Abstract
The outbreak of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused the unprecedented COVID-19 pandemic, which has infected over 178 million people worldwide. Even with new vaccines, global herd immunity will not be reached soon. New cases and viral variants are being reported at an alarming rate. Effective antiviral treatment is urgently needed. Patients with severe COVID-19 suffer from life-threatening respiratory failure due to acute respiratory distress syndrome in their lungs, a leading cause of COVID-19 mortality. This lung hyper-inflammation is induced by virus-caused massive tissue damage that is associated with uncontrolled cytokine release, known as a cytokine storm, through JAK/STAT signaling pathways. Here, we review the FDA-approved JAK inhibitors that are being clinically evaluated and repurposed for the treatment of patients with severe COVID-19 by calming SARS-CoV-2 infection.
Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; CRS, cytokine release syndrome; JAK, Janus kinase; STAT3, Signal transducer and activator of transcription 3
Keywords: COVID-19, Drug discovery, SARS-CoV-2
Introduction
Coronavirus disease-19 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which has infected more than 178 million people and caused 3.9 million fatalities worldwide as of mid-June 2021.1 SARS-CoV-2 was first identified in the respiratory tract of patients with pneumonia in Wuhan, China in December 2019.2 The COVID-19 symptoms that typically appear 2–14 days after viral exposure include fever, cough, shortness of breath and pneumonia.3., 4., 5. COVID-19 can reportedly range from a mild to a severe condition, and the latter can lead to death.5 Age and health problems such as chronic pulmonary or cardiac disease are risk factors that determine the mortality rate.4., 5., 6.
Newly introduced vaccines against SARS-CoV-2, most notably those developed by Oxford-AstraZeneca, Pfizer-BioNTech, Sinopharm-Beijing, and Moderna, have been administrated under Emergency Use Authorization in several countries, and have been shown to be effective in preventing the rapid spread of viral infection.7., 8., 9. However, worldwide COVID-19 herd immunity will probably not be reached for several years because low-to-middle income countries, including many in Africa, have an extremely low vaccination rate (currently 0.3%) or are yet to begin mass vaccination campaigns.7 New cases and viral variants are still being reported at an alarming rate, no specific treatment for COVID-19 exists, and the available clinical options are limited. Therefore, the development of effective treatments is an urgent need, especially for the life-threatening severe cases.
SARS-CoV-2 is an enveloped virus that contains single-stranded positive-sense RNA (+ssRNA), with 5-cap structure and a 3-poly-A tail, and belongs to the Betacoronavirinae subfamily of the Coronaviridae family, which causes illness in birds, mammals and humans.8 The viral genome is 27–32 kb that encodes both structural and non-structural proteins.9 SARS-CoV-2 comprises four main structural proteins, the spike (S) glycoprotein, the small envelope (E) glycoprotein, the membrane (M) glycoprotein, and the nucleocapsid (N) protein, as well as several accessory proteins. The spike protein, which is key for viral entry and replication in infected host cells,8., 9. is composed of two functional subunits. The S1 subunit binds to host cell receptors, whereas the S2 subunit mediates the fusion of the viral and host cellular membranes. The distal part of the S1 subunit contains the receptor binding domain (RBD) that directly binds to the peptidase domain of angiotensin converting enzyme 2 (ACE2) of the host cell.10., 11.
Upon entry into a host cell, SARS-CoV-2 releases its genetic material into the cytoplasm. The viral RNA is translated into polyproteins PP1a and PP1ab, which are subsequently cleaved into functional proteins by viral proteases. Sub-genomic templates for mRNA synthesis and the translation of viral structural proteins are formed through discontinuous transcription. Viral genome replication is mediated by a complex consisting of an RNA-dependent RNA polymerase (RdRp), a helicase, exonuclease N, and other accessory proteins. The assembly of viral nucleocapsids from the packaged viral genome and viral structural proteins takes place at the endoplasmic reticulum – Golgi intermediate compartment. The infectious virions are released from the cell through exocytosis.12
Immune response and cytokine storm in COVID-19 patients
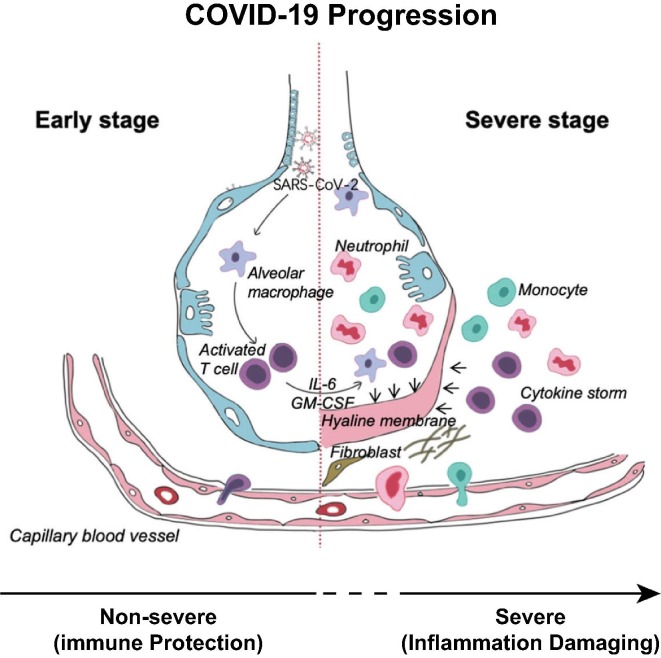
Clinically, host cells in the lungs elicit a two-phased response to SARS-CoV-2 infection (Fig. 1 ).13 In an early incubation and non-severe stage, alveolar macrophages detect the virus and produce cytokines (such as interferons) and chemokines that activate antiviral gene expression and recruit innate response cells (such as leukocytes, monocytes, natural killer (NK) cells, and dendritic cells) and adaptive immune cells to eliminate the virus and prevent disease progression.14., 15., 16. When this effort fails, or a protective immune response is impaired, the virus propagates. The disease transitions to a severe stage in which innate inflammation is induced by virus-caused massive tissue damage that is associated with uncontrolled cytokine release, known as a cytokine storm, from inflammatory macrophages and granulocytes. This results in acute respiratory distress syndrome (ARDS) in the lungs. COVID-19 patients in intensive care units (ICU) have been found to have higher concentrations of cytokines in their plasma than non-ICU patients, linking cytokines to disease severity.17., 18. These proinflammatory cytokines and chemokines include tumor necrosis factor α (TNF-α), interleukin 1β (IL-1β), IL-6, IL-10, IL-17, granulocyte/macrophage colony stimulating factor (GM-CSF), interferon γ (IFN-γ), monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein 1-α (MIP-1α).[19–22 They trigger immune cells to release a large number of free radicals that can cause pneumonia and ARDS, cumulating in systemic inflammation and ultimately multi-system organ failure.15., 23., 24. Thus, the cytokine storm plays a major role in the immunopathology of SARS-CoV-2 and is likely to be the main cause of the life-threatening respiratory disorders seen in patients with severe COVID-19.4., 16., 21., 22., 25.
Figure 1.
A hyperactive immune response, also known as cytokine storm, exemplified here in the alveoli of the lungs, has been recognized as the major cause of COVID-19 mortality. Adapted with modifications from Shi et al. 2020.13
Elevated IL-6 and GM-CSF levels in severe COVID-19 patients
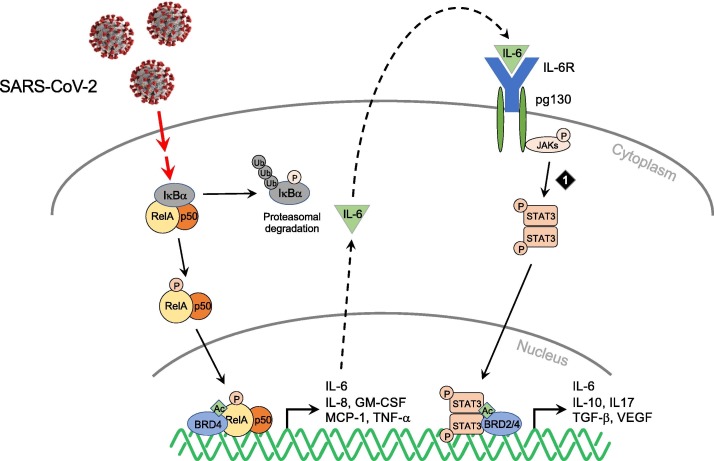
It has been suggested that SARS-CoV-2-induced hyperactivation of proinflammatory cytokines is achieved through the NF-κB and IL-6–JAK–STAT3 signaling pathways (Fig. 2 ).5 Studies of SARS and Middle East Respiratory Syndrome (MERS) coronaviruses that are closely related to SARS-CoV-2 have firmly established the role of the NF-κB pathway in human coronavirus infections.20., 26., 27., 28. Evidence that viral proteins can activate NF-κB came from studies showing that the envelop (E) protein of SARS-CoV acts as an ion channel in the virus,25., 26. and is critical to viral induction of increased NF-κB activity that leads to the overproduction of inflammatory cytokines in the infected host cells.20., 29., 30. A recently reported mapping of SARS-CoV-2 and host protein–protein interactions showed that the SARS-CoV-2 E protein, which shares 96.1% sequence identity with the SARS-CoV E protein, binds BET proteins BRD2/4 in human host cells.31 BRD2/4 are well known to facilitate NF-kB activity in the transcriptional activation of proinflammatory cytokines.32
Figure 2.
SARS-CoV-2 induction of NF-κB and IL-6–JAK–STAT3 signaling leading to over-production of pro-inflammatory cytokines in cytokine storm including IL-6 and GM-CSF. Targeted chemical intervention against JAKs is indicated by numbered diamond. Adapted with modifications from Lim et al.97 BRD2/4, Bromodomain-containing protein 2/4; GM-CSF, granulocyte/macrophage colony stimulating factor; IL6, Interleukin 6; JAK, Janus kinase; STAT3, Signal transducer and activator of transcription 3; TGF-β, Transforming growth factor β; VEGF, Vascular endothelial growth factor.
The JAK–STAT signaling pathway plays a major role in intracellular signaling induced by IFN in hematopoietic and immune cells. It transduces extracellular signals that are transmitted by a large number of cytokines, lymphokines and growth factors.33 IL-6 is one of major activators of JAK–STAT signaling and has been shown to be increased substantially in COVID-19 patients, strongly implying its involvement in acute inflammation and cytokine storm.34 The elevated levels of IL-6 stimulate various cell types that express the membrane-bound IL-6 receptor and the glycoprotein (gp130) receptor, as well as a soluble form of the IL-6 receptor that interacts with gp130, leading to constitutive activation of JAK–STAT signaling (Fig. 2).33., 35. Notably, the ability of STAT3 to promote IL6 gene expression results in an autocrine feed-forward loop, which amplifies cytokine expression.35 Although the mechanistic details await further study, these studies suggest that the activation of host NF-κB and IL-6–JAK–STAT signaling pathways by SARS-CoV-2 viral proteins is probably a crucial determinant of virulence, acting to promote the overexpression of proinflammatory cytokines, viral replication and pathogenicity.
IL-6 blockade has emerged as a potentially promising approach to control SARS-CoV-2-associated cytokine release syndrome (CRS) (i.e. cytokine storm). Tocilizumab is an FDA approved monoclonal antibody against IL-6 that is used for the treatment of rheumatoid arthritis (RA) and CRS accompanying CAR-T therapy for cancer, a syndrome akin to the hyperinflammatory phase of COVID-19.30., 36., 37. COVID-19 patients with severe and critical COVID-19 showed decreased counts of white blood cell and lymphocytes after receiving a 5-day treatment of tocilizumab.38., 39. Accordingly, tocilizumab was approved in China for patients affected by severe SARS-CoV-2 pulmonary complications.40 Sarilumab, another IL-6 receptor antagonist that has been approved for the treatment of RA in patients with COVID-19,41., 42. was shown to block IL-6 and to exert positive effects in COVID-19 patients with severe disease and high IL-6 levels. At present, the clinical trial involving Sarilumab for the treatment of severe COVID-19 is ongoing in the USA.43
GM-CSF is a cytokine that is critical for healthy pulmonary function and is necessary for the maturation and maintenance of alveolar macrophages. Higher levels of GM-CSF were observed in the early phase of COVID-19 (1–3 days), with a progressive decrease the late stage (day 14) of the disease. GM-CSF may contribute to ARDS indirectly by suppressing neutrophil apoptosis, as activated neutrophils can cause microvascular damage that results in lung injury.20 The inhibition of GM-CSF signaling may be beneficial in reducing hyperinflammation-related lung damage in the most severe cases of COVID-19.21 This blockade can be achieved by antagonizing the GM-CSF receptor or circulating GM-CSF. A recombinant human GM-CSF (Sargramostim) shows promising effects in improving oxygen levels in the blood of COVID patients. COVID-19 patients with hypoxic respiratory failure (saturation below 93%) are enrolled for Sargramostim treatment as a nebulized inhalation, which is administered alongside standard of care treatment. For patients with more severe COVID-19 who require mechanical ventilatory support, intravenous administration of Sargramostim will be used in the clinical study (NCT04326920).44 In addition, Mavrilimumab, a monoclonal antibody that binds GM-CSF receptor α, results in an improvement in oxygenation and shorter hospitalization.45 The anti-GM-CSF monoclonal antibodies TJ003234 and Gimsilumab will be tested in clinical trials in COVID-19 patients, whereas Lenzilumab has received FDA approval for compassionate use.20 Collectively, these studies support the notion that targeting IL-6 or GM-CSF and their receptors is an attractive strategy that warrants investigation as part of the current search for effective COVID-19 therapeutics.
JAK inhibitors against human diseases
In addition to antibodies against IL-6 and GM-CSF, FDA-approved small-molecule drugs that inhibit IL-6–JAK–STAT signaling (Fig. 3 ) represent a valuable clinical option for the treatment of COVID-19.44 JAK inhibition can affect both inflammation and cellular viral entry in COVID-19.45 JAK pathways are critically important for immune and hematopoietic cells, affecting processes including growth, survival, development and differentiation. Each of the JAK kinases has specificity for a different set of cytokine receptors, and thus is functionally linked to specific cytokines that bind these receptors.
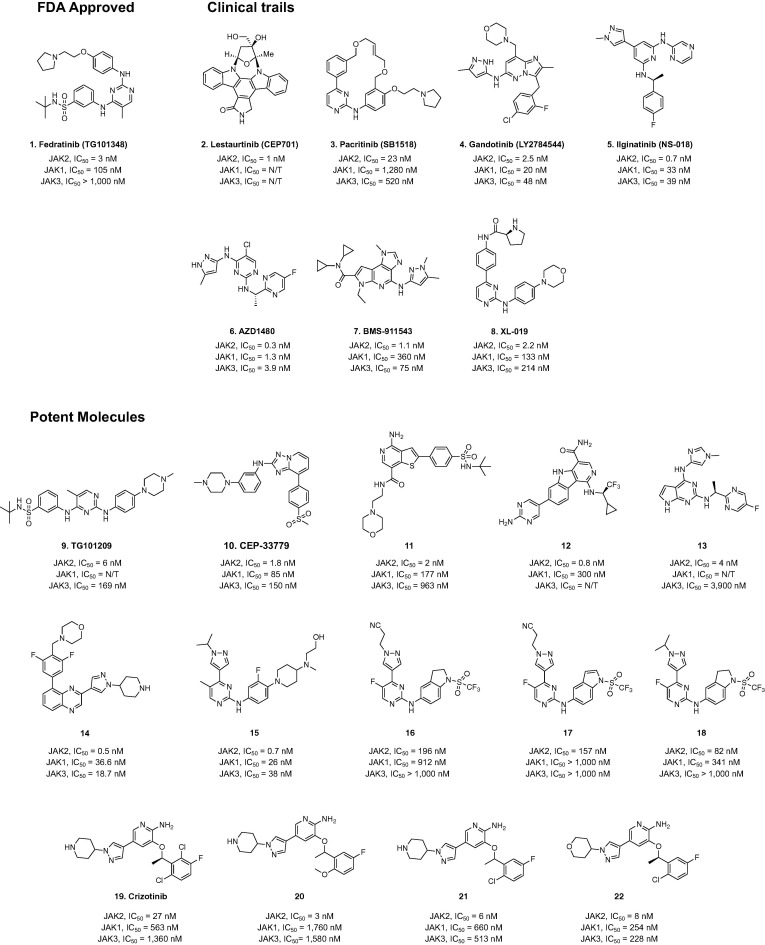
Figure 3.
Chemical structures of JAK2 inhibitors that are either approved by the US FDA or being evaluated in human clinical trials.
Targeting of the cytokine signaling pathway with JAK inhibitors is an exciting opportunity for the treatment of immunologic and hematopoietic diseases. At present, a number of JAK inhibitors have been approved as therapeutics or are being tested in clinical trials (Table 1 and Supplementary Table 1). This section provides an overview of the clinical developments relating to the use of JAK1–3 inhibitors for the treatment of cancers and autoimmune dysfunctions.
Table 1.
JAK2 inhibitors that are approved by the US FDA or being evaluated in human clinical trials.
| Compounds | Sponsor | Indication | Phase/Status/Start date | NCT Identifier |
|---|---|---|---|---|
| Fedratinib (Inrebic) | Sanofi | Myelofibrosis (MF) | FDA Approved/August 2019 | |
| Hepatic impairment | 1/Recruiting/June 2019 | NCT03983161 | ||
| Lestaurtinib (CEP701) | Cephalon | Leukemia, MF | 2/Completed/June 2015 | NCT00494585 |
| Neuroblastoma | 1/Completed/August 2014 | NCT00084422 | ||
| Acute myeloid leukemia (AML) | 2/Completed/June 2016 | NCT00079482 | ||
| Psoriasis | 2/Completed/August 2012 | NCT00236119 | ||
| Pacritinib (SB1518) | AbbVie | Primary myelofibrosis (PMF)/post-polycythemia vera MF, post-essential thrombocythemia MF | 3/Recruiting/May 2017 | NCT03165734 |
| AML | 2/Terminated/August 2015 | NCT02532010 | ||
| Gandotinib (LY2784544) | Eli Lilly | Myeloproliferative disorders, essential thrombocythemia, polycythemia vera, PMF | 1/Completed/April 2018 | NCT01134120 |
| Ilginatinib (NS-018) | NS Pharma, Inc. | PMF, post-polycythemia vera MF, post-essential thrombocythemia MF | 1 & 2/Active, not recruiting/August 2011 | NCT01423851 |
| AZD1480 | AstraZeneca | PMF, post-polycythaemia vera, essential thrombocythaemia MF | 1/Completed/April 2017 | NCT00910728 |
| BMS-911543 | Bristol-Myers Squibb | Cancer | 1 & 2/Terminated/November 2010 | NCT01236352 |
| XL019 | Exelixis | Myeloproliferative disorders, MF, polycythemia vera, essential thrombocythemia | 1/Terminated/August 2007 | NCT00522574 |
Baricitinib (also known as Olumiant, LY3009104 and INCB028050) (Fig. 4 ) is a first-generation JAK inhibitor that is active against JAK1 and JAK2. It is used for the treatment of RA and other inflammatory disorders, such as plaque psoriasis and chronic atypical neutrophilic dermatosis with lipodystrophy and promoted temperatures.46 Tofacitinib is another first-generation highly potent JAK inhibitor, developed by Pfizer for the treatment of autoimmune diseases. It inhibits JAK1 and JAK3, and to a lesser extent, JAK2 and TYK2. In cells, Tofacitinib preferentially inhibits signaling by cytokine receptors associated with JAK3 and/or JAK1 with selectivity over receptors paired with JAK2.47 Peficitinib is a JAK inhibitor that is approved in Japan for the treatment of RA. This drug inhibits JAK1, JAK2, JAK3 and Tyk2 enzyme activity with IC50 values of 3.9, 5.0, 0.71 and 4.8 nM, respectively. Peficitinib also inhibits IL-2-induced proliferation of human T cells (IC50 = 18 nM) and was 14-fold more potent against JAK3 or JAK1 than against JAK2 in the suppression of erythropoietin-induced proliferation of human leukemia.48., 49.
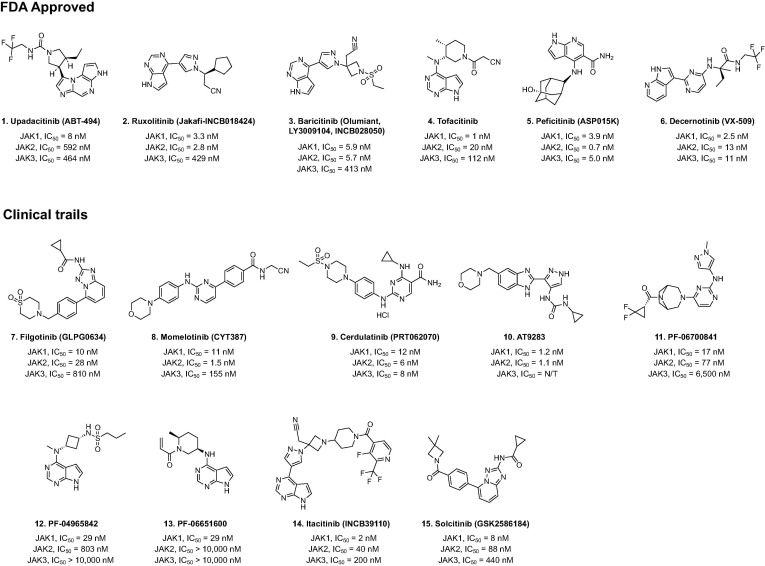
Figure 4.
Chemical structures of JAK1–3 inhibitors that are either approved by the US FDA or being evaluated in human clinical trials.
Ruxolitinib (Fig. 4) is the first FDA-approved JAK inhibitor for the treatment for myelofibrosis, post-polycythemia vera myelofibrosis, post-essential thrombocythemia myelofibrosis, and acute graft-versus-host disease (GvHD). It has shown promising effects in autoimmune diseases such as RA, psoriasis, alopecia areata, dermatomyositis and lupus erythematosus.48 Fedratinib (Fig. 3) is an orally administrated JAK inhibitor developed by Celgene for the treatment of intermediate-2 or high-risk primary or secondary myelofibrosis, and was second drug to be approved for the treatment of myelofibrosis after Ruxolitinib.50 Fedratinib, a selective JAK2 inhibitor, disrupts JAK–STAT signaling, which is overactive in patients with myelofibrosis due to JAK2V617F, CALR, or MPL mutations.51
Upadacitinib or ABT494 (Fig. 4) selectively inhibits JAK1, and is approved by the US FDA for the treatment of moderate-to-severe active RA in patients who have an inadequate response to or intolerance of methotrexate.52 Decernotinib is a newer JAK inhibitor that has ∼ five-fold selectivity toward JAK3 over JAK1, JAK2, and Tyk2 for RA. Nevertheless, the observed adverse effect of neutropenia raises the possibility that this drug inhibits other JAKs besides JAK3.53 Oclacitinib (PF03394197) was licensed in the EU and the US for the control of pruritus related to canine atopic dermatitis (AD) and allergic dermatitis.54 It selectively inhibits JAK1 in signaling pathways that induce many pro-inflammatory cytokines and has minimal effects against JAK2, which is involved in normal haematopoiesis.55
Filgotinib (GLPG0634) (Fig. 4) is a reversible JAK1 preferential inhibitor, with 30- and 80-fold selectivity over JAK2 and JAK3, respectively, as determined in cellular and whole blood assays.56 In September 2020, Filgotinib received its first approvals in the EU and Japan for the treatment of moderate-to-severe RA in adults.51 Filgotinib is currently under investigation for the treatment of RA, ulcerative colitis (UC), psoriatic arthritis (PsA) and Crohn’s disease.57., 58. In addition, numerous new small molecules are in the clinical and preclinical stages of development for the treatment of RA and myelofibrosis. For example, Cerdulatinib (PRT062070), Itacitinib (INCB39110), AT9283, PF06700841, PF04965842, and PF06651600 are potent molecules that are at different stages of evaluation in clinical trials. In particular, Cerdulatinib is multi-target tyrosine kinase inhibitor with IC50 of 12, 6, 8, 0.5, 32 nM for JAK1, JAK2, JAK3, TYK2 and SYK, respectively, whereas PF06651600, Decernotinib, Itacitinib and PF-04965842 selectively inhibit JAK1 over other members of the JAK family (Fig. 4).59., 60., 61., 62., 63.
Clinical investigation of JAK inhibitors in COVID-19 patients
JAK inhibitors including Baricitinib, Ruxolitinib, Tofacitinib, Peficitinib, and Fedratinib are under clinical investigation for the treatment of COVID-19 patients who are a risk of cytokine storm (Table 2 ). These JAK-inhibitors, most notably Baricitinib, show promising effects in early-stage COVID-19 patients, decreasing the use of invasive mechanical ventilation and increasing survival.64 Specifically, Baricitinib treatment attenuates cytokine storm by reducing the expression levels of IL-2, IL-6, IL-10, IFN-g, and GM-CSF, resulting in rapid decline in SARS-CoV-2 viral load and improved lymphocyte counts in severely ill elderly patients with COVID-19.65., 66. Baricitinib inhibits AP2-associated protein kinase 1, which is required for SARS-CoV-2 cellular entry and infectivity.60 Baricitinib exerts rapid inhibition of host numb-associated kinases and reduces viral infectivity in human primary liver spheroids. Furthermore, Baricitinib also prevents type-1 IFN-mediated increase of ACE2 expression, and significantly reduces Tyr705-phosphorylated STAT3 (pSTAT3) level in T lymphocytes, NK cells, monocytes, and neutrophils, regularizing the immune response in COVID-19 patients.67., 68. Baricitinib is generally well tolerated and produces a reduction in inflammation and improved outcomes. However, as a potent immunosuppressant, Baricitinib can lead to an additive risk of infection in severely ill patients. Baricitinib combination with Remdesivir is better than Baricitinib alone in accelerating recovery time and in improving the clinical status of COVID-19 patients on high-flow oxygen or noninvasive ventilation,69 and has fewer adverse events.
Table 2.
Ongoing clinical studies of JAK inhibitors for COVID-19.
| Compounds | Organization | Combination | Phase/Status/Start date | NCT identifier |
|---|---|---|---|---|
| Ruxolitinib | Novartis | – | 3/Completed/November 2020 | NCT04362137 |
| Vanderson Geraldo Rocha | – | 2 & 3/Terminated/April 2021 | NCT04477993 | |
| Incyte | – | 3/Terminated/March 2021 | NCT04377620 | |
| Incyte | – | Temporarily not available/May 2021 | NCT04355793 | |
| University of Colorado | – | 2 & 3/Withdrawn/March 2021 | NCT04348071 | |
| Azienda Usl Toscana Nord Ovest | – | Not yet recruiting/April 2020 | NCT04361903 | |
| Marcelo Iastrebner | – | Phase 2/Not yet recruiting/June 2020 | NCT04414098 | |
| Fundación de investigación HM | Simvastatin | 2/Recruiting/April 2020 | NCT04348695 | |
| Washington University School of Medicine | – | 2/Withdrawn/2020 | NCT04354714 | |
| University of Jena | – | 2/Recruiting/January 2021 | NCT04338958 | |
| Grupo Cooperativo de Hemopatías Malignas | – | 1 & 2/Recruiting/February 2021 | NCT04334044 | |
| Prisma Health-Upstate | – | 2/Completed/March 2021 | NCT04374149 | |
| Philipps University Marburg Medical Center | – | 2/Active not recruited/December 2020 | NCT04359290 | |
| Assistance Publique Hopitaux De Marseille | Anakinra and Tocilizumab | 3/Not yet recruited/ June 2020 | NCT04424056 | |
| Lomonosov Moscow State University | Colchicine and Secukinumab | 2/Recruiting/May 2020 | NCT04403243 | |
| Novartis Pharmaceuticals | – | No longer available/January 2021 | NCT04337359 | |
| Centre Hospitalier Intercommunal de Toulon La Seyne sur Mer | Anakinra | 2/Terminated/December 2020 | NCT04366232 | |
| University Health Network, Toronto | – | Not yet recruited/April 2020 | NCT04331665 | |
| Baricitinib | NIAID | Remdesivir | 3/Completed/December 2020 | NCT04280705 |
| Cambridge University Hospitals NHS Foundation Trust | Ravulizumab | 4/Recruiting/May 2020 | NCT04390464 | |
| Fabrizio Cantini | – | 2 & 3/Completed | NCT04358614 | |
| Hospital of Prato | – | 2 & 3/Not yet recruiting | NCT04320277 | |
| University of Colorado | – | 2 & 3/Withdrawn/ March 2021 | NCT04340232 | |
| University of Southern California | Hydroxychloroquine | 2/Recruiting/June 2020 | NCT04373044 | |
| Eli Lilly and Company | – | 3/Active, not recruiting/May 2021 | NCT04421027 | |
| M Abdur Rahim Medical College & Hospital | Remdesivir and Tocilizumab | 3/Recruiting/January 2021 | NCT04693026 | |
| Azienda Ospedaliero | – | 2/Not yet recruiting/May 2020 | NCT04393051 | |
| IRCCS Policlinico S. Matteo | – | 2/Not yet recruiting/May 2020 | NCT04399798 | |
| ASST Fatebenefratelli Sacco | Remdesivir and Dexamethasone | 3/Not yet recruiting/April 2021 | NCT04832880 | |
| Hospital Universitario de Fuenlabrada | Imatinib | 2/Recruiting/February 2021 | NCT04346147 | |
| ComplejoHospitalario Universitario de Albacete | – | Recruiting/April 2020 | NCT04362943 | |
| Lisa Barrett | – | 2/Recruiting/June 2020 | NCT04321993 | |
| Tofacitinib | Yale University | – | 2/Recruiting/February 2021 | NCT04415151 |
| Pfizer | – | 2/Withdrawn/February 2021 | NCT04412252 | |
| Hospital Israelita Albert Einstein | – | 2/Active, not recruiting/February 2021 | NCT04469114 | |
| I.M. Sechenov First Moscow State Medical University | – | 2/Completed/February 2021 | NCT04750317 | |
| Università Politecnica delle Marche | Hydroxychloroquine | 2/Not yet recruiting/May 2020 | NCT04390061 | |
| Università Politecnica delle Marche | – | 2/Not yet recruiting/April 2020 | NCT04332042 | |
| Pacritinib | CTI BioPharma | – | 3/Not yet recruiting/April 2021 | NCT04404361 |
Notes: ‘–‘, no combination.
Ruxolitinib has robust activity in inhibiting JAK–STAT signaling and significantly suppresses the elevation of IL-6 and TNF-α levels in COVID-19 patients. When compared to a placebo (100 mg vitamin C, twice a day), Ruxolitinib treatment (5 mg, twice a day, a treatment dose for autoimmune or inflammatory conditions) resulted in markedly improved chest computed tomography and faster recovery from lymphopenia.70 A low dose of Ruxolitinib plus steroid reduced mortality and resulted in a 75% recovery rate in COVID-19 patients enrolled in the MAP program.41 The observation of a faster decline in CRP levels and disappearance of fever in treated patients suggests that steroids may play a synergistic role with Ruxolitinib in dampening the immune over-reactivity. Although Ruxolitinib is safe in COVID-19 patients with severe systemic hyper-inflammation, it failed to reduce inflammation significantly in those COVID-19 patients who died or who experienced respiratory failure or admission to the intensive care unit in the Phase III trial.
Tofacitinib, another JAK inhibitor that suppresses inflammatory signaling that is important for the pathological progression of severe lung disease and ARDS, has shown promising results either alone or in combination with hydroxychloroquine in a clinical study of COVID-19 patients.71 Pacritinib is also being investigated in hospitalized patients with severe COVID-19 with or without cancer (NCT04404361).52
Potential of JAK2 inhibitors to block IL-6 receptor signaling and prevent SARS-CoV-2
One concern in using pan-JAK inhibitors for COVID-19 is that such inhibitors may interfere with antibacterial and antiviral responses that are mediated by type I and type II interferons.72., 73. Type I interferons have important antiviral activity through their ability to inhibit viral replication in infected cells. They protect uninfected cells from infection and stimulate antiviral immunity by CD8+ lymphocytes and NK cells. By contrast, type II interferons are produced primarily by T cells and NK cells and help to fight against certain bacteria and to inhibit viral replication. Notably, because JAK2 is not involved in cell signaling that regulates type I interferons, and is reportedly not absolutely required for signaling of type II and type III interferons in host immunity due to functional redundancy with JAK1,74., 75., 76. JAK2 selective inhibitors may be preferred over other JAK inhibitors for blocking signaling by cytokines such as IL-6 and GM-CSF, leading to the suppression of COVID-19-associated CRS.
The development of JAK inhibitors for the treatment of COVID-19-associated CRS is an active area of investigation, with multiple ongoing clinical trials.77 Recent studies have shown that the IL-6/GM–CSF–JAK–STAT axis is closely associated with the development of severe COVID-19 (Fig. 3).78., 79. Antibodies that target IL-6 or GM-CSF normally target only one cytokine, whereas JAK inhibitors can simultaneously target the actions of multiple cytokines, including IL-2, IL-6/GM-CSF IL-4, and IFN-γ.80., 81., 82. The hypothetical benefits of JAK2 inhibition in the management of COVID-19-associated CRS are being evaluated using the FDA-approved JAK2 inhibitors. These benefits may also be provided by the improved JAK2 inhibitors that are currently being evaluated in clinical trials for other disease indications, which may be repurposed for COVID-19 in the future.
Fedratinib (1, TG101348) (Fig. 3) is an FDA-approved JAK2 inhibitor that exhibits nanomolar activity in the treatment of myleofibrosis (MF).83 Fedratinib has also been reported to prevent the deteriorating outcomes that occur with Th17-associated cytokine storm in COVID-19 and other severe viral infections.84 Some JAK2 inhibitors are currently being studied clinically for the treatment of various human diseases. For example, CEP701 (2, Lestaurtinib) (Fig. 2) is a potent JAK2 inhibitor, originally developed by Cephalon, that is being assessed in trials in multiple phases for acute myeloid leukemia (Phase 2), MF (Phases 1/2) and psoriasis (Phase 2).85
Pacritinib (3, SB1518) (Fig. 3) is a JAK2/FLT3 inhibitor that has a very potent JAK2 inhibitory activity without myelosuppressive effects.86 In Phase 2 clinical trials, it has been shown to improve the condition of MF patients and to decrease spleen size and possibly GvHD.86 Gandotinib (4, LY27845544) (Fig. 3), another potent JAK2 inhibitor, is also in Phase 2 clinical trials, in this case for myeloproliferative neoplasms including polycythemia vera, essential thrombocythemia, MF and hematologic disorders.38
Ilginatinib (5, NS018) (Fig. 3), a JAK2 inhibitor, is in Phase 1/2 clinical trials for MF.39 The JAK2 inhibitors AZD1480 (6),41 BMS911543 (7),57 and XL019 (8)87 (Fig. 3) are in advanced clinical studies for inflammatory disorders such as MF, RA, psoriatic arthritis, and ulcerative colitis. Heterocyclic compounds TG101209 (9),88 CEP33779 (10),86 11 (4-amino-2-(4-(N-(tert-butyl)sulfamoyl) phenyl)-N-(2-morpholinoethyl) thieno3., 2., 3., 4., 5., 6., 7., 8., 9., 10., 11., 12., 13., 14., 15., 16., 17., 18., 19., 20., 21., 22., 23., 24., 25., 26., 27., 28., 29., 30., 31., 32., 33., 34., 35., 36., 37., 38., 39., 40., 41., 42., 43., 44., 45., 46., 47., 48., 49., 50., 51., 52., 53., 54., 55., 56., 57., 58., 59., 60., 61., 62., 63., 64., 65., 66., 67., 68., 69., 70., 71., 72., 73., 74., 75., 76., 77., 78., 79., 80., 81., 82., 83., 84., 85., 86., 87., 88., 89., 90., 91., 92., 93., 94., 95., 96., 97. pyridine-7-carboxamide),89 12 ((R)-7-(2-amino-pyrimidin-5-yl)-1-((1-cyclo-propyl-2,2,2-trifluoroethyl) amino)-5H-pyrido4., 3., 4., 5., 6., 7., 8., 9., 10., 11., 12., 13., 14., 15., 16., 17., 18., 19., 20., 21., 22., 23., 24., 25., 26., 27., 28., 29., 30., 31., 32., 33., 34., 35., 36., 37., 38., 39., 40., 41., 42., 43., 44., 45., 46., 47., 48., 49., 50., 51., 52., 53., 54., 55., 56., 57., 58., 59., 60., 61., 62., 63., 64., 65., 66., 67., 68., 69., 70., 71., 72., 73., 74., 75., 76., 77., 78., 79., 80., 81., 82., 83., 84., 85., 86., 87., 88., 89., 90., 91., 92., 93., 94., 95., 96., 97.indole-4-carboxamide),90 13 ((S)-N-(1-(5-fluoro-pyrimidin-2-yl) ethyl)-4-((1-methyl-1H-imidazol-4-yl)methyl)-7H-pyrrolo2., 3., 4., 5., 6., 7., 8., 9., 10., 11., 12., 13., 14., 15., 16., 17., 18., 19., 20., 21., 22., 23., 24., 25., 26., 27., 28., 29., 30., 31., 32., 33., 34., 35., 36., 37., 38., 39., 40., 41., 42., 43., 44., 45., 46., 47., 48., 49., 50., 51., 52., 53., 54., 55., 56., 57., 58., 59., 60., 61., 62., 63., 64., 65., 66., 67., 68., 69., 70., 71., 72., 73., 74., 75., 76., 77., 78., 79., 80., 81., 82., 83., 84., 85., 86., 87., 88., 89., 90., 91., 92., 93., 94., 95., 96., 97.pyrimidin-2-amine),91 and 14 (4-(2,6-difluoro-4-(3-(1-(piperidin-4-yl)-1H-pyrazol-4-yl)quinoxalin-5-yl)benzyl)morpholine)92 (Fig. 3) exhibit significant inhibition of JAK2 in pre-clinical studies. Recently, it has been found that pyrimidine derivatives 15–18 have 5–7-fold selectivity for JAK2 over JAK1 and JAK3.93 In addition, several 2-aminopyridine scaffold compounds have also been reported as promising new JAK2 inhibitors; for example, Crizotinib (19) and its analogs 20–22 exhibit nanomolar inhibitory activity and high selectivity for JAK2, and display remarkable anticancer proliferation activity.94., 95.
Given that JAK2 inhibitors probably do not interfere with the type I interferon response in immunity, but inhibit cytokines including IL-6 and GM-CSF in COVID-19-associated CRS, JAK2 inhibition is likely to offer an attractive therapeutic option for blocking cytokine storm in COVID-19.
Conclusions
Recent clinical observations have led to the rapid recognition of the major role played by cytokine storm in the deterioration of SARS-CoV-2 patients from pneumonia through ARDS, to systemic inflammation and ultimately multi-system organ failure. Pharmacological inhibition of the JAK–STAT signaling pathway is an attractive therapeutic option for the management of COVID-19, which involves many cytokines including IL-6 and GM-CSF. Drugs such as Tocilizumab and Sarilumab have been shown to target IL-6, and consequently improve the respiratory and laboratory parameters of patients with severe and critical COVID-19. Small molecule JAK inhibitors have added advantages as therapeutics as they can target the actions of multiple cytokines, including IL-6 and GM-CSF, simultaneously. JAK inhibitors such as Baricitinib have been shown to minimize the cytokine storm effectively via inhibition of the JAK–STAT signaling pathway and may reduce both viral replication and aberrant host inflammatory response. Given that JAK2 inhibitors have been shown not to interfere with cell signaling by type I interferons, which is essential for the anti-viral immunity of the host cells,96 Fedratinib, an FDA-approved JAK2 inhibitor, could be used to prevent COVID-19-associated cytokine storm with minimal effects on the host immune system. Several potent JAK2 inhibitors that are currently being evaluated in human clinical trials, as well as drugs that have already been FDA approved, have been shown to be moderately effective in controlling host antiviral and anti-bacterial immunity responses. Lestaurtinib (CEF701), Pacritinib, AZD1480, BMS-911543, Ilginatinib (NS108), TG101209 and Gandotinib are among the JAK2-selective inhibitors that have been studied in clinical and preclinical trials for MF, RA and other inflammatory disorders. These JAK2 inhibitors can also be tested for the treatment of COVID-19, either as monotherapies or in combination with IL-6 or IL-6R antagonists. Therefore, these JAK2 inhibitors represent an attractive therapeutic option that we hope will be developed into much-needed therapeutics to treat and prevent the devastating effects of COVID-19.
Acknowledgements
We thank members of the Zhou group for helpful discussions. This work was supported in part by research grants from the National Institutes of Health (R01AI124465 to M.-M.Z.).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.drudis.2021.10.016.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.WHO. Coronavirus disease (COVID-19). Situation Report – 199. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200806-covid-19-sitrep-199.pdf?sfvrsn=6b9d262d_2. Published August 6, 2020 [accessed May 28, 2021].
- 2.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yi Y., Lagniton P.N.P., Ye S., Li E., Xu R.H. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int J Biol Sci. 2020;16:1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Draft landscape and tracker of COVID-19 candidate vaccines [press release]. https://www.who.int/docs/default-source/coronaviruse/novel-coronavirus-landscape-covid-19cf1952c105464714aaaf8c7cd5c5cc8b.pdf. Published October 15, 2020 [accessed May 28, 2021].
- 8.Ritchie H, Mathieu E, Rodés-Guirao L, Appel C, Giattino C, Ortiz-Ospina E, et al. Coronavirus Pandemic (COVID-19). https://ourworldindata.org/coronavirus. Published 2020 [accessed May 28, 2021].
- 9.Zimmer C, Corum J, Wee S-L. Coronavirus vaccine tracker. https://www.nytimes.com/interactive/2020/science/coronavirus-vaccine-tracker.html. Published 2020 [accessed May 29, 2021].
- 10.Chen Y., Liu Q., Guo D. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020;92:418–423. doi: 10.1002/jmv.25681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tahir ul Qamar M, Alqahtani SM, Alamri MA, Chen L-L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. J Pharm Anal 2020;10:313–9. doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed]
- 12.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shi Y., Wang Y., Shao C., Huang J., Gan J., Huang X., et al. COVID-19 infection: the perspectives on immune responses. Cell Death Differ. 2020;27:1451–1454. doi: 10.1038/s41418-020-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sallard E., Lescure F.X., Yazdanpanah Y., Mentre F., Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merad M., Martin J.C. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García L.F. Immune response, inflammation, and the clinical spectrum of COVID-19. Front Immunol. 2020;11:1441. doi: 10.3389/fimmu.2020.01441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallagher T.M., Buchmeier M.J. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279:371–374. doi: 10.1006/viro.2000.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirchdoerfer R.N., Cottrell C.A., Wang N., Pallesen J., Yassine H.M., Turner H.L., et al. Pre-fusion structure of a human coronavirus spike protein. Nature. 2016;531:118–121. doi: 10.1038/nature17200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Y.R., Cao Q.D., Hong Z.S., Tan Y.Y., Chen S.D., Jin H.J., et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak — an update on the status. Mil Med Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giamarellos-Bourboulis E.J., Netea M.G., Rovina N., Akinosoglou K., Antoniadou A., Antonakos N., et al. Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 2020;27:992–1000. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Melenotte C., Silvin A., Goubet A.G., Lahmar I., Dubuisson A., Zumla A., et al. Immune responses during COVID-19 infection. Oncoimmunology. 2020;9:1807836. doi: 10.1080/2162402X.2020.1807836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walls A.C., Tortorici M.A., Bosch B.J., Frenz B., Rottier P.J.M., DiMaio F., et al. Cryo-electron microscopy structure of a coronavirus spike glycoprotein trimer. Nature. 2016;531:114–117. doi: 10.1038/nature16988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vabret N., Britton G.J., Gruber C., Hegde S., Kim J., Kuksin M., et al. Immunology of COVID-19: current state of the science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gubernatorova E.O., Gorshkova E.A., Polinova A.I., Drutskaya M.S. IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020;53:13–24. doi: 10.1016/j.cytogfr.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 28.Ye Q., Wang B., Mao J. The pathogenesis and treatment of the 'Cytokine Storm' in COVID-19. J Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaware N., Zhou M.M. Bromodomain biology and drug discovery. Nat Struct Mol Biol. 2019;26:870–879. doi: 10.1038/s41594-019-0309-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dosch S.F., Mahajan S.D., Collins A.R. SARS coronavirus spike protein-induced innate immune response occurs via activation of the NF-kappaB pathway in human monocyte macrophages in vitro. Virus Res. 2009;142:19–27. doi: 10.1016/j.virusres.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fung S.Y., Yuen K.S., Ye Z.W., Chan C.P., Jin D.Y. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect. 2020;9:558–570. doi: 10.1080/22221751.2020.1736644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lim Y.X., Ng Y.L., Tam J.P., Liu D.X. Human Coronaviruses: a review of virus-host interactions. Diseases. 2016;4:26. doi: 10.3390/diseases4030026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeDiego M.L., Nieto-Torres J.L., Regla-Nava J.A., Jimenez-Guardeño J.M., Fernandez-Delgado R., Fett C., et al. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J Virol. 2014;88:913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoeman D., Fielding B.C. Coronavirus envelope protein: current knowledge. Virol J. 2019;16:69. doi: 10.1186/s12985-019-1182-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DeDiego M.L., Nieto-Torres J.L., Jimenez-Guardeño J.M., Regla-Nava J.A., Castaño-Rodriguez C., Fernandez-Delgado R., et al. Coronavirus virulence genes with main focus on SARS-CoV envelope gene. Virus Res. 2014;194:124–137. doi: 10.1016/j.virusres.2014.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gordon DE, Jang GM, Bouhaddou M, Xu J, Obernier K, O'Meara MJ, et al. A SARS-CoV-2-human protein-protein interaction map reveals drug targets and potential drug-repurposing. bioRxiv 2020; 2020.03.22.002386. doi: 10.1101/2020.03.22.002386.
- 37.Zhang G., Liu R., Zhong Y., Plotnikov A.N., Zhang W., Zeng L., et al. Down-regulation of NF-κB transcriptional activity in HIV-associated kidney disease by BRD4 inhibition. J Biol Chem. 2012;287:28840–28851. doi: 10.1074/jbc.M112.359505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gadina M., Le M.T., Schwartz D.M., Silvennoinen O., Nakayamada S., Yamaoka K., O'Shea J.J. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology. 2019;58(Suppl 1):i4–i16. doi: 10.1093/rheumatology/key432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kang S., Tanaka T., Narazaki M., Kishimoto T. Targeting Interleukin-6 signaling in clinic. Immunity. 2019;50:1007–1023. doi: 10.1016/j.immuni.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 40.Zhang C., Wu Z., Li J.W., Zhao H., Wang G.Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., et al. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Johnson D.E., O'Keefe R.A., Grandis J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol. 2018;15:234–248. doi: 10.1038/nrclinonc.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sciascia S., Aprà F., Baffa A., Baldovino S., Boaro D., Boero R., et al. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol. 2020;38:529–532. [PubMed] [Google Scholar]
- 44.Xu X., Han M., Li T., Sun W., Wang D., Fu B., et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J Med Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Della-Torre E., Campochiaro C., Cavalli G., De Luca G., Napolitano A., La Marca S., et al. Interleukin-6 blockade with sarilumab in severe COVID-19 pneumonia with systemic hyperinflammation: an open-label cohort study. Ann Rheum Dis. 2020;79:1277–1285. doi: 10.1136/annrheumdis-2020-218122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lang F.M., Lee K.M., Teijaro J.R., Becher B., Hamilton J.A. GM-CSF-based treatments in COVID-19: reconciling opposing therapeutic approaches. Nat Rev Immunol. 2020;20:507–514. doi: 10.1038/s41577-020-0357-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonaventura A., Vecchié A., Wang T.S., Lee E., Cremer P.C., Carey B., et al. Targeting GM-CSF in COVID-19 pneumonia: rationale and strategies. Front Immunol. 2020;11:1625. doi: 10.3389/fimmu.2020.01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bosteels C., Maes B., Van Damme K., De Leeuw E., Declercq J., Delporte A., et al. Sargramostim to treat patients with acute hypoxic respiratory failure due to COVID-19 (SARPAC): a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21:491. doi: 10.1186/s13063-020-04451-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiang X., Zhu S., Li J., Wang P., Tracey K.J., Wang H. Monoclonal antibodies capable of binding SARS-CoV-2 spike protein receptor-binding motif specifically prevent GM-CSF induction. J Leukoc Biol. 2021 doi: 10.1002/JLB.3COVCRA0920-628RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ajayi S., Becker H., Reinhardt H., Engelhardt M., Zeiser R., von Bubnoff N., Wäsch R. Ruxolitinib. Recent Results Cancer Res. 2018;212:119–132. doi: 10.1007/978-3-319-91439-8_6. [DOI] [PubMed] [Google Scholar]
- 52.Stebbing J., Sánchez Nievas G., Falcone M., Youhanna S., Richardson P., Ottaviani S., et al. JAK inhibition reduces SARS-CoV-2 liver infectivity and modulates inflammatory responses to reduce morbidity and mortality. Sci Adv. 2021;7:eabe4724. doi: 10.1126/sciadv.abe4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Al-Salama Z.T., Scott L.J. Baricitinib: a review in rheumatoid arthritis. Drugs. 2018;78:761–772. doi: 10.1007/s40265-018-0908-4. [DOI] [PubMed] [Google Scholar]
- 54.Dhillon S. Tofacitinib: a review in rheumatoid arthritis. Drugs. 2017;77:1987–2001. doi: 10.1007/s40265-017-0835-9. [DOI] [PubMed] [Google Scholar]
- 55.Markham A., Keam S.J. Peficitinib: first global approval. Drugs. 2019;79:887–891. doi: 10.1007/s40265-019-01131-y. [DOI] [PubMed] [Google Scholar]
- 56.Ito M., Yamazaki S., Yamagami K., Kuno M., Morita Y., Okuma K., et al. A novel JAK inhibitor, peficitinib, demonstrates potent efficacy in a rat adjuvant-induced arthritis model. J Pharmacol Sci. 2017;133:25–33. doi: 10.1016/j.jphs.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 57.Blair H.A. Fedratinib: first approval. Drugs. 2019;79:1719–1725. doi: 10.1007/s40265-019-01205-x. [DOI] [PubMed] [Google Scholar]
- 58.Santos F.P., Verstovsek S. JAK2 inhibitors for myelofibrosis: why are they effective in patients with and without JAK2V617F mutation? Anticancer Agents Med Chem. 2012;12:1098–1109. doi: 10.2174/187152012803529727. [DOI] [PubMed] [Google Scholar]
- 59.Duggan S. Keam SJ. Upadacitinib: first approval. Drugs. 2019;79:1819–1828. doi: 10.1007/s40265-019-01211-z. [DOI] [PubMed] [Google Scholar]
- 60.Gadina M., Schwartz D.M., O'Shea J.J. Decernotinib: a next-generation Jakinib. Arthritis Rheumatol. 2016;68:31–34. doi: 10.1002/art.39463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haugh I.M., Watson I.T., Alan Menter M. Successful treatment of atopic dermatitis with the JAK1 inhibitor oclacitinib. Proc (Bayl Univ Med Cent) 2018;31:524–525. doi: 10.1080/08998280.2018.1480246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gonzales A.J., Bowman J.W., Fici G.J., Zhang M., Mann D.W., Mitton-Fry M. Oclacitinib (APOQUEL®) is a novel Janus kinase inhibitor with activity against cytokines involved in allergy. J Vet Pharmacol Ther. 2014;37:317–324. doi: 10.1111/jvp.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Rompaey L., Galien R., van der Aar E.M., Clement-Lacroix P., Nelles L., Smets B., et al. Preclinical characterization of GLPG0634, a selective inhibitor of JAK1, for the treatment of inflammatory diseases. J Immunol. 2013;191:3568–3577. doi: 10.4049/jimmunol.1201348. [DOI] [PubMed] [Google Scholar]
- 64.Dhillon S., Keam S.J. Filgotinib: first approval. Drugs. 2020;80:1987–1997. doi: 10.1007/s40265-020-01439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Taylor P.C., Abdul Azeez M., Kiriakidis S. Filgotinib for the treatment of rheumatoid arthritis. Expert Opin Investig Drugs. 2017;26:1181–1187. doi: 10.1080/13543784.2017.1372422. [DOI] [PubMed] [Google Scholar]
- 66.Labetoulle R., Paul S., Roblin X. Filgotinib for the treatment of Crohn's disease. Expert Opin Investig Drugs. 2018;27:295–300. doi: 10.1080/13543784.2018.1442433. [DOI] [PubMed] [Google Scholar]
- 67.Coffey G., Betz A., DeGuzman F., Pak Y., Inagaki M., Baker D.C., et al. The novel kinase inhibitor PRT062070 (Cerdulatinib) demonstrates efficacy in models of autoimmunity and B-cell cancer. J Pharmacol Exp Ther. 2014;351:538–548. doi: 10.1124/jpet.114.218164. [DOI] [PubMed] [Google Scholar]
- 68.Telliez J.B., Dowty M.E., Wang L., Jussif J., Lin T., Li L., et al. Discovery of a JAK3-selective inhibitor: functional differentiation of JAK3-selective inhibition over pan-JAK or JAK1-selective inhibition. ACS Chem Biol. 2016;11:3442–3451. doi: 10.1021/acschembio.6b00677. [DOI] [PubMed] [Google Scholar]
- 69.Kettle J.G., Åstrand A., Catley M., Grimster N.P., Nilsson M., Su Q., Woessner R. Inhibitors of JAK-family kinases: an update on the patent literature 2013–2015, part 1. Expert Opin Ther Pat. 2017;27:127–143. doi: 10.1080/13543776.2017.1252753. [DOI] [PubMed] [Google Scholar]
- 70.Vazquez M.L., Kaila N., Strohbach J.W., Trzupek J.D., Brown M.F., Flanagan M.E., et al. Identification of N-{cis-3-[Methyl(7H-pyrrolo[2,3-d]pyrimidin-4-yl)amino]cyclobutyl}propane-1-sulfonamide (PF-04965842): a selective JAK1 clinical candidate for the treatment of autoimmune diseases. J Med Chem. 2018;61:1130–1152. doi: 10.1021/acs.jmedchem.7b01598. [DOI] [PubMed] [Google Scholar]
- 71.Stebbing J., Krishnan V., de Bono S., Ottaviani S., Casalini G., Richardson P.J., et al. Mechanism of baricitinib supports artificial intelligence-predicted testing in COVID-19 patients. EMBO Mol Med. 2020;12 doi: 10.15252/emmm.202012697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoang TN, Pino M, Boddapati AK, Viox EG, Starke CE, Upadhyay AA, et al. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell 2021;184:460–75.e21. doi: 10.1016/j.cell.2020.11.007. [DOI] [PMC free article] [PubMed]
- 73.Bronte V., Ugel S., Tinazzi E., Vella A., De Sanctis F., Canè S., et al. Baricitinib restrains the immune dysregulation in patients with severe COVID-19. J Clin Invest. 2020;130:6409–6416. doi: 10.1172/JCI141772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., et al. Baricitinib plus Remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795–807. doi: 10.1056/NEJMoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chow K.T., Gale M., Jr. SnapShot: interferon signaling. Cell. 2015;163:1808–1808.e1. doi: 10.1016/j.cell.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 76.Platanias L.C. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol. 2005;5:375–386. doi: 10.1038/nri1604. [DOI] [PubMed] [Google Scholar]
- 77.Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol 2020;146:137–46.e3. doi: 10.1016/j.jaci.2020.05.019 [DOI] [PMC free article] [PubMed]
- 78.D'Alessio A., Del Poggio P., Bracchi F., Cesana G., Sertori N., Di Mauro D., et al. Low-dose ruxolitinib plus steroid in severe SARS-CoV-2 pneumonia. Leukemia. 2021;35:635–638. doi: 10.1038/s41375-020-01087-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Agrawal M., Brenner E.J., Zhang X., Modesto I., Woolcott J., Ungaro R.C., et al. Characteristics and outcomes of IBD patients with COVID-19 on tofacitinib therapy in the SECURE-IBD registry. Inflamm Bowel Dis. 2021;27:585–589. doi: 10.1093/ibd/izaa303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Betts B.C., Young J.W. Less can be more when targeting Interleukin-6-mediated cytokine release syndrome in Coronavirus Disease 2019. Crit Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cameron M.J., Bermejo-Martin J.F., Danesh A., Muller M.P., Kelvin D.J. Human immunopathogenesis of severe acute respiratory syndrome (SARS) Virus Res. 2008;133:13–19. doi: 10.1016/j.virusres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Channappanavar R., Fehr A.R., Zheng J., Wohlford-Lenane C., Abrahante J.E., Mack M., et al. IFN-I response timing relative to virus replication determines MERS coronavirus infection outcomes. J Clin Invest. 2019;129:3625–3639. doi: 10.1172/JCI126363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kotenko S.V., Izotova L.S., Pollack B.P., Muthukumaran G., Paukku K., Silvennoinen O., et al. Other kinases can substitute for Jak2 in signal transduction by interferon-gamma. J Biol Chem. 1996;271:17174–17182. doi: 10.1074/jbc.271.29.17174. [DOI] [PubMed] [Google Scholar]
- 84.Spinelli F.R., Conti F., Gadina M. HiJAKing SARS-CoV-2? The potential role of JAK inhibitors in the management of COVID-19. Sci Immunol. 2020;5:eabc5367. doi: 10.1126/sciimmunol.abc5367. [DOI] [PubMed] [Google Scholar]
- 85.Atal S., Fatima Z. IL-6 inhibitors in the treatment of serious COVID-19: a promising therapy? Pharmaceut Med. 2020;34:223–231. doi: 10.1007/s40290-020-00342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tremblay D., Mascarenhas J. Pacritinib to treat myelofibrosis patients with thrombocytopenia. Expert Rev Hematol. 2018;11:707–714. doi: 10.1080/17474086.2018.1500456. [DOI] [PubMed] [Google Scholar]
- 87.Wu D., Yang X.O. TH17 responses in cytokine storm of COVID-19: an emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53:368–370. doi: 10.1016/j.jmii.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hexner E.O., Serdikoff C., Jan M., Swider C.R., Robinson C., Yang S., et al. Lestaurtinib (CEP701) is a JAK2 inhibitor that suppresses JAK2/STAT5 signaling and the proliferation of primary erythroid cells from patients with myeloproliferative disorders. Blood. 2008;111:5663–5671. doi: 10.1182/blood-2007-04-083402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berdeja J., Palandri F., Baer M.R., Quick D., Kiladjian J.J., Martinelli G., et al. Phase 2 study of gandotinib (LY2784544) in patients with myeloproliferative neoplasms. Leukemia Res. 2018;71:82–88. doi: 10.1016/j.leukres.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 90.Nakaya Y., Shide K., Naito H., Niwa T., Horio T., Miyake J., Shimoda K. Effect of NS-018, a selective JAK2V617F inhibitor, in a murine model of myelofibrosis. Blood Cancer J. 2014;4 doi: 10.1038/bcj.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Derenzini E., Lemoine M., Buglio D., Katayama H., Ji Y., Davis R.E., et al. The JAK inhibitor AZD1480 regulates proliferation and immunity in Hodgkin lymphoma. Blood Cancer J. 2011;1 doi: 10.1038/bcj.2011.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Pomicter A.D., Eiring A.M., Senina A.V., Zabriskie M.S., Marvin J.E., Prchal J.T., et al. Limited efficacy of BMS-911543 in a murine model of Janus kinase 2 V617F myeloproliferative neoplasm. Exp Hematol. 2015;43(537–45):e1–e11. doi: 10.1016/j.exphem.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verstovsek S., Tam C.S., Wadleigh M., Sokol L., Smith C.C., Bui L.A., et al. Phase I evaluation of XL019, an oral, potent, and selective JAK2 inhibitor. Leukemia Res. 2014;38:316–322. doi: 10.1016/j.leukres.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pardanani A., Hood J., Lasho T., Levine R.L., Martin M.B., Noronha G., et al. TG101209, a small molecule JAK2-selective kinase inhibitor potently inhibits myeloproliferative disorder-associated JAK2V617F and MPLW515L/K mutations. Leukemia. 2007;21:1658–1668. doi: 10.1038/sj.leu.2404750. [DOI] [PubMed] [Google Scholar]
- 95.Stump K.L., Lu L.D., Dobrzanski P., Serdikoff C., Gingrich D.E., Dugan B.J., et al. A highly selective, orally active inhibitor of Janus kinase 2, CEP-33779, ablates disease in two mouse models of rheumatoid arthritis. Arthritis Res Ther. 2011;13:R68. doi: 10.1186/ar3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Schenkel L.B., Huang X., Cheng A., Deak H.L., Doherty E., Emkey R., et al. Discovery of potent and highly selective thienopyridine Janus kinase 2 inhibitors. J Med Chem. 2011;54:8440–8450. doi: 10.1021/jm200911r. [DOI] [PubMed] [Google Scholar]
- 97.Lim J., Taoka B., Otte R.D., Spencer K., Dinsmore C.J., Altman M.D., et al. Discovery of 1-amino-5H-pyrido[4,3-b]indol-4-carboxamide inhibitors of Janus kinase 2 (JAK2) for the treatment of myeloproliferative disorders. J Med Chem. 2011;54:7334–7349. doi: 10.1021/jm200909u. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.