Editor
We read with interest the article by Amarnath Sen suggesting serotonin deficiency as a mechanism for anosmia, ageusia, chemesthesis dysfunction and COVID-19 severity [1]. Although, we concur with the proposed pathophysiological model, we believe, the additional pathways of tryptophan (TRP) metabolism that are closely linked with serotonin synthesis – the kynurenine (KYN) pathways – may have a greater pathophysiological role in the COVID-19 symptomatology and severity.
The evidence from the recent longitudinal metabolomic studies in the COVID-19 patients demonstrated deregulated kynurenine metabolism as a key signature for disease severity [2], [3]. The depletion of TRP is associated with increased kynurenine levels, suggesting increased indoleamine-pyrrole 2,3-dioxygenase (IDO) expression, instead of reduced intestinal absorption of TRP [1], as a key mechanism for TRP depletion in COVID-19 patients. This may eventually result in decreased TRP availability for serotonin and melatonin synthesis. However, the reduction in serotonin levels observed in SARS-CoV-2 were not significant [2], while the neurotoxic metabolites of kynurenine, 3-hydroxykynurenine (3-HK) and quinolinic acid (QA) were significantly elevated [2], [3]. Additionally, neuroprotective metabolites such as kynurenic acid (KA), xanthurenic acid and nicotinamide adenine dinucleotide (NAD+) are decreased. Further, the increased ratios of 3HK/KYN. 3HK/KA, QA/KYN and KYN/TRP significantly correlates with COVID-19 severity [2], [3].
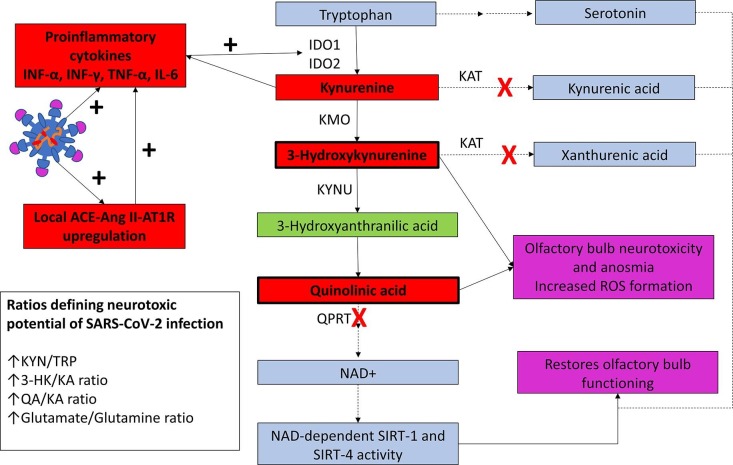
Following SARS-CoV-2-induced injury, infected sustentacular cells release interferon-γ to induce increase expression of IDO in epithelial and endothelial cells [4] in the nasal and olfactory epithelium resulting in the above described perturbations in the KYN metabolism. As 3-HK and QA have strong glutaminergic activity [2] and olfactory bulbs have high NMDA receptor expression [5], we believe that these metabolites diffuse from the olfactory epithelium transmucosally to reach the olfactory bulbs, producing neurotoxic injury via NMDA agonistic activity. Our hypothesis is supported by the finding that in an air–liquid interface model, intranasal glutamate administration induces olfactory bulb neurotoxic anosmia [5]. Importantly, the timing and duration of anosmia observed in this model correlates with COVID-19 associated anosmia [1], [5]. Also, the histological changes of glutamate-induced neuronal loss in olfactory bulbs correlates with the COVID-19 related necrotizing olfactory bulbitis reported by Stoyanov et al. [6]. Notably. recovery of olfactory function in the acute lung injury model correlates with increased sirtuin deacetylase (SIRT)-1 (SIRT-1) and SIRT-4 activity, a family of NAD+ dependent histone deacetylases. Interestingly, severe COVID-19 is associated with reduced quinolinate phosphoribosyltransferase expression and reduced production of NAD+ [7]. As, QA inhibits SIRT-1 activity, raised QA levels and low NAD+ levels in COVID-19 may be responsible for anosmia (Fig. 1 ).
Fig. 1.
Schematic presentation of the systematic perturbation in TRP and KYN metabolism during SARS-CoV-2 infection. SARS-CoV-2 infection of the nasal-olfactory epithelium is associated with downregulation of ACE2 and upregulation of ACE-Ang II-AT1R activity along with local innate immune response and release of proinflammatory cytokines. This results in increased expression of IDO activity in the nasal epithelium resulting in perturbation of TRP metabolism towards KYN metabolites, while serotonin synthesis is reduced. Blue boxes indicate neuroprotective KYN metabolites that decreases in SARS-CoV-2 infected patients; red boxes indicate the neurotoxic metabolites that significantly increase following SARS-CoV-2 infection. Abbreviations: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; ACE, angiotensin converting enzyme; Ang II, angiotensin II; AT1R, angiotensin type 1 receptor; IFN-γ, interferon-γ; IFN- α, interferon-α; TNF-α, tumor necrosis factor-α; IDO1, indoleamine-2,3-dioxygenase 1; IDO2, indoleamine-2,3-dioxygenase 2; TRP, tryptophan; KYN, kynurenine; 3-HK, 3-hydroxykynurenine; KA, kynurenic acid; QA, quinolinic acid; NAD, nicotinamide adenine dinucleotide; KAT, kynurenine aminotransferase; KMO, kynurenine 3-monooxygenase; KYNU, kynurenine hydroxylase; QPRT, quinolinic acid phosphoribosyltransferase; SIRT, sirtuin deacetylase. Solid lines represent pathways that are predominant and dash lines represent pathways with reduced activity. Red cross represents enzymatic activities that are specifically reduced in SARS-CoV-2 infection.
Peripheral alteration in the KYN metabolism can similarly be responsible for COVID-19 disease severity and its neurocognitive manifestations. Notably, plasma KYN and 3-HK, in contrast to plasma KA, can readily cross the blood–brain barrier; thus, high plasma levels of KYN and 3-HK can generate high brain QA levels to induce neuronal inflammation, oxidative stress, hippocampal injury and neurocognitive impairment.
Funding statement
Support was provided solely from institutional and/or departmental sources
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Sen A. Does serotonin deficiency lead to anosmia, ageusia, dysfunctional chemesthesis and increased severity of illness in COVID-19? Med Hypotheses. 2021;153:110627. doi: 10.1016/j.mehy.2021.110627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lawler N.G., Gray N., Kimhofer T., Boughton B., Gay M., Yang R., et al. Systemic perturbations in amine and kynurenine metabolism associated with acute SARS-CoV-2 infection and inflammatory cytokine responses. J Proteome Res. 2021;20(5):2796–2811. doi: 10.1021/acs.jproteome.1c00052. [DOI] [PubMed] [Google Scholar]
- 3.Marín-Corral J., Rodríguez-Morató J., Gomez-Gomez A., Pascual-Guardia S., Muñoz-Bermúdez R., Salazar-Degracia A., et al. Metabolic signatures associated with severity in hospitalized COVID-19 patients. Int J Mol Sci. 2021;22(9):4794. doi: 10.3390/ijms22094794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pozharskaya T., Lane A.P. Interferon gamma causes olfactory dysfunction without concomitant neuroepithelial damage. Int Forum Allergy Rhinol. 2013;3(11):861–865. doi: 10.1002/alr.21226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Marin C., Langdon C., Alobid I., Fuentes M., Bonastre M., Mullol J. Recovery of olfactory function after excitotoxic lesion of the olfactory bulbs is associated with increases in bulbar SIRT1 and SIRT4 expressions. Mol Neurobiol. 2019;56(8):5643–5653. doi: 10.1007/s12035-019-1472-y. [DOI] [PubMed] [Google Scholar]
- 6.Stoyanov G.S., Petkova L., Dzhenkov D.L., Sapundzhiev N.R., Todorov I. Gross and histopathology of COVID-19 with first histology report of olfactory bulb changes. Cureus. 2020;12 doi: 10.7759/cureus.11912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heer C.D., Sanderson D.J., Voth L.S., Alhammad Y.M.O., Schmidt M.S., Trammell S.A.J., et al. Coronavirus infection and PARP expression dysregulate the NAD metabolome: an actionable component of innate immunity. J Biol Chem. 2020;295(52):17986–17996. doi: 10.1074/jbc.RA120.015138. [DOI] [PMC free article] [PubMed] [Google Scholar]