Abstract
Background
Studies evaluating neutralizing antibody (NAb) after BNT162b2 vaccine are scarce. We therefore compared NAb using the plaque reduction neutralization test (PRNT) in vaccinated subjects, with those from five chemiluminescent (CLIA) assays, two targeting ACE and S-RBD interaction.
Methods
Sera from 174 completely Comirnaty/BNT162b2 vaccinated healthcare workers (HCW) were evaluated at t12 and t28. NAb titers at low (PRNT50) or high (PRNT90) stringency were compared with: Liaison SARS-CoV-2 Trimeric-S IgG, Elecsys S-RBD Ab, Maglumi SARS-CoV-2 S-RBD IgG and SARS-CoV-2 Nab; iFlash 2019-nCoV NAb.
Results
Neither PRNT50 nor PRNT90 correlated with age (range, 24–65 years); no significant differences were found for gender. PRNT50 and PRNT90 seropositive titers (≥1:20) were 43 (24.7%) and 15 (8.6%) at t12 and 167 (95.9%) and 149 (85.6%) at t28. CLIA results at t28 were uncorrelated with age, apart from Elecsys S-RBD Ab (r = -0.164, p = 0.046). Gender differences were found for Maglumi SARS-CoV-2 S-RBD IgG (p = 0.037) and Maglumi NAb (p = 0.046). Considering PRNT50 at thresholds of 1:20 (or 1:40) and 1:160 (or 1:320), corresponding to different immune protective levels, CLIA cut-offs have been identified.
Conclusions
Comirnaty/BNT162b2 elicits strong NAb production, especially 28 days after first inoculum. Differences in correlation between Nab titers and circulating antibodies measured by 5 immunoassays have been found, being stronger the correlation for Maglumi Nab.
Keywords: Antibody, BNT162b2, Comirnaty, COVID-19, Immunoassays, Immunological response, SARS-CoV-2 vaccine, Serology
1. Introduction
Comirnaty (BNT162b2 mRNA, BioNTech-Pfizer, Mainz, Germany/New York, United States (US)) vaccine received emergency use authorization (EUA) by the Food and Drug Administration in December 2020, and full, final approval on August 2021 (https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine). Results from clinical trials demonstrate its efficacy in preventing symptomatic coronavirus disease 2019 (COVID-19) [1] as well as the increase achieved in detectable anti-SARS-CoV-2 antibodies in the serum of vaccinated individuals [2].
Scientific knowledge of the immunological parameters required for protecting subjects from SARS-CoV-2 is incomplete, albeit rapidly evolving, and antibody-mediated viral neutralization is still considered the gold standard in determining immune protection against COVID-19 [3]. Until now, the humoral response elicited by this vaccine has mainly been demonstrated after the second dose, by measuring binding antibody (bAb) titers with commercially available assays, often based on chemiluminescent technology targeting different forms of Spike proteins or its RBD portions [4]. Few studies have focused on the neutralization abilities of antibodies (Ab) developed after the first and second dose of vaccine [5], [6], [7]. Both time points appear of particular interest, since it has been shown that BNT162b2 has an efficacy of around 93% as from 14 days after dose 1 to before dose 2, against confirmed COVID-19 [8], despite a weak neutralization activity being found following a single dose [9]. A further issue hindering consensus over the strength and the timing of anti-SARS-CoV-2 bAb determination regards agreement among commercial assays, which is somewhat low [10]. Indeed, the bAb values obtained from different test systems are not completely interchangeable, even when converted to binding antigen units (BAU) per milliliter using the WHO international standard for SARS-CoV-2 immunoglobulin [11]. Furthermore, differences between assay results have been found in convalescent individuals and naïve subjects with vaccine-induced Ab against SARS-CoV-2 [9], [12].
In this study, we describe the neutralizing response of sera from healthcare workers without and with prior SARS-CoV-2 infection following a first and a second vaccine dose of Comirnaty/BNT162b2, measured with the plaque reduction neutralization test (PRNT), which is considered the gold standard method for determining anti-SARS-CoV-2 NAb [13]. Measuring NAb titers is of utmost importance, especially for evaluating the humoral response to vaccine. Indeed, despite bAb determinations could be useful for sero-surveillance surveys and for diagnosing a previous COVID-19 infection (late diagnosis), their levels do not indicate whether an individual is immune to SARS-CoV-2 infection [13]. Further, PRNT determination is a labour-intensive technique, has an elevated turnaround time and requires a bio-safety level 3 (BSL-3) containment, which is not available in many laboratories. Hence, it could be interesting to explore whether a correlation from bAb and NAb exists, not only to improve assays development and for a rationale adoption in clinical practice, but also to eventually identify PRNT-derived assay-specific protective levels. To achieve this goal, PRNT results were compared with two commercially-available chemiluminescent (CLIA) assays measuring specific interactions between SARS-CoV-2 and host cells with high affinity to Ab neutralization activity of Ab, also defined as surrogate viral neutralization tests (sVNT) [14], and with three CLIA assays measuring anti-SARS-CoV-2 bAb, having as targets either the RBD portions or the trimeric form of the viral Spike Protein.
2. Materials and methods
A cohort of 174 healthcare workers (HCW) of the Padua University Hospitals, who underwent complete vaccination (first dose followed by a second after 21 days) between December 26th 2020 and March 10th 2021, were included in the study. They were consecutively enrolled from the Emergency Department, the Infectious Disease and the Laboratory Medicine wards of University-Hospital of Padova. All subjects underwent periodical nasopharyngeal swab testing (every 1 week) from March 2020 to March 2021, while their immunological status for SARS-CoV-2 was determined weekly between April 8th and May 29th, 2020, as described elsewhere [15]. A total of 38 post-graduate medical trainee participants were included later in the cohort. Ten HCW have been previously diagnosed to be affected by COVID-19 natural infection on the basis of at least one positive nasopharyngeal swab test and clinical confrmation; the time elapsed after infection was ranging from 3 to 9 months. Overall, the percentages of subjects within the following age classes < 30 yrs, 30/40 yrs, 40/50 yrs, 50/60 yrs and > 60 yrs were: 43 (24.7%), 39 (22.4%), 37 (21.3%), 51 (29.3%) and 4 (2.2%), respectively.
All HCW were asked to collect two serum samples for determining Ab 12 (t12) and 28 (t28) days after the first Comirnaty/BNT162b2 inoculum; all subjects underwent a second vaccine administration after 21 days from the first dose, with the exception of 10, who had a single dose, being non-naïve to SARS-CoV-2 infection. A pre-vaccination sample (t0) was collected 24 h to 0 h only from the 38 residents before vaccination. PRNT assays were performed with Vero E6 cells, in 96-wells plates as described elsewhere [16], using hCoV-19/Italy/PD_20VIR1935i-P4-L/2020 virus. The serum neutralization titer was defined as the reciprocal of the highest dilution resulting in a reduction of the control plaque count > 50% (PRNT50) or > 90% (PRNT90). A titer of ≥ 1:20 was considered the seropositive threshold. An evaluation was also made of the levels of antibodies against different chemiluminescent assays (CLIA) (Table 1 ). SARS-CoV-2 Trimeric-S IgG was determined using Liaison XL (Diasorin, Sallugia, VC, Italy), S-RBD Ab were measured by Cobas C-8000 (Roche, Basel, Switzerland), SARS-CoV-2 S-RBD IgG by Maglumi 2000 plus (Snibe Diagnostics, Shenzhen, China), sVNT NAb by iFlash 1800 (Shenzhen Yhlo biotech Co, China) and Maglumi 2000 plus (Snibe Diagnostics, Shenzhen, China). Due to limitations in the availability of reagents, different numbers of samples were evaluated for each CLIA assay (Table 1). The sensitivity and specificity of the assays, as declared by manufacturers, ranged from 91.7% to 100% and from 99.5% to 100%, respectively. The GraphPad Prism version 9.1 for Windows (GraphPad Software, LLC) was employed, using non-parametric tests (Kruskall-Wallis test and Spearman’s correlation). Stata 16.1 (Statacorp, Lakeway Drive, TX, USA) was employed for multivariate analyses, performed using log transformed PRNT titers, and Ab values and for non-parametric ROC analyses, the Youden index being used for identifying the best cut-off. All subjects gave their fully informed written consent to participate in the study, which was conducted in accordance with the Declaration of Helsinki, and the Institutional Review Board of the University of Padua (protocol nr 7862).
Table 1.
Characteristics of the chemiluminescent SARS-CoV-2 Antibodies assays investigated in this study, given by the manufacturers.
| Manufacturer | Roche Diagnostics | Diasorin Inc | Snibe diagnostics | Yhlo | |
|---|---|---|---|---|---|
| Commercial Name | Anti-SARS-CoV-2 S | Liaison SARS-CoV-2 Trimeric-S IgG | SARS-CoV-2 S-RBD IgG | SARS-CoV-2 Neutralizing Antibody | iFlash-2019-nCov Neutralizing Antibody |
| Platform | Cobas e analysers | LIAISON XL Analyzer | Maglumi series | Maglumi series | iFlash analysers |
| Method | Elettro-ChemiLuminescent (ECLIA) | Chemiluminescent immunoassay (CLIA) | Chemiluminescent immunoassay (CLIA) | Chemiluminescent immunoassay (CLIA) | Chemiluminescent immunoassay (CLIA) |
| Detection | Total Antibodies (included IgG) | IgG antibodies | IgG antibodies | Neutralizing antibodies | Neutralizing antibodies |
| Antigen Target | Spike RBD portion | Trimeric Spike protein | Spike RBD portion | Spike RBD-ACE2 protein interaction | Spike RBD-ACE2 protein interaction |
| Results | kBAU/L | kBAU/L | kBAU/L | mg/L | kAU/L |
| Interpretation | < 0.8 Negative ≥ 0.8 Positive |
< 33.8 Negative ≥ 33.8 Positive |
< 4.33 Negative ≥ 4.33 Positive |
< 0.3 Negative ≥ 0.3 Positive |
< 10 Negative ≥ 10 Positive |
| Patients with evaluation both at t12 and t28 | 169 | 148 | 171 | 150 | 35 |
| Samples evaluated (n)* | 376 | 331 | 386 | 343 | 104 |
Calculated including samples at t0, t12 and t28.
3. Results
Among the 174 HCW included in the study, 120 (69.0%) were females and 54 males (31%), with a mean (±SD) age of 41.8 ± 11.54 (range 24–65) years. Age differed between genders (mean ± SD of 42.2 ± 11.6 yrs, range 24–65 for females, mean ± SD of 39.4 ± 11.9 yrs, range 25–61 for males). A total of 17 individuals (9.7%) presented one or more comorbidities [11 had cardiovascular diseases without or in association with diabetes (n = 1), respiratory diseases (n = 1) or severe obesity (n = 7); 3 had respiratory diseases; 1 had diabetes; 2 had past or present cancer]. Among the 10 individuals that were not naïve to SARS-CoV-2 infection 3 were males, 7 females. Two individuals with previous SARS-CoV-2 infection had samples at t0 (PRNT50 titers were 0 and 40, whilst PRNT90 titers were both < 20). In view of the limited number of individuals with previous SARS-CoV-2, their results were not assessed separately in the analyses.
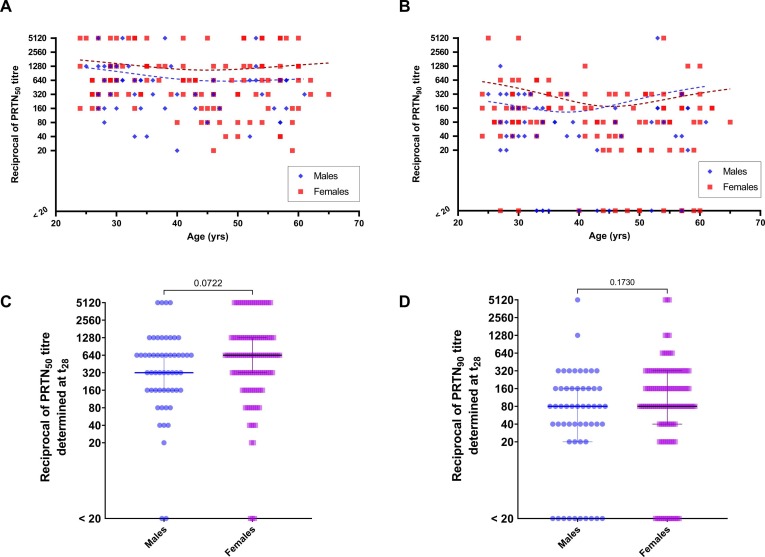
Considering only results at t28, age was significantly correlated neither with PRNT50 (Spearman’s rho = -0.135, p = 0.076) nor with PRNT90 (Spearman’s rho = -0.108, p = 0.157) (Fig. 1 , panels A and B), whereas at t12 age was significantly inversely correlated with PRNT50 (Spearman’s Rho = -0.386, p < 0.001), but not with PRNT90 (Spearman’s rho = -0.145, p = 0.006). No significant differences for PRNT50 and PRNT90 were found at t28 by gender (p = 0.072 and p = 0.173, respectively) (Fig. 1, panels C and D). At t12, PRNT50, but not PRNT90 (p = 0.641), differed slightly according to gender (p = 0.039).
Fig. 1.
Reciprocal of plaque reduction neutralization test results at low (PRNT50) or high (PRNT90) stringency thresholds and age (panels A and B) or subdivided by gender (C and D).
The PRNT50 and PRNT90 titers at t0, t12 and t28 are reported in Fig. 2 (panels A and B). PRNT50 seropositive titers (≥1:20) were 43 (24.7%) at t12 and 167 (95.9%) at t28, while PRNT90 seropositive titers were 15 (8.6%) at t12 and 149 (85.6%) at t28. In subjects with previous SARS-CoV-2 infection, PRNT50 titers were positive at t12 in 9/10 (8/10 had titers of > 1:1280), while PRNT50 titers at t28 were above 1:1280 in 10/10; PRNT90 titers at t12 were positive in 8/10 (6/10 had titers of > 1:1280), while PRNT90 titers at t28 were positive in 10/10 (7/10 had titers of > 1:1280).
Fig. 2.
Dot-plots of PRNT50 and PRNT90 results (panels A and B) and Liaison SARS-CoV Trimeric-S IgG (panel C), Elecsys SARS-CoV-2 S-RBD Ab (panel D), Maglumi SARS-CoV-2 S-RBD IgG (E), Maglumi SARS-CoV-2 Neutralizing Ab (NAb) (F) and iFlash nCoV-2019 NAb (G), at different times from the first Comirnaty/BNT162b2 inoculum.
The comparison between PRNT and CLIA Ab results is reported in Fig. 2, Fig. 3 . CLIA anti-SARS-CoV-2 Ab results at t28 did not significantly correlate with age, with the exception of Elecsys S-RBD Ab (Spearman’s r = -0.164, p = 0.046). Differences between CLIA anti-SARS-CoV-2 Ab results by gender were found only for Maglumi SARS-CoV-2 S-RBD [median levels (IQR) were 1553 (503.6–2876.8) kBAU/L and 2210.5 (802–4045.5) kBAU/L for males and females, respectively, p = 0.037] and Maglumi SARS-CoV-2 NAb [median levels (IQR) were 3.12 (1.19–5.95) mg/L and 4.45 (2.15–11.95) mg/L for males and females, respectively, p = 0.046].
Fig. 3.
Comparison of PRNT50 (panels A, B, C, D, E) and PRNT90 (panels F, G, H, I, L) results and immunoassay results. A) and F), Liaison SARS-CoV Trimeric-S IgG; B) and G) Elecsys SARS-CoV-2 S-RBD Ab; C) and H) Maglumi SARS-CoV-2 S-RBD IgG; D) and I) Maglumi SARS-CoV-2 Neutralizing Ab (NAb); E) and L) iFlash nCoV-2019 NAb.
Fig. 4 shows in detail how PRNT50 and PRNT90 titers were associated with CLIA Ab levels using t28 values, reporting the pairwise comparisons of different assays results for each HCW subject. Multiple linear regression analyses, including age and gender as covariates, were performed using t28 values to assess the correlation between the PRNT titers and CLIA results (Table 2 ).
Fig. 4.
Dot-plots and pairwise comparison of PRNT50 and PRNT90 results and immunoassay results.
Table 2.
Correlation coefficients from multivariate linear regression analyses, obtained using CLIA assays, PRNT50 or PRNT90 results, age and gender.
| CLIA assays | PRNT50 correlation coefficient from multivariate analysis ( | PRNT90 correlation coefficient from multivariate analysis ( |
|---|---|---|
| Trimeric-S IgG, Liaison | 0.836 | 0.799 |
| S-RBD IgG, Maglumi | 0.820 | 0.828 |
| S-RBD Ab, Elecsys | 0.833 | 0.850 |
| hACE-S-RBD NAb, iFlash | 0.818 | 0.851 |
| ACE-S-RBD NAb, Maglumi | 0.839 | 0.872 |
To investigate the relationship between CLIA SARS-CoV-2 Ab levels and vaccine-induced immunogenicity, two PRNT50 thresholds were chosen, the first established at 1:20 or 1:40 and the second at 1:160 or 1:320, corresponding to different immune protective levels and results previously obtained for PRNT50 in our previous study [14], [16]. Based on these PRNT50 thresholds, the corresponding CLIA SARS-CoV-2 protective Ab values were identified by ROC analyses (Table 3 ).
Table 3.
Optimal thresholds identified by Youden index for each CLIA assays with respect to different PRNT50 levels by non-parametric ROC analyses. The best combination of Sensitivity and Specificity found by Youden index is also reported for any PRNT50 levels and CLIA assay.
| PRNT50 levels | CLIA assays | Optimal Threshold | AUC | Sensitivity/Specificity (%) |
|---|---|---|---|---|
| 20 | Trimeric-S IgG, Liaison | 535 kBAU/L | 0.962 | 87.0/93.0 |
| 40 | 606 kBAU/L | 0.980 | 90.0/97.0 | |
| 160 | 860 kBAU/L | 0.987 | 95.2/97.6 | |
| 320 | 1335 kBAU/L | 0.984 | 92.0/96.0 | |
| 20 | S-RBD IgG, Maglumi | 90 kBAU/L | 0.984 | 92.0/95.0 |
| 40 | 94 kBAU/L | 0.989 | 97.0/95.0 | |
| 160 | 360 kBAU/L | 0.990 | 96.0/96.0 | |
| 320 | 585 kBAU/L | 0.986 | 97.0/94.0 | |
| 20 | S-RBD Ab, Elecsys | 23 kBAU/L | 0.973 | 0.91/0.94 |
| 40 | 33 kBAU/L | 0.984 | 0.94/0.94 | |
| 160 | 205 kBAU/L | 0.990 | 0.98/0.95 | |
| 320 | 435 kBAU/L | 0.984 | 0.93/0.93 | |
| 20 | ACE-S-RBD NAb, iFlash | 18 kAU/L | 0.958 | 0.98/0.96 |
| 40 | 32 kAU/L | 0.981 | 0.91/0.95 | |
| 160 | 96 kAU/L | 1.000 | 1.00/1.00 | |
| 320 | 138 kAU/L | 1.000 | 1.00/1.00 | |
| 20 | ACE-S-RBD NAb, Maglumi | 0.27 mg/L | 0.981 | 0.93/0.95 |
| 40 | 0.38 mg/L | 0.989 | 0.95/0.96 | |
| 160 | 0.96 mg/L | 0.995 | 0.99/0.95 | |
| 320 | 1.90 mg/L | 0.995 | 0.96/0.98 |
4. Discussion
The nature of the protective immune response to SARS-CoV-2 after vaccine, a widely debated issue, is thought to involve both cellular and humoral immunity [17]. In the present study we characterized the humoral response of antibodies against SARS-CoV-2, developed after the first and the second dose of Comirnaty/BNT162b2. Using vital virus, the NAb was measured by the gold standard, the plaque reduction neutralization test (PRNT), at low (PRNT50) or high (PRNT90) stringency thresholds, and the results compared with those from five different CLIA assays: three assays measured binding antibodies, and two sVNT that determined NAb (based on ACE2 and S-RBD interaction).
To obtain reliable insight on immunological status after SARS-CoV-2 vaccination, vaccine-induced NAb were tested in a cohort of HCW, who were followed up for SARS-CoV-2 infection every 1 week as from March 2020 until March 2021. Assessment of previous SARS-CoV-2 infection is of utmost importance in understanding the humoral response to vaccination. In this cohort, a total of 10/174 (5.7%) individuals had previous SARS-CoV-2 infection. All subjects were further interviewed to collect data on comorbidities, which affected only a limited number of individuals, the impact on SARS-CoV-2 Ab production being marginal.
PRNT results underlined that, in the age range studied (24–65 yrs), no significant differences were identified on considering age, although a slight decrease of the titer was visible, especially in the case of PRNT90 (Fig. 1). Likewise, no gender differences were identified after 28 days following the first inoculum, while PRNT50 titers marginally differed at t12, and positive titers (≥1:20) were 37/120 (30.1%) for females and 6/54 (11.1%) for males, although titers for both groups were mainly in the range 1:20 – 1:160. These results are in agreement with our previous findings on PRNT50 in SARS-CoV-2 convalescent individuals [16]. The effect of age and gender are still a controversial issue. Recently, Israel and colleagues reported that antibody titers decreased with older age, chronic renal disease, underweight and solid malignancy, while they are increased in females [18], but further studies should be performed to confirm these data.
SARS-CoV-2 immune protection related to antibody-mediated viral neutralization is considered of utmost importance, and an efficacy of up to 92% has been attributed to a single dose of Comirnaty/BNT162b2 and Moderna vaccine after 14 days from inoculum [8]. However, in the present study PRNT50 and PRNT90 results underline that 12 days after the first dose, NAb production is weak, although the response improves after second vaccination, both in terms of the total number of seropositive individuals and of NAb titers (Fig. 2). These results agree with findings from Trougakos et al. and Pratesi et al., obtained using a different analytical method, not measuring PRNT titers [9], [19]. Interestingly, since all CLIA assays were able to detect a significant increase in Ab titers after 12 days, the data reported support the hypothesis that early-produced humoral immune response is sustained by heterogeneous Ab types recognizing various RBD epitopes of the Spike protein, being only marginally efficient in neutralizing viral entry to the cells. Based on these data, it appears reasonable to suggest that these early produced heterogenous Ab, in addition to cell mediated immune response, might be able to induce the protection described in vaccine efficacy studies (by Fc-mediated effector functions including antibody-dependent phagocytosis, antibody-dependent cellular cytotoxicity and antibody- dependent natural killer cell activation) [8], [20].
A relevant issue compromising consensus over anti-SARS-CoV-2 Ab determination with CLIA commercial assays, is that results obtained using the different test systems are not completely interchangeable, even when converted to BAU per milliliter. In this study, rather than comparing CLIA methods, we evaluated five different commercially available assays with respect to PRNT50 and PRNT90 titers (Table 1). Liaison anti-SARS-CoV-2 Trimeric-S IgG, Maglumi anti-SARS-CoV-2 S-RBD IgG and Elecsys anti-SARS-CoV-2 S-RBD presented a similar pattern of results at t12 and t28 (Fig. 2). However, quite different scales of values are appreciable among assays, even if all methods reported results in kBAU/L. The same pattern among all the assays is indicated in Fig. 3, and Fig. 4, which reports the pairwise comparison between PRNT50 and PRNT90 titers. Fig. 3 and Table 2 also show that NAb Maglumi has a greater correlation with PRNT50 than other CLIA assays, also demonstrating a wide dynamic range of results. Differently, Fig. 4 shows that CLIA assays can produce discordant results, particularly when predicting negative PRNT50 results (PRNT50 < 20). This fact supports the hypothesis that, already at t28 and at least for some subjects, Comirnaty/BNT162b2 elicits discrete Ab production, while these immunoglobulins do not exert SARS-CoV-2 neutralization activity.
NAbs titers, a key factor in convalescent plasma therapy, are, above all, predictive of immune protection from symptomatic or asymptomatic SARS-CoV-2 infection [21]. To assess vaccination immunogenicity and protection, four different levels of PRNT50 titers were identified at low and high immunogenicity (1:20 and 1:40 or 1:160 and 1:320, respectively) and used to identify the best corresponding CLIA thresholds (Table 3). These PRNT levels are in agreement with that proposed, by mathematical extrapolation, as levels protective against severe infection (1:20 or 1:40) or protective against any infection (1:160 or 1:320), using estimated equations from Khoury et al. and PRNT50 data from our previous study [14], [16], [21]. Results underlined that with PRNT50 at 1:20 and at 1:40, CLIA assay thresholds were quite different, with the exception of the two assays measuring ACE-S-RBD interactions. However, overall, the CLIA assays derived thresholds at 1:20 and at 1:40 are quite different, and even if a threshold above 500 kBAU/L for Maglumi and Elecsys might be considered highly protective, this is not the case for Liaison, which requires values above 1300 kBAU/L. Overall, these results underline that the transition from manufacturers specific and arbitrary units against the WHO international standard for SARS-CoV-2 immunoglobulin is still incomplete, and more efforts are required in order to achieve harmonization of results.
The present study has some limitations. First, the number of HCW with a previous infection is limited, although our data are consistent, and confirm previously reported patterns [15]. In addition, we evaluated only an early antibody response after vaccination, while a long-term response study is still in progress. Second, the full spectrum of analytical performances of CLIA methods (except for Maglumi SARS-CoV-2 S-RBD IgG [22]) was not verified. Third, the study focused on circulating antibodies (particularly Nab), while other components of the adaptive immune responses were not investigated. Finally, data on the side effects of Comirnaty/BNT162b2 were not collected and assessed with respect to NAb. However, this study also presents some strengths. First, all HCW have been followed every week with molecular testing to eventually identify an early infection. Second, our data regarding NAb titers are developed using vital virus (PRNT), as this method is consensually accepted as a valuable tool for appropriately estimating the risk of re-infection and protection against SARS-CoV-2.
5. Conclusions
The results of the present study conducted on a cohort of HCW confirm that Comirnaty/BNT162b2 elicits a strong production of NAb, significantly quantifiable after 28 days from the first inoculum. In subjects aged 24 to 65 years, no significant differences between males and females were found at t28. Despite all CLIA assays enabling the identification of circulating Ab induced by Comirnaty/BNT162b2 after 12 days from first inoculum, commercially available assays targeting the interaction between ACE and S-RBD more accurately identify measurable NAb levels. However, further standardization efforts for SARS-CoV-2 Antibody assays are urgently needed, in order to improve upon both the comparability of data and our understanding as to which values should be considered predictive of immune protection.
Funding
This research received no external funding.
CRediT authorship contribution statement
Andrea Padoan: Conceptualization, Formal analysis, Writing – original draft. Chiara Cosma: Methodology. Francesco Bonfante: Methodology. Foscarina della Rocca: . Francesco Barbaro: . Claudia Santarossa: Methodology. Luigi Dall'Olmo: Methodology. Matteo Pagliari: . Alessio Bortolami: . Annamaria Cattelan: Visualization. Vito Cianci: Visualization. Daniela Basso: . Mario Plebani: Conceptualization, Writing – review & editing, Visualization, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The Authors thank Daniela Rinaldi (medical laboratory scientists) for their valuable technical support, and Diasorin Diagnostics, Roche Diagnostics, Snibe Diagnostics and Yhlo Diagnostics for kindly supplying reagents without in any way influencing the study design and data analysis.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson M., Stec M., Rewane A., Landay A., Cloherty G., Moy J. SARS-CoV-2 Antibody Responses in Infection-Naive or Previously Infected Individuals after 1 and 2 Doses of the BNT162b2 Vaccine. JAMA Netw. Open. 2021;4(8):e2119741. doi: 10.1001/jamanetworkopen.2021.19741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damani-Yokota P., Yeung S.T., Khanna K.M. Beyond neutralization for BNT162b2 mRNA vaccination. Cell Host Microbe. 2021;29(7):1033–1035. doi: 10.1016/j.chom.2021.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perkmann T., Perkmann-Nagele N., Koller T., Mucher P., Radakovics A., Marculescu R., Wolzt M., Wagner O.F., Binder C.J., Haslacher H., Powell E.A. Anti-Spike Protein Assays to Determine SARS-CoV-2 Antibody Levels: a Head-to-Head Comparison of Five Quantitative Assays. Microbiol Spectr. 2021;9(1) doi: 10.1128/Spectrum.00247-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Demonbreun A.R., Sancilio A., Velez M.P., Ryan D.T., Saber R., Vaught L.A., Reiser N.L., Hsieh R.R., Aquila R.T.D., Mustanski B., Mcnally E.M., Mcdade T.W. Comparison of IgG and neutralizing antibody responses after one or two doses of COVID-19 mRNA vaccine in previously infected and uninfected individuals. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J., Liu Y., Xia H., Zou J., Weaver S.C., Swanson K.A., Cai H., Cutler M., Cooper D., Muik A., Jansen K.U., Sahin U., Xie X., Dormitzer P.R., Shi P.-Y. BNT162b2-elicited neutralization of B.1.617 and other SARS-CoV-2 variants. Nature. 2021;596(7871):273–275. doi: 10.1038/s41586-021-03693-y. [DOI] [PubMed] [Google Scholar]
- 7.Edara V.-V., Pinsky B.A., Suthar M.S., Lai L., Davis-Gardner M.E., Floyd K., Flowers M.W., Wrammert J., Hussaini L., Ciric C.R., Bechnak S., Stephens K., Graham B.S., Bayat Mokhtari E., Mudvari P., Boritz E., Creanga A., Pegu A., Derrien-Colemyn A., Henry A.R., Gagne M., Douek D.C., Sahoo M.K., Sibai M., Solis D., Webby R.J., Jeevan T., Fabrizio T.P. Infection and Vaccine-Induced Neutralizing-Antibody Responses to the SARS-CoV-2 B.1.617 Variants. N. Engl. J. Med. 2021;385(7):664–666. doi: 10.1056/NEJMc2107799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skowronski D.M., De Serres G., Vergnes J.-N. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2021;384:1577. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- 9.Trougakos I.P., Terpos E., Zirou C., Sklirou A.D., Apostolakou F., Gumeni S., Charitaki I., Papanagnou E.-D., Bagratuni T., Liacos C.-I., Scorilas A., Korompoki E., Papassotiriou I., Kastritis E., Dimopoulos M.A. Comparative kinetics of SARS-CoV-2 anti-spike protein RBD IgGs and neutralizing antibodies in convalescent and naïve recipients of the BNT162b2 mRNA vaccine versus COVID-19 patients. BMC Med. 2021;19:208. doi: 10.1186/s12916-021-02090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jeong S., Lee N., Lee S.K., Cho E.-J., Hyun J., Park M.-J., Song W., Jung E.J., Woo H., Seo Y.B., Park J.J., Kim H.S., Tang Y.-W. Comparing Results of Five SARS-CoV-2 Antibody Assays Before and After the First Dose of ChAdOx1 nCoV-19 Vaccine among Health Care Workers. J. Clin. Microbiol. 2021;59(9) doi: 10.1128/JCM.01105-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kristiansen P.A., Page M., Bernasconi V., Mattiuzzo G., Dull P., Makar K., Plotkin S., Knezevic I. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet. 2021;397(10282):1347–1348. doi: 10.1016/S0140-6736(21)00527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plebani M., Padoan A., Negrini D., Carpinteri B., Sciacovelli L. Diagnostic performances and thresholds: the key to harmonization in serological SARS-CoV-2 assays? Clin. Chim. Acta. 2020;509:1–7. doi: 10.1016/j.cca.2020.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi A.C., Ren P. SARS-CoV-2 serology testing: Progress and challenges. J. Immunol. Methods. 2021;494:113060. doi: 10.1016/j.jim.2021.113060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y., Wang J., Li Q., Hu H., Lu J., Chen Z. Advances in Neutralization Assays for SARS-CoV-2. Scand. J. Immunol. 2021;94:1–15. [Google Scholar]
- 15.Padoan A., Dall'Olmo L., Rocca F.D., Barbaro F., Cosma C., Basso D., Cattelan A., Cianci V., Plebani M. Antibody response to first and second dose of BNT162b2 in a cohort of characterized healthcare workers. Clin. Chim. Acta. 2021;519:60–63. doi: 10.1016/j.cca.2021.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Padoan A., Bonfante F., Pagliari M., Bortolami A., Negrini D., Zuin S., Bozzato D., Cosma C., Sciacovelli L., Plebani M. Analytical and clinical performances of five immunoassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity. EBioMedicine. 2020;62 doi: 10.1016/j.ebiom.2020.103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A. Israel, Y. Shenhar, I. Green, E. Merzon, A. Golan-Cohen, A.A. Schäffer, E. Ruppin, S. Vinker, E. Magen, Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. medRxiv (Preprint). 2021. doi: 10.1101/2021.08.19.21262111. [DOI] [PMC free article] [PubMed]
- 19.Pratesi F., Caruso T., Testa D., Tarpanelli T., Gentili A., Gioè D., Migliorini P. Bnt162b2 mRNA sars-cov-2 vaccine elicits high avidity and neutralizing antibodies in healthcare workers. Vaccines. 2021;9:1–9. doi: 10.3390/vaccines9060672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sadarangani M., Marchant A., Kollmann T.R. Immunological mechanisms of vaccine-induced protection against COVID-19 in humans. Nat. Rev. Immunol. 2021;21(8):475–484. doi: 10.1038/s41577-021-00578-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., Subbarao K., Kent S.J., Triccas J.A., Davenport M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021;27(7):1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 22.Padoan A., Bonfante F., Cosma C., Di Chiara C., Sciacovelli L., Pagliari M., Bortolami A., Costenaro P., Musso G., Basso D., Giaquinto C., Plebani M. Analytical and clinical performances of a SARS-CoV-2 S-RBD IgG assay: comparison with neutralization titers. Clin. Chem. Lab. Med. 2021;59(8):1444–1452. doi: 10.1515/cclm-2021-0313. [DOI] [PubMed] [Google Scholar]