Supplemental Digital Content is available in the text.
Keywords: cardiac arrest, intensive care unit, preventable harm, quality improvement
OBJECTIVES:
Although patients in the ICU are closely monitored, some ICU cardiac arrest events may be preventable. In this study, we sought to reduce the rate of cardiac arrests occurring in the ICU through a quality improvement initiative.
DESIGN:
Prospective, observational study.
SETTING:
ICUs of a single tertiary care center.
PATIENTS:
Patients hospitalized in the ICUs between August 2017 and November 2019.
INTERVENTIONS:
A comprehensive trigger and response tool.
MEASUREMENT AND MAIN RESULTS:
Forty-three patients experienced an ICU cardiac arrest in the preintervention epoch (6.79 arrests per 1,000 discharges), and 59 patients experienced an ICU cardiac arrest in the intervention epoch (7.91 arrests per 1,000 discharges). In the intervention epoch, the clinical trigger and response tool was activated 106 times over a 1-year period, most commonly due to unexpected new/worsening hypotension. There was no step change in arrest rate (2.24 arrests/1,000 patients; 95% CI, –1.82 to 6.28; p = 0.28) or slope change (–0.02 slope of arrest rate; 95% CI, –0.14 to 0.11; p = 0.79) comparing the preintervention and intervention time epochs. Cardiac arrests in the preintervention epoch were more likely to be “potentially preventable” than that in the intervention epoch (25.6% vs 12.3%, respectively; odds ratio, 0.58; 95% CI, 0.20–0.88; p < 0.01).
CONCLUSIONS:
A novel trigger-and-response tool did not reduce the frequency of ICU cardiac arrest. Additional investigation is needed into the optimal approach for ICU cardiac arrest prevention.
Inhospital cardiac arrest (IHCA) is a relatively common and often devastating event, affecting nearly 300,000 adult inpatients in the United States each year (1). Of these IHCA events, more than half occur in the ICU (2). Although overall survival following IHCA has improved modestly over time (3), mortality remains high, and most IHCA victims do not survive to hospital discharge.
Although efforts to improve intra-arrest and postarrest care of IHCA victims continue to provide incremental gains in survival, prevention of IHCA is likely to result in the greatest reduction in morbidity and mortality. To date, substantial investments have been made in the recognition of early deterioration on the hospital ward to allow for rapid intervention and prevention of IHCA (4, 5). Most investigators, however, have focused either on ward patients only or on hospitalwide cardiac arrest, with little published data focusing specifically on the causes and preventability of arrests that occur in the ICU (6–8). This is likely due both to unique ICU-specific challenges (e.g., determining an appropriate cutoff for vital signs to trigger an alert in a setting where abnormal vital signs are common) and to the assumption that patients in the ICU are already optimally monitored and managed (9).
In previously published work, we demonstrated that many cardiac arrests in the ICU (ICU-CAs) are potentially preventable and identified several recurring themes of preventability (10). In the present study, we set out to test the effects of an intervention aimed at reducing both the number and the preventability of ICU-CAs at our institution.
MATERIALS AND METHODS
Study Design, Setting, and Data Collection
This was a prospective, prepost intervention study of cardiac arrests occurring in the ICUs of a single, urban tertiary-care center. The study site includes 77 ICU beds divided into medical, surgical, neuroscience, cardiac, trauma, and cardiac surgery units, with one mixed medical/surgical unit. Supervised trainees (interns, residents, and fellows) are commonly at the frontline of care. Patients in surgical ICUs are comanaged between the intensivist and surgical teams. Boarding patients (e.g., a patient on a medical ICU service but physically in a surgical ICU bed due to lack of beds in the medical ICU) were counted as being in the ICU in which they were physically located. Patients admitted to an ICU who experienced an ICU-CA and underwent chest compressions were included. Nonindex ICU-CAs and ICU-CAs occurring within 1 hour of arrival to the ICU were excluded.
The study was divided into a number of time epochs. The preintervention period was from August 1, 2017, to May 31, 2018. This was followed by an intervention development period from June 1, 2018, to October 21, 2018. During this time, the intervention was developed as described below using data collected during the preintervention period. The intervention was piloted in one ICU from October 22, 2018, to November 30, 2018. Data collection for the intervention period began on December 1, 2018, and continued through November 30, 2019 (Fig. 1).
Figure 1.
Study timeline.
All ICU-CAs occurring during the preintervention and intervention phases were reviewed by an expert multidisciplinary and multispecialty panel. This panel consisted of attending physicians from medical critical care, anesthesia critical care, emergency medicine, and cardiology. Nursing representation included senior-level critical care nurses, all with at least 10 years of critical care nursing experience. The composition and procedures for these reviews can be found in our previously published work on this topic (10). The study was reviewed and approved by the Beth Israel Deaconess Medical Center Committee on Clinical Investigation (Institutional Review Board protocol 2012P-000127). Funding for this study was provided by the Controlled Risk Insurance Company.
Comprehensive Trigger and Response Tool Development and Implementation
The comprehensive trigger and response (CTRS) tool was designed by the expert panel. The panel identified key themes of preventability based on review of the ICU-CAs in the preintervention period and created a data-driven intervention targeted at these themes. The final CTRS tool can be found in the data supplement. In short, the CTRS tool consisted of four 5-inch × 9-inch cards held together by a ring. Each card contained a trigger criterion and a flow diagram describing trigger and response procedures. The four trigger criteria were based on previously identified themes of preventability and included worsening respiratory insufficiency, worsening hypotension, new/unexpected anxiety or agitation, and a final category of marked clinician concern. Tips and reminders to assist medical decision-making appeared on the back of each card. (Fig. S1, http://links.lww.com/CCX/A817). A trigger could be initiated by a nurse or physician. Once initiated, clinical team members including the nurse and the resident or advanced care provider were then called or paged to the bedside depending on proximity. At the bedside, the patient was assessed, relevant decision aids were reviewed, and a plan of care was made. The attending physician was made aware of the trigger but was not required to be at bedside. An event note documenting the trigger was entered into the electronic medical record. Trigger events were captured by the study team electronically through a marker placed in the ICU electronic medical record (Fig. S2, http://links.lww.com/CCX/A818).
The CTRS tool was implemented across the study-site ICUs using a multimodal approach that included: 1) identification and empowerment of nurse champions in each ICU; 2) presentations to nurses, respiratory therapists, house staff, fellows, and attending physicians; and 3) placement of large informational posters in each ICU. In addition, the trigger tool itself was affixed to the computer workstation in each ICU patient room.
Outcome Definitions
The primary outcome measure was the ICU-CA rate, defined as the number of ICU-CAs divided by the total number of patients who were in the ICU. This rate was calculated for each 1-week period during the study. The secondary outcome was the expert rating of preventability, dichotomized as “potentially preventable” versus “not potentially preventable.” This dichotomization mirrors our prior publication on this topic (10). A “potentially preventable” arrest was one in which at least half of all expert panel raters assigned a score of 3 or higher on a 5-point scale.
Statistical Analysis
The study power was determined a priori for the primary outcome of ICU-CA event rate. Between January 2010 and December 2015, there were an average of 5.7 cardiac arrests per 1,000 unique ICU discharges at the study site. When a rapid response system was implemented on hospital floors at the same institution, there was a 65% reduction in unexpected mortality outside of the ICU (11). We estimated a 46% reduction in ICU-CA frequency, which provided us with 80% power using a two-sided alpha of 0.05.
Descriptive statistics for continuous variables are reported with means and standard deviations or medians and interquartile ranges. Categorical variables are reported as counts with frequencies. The primary outcome was analyzed using an interrupted time series approach to evaluate the change in unadjusted ICU-CA rates before-and-after implementation of the CTRS intervention. For this primary analysis, study time was divided into three epochs as above. The primary outcome was the step change and slope change in ICU-CA rate between the preintervention/CTRS development period and intervention time epochs, excluding the CTRS pilot period. Autocorrelation was tested for using the Durbin-Watson test with values less than 1.5 or greater than 2.5 considered suggestive of significant first-order autocorrelation.
The secondary outcome focusing on arrest preventability was assessed through hierarchical, multivariable logistic regression with generalized estimating equations. The outcome variable was expert rating, dichotomized as “potentially preventable” or “not potentially preventable.” The primary exposure variable was the period in which the event occurred (preintervention vs intervention period). Covariates included in the model were patient age, biologic sex, ICU-CA location, and initial rhythm. As multiple experts provided a rating for each patient, clustering was accounted for at the patient level. A robust variance-covariance structure was chosen.
Two additional post hoc sensitivity analyses were performed. In the first, only events occurring in the medical ICUs were considered. This analysis was performed as the greatest number of CTRS activations occurred in the medical ICUs. Second, to explore whether differences seen were a result of changes not related to the CTRS, we performed a difference-in-difference analysis comparing rates of ICU-CA to rates of cardiac arrest events occurring on the hospital ward. The difference in step change and slope change was assessed through the inclusion of relevant interaction terms in the model.
For all analyses, p values less than 0.05 were considered statistically significant. All statistics were performed using STATA Version 15.1 (StataCorp LP, College Station, TX), and some graphics were created using R Version 3.0.2 (The R Foundation for Statistical Computing).
RESULTS
Cohort Characteristics
A total of 6,327 (from August 1, 2017, to May 31, 2018) and 7,453 (from December 1, 2018, to November 30, 2019) patients were discharged from the ICU in the preintervention and intervention epochs, respectively. During the preintervention epoch, there were 43 patients with ICU-CA meeting all inclusion and no exclusion criteria (6.79 arrests per 1,000 discharges). During the intervention epoch, there were 59 arrests meeting these criteria (7.91 arrests per 1,000 discharges). Complete details can be found in Table 1.
TABLE 1.
Characteristics of ICU-Cardiac Arrests Before and After the Comprehensive Trigger and Response Intervention
| Variable | Preintervention (n = 43) | Intervention Period (n = 59) | p |
|---|---|---|---|
| Demographics | |||
| Age (mean [sd]) | 66.5 (15.1) | 68.6 (16.3) | 0.51 |
| Sex (n [%], female) | 15 (34.9) | 16 (27.1) | 0.51 |
| Race (n [%])a | |||
| White | 22 (64.7) | 28 (62.2) | 0.08 |
| Black | 10 (29.4 | 7 (15.6) | |
| Other | 2 (5.9) | 10 (22.2) | |
| Arrest characteristics | |||
| Location (n [%]) | |||
| Cardiac care unit eight beds | 10 (23.3) | 19 (32.2) | 0.29 |
| Cardiac surgery | 2 (4.7) | 6 (10.2) | |
| Medical ICU 28 beds | 18 (41.9) | 24 (40.7) | |
| Neuroscience ICU eight beds | 1 (2.3) | 1 (1.7) | |
| Surgical ICU eight beds | 8 (18.6) | 3 (5.1) | |
| Trauma surgical ICU 10 beds | 4 (9.3) | 6 (10.2) | |
| Initial rhythm (n [%], shockable)b | 11 (25.6) | 10 (17.5) | 0.46 |
| Duration of resuscitative efforts (min, median [IQR]) | 12 (4–27) | 9 (4–20) | 0.33 |
| Time from ICU admission until arrest (hr, median [IQR]) | 50.0 (9.5–156.8) | 43.3 (9.2–119.3) | 0.92 |
| Outcomes | |||
| Return of spontaneous circulation (n [%], achieved) | 27 (62.8) | 40 (67.8) | 0.68 |
| Survival to hospital discharge (n [%]) | 10 (23.3) | 14 (23.8) | > 0.999 |
IQR = interquartile range.
aNine missing values (21.0%) of race in the preintervention data, and 14 (23.8%) missing values of race in the intervention data excluded from calculation of percentages in table.
bTwo missing values (3.4%) of shock status in the intervention data omitted from calculation of percentages in table.
Use of the Clinical Trigger and Response System
During the intervention period, the CTRS was initiated 106 times. The CTRS was most commonly activated in the medical ICUs, accounting for 64 activations (60.4%). The reason for activation was most commonly hypotension (47 activations [44.3%]) and respiratory insufficiency (35 activations [33.0%]). Following CTRS activation, 62 patients (58.5%) had a change in medications ordered, 42 (39.6%) had a change in the monitoring or follow-up plan, and 27 (25.5%) had a procedure performed. Thirteen (12.3%) had a change in their code status (e.g., change from “full code” to “do not resuscitate”) (Table 2). Among those patients for whom the CTRS was activated, three (2.9%) suffered an ICU-CA within 48 hours.
TABLE 2.
Use of the Clinical Trigger and Response System
| Trigger Details | Total Triggers (n = 106) |
|---|---|
| Trigger location (n [%]) | |
| Cardiac care unit eight beds | 25 (23.6) |
| Cardiac surgery 15 beds | 1 (1.0) |
| Medical ICU 28 beds | 64 (60.4) |
| Neuroscience ICU eight beds | 3 (2.8) |
| Surgical ICU eight beds | 4 (3.8) |
| Trauma surgical ICU 10 beds | 9 (8.5) |
| Primary trigger initiation criteria | |
| Hypotension | 47 (44.3) |
| Respiratory insufficiency | 35 (33.0) |
| Change in mental status | 11 (10.4) |
| Arrhythmia | 6 (5.7) |
| Nursing concern | 7 (6.6) |
| Trigger responsea | |
| Change in medications | 62 (58.5) |
| Change in monitoring/follow-up plan | 42 (39.6) |
| Procedure performed | 27 (25.5) |
| New diagnostic imaging | 14 (13.2) |
| Change in code status | 13 (12.3) |
| New consult placed | 6 (5.7) |
| Trigger documentation | |
| Nurse event documentation | 106 (100) |
| Physician event documentation | 72 (67.9) |
| ICU cardiac arrest after trigger | |
| Yes | 3 (2.9) |
aMore than one can be selected for each trigger.
Primary Outcome and Related Sensitivity Analyses
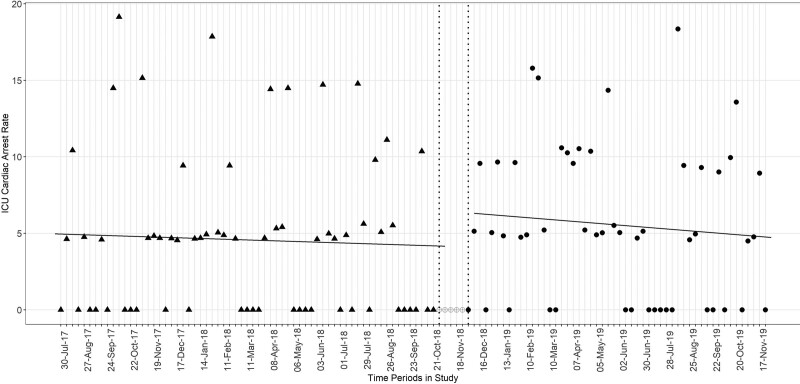
At the start of the study, the predicted (model y-intercept) ICU-CA frequency rate was 5.11/1,000 patients in the ICU (95% CI, 2.21–8.01). Over the preintervention period and CTRS development period, there was no significant change in arrest rate over time (slope, –0.01; 95% CI, –0.09 to 0.06; p = 0.69). As compared with the preintervention and development periods, there was no significant step change (step change in arrest rate of 2.24, 95% CI, –1.82 to 6.28; p = 0.28) or slope change (difference in slope as compared with preintervention, –0.02; 95% CI, –0.14 to 0.11; p = 0.79) (Fig. 2) in the intervention period. There was no substantial first-order autocorrelation (Durbin-Watson statistic 1.84).
Figure 2.
Interrupted time series analysis of ICU-CAs. Cardiac arrest rate per 1,000 active ICU patients per 1-wk period. Filled triangles represent preintervention and development epoch. Open circles represent the wash-out period. Filled circles represent the intervention period.
When restricted to patients who were hospitalized in the medical ICU, there was likewise no identified effect of the intervention in the interrupted time series analysis (step change, 0.39; 95% CI, –7.30 to 8.07; p = 0.92; slope change, 0.03; 95% CI, –0.20 to 0.26; p = 0.79).
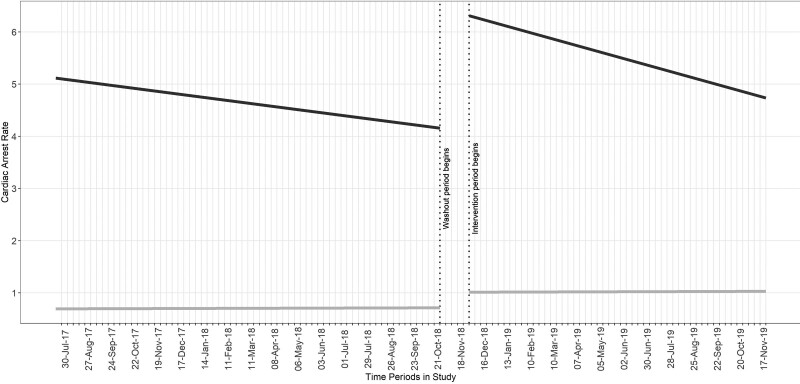
In the difference-in-difference sensitivity analysis comparing ICU-CA rates to inhospital arrest rates on the hospital ward, there was no difference comparing intercepts or slopes between the ICU arrest rate and the ward arrest rate (difference in step change, 2.02; 95% CI, –2.10 to 6.14; p = 0.34; difference in slope change, –0.03; 95% CI, –0.12 to 0.05) (Fig. 3).
Figure 3.
Difference-in-difference analysis comparing ICU-CA to CA on the hospital ward. Cardiac arrest rate per 1,000 active ICU patients per 1-wk period. Dark line represents ICU-CA. Light line represents ward CA.
Secondary Outcome of Arrest Preventability
In the preintervention period, there were 11 “potentially preventable” arrests out of a total of 43 arrests (25.6%) rated. In the intervention period, there were seven “potentially preventable” arrests out of a total of 57 arrests (12.3%) rated. Using hierarchical multivariable logistic regression analysis, there was a 58% lower odds (odds ratio [OR], 0.42; 95% CI, 0.20–0.88; p = 0.02) of an arrest in the intervention period being “potentially preventable” as compared with an arrest in the preintervention period. In the adjusted analysis, there was a 76% lower odds (OR, 0.24; 95% CI, 0.10–0.60; p < 0.01) of the arrest being “potentially preventable” in the intervention period.
DISCUSSION
In this prospective study of cardiac arrests occurring in the ICU, the introduction of a CTRS system was feasible and was frequently used by frontline critical care staff (approximately 10 activations per month). The most common reasons for CTRS activation were hypotension and respiratory insufficiency, and most patients underwent a change in the diagnostic and/or management plan in response to the activation. Although the most common result of the CTRS activation was a change in prescribed medications, 13% of activations resulted in a change in patient code status. With regard to the prespecified primary outcome of change in ICU-CA rate in the period prior to the intervention as compared with afterward, there was no significant effect of the CTRS. There was likewise no effect seen in two post hoc sensitivity analyses. Of ICU-CAs assessed, those occurring in the intervention period were less likely to be “potentially preventable” as compared with those in the preintervention period. As detailed below, the failure of the CTRS tool to reduce ICU-CA rates likely stemmed from a number of factors including design elements (e.g., incomplete capture of prodromes leading to ICU-CA), inadequate statistical power to detect an effect, implementation missteps including failure to recognize and adequately mitigate reluctance of nursing staff and physicians to activate the system due to fear of breach in medical team cohesiveness, and lack of clear criteria for activating the CTRS and reliance on clinician judgment in that regard.
ICU-CA has historically not been considered preventable given high nurse and physician to patient ratios, close monitoring of patients, and the overall high severity-of-illness in the ICU (9). In previous work, we challenged that paradigm and found that many ICU-CAs have preventable elements with common themes of preventability (10). Further, these themes of preventability suggested possible targets for quality improvement and educational interventions. These themes include inadequate communication, misattribution/diagnostic bias, and failure to treat or to escalate treatments promptly. The data-driven CTRS tool was an intervention developed based to address the issue of preventability of ICU-CAs. This was a novel intervention in that the CTRS tool was more than just a “trigger” system but combined an alert with a decision aid for on-the-ground management of critically ill patients.
Numerous tools have been developed to detect and alert clinical teams to impending deterioration on hospital wards. These tools commonly rely on structured clinical data from the electronic medical record or bedside flow sheets. Results are mixed as to whether these alerts improve patient outcomes (12, 13), although a recent large study combining a data-driven alert tool with clinician filtering, and a clear efferent response plan (that included the option of calling for palliative care consultation) was associated with an improvement in survival (4). In general, the afferent arms of these tools are better developed than the efferent arms, wherein clinicians alerted to impending decompensation are not provided with tools or decision aids to guide subsequent management (11, 14–16). As above, the CTRS attempts to mitigate that afferent/efferent imbalance by providing bedside decision aids for review by the entire team (nurses, respiratory therapists, and physicians) at the time of an unexpected change in clinical status. To our knowledge, this is the first published description of an alert-and-response tool for the prevention of cardiac arrest in the adult ICU setting. One study in PICUs did find that implementation of a cardiac arrest prevention bundle reduced ICU-CA rates—especially among surgical populations (17).
The finding that introduction of our CTRS tool did not reduce ICU-CA may be explained by incomplete implementation, suboptimal design of the intervention, lack of power owing to fewer preventable arrests than expected, or a combination thereof. In terms of implementation, one concern commonly cited by nursing and physician house staff was that activating the CTRS would be seen as punitive and detrimental to the nursing-physician relationship. This was addressed by study staff on a number of occasions and in a number of forums; however, concerns in this area persisted throughout the study period and may have resulted in fewer CTRS activations. Perhaps a more automated CTRS-activation approach with physician filtering may have ensured that the afferent limb was not affected by concerns regarding perceived punitive action. Additional challenges to implementation included a frequently changing physician workforce with rotation of resident teams, an electronic medical record in transition that did not allow for seamless CTRS integration for ease of decision aid review and documentation, and variable involvement of unit champions to advance the CTRS tool and remind frontline staff of its intended use.
With respect to design of the intervention, it is possible that the low activation rate of the CTRS and the high frequency of ICU-CA without preceding CTRS activation resulted from incomplete capture of prodromes leading up to the arrest. In particular, acute neurologic decompensation manifesting not as agitation/anxiety but in a more hypoactive state was not fully captured by any of the activation criteria aside from “marked clinician concern.” Although additional prodromes leading up to ICU-CA were not identified in expert review of the cases, other prearrest prodromes may exist and could have contributed to the low CTRS activation rate preceding ICU-CA. Overall, however, the opinion of the authors is that the intervention failed with respect to the primary outcome largely due to deficiencies in implementation and not design. Commonly, during review of an ICU-CA by the expert panel, it was clear that an opportunity for CTRS activation existed and was indicated but was not carried through by the frontline team.
Although we did not find an overall reduction in the primary outcome of the number of ICU arrests after the intervention, the relatively small percentage of arrests thought to be potentially preventable may have meant that our study was significantly underpowered for the primary outcome. Indeed, 25.6% of arrests in the preintervention period were “potentially preventable,” but we were powered to detect a 46.0% reduction in ICU-CA rate. The finding that arrests occurring in the intervention period had lower odds of being “potentially preventable” than those in the preintervention period suggests a possible benefit of the intervention and may warrant further investigation either in a larger study or one specifically focused on the potentially preventable arrests. It should be noted, however, that the reduction in ICU-CA preventability is hypothesis-generating only and carries risk of bias as a result of the expert panel evaluating cases during both the preintervention and intervention periods.
This study had a number of strengths. First, the prospective nature of the study ensured capture of all ICU-CAs occurring during the study period and allowed expert reviewers to assess data in real time without reliance on archived data sources. In addition, the multidisciplinary and multispecialty nature of the expert panel provided a comprehensive review of each arrest and allowed for the development of a CTRS that reflected priorities of a number of stakeholders. In being the first study, to our knowledge, that systematically assessed a tool created to prevent ICU-CAs, there were also a number of important limitations. First, without a clear control group, it is difficult to know whether the patients admitted to the ICU in the intervention period were at similar risk of ICU-CA as those in the period prior to implementation. Furthermore, since our CRTS tool relied on human activation rather than on an automated system, we cannot assess the reliability of activation as a percent of total trigger opportunities. Another important limitation is that the same expert review panel assessed arrests throughout the study period; it is, therefore, possible that subconscious bias affected preventability ratings in the intervention period. A third limitation is that there were other ongoing quality improvement projects in the ICUs unrelated to cardiac arrest during the trial, and the impact of those interventions on the effectiveness of the CTRS is not clear.
CONCLUSIONS
ICU-CAs commonly have elements of preventability. The introduction of a CTRS system was feasible but did not result in a reduction in the frequency of cardiac arrests occurring in the ICU. Arrests occurring in the ICU after the introduction of the trigger-and-response system were deemed less likely to be “potentially preventable” than those occurring in the preimplementation period, although this finding is hypothesis-generating only due to the risk of observer bias. Additional work is needed to identify the optimal approach to mitigation of preventable cardiac arrests in the critically ill.
ACKNOWLEDGMENT
Donna Williams, RN, participated in the early phases of study development and implementation.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Supported, in part, by a grant from the Controlled Risk Insurance Company.
Drs. Moskowitz and Berg are additionally funded through National Institutes of Health grants K23GM128005 and K23 HL128814, respectively. The remaining authors have disclosed that they do not have any potential conflicts of interest.
The funding sources had no role in the collection, analysis and/or interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
REFERENCES
- 1.Holmberg MJ, Ross CE, Fitzmaurice GM, et al. ; American Heart Association’s Get With The Guidelines–Resuscitation Investigators. Annual incidence of adult and pediatric in-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes. 2019; 12:e005580. [PMC free article] [PubMed] [Google Scholar]
- 2.Perman SM, Stanton E, Soar J, et al. ; American Heart Association’s Get With the Guidelines®—Resuscitation (formerly the National Registry of Cardiopulmonary Resuscitation) Investigators. Location of in-hospital cardiac arrest in the United States-variability in event rate and outcomes. J Am Heart Assoc. 2016; 5:e003638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiberg S, Holmberg MJ, Donnino MW, et al. ; American Heart Association’s Get With The Guidelines®-Resuscitation Investigators. Age-dependent trends in survival after adult in-hospital cardiac arrest. Resuscitation. 2020; 151:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Escobar GJ, Liu VX, Schuler A, et al. Automated identification of adults at risk for in-hospital clinical deterioration. N Engl J Med. 2020; 383:1951–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerry S, Bonnici T, Birks J, et al. Early warning scores for detecting deterioration in adult hospital patients: Systematic review and critical appraisal of methodology. BMJ. 2020; 369:m1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galhotra S, DeVita MA, Simmons RL, et al. ; Members of the Medical Emergency Response Improvement Team (MERIT) Committee. Mature rapid response system and potentially avoidable cardiopulmonary arrests in hospital. Qual Saf Health Care. 2007; 16:260–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodgetts TJ, Kenward G, Vlackonikolis I, et al. Incidence, location and reasons for avoidable in-hospital cardiac arrest in a district general hospital. Resuscitation. 2002; 54:115–123 [DOI] [PubMed] [Google Scholar]
- 8.Dewan M, O’Halloran A, Kleinman M, et al. eStablish and formalize expert criteria for avoidable resuscitation review (SAFECARR) electronic Delphi: Development of a consensus framework for classifying and reviewing cardiac arrests within the PICU. Pediatr Crit Care Med. 2020; 21:992–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Littlewood S, Snow TA. Reporting the incidence of cardiac arrest on the ICU: Are we still waiting for ROSC? A letter in response to: ‘Armstrong RA, Kane C, Oglesby F, et al. The incidence of cardiac arrest in the intensive care unit: A systematic review and meta-analysis. JICS 2018. In press’. J Intensive Care Soc. 2019; 20:NP4–NP5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moskowitz A, Berg KM, Cocchi MN, et al. Cardiac arrest in the intensive care unit: An assessment of preventability. Resuscitation. 2019; 145:15–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howell MD, Ngo L, Folcarelli P, et al. Sustained effectiveness of a primary-team-based rapid response system. Crit Care Med. 2012; 40:2562–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JR, Kim EM, Kim SA, et al. A systematic review of early warning systems’ effects on nurses’ clinical performance and adverse events among deteriorating ward patients. J Patient Saf. 2020; 16:e104–e113 [DOI] [PubMed] [Google Scholar]
- 13.Smith ME, Chiovaro JC, O’Neil M, et al. Early warning system scores for clinical deterioration in hospitalized patients: A systematic review. Ann Am Thorac Soc. 2014; 11:1454–1465 [DOI] [PubMed] [Google Scholar]
- 14.Kollef MH, Chen Y, Heard K, et al. A randomized trial of real-time automated clinical deterioration alerts sent to a rapid response team. J Hosp Med. 2014; 9:424–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Priestley G, Watson W, Rashidian A, et al. Introducing critical care outreach: A ward-randomised trial of phased introduction in a general hospital. Intensive Care Med. 2004; 30:1398–1404 [DOI] [PubMed] [Google Scholar]
- 16.Bedoya AD, Clement ME, Phelan M, et al. Minimal impact of implemented early warning score and best practice alert for patient deterioration. Crit Care Med. 2019; 47:49–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alten J, Klugman D, Cooper DS, et al. Abstract 11798: Cardiac arrest prevention quality improvement project from the pediatric cardiac critical care consortium. Circulation. 2019; 140:A11798 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.