Background:
The cochlear implant (CI) procedure is one of the most efficient surgical options for the management of patients suffering from severe bilateral sensorineural hearing loss. Notably, CI exposure is one of the most commonly reported complications. Herein, we report our experience in the management of three patients with CIs complicated by implant exposure.
Methods:
We present a retrospective review-based case series of three patients with exposed CIs requiring soft tissue coverage who were referred to plastic surgery care. These patients underwent their CI procedures at a university hospital specializing in ear, nose, and throat, after which they were referred for plastic surgery care at our university hospital for reconstruction after exposure. Each patient was managed through different surgical techniques based on the size, site, and condition of the surrounding tissue. The management options are discussed in this article.
Results:
Three patients with exposed cochlear implants were treated with different surgical techniques. During the 1-year follow-up period, the patients made an uneventful recovery with fully functional cochlear implants.
Conclusions:
The management of CI extrusion with local scalp flaps can constitute an effective and reliable option to salvage CI with a good prognosis and a lower incidence of exposure recurrence. Optimal results for establishing effective soft tissue coverage can be achieved by choosing the appropriate technique according to the clinical presentation.
INTRODUCTION
Cochlear implantation is one of the most efficient surgical options for the management of patients suffering from severe to profound bilateral sensorineural hearing loss. Cochlear implants (CIs) comprise a breakthrough treatment modality that was first introduced in 1972 as a single-channel CI. Since their introduction, CIs have been increasingly used in thousands of patients, which increased the incidence of their complications and, subsequently, the number of revision surgeries. The incidence of postoperative complications ranges broadly from 1.4% to 8.2%.1 However, the cochlear implantation safety profile is not in question; it is still considered to be a safe procedure. Moreover, CIs have relatively long survival rates, estimated to be 91.9% in a 10-year period.2
CI complications are generally classified as minor or major. Minor complications only require conservative management, whereas major complications necessitate revision surgeries or hospitalization for medical treatment. The possible complications include device extrusion, overlying skin necrosis, wound dehiscence, and local or systematic infections. Children aged 1–2 years are at a higher risk for repeated infections.1 Notably, CI exposure is the most common complication reported, with an incidence that reaches up to 5.4%.3
In this article, we present a case series of three patients to demonstrate the different available options for soft tissue coverage of CIs, including transposition and rotational flaps. Moreover, we report our experience of managing the condition of patients with exposed CIs at our teaching university hospital.
METHODS
The case series involves three patients aged between 3 and 12 years. All patients underwent surgery to salvage the CI-contaminated device extrusions in a tertiary care university hospital. The consent of each patient′s guardian was obtained before the study. The subjects were followed up for 1 year after the surgery. The follow-up consisted of incision assessment, measurement, and photography.
CASES
Case 1
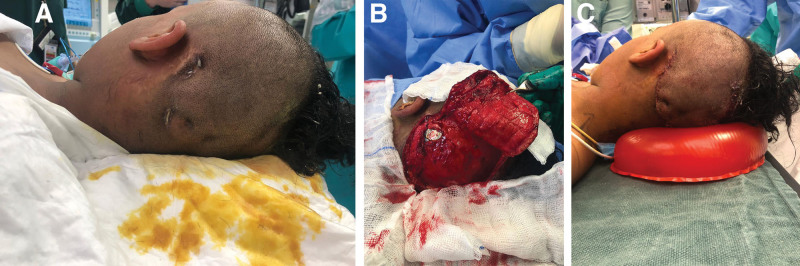
A 12-year-old girl who was otherwise medically free underwent a CI procedure at the age of 2 years. She presented with three small areas of exposed wirings on the dorsal edge of her left ear CI with wound dehiscence. Upon examination, purulent discharge and partial extrusion of the device were noted. She reported a history of trauma on the implant site before presentation to our service. The wound culture was positive for Staphylococcus aureus. Thus, the patient was administered with the appropriate culture-sensitive antibiotic therapy. Intraoperatively, the necrotic tissue was debrided to healthy tissue. The wound was irrigated with 1 L of antibiotic solution and the CI was repositioned by the ear, nose, and throat surgeon. A wide superiorly based rotational flap was designed. The rotation flap was measured with a template and designed accordingly. Dissection of the flap was conducted on the subglacial plane. Scoring of the galea was done to increase the surface area and decrease the tension on the flap. Finally, a three-layer closure was achieved (Fig. 1). The patient was treated with amoxicillin-clavulanate for another 10 days postoperatively based on the culture results. During the 1-year follow-up period, the patient made an uneventful recovery with a fully functional CI.
Fig. 1.
Rotational flap of exposed CIs. A, Exposed cochlear device. B, Rotational flap with scoring of the galea. C, Wound after closure.
Case 2
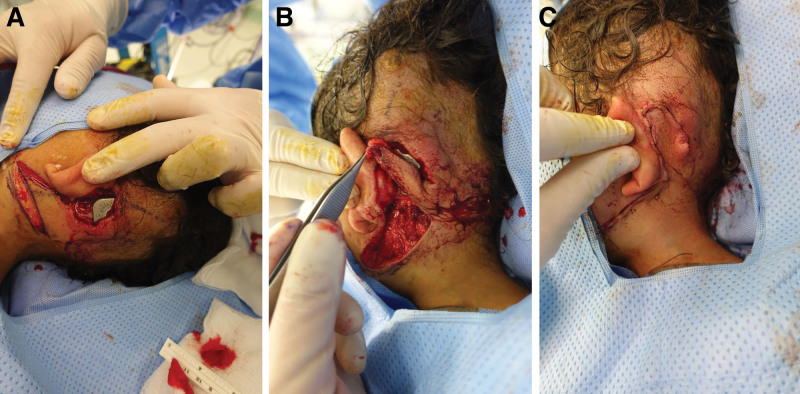
A 3-year-old girl with bilateral congenital sensorineural hearing loss who was otherwise medically free presented with a 1 × 2 cm wound with granulation tissue. Upon examination, the left CI was found to be exposed superiorly. The culture was positive for Staphylococcus aureus, so the patient was treated with appropriate culture-sensitive antibiotics. The decision was made to manage her condition with a local well-vascularized transposition flap designed inferiorly. The device contamination was managed through irrigation with 1 L of antibiotic solution intraoperatively. The wound edges were debrided to healthy and bleeding tissues. Based on the defect that resulted after extensive debridement, a 5 × 3 cm fasciocutaneous transposition flap was designed. Through reverse planning, the flap was designed with a template of the defect. Areas of laxity on the upper neck were marked. The transposition flap was planned with a wide base to ensure adequate blood perfusion. The flap was elevated down to the platysma muscle to cover the defect completely (Fig. 2). Thereafter, layer closure was performed. The patient was treated with amoxicillin-clavulanate for another 10 days postoperatively based on the culture results. During the 1-year follow-up period, the patient made an uneventful recovery with a fully functional CI.
Fig. 2.
Transposition flap of exposed CIs. A, preoperative photograph of the exposed CIs. B, Transposition flap designing. C, Wound closure.
Case 3
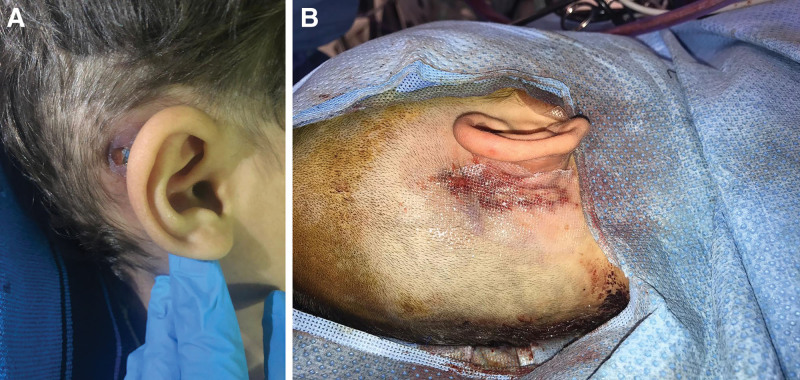
A three-year-old boy presented with bilateral sensorineural hearing loss and underwent right side CI without any complications. At the age of 5 years, he presented with right postauricular area (size 2 × 1 cm) of necrotic tissue, wound dehiscence, and purulent discharge with exposed CI. The culture was positive for Staphylococcus aureus. He had a history of trauma on the site of the CI 6 months before his presentation. The concurrent medical problems were type 1 diabetes mellitus, iron deficiency anemia, and prolonged QT syndrome (proven KCNQ1 mutation). The patient’s medical conditions were optimized preoperatively. The patient received the appropriate antibiotic treatment and was optimized for surgery. The device contamination was managed through irrigation with 1 L of antibiotic solution intraoperatively. The edges of the wounds were freshened to healthy tissue, and necrotic tissue was excised. The wound was then thoroughly irrigated, after which the CI was repositioned by the ear, nose, and throat team. The edges of the skin flap were undermined around the implant. The wound was then closed primarily in layers in a tension-free manner (Fig. 3). The patient was treated with amoxicillin-clavulanate for another 10 days postoperatively based on the culture results. During the 1-year follow-up period, the patient made an uneventful recovery with a fully functional CI.
Fig. 3.
Primary closure of exposed CIs. A, Exposed cochlear device. B, Primary closure wound.
DISCUSSION
There are many local and systematic risk factors that might play a role in CI exposure, including factors related to the surgical technique, the patient′s medical history, and/or the postoperative course. Although causative relationship has not been well established, a number of correlations have been suggested. The pressure of the implant magnet on the skin can cause skin necrosis. Additionally, tight compression dressings, head trauma, or hematoma collection may also induce flap necrosis, leading to exposure of the CI.4 Two of our cases reported a history of trauma before CI exposure. Furthermore, some studies have suggested that the pressure created by external devices, such as hats or eyeglass frames, may contribute to CI device extrusion.3,5 In all of the cases, infections were reported. Infections can predispose to device extrusion by generating internal pressure due to the active formation of granulation tissue.3
CI insertion complications are treated with antibiotics as the first step in all patients, followed by revision surgeries with or without explantation or reimplantation. Some patients may need multiple operations to salvage the high-cost devices. Implant explantation is advised only in severe cases wherein primary revision surgery has failed or when severe allergic reaction/infection or device failure has occurred.6
Extrusions have been minimized with the advancements in implant design and surgical approaches, avoidance of excessive scalp thinning, and the use of smaller incisions.7 Additionally, the placement of the implant near the incision is avoided. Pocket fashioning during implant insertion may also play a major role in minimizing device extrusion.
The high cost of CIs has led to the development of many successful surgical techniques to salvage these implants and avoid their removal. Moreover, reimplantation is surgically more complex and may not achieve the hearing results that were obtained from the first implantation. Early intervention with flap coverage is necessary to salvage the CI. Coverage of the exposed CI with well-vascularized flaps helps in treating infections and facilitates the delivery of antibiotics.8
Several types of flaps have been used to salvage CIs. Locoregional reconstruction includes postauricular pedicled skin flaps, rotational scalp flaps, pericranial flaps, pedicled temporalis muscle flaps, and temporoparietal fascia flaps.3,9,10 Additionally, the use of microvascular free tissue transfer has been reported to be successful, especially for larger defects.11 Moreover, the utilization of rotation flaps from the nape has been reported.12 However, the indications for using each type of flap to cover CI device extrusion scalp defects are not well defined. Various factors, such as proximity, vascularity, thinness, and ease of reach, are considered in choosing the flap type.
In our first and second cases, we used the fasciocutaneous flap, as the defect area was relatively large after extensive debridement. Thus, a distance fasciocutaneous flap from the neck and scalp was chosen because of the skin laxity and the ability to close the donor site primarily to ensure that the wire of the CI remains undamaged. The fasciocutaneous flap has many advantages: for example, it is thin and versatile. Moreover, it provides a wide base that adheres to the 1:2 principle for random flaps. However, it posed a few disadvantages with regard to our two cases, such as difficulty in drawing the flap geometry and angle and deciding the donor site.
For the third case, primary closure was used given the small size of the wound. The edges were refreshed to healthy bleeding tissue, and then the implant was repositioned by the ear, nose, and throat team. After undermining the skin flaps around the implant, it was felt that there was enough laxity in the skin to allow for primary closure without the need for any local flap. In our case, primary closure was preferred due to its simplicity and to limit the need to burn bridges for future flaps.
The use of scalp flaps to cover scalp defects in patients with CIs is uncommon. Only a limited number of cases that were managed by scalp flaps have been reported.13–16 The hair-bearing characteristics are unique to the scalp tissue and cannot be approximated by other body tissues. Therefore, scalp flaps may be considered as the best substitution for scalp defects, as they yield more cosmetically acceptable results. Patients who receive rotational flaps usually experience shorter operative times and hospital stays compared with patients who were administered with more complex flap types.
The three main types of scalp flaps are advancement, rotational, and transposition. The limited elasticity of the scalp is its main disadvantage, which constrains the use of advancement flaps. Scalp flaps have a robust blood supply, which facilitates harvesting and promotes healing. These flaps can be based anteriorly (supratrochlear artery), laterally (superficial temporal artery), or posteriorly (occipital artery).
There is a dearth of literature on the management of CI exposure. Hariharan et al reported the successful salvaging of five cases with a two-layer coverage consisting of an inner temporoparietal fascial flap and an outer scalp skin flap.17 Low et al reported that the device was successfully salvaged in six patients. The salvage surgery involved either skin flap reconstruction or transposition of the CI body to a new location, or both. Moreover, they concluded that the salvage surgery was more likely to succeed in patients with positive cultures.18 Meanwhile, Leonhard et al were able to save the device in five patients with the use of temporoparietal fascia flap. The patients had major soft tissue and skin complications after cochlear implantation, and only one experienced delayed failure due to another unrelated surgery by a different provider that involved direct injury to the flap. Furthermore, they asserted that temporoparietal fascia flaps are of particular importance to patients with comorbidities.19
CONCLUSIONS
Based on our experience, scalp flaps can be considered as an effective and reliable option in the management of CI extrusions to salvage the CI with good prognosis and lower incidence of exposure recurrence. Small defects with minimal exposure and adequate debridement can be closed primarily as well. We believe that attempting to salvage the implant is preferable and should always be considered when possible due to the high cost of surgery and rehabilitation regimens.
Footnotes
Published online 28 October 2021.
Disclosure: The authors have no financial interest to declare in relation to the content of this article.
REFERENCES
- 1.Lander DP, Durakovic N, Kallogjeri D, et al. Incidence of infectious complications following cochlear implantation in children and adults. JAMA. 2020;323:182–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karamert R, Düzlü M, Tutar H, et al. Assessment of cochlear implant revision surgeries in a cohort of 802 patients. Otol Neurotol. 2019;40:464–470. [DOI] [PubMed] [Google Scholar]
- 3.Leach J, Kruger P, Roland P. Rescuing the imperiled cochlear implant: a report of four cases. Otol Neurotol. 2005;26:27–33. [DOI] [PubMed] [Google Scholar]
- 4.Kim CS, Oh SH, Chang SO, et al. Management of complications in cochlear implantation. Acta Otolaryngol. 2008;128:408–414. [DOI] [PubMed] [Google Scholar]
- 5.Telian SA, El-Kashlan HK, Arts HA. Minimizing wound complications in cochlear implant surgery. Am J Otol. 1999;20:331–334. [PubMed] [Google Scholar]
- 6.Gawęcki W, Karlik M, Borucki Ł, et al. Skin flap complications after cochlear implantations. Eur Arch Otorhinolaryngol. 2016;273:4175–4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ray J, Gibson W, Sanli H. Surgical complications of 844 consecutive cochlear implantations and observations on large versus small incisions. Cochlear Implants Int. 2004;5:87–95. [DOI] [PubMed] [Google Scholar]
- 8.Tan KJ, Lim CT, Lim AY. The use of muscle flaps in the salvage of infected exposed implants for internal fixation. J Bone Joint Surg Br. 2010;92:401–405. [DOI] [PubMed] [Google Scholar]
- 9.Karimnejad K, Akhter AS, Walen SG, et al. The temporoparietal fascia flap for coverage of cochlear reimplantation following extrusion. Int J Pediatr Otorhinolaryngol. 2017;94:64–67. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham CD, III, Slattery WH, III, Luxford WM. Postoperative infection in cochlear implant patients. Otolaryngol Head Neck Surg. 2004;131:109–114. [DOI] [PubMed] [Google Scholar]
- 11.Lindquist NR, Vinh DB, Appelbaum EN, et al. Microvascular free tissue transfer and cochlear implants: a case series and literature review. Laryngoscope. 2020;130:1552–1557. [DOI] [PubMed] [Google Scholar]
- 12.Seo BF, Park SW, Han HH, et al. Salvaging the exposed cochlear implant. J Craniofac Surg. 2015;26:e749–e752. [DOI] [PubMed] [Google Scholar]
- 13.Harrison HC, Gibson WP, Thompson PG. Cochlear implant extrusion in a young child–a preventive procedure. J Laryngol Otol. 1995;109:425–428. [DOI] [PubMed] [Google Scholar]
- 14.el-Naggar M, Hawthorne M. Delayed extrusion of a cochlear implant: a case report of an implant extruding 21 months after the original operation. J Laryngol Otol. 1995;109:56–57. [DOI] [PubMed] [Google Scholar]
- 15.Harada T, Ishida K, Endo M, et al. Recurrent extrusion of cochlear implant at an interval of 5 years. Otol Neurotol. 2003;24:83–85. [DOI] [PubMed] [Google Scholar]
- 16.Costa DJ, Walen S, Varvares M, et al. Scalp rotation flap for reconstruction of complex soft tissue defects. J Neurol Surg B Skull Base. 2016;77:32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hariharan NC, Muthukumar R, Sridhar R, et al. Ideal flap cover for the salvage of exposed/infected cochlear implants: a case series and literature review. Indian J Otolaryngol Head Neck Surg. 2020;72:292–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Low WK, Rangabashyam M, Wang F. Management of major post-cochlear implant wound infections. Eur Arch Otorhinolaryngol. 2014;271:2409–2413. [DOI] [PubMed] [Google Scholar]
- 19.Leonhard L, Roche J, Wieland A, et al. The temporoparietal fascia flap is an effective strategy for cochlear implant wound coverage. Ann Otol Rhinol Laryngol. 2020;129:135–141. [DOI] [PubMed] [Google Scholar]