Abstract
Objective
Surgical site infection (SSI) is the second most prevalent hospital-based infection and affects the surgical therapeutic outcomes. However, the factors of SSI are not uniform. The main purpose of this study was to understand the risk factors for the different types of SSI in patients undergoing colorectal surgery (CRS).
Methods
PubMed, EMBASE, and Cochrane Library databases were searched using the relevant search terms. The data extraction was independently performed by two investigators using a standardized format, following the pre-agreed criteria. Meta-analysis for the risk factors of SSI in CRS patients was carried out using Review Manager 5.3 (RevMan 5.3) and Stata 15.1 software. The quality of evidence was evaluated using total sample size, Egger’s P-value, and intergroup heterogeneity, which contained three levels: high-quality (Class I), moderate-quality (Class II/III), and low-quality (Class IV). The publication bias of the included studies was assessed using funnel plots, Begg’s test, and Egger’s test.
Results
Of the 2660 potentially eligible studies, a total of 31 studies (22 retrospective and 9 prospective cohort studies) were included in the final analysis. Eventually, the high-quality evidence confirmed that SSI was correlated with obesity (RR = 1.60, 95% confidence interval (CI): 1.47–1.74), ASA score ≥3 (RR = 1.34, 95% CI: 1.19–1.51), and emergent surgery (RR = 1.36, 95% CI: 1.19–1.55). The moderate-quality evidence showed the correlation of SSI with male sex (RR = 1.30, 95% CI: 1.14–1.49), diabetes mellitus (RR = 1.65, 95% CI: 1.24–2.20), inflammatory bowel disease (RR = 2.12, 95% CI: 1.24–3.61), wound classification >2 (RR = 2.65, 95% CI: 1.52–4.61), surgery duration ≥180 min (RR = 1.88, 95% CI: 1.49–2.36), cigarette smoking (RR = 1.38, 95% CI: 1.14–1.67), open surgery (RR = 1.81, 95% CI: 1.57–2.10), stoma formation (RR = 1.89, 95% CI: 1.28–2.78), and blood transfusion (RR = 2.03, 95% CI:1.34–3.06). Moderate-quality evidence suggested no association with respiratory comorbidity (RR = 2.62, 95% CI:0.84–8.13) and neoplasm (RR = 1.24, 95% CI:0.58–2.26). Meanwhile, the moderate-quality evidence showed that the obesity (RR = 1.28, 95% CI: 1.24–1.32) and blood transfusion (RR = 2.32, 95% CI: 1.26–4.29) were independent risk factors for organ/space SSI (OS-SSI). The high-quality evidence showed that no correlation of OS-SSI with ASA score ≥3 and stoma formation. Furthermore, the moderate-quality evidence showed that no association of OS-SSI with open surgery (RR = 1.37, 95% CI: 0.62–3.04). The high-quality evidence demonstrated that I-SSI was correlated with stoma formation (RR = 2.55, 95% CI: 1.87–3.47). There were some certain publication bias in 2 parameters based on asymmetric graphs, including diabetes mellitus and wound classification >2. The situation was corrected using the trim and fill method.
Conclusions
The understanding of these factors might make it possible to detect and treat the different types of SSI more effectively in the earlier phase and might even improve the patient’s clinical prognosis. Evidence should be continuously followed up and updated, eliminating the potential publication bias. In the future, additional high-level evidence is required to verify these findings.
Introduction
Surgical site infection (SSI), which might be either at the site of incision (superficial incisional SSI (SSSI) or deep incisional SSI (DSSI)) or any organ or space infections (OS-SSI), is a serious national health problem, affecting approximately 500,000 people in the United States each year [1]. It is the second most frequent nosocomial infection, which accounts for 40% of all the healthcare-related infections in patients undergoing surgery [2]. SSI is correlated with staying at a hospital for a long time, high readmission rates, poor quality of life, and huge healthcare costs [3–5]. It is one of the important indices of medical safety evaluation.
In general, the patients undergoing surgery, particularly those who undergo surgery for colorectal diseases, are more likely to develop SSI [6, 7]. Although their etiology is multifactorial, the majority of SSIs are preventable [8]. Multiple factors can affect the development of SSI, including patient-related factors (such as obesity, diabetes mellitus, age, gender, and smoking) and treatment-related factors (such as laparoscopic procedure, prophylactic antibiotics, and stoma creation) [5, 8–10]. Unsatisfactorily, few risk factors are generally accepted and some findings on these factors in medical literature are often contradictory. Accordingly, many perioperative interventions are supported by very limited literature evidence. More importantly, many scholars have realized that the risk factors are different for the different types of SSI. The understanding of these risk factors might better prevent and treat SSI. Besides, SSI reduces the benefits of surgical treatment. Therefore, systematically assessing the common factors of the different types of SSI is a priority. In our previous report [10], some risk factors of SSI in the patients with colorectal cancer (CRC) were presented and the associated concomitant changes and explanatory reasons were also provided. However, some patients, undergoing colorectal surgery (CRS), were excluded, which included patients with benign lesions, diverticular disease, ulcerative colitis, Crohn’s disease, volvulus, bowel obstruction, or other conditions. Therefore, this meta-analysis was conducted to review the potential risk factors of SSIs, incisional SSI (I-SSI), and OS-SSI in the patients undergoing surgery, thereby further assessing the evidence grading and aiming to offer help to clinical treatments.
Method
The study protocol was registered with the PROSPERO database (registration ID: CRD42020178270), which is an international perspective registry for systematic reviews.
Search strategy
Peer-reviewed literature in the PubMed, Cochrane Library and EMBASE databases were thoroughly and systematically searched using a similar strategy for each database from inception to May 2020 (cut-off date 1st May 2020). The exact search strategy included searching the following mesh terms in each database: (colorectal surgery, colectom*, or proctectom*); ([colon, sigmoid, rectum, large bowel, bowel, colonic, rectal, or colorectal] and [excision, resection, surgical, surgically, surgery, or procedure]); (surgical site infection*, surgical wound infection*, or postoperative wound infection); and (risk factor*). The reference list of all the included studies was screened to extend the search. The detailed search strategy for each database is provided in the S1 Table.
Inclusion and exclusion criteria
The study inclusion criteria were defined by PICOS (population, interventions, comparator, outcomes, and study design) categories [11].
Studies that reported the following were included in this study: (a) Patients who underwent CRS; (b) Related risk factor interventions were reported; (c) The main outcome was the incidence of SSI; (d) Studies providing effect estimates (relative risks (RRs) or odds ratios (ORs)) with corresponding 95% confidence interval (CIs). (e) Case-control or cohort studies.
Review articles, conference abstracts, unpublished gray literature, study protocols, letters, animal experiments, and studies with insufficient and overlapping data (when using the same data source and overlapping search period, there was overlapping data, which was avoided by only selecting the most recent or high-quality articles) were excluded from the current study.
Data extraction
Two researchers (ZhaoHui Xu and Hui Qu) independently extracted the details of the included studies, including the name of the first author, publication year, year of the study, study design, study size, country, risk factors, surgical types, definition and classification of SSI, the number of patients and average follow-up time, and quality score of Newcastle–Ottawa Quality Assessment Scale (NOS). The multivariate RRs/ORs with 95% CIs were preferred rather than univariate results. Any disagreement, if found, was resolved based on the assessment of a senior investigator (Xin Chen).
Quality assessment
Quality was assessed by scoring the 3 evaluating indicators of NOS, which included the selection of study groups, inter-comparability of groups, and outcomes, with a maximum score of 9 stars [12]. The score of each included study was also evaluated. The studies having scores of ≥6 stars were considered to be of relatively higher quality; the final results are provided in the S2 Table.
This study was conducted in strict conformity with the Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines [13] and Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [14].
Assessment of the strength of evidence
The strength of evidence was evaluated using the total sample size >1000, Egger’s P-value >0.1, and intergroup inconsistency (I2) <50%. The Class I (high-quality) evidence referred to when the three conditions were met simultaneously. Class II (moderate-quality) and Class III (moderate-quality) evidence were defined as satisfying the two and one conditions of the three conditions, respectively. Class IV (low-quality) evidence was defined as satisfying none of these conditions [15].
Statistical analyses
All the statistical analyses were performed using Review Manager (RevMan) software (version 5.3; The Nordic Cochrane Centre, Copenhagen, Denmark) and Stata software (version 15.1). The pooled RRs and 95% Cis from the studies were analyzed using the DerSimonian–Laird random-effects model [16]. A two-tail P value of 0.05 or below was considered statistically significant.
The inter-group heterogeneity was examined using Cochran’s Q (χ2) test and quantified by the I2 statistic. The heterogeneities were categorized into three groups based on the I2 value (low group <50%, moderate group 50–74%, and high group ≥75%) [17]. Sensitivity analyses were performed to recognize the potential sources of heterogeneities by changing the effect model or removing one study at a time. Publication bias was evaluated using funnel plots, Begg’s test [18], and Egger’s test [19]. The funnel plot asymmetry was further corrected using the trim and fill method.
Results
Study characteristics
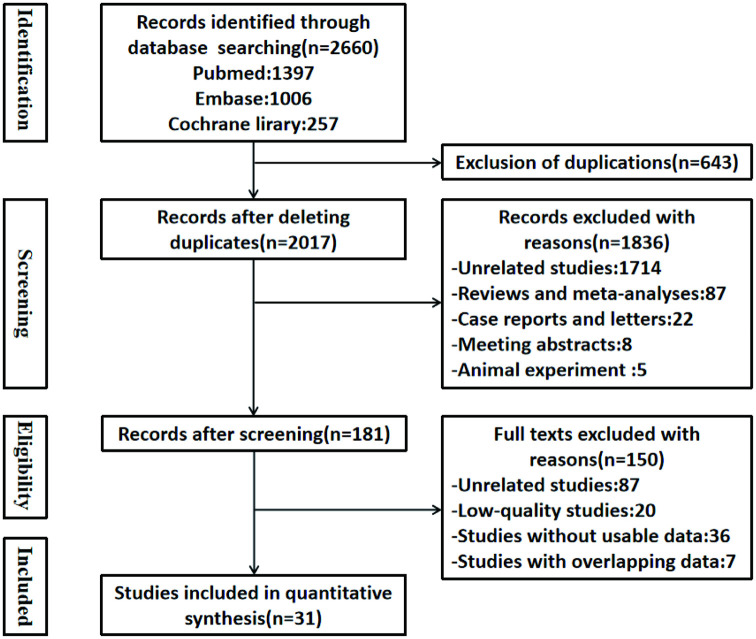
In the initial search, a total of 2660 potentially eligible studies were identified, of which 643 duplicated studies were excluded. A total of 1836 articles, including review studies, case reports, letters, animal studies, or unrelated studies, were excluded, leaving only 181 studies reviewed by the two independent investigators for full text. Thus, 31 studies were finally included. Nine of these studies were prospective cohort studies, while the remaining were retrospective cohort studies. The baseline characteristics of the included articles are presented in Table 1. The flow diagram for the study procedure is presented in Fig 1.
Table 1. General characteristics of the included studies in the meta-analysis.
| Study | nation | Data sources | Recruited period | Procedures | No.of patients | Study type | Risk factors |
|---|---|---|---|---|---|---|---|
| Kwaan201338 | USA | the University of Minnesota | 2008-2009 | CRS | 143 | retrospective cohort study | 1.2.29.30 |
| Kwaan201535 | USA | the ACS NSQIP database | 2005-2012 | CRS | 112,282 | retrospective cohort study | 1.3.4.5.6.7.8.24.25 |
| Bot201321 | France | the Lille University Hospital and a private hospital | June 2004-Dec 2011 | CRC | 740 | retrospective cohort study | 4.7.8.9.10.11.16.19.20.31 |
| Bert201739 | Italy | the SNICh database | Jan 2012-Dec 2012 | CS | 1322 | retrospective cohort study | 1.7.10.12 |
| Poeran201625 | USA | the Premier Perspective database | Jan 2006-Dec 2013 | CS | 90725 | retrospective cohort study | 3.9.12.13.21.22.23.25.26.27 |
| Guzman-Pruneda 201834 | USA | the Ohio State University Wexner Medical Center | Jan 2010-Dec 2016 | CRS | 469 | retrospective cohort study | 2.3.4.7.11.21 |
| Ho201140 | USA | The NewYork-Presbyterian Hospital /Weill Cornell Medical Center | June 2001-July 2008 | CRS | 650 | retrospective cohort study | 1.6.7.10.11 |
| Nakamura200841 | Japan | the Kitasato University Hospital | Jan 2004-Dec 2005 | CRC | 144 | retrospective cohort study | 7 |
| Hennessey201542 | Ireland | 3 institutions | 2007–2009 | CRS | 386 | retrospective cohort study | 7.12.14.25 |
| Uchino201343 | Japan | the Hyogo College of Medicine | Jan 2008-Dec 2011 | CD | 405 | prospective cohort study | 11.25 |
| Tang 200132 | China | the Chang Gung Memorial Hospital | Feb 1995-Dec 1998 | CRS | 2809 | prospective cohort study | 11.13.15.16 |
| Biondo201228 | Spain | the Spanish Rectal Cancer Project | May 2006-May 2009 | RC | 2131 | retrospective cohort study | 7.10.13.15.18.25 |
| Bislenghi201920 | Belgium | the University Hospitals Leuven | Oct 2016-Jan 2017 | CRS | 287 | prospective cohort study | 5.9.14.21.37 |
| Itatsu201344 | Japan | 19 affiliated hospitals | Nov 2009-Feb 2011 | CRC | 1980 | prospective cohort study | 1.11.28.32.33 |
| Hibbert201523 | Saudi Arab | the King Faisal Specialist Hospital & Research Centre | not involved | CRS | 296 | prospective cohort study | 9 |
| Hubner201129 | Switzerland | 9 secondary and tertiary care public Swiss hospitals, | Mar 1998-Dec 2008 | CS | 2393 | prospective cohort study | 7.12.13.14 |
| Wick201127 | USA | 8 different BCBS insurance plans | Jan 2000-Dec 2008 | CRS | 7020 | retrospective cohort study | 7.9.13 |
| Blumetti200745 | USA | a single tertiary care institution | Jan 2002-Dec 2005 | CRS | 428 | retrospective cohort study | 11.12 |
| Tserenpuntsag201426 | USA | the 174 NYS hospitals | 2009–2010 | CS | 2656 | retrospective cohort study | 9.10.13.15.27.38 |
| Imai200830 | Japan | the Keio University Hospital | Aug 1997-Dec 2005 | CC | 801 | retrospective cohort study | 4.7.10.13. |
| Colas-Ruiz 201846 | Spain | the HUFA in Madrid | Jan 2013-Dec 2016 | RS | 154 | prospective cohort study | 15.16.21 |
| Park 201536 | Korea | the Kyung Hee University Hospital, Gangdong | Jan 2010-May 2014 | CRC | 327 | retrospective cohort study | 4.5.7.10.14.15.19.35 |
| Silvestri201737 | Italy | the University Hospital of Trieste | June 2010-July 2014 | CRS | 687 | retrospective cohort study | 1.4.12 |
| Cima201722 | USA | the Mayo Clinic Hospital | Apr 2006-June 2014 | CRS | 2376 | retrospective cohort study | 3.4.7.9.10.17.22 |
| Watanabe201547 | Japan | the Nippon Medical School Musashikosugi Hospital | July 2005-May 2010 | CRS | 538 | retrospective cohort study | 1.7.10 |
| Mason201631 | UK | the Colchester University Hospital | Sep 2012-July 2014 | CRS | 246 | retrospective cohort study | 3.4.13.18.21.39 |
| Mik201624 | Poland | the Medical University of Lodz and the Centre for Treatment of Bowel Diseases Hospital in Brzeziny | Jan 2008-Dec 2015 | CRC | 2240 | retrospective cohort study | 6.9.12.14.25 |
| Olmez201948 | Turkey | the Kosuyolu Resarch and Education Hospital | Jan 2013-July 2019 | CRC | 209 | retrospective cohort study | 7.14 |
| Uchino200949 | Japan | the Hyogo College of Medicine | Mar 2006-Dec 2007 | CRS | 562 | prospective cohort study | 10.11.22.25.36 |
| Ghuman201533 | Canada | The St. Paul’s Hospital | Dec 2012-July 2014 | CS | 205 | retrospective cohort study | 3.4 |
| Poon 200950 | China | the Queen Mary Hospital, | Jan2002-Dec 2006 | CRC | 1011 | prospective cohort study | 7.15.34 |
Remark: 1. Wound classification≥3; 2.Oral antibiotics; 3.Cigarette smoking; 4.Diabetes mellitus; 5.Pulmonary comorbidities; 6.Radiation therapy; 7.Open vs minimally invasive surgery (MIS); 8.Advanced tumors; 9.Obesity; 10.ASA grade ≥3; 11.Ostomy creation; 12.Emergent surgery; 13.Male gender; 14.Operation time (≥180 min); 15. Blood transfusion; 16.Intra-abdominal drain; 17.Steroid use; 18.Converted to open procedure; 19.Hemoglobin level<10g/dL; 20.Blood loss≥500 mL; 21.Neoplasm; 22.Inflammatory Bowel Disease; 23.Diverticular Disease; 24.Cardiac comorbidity; 25.Resection procedure (Abdominoperineal resection, pelvic exenteration, extended resection, etc); 26.Hospital location; 27.Hospital Teaching Status; 28.Chronic liver disease; 29.Abdominal wall thickness (AW2); 30.History of soft tissue infection; 31.Malnutrition; 32. Previous laparotomy; 33.Wound length; 34. Anastomotic leakage; 35. Estimated blood loss (≥100 mL); 36. Preoperative hospital stay>6 days; 37. Preoperative stoma; 38.Bed size>500 vs ≤500; 39. Use of CO2 conditioner.
Abbreviations: BCBS: Blue Cross and Blue Shield; CC: colon cancer; CD: Crohn’s disease; CRC: colorectal cancer; CRS: colorectal surgery; CS: colon surgery; HUFA: Hospital Universitario Fundación Alcorcón; NYS: New York State; RC: rectal cancer; RS: rectal surgery; SNICh: the National System of Surveillance of Surgical Site Infections.
Fig 1. Flow chart of literature search and data extraction.
Risk factors of SSI
A total of 39 risk factors were found from the selected 31 articles. Among them, 25 factors could not be quantitatively analyzed in the study without adequate data sources and were excluded. Finally, 14 risk factors, reported in more than 2 studies, were included in this study, on which the meta-analysis was performed.
Unmodifiable factors
Male sex
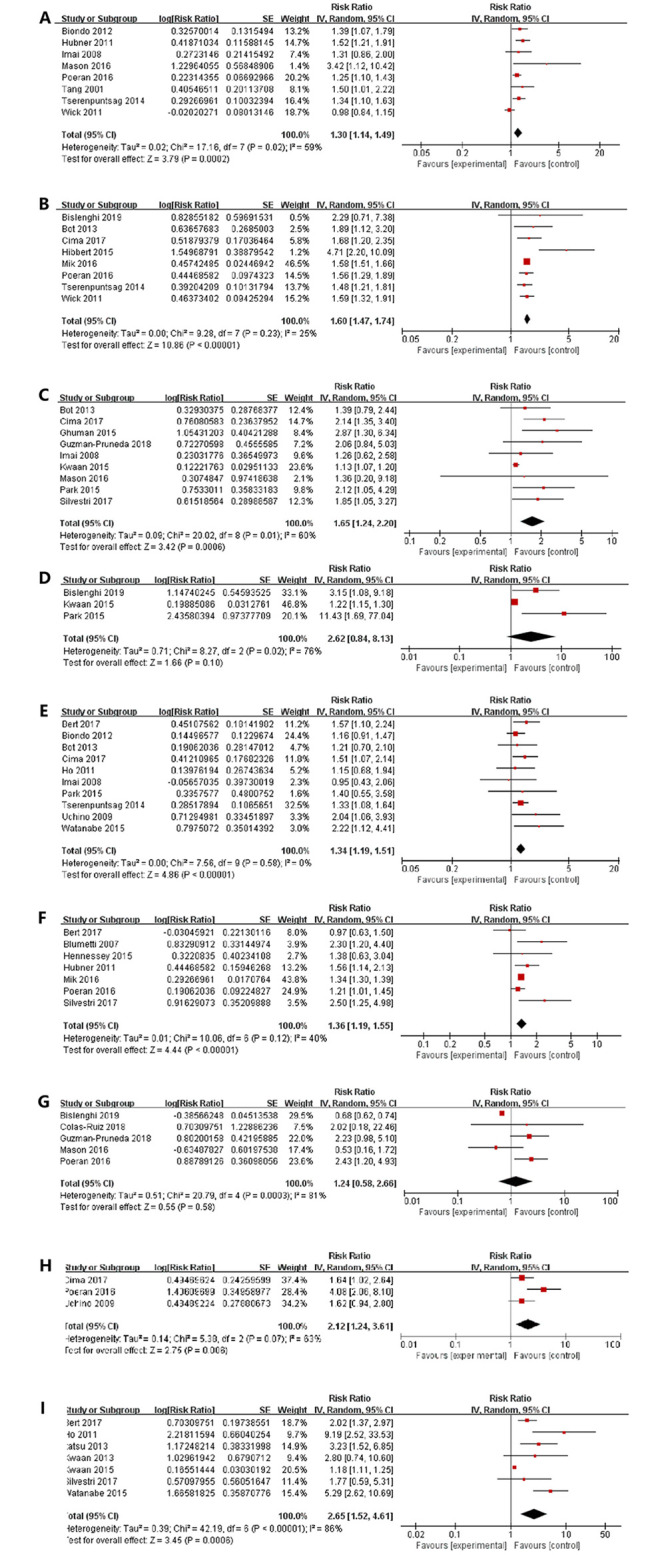
Eight studies [5, 9, 20–25] were identified in the present study, showing that the male sex was a statistically significant risk factor for SSI (RR = 1.30, 95% CI: 1.14–1.49, I2 = 59%) (Fig 2A).
Fig 2. The forest plots showed that the correlations between the risk of SSIs with (A) male sex, (B) obesity, (C) diabetes mellitus, (D) respiratory disease, (E) ASA classification, (F) emergent status, (G) neoplasm, (H) inflammatory bowel disease, (I) wound classification>2.
Obesity
The World Health Organization (WHO) definition for obesity was used, which defines obesity as body mass index (BMI) greater than 30 kg/m2 [26]. A meta-analysis of the eight studies [21, 23, 24, 27–31], which reported obesity, showed that the obese patients were positively correlated with the rate of SSI (RR = 1.60, 95% CI: 1.47–1.74, I2 = 25%) (Fig 2B).
Diabetes mellitus
Nine studies [20, 25, 27, 30, 32–36], including 118,133 patients, showed that there was a positive linear proportional correlation between the diabetes mellitus and rate of SSIs in the patients undergoing CRS (RR = 1.65, 95% CI: 1.24–2.20, I2 = 60%) (Fig 2C).
Respiratory comorbidity
Three studies [31, 33, 34] reported the connection between respiratory comorbidity and SSI. The synthetic results of these studies showed that there was a significant correlation between them in the patients undergoing CRS (RR = 2.62, 95% CI: 0.84–8.13, I2 = 76%) (Fig 2D).
American Society of Anesthesiologists (ASA) classification
Among ten studies [20, 22, 23, 27, 30, 34, 37–40], the combined results of meta-analysis revealed that the ASA score of higher than or equal to 3 showed an increased risk of developing SSI (RR = 1.34, 95% CI: 1.19–1.51, I2 = 0%) (Fig 2E).
Emergent surgery
The results of seven studies [5, 24, 29, 35, 40–42] showed that the emergent status could increase the risk of SSI by 36% (RR = 1.36, 95% CI: 1.19–1.55, I2 = 40%) (Fig 2F).
Neoplasm
The meta-analysis of five studies [24, 25, 31, 36, 43] found that there was not a significant correlation between neoplasm and SSI (RR = 1.24, 95% CI: 0.58–2.26, I2 = 81%) (Fig 2G).
Inflammatory Bowel Disease(IBD)
The meta-analysis of three studies [24, 30, 37], reporting IBD, indicated that IBD could increase the SSI rate (RR = 2.12, 95% CI: 1.24–3.61, I2 = 63%) (Fig 2H).
Wound classification>2
The association between wound classification and SSI was investigated in seven studies [33, 35, 38–40, 44, 45]. The pooled results indicated that the wound classification >2 might increase the occurrence of SSI (RR = 2.65, 95% CI: 1.52–4.61, I2 = 86%) (Fig 2I).
Modifiable factors
Operative time (≥180 min)
Six studies [5, 29, 31, 34, 42, 46] focused on the effects of surgery duration. There was an 88% increase in the risk of SSI for the surgeries having the duration of longer than 180 min (RR = 1.88, 95% CI: 1.49–2.36, I2 = 58%) (Fig 3A).
Fig 3. The forest plots showed that the correlations between the risk of SSIs with (A) operative time (≥180min), (B) cigarette smoking, (C) open surgery, (D) stoma formation, (E) blood transfusion.
Cigarette smoking
The pooled data from six studies [24, 25, 30, 32, 33, 36] showed that the smoking patients had a 1.22-fold increased risk of developing SSI as compared to the non-smoking patients (RR = 1.38, 95% CI: 1.14–1.67, I2 = 64%) (Fig 3B).
Open surgery
A meta-analysis of sixteen studies [5, 20–22, 27, 30, 33, 34, 36, 38–40, 42, 46–48] showed that the patients who accepted laparotomy had a 1.81-fold increased risk of developing SSI as compared to the patients with laparoscopic surgery (RR = 1.81, 95% CI: 1.57–2.10, I2 = 69%) (Fig 3C).
Stoma formation
The pooled analysis of eight studies [9, 27, 36–38, 41, 45, 49] suggested that the risk of SSI in the patients having in-hospital stoma formation was 1.89 times higher than those who did not have one (RR = 1.89, 95% CI: 1.28–2.78, I2 = 69%) (Fig 3D).
Blood transfusion
The pooled results of five studies [22, 23, 34, 43, 48] indicated that the perioperative blood transfusion increased the risk of developing SSIs by 103% (RR = 2.03, 95% CI:1.34–3.06, I2 = 74%) (Fig 3E).
Risk factors of I-SSI and OS-SSI
The risk factors for OS-SSI were also identified (Table 2). These risk factors included: obesity (RR = 1.63, 95% CI: 1.48–1.80, P <0.00001); ASA score ≥3 (RR = 1.14, 95% CI: 0.90–1.46, P = 0.28); open surgery (RR = 1.37, 95% CI: 0.62–3.04, P = 0.44); stoma creation (RR = 1.19, 95% CI: 0.95–1.49, P = 0.12); and blood transfusion (RR = 2.32, 95% CI: 1.26–4.29, P = 0.007).
Table 2. Risk factors of SSIs, I-SSI, and O-SSI in patients undergoing CRS.
| Significant factors | No. of studies | No. of patients | I2(%) | PEgger’S | PBegg-Mazumdar’S | RR | Evidence grading |
|---|---|---|---|---|---|---|---|
| SSIs | |||||||
| Male sex | 8 | 109727 | 59 | 0.106 | 0.174 | 1.30(1.14–1.49) | Class II (moderate-quality) |
| Obesity | 8 | 106,340 | 25 | 0.161 | 0.063 | 1.60(1.47–1.74) | Class I (high-quality) |
| Diabetes mellitus | 9 | 118,133 | 60 | 0.006 | 0.971 | 1.65(1.24–2.20) | Class III (moderate-quality) |
| ASA score≧3 | 10 | 13,049 | 0 | 0.415 | 0.474 | 1.34(1.19–1.51) | Class I (high-quality) |
| Emergent surgery | 7 | 98,181 | 40 | 0.55 | 0.548 | 1.36(1.19–1.55) | Class I (high-quality) |
| IBD | 3 | 93,663 | 63 | 0.262 | 0.296 | 2.12(1.24–3.61) | Class II (moderate-quality) |
| Wound classification | 7 | 117,602 | 86 | 0.007 | 1.000 | 2.65(1.52–4.61) | Class III (moderate-quality) |
| Operative Time(≧180min) | 6 | 5,842 | 58 | 0.195 | 0.133 | 1.88(1.49–2.36) | Class II (moderate-quality) |
| Cigarette smoking | 6 | 206,303 | 64 | 0.129 | 0.260 | 1.38(1.14–1.67) | Class II (moderate-quality) |
| Open surgery | 16 | 133,745 | 69 | 0.707 | 0.137 | 1.81(1.57–2.10) | Class II (moderate-quality) |
| Stoma creation | 8 | 8,043 | 69 | 0.424 | 0.536 | 1.89(1.28–2.78) | Class II (moderate-quality) |
| Blood transfusion | 5 | 6,279 | 74 | 0.567 | 0.806 | 2.03(1.34–3.06) | Class II (moderate-quality) |
| Respiratory comorbidity | 3 | 112,896 | 76 | 0.079 | 0.296 | 2.62(0.84–8.13) | Class III (moderate-quality) |
| Neoplasm | 5 | 91,881 | 81 | 0.466 | 0.462 | 1.24(0.58–2.26) | Class II (moderate-quality) |
| I-SSI | |||||||
| Stoma | 5 | 5,933 | 0 | 0.197 | 0.221 | 2.55(1.87–3.47) | Class I (high-quality) |
| O-SSI | |||||||
| Obesity | 3 | 7,272 | 17 | 0.002 | 0.296 | 1.63(1.48–1.80) | Class II (moderate-quality) |
| Blood transfusion | 3 | 5,215 | 86 | 0.900 | 1.000 | 2.32(1.26–4.29) | Class II (moderate-quality) |
| ASA score≧3 | 3 | 5,157 | 0 | 0.821 | 1.000 | 1.14(0.90–1.46) | Class I (high-quality) |
| Open surgery | 3 | 5,157 | 84 | 0.676 | 1.000 | 1.37(0.62–3.04) | Class II (moderate-quality) |
| Stoma creation | 3 | 3,452 | 15 | 0.292 | 0.296 | 1.19(0.95–1.49) | Class I (high-quality) |
The risk factors for developing I-SSI were also explored. It was found that the risk factors of each study were not completely consistent (S3 Table). The classifications were not completely consistent even for the same indicators but still, the stoma creation was found to be a statistically significant risk factor for developing I-SSI (Table 2) (RR = 2.55, 95% CI: 1.87–3.47, P <0.05).
Sensitivity analyses
Further sensitivity analyses were conducted due to varying degrees of heterogeneities in the study. The merging direction of any risk factor was not significantly influenced using the fixed-effect models or random-effects (S4 Table). The pooled RR for the remaining studies remained unchanged in the above analysis after sequentially omitting any single study (S5 Table). Only the removal of Kwaan’s [33] study from the analysis of respiratory comorbidity changed the overall conclusion (RR ranged from 2.62 (95% CI: 0.84–8.13) to 4.66 (95% CI: 1.46–14.89). Consequently, the results of this study regarding all the other risk factors might be stable.
Assessment of publication bias
The funnel plot was used to qualitatively assess the publication bias (S1 Fig). There was a publication bias in some analyses due to asymmetric graphs.
The P-value, in the analysis of diabetes mellitus, was less than 0.05 based on the results of Egger’s test, indicating a certain publication bias (Table 2). Accordingly, the funnel plot asymmetry was corrected using the trim and fill method. The five squared dots represented the effective quantities condition of included studies in the future and the corrected estimates of the intervention effects of 14 studies were 1.163 (95% CI = 0.901–1.501) (S2A Fig).
In the analysis of wound classification >2, the P-value was less than 0.05 based on the results of Egger’s test, indicating a certain publication bias (Table 2). Accordingly, the funnel plot asymmetry was corrected using the trim and fill method. The four squared dots represented the effective quantities condition of included studies in the future and the corrected estimated of intervention effects of 11studies was 1.310 (95% CI = 0.790–2.173) (S2B Fig). Taken together, the relevant evidence should be continuously followed up and updated, eliminating the potential publication bias.
Discussion
The factors in this study could be divided into two categories: modifiable and unmodifiable factors. The clinicians should monitor SSI earlier to achieve early prevention, intervention, and even effective treatment by targeting the unmodifiable factors. For the modifiable factors, the indicators can be adjusted throughout the perioperative period to further reduce the occurrence of SSI.
The commonly investigated unmodifiable risk factors, at least in the short term, including gender, obesity, ASA score, and primary disease diagnosis, can affect the incidence of SSI. Male sex is prone to develop SSI (RR = 1.30) due to abdominal visceral obesity. This might lead to a more complicated procedure with a longer surgical duration and incision, thereby increasing the SSI rate [50]. Obesity is commonly perceived to be associated with adverse outcomes, such as SSI [50]. It is worth noting that an appropriate definition of reasonable obesity might take into account the differences in visceral fats and ethnicity [50]. As reported, the BMI and SSI might be linearly correlated [51, 52]. Interestingly, the BMI <20 kg/m2 is also an independent risk factor for SSI, reflecting the patient’s malnutrition [31].
For unclear reasons, in this study, IBD but not cancer was strongly correlated with SSI. Increasing studies [51, 53] have found that the types of SSI were correlated with underlying disease diagnosis. For instance, the patients with diverticular developed more SSSI. Strikingly, patients with IBD had more DSSI and OS-SSI. The intrinsic mechanism for the increased correlation of disease diagnosis with SSI has not been emphasized yet in the medical literature. However, the SSI surveillance of CRS should take the surgical site and disease classification into account to more effectively identify the risk factors and reduce the occurrence of SSI. Meanwhile, more attention should be paid to the patients having one or more of the above risk factors in the postoperative follow-up period.
In this study, some modifiable factors also merit attention. The laparoscopic approach was correlated with the reduced occurrence of SSI. This has been already proven in previous studies [7, 54]. Notably, in this study, laparoscopic surgery could reduce the overall SSI rate but not OS-SSI. Unsurprisingly, this study showed that the long duration of surgery (≥180 min) was an independent predictor of SSI (RR = 1.88), which was consistent with a previous studies [31]. Moreover, the likelihood of SSI can be increased by increasing the duration of surgery [55]. The long-duration [56] is usually a reliable symbol of the complexity of the surgical procedure, with possible accidental local tissue injuries. A previous study [57] showed that the in-hospital stoma formation was a risk factor for SSSI and DSSI but that study did not investigate OS-SSI. Similarly, this study found an 89% increase in the risk of SSI in patients having in-hospital stoma formation. The colostomy closure could lead to SSI in the patients having CRS [58, 59]. Perioperative blood transfusion is related to immunomodulation that could explain the increase in SSI rate [43]. Therefore, it is advised for the clinicians to focus on their surgical skills, shorten surgery duration, and reduce intraoperative blood loss and perioperative blood transfusion. In the meantime, the clinicians should suitably control the indicators of stoma formation and avoid unnecessary ones.
Cigarette smoking can delay wound healing, even for a minor and clean wound, thereby increasing the risk of SSI [8, 33, 36, 60]. This study found that the smokers had a 1.38-fold increased risk of developing SSI in comparison with the nonsmokers, which was consistent with the NNIS guidelines [58]. Smoking cessation instead of decreasing the level of smoking should be a routine as a part of perioperative management but there is often a time-limitation. Four weeks of abstinence from smoking before surgery might be appropriate [60]. A standard time to achieve smoking cessation as a part of perioperative management needs to be evaluated in more prospective studies.
The existence of underlying basic diseases can easily lead to the occurrence of SSI. Numerous studies [6, 61, 62] have confirmed that postoperative hyperglycemia is an independent risk factor for SSI and is also independent of diabetes. The uses of perioperative glycemic controls vary by country in the patients undergoing surgery. Many patients might adjust according to their actual situation. Further prospective studies are needed to verify the ideal perioperative glycemic regimens and optimal hemoglobin A1C target levels. Respiratory diseases are found to be associated with poor postoperative outcomes after CRS [63] but its correlation with SSI was not found in this study.
It is not appropriate to perform only preoperative mechanical bowel preparation in the patients undergoing CRS. Many antibiotic regimens have been studied to prevent SSI in patients undergoing CRS but there is no consensus on which antibiotic is the best [38]. Mechanical and oral antibiotic bowel preparation is widely used to reduce the risk of SSI after CRS [1, 64–66], which is well-accepted among many clinicians. Microorganisms in the intestinal lumen during surgery are still the potential infection sources of the surgical area; a joint plan is essential. On contrary, this view is suspected by more than 50% of American clinicians [67]. There are even calls for a reconsideration of this recommendation [68]. It should also be noted that the type of bowel preparation regimens cannot replace intravenous prophylactic antibiotics preoperatively. Many guidelines [69, 70] usually state that it should be administered within 60 min of an incision. The intra-operative re-dosing depends on the half-life of the drug and can be extended up to postoperative 24 h but this recommendation has not been tested rigorously. Owing to the lack of data, this aspect was not analyzed in this study.
There were different degrees of heterogeneities among the included studies, which were due to the differences in various clinical factors and parameters. First, the specific surgical procedures and the surgeon’s surgical skills in each study were not completely consistent. Second, there were differences in cultural backgrounds. There were more or fewer differences in age, sex, education level, and national region among the patients in each trial. Thirdly, the methodological heterogeneity was caused by different studies. Nevertheless, this reflected a real-life situation and the results were trustworthy.
This study will be useful in future studies regarding SSI. This study aimed to provide data to solidify some risk factors but there are still some shortcomings. First, an important factor in preventing SSI is the surgeon’s competence and skills [71], which is a variable factor that is difficult to quantify. The surgeon’s experience could not be quantified in this study. Secondly, there might be inherent bias due to the nature of retrospective or prospective cohort studies. Therefore, more studies and randomized controlled trials are still needed. Thirdly, the information of all the risk factors for I-SSI and OS-SSI could not be integrated due to the non-identical factor profiles of different studies. These differences showed that the two subtypes might have distinct pathogenesis and risk factors.
Conclusions
The study showed that 12 factors (male sex, diabetes mellitus, obesity, ASA score ≥3, cigarette smoking, wound classification >2, IBD, open surgery, stoma formation, emergent surgery, operative time ≥180 min, and perioperative blood transfusion) were the significant risk factors for SSI. Moreover, two factors (obesity and blood transfusion) and one factor (stoma formation) were the significant risk factors for OS-SSI and I-SSI, respectively. A better understanding of these issues can lead to carrying out the precise intervention. There were some certain publication bias in 2 parameters based on asymmetric graphs, including diabetes mellitus and wound classification >2. Evidence should be continuously followed up and updated, eliminating the potential publication bias. In the future, additional high-level studies (such as well-designed randomized controlled trials or high-strength evidence according to the different grading systems) are needed to verify these results.
Supporting information
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(A: Diabetes mellitus; B: wound classification>2).
(DOC)
(PDF)
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infection control and hospital epidemiology. 1999;20(4):250–278. doi: 10.1086/501620 [DOI] [PubMed] [Google Scholar]
- 2.Weiss CA 3rd, Statz CL, Dahms RA, Remucal MJ, Dunn DL, Beilman GJ. Six years of surgical wound infection surveillance at a tertiary care center: review of the microbiologic and epidemiological aspects of 20,007 wounds. Archives of surgery (Chicago, Ill: 1960). 1999;134(10):1041–1048. doi: 10.1001/archsurg.134.10.1041 [DOI] [PubMed] [Google Scholar]
- 3.Kirkland KB, Briggs JP, Trivette SL, Wilkinson WE, Sexton DJ. The impact of surgical-site infections in the 1990s: attributable mortality, excess length of hospitalization, and extra costs. Infection control and hospital epidemiology. 1999;20(11):725–730. doi: 10.1086/501572 [DOI] [PubMed] [Google Scholar]
- 4.Anthony T, Long J, Hynan LS, S GA Jr, Nwariaku F, Huth J, et al. Surgical complications exert a lasting effect on disease-specific health-related quality of life for patients with colorectal cancer. Surgery. 2003;134(2):119–125. doi: 10.1067/msy.2003.212 [DOI] [PubMed] [Google Scholar]
- 5.Hubner M, Diana M, Zanetti G, Eisenring MC, Demartines N, Troillet N. Surgical site infections in colon surgery: the patient, the procedure, the hospital, and the surgeon. Archives of surgery (Chicago, Ill: 1960). 2011;146(11):1240–1245. doi: 10.1001/archsurg.2011.176 [DOI] [PubMed] [Google Scholar]
- 6.Ata A, Valerian BT, Lee EC, Bestle SL, Elmendorf SL, Stain SC. The effect of diabetes mellitus on surgical site infections after colorectal and noncolorectal general surgical operations. The American surgeon. 2010;76(7):697–702. [PubMed] [Google Scholar]
- 7.Kiran RP, El-Gazzaz GH, Vogel JD, Remzi FH. Laparoscopic approach significantly reduces surgical site infections after colorectal surgery: data from national surgical quality improvement program. Journal of the American College of Surgeons. 2010;211(2):232–238. doi: 10.1016/j.jamcollsurg.2010.03.028 [DOI] [PubMed] [Google Scholar]
- 8.Nasser H, Ivanics T, Leonard-Murali S, Stefanou A. Risk Factors for Surgical Site Infection After Laparoscopic Colectomy: An NSQIP Database Analysis. Journal of Surgical Research. 2020;249:25–33. doi: 10.1016/j.jss.2019.12.021 [DOI] [PubMed] [Google Scholar]
- 9.Tang R, Chen HH, Wang YL,Changchien CR, Chen JS, Hsu KC, et al. Risk factors for surgical site infection after elective resection of the colon and rectum: A single-center prospective study of 2,809 consecutive patients. Annals of surgery. 2001;234(2):181–189. doi: 10.1097/00000658-200108000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu Z, Qu H, Kanani G, Guo Z, Ren Y, Chen X. Update on risk factors of surgical site infection in colorectal cancer: a systematic review and meta-analysis. Int J Colorectal Dis. 2020;35(12):2147–2156. doi: 10.1007/s00384-020-03706-8 [DOI] [PubMed] [Google Scholar]
- 11.Hang C, Clymer JW, Brian PHC, Behnam S, Ferko NC, Cameron CG, et al. Prolonged operative duration is associated with complications: a systematic review and meta-analysis. Journal of Surgical Research. 2018;229:134–144. doi: 10.1016/j.jss.2018.03.022 [DOI] [PubMed] [Google Scholar]
- 12.Andreas Stang. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z [DOI] [PubMed] [Google Scholar]
- 13.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA: The Journal of the American Medical Association. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009; 18(3):e123. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mei ZB, Wang QM, Zhang Y, Liu P, Ge MJ, Du PX, et al. Risk Factors for Recurrence after Anal Fistula Surgery: A Meta-analysis. Int J Surg.2019;69:153–164. doi: 10.1016/j.ijsu.2019.08.003 [DOI] [PubMed] [Google Scholar]
- 16.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency In Meta-Analyses. BMJ: British Medical Journal. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101. [PubMed] [Google Scholar]
- 19.Egger M, Smith GD, Schneider M, Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ: British Medical Journal. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imai E, Ueda M, Kanao K, Kubota T, Hasegawa H, Omae K, et al. Surgical site infection risk factors identified by multivariate analysis for patient undergoing laparoscopic, open colon, and gastric surgery. American journal of infection control. 2008;36(10):727–731. doi: 10.1016/j.ajic.2007.12.011 [DOI] [PubMed] [Google Scholar]
- 21.Wick EC, Hirose K, Shore AD, Clark JM, Gearhart SL, Efron J, et al. Surgical site infections and cost in obese patients undergoing colorectal surgery. Archives of Surgery. 2011;146(9):1068–1072. doi: 10.1001/archsurg.2011.117 [DOI] [PubMed] [Google Scholar]
- 22.Biondo S, Kreisler E, Fraccalvieri D, Basany EE, Codina-Cazador A, Ortiz H. Risk factors for surgical site infection after elective resection for rectal cancer. A multivariate analysis on 2131 patients. Colorectal Disease. 2012;14(3):e95–e102. doi: 10.1111/j.1463-1318.2011.02798.x [DOI] [PubMed] [Google Scholar]
- 23.Tserenpuntsag B, Haley V, Van Antwerpen C, Doughty D, Gase KA, Hazamy PA, et al. Surgical site infection risk factors identified for patients undergoing colon procedures, New York State 2009–2010. Infection control and hospital epidemiology. 2014;35(8):1006–1012. doi: 10.1086/677156 [DOI] [PubMed] [Google Scholar]
- 24.Poeran J, Wasserman I, Zubizarreta N, Mazumdar M. Characteristics of Antibiotic Prophylaxis and Risk of Surgical Site Infections in Open Colectomies. Diseases of the colon and rectum. 2016;59(8):733–742. doi: 10.1097/DCR.0000000000000633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mason SE, Kinross JM, Hendricks J, Arulampalam TH. Postoperative hypothermia and surgical site infection following peritoneal insufflation with warm, humidified carbon dioxide during laparoscopic colorectal surgery: a cohort study with cost-effectiveness analysis. Surgical endoscopy. 2017;31(4):1923–1929. doi: 10.1007/s00464-016-5195-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organization technical report series. 2000;894(1):18–30. [PubMed] [Google Scholar]
- 27.Bot J, Piessen G, Robb WB, Roger V, Mariette C. Advanced tumor stage is an independent risk factor of postoperative infectious complications after colorectal surgery: Arguments from a case-matched series. Diseases of the colon and rectum. 2013;56(5):568–576. doi: 10.1097/DCR.0b013e318282e790 [DOI] [PubMed] [Google Scholar]
- 28.Hibbert D, Abduljabbar AS, Alhomoud SJ, Ashari LH, Alsanea N. Risk factors for abdominal incision infection after colorectal surgery in a Saudi Arabian population: The method of surveillance matters. Surgical infections. 2015;16(3):254–262. doi: 10.1089/sur.2013.208 [DOI] [PubMed] [Google Scholar]
- 29.Mik M, Berut M, Trzcinski R, Dziki L, Buczynski J, Dziki A. Preoperative oral antibiotics reduce infections after colorectal cancer surgery. Langenbeck’s archives of surgery / deutsche gesellschaft fur chirurgie. 2016;401(8):1153‐1162. doi: 10.1007/s00423-016-1513-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cima RR, Bergquist JR, Hanson KT, Thiels CA, Habermann EB. Outcomes are Local: Patient, Disease, and Procedure-Specific Risk Factors for Colorectal Surgical Site Infections from a Single Institution. Journal of gastrointestinal surgery: official journal of the Society for Surgery of the Alimentary Tract. 2017;21(7):1142–1152. doi: 10.1007/s11605-017-3430-1 [DOI] [PubMed] [Google Scholar]
- 31.Bislenghi G, Vanhaverbeke A, Fieuws S, Overstraeten AdBv, D’Hoore A, Schuermans A, et al. Risk factors for surgical site infection after colorectal resection: a prospective single centre study An analysis on 287 consecutive elective and urgent procedures within an institutional quality improvement project. Acta chirurgica Belgica. 2019:1–24. doi: 10.1080/00015458.2019.1675969 [DOI] [PubMed] [Google Scholar]
- 32.Ghuman A, Brown C, Karimuddin A, Raval M, Phang T. Surgical site infection rates following implementation of a colorectal closure bundle in elective colorectal surgeries. Diseases of the colon and rectum. 2015;58(5):133–135. doi: 10.1097/DCR.0000000000000455 [DOI] [PubMed] [Google Scholar]
- 33.Kwaan MR, Melton GB, Madoff RD, Chipman JG. Abdominoperineal Resection, Pelvic Exenteration, and Additional Organ Resection Increase the Risk of Surgical Site Infection after Elective Colorectal Surgery: An American College of Surgeons National Surgical Quality Improvement Program Analysis. Surgical infections. 2015;16(6):675–683. doi: 10.1089/sur.2014.144 [DOI] [PubMed] [Google Scholar]
- 34.Park YY, Kim CW, Park SJ, Lee Y, Lee JJ, Lee HO, et al. Influence of shorter duration of prophylactic antibiotic use on the incidence of surgical site infection following colorectal cancer surgery. Annals of coloproctology. 2015;31(6):235–242. doi: 10.3393/ac.2015.31.6.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Silvestri M, Dobrinja C, Scomersi S, Giudici, Turoldo, Princic E, et al. Modifiable and non-modifiable risk factors for surgical site infection after colorectal surgery: a single-center experience. Surgery today. 2018;48(3):338–345. doi: 10.1007/s00595-017-1590-y [DOI] [PubMed] [Google Scholar]
- 36.Guzman‐Pruneda FA, Husain SG, Jones CD, Beal W, Porter, Grove M, et al. Compliance with preoperative care measures reduces surgical site infection after colorectal operation. Journal of surgical oncology. 2019;119(4):497–502. doi: 10.1002/jso.25346 [DOI] [PubMed] [Google Scholar]
- 37.Uchino M, Ikeuchi H, Tsuchida T, Nakajima K, Tomita N, Takesue Y. Surgical site infection following surgery for inflammatory bowel disease in patients with clean-contaminated wounds. World journal of surgery. 2009;33(5):1042–1048. doi: 10.1007/s00268-009-9934-4 [DOI] [PubMed] [Google Scholar]
- 38.Ho VP, Barie PS, Stein SL, Trencheva K, Milsom JW, Lee SW, et al. Antibiotic regimen and the timing of prophylaxis are important for reducing surgical site infection after elective abdominal colorectal surgery. Surgical infections. 2011;12(4):255–260. doi: 10.1089/sur.2010.073 [DOI] [PubMed] [Google Scholar]
- 39.Watanabe M, Suzuki H, Nomura S, Hanawa H, Chihara N, Mizutani S, et al. Performance Assessment of the Risk Index Category for Surgical Site Infection after Colorectal Surgery. Surgical infections. 2015;16(1):84–89. doi: 10.1089/sur.2013.260 [DOI] [PubMed] [Google Scholar]
- 40.Bert F, Giacomelli S, Amprino V, Pieve G, Ceresetti D, Testa M et al. The “bundle” approach to reduce the surgical site infection rate. Journal of evaluation in clinical practice. 2017;23(3):642–647. doi: 10.1111/jep.12694 [DOI] [PubMed] [Google Scholar]
- 41.Blumetti J, Luu M, Sarosi G, Hartless K, McFarlin J, Parker B, et al. Surgical site infections after colorectal surgery: Do risk factors vary depending on the type of infection considered? Surgery. 2007;142(5):704–711. doi: 10.1016/j.surg.2007.05.012 [DOI] [PubMed] [Google Scholar]
- 42.Hennessey DB, Burke JP, Ni-Dhonochu T, Shields C, Winter DC, Mealy K. Risk factors for surgical site infection following colorectal resection: a multi-institutional study. International journal of colorectal disease. 2016;31(2):267–271. doi: 10.1007/s00384-015-2413-5 [DOI] [PubMed] [Google Scholar]
- 43.Colas-Ruiz E, Del-Moral-Luque JA, Gil-Yonte P, Fernández-Cebriá n M, Alonso-García M, Villar-Del-Campo MC, et al. Incidence of surgical site infection and risk factors in rectal surgery: A prospective cohort study. Cirugia espanola. 2018;96(10):640–647. doi: 10.1016/j.ciresp.2018.06.007 [DOI] [PubMed] [Google Scholar]
- 44.Kwaan MR, Sirany AM, Rothenberger DA, Madoff RD. Abdominal wall thickness: is it associated with superficial and deep incisional surgical site infection after colorectal surgery? Surgical infections. 2013;14(4):363–368. doi: 10.1089/sur.2012.109 [DOI] [PubMed] [Google Scholar]
- 45.Itatsu K, Sugawara G, Kaneoka Y, Kato T, Takeuchi E, Kanai M, et al. Risk factors for incisional surgical site infections in elective surgery for colorectal cancer: Focus on intraoperative meticulous wound management. Surgery today. 2014;44(7):1242–1252. doi: 10.1007/s00595-013-0677-3 [DOI] [PubMed] [Google Scholar]
- 46.Olmez T, Karakose E, Keklikkiran ZZ, Ofluoglu B, Bas, Uzun O, et al. Relationship between Sarcopenia and Surgical Site Infection in Patients Undergoing Colorectal Cancer Surgical Procedures. Surgical infections. 2019. doi: 10.1089/sur.2019.285 [DOI] [PubMed] [Google Scholar]
- 47.Nakamura T, Mitomi H, Ihara A, Onozato W, Sato T, Ozawa H, et al. Risk factors for wound infection after surgery for colorectal cancer. World journal of surgery. 2008;32(6):1138–1141. doi: 10.1007/s00268-008-9528-6 [DOI] [PubMed] [Google Scholar]
- 48.Poon JT, Law WL, Wong IW, Ching PT, Wong LM, Fan JKM, et al. Impact of laparoscopic colorectal resection on surgical site infection. Annals of surgery. 2009;249(1):77–81. doi: 10.1097/SLA.0b013e31819279e3 [DOI] [PubMed] [Google Scholar]
- 49.Uchino M, Ikeuchi H, Matsuoka H, Bando T, Ichiki K, Nakajima K, et al. Risk factors for surgical site infection and association with infliximab administration during surgery for Crohn’s disease. Diseases of the colon and rectum. 2013;56(10):1156–1165. doi: 10.1097/DCR.0b013e31829f682c [DOI] [PubMed] [Google Scholar]
- 50.Makino T, Shukla PJ, Rubino F, Milsom JW. The Impact of Obesity on Perioperative Outcomes After Laparoscopic Colorectal Resection. Annals of surgery. 2012;255(2):228–236. doi: 10.1097/SLA.0b013e31823dcbf7 [DOI] [PubMed] [Google Scholar]
- 51.Bhakta A, Tafen M, Glotzer O, Ata A, Chismark AD, Valerian BT, et al. Increased Incidence of Surgical Site Infection in IBD Patients. Diseases of the colon and rectum. 2016;59(4):316–322. doi: 10.1097/DCR.0000000000000550 [DOI] [PubMed] [Google Scholar]
- 52.Gurunathan U, Ramsay S, Mitrić G, Way M, Wockner L, Myles P. Association Between Obesity and Wound Infection Following Colorectal Surgery: Systematic Review and Meta-Analysis. Journal of Gastrointestinal Surgery. 2017;21(10):1700–1712. doi: 10.1007/s11605-017-3494-y [DOI] [PubMed] [Google Scholar]
- 53.Pendlimari R, Cima RR, Wolff BG, Pemberton JH, Huebner M. Diagnoses Influence Surgical Site Infections (SSI) in Colorectal Surgery: A Must Consideration for SSI Reporting Programs? Journal of the American College of Surgeons. 2012;214(4):574–580. doi: 10.1016/j.jamcollsurg.2011.12.023 [DOI] [PubMed] [Google Scholar]
- 54.Gandaglia G, Ghani KR, Sood A, Meyers R, Sammon D, Schmid M, et al. Effect of minimally invasive surgery on the risk for surgical site infections: results from the National Surgical Quality Improvement Program (NSQIP) Database. JAMA surgery. 2014;149(10):1039–1044. doi: 10.1001/jamasurg.2014.292 [DOI] [PubMed] [Google Scholar]
- 55.Cheng H, Chen PH, Soleas IM, Ferko NC, Cameron CG, Hinoul P. Prolonged Operative Duration Increases Risk of Surgical Site Infections: A Systematic Review. Surgical infections. 2017;18(6):722–735. doi: 10.1089/sur.2017.089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Culver DH, Horan TC, Gaynes RP, Martone WJ, Jarvis WR, Emori TG, et al. Surgical wound infection rates by wound class, operative procedure, and patient risk index. National Nosocomial Infections Surveillance System. The American journal of medicine. 1991;91(3b):152s–157s. doi: 10.1016/0002-9343(91)90361-z [DOI] [PubMed] [Google Scholar]
- 57.Ricciardi R, Roberts PL, Hall JF, Read TE, Marcello PW. What Is the Effect of Stoma Construction on Surgical Site Infection After Colorectal Surgery? Journal of Gastrointestinal Surgery. 2014;18(4):789–795. doi: 10.1007/s11605-013-2439-3 [DOI] [PubMed] [Google Scholar]
- 58.Konishi T, Watanabe T, Kishimoto J, Nagawa H. Elective colon and rectal surgery differ in risk factors for wound infection Results of prospective surveillance. Annals of surgery. 2006;244(244):758–763. doi: 10.1097/01.sla.0000219017.78611.49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hackam D, Rotstein O. Stoma closure and wound infection: an evaluation of risk factors. Canadian Journal of Surgery Journal Canadien De Chirurgie. 1995;38(2):144–148. [PubMed] [Google Scholar]
- 60.Sorensen LT, Karlsmark T, Gottrup F. Abstinence from smoking reduces incisional wound infection: a randomized controlled trial. Annals of surgery. 2003;238(1):1–5. doi: 10.1097/01.SLA.0000074980.39700.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiran RP, Turina M, Hammel J, Fazio V. The clinical significance of an elevated postoperative glucose value in nondiabetic patients after colorectal surgery: evidence for the need for tight glucose control? Annals of surgery. 2013;258(4):599–604; discussion 604–595. doi: 10.1097/SLA.0b013e3182a501e3 [DOI] [PubMed] [Google Scholar]
- 62.Kwon S, Thompson RE, Dellinger P, Rogers T, D F. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Annals of surgery. 2013;172(2):274. doi: 10.1097/SLA.0b013e31827b6bbc [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Baré M, Montón C, Mora L, Redondo, Pont, Escobar A, et al. COPD is a clear risk factor for increased use of resources and adverse outcomes in patients undergoing intervention for colorectal cancer: a nationwide study in Spain. International Journal of Chronic Obstructive Pulmonary Disease. 2017;12:1233–1241. doi: 10.2147/COPD.S130377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koller SE, Bauer KW, Egleston BL,Smith, Philp M, Ross HM, et al. Comparative Effectiveness and Risks of Bowel Preparation Before Elective Colorectal Surgery. Annals of surgery. 2017;267(4):734–742. doi: 10.1097/SLA.0000000000002159 [DOI] [PubMed] [Google Scholar]
- 65.Klinger AL, Green H, Monlezun DJ, Beck, Kann, Vargas HD, et al. The Role of Bowel Preparation in Colorectal Surgery: Results of the 2012–2015 ACS-NSQIP Data. Annals of surgery. 2019;269(4):671–677. doi: 10.1097/SLA.0000000000002568 [DOI] [PubMed] [Google Scholar]
- 66.Migaly J, Bafford AC, Francone TD, Gaertner B, Eskicioglu, Bordeianou L, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Use of Bowel Preparation in Elective Colon and Rectal Surgery. Diseases of the colon and rectum. 2019;62(1):3–8. doi: 10.1097/DCR.0000000000001238 [DOI] [PubMed] [Google Scholar]
- 67.Zmora O, Wexner SD, Hajjar L, Park T, Weiss EG. Trends in preparation for colorectal surgery: Survey of the members of the American Society of Colon and Rectal Surgeons. J Am Surg. 2003;69(2):150–154. [PubMed] [Google Scholar]
- 68.Laura K, Taru L, Selja K, Rasilainen, Klintrup, Ehrlich A, et al. Mechanical and oral antibiotic bowel preparation versus no bowel preparation for elective colectomy (MOBILE): a multicentre, randomised, parallel, single-blinded trial. The Lancet. 2019;394(10201):840–848. doi: 10.1016/S0140-6736(19)31269-3 [DOI] [PubMed] [Google Scholar]
- 69.Fry DE. Preventive Systemic Antibiotics in Colorectal Surgery. Surgical infections. 2008;9(6):547–552. doi: 10.1089/sur.2008.9956 [DOI] [PubMed] [Google Scholar]
- 70.Steinberg JP, Braun BI, Hellinger WC, Kusek L, Bozikis MR, Bush AJ, et al. Timing of Antimicrobial Prophylaxis and the Risk of Surgical Site Infections. Annals of surgery. 2009;250(1):10–16. doi: 10.1097/SLA.0b013e3181ad5fca [DOI] [PubMed] [Google Scholar]
- 71.Mchugh SM, Hill ADK, Humphreys H. Intraoperative technique as a factor in the prevention of surgical site infection. Journal of Hospital Infection. 2011;78(1):1–4. doi: 10.1016/j.jhin.2011.01.011 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(DOC)
(A: Diabetes mellitus; B: wound classification>2).
(DOC)
(PDF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting information files.