Abstract
BACKGROUND:
LMB-100 is an antibody-toxin conjugate with an anti-mesothelin Fab linked to a 24 kDa portion of Pseudomonas exotoxin A with mutations that decrease immunogenicity. The objective of this first-in-human Phase I study was to determine the maximum tolerated dose (MTD) and safety in patients with advanced solid tumors expressing mesothelin.
METHODS:
Cohorts of 1–7 patients received intravenous LMB-100 at 7 dose levels from 40 to 250 mcg/kg intravenously on days 1, 3 and 5 of a 21-day cycle.
RESULTS:
Of 25 subjects accrued, 17 had mesothelioma, 3 each had ovarian or pancreatic cancer and 2 had gastric cancer. Dose limiting toxicity (DLT) occurred in 2 of 4 treated at 250 mcg/kg (capillary leak syndrome) and 3 of 7 treated at 170 mcg/kg (creatinine increase). The MTD of LMB-100 was 140 mcg/kg. Of 10 mesothelioma patients treated at 170 or 140 mcg/kg, 8 had stable disease and 2 progressive disease. Peak LMB-100 plasma concentrations were dose-dependent during cycle 1. Development of anti-drug antibodies (ADAs) decreased LMB-100 blood levels in 8 of 21 (38%) patients who received cycle 2 and 9 of 11 patients (81.8%) receiving cycle 3.
CONCLUSIONS:
The MTD for single agent LMB-100 is 140 mcg/kg given on a QOD x 3 schedule. Although less immunogenic than the first generation anti-mesothelin immunotoxin SS1P, the majority of patients developed ADAs after 2 cycles and LMB-100 has limited anti-tumor efficacy as a single agent. Phase II studies of LMB-100 plus pembrolizumab are ongoing for patients with mesothelioma and lung cancer.
Keywords: immunotoxin, mesothelin, mesothelioma, LMB-100
Lay Summary:
Mesothelin, a cell surface antigen is an attractive target for cancer therapy, given its limited expression on normal human tissues and high expression in many human cancers. LMB-100 is a recombinant anti-mesothelin immunotoxin consisting of a humanized anti-mesothelin antibody fragment fused to a truncated Pseudomonas exotoxin A (PE). In this study, the authors have determined the safety, maximum tolerated dose and pharmacokinetics of LMB-100, as well as the generation of anti-drug antibodies. Ongoing phase II clinical trials are evaluating the combination of LMB-100 plus pembrolizumab in patients with treatment refractory mesothelioma and non-small cell lung cancer.
Precis:
Mesothelin targeting immunotoxin LMB-100 is well tolerated with manageable adverse effects. Based on anti-tumor efficacy seen in preclinical studies, the combination of LMB-100 plus pembrolizumab is currently being evaluated in the clinic.
INTRODUCTION
LMB-100 is a second-generation recombinant immunotoxin that targets the cell surface glycoprotein mesothelin expressed on cancer cells.1, 2 Mesothelin expression on normal human tissues is limited to the mesothelial cells of the pleura, peritoneum and pericardium.3, 4 About 30% of human tumors express mesothelin, including pancreatic,5, 6 gastric,7 and non-small cell lung cancer (NSCLC).8–10 Mesothelin is also highly expressed in the vast majority of patients with malignant mesothelioma.11–13 Due to its high expression on tumor cells as well as limited expression on normal tissues, mesothelin is an attractive target for anti-cancer therapeutics.14, 15
Recombinant immunotoxins are antibody-based biologics which carry a protein toxin as payload.16 LMB-100 contains a modified Pseudomonas exotoxin A (PE) payload.1, 2, 17 PE kills cells by irreversibly modifying elongation factor-2 to halt protein synthesis and induce apoptosis, a unique mechanism of action among anti-cancer agents. PE activity requires toxin internalization by the target cell through binding to a surface receptor. By replacing the native binding domain of PE with an alternative targeting moiety, the specificity of this activity can be rationally directed.18 Moxetumomab pasudotox, a PE-based immunotoxin targeted against CD22, was recently FDA approved for the treatment of hairy cell leukemia after demonstrating a high rate of durable complete responses.19 Achieving similar success against solid tumors has been more difficult. SS1P, a first generation mesothelin-targeted immunotoxin showed little activity as a single agent despite having an acceptable safety profile.20 In solid tumor patients, neutralizing anti-drug antibodies (ADAs) directed against PE develop after only three infusions of SS1P and decrease its serum levels. To overcome ADA formation, SS1P was combined with a pre-conditioning regimen of lymphocyte depleting chemotherapy to delay development of high-titer ADAs. With the combination, patients were able to receive more cycles of treatment, and SS1P produced deep and durable objective clinical responses in several mesothelioma patients, thus demonstrating proof of principle for immunotoxin efficacy in solid tumors.21
LMB-100 was engineered to have reduced immunogenicity and toxicity compared to SS1P.2 In LMB-100, domain II of PE, containing B and T cell epitopes was removed, and the remaining protein (PE24) was deimmunized to remove most B cell recognition epitopes as well as 2 of 6 T-cell epitopes. In addition, LMB-100 bears a fully humanized anti-MSLN Fab, rather than the murine dsFv antibody fragment in SS1P.2 In most established and early passage mesothelioma cell lines, LMB-100 was more potent than SS1P and it also showed efficacy against patient-derived xenografts.22 A single cycle of LMB-100 significantly shrank large human lung carcinoma tumors in mice.2 The immunotoxin also demonstrated activity against mesothelin expressing xenograft murine models of mesothelioma,22 gastric, breast and pancreatic cancers.17, 18, 23 Based on these findings of anti-tumor activity, a first in human study of LMB-100 was initiated to determine the safety and tolerability of LMB-100 in patients with advanced mesothelin expressing cancers.
METHODS
Patients
Initial testing of LMB-100 (previously RG7787 and RO6927005) was performed in a multicenter, international, single-arm, open-label first in human trial (NCT02317419) sponsored by F. Hoffmann-La Roche Ltd, Basel, Switzerland. Participants included those with metastatic or locally advanced malignant solid tumors known to express mesothelin including mesothelioma, pancreatic cancer, ovarian cancer, gastric cancer and NSCLC. Evaluation of mesothelin expression was performed retrospectively for initial cohorts but was prospectively assessed centrally (Ventana) in later multi-patient cohorts. Moderate (2+) or strong (3+) membrane staining in ≥ 1% of tumor cells from archival tumor samples was required for eligibility. After F. Hoffmann-La Roche Ltd decided to stop the trial, LMB-100 IND was transferred to the National Cancer Institute (NCI) where the phase I evaluation was completed on a separate clinical protocol (NCT02798536). Eligible participants were required to have mesothelioma with epithelioid subtype or at least 50% epithelioid component for biphasic subtype. Other eligibility criteria shared by both studies included at least one prior systemic treatment, Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2, adequate organ and bone marrow function and measurable disease. Patients with a prior history of pneumonectomy, use of high dose steroids within the last 7 days, active HIV, or Hepatitis B or C infection were not eligible for enrollment. The study was conducted in accordance with the principles of the International Conference on Harmonisation - Good Clinical Practice (ICH-GCP) guidelines. The study protocol was approved by the local Institutional Review Boards before patients were enrolled on the study. All patients provided written informed consent prior to treatment.
Study design and treatment
Participants in the multi-center phase I study were enrolled between December 2014 and August 2015 at five centers across Europe and North America. Treatment was initiated at a dose of 45 mcg/kg given as a 30-minute intravenous (IV) infusion on days 1, 3 and 5 of a 21-day cycle, the maximum tolerated dose (MTD) of SS1P, the predecessor MSLN-targeted immunotoxin.20 The dose-limiting toxicity (DLT) period continued for 2 weeks beyond the last administration of study drug during cycle 1. The dose was escalated using modified Continual Reassessment Method with Overdose Control (mCRM with EWOC) aimed at reaching MTD.24, 25 No intra-patient dose escalation was permitted. Following completion of the safety evaluation for a dose level, investigators and the sponsor study team evaluated the data available and set the next dose level. Initial dose levels were assessed with a single participant until a first DLT or grade 2 toxicity related to study drug was encountered, and then cohorts were expanded to at least 3 participants. Pre-medications to prevent infusion-related reaction (IRR) were not routinely administered. F. Hoffmann-La Roche Ltd terminated the study before an MTD was determined. Phase I testing to establish the MTD of LMB-100 was continued at NCI between July 2016 and February 2017 using a dose de-escalation schedule with a standard 3+3 design, starting at 170 mcg/kg. If 2 or more patients experienced DLT within 21 days of LMB-100 administration, the dose could be reduced to the next dose level, i.e. 140 mcg/kg. MTD was defined as the highest dose level that caused DLT in ≤ 1 of 6 participants. After IRR were observed in the initial patients treated on the NCI part of the phase I study, all patients were pre-medicated prior to each administration of study drug with acetaminophen, diphenhydramine and ranitidine to prevent IRR. In case a patient developed IRR, they could receive dexamethasone to manage it. For the NCI Phase I part of the study participants were treated until progression, unacceptable toxicity, or for a maximum of 4 cycles.
Assessments
Adverse events were assessed using the NCI Common Terminology Criteria for Adverse Events (CTCAE), version 4.0. Clinical response was assessed by CT scan every 2 cycles (+/− 7 days). For participants with pleural mesothelioma, response was evaluated using modified Response Evaluation Criteria in Solid Tumors (mRECIST) for mesothelioma.26 For all others, RECIST 1.1 was used.27
Tumor and serum mesothelin
Immunohistochemistry (IHC) was used to evaluate mesothelin expression in tumor samples. For the multicenter part of the study, Ventana MSLN (SP74) assay (Ventana Medical Systems, Inc. Tucson, USA) was used. Mesothelin expression of the tumor cell membrane was reported as H score, that incorporate staining intensity and percent of positive cells and ranged from 0 to 300; with 100% of tumor cells having 3+ staining scored as 300.28 For patients treated on the NCI phase I study tumor mesothelin expression was evaluated by IHC as previously described.29 The slides were reviewed by pathologists with expertise in IHC and the results were described as either absent or positive membranous staining on tumor cells. In case of positive mesothelin staining, the percent of tumor cells expressing mesothelin was estimated and the intensity of staining [mild (1+), moderate (2+) or strong (3+)] was assessed. Serum mesothelin levels were evaluated using the Mesomark (Fujirebio Diagnostics, Inc., Malvern, PA) enzyme-linked immunosorbent assay (ELISA) kit as previously described and following the manufacturer’s instructions.29
Pharmacokinetic (PK) analysis
Plasma concentration of free LMB-100 were measured with a validated ELISA with a lower limit of quantification of 2.1 ng/mL. Samples for PK analysis were obtained from patients at pre-dose, end of infusion (EOI; 30-min post start of LMB-100 infusion), and 1, 2, 3, 4, 6 and (in some cycles) 24 hours post EOI. Concentration data for each dose was plotted over time to assess the impact of increasing ADAs that are generated in response to LMB-100 exposure. Additionally, maximum concentration (Cmax), area under the curve to the last quantifiable time point (AUCLAST), AUC extrapolated to time infinity (AUCINF), half-life (T1/2), clearance (CL), and volume of distribution (Vz), were calculated using noncompartmental methods. Additionally, correlations were assessed between Cmax or AUC and renal function [eGFR, calculated by the Modification of Diet in Renal Disease Study (MDRD) equation], ADAs, baseline serum mesothelin and the percentage of mesothelioma tumor cells expressing mesothelin. Exposure metrics (Cmax and AUCINF) were normalized to dose for comparisons between dose levels. Phoenix WinNonlin v7.0 (Certara, Cary, NC) was used to perform PK analyses; all plots and statistical tests (alpha=0.05) were performed in GraphPad Prism (GraphPad Software Inc., La Jolla, CA) and R v3.6.3. (RStudio, Boston, MA)
Detection of anti-drug antibody (ADA)
Plasma samples for ADA analysis were collected at baseline and at the beginning of each cycle, prior to LMB-100 infusion. ADAs were identified using a validated screening ELISA in which positive samples were those that had mean assay signal above the cutoff point (OD 0.05). Positive screened samples were then run in duplicate (two aliquots) in a confirmatory assay, one sample with and the other without spiked LMB-100 (32 mcg/mL final concentration) to assess induction of ADAs. The percent difference between the mean assay signals among the aliquots is the percent inhibition.
Statistical methods
Among patients with data on ADA at cycle 1 and 2 as well as Cmax after cycle 2, the ADA values at cycle 1 and cycle 2 were evaluated for their potential predictive ability for determining if Cmax after cycle 2 is <100 ng/ml or >100 ng/ml as follows. Initially, a screening was performed to test if the ADA levels at the two cycles or their difference, as well as the dose level of LMB-100 differed between those patients with Cmax cycle 2 <100 ng/ml or >100 ng/ml using an exact two-tailed Wilcoxon rank sum test with unadjusted p-values. After the initial screening, logistic regression analysis was used to develop a model to predict Cmax cycle 2 based on ADA levels at cycle 1, cycle 2, or their difference.
Overall survival (OS) was determined from the date LMB-100 treatment was initiated until the date of death or date of last follow-up (November 1, 2019). Progression free survival (PFS) was determined from the date LMB-100 treatment was initiated until the date of progression or death. The probability of OS and PFS as a function of time were determined by the Kaplan-Meier method.
RESULTS
Patient characteristics
Twenty-five patients received at least one infusion of LMB-100 and were evaluable for safety assessment (Table 1). The median age of patients was 62.5 years and 14 were females. Seventeen patients had mesothelioma, 2 gastric cancer and 3 each ovarian and pancreatic cancer. Fifteen patients were treated on the multicenter Phase I, and 10 on the NCI Phase I part of the study. The median number of prior treatments for the mesothelioma patients treated at NCI was 2.5 (range 1–5).
Table 1.
Patient Demographics and Clinical Characteristics
| Characteristics | Patients (n = 25) |
|---|---|
| Sex | |
| Male | 11 |
| Female | 14 |
| Age (years) | |
| Median (range) | 62.5 (37–79) |
| ECOG Performance Status | |
| 0 | 9 |
| 1 | 16 |
| Diagnosis | |
| Malignant Mesothelioma | 17 |
| Gastric Cancer | 2 |
| Ovarian Cancer | 3 |
| Pancreatic Cancer | 3 |
| Phase I cohorts | |
| Multicenter Phase I | 15 |
| NCI Phase I | 10 |
| LMB-100 Treatment Cycles | |
| Median (range) | 2 (1–7) |
Phase I dose escalation
Seven different dose levels of LMB-100 were evaluated ranging from 45 to 250 mcg/kg (Table 2). On the multi-center part of the phase I study doses of 45, 65, 100, and 170 mcg/kg were evaluated before DLT (capillary leak syndrome, CLS) was observed in 2 of 4 patients treated at 250 mcg/kg. Subsequently, the dose was de-escalated, and 2 additional participants were enrolled at a 200 mcg/kg dose level. However, these two patients were not evaluable for DLT evaluation since they received only a single infusion of LMB-100 before the study was terminated. For the NCI part of the phase I study, treatment began at 170 mcg/kg and 3 of 3 patients experienced creatinine increase (grade 2) during the first cycle. This led to a delay in cycle 2 treatment, an investigator-defined DLT. Kidney function of all 3 participants recovered to baseline. The protocol was amended to allow intra-patient dose-de-escalation so that these patients were able to receive Cycle 2 of therapy at 140 mcg/kg without any DLT. Seven participants were then treated at reduced dose level of 140 mcg/kg, which was found to be the MTD. Asymptomatic, grade 3 hyponatremia in the setting of CLS was noted in 1 patient at the 140 mcg/kg dose level which was a DLT.
Table 2:
Dose escalation scheme of LMB-100 and DLTs
| Dose Level | LMB-100 Dose (mcg/kg) | No. of patients treated | Patients with DLT* | DLT* |
|---|---|---|---|---|
| Multicenter Phase I | ||||
| 1 | 45 | 1 | 0 | - |
| 2 | 65 | 1 | 0 | - |
| 3 | 100 | 3 | 0 | - |
| 4 | 170 | 4 | 0 | - |
| 5 | 200 | 2 | NE | - |
| 6 | 250 | 4 | 2 | CLS |
| NCI Phase I | ||||
| 4‡ | 170 | 3 | 3 | Creatinine Increase |
| 7 | 140 | 7 | 1 | Hyponatremia |
DLT, dose-limiting toxicity; NE, not evaluable; CLS, capillary leak syndrome
received cycle 1 at dose level 4 and subsequent cycles at dose level 7.
Safety
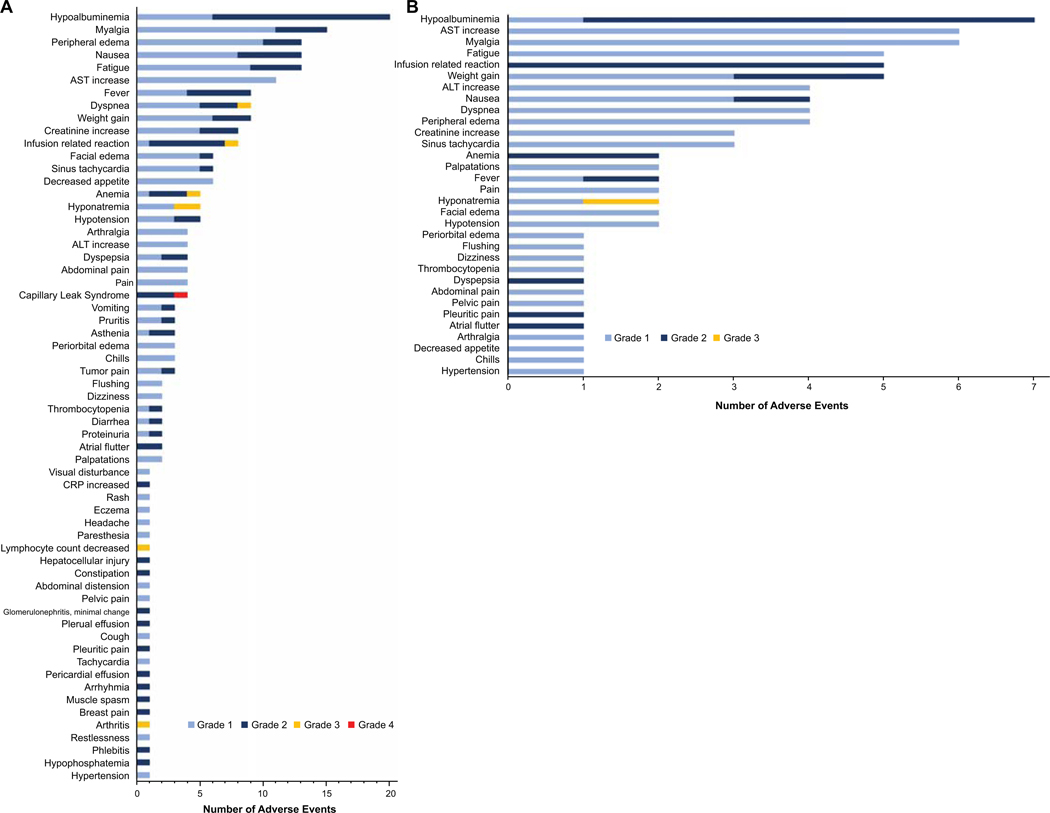
All adverse events possibly, probably or definitely related to LMB-100 treatment are shown in Figure 1. The most common toxicities seen across all dose levels included hypoalbuminemia (80%), myalgia (60%), peripheral edema (52%), weight gain (36%) and creatinine increase (32%) as manifestation of CLS (Fig.1A). Other frequent toxicities were nausea (52%), fatigue (52%), dyspnea (36%) and IRR (32%). Most of the toxicities were grade 1 and 2. Grade 3 toxicities included dyspnea (1 patient), IRR (1 patient), anemia (1 patient), hyponatremia (2 patients), decreased lymphocyte count (1 patient) and arthritis (1 patient). Two patients treated at the 250 mcg/kg dose level had DLT due to a grade 4 CLS in one patient and a grade 2 CLS in the other patient that led to discontinuation of the third dose of LMB-100 during cycle 1. The adverse events observed in the seven patients treated at the LMB-100 MTD of 140 mcg/kg are shown in figure 1B. One patient had grade 3 hyponatremia which was considered as DLT. Based on the high incidence of IRR we instituted routine prophylaxis with acetaminophen, diphenhydramine and ranitidine before each dose of LMB-100 for all patients treated at NCI. Of these 10 patients, 6 had a Grade 2 IRR at some point during their treatment. Of the 7 patients treated at the MTD, 5 had IRR. These reactions were observed at various points in the treatment course occurring during cycle 2 (n=2), cycle 3 (n=2) or cycle 4 (n=2), with one patient having reactions during cycles 3 and 4. The recurrence of IRR despite prophylactic medications resulted in treatment discontinuation during cycle 4 in 2 patients.
Figure 1-. LMB-100 related adverse events.
LMB-100 related adverse events (AEs) that were possibly, probably or definitively related to LMB-100 are shown (A) AE seen at all doses of LMB-100 evaluated. (B) AEs seen at the MTD of LMB-100.
Pharmacokinetics of LMB-100
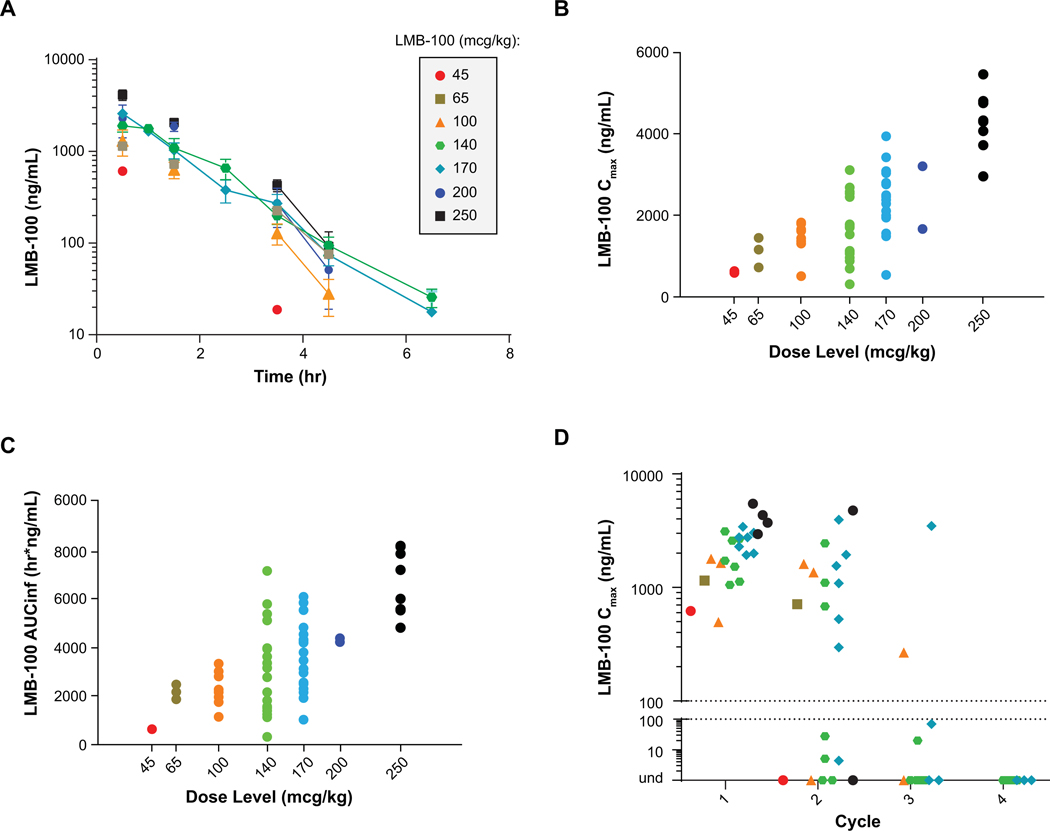
The change in LMB-100 exposure over time at the different dose levels during cycle 1 day 1 infusion is shown in figure 2A. There was a dose-proportional increase in both the Cmax and AUCINF from 45 mcg/kg to 250 mcg/kg (Fig. 2B and C). In patients who were treated at the LMB-100 MTD of 140 mcg/kg, the mean Cmax was 1974 ng/ml, mean AUCINF 3709, mean half-life 0.87 hour (53 minutes), mean clearance 3.98 L/hour and the mean volume of distribution was 5.90 L. The Cmax of LMB-100 after day 1 infusion of LMB-100 during cycles 1, 2, 3 and 4 are shown in figure 2D. In cycle 1 all patients had Cmax >100 ng/mL; in cycle 2, 13 of 21 (62%) patients who received LMB-100 had Cmax >100 ng/mL; during Cycle 3 this was reduced to 2 of 11 (18%) patients. All 9 patients who received Cycle 4 had undetectable plasma drug concentration after LMB-100 infusion (Fig. 2D; Table 3). Of the 10 mesothelioma patients treated at NCI with available data for the covariates of eGFR, tumor mesothelin expression, serum mesothelin (sMSLN) levels, dose levels, and ADAs, multiple linear regression determined that only ADAs significantly impacted both Cmax (p=0.0036) and AUC (p=0.0045) when pooling both cycle 1 and 2 PK data. To study the effect of LMB-100 on renal function, we analyzed eGFR of all 25 patients but did not find any significant co-relation with cycle 1 Day 1 (C1D1) PK Cmax or AUCLAST (Supporting Fig. S1A). There was no significant relationship of C1D1 Cmax (p=0.66) or AUC (p=0.76) (Supporting Fig. S1B) with tumor mesothelin expression, based on percentage of mesothelin-expressing mesothelioma cells. While there was a negative correlation between baseline serum mesothelin and C1D1 Cmax (greater sMSLN led to lower Cmax), statistical significance was not reached (p=0.23); the same was true for AUCLAST (p=0.12) (Supporting Fig. S1C). It is possible that greater shed mesothelin could be acting as a sink in the serum to bind LMB-100 and lower the systemic exposure.
Figure 2-. Pharmacokinetics of LMB-100.
(A) Change in LMB-100 exposure over time after first infusion. (B) Dose-proportional relationship of Cmax and (C) AUCINF versus dose level. (D) Change in Cmax by cycle of LMB-100 administered.
Table 3:
Effect of ADA on LMB-100 blood levels
| Patient ID+ | LMB-100 Dose (mcg/kg) | Cycle 1 | Cycle 2 | Cycle 3 | Cycle 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| ADAa | Cmaxb (ng/ml) | ADAa | Cmaxb (ng/ml) | ADAa | Cmaxb (ng/ml) | ADAa | Cmaxb (ng/ml) | |||
|
| ||||||||||
| Multicenter Phase I | 1 | 45 | 0.436 | 620 | 2.996 | 2.1 | - | - | - | - |
| 2 | 65 | 0.025 | 1150 | 0.727 | 711 | - | - | - | - | |
| 3 | 100 | 0.848 | 495 | 3.094 | 2.1 | - | - | - | - | |
| 4 | 100 | 0.02 | 1790 | 0.024 | 1610 | 2.431 | 267 | 3.331 | - | |
| 5 | 100 | 0.025 | 1650 | 0.052 | 1360 | 2.418 | 2.1 | 3.44 | - | |
| 6 | 170 | 0.037 | 3040 | 0.02 | 1940 | - | - | - | - | |
| 7 | 170 | 0.028 | 3430 | 3.308 | 527 | - | - | - | - | |
| 8 | 170 | 0.024 | 1930 | 0.227 | 1550 | - | - | - | - | |
| 9 | 170 | 0.024 | 2760 | 0.105 | 3950 | 0.292 | 3490 | 1.604 | 2.1 | |
| 12 | 250 | 0.387 | 2960 | 3.286 | - | - | - | - | - | |
| 13 | 250 | 0.02 | 5480 | 0.049 | 4770 | - | - | - | - | |
| 14 | 250 | 0.019 | 4340 | - | - | - | - | - | - | |
| 15 | 250 | 0.454 | 3730 | 3.232 | 2.1 | - | - | - | - | |
| NCI Phase I | 16 | 170 | 0.007 | 1991 | 1.846 | 1091 | 2.67 | 2.1 | 2.663 | 2.1 |
| 17 | 170 | 0.057 | 2286 | 2.466 | 297 | 2.69 | 70 | 2.681 | 2.1 | |
| 18 | 170 | 0.392 | 2760 | 2.721 | 4.4 | 2.619 | 2.1 | 2.661 | 2.1 | |
| 19 | 140 | 0.05 | 1054 | 0.164 | 681 | 2.758 | 2.1 | 2.744 | 2.1 | |
| 20 | 140 | 0.446 | 1124 | x | 2.1 | - | - | - | - | |
| 21 | 140 | 0.027 | 3118 | 2.31 | 2450 | 3.389 | 2.1 | 3.277 | 2.1 | |
| 22 | 140 | 0.007 | 2584 | 2.394 | 1099 | 3.432 | 2.1 | 3.433 | 2.1 | |
| 23 | 140 | 0.012 | 1721 | 3.236 | 2.1 | 3.562 | 2.1 | 3.743 | 2.1 | |
| 24 | 140 | 0.174 | 1526 | 3.598 | 27.8 | 3.623 | 20 | 3.639 | 2.1 | |
| 25 | 140 | 1.476 | 2689 | 2.863 | 5.1 | 2.967 | - | 2.979 | - | |
Two patients who received only a single dose of LMB-100 at 200 mcg/kg are not included
Anti-LMB-100 antibodies (ADA) were measured prior to infusion
LMB-100 blood levels
ADA assay not performed
Patients did not receive the 2nd, 3rd or 4th cycle of LMB-100
Human anti-LMB-100 antibodies
The presence of ADA was assessed retrospectively, and this information was not available to guide decisions concerning administration of LMB-100. Presence of ADA, of any titer, at baseline i.e. prior to receiving first cycle of LMB-100 did not affect the LMB-100 blood levels during cycle 1, since all 23 patients had LMB-100 Cmax levels ≥ 495 ng/ml on the day 1 infusion. During cycle 2, 13 of 21 patients who received LMB-100 achieved good blood levels (arbitrarily defined as levels >100 ng/ml; in-vitro most mesothelin expressing patient derived mesothelioma cell lines are sensitive to LMB-100 at concentrations of <10 ng/ml),22 and this was associated with low-titer ADA. Specifically, by logistic regression analysis, it was determined that a rule in which ADA cycle 2 <2.63 would predict Cmax cycle 2 >100 ng/ml (and that ADA cycle 2 >2.63 would predict Cmax cycle 2 <100 ng/ml) could apply to the data. Using this rule, 7/7 (100%) patients of those with Cmax cycle 2 <100 ng/ml were predicted correctly and 12/13 (92.3%) patients of those with Cmax cycle 2 >100 ng/ml were predicted correctly. One patient with ADA cycle 2 of 3.308 had Cmax cycle 2 of 527 ng/ml and was the only one not predicted correctly; one patient with Cmax cycle 2 <100 ng/ml did not have a value for ADA at cycle 2.
In 8 patients with ADA determined before cycle 2 > 2.63 there were no detectable LMB-100 blood levels in 7 of them, and all 12 patients with ADA levels <2.63 had detectable LMB-100 blood levels. Pre-cycle 1 ADA levels were also clearly associated with LMB-100 cycle 2 Cmax; all 13 patients with cycle 2 Cmax >100 ng/ml had ADA pre-cycle 1 <0.10 (100%), while 7/8 of those with cycle 2 Cmax <100 ng/ml had ADA pre-cycle 1 >0.10 (87.5%). Similarly, high titer ADA before cycle 3 or cycle 4 LMB-100 infusion resulted in undetectable LMB-100 blood levels. These results show that presence of ADA does not affect LMB-100 blood levels during cycle 1, and in cycle 2 about 60% of patients have good blood levels despite low titer ADA. However, the majority of patients develop high titer antibodies before cycle 3 which greatly reduces meaningful LMB-100 blood levels during cycles 3 and 4.
Tumor and serum mesothelin
Archival tumor samples of the 15 patients treated on the multicenter phase I part of the study were analyzed for mesothelin expression using membrane H scoring.28 The median H score for patients with gastric cancer was 3.5 (range 0–7, n=2), mesothelioma 235 (range 21–290, n=7), ovarian cancer 249 (range 152–266, n=3) and for pancreatic cancer was 24 (range 10–110, n=3) (Supporting Fig. S2). Of the ten mesothelioma patients treated at NCI, the assessment of tumor histology and mesothelin expression was based on surgically resected tumor for 6 cases and by needle biopsy for 4 cases. Since fresh tumor biopsy was not mandated by protocol, pathologic analysis was done using archival tissue for 7 patients and 3 patients underwent tumor biopsy. All 10 mesothelioma patients treated on the NCI part of the study were mesothelin positive with staining intensity of 3+ in ≥ 50% of tumor cells (Table 4). Out of them, 8 had epithelioid mesothelioma and for two cases (which were needle biopsies), the histologic sub-type was not specified in the pathology report. Both of these cases (P04 and P10) were strongly mesothelin positive, and therefore were most likely of epithelioid subtype (Table 4). Since the primary antibodies used to detect tumor mesothelin expression were different in the multicenter phase I study and NCI Phase I trial, we cannot directly compare the tumor mesothelin expression levels across all patients enrolled on this study. Serum mesothelin levels were also evaluated in these patients and the baseline levels ranged from 2.1 to 30.3 nM with a median value of 6.93 nM. The baseline serum mesothelin levels of the individual patients is shown in Supporting Table S1.
Table 4:
Clinical characteristics and treatment response in mesothelioma patients treated at the 170 and 140 mcg/kg dose levels.
| Pt. ID | Age/Sex | Site | Histologic Subtype | Tumor MSLN¶ | Radiologic Response* | PFS (months) | OS (months) | % Change in serum MSLN (Post C2) | % Change in serum MPF (Post C2)# |
|---|---|---|---|---|---|---|---|---|---|
| ^P01 | 37/F | Peri | E | 2- 3+>90% | SD post C4 | 2.7 | 34.6 | 16.6 | 70.22 |
| ^P02 | 72/ M | Peri | E | 3+ 100% | SD post C4 | 3.0 | 27.7 | 15.2 | −31.43 |
| ^P03 | 66/ M | Pleu | E | 3+ 90% | SD post C2 | 2.7 | 33.5 | 3.3 | 23.42 |
| P04 | 67/F | Peri | NS | 90% | SD after C2 | 2.9 | 9.4 | −27.0 | −42.57 |
| P05 | 68/ M | Pleu | E | 3+95% | PD post C2 | 1.5 | 37.0 | 70.1 | 104.67 |
| P06 | 46/F | Peri | E | 3+>50% | SD post C4 | 3.1 | 16.9 | 6.1 | 96.94 |
| P07 | 48/F | Peri | E | 3+ 100% | SD post C4 | 2.7 | 36.8 | −12.9 | −1.04 |
| P08 | 68/ M | Pleu | E | 3+ 50% | PD post C4 | 3.2 | 6.8 | −59.4 | 141.38 |
| P09 | 40/F | Peri | E | 3+ 100% | SD post C3 | 2.7 | 11.3 | −7.5 | −11.34 |
| P10 | 59/F | Peri | NS | 3+100% | SD post C4 | 2.8 | 19.1 | −31.8 | 107.32 |
Pt., patient; Peri, peritoneal mesothelioma; Pleu, pleural mesothelioma; E, epithelioid; NS, histologic subtype of mesothelioma not specified in the pathologist’s report; MSLN, mesothelin; SD, Stable Disease; PD, Progression of Disease; PFS, progression free survival; OS, overall survival; C, cycle
Patients received LMB-100 170 mcg/kg during cycle 1 and 140 mcg/kg during subsequent cycles.
MSLN expression determined by IHC
Best response at completion of LMB-100 treatment
alive
Change in serum mesothelin at end of two cycles of LMB-100
Efficacy
Of the 25 patients enrolled on the study 20 were evaluable for response. Response data were unavailable for the 5 patients (2 patients who received single dose of LMB-100 at 200 mcg/kg dose level and 3 patients treated at the 250 mcg/kg dose level) on the multi-center phase I study who were taken off treatment due to study discontinuation. Of the 20 patients evaluable for tumor response, 10 had stable disease and 10 had progressive disease as their best response. There were no objective radiologic partial or complete responses.
The characteristics of the 10 mesothelioma patients treated on the NCI phase I study are shown in table 4. Patients P01, P02 and P03 received LMB-100 at 170 mcg/kg during cycle 1 and 140 mcg/kg during subsequent cycles, whereas patients P04 to P10 received 140 mcg/kg of LMB-100 during all cycles of treatment. Three patients had pleural mesothelioma and 7 had peritoneal mesothelioma. Tumors from all these patients had high mesothelin expression with at least ≥50% tumor cells expressing it with a 2–3+ staining intensity. Eight patients had stable disease and 2 had progressive disease. The 2 patients with progressive disease had pleural mesothelioma. The median PFS was 2.8 months (95% CI: 1.5 −3.0 months) with all patients progressing within approximately 3 months of starting LMB-100 treatment; median overall survival was 23.4 months (95% CI: 6.8 – 34.6 months). Four patients had survival of greater than 30 months and out of these, 2 patients are alive at last follow-up (November 1, 2019). Five of 10 patients had a decrease in serum mesothelin levels after 2 cycles of LMB-100 as compared to pre-treatment serum mesothelin. However, given the small number of patients treated, it is difficult to draw any conclusions correlating stable disease with changes in serum mesothelin levels.
DISCUSSION
In this first in human study of a second generation anti-mesothelin immunotoxin, LMB-100, we have established its tolerability, MTD, immunogenicity and pharmacokinetics. The MTD of LMB-100 was 140 mcg/kg and the DLT was CLS. All patients including those with pre-existing ADA at baseline had good blood levels during cycle 1 and 60% of patients had good LMB-100 blood levels during cycle 2. Development of ADA prevented good LMB-100 levels during cycles 3 and 4. Of the 20 patients evaluable for response, 10 had stable disease as best response and 10 had progressive disease. Of the 10 mesothelioma patients treated at MTD, 8 had stable disease and 2 had progressive disease. We did not observe any objective radiologic responses. Two out of three pleural mesothelioma patients had progressive disease, which could be due to the biology of the disease, since pleural mesothelioma is more aggressive than peritoneal mesothelioma.
Our data demonstrate that LMB-100 has an acceptable safety profile and the observed adverse event of CLS was also seen with the first-generation mesothelin-targeted immunotoxin SS1P as well as the CD22-targeted immunotoxin moxetumomab pasudotox.19 Interestingly, pleuritis, the DLT for SS1P and an anticipated on-target off-tumor toxicity of anti-mesothelin therapeutics, was observed in only one patient treated with LMB-100. Renal toxicity seen with LMB-100 were not reported in studies of single agent SS1P but have been seen with moxetumomab pasudotox. It is not clear why LMB-100 affects the kidney differently than SS1P. As expected, hemolytic uremic syndrome was not observed with LMB-100. This toxicity of moxetumomab pasudotox is believed to be related to an off-target effect of the anti-CD22 binding moiety of this immunotoxin. Overall, toxicity of LMB-100 was manageable at the MTD of 140 mcg/kg.
LMB-100 was designed to have decreased immunogenicity compared to prior PE-based therapeutics like SS1P. Our data show that even patients with pre-existing ADAs against LMB-100 (presumably from prior exposure to Pseudomonas) achieved expected circulating concentrations of LMB-100 during the first cycle. In addition, more than half of patients receiving a second cycle had detectable plasma drug concentrations. These results compare favorably to SS1P. For the SS1P clinical trials, patients were prospectively evaluated for the presence of neutralizing antibodies, and if present, these patients were not included in the studies.20, 30 Greater than 90% of patients lacking pre-existing ADAs to SS1P had undetectable drug levels during cycle 2.20, 30 Although LMB-100 is less immunogenic than SS1P, all patients do develop neutralizing antibodies to LMB-100 with repeated administration such that most patients have poor drug levels beyond cycle 2 and high rates of IRRs. For reasons that are presently unclear the mean half-life of LMB-100 of 53 minutes was shorter than that of SS1P which has a mean half-life of 727 minutes at MTD.29 It is possible that the shorter half-life and the inability to deliver multiple courses of LMB-100 limits its efficacy, a conclusion consistent with the limited anti-tumor activity we observed in mesothelioma patients despite their high tumor mesothelin expression. These data suggest that single agent LMB-100 will have limited therapeutic potential.
Although LMB-100 by itself has limited clinical activity, preclinical studies support its development as part of combination studies which are currently in the clinic. The two main approaches being explored include, studies in combination with immune checkpoint inhibitors and studies to decrease antibody formation to LMB-100. Preclinical studies of LMB-100 show remarkable synergistic anti-tumor efficacy with anti-CTLA4 as well as anti-PD-1 antibodies using immunocompetent mouse models.31 Additionally, amongst the patients who received anti-PD1 antibodies after LMB-100, durable responses of >12 months were seen in 4 of 7 evaluable patients, including one with on-going complete response at 36 months32. Analysis of tumor biopsies from six patients taken before and after LMB-100 treatment showed increased CD8+ T-cells in four of these patients indicating LMB-100 induced immune infiltration in the tumors32. In addition, in pre-clinical studies LMB-100 treatment leads to immunogenic cell death.33 This has led to two Phase II clinical trials testing sequential LMB-100 followed by pembrolizumab in patients with refractory mesothelioma (NCT03644550) and non-small cell lung cancer (NCT04027946). The patients are treated with 2 cycles of LMB-100 (140 mcg/kg QOD x 3 doses every 3 weeks), followed by pembrolizumab 200 mg every 3 weeks until disease progression, unacceptable toxicity or maximum of 2 years.
Studies to decrease the formation of antibodies to LMB-100 are also being pursued.34 Pre-clinical studies in murine models have shown successful suppression of high-titer ADAs using the JAK/STAT inhibitor tofacitinib. In addition, tofacitinib co-administration improves half-life and anti-tumor effect of LMB-100 in murine models.35 Based on this, the combination of LMB-100 with tofacitinib is being evaluated in patients with treatment refractory pancreatic cancer and extrahepatic cholangiocarcinoma (NCT04034238). This trial has a dose-escalation phase followed by dose-expansion phase. For the dose-escalation part of the study, patients with other solid tumors that express mesothelin are eligible, while as the dose expansion part of the study will enroll patients with pancreatic cancer and cholangiocarcinoma. If tofacitinib decrease anti-drug antibodies, this combination treatment could potentially be expanded to other tumor types that express mesothelin.
In summary LMB-100 demonstrates an acceptable safety profile and although it is less immunogenic than SS1P almost all patients develop anti-drug antibodies after 2 cycles. Single agent LMB-100 has limited anti-tumor efficacy but ongoing studies of LMB-100 with immune checkpoint inhibitors in mesothelioma and lung adenocarcinoma as well as studies to decrease the development of anti-LMB-100 antibodies with tofacitinib will further define its role as an anti-cancer therapeutic.
Supplementary Material
Acknowledgements:
The authors thank all patients who participated in this study and their caregivers. The authors would also like to thank the participating sites and the Roche pRED oncology clinical and management teams, specifically Ernesto Guarin (clinical scientist), David Dejardin (biostatistician) and Roland Staack (bioanalytical leader). This study was sponsored and funded by the NIH, NCI, Center for Cancer Research Intramural Research Program and F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Funding: This research was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research (Z01-BC-006150) and F. Hoffmann-La Roche Ltd, Basel, Switzerland.
Footnotes
Disclosure of Potential Conflicts of Interest
RH: has received funding for conduct of clinical trials via a cooperative research and development agreement between NCI and Bayer AG, Aduro BioTech, TCR2 Therapeutics and Morphotek Inc.
AS: Consultant for (Advisory Board): Merck, Bristol-Myers Squibb, Novartis, Oncorus, and Janssen. Grant/Research support from (Clinical Trials): Novartis, Bristol-Myers Squibb, Symphogen, AstraZeneca/Medimmune, Merck, Bayer, Surface Oncology, Northern Biologics, Janssen Oncology/Johnson & Johnson, Roche, Regeneron, Alkermes, Array Biopharma and GSK
LS: Consultant for: Merck, Pfizer, Celgene, AstraZeneca/Medimmune, Morphosys, Roche, GeneSeeq, Loxo, Oncorus, Symphogen, Seattle Genetics, GSK, Voronoi, Treadwell Therapeutics and Arvinas. Grant/Research support from (Clinical Trials for institution): Novartis, Bristol-Myers Squibb, Pfizer, Boerhinger-Ingelheim, GlaxoSmithKline, Roche/Genentech, Karyopharm, AstraZeneca/Medimmune, Merck, Celgene, Astellas, Bayer, Abbvie, Amgen, Symphogen, Intensity Therapeutics, Mirati, Shattucks, and Avid Stockholder in: Agios (spouse)
CGR: Consultant for BMS; Roche/Genentech; Pierre Fabre, Erytech, MSD, Astra-Zeneca, Sanofi-Aventis, and Novartis. Grant/Research support from BMS; Roche/Genentech
JPD: Consulting or Advisory Role: Novartis, Roche/Genentech, Bristol-Myers Squibb, MSD Oncology. Research Funding to Institute: Genentech, Bristol-Myers Squibb, and MSD Oncology
AI: Advisory board/consulting: Astra Zeneca, Bayer, Daiichi/sankyo, Epizyme, Ipsen, Merck, Roche, and Springworks. Research grant/support: Astra Zeneca, Bayer, Chugai, Novartis, Pharmamar, Merck, MSD, Roche, Transgene
JCS: Receipt of honoraria or consultation fees: Astex, AstraZeneca, Bayer, Blend Therapeutics, Boehringer-Ingelheim, Clovis, Eli Lilly, Gammamabs, Merus, Mission Therapeutics, Pfizer, Pharmamar, Pierre Fabre, Roche, Sanofi, Servier, Symphogen, and Tarveda. Stock shareholder: Gritstone. JCS was a full time employee of ASTRAZENECA from Sept 2017 to Dec 2019
VMN: is an employee of Roche Inc.
IP: is an inventor on patents on LMB-100, all of which have been assigned to NIH.
The other authors declare that they have no competing interests.
REFERENCES:
- 1.Hollevoet K, Mason-Osann E, Liu XF, Imhof-Jung S, Niederfellner G, Pastan I. In vitro and in vivo activity of the low-immunogenic antimesothelin immunotoxin RG7787 in pancreatic cancer. Mol Cancer Ther. 2014;13: 2040–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bauss F, Lechmann M, Krippendorff BF, et al. Characterization of a re-engineered, mesothelin-targeted Pseudomonas exotoxin fusion protein for lung cancer therapy. Mol Oncol. 2016;10: 1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang K, Pastan I, Willingham MC. Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer. 1992;50: 373–381. [DOI] [PubMed] [Google Scholar]
- 4.Chang K, Pastan I. Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A. 1996;93: 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Argani P, Iacobuzio-Donahue C, Ryu B, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res. 2001;7: 3862–3868. [PubMed] [Google Scholar]
- 6.Hassan R, Laszik ZG, Lerner M, Raffeld M, Postier R, Brackett D. Mesothelin is overexpressed in pancreaticobiliary adenocarcinomas but not in normal pancreas and chronic pancreatitis. Am J Clin Pathol. 2005;124: 838–845. [PubMed] [Google Scholar]
- 7.Illei PB, Alewine C, Zahurak M, et al. Mesothelin Expression in Advanced Gastroesophageal Cancer Represents a Novel Target for Immunotherapy. Appl Immunohistochem Mol Morphol. 2016;24: 246–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho M, Bera TK, Willingham MC, et al. Mesothelin expression in human lung cancer. Clin Cancer Res. 2007;13: 1571–1575. [DOI] [PubMed] [Google Scholar]
- 9.Thomas A, Chen Y, Steinberg SM, et al. High mesothelin expression in advanced lung adenocarcinoma is associated with KRAS mutations and a poor prognosis. Oncotarget. 2015;6: 11694–11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kachala SS, Bograd AJ, Villena-Vargas J, et al. Mesothelin overexpression is a marker of tumor aggressiveness and is associated with reduced recurrence-free and overall survival in early-stage lung adenocarcinoma. Clin Cancer Res. 2014;20: 1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mutti L, Peikert T, Robinson BWS, et al. Scientific Advances and New Frontiers in Mesothelioma Therapeutics. J Thorac Oncol. 2018;13: 1269–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ordonez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol. 2003;16: 192–197. [DOI] [PubMed] [Google Scholar]
- 13.Inaguma S, Wang Z, Lasota J, et al. Comprehensive immunohistochemical study of mesothelin (MSLN) using different monoclonal antibodies 5B2 and MN-1 in 1562 tumors with evaluation of its prognostic value in malignant pleural mesothelioma. Oncotarget. 2017;8: 26744–26754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hassan R, Thomas A, Alewine C, Le DT, Jaffee EM, Pastan I. Mesothelin Immunotherapy for Cancer: Ready for Prime Time? J Clin Oncol. 2016;34: 4171–4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hassan R, Bera T, Pastan I. Mesothelin: a new target for immunotherapy. Clin Cancer Res. 2004;10: 3937–3942. [DOI] [PubMed] [Google Scholar]
- 16.Pastan I, Hassan R, Fitzgerald DJ, Kreitman RJ. Immunotoxin therapy of cancer. Nat Rev Cancer. 2006;6: 559–565. [DOI] [PubMed] [Google Scholar]
- 17.Alewine C, Xiang L, Yamori T, Niederfellner G, Bosslet K, Pastan I. Efficacy of RG7787, a next-generation mesothelin-targeted immunotoxin, against triple-negative breast and gastric cancers. Mol Cancer Ther. 2014;13: 2653–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weldon JE, Pastan I. A guide to taming a toxin--recombinant immunotoxins constructed from Pseudomonas exotoxin A for the treatment of cancer. FEBS J. 2011;278: 4683–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kreitman RJ, Dearden C, Zinzani PL, et al. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia. 2018;32: 1768–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hassan R, Bullock S, Premkumar A, et al. Phase I study of SS1P, a recombinant anti-mesothelin immunotoxin given as a bolus I.V. infusion to patients with mesothelin-expressing mesothelioma, ovarian, and pancreatic cancers. Clin Cancer Res. 2007;13: 5144–5149. [DOI] [PubMed] [Google Scholar]
- 21.Hassan R, Miller AC, Sharon E, et al. Major cancer regressions in mesothelioma after treatment with an anti-mesothelin immunotoxin and immune suppression. Sci Transl Med. 2013;5: 208ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang J, Khanna S, Jiang Q, et al. Efficacy of Anti-mesothelin Immunotoxin RG7787 plus Nab-Paclitaxel against Mesothelioma Patient-Derived Xenografts and Mesothelin as a Biomarker of Tumor Response. Clin Cancer Res. 2017;23: 1564–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolyvas E, Rudloff M, Poruchynsky M, et al. Mesothelin-targeted immunotoxin RG7787 has synergistic anti-tumor activity when combined with taxanes. Oncotarget. 2017;8: 9189–9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babb J, Rogatko A, Zacks S. Cancer phase I clinical trials: efficient dose escalation with overdose control. Stat Med. 1998;17: 1103–1120. [DOI] [PubMed] [Google Scholar]
- 25.Le Tourneau C, Lee JJ, Siu LL. Dose escalation methods in phase I cancer clinical trials. J Natl Cancer Inst. 2009;101: 708–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Byrne MJ, Nowak AK. Modified RECIST criteria for assessment of response in malignant pleural mesothelioma. Ann Oncol. 2004;15: 257–260. [DOI] [PubMed] [Google Scholar]
- 27.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45: 228–247. [DOI] [PubMed] [Google Scholar]
- 28.Parinyanitikul N, Blumenschein GR, Wu Y, et al. Mesothelin expression and survival outcomes in triple receptor negative breast cancer. Clin Breast Cancer. 2013;13: 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hassan R, Sharon E, Thomas A, et al. Phase 1 study of the antimesothelin immunotoxin SS1P in combination with pemetrexed and cisplatin for front-line therapy of pleural mesothelioma and correlation of tumor response with serum mesothelin, megakaryocyte potentiating factor, and cancer antigen 125. Cancer. 2014;120: 3311–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreitman RJ, Hassan R, Fitzgerald DJ, Pastan I. Phase I trial of continuous infusion anti-mesothelin recombinant immunotoxin SS1P. Clin Cancer Res. 2009;15: 5274–5279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leshem Y, O’Brien J, Liu X, et al. Combining Local Immunotoxins Targeting Mesothelin with CTLA-4 Blockade Synergistically Eradicates Murine Cancer by Promoting Anticancer Immunity. Cancer Immunol Res. 2017;5: 685–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Q, Ghafoor A, Mian I, et al. Enhanced efficacy of mesothelin-targeted immunotoxin LMB-100 and anti-PD-1 antibody in patients with mesothelioma and mouse tumor models Science Translational Medicine. 2020. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leshem Y, King EM, Mazor R, Reiter Y, Pastan I. SS1P Immunotoxin Induces Markers of Immunogenic Cell Death and Enhances the Effect of the CTLA-4 Blockade in AE17M Mouse Mesothelioma Tumors. Toxins (Basel). 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mazor R, King EM, Pastan I. Strategies to Reduce the Immunogenicity of Recombinant Immunotoxins. Am J Pathol. 2018;188: 1736–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Onda M, Ghoreschi K, Steward-Tharp S, et al. Tofacitinib suppresses antibody responses to protein therapeutics in murine hosts. J Immunol. 2014;193: 48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.