Abstract
Purpose
Diabetes is one of the major comorbidities associated with COVID-19. We aimed to determine the clinical and epidemiological factors associated with the mortality of COVID-19 in diabetic patients in Iran, and also the impact of prescribed antiviral and antibiotics on patients’ status.
Methods
In this study, we used the national registry of hospitalized patients with Severe Acute Respiratory Syndrome (SARS) Symptoms with diabetes from February 18, 2020, to December 22, 2020. Demographic, clinical features, treatments, concurrent comorbidities, and their associations with mortality and severity outcomes were assessed using logistic regression.
Results
78,554 diabetic in-patients with SARS symptoms were included from 31 provinces of whom 37,338 were PCR positive for COVID-19. Older age and male gender are associated with COVID-19 mortality in diabetic patients. CVD is the most frequent comorbidity (42%). CVD, kidney disease, liver disease, and COPD are associated comorbidities which increased the risk of mortality. The mortality rate is higher in diabetic patients comparing to patients with no comorbidities, particularly in younger age groups. The frequency of antiviral, and antibiotics in COVID-19 positive patients was 34%, and 31%, respectively. Antibiotic treatment has no association with mortality in COVID-19 patients.
Conclusions
Diabetic patients indicate higher mortality comparing to patients without any underlying comorbidities. Restrict strategies on increasing effective health care utilization must be considered in diabetic patients, especially in those with parallel underlying comorbidities. Regarding the antibiotic resistance issue and the noticeable use of antibiotics in diabetic patients, it is recommended to prioritize an antibiotic guideline prescription in COVID-19 patients for better stewardship by countries.
Keywords: COVID-19, Diabetes, Comorbidities, Antibiotics, Antiviral
Introduction
Diabetes is one of the major comorbidities associated with COVID-19 [1]. Despite the higher propensity of diabetic patients to other coronaviruses infections in previous epidemics, the prevalence of diabetes among COVID-19 patients seems to be similar to the general population [2]. It is currently unknown whether diabetes predisposes people to COVID-19. However, it makes patients more vulnerable to severe forms of the disease resulting in a higher proportion of intensive care unit admission besides more mortality [3, 4]. The higher mortality rate and poor prognosis among diabetic patients with COVID-19 have been reported in different studies [5, 6]. Findings from a study conducted in the USA indicated a four-fold higher in-hospital mortality rate among diabetic and uncontrolled hyperglycemic patients. Additionally, in a Chinese study, the mortality rate in diabetic patients was 7.3% vs 2.3% for COVID-19 patients overall [7–9].
Age, sex, ethnicity, and low socioeconomic status, as well as concomitant medical conditions like renal impairment, may affect the prognosis of diabetic patients [2]. Several studies showed that poor glycemic control in diabetic patients increases the risk of complications [10, 11]. Furthermore, the impacts of glucose-lowering medication on the severity and fatality of COVID-19 have been reported [6, 12]. Although there are limited studies on antibiotics and antiviral treatment and the interactions with diabetes medications and a unique guild line on this issue, recent studies pointed to the specific effects of treatments for COVID-19 in diabetic patients, e.g. hydroxychloroquine reduces plasma glucose level leading to improve glycemic control besides the higher risk of hypoglycemia which needs special considerations in patients with diabetes [13, 14].
Thus, worse outcomes of COVID-19 in diabetic patients are more likely due to multiple factors that need to be more evaluated in different populations worldwide [2]. Regardless of the special attention to potential characteristics of antiviral and antibacterial drugs to treat COVID-19 [15], there is a lack of information about the impacts of these drugs on the outcomes of diabetic patients with COVID-19. We aimed to determine the clinical and epidemiological characteristics as well as the risk factors associated with the mortality of COVID-19 in diabetic patients in Iran and also the impact of prescribed antiviral and antibiotic on patients’ mortality.
Methods
Overview
In this study, we used a national registry of hospitalized patients with Severe Acute Respiratory Syndrome (SARS) symptoms who are diagnosed with diabetes including patients with confirmed positive COVID-19 PCR from February 18, 2020, to December 22, 2020.
Case definitions
Diabetes is a self-report in this study. COVID-19 confirmed cases are defined as a positive result of laboratory tests for the COVID-19 from the respiratory specimens by the Real-Time Reverse Transcription Polymerase Chain Reaction (RT-PCR) assay. SARS cases were defined as diabetic patients with the following criteria: fever, respiratory symptoms, radiographic evidence of pneumonia, low or normal white-cell count with low lymphocyte count; with a history of travel to contaminated cities or direct contact with patients who have a fever or respiratory symptoms within 14 days before illness. All of these criteria were considered for hospitalization, but not necessarily all of them were required, and finally, the physician decided whether or not to hospitalize according to the patient’s clinical condition. Severe cases are defined as diabetic patients who are admitted to ICU or needs a ventilator.
Collecting data
Demographics consist of gender (male/female) and age, comorbidities including cardiovascular diseases, chronic pulmonary diseases, kidney diseases, liver diseases, immune deficiency diseases, and malignancies (Yes/No). Signs and symptoms consist of fever, cough, dyspnea, myalgia, diarrhea, sore throat, and headache (Yes/No). Pregnancy status, Intensive Care Unit (ICU) admission status, ventilator assistance, COVID-19 PCR diagnostic test result, and clinical outcome, including death, under treatment, severe condition, and recovered were recorded using electronic forms from Hospital Information System (HIS). Moreover, there is a sub-sample of patients who have received prescribed drugs including antibiotics and antiviral treatment during a period of 4 months from initial point of pandemic. All electronic questionnaires were completed by skilled medical staff or physicians.
Statistical analysis
Data cleaning
The cleaning process was done, including managing duplicates cases, addressing missing data problems, recoding variables, and handling garbage records. Also, we had written text for prescribed drugs. So we used data mining via Python to recode them into variables, to calculate the association with outcomes.
Descriptive statistics
Frequency tables were used to describe data on comorbidities, signs, and symptoms, and treatment. Continuous variables (age and body temperature) have not normal distribution, so we reported a median ± interquartile range of 5 to 95%. An appropriate map was used for showing the geographic distribution of COVID-19 mortality rates in patients with diagnosed diabetes.
Analytical statistics
First, to evaluate univariate analysis between mortality, severity, and recovery as outcome dependent variables and the demographic, comorbidities, signs and symptoms, pregnancy, antiviral, and antibiotics as independent variables, the models were built distinctly. The crude, as well as adjusted ORs, were calculated. The univariate analysis was used for calculating crude odds ratio (OR) and 95% confidence interval (CI) and eligibility to enter the final model. To conclude, the exposure factors with a p value of less than 0.2 were entered in the multiple logistic regression model. Consequently, a backward stepwise selection method was used to build multiple models that contained the independent variable groups of comorbidities, signs, and symptoms, that were independently associated with mortality. Adjusted ORs with p values of less than 0.05 and 95% CIs were reported for significant variables that were associated with outcomes. Confounding bias was identified as a consequence of the change in OR before and after adjustment for the confounding variable. Adjustment variables were different in each model based on the logic of the confounding role of the variables in the model. Age, sex, ICU, ventilator assistance, and treatment were variables that were used for adjustment in the multiple regression model. Consequently, the treatment was divided into four groups, antibiotics, antiviral, and pneumonia antibiotics protocol by NICE [16] and hydroxychloroquine. Finally, univariate and multiple logistic analysis was performed for the above-mentioned. The level of significance was considered as p < 0.05. The analyses have been done using R version 3.5.2.
Results
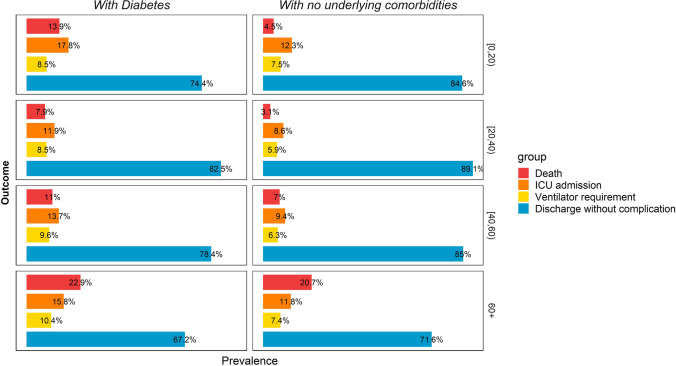
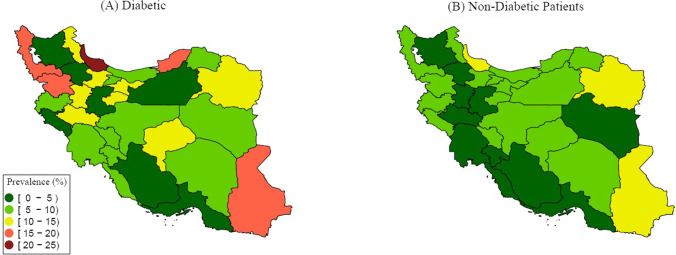
Between February 18, 2020, and December 22, 2020, 78,554 diabetic in-patients with SARS symptoms were registered in the ministry of health registry of Iran from 31 provinces of whom 37,338 were PCR positive for COVID-19 (47.5%). Median age (5–95 interquartile range) for hospitalized, and COVID-19 positive cases were 66(40–86), and 64(40–85), respectively. Females were slightly more than males in both hospitalized and COVID-19 PCR positive patients. Only 36% of pregnant hospitalized patients were PCR positive. The most prevalent comorbidities were CVD, kidney disease, and COPD. The most prevalent signs and symptoms were dyspnea, coughs, and myalgia. These were similar in PCR positive cases. Of patients who were ICU admitted, only 41% were PCR positive. This raised to nearly 48% in ventilator assistance. About 15% of registered patients were deceased, 56% of patients were recovered and 29% were under treatment and about 16% had a severe condition. The mortality rate is higher in diabetic patients comparing to patients with no comorbidities, particularly in younger age groups (13.9% vs 4.5% in under 20 year old, 7.8% vs 3.1% in 20–40 years old, 11% vs 7% in 40–60 years old, and 22.9% vs 20.7% in over 60 years old). This pattern is seen, as well, in ICU admission and ventilation requirement. More detail is determined in Table 1 and Fig. 1. The geographical pattern of mortality in diabetic in-patients with PCR positive COVID-19 was illustrated in Fig. 2.
Table 1.
Descriptive data of diabetic and non-diabetic inpatients with COVID-19
| SARS Positive Diabetic Patients (N = 78,554) |
PCR Positive Diabetic Patients (N = 37,338) |
|
|---|---|---|
| Age, N (%) | ||
| (Mean ± SD) | 64.6 ± 14.0 | 63.6 ± 13.6 |
| <20 yrs. old | 445 (0.6%) | 129 (0.3%) |
| 20 to 40 yrs. old | 3245 (4.1%) | 1, 6137 (4.3%) |
| 40 to 60 yrs. old | 21,728 (27.6%) | 11,618 (31.1%) |
| >60 yrs. old | 53,136 (67.6%) | 243,978 (64.2%) |
| Sex, N (%) | ||
| Female | 43,451 (55.3%) | 20,368 (54.5%) |
| Pregnancy, N(%) | 332 (0.4%) | 119 (0.3%) |
| Comorbidity, N(%) | ||
| CVD | 33,746 (42.9%) | 14,790 (39.6%) |
| COPD | 4.733(6.03%) | 1831(4.9%) |
| Kidney disease | 5928 (7.5%) | 2168 (5.8%) |
| Malignancy | 1215 (1.5%) | 401 (1.1%) |
| Liver disease | 1153 (1.4%) | 488 (1.3%) |
| Immune deficiency disorders | 345 (0.4%) | 150 (0.4%) |
| Sign &symptoms, N(%) | ||
| Dyspnea | 45,754 (58.2%) | 22,344 (59.8%) |
| Cough | 35,017 (44.5%) | 18,973 (50.8%) |
| Body temp (Mean ± SD) | 37.9 ± 0.8 | 38.0 ± 0.8 |
| Fever | 19,371 (24.6%) | 10,271 (27.5%) |
| Myalgia | 20,127 (25.6%) | 10,923 (29.3%) |
| Sore throat | 6529 (8.3%) | 3469 (9.3%) |
| Headache | 7937 (10.1%) | 4262 (11.4%) |
| Diarrhea | 3716 (4.7%) | 1910 (5.1%) |
| ICU admission | 8358 (10.6%) | 3426 (9.2%) |
| Non-invasive ventilation | 7867 (10.0%) | 3771 (10.1%) |
| Outcome, N(%) | ||
| Death | 11,428 (14.5%) | 6920 (18.5%) |
| Recovered | 44,042 (56.1%) | 20,214 (54.1%) |
| Under treatment | 23,084 (29.3%) | 10,204 (27.3%) |
| Sever Condition | 12,820 (16.3%) | 5593 (14.9%) |
Fig. 1.
Outcome of diabetic and non-diabetic inpatients with COVID-19
Fig. 2.
Geographical pattern of age-standardized prevalence of death in diabetic and non-diabetic inpatients with PCR positive COVID-19
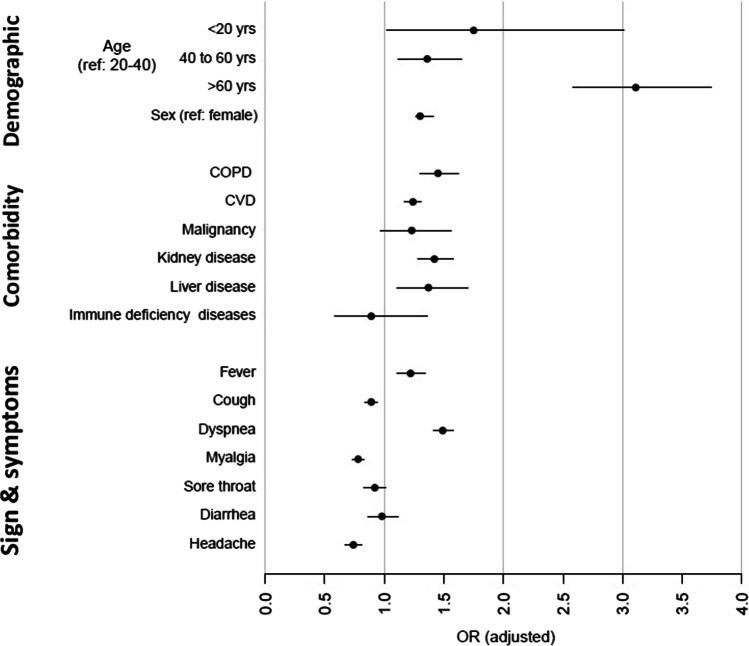
In diabetic patients with COVID-19, the odds of mortality in patients aged more than 60, and under 20 are 3.4 and 1. 9 times higher than the 20 to 40 age group, respectively, which demonstrated the higher risk of mortality in adults and children with diabetes. Males’ odd of mortality is 1.34 times more than females. Mortality in COVID-19 patients are higher in COPD (OR = 1.45, 95%CI: 1.30–1.62), kidney disease (1.42, 1.28–1.57), liver disease (1.37, 1.11–1.70), and CVD (1.23, 1.17–1.30), respectively. Dyspnea (1.48(1.39–1.56)) was associated with higher mortality in diabetic patients, in contrast cough, fever, myalgia, and headache were associated with lower mortality. Although the odds ratio in non-diabetic patients were lower in many aspects comparing to diabetic patients, (such as CVD: 1.16, 1.12–1.20), but we could not compare the magnitude of the mortality effect size between two groups as the CI95% cross each other. The detail on OR crude (CI95%) is available in Table 2, Fig. 3.
Table 2.
Odds ratio of mortality In diabetic and non-diabetic patients with positive COVID-19 PCR
| Demographic | Diabetic with positive COVID-19 PCR (n = 37,338) | Non-diabetic with positive COVID-19 PCR (n = 233,611) | ||||||
|---|---|---|---|---|---|---|---|---|
| OR crude (CI 95%) | PV | OR adjusted (CI 95%) | PV | OR crude (CI 95%) | PV | OR adjusted (CI 95%) | PV | |
| Age 20–40 | Reference | |||||||
| <20 | 1.90(1.12–3.23) | 0.017 | 1.73(1.00–2.97)** | 0.047 | 1.54(1.37-1.72) | <0.001 | 1.42(1.27–1.60)** | <0.001 |
| 40-60 | 1.45(1.20–1.75) | <0.001 | 1.36(1.12–1.65)** | 0.001 | 2.35(2.23-2.49) | <0.001 | 2.26(2.14–2.39)** | <0.001 |
| >60 | 3.49(2.90–4.19) | <0.001 | 3.11(2.58–3.74)** | <0.001 | 7.75(7.36-8.15) | <0.001 | 7.13(6.77–7.51)** | <0.001 |
| Sex | 1.33(1.26–1.40) | <0.001 | 1.34(1.26–1.41)** | <0.001 | 1.27(1.24-1.30) | <0.001 | 1.29(1.24–1.33)** | <0.001 |
| Comorbidity | ||||||||
| Cardiovascular diseases | 1.45(1.37–1.52) | <0.001 | 1.23(1.17–1.30)* | <0.001 | 1.92(1.86-1.98) | <0.001 | 1.16(1.12–1.20)* | <0.001 |
| COPD | 1.65(1.48–1.83) | <0.001 | 1.45(1.30–1.62)* | <0.001 | 2.05(1.94-2.17) | <0.001 | 1.48(1.40–1.57)* | <0.001 |
| Malignancy | 1.46(1.17–1.84) | 0.001 | 1.23(0.97–1.56)* | 0.085 | 2.95(2.71-3.21) | <0.001 | 2.51(2.29–2.75)* | <0.001 |
| Kidney diseases | 1.66(1.51–1.83) | <0.001 | 1.42(1.28–1.57)* | <0.001 | 1.96(1.82-2.11) | <0.001 | 1.47(1.36–1.59)* | <0.001 |
| Liver disease | 1.49(1.21–1.83) | <0.001 | 1.37(1.11–1.70)* | 0.003 | 1.82 (1.58–2.10) | <0.001 | 1.61(1.38–1.87)* | <0.001 |
| Immune deficiency disorders | 1.01(0.67–1.52) | 0.963 | 0.90(0.59–1.38)* | 0.650 | 1.52(1.28-1.80) | <0.001 | 1.4(1.12–1.61)* | 0.001 |
| Sign & symptoms | ||||||||
| Fever | 0.82(0.74–0.90) | <0.001 | 0.86(0.80–0.91)* | <0.001 | 0.78(0.74-0.81) | <0.001 | 0.88(0.86–0.91)* | <0.001 |
| Cough | 0.86(0.81–0.90) | <0.001 | 0.89(0.80–0.94)* | <0.001 | 0.86(0.84-0.88) | <0.001 | 0.87(0.85–0.89)* | <0.001 |
| Dyspnea | 1.56(1.48–1.65) | <0.001 | 1.48(1.39–1.56)* | <0.001 | 1.66(1.62-1.70) | <0.001 | 1.44(1.40–1.47)* | <0.001 |
| Myalgia | 0.73(0.69–0.77) | <0.001 | 0.79(0.73–0.83)* | <0.001 | 0.68(0.66-0.71) | <0.001 | 0.75(0.73–0.78)* | <0.001 |
| Sore throat | 0.83(0.76–0.92) | <0.001 | 0.92(0.83–1.01)* | 0.108 | 0.76(0.73-0.80) | <0.001 | 0.91(0.87–0.95)* | <0.001 |
| Diarrhea | 0.85(0.75–0.97) | 0.014 | 1.00(0.88–1.13)* | 0.978 | 0.60(0.56-0.64) | <0.001 | 0.78(0.73–0.84)* | <0.001 |
| Headache | 0.62(0.57–0.69) | <0.001 | 0.74(0.67–0.832)* | <0.001 | 0.59(0.56-0.62) | <0.001 | 0.75(0.71–0.78)* | <0.001 |
*adjusted for age, sex, ICU admitting, Ventilator aid
**adjusted for ICU admitting, Ventilator aid, comorbidities
Fig. 3.
Odds ratio of mortality in diabetic patients with positive COVID-19
During the first four months, nearly 34% of registered patients have used antibiotics and 24% used antiviral therapies. This was followed by 34% and 31% in PCR positive patients. The most prevalent prescribed antibiotic was from the cephalosporin drug category followed by macrolides and Broad-Spectrum hospital antibiotics like vancomycin. Remarkably, the Fluoroquinolones were used less than 4%, although they are the main drug for lung pneumonia infections. Kaletra followed by Oseltamivir are the two main antiviral prescribed drugs. The percent of prescribed Cefteriaxson, Ceftizoxime, Cefepime, Ceftazidime, Cefixime, Cefazolin, Cefotaxime, CoAmoxiclave, Ampicillin were17%,0.3%,0.3%,0.2%,0.2%,0.1%, 0.06%, 0.05%,0.01%, respectively from cepharosporin group in hospitalized patients with diabetes. This is 13.5% for Azithromycin and 0,08% for Clarithromycin/Erythromycin from macrolid group, 7.6% for Meropenem/Imipenem, 6.1% for Vancomycin, and 0.2% for TazocinPiperacillin/Tazobactam. The Recommended treatment for patients with pneumonia by NICE protocol includes Tavanex/Levofloxacin or TazocinPiperacillin/Tazobactam or Ceftazidime or Vancomycin or Amoxicillin/CoAmoxiclav or Clarithromycin/Erythromycin was prescribed for less than 10% of patients. More information could be noticed in Table 3. Antibiotic treatment has no association with mortality.
Table 3.
Antibiotic and anti-viral treatment in diabetic patients with SARS symptoms, hospitalized, COVID positive PCR during 4 months
| Diabetic Patients With SARS Symptoms | Hospitalized (N = 21,247) | COVID PCR Positive (N = 8235) |
|---|---|---|
| Antibiotic treatment | 7267(34.20) | 2845(34.55) |
| Anti-viral treatment | 5133 (24.16) | 2633(31.97) |
| Types of antibiotics | ||
| Cephalosporin | 3892(18.32) | 1467(17.81) |
| Macrolide | 2894(13.62) | 1276(15.49) |
| Broad-Spectrum Antibiotic | 2228(10.49) | 854(10.37) |
| Fluoroquinolone | 701(3.30) | 341 (3.97) |
| Clindamycin | 209(0.98) | 53 (0.64) |
| Metronidazole | 44(0.21) | 2(0.02) |
| Aminoglycoside | 27(0.13) | 6(0.07) |
| Other Drug | ||
| Hydroxychloroquine | 5916(27.84) | 2689(32.65) |
| Antiviral Treatment | ||
| Kaletra | 3312(15.59) | 1674(20.33) |
| Oseltamivir | 2593(12.20) | 1455(17.67) |
| Ribavirin | 235(1.11) | 117(1.42) |
| Atazanavir | 19(0.09) | 12(0.15) |
| Interferon | 5(0.02) | 4(0.05) |
| Recommended treatment for patients with pneumoniaa | 1762 (8.29) | 755(9.17) |
aTavanex/Levofloxacin or TazocinPiperacillin/Tazobactam or Ceftazidime or Vancomycin or Amoxicillin/ CoAmoxiclav or Clarithromycin/ Erythromycin
Discussion
In diabetic patients with COVID-19, patients aged more than 60, and under 20 had 3.4 and 1. 9 time’s higher odds of mortality compared to the 20 to 40 age group, which indicated the higher risk of mortality in elderly and young patients with diabetes. The odds of mortality in diabetic male patients was 34% more than female. COPD, kidney disease, liver disease, and CVD are associated comorbidities with higher mortality. The frequency of antiviral, and antibiotics were 34%, 31% respectively. Antibiotic treatment has no association with mortality in COVID patients.
Several studies pointed to age as a no modifiable risk factor for COVID-19 prognosis [5, 17]. The results of a retrospective study in Wuhan demonstrated that in diabetic people older than 70 years old, age is an independent risk factor for in-hospital death (HR 2.39, 95% CI 1.03–5.56) [3]. These data were consistent with similar studies showing that older age was associated with a higher mortality rate (Age ≥ 80 HR 4·79, 95% CI 3·62–6·32) of COVID-19 in diabetic patients [10]. Our study indicated that the diabetic in-patients with symptoms are more likely to be in the older age group parallel to similar other studies [2, 10]. Older age is likely to decline the immune responses leading to more susceptibility to complications of respiratory infection like acute respiratory distress syndrome and death [18]. Moreover, having diabetes for a longer period in older patients may lead to developing more complications of diabetes contributing to poor prognosis in COVID-19 [2].
The effect of being male on the mortality rate of COVID-19 is more significant in diabetic than non-diabetic patients [10, 19]. Data from NHS England introduced the male sex as a risk factor for the COVID-19 mortality rate (HR 2.39, 95% CI 1.53–1.65) [20]. These findings are parallel with our study results (OR = 1.34(1.26–1.41)). Lifestyle, type of occupation, habitual history (such as smoking and alcohol use), underlying disorders, and medications probably cause these remarkable differences between males and females. Thus, more special attention should be paid to this group to tackle this gender inequity in COVID-19 health care.
COPD with increased mucous production and obstacle of airways can lead to the development of hypoxemia. The inflammatory response, weak immunity, constant mucus production, use of respiratory corticosteroids, and structural damages of air paths are observed in COPD patients [21] which make the outcome of COVID worse when they are associated with diabetes. A study in China reported 50–52.3% of the total ICU admitted COVID-19 cases had COPD [21, 22]. The results of this study show a positive association between COPD and mortality in diabetic patients with COVID-19.
Individuals with cardiovascular comorbidities tend to develop a more severe form of COVID-19 resulting in a higher mortality rate [23]. Greater prevalence of cardiovascular diseases among diabetic patients leads to a higher rate of death associated with diabetes. In this study, 43% of diabetic patients suffer from CVD. This comorbidity is significantly higher than the second common comorbidity, kidney disease, with 7.5% of diabetic patients. Underlying comorbidities contribute to worsening the outcome of diabetic patients infected by novel coronavirus COVID- 19 and increasing the death rate. The role of some comorbidities like cardiovascular disease, hypertension, and chronic pulmonary disease is so important that the higher mortality rate among diabetic patients is attributed to these factors independently [3]. However, a study reported that the rate of death among diabetic patients remained higher than non-diabetic people after adjustment for cardiovascular comorbidities [8]. Patients with diabetic kidney diseases are more prone to infection complications due to chronic inflammation and subsequent immunosuppression. Also, renal impairment plays a role in developing cardiovascular diseases [24]. Moreover, the novel coronavirus COVID- 19 can directly affect the kidneys by cellular injury or sepsis. This may lead to a cytokine storm, as well [25]. The prevalence of these comorbidities among diabetic patients is higher than the general population probably due to the nature of diabetes and consequent complications [19]. Thus, according to our results, more attention must be focused on diabetic patients with kidney disease.
A systematic review and meta-analysis on clinical symptoms of COVID19 reveal fever (88.7%, 95%CI 84.5–92.9%), cough (57.6%, 95%CI 40.8–74.4%), and dyspnea (45.6%, 95%CI 10.9–80.4%) were the most prevalent symptoms [26]. It seems dyspnea symptoms are more prevalent in diabetic in-patients in this study (45.6% vs 58.25) but this difference is not significant. Because it is located in a confidence interval of 95% of the prevalence of meta-analysis. However, other symptoms are less than recent reported studies (fever: 24.6% vs 88.7%, cough: 44.58% vs 57.6%). Only fever is significantly lower in diabetic patients of this study comparing to the result of the meta-analysis which could be a result of the immune response.
Of note, amid the COVID pandemic, health care utilization has been reduced especially for patients with chronic medical conditions leading to more morbidity and mortality [27] which is parallel with the mortality rate in this study (22%). Diabetes is a complex disease that should be managed continually by health care providers and patients closely [28] which needs health care infrastructures to facilitate patients management during COVID pandemics. According to our results, the high mortality rate of diabetic patients with COVID-19 might raise concerns for policymakers in Iran to improve health care utilization for these patients.
During the COVID-19 pandemic, many efforts have been made to find appropriate and cost-effective available treatments [29, 30]. Despite the higher mortality risk in patients with specific comorbidities, there is still a paucity of information on the potential effects of recommended treatments on the prognosis of COVID-19 in diabetic patients [29, 31]. Due to the anti-inflammatory, anti-thrombotic, and anti-viral effects of chloroquine or hydroxychloroquine especially on SARS-CoV-2 in vitro, these drugs have been initially approved by the United States food and drug administration (FDA) in treating COVID-19 hospitalized patients [32]. However, subsequent analysis revealed that hydroxychloroquine has no beneficial effects on post-exposure prophylaxis and treatment in COVID-19 [33]. Moreover, several side effects have been reported for hydroxychloroquine e.g. cardiac toxicity including cardiomyopathy, heart failure, and rhythm disturbance, retinopathy, kidney injury, and liver problems [34]. Hydroxychloroquine may also cause hypoglycemia, particularly in patients on insulin or sulfonylureas [2]. Thus, this drug should be used with caution especially in diabetic patients. Despite the several side effects of this drug in diabetic patients as well as the lack of effectiveness in COVID-19 treatment, hydroxychloroquine is widely used in Iran. Our results show that about 30% of COVID-19 positive diabetic patients have been received hydroxychloroquine during their hospitalization. It seems that national COVID-19 treatment guidelines in Iran need to be frequently updated. Moreover, the results of this study reveal that antibiotics have no association with mortality in COVID patients. Also, we have a high-frequency use of high-cost antibiotics like imipenem and vancomycin. Studies reveal a wild range of antibiotic use in COVID patients worldwide [15, 35, 36]. Stewardship based on international guidelines and antibiogram performance [37] is needed to reduce antibiotic resistance in the age of COVID. Furthermore, this will support shrinking the economic burden of COVID-19 on individuals and governments. Although this is a cross-sectional registry and the absolute effect of any drug needs setting up clinical trials, but this result could help to develop a hypothesis on antibiotic resistance neglected issues during the COVID pandemic.
Of the strengths of this study is the large sample size of diabetic in-patient from all provinces of Iran which is the most attacked country by COVID-19. The data include most epidemiologic and clinical features with the utmost important outcomes which enable us to report a noble demonstration of the condition. Also, diabetes is a concern among non-communicable diseases in many countries. These make the result of this study valuable for future research and policymaking on these issues. Another advantage of this study is the duration of gathering data which covers a long period of the COVID epidemic in Iran. The main limitation of our study is that our registry data is limited to hospital cases and may not identify the underlying cause of death, particularly in the absence of extensive COVID-19 testing. In addition, the registry data included comorbidities such as diabetes, based on self-report as a binary variable. Although, self-report is acceptable in the field of research, but clinically, it may be responsible for some sort of residual bias which could not be avoided. Moreover, lab data on glycemic control of diabetic patients were not provided in the registry. Future population-based longitudinal studies are needed for resolving these issue. Also, the data on diabetes drugs were not available in the registry and the data on antibiotic and antiviral treatment were in Persian text and only available during a subset of early 4 month period of pandemic, although Python text mining enables us to report these data.
Given the nature of diabetes which is multifactorial and syndemic, the inter-relationship between diabetes and COVID-19 should trigger more research to understand specific mechanisms of the virus that might contribute to worsening symptoms and outcomes in diabetic patients. The demographic and social factors may affect both diabetes and COVID, simultaneously. There is a huge chasm between developing and developed countries in this regard. So, increasing the capability of controlling this visues cycle is needed to be focused in low and middle-income countries which are more prone to inequity in the quality of care and health utilization.
Regarding the antibiotic resistance issue and the diversity of antibiotic and antiviral treatment in the COVID pandemic worldwide, and the noticeable use of antibiotics in diabetic patients, it is recommended to prioritize an antibiotic guideline prescription in COVID-19 patients for better stewardship by countries. This is true in the case of antiviral therapy.
Acknowledgments
The authors would like to thank the National Institute for Medical Research Development (NIMAD) for their support of this study (grant number: 995531). We also like to thank the Non-Communicable Diseases Research Center’s staff of the Endocrinology and Metabolism Population Sciences Institute of Tehran University of Medical Sciences, Barakat pharmaceutical group and Iran Ministry of Health and Medical Education for their wholehearted cooperation. The authors declare that this article has not been submitted for publication elsewhere and the content of this manuscript has not been presented as an abstract previously.
Funding
This work was supported by National Institute for Medical Research Development (NIMAD).
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Ethics approval and consent to participate
The study was ethically approved by the Ethical Committee of National Institute for Medical Research Development, Tehran, Iran (ID: IR.NIMAD.REC.1399.185).
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Farshad Farzadfar, Email: farzadfar3@yahoo.com, Email: f-farzadfar@tums.ac.ir.
Bagher Larijani, Email: Emrc@tums.ac.ir.
References
- 1.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. N Engl J Med. 1999;341:1906–1912. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 2.Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. The Lancet Diabetes & Endocrinology. 2020;8:782–792. doi: 10.1016/s2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi Q, Zhang X, Jiang F, Zhang X, Hu N, Bimu C, et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center. Retrospective Study Diabetes Care. 2020;43:1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 4.Huang I, Lim MA, Pranata R. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19 . The COVID-19 resource centre is hosted on Elsevier Connect , the company ' s public news and information 2020.
- 5.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. OpenSAFELY: factors associated with COVID-19 death in 17 million patients. Nature. 2020;584 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed]
- 6.Chen Y, Yang D, Cheng B. al. e. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43:1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 7.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72 314 Cases From the Chinese Center for Disease Control and Prevention. JAMA. 2020 Apr 7;323(13):1239-1242. 10.1001/jama.2020.2648. PMID: 32091533. [DOI] [PubMed]
- 8.Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020; 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed]
- 9.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 10.Holman N, Knighton P, Kar P, O'Keefe J, Curley M, Weaver A, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020; 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed]
- 11.Bode B, Garrett V, Messler J, McFarland R, Crowe J, Booth R, et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14:813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? The Lancet Respiratory Medicine. Lancet Publishing Group; 2020. p. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korytkowski M, Antinori-Lent K, Drincic A, Hirsch IB, McDonnell ME, Rushakoff R, Muniyappa R. A Pragmatic Approach to Inpatient Diabetes Management during the COVID-19 Pandemic. J Clin Endocrinol Metab. 2020 Sep 1;105(9):dgaa342. 10.1210/clinem/dgaa342. PMID: 32498085; PMCID: PMC7313952. [DOI] [PMC free article] [PubMed]
- 14.Infante M, Ricordi C, Fabbri A. Antihyperglycemic properties of hydroxychloroquine in patients with diabetes: risks and benefits at the time of COVID-19 pandemic. J Diabetes. 2020;12(9):659–667. doi: 10.1111/1753-0407.13053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antimicrobial resistance in the age of COVID-19. Nature Microbiology. 2020;5(6):779-. 10.1038/s41564-020-0739-4. [DOI] [PubMed]
- 16.[NG173] Ng: COVID-19 rapid guideline: antibiotics for pneumonia in adults in hospital available on https://www.nice.org.uk/guidance/ng173. Accessed date 16 Sept 2020. 2020. [PubMed]
- 17.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu C, Chen X, Cai Y, Ja X, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020 Sep;8(9):782-792. doi: 10.1016/S2213-8587(20)30238-2. Epub 2020 Jul 17. Erratum in: Lancet Diabetes Endocrinol. 2020 Oct;8(10):e5. Erratum in: Lancet Diabetes Endocrinol. 2020 Nov;8(11):e6. PMID: 32687793; PMCID: PMC7367664. [DOI] [PMC free article] [PubMed]
- 20.Bhopal SS, Bhopal R. Sex differential in COVID-19 mortality varies markedly by age. Lancet. 2020;396(10250):532–533. doi: 10.1016/S0140-6736(20)31748-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu H, Tong Z, Ma P, Hu M, Peng Z, Wu W, et al. Intensive care during the coronavirus epidemic. Belin: Springer; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu W, Tao Z-W, Wang L, Yuan M-L, Liu K, Zhou L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020; [DOI] [PMC free article] [PubMed]
- 23.Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17(9):543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.D'Marco L, Puchades MJ, Romero-Parra M, Gorriz JL. Diabetic Kidney Disease and COVID-19: The Crash of Two Pandemics. Frontiers in Medicine. 2020;7(199). 10.3389/fmed.2020.00199. [DOI] [PMC free article] [PubMed]
- 25.Sun J, Zhu A, Li H, Zheng K, Zhuang Z, Chen Z, et al. Isolation of infectious SARS-CoV-2 from urine of a COVID-19 patient. Emerging microbes & infections. 2020;9(1):991–993. doi: 10.1080/22221751.2020.1760144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Morales AJ, Cardona-Ospina JA, Gutiérrez-Ocampo E, Villamizar-Peña R, Holguin-Rivera Y, Escalera-Antezana JP, et al. Clinical, laboratory and imaging features of COVID-19: a systematic review and meta-analysis. Travel Med Infect Dis. 2020;34:101623. doi: 10.1016/j.tmaid.2020.101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Malhotra C, Chaudhry I, Ozdemir S, Finkelstein EA. Reduced health-care utilization among people with chronic medical conditions during coronavirus disease 2019. Proceedings of Singapore Healthcare. 2020:2010105820964533. 10.1177/2010105820964533.
- 28.Standards of Medical Care for Patients With Diabetes Mellitus. Diabetes Care. 2003;26(suppl 1):s33. 10.2337/diacare.26.2007.S33. [DOI] [PubMed]
- 29.Ali MJ, Hanif M, Haider MA, Ahmed MU, Sundas F, Hirani A, et al. Treatment options for COVID-19: a review. Frontiers in Medicine. 2020;7(480). 10.3389/fmed.2020.00480. [DOI] [PMC free article] [PubMed]
- 30.Oldenburg CE, Doan T. Azithromycin for severe COVID-19. Lancet. 10.1016/S0140-6736(20)31863-8. [DOI] [PMC free article] [PubMed]
- 31.Poston JT, Patel BK, Davis AM. Management of critically ill adults with COVID-19. Jama. 2020;323(18):1839–1841. doi: 10.1001/jama.2020.4914. [DOI] [PubMed] [Google Scholar]
- 32.Li X, Wang Y, Agostinis P, Rabson A, Melino G, Carafoli E, et al. Is hydroxychloroquine beneficial for COVID-19 patients? Cell Death Dis. 2020;11(7):512. doi: 10.1038/s41419-020-2721-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jorge A. Hydroxychloroquine in the prevention of COVID-19 mortality. Lancet Rheumatol. 2021;3(1):e2–e3. doi: 10.1016/S2665-9913(20)30390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.La A, Alzoughool F, Atoum M. Risk of using hydroxychloroquine as a treatment of COVID-19. Int J Risk Safety Med. 2020;31(3):111–116. doi: 10.3233/JRS-200024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020 Mar 28;395(10229):1054-1062. doi: 10.1016/S0140-6736(20)30566-3. Epub 2020 Mar 11. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. Erratum in: Lancet. 2020 Mar 28;395(10229):1038. PMID: 32171076; PMCID: PMC7270627. [DOI] [PMC free article] [PubMed]
- 36.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical characteristics of Covid-19 in New York City. N Engl J Med. 2020;382(24):2372–2374. doi: 10.1056/NEJMc2010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verroken A, Scohy A, Gérard L, Wittebole X, Collienne C, Laterre P-F. Co-infections in COVID-19 critically ill and antibiotic management: a prospective cohort analysis. Crit Care. 2020;24(1):1–3. doi: 10.1186/s13054-020-03135-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.