Abstract
Background
Patients with Coronavirus disease 2019 (COVID-19)-related acute respiratory disease (ARDS) increasingly receive extracorporeal membrane oxygenation (ECMO) support. While ECMO has been shown to increase risk of stroke, few studies have examined this association in COVID-19 patients.
Objective
We conducted a systematic review to characterise neurological events during ECMO support in COVID-19 patients.
Design
Systematic review of cohort and large case series of COVID-19 patients who received ECMO support.
Data Sources
Studies retrieved from PubMed, EMBASE, Cochrane, Cochrane COVID-19 Study Register, Web of Science, Scopus, Clinicaltrials.gov, and medRχiv from inception to November 11, 2020.
Eligibility Criteria
Inclusion criteria were a) Adult population (>18 year old); b) Positive PCR test for SARS-CoV-2 with active COVID-19 disease; c) ECMO therapy due to COVID-19 ARDS; and d) Neurological events and outcome described while on ECMO support. We excluded articles when no details of neurologic events were available.
Results
1,322 patients from 12 case series and retrospective cohort studies were included in our study. The median age was 49.2, and 75% (n=985) of the patients were male. Diabetes mellitus and dyslipidaemia were the most common comorbidities (24% and 20%, respectively). Most (95%, n=1,241) patients were on venovenous ECMO with a median P:F ratio at the time of ECMO cannulation of 69.1. The prevalence of intracranial haemorrhage (ICH), ischaemic stroke, and hypoxic ischaemic brain injury (HIBI) was 5.9% (n=78), 1.1% (n=15), and 0.3% (n=4), respectively. The overall mortality of the 1,296 ECMO patients in the 10 studies that reported death was 36% (n=477), and the mortality of the subset of patients who had a neurological event was 92%.
Conclusions
Neurological injury is a concern for COVID-19 patients who receive ECMO. Further research is required to explore how neuromonitoring protocols can inform tailored anticoagulation management and improve survival in COVID-19 patients with ECMO support.
Keywords: ECMO, COVID-19, Stroke, Intracranial haemorrhage
Introduction
The use of extracorporeal membrane oxygenation (ECMO) support in Coronavirus disease 2019 (COVID-19)-related acute respiratory distress syndrome (ARDS) has increased throughout the pandemic due to its survival benefit as a rescue therapy, similar to those of pre-COVID-19 veno-venous (VV) ECMO [1,2]. ECMO support has been frequently associated with neurological events, such as ischaemic stroke and intracranial haemorrhage (ICH), independent of COVID-19 infection [3,4]. COVID-19 has been associated with endothelial dysregulation and a pro-thrombotic state, which may contribute to increased risk of neurological events [5]. One autopsy study found that cerebral haemorrhage was the most common gross abnormality in brain samples of COVID-19 patients, suggesting a high prevalence of ICH independent of ECMO support [6]. Although it has been perceived anecdotally that neurological events were more common in COVID-19 ECMO patients than uninfected ECMO patients, there are only few studies specifically describing neurological events in the former population. Herein, we conducted a systematic review and meta-analysis to report and characterise neurological events during ECMO support in COVID-19 patients during the pandemic.
Material and Methods
Search Strategy
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guidelines (Appendix A) [7,8]. We performed a literature search with Medline via PubMed, EMBASE, Cochrane Library, Web of Science, Scopus, clinicaltrials.gov, and medRχiv using controlled vocabulary and keywords related to ECMO and COVID-19. Search terms are detailed in Appendix B. Our search identified 12 unique case series and observational studies published from inception to 11 November 2020. The review was not registered and protocol was not prepared.
Study Eligibility: Inclusion and Exclusion Criteria
Inclusion criteria were: a) Adult population (>18 year old); b) Positive PCR test for SARS-CoV-2 with active COVID-19 disease; c) ECMO therapy due to COVID-19 ARDS; and d) Neurological events and outcome described while on ECMO support. Conference papers, posters, author’s replies, editorials, commentaries, systematic review articles, review articles, case reports, and letters to the editor were excluded. We excluded case series with less than 10 patients. We excluded articles when no details of neurologic events were available. The articles were discussed in detail among authors (N.V.K. and S.M.C.) before being excluded.
Study Selection and Data Extraction
Two (2) independent reviewers (N.V.K. and M.J.) screened all studies based on titles and abstracts. Disagreements were resolved through consensus or referral to a third reviewer (S.M.C). Each study was evaluated independently. Then data were extracted from eligible studies into a shared Excel spreadsheet (Microsoft, Redmond, WA, USA) by one reviewer (N.V.K.). We collected demographic characteristics, past medical history, neurological events, laboratory abnormalities, anti-coagulation status, ECMO variables, and survival outcome.
Definitions of Outcomes
Primary outcomes were new neurological events in ECMO patients. Neurological events of interest included ischaemic stroke, ICH, and hypoxic ischaemic brain injury (HIBI). Secondary outcome was mortality. Pooled prevalence and weighted average of median values were calculated for variables of COVID-19 patients receiving ECMO treatment.
Risk of Bias
The quality of included cohort studies was assessed using the Newcastle-Ottawa scale (NOS) in order to evaluate the level of bias in the non-randomised observational studies [9]. The maximal score is 9, comprised of three domains: study group selection (maximal score: 4), comparability of groups (maximal score: 2), and ascertainment of exposure and outcomes (maximal score: 3). Murad et al.’s NOS scale (maximal score: 6) was used to evaluate the methodological quality of the case-series studies [10]. As suggested by Murad et al., a holistic judgement on the score was made in addition to the quantitative summation. Both were then compared to obtain a final rating. Two (2) reviewers (N.V.K. and L.P.) assessed quality independently and discussed each study in detail before arriving at a consensus.
Data Analysis
For each study, categorical variables were reported as proportions and continuous variables were reported as medians with interquartile range if available. Summative data was reported as pooled prevalence for categorical variables and weighted average of the median values for continuous variables. These values were calculated using Stata Release 15 (StataCorp LLC, College Station, TX, USA). All missing values were not included in the table, and the summative data was calculated accordingly.
Results
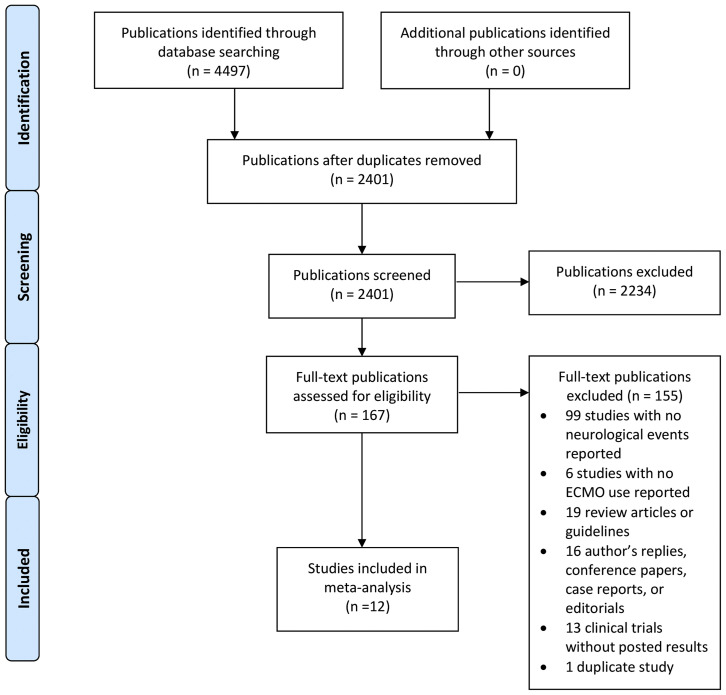
The search identified 2,401 publications, after removal of duplicates, of which 167 were selected for full-text review after the abstract screening (Figure 1 ). Twelve (12) publications (10 cohort studies and 2 large case series) with a combined population of 1,322 COVID-19 ECMO patients were included (Appendix C). All cohort studies were judged to be of high quality with median scores of 8 on the NOS and 5 on the Murad’s NOS, respectively. The quality assessment also showed that all case series had adequate data on case selection, exposure and outcome ascertainment, causality regarding outcomes, and sufficient detail to make inference related to clinical practice. Supplemental Table 1 depicts the details of the quality assessment.
Figure 1.
Flow diagram for systematic review of COVID-19 patients on ECMO developing neurological complications. Abbreviation: ECMO, extracorporeal membrane oxygenation.
The median age was 49.2, and 75% (n=985) of the patients were male (Table 1 ). Out of the 1,091 patients whose race or ethnicity was reported, 15% (n=165), 22% (n=225) and 35% (n=361) were Black, Hispanic, and White, respectively. The median body mass index (BMI) was 30.2 kg/m2. Diabetes mellitus was the most common comorbidity (24%), followed by dyslipidaemia (20%), hypertension (8%), chronic obstructive pulmonary disease (4%), and coronary artery disease (2%).
Table 1.
Demographic information, comorbidities, and ICU course of COVID-19 patients receiving ECMO therapy.
| Author | Study Type | n | Age, Years, Median (IQR) | Sex, Male, n (%) | Race and Ethnicity, Black, Hispanic, Shite, n (%) |
BMI, kg/m2, Median (IQR), | DM, n (%) | HTN, n (%) | CAD, n (%) | DL, n (%) | COPD/Asthma, n (%) | Smoker, n (%) | ECMO Type, VV, n (%) | P:F Ratio, Median (IQR) | Goal Anti-Xa Level (IU/mL) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alnababteh et al. | Retrospective cohort | 13 | 44.5 | 8 (62) | 34.4 [28–41] | 4 (31) | 5 (39) | 0 (0) | 0 (0) | 0 (0) | 13 (100) | 98∗∗ | 0.3–0.5 | ||
| Barbaro et al. | Retrospective cohort | 1,035 | 49 [41–57] | 764 (74) | 150 (14) 218 (21) 346 (33) |
31 [27–37] | 245 (24) | 29 (3) | 973 (94) | 72 (59–94)∗∗ | |||||
| Cousin et al. | Retrospective cohort | 30 | 57 [47–62] | 24 (80) | 33 [29–38] | 10 (33) | 16 (53) | 0 (0) | 7 (23) | 3 (10) | 1 (3) | 30 (100) | 69 (63–75)∗ | 0.3–0.5 | |
| Falcoz et al. | Retrospective cohort | 17 | 56 | 16 (94) | 3 (18) | 9 (53) | 16 (94) | 71∗∗ | 0.5–0.7 | ||||||
| Guihaire et al. | Retrospective cohort | 24 | 48.8 | 20 (83) | 29.4 [22–44] | 5 (21) | 5 (21) | 4 (17) | 2 (8) | 24 (100) | 67 (52–78)∗∗ | 0.4–0.6 | |||
| Jang et al. | Retrospective cohort | 19 | 63 [60–66] | 15 (79) | 26.7 [26–28] | 8 (42) | 11 (58) | 1 (5) | 1 (5) | 16 (84) | 92 (62–139)∗ | None mentioned | |||
| Kon et al. | Retrospective cohort | 27 | 40 [31–47] | 23 (85) | 32 [–37] | 4 (15) | 5 (19) | 1 (4) | 2 (7) | 27 (100) | 84 (70-118) | >0.15 | |||
| Masur et al. | Case series | 12 | 59 [50–62] | 8 (67) | 4 (33) | 5 (42) | 4 (33) | 12 (100) | None mentioned | ||||||
| Osho et al. | Retrospective cohort | 6 | 47 [43–53] | 5 (83) | 31.2 [31–35] | 4 (67) | 3 (50) | 0 (0) | 0 (0) | 2 (33) | 6 (100) | 94.5∗∗ | None mentioned | ||
| Parzy et al. | Retrospective cohort | 13 | 50 [43–62] | 8 (70) | 5 (39) 8 (62) 0 (0) |
31 [27–34] | 2 (15) | 1 (8) | 13 (100) | 0.3–0.6 | |||||
| Schmidt et al. | Retrospective cohort | 83 | 49 [41–56] | 61 (73) | 30.4 [28–34] | 26 (31) | 32 (39) | 9 (11) | 2 (2) | 81 (97) | 60 (54–68)∗ | 0.3–0.5 | |||
| Zhang et al. | Case series | 43 | 46 [36–53] | 33 (77) | 10 (23) 0 (0) 15 (35) |
29 [27–34] | 8 (19) | 10 (23) | 5 (12) | 43 (100) | 67.5 (53–76)∗ | 0.3–0.7 | |||
| Weighted average/Pooled prevalence | 1,322 | 49.2 | 985 (75) |
165 (15) 226 (22) 361 (35) |
30.2 | 323 (24) | 101 (8) | 2 (2) | 11 (20) | 54 (4) | 7 (4) | 1,254 (95) | 69.1 | N/A |
Abbreviations: BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; DL, dyslipidaemia; DM, diabetes mellitus; ECMO, extra-corporeal membrane oxygenation; HTN, hypertension; ICU, intensive care unit; IQR, interquartile range.
During the hospitalisation course, of those that reported specific treatments, 30% (n=323) and 41% (n=455) received anti-interleukin-6 treatment and corticosteroid treatment, respectively (Supplemental Table 2). The median Sequential Organ Failure Assessment (SOFA) score among the studies that reported this value was 9.8. Most (95%, n=1,241) patients were on venovenous (VV)-ECMO with a median ECMO flow of 5.0 L/min on day 1 of ECMO support (Table 1 and Supplemental Table 2). The median P:F ratio at the time of ECMO cannulation was 69.1 (Table 1). While three studies did not mention an anticoagulation protocol during ECMO therapy, the remaining nine reported using unfractionated heparin with a specific anti-Xa goal. The goals ranged from 0.15 to 0.7 IU/mL. Only two studies mentioned utilising a neuromonitoring protocol or standardised neurological assessment during ECMO therapy.
The prevalence of ICH, ischaemic stroke, and HIBI was 5.9% (n=78), 1.1% (n=15), and 0.3% (n=4), respectively (Table 2 ). In a sensitivity analysis, excluding the study by Barbaro et al. (n=1,035) due to the large size of the study, the prevalence of ICH, ischaemic stroke, and HIBI were similar with 7.7% (n=22), 2.8% (n=8), and 1.3% (n=4), respectively (Supplemental Table 3). The overall mortality of the 1,296 ECMO patients in the 10 studies that reported death was 36% (n=477), which was similar in the sensitivity analysis (34%). Only four studies specifically reported the mortality for patients with neurological events and the pooled mortality proportion was 92% (n=11). No studies specifically identified risk factors for stroke in this patient population.
Table 2.
Neurological events during ECMO course in COVID-19 patients.
| Author | n | ICH, n (%) | Ischaemic Stroke, n (%) | HIBI, n (%) | Overall Mortality, n (%) | Mortality in Patients With Neurological Events, n (%)∗ |
|---|---|---|---|---|---|---|
| Alnababteh et al. | 13 | 0 (0) | 0 (0) | 0 (0) | 6 (46) | |
| Barbaro et al. | 1,035 | 56 (6) | 7 (0.7) | 0 (0) | 380 (37) | |
| Cousin et al. | 30 | 3 (10) | 1 (3.3) | 0 (0) | 16 (53) | |
| Falcoz et al. | 17 | 0 (0) | 1 (5.9) | 0 (0) | 6 (35) | |
| Guihaire et al. | 24 | 1 (4) | 0 (0) | 0 (0) | 7 (29) | 1 (100) |
| Jang et al. | 19 | 0 (0) | 0 (0) | 4 (21) | 9 (58) | |
| Kon et al. | 27 | 0 (0) | 0 (0) | 0 (0) | 1 (4) | 0 (0) |
| Masur et al. | 12 | 5 (42) | 2 (17) | 0 (0) | 5 (100) | |
| Osho et al. | 6 | 1 (17) | 0 (0) | 0 (0) | 1 (17) | |
| Parzy et al. | 13 | 1 (8) | 0 (0) | 0 (0) | ||
| Schmidt et al. | 83 | 4 (5) | 1 (1) | 0 (0) | 30 (36) | 4 (80) |
| Zhang et al. | 43 | 7 (16) | 3 (7) | 0 (0) | 14 (33) | |
| Weighted average/Pooled prevalence | 1,322 | 78 (5.9) | 15 (1.1) | 4 (0.3) | 477 (36) | 11 (92) |
Abbreviations: ECMO, extra-corporeal membrane oxygenation; HIBI, hypoxic-ischaemic brain injury; ICH, intracranial haemorrhage.
Percentage reported as proportion of patients with neurological injury who were deceased.
Discussion
In this study, we performed a comprehensive review and meta-analysis to report the prevalence of neurologic events in COVID-19 patients on ECMO therapy. We demonstrated an overall prevalence of 5.9% for ICHs, 1.1% for ischaemic stroke and 0.3% for HIBI among 1,322 COVID-19 patients requiring ECMO support. Previously, a large cohort study using the Extracorporeal Life Support Organization registry database demonstrated that patients with VV-ECMO support for acute respiratory failure, without COVID-19, had 3.1% ICHs and 1.4% ischaemic strokes [11]. Similarly, a recent meta-analysis, pooling ECMO patient data from 1992–2015, showed a 7.1% overall prevalence of neurological events, with 3.6% of patients with ICH and 1.7% with ischaemic stroke [12]. While these values were similar to the ischaemic stroke prevalence in our meta-analysis (1.1%), it is interesting to note that the proportion of ICH (5.9%) in our study was higher than previously reported for VV-ECMO patients. In the absence of a comparative study with an appropriate control group, we cannot definitively state that COVID-19 infection modifies risk of neurological events, especially ICH, for patients with ECMO support. However, the findings of our study may suggest that acute COVID-19 infection with aggressive anticoagulation in this population may confer a higher risk of ICH. It is well established that bleeding events in ECMO are associated with acquired von Willebrand syndrome, due to the high sheer stress of the ECMO circuit, and aggressive anticoagulation as well as thrombocytopaenia [13,14]. It is unclear, at this time of the review, if COVID-19 infection confers additional risk of cerebral endothelial injury leading to a higher prevalence of neurological events [15].
It is important to note that acute COVID-19 infection is known to confer an increased risk of thrombotic complications including ischaemic stroke as well as a pro-haemorrhagic state with coagulopathy and aggressive anticoagulation. The current understanding of COVID-19 pathophysiology involves a pro-coagulable state and infection-related thrombosis, requiring higher intensity anticoagulation during acute hospitalisation [16,17]. On the other hand, aggressive anticoagulation may have led to a higher bleeding events in this population [18]. Sepsis-induced coagulopathy, caused in part by elevated D-dimer and fibrinogen levels, endothelial damage due to viral entry, and the cytokine storm have all been associated with pro-thrombotic states in COVID-19 patients. One prior study utilised a control group of 1,486 influenza-infected patients to compare to the ischaemic stroke risk in 1,916 COVID-19 patients and found that COVID-19 conferred eight times higher odds of ischaemic stroke [19]. However, there is limited evidence comparing the risk of ICH in patients with COVID-19 vs. without. Nannoni et al., in their meta-analysis including over 100,000 COVID-19 patients, found the overall frequency of stroke to be 1.4%. Within this stroke group, acute ischaemic stroke was much more common than ICH (1.2% and 0.2%, respectively) [20]. Another multicentre study including over 17,000 COVID-19 patients showed ischaemic stroke and ICH incidence rates of 0.7% and 0.2%, respectively [21]. Given the higher risk of ischaemic stroke in COVID-19 patients, an addition of ECMO support as well as aggressive anticoagulation may have led to the higher frequency of ICHs as a synergistic shift to a haemorrhagic state in COVID-19 patients with ECMO.
Our meta-analysis showed an overall mortality of 36%, and this increased to 92% in patients with neurological events. A previous study, including 15,872 VV-ECMO patients (non COVID-19), showed a similar overall mortality (34%) and 73% mortality for patients with ICH, which is consistent with the findings from our study [11]. While it is not surprising that the presence of neurological events increased the risk of mortality, it is essential to emphasise the importance of a standardised neuromonitoring protocol and frequent neurological assessment for ECMO patients regardless of COVID-19 status [22].
Strengths of this study include the relatively large number of cohorts included in the analysis on this particular topic. It is also the only meta-analysis to examine each type of neurological event associated with COVID-19 patients on ECMO. However, our study has several limitations. First, many studies did not report the details of neurological events and most studies lacked reports on standardised monitoring for neurological events. Given that these patients are usually heavily sedated and paralysed, this may lead to an underestimation of true prevalence of neurological events. Additionally, studies did not report data on the subset of patients who had neurological events versus those who did not have neurological events, limiting a comparative analysis. Second, there is a substantial heterogeneity in the definition of each neurological event, and it is possible that not all included patients received neuroimaging studies. Thus, although mortality was more than doubled in patients with neurological events, this represents an associative rather than causative relationship. Lastly, modifications to patient management during the pandemic may affect the relevance of the findings presented. As a note, no study compared COVID-19 ECMO patients without neurological events to those with events to determine risk factors. Despite the limitations, our study represents the largest data to-date on the topic of neurological events in COVID-19 patients with ECMO support. Further research that describes prevalence, risk factors and outcomes for neurological complications in the critically ill COVID-19 population is required to corroborate our findings and inform management decisions.
Conclusions
We found that the prevalence of ICH, ischaemic stroke, and HIBI were 5.9%, 1.1%, and 0.3%, respectively. Overall mortality for COVID-19 patients on ECMO was 36%, and the mortality was more than double in patients with neurological events. Future studies are required to explore risk factors and how neuromonitoring protocols, including routine neurological assessment for early detection of stroke, can inform tailored anticoagulation management and improve survival in COVID-19 patients with ECMO support.
Conflict of Interest/Financial Statement
The authors have nothing to disclose.
Footnotes
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.hlc.2021.10.007.
Appendices. Supplementary Data
References
- 1.Badulak J., Antonini M.V., Stead C.M., Shekerdemian L., Raman L., Paden M.L., et al. Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the extracorporeal Life support Organization. ASAIO J. 2021;67(5):485–495. doi: 10.1097/MAT.0000000000001422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbaro R.P., MacLaren G., Boonstra P.S., Iwashyna T.J., Slutsky A.S., Fan E., et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396(10257):1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Migdady I., Rice C., Deshpande A., Hernandez A.V., Price C., Whitman G.J., et al. Brain injury and neurologic outcome in patients undergoing extracorporeal cardiopulmonary resuscitation: a systematic review and meta-analysis. Crit Care Med. 2020;48(7):e611. doi: 10.1097/CCM.0000000000004377. [DOI] [PubMed] [Google Scholar]
- 4.Shoskes A., Migdady I., Rice C., Hassett C., Deshpande A., Price C., et al. Brain injury is more common in venoarterial extracorporeal membrane oxygenation than venovenous extracorporeal membrane oxygenation: a systematic review and meta-analysis. Crit Care Med. 2020;48(12):1799–1808. doi: 10.1097/CCM.0000000000004618. [DOI] [PubMed] [Google Scholar]
- 5.Bernard I., Limonta D., Mahal L.K., Hobman T.C. Endothelium infection and dysregulation by SARS-CoV-2: evidence and caveats in COVID-19. Viruses [Internet] 2020;13(1):29. doi: 10.3390/v13010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mukerji S.S., Solomon I.H. What can we learn from brain autopsies in COVID-19? Neurosci Lett. 2021;742:135528. doi: 10.1016/j.neulet.2020.135528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ [Internet] 2009;339:b2535. [PMC free article] [PubMed] [Google Scholar]
- 8.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162(11):777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 9.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 10.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid-Based Med. 2018;23(2):60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cho S.-M., Canner J., Caturegli G., Choi C.W., Etchill E., Giuliano K., et al. Risk Factors of ischemic and hemorrhagic strokes during venovenous extracorporeal membrane oxygenation: analysis of data from the Extracorporeal Life Support Organization Registry. Crit Care Med. 2021;49(1):91–101. doi: 10.1097/CCM.0000000000004707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lorusso R., Gelsomino S., Parise O., Di Mauro M., Barili F., Geskes G., et al. Neurologic injury in adults supported with veno-venous extracorporeal membrane oxygenation for respiratory failure: findings from the Extracorporeal Life Support Organization Database. Crit Care Med. 2017;45(8):1389–1397. doi: 10.1097/CCM.0000000000002502. [DOI] [PubMed] [Google Scholar]
- 13.Kalbhenn J., Schlagenhauf A., Rosenfelder S., Schmutz A., Zieger B. Acquired von Willebrand syndrome and impaired platelet function during venovenous extracorporeal membrane oxygenation: Rapid onset and fast recovery. J Heart Lung Transplant. 2018;37(8):985–991. doi: 10.1016/j.healun.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Uriel N., Pak S.-W., Jorde U.P., Jude B., Susen S., Vincentelli A., et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56(15):1207–1213. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosovsky R.P., Sanfilippo K.M., Wang T.F., Rajan S.K., Shah S., Martin K.A., et al. Anticoagulation practice patterns in COVID-19: a global survey. Res Pract Thromb Haemost. 2020;4(6):969–983. doi: 10.1002/rth2.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olson S.R., Murphree C.R., Zonies D., Meyer A.D., Mccarty O.J.T., Deloughery T.G., et al. Thrombosis and bleeding in extracorporeal membrane oxygenation (ECMO) without anticoagulation: a systematic review. ASAIO J. 2021;67(3):290–296. doi: 10.1097/MAT.0000000000001230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Merkler A.E., Parikh N.S., Mir S., Gupta A., Kamel H., Lin E., et al. Risk of ischemic stroke in patients with Coronavirus Disease 2019 (COVID-19) vs patients with influenza. JAMA Neurol. 2020;77(11):1366–1372. doi: 10.1001/jamaneurol.2020.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nannoni S., de Groot R., Bell S., Markus H.S. Stroke in COVID-19: a systematic review and meta-analysis. Int J Stroke. 2020;26 doi: 10.1177/1747493020972922. 1747493020972922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shahjouei S., Naderi S., Li J., Khan A., Chaudhary D., Farahmand G., et al. Risk of stroke in hospitalized SARS-CoV-2 infected patients: a multinational study. EBioMedicine. 2020;59:102939. doi: 10.1016/j.ebiom.2020.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cho S.-M., Ziai W., Mayasi Y., Gusdon A.M., Creed J., Sharrock M., et al. Noninvasive neurological monitoring in extracorporeal membrane oxygenation. ASAIO J. 2020;66(4):388–393. doi: 10.1097/MAT.0000000000001013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.