Abstract
Introduction
Prostate-specific membrane antigen (PSMA) positron emission tomography/ computed tomography (PET-CT) is widely used as a staging tool for patients with prostate cancer (PCa). The objective of the study is to assess the diagnostic accuracy of 68Ga-PSMA-PET/CT for PCa, which may help us avoid unnecessary biopsies in patients with intermediate prostate-specific antigen (PSA) levels.
Material and methods
In this prospective study, 81 patients suspected of PCa, with either raised PSA between 4–20 ng/ml or abnormal digital rectal examination (DRE) findings were included. 68Ga-PSMA-PET/CT was performed for all patients followed by transrectal ultrasound (TRUS) guided prostate biopsy. SUVmax (maximum standardized uptake value) was measured and correlated with biopsy results.
Results
Out of 81 patients, 31 (38.3%) patients were found to have malignancy on biopsy. Median SUVmax of biopsy positive patients was 10.4 (IQR 6.5–16.1) and biopsy negative patients (n=50) was 3.5 (IQR 1–4.9), (p <0.001). At a cut-off of 6.15, 68GA-PSMA-PET/CT demonstrated sensitivity of 84%, specificity of 80%, positive predictive value of 72.2%, negative predictive value of 88.9% and accuracy of 81.5% with an AUC of 0.876 (95% CI: 0.799–0.953, p <0.001).
Conclusions
The 68Ga-PSMA-PET/CT helps to localize suspicious lesions and improving the detection of primary prostate cancer. Our findings indicate a significant correlation of SUVmax values with biopsy results. We were also able to determine a cut-off value of SUVmax below which prostate biopsy can be avoided in selected patients.
Keywords: biopsy, 68Ga-PSMA ligand, positron emission tomography/computed tomography, prostate cancer
INTRODUCTION
Prostate cancer (PCa) is the most common solid cancer in men and is the second most common cause of death in developed countries [1]. According to current guidelines, diagnosis of prostate cancer is most commonly made by transrectal ultrasound (TRUS) guided prostate biopsy and the detection rate is 30% [2, 3]. To improve detection of clinically significant cancer, various imaging modalities have emerged for local staging and biopsy guidance. Multiparametric magnetic resonance imaging (mpMRI) shows promising results for localizing prostate cancer and improves the accuracy of ultrasound-guided biopsy [4, 5]. Despite important advances such as standardized reporting, considerable interobserver variability remains a major drawback for MRI [6], resulting in overall heterogeneous findings for accuracy in the literature [7].
Literature demonstrated that 18F fluoroethylcholine positron emission tomography (PET) had higher accuracy for the detection of primary prostate cancer when compared to MRI, however specificity was limited due to choline uptake in benign lesions [8]. PET with ligands for the prostate-specific membrane antigen (PSMA) might overcome this limitation. Indeed, PET with Gallium-68 labelled PSMA-HBED-CC (68Ga-PSMA) demonstrated superior tumor-to-background signal intensity and substantially higher detection rates in patients with primary PCa [9, 10] and biochemical recurrence even with low total prostate-specific antigen (tPSA) levels [11, 12]. Studies showed that in spite of additional sites of uptake being detected on PSMA-PET/CT as compared to mpMRI, no additional biopsies are required [13]. Based on the present literature, the purpose of the current study was to validate the diagnostic performance of 68Ga-PSMA-PET/CT in the detection of suspected treatment-naive PCa by correlating SUVmax (maximum standardized uptake value) values obtained from 68Ga-PSMA-PET/CT with biopsy.
MATERIAL AND METHODS
Study Design
This was a cross sectional observational study conducted from 1st November 2017 to 31st May 2019 in the Department of Urology & Renal Transplant, ABVIMS & Dr. RML Hospital, New Delhi after obtaining approval from institutional ethical committee [TP (DM/M.Ch) (19/2016)/IEC/PGIMER/RMLH- 7967/16]. Inclusion criteria were as follows: (1) elevated tPSA level between 4–20 ng/ml; (2) suspicious digital rectal examination (DRE) findings; (3) treatment naive suspected PCa patients. The exclusion criteria were: (1) history of prostate biopsy within the past 6 weeks; (2) history of prostate surgery within the past 3 months; (3) painful anorectal conditions; (4) refusal to undergo PSMA-PET/CT; (5) refusal to enroll in the study.
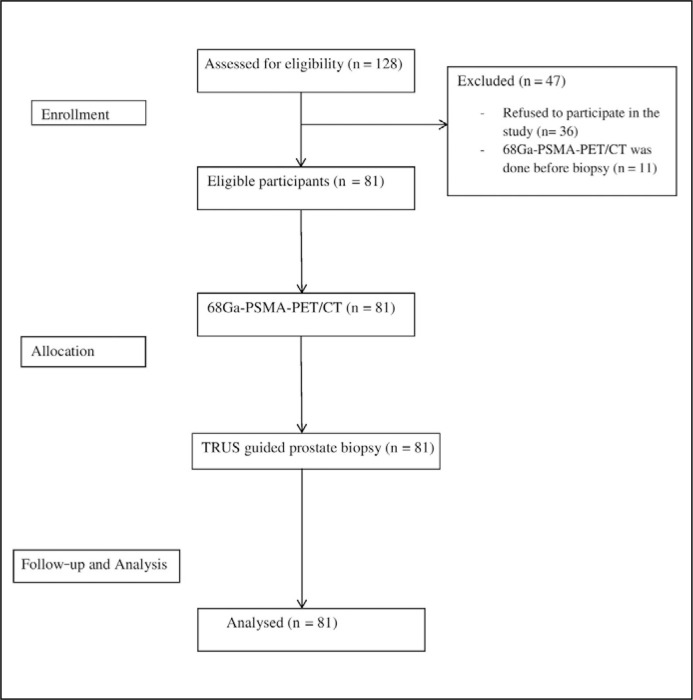
Overall, 128 patients were enrolled into the study, of which 81 patients with suspected PCa were included in the study (Figure 1). The written informed consent for PSMA-PET/CT and biopsy was obtained from all of the participants included in the study. The primary objective was to correlate the SUVmax used in 68Ga-PSMA-PET/CT with the TRUS-guided prostate biopsies. The secondary objective was to evaluate the specificity, sensitivity, accuracy and predictive value of the PSMA-PET/CT at optimal cut-off SUVmax value.
Figure 1.
Study design.
TRUS – transrectal ultrasound biopsy; n- number; 68-Ga-PSMA-PET/CT – Gallium-68 labelled prostate-specific membrane antigen positron emission tomography/computer tomography
All included patients underwent Ga68-PSMA PET/CT scan for initial diagnosis and staging. The conclusive results were pathologically confirmed by TRUS-guided prostate biopsy of all patients.
68Ga-PSMA PET/CT acquisition and image interpretation
The whole body Ga68-PSMA-PET/CT scan was performed after injection of 1.8–2.2 MBq per kg body weight of Ga68 PSMA and patients were allowed to relax for 60 minutes in a shielded room. Imaging was performed on an integrated PET/CT scanner (Siemens system, Tennessee, USA). Non-contrast CT scan was performed with following parameters: 2.5 mm axial reconstruction, tube voltage of 120 kVp and current modulation of 80–120 mAs. PET scans were acquired in three-dimensional mode with an acquisition time of 3–4 minutes per bed position, with a 780 mm field of vision (FOV) and 200 × 200 matrix. The emission data was attenuation corrected along with scattering, random and decay correction. Reconstruction was conducted from 2 iterations and 21 subsets using the ordered subset expectation maximization (OSEM) algorithm. CT images were analyzed for attenuation correction and anatomic localization followed by PET images from skull to mid-thigh.
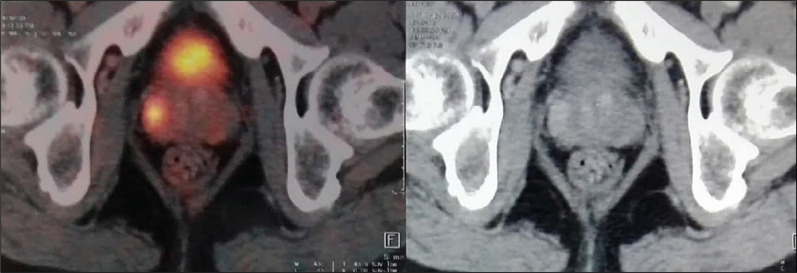
The Ga68-PSMA-PET/CT images were transferred to a multimodal workstation for data analysis. Images were reviewed independently by two experienced nuclear medicine specialists. The scan findings were interpreted as positive if focal uptake of the 68Ga-labeled PSMA ligand was greater than the background activity (surrounding prostate gland activity) (Figure 2). A semiquantitative analysis of PSMA uptake was performed by calculating SUVmax value corrected for dose administered and patients’ lean body mass. SUVmax cutoff was calculated as the value with maximal Youden index on ROC curve.
Figure 2.
Shows two PSMA avid lesion with SUVmax 15.3 in right lateral peripheral zone and other of SUV max 6.1 in left mid peripheral zone posterolaterally.
AUC – area under curve; 68-Ga-PSMA-PET/CT – Gallium-68 labelled prostate-specific membrane antigen positron emission tomography/computer tomography
Histopathological examination
All the patients underwent TRUS-guided 12-core prostate biopsy with additional cores taken from suspicious lesions identified during Ga68-PSMA-PET/CT by the cognitive fusion technique. The histopathological examination (HPE) was conducted by a senior uropathologist. Biopsy results were defined positive in case of malignancy and negative in benign conditions.
The region of interests (ROIs) identified on Ga68-PSMA-PET/CT were marked on a 24 segment sector map of the prostate. Initially, the gland was divided into lobes, right and left, and regions, base, mid, and apex. Each lobe was then sub-divided into medial and lateral parts by a sagittal plane running across the center of each lobe and into anterior and posterior parts by a coronal plane running across the center. Thus, 24 segments were formed, 12 in each lobe. During the biopsy procedure, the prostate and the ROIs PET/CT derived image contours were fused in real time with the TRUS image stack and ROIs were targeted. TRUS-guided 12-core systematic biopsy with additional targeted biopsy (2 cores) if required were obtained by cognitive fusion technique by an experienced Urologist.
Statistical analysis
The data were analysed by IBM SPSS statistics software, version 22.0 (IBM, Inc., Chicago, IL, USA).
All the variables were tested for normality using the Kolmogorov-Smirnov test. Categorical variables were summarized as frequencies (percentages), while continuous variables as means ± standard deviations or medians (IQR, interquartile range). Univariate association of variables with biopsy results and other outcome measures was assessed using the chi-square statistic or Fischer exact test for categorical variables and the Student’s t-test or Mann-Whitney U test for continuous variables. Correlation of variables was assessed using Spearman’s rank correlation. Diagnostic accuracy of the 68Ga-PSMA-PET/CT with the biopsy results was compared by calculating area under curve (AUC) using the receiver operating characteristic curve (ROC). Prediction accuracy was evaluated by the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy. The p value <0.05 was considered statistically significant.
RESULTS
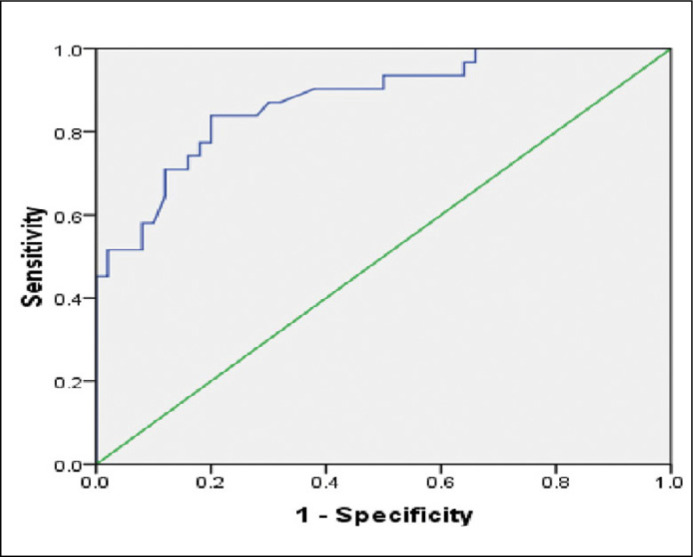
A total of 81 patients with a mean age of 68.4 ±8.1 years were included in the study. The mean maximum standard unit value (SUVmax) was 7 ± 6.5 and median was 4.3 (IQR 3.2–9.4). The mean tPSA was 10.5 ±4.6 ng/ml. In total, 31 (38.3%) of 81 patients were positive for malignancy on HPE (Table 1). The additional targeted biopsy (2 cores) was performed in 65 out of 81 patients. Out of 65 patients who underwent targeted biopsies, 27 (41.5%) were found to be positive. In comparison to histopathology, the median SUVmax on 68Ga-PSMA-PET/CT were significantly higher for PCa than for benign conditions (Table 2). Most of the patients with Gleason ≥7 had a SUVmax value >10 but the correlation was not significant (p = 0.759; Table 3). None of the patients had Gleason score 6. The diagnostic performance of 68Ga-PSMA-PET/CT was proved to be significant (p <0.001) based on sensitivity, specificity, PPV, NPV, accuracy at cut-off SUVmax value of 6.15 (Table 4) and ROC analysis (AUC- 0.876; Figure 3).
Table 1.
Summary of patient characteristics
| Parameter | Results |
|---|---|
| Median age, years (IQR) | 68 (62.3–74) |
| Median PSA, ng/ml (IQR) | 9.9 (6.9–13.1) |
| Positive TRUS prostate biopsy, n (%) | 31 (38.3) |
| Median SUVmax, (IQR) | 4.3 (3.2–9.4) |
IQR – interquartile range; PSA – prostate-specific antigen; TRUS – transrectal ultrasound, SUVmax – maximum standardized uptake value; n – number
Table 2.
SUVmax in relation with the biopsy results
| Parameter | Biopsy | Number of patients (%) | Median (IQR) | p value |
|---|---|---|---|---|
| SUVmax | Positive | 31 (38.3) | 10.4 (6.5–16.1) | <0.001 |
| Negative | 50 (61.7) | 3.5 (1–4.9) |
IQR – interquartile range, SUVmax – maximum standardized uptake value
Table 3.
Correlation of Gleason score with stratified SUVmax values
| SUVmax | Gleason score | Total | p value | ||
|---|---|---|---|---|---|
| <7 | 7 | >7 | |||
| <4 | 0 (0%) | 2 (13.3%) | 1 (6.3%) | 3 (9.7%) | 0.759 |
| 4-10 | 0 (0%) | 6 (40%) | 6 (37.5%) | 12 (38.7%) | |
| >10 | 0 (0%) | 7 (46.7%) | 9 (56.3%) | 16 (51.6%) | |
| Total | 0 (0%) | 15 (100%) | 16 (100%) | 31 (100%) | |
SUVmax – maximum standardized uptake value
Table 4.
Sensitivity, specificity, PPV, NPV, accuracy of 68Ga-PSMA-PET/CT in the detection of prostate cancer at cut off SUVmax value – 6.15
| Parameter | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) |
|---|---|---|---|---|---|
| 68Ga-PSMA-PET/CT | 84 | 80 | 72.2 | 88.9 | 81.5 |
PPV – positive predictive value; NPV – negative predictive value; 68-Ga-PSMA-PET/CT Gallium-68 labelled prostate-specific membrane antigen positron emission tomography/computed tomography; SUVmax – maximum standardized uptake value
Figure 3.
Receiver operating characteristic (ROC) curve of 68Ga-PSMA-PET/CT for detecting primary prostate cancer.
DISCUSSION
The incidence rate of prostate cancer in India is lower than the western population [14]. The diagnosis of PCa usually commences with abnormal DRE and raised PSA levels. The elevated PSA measurements are organ specific and not disease specific [14]. The various PSA parameters have been studied but none of them optimally guided in diagnosing PCa [15, 16]. A study showed that PSMA-PET/MRI had higher accuracy for detecting significant PCa (90%) with sensitivity of 96% and specificity of 81% than mpMRI [17]. Nevertheless, until now no imaging modality is considered as standard in the diagnosis of PCa in comparison with prostate biopsy [18]. The 68Ga-PSMA-PET/CT has brought drastic change in the management of recurrent PCa [11, 12] but the data evaluating 68Ga-PSMA-PET/CT for the primary diagnosis of PCa is still insufficient. The present study assessed the role of 68Ga-PSMA-PET/CT in patients suspected of prostate carcinoma and to determine a cut-off SUVmax value to predict the outcome of biopsy with high diagnostic accuracy.
The literature showed that SUVmax of primary PCa was significantly higher than normal prostate tissue, and enables the detection of PCa with high sensitivity and specificity [19, 20]. Fendler et al., evaluated twenty-one patients for the accuracy of 68Ga-PSMA- PET/CT to localize tumor in the prostate and surrounding tissue. They found a significantly higher SUVmax in histopathologically positive segments (11.8 ±7.6) compared to negative segments (4.9 ±2.9; p <0.001) [21]. Our study was also consistent with the previous studies and significantly correlated the SUVmax with the prostate biopsy reports.
Literature based on histopathology, reported cut-off SUVmax value of 6.5 for discrimination between positive and negative segments (area under the curve, AUC: 0.84; p <0.001) with sensitivity (67%), specificity (92%), PPV (97%), NPV (42%) and accuracy (72%) [21]. They concluded that 68Ga-PSMA-11 PET/CT accurately detects location and extent of primary prostate cancer and might be a promising tool for non-invasive tumor characterization and biopsy guidance. Consistent with the previous study, our results also recommended superior diagnostic performance of 68Ga-PSMA- PET/CT. However, our study showed higher sensitivity (84%) than specificity (80%) at a cut-off SUVmax of 6.15. Nevertheless, it is noteworthy that our study only included patients with tPSA (4–20 ng/ml). In this range, compared with the study of tPSA greater than 50 ng/ml, there would be lower prevalence of PCa and higher prevalence of false-positive cases [22]. Hence, this could account for relatively low specificity in the current study. Moreover, high negative predictive value (88.9%) in our study would help to avoid prostate biopsies in patients with equivocal parameters or with previous negative biopsy. A study by Kalapara et al. showed that sensitivity and NPV of 68Ga-PSMA-PET/CT can be increased by combining mpMRI especially for intermediate-risk prostate cancer [23]. This results in both early diagnosis and management.
Uprimny [24] found that the Gleason score (GS) and PSA level correlated with the intensity of tracer accumulation in the primary tumors of PCa patients on 68Ga-PSMA-PET/CT. Tumors with GS of 6, 7a (3+4) and 7b (4+3) showed significantly lower 68Ga-PSMA-11 uptake, with median SUVmax of 5.9, 8.3 and 8.2, respectively, compared to patients with GS >7 (median SUVmax: 21.2; p <0.001). PCa patients with PSA ≥10 ng/ml exhibited significantly higher uptake than those with PSA levels <10 ng/ml (median SUVmax: 17.6 versus 7.7; p <0.001). In contrast to the previously mentioned study, the correlation of SUVmax with the Gleason score was not clinically significant in our study. This may be explained by the fact that most of our patients had a GS ≥7 as opposed to western countries which have a substantial number of patients with GS <7, owing to screening protocols, genetic and socio-economic differences [25, 26].
The 68Ga-PSMA-PET/CT is a non-invasive technique with a small radiation exposure. Another major advantage is that initial staging workup is completed in the patients of organ-confined PCa in whom no surgical procedure is planned. Although the excellent diagnostic ability of 68Ga-PSMA-PET/CT has been proved, it can also be used as a PET-based TRUS-guided prostate biopsy. Our preliminary data must encourage further prospective studies to evaluate the role of PSMA-PET/CT for initial tumor staging, biopsy guidance and more importantly to help us avoid unnecessary biopsies especially when other parameters are equivocal.
Limitations
In this study, cognitive TRUS biopsy was performed which might have led to inaccurate sampling of suspicious lesion. For this, similar to MRI-fusion biopsy, suspicious lesions can be traced with PSMA PET/MRI fusion platform to avoid false-negative results or under staging of the tumor. Furthermore, the findings of the present study cannot be generalized to other populations due to absence of any patient with GS 6 prostate cancer on HPE. Moreover, the sample size was small and further multicentric studies are required with large sample size.
CONCLUSIONS
This study illustrates the role of 68Ga-PSMA-PET/CT as a predictor of outcome of biopsy. The 68Ga-PSMA-PET/CT helps to localize suspicious lesions and improving the detection of primary prostate cancer. We were also able to determine a cut-off value of SUVmax below which prostate biopsy can be avoided in selected patients.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
References
- 1.Brawley OW. Prostate cancer epidemiology in the United States. World J Urol. 2012;30:195–200. doi: 10.1007/s00345-012-0824-2. [DOI] [PubMed] [Google Scholar]
- 2.American Urological Association Clinically localized prostate cancer: AUA/ASTRO/SUO Guideline (2017) [Internet] [cited 2021 Jan 30]. Available from: https://www.auanet.org/guidelines/prostate-cancer-clinically-localized-guideline.
- 3.Mottet N, Van den Bergh RCN, Briers E, et al. European Association of Urology. EAU - ESTRO - ESUR - SIOG Guidelines on Prostate Cancer (2018) [Internet] [cited 2021 Jan 30]. Available from: https://uroweb.org/wp-content/uploads/EAU-ESUR-ESTRO-SIOG-Guidelines-on-Prostate-Cancer-large-text-V2.pdf.
- 4.Wu LM, Xu JR, Ye YQ, Lu Q, Hu JN. The clinical value of diffusion-weighted imaging in combination with T2-weighted imaging in diagnosing prostate carcinoma: a systematic review and meta-analysis. AJR Am J Roentgenol. 2012;199:103–110. doi: 10.2214/AJR.11.7634. [DOI] [PubMed] [Google Scholar]
- 5.Sonn GA, Chang E, Natarajan S, et al. Value of targeted prostate biopsy using magnetic resonance-ultrasound fusion in men with prior negative biopsy and elevated prostate-specific antigen. Eur Urol. 2014;65:809–815. doi: 10.1016/j.eururo.2013.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller BG, Shih JH, Sankineni S, et al. Prostate cancer: Interobserver Agreement and Accuracy with the Revised Prostate Imaging Reporting and Data System at Multiparametric MR Imaging. Radiology. 2015;277:741–750. doi: 10.1148/radiol.2015142818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mowatt G, Scotland G, Boachie C, et al. The diagnostic accuracy and cost-effectiveness of magnetic resonance spectroscopy and enhanced magnetic resonance imaging techniques in aiding the localisation of prostate abnormalities for biopsy: a systematic review and economic evaluation. Health Technol Assess. 2013;17:vii–xix. doi: 10.3310/hta17200. 1-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hartenbach M, Hartenbach S, Bechtloff W, et al. Combined PET/MRI improves diagnostic accuracy in patients with prostate cancer: a prospective diagnostic trial. Clin Cancer Res. 2014;20:3244–3353. doi: 10.1158/1078-0432.CCR-13-2653. [DOI] [PubMed] [Google Scholar]
- 9.Lopci E, Lughezzani G, Castello A, et al. Prospective Evaluation of 68Ga-labeled Prostate-specific Membrane Antigen Ligand Positron Emission Tomography/Computed Tomography in Primary Prostate Cancer Diagnosis. Eur Urol Focus. 2020 doi: 10.1016/j.euf.2020.03.004. S2405-4569(20)30092-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Satapathy S, Singh H, Kumar R, Mittal BR. Diagnostic Accuracy of 68Ga-PSMA PET/CT for Initial Detection in Patients With Suspected Prostate Cancer: A Systematic Review and Meta-Analysis. AJR Am J Roentgenol. 2021;216:599–607. doi: 10.2214/AJR.20.23912. [DOI] [PubMed] [Google Scholar]
- 11.Herlemann A, Wenter V, Kretschmer A, et al. 68)Ga-PSMA positron emission tomography/computed tomography provides accurate staging of lymph node regions prior to lymph node dissection in patients with prostate cancer. Eur Urol. 2016;70:553–557. doi: 10.1016/j.eururo.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 12.Virgolini I, Decristoforo C, Haug A, Fanti S, Uprimny C. Current status of theranostics in prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:471–495. doi: 10.1007/s00259-017-3882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roach PJ, Francis R, Emmett L, et al. The impact of (68)Ga-PSMA PET/CT on management intent in prostate Cancer: results of an Australian prospective multicentre study. J Nucl Med. 2018;59:82–88. doi: 10.2967/jnumed.117.197160. [DOI] [PubMed] [Google Scholar]
- 14.Hariharan K, Padmanabha V. Demography and disease characteristics of prostate cancer in India. Indian J Urol. 2016;32:103–108. doi: 10.4103/0970-1591.174774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Croswell JM, Kramer BS, Crawford ED. Screening for prostate cancer with PSA testing: current status and future directions. Oncology (Williston Park) 2011;25:452–460. [PubMed] [Google Scholar]
- 16.Louie KS, Seigneurin A, Cathcart P, Sasieni P. Do prostate cancer risk models improve the predictive accuracy of PSA screening? A meta-analysis. Ann Oncol. 2015;26:848–864. doi: 10.1093/annonc/mdu525. [DOI] [PubMed] [Google Scholar]
- 17.Ferraro DA, Becker AS, Kranzbühler B, et al. Diagnostic performance of 68Ga-PSMA-11 PET/MRI-guided biopsy in patients with suspected prostate cancer: a prospective single-center study. Eur J Nucl Med Mol Imaging. 2021;48:3315–3324. doi: 10.1007/s00259-021-05261-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blomqvist L, Carlsson S, Gjertsson P, et al. Limited evidence for the use of imaging to detect prostate cancer: a systematic review. Eur J Radiol. 2014;83:1601–1606. doi: 10.1016/j.ejrad.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 19.Woythal N, Arsenic R, Kempkensteffen C, et al. Immunohistochemical validation of PSMA-expression measured by Ga-68-PSMA PET/CT in primary prostate cancer. J Nucl Med. 2017;59:238–243. doi: 10.2967/jnumed.117.195172. [DOI] [PubMed] [Google Scholar]
- 20.Koerber SA, Utzinger MT, Kratochwil C, et al. 68Ga-PSMA11-PET/CT in Newly Diagnosed Carcinoma of the Prostate: Correlation of Intraprostatic PSMA Uptake with Several Clinical Parameters. J Nucl Med. 2017;58:1943–1948. doi: 10.2967/jnumed.117.190314. [DOI] [PubMed] [Google Scholar]
- 21.Fendler WP, Schmidt DF, Wenter V, et al. 68Ga-PSMA-HBED-CC PET/CT detects location and extent of primary prostate cancer. J Nucl Med. 2016;57:1720–1725. doi: 10.2967/jnumed.116.172627. [DOI] [PubMed] [Google Scholar]
- 22.Jang JY, Kim YS. Is prostate biopsy essential to diagnose prostate cancer in the older patient with extremely high prostate-specific antigen? Korean J Urol. 2012;53:82–86. doi: 10.4111/kju.2012.53.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kalapara AA, Ballok ZE, Ramdave S, et al. Combined Utility of 68Ga-Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography and Multiparametric Magnetic Resonance Imaging in Predicting Prostate Biopsy Pathology. Eur Urol Oncol. 2021 Mar;16 doi: 10.1016/j.euo.2021.02.006. S2588-9311(21)00040-7 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Uprimny C, Kroiss AS, Decristoforo C, et al. 68)Ga-PSMA11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging. 2017;44:941–949. doi: 10.1007/s00259-017-3631-6. [DOI] [PubMed] [Google Scholar]
- 25.Ghagane SC, Nerli RB, Hiremath MB, Wagh AT, Magdum PV. Incidence of prostate cancer at a single tertiary care center in North Karnataka. Indian J Cancer. 2016;53:429–431. doi: 10.4103/0019-509X.200671. [DOI] [PubMed] [Google Scholar]
- 26.Zeigler-Johnson CM, Rennert H, Mittal RD, et al. Evaluation of PCa characteristics in four populations worldwide. Can J Urol. 2008;15:4056–4064. [PMC free article] [PubMed] [Google Scholar]