Expert Opinion
The tumor microenvironment, which includes the vasculature, is critical to tumor progression. The vasculature architecture can also influence therapeutic response by modulating the delivery of chemotherapy. Only a few studies have looked at vascular biology in medulloblastoma tumors, although existing studies suggest that neurodevelopmental pathways and chromatin-remodelers may contribute to angiogenic growth in these tumors. Clinical studies with bevacizumab in combination with radiation or chemotherapy have not been very successful. Chromatin remodelers can be pharmacologically targeted, and should be evaluated in pre-clinical models for their anti-angiogenic activity.
Keywords: medulloblastoma, angiogenesis, vasculature, tumor-microenvironment, REST, VEGF
1. Introduction
Medulloblastoma is the most common malignant brain cancer in children, and most frequently arises in the cerebellum [1–3]. Molecular profiling has allowed their classification into four subgroups: Wingless (WNT), Sonic Hedgehog (SHH), Group 3, and Group 4 [4, 5]. Although the 5-year survival for medulloblastoma is around 72%, retrospective studies show that long-term outcomes are subgroup-specific. WNT sub-group patients have a better prognosis with a 5-year survival rate of more than 90% [6]. Group 3 and Group 4 medulloblastoma patients have a 5-year survival rate of 40–60% and 75–80%, respectively. The survival of SHH-medulloblastoma patients is defined by the p53 status of their tumors, with p53 mutations (SHH-1) driving down survival to 41%, in contrast to a 5-year survival of 81% in patients with wild type p53 (SHH-2) [6, 7]. Based on multivariate analysis, it is accepted that p53 status is the most important risk factor for children with SHH-1 medulloblastoma, with 76% of deaths associated with p53 mutations or loss of function [7]. Despite this intertumoral survival heterogeneity, current treatments for all medulloblastomas include surgical resection, craniospinal radiation therapy, and multiagent chemotherapy. Survival has certainly improved, however, recurrence, metastasis and long-term cognitive deficits due to treatment-related toxicity to the normal brain continue to be major clinical challenges [8]. Therefore, a better understanding of tumor biology will allow a more targeted treatment approach and spare the normal brain. Here, we focus our discussions on molecular differences in tumor vasculature between the medulloblastoma subgroups, and how variability in their structure and architecture may influence survival and therapeutic responses.
2. Molecular basis for medulloblastoma vascular diversity
Tumor vasculature is an important component of the tumor microenvironment and plays a key role in tumor growth and progression [9, 10]. Vascular growth in brain tumors has been shown to occur through co‐option, angiogenesis, vasculogenesis, and vascular mimicry (VM) [11, 12]. Tumor blood vessels are structurally and functionally abnormal [11, 13]. Increased endothelial cell proliferation and high angiogenic activity have been described in other brain tumors [14]. In general, tumor vessels are larger in diameter, have thicker basement membranes, and tend to be tortuous [15, 16]. They also exhibit defects in their endothelial cell wall, pericyte coverage, and basement membrane associated with abnormal cell-cell associations and a leaky vascular structure [17]. These characteristics not only uniquely alter tumor growth and progression but also impact permeability to chemotherapy and therapeutic response. Although previous studies have demonstrated that medulloblastoma tumor cells secrete various proangiogenic molecules, a methodical sub-group-specific investigation is somewhat limited [18]. As discussed later, the identification of such angiogenic drivers may allow the design of pharmacological agents to normalize tumor vasculature or even target the tumor vasculature, and although difficult, potentially in a manner that can spare the normal vasculature.
The WNT group of medulloblastomas is driven by ectopic activation of its namesake WNT developmental signaling pathway [19] (Figure 1A). Somatic mutations in the β-catenin gene (CTNNB1) occur in a subset of sporadic WNT medulloblastomas [20–22]. Morphologically, the vasculature in WNT medulloblastomas is highly hemorrhagic, thick‐walled arterial‐type, and also includes numerous small venous and capillary structures lacking an intact blood-brain barrier (BBB) [16, 23]. This was captured in a case report of a patient with WNT medulloblastoma which describes the identification of tumor structures with dilated arteriolar vessels with slow flow and lack of venous drainage [23]. Although mechanistic studies are needed in WNT medulloblastomas, work from other systems has implicated β-catenin in the direct regulation of endothelial cell-cell adhesions and maintaining vascular barrier function during embryonic and postnatal development [24, 25]. So, it is not entirely surprising that the vascular architecture of WNT-driven medulloblastomas is aberrant. WNT antagonists, WNT inhibitor factor 1, and Dickkopf 1, which can disrupt endothelial cell-cell interaction, are also found at higher levels in WNT MB [26].
Figure 1.
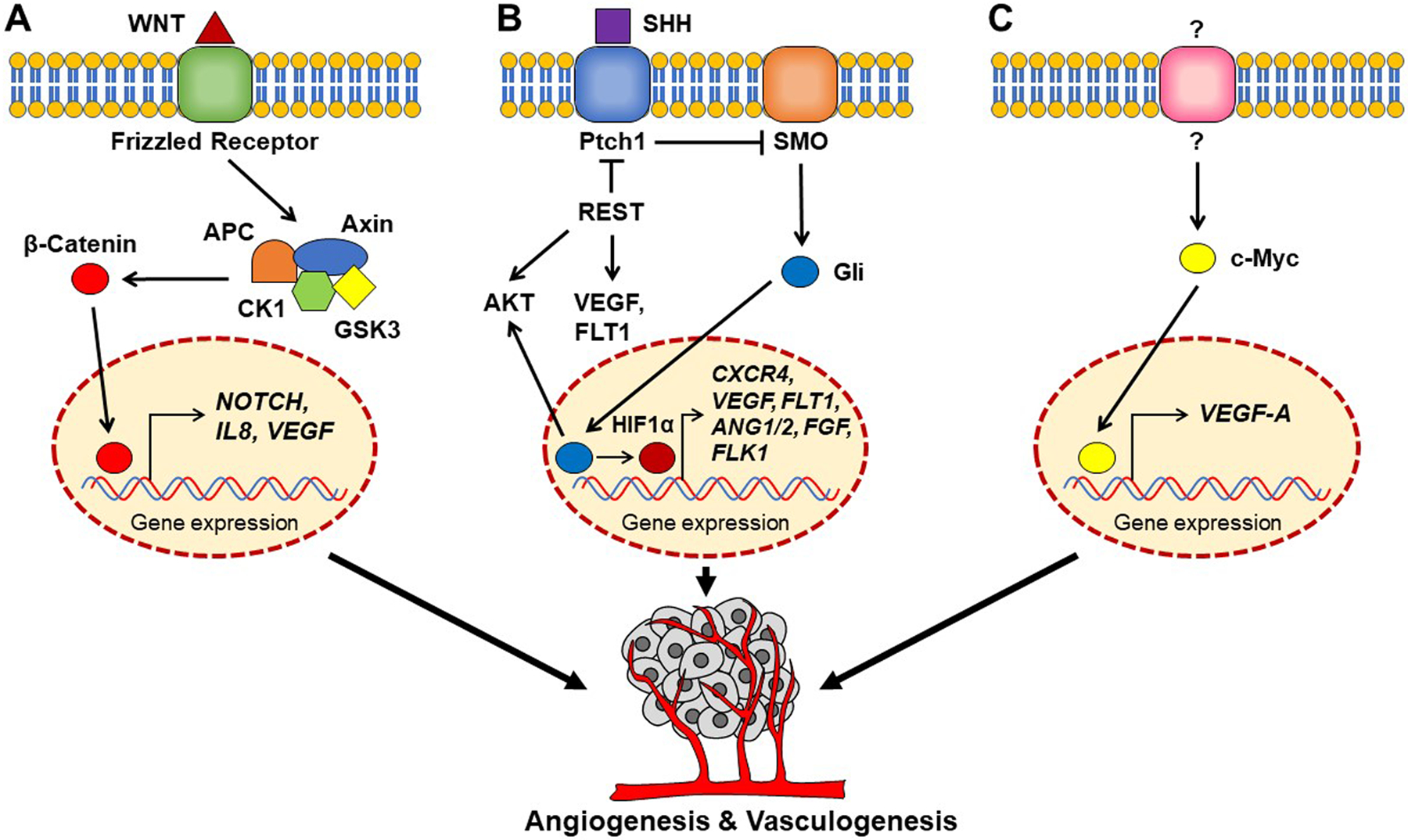
Schematic representation of angiogenic pathways in WNT, SHH and Group 3 medulloblastomas. (A) WNT ligand binding to FRIZZLED (Frz) receptor activates downstream signaling and β-Catenin activation, which is known to increase the expression of VEGF either directly [30], or through activation of NOTCH signaling [28]. β-Catenin can also induce IL8 and VEGF expression [30]. (B) In SHH medulloblastoma, loss of the tumor suppressor PTCH1 promotes oncogenic Smoothened (SMO) activity and downstream signaling through Glioma associated oncogene (GLI)-1 transcription factor. Gli1 can upregulate HIF1-α and AKT and activate pro-angiogenic CXCR4 signaling. HIF1-α can also directly upregulate the expression of other pro-angiogenic molecules such as VEGF, FLT1, FLK1, ANG1, ANG2 and FGF. REST elevation in SHH medulloblastoma cells has been shown to elevate AKT, FLT1 and VEGF levels [38, 39]. (C) In Group 3 medulloblastoma, MYC elevation in response to an unknown extracellular signal (?) can upregulate VEGFA expression to promote vascular growth [47].
Loss of β-catenin expression and activity in endothelial cells has been shown to affect endothelial cell-cell junctions in CTNN1B−/− mice and cause increased fragility and permeability during vascular development [27]. Cross-talk between WNT and NOTCH signaling demonstrated in other systems, and activation of the NOTCH signaling in endothelial cells of patients with WNT-medulloblastomas has the potential to promote vascular sprouting and remodeling of existing arterial and venous networks [28] (Figure 1A). NOTCH signaling is also shown to regulate vascular barrier function and the maintenance of BBB during early and post-natal development by regulating adherens junctions [29]. Non-canonical WNT pathway activation in endothelial cells can also regulate vascular development, which involves a direct β-catenin -mediated upregulation of interleukin (IL)-8 and vascular endothelial growth factor (VEGF) and increased VEGF signaling [30] (Fig.1A). Based on these observations, dysregulation of WNT signaling in medulloblastoma should be expected to affect vascular and BBB integrity, in a similar manner [31]. It is important to note that the above findings may be more relevant to medulloblastoma patients with inherited mutations in WNT/ β-catenin signaling.
In contrast to WNT medulloblastomas, the vasculature in Sonic hedgehog (SHH) tumors, driven by constitutive activation of the SHH developmental pathway, has an intact BBB [16]. It has provided the basis for the assumption that these tumors exhibit a more selective permeability to chemotherapy. Indeed, a recent dynamic contrast enhancement magnetic resonance imaging (MRI) study revealed functional differences in the integrity of the blood-brain tumor barrier (BBTB) and tumor vessel phenotype between various medulloblastoma mouse models [32]. Canonical and non-canonical SHH signaling pathways play important roles in promoting angiogenesis and vasculogenesis [33]. Chemokine signaling through the pro-angiogenic C-X-C motif receptor 4 (CXCR4) and stromal-derived factor-1 (SDF)-1 axis, is upregulated in SHH medulloblastomas and is associated with poor patient survival [34–36]. Increased hypoxia-inducible factor 1-alpha (HIF1α) expression, frequently noted in SHH medulloblastomas, may be a likely cause of CXCR4 upregulation in these tumors (Fig 1B) [37]. HIF1α may also upregulate the expression of VEGF and its cognate receptors, Fms-related receptor tyrosine kinase 1 (Flt-1) and, fetal liver kinase 1 (FLK1); angiopoietin (ANG)-1 and −2 and their receptor tyrosine protein kinase receptor-TIE-2 as well as fibroblast growth factor (FGF) to drive vascular growth, though not demonstrated in SHH medulloblastomas (Fig 1B) [38, 39]. Finally, elevation of the pro-angiogenic placental growth factor (PGF) in SHH medulloblastomas was shown to be associated with a surprising lack of effect on vascular growth, although an increase in tumor cell growth was seen (Fig 1B) [40]. Non-canonical SHH signaling pathway activity is also known to affect vascular growth by promoting endothelial cells differentiation and vessel maturation [41].
More recently, the RE1 Silencing transcription factor (REST), a regulator of neurogenesis was implicated in vascular remodeling in SHH-driven medulloblastomas [42]. A combination of in vitro and in vivo experiments with genetically engineered mice and xenograft models showed that REST elevation in subgroups of SHH medulloblastomas upregulated HIF1α, PGF, and VEGF expression and increased vascular growth [42, 43]. Surprisingly, a REST-dependent increase in the expression of v-ets erythroblastosis virus E26 oncogene homolog 1 (ETS1) and FLT1/VEGF receptor 1 (VEGFR1) was also noted in tumor cells, suggesting the existence of an autocrine loop and possibly, VM [42, 44]. An in-depth investigation of the mechanisms underlying this phenomenon is needed. In a separate body of work, REST elevation in SHH medulloblastomas was shown to increase pro-angiogenic AKT signaling [2]. AKT overexpression is known to promote VEGF secretion and upregulate HIF1α expression [45, 46].
The vascular architecture in Group 3/4 medulloblastomas is not well defined. At the molecular level, Myc elevation, a hallmark of Group 3 medulloblastoma, has been shown to upregulate VEGFA expression (Fig 1C) [47]. Group 3 medulloblastomas also exhibit increased expression of Ribonuclease/Angiogenin Inhibitor 1 (RNH1), Secretogranin II (SCG2), angiogenic factor with G-patch and FHA Domains 1 (AGGF1), and prokineticin 2 (PROK2), molecules with known roles in angiogenesis. [48]. Finally, the increase in VEGFA in Group 3 tumors and its correlation with increased vessel density and poor survival in patients and mouse models, argues for a role for vascular remodeling in disease progression [48]. Information on the molecular characteristics and architecture of Group 4 medulloblastoma vasculature is lacking.
3. Targeting the medulloblastoma vasculature
A systematic molecular group-specific assessment of angiogenesis targeting therapy has not been conducted in medulloblastoma patients. However, a critical review of the published literature on preclinical studies may offer guidance on how this could be facilitated. In WNT medulloblastomas, the increase in the number of tumor vessels and their leaky morphology was associated with responsiveness to the vincristine, which was taken as evidence that a leaky vasculature is a good predictor of therapeutic response [16]. However, work from other groups has suggested that a leaky vasculature can cause spatial and temporal heterogeneity in tumor blood flow along with an increase in tumor interstitial fluid pressure, to disrupt targeted drug delivery [49]. Normalization of tumor vessels by anti-VEGF therapy is proposed as an alternative approach to improve therapeutic efficacy. Indeed, better-targeted delivery of paclitaxel was demonstrated in human xenograft models of ovarian and colon carcinoma [50]. Consistent with these observations, the combination of anti-VEGF and standard chemotherapy was associated with improved survival in patients with colorectal cancer and lung cancer [51, 52]. This needs to be explored in brain cancers especially in tumors with leaky vasculature.
WNT and NOTCH pathways regulate vascular growth and inhibition of these pathways either alone or in combination may have potential applications in WNT medulloblastomas. In support of this possibility, preclinical studies with renal cancer cell xenograft models have demonstrated that NOTCH inhibitor (DAPT) and WNT inhibitor (ICG-001) markedly reduced tumor growth likely through an effect on tumor vasculature [53].
Pre-clinical work with SHH medulloblastoma models has shown the feasibility of targeting CXCR4 signaling in mice [34, 35, 54, 55]. These studies employed the CXCR4 inhibitor-AMD3100 either as a single agent or in combination with the Smoothened inhibitor-GDC-0449/vismodegib to demonstrate a significant reduction in tumor burden in the SmoA1 medulloblastoma mouse model [34, 56]. However, their effect on tumor vasculature was not investigated. Work by Bai et al. showed that the anti-parasitic agent mebendazole (MBZ) could inhibit angiogenesis and tumor growth in genetically engineered mouse models of SHH medulloblastomas by inhibiting VEGFR2 signaling [57]. REST-associated chromatin remodelers could also be potential therapeutic targets. Germane to this idea, pre-clinical studies have shown G9a inhibition to block tumor growth in vivo [58]. Histone deacetylase (HDAC) inhibitors such as valproic acid (VPA), vorinostat, and MS275 were shown to reduce levels or target REST activity in vitro [59]. Callegari et al showed the feasibility of targeting REST activity using LSD1 inhibitors [43]. However, a careful investigation of their ability to reduce vascular growth in mouse models remains to be conducted. Such studies would be especially pertinent to patients with SHH (Group 1) tumors, who are typically infants, and where improving chemosensitivity through vascular remodeling may circumvent the need for radiation and prevent late-effects.
In Group 3 and 4 medulloblastoma xenograft models, intra-tumoral injection of measles viruses expressing the angiogenesis inhibitors endostatin and angiostatin cells promoted a significant reduction in endothelial cell migration in vitro, and reduced tumor-associated blood vessels and tumor growth in mice [60]. The angiogenesis inhibitor, thrombospondin-1 (TSP-1) showed effectiveness in reducing AKT signaling and metastasis, and promoted chemo- and radio-sensitivity to increase the survival of tumor-bearing mice [61]. MBZ could also reduce vasculature and tumor burden in Group 3 medulloblastoma mouse models.
Clinical evaluation of anti-angiogenic therapy in patients with brain tumors including medulloblastoma has been somewhat focused on the evaluation of the anti-VEGF antibody, bevacizumab. In combination with irinotecan or radiation, bevacizumab promoted an initial antitumor activity in human clinical studies [62–64]. However, tumor recurrence and resistance to bevacizumab were common, and overall survival was not significantly improved [62–64]. However, pre-clinical and clinical follow-up studies are needed to ascertain the molecular basis for this poor response.
Funding
The research of the authors was supported by funding from the Cancer Prevention Research Institutes of Texas (CPRIT-RP150301), NIH (R01NS079715) and Addis Faith Foundation to VG.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants, or patents received or pending, or royalties.
LIST OF ABBREVIATIONS
- WNT
Wingless
- SHH
Sonic Hedgehog
- BBB
Blood-brain barrier
- IL8
Interluekin 8
- VEGF
Vascular endothelial growth factor
- CXCR4
C-X-C motif receptor 4
- SDF-1
Stromal-derived factor-1
- HIF1-α
Hypoxia-inducible factor 1 alpha
- Flt-1
Fms related receptor tyrosine kinase 1
- Flk-1
Fetal Liver Kinase 1
- Ang)-1
Angiopoietin-1
- Ang)-2
Angiopoietin-2
- FGF
Fibroblast growth factor
- REST
RE1 Silencing transcription factor
- ETS1
Proto-Oncogene 1
- VEGFR1
vascular endothelial growth factor receptor 1
- Akt
Protein Kinase B
- VEGF-A
Vascular endothelial growth factor-A
- RNH1
Ribonuclease/Angiogenin Inhibitor 1
- SCG2
Secretogranin II (SCG2)
- AGGF1
Angiogenic factor with G-patch and FHA Domains 1
- PROK2
Prokineticin 2
- Notch
Notch homolog 1, translocation-associated
- FGF
Fibroblast growth factor
- PGF
Placental growth factor
- MBZ
Mebendazole
- VEGFR2
Vascular Endothelial Growth Factor Receptor-2
- HDAC
Histone deacetylase
- VPA
Valproic acid
- TSP1
Thrombospondin 1
Footnotes
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers
- 1.Northcott PA, Robinson GW, Kratz CP, et al. Medulloblastoma. Nat Rev Dis Primers 2019. February 14;5(1):11. [DOI] [PubMed] [Google Scholar]
- 2.Dobson THW, Tao RH, Swaminathan J, et al. Transcriptional repressor REST drives lineage stage-specific chromatin compaction at Ptch1 and increases AKT activation in a mouse model of medulloblastoma. Sci Signal 2019. January 22;12(565). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahapatra S, Amsbaugh MJ. Medulloblastoma. StatPearls. Treasure Island (FL) 2021:1. [Google Scholar]
- 4.Cavalli FMG, Remke M, Rampasek L, et al. Intertumoral heterogeneity within medulloblastoma subgroups. Cancer Cell 2017. June 12;31(6):737–54 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Northcott PA, Buchhalter I, Morrissy AS, et al. The whole-genome landscape of medulloblastoma subtypes. Nature 2017. July 19;547(7663):311–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menyhart O, Giangaspero F, Gyorffy B. Molecular markers and potential therapeutic targets in non-WNT/non-SHH (group 3 and group 4) medulloblastomas. J Hematol Oncol 2019. March 15;12(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhukova N, Ramaswamy V, Remke M, et al. Subgroup-specific prognostic implications of TP53 mutation in medulloblastoma. J Clin Oncol 2013. August 10;31(23):2927–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coluccia D, Figuereido C, Isik S, et al. Medulloblastoma: Tumor biology and relevance to treatment and prognosis paradigm. Curr Neurol Neurosci Rep 2016. May;16(5):43. [DOI] [PubMed] [Google Scholar]
- 9.Quail DF, Joyce JA. The microenvironmental landscape of brain tumors. Cancer Cell 2017. March 13;31(3):326–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magnussen AL, Mills IG. Vascular normalisation as the stepping stone into tumour microenvironment transformation. Br J Cancer 2021. April 7;125:324–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RK Jain, di Tomaso E, Duda DG, et al. Angiogenesis in brain tumours. Nat Rev Neurosci 2007. August;8(8):610–22. [DOI] [PubMed] [Google Scholar]; **Mechanisms of angiogenesis in brain tumors and effect of vessel normalization on therapeutic response.
- 12.Rosinska S, Gavard J. Tumor vessels fuel the fire in glioblastoma. Int J Mol Sci 2021. June 17;22(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arvanitis CD, Ferraro GB, Jain RK. The blood-brain barrier and blood-tumour barrier in brain tumours and metastases. Nat Rev Cancer 2020. January;20(1):26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahir BK, Engelhard HH, Lakka SS. Tumor development and angiogenesis in adult brain tumor: Glioblastoma. Mol Neurobiol 2020. May;57(5):2461–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagy JA, Chang SH, Dvorak AM, et al. Why are tumour blood vessels abnormal and why is it important to know? Br J Cancer 2009. March 24;100(6):865–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phoenix TN, Patmore DM, Boop S, et al. Medulloblastoma genotype dictates blood brain barrier phenotype. Cancer Cell 2016. April 11;29(4):508–22. [DOI] [PMC free article] [PubMed] [Google Scholar]; **Key study that identified genotype specific differences in the medulloblastoma blood brain barrier.
- 17.Zanotelli MR, Reinhart-King CA. Mechanical forces in tumor angiogenesis. Adv Exp Med Biol 2018;1092:91–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huber H, Eggert A, Janss AJ, et al. Angiogenic profile of childhood primitive neuroectodermal brain tumours/medulloblastomas. Eur J Cancer 2001. November;37(16):2064–72. [DOI] [PubMed] [Google Scholar]
- 19.Zhan T, Rindtorff N, Boutros M. Wnt signaling in cancer. Oncogene 2017. March;36(11):1461–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marino S Medulloblastoma: developmental mechanisms out of control. Trends Mol Med 2005. January;11(1):17–22. [DOI] [PubMed] [Google Scholar]
- 21.Guessous F, Li Y, Abounader R. Signaling pathways in medulloblastoma. J Cell Physiol 2008. December;217(3):577–83. [DOI] [PubMed] [Google Scholar]
- 22.Roussel MF, Hatten ME. Cerebellum development and medulloblastoma. Curr Top Dev Biol 2011;94:235–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Di Giannatale A, Carai A, Cacchione A, et al. Anomalous vascularization in a Wnt medulloblastoma: a case report. BMC Neurol 2016. July 15;16:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liebner S, Corada M, Bangsow T, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol 2008. November 3;183(3):409–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guo M, Breslin JW, Wu MH, et al. VE-cadherin and beta-catenin binding dynamics during histamine-induced endothelial hyperpermeability. Am J Physiol Cell Physiol 2008. April;294(4):C977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guerit S, Liebner S. Blood-brain barrier breakdown determines differential therapeutic outcome in genetically diverse forms of medulloblastoma. Cancer Cell 2016. April 11;29(4):427–29. [DOI] [PubMed] [Google Scholar]; **Blood-brain barrier phenotypes in medulloblastoma sub-groups affect therapeutic response.
- 27.Cattelino A, Liebner S, Gallini R, et al. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol 2003. September 15;162(6):1111–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Corada M, Nyqvist D, Orsenigo F, et al. The Wnt/beta-catenin pathway modulates vascular remodeling and specification by upregulating Dll4/Notch signaling. Dev Cell 2010. June 15;18(6):938–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Polacheck WJ, Kutys ML, Yang J, et al. A non-canonical Notch complex regulates adherens junctions and vascular barrier function. Nature 2017. December 14;552(7684):258–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lyle CL, Belghasem M, Chitalia VC. c-Cbl: An Important regulator and a target in angiogenesis and tumorigenesis. Cells 2019. May 23;8(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Braune EB, Seshire A, Lendahl U. Notch and Wnt dysregulation and its relevance for breast cancer and tumor initiation. Biomedicines 2018. November 1;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Genovesi LA, Puttick S, Millar A, et al. Patient-derived orthotopic xenograft models of medulloblastoma lack a functional blood-brain barrier. Neuro Oncol 2021. May 5;23(5):732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nagase T, Nagase M, Machida M, et al. Hedgehog signalling in vascular development. Angiogenesis 2008;11(1):71–7. [DOI] [PubMed] [Google Scholar]
- 34.Sengupta R, Dubuc A, Ward S, et al. CXCR4 activation defines a new subgroup of Sonic hedgehog-driven medulloblastoma. Cancer Res 2012. January 1;72(1):122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan L, Zhang H, Liu J, et al. Growth factor receptor-Src-mediated suppression of GRK6 dysregulates CXCR4 signaling and promotes medulloblastoma migration. Mol Cancer 2013. March 5;12:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res 2010. June 1;16(11):2927–31. [DOI] [PubMed] [Google Scholar]
- 37.Guan G, Zhang Y, Lu Y, et al. The HIF-1alpha/CXCR4 pathway supports hypoxia-induced metastasis of human osteosarcoma cells. Cancer Lett 2015. February 1;357(1):254–64. [DOI] [PubMed] [Google Scholar]
- 38.Giatromanolaki A, Koukourakis MI, Sivridis E, et al. Relation of hypoxia inducible factor 1 alpha and 2 alpha in operable non-small cell lung cancer to angiogenic/molecular profile of tumours and survival. Br J Cancer 2001. September 14;85(6):881–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zimna A, Kurpisz M. Hypoxia-Inducible Factor-1 in Physiological and pathophysiological angiogenesis: Applications and therapies. Biomed Res Int 2015;2015:549412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Snuderl M, Batista A, Kirkpatrick ND, et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell 2013. February 28;152(5):1065–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salybekov AA, Salybekova AK, Pola R, et al. Sonic hedgehog signaling pathway in endothelial progenitor cell biology for vascular medicine. Int J Mol Sci 2018. October 5;19(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaik S, Maegawa S, Haltom AR, et al. REST promotes ETS1-dependent vascular growth in medulloblastoma. Mol Oncol 2021. May;15(5):1486–506. [DOI] [PMC free article] [PubMed] [Google Scholar]; ***Epigenetic modulators regulate vascular growth in SHH medulloblastoma tumors by increased expression of pro-angiogenic growth factors
- 43.Callegari K, Maegawa S, Bravo-Alegria J, et al. Pharmacological inhibition of LSD1 activity blocks REST-dependent medulloblastoma cell migration. Cell Commun Signal 2018. September 18;16(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]; *REST-LSD1 converges on HIF1 activity, a major regulator of vascular growth.
- 44.Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci 2011;4:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xia C, Meng Q, Cao Z, et al. Regulation of angiogenesis and tumor growth by p110 alpha and AKT1 via VEGF expression. J Cell Physiol 2006. October;209(1):56–66. [DOI] [PubMed] [Google Scholar]
- 46.Jiang BH, Zheng JZ, Aoki M, et al. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci U S A 2000. February 15;97(4):1749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Baudino TA, McKay C, Pendeville-Samain H, et al. c-Myc is essential for vasculogenesis and angiogenesis during development and tumor progression. Genes Dev 2002. October 1;16(19):2530–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thompson EM, Keir ST, Venkatraman T, et al. The role of angiogenesis in Group 3 medulloblastoma pathogenesis and survival. Neuro Oncol 2017. September 1;19(9):1217–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Goel S, Duda DG, Xu L, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev 2011. July;91(3):1071–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cesca M, Morosi L, Berndt A, et al. Bevacizumab-induced inhibition of angiogenesis promotes a more homogeneous intratumoral distribution of paclitaxel, improving the antitumor response. Mol Cancer Ther 2016. January;15(1):125–35. [DOI] [PubMed] [Google Scholar]
- 51.Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008. April 20;26(12):2013–9. [DOI] [PubMed] [Google Scholar]
- 52.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol 2009. March 10;27(8):1227–34. [DOI] [PubMed] [Google Scholar]
- 53.Fendler A, Bauer D, Busch J, et al. Inhibiting WNT and NOTCH in renal cancer stem cells and the implications for human patients. Nat Commun 2020. February 17;11(1):929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Buss MC, Remke M, Lee J, et al. The WIP1 oncogene promotes progression and invasion of aggressive medulloblastoma variants. Oncogene 2015. February 26;34(9):1126–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amarante MK, Vitiello GAF, Rosa MH, et al. Potential use of CXCL12/CXCR4 and sonic hedgehog pathways as therapeutic targets in medulloblastoma. Acta Oncol 2018. September;57(9):1134–42. [DOI] [PubMed] [Google Scholar]
- 56.Ward SA, Warrington NM, Taylor S, et al. Reprogramming medulloblastoma-propagating cells by a combined antagonism of sonic hedgehog and CXCR4. Cancer Res 2017. March 15;77(6):1416–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bai RY, Staedtke V, Rudin CM, et al. Effective treatment of diverse medulloblastoma models with mebendazole and its impact on tumor angiogenesis. Neuro Oncol 2015. April;17(4):545–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dobson THW, Hatcher RJ, Swaminathan J, et al. Regulation of USP37 expression by REST-associated G9a-dependent histone methylation. Mol Cancer Res 2017. August;15(8):1073–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor P, Fangusaro J, Rajaram V, et al. REST is a novel prognostic factor and therapeutic target for medulloblastoma. Mol Cancer Ther 2012. August;11(8):1713–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hutzen B, Bid HK, Houghton PJ, et al. Treatment of medulloblastoma with oncolytic measles viruses expressing the angiogenesis inhibitors endostatin and angiostatin. BMC Cancer 2014. March 19;14:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chan TSY, Picard D, Hawkins CE, et al. Thrombospondin-1 mimetics are promising novel therapeutics for MYC-associated medulloblastoma. Neurooncol Adv 2021. Jan-Dec;3(1):vdab002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Okada K, Yamasaki K, Tanaka C, et al. Phase I study of bevacizumab plus irinotecan in pediatric patients with recurrent/refractory solid tumors. Jpn J Clin Oncol 2013. November;43(11):1073–9. [DOI] [PubMed] [Google Scholar]
- 63.Bonney PA, Santucci JA, Maurer AJ, et al. Dramatic response to temozolomide, irinotecan, and bevacizumab for recurrent medulloblastoma with widespread osseous metastases. J Clin Neurosci 2016. April;26:161–3. [DOI] [PubMed] [Google Scholar]
- 64.Zhao M, Wang X, Fu X, et al. Bevacizumab and stereotactic radiosurgery achieved complete response for pediatric recurrent medulloblastoma. J Cancer Res Ther 2018. September;14(Supplement):S789–S92. [DOI] [PubMed] [Google Scholar]


