Abstract
Objective:
This study aimed to establish a mammography-based radiomics model for predicting the risk of estrogen receptor (ER)-positive, lymph node (LN)-negative invasive breast cancer recurrence based on Oncotype DX and validated it by using multicenter data.
Methods:
A total of 304 potentially eligible patients with pre-operative mammography images and available Oncotype DX score were retrospectively enrolled from two hospitals. The patients were grouped as training set (168 patients), internal test set (72 patients), and external test set (64 patients). Radiomics features were extracted from the mammography images of each patient. Spearman correlation analysis, analysis of variance, and least absolute shrinkage and selection operator regression were performed to reduce the redundant features in the training set, and the least absolute shrinkage and selection operator algorithm was used to construct the radiomics signature based on selected features. Multivariate logistic regression was utilized to construct classification models that included radiomics signature and clinical risk factors to predict low vs intermediate and high recurrence risk of ER-positive, LN-negative invasive breast cancer in the training set. The models were evaluated with the receiver operating characteristic curve in the training set. The internal and external test sets were used to confirm the discriminatory power of the models. The clinical usefulness was evaluated by using decision curve analysis.
Results:
The radiomics signature consisting of three radiomics features achieved favorable prediction performance. The multivariate logistic regression model including radiomics signature and clinical risk factors (tumor grade and HER 2) showed good performance with areas under the curve of 0.92 (95% confidence interval [CI] 0.86 to 0.97), 0.88 (95% CI 0.75 to 1.00), and 0.84 (95% CI 0.69 to 0.99) in the training, internal and external test sets, respectively. The DCA indicated that when the threshold probability is ranges from 0.1 to 1.0, the radiomics model adds more net benefit than the “treat all” or “treat none” scheme in internal and external test sets.
Conclusion:
As a non-invasive pre-operative prediction tool, the mammography-based radiomics model incorporating radiomics and clinical factors show favorable predictive performance for predicting the risk of ER-positive, LN-negative invasive breast cancer recurrence based on Oncotype DX.
Advances in knowledge:
The mammography-based radiomics model incorporating radiomics and clinical factors shows favorable predictive performance for predicting the risk of ER-positive, LN-negative invasive breast cancer recurrence.
Introduction
Breast cancer is the most common tumor and one of the leading causes of cancer deaths in females.1 Targeted adjuvant therapy based on estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor Type 2 (HER2) expression can effectively improve the prognosis of patients with breast cancer. Adjuvant chemotherapy can reduce the risk of breast cancer recurrence. However, increasing chemotherapy on the basis of endocrine therapy may not be suitable for all patients with ER-positive and lymph node (LN)-negative breast cancer.2 The risk of recurrence in 10 years after tamoxifen treatment alone is only about 15%. If adjuvant chemotherapy is applied to all patients, then at least 85% of them are over treated.3,4 The 21-gene recurrence risk score test (Oncotype DX) is a model that provides outcome prediction information for patients with ER-positive and LN-negative breast cancer by measuring specific breast cancer genes.5,6 When the recurrence risk score is high (≥31), patients are predicted to benefit from adjuvant chemotherapy. When the recurrence risk score is low (<18), the distant recurrence rate within 10 years is extremely low and the patient is unlikely to be affected by adjuvant chemotherapy.7 The Oncotype DX was approved by the US FDA in 2005 and is the only multigene test project jointly recommended by the American Society of Clinical Oncology and the National Comprehensive Cancer Network.6,8 However, such tests are expensive, costing about $4000 per patient. Therefore, developing a non-invasive and accurate method for predicting the recurrence risk of breast cancer is of great clinical value.
Mammography is one of the common methods for breast cancer screening. However, conventional mammography cannot accurately evaluate the recurrence risk of breast cancer. With the development of analytical techniques, the potential of radiomics in oncology decision support has increased.9–11 Our previous research and other researchers’ results indicated that radiomics can decode clinical information including diagnosis and evaluation for breast cancer.12–17 Recent studies have explored the use of radiomic features in distinguishing benign and malignant lesions, predicting axillary lymph node metastasis or molecular subtype based on mammography.18–20 To the best of our knowledge, no mammography-based radiomics studies has been conducted for predicting breast cancer recurrence.
In this study, we developed a mammography-based radiomics model for predicting the risk of ER-positive, LN-negative invasive breast cancer recurrence and validated it by using multicenter data.
Methods and materials
Patients
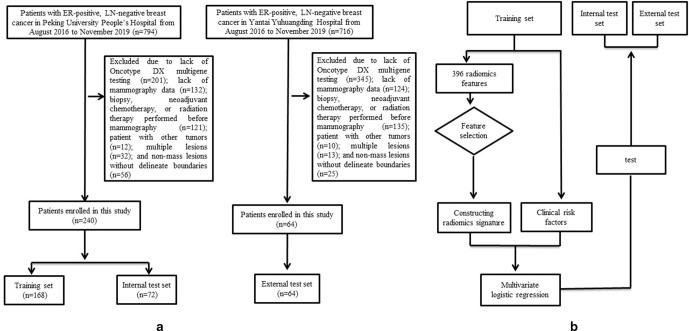
Figure 1 presents the study flowchart. This retrospective study collected patients who underwent mammography in Peking University People’s Hospital and Yantai Yuhuangding Hospital from August 2016 to November 2019. The inclusion criteria were as follows: (1) available Oncotype DX multigene testing; (2) pathologically confirmed ER-positive, LN-negative invasive breast cancer; and (3) available mammography data. The exclusion criteria were as follows: (1) biopsy, neoadjuvant chemotherapy, or radiation therapy performed before mammography; (2) patient with other tumors; (3) multiple lesions and (4) non-mass lesions without delineate boundaries. Finally, 240 patients from Peking University People’s Hospital were included and divided into training set (168 patients) and internal test set (72 patients) according to the ratio of 7:3. External test set consisting of 64 patients were from Yantai Yuhuangding Hospital. This study was approved by the ethics committees of the two hospitals. Informed consent from the patients was waived.
Figure 1.
The study flowchart. Flow chart of the study population selection (a) and study design (b).
Oncotype DX test
Oncotype DX is a detection method used to design the best individualized treatment for patients with ER-positive and LN-negative breast cancer. Combined with other clinical information, breast cancer Oncotype DX can help to judge whether chemotherapy is needed or not, and it also helps doctors to judge the risk of recurrence to avoid overtreatment, effectively intervene and manage the entire disease process, and improve survival benefits. In this study, Oncotype DX score was used to evaluate the risk of recurrence and analyze the benefits of chemotherapy by examining the expression of 21 genes in breast cancer tissues. These 21 genes include 16 tumor-related genes and 5 reference genes.3,4 The expression levels of the 21 genes were converted to recurrence risk index by a special algorithm (0–100 points). The risk was divided into three levels: low (<18), medium (18–30), and high (>31).3 The risk of recurrence was 6.8%, 14.3%, and 30.5%, respectively. Our prediction task was low versus medium and high recurrence risk to identify patients with low risk of recurrence.
Pathological evaluation
We obtained histopathological information including tumor grade as well as ER, PR, HER 2, and Ki-67 status through medical record systems. Threshold values were ≤1% for the ER and PR levels and ≤20% for Ki-67.21
Mammography image acquisition
The patients underwent mammography with GE Senographe Essential (GE Healthcare, Milwaukee, WI). The mammography images were at 14-bit quantization with a pixel size of 100 × 100 µm. All DICOM data were exported from Picture Archiving and Communication Systems.
Image segmentation and radiomics feature extraction
Region of interest (ROI) was segmented by two senior radiologists who have 5 and 10 years of experience in medical imaging diagnosis. They were blinded to the pathology reports and segmented manually the mammography images of CC view for further analysis (Figure 2). Image preprocessing, which was needed before feature extraction, included three steps: (1) Gray level standardization adopted the μ ± 3σ method and the gray values greater than μ ± 3σ were set to 022; (2) discretization of the gray level23 ; and (3) the image resampling was performed, and the anisotropic voxels were converted into 0.1× 0.1 mm.24 We extracted 396 radiomics features from each image, including shape-and size-based, first-order statistical, and texture features. The process was performed using AK software (Artificial Intelligence Kit; GE Healthcare, China, Shanghai).
Figure 2.
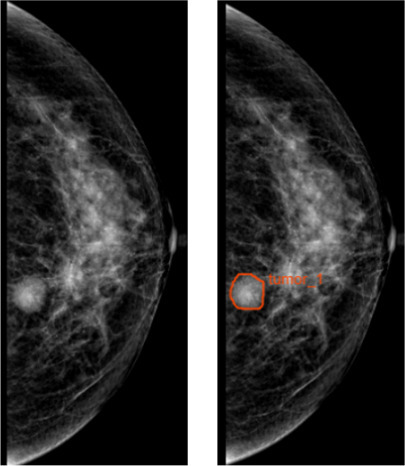
ROI segmentation. The ROI segmentation of breast lesion on CC view of mammography. CC, craniocaudal; ROI, region of interest.
Inter- and intracorrelation coefficients (ICCs) were calculated to assess the reproducibility of the radiomics features. First, the two radiologists randomly segmented 30 images. After 2 weeks, radiologist 1 repeated the same procedure. Subsequently, radiologist 1 segmented the remaining images. An ICC >0.8 indicates good agreement with the feature extraction.
Radiomics feature selection and radiomics signature building
First, features with ICC >0.8 were retained in the training set. Second, Spearman correlation analysis was used to exclude highly correlated features. One feature in the paired features with correlation coefficients > 0.9 was excluded. Third, features were further selected using analysis of variance (ANOVA). The features with p < 0.001 were further selected. ANOVA was a filtering feature selection method, which could be used to select the significant feature between two classes, and the significant features might have more discriminative ability, besides, ANOVA would reduce the number of the features, which was good for least absolute shrinkage and selection operator (LASSO) to reduce the calculation consuming. Fourth, we applied the LASSO regression for selecting the key radiomics features. LASSO method, which is suitable for the regression of high-dimensional data. When LASSO was performed, the hyperparameter (λ) in the LASSO model was determined at first using 10-fold cross-validation based on minimum criteria, binomial deviances. The optimized λ was corresponding to the least binomial deviance. Secondly, after the λ was determined, the coefficients of the feature were determined too. At this time, the features with non-zero coefficients were kept as the final feature subset. The radiomics signature score (Rad-score) was then computed using a linear combination of the key features weighted by their LASSO coefficients.
Development of a multivariate regression model
Univariate and multivariate logistic regression analyses were performed to analyze risk factors related to the identification of recurrence risk of breast cancer. These factors, which included Rad-score, age, tumor size, tumor grade, PR, HER 2, and Ki-67, were integrated into the multivariate logistic regression analysis of backward-stepwise selection. A multivariate logistic regression model including radiomics signature and clinical risk factors was constructed to identify patients with a low risk of recurrence. For comparison, we also constructed a clinical model based on clinical risk factors. Variance inflation factor (VIF) was used to eliminate collinearity. The stepwise process was stopped when the Akaike information criterion (AIC) is minimum.
Assessment and validation of multivariate logistic regression model
The performance of multivariate logistic regression models was assessed with the ROC curve in the training set. Decision curve analysis (DCA) was conducted to determine the clinical usefulness of the radiomics models by quantifying the net benefits at different threshold probabilities. The models were validated using internal and external test sets.
Statistical analysis
R software (v. 3.4.4, https://www.r-project.org/) was used for statistical analysis. p < 0.05 was considered statistically significant. Logistic regression was used to establish the prediction model for predicting the risk of ER-positive, LN-negative invasive breast cancer recurrence. LASSO logistic regression was established with 10-fold cross-validation. DeLong’s test was used to compare the differences of ROC curves. Logistic regression analysis was conducted by “glmnet” software package, and ROC curve analysis was performed with “pROC” software package. DCA was performed with “rmda” package.
Results
Clinical characteristics
The patient characteristics in the training set, internal and external test sets are shown in Table 1. No significant difference in the Oncotype DX score was found among the three sets (p = 0.845). Clinicopathological factors were not significantly different between the three sets.
Table 1.
Clinical characteristics of the patients included in this study
| Characteristics | Training set | Internal test set | External test set | p |
|---|---|---|---|---|
| Number | 168 | 72 | 64 | |
| Age, year (SD) | 48.10 ± 8.1 | 47.51 ± 9.1 | 47.90 ± 10.5 | 0.550 |
| Tumor size, cm (SD) | 2.48 ± 0.9 | 2.39 ± 1.1 | 2.45 ± 1.2 | 0.323 |
| Oncotype DX score (%) | ||||
| Low | 80 (48.62) | 35 (48.61) | 37 (57.81) | 0.317 |
| Medium | 56 (33.33) | 24 (33.33) | 22 (34.38) | |
| High | 32 (18.05) | 13 (18.06) | 5 (7.81) | |
| Tumor grade (%) | ||||
| I | 45 (26.79) | 22 (30.56) | 20 (31.25) | 0.923 |
| II | 95 (56.55) | 44 (61.11) | 34 (53.13) | |
| III | 28 (16.66) | 10 (13.89) | 10 (15.63) | |
| PR status (%) | 0.317 | |||
| Positive | 70 (71.43) | 30 (46.67) | 20 (31.25) | |
| Negative | 98 (58.33) | 42 (58.33) | 44 (68.75) | |
| HER 2 status (%) | ||||
| Positive | 38 (22.62) | 16 (22.22) | 14 (21.88) | |
| Negative | 130 (77.38) | 56 (77.78) | 50 (78.12) | |
| Ki-67 status (%) | ||||
| Positive | 131 (77.98) | 59 (81.94) | 49 (76.56) | |
| Negative | 37 (22.02) | 13 (18.06) | 15 (23.44) | |
HER, human epidermal growth factor receptor; PR, progesterone receptor; SD, standadrd deviation.
Feature selection and development of multivariate regression model
The substantial reproducibility of radiomics feature extraction were achieved with the intra- and interobserver ICCs from 0.861 to 0.973. After correlation analysis, 189 features were selected. After ANOVA, 99 features were selected. After conducting LASSO method, three features, namely, HaralickCorrelation_angle45_offset7, GLCMEntropy_AllDirection_offset1_SD, and LongRunLowGreyLevelEmphasis_AllDirection_offset4_SD were selected. The final formula of Rad-score is as follow:
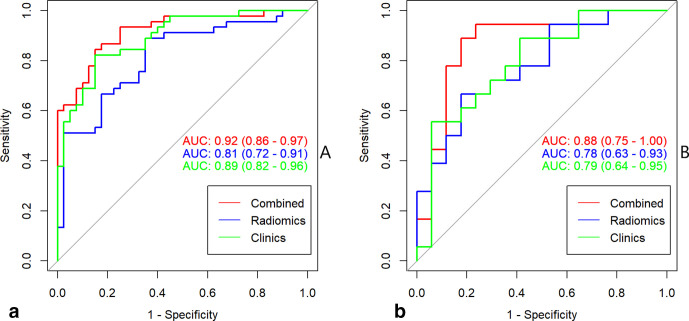
Rad-score = 0.919×HaralickCorrelation_angle45_offset7 + 0.089×GLCMEntropy_AllDirection_offset1_SD + 0.061×LongRunLowGreyLevelEmphasis_AllDirection_offset4_SD + 0.186. The radiomics signature yielded AUCs of 0.81 (95% CI, 0.72–0.91) in training set and 0.78 (95% CI, 0.63–0.93) in internal test set (Figure 3).
Figure 3.
The ROC curves of multivariate logistic regression in training (a) and internal (b) test sets. ROC, ROC, receiver operating characteristic
Table 2 shows clinical risk factors related to identifying the recurrence risk of breast cancer in the training cohort. Based on the uni- and multivariate logistic regression analyses, Rad-score, tumor grade, and HER 2 were proved to be the independent clinical risk factors of tumor recurrence. The clinical model yielded AUCs of 0.89 (95% CI, 0.82–0.96) in training set and 0.79 (95% CI, 0.64–0.95) in internal test set (Figure 3).
Table 2.
The relationship between clinical factors and Oncotype DX
| Variable | Univariate logistic regression | Multivariate logistic regression | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| Age, years | 0.987 (0.953–1.021) | 0.465 | NA | NA |
| Tumor size | 1.013 (0.788–1.314) | 0.917 | NA | NA |
| Tumor grade | 2.858 (1.367–3.989) | 0.011 | 2.110 (1.137–3.957) | 0.018a |
| PR | 2.258 (0.067–0.489) | 0.502 | NA | NA |
| HER 2 | 2.186 (1.162–4.165) | 0.016a | 2.002 (1.206–3.823) | 0.016a |
| Ki-67 | 0.902 (0.543–1.301) | 0.545 | NA | NA |
| Rad-score | 5.186 (4.262–6.386) | 0.006a | 6.002 (3.947–7.002) | 0.008a |
CI, confidence interval; HER 2, Human epidermal growth factor receptor type 2; NA, Not available; OR, Odds ratio; PR, Progesterone receptor.
These variables were eliminated in the multivariate logistic regression model in training set, so the OR and p values were not available.
p < 0.05.
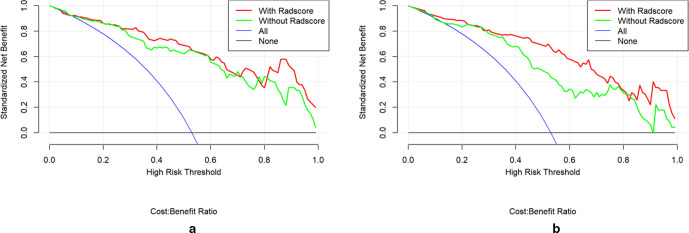
The multivariate logistic regression model including radiomics signature and clinical risk factors yielded AUCs of 0.92 (95% CI, 0.86–0.97) in the training set, 0.88 (95% CI, 0.75–1.00) in the internal test set (Figure 3). A significant difference was observed between the differences of ROC curves in the two sets (p < 0.001). The DCA indicated that when the threshold probability is ranges from 0.1 to 1.0 in internal test set the radiomics model to predict recurrence risk adds more net benefit than the “treat all” or “treat none” scheme (Figure 4a).
Figure 4.
The DCA of multivariate logistic regression model in internal (a) and external (b) test sets. DCA, decision curve analysis.
Multicenter verification of multivariate logistic regression model
The multivariate logistic regression model yielded AUC of 0.84 (95% CI, 0.69–0.99) in external test set (Figure 5). The DCA indicated that when the threshold probability is ranges from 0.1 to 1.0 in external test set the radiomics model to predict recurrence risk adds more net benefit than the “treat all” or “treat none” scheme (Figure 4b).
Figure 5.
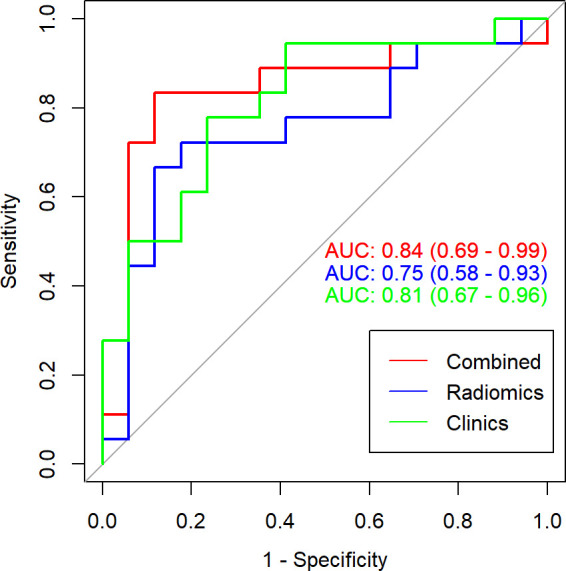
The ROC curves of multivariate logistic regression in external test set. AUC, area under the curve; ROC, receiver operating characteristic.
Discussion
In the present study, the proposed multivariate logistic regression model including radiomics and clinical risk factors shows favorable predictive performance in discriminating low versus intermediate and high recurrence risk of ER-positive, LN-negative invasive breast cancer. This multicenter study showed that the model had good performance, which provided a higher level for the application of the model.
In this study, the prediction task was low vs medium and high recurrence risk to identify patients with low risk of recurrence. The choice of classification task mainly refers to previous literature, which focused on identifying patients with high vs medium and low risk.25,26 However, studies have shown that identifying patients with a low risk of recurrence is important for decision-making in chemotherapy because the risk of recurrence is low and it is possible to avoid chemotherapy. A previous study reported that MRI-based radiomics model can predict the recurrence risk of breast cancer.27 Mammography has the advantages of lower cost and less time consumption compared with MRI and thus has great clinical application value. Therefore, we constructed a mammography-based radiomics model. It is the same as the previous study.27 Our study also reported that texture features which indicated tumor heterogeneity have important significance for predicting the recurrence risk of breast cancer. Three texture features remained and were combined to build the radiomics signature. three features were HaralickCorrelation_angle45_offset7, GLCMEntropy_AllDirection_offset1_SD, and LongRunLowGreyLevelEmphasis_AllDirection_offset4_SD, respectively. HaralickCorrelation_angle45_offset7 reflected the linear dependency of gray level values to their respective voxels in the GLCM, GLCMEntropy_AllDirection_offset1_SD meant a sum of neighborhood intensity value differences and LongRunLowGreyLevelEmphasis represented the joint distribution of long run lengths with lower gray-level values. And, we also found that the three texture features values in the medium and high recurrence risk group were higher than those in the low recurrence risk group, indicating that the increase of the three texture features values will increase the recurrence risk of breast patients.
The combination of a set of biomarkers rather than a single marker is the most promising method, which may improve the prediction performance of the model.28–30 Based on the logistic regression analysis, tumor grade and HER 2 were proved to be the independent risk factors of tumor recurrence. This finding is consistent with the reports of previous studies, which constructed a model based on clinicopathological factors for predicting the recurrence risk of breast cancer.28,31
Another advantage of this study is our multicenter verification. The lack of external verification may lead to the accidental acquisition of a high correlation between radiomics and genomic characteristics, but the conclusion lacks credibility. This multicenter study provided additional evidence of radiomics in predicting the recurrence risk of breast cancer.
Our study has several limitations. First, the data set is small. Most patients cannot obtain Oncotype DX score because of the high cost of Oncotype DX. Large sample size is needed to improve the prediction performance of the model. Second, the predictive model is fit to, and its performance is evaluated against, the Oncotype DX score, which is an imperfect measure of recurrence risk.3,4,32–35 Furthermore, the DCA shows that the radiomics model adds more net benefit than the “treat all” or “treat none” scheme, but to show clinical usability the true recurrence outcome would need to be known. Third, ROIs were annotated manually, although we sought to analyze inter- and intraobserver variabilities among radiologists by utilizing ICCs and satisfactory ICCs is achieved. Fourth, data acquired from a single model of the imaging device, which means that the generalizability beyond this device cannot be determined despite the use of a data set from a different institution. Fifth, the radiomics features were extracted with proprietary analysis software, which creates a significant barrier to reproducibility. We will continue our research in the future.
In summary, the proposed multivariate logistic regression model including radiomics and clinical risk factors is a non-invasive predictive tool that shows good application prospects in predicting the risk of ER-positive, LN-negative invasive breast cancer recurrence. The combination of radiomics and genomic data is needed to improve the prediction performance of the model.
Footnotes
Acknowledgments: We thank GE Healthcare for providing technical support in this study.
Funding: This study was supported by the National Natural Science Foundation of China (82001775) and “Taishan Scholar” Project (No. ts20190991).
The authors Ning Mao and Ping Yin contributed equally to the work.
Data sharing statement: Data are available upon request from the corresponding author.
Contributor Information
Ning Mao, Email: maoning@pku.edu.cn.
Ping Yin, Email: yinping915@pku.edu.cn.
Haicheng Zhang, Email: haicheng92@126.com.
Kun Zhang, Email: zkzkzkhp@126.com.
Xicheng Song, Email: drxchsong@163.com.
Dong Xing, Email: 18660505166@163.com.
Tongpeng Chu, Email: chutongpeng@163.com.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 2.Boyages J, Taylor R, Chua B, Ung O, Bilous M, Salisbury E, et al. A risk index for early node-negative breast cancer. Br J Surg 2006; 93: 564–71. doi: 10.1002/bjs.5207 [DOI] [PubMed] [Google Scholar]
- 3.Paik S, Shak S, Tang G, Kim C, Baker J, Cronin M, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med 2004; 351: 2817–26. doi: 10.1056/NEJMoa041588 [DOI] [PubMed] [Google Scholar]
- 4.Cronin M, Sangli C, Liu M-L, Pho M, Dutta D, Nguyen A, et al. Analytical validation of the Oncotype DX genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin Chem 2007; 53: 1084–91. doi: 10.1373/clinchem.2006.076497 [DOI] [PubMed] [Google Scholar]
- 5.Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. JCO 2008; 26: 721–8. doi: 10.1200/JCO.2007.15.1068 [DOI] [PubMed] [Google Scholar]
- 6.Carlson RW, Allred DC, Anderson BO, Burstein HJ, Carter WB, Edge SB, et al. Breast cancer. clinical practice guidelines in oncology. J Natl Compr Canc Netw 2009; 7: 122–92. [DOI] [PubMed] [Google Scholar]
- 7.Sparano JA, Gray RJ, Makower DF, Pritchard KI, Albain KS, Hayes DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 2018; 379: 111–21. doi: 10.1056/NEJMoa1804710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dinan MA, Mi X, Reed SD, Hirsch BR, Lyman GH, Curtis LH. Initial trends in the use of the 21-gene recurrence score assay for patients with breast cancer in the Medicare population, 2005-2009. JAMA Oncol 2015; 1: 158–66. doi: 10.1001/jamaoncol.2015.43 [DOI] [PubMed] [Google Scholar]
- 9.Gillies RJ, Kinahan PE, Hricak H. Radiomics: images are more than pictures, they are data. Radiology 2016; 278: 563–77. doi: 10.1148/radiol.2015151169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RGPM, Granton P, et al. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 2012; 48: 441–6. doi: 10.1016/j.ejca.2011.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aerts HJWL, Velazquez ER, Leijenaar RTH, Parmar C, Grossmann P, Carvalho S, et al. Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 2014; 5: 4006. doi: 10.1038/ncomms5006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parekh VS, Jacobs MA. Integrated radiomic framework for breast cancer and tumor biology using advanced machine learning and multiparametric MRI. NPJ Breast Cancer 2017; 3: 43. doi: 10.1038/s41523-017-0045-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu Z, Li Z, Qu J, Zhang R, Zhou X, Li L, et al. Radiomics of multiparametric MRI for pretreatment prediction of pathologic complete response to neoadjuvant chemotherapy in breast cancer: a multicenter study. Clin Cancer Res 2019; 25: 3538–47. doi: 10.1158/1078-0432.CCR-18-3190 [DOI] [PubMed] [Google Scholar]
- 14.Antunovic L, De Sanctis R, Cozzi L, Kirienko M, Sagona A, Torrisi R, et al. Pet/Ct radiomics in breast cancer: promising tool for prediction of pathological response to neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging 2019; 46: 1468–77. doi: 10.1007/s00259-019-04313-8 [DOI] [PubMed] [Google Scholar]
- 15.Park H, Lim Y, Ko ES, Cho H-ho, Lee JE, Han B-K, et al. Radiomics signature on magnetic resonance imaging: association with disease-free survival in patients with invasive breast cancer. Clin Cancer Res 2018; 24: 4705–14. doi: 10.1158/1078-0432.CCR-17-3783 [DOI] [PubMed] [Google Scholar]
- 16.Mao N, Yin P, Wang Q, Liu M, Dong J, Zhang X, et al. Added value of Radiomics on mammography for breast cancer diagnosis: a feasibility study. J Am Coll Radiol 2019; 16(4 Pt A): 485-491. doi: 10.1016/j.jacr.2018.09.041 [DOI] [PubMed] [Google Scholar]
- 17.Mao N, Yin P, Li Q, Wang Q, Liu M, Ma H, et al. Radiomics nomogram of contrast-enhanced spectral mammography for prediction of axillary lymph node metastasis in breast cancer: a multicenter study. Eur Radiol 2020; 30: 6732–9. doi: 10.1007/s00330-020-07016-z [DOI] [PubMed] [Google Scholar]
- 18.Ma W, Zhao Y, Ji Y, Guo X, Jian X, Liu P, et al. Breast cancer molecular subtype prediction by mammographic radiomic features. Acad Radiol 2019; 26: 196–201. doi: 10.1016/j.acra.2018.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Mendel KR, Lan L, Sheth D, Giger ML. Digital mammography in breast cancer: additive value of Radiomics of breast parenchyma. Radiology 2019; 291: 15–20. doi: 10.1148/radiol.2019181113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan H, Wu Y, Bao F, Zhou J, Wan J, Tian J, et al. Mammography-based radiomics nomogram: a potential biomarker to predict axillary lymph node metastasis in breast cancer. Br J Radiol 2020; 93: 20191019. doi: 10.1259/bjr.20191019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, et al. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen international expert consensus on the primary therapy of early breast cancer 2013. Annals of Oncology 2013; 24: 2206–23. doi: 10.1093/annonc/mdt303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Collewet G, Strzelecki M, Mariette F. Influence of MRI acquisition protocols and image intensity normalization methods on texture classification. Magn Reson Imaging 2004; 22: 81–91. doi: 10.1016/j.mri.2003.09.001 [DOI] [PubMed] [Google Scholar]
- 23.Gibbs P, Turnbull LW. Textural analysis of contrast-enhanced Mr images of the breast. Magn Reson Med 2003; 50: 92–8. doi: 10.1002/mrm.10496 [DOI] [PubMed] [Google Scholar]
- 24.Depeursinge A, Foncubierta-Rodriguez A, Van De Ville D, Müller H. Three-Dimensional solid texture analysis in biomedical imaging: review and opportunities. Med Image Anal 2014; 18: 176–96. doi: 10.1016/j.media.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 25.Ashraf AB, Daye D, Gavenonis S, Mies C, Feldman M, Rosen M, et al. Identification of intrinsic imaging phenotypes for breast cancer tumors: preliminary associations with gene expression profiles. Radiology 2014; 272: 374–84. doi: 10.1148/radiol.14131375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Zhu Y, Burnside ES, Drukker K, Hoadley KA, Fan C, et al. MR imaging radiomics signatures for predicting the risk of breast cancer recurrence as given by research versions of MammaPrint, Oncotype DX, and PAM50 gene assays. Radiology 2016; 281: 382–91. doi: 10.1148/radiol.2016152110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li H, Zhu Y, Burnside ES, Drukker K, Hoadley KA, Fan C, et al. MR imaging Radiomics signatures for predicting the risk of breast cancer recurrence as given by research versions of MammaPrint, Oncotype DX, and PAM50 gene assays. Radiology 2016; 281: 382–91. doi: 10.1148/radiol.2016152110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klein ME, Dabbs DJ, Shuai Y, Brufsky AM, Jankowitz R, Puhalla SL, et al. Prediction of the Oncotype DX recurrence score: use of pathology-generated equations derived by linear regression analysis. Mod Pathol 2013; 26: 658–64. doi: 10.1038/modpathol.2013.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gage MM, Rosman M, Mylander WC, Giblin E, Kim H-seok, Cope L, et al. A Validated Model for Identifying Patients Unlikely to Benefit From the 21-Gene Recurrence Score Assay. Clin Breast Cancer 2015; 15: 467–72. doi: 10.1016/j.clbc.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang P, Wang J, Hicks DG, Wang X, Schiffhauer L, McMahon L, et al. A lower Allred score for progesterone receptor is strongly associated with a higher recurrence score of 21-gene assay in breast cancer. Cancer Invest 2010; 28: 978–82. doi: 10.3109/07357907.2010.496754 [DOI] [PubMed] [Google Scholar]
- 31.Dialani V, Gaur S, Mehta TS, Venkataraman S, Fein-Zachary V, Phillips J, et al. Prediction of low versus high recurrence scores in estrogen Receptor–Positive, lymph Node–Negative invasive breast cancer on the basis of radiologic-pathologic features: comparison with Oncotype DX test recurrence scores. Radiology 2016; 280: 370–8. doi: 10.1148/radiol.2016151149 [DOI] [PubMed] [Google Scholar]
- 32.van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AAM, Mao M, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 2002; 415: 530–6. doi: 10.1038/415530a [DOI] [PubMed] [Google Scholar]
- 33.van de Vijver MJ, He YD, van 't Veer LJ, Dai H, Hart AAM, Voskuil DW, et al. A gene-expression signature as a predictor of survival in breast cancer. New England Journal of Medicine 2002; 347: 1999–2009. doi: 10.1056/NEJMoa021967 [DOI] [PubMed] [Google Scholar]
- 34.Paik S, Tang G, Shak S, Kim C, Baker J, Kim W, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen Receptor–Positive breast cancer. J Clin Oncol 2006; 24: 3726–34. doi: 10.1200/JCO.2005.04.7985 [DOI] [PubMed] [Google Scholar]
- 35.Parker JS, Mullins M, Cheang MCU, Leung S, Voduc D, Vickery T, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. JCO 2009; 27: 1160–7. doi: 10.1200/JCO.2008.18.1370 [DOI] [PMC free article] [PubMed] [Google Scholar]