Abstract
Restriction fragment length polymorphism (RFLP) analysis with probes derived from the insertion element IS6110, the direct repeat sequence, and the polymorphic GC-rich sequence (PGRS) and a PCR-based typing method called spacer oligonucleotide typing (spoligotyping) were used to strain type Mycobacterium bovis isolates from the Republic of Ireland. Results were assessed for 452 isolates which were obtained from 233 cattle, 173 badgers, 33 deer, 7 pigs, 5 sheep, and 1 goat. Eighty-five strains were identified by RFLP analysis, and 20 strains were identified by spoligotyping. Twenty percent of the isolates were the most prevalent RFLP type, while 52% of the isolates were the most prevalent spoligotype. Both the prevalent RFLP type and the prevalent spoligotype were identified in isolates from all animal species tested and had a wide geographic distribution. Isolates of some RFLP types and some spoligotypes were clustered in regions consisting of groups of adjoining counties. The PGRS probe gave better differentiation of strains than the IS6110 or DR probes. The majority of isolates from all species carried a single IS6110 copy. In four RFLP types IS6110 polymorphism was associated with deletion of fragments equivalent in size to one or two direct variable repeat sequences. The same range and geographic distribution of strains were found for the majority of isolates from cattle, badgers, and deer. This suggests that transmission of infection between these species is a factor in the epidemiology of M. bovis infection in Ireland.
Mycobacterium bovis infection in animals is usually a slow progressive disease in which clinical signs are not apparent until late in the disease process. In farm animals infection is usually diagnosed by tuberculin testing or by abattoir postmortem examination (5, 15). A prolonged time interval between the establishment of infection and diagnosis can make tracing of the source of the outbreak difficult.
A test and slaughter program to eradicate M. bovis infection from cattle herds has been in operation in the Irish Republic since 1954, but a low level of infection still persists (4). There is a considerable amount of movement of cattle between herds. Frequently, beef cattle pass through three or more different herds before slaughter (4). An effective system for tracing animal movements is in place, however, and all cattle can be individually identified. M. bovis infection is endemic in badgers and is also present in farmed and feral deer (13, 15). A thorough epidemiological analysis of every major herd breakdown is routinely carried out. A database of M. bovis strains related to geographical location and species will provide a useful tool in such epidemiological investigations. In recent years, DNA fingerprinting of M. bovis isolates has proven useful in epidemiological investigations of tuberculosis outbreaks in deer, elk, and coyotes in the United States (26), in deer in Sweden (22), and in cattle in The Netherlands (24).
Restriction fragment length polymorphism (RFLP) analysis with probes derived from the insertion element IS6110, the polymorphic GC-rich sequence (PGRS), and the direct repeat (DR) sequence has proved to be the most useful method of differentiating M. bovis strains (2, 6, 9, 16, 21, 24). Compared to Mycobacterium tuberculosis, most isolates of M. bovis contain relatively few copies of IS6110 (7). Nevertheless, this element has proved useful for differentiation of strains (6, 12, 14, 16, 21, 24). There are approximately 30 copies of PGRS in the M. bovis genome (19). Generally, only the larger PGRS fragments are analyzed, as these show the greatest degree of polymorphism and resolution of the low-molecular-weight fragments is difficult (2, 21). PGRS has usually given greater differentiation of strains than either IS6110 or DR (2, 6, 24). The DR region consists of a series of virtually identical DR sequences, each 36 bp long, interspersed with variable spacer sequences from 35 to 41 bp long (8, 10). Each DR sequence and the adjacent variable spacer sequence is termed a direct variable repeat (DVR) (8). The results of previous studies indicate that the single DR region is unique to the M. tuberculosis complex and occurs in all isolates (8, 11).
Spacer oligonucleotide typing (spoligotyping) is a more recent development which is faster and less labor intensive than the RFLP procedure (11). It is a PCR-based method which detects polymorphisms in the spacer sequences of the DR region. The spacer sequences of the isolates being typed are amplified and labelled by PCR and are superimposed on a series of known spacer sequences attached to a membrane. The pattern of hybridization will depend on the number of membrane-bound spacer sequences that are also present in the isolate being typed. The membranes presently in use have 37 different spacer sequences from M. tuberculosis H37Rv and 6 spacer sequences from M. bovis BCG (11). The spoligotypes identified by different laboratories can be readily compared as spoligotyping detects the presence or absence of specific spacer sequences and does not require measurement of fragment size. This method does not give the same degree of differentiation between strains as RFLP typing (6, 18); nevertheless, certain groups of strains have shown highly unique spoligotypes, e.g., caprine strains of M. bovis from Spain (1) and the Beijing strains of M. tuberculosis (25).
The objective of this study was to determine the geographic and species distributions of M. bovis strains from the Republic of Ireland by RFLP analysis with IS6110, PGRS, and DR probes and by spoligotyping. The relative ability of these typing methods to differentiate strains was assessed. We show that while a large number of strains were identified, the majority of isolates from all species were grouped into a small number of RFLP types and spoligotypes. Isolates of some of these prevalent types were widely distributed, while isolates of other types were clustered in specific regions.
MATERIALS AND METHODS
M. bovis isolates.
A total of 734 M. bovis isolates were typed by RFLP analysis and spoligotyping. The isolates were obtained from animals from all areas of the Republic of Ireland during the period from 1993 to 1998. A total of 515 isolates were cultured from lymph glands from cattle which had been slaughtered after showing positive reactions to the single intradermal comparative tuberculin test. The bovine isolates were recovered from cattle from 210 different herds, with a range of 1 to 18 isolates per herd. A total of 173 of the isolates were from badgers, 33 were from deer, 7 were from pigs, 5 were from sheep, and 1 was from a goat.
Samples were cultured initially on Stonebrinks or Lowenstein-Jensen medium, and isolates were identified as M. bovis by standard biochemical tests as described previously (5). Isolates were then subcultured on 50 ml of Middlebrook 7H9 medium with ADC (albumen, dextrose, citric acid) enrichment (Gibco BRL) for 4 weeks at 37°C. The cultures were harvested after inactivation by heating at 75°C for 1 h.
RFLP method.
DNA was extracted as described previously (20). DNA was digested with PvuII for the IS6110 probe and AluI for the PGRS and DR probes as described previously (20). Electrophoresis and Southern blotting were carried out by standard methods (20). External molecular size markers and M. bovis NCO5693 were run on each gel. The IS6110 probe consisted of the entire 1,358-bp sequence prepared by PCR as described previously (20). This probe hybridized to both sides of the PvuII site, which is present in IS6110, identifying two fragments for every copy of IS6110 present in the genome. The PGRS probe was plasmid pTBN12 (19), which was kindly provided by Bruce Ross, Fairfield Hospital, Fairfield, Victoria, Australia. The 36-bp DR probe was obtained as a commercially prepared oligonucleotide. The IS6110 and PGRS probes were labelled with a Digoxigenin High Prime Labelling Kit (Boehringer Mannheim). The DR probe was labelled with a Digoxigenin Oligonucleotide 3′-End Labelling Kit (Boehringer Mannheim). Hybridization was performed by the procedure recommended by Boehringer Mannheim. Bound probes were detected with a Digoxigenin Luminescent Detection Kit (Boehringer Mannheim). GelCompar, version 3.1, Applied Maths, Kortrijk, Belgium, was used to determine the molecular weights of the probed DNA fragments.
Spoligotyping.
Membranes to which 37 spacer sequences from M. tuberculosis H37Rv and 6 spacer sequences from M. bovis BCG were covalently bound (11) were kindly provided by J. van Embden, Institute of Public Health, Bilthoven, The Netherlands. The procedure was as described by Kamerbeek and colleagues (11), except that a digoxigenin labelling and detection system was used. One of the primers was 5′ end labelled with digoxigenin by Genosys Biotechnologies Ltd. A Digoxigenin Luminescent Detection Kit (Boehringer Mannheim) was used. Spoligotype patterns were read visually. Weak hybridization reactions were classified as positive.
Nomenclature.
There is no recognized standard method of nomenclature for strain types identified by DNA fingerprinting. Two alphanumeric characters (a letter and a number) were used to designate each IS6110 type; similarly, a letter and a number were used to designate each PGRS type, and a letter was used to designate each DR type. Thus, each RFLP type was designated by five alphanumeric characters in the order IS6110, PGRS, and DR. Spoligotypes were also described by a letter and a number.
RESULTS
A total of 734 M. bovis isolates obtained from 515 cattle, 173 badgers, 33 deer, 7 pigs, 5 sheep, and 1 goat were typed by RFLP analysis and spoligotyping. The 515 cattle belonged to 210 herds, and isolates were obtained from a number of animals from some herds. As all of the isolates from a herd usually had the same RFLP and spoligotyping patterns, results were assessed for only 233 of the cattle isolates which were representative of the strain (or strains) found in each of the 210 herds. All of the 219 isolates from badgers, deer, pigs, sheep, and a goat were included in the assessment of the results. Therefore, a total of 452 isolates from all species were included in the analysis of the results.
RFLP analysis.
A total of 85 RFLP types were identified among the 452 M. bovis isolates assessed in this study. The 233 cattle isolates were divided into 62 types, while the 219 isolates from badgers, deer, sheep, pigs, and a goat were divided into 44 types. Twenty-one of the 85 RFLP types were identified both in bovine isolates and in isolates from the other species. These 21 types represented 180 (77.3%) of the cattle isolates and 186 (84.9%) of the isolates from the other species. A total of 41 RFLP types were identified only among isolates from cattle, and 23 RFLP types were identified only among isolates from the other species.
A total of 88 (19.5%) of the 452 isolates were found to be one RFLP type (type A1,A1,A). Isolates of this type were present in all the species from which isolates were obtained for typing (Table 1) and were found in all areas of the country. A further eight RFLP types were represented by 10 or more isolates. Six of these types were clustered in geographic regions, typically consisting of groups of adjoining counties (Table 1). For example, RFLP type B1,C1,C was isolated from cattle and badgers in counties Monaghan, Cavan, and Meath, and RFLP type A1,A3,A was isolated from cattle and badgers in counties Cork, Kerry, and Limerick. Sixty-three of the remaining 76 RFLP types were represented by only one or two isolates.
TABLE 1.
Further differentiation of RFLP types (10 or more isolates of each type) by spoligotyping showing the species distribution of the different spoligotypes identified within each RFLP type and the geographic distribution of the RFLP types
| RFLP type (no. of isolates) | Spoligotype (no. of isolates) | Species distribution of spoligotypes (species, no. of animals) | Geographic distribution of RFLP types
|
|
|---|---|---|---|---|
| No. of adjoining counties in which RFLP type was clustereda | % of isolates with RFLP type in region | |||
| A1,A1,A (88) | A1 (86) | Cattle, 48; badger, 21; deer, 8; other isolates, 9b | Not clustered | |
| A9 (1) | Badger, 1 | |||
| B4 (1) | Deer, 1 | |||
| C1,H1,J (56) | D1 (56) | Cattle, 22; badger, 32; deer, 2 | 7 | 98 |
| B1,C1,C (54) | A2 (50) | Cattle, 26; badger, 24 | 3 | 94 |
| A1 (3) | Badger, 3 | |||
| B2 (1) | Badger, 1 | |||
| A1,A5,A (39) | A1 (38) | Cattle, 14; badger, 20; deer, 3; other isolates, 1c | 1 | 67 |
| B1 (1) | Pig, 1 | |||
| A2,A1,B (27) | A1 (19) | Cattle, 11; badger, 7; sheep, 1 | Not clustered | |
| B2 (6) | Cattle, 5; badger, 1 | |||
| B4 (1) | Cattle, 1 | |||
| B1 (1) | Badger, 1 | |||
| A1,A3,A (20) | A1 (20) | Cattle, 8; badger, 12 | 3 | 95 |
| J1,A1,A (13) | A1 (12) | Cattle, 7; badger, 5 | 5 | 100 |
| B1 (1) | Badger, 1 | |||
| A1,A1,F (10) | A8 (10) | Cattle, 8; badger, 2 | 3 | 70 |
| A1,B1,D (10) | A1 (8) | Cattle, 5; badger, 1; deer, 2 | Not clustered | |
| A2 (1) | Deer, 1 | |||
| B2 (1) | Badger, 1 | |||
The mean area of a county is 2,650 km2.
Pig, 6; sheep, 2; goat, 1.
Sheep, 1.
The PGRS probe gave better differentiation of strains than the IS6110 and DR probes, identifying 48 types among the 452 isolates. The most prevalent PGRS type was designated A1 and 155 (34.3%) of the isolates were this type. Only PGRS fragments greater than 1.9 kb were analyzed; the number of such fragments varied between 12 and 17. Twenty-three types were identified with the DR probe. The most prevalent DR type was designated A, and 201 (44.5%) of the isolates were this DR type. The number of fragments that hybridized to the DR probe varied between four and six. A combination of PGRS and DR probes identified 66 strains. A total of 108 (23.9%) of the isolates were of the most prevalent type.
Thirty-six types were identified with the IS6110 probe. The most prevalent IS6110 type was designated A1, and 198 (43.8%) of the isolates were this type. Isolates of this type contained a single IS6110 copy with a 1.9-kb fragment and a 3.6-kb fragment. Thirteen types, representing 202 (86.7%) of the isolates from cattle, 157 (90.8%) of the isolates from badgers, 27 (81.8%) of the isolates from deer, 4 of the isolates from sheep, and all of the pig and goat isolates, contained a single IS6110 copy. With the exception of isolates of RFLP type J1,A1,A, which contained two IS6110 copies, isolates of all of the prevalent RFLP types contained a single IS6110 copy. Seven of the strain types with a single copy representing 317 isolates, contained a 1.9-kb fragment. Thirteen strain types, representing 40 isolates, contained two IS6110 copies; 8 strain types, representing 12 isolates, contained three copies; 1 bovine isolate contained four copies; and one isolate from a badger had six IS6110 copies.
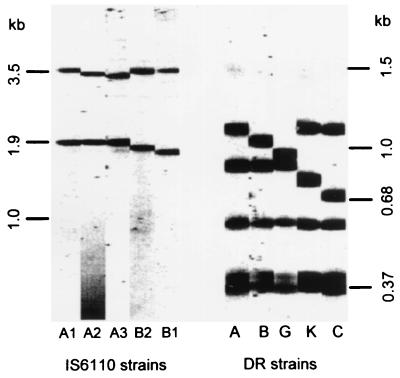
In isolates of four RFLP types, IS6110 polymorphism appeared to be caused by the deletion of fragments, equivalent in size to either one or two DVRs, from the prevalent RFLP type (type A1,A1,A). A correlation between IS6110 and DR types in these four RFLP types was associated with a single IS6110 fragment and a single DR fragment that differed from the corresponding fragments in the prevalent RFLP type by a number of base pairs equivalent to one or two DVRs. As a result of the deletion of a fragment, which corresponded in size to a single DVR, the largest fragment of DR pattern B was approximately 70 bp less than the largest fragment of the prevalent DR pattern A, while the larger fragment of IS6110 pattern A2 was approximately 70 bp less than the larger (3.6-kb) fragment of the prevalent IS6110 pattern A1 (Fig. 1). A total of 35 isolates were IS6110 type A2 and DR type B. These came from different sources and species and were subdivided into six different strains with the PGRS probe. There was also a correlation between IS6110 type B1 and DR type C which was associated with the deletion of a fragment corresponding in size to two DVRs. The second largest fragment of DR pattern C was approximately 140 bp less than the second largest fragment of the prevalent DR pattern A, while the smaller fragment of IS6110 pattern B1 was approximately 140 bp less than the smaller (1.9-kb) fragment of the prevalent IS6110 pattern A1 (Fig. 1). The 58 isolates, from different sources and species, which were IS6110 type B1 and DR type C were subdivided into three different strains with the PGRS probe. Similarly, there was a correlation between three isolates which were IS6110 pattern B2 and DR pattern K, which was associated with the deletion of a fragment equivalent in size to one DVR, and between four isolates which were IS6110 pattern A3 and DR pattern G, which was associated with the deletion of a fragment equivalent in size to two DVRs.
FIG. 1.
Correlation between IS6110 and DR types associated with deletion of fragments corresponding in size to one or two DVRs. IS6110 type A1 was the most prevalent IS6110 type. The larger fragments of IS6110 patterns A2 and A3 were approximately 70 and 140 bp, respectively, less than the 3.6-kb fragment of IS6110 pattern A1. The smaller fragments of IS6110 patterns B2 and B1 were approximately 70 and 140 bp, respectively, less than the 1.9-kb fragment of IS6110 pattern A1. DR type A was the most prevalent DR type. The largest fragments of DR patterns B and G were approximately 70 and 140 bp, respectively, less than the largest fragment of DR pattern A. The second largest fragments of DR patterns K and C were approximately 70 and 140 bp, respectively, less than the second largest fragment of DR pattern A. There was a correlation between IS6110 type A2 and DR type B, between IS6110 type A3 and DR type G, between IS6110 type B2 and DR type K, and between IS6110 type B1 and DR type C.
Twenty-one (10%) of the 210 cattle herds harbored more than one strain on the basis of the results of RFLP analysis. Nineteen herds had two strains, while two herds had three strains. In six of the herds which had two strains the difference between the RFLP patterns was confined to a change involving a single fragment that hybridized to one of the probes.
Spoligotyping.
Twenty spoligotypes were identified among the 452 M. bovis isolates. The 233 cattle isolates were divided into 17 spoligotypes, while the 219 isolates from badgers, deer, sheep, pigs, and a goat were divided into 18 spoligotypes. Fifteen of the 20 spoligotypes were present both in cattle and in the other species. These 15 spoligotypes represented 230 (98.7%) of the cattle isolates and 214 (97.7%) of the isolates from the other species. A total of two spoligotypes were identified only among isolates from cattle, and three spoligotypes were identified only among isolates from the other species. The most prevalent spoligotype was designated A1, and 234 (51.8%) of the isolates were this spoligotype. Isolates of this spoligotype were found in cattle, badgers, and deer from all areas of the country. All of the sheep and goat isolates and six of the seven isolates from pigs were spoligotype A1. A further six spoligotypes were represented by 10 or more isolates. One of these six spoligotypes was widely distributed, while five were clustered in groups of adjoining counties (Table 2). Thirteen (6.2%) of the 210 herds had more than one spoligotype. Twelve herds had isolates of two different spoligotypes, while three different spoligotypes were identified in one herd.
TABLE 2.
Species and geographic distribution of strain typesa identified by spoligotyping
| Spoligotype | No. of isolates from the following species:
|
Geographic distribution
|
|||||
|---|---|---|---|---|---|---|---|
| Cattle | Badgers | Deer | Other | Total | No. of adjoining counties in which spoligotype was clustered | % of isolates with spoligotype in region | |
| A1 | 124 | 79 | 19 | 12 | 234 | Not clustered | |
| D1 | 27 | 39 | 2 | 1 | 69 | 7 | 96 |
| A2 | 32 | 26 | 1 | 59 | 3 | 93 | |
| D3 | 5 | 7 | 2 | 14 | 2 | 86 | |
| B2 | 5 | 4 | 3 | 12 | 4 | 66 | |
| A8 | 10 | 1 | 11 | Not clustered | |||
| D2 | 8 | 2 | 10 | 3 | 89 | ||
Spoligotypes for which 10 or more isolates were of each strain type.
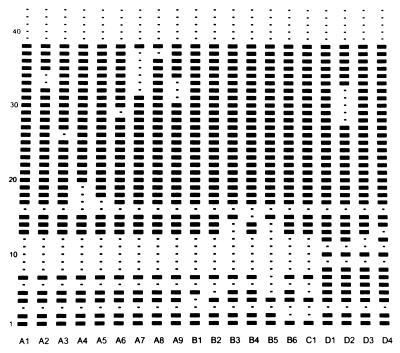
All of the spoligotypes lacked the five spacer sequences at the 3′ end of the DR locus and spacers 3, 9, 11, and 16, while spacers 1, 20 to 25, 27, and 38 were present in all spoligotypes (Fig. 2). Fourteen spoligotypes differed from the prevalent spoligotype by deletion of a single spacer sequence or a single block of spacer sequences. One spoligotype (spoligotype C1) differed from the prevalent spoligotype by deletion of two nonadjoining spacer sequences. Four spoligotypes (D1, D2, D3, and D4) differed from other spoligotypes in having extra spacers. Spoligotypes D1 and D2 were present principally in the south, while spoligotype D3 was clustered in the adjoining south midlands.
FIG. 2.
Schematic representation of 20 spoligotype patterns identified among 452 M. bovis isolates. The 43 spacer oligonucleotides are numbered as described by Kamerbeek et al. (11).
Combined RFLP and spoligotyping.
A combination of RFLP analysis with three probes and spoligotyping identified 98 strain types among the 452 isolates. The 233 cattle isolates were divided into 64 strain types, while the 219 isolates from badgers, deer, sheep, pigs, and a goat were divided into 56 strain types. Twenty-two of the 98 strain types were present both in cattle and in the other species. These 22 strain types represented 172 (73.8%) of the cattle isolates and 174 (79.5%) of the isolates from the other species. A total of 42 strain types were identified among isolates only from cattle and 34 strain types were identified among isolates only from the other species. A combination of RFLP analysis with PGRS probe and spoligotyping identified 70 strain types. A total of 28.1% of the isolates were found to be the most prevalent strain type. A combination of RFLP analysis with both PGRS and IS6110 probes and spoligotyping identified 94 strain types. The most prevalent strain type was detected in 19.9% of the isolates.
Spoligotyping was of limited value for further subdivision of RFLP types (Table 1). For example, the 88 isolates of the most prevalent RFLP type were subdivided into three strains by spoligotyping; however, 86 of the 88 isolates were one strain type (spoligotype A1, RFLP type A1,A1,A), while the 56 isolates of the second most prevalent RFLP type (RFLP type C1,H1,J) were not further differentiated by spoligotyping (Table 1). There were 26 RFLP types which were represented by between two and nine isolates. Only two of these were further subdivided by spoligotyping.
RFLP analysis subdivided the 234 isolates contained in the prevalent spoligotype, spoligotype A1, into 42 different strains. The remaining 19 spoligotypes, comprising 218 isolates, were subdivided into 56 strains by RFLP analysis.
DISCUSSION
RFLP analysis and spoligotyping were used to assess the geographic and species distributions of M. bovis strains in the Republic of Ireland. RFLP analysis identified one prevalent type (RFLP type A1,A1,A) with a wide geographic distribution; 19.5% of the isolates were this type. Isolates of this type were present in cattle, badgers, and deer. The majority of the isolates from sheep and pigs were also this type. As M. bovis is infrequently isolated from sheep and pigs (13), the predominance of this type suggests that it has a relatively high degree of virulence in many species. This is the RFLP type which has been most frequently identified in isolates from Northern Ireland (21). Most of the other frequently occurring RFLP types were clustered in regions consisting of a number of adjoining counties. One of these types (RFLP type C1,H1,J) was spread over seven counties in the south. Two frequently occurring RFLP types (types A2,A1,B and A1,B1,D) as well as the predominant type (type A1,A1,A) were not clustered. All of the geographic clusters identified to date extend over relatively large areas. As more isolates are examined, it is possible that strains which are clustered in smaller areas will be identified. Seventy-six of the 85 RFLP types were identified in too few isolates to assess their geographic distribution. All of the RFLP types that were clustered in particular areas were present in both cattle and badgers in those localities. This finding is in agreement with a previous study (3), in which 20 M. bovis isolates from the Republic of Ireland were typed by DNA restriction fragment analysis. While different restriction types were present in different areas, isolates of the same restriction type were present in both cattle and badgers from six localities. In the present study, 21 RFLP types were identified both in bovine isolates and in isolates from badgers or deer. Over 75% of the isolates from cattle, badgers, and deer were one of these 21 types. All of the 41 RFLP types, which were identified only in bovine isolates, and the 23 RFLP types, which were identified only in isolates from the other species, were represented by fewer than four isolates. The low number of isolates in which these types were identified may be the reason why they were associated with particular species in the present study. Alternatively, virulence factors may tend to confine some strains to a particular species. It will require a larger survey of isolates to determine if species-specific strains exist.
The PGRS probe showed the greatest polymorphism, identifying over twice as many strains as the DR probe. This is probably due to the wide distribution of PGRS throughout the genome (19). Cousins et al. (6) also found that PGRS identified approximately twice as many strains as the DR probe among 273 isolates obtained mainly from Australian cattle. However, a number of other studies (9, 16, 21) found little difference in the ability of PGRS and DR to differentiate strains. This may be due to analysis of a lower number of PGRS fragments and a smaller range and distribution of isolates examined.
We used a 1,358-bp probe which hybridized to both sides of the PvuII site which is present in IS6110. This probe identified more strains than the DR probe and fewer strains than the PGRS probe. Skuce et al. (21) found that an IS6110 probe which hybridized to both sides of the PvuII site identified approximately the same number of strains as the PGRS and DR probes. Other studies have shown that IS6110 probes which hybridize to only one side of the PvuII site have identified fewer strains than the PGRS probe (16) or DR probe (6, 14, 16). Our findings are in agreement with most previous studies which have shown that the majority of isolates from cattle carry a single IS6110 copy (7). We also found that the majority of isolates from other species including deer, sheep, and a goat harbor only a single IS6110 copy. This differs from previous studies which have associated multiple IS6110 copies with isolates from goats and sheep (9, 12) and from deer and antelope (22, 24).
The insertion sequence IS6110 is preferentially integrated in the DR region of M. tuberculosis complex strains (10). Therefore, it may be expected that deletion or insertion of DVRs will result in polymorphism of IS6110 fragments as well as DR fragments. In the present study, a single IS6110 fragment and a single DR fragment from each of four RFLP types differed from the prevalent RFLP pattern by a number of base pairs corresponding in size to either one or two DVRs. IS6110 polymorphism in 100 (25%) of the 398 isolates with a single IS6110 copy was associated with the deletion of a fragment equivalent in size to one or two DVRs. This is a factor to be considered in assessing the relationship between strains involved in an outbreak, as a single event, such as deletion of a DVR or a block of DVRs, may result in changes to both IS6110 and DR patterns.
Spoligotyping did not differentiate strains as well as any of the individual probes used in RFLP typing did because 51.8% of the isolates were identified as a single type (spoligotype A1). This spoligotype was the same as that for 68.2% of 211 Australian M. bovis isolates (6) and 61.5% of 52 M. bovis isolates from Northern Ireland (17). A small number of M. bovis isolates from Iran examined by Cousins et al. (6) were also this type. Our spoligotype A1 was not detected among M. bovis isolates obtained from 129 cattle and 44 goats in Spain (1). Most of the other spoligotypes seen in the present study differed from spoligotype A1 by the deletion of a single spacer sequence or a single block of spacer sequences. However, four spoligotypes (spoligotypes D1, D2, D3, and D4) were unique in that they had three or four spacer sequences which were not present in spoligotype A1. These four spoligotypes were clustered in a large area in the South and South Midlands. Six of the seven isolates from pigs, the isolate from a goat, and all of the sheep isolates were characterized as spoligotype A1. In contrast to our findings, M. bovis strains isolated from goats and sheep in Spain had a highly unique spoligotype which was not found among strains from cattle (1). Overall, the range of spoligotype patterns more closely resembled those described for Australian M. bovis isolates (6) than those reported for Spanish isolates (1).
Spoligotyping resulted in only limited differentiation of the more prevalent RFLP types, while only two of the less prevalent RFLP types were further differentiated. A study in Northern Ireland also found that the prevalent RFLP type was only marginally subdivided by spoligotyping (18). Most of the RFLP types which were further differentiated by spoligotyping in the present study were from badgers and deer. Twelve extra strains were identified among the nonbovine isolates when spoligotyping was combined with RFLP analysis, whereas only two extra strains were identified among the bovine isolates.
A standardized method is widely used for RFLP analysis of M. tuberculosis isolates (23). A number of strategies for DNA fingerprinting of M. bovis isolates have been suggested. Cousins et al. (7) recommended a method for the typing of M. bovis isolates from populations which contain low copy numbers of IS6110 on the basis of an initial analysis by spoligotyping followed by RFLP typing of the more prevalent spoligotypes with the PGRS probe. Aranaz et al. (2) also recommended initial screening by spoligotyping followed by further differentiation of the prevalent spoligotypes by RFLP analysis with the IS6110 or the PGRS probe. Spoligotyping has advantages as an initial screening test as only small amounts of DNA are required, the procedure can be carried out quickly, and results are easy to analyze (11). However, the results of the present study show that considerable differentiation of even the less prevalent spoligotypes can be achieved by RFLP analysis. The 218 isolates which were not the prevalent spoligotype (spoligotype A1) were divided into 19 strains by spoligotyping and into 56 strains by RFLP analysis. Therefore, the best differentiation of strains can be achieved only by RFLP analysis of all isolates. The present study and previous studies (2) have shown that PGRS and IS6110 are the most effective combination of probes. Further differentiation can be achieved by spoligotyping, which proved to be marginally more discriminatory than typing with the DR probe in the present study. Future development of the spoligotyping technique with the incorporation of more spacer sequences from M. bovis should enhance its potential as an initial screening method for strain typing of isolates.
The same range and geographic distribution of strains were found for the majority of cattle, badger, and deer isolates. This suggests that transmission of infection between these species is a factor in the epidemiology of M. bovis infection in Ireland. Detailed studies of selected areas will be required to more clearly define the relationship between strains from different herds and from wildlife species. In the present study, RFLP analysis and, to a lesser extent, spoligotyping achieved good differentiation of strains obtained from many areas of the country. However, detailed studies of local areas, where many isolates may have a recent clonal origin, will require additional methods for the subdivision of strains. Such methods may include the use of additional probes for RFLP analysis and also automated PCR-based techniques which will provide advantages such as easier analysis of results and lower labor requirements compared to those for RFLP analysis.
ACKNOWLEDGMENTS
We thank Anthony Gogarty and John McGuirk for culture of samples. We gratefully appreciate the contribution of staff at meat plants, Regional Veterinary Laboratories, and District Veterinary Offices. We thank Michael Sheridan and Ian O’Boyle for contribution to the organization of this study. We thank Martin Hill for photographic reproduction.
REFERENCES
- 1.Aranaz A, Liébana E, Mateos A, Dominguez L, Vidal D, Domingo M, Gonzolez O, Rodriguez-Ferri E F, Bunschoten A E, van Embden J D A, Cousins D. Spacer oligonucleotide typing of Mycobacterium bovis strains from cattle and other animals: a tool for studying epidemiology of tuberculosis. J Clin Microbiol. 1996;34:2734–2740. doi: 10.1128/jcm.34.11.2734-2740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aranaz A, Liébana E, Mateos A, Domínguez L, Cousins D. Restriction fragment length polymorphism and spacer oligonucleotide typing: a comparative analysis of fingerprinting strategies for Mycobacterium bovis. Vet Microbiol. 1998;61:311–324. doi: 10.1016/s0378-1135(98)00192-8. [DOI] [PubMed] [Google Scholar]
- 3.Collins D M, de Lisle G W, Collins J D, Costello E. DNA restriction fragment typing of Mycobacterium bovis isolates from cattle and badgers in Ireland. Vet Rec. 1994;134:681–682. doi: 10.1136/vr.134.26.681. [DOI] [PubMed] [Google Scholar]
- 4.Collins J D. Ireland. In: Thoen C O, Steele J H, editors. Mycobacterium bovis infection in animals and humans. 1st ed. Ames: Iowa State University Press; 1995. pp. 224–238. [Google Scholar]
- 5.Costello E, Quigley F, Flynn O, Gogarty A, McGuirk J, Murphy A, Dolan L. Laboratory examination of suspect tuberculous lesions detected on abattoir post mortem examination of cattle from non-reactor herds. Ir Vet J. 1998;51:248–250. [Google Scholar]
- 6.Cousins D, Williams S, Liébana E, Aranaz A, Bunschoten A, van Embden J, Ellis T. Evaluation of four DNA typing techniques in epidemiological investigations of bovine tuberculosis. J Clin Microbiol. 1998;36:168–178. doi: 10.1128/jcm.36.1.168-178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cousins D V, Skuce R A, Kazwala R R, van Embden J D A. Towards a standardized approach to DNA fingerprinting of Mycobacterium bovis. Int J Tuberc Lung Dis. 1998;2:471–478. [PubMed] [Google Scholar]
- 8.Groenen P M A, Bunschoten A E, van Soolingen D, van Embden J D A. Nature of DNA polymorphism in the direct repeat cluster of Mycobacterium tuberculosis; application for strain differentiation by a novel typing method. Mol Microbiol. 1993;10:1057–1065. doi: 10.1111/j.1365-2958.1993.tb00976.x. [DOI] [PubMed] [Google Scholar]
- 9.Gutierréz M, Samper S, Gavigan J-A, García Marín J F, Martín C. Differentiation by molecular typing of Mycobacterium bovis strains causing tuberculosis in cattle and goats. J Clin Microbiol. 1995;33:2953–2956. doi: 10.1128/jcm.33.11.2953-2956.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hermans P W M, van Soolingen D, Bik E M, de Haas P E W, Dale J W, van Embden J D A. Insertion element IS987 from Mycobacterium bovis BCG is located in a hot-spot integration region for insertion elements in Mycobacterium tuberculosis complex strains. Infect Immun. 1991;59:2695–2705. doi: 10.1128/iai.59.8.2695-2705.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamerbeek J, Schouls L, Kolk A, van Agterveld M, van Soolingen D, Kuijper S, Bunschoten A, Molhuizen H, Shaw R, Goyal M, van Embden J. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J Clin Microbiol. 1997;35:907–914. doi: 10.1128/jcm.35.4.907-914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liébana E, Aranaz A, Dominguez L, Mateos A, González-Llamazares O, Rodriguez-Ferri E F, Domingo M, Vidal D, Cousins D. The insertion element IS6110 is a useful tool for DNA fingerprinting of Mycobacterium bovis isolates from cattle and goats in Spain. Vet Microbiol. 1997;54:223–233. doi: 10.1016/s0378-1135(96)01282-5. [DOI] [PubMed] [Google Scholar]
- 13.O’Reilly L M, Daborn C J. The epidemiology of Mycobacterium bovis infections in animals and man: a review. Tubercle Lung Dis. 1995;76(Suppl. 1):1–46. doi: 10.1016/0962-8479(95)90591-x. [DOI] [PubMed] [Google Scholar]
- 14.Perumaala V S, Adams L G, Payeur J B, Jarnagin J L, Baca D R, Güemes F S, Ficht T A. Molecular epidemiology of Mycobacterium bovis in Texas and Mexico. J Clin Microbiol. 1996;34:2066–2071. doi: 10.1128/jcm.34.9.2066-2071.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quigley F C, Costello E, Flynn O, Gogarty A, Mc. Guirk J, Murphy A, Egan J. Isolation of mycobacteria from lymph node lesions in deer. Vet Rec. 1997;141:516–518. doi: 10.1136/vr.141.20.516. [DOI] [PubMed] [Google Scholar]
- 16.Romano M I, Alito A, Fisanotti J C, Bigi F, Kantor I, Cicuta M E, Cataldi A. Comparison of different genetic markers for molecular epidemiology of bovine tuberculosis. Vet Microbiol. 1996;50:59–71. doi: 10.1016/0378-1135(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 17.Roring S, Hughes M S, Beck L-A, Skuce R A, Neill S D. Rapid diagnosis and strain differentiation of Mycobacterium bovis in radiometric culture by spoligotyping. Vet Microbiol. 1998;61:71–80. doi: 10.1016/s0378-1135(98)00167-9. [DOI] [PubMed] [Google Scholar]
- 18.Roring S, Brittain D, Bunschoten A E, Hughes M S, Skuce R A, van Embden J D A, Neill S D. Spacer oligotyping of Mycobacterium bovis isolates compared to typing by restriction fragment length polymorphism using PGRS, DR and IS6110 probes. Vet Microbiol. 1998;61:111–120. doi: 10.1016/s0378-1135(98)00178-3. [DOI] [PubMed] [Google Scholar]
- 19.Ross B C, Raios K, Jackson K, Dwyer B. Molecular cloning of a highly repeated DNA element from Mycobacterium tuberculosis and its use as an epidemiological tool. J Clin Microbiol. 1992;30:942–946. doi: 10.1128/jcm.30.4.942-946.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skuce R A, Brittain D, Hughes M S, Beck L-A, Neill S D. Genomic fingerprinting of Mycobacterium bovis from cattle by restriction fragment length polymorphism analysis. J Clin Microbiol. 1994;32:2387–2392. doi: 10.1128/jcm.32.10.2387-2392.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skuce R A, Brittain D, Hughes M S, Neill S D. Differentiation of Mycobacterium bovis isolates from animals by DNA typing. J Clin Microbiol. 1996;34:2469–2474. doi: 10.1128/jcm.34.10.2469-2474.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szewzyk R, Svenson S B, Hoffner S E, Bölske G, Wahlström H, Englund L, Engvall A, Källenius G. Molecular epidemiological studies of Mycobacterium bovis infections in humans and animals in Sweden. J Clin Microbiol. 1995;33:3183–3185. doi: 10.1128/jcm.33.12.3183-3185.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Embden J D A, Cave M D, Crawford J T, Dale J W, Eisenach K D, Gicquel B, Hermans P, Martin C, McAdam R, Shinnick T M, Small P M. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J Clin Microbiol. 1993;31:406–409. doi: 10.1128/jcm.31.2.406-409.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Soolingen D, de Haas P E W, Haagsma J, Eger T, Hermans P W M, Ritacco V, Alito A, van Embden J D A. Use of various genetic markers in differentiation of Mycobacterium bovis strains from animals and humans and for studying epidemiology of bovine tuberculosis. J Clin Microbiol. 1994;32:2425–2433. doi: 10.1128/jcm.32.10.2425-2433.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Soolingen D, Qian L, de Haas P E W, Douglas J T, Traore H, Portaels F, Qing H Z, Enkhsaikan D, Nymadawa P, van Embden J D A. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J Clin Microbiol. 1995;33:3234–3238. doi: 10.1128/jcm.33.12.3234-3238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Whipple D L, Ryan Clarke P, Jarnagin J L, Payeur J B. Restriction fragment length polymorphism analysis of Mycobacterium bovis isolates from captive and free-ranging animals. J Vet Diagn Invest. 1997;9:381–386. doi: 10.1177/104063879700900407. [DOI] [PubMed] [Google Scholar]