammopharmacology. 2017;25:451–458. doi: 10.1007/s10787-017-0343-2. [DOI] [PubMed] [Google Scholar]
- 78.Di Silverio F, Bosman C, Salvatori M, et al. Combination therapy with rofecoxib and finasteride in the treatment of men with lower urinary tract symptoms (LUTS) and benign prostatic hyperplasia (BPH) Eur Urol. 2005;47:72–79. doi: 10.1016/j.eururo.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 79.Goodarzi D, Cyrus A, Vishtec HRK, Solhi H, Shirinkar Effect of celecoxib on benign prostatic hyperplasia: Results of a preliminary study. Urol Sci. 2011;22:147–150. [Google Scholar]
- 80.Jhang JF, Jiang YH, Kuo HC. Adding cyclooxygenase-2 inhibitor to alpha blocker for patients with benign prostate hyperplasia and elevated serum prostate specific antigen could not improve prostate biopsy detection rate but improve lower urinary tract symptoms. Int J Clin Pract. 2013;67:1327–1333. doi: 10.1111/ijcp.12220. [DOI] [PubMed] [Google Scholar]
- 81.Page ST, Hirano L, Gilchriest J, et al. Dutasteride reduces prostate size and prostate specific antigen in older hypogonadal men with benign prostatic hyperplasia undergoing testosterone replacement therapy. J Urol. 2011;186:191–197. doi: 10.1016/j.juro.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kacker R, Harisaran V, Given L, Miner M, Rittmaster R, Morgebtaler A. Dutasteride in men receiving testosterone therapy: a randomised, double-blind study. Andrologia. 2015;47:148–152. doi: 10.1111/and.12237. [DOI] [PubMed] [Google Scholar]
- 83.Chung SD, Chang HC, Chiu B, Liao CH, Kuo HC. The efficacy of additive tolterodine extended release for 1-year in older men with storage symptoms and clinical benign prostatic hyperplasia. Neurourol Urodyn. 2011;30:568–571. doi: 10.1002/nau.20923. [DOI] [PubMed] [Google Scholar]
- 84.Okutsu H, Matsumoto S, Hanai T, et al. Effects of tamsulosin on bladder blood flow and bladder function in rats with bladder outlet obstruction. Urology. 2010;75:235–240. doi: 10.1016/j.urology.2009.05.045. [DOI] [PubMed] [Google Scholar]
- 85.Pinggera GM, Mitterberger M, Pallwein L, et al. alpha-blockers improve chronic ischaemia of the lower urinary tract in patients with lower urinary tract symptoms. BJU Int. 2008;101:319–324. doi: 10.1111/j.1464-410X.2007.07339.x. [DOI] [PubMed] [Google Scholar]
- 86.Haggstrom S, Lissbrant IF, Bergh A, Damber JE. Testosterone induces vascular endothelial growth factor synthesis in the ventral prostate in castrated rats. J Urol. 1999;161:1620–1625. [PubMed] [Google Scholar]
- 87.Rittmaster RS, Norman RW, Thomas LN, Rowden G. Evidence for atrophy and apoptosis in the prostates of men given finasteride. J Clin Endocrinol Metab. 1996;81:814–819. doi: 10.1210/jcem.81.2.8636309. [DOI] [PubMed] [Google Scholar]
- 88.Kravchick S, Cytron S, Mamonov A, Peled R, Linov L. Effect of short-term dutasteride therapy on prostate vascularity in patients with benign prostatic hyperplasia: a pilot study. Urology. 2009;73:1274–1278. doi: 10.1016/j.urology.2008.08.461. [DOI] [PubMed] [Google Scholar]
- 89.Mitterberger M, Pinggera G, Horninger W, et al. Dutasteride prior to contrast enhances colour Doppler ultrasound prostate biopsy increases prostate cancer detection. Eur Urol. 2008;53:112–117. doi: 10.1016/j.eururo.2007.02.031. [DOI] [PubMed] [Google Scholar]
- 90.Guh JH, Hwang T, Ko FN, Chueh SC, Lai MK, Teng CM. Antiproliferative effect in human prostatic smooth muscle cells by nitric oxide donor. Mol Pharmacol. 1998;53:467–474. doi: 10.1124/mol.53.3.467. [DOI] [PubMed] [Google Scholar]
- 91.Kedia GT, Uckert S, Jonas U, Kuczyk MA, Burchardt M. The nitric oxide pathway in the human prostate: Clinical implications in men with lower urinary tract symptoms. World J Urol. 2008;26:603–609. doi: 10.1007/s00345-008-0303-y. [DOI] [PubMed] [Google Scholar]
- 92.Bertolotto M, Trincia E, Zappetti R, Bernich R, Savoca G, Cova MA. Effect of tadalafil on prostate haemodynamics: preliminary evaluation with contrast-enhanced US. Radiol Med. 2009;114:1106–1114. doi: 10.1007/s11547-009-0449-8. [DOI] [PubMed] [Google Scholar]
- 93.Rosano GM, Aversa A, Vitale C, Fabbri A, Fini M, Spera G. Chronic treatment with tadalafil improves endothelial function in men with increased cardiovascular risk. Eur Urol. 2005;47:214–220. doi: 10.1016/j.eururo.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 94.Sahinkanat T, Efe E, Ekerbicer HC, Kucukdurmaz F. Effects of a single dose 20 mg tadalafil on resistive index value of prostate zones. Eur Res J. 2018;4:320–325. [Google Scholar]
- 95.Morelli G, Pagni R, Mariani C, et al. Results of vardenafil mediated power Doppler ultrasound, contrast enhanced ultrasound and systemaic random biopsies to detect prostate cancer. J Urol. 2011;185:2126–2131. doi: 10.1016/j.juro.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 96.Vela-Navarrete R, Alcatraz A, Rodriguez-Antolin A, et al. Efficacy and safety of a hexanic extract of Serenoa repens (Permixon) for the treatment of lower urinary tract symptoms associated with benign prostatic hyperplasia (LUTS/BPH): systematic review and meta-analysis of randomised controlled trials and observational studies. BJU Int. 2018;122:1049–1065. doi: 10.1111/bju.14362. [DOI] [PubMed] [Google Scholar]
- 97.Majima T, Matsukawa Y, Funahashi Y, Kato M, Yamamoto T, Gotoh M. The effect of mirabegron on bladder blood flow in a rat model of bladder outlet obstruction. World J Urol. 2019;38:2021–2027. doi: 10.1007/s00345-019-02939-9. [DOI] [PubMed] [Google Scholar]
- 98.Roerhborn CG, Perez IO, Roos EPM, et al. Efficacy and safety of a fixed-dose combination of dutasteride and tamsulosin treatment (Duodart) compared with watchful waiting with initiation of tamsulosin therapy if symptoms do not improve, both provided with lifestyle advice, in the management of treatment-naive men with moderately symptomatic benign prostatic hyperplasia: 2-year CONDUCT study results. BJU Int. 2015;116:450–459. doi: 10.1111/bju.13033. [DOI] [PubMed] [Google Scholar]
- 99.Griwan S, Karthikeyan YR, Kumar M, Singh BJ, Singh SK. Comparative evaluation of naftopidil and tamsulosin in the treatment of patients with lower urinary tract symptoms with bening prostatic hyperplasia. Urol Ann. 2014;5:181–186. doi: 10.4103/0974-7796.134254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Persu C, Braschi E, Lavelle J. A review of prospective Clinical Trials for neurogenic bladder: Pharmaceuticals. Cent European J Urol. 2014;67:264–269. doi: 10.5173/ceju.2014.03.art11. [DOI] [PMC free article] [PubMed] [Google Scholar]