Abstract
Introduction
Infectious complications are among the most frequent and significant complications in retrograde intrarenal lithotripsy. To date, review articles have covered complications after a ureteroscopy, but not after retrograde intrarenal surgery (RIRS), specifically. Because the complications and risk factors are different for a ureteroscopy and RIRS, we aimed to identify variables related to the occurrence of infectious complications post-RIRS.
Material and methods
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement. We included original studies that described 100 or more procedures published in 2014–2021. We extracted data and performed a narrative synthesis to explore and interpret differences between the studies.
Results
We selected 17 studies for analysis, including 10 from 2019–2021. Infectious complications after RIRS were observed in 2.8–7.5% of patients (mean 7.1%). We found seven independent risk factors associated with infectious complications after RIRS: long operative time, recent history of positive urine culture or urinary tract infection or antibiotic use, pyuria/nitrites, small caliber of ureteral access sheath, struvite stone, high irrigation rate, and comorbidities.
Conclusions
If an increased rate of infectious complications is found at a RIRS center, countermeasures should include restrictions on operative time and irrigation rate, and consideration of larger access sheaths, especially for patients with abnormal urine results or with struvite stones or with a history of urinary tract infection or co-morbidities.
Keywords: flexible ureteroscopy, nephrolithiasis, fever, infection, sepsis, systemic inflammatory response syndrome
INTRODUCTION
With the development of laser and endoscopic technology, retrograde intrarenal surgery (RIRS) is gaining new indications, and the number of procedures is constantly growing [1]. However, RIRS is associated with complications, and infectious complications are among the most common. Infectious complications result from the combination of increased pressure in the collecting system and the presence of bacteria in the urinary tract. The identification of independent predictors should reduce the risk of infectious complications. To date, most studies on the risks associated with a ureteroscopy (URS) have analyzed complications after ureteral stone surgeries or combined ureteral and intrarenal lithotripsies [2, 3]. However, intraureteral and intrarenal lithotripsies have different characteristics. Thus, this study aimed to summarize the available data from multivariate analyses on risk factors for infectious complications after RIRS. We divided the factors identified into (a) well-established, independent predictors of infection, (b) potential independent predictors that require complementary studies, and (c) factors that, based on the vast majority of evidence, are irrelevant or dependent on other, truly independent factors.
MATERIAL AND METHODS
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [4]. We created a Population, Intervention, Comparison and Outcomes review protocol, which was registered in the PROSPERO registry (ID: CRD42021249976). The literature search was restricted to 2014–2021. We did not restrict the language. We searched Medline, Scopus, and the Cochrane Library with the following search strategy: (septic OR infection OR sepsis OR infectious OR SIRS OR systemic inflammatory OR urosepsis) AND (RIRS OR retrograde OR intrarenal OR ‘flexible ureteroscopy’). We searched Google Scholar with the following string: (‘flexible ureteroscopy’ OR ‘retrograde intrarenal’) (fever OR infection OR inflammatory) (factors OR predictors). We also searched relevant individual urologic journals. We scrutinized references and citations of each relevant study to extend the search range. The search was completed on April 21st, 2021. We included all original studies with data on preoperative risk factors for infectious complications of retrograde intrarenal stone lithotripsy performed with flexible ureteroscopes. Inclusion criteria were: retrospective, prospective, observation, and controlled trial studies that included more than 100 procedures. We excluded studies that lacked a multivariate analysis of risk factors for infectious complications; evaluated only one risk factor; studied a pediatric population; or included ureteroscopies for non-stone diseases.
Two reviewers extracted all data and discussed any differences. Patient demographics, the surgery technique, stone characteristics, the type and incidence of infectious complications, and the results of univariate and multivariate analyses of risk factors were recorded with Microsoft Excel 2019. Each relevant study was assessed for quality by carefully evaluating the published results, the compliance of results with our objectives, and the study methodology. We evaluated non-randomized studies with the methodological index for non-randomized studies (MINORS) questionnaire [5]. Due to the heterogeneous nature of the data available in the source publications, the various measures of variation, and the variable ranges of the parameters studied, we decided to perform a narrative synthesis, instead of a meta-analysis [6].
RESULTS
Characteristics of the included studies
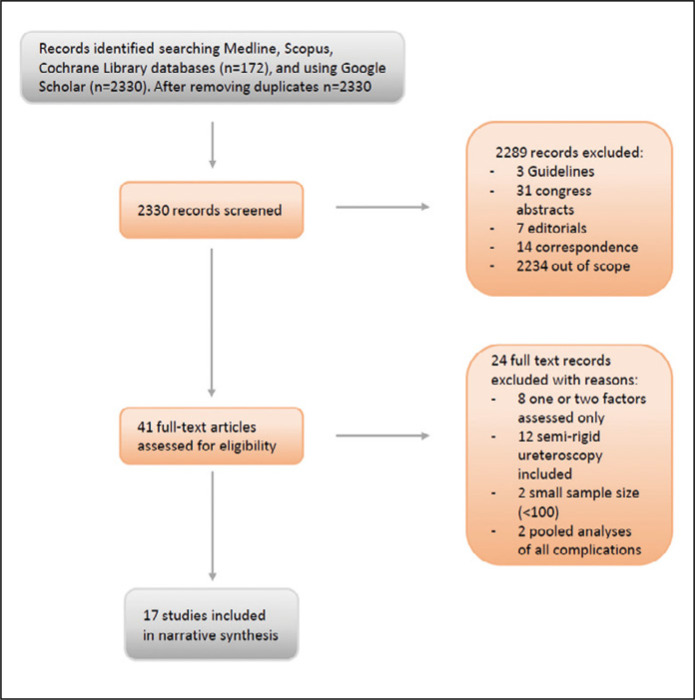
We initially identified 2330 articles and processed them according to the PRISMA guidelines (Figure 1). Seventeen full-text English language articles were included in this systematic review (Table 1) [7–23]. The studies were published in 2015–2021, most (10/17) in the last 30 months (January 2019 to May 2021). Two studies [9, 16] were conducted prospectively. All but one [9] study described a single-center experience. We included a total of 7662 RIRS procedures.
Figure 1.
PRISMA flowchart of the included studies.
Table 1.
Details of the studies included in the systematic review. All risk factors of post-RIRS infectious complications identified in univariate analyses are listed. Factors found significant in multivariate analyses are bolded
| Study | Sample size | Technique variations | Mean operative time (min.) | Type/grade of infectious complications and morbidity | Risk factors |
|---|---|---|---|---|---|
| Zhong et al. [7], 2015 | 260 | srURS | 45 | SIRS – 8.1% | Stone size Irrigation rate Stone composition UAS caliber Gender Irrigation volume |
| Fan et al. [8], 2015 | 227 | Prestenting | 75 | SIRS/Fever – 8.4% | Operative time Pyuria Infection stones Stone size |
| Berardinelli et al. [9], 2016 | 403 | Gravity irrigation (40–60 cm H2O) Multicenter study |
62 | SIRS/Fever – 7.7% | Coronary heart disease Hyperlipidemia Anticoagulation Residual stones |
| Alezra et al. [10], 2016 | 325 | No details | 75 | Fever – 7.4% | Antibiotics within a week prior to surgery Stone >14 mm Operative time >70 min |
| Xu et al. [11], 2018 | 322 | srURS Irrigation with pressure- -controlling pump |
36 | Fever – 13.4% | Operative time Irrigation rate (+) urine culture Stone size |
| Li et al. [12], 2018 | 337 | Prestenting | 57 | Fever – 17.5% SIRS – 6.5% |
Operative time Diabetes mellitus Preoperative elevated CRP Stone size (+) stone culture Pelvic urine culture |
| Senocak et al. [13], 2018 | 492 | Gravity + manual irrigation | 57 | Fever/SIRS – 8.5% | (+) urine culture Operative time Diabetes mellitus Stone size |
| Demir et al. [14], 2019 | 189 | Prestenting in 30% Operative time <120 min. |
58 | Fever/SIRS – 10.5% | Operative time ASA score 3 Stone size |
| Baseskioglu [15], 2019 | 111 | Gravity irrigation (40 cmH2O) | 49 | Fever – 12.6% | Preoperative UTI Comorbidities |
| Ozgor et al. [16], 2019 | 494 | srURS Gravity irrigation (<100 cm H2O) No manual pump |
49 | Fever/SIRS – 6.3% | Operative time >60 min. Age ≤40 Renal abnormality |
| Mi et al. [17], 2020 | 216 | Prestenting srURS |
57 | SIRS – 9.7% | Operative time (+) urine culture Stone size UAS size |
| Baboudjian et al. [18], 2020 | 604 | Irrigation with pressure- -controlling pump (60–70 cmH2O) |
61 | Fever – 6.7% | Operative time UTI history (+) urine culture >1 species Gender (female) Stone size History of antibiotics |
| Zhang et al. [19], 2020 | 602 | srURS Irrigation unknown |
62 | Fever – 10.6% SIRS – 7.1% |
Operative time Stone >20 mm (+) urine culture |
| Peng et al. [20], 2020 | 1493 | Syringe manual irrigation | 30 | Fever – 4.9% Sepsis – 0.5% Septic shock – 0.3% |
Gender Urine test (+) Infection stone Postoperative neutrophil ratio >75% |
| Jian et al. [21], 2020 | 1095 | Prestenting “Control of time and irrigation pressure” |
– | Fever – 2.8% | Operative time >30 min. (+) nitrites (+) urine culture Albumin/Globulin ratio |
| Kazan et al. [22], 2020 | 289 | srURS Gravity irrigation (<100 cmH2O) |
60 | UTI – 6.9% | Operative time (+) nitrites UTI history |
| Kim et al. [23], 2021 | 150 | Gravity irrigation | 74 | Fever – 11.3% | Preoperative pyuria |
RIRS – retrograde intrarenal surgery; srURS – ureter inspection with a semi-rigid ureteroscope prior to RIRS; SIRS – systemic inflammatory response syndrome; UAS – ureteral access sheath; UTI – urinary tract infection
Study quality was assessed with the MINORS questionnaire, which included eight items, with a maximum score of 16 (Table 2) [5]. All studies clearly stated the aims and adequately defined the endpoints. No study mentioned using an intention-to-treat analysis, but we did not deduct points for this item, due to the retrospective nature of most studies. Although no studies described blinded evaluations of the study endpoints, we assumed that all endpoints were based on objective measurements performed by a staff nurse that was not involved in the research; consequently, all studies received 2 points for this item. Two studies received 2 points each for their prospective study design. One point was deducted for lack of exclusion criteria in four studies. Two points were given when the specified follow-up exceeded the hospitalization time, and only one point was given when the follow-up was restricted to the hospital stay. In three studies, one point was deducted for overly high exclusion rates or overly restrictive exclusion criteria. No study had estimated the statistical power of the study or the appropriate sample size. However, one point was given to studies that included more than 400 patients, and a second point was given to studies that provided the odds ratios and 95% confidence intervals for independent prognostic factors. Four publications scored 14 or 15 points [9, 13, 16, 18] and four studies scored 13 points [10, 11, 14, 20]. However, articles that scored high were not distinctively different from those that scored low in terms of factors analyzed and results.
Table 2.
Individual MINORS score for studies included in the systematic review
| Clearly stated aim | Consecutive patients | Prospective data collection | Endpoints appropriate to study aim | Unbiased assessment of endpoint | Follow-up appropriate to study aim | Lost to follow-up <5% | Study size prospective calculation | TOTAL | |
|---|---|---|---|---|---|---|---|---|---|
| Zhong et al. [7] | 2 | 1 | 0 | 2 | 2 | 1 | 2 | 1 | 11 |
| Fan et al. [8] | 2 | 1 | 0 | 2 | 2 | 1 | 2 | 2 | 12 |
| Berardinelli et al. [9] | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 14 |
| Alezra et al. [10] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 1 | 13 |
| Xu et al. [11] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 1 | 13 |
| Li et at. [12] | 2 | 2 | 0 | 2 | 2 | 1 | 2 | 1 | 12 |
| Senocak et al. [13] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 14 |
| Demir et al. [14] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 1 | 13 |
| Baseskioglu [15] | 2 | 2 | 0 | 2 | 2 | 2 | 1 | 1 | 12 |
| Ozgor et al. [16] | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 15 |
| Mi et al. [17] | 2 | 1 | 0 | 2 | 2 | 1 | 1 | 1 | 10 |
| Baboudjian et al. [18] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 14 |
| Zhang et al. [19] | 2 | 2 | 0 | 2 | 2 | 1 | 2 | 1 | 12 |
| Peng et al. [20] | 2 | 2 | 0 | 2 | 2 | 1 | 2 | 2 | 13 |
| Jian et al. [21] | 2 | 2 | 0 | 2 | 2 | 2 | 2 | 2 | 12 |
| Kazan et al. [22] | 2 | 2 | 0 | 2 | 2 | 1 | 1 | 2 | 12 |
| Kim et al. [23] | 2 | 2 | 0 | 2 | 2 | 1 | 1 | 1 | 11 |
MINORS – methodological index for non-randomized studies
RIRS technique
All studies that described their technique used perioperative antibiotic prophylaxis, ureteral access sheaths (UAS), postoperative JJ stenting, and holmium laser for intrarenal lithotripsy (the power and other settings were typically not provided). Variations in the RIRS technique (Table 1) included:
prestenting (routinely used in four centers);
initial semi-rigid ureteroscopy (srURS); six centers routinely used srURS; one center routinely used both prestenting and srURS; technical details were given in 9 of 14 studies, and ureteral dilatation was always obtained with either prestenting or srURS;
the UAS diameter (not specified by most authors);
the ureteroscope type and diameter;
the irrigation type and pressure (not specified in eight studies, gravity used in six studies, manually applied with a syringe in one study, and pressure-controlling pump in two studies); the effect of the irrigation type was not verified.
Endpoints and risk factors
The studies used one of two primary endpoints: systemic inflammatory response syndrome (SIRS, in three studies [7, 17, 19]) and fever (in 14 studies). Despite differences in endpoint definitions, the incidence rates were similar: fever was observed in 2.8–7.5% of patients (mean 7.1%), and the mean incidence of SIRS was 7.9%. No study described the effects of surgeons’ experience on the incidence of infectious complications: the rates of postoperative infections were similar between large cohorts (>400 patients) and small cohorts (≤400; p = 0.12, U-Mann-Whitney test). No study found a correlation between the mean duration of the RIRS procedure and the rate of infectious complications (correlation coefficient rxy = 0.02).
All investigators performed logistic regression analyses to identify preoperative parameters associated with infectious complications. The choice of examined factors varied (Table 3), but some parameters were examined in all or most studies, including: sex, age, body mass index, diabetes, presence of hydronephrosis, stone location, preoperative bacteriuria, stone size, and operative time. Four to 10 centers examined the following: prestenting, anatomical anomaly of the kidney, a history of urinary tract infection, stone composition, UAS caliber, a history of stone surgery, and a preoperative urinalysis. One to three studies examined comorbidity scores, preoperative C-reactive protein, a stone or collecting system urine culture, the irrigation rate, and antibiotic therapy.
Table 3.
Risk factors for infectious complications after retrograde intrarenal surgery and their significance in univariate and multivariate analysis
| Zhong et al. [7] | Fan et al. [8] | Berardinelli et al. [9] | Alezra et al. [10] | Xu et al. [11] | Li et al. [12] | Demir et al. [13] | Senocak et al. [14] | Baseskioglu et al. [15] | Mi et al. [16] | Baboudjian et al.17 | Ozgor et al. [18] | Zhang et al. [19] | Peng et al. [20] | Jian et al. [21] | Kazan et al. [22] | Kim et al. [23] | MV+ | UV+ | No. of studies | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Strong evidence | ||||||||||||||||||||
| Operative time | – | ! | – | + | ! | ! | ! | + | – | ! | ! | ! | ! | + | ! | ! | – | 10 | 13 | 17 |
| Urine culture (+) | – | + | ! | – | ! | – | ! | ! | + | + | ! | – | – | 5 | 8 | 13 | ||||
| Stone size | ! | + | – | + | + | ! | + | + | – | ! | + | – | ! | + | + | – | – | 4 | 11 | 17 |
| Sex | + | – | – | – | – | – | – | – | – | – | ! | – | – | ! | – | – | – | 2 | 3 | 17 |
| Age | – | – | – | – | – | – | – | – | – | – | – | ! | – | + | – | – | – | 1 | 2 | 17 |
| Diabetes | – | – | ! | – | + | – | – | – | – | – | – | 1 | 2 | 11 | ||||||
| Body mass | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 0 | 0 | 15 | |||
| Hydronephrosis | – | – | – | – | – | – | – | – | – | – | – | 0 | 0 | 11 | ||||||
| Lower pole stone | – | – | – | – | – | – | – | – | – | – | – | 0 | 0 | 11 | ||||||
| Moderate evidence | ||||||||||||||||||||
| Urinalysis (+) | + | – | – | ! | ! | ! | ! | 4 | 5 | 7 | ||||||||||
| UTI history | – | + | – | – | ! | ! | ! | 3 | 4 | 7 | ||||||||||
| UAS caliber | ! | – | – | ! | + | – | 2 | 3 | 6 | |||||||||||
| Stone composition | ! | – | – | + | – | ! | 2 | 3 | 6 | |||||||||||
| History of stone surgery | – | – | – | – | – | – | – | – | – | – | 0 | 0 | 10 | |||||||
| Solitary kidney | – | – | – | – | – | – | – | – | 0 | 0 | 8 | |||||||||
| Preoperative JJ | – | – | – | – | – | – | 0 | 0 | 6 | |||||||||||
| Limited evidence | ||||||||||||||||||||
| Irrigation rate | ! | ! | 2 | 2 | 2 | |||||||||||||||
| Antibiotics | ! | + | 1 | 2 | 2 | |||||||||||||||
| Comorbidity score | ! | + | – | 1 | 2 | 3 | ||||||||||||||
| Pelvic urine culture | + | 0 | 1 | 1 | ||||||||||||||||
| Preoperative CRP | + | – | 0 | 1 | 2 | |||||||||||||||
| Stone culture | – | 0 | 0 | 1 | ||||||||||||||||
– insignificant; + – significant in univariate analysis; ! – significant in multivariate analysis; MV+ – number of studies showing significance in multivariate analysis; UV+ – number of studies showing significance in univariate analysis
Only four studies found no relationship between the operative time and infectious complications. Of those, three did not find any well-known predictors, [9, 15, 23] and one reported on rarely investigated factors, such as the irrigation rate, stone composition, and UAS caliber [7]. The other 13 studies found that the operative time was related to postoperative infections. Most studies analyzed time as a continuous variable, but four used thresholds. Among these, significant relationships were found at thresholds of 70 min [10], 60 min [16], and 30 min [21]; but the relationship was insignificant with a threshold of 120 min [7]. Most studies found that stone size and preoperative urine culture were significantly related to postoperative infections, although most performed univariate, not multivariate analyses. Some studies found that postoperative infections were significantly related the stone composition (infected stone), abnormal urinalysis, or recent antibiotic use abnormalities, but not the urine culture. Only three studies [9, 14, 16] showed no correlation between a fever/SIRS and an abnormal urine culture, urinalysis, the presence of infection or struvite stone, or antibiotic use. In two studies [14, 16], highly restrictive criteria were used to exclude patients with an infection or bacteriuria, and none of the above parameters were included in the analysis. However, the results of those studies may be important for patients without bacteriuria and prolonged surgery times. Three studies highlighted the importance of comorbidities, although they reported postoperative infection rates as low as <8%. Jian et al. and Peng et al. [20, 21] showed even better statistics, but it was difficult to find a single common element between these studies, apart from the country of origin and the large numbers of patients included. They were both recent studies that showed that the preoperative urinalysis was more important than a urine culture as an independent predictor of complications. The UAS caliber, stone composition (one author proposed an intraoperative analysis of stone composition), irrigation rate, and comorbidities are among potentially influencing factors that have been insufficiently studied in the context of multivariate analyses. Most authors included age, gender, diabetes mellitus, body mass, hydronephrosis, and stone location in their analyses, but the vast majority of studies found insignificant relationships to infectious complications of RIRS.
The results of two studies were considered debatable. In the only multicenter study [9], no prognostic factor was significantly associated with infectious complications in the multivariate analysis, but the univariate analysis showed an association with comorbidities. In the study by Mi et al. some differences seem unlikely to be significant; e.g., the surgery times differed by one minute and the stone sizes differed by a few millimeters between groups. Nevertheless, we did not exclude these works from the analysis. Our choice of a narrative synthesis and our reliance on the results shown in Table 3 ensured that the results of these works did not affect the conclusions of the entire analysis.
DISCUSSION
This systematic review was the first to specifically focus on infectious complications after a RIRS. However, a number of review articles have described infectious complications after an unspecified ureteroscopy. Moreover, in the present study, most original reports were published in the last 2.5 years, and thus, they were not included in previous systematic reviews. Unlike reviews on the semi-rigid ureteroscopy, we did not perform a meta-analysis, because this method would significantly limit the scope of the evidence, and it would have excluded recently emerging factors that are supported by little evidence. Indeed, the latest research has revealed several new factors that were not studied previously.
We estimated that the average incidence of fever after RIRS was about 7%, which was higher than that found in studies on semi-rigid ureteroscope procedures (2–3.8%) [3, 24]. This finding confirmed the notion that patients undergoing RIRS might be at higher risk of infectious complications than patients undergoing semi-rigid ureteroscopy. Thus, these two procedures should be analyzed separately. This study could not confirm the value of many factors previously recognized as important in ureteroscopy; e.g., age, sex, body mass, diabetes, or hydronephrosis. Additionally, some variables, such as the JJ stent dwell time or lower urinary tract neurogenic dysfunction, were not analyzed in any of the studies we examined.
We found that, operative time was a risk factor for post-RIRS infections. It was included in all 17 articles, and it was considered significant by the vast majority of authors. Based on studies by Alezra et al., Ozgor et al., and other specific analyses, an operating time over 60 min is currently considered to increase the risk of infectious complications. Although stone size seems to affect the operating time in fact it was not found an independent predictior by majority of researchers. It is because the time is rather determined by the surgeon's decision to halt RIRS, the individual skills of the operator, and the composition of the stone. Most of the analyzed studies (13 of 17) revealed that, apart from the operating time, various factors related to presurgical urinary tract infections were independent predictors. These factors included a positive urine culture, stone composition, and the use of antibiotics before surgery. In the most recent studies, an abnormal presurgical urinalysis was also identified as an independent predictor of infectious complications [20, 23].
The intrarenal pressure applied during surgery can allow bacteria to penetrate the collecting ducts and migrate deeper into the kidney parenchyma. When irrigation is performed with a pressure pump set to 80 cmH2O or a gravity-driven irrigation bag, the pressure in the system is constantly increased to 60 cmH2O [25]. Each additional manual maneuver can cause a short-term, but strong pressure rise that boosts the intrarenal reflux. Because pelvic pressure is difficult to measure directly, multivariate analyses typically include factors that influence the pressure, like the type and rate of irrigation (inflow) or the UAS caliber (outflow). However, the latter has not been sufficiently tested in multivariate analyses for its effect on RIRS. Additionally, preparing the ureter with prestenting or dilatation with a srURS (outflow) might prove to be beneficial. All 17 included studies used the same type of irrigation between study groups; therefore, we could not assess the effects of different types. Only two studies assessed the effect of the irrigation rate on infectious complications, and both confirmed its independent predictive value [7, 11]. It is well known that irrigation is an important parameter; the trade-off between visibility and low irrigation pressure remains an unresolved dilemma for endourologists.
If we could have eliminated all contaminated cases, we might have found that comorbidities were important factors for predicting infectious complications. Indeed, comorbidities were previously associated with infectious complications related to percutaneous nephrolithotomies [26] and semi-rigid ureteroscopic lithotripsies [27].
Previous studies have shown that the ureteral stent dwell time prior to ureteroscopic surgery contributed to bacteriuria [28]. This association was not analyzed in the studies included in this review. However, we found that short-term prestenting was irrelevant to the risk of complications. Additionally, a history of urolithiasis treatment was irrelevant, even in univariate analyses; thus, it was not investigated further.
We might expect the incidence of fever after RIRS to decrease with the surgeon’s experience. A previous propensity score analysis confirmed that surgeon experience was a significant independent factor for the risk of complications after a ureteroscopy [29]. However, none of the studies we included analyzed surgeon experience. Therefore, we could not address this factor. Consequently, the effect of surgeon experience on infectious complications after RIRS remains to be determined.
The distribution of parameters studied here clearly indicated a group of factors that were significantly associated with the occurrence of infectious complications after RIRS, although not all were well studied (Table 4). The remaining parameters listed in Table 3 were mostly considered insignificant. We could not investigate why they were considered significant in individual studies, because we lacked access to source data.
Table 4.
Strength of evidence for independent predictors of infectious complications after retrograde intrarenal lithotripsy
| Strong evidence |
|---|
| Operative time |
| Parameters related to urinary tract infection (positive urine culture/history of infection or antibiotics before surgery) |
| Moderate evidence |
| Comorbidity indices or selected comorbidities |
| Irrigation – type and rate |
| Ureteral access sheath – presence and size |
| Emerging evidence |
| Urinalysis: pyuria/nitrites |
| Stone composition: struvite/infection stone |
| Not investigated in multivariate analyses |
| Surgical experience |
| Long ureteral stent dwell time |
CONCLUSIONS
This systematic review confirmed that the operative time and a history of bacteriuria/infection were best studied independent risk factors in the incidence of infectious complications after RIRS. The most recent studies suggest that preoperative urinalysis may have higher predictive power than urine culture. Future multivariate analyses should focus on irrigation parameters, surgeon experience, comorbidities, long-term ureter stenting, and the interdependence of these variables.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Karagöz MA, Erihan IB, Doluoğlu ÖG, et al. Efficacy and safety of fURS in stones larger than 20 mm: is it still the threshold? Cent European J Urol. 2020;73:49–54. doi: 10.5173/ceju.2020.0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chugh S, Pietropaolo A, Montanari E, Sarica K, Somani BK. Predictors of Urinary Infections and Urosepsis After Ureteroscopy for Stone Disease: a Systematic Review from EAU Section of Urolithiasis (EULIS) Curr Urol Rep. 2020;21:16. doi: 10.1007/s11934-020-0969-2. [DOI] [PubMed] [Google Scholar]
- 3.Sun J, Xu J, OuYang J. Risk Factors of Infectious Complications following Ureteroscopy: A Systematic Review and Meta-Analysis. Urol Int. 2020;104:113–124. doi: 10.1159/000504326. [DOI] [PubMed] [Google Scholar]
- 4.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73:712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 6.Popay J, Roberts HM, Sowden A, et al. Guidance on the conduct of narrative synthesis in systematic reviews. London: Institute for Health Research; 2006. p. 92. https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf. [Google Scholar]
- 7.Zhong W, Leto G, Wang L, Zeng G. Systemic inflammatory response syndrome after flexible ureteroscopic lithotripsy: a study of risk factors. J Endourol. 2015;29:25–28. doi: 10.1089/end.2014.0409. [DOI] [PubMed] [Google Scholar]
- 8.Fan S, Gong B, Hao Z, et al. Risk factors of infectious complications following flexible ureteroscope with a holmium laser: a retrospective study. Int J Clin Exp Med. 2015;8:11252–11259. [PMC free article] [PubMed] [Google Scholar]
- 9.Berardinelli F, De Francesco P, Marchioni M, et al. Infective complications after retrograde intrarenal surgery: a new standardized classification system. Int Urol Nephrol. 2016;48:1757–1762. doi: 10.1007/s11255-016-1373-1. [DOI] [PubMed] [Google Scholar]
- 10.Alezra E, Lasselin J, Forzini T, François T, Viart L, Saint F. Facteurs favorisants les infections sévères après urétéroscopie souple: intérêt de l'ECBU systématique la veille de l'intervention. Prog Urol. 2016;26:65–71. doi: 10.1016/j.purol.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Xu Y, Min Z, Wan SP, Nie H, Duan G. Complications of retrograde intrarenal surgery classified by the modified Clavien grading system. Urolithiasis. 2018;46:197–202. doi: 10.1007/s00240-017-0961-6. [DOI] [PubMed] [Google Scholar]
- 12.Li T, Sun XZ, Lai DH, Li X, He YZ. Fever and systemic inflammatory response syndrome after retrograde intrarenal surgery: Risk factors and predictive model. Kaohsiung J Med Sci. 2018;34:400–408. doi: 10.1016/j.kjms.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senocak C, Ozcan C, Sahin T, et al. Risk factors of infectious complications after flexible uretero-renoscopy with laser lithotripsy. Urol J. 2018;15:158–163. doi: 10.22037/uj.v0i0.3967. [DOI] [PubMed] [Google Scholar]
- 14.Demir DO, Doluoglu OG, Yildiz Y, Bozkurt S, Ayyildiz A, Demirbas A. Risk factors for infectious complications in patients undergoing retrograde intrarenal surgery. J Coll Physicians Surg Pak. 2019;29:558–562. doi: 10.29271/jcpsp.2019.06.558. [DOI] [PubMed] [Google Scholar]
- 15.Baseskioglu B. The prevalence of urinary tract infection following flexible ureterenoscopy and the associated risk factors. Urol J. 2019;16:439–442. doi: 10.22037/uj.v0i0.4340. [DOI] [PubMed] [Google Scholar]
- 16.Ozgor F, Sahan M, Cubuk A, Ortac M, Ayranci A, Sarilar O. Factors affecting infectious complications following flexible ureterorenoscopy. Urolithiasis. 2019;47:481–486. doi: 10.1007/s00240-018-1098-y. [DOI] [PubMed] [Google Scholar]
- 17.Mi Q, Meng X, Meng L, Chen D, Fang S. Risk factors for systemic inflammatory response syndrome induced by flexible ureteroscope combined with holmium laser lithotripsy. Biomed Res Int. 2020;2020:6842479. doi: 10.1155/2020/6842479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baboudjian M, Gondran-Tellier B, Abdallah R, et al. Predictive risk factors of urinary tract infection following flexible ureteroscopy despite preoperative precautions to avoid infectious complications. World J Urol. 2020;38:1253–1259. doi: 10.1007/s00345-019-02891-8. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H, Jiang T, Gao R, et al. Risk factors of infectious complications after retrograde intrarenal surgery: a retrospective clinical analysis. J Int Med Res. 2020;4 8:300060520956833. doi: 10.1177/0300060520956833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng L, Xu Z, Wen J, Zhong W, Zeng G. A quick stone component analysis matters in postoperative fever: a propensity score matching study of 1493 retrograde intrarenal surgery. World J Urol. 2021;39:1277–1285. doi: 10.1007/s00345-020-03268-y. [DOI] [PubMed] [Google Scholar]
- 21.Jian ZY, Ma YC, Liu R, Li H, Wang K. Preoperative positive urine nitrite and albumin-globulin ratio are independent risk factors for predicting postoperative fever after retrograde Intrarenal surgery based on a retrospective cohort. BMC Urol. 2020;20:50. doi: 10.1186/s12894-020-00620-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kazan HO, Cakici CM, Ferhat K, Efiloglu O, Yildirim A, Atis G. Factors for predicting early infection after retrograde intrarenal surgery (RIRS) in 1-2 cm renal stones. J Urol. 2020;203:e1048–e1049. [Google Scholar]
- 23.Kim DS, Yoo KH, Jeon SH, Lee SH. Risk factors of febrile urinary tract infections following retrograde intrarenal surgery for renal stones. Medicine (Baltimore) 2021;100:e25182. doi: 10.1097/MD.0000000000025182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagenius M, Rydberg M, Popiolek M, Forsvall A, Stranne J, Linder A. Ureteroscopy: a population based study of clinical complications and possible risk factors for stone surgery. Cent European J Urol. 2019;72:285–295. doi: 10.5173/ceju.2019.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doizi S, Letendre J, Cloutier J, Ploumidis A, Traxer O. Continuous monitoring of intrapelvic pressure during flexible ureteroscopy using a sensor wire: a pilot study. World J Urol. 2021;39:555–561. doi: 10.1007/s00345-020-03216-w. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia VP, Aro T, Smith SM, et al. Frailty as predictor of complications in patients undergoing percutaneous nephrolithotomy (PCNL) World J Urol. 2021 Apr;:2. doi: 10.1007/s00345-021-03681. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Sugihara T, Yasunaga H, Horiguchi H, et al. A nomogram predicting severe adverse events after ureteroscopic lithotripsy: 12 372 patients in a Japanese national series. BJU Int. 2013;111:459–466. doi: 10.1111/j.1464-410X.2012.11594.x. [DOI] [PubMed] [Google Scholar]
- 28.Nevo A, Mano R, Baniel J, Lifshitz DA. Ureteric stent dwelling time: a risk factor for post-ureteroscopy sepsis. BJU Int. 2017;120:117–122. doi: 10.1111/bju.13796. [DOI] [PubMed] [Google Scholar]
- 29.Berardinelli F, Cindolo L, De Francesco P, et al. The surgical experience influences the safety of retrograde intrarenal surgery for kidney stones: a propensity score analysis. Urolithiasis. 2017;45:387–392. doi: 10.1007/s00240-016-0919-0. [DOI] [PubMed] [Google Scholar]