Abstract
Radiation therapy has the potential to modulate the immune system in a variety of ways, and given the critical role of the immune system in cancer elimination, it is becoming increasingly important to understand how radiation can be strategically implemented in conjunction with approved immunotherapies to improve the cancer patient’s chance of cure and/or quality of life. Current successful, approved cancer immunotherapies fall into two broad classes: antibodies and cellular therapies. Approved cellular therapies thus far consist of Chimeric Antigen Receptor (CAR) T-cells targeting CD19 for refractory non-Hodgkin lymphoma and relapsed or refractory acute lymphoblastic leukemia. Part of the ardor surrounding CAR T-cells stems from the fact that the survival curve of treated patients has a clear plateau, meaning that a number of patients with aggressive, disseminated disease who would have otherwise died rather rapidly appear to now be cured, commonly after just one dose. Despite an encouraging number of these durable remissions, the majority do still relapse. In this review, we discuss the potential for strategically utilizing radiation to further improve CAR T-cell patient outcomes. Given that there are currently over 750 cellular therapies in development, half of which are now in clinical trial, CAR T-cell usage will inevitably expand; as the field grows in importance and effectiveness, radiation oncology has the opportunity to coevolve symbiotically and steer these novel, exciting live therapies to new depths.
The CAR T-cell lymphoma landscape
Being the most common hematologic malignancy, non-Hodgkin lymphoma (NHL) accounts for 4–5% of all cancers in the United States.1 Of the >90 subtypes of NHL, diffuse large B-cell lymphoma (DLBCL) is the most common,2 accounting for 30–40% of cases. First-line therapy for DLBCL remains R-CHOP chemoimmunotherapy (the anti-CD20 monoclonal antibody rituximab with cyclophosphamide, vincristine, doxorubicin, and prednisone) with or without radiation therapy. Although this regimen is successful for the majority of patients, 10–30% are expected to relapse 31170029.
Patients who relapse after first-line therapy generally undergo second-line chemotherapy followed by high-dose chemotherapy and autologous hematopoietic cell transplant.3 Patients who achieve a complete metabolic response to second-line chemotherapy fare better than those who do not, with an overall relapse rate of 20–50%, respectively.4
At this juncture, patients who are not free of tumor may have the option of further third-line chemotherapy, allogeneic stem cell transplant, or Chimeric Antigen Receptor (CAR) T-cell therapy. Given its favorable side-effect profile and relatively strong efficacy, many will choose CAR T-cell therapy if available. CAR T-cells achieve a complete remission in 40–52% in this group of patients after a single dose, many of which appear to be lasting.5–7
Despite impressive success by historical standards, still the majority of patients will fail CAR T-cells. This begs the question, how do we improve the durability of response to CAR T-cells? What can we offer patients who relapse after CAR T-cells?
CAR T-cell mechanism
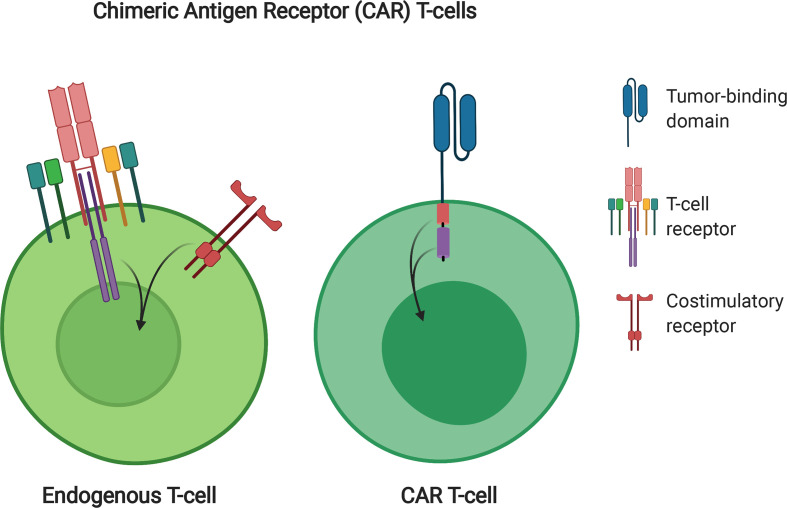
Uniform understanding of what CAR T-cells are and why they function is imperative to rationalizing concurrent or sequential treatment regimens. After it became known T-cells’ killing capacity relied upon engagement of the T-cell receptor (TCR) with a tumor cell, and the structure of the TCR become known, first-generation CARs were developed in 1989 consisting of a TCR stimulatory domain attached to a single chain variable antibody fragment that recognized a tumor antigen.8 However, T-cells containing these chimeric receptors did not exert powerful long-term effects, and the therapy did not gain traction. After it became known that optimal T-cell function critically relies on both TCR activation as well as costimulatory receptor activation, the experiment was performed in which an internal costimulatory domain (originally CD28) was cloned in frame with an intracellular stimulatory domain from the TCR, as well as an extracellular tumor-binding domain.9 Somewhat remarkably, tumor binding achieved full activation of both the TCR and costimulatory receptor domains simultaneously, leading to tumor killing through the TCR fragment and enhanced T-cell expansion and function through the costimulatory domain. This “second generation” CAR became the template for further CAR therapies. 2 years later, the CD28 costimulatory domain was replaced with the 4-1BB costimulatory domain,10 again with excellent results. Further studies using different costimulatory domains11 or a combination of two costimulatory domains together to make a “third generation” CAR12,13 have since been performed, although without consistently improved results, so second generation CAR T-cells remain the most common choice in clinical trials, and the only FDA approved version. CD28 costimulation is used in Axicabtagene ciloleucel, and 4-1BB in Tisagenlecleucel and Lisocabtagene maraleucel (Figure 1).
Figure 1.
CAR T-cell design. Endogenous T-cells require stimulation through the TCR as well as costimulatory signaling to become fully functional. CARs combine signaling domains from both to create one simplified receptor. This receptor consists of an extracellular tumor-binding domain (blue), most commonly derived from an antibody fragment, an extracellular linker, a transmembrane domain, an intracellular costimulatory domain (yellow), and an intracellular stimulatory domain, most commonly derived from the ζ domain of the TCR (purple). CAR, Chimeric Antigen Receptor; TCR, T-cell receptor.
When CAR T-cells cease to control disease
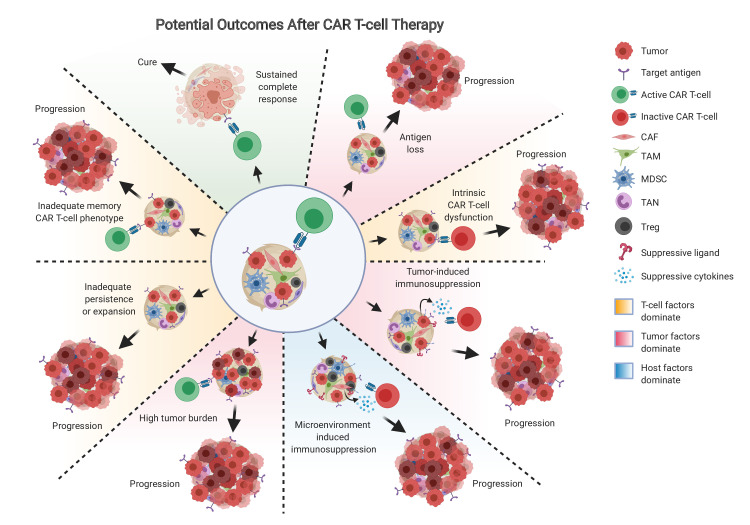
In designing strategies for improving the durability of CAR T-cell responses, it is helpful to first understand what limits their ability to achieve lasting remissions. When the first patients began relapsing after CAR T-cell therapy, it was tantalizing to speculate the reason for relapse would be predictable and simple, such as loss of target antigen expression. Although this is the cause of relapse in a minority of lymphoma patients, a number of additional factors are thought to contribute to the remaining relapses. These factors can be broken down into three categories: tumor-related, T-cell-related, and host-related (Figure 2).
Figure 2.
Potential outcomes after CAR T-cells. Cancer may progress or relapse after CAR T-cells due to tumor-related factors (red background), such as antigen escape, tumor-induced immunosuppression such as PD-L1 or suppressive cytokine production, or high tumor burden relative to functional CAR T-cells; T-cell factors may dominate (orange background), such as intrinsic CAR T-cell dysfunction, inadequate persistence or expansion of the CAR T-cells in vivo, or inadequate memory phenotype achieved by the CAR T-cells; host factors (blue background) may impel resistance to CAR T-cells, such as microenvironment-induced immunosuppression driven by CAF, TAM, MDSC, TAN, or Treg. CAFs, cancer-associated fibroblasts; MDSCs, marrow-derived suppressor cells; TAMs, tumor-associated macrophages; TAN, tumor-associated neutrophils; Tregs, regulatory T cells.
Tumor-related factors beyond loss of the target antigen include expression of inhibitory molecules such as immune checkpoint ligands, or expression of inhibitory cytokines such as TGF β or IL-10. Loss of the target antigen, specifically CD19, occurred in 27% (3 of 11 patients) of DLBCL patients treated on ZUMA-1.7 Mechanistically, antigen loss has been best studied in leukemia where it affects 10–20% of patients, and occurs through the generation of exon splice variants that eliminate the extracellular CAR binding domain, as well as tumor lineage switch to a myeloid phenotype, and outgrowth of cells with acquired CD19 mutations or loss of heterozygosity.14–18 In addition to modifying the target antigen, tumor cells can express receptors to inhibit CAR T-cell function. These immune checkpoint molecules, such as PD-1, Tim3, Lag3, and CTLA4, are best known for their function in quenching the endogenous T-cell response, although CAR T-cell specific activities have also been documented. In mice, inhibiting PD-1 pathway activation improves CAR T-cell mediated solid tumor clearance.19 A limited number of CD19 CAR-T cell-refractory DLBCL patients have been described that achieved significant CAR-T cell expansion, antitumor response, and decreased tumor burden after treatment with PD-1 blocking antibody.20–22 In an analysis performed on final CAR products going into DLBCL patients, Tim3 and Lag3 co-expression on CAR T-cells pre-infusion was found to significantly predict for negative response, suggesting that CAR T-cell exhaustion status may be largely ordained even before the cells enter the tumor.23
Since there is a finite number of tumor cells the average CAR T-cell can kill, a simple mechanism for relapse is likely the be excessive tumor burden relative to CAR T-cell number. Long-term follow-up of ALL patients treated with CAR T-cells observed a clear difference in survival between patients starting with low vs high tumor burden; in fact, the only patients who survived long-term were those who started with low tumor burden.24 In DLBCL patients treated on ZUMA-1, low baseline tumor burden also strongly predicted for durable response.25 Even among patients who achieved comparable peak CAR T-cell levels, those with higher baseline tumor burden had fewer durable responses than those with lower baseline tumor burden, and the CAR T-cell/tumor volume ratio was predictive.25
T-cell-related factors include failure to achieve adequate expansion and failure to adequately kill. These impairments can be attributed to T-cell anergy, exhaustion, or senescence. Although functionally these conditions overlap, T-cell anergy is a hyporesponsive state in T-cells which is triggered by activation of TCR signaling in the absence of adequate costimulation through CD28; T-cell exhaustion occurs when T-cells achieve adequate stimulation and costimulation, but repeated activation during chronic infection or tumor progression eventually silences their function, and T-cell senescence is characterized by growth arrest after excessive proliferation with maintained viability and metabolic activity.26 CAR T-cell exhaustion, anergy, and senescence may reflect the patient’s baseline T-cell status, and may be influenced by treatment history and the CAR manufacturing process. Like endogenous T-cells, CAR T-cells become exhausted by repetitive (tonic) signaling, which can be antigen-induced or antigen-independent.27 Over expression of the transcription factors associated with further costimulation, such as c-Jun, may ameliorate CAR T-cell exhaustion.28
The importance of CAR T-cell proliferation, which is negatively affected by anergy, exhaustion, and senescence, in achieving durable responses is well documented. CAR T-cell growth kinetics measured prior to infusion in DLBCL patients treated on ZUMA-1 showed that those with a short doubling time were significantly more likely to achieve an objective response than patients with slower CAR T-cell division in the same growth media.25 The CAR T-cell phenotype most associated with effective doubling time in this analysis was the stem-like memory cells (defined here as CCR7 +CD45RA + CD8 T-cells). Consistently, in CLL patients treated with CAR T-cells, sustained remission was associated with an elevated frequency of CD27+CD45RO-CD8+ T cells before CAR T-cell generation, and these lymphocytes possessed memory-like characteristics.29
Although long-term CAR T-cell persistence might rationally be assumed to be important to maintaining a durable response, the actual importance of CAR T-cell persistence beyond the first several months currently appears to be largely insignificant; long-term follow-up from ZUMA-1 showed that 3/4 of patients with ongoing responses had B cell recovery5 and that CAR T-cell levels at 4 weeks but not at 3 months or beyond predicted for objective and durable response25 ; data from JULIET showed no link between absolute T-cell concentration and clinical outcomes, suggesting the CAR T-cell functionality, and not the absolute numbers over time, are most meaningful.6 However, achieving a supportive environment for initial CAR T-cell engraftment, expansion and function is still critical, and currently supported by conditioning chemotherapy prior to CAR T-cell infusion.
Host factors that affect relapse after CAR T-cell therapy are largely related to the presence and dominance of immunosuppressive cells in the tumor microenvironment, such as tumor-associated macrophages (TAMs), marrow-derived suppressor cells (MDSCs), tumor-associated neutrophils (TANs), cancer-associated fibroblasts (CAFs) and regulatory T cells (Tregs). These cells are known to inhibit T-cell function in a plethora of ways, however the exact contribution of each of these cell types to CAR T-cell hyporesponsiveness remains to be studied in vivo, and may also depend on the cancer type, location, and patient.
In DLBCL patients treated with CAR T-cells in which biopsy was performed before treatment and analyzed by RNAseq, patients who went on to only achieve PR had a tumor profile upfront suggestive of more MDSCs (defined by CD33, CD14), tumor-associated fibroblasts (FAP, TNC, CSPG4, PDGFRA, S100A4, ASPN, STC1, ITGAM), and immunosuppressive cytokines (IL10, TGF-β1), compared with those who went on to achieve a complete response (CR).30 Determining the individual contribution of each of these cell types in CAR T-cell mouse models requires an immunocompetent syngeneic system with genetic knockout capacity, which has been limited primarily due to difficulty in effectively generating mouse CAR T-cells.
Clinical predictors of relapse
In an analysis of risk factors for recurrence post-CAR T-cells, total metabolic tumor volume and two or more extranodal sites of disease were most highly associated with recurrence on multivariable analysis.31 Total metabolic tumor volume was more discriminative than bulky mass (defined either as >5 cm or >10 cm), which did not independently predict progression or relapse. Other risk factors analyzed, which were not as predictive as extranodal sites or total metabolic tumor volume, were ECOG performance status ≥2, Stage III/IV disease, elevated lactate dehydrogenase (LDH), increased C-reactive protein, and high International Prognostic Index.31
Current use of RT with CAR T-cells
After recognizing that most patients treated with CAR T-cells alone will eventually relapse, and understanding the known potential mechanisms of relapse from CAR T-cells, we may envision a number of potential roles for RT in improving patient outcomes. Currently, radiation with CAR T-cell therapy may be used prior to CAR T-cells as “bridging” therapy32–34 or in the post-CAR T-cell relapse salvage setting.35
RT bridging prior to CAR T-cells
Given that tumor burden is significantly associated with relapse as well as with toxicity24,25,36 and that RT is particularly effective at debulking hematological malignancies, a natural starting point for incorporating RT into CAR T-cell therapy is just prior to CAR T-cell injection with the goal of tumor debulking. Although promising, the benefit of tumor debulking RT on patient survival or toxicity remains to be conclusively tested and established. Retrospective analyses have however thus far found a significant association between bridging RT use and reduced post-CAR T-cell hospitalization rates,34 as well as increased likelihood of receiving CAR T-cells by decreasing the chance of progression between leukapheresis and CAR T-cell delivery.33 In a retrospective comparison of bridging RT vs systemic therapy, baseline patient characteristics were similar but RT was associated with a higher CR rate (82% vs 35%, p = 0.01), as well as higher ORR (100% vs 67%, p = 0.03) compared with systemic therapy.33 Further, compared with those given systemic therapy bridging, patients who received RT bridging had improved PFS.33 From this analysis, presumably all patients undergoing bridging therapy with disease that could feasibly be treated by either RT or systemic therapy may benefit most from RT. However, being retrospective data these are weak recommendations, and prospective trials are needed to definitively determine which patients may benefit most from RT vs systemic therapy or combined therapy bridging.
Current indications for RT bridging
Currently, it is very reasonable to treat areas at high-risk for recurrence or progression, such as extranodal sites of disease, areas of high metabolic tumor volume or bulk, or to target limited sites of persistent or progressive disease prior to CAR T-cell delivery. To establish the efficacy of these approaches, they should be done on clinical trial when available. Additional indications for RT, as always, are to palliate pain, bleeding, dysphagia, SVC syndrome, or other symptoms. Further, RT may be used during bridging to control growth in particularly problematic or dangerous areas, such as around the great vessels, esophagus, biliary tree, major nerves, the CNS, or spinal cord, prior to CAR T-cells.
Bridging RT dose
It should first be noted that since bridging RT has yet to demonstrate an advantage in terms of survival or toxicity, the optimal doses for bridging RT are far from established. Conceptually speaking, bridging RT may be utilized with CAR T-cells to achieve one of three main goals, and we may see the emergence of one or more of these approaches become more standard in the future: (1) tumor debulking RT, (2) conditioning RT, and (3) tumor sensitizing RT. The degree of tumor debulking correlates with dose and is somewhat variable by patient and histology, but generally some degree of debulking is achieved with even low palliative doses while complete local debulking often requires 45–50 Gy in 2 Gy fractions (or an equivalent regimen). However, it is important to not utilize a dose regimen that results in delay of CAR T-cell delivery, either due to prolonged fractionation or the induction of side-effects that must subside before CAR T-cells are administered. For these reasons, hyper- or hypofractionated RT may be preferred, such as BID fractionation or 4–6 Gy x 5 fractions. Conditioning RT consists of low dose RT to the total body, or to a large area, to facilitate CAR T-cell engraftment; this approach currently has very little clinical data for CAR T-cells but has previously been used successfully in transplant and in mouse CAR T-cell models.37 Tumor sensitizing RT utilizes subdefinitive doses, such that RT alone does not substantially eliminate the tumor in field, but induces apoptotic pathway expression that leaves the tumors more susceptible to being killed by the CAR T-cells, which is clinically significant in animals down to doses of 1.8 Gy [unpublished and DeSelm et al37 ]. This approach allows for larger areas of disease to be targeted in regions with more sensitive normal tissues when definitive doses to the region may be problematic. Table 1 below summarizes previously published doses and ranges used for CAR T-cell bridging.
Table 1.
Mean dose, dose range, number of patients treated, and CAR T-cell products used in published reports of bridging RT regimens used in patients awaiting CAR T-cell delivery
| Mean dose | Dose range | # Patients treated | CAR T-cell product | Institution | Ref |
|---|---|---|---|---|---|
| 37.5 Gy | 20–45 Gy in 2.2–4 Gy fractions | 5 | 3 Tisa-cel, 2 Axi-cel | UPenn | 34 |
| 20 Gy | 6–36.5 Gy in 2–4 Gy fractions; half received either 4 Gy x 5 or 3 Gy x 10 | 12 | Axi-cel | Moffitt | 32 |
| 35.2 Gy | 10–45 Gy in 1.8–2.6 Gy fractions | 17 | Axi-cel | MDACC | 33 |
CAR, Chimeric Antigen Receptor.
Bridging RT timing
As a bridge to CAR T-cells, the most established time to treat patients with radiation is after leukapheresis and before CAR T-cell delivery, which typically provides a 2–4 week interval. Radiation before leukapheresis may deplete the T-cells that need to be collected for CAR T-cell generation.32 Radiation after CAR T-cell delivery theoretically risks destroying the cytotoxic cells, which accumulate in tumor sites after days to weeks and persist for weeks to years, depending on the patient and CAR type. 4-1BB based receptors (such as Tisa-cel) persists longer and have slower killing kinetics than CD28 based receptors (such as Axi-cel). Radiation before CD19 targeting CAR T-cell therapy has not increased toxicity in clinical series thus far, and radiation does not appear to affect CD19 expression levels.37
RT salvage after CAR T-cell relapse
Despite the fact that the majority of current CAR T-cell treated patients have relapsed or will relapse, the optimal salvage treatment at this juncture has not been established and remains a challenging clinical decision.
Current role of salvage RT
As in other scenarios, the presence of localized vs systemic disease bears significantly on the patient’s ultimate outcome, and on the potential impact of RT. One fascinating concept, which remains to be validated, is that a patient with diffuse systemic disease may undergo CAR T-cell therapy that effectively eliminates all disease except for a small number of isolated tumor deposits that recur due to one or more factors described above; if eliminated by other methods, such as RT, conceivably these patients may still achieve durable response or cure. In an analysis of 14 DLBCL patients treated with RT after CAR T-cell relapse, the only ones that maintained ongoing response at last follow-up were those who had localized recurrences; all patients with advanced relapse succumbed within a year of relapse and of RT.35 While median OS was not reached among patients with a localized recurrence post CAR T-cells treated with definitive-dose RT (who often also went on to receive a stem cell transplant), it was only 2.6 months in those with systemic recurrence treated with local palliative RT. Although these numbers are small and universal conclusions cannot be drawn, the patients who appear to benefit most, from a disease status standpoint, are those with localized or perhaps oligometastatic recurrence. Allogeneic stem cell transplant should be considered in any patients who received a CR after salvage RT or other therapy.
Relapse kinetics may impact treatment decisions
Patient outcomes after CAR T-cells fall into five categories: primary resistance, early relapse (<3 months), late relapse (>3 months), stable disease, and objective response. For patients who are responding to CAR T-cells, even partially, current evidence does not support maintenance therapy, consolidative RT, stem cell transplant, or other intervention, as many of these patients will achieve long-term remission without any further therapy. However for all other patients, the consensus is less clear.
Primary resistance
Patients who have no response to CAR T-cells, which represented 12% of ZUMA-1 patients,7 have a very poor prognosis regardless of further salvage attempts. If only a single or a small number of disease sites are present, salvage definitive-dose RT is a very reasonable option. In the face of systemic progression, chemotherapy may provide a short interval of benefit for some patients,38 and generally RT is reserved for palliative measures, such as to relieve pain, neurological compromise, or other symptoms.
Early relapse
Unlike patients who immediately progress, patients with early relapse (<3 months) have an initial response that is short lived. Since these patients have evidence of functional CAR T-cells, checkpoint blocking antibodies are a rational therapy. Although off-label in this context, PD-1 blocking antibodies have revitalized CAR T-cell function and expansion leading to clinical response in DLBCL case reports20,21 ; in both of these cases PD-1 inhibitor therapy was initiated quickly after relapse (within 30 days). A separate case series found an ORR of 36% in 11 DLBCL patients treated with nivolumab at progression after CAR T-cells.22 If disease relapse is localized, definitive dose RT is a very reasonable option.35
Late relapse
If patients achieve a PR or CR, then progress or relapse >3 months after CAR T-cell therapy, they are considered a late relapse and have little likelihood of continuing to achieve a benefit from their CAR T-cells, at least in the site(s) of relapse. Thus, salvage therapy should be initiated. If the relapse is localized or oligometastatic, definitive dose RT is again an appropriate strategy.35 If the relapse is systemic and not addressable with local RT, systemic therapy should be initiated. If the patient’s tumor remains CD19+ on post-relapse biopsy, readministration of CAR T-cells is an option, ideally with an additional form of therapy to further augment the tumor or CAR T-cells. This additional therapy could be cytoreductive or bridging RT to major sites of disease prior to CAR T-cell retreatment, or could be a concurrent immunomodulatory agent, such as checkpoint blockade. In ZUMA-1, nine patients with an initial response for >3 months and CD19-positive relapse were retreated with axi-cel at disease progression, resulting in five responses (2 CRs and 3 PRs).7
Stable disease
Most patients will either respond to CAR T-cells rapidly, or will progress at first follow-up; however, a minority will have stable disease. It is important to note that these patients may have further response over time. In ZUMA-1, 48% (12/25) of patients with stable disease had improved response over time without further intervention, and several JULIET patients with stable disease eventually achieved a CR.7,39 However, stable disease that does not respond at 3 months becomes very high risk for eventual progression; only 22% of ZUMA-1 patients with stable disease at 3 months had not progressed by 2 years. The optimal management of these patients should be studied on clinical trial to ascertain what is impairing the presumably functional CAR T-cells from fully performing. If one site remains stable while all other sites have responded at 3 months or more, it remains reasonable to eliminate the stable site with targeted definitive dose radiation. In the future, a to-be-established lower dose RT may be appropriate for patients with stable disease to tip the balance toward CAR T-cell success without killing them off (further described below).
Salvage RT dose
While it is true in the limited data that exist that patients salvaged with RT after localized recurrence post-CAR T-cells have fared better than those treated with RT after systemic recurrence, there are many differences in the way locally recurrent vs systemically recurrent patients are treated, one of which is RT dose. In the retrospective study of 14 DLBCL patients treated with RT after CAR T-cell relapse, e.g. the ORR to RT was 100% in field for those with local relapse, however none of the seven patients with systemic relapse treated with RT achieved a CR within the RT field, reflective of less aggressive doses used (generally 4 Gy x 5 or 3 Gy x 10).35 For patients with localized or oligometastatic recurrences, definitive doses should be used to achieve disease elimination, recognizing refractory disease at this stage is likely more resistant than earlier stage disease. Thus, doses of at least 36 Gy, and generally 45–50.4 Gy should generally be considered if possible.
Future role of RT with CAR T-cells
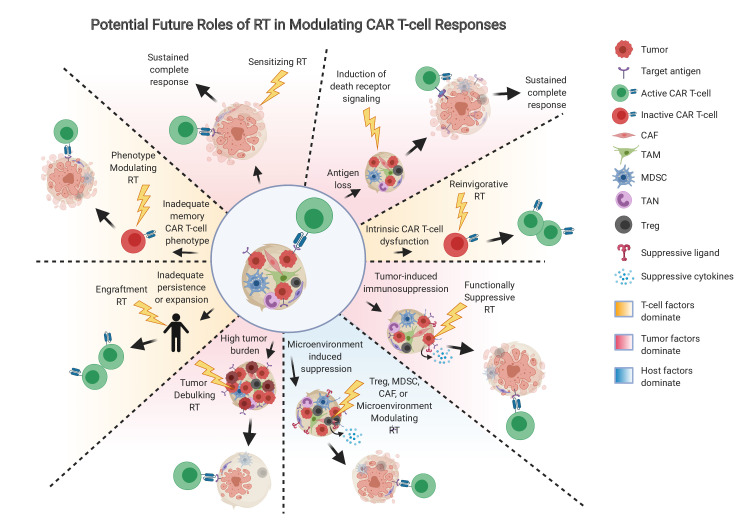
As in other cancer circumstances, the current role of RT relates to addressing local disease, and is thus mostly applicable to giving a tumoricidal dose to areas that are likely to relapse after CAR T-cells, or to patients fortunate enough to have limited sites of disease prior to CAR T-cells. In the future, the role of RT may expand to include addressing the underlying mechanistic causes of CAR T-cell failure (Figure 2), which would apply to a much larger patient population.
For patients who relapse after CAR T-cells with antigen-positive disease who are eligible for retreatment with CAR T-cells, and for patients receiving CAR T-cells for the first time, neoadjuvant RT may be utilized to address the main functional limitations outlined in Figure 3. For example, RT may be utilized to specifically kill off MDSCs, Tregs, CAFs, or other immunosuppressive cells that are often rich in the tumor microenvironment.40 The precise dose of RT required to specifically ablate these cell types requires further investigation, but likely would be much less than the tumoricidal dose, and thus may be more safely deliverable to larger regions. These cells may be stained and quantified in biopsy specimens or analyzed by flow or RNAseq to predict their likely contribution to CAR T-cell resistance in advance.
Figure 3.
Potential future roles of RT in modulating CAR T-cell outcomes. Beyond simple tumor debulking, RT may be utilized in the future at appropriate doses to address tumor-related factors (red background), T-cell factors (orange background), or host factors (blue background) that are operative in driving resistance to CAR T-cells. CAF, cancer associated fibroblasts; MDSC, myeloid derived suppressive cells; RT, radiation therapy; TAM, tumor associated macrophages; TAN, tumor associated neutrophils; Treg, regulatory T-cells.
While tumor-debulking RT prior to CAR T-cell infusion is appealing and should be studied more precisely on trial, disease often progresses after CAR T-cells and the dilemma of whether or not to use post-CAR RT arises. RT is generally avoided after CAR T-cell therapy for the same reason steroids were initially avoided; there is concern the therapy may impair or kill the cytotoxic cells. However, just as moderate steroid use has thus far not had a clearly inhibitory effect on CAR T-cell function in patients 32029707, it may also be true that moderate focal RT does not significantly affect CAR T-cell efficacy. For example, a multiple myeloma patient treated with 4 Gy x 5 fractions of palliative RT to the brain and spine shortly after progressing clinically and biochemically after CAR T-cells exhibited a significant expansion of T-cell clones post-RT, as well as a systemic response that was durable at last follow-up.41 It is possible that RT doses that do not kill CAR T-cells modulate their phenotype in a clinically relevant manner. It is still unclear whether the RT sensitivity of CAR T-cells is different from endogenous T-cells, and what the “CAR T-cidal” dose is. It is also unknown how lower, non-lethal doses of RT affect CAR T-cell function, specifically their tumor killing-capacity, persistence, or expansion. Tumor cells sometimes undergo a phenomenon of accelerated repopulation if they do not die after RT, and RT can independently induce inflammatory signaling in a number of cells; potentially conserved mechanisms may exist in CAR T-cells that could be harnessed to the patient’s benefit by utilizing the correct dose regimen in a form of “reinvigorative RT”.
Both CD4 and CD8 cells can develop a number of different phenotypes that subsequently affect their ability to respond to further antigen stimulation. Several studies have found associations between the memory phenotype present in patient’s CAR T-cell populations and their chance of attaining a durable tumor response.23,29,42 Thus, attention has been placed on inducing the appropriate memory T-cell phenotype in CAR-modified cells prior to infusion.43,44 Phenotype can also be plastic, and the phenotype of an injected CAR T-cell may not durably maintain in vivo over time. It is currently unknown whether low-dose RT influences CAR T-cell phenotype. In the future, if such is the case, RT may be given to patients failing CAR T-cells to re-establish a favorable phenotype and extend the potential for response.
In patients with heterogenous antigen expression, “sensitizing RT” may be utilized, which may consist of a low dose (~2 Gy) of RT to a large area to encompass all sites of disease. This approach in mice results in CAR T-cells more effectively killing antigen-positive cells, as well as nearby antigen-negative tumor cells through death receptor ligand naturally induced on activated CAR T-cells that interact with death receptor pathway molecules upregulated on and within tumor by low dose RT.37 This approach requires further validation in patients, but may be a way to improve responses in those likely to fail due to partial antigen loss.
Given the growing importance of CAR T-cell therapy and the wide array of potentially synergistic effects of RT with CAR T-cells, the next 5–10 years may be a particularly exciting time for the field of radiation oncology and cellular immune therapy. Carefully executed, well-designed clinical trials should be performed to document and test mechanistic hypothesis behind various potentially synergistic RT regimens. In doing so, RT as a localized modality may eventually achieve the elusive goal of improving cure rates of patients with metastatic disease, a goal that has thus far been unachievable.
REFERENCES
- 1.Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin 2020; 70: 145–64. doi: 10.3322/caac.21601 [DOI] [PubMed] [Google Scholar]
- 2.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the world Health organization classification of lymphoid neoplasms. Blood 2016; 127: 2375–90. doi: 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philip T, Guglielmi C, Hagenbeek A, Somers R, Van Der Lelie H, Bron D, et al. Autologous Bone Marrow Transplantation as Compared with Salvage Chemotherapy in Relapses of Chemotherapy-Sensitive Non-Hodgkin’s Lymphoma. N Engl J Med 1995; 333: 1540–5. doi: 10.1056/NEJM199512073332305 [DOI] [PubMed] [Google Scholar]
- 4.Sauter CS, Matasar MJ, Meikle J, Schoder H, Ulaner GA, Migliacci JC, et al. Prognostic value of FDG-PET prior to autologous stem cell transplantation for relapsed and refractory diffuse large B-cell lymphoma. Blood 2015; 125: 2579–81. doi: 10.1182/blood-2014-10-606939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Locke FL, Ghobadi A, Jacobson CA, Miklos DB, Lekakis LJ, Oluwole OO, et al. Long-Term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 2019; 20: 31–42. doi: 10.1016/S1470-2045(18)30864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuster SJ, Bishop MR, Tam CS, Waller EK, Borchmann P, McGuirk JP, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019; 380: 45–56. doi: 10.1056/NEJMoa1804980 [DOI] [PubMed] [Google Scholar]
- 7.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA. Axicabtagene Ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med 2017;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 1989; 86: 10024–8. doi: 10.1073/pnas.86.24.10024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maher J, Brentjens RJ, Gunset G, Rivière I, Sadelain M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ /CD28 receptor. Nat Biotechnol 2002; 20: 70–5. doi: 10.1038/nbt0102-70 [DOI] [PubMed] [Google Scholar]
- 10.Imai C, Mihara K, Andreansky M, Nicholson IC, Pui C-H, Geiger TL, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004; 18: 676–84. doi: 10.1038/sj.leu.2403302 [DOI] [PubMed] [Google Scholar]
- 11.Yvon E, Del Vecchio M, Savoldo B, Hoyos V, Dutour A, Anichini A, et al. Immunotherapy of metastatic melanoma using genetically engineered GD2-specific T cells. Clin Cancer Res 2009; 15: 5852–60. doi: 10.1158/1078-0432.CCR-08-3163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Jensen M, Lin Y, Sui X, Chen E, Lindgren CG, et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum Gene Ther 2007; 18: 712–25. doi: 10.1089/hum.2007.028 [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Wang QJ, Yang S, Kochenderfer JN, Zheng Z, Zhong X, et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J Immunol 2009; 183: 5563–74. doi: 10.4049/jimmunol.0900447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sotillo E, Barrett DM, Black KL, Bagashev A, Oldridge D, Wu G, et al. Convergence of Acquired Mutations and Alternative Splicing of CD19 Enables Resistance to CART-19 Immunotherapy. Cancer Discov 2015; 5: 1282–95. doi: 10.1158/2159-8290.CD-15-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer J, Paret C, El Malki K, Alt F, Wingerter A, Neu MA, et al. Cd19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother 2017; 40: 187–95. doi: 10.1097/CJI.0000000000000169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gardner R, Wu D, Cherian S, Fang M, Hanafi L-A, Finney O, et al. Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood 2016; 127: 2406–10. doi: 10.1182/blood-2015-08-665547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z, Chen X, Tian Y, Li F, Zhao X, Liu J, et al. Point mutation in CD19 facilitates immune escape of B cell lymphoma from CAR-T cell therapy. J Immunother Cancer 2020; 8: e001150. doi: 10.1136/jitc-2020-001150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Orlando EJ, Han X, Tribouley C, Wood PA, Leary RJ, Riester M, et al. Genetic mechanisms of target antigen loss in CAR19 therapy of acute lymphoblastic leukemia. Nat Med 2018; 24: 1504–6. doi: 10.1038/s41591-018-0146-z [DOI] [PubMed] [Google Scholar]
- 19.Cherkassky L, Morello A, Villena-Vargas J, Feng Y, Dimitrov DS, Jones DR, et al. Human CAR T cells with cell-intrinsic PD-1 checkpoint blockade resist tumor-mediated inhibition. J Clin Invest 2016; 126: 3130–44. doi: 10.1172/JCI83092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chong EA, Melenhorst JJ, Lacey SF, Ambrose DE, Gonzalez V, Levine BL, et al. PD-1 blockade modulates chimeric antigen receptor (CAR)–modified T cells: refueling the CAR. Blood 2017; 129: 1039–41. doi: 10.1182/blood-2016-09-738245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill BT, Roberts ZJ, Xue A, Rossi JM, Smith MR. Rapid tumor regression from PD-1 inhibition after anti-CD19 chimeric antigen receptor T-cell therapy in refractory diffuse large B-cell lymphoma. Bone Marrow Transplant 2020; 55: 1184–7. doi: 10.1038/s41409-019-0657-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, et al. Nivolumab in patients with relapsed or refractory hematologic malignancy: preliminary results of a phase Ib study. JCO 2016; 34: 2698–704. doi: 10.1200/JCO.2015.65.9789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng Q, Han G, Puebla-Osorio N, Ma MCJ, Strati P, Chasen B, et al. Characteristics of anti-CD19 CAR T cell infusion products associated with efficacy and toxicity in patients with large B cell lymphomas. Nat Med 2020; 26: 1878–87. doi: 10.1038/s41591-020-1061-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JH, Rivière I, Gonen M, Wang X, Sénéchal B, Curran KJ, et al. Long-Term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N Engl J Med 2018; 378: 449–59. doi: 10.1056/NEJMoa1709919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Locke FL, Rossi JM, Neelapu SS, Jacobson CA, Miklos DB, Ghobadi A, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv 2020; 4: 4898–911. doi: 10.1182/bloodadvances.2020002394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kasakovski D, Xu L, Li Y, cell senescence T. T cell senescence and CAR-T cell exhaustion in hematological malignancies. J Hematol Oncol 2018; 11: 91. doi: 10.1186/s13045-018-0629-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4-1Bb costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 2015; 21: 581–90. doi: 10.1038/nm.3838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lynn RC, Weber EW, Sotillo E, Gennert D, Xu P, Good Z, et al. C-Jun overexpression in car T cells induces exhaustion resistance. Nature 2019; 576: 293–300. doi: 10.1038/s41586-019-1805-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraietta JA, Lacey SF, Orlando EJ, Pruteanu-Malinici I, Gohil M, Lundh S, et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat Med 2018; 24: 563–71. doi: 10.1038/s41591-018-0010-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan Z-X, Li L, Wang W, OuYang B-S, Cheng S, Wang L, et al. Clinical Efficacy and Tumor Microenvironment Influence in a Dose-Escalation Study of Anti-CD19 Chimeric Antigen Receptor T Cells in Refractory B-Cell Non-Hodgkin’s Lymphoma. Clin Cancer Res 2019; 25: 6995–7003. doi: 10.1158/1078-0432.CCR-19-0101 [DOI] [PubMed] [Google Scholar]
- 31.Vercellino L, Di Blasi R, Kanoun S, Tessoulin B, Rossi C, D'Aveni-Piney M, et al. Predictive factors of early progression after car T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv 2020; 4: 5607–15. doi: 10.1182/bloodadvances.2020003001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sim AJ, Jain MD, Figura NB, Chavez JC, Shah BD, Khimani F, et al. Radiation therapy as a bridging strategy for CAR T cell therapy with Axicabtagene Ciloleucel in diffuse large B-cell lymphoma. Int J Radiat Oncol Biol Phys 2019; 105: 1012–21. doi: 10.1016/j.ijrobp.2019.05.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pinnix CC, Gunther JR, Dabaja BS, Strati P, Fang P, Hawkins MC, et al. Bridging therapy prior to axicabtagene ciloleucel for relapsed/refractory large B-cell lymphoma. Blood Adv 2020; 4: 2871–83. doi: 10.1182/bloodadvances.2020001837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wright CM, LaRiviere MJ, Baron JA, Uche C, Xiao Y, Arscott WT, et al. Bridging radiation therapy before commercial chimeric antigen receptor T-cell therapy for relapsed or refractory aggressive B-cell lymphoma. Int J Radiat Oncol Biol Phys 2020; 108: 178–88. doi: 10.1016/j.ijrobp.2020.05.014 [DOI] [PubMed] [Google Scholar]
- 35.Imber BS, Sadelain M, DeSelm C, Batlevi C, Brentjens RJ, Dahi PB. Early experience using salvage radiotherapy for relapsed/refractory non-Hodgkin lymphomas after CD19 chimeric antigen receptor (CAR) T cell therapy. Br J Haematol 2020;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qu C, Ping N, Kang L, Liu H, Qin S, Wu Q, et al. Radiation priming chimeric antigen receptor T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma with high tumor burden. J Immunother 2020; 43: 32–7. doi: 10.1097/CJI.0000000000000284 [DOI] [PubMed] [Google Scholar]
- 37.DeSelm C, Palomba ML, Yahalom J, Hamieh M, Eyquem J, Rajasekhar VK, et al. Low-Dose radiation conditioning enables CAR T cells to mitigate antigen escape. Molecular Therapy 2018; 26: 2542–52. doi: 10.1016/j.ymthe.2018.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chow VA, Gopal AK, Maloney DG, Turtle CJ, Smith SD, Ujjani CS, et al. Outcomes of patients with large B‐cell lymphomas and progressive disease following CD19‐specific CAR T‐cell therapy. Am J Hematol 2019; 94: E209–13. doi: 10.1002/ajh.25505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goto H, Makita S, Kato K, Tokushige K, Fujita T, Akashi K, et al. Efficacy and safety of tisagenlecleucel in Japanese adult patients with relapsed/refractory diffuse large B-cell lymphoma. Int J Clin Oncol 2020; 25: 1736–43. doi: 10.1007/s10147-020-01699-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu J, Green MD, Li S, Sun Y, Journey SN, Choi JE, et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 2021; 27: 152–64. doi: 10.1038/s41591-020-1131-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Smith EL, Mailankody S, Staehr M, Wang X, Senechal B, Purdon TJ, et al. BCMA-Targeted CAR T-cell therapy plus radiotherapy for the treatment of refractory myeloma reveals potential synergy. Cancer Immunol Res 2019; 7: 1047–53. doi: 10.1158/2326-6066.CIR-18-0551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sommermeyer D, Hudecek M, Kosasih PL, Gogishvili T, Maloney DG, Turtle CJ, et al. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia 2016; 30: 492–500. doi: 10.1038/leu.2015.247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turtle CJ, Hanafi L-A, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR–T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016; 126: 2123–38. doi: 10.1172/JCI85309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abramson JS, Palomba ML, Gordon LI, Lunning MA, Wang M, Arnason J, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. The Lancet 2020; 396: 839–52. doi: 10.1016/S0140-6736(20)31366-0 [DOI] [PubMed] [Google Scholar]