Abstract
In this article a syringol-π-benz[e]indolium based donor-acceptor fluorophore has been reported. The fluorophore shows a solvent polarity dependent change in the absorption and emission spectra in solution. A combined spectroscopic and time dependent density functional theory (TDDFT) studies reveal higher dipole moment of the fluorophore in the excited state, resulting positive solvatochromism. In physiological pH, the phenol group in the fluorophore is easily deprotonated owing to the electron pulling effect of the substituents. Consequently, the phenolate (PhO-) becomes a strong active donor in the new donor-acceptor pair. In aqueous solution, the new phenolate fluorochrome shows negligible fluorescence due to energy loss via non-radiative pathways from the low-lying polar excited states. The fluorochrome can detect human and bovine serum albumins in physiological buffer solution with high selectivity. The underlying mechanism of human serum albumin (HSA) detection was estimated to be strong (1.46 × 105 M−1, ΔG = −7.05 kcal/mol) supramolecular complexation between the fluorophore and albumin in hydrophobic binding site III-B. The linear relationship between fluorescence intensity and HSA concentration extends from 40 mg/L to an impressive upper limit (540 mg/L), thereby opening an opportunity for albumin detection in a broad range of health conditions. The practical applicability of the fluorophore was tested in spiked urine samples and a good correlation was observed between fluorescence intensity and the concentration of human serum albumin in neutral aqueous samples.
Keywords: Donor-acceptor fluorophore, Intramolecular charge transfer, Turn-on fluorescence, Human Serum Albumin
Introduction
Phenolic compounds are amongst the most important class of aromatic compounds that have found applications in many areas of our everyday lives [1-5]. Phenol and its derivatives are important precursors for production of polymers, plastics, detergents, disinfectants, herbicides, antibiotics, pharmaceutical drugs, food flavoring agents, and cosmetic products. Phenol is weakly acidic (pKa ~ 10). But, substantial lowering of acid dissociation constant is achieved by introducing electron-withdrawing groups [6]. These groups stabilize the phenolate conjugate base by negative inductive and/or mesomeric effects. Moreover, the optical properties of a phenol-phenolate pair can be tuned by altering the stereoelectronic properties of the molecular core. In chemistry and biology, the distinct optical property of the phenol-phenolate pair has been widely utilized to develop acidochromic dyes for various applications, including ratiometric pH sensing in organelles [7-11]. Given the importance of the phenolic compounds in optical sensing and detection, finding new simple dyes for specific application needs is of significant interest. Moreover, easy access to numerous derivatives of phenols makes the synthesis process less troublesome, and new donor groups other than dimethylamino, diethylamino and similar groups of cyclic nature are also highly desirable for various applications [12, 13].
In this article, we have reported a fluorogenic dye (1) consisting a phenol core substituted with two alkoxy groups. The acid-base equilibrium between phenol and phenolate has been exploited to develop a strongly colored (ε = 118,800 M−1cm−1) charge transfer (CT) fluorophore for potential biological applications [7, 14-15]. Fluorophore 1 is composed of a syringol moiety that functions as a weak donor in conjugation with a benz[e]indolium acceptor. In aqueous solution of near neutral pH, deprotonation of the ─OH group results a strong active phenolate donor which donates a pair of electrons to the conjugated acceptor. The enhanced intramolecular charge transfer results a low-lying singlet state and weak fluorescence in aqueous solution [16, 17]. The fluorescence is dramatically enhanced once the new zwitterionic fluorophore binds with serum albumins. This phenomenon enables 1 to function as a turn-on fluorescence probe by simple adding and mixing with albumins in aqueous solution. The purpose of two -OCH3 groups is two-fold; they lower the acid dissociation constant of the phenolic ─OH by –I effect and at the same time the positive mesomeric effect (+M) lowers the energy gap between the conjugated molecular orbitals, thus extending the absorption wavelength to the red region of the electromagnetic spectrum [13, 16]. Moreover, the zwitterionic phenolate fluorochrome would remain soluble in water due to polar solute-solvent interactions via hydrogen bonds [18]. These two properties are highly desirable for biological applications [19].
Herein, we have examined the photophysical properties of 1 by UV-vis and fluorescence spectroscopy, employed quantum chemical calculations to gain insights into the frontier molecular orbitals, the charge transfer states, the ground and excited state dipole moments of the ionized and non-ionized forms, and determined the ground and excited state acid dissociation constants from UV-vis and fluorescence spectroscopy. After establishing positive solvatochromism and the conditions under which the protonated and deprotonated forms exist in aqueous solution, we explored the application potential of 1 for selective detection of human serum albumin (HSA) in urine samples. In healthy individuals, HSA is excreted with urine below 20 mg/L [20-23]. However, this amount might increase due to adverse health conditions. Amount of HSA in urine between 20-300 mg/L (or excretion 20-200 μg/min) has been linked to the risk of cardiovascular or early stages of kidney disease —this condition in medical term is known as microalbuminuria [20-23]. Amount of HSA more than 300 mg/L in urine is diagnosed as macroalbuminuria—a condition when a kidney disease has progressed to an irreversible kidney failure [20-23]. Moreover, when the amount of HSA in plasma is too low, it can indicate liver ailments, liver failure, or chronic hepatitis [24]. Hence, determining the concentration of HSA in biological samples is of extreme importance for diagnosis of kidney and cardiovascular diseases.
Results and discussion
Our design involves incorporation of a substituted phenol to a cationic benzo[e]indole acceptor by an alkenyl group (Scheme 1). As shown in scheme 1, unsubstituted phenol is weakly acidic with a pKa value of ~10.0. Substitution of an aldehyde group at the para position lowers the pKa of phenol by ~1.6 unit. Further substitution of two methoxy groups at the ortho positions lowers the pKa by an additional ~1.4 unit. Overall, the pKa of the phenolic ─OH becomes 3 unit lower in 4-hydroxy-3,5-dimethoxybenzaldehyde than the parent phenol. A similar observation was made in our previous studies for dimethoxy substituted tricyanofuran (TCF) dyes (scheme 1) [12, 13]. One tricyanofuran group lowers the pKa of the unsubstituted phenol by ~2.3 unit, and incorporation of two methoxy groups at the ortho positions results an overall 3.3 unit lower pKa value than the parent phenol. Based on these higher pKa values of the phenol derivatives and findings from our previous studies, we thought that attaching a strong electron withdrawing group at the para position of syringol could result a near neutral or even lower pKa for the phenolic ─OH group. Moreover, two ortho methoxy groups could push the absorption and emission of charge transfer (CT) dyes to the red region of the electromagnetic spectrum [12, 25].
Scheme 1.
Synthesis of the fluorophore 1 (top). Molecular structures of derivatives of phenol and TCF dyes (bottom) [12].
With this design principle generation of a phenolate charge transfer (CT) compound would be possible by adding and mixing the parent phenol dye in neutral aqueous solution. A phenolate CT dye will have some added advantages over a phenol dye. Phenolate dye would absorb and emit in the longer wavelength than the parent phenol dye [26]. Therefore, interference from absorption and emission of tryptophan in protein can be excluded. Moreover, phenolate would result well-resolved absorption and emission spectra than phenol as excited state proton transfer event often results poorly resolved dual emission for aromatic alcohols depending on the pH of the solution [27, 28]. Moreover, a phenolate CT dye would exhibit better intramolecular charge transfer interaction than its phenol component [26, 29]. Therefore, a low level of background fluorescence can be achieved due to energy loss from the low-lying fluorescence states, which is an important property for a turn-on fluorescent probe.
As a part of our continuous research effort in designing and developing CT fluorophores for detection of chemical analytes and biological molecules we have previously developed dyes with phenol and its derivatives. A strong electron withdrawing tricyanofuran (TCF) group was selected to achieve the desired CT interactions. It was attached with the phenol moieties with alkenyl or dialkenyl groups [12, 13]. Herein, a cationic benzoindolium group was chosen as an electron withdrawing group. Facile synthesis and ease of purification make it an ideal acceptor group for a push-pull CT compound. It can serve as an alternative to TCF group as it can also lower the pKa of syringol to same degree. For supramolecular complexation based studies and detection of biomolecules the cationic charge could provide additional electrostatic interaction and stronger complexation affinity. The nonpolar naphthalene moiety could facilitate the spontaneous complexation event by establishing hydrophobic interactions with the nonpolar residues. Moreover, polarity and steric property of the benzoindolium acceptor can be tuned by simple synthesis reactions.
Following the aforementioned design strategies we developed CT compound 1 in two simple steps. It was synthesized from Knoevenagel condensation of syringaldehyde and 3-ethyl-1,1,2-trimethyl-1H-benzo[e]indol-3-ium iodide in ethanol (Fig. S1-S5). It was purified via recrystallization from ethanol (pure yield ~35%). In the 1H NMR spectra, the signals at 9.92 ppm (singlet, 1H) and 3.89 ppm (singlet, 6H) indicate presence of ─OH and two methoxy (─OCH3) groups in the aromatic ring, respectively. Quaternization of the benzo[e]indole was confirmed from the large chemical shifts (~4.80 ppm) of the methylene hydrogens in the alkyl chain. Moreover, selectivity to all trans isomers in the Knoevenagel condensation reaction was confirmed from the NMR coupling constants (Jvinylic H = 16 Hz) [30].
Solvatochromic behavior of 1 was examined in order to evaluate the effect of microenvironment on the relative polarities of the ground and excited states. Figure 1 depicts steady state absorption and fluorescence spectra of 1 in nine different solvents. In nonpolar solvents such as ethyl acetate (ε = 6.02) and dichloromethane (ε = 8.93) a broad absorption band was observed at around 500 nm and 517 nm, respectively. Upon increasing the polarity, the absorption maxima shifted to the longer wavelengths, up to 600 nm in ethanol (Fig. 1a). As expected, the fluorescence spectra were broad and structureless in all the solvents. A shoulder band was visible at longer wavelengths in the polar protic solvents and in dimethyl sulfoxide (Fig. 1b). Excitation spectra were concurrently recorded in all the solvents (Fig. S6). Fluorescence maxima was selected for collecting the excitation spectra. A clear resemblance to absorption profiles was observed, thus ruling out presence of any secondary fluorescent species in the solution. Interestingly, in DMSO the fluorescence was most likely originating from two separate energy states (Fig. S6). In fluorescence spectra, excitation of 1 in DMSO at the higher energy shoulder (485 nm) band resulted dual emissions, at 575 nm and 625 nm with a ratio of I625 nm/I575 nm = 2.4. Similarly, the excitation spectra showed that the origin of the fluorescence band at 575 nm and 625 nm was predominately from the higher energy state at 485 nm and the low-lying state at 625 nm, respectively.
Figure 1.
(a) Normalized absorption spectra of 1 (10 μM) in various solvents. Each solution contains 1% DMSO (v/v) (b) Normalized fluorescence spectra of 1 (10 μM) in various solvents. Each solution contains 1% DMSO (v/v).
Positive solvatochromism was observed in absorption and fluorescence spectra. Both absorption and fluorescence maxima shifted bathochromically as polarity of the solvents increased. Bathochromic shift around 100 nm in absorption spectra and 35 nm in fluorescence spectra were recorded (Fig. 1 and Table S1). Moreover, from the red shift in absorption and emission spectra it is evident that the ground state of 1 is affected more by the polarity of the solvents than the excited state. Positive solvatochromism indicates a higher excited state dipole moment which is possible due to photoinduced intramolecular charge transfer from the syringol donor to the benz[e]indolium acceptor [31]. Our theoretical calculation with time-dependent density functional theory (TD-DFT) at the RB3LYP/aug-cc-PVDZ level resulted an excited state dipole moment (μe) of 7.61 D in the gas phase, whereas DFT calculation resulted a ground state dipole moment of 2.66 D. This higher dipole moment in the excited state agrees well with the positive solvatochromism.
Experimentally, the greater dipolar character of 1 in the excited state can be tentatively assessed from the plot of emission maxima (λem) vs Reichardt’s solvent ET (30) parameters [32]. A positive slope was observed for all the solvents studied. Moreover, as can be seen in figure 2a, two distinct fits are apparent from the linear regression analysis, one for the aprotic solvents and another for the protic solvents, suggesting both dipolar and hydrogen bonding interactions are prevalent in the excited state of 1. A reasonable linear relationship was observed between emission wavenumber (νe) and Kamlet-Taft hydrogen bond parameter (α) of the protic solvents (Fig. 2b). In both aprotic and protic solvents, the excited emitting state becomes more stable as polarity and hydrogen bond donor ability of the solvents increase. Lippert-Mataga plot of Stokes shift vs solvent orientation polarizability (Δf) also resulted a reasonable linearity (r2 = 0.84) in the aprotic solvents [33, 34]. However, a decrease in Stokes shift was observed as the solvent polarizability increased from ethyl acetate to acetonitrile (Fig. 2c). From the relationship of slope = 2 (Δμ)2/4πε0hcr3 the dipole moment difference (Δμ) between ground and excited state was calculated; the value of Onsager cavity radius (r) was estimated from the 40% of the distance between two farthest atoms in the molecule [35]. The value of Δμ = ∣μe - μg∣ was found to be 7.32 D in aprotic solvents. Using 2.66 D as the ground state dipole moment (μg, estimated from hybrid DFT calculation with B3LYP functional in gas phase; table S2) the excited dipole moment (μe) was calculated to be 9.98 D. Although these values are approximate, the μe compares well with the dipole moment obtained from the TD-DFT calculation and the observed positive solvatochromism.
Figure 2.
(a) Dependence of fluorescence maxima on solvent polarity ET (30) value. (b) Plot of fluorescence wavenumbers (νemmax, cm−1) against the hydrogen bonding parameter (α) of the solvents. (c) Plot of Stokes shifts as a function of orientation polarizability of the solvents (Lippert-Mataga equation).
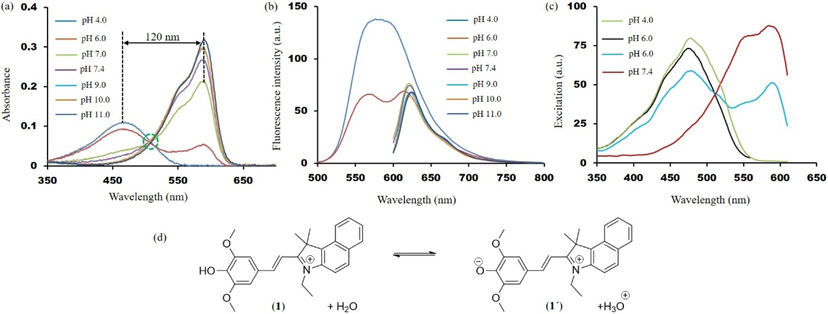
The acid dissociation constant (pKa) of 1 was estimated from the acid-base equilibria in different pH values, ranging from pH 4.0 to pH 11.0. As depicted in figure 3a, 1 produces one broad absorption band at 470 nm. Upon gradually increasing the pH of the solution, a new band arises at 590 nm with concomitant decrease in the peak at 470 nm. A clear isobestic point appears at 511 nm, indicating a transition from the phenol to the deprotonated phenolate form. The pKa was calculated from the ratio of absorbance values (Aphenol/Aphenolate), and it was found to be 6.5 (Fig. S7) [26]. The acid-base equilibrium of 1 has been shown in figure 3d. The deprotonated phenolate form (1΄) predominately exits in neutral pH solution. Therefore, all the studies reported in this article in neutral or higher pH buffer solutions indicate the phenolate form of the dye as the active fluorochrome.
Figure 3.
(a) Absorption spectra of 1 in different pH values (green dotted circle indicates the isobestic point). (b) Fluorescence spectra of 1 in different pH values (excited at λmax in the absorption spectra). (c) Excitation spectra of 1 in different pH values (pH 4.0 green line—excited at 625 nm; pH 6.0 black line— excited at 570 nm; pH 6.0 blue line—excited at 625 nm; pH 7.4—excited at 625 nm). (d) Acid-base equilibrium of 1 in water.
Figure 3b exhibits fluorescence spectra of 1 in both acidic and basic pH values. Upon exciting the protonated phenol form at pH 4.0 (λmax = 470 nm), a broad fluorescence spectrum centered at ~585 nm was observed. In contrast, two emission maxima (λmaxem = 570 nm and λmaxem = 625 nm) of near equal intensity were observed in pH 6.0 solution. Deconvolution (χ2 = 4.76; Adj. R2 = 0.992) of the spectrum confirms that it consists two peaks — at ~570 nm and ~ 625 nm (Fig. S8). Excitation of the deprotonated phenolate form (1΄) at 590 nm resulted a narrow fluorescence band at 625 nm. Based on these two studies, we attribute the origin of the fluorescence signals at 570 nm and 625 nm to the protonated phenol form and deprotonated phenolate form, respectively.
Figure 3c shows the steady state excitation spectra of 1 in both acidic and basic solutions. In slightly basic solution (pH = 7.4), when excited at the fluorescence maxima (λmaxex = 625 nm), 1 exhibited an excitation spectrum resembling to the absorption spectrum in pH 7.4 value. In pH 6.0 solution, however, two bands were observed at ~480 nm and ~590 nm when excited at 625 nm, resembling to the absorption spectrum of protonated phenol and deprotonated phenolate form, respectively. Interestingly, only one signal centered at ~480 nm appeared when 1 was excited at 625 nm in pH 4.0 solution. Similarly, excitation at 570 nm in pH 6.0 solution resulted a spectrum centered at ~480 nm. In addition, 10 nm blue shift was observed in the fluorescence maxima of the deprotonated form when pH value was gradually lowered from 10 to 6 (Fig. 3b). Based on these studies we ascribe the origin of the long wavelength fluorescence (λmax = 625 nm) to the phenolate form [36-38].
The concomitant observation of absorption and emission spectra of protonated phenol and deprotonated phenolate forms allowed us to determine the excited state acid dissociation constant (pKa*) of 1. The pKa* of 1 was estimated to be 3.3 according to the Förster equation pKa* = pKa + 0.002142 (~νD — ~νDH); ~νD is 0.5(~νabsmax - ~νemmax) of the deprotonated form at 0,0 transition and ~νDH is 0.5(~νabsmax - ~νemmax) of the protonated from at 0,0 transition. Therefore, from the steady state fluorescence and absorption spectra and the pKa value estimated from the thermodynamic Förster cycle, it can be concluded that 1 becomes more acidic upon photoexcitation and an intermolecular proton transfer is most likely taking place between the phenolic ─OH and the surrounding water molecules [27, 36-38].
Our theoretical calculations support the observed solvatochromism of 1 and predicts the molecular orbitals that contribute to the low-lying singlet states. As can be seen in figure 4a, the HOMO orbital is polarized toward the syringol ring, while the LUMO is mainly distributed on the nitrogen center and at the connecting ─C=C- moiety of the benzo[e]indolium. From the HOMO-LUMO overlap and transition oscillator strength a fully allowed S0 → S1 transition can be suggested, and the redistribution of electron charge density between HOMO and LUMO most likely results the observed solvatochromicity. Moreover, the calculations predict the S0 → S1 transition (calculated λmax = 512 nm; f =0.6090; CI = 0.664) with high configuration interaction value as the dominant orbital transition in low-lying singlet state, which is in good agreement with the energy differences of absorptions in low dielectric aprotic solvents such as ethyl acetate and dichloromethane (λmax = 502 nm in ethyl acetate; λmax = 517 nm in DCM; Table S1). However, for HOMO-2 to LUMO transition (f = 0.3857; λ = 433 nm) no prominent band was identified in the experimental spectra. In the case of the phenolate form (1΄), the HOMO is distributed along the entire molecule, but the LUMO is mainly concentrated in the benzene ring and on the nitrogen center of the benzo[e]indolium (Fig. 4b]. The S0 → S1 transition (f=1.7272; CI = 0.703) appears to be the dominant transition among all three calculated excitations. The calculated excitation energy (S0 → S1; λmax = 562 nm) correlates well with the experimental absorption maximum (λmax = 590 nm) in water. Moreover, the strong HOMO-LUMO mixing also indicates that the emissive state of 1΄ is of ICT nature.
Figure 4.
Possible transitions calculated from TD-DFT calculations for (a) 1 (in gas phase) and (b) 1΄ (in implicit water).
Fluorescence of the active fluorochrome (1΄) dramatically increased in high viscous liquids such as ethylene glycol and glycerol. Ten-fold fluorescence enhancement was observed in 9:1/glycerol: water (v/v) mixture, indicating significant reduction of non-radiative energy loss in viscous media (Fig. S9) [39]. We then investigated binding of 1΄ with human serum albumin by spectroscopic studies. Upon addition of increased amount of HSA, the λmaxabs of 1΄ bathochromically shifted (10 nm), indicating environment induced conformational change of 1΄in low dielectric environment of the protein matrix (Fig. S10) [40, 41]. Concentration-dependent complexation study of 1΄ with HSA revealed a linear increase (r2 = 0.9952) of fluorescence upon gradual addition of HSA in physiological buffer solution, reaching a saturation with 1.5 equivalents of HSA (Fig. 5a). From the titration study binding affinity (Benesi-Hildebrand equation) was calculated and it was found to be 1.46 × 105 M−1 (ΔG = −7.05 kcal/mol), indicating a tight supramolecular complexation between 1΄ and HSA in aqueous solution (Fig. 5b) [42].
Figure 5.
(a) Fluorescence spectra of 1 in presence of HSA (up to 25 μM of HSA) in phosphate buffer (pH 7.4). (b) Benesi-Hildebrand plot of 1/[I-I0] vs 1/[HSA] (M−1) for binding of 1 with HSA in phosphate buffer (pH 7.4).
Fluorochrome 1΄ formed complexes with both human and bovine serum albumin as evidenced by the same amount fluorescence enhancement in physiological buffer solution (Fig. 6a). This result is expected considering the fact that bovine serum albumin shares ~75% identity in amino acid sequence with human serum albumin [43, 44]. However, it showed negligible complexation with other biomolecules and metabolites such as hemoglobin, trypsin, transferrin, lysozyme, insulin, γ-globulin, α1- GS-glycoprotein, α1- acid-glycoprotein, α1- antitrypsin, α1- antichymotrypsin, and creatinine, as well as ct-DNA (Fig. 6a).
Figure 6.
Fluorescence response of 1΄ (10 μM) in presence of HSA and other biomacromolecules in phosphate buffer (pH 7.4). (b) Fluorescence of 1΄@HSA complex upon addition of different amounts of competitive binders.
In human serum albumin, three major ligand binding domains (domain I, II, and III) exist as identified by numerous studies in the past [43, 45]. Each domain consists of two subdomains (subdomain A and B). Fluorochrome 1΄ preferentially binds to subdomain IIIB at propofol binding site as concluded from the spectroscopic and molecular docking analysis [46]. Titration of 1:1 complex of 1΄@HSA with up to ten equivalents of competitive ligands such as Ibuprofen (site IIIA binder), phenylbutazone (site IIA binder) and salicylic acid (site IB and IIA binder) resulted insignificant fluorescence change, indicating minimal or no ligand displacement (Fig. 6b). However, with salicylic acid 12% decrease of fluorescence was recorded in aqueous buffer (pH 7.4). We tentatively attribute this phenomenon to the binding of 1΄ in the secondary site IB. Additionally, titration of 1΄@HSA complex (1:1) with non-ionic surfactant Triton X-100 resulted significant decrease of fluorescence intensity, indicating gradual release of 1΄ from the binding pocket due to disruption of secondary structure of the protein by the surfactant (Fig. S11) [47].
Inspection of docked conformers of 1΄ with HSA reveals that it predominately binds at site IIIB (average binding energy = −9.2 kcal/mol), and ~20% of the conformers bind at subdomain IB with binding energy ranging from −7.2 kcal/mol to −8.5 kcal/mol. However, the most thermodynamically stable conformer (binding energy −9.5 kcal/mol) was located within the site IIIB surrounded by hydrophobic as well as polar side chains (Fig. 7a). The entrance of the binding pocket is quite polar with a number of polar residues in close proximity. The aromatic ring of 1΄ was sandwiched between Phe 502 and Leu 532, similar to complexation of propofol at site IIIB [48, 49]. The aliphatic portion of Glu-531 and the side chain of His-535 situated approximately 5 Å from the phenyl ring of 1΄, thereby closing off the lower end of the pocket. Residues Phe 507, Phe 509, Ile 513, Arg 521, Lys 524, Lys 525, Phe 551 and Val 555 were involved in hydrophobic interactions with naphthalene and benzene rings of 1΄, thereby supporting our design concept. Ser 579 made H-bonding interaction with the ─OCH3 group of 1΄, thus making the supramolecular association stronger. Therefore, based on the competitive ligand displacement assays and molecular docking analysis we conclude that 1΄ binds in the subdomain IIIB, and the increase of fluorescence is probably resulting from the strong supramolecular association within the low dielectric environment of the binding site. Rotational restrictions, resulting from the complexation within a binding pocket surrounded by several residues in close proximity, is most likely minimizing the non-radiative energy loss from the excited state. Moreover, reduced solute-solvent interactions (H-bond type) in the hydrophobic microenvironment of IIIB could increase the fluorescence of 1΄ in the complexed state.
Figure 7.
(a) Docking conformation of 1΄@HSA and various interactions within site IIIB¸ (b) Linear relationship between fluorescence intensity ([1] = 10 μM) with amount of HSA in synthetic urine samples (pH 7.0).
Concentration-dependent study revealed a linear relationship of binding between 1΄ and HSA (vide supra). To assess the practical applicability of fluorophore 1 as HSA detection probe, we first developed a standard curve in simulated urine samples ([1] = 10 μM; pH 7.0; Fig. 7b). A linear relationship (r2 = 0.9916) was obtained up to 8.16 μM of HSA (540 mg/L). The slope of the curve (m= 2 × 107) was identical to that of the study carried out in physiological pH buffer solutions. Next, two urine samples (sample 1 at pH 7.01 and sample 2 at pH 7.06) were tested with 1. The urine samples were free of any residual HSA as confirmed by the near zero intrinsic fluorescence of HSA when it was excited at 270 nm. Moreover, identical fluorescence intensity of 1 in the urine samples and in the aqueous buffer (pH 7.0) indicated that no HSA was present in the urine samples. Two studies were performed for each sample and the average value is reported here. The first sample was spiked with 3.60 μM of HSA, incubated for five minutes and then fluorescence was recorded with 10 μM of 1. Fitting the fluorescence intensities with the standard curve resulted 4.08 μM of HSA (13.3% error). Similarly, the second sample was spiked with 3.56 μM of HSA, and fitting to the standard curve resulted 3.13 μM of HSA (12.1% error).
The molar concentrations of HSA in the spiked urine samples were within the lower and upper limit of the linear standard graph. The limit of detection (3σ/k; σ is standard deviation from ten blank titrations and k is slope of the titration curve) was found to be 0.6 μM (40 mg/L) [50]. This limit of detection is in the lower end of the microalbuminuria range (30 mg/L to 300 mg/L) [20-23]. Though other compounds have been reported in the literature with greater sensitivity, the detection potential of 1 spans from a modest lower limit to an impressive upper limit (540 mg/L), reaching to the upper end of the macroalbuminuria (>300 mg/L) range [20-23, 51-54]. The fluorophore reported here exhibits a lower LOD value than our previously reported phenol CT dyes, however, it displays a linear relationship with concentrations of HSA up to 540 mg/L, which is highest among all the dyes we have studied thus far [12, 13, 51]. Moreover, the selectivity to HSA appears to improve significantly with two ortho methoxy groups in the phenol ring [12]. A comparison of the current phenol/phenolate fluorophore with relevant fluorophores of phenols and naphthols shows that the excitation and emission wavelengths for 1΄ are in the red region of the electromagnetic spectrum, which is highly desirable considering HSA displays a strong spectral signature in the blue/green region [53]. Additionally, this study shows that other drug binding sites of HSA can be explored for development of HSA detection assay [53, 54].
In this context it is also important to note that pKa of 1 is 6.3, which is in the acidic range of the pH scale. However, pH of human urine could vary from 4 to 8, depending on the health conditions of the individuals. At low pH, compound 1 underestimates (~ 53% less in pH 6.0) the true HSA concentration in the samples. To avoid this a dilute aqueous base such as sodium hydroxide can be used to adjust the pH of the sample to near neutral value. In our study, adjusting the pH of a second urine sample from 6.02 to 7.10 with 125 mM aqueous NaOH stock and fitting the fluorescence intensities to the standard curve at pH 7.0 ([1] = 10 μM in simulated urine) resulted near identical HSA estimation (error 15%).
Performance of this class of fluorophores for a wide range of pH values and the sensitivity for practical applications can be improved by carefully choosing the substituents on the phenyl ring and on the benzoindolium moiety. Lower ground state pKa or greater ΔpKa* could lead to generation of a charge transfer fluorophore with weakened fluorescence in aqueous samples, which can be considered as an ideal system for applications as turn-on sensors, including for highly acidic environments. However, while choosing the substituents for the phenyl ring, electron withdrawing and donating groups must be carefully selected. Strong electron withdrawing groups could decrease the degree of charge transfer between the donor-acceptor pairs. Similarly, electron donating groups could increase the pKa of the phenolic -OH, making the fluorophores impractical as ‘turn-on’ sensors for biological applications. Development of various donor-acceptor derivatives of 1 with the core phenol moiety as donors and benzoindolium as acceptor is underway in our laboratory.
Conclusions
In conclusion, we have developed a syringol-π- benz[e]indolium based fluorogenic dye for probing HSA in aqueous solution. The dye was easily prepared from the Knoevenagel condensation between syringaldehyde and 3-ethyl-1,1,2-trimethyl-1H-benzo[e]indol-3-ium iodide. By employing spectroscopic and theoretical studies we have established that the dye undergoes an intramolecular charge transfer and exhibits positive solvatochromism. In aqueous solution, the aromatic ─OH deprotonates near physiological pH (pKa = 6.3) and produces a zwitterionic phenolate fluorochrome with higher dipole moment (19.3 D) and an impressive molar extinction co-efficient (ε = 118,800 M−1cm−1). The new phenolate fluorochrome shows predictable spectroscopic properties and exhibits fast turn-on response to human and bovine serum albumins with high selectivity. The quantitative turn-on response with HSA has also been observed in spiked urine samples. Molecular docking and drug binding studies reveal that the dye preferentially binds at hydrophobic site IIIB. Restriction of intramolecular rotations within the nonpolar site most likely results fluorescence enhancement and the subsequent turn-on response. Overall, the findings reported in this article suggest that this new fluorogenic dye can be used for quantification of HSA in aqueous biological samples.
Supplementary Material
A syringol derived red light emitting hemicyanine dye as fluorescent probe
pH dependent modulation of intramolecular charge transfer
Selective detection of serum albumins in ‘turn-on’ fashion
Detection of human serum albumin in urine samples
Acknowledgements
This work was supported by the IDeA Networks of Biomedical Research Excellence (INBRE) grant (grant number P20GM 103429 to R.C.). A.K.S. acknowledges the support of the National Science Foundation (CHE-1708635) and the Extreme Science and Engineering Discovery Environment (XSEDE) resource COMET at San Diego Supercomputer Center through allocation TG-CHE180054. R.C. thanks Dr. Anindya Ghosh and Dr. Soumyadip Nemu at UALR for providing the chemical characterization data for the fluorophore. R.C. thanks Dr. Mrigendra Rajput at Arkansas Tech University for assisting with the biological studies.
Footnotes
Supplementary data
Details of the synthesis of the fluorophore, 1H and 13C NMR, molecular docking, DFT and TD-DFT calculations, and additional spectra. This material is available online via www.elsevier.com.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Boudet AM. Phytochem. 2007; 68: 2722–2735. [DOI] [PubMed] [Google Scholar]
- 2.Pereira DM, Valentão P, Pereira JA, Andrade PB. Molecules. 2009; 14(6): 2202–2211. [Google Scholar]
- 3.Ozcan T, Akpinar-Bayizit A, Yilmaz-Ersan L, Delikanli B. IJCEA. 2014; 5: 393–396. [Google Scholar]
- 4.Kumar N, Goel N. Biotechnol. Rep 2019; 24: e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goufo P, Singh RK, Cortez I. Antioxidants. 2020; 9:1–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liptak MD, Gross KC, Seybold PG, Feldgus S, Shields GC. J. Am. Chem. Soc 2002; 124: 6421–6427. [DOI] [PubMed] [Google Scholar]
- 7.Han J, Burgess K. Chem. Rev 2010; 110: 2709–2728. [DOI] [PubMed] [Google Scholar]
- 8.Kurtz I, Balaban RS. Biophys. J 1985; 48: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhujun Z, Seitz WR. Anal. Chim. Acta 1984; 160: 47–55. [Google Scholar]
- 10.Rink TI, Tsien RY, Pozzan T. J. Cell Biol 1982; 95: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whitaker JE, Haugland RP, Prendergast FG. Anal. Biochem 1991; 194: 330–344. [DOI] [PubMed] [Google Scholar]
- 12.Choudhury R, Patel SR, Ghosh A. J. Photochem. Photobiol. A 2019; 376: 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhury R, Quattlebaum B, Conkin C, Patel SR, Mendenhall K. Spectrochim. Acta A Mol. Biomol. Spectrosc 2020; 235: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varadi A, Rutter GA. Endocrinology, 2004; 145: 4540. [DOI] [PubMed] [Google Scholar]
- 15.Martinez GM, Gollahon LS, Shafer K, Omman SK, Busch C, Martinez-Zaguilan R. Proc. SPIE, Int. Soc. Opt. Eng 2001; 4259:144. [Google Scholar]
- 16.Karton-Lifshin N, Albertazzi L, Bendikov M, Baran PS, Shabat D. J. Am. Chem. Soc 2012; 134:50, 20412–20420. [DOI] [PubMed] [Google Scholar]
- 17.Krauss M, Jensen JO, Hameka HF. J. Phys. Chem 1994; 98: 9955–9959. [Google Scholar]
- 18.Kumar SS, Nangia A. Cryst. Growth Des 2014; 14: 1865–1881. [Google Scholar]
- 19.Staudinger C, Borisov SM, Methods Appl. Fluoresc 2015; 042005. [DOI] [PubMed] [Google Scholar]
- 20.Doumas BT, Peters T. Clin. Chim. Acta 1997; 258: 3–20. [DOI] [PubMed] [Google Scholar]
- 21.Arques S, Ambrosi P. J. Card. Failure 2011; 17: 451–458. [DOI] [PubMed] [Google Scholar]
- 22.Hoogenberg K, Sluiter WJ, Dullaart RP. Acta Endocrinol, 1993; 129: 151–157. [DOI] [PubMed] [Google Scholar]
- 23.Amin R, Widmer B, Prevost AT, Schwarze P, Coope J, Edge JL, Marcovecchio A. Neil RN, Dalton DB, Br. Med. J 2008; 336: 697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinlan GJ, Martin GS, Evans TW, Hepatology, 2005; 41: 1211–1219. [DOI] [PubMed] [Google Scholar]
- 25.Marder SR, Cheng LT, Tieman BG, J Chem Soc., Chem. Commun, 1992; 672–674. [Google Scholar]
- 26.Ipuy M, Billon C, Micouin G, Samarut J, Andraud C, Bretonniere Y. Org. Biomol. Chem, 2014; 12: 3641–3648. [DOI] [PubMed] [Google Scholar]
- 27.Agmon N J. Phys. Chem. A 2005; 109: 13–35. [DOI] [PubMed] [Google Scholar]
- 28.Sanborn CD, Chacko JV, Digman M, Ardo S. Chem. 2019; 5(6): 1648–1670 [Google Scholar]
- 29.Zhao GJ, Han KL. Phys. Chem. Chem Phys 2010; 12: 8914–8918. [DOI] [PubMed] [Google Scholar]
- 30.Pretsch PDE; Clerc T; Seibl J; Simon W Spectral Data for Structure Determination of Organic Compounds, Springer-Verlag, Heidelber, 1989. [Google Scholar]
- 31.Jacques P J. Phys. Chem 1986; 90: 5535–5539. [Google Scholar]
- 32.Reichardt C Angew. Chem. Int. Ed 1979; 18: 98–110. [Google Scholar]
- 33.Forster TZ Elektrochem 1950; 54: 42. [Google Scholar]
- 34.Bartok W, Lucchesi PJ, Snider NS. J. Am. Chem. Soc 1962; 84: 1842–1844. [Google Scholar]
- 35.Song SM, Ju D, Li JF, Li DX, Wei YL, Dong C, Peihua L, Shaomin S, Talanta, 2009; 77: 1707–1714. [DOI] [PubMed] [Google Scholar]
- 36.Clay A, Krishnan R, Sibi M, Webster D, Jockusch S, Sivaguru J. J. Photochem. Photobiol. Chem. A 2018; 355: 38–41. [Google Scholar]
- 37.Tolbert L, Solntsev KM. Acc. Chem. Res 2002; 35: 19–27. [DOI] [PubMed] [Google Scholar]
- 38.Simkovitch R, Shomer S, Gepshtein R, Shabat D, Huppert D. J. Phys. Chem. A 2014; 118: 1832–1840. [DOI] [PubMed] [Google Scholar]
- 39.Grabowski ZR, Rotkiewicz K Chem. Rev 2003; 103: 3899–4031. [DOI] [PubMed] [Google Scholar]
- 40.Tatikolov AS, Costa SMB. Biophys. Chem 2004; 107: 33–49. [DOI] [PubMed] [Google Scholar]
- 41.Banerjee M, Pal U, Subudhhi A, Chakrabarti A, Basu S. J. Photochem. Photobiol. B 2012; 108: 23–33. [DOI] [PubMed] [Google Scholar]
- 42.Benesi HA, Hildebrand JH. J. Am. Chem. Soc 1949; 71: 2703–2707. [Google Scholar]
- 43.Petitpas I, Bhattacharya AA, Twine S, East M, Curry S, J. Biol. Chem, 2001; 276: 22804–22809. [DOI] [PubMed] [Google Scholar]
- 44.Akdogan Y, Reichenwallner J, Hinderberger D. PLOS One, 2012; 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Basken NE, Mathias GJ, Green MA. J. Pharm. Sci 2009; 98: 2170–2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trott O, Olson AJ. J. Comput. Chem 2009; 31: 455–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh SK, Kishore N. J. Phys. Chem. B 2006; 110: 9728–9737. [DOI] [PubMed] [Google Scholar]
- 48.Fasano M, Curry S, Terreno E, Galliano M, Gabriella F, Narciso P, Notari S, Ascenz P. IUBMB Life, 2005; 57: 787 – 796. [DOI] [PubMed] [Google Scholar]
- 49.Ghuman J, Zunszain PA, Petitpas I, Bhattacharya AA, Otagiri M, Curry S. J. Mol. Biol 2005; 353: 38–52. [DOI] [PubMed] [Google Scholar]
- 50.Long L, Winefordner JD. Anal. Chem 1983; 55: 712–724. [Google Scholar]
- 51.Choudhury R, Parker HE, Cendejas CM, Mendenhall K. Tet. Lett 2018; 59: 3020–3025. [Google Scholar]
- 52.Samanta S, Halder S, Das G. Anal. Chem 2018; 90: 7561–7568. [DOI] [PubMed] [Google Scholar]
- 53.Xu J-F, Yang Y-S, Jiang A-Q, Zhu H-L Crit. Rev. Anal. Chem 2020; 1–21, 10.1080/10408347.2020.1789835. [DOI] [Google Scholar]
- 54.Ronzetti M, Baljinnyam B, Yasgar A, Simeonov A. 2018; 1005–1014. 10.1080/17460441.2018.1534824. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.