Supplemental Digital Content is available in the text.
Keywords: coronavirus disease 2019, intensive care unit, interhospital transfer, mechanical ventilation, mortality, outcomes
IMPORTANCE:
Studying interhospital transfer of critically ill patients with coronavirus disease 2019 pneumonia in the spring 2020 surge may help inform future pandemic management.
OBJECTIVES:
To compare outcomes for mechanically ventilated patients with coronavirus disease 2019 transferred to a tertiary referral center with increased surge capacity with patients admitted from the emergency department.
DESIGN, SETTING, PARTICIPANTS:
Observational cohort study of single center urban academic medical center ICUs. All patients admitted and discharged with coronavirus disease 2019 pneumonia who received invasive ventilation between March 17, 2020, and October 14, 2020.
MAIN OUTCOME AND MEASURES:
Demographic and clinical variables were obtained from the electronic medical record. Patients were classified as emergency department admits or interhospital transfers. Regression models tested the association between transfer status and survival, adjusting for demographics and presentation severity.
RESULTS:
In total, 298 patients with coronavirus disease 2019 pneumonia were admitted to the ICU and received mechanical ventilation. Of these, 117 were transferred from another facility and 181 were admitted through the emergency department. Patients were primarily male (64%) and Black (38%) or Hispanic (45%). Transfer patients differed from emergency department admits in having English as a preferred language (71% vs 56%; p = 0.008) and younger age (median 57 vs 61 yr; p < 0.001). There were no differences in race/ethnicity or primary payor. Transfers were more likely to receive extracorporeal membrane oxygenation (12% vs 3%; p = 0.004). Overall, 50 (43%) transferred patients and 78 (43%) emergency department admits died prior to discharge. There was no significant difference in hospital mortality or days from intubation to discharge between the two groups.
CONCLUSIONS AND RELEVANCE:
In a single-center retrospective cohort, no significant differences in hospital mortality or length of stay between interhospital transfers and emergency department admits were found. While more study is needed, this suggests that interhospital transfer of critically ill patients with coronavirus disease 2019 can be done safely and effectively.
Interhospital transfers account for 8.7% of critical care admissions (1), with the most frequent reasons for transfer including access to higher quality of care, availability of specialized tests or procedures not available in the referring hospital, and to increase the patient’s likelihood of survival (2). Prior research has found that transferred patients are more severely ill (3–5), more likely to be White (6), and that these transfers may be associated with adverse patient outcomes, including delays in care, increased likelihood of ICU stay, higher hospital mortality, and longer lengths of stay (7–11). However, other studies found that transferred patients have lower adjusted risk of mortality (3, 12). In the coronavirus disease 2019 (COVID-19) population, interhospital transfers have been described but results are limited (13–15).
In the spring of 2020 during the initial COVID-19 surge, the U.S. medical system was overwhelmed with ICU needs. Hospitals struggled with shortages of intensive care beds, ventilators, and staff available to care for these critically ill patients (16, 17). Early COVID-19 surge plans included centralized critical care via interhospital transfer to better offer access to specialized therapies (18, 19) including extracorporeal membrane oxygenation (ECMO), proning (20–23), and investigative medications such as tocilizumab and remdesivir (24–26). Our institution, a large, urban academic medical center in Chicago, IL, implemented an aggressive surge plan, increasing available ICU beds and ventilators. Adult surgical, cardiac, and neurologic ICU beds, as well as PICU beds accepted COVID-19 admissions. Post-anesthesia care beds were used as ICUs since fewer surgeries were being performed. Physicians, housestaff, advanced practice providers, and nurses were deployed from the above ICUs, operating rooms, and procedural roles. Ventilators were increased by using operating room ventilators and borrowing or renting from regional facilities. Critically ill patients from community hospital emergency departments (EDs) and ICUs were accepted in transfer. The purpose of this study was to compare demographics and hospital outcomes for patients admitted from the ED to the ICU with patients transferred from other acute care hospitals. We hypothesized that ICU patients who were transferred from other facilities and received invasive ventilation would have higher severity of illness and higher hospital mortality compared with patients admitted through the ED, after controlling for patient sociodemographic characteristics.
MATERIALS AND METHODS
Study Design
A retrospective cohort analysis was conducted on data collected from the electronic medical record (EMR) at Rush University Medical Center (RUMC) from patients who were admitted and discharged between March 17, 2020, and October 14, 2020, capturing the first surge of the COVID-19 pandemic. This study was approved by the RUMC Institutional Review Board (20041014-IRB01). The reporting of this study adheres to the STrengthening and Reporting of OBservational studies in Epidemiology statement (27).
Setting
RUMC is a 671-bed urban tertiary care academic institution with 112 adult ICU beds (28). In March of 2020, adult ICU capacity was dynamically expanded through an extensive institutional surge plan as described above.
Participants
The study cohort included all intubated patients with COVID-19 pneumonia. Patients admitted to RUMC and had a positive test for severe acute respiratory syndrome coronavirus 2 during their hospitalization or in the week prior to admission to RUMC were considered for inclusion. These patients were then dichotomized into patients who were admitted through the RUMC ED (ED admits) versus transferred from another acute care hospital (interhospital transfers). Patients were further limited to those who were admitted to an ICU and received invasive mechanical ventilation at any time during their hospital stay.
The EMR data were validated using two separate processes. First, we compared abstracted data to internal standardized reports used for quality improvement to ensure that all patient records meeting inclusion criteria were in our database. Second, patient records were randomly selected for a manual review of provider documentation by two independent physicians (E.C., S.K.M.) who verified the data separately; differences were settled by consensus among the research team.
Variables
Data included sociodemographic details, hospital administrative data, principal and secondary International Classification of Diseases, 10th Revision diagnosis codes, vital signs, laboratory tests, ventilator settings, orders, and clinical notes. To account for transferred patients having care at another hospital prior to transfer that was not included in our database, the first date of comparison (D0) was the first date of invasive ventilation or, for interhospital transfers who were already receiving invasive mechanical ventilation at the time of arrival, ICU admission date.
Outcomes
The primary outcome was hospital mortality, including discharge to hospice, versus survival to hospital discharge. A secondary analysis used the number of days from initial intubation time in our institution to hospital discharge or hospital mortality, censored at discharge.
Covariates
Patient sociodemographic characteristics included sex, patient-reported race/ethnicity (White, Black/African American, Hispanic or Latino, other), preferred language (English or Spanish/Other), marital status, primary payor, and age. Admission month was dichotomized into two time periods: March–April, when hospital operations were restricted in response to a statewide shelter-in-place order (29), versus May–September, when routine hospital operations resumed. The number of comorbid medical conditions was determined using the Clinical Classification System Refined from the Agency for Healthcare Research and Quality (30). Comorbidities were taken from a Centers for Disease Control and Prevention list of conditions associated with a higher level of severity and included kidney disease, chronic obstructive pulmonary disease (COPD), diabetes, heart disease, sickle cell, asthma, cystic fibrosis, human immunodeficiency viruses, cerebrovascular disease, obesity, hypertension, neurologic conditions, thalassemia, and liver disease (31). Count of comorbidity was calculated by taking the count of comorbidities a patient had from the above list. Additional clinical characteristics included the presence of specific comorbidities listed above, the ratio of Pao2/Fio2, and orders for prone positioning and dialysis.
Statistical Methods
Frequency distributions and medians with interquartile ranges (IQRs) (as all continuous variables were non-normally distributed) were used to describe the sample. chi-square tests and Mann-Whitney U tests were used to assess differences in patient characteristics by transfer status. Univariate logistic regression was used to examine the association between hospital mortality and each covariate separately. Baseline characteristics were compared between patients with at least one variable missing and patients with complete data to examine the potential impact of missing data.
To answer the question of mortality difference between the two groups, a binary logistic regression was used to test the association between hospital mortality and transfer status adjusting for patient sociodemographic characteristics, admission month, and comorbid medical conditions. For the comorbid conditions, only those that had a significant difference in the probability of death were displayed in the tables and used in the models.
Since the time a patient spent receiving treatment before being transferred was not readily available, a time-to-event analysis was conducted to get another perspective on severity of illness. A multivariable Cox regression model was constructed to compare time to death by transfer status, adjusted for the same covariates as the binary logistic regression model, as well as using a Kaplan-Meier curve to visually display the time to death difference between the two groups.
To try to adjust for possible selection bias, a sensitivity analysis was included using inverse probability of treatment weights (IPTWs) to balance characteristics between ED admits and transfers (32). The binary logistic and multivariable Cox regression models were then repeated as described above, adjusted for the IPTW, resulting in weighted adjusted odds ratios (ORs) and weighted adjusted hazards ratios, respectively. First, binary logistic regression was used to estimate the probability of being transferred from another facility and included all the covariates used in the previous models as predictors. The IPTW was then calculated using the inverse of the predicted probabilities for the weight assigned transfers and the inverse of one minus the predicted probability as the weight for ED admits. The propensity score model was evaluated for the goodness of fit by using the Hosmer-Lemeshow test (33). To evaluate the propensity score’s effectiveness in balancing the covariates between the transfers and ED admits, the absolute standardized difference was calculated for each covariate and plotted for easy comparison. Sensitivity analysis was also performed using laboratory values for comparison. All statistical analyses were performed in SAS 9.4 (SAS Institute, Cary, NC). A two-sided p value of less than 0.05 was defined as statistically significant.
RESULTS
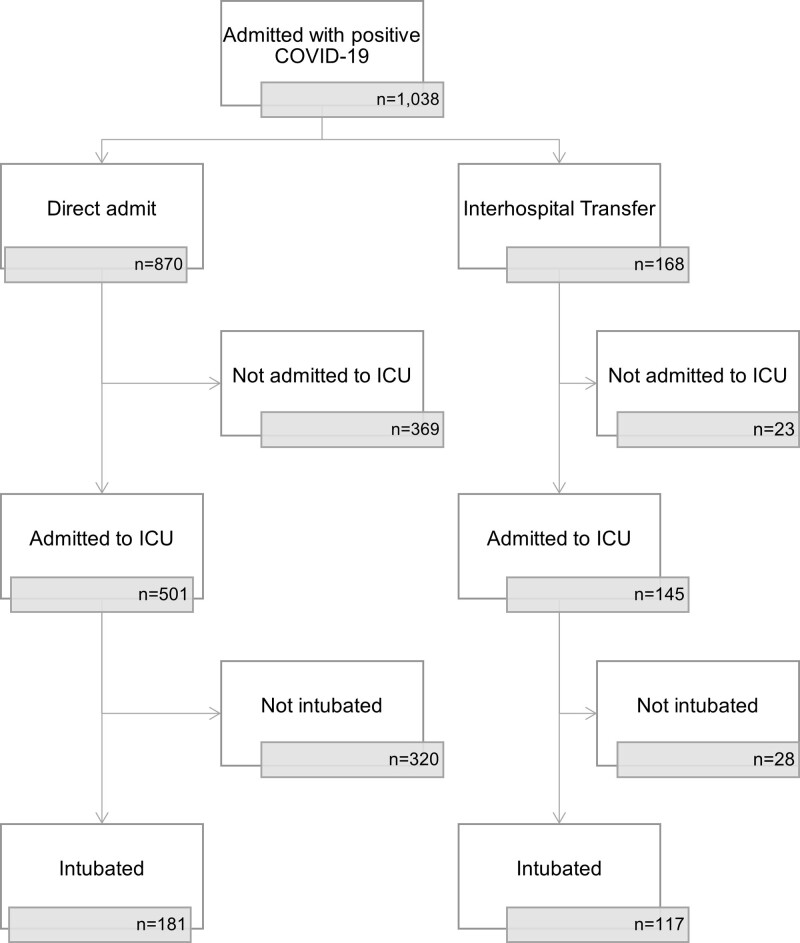
Of 1,038 patients admitted to RUMC with COVID-19 between March 2020 and September 2020, 117 interhospital transfers and 181 ED admits were admitted to the ICU and intubated, and included for analysis (Fig. 1). Note that of all patients admitted to the ICU, 21% of ED admits (181/870) and 70% of interhospital transfers (117/168) met inclusion criteria.
Figure 1.
Flowchart of patient inclusion. COVID-19 = coronavirus disease 2019.
Patient Characteristics
Patients in this analysis had a median age of 59 years (IQR, 50–68 yr) and were male (64%), obese (73%), and primarily Black (38%) or Hispanic/Latino (45%) (Table 1). Patients had a median of 4 (IQR, 3–5) comorbid conditions. Most patients had an order for proning (78%), and nearly half had an order for dialysis (45%). The median duration of mechanical ventilation was 9 days (IQR, 5–16 d) and median ICU stay was 17 days (IQR, 8–26 d).
TABLE 1.
Patient Characteristics by Interhospital Transfer Status (n = 298)
| Characteristic | Total (n = 298) | Transfers (n = 117) | Emergency Department Admits (n = 181) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age | 59 (50–68) | 57 (45–64) | 61 (52–69) | < 0.001 |
| Sex (male) | 191 (64.1) | 77 (65.8) | 114 (63.0) | 0.619 |
| Race/ethnicity | 0.276 | |||
| White | 29 (9.9) | 14 (12.1) | 15 (8.5) | |
| Black/African American | 111 (37.9) | 49 (42.2) | 62 (35.0) | |
| Hispanic or Latino | 131 (44.7) | 44 (37.9) | 87 (49.2) | |
| Other | 22 (7.5) | 9 (7.8) | 13 (7.3) | |
| Married | 135 (47.0) | 50 (43.5) | 85 (49.4) | 0.323 |
| Primary payor | 0.194 | |||
| Commercial | 87 (29.2) | 41 (35.0) | 46 (25.4) | |
| Medicare | 101 (33.9) | 32 (27.4) | 69 (38.1) | |
| Medicaid | 80 (26.9) | 32 (27.4) | 48 (26.5) | |
| Other | 30 (10.1) | 12 (10.3) | 18 (9.9) | |
| Spanish/other preferred language | 114 (38.3) | 34 (29.1) | 80 (44.2) | 0.008 |
| Clinical characteristics | ||||
| Admission month | 0.002 | |||
| March–April | 199 (66.8) | 90 (76.9) | 109 (60.2) | |
| May–September | 99 (33.2) | 27 (23.1) | 72 (39.8) | |
| Comorbidities | ||||
| Kidney disease | 96 (32.2) | 37 (31.6) | 59 (32.6) | 0.861 |
| Liver disease | 45 (15.1) | 18 (15.4) | 27 (14.9) | 0.912 |
| Heart disease | 283 (95.0) | 111 (94.9) | 172 (95.0) | 0.952 |
| Obesity | 216 (72.5) | 90 (76.9) | 126 (69.6) | 0.167 |
| Chronic obstructive pulmonary disease | 47 (15.8) | 18 (15.4) | 29 (16.0) | 0.883 |
| Diabetes | 158 (53.0) | 59 (50.4) | 99 (54.7) | 0.471 |
| Number of comorbid conditions | 4 (3–5) | 4 (3–5) | 4 (3–5) | 0.839 |
| Pao2/Fio2 ratio, severe (< 150) | 166 (55.9) | 55 (47.4) | 111 (61.3) | 0.019 |
| d-dimer, severe (> 4) | 126 (46.0) | 76 (69.7) | 50 (30.3) | < 0.001 |
| C-reactive protein, severe (> 50) | 257 (91.1) | 95 (88.8) | 162 (92.6) | 0.278 |
| Troponin, severe (> 0.4) | 31 (11.0) | 23 (21.1) | 8 (4.6) | < 0.001 |
For categorical variables, n (%) is shown; for continuous variables, median (interquartile range) is shown.
Interhospital transfers and ED admits differed on several key characteristics (Table 1). Transferred patients were younger (median age, 57 vs 61 yr; p < 0.001) and were more likely to have English as their preferred language (71% vs 56%; p = 0.008). A higher proportion of interhospital transfers received ECMO (12% vs 3%; p = 0.004). While 77% of interhospital transfers occurred in March/April, only 60% of ED admits occurred in the same period (p = 0.002). There were no significant differences noted in sex, race/ethnicity, primary payor, comorbid conditions, and use of proning and dialysis between interhospital transfers and ED admits.
Overall, there was no difference in hospital mortality between groups: 43% of interhospital transfers and 43% of ED admits died prior to discharge or were discharged to hospice (p = 0.95; Table 1). Additionally, the number of days from ventilation (D0) to discharge did not differ between interhospital transfers (median, 17; IQR, 8–31) and ED admits (median, 20; IQR, 9–28). All patients (n = 5) who discharged to hospice were ED admits. For patients who survived to discharge, discharge destinations are listed in Table 2.
TABLE 2.
Patient Outcomes by Interhospital Transfer Status (n = 298)
| Hospitalization Outcome | Total (n = 298) | Transfers (n = 117) | Emergency Department Admits (n = 181) | p |
|---|---|---|---|---|
| Extracorporeal membrane oxygenation order | 20 (6.7) | 14 (12.0) | 6 (3.3) | 0.004 |
| Prone order | 231 (77.5) | 90 (76.9) | 141 (77.9) | 0.844 |
| Dialysis order | 133 (44.6) | 56 (47.9) | 77 (42.5) | 0.367 |
| Time (in d) from first ventilation day to discharge | 19 (9–28) | 17 (8–31) | 20 (9–28) | 0.918 |
| ICU days | 17 (8–26) | 14 (6–27) | 18 (9–25) | 0.069 |
| Ventilation days | 9 (5–16) | 8 (5–15) | 10 (5–16) | 0.786 |
| Hospital mortality | 123 (41.3) | 50 (42.7) | 73 (40.3) | 0.681 |
| Discharge destination | 0.143 | |||
| Mortality or discharge to hospice | 128 (43.0) | 50 (42.7) | 78 (43.1) | |
| Discharge to home | 41 (13.8) | 17 (14.5) | 24 (13.2) | |
| Discharge to rehabilitation | 74 (25.8) | 28 (23.9) | 46 (25.4) | |
| Discharge to skilled nursing facility | 10 (3.4) | 5 (4.3) | 5 (2.8) | |
| Discharge to long-term acute care hospital | 41 (13.8) | 13 (11.1) | 28 (15.5) | |
| Discharge to short-term acute care hospital | 4 (1.3) | 4 (3.4) | 0 |
For categorical variables, n (%) is shown; for continuous variables, median (interquartile range) is shown.
Odds of Hospital Mortality
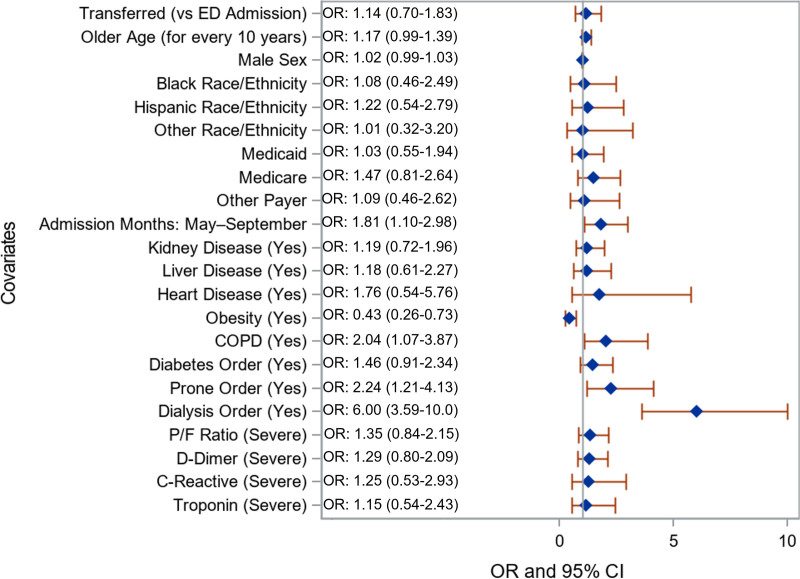
Prior to adjustment, there was no difference in odds of mortality between interhospital transfers or ED admits (OR, 1.1; 95% CI, 0.7–1.8; Fig. 2). Regardless of transfer status, admission later in the study period (May to September; as compared with March and April; OR, 1.8; 95% CI, 1.1–3.0), patients with COPD (OR, 2.0; 95% CI, 1.1–3.9), and having an order for proning (OR, 2.2; 95% CI, 1.2–4.1) or dialysis (OR, 6.0; 95% CI, 3.6–10.0) were more likely to die. After adjusting for covariates, there remained no significant difference in hospital mortality by transfer status (OR for interhospital transfers, 1.5; 95% CI, 0.9–2.4), race/ethnicity, or primary payor (Table 3). Among this cohort, obesity was associated with lower odds of mortality (OR, 0.5; 95% CI, 0.3–0.8).
Figure 2.
Unweighted unadjusted odds ratios (ORs) comparing patients who died in the hospital versus discharged alive. Gray reference line is equal to an OR of 1. Reference categories include: emergency department admit, female sex, White race/ethnicity, commercial payor, March–April admission month, no disease, no order for proning, did not receive dialysis, nonsevere laboratory levels. COPD = chronic obstructive pulmonary disease, P/F ratio = ratio of Pao2/Fio2.
TABLE 3.
Odds of Mortality Versus Discharge Alive in Unweighted and Weighted Adjusted Models
| Variables | Unweighted Adjusted ORsa | Weighted Adjusted ORsa |
|---|---|---|
| OR (95% CI) | OR (95% CI) | |
| Transferred | 1.45 (0.86–2.43) | 1.22 (0.87–1.73) |
| Age (per 10 yr older) | 1.03 (0.81–1.31) | 0.91 (0.77–1.07) |
| Male sex | 1.10 (0.64–1.90) | 1.79b (1.20–2.66) |
| Black race/ethnicity | 1.36 (0.55–3.36) | 1.44 (0.74–2.79) |
| Hispanic race/ethnicity | 1.40 (0.57–3.45) | 1.86 (0.96–3.60) |
| Other race/ethnicity | 1.54 (0.45–5.19) | 1.69 (0.71–4.01) |
| Medicaid | 0.84 (0.42–1.70) | 0.55b (0.34–0.90) |
| Medicare | 1.01 (0.50–2.07) | 1.01 (0.61–1.66) |
| Other payor | 0.92 (0.36–2.35) | 0.75 (0.38–1.47) |
| Admission month May–September | 1.83b (1.05–3.18) | 1.63b (1.11–2.38) |
| Kidney disease | 1.07 (0.61–1.90) | 0.80 (0.53–1.21) |
| Liver disease | 1.22 (0.60–2.48) | 1.29 (0.79–2.12) |
| Heart disease | 1.41 (0.40–4.98) | 0.89 (0.39–2.03) |
| Obesity | 0.47b (0.27–0.83) | 0.60b (0.40–0.88) |
| Chronic obstructive pulmonary disease | 1.78 (0.87–3.64) | 3.02b (1.79–5.10) |
| Diabetes | 1.40 (0.84–2.36) | 1.79b (1.24–2.58) |
| Area under the curve | 0.65 (0.59–0.72) | 0.64 (0.57–0.70) |
OR = odds ratio.
aORs are adjusted for all other listed variables.
bp < 0.05.
Reference categories include emergency department admit, female sex, White race/ethnicity, commercial payor, March–April admission month, no disease.
Time-to-Event Analysis
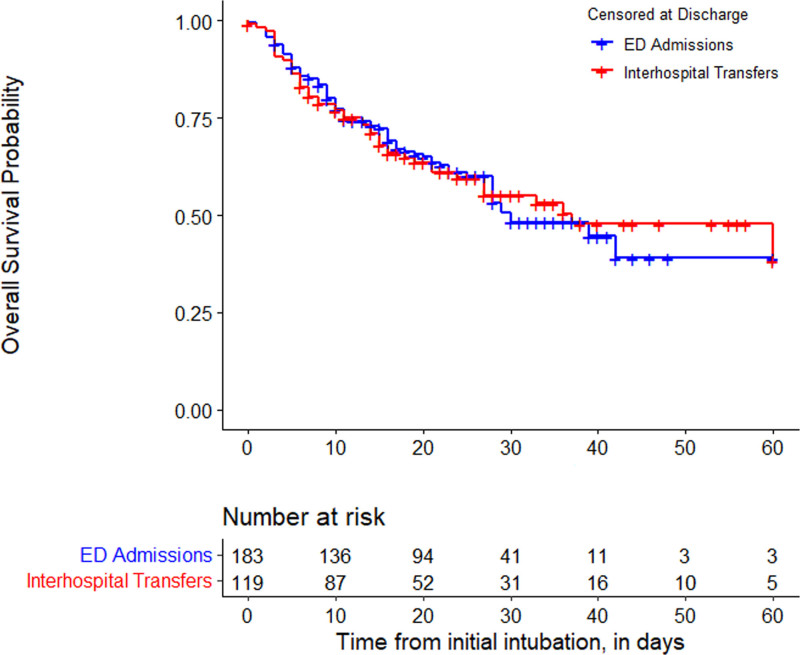
After censoring patients who were discharged alive, the median survival time was 37 days (IQR, 13–118 d) for interhospital transfers and 30 days (IQR, 11–42 d) for ED admits. According to the log-rank test, there was no difference between interhospital transfers and ED admits in survival time. In the adjusted Cox regression model, there was also no difference by interhospital transfer status (hazard ratio [HR], 1.1; 95% CI, 0.8–1.7) (e-Table 1, http://links.lww.com/CCX/A820).
Sensitivity Analysis
After IPTW adjustment, the distribution of the transferred and ED admitted patients among the baseline characteristics were similar (e-Fig. 1, http://links.lww.com/CCX/A819) (34). The IPTW-adjusted logistic regression also showed no difference in hospital mortality between interhospital transfers and ED admits. Figure 3 shows the IPTW weighted Kaplan-Meier survival curve. According to the IPTW-adjusted log-rank test, there was no difference between transfers and ED admits in survival time. In the IPTW-adjusted Cox regression (e-Table 1, http://links.lww.com/CCX/A820), there was also no difference by interhospital transfer status (HR, 1.0; 95% CI, 0.8–1.3).
Figure 3.
Weighted Kaplan-Meier curve of comparing the time to death between interhospital transfers versus emergency department (ED) admits. Using the inverse probability of treatment weight-adjusted log-rank test of the Kaplan-Meier curve, there was no difference between interhospital transfers and ED admits in time to death.
DISCUSSION
In a retrospective cohort of critically ill, mechanically ventilated patients with COVID-19 pneumonia, we did not find a significant difference in hospital mortality between ED admits and interhospital transfers in a large, urban, tertiary referral center during the initial surge of 2020. To our knowledge, this is the largest study to date examining interhospital transfer outcomes in patients with COVID-19 (13–15). Prior studies of non-COVID–related ICU transfers from community hospitals to tertiary centers reported substantially higher severity of illness and mortality in the transfer group compared with the nontransfers (4, 7, 8, 11). The cohorts in these studies were quite heterogeneous. We compared only the most critically ill cohort of patients, with the same primary diagnosis (COVID-19 pneumonia), who received mechanical ventilation. Many more ED admits (79%) than interhospital transfers (30%) were excluded from our analysis (Fig. 1), suggesting that interhospital transfers had a higher severity of illness than the general population of ED admits.
Almost 83% of our critically ill COVID-19 patients were Black or Hispanic, despite comprising only 58% of the population of Chicago (35). Similar to other analyses (36), our study did not find that race was an independent predictor of mortality in COVID-19 pneumonia (Table 3). Prior research on interhospital transfer has also suggested that White patients are more likely to be transferred compared with Black and Hispanic patients (6, 7). In our cohort, the racial and ethnic breakdowns between interhospital transfers and ED admits were similar (Table 1).
As expected, interhospital transfers were more likely to have received ECMO as evaluation for ECMO may have been the primary reason for transfer. This may account for why no interhospital transfers elected hospice and that the average age of transferred patients was less than that of ED admits. Factoring patient selection biases, it is notable that interhospital transfers had equivalent comorbid conditions as ED admits, as patients “most likely to benefit” may have been expected to have fewer comorbid conditions.
Timing of interhospital transfers and deaths reflect many complexities of the COVID-19 pandemic. Because pretransfer ED duration, ICU duration, hospital length of stay, transport time, and timing of intubation could not be obtained in our electronic chart abstraction, we are unable to better characterize how these potential delays in care may have affected outcomes. A significantly larger proportion or interhospital transfers occurred in March and April, compared with ED admits, coinciding with the initial U.S. surge in COVID-19 infections (19) and our hospital ICU surge plan. Odds of mortality were higher for all patients in our cohort from May to September, as compared with March and April. In addition to epidemiologic factors noted above, practice patterns in management of COVID-19 pneumonia changed significantly during the study period. Evolution of care led to increased access to and evidence for supportive therapies such as remdesivir (37) and dexamethasone (38), as well as increased use of noninvasive ventilation and delayed intubation (39). These factors have led to fewer patients requiring invasive ventilation and a higher severity of illness at the time of intubation. Additionally, as community ICU beds became available, and education and comfort with COVID-19 grew, fewer transfers were requested.
Our study had several limitations. We performed a retrospective cohort review using electronic chart extraction. Subtleties of care or differences in outcome could have been better gleaned from subjective chart review; however, the objective nature of our extraction may have prevented investigator bias. Further, our analysis was limited to data routinely collected for all patients. While more advanced disease severity scoring (such as Acute Physiology and Chronic Health Evaluation or Simplified Acute Physiology Score [40]) was not performed due to missing data in a significant portion of our cohort, we used laboratory values and comorbidities as surrogate markers to adjust for severity of illness. Finally, we were unable to analyze data from the hospital of origin for transfer patients. To account for this, we compared the day of transfer to the first day of intubation in the ED admits. As a result, our data may have captured some transferred patients later in their disease course, explaining their relatively higher disease severity. We note that five of the patients had missing data in race/ethnicity and were excluded from the regression analyses. There were no significant differences in mortality between patients with missing versus complete data, and no differences found in our sensitivity analyses. As this is a small single-center study, it is possible that our sample size was insufficient to exclude a significant mortality difference; a larger multi-institutional sample would be beneficial.
Ideally, we would compare the care the patients received with the hypothetical care they would have received without interhospital transfer. Even if referring hospital data were available, factors such as patient selection for transfer request, acceptance of request, death prior to transfer, and timing of available ICU beds or ECMO circuits would confound this potential evaluation. This study characterizes the outcomes of interhospital transfer in a single tertiary care institution and suggests that interhospital transfer of critically ill patients with COVID-19 can be done safely and effectively. To build on this study, comprehensive multi-institutional investigations could help to better understand the outcomes of interhospital transfer of patients with COVID-19. This might span from the time of presentation in a community ED, through the tertiary hospital stay and long-term acute care or post-acute care stay, until return home. We suggest a regional collaboration amongst such institutions to account for the complex web of transfers, in order to consider centralized regional transfer protocols in future surges and pandemics.
CONCLUSIONS
Critically ill, mechanically ventilated patients with COVID-19 pneumonia were frequently transferred from community hospitals to our tertiary care institution during the first surge of COVID-19 in Chicago, IL. The proportion of both ED admits and interhospital transfers who were Black or Hispanic was significantly higher than in the local population in general. While interhospital transfers were younger than ED admits, hospital mortality, ICU days, and ventilation days were similar. Interhospital transfers received more aggressive care, with higher rates of ECMO and lower utilization of hospice. Interhospital transfer of critically ill patients with COVID-19 pneumonia is an important aspect of the care of these patients and deserves further study.
Supplementary Material
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
Drs. Longcoy, Lange-Maia, Avery, and Johnson performed the statistical data analysis and interpretation. Dr. Chen is the guarantor for the article. All authors made substantial contributions to the conception or design of the work, drafted the work or revised it critically for important intellectual content, and approved the final version.
Supported, in part, by the Rush Coronavirus Research Fund.
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Iwashyna TJ, Kramer AA, Kahn JM. Intensive care unit occupancy and patient outcomes. Crit Care Med. 2009; 37:1545–1557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner J, Iwashyna TJ, Kahn JM. Reasons underlying interhospital transfers to an academic medical intensive care unit. J Crit Care. 2013; 28:202–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flabouris A. Patient referral and transportation to a regional tertiary ICU: Patient demographics, severity of illness and outcome comparison with non-transported patients. Anaesth Intensive Care. 1999; 27:385–390 [DOI] [PubMed] [Google Scholar]
- 4.Golestanian E, Scruggs JE, Gangnon RE, et al. Effect of interhospital transfer on resource utilization and outcomes at a tertiary care referral center. Crit Care Med. 2007; 35:1470–1476 [DOI] [PubMed] [Google Scholar]
- 5.Schiff RL, Ansell DA, Schlosser JE, et al. Transfers to a public hospital. A prospective study of 467 patients. N Engl J Med. 1986; 314:552–557 [DOI] [PubMed] [Google Scholar]
- 6.Tyler PD, Stone DJ, Geisler BP, et al. Racial and geographic disparities in interhospital ICU transfers. Crit Care Med. 2018; 46:e76–e80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arthur KR, Kelz RR, Mills AM, et al. Interhospital transfer: An independent risk factor for mortality in the surgical intensive care unit. Am Surg. 2013; 79:909–913 [DOI] [PubMed] [Google Scholar]
- 8.Duke GJ, Green JV. Outcome of critically ill patients undergoing interhospital transfer. Med J Aust. 2001; 174:122–125 [DOI] [PubMed] [Google Scholar]
- 9.Sokol-Hessner L, White AA, Davis KF, et al. Interhospital transfer patients discharged by academic hospitalists and general internists: Characteristics and outcomes. J Hosp Med. 2016; 11:245–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faine BA, Noack JM, Wong T, et al. Interhospital transfer delays appropriate treatment for patients with severe sepsis and septic shock: A retrospective cohort study. Crit Care Med. 2015; 43:2589–2596 [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg AL, Hofer TP, Strachan C, et al. Accepting critically ill transfer patients: Adverse effect on a referral center’s outcome and benchmark measures. Ann Intern Med. 2003; 138:882–890 [DOI] [PubMed] [Google Scholar]
- 12.Hill AD, Vingilis E, Martin CM, et al. Interhospital transfer of critically ill patients: Demographic and outcomes comparison with nontransferred intensive care unit patients. J Crit Care. 2007; 22:290–295 [DOI] [PubMed] [Google Scholar]
- 13.Painvin B, Messet H, Rodriguez M, et al. Inter-hospital transport of critically ill patients to manage the intensive care unit surge during the COVID-19 pandemic in France. Ann Intensive Care. 2021; 11:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Allen R, Wanersdorfer K, Zebley J, et al. Interhospital transfer of critically ill patients because of coronavirus disease 19-related respiratory failure. Air Med J. 2020; 39:498–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salman S, Saleem SG, Khatri A, et al. Inter-hospital communication and transfer practices during COVID-19 Pandemic in Karachi, Pakistan. A brief overview. Pak J Med Sci. 2020; 36:S118–S120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wahlster S, Sharma M, Lewis AK, et al. The coronavirus disease 2019 pandemic’s effect on critical care resources and health-care providers. Chest. 2020; 159:619–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maves RC, Downar J, Dichter JR, et al. ; ACCP Task Force for Mass Critical Care. Triage of scarce critical care resources in COVID-19 an implementation guide for regional allocation: An expert panel report of the Task Force for Mass Critical Care and the American College of Chest Physicians. Chest. 2020; 158:212–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu H, Tong Z, Ma P, et al. ; China Critical Care Clinical Trials Group (CCCCTG). Intensive care during the coronavirus epidemic. Intensive Care Med. 2020; 46:576–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abir M, Nelson C, Chan EW, et al. Critical care surge response strategies for the 2020 COVID-19 outbreak in the United States. Rand Corporation, 2020. 10.7249/RRA164-1 [DOI]
- 20.Guérin C, Reignier J, Richard JC, et al. ; PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013; 368:2159–2168 [DOI] [PubMed] [Google Scholar]
- 21.Combes A, Hajage D, Capellier G, et al. ; EOLIA Trial Group, REVA, and ECMONet. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018; 378:1965–1975 [DOI] [PubMed] [Google Scholar]
- 22.Peek GJ, Mugford M, Tiruvoipati R, et al. ; CESAR trial collaboration. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): A multicentre randomised controlled trial. Lancet. 2009; 374:1351–1363 [DOI] [PubMed] [Google Scholar]
- 23.Weiss TT, Cerda F, Scott JB, et al. Prone positioning for patients intubated for severe acute respiratory distress syndrome (ARDS) secondary to COVID-19: A retrospective observational cohort study. Br J Anaesth. 2021; 126:48–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020; 18:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Zhang D, Du G, et al. Remdesivir in adults with severe COVID-19: A randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020; 395:1569–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spinner CD, Gottlieb RL, Criner GJ, et al. ; GS-US-540-5774 Investigators. Effect of remdesivir vs standard care on clinical status at 11 days in patients with moderate COVID-19: A randomized clinical trial. JAMA. 2020; 324:1048–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von EE, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007; 370:1453–1457 [DOI] [PubMed] [Google Scholar]
- 28.Rush System. About Our System. 2021. Available at: https://www.rush.edu/about-us/about-our-system. Accessed February 24, 2021
- 29.Illinois.gov. Executive Order 2020-10. 2020. Available at: https://www2.illinois.gov:443/Pages/Executive-Orders/ExecutiveOrder2020-10.aspx. Accessed February 24, 2021
- 30.Clinical Classifications Software Refined (CCSR). Healthcare Cost and Utilization Project (HCUP). Rockville, MD, Agency for Healthcare Research and Quality. 2020. Available at: https://www.hcup-us.ahrq.gov/toolssoftware/ccsr/ccs_refined.jsp. Accessed February 25, 2021 [Google Scholar]
- 31.Centers for Disease Control and Prevention. COVID-19 and Your Health. 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/need-extra-precautions/people-with-medical-conditions.html. Accessed February 24, 2021 [PubMed]
- 32.Pirracchio R, Resche-Rigon M, Chevret S. Evaluation of the propensity score methods for estimating marginal odds ratios in case of small sample size. BMC Med Res Methodol. 2012; 12:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lemeshow S, Hosmer DW, Jr. A review of goodness of fit statistics for use in the development of logistic regression models. Am J Epidemiol. 1982; 115:92–106 [DOI] [PubMed] [Google Scholar]
- 34.Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011; 46:399–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S. Census Bureau QuickFacts. Chicago City, Illinois. 2021. Available at: https://www.census.gov/quickfacts/chicagocityillinois. Accessed February 24, 2021
- 36.Price-Haywood EG, Burton J, Fort D, et al. Hospitalization and mortality among black patients and white patients with COVID-19. N Engl J Med. 2020; 382:2534–2543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beigel JH, Tomashek KM, Dodd LE, et al. ; ACTT-1 Study Group Members. Remdesivir for the treatment of COVID-19 - final report. N Engl J Med. 2020; 383:1813–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19 — preliminary report. N Engl J Med. 2020; 384:693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreyro BL, Angriman F, Munshi L, et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: A systematic review and meta-analysis. JAMA. 2020; 324:57–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vasilevskis EE, Kuzniewicz MW, Cason BA, et al. Mortality probability model III and simplified acute physiology score II: Assessing their value in predicting length of stay and comparison to APACHE IV. Chest. 2009; 136:89–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.