Abstract
Objectives:
This study aimed to elucidate the actual state of anal incontinence (AI), fecal incontinence (FI), and the associated factors in Japanese medical personnel.
Methods:
A questionnaire was completed by Japanese medical personnel after listening to lectures on AI. AI was defined as involuntary loss of feces or flatus.
Results:
A total of 463 persons (mean age, 35.6 years; range, 20-91; male/female/no answer, 132/324/7) participated in the questionnaire. AI occurred in 34.4% of 450 participants (flatus/liquid stool/solid stool: 30.4%/3.6%/0.4%). AI was significantly more prevalent in females (male/female: 15.5%/42.7%, p < 0.001). AI and FI occurred significantly more prevalent in participants aged ≧40 years (p < 0.024). AI was significantly associated with childbirth, frequency of childbirth (more than three times), vaginal delivery, urinary incontinence, the style of urination/defecation, and a history of gynecologic surgery and systemic diseases (p < 0.05). Female gender and age as well as urinary incontinence and inability to defecate separately in female and previous colorectal disease and/or surgery in male were risk factors of AI by multivariate analysis (p < 0.05). FI was correlated with urinary incontinence.
Conclusions:
AI and FI occurred in 34.4% and 4.0% of Japanese medical personnel, respectively. Gas incontinence was common in every age group. AI was associated with female gender, higher age group, urinary incontinence, the style of urination and defecation in female, and previous colorectal disease and/or surgery in male. FI was associated with urinary incontinence.
Keywords: anal incontinence, fecal incontinence, Japanese population, child birth, urinary incontinence
Introduction
Fecal incontinence (FI) is defined as involuntary or uncontrollable loss of feces and gas incontinence as voluntary or uncontrollable loss of flatus[1]. Anal incontinence (AI) is defined as involuntary loss of feces or flatus[1]. The prevalence of FI is reported to range widely (2.2%-25%)[1]. Most of the reports so far have investigated AI in older age groups[2-5], and the prevalence of AI in younger age groups has rarely been documented[6-9]. Furthermore, the prevalence of AI and FI in the Japanese population has not been highly documented[5,6].
It has been reported that risk considerations for FI include physiological factors, such as age and gender; comorbidities, such as diabetes and irritable bowel syndrome; and obstetric factors, such as multiple deliveries, home delivery, first vaginal delivery, and forceps delivery[1]. However, most of these reports are based on data from the USA and Europe[2-4,7,9,10-18], and risk factors of AI have rarely been documented. There is also limited documentation concerning risk factors of AI and FI for the Japanese population[5], especially in the younger population.
Therefore, we administered a questionnaire to a Japanese sample to study bowel habit, AI, and the associated factors. Bowel habits and the associated factors have already been surveyed and analyzed[19]. In this study, we investigated AI and FI, as well as the associated factors, to elucidate the aforementioned conditions in Japan, especially in the younger population and in females.
Methods
A questionnaire was completed by Japanese medical personnel after they had listened to lectures on bowel disorders between 2007 and 2019. The medical personnel were selected to complete the questionnaire as they had a good understanding of bowel disorders and the significance of the current study. The lectures included topics on anatomy, physiology concerning defecation, definitions, epidemiology, pathophysiology, and diagnosis and treatment for bowel disorders and incontinence. The lectures were held 12 times in total to 502 participants by KM. Of these 12 lectures, 8 were held for 12 to 35 participants and 4 for 70 to 100 participants. The questionnaire, which is presented in Table 1, was distributed by a clerk to all 502 participants prior to the lecture. FI was defined as involuntary or uncontrollable loss of feces and gas incontinence as voluntary or uncontrollable loss of flatus[1]. AI was defined as involuntary loss of feces or flatus[1] in the lecture. Only those who provided approval for the use of de-identified data were asked to participate in the questionnaire. The questionnaires were collected immediately after the lecture by a clerk. All the collected data was summarized and analyzed with the approval of the Institutional Review Board of Fujita Health University (HM20-197), and the study was conducted in accordance with the 1964 World Medical Association's Declaration of Helsinki and its later amendments.
Table 1.
Questionnaire on Anal Incontinence.
| 1, Age 2, Gender 3, Occupation | |
| 4, Did you experience anal incontinence recently? | Never/gas/liquid/solid stool |
| 5, What is the frequency of the anal incontinence? | Daily/weekly/monthly/yearly |
| 6, Did you experience urinary incontinence recently? | Yes/No |
| 7, Have you given birth? | Yes/No |
| 8. How many times have you given birth? | 1/2/3~ |
| 9, Vaginal delivery/Cesarean section/both? | |
| 10, Defecation and urination (must be done at the same time/can be done separately). | |
| 11, Have you ever had gynecologic surgery (only applicable to the removal of ovary and/or uterus)? | |
| Yes/ No | |
| 12, Have you ever had a colorectal disease and/or surgery? | Yes/No |
| 13, The colorectal diseases/surgery was ( ). | |
| 14, Have you ever had an anal disease? | Yes/No |
| 15, Have you previously had anal surgery? | Yes/No |
| 16, The anal surgery was ( ). | |
| 17, Do you undergo any treatment for systemic disease? | Yes/No |
| 18, The treatment is for ( ). | |
The Wilcoxon rank-sum test was used for age difference analysis and Fisher's exact test or the chi-squared test for the other statistical analysis. Logistic regression analysis was employed for multivariate analysis. The differences were considered significant at p < 0.05.
Results
A total of 463 of 502 participants responded to the questionnaire (response rate, 92.2%), and 12 participants did not provide their age. The mean age of the remaining 451 participants was 35.6 years (range, 20-91). The mean age of females (39 years; range, 20-91) was significantly higher than that of males (28 years; range, 20-70) (p < 0.0001). The characteristics of the participants are presented in Table 2.
Table 2.
Characteristics of the Participants (n = 463).
| Age (n = 451) | 35.6 (20–91) | |
| M/F (n = 440) | 132/324 | |
| Occupation (n = 440) | Medical students 224/Nurses 120/Others 96 | |
| Anal incontinence (n = 450) | 155 (34.4%) | |
| Fecal incontinence (n = 450) | 18 (4.0%) | |
| Incontinence to gas only: 137 (30.4%) | ||
| Incontinence to liquid stool: 16 (3.6%) | ||
| Incontinence to solid stool: 2 (0.4%) | ||
| Frequency of anal incontinence | Daily: 26 (22.2%) | |
| (n = 117) | Weekly: 32 (27.4%) | |
| Monthly: 25 (21.4%) | ||
| Yearly: 34 (29.1%) | ||
| Obstetric history (n = 300) | Nulliparous: 151 | |
| Parous: 149 | ||
| Mode of delivery (n = 136) | Vaginal delivery only: 121 | |
| Cesarean section only: 9 | ||
| Both: 6 | ||
| Number of birth (n = 141) | 1X: 17 | |
| 2X: 71 | ||
| 3X~: 53 | ||
| Urinary incontinence (n = 456) | 101 (22.1%) | |
| Treatment for concomitant diseases | 42 (9.4%) | |
| (n = 448) | Hypertension: 13 (2.9%) | |
| Hyperlipidemia: 5 (1.1%) | ||
| Allergic dermatitis: 4 (0.9%) | ||
| Diabetes mellitus: 3 (0.7%) | ||
| Medical history | Gynecologic surgery (n = 310) | 20 (6.5%) |
| Colorectal disease/surgery (n = 452) | 13 (2.9%) | |
| Anal diseases (n = 458) | 30 (7.0%) | |
| Anal surgery (n = 449) | 6 (1.3%) | |
(n = number of participants who answered the question)
Prevalence of AI and FI
AI was confirmed in 155 of 450 participants (34.4%) who responded to this question; gas incontinence in 137 participants (30.4%), incontinence to liquid stool in 16 participants (3.6%), and incontinence to solid stool in 2 participants (0.4%) (Table 2).
The prevalence of AI was reported daily by 26 (22.2%) of 117 participants who responded to this question, weekly by 32 participants (27.4%), monthly by 25 participants (21.4%), and yearly by 34 participants (29.1%) (Table 2).
Prevalence of AI and FI according to gender
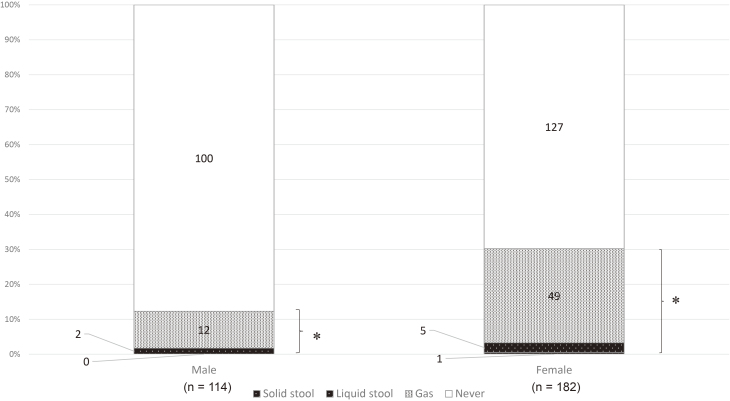
Gas incontinence was reported in 13 of 126 males (10.3%) and in 118 of 309 females (38.2%), incontinence to liquid stool in 3 males (2.4%) and in 12 females (3.1%), and incontinence to solid stool in 0 males (0%) and 2 females (0.6%). AI and FI were reported in 16 (15.5%) and 3 (2.9%) of 126 males and 132 (42.7%) and 14 (4.5%) of 309 females, respectively. AI was significantly more prevalent in females than in males (p < 0.001) (Table 3). However, no significant difference was observed in the occurrence of FI between males and females (p = 0.294) (Table 3). When analyzed for the participants of 20 to 39 years of age (Figure 1), AI was also significantly more prevalent in females than in males (55/182 females (30.2%), 14/114 males (12.3.%), p < 0.001), but no significant difference was observed in the occurrence of FI between males and females (2/114 males (1.8%), 6/182 females (3.3%), p = 0.426).
Table 3.
Prevalence of Anal and Fecal Incontinence for Various Parameters.
| Parameter | Prevalence of AI | p value | Prevalence of FI | p value |
|---|---|---|---|---|
| Gender | ||||
| Male | 15.5% (16/126) | p < 0.001 | 2.9% (3/126) | p = 0.294 |
| Female | 42.7% (132/309) | 4.5% (14/309) | ||
| Age (years) | ||||
| <40 | 20.3% (69/296) | p < 0.001 | 2.7% (8/296) | p = 0.024 |
| ≧40 | 56.8% (79/139) | 6.5% (9/139) | ||
| Childbirth | ||||
| None | 31.3% (47/150) | p < 0.001 | 4.7% (7/150) | p = 0.509 |
| Yes | 61.7% (87/141) | 5.7% (8/141) | ||
| Number of birth | ||||
| 1X | 46.7% (7/15) | p < 0.05 | 0% (0/15) | p = 0.113 |
| 2X | 54.4% (37/68) | 10.3% (7/68) | ||
| ≧3X | 74.0% (37/50) | 2.0% (1/50) | ||
| Mode of delivery | ||||
| Vaginal | 64.7% (75/116) | p < 0.03 | 6.0% (7/116) | p = 0.419 |
| Cesarean | 22.2% (2/9) | 0% (0/9) | ||
| Both | 66.7% (4/6) | 16.7% (1/6) | ||
| Urinary incontinence | ||||
| None | 26.0% (91/350) | p = 0.001 | 2.9% (10/350) | p = −0.008 |
| Yes | 65.6% (63/96) | 8.3% (8/96) | ||
| Ability to separate defecation | ||||
| Yes | 30.2% (109/361) | p = 0.001 | 4.4% (16/361) | p = 0.474 |
| None | 50.0% (40/80) | 2.5% (2/80) | ||
| Previous gynecologic surgery | ||||
| None | 40.3% (117/290) | p = 0.006 | 5.2% (15/290) | p = 0.315 |
| Yes | 70.0% (14/20) | 0% (0/20) | ||
| Previous colorectal disease and/or surgery | ||||
| None | 33.7% (148/439) | p = 0.751 | 4.1% (18/439) | p = 0.455 |
| Yes | 38.5% (5/13) | 0% (0/13) | ||
| Previous anal disease | ||||
| None | 33.2% (142/428) | p = 0.242 | 3.5% (15/428) | p = 0.083 |
| Yes | 40.0% (12/30) | 10.0% (3/30) | ||
| History of anal surgery | ||||
| None | 33.6% (149/443) | p = 1.000 | 3.8% (17/443) | p = 0.220 |
| Yes | 33.3% (2/6) | 16.7% (1/6) | ||
| History of systemic disease | ||||
| None | 29.8% (121/406) | p < 0.001 | 3.7% (15/406) | p = 0.242 |
| Yes | 66.7% (28/42) | 7.1% (3/42) |
(n = number of participants who answered the question)
Figure 1.
Anal incontinence in participants aged 20 to 39 years according to gender (n = 296).
(* p < 0.001, chi-squared test)
Prevalence of AI and FI according to age group
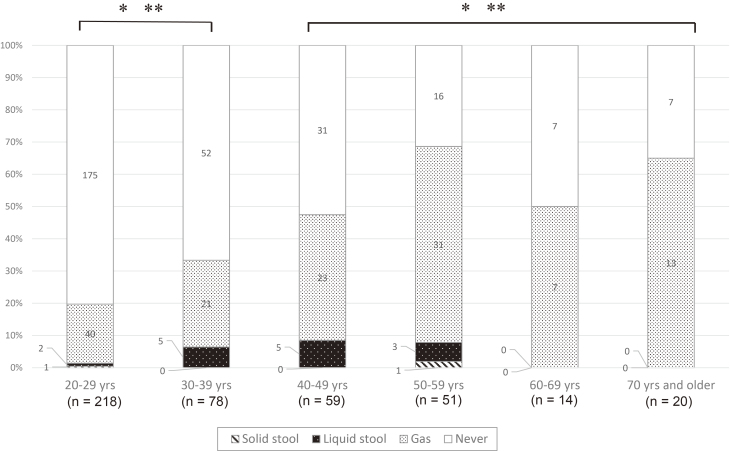
AI and FI according to age group is presented in Figure 2. AI and FI were significantly more prevalent in participants in their 40s or older than in participants in their 30s or younger (AI, p < 0.001; FI, p = 0.024) (Table 3). When analyzing AI and FI of females according to age group, AI was significantly more prevalent in participants in their 40s or older than in participants in their 30s or younger (p < 0.001). However, the prevalence of FI was not significantly different between participants in their 40 or older and participants in their 30s or younger (p = 0.274).
Figure 2.
Anal incontinence by age group (n = 451).
(AI: * p < 0.001, FI: * p = 0.024, chi-squared test)
AI and FI and childbirth
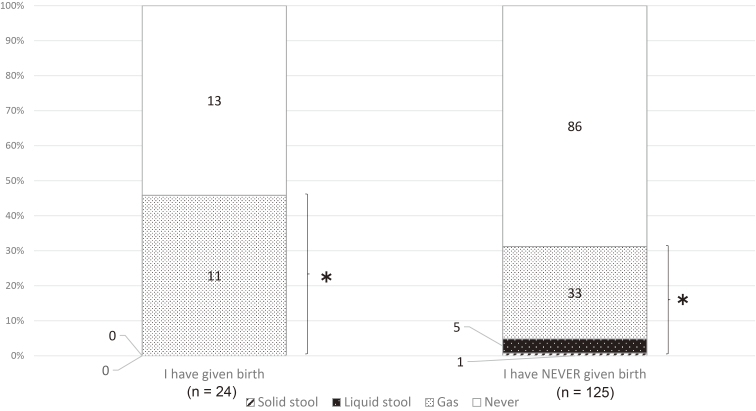
AI and FI occurred in 87 (61.7%) and 8 (5.7%) of 141 females who had given birth and in 47 (31.3%) and 7 (4.7%) of 150 females who had not given birth, respectively (Table 3). AI was significantly more prevalent in females who had given birth than in those who had not (p < 0.001); however, the prevalence of FI was not significantly different between females with and without a childbirth history (p = 0.509). An analysis of the data of the participants aged 20 to 39 years revealed that AI and FI occurred in 11 (45.8%) and 0 of 24 female participants who had given birth and in 39 (31.2%) and 6 (4.8%) of 125 female participants who had not given birth, respectively (Figure 3). The prevalence of AI and FI was not significantly different in female participants aged 20 to 39 years with or without a history of childbirth (AI, p = 0.164; FI, P = 0.590).
Figure 3.
Childbirth and anal incontinence in females aged 20 to 39 years (n = 150).
(* p < 0.164, chi-squared test)
AI and FI according to number of birth are presented in Table 3. The prevalence of AI was significantly associated with the frequency of childbirth (once versus three times and more, p = 0.047; twice versus three times and more, p = 0.037). FI was not associated with an occurrence of childbirth.
AI and FI according to mode of delivery are presented in Table 3. AI was significantly associated with vaginal delivery (vaginal versus Cesarean, p = 0.011, vaginal + both versus Cesarean, p = 0.026), but vaginal delivery was not associated with FI.
Urinary incontinence and AI and FI
Urinary incontinence was reported in 101 of 456 participants (22.1%) (Table 2). AI and FI were reported in 63 (65.6%) and 8 (8.3%) of 96 participants with urinary incontinence and 91 (26.0%) and 10 (2.9%) of 350 participants without urinary incontinence, respectively (Table 3). AI and FI were significantly more prevalent in participants with urinary incontinence than in those without urinary incontinence (p < 0.001 and p = 0.008).
Style of defecation and urination and AI and FI
It was reported that defecation and urination had to be done at the same time in 81 of 452 participants (17.9%) and could be done separately in 371 participants (82.0%)[19]. AI and FI according to the ability to defecate separately are presented in Table 3. AI was significantly more prevalent in participants who had to defecate and urinate at the same time than in participants who could defecate and urinate separately (p = 0.001). However, the prevalence of FI was not affected by the style of defecation and urination (p = 0.474). When analyzing AI and FI of each gender according to the style of defecation and urination, AI was significantly prevalent only in female participants (p = 0.009).
Medical history and AI and FI
Of 310 females, 20 (6.5%) previously underwent gynecologic surgery (Table 2). AI was significantly more prevalent in participants who had gynecologic surgery than in participants who had not (p = 0.006). However, the occurrence of FI was not influenced by a history of gynecologic surgery (p = 0.315) (Table 3).
Of 452 participants, 13 (2.9%) experienced colorectal disease and/or previously underwent surgery (Table 2). The most frequent colorectal diseases and/or surgery included colorectal polyp (n = 4), followed by intussusception (n = 2) and then irritable bowel syndrome (n = 2). Previous colorectal disease and/or surgery did not affect the occurrence of AI (p = 0.751) or FI (p = 0.455) (Table 3).
Of 458 participants, 30 (7.0%) previously had an anal disease (Table 2). The previous anal disease did not significantly affect the occurrence of AI (p = 0.242) or FI (p = 0.083) (Table 3).
Of 449 participants, 6 (1.3%) had anal surgery for hemorrhoids (Table 2). The previous history of anal surgery did not affect the occurrence of AI (p = 1.000) or FI (p = 0.22) (Table 3).
Of 448 participants, 42 (9.4%) underwent some treatment for systemic disease (Table 2). The most frequent diseases are presented in Table 2. AI was significantly more prevalent in participants who underwent any treatment for systemic diseases than in participants who did not (p < 0.001). However, the occurrence of FI was not affected by a history of systemic disease (p = 0.242) (Table 3).
Multivariate analysis of risk factors of AI and FI
Multivariate analysis revealed that gender and age were significant factors of AI (p < 0.001) (Table 4). Therefore, further analysis was conducted according to gender. In female, urinary incontinence and inability to defecate separately were significant risk factors of AI (Table 4). Although the previous colorectal diseases and/or surgery was identified as a risk factor of AI in male, the 95% confidence interval was wide due to the small number of persons concerned. Urinary incontinence was a significant risk factor of FI in female (Table 5).
Table 4.
Multivariate Analysis of Risk Factors for Anal Incontinence.
| Participants | Factors | Odd ratio | 95% Confidence interval |
P-value | |
|---|---|---|---|---|---|
| All participants | |||||
| Gender | 3.50 | 1.93 | 6.34 | p < 0.001 | |
| Age | 1.04 | 1.03 | 1.06 | p < 0.001 | |
| Female | |||||
| Urinary incontinence | 2.07 | 1.04 | 4.11 | p = 0.039 | |
| Inability to separate defecation | 2.03 | 1.03 | 3.97 | p = 0.040 | |
| History of systemic disease | 2.72 | 1.00 | 7.39 | p = 0.050 | |
| Male | |||||
| Previous colorectal disease and/or surgery | 11.24 | 1.61 | 78.65 | p = 0.015 | |
Table 5.
Multivariate Analysis of Risk Factor for Fecal Incontinence.
| Participants | Factors | Odd ratio | 95% Confidence interval |
P-value | |
|---|---|---|---|---|---|
| All participants | |||||
| Gender | 1.64 | 0.44 | 6.13 | p = 0.462 | |
| Age | 1.02 | 0.99 | 1.04 | p = 0.307 | |
| Female | |||||
| Urinary incontinence | 4.63 | 1.11 | 19.39 | p = 0.036 | |
Discussion
The prevalence of AI and FI widely differs according to the definition and the way of research; relevant groups also need to be classified[1]. The prevalence of AI and FI is documented to be 2% to 24% and 0.4% to 18%, respectively, in community-dwelling adults[20], and the prevalence of FI increases up to more than 50% in nursing home residents[21]. Most studies on the prevalence of FI and AI have been performed for predominantly investigating the aged population[2-5,21]. Whitehead et al.[8] conducted a questionnaire survey through telephone interview in the USA and reported that the prevalence of FI in males and females aged 29 years or more was 7.7% (6.0%-9.4%) and 8.9% (7.2%-10.5%), respectively. In Taiwan, the report of at-home interviews[9] revealed the prevalence of FI and gas incontinence to be 2.8% and 8.6% of 1,253 females, respectively. The current study found that the prevalence of FI and AI in participants who were 20 years or older was 4.0% and 34.4%, respectively, and the prevalence of FI of males and females was 2.9% and 4.5%, respectively. Mimura et al.[6] reported that the prevalence of FI occurring more than once per month in a healthy Japanese working population of 20 to 65 years of age was 4.0%. The prevalence of FI was equivalent to the report from Taiwan; however, gas incontinence was more common in every age group (18%-65%), especially in females (24%-68%) in the current study.
Epidemiological studies including adults of all ages demonstrated that there was a concrete relationship between age and FI[1-3,5,7,10,11]. In the current study, AI and FI increased with age group, and AI and FI were significantly more prevalent in participants in their 40s or older than in participants in their 30s or younger (p < 0.001 and p = 0.024). However, multivariate analysis revealed that age was a risk factor of AI but not of FI. Gender is considered to be a relatively weak risk factor of FI[1], with several studies having reported that FI is more prevalent in women than in men[2,3,7,11,12] and with other studies having shown no significant difference between genders[3,5,13,14]. In the current study, AI was significantly more prevalent in females than in males, even in the 20-39 age group. However, no significant difference was observed in FI between females and males.
Certain obstetric conditions, such as multiple child deliveries[8,10], home child delivery[22], first vaginal child delivery[17,18], and forceps child delivery[23], are reported to be risk factors of FI. AI was significantly more prevalent in females who had given birth than in females without a history of childbirth, whereas a significant difference was not observed in FI. Conversely, the prevalence of AI and FI was not significantly different between the presence and absence of childbirth in females aged 20 to 39 years. This might mean that the effect of pudendal neuropathy induced by pressure and traction during the delivery, causing FI[24], appears at a later age in people with a history of childbirth. This might be one of the reasons that the prevalence of AI and FI increases with age in females. The frequency of childbirth and vaginal delivery influenced the occurrence of AI in the current study, whereas these factors did not affect FI. Furthermore, childbirth was not a risk factor of AI by multivariate analysis. These results might be due to the fact that the current study only included younger females.
Roberts et al.[2] and Fornell et al.[14] described the association between urinary incontinence and FI. Suzuki et al. studied 2,517 Japanese residents aged ≧65 years living in long-term care facilities and concluded that 61% of residents with urinary incontinence had FI[25]. In the current study, 65.6% and 8.3% of participants with urinary incontinence had AI and FI, respectively, and AI and FI were significantly more prevalent in participants with urinary incontinence than in those without. Furthermore, urinary incontinence was a risk factor of AI and FI in female by multivariate analysis. Interestingly, AI was significantly more prevalent in participants who had to defecate and urinate at the same time than in participants who could defecate and urinate separately (p = 0.001, chi-squared test). Furthermore, the inability to defecate separately was a risk factor of AI in female by multivariate analysis. The relationship between urinary incontinence and AI and the style of defecation and urination might be associated with the fact that the neural control of urination and defecation is managed by the same sacral nerves (S2-4).
Comorbidities, such as diabetes[5,16], irritable bowel syndrome[26], and inflammatory bowel diseases[15], are reported to be risk factors of FI, and anal surgery can be a cause of FI[27]. In the current study, AI was significantly more prevalent in participants who had a history of gynecologic surgery (p = 0.006) and systemic diseases (p < 0.006). Removal of ovary and/or uterus might affect the functioning of the pelvic floor. In the current study, the number of systemic diseases, such as diabetes that is reported to affect FI, was small. However, the presence of any systemic diseases was considered to affect the occurrence of AI, especially in female (Table 4). Although the presence or absence of previous colorectal disease and/or surgery did not show a significant difference in AI and FI by chi-squared test, the previous colorectal disease was a significant risk factor of AI in male by multivariate analysis. No clear correlations with AI and other comorbidities were observed in the current study. This might be because the number of participants who had both these diseases and surgery was low due to the younger age of patients.
The limitation of this study is the small sample size and the fact that the questionnaire was undertaken over a long period of time. The prevalence of AI and FI reported in this study was based on the recent memory of the participants and not on some bowel diary. The survey was mainly completed by people of relatively younger age, and the number of males was limited. Majority of the participants were medical students and nurses; other professionals were not included. Furthermore, the grade of incontinence was not evaluated using symptom scores, such as the Cleveland Clinic Florida Fecal Incontinence score[28] and St Mark's score[29]. While more participants were intended to be invited to complete the questionnaire, the chance of conducting lectures to the target cohort was limited. However, the analysis of each survey questions was not impacted by different sample sizes (100 cases and 300 cases). Therefore, it is believed that this report shows the actual state of AI, especially in Japanese younger females.
In conclusion, AI and FI occurred in 34.4% and 4.0% of Japanese medical personnel, respectively. AI was significantly more prevalent in females, and the prevalence of AI and FI increased with age, especially from the age of 40 and older. AI, especially incontinence to flatus, was common in every age group (18%-65%), especially in females (24%-68%). AI was associated with female gender, higher age, urinary incontinence, style of urination and defecation in female, and previous colorectal disease and/or surgery in male. FI was associated with urinary incontinence.
Conflicts of Interest
There are no conflicts of interest.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Kotaro Maeda. The first draft of the manuscript was written by Kotaro Maeda, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Approval by Institutional Review Board (IRB)
Fujita Health University HM20-197
Disclaimer
Hidetoshi Katsuno is one of the Associate Editors of Journal of the Anus, Rectum and Colon and on the journal's Editorial Board. He was not involved in the editorial evaluation or decision to accept this article for publication at all.
Acknowledgements
We would like to thank Ms. Fusae Nojima for her secretarial work and English language editing.
References
- 1.Maeda K, Yamana T, Takao Y, et al. Japanese practice guidelines for fecal incontinence Part 1- Definition, epidemiology, pathophysiology and causes, risk factors, clinical evaluation, and symptomatic scores and Qol, questionnaire for clinical evaluations- English version. J Anus Rectum Colon. 2021 Jan; 5(1): 52-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roberts RO, Jacobsen SJ, Reilly WT, et al. Prevalence of combined fecal and urinary incontinence: a community-based study. J Am Geriatr Soc. 1999 Jul; 47(7): 837-41. [DOI] [PubMed] [Google Scholar]
- 3.Perry S, Shaw C, McGrother C, et al. Prevalence of faecal incontinence in adults aged 40 years or more living in the community. Gut. 2002 Apr; 50(4): 480-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teunissen TA, van den Bosch WJ, van den Hoogen HJ, et al. Prevalence of urinary, fecal and double incontinence in the elderly living at home. Int Urogynecol J Pelvic Floor Dysfunct. 2004 Jan-Feb; 15(1): 10-3. [DOI] [PubMed] [Google Scholar]
- 5.Nakanishi N, Tatara K, Naramura H, et al. Urinary and fecal incontinence in a community-residing older population in Japan. J Am Geriatr Soc. 1997 Feb; 45(2): 215-9. [DOI] [PubMed] [Google Scholar]
- 6.Mimura T, Ohmi T, Yago J, et al. Study of fecal incontinence in Japanese working population. J Japan Surg Soc. 2003 Nov; 104: 536 (Abstract, in Japanese). [Google Scholar]
- 7.Nelson R, Norton N, Cautley E, et al. Community-based prevalence of anal incontinence. JAMA. 1995 Aug; 274(7): 559-61. [PubMed] [Google Scholar]
- 8.Whitehead WE, Borrud L, Goode PS, et al. Fecal incontinence in US adult: epidemiology and risk factors. Gastroenterol. 2009 Aug; 137(2): 512-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen GD, Hu SW, Chen YC, et al. Prevalence and correlations of anal incontinence and constipation in Taiwanese women. Neurourol Urodyn. 2003 Oct; 22(7): 664-9. [DOI] [PubMed] [Google Scholar]
- 10.MacLennan AH, Taylor AW, Wilson DH, et al. The prevalence of pelvic floor disorders and their relationship to gender, age, parity and mode of delivery. BJOG. 2000 Aug; 107(12): 1460-70. [DOI] [PubMed] [Google Scholar]
- 11.Walter S, Hallbook O, Gotthard R, et al. A population-based study on bowel habits in a Swedish community: prevalence of faecal incontinence and constipation. Scand J Gastroenterol. 2002 Aug; 37(8): 911-6. [DOI] [PubMed] [Google Scholar]
- 12.Damon H, Guye O, Seigneurin A, et al. Prevalence of anal incontinence in adults and impact on quality-of-life. Gastroenterol Clin Biol. 2006 Jan; 30(1): 37-43. [DOI] [PubMed] [Google Scholar]
- 13.Goode PS, Burgio KL, Halli AD, et al. Prevalence and correlates of fecal incontinence in community-dwelling older adults. J Am Geriatr Soc. 2005 Apr; 53(4): 629-35. [DOI] [PubMed] [Google Scholar]
- 14.Fornell EU, Wingren G, Kjolhede P. Factors associated with pelvic floor dysfunction with emphasis on urinary and fecal incontinence and genital prolapse: an epidemiological study. Acta Obstet Gynecol Scand. 2004 Apr; 83(4): 383-9. [DOI] [PubMed] [Google Scholar]
- 15.Varma MG, Brown JS, Creasman JM, et al. Fecal incontinence in females older than aged 40 years: who is at risk? Dis Colon Rectum. 2006 Jun; 49(6): 841-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bytzer P, Talley NJ, Leemon M, et al. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a populationbased survey of 15,000 adults. Arch Intern Med. 2001 Sep; 161(16): 1989-96. [DOI] [PubMed] [Google Scholar]
- 17.Zetterstrom J, Lopez A, Anzen B, et al. Anal sphincter tears at vaginal delivery: risk factors and clinical outcome of primary repair. Obstet Gynecol. 1999 Jul; 94(1): 21-8. [PubMed] [Google Scholar]
- 18.Borello-France D, Burgio KL, Richter HE, et al. Fecal and urinary incontinence in primiparous women. Obstet Gynecol. 2006 Oct; 108(4): 863-72. [DOI] [PubMed] [Google Scholar]
- 19.Maeda K, Koide Y, Katsuno H, et al. Questionnaire survey of bowel habit in Japanese medical personnel. J Anus Rectum Colon. 2021 Jul; 5(3): 297-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macmillan AK, Merrie AEH, Marshall RJ, et al. The prevalence of fecal incontinence in community-dwelling adults: systemic review of the literature. Dis Colon Rectum. 2004 Sep; 47(8): 1341-9. [DOI] [PubMed] [Google Scholar]
- 21.Nelson RC. Epidemiology of fecal incontinence. Gastroenterology. 2004 Jan; 126(1): S3-7. [DOI] [PubMed] [Google Scholar]
- 22.Roman H, Robillard PY, Payet E, et al. Factors associated with fecal incontinence after childbirth. Prospective study in 525 women. J Gynecol Obstet Biol Reprod (Paris). 2004 Oct; 33(6): 497-505. [DOI] [PubMed] [Google Scholar]
- 23.Fenner DE, Genberg B, Brahma, et al. Fecal and urinary incontinence after vaginal delivery with anal sphincter disruption in an obstetrics unit in the United States. Am J Obstet Gynecol. 2003 Dec; 189(6): 1543-9. [DOI] [PubMed] [Google Scholar]
- 24.Kamm MA. Faecal incontinence. BMJ. 1998 Feb; 316(7130): 528-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Suzuki M, Okochi J, Murata K, et al. Nationwide survey of continence status among older adult residents living in long-term care facilities in Japan: The prevalence and associated risk factors of incontinence and effect of comprehensive care on continence status. Geriatr Gerontol Int. 2020 Apr; 20(4): 285-90. [DOI] [PubMed] [Google Scholar]
- 26.Drossman DA, Sander RS, Broom CM, et al. Urgency and fecal soiling in people with bowel dysfunction. Dig Dis Sci. 1986 Nov; 31(11): 1221-5. [DOI] [PubMed] [Google Scholar]
- 27.Walker WA, Rothenberger DA, Goldberg SM. Morbidity of internal sphincterotomy for anal fissure and stenosis. Dis Colon Rectum. 1985 Nov; 28(11): 832-5. [DOI] [PubMed] [Google Scholar]
- 28.Jorge JM, Wexner SD. Rtiology and management of fecal incontinence. Dis Colon Rectum. 1993 Jan; 36(1): 77-97. [DOI] [PubMed] [Google Scholar]
- 29.Vaizey CJ, Carapeti E, Cahill JA, et al. Prospective comparison of faecal incontinence grading system. Gut. 1999 Jan; 44(1): 77-80. [DOI] [PMC free article] [PubMed] [Google Scholar]