Abstract
Background
Coronavirus disease-2019 (COVID-19) cases continue to increase globally. Poor outcomes in patients with COVID-19 and cirrhosis have been reported; predictors of outcome are unclear. The existing data is from the early part of the pandemic when variants of concern (VOC) were not reported.
Aims
We aimed to assess the outcomes and predictors in patients with cirrhosis and COVID-19. We also compared the differences in outcomes between the first wave of pandemic and the second wave.
Methods
In this retrospective analysis of a prospectively maintained database, data on consecutive cirrhosis patients (n = 221) admitted to the COVID-19 care facility of a tertiary care center in India were evaluated for presentation, the severity of liver disease, the severity of COVID-19, and outcomes.
Results
The clinical presentation included: 18 (8.1%) patients had compensated cirrhosis, 139 (62.9%) acute decompensation (AD), and 64 (29.0%) had an acute-on-chronic liver failure (ACLF). Patients with ACLF had more severe COVID-19 infection than those with compensated cirrhosis and AD (54.7% vs. 16.5% and 33.3%, P < 0.001). The overall mortality was 90 (40.7%), the highest among ACLF (72.0%). On multivariate analysis, independent predictors of mortality were high leukocyte count, alkaline phosphatase, creatinine, child class, model for end-stage liver disease (MELD) score, and COVID-19 severity. The second wave had more cases of severe COVID-19 as compared to the first wave, with a similar MELD score and Child score. The overall mortality was similar between the two waves.
Conclusion
Patients with COVID-19 and cirrhosis have high mortality (40%), particularly those with ACLF (72%). A higher leukocyte count, creatinine, alkaline phosphatase, Child class, and MELD score are predictors of mortality.
Keywords: portal hypertension, alcohol, HBV, HCV, NAFLD
Abbreviations: ACLF, acute-on-chronic liver failure; AD, acute decompensation; AIH, autoimmune hepatitis; ALT, alanine aminotransferase; Alk P, alkaline phosphatase; AST, aspartate aminotransferase; COVID-19, Coronavirus disease-2019; CTP, Child-Turcotte-Pugh; EHPVO, extrahepatic portal vein obstruction; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; MELD, model for end-stage liver disease; MODS, multiorgan dysfunction syndrome; NAFLD, nonalcoholic fatty liver disease; NAAT, Nucleic Acid Amplification Test; NCPF, noncirrhotic portal fibrosis; TLC, Total leukocyte count; VOC, variants of concern; VOI, variants of interest
Globally, over 3,927,222 lives have been lost due to the ongoing Coronavirus disease 2019 (COVID-19) pandemic, and the number is still increasing.1 In addition to its tropism for respiratory tract epithelium, the severe acute respiratory syndrome coronavirus 2 (SARS CoV2) also has an affinity for the gastrointestinal tract.2 The SARS-CoV-2 is well-known to have high mortality rates in people with older age, obesity, diabetes, or coronary artery disease.2 Large registries and data from individual centers suggest that the presence of chronic liver disease (CLD) is associated with poor outcomes, and the risk of mortality is close to 30%–40%.3, 4, 5 Among those with CLD, mortality is higher in cirrhotics as compared to noncirrhotics.3, 4, 5
The data available on the influence of COVID-19 in patients with CLD has a few limitations. First, the data are controversial about the outcome following COVID-19 infection in patients with CLD.6 Second, there is an under-representation of data from resource constraint developing world where the cause of CLD and access to the health care resource might be different from those in developed countries. Third, data from India are extremely limited.5,7 Fourth, the previous data were collected during the early part of the pandemic when variants of concern (VOC) and variants of interest (VOI) were not prevalent; these newly identified Corona variants, which have faster transmission and a higher risk of severe disease, may pose a higher risk for CLD patients. Fifth, data are heterogeneous as it included data from CLD patients with or without cirrhosis.
We retrospectively reviewed our database to study the in-hospital outcomes of COVID-19 infected cirrhosis patients and to identify the predictors of mortality in them. We also attempted to compare the two cohorts of those patients admitted between the first and the second waves of the COVID-19 pandemic in India.
Patients and methods
Patient Population
Our database prospectively collected data from all consecutive cirrhosis patients who were admitted with COVID-19 between April 2020 and June 2021 in the COVID-19 care facility of the All India Institute of Medical Sciences, New Delhi, India. Patients were followed till death or discharge. Ethical clearance was obtained from the Institutional Ethics Committee of All India Institute of Medical Sciences, New Delhi (Ref No: IEC-253/17.04.2020). Since this was a retrospective analysis, the need for informed consent was waived off.
Patient Evaluation and Management
Patients with features of cirrhosis who presented any sign or symptoms of COVID-19 were screened for SARS-CoV2 infection. COVID-19 infection was confirmed with a positive nucleic acid amplification test (NAAT) on nasal/oropharyngeal swabs. The severity of COVID-19 was graded as per the Ministry of Health and Family Welfare (MOHFW) guidelines.8 Asymptomatic patients or those with only upper respiratory tract symptoms and normal oxygen saturation on room air were defined as having a mild disease. Those with lower respiratory tract involvement in the form of pneumonia and saturation between 90% and 94% on room air and/or respiratory rate (RR) between 24 and 30/minute were defined as having moderate COVID-19. Patients having saturation <90% on room air and/or a RR > 30/minute or severe acute respiratory illness were classified as severe disease.9 Mild COVID-19 patients were treated symptomatically with paracetamol as and when needed for fever and given hydroxychloroquine/ivermectin/budesonide metered-dose inhaler. Moderate cases were additionally given prophylactic enoxaparin 0.5 mg/kg subcutaneously (SC) once a day and intravenous (IV) methylprednisolone 0.5–1 mg/kg/day in two divided doses for 5 days according to the treating physician’s discretion. Supplemental oxygen was given to these patients via nasal cannula or facemask to maintain oxygen saturation between 92% and 96%. Severe COVID-19 patients were managed in intensive care unit setting with mechanical ventilation or high-flow nasal oxygen as required.
Cirrhosis and associated complications were managed as per the international consensus and guidelines.13 The only deviation being those presenting with bleeding were managed conservatively with splanchnic vasoconstrictors, restrictive transfusion, prophylactic antibiotics, and endoscopy only when the need arose in accordance with our previously published experience.7
Definitions
Cirrhosis was defined based on imaging evidence of an irregular liver outline, with evidence of portal hypertension as clinical decompensation or imaging evidence of dilated splenoportal axis and/or splenomegaly. Patients were divided into three groups - compensated cirrhosis, decompensated cirrhosis, and ACLF. Decompensation was defined as the presence of ascites, jaundice, variceal bleed, or hepatic encephalopathy. The severity of cirrhosis was assessed using standard prognostic scores such as the Child-Turcotte-Pugh (CTP) score and model for end-stage liver disease (MELD).10,11 Acute-on-chronic liver failure (ACLF) was defined according to the European Association for the Study of Liver (EASL) chronic liver failure (CLIF) guidelines as the presence of a hepatic or extrahepatic insult in a patient with cirrhosis leading to organ failures and high short-term mortality.12 Cirrhosis and associated complications were managed as per the international consensus and guidelines13 with the only deviation being those presenting with bleeding were managed conservatively with splanchnic vasoconstrictors, restrictive transfusion, and prophylactic antibiotics and endoscopy only when the need arose in accordance with our previously published experience.7
For comparing the possible effects of viral variants on clinical outcome, the study duration was empirically divided into those who fell ill during the first wave (April 23, 2020 to March 14, 2021; n = 188) or second wave (March 15, 2021 to June 30, 2021; n = 33).14
All the relevant health care records of the included patients were reviewed, and the information was retrieved. The data relating to liver disease statuses, such as its cause, severity, and complications, were retrieved along with manifestations, management, and outcome of superadded COVID-19 infection.
Statistical analysis
Qualitative data were expressed as ratios and proportions. Quantitative data were expressed as median (interquartile range). Qualitative data were compared using the Chi-square test or Fisher’s Exact test, as appropriate, while quantitative data were compared using Mann–Whitney/Kruskal Wallis test. A multivariate analysis was done using the Cox-regression method. Variables with P < 0.10 were included in the multivariate analysis. A P-value of <0.05 was considered significant. Statistical calculations were made using the statistical package for social sciences (SPSS) version 20.0 (IBM, Chicago, IL, USA). MedCalc Statistical Software version 18.2.1 (MedCalc Software bvba, Ostend, Belgium) was used to generate Kaplan–Meier curves for 28-day survival.
Results
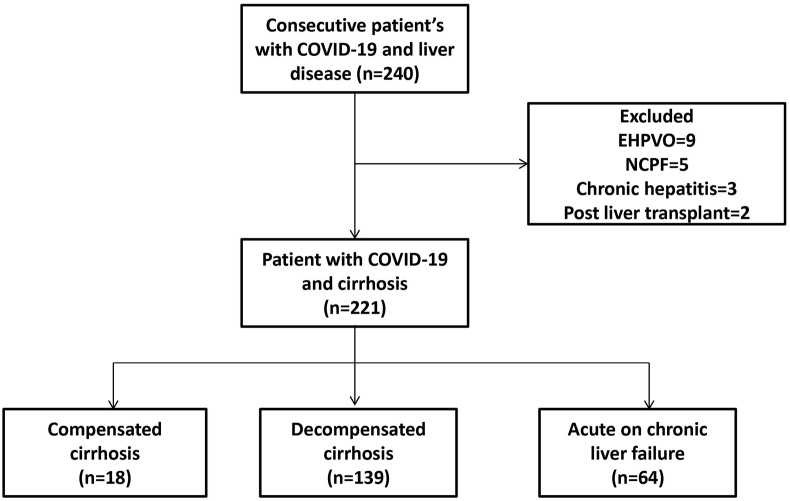
A total of 240 patients with liver disease were admitted to our COVID-19 care facility during the study period. We excluded 19 patients without chronic liver disease, including 9 with extrahepatic portal vein obstruction (EHPVO), 5 with noncirrhotic portal fibrosis (NCPF), 3 patients with chronic hepatitis and two postliver transplants (Figure 1). Of the 221 cirrhosis patients included in our study, at presentation, 18 (8.1%) had compensated cirrhosis, 139 (62.9%) had decompensated cirrhosis, and 64 (29.0%) had ACLF. Eight (3.6%) patients had hepatocellular carcinoma.
Figure 1.
Flowchart of patient inclusion and exclusion.
Comparison of Patients with Compensated, Decompensated Cirrhosis and ACLF
Patients with ACLF, when compared to compensated and decompensated cirrhosis, had higher bilirubin, total leucocyte count (TLC), international normalized ratio (INR), and prognostic scores, including CTP and MELD. The proportion of patients with severe COVID-19 disease was higher in ACLF cases than patients with compensated and decompensated cirrhosis (54.7% vs. 33.3 and 16.5%; P < 0.001).
Patients with alcohol-related liver disease (ALD) were more likely to present with ACLF (P = 0.002). There were no differences in age, gender, and comorbidities between the three groups (Table 1).
Table 1.
Comparison of Characteristics of Patients With Compensated Cirrhosis, Decompensated Cirrhosis, and Acute-on-Chronic Liver Disease.
| Characteristics | Overall cohort (n = 221) | Compensated cirrhosis (n = 18) | Decompensated cirrhosis (n = 139) | ACLF (n = 64) | P-value |
|---|---|---|---|---|---|
| Mean Age (years) | 46 (37–55) | 52 (32–59) | 47 (37–54) | 44 (37–55) | 0.503 |
| Sex, male n (%) | 171 (77.4%) | 11 (61.1%) | 108 (77.7%) | 52 (81.3%) | 0.194 |
| Diabetes | 48 (21.7%) | 4 (22.2%) | 33 (23.7%) | 11 (17.2%) | 0.574 |
| Etiology of cirrhosis | 0.014 | ||||
| Alcohol | 102 (46.2%) | 5 (27.8%) | 56 (40.3%) | 41 (64.1%) | |
| HBV | 24 (10.9%) | 0 | 15 (10.8%) | 9 (14.1%) | |
| HCV | 15 (6.8%) | 3 (16.7%) | 9 (6.5%) | 3 (4.7%) | |
| AIH | 12 (5.4%) | 1 (5.6%) | 9 (6.5%) | 2 (3.1%) | |
| NAFLD | 22 (10.0%) | 4 (22.2%) | 16 (11.5%) | 2 (3.1%) | |
| Cryptogenic | 36 (16.3%) | 3 (16.7%) | 27 (19.4%) | 6 (9.4%) | |
| Others | 10 (4.5%) | 2 (11.1%) | 7 (5.0%) | 1 (1.6%) | |
| Etiology Alcohol: Others | 102 (46.2%): 119 (53.8%) | 5 (27.8%): 13 (72.2%) | 56 (40.3%): 83 (59.7%) | 41 (64.1%): 23 (35.9%) | 0.002 |
| Hemoglobin | 8.4 (7.1–10.2) | 9.7 (7.4–11.1) | 8.5 (7.2–10.4) | 8.0 (7.0–9.9) | 0.204 |
| TLC | 6850 (4400–10800) | 5800 (2610–8250) | 6350 (3860–9650) | 9550 (6200–17427) | <0.001 |
| Platelet count | 72 (48–120) | 131 (54–209) | 71 (47–113) | 67 (49–99) | 0.161 |
| INR | 1.6 (1.3–2.3) | 1.3 (1.2–1.6) | 1.5 (1.3–1.9) | 2.5 (1.8–3.4) | <0.001 |
| Total bilirubin | 2.7 (1.2–5.9) | 1.6 (0.8–2.8) | 2.3 (1.1–4.1) | 10.1 (2.6–20.1) | <0.001 |
| Creatinine | 1.1 (0.7–1.9) | 0.8 (0.6–1.2) | 0.9 (0.7–1.3) | 2.4 (1.1–3.1) | <0.001 |
| AST | 54 (38–82) | 54 (40–74) | 46 (36–70) | 72 (54–142) | <0.001 |
| ALT | 37 (26–54) | 33 (27–53) | 34 (25–48) | 48 (29–68) | 0.003 |
| Alk P | 129 (92–148) | 130 (84–162) | 129 (97–144) | 116 (82–166) | 0.926 |
| Albumin | 2.6 (2.3–3.0) | 2.8 (2.5–3.3) | 2.8 (2.4–3.1) | 2.4 (2.0–2.8) | <0.001 |
| CTP | 9 (1–11) | 6.5 (5.0–8.2) | 8 (7–10) | 11 (10–13) | <0.001 |
| MELD | 19.9 (12.3–26.3) | 12.7 (9.0–22.4) | 16.7 (11.7–22.2) | 30.3 (23.2–37.4) | <0.001 |
| COVID severity | <0.001 | ||||
| Mild | 136 (61.5%) | 11 (61.1%) | 101 (72.7%) | 24 (37.5%) | |
| Moderate | 21 (9.5%) | 1 (5.6%) | 15 (10.8%) | 5 (7.8%) | |
| Severity | 64 (29.0%) | 6 (33.3%) | 23 (16.5%) | 35 (54.7%) | |
| Hospital stay | 8 (6–12) | 7 (5–13) | 8 (6–12) | 7 (5–12) | 0.312 |
| Died | 90 (40.7%) | 4 (22.2%) | 40 (28.8%) | 46 (71.9%) | <0.001 |
Note: all data are presented as median (IQR) unless specified. Albumin (G/dl), AST/ALT (iu/L), Alk P (iu/L), Bilirubin (mg/dl), creatinine (mg/dl), Hemoglobin: (g/dl), Platelets (x109/L), TLC (cells per Cmm).
Abbreviation: AIH, autoimmune hepatitis; ALT, alanine aminotransferase; Alk P, alkaline phosphatase; AST, aspartate aminotransferase; CTP, Child-Turcotte-Pugh; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; MELD, model for end-stage liver disease; NAFLD, nonalcoholic fatty liver disease; TLC, Total leukocyte count.
The median hospital stay was similar in all three subgroups, but as expected, patients with ACLF had higher in-hospital mortality.
Interaction of Severity of Liver Disease and Severity of COVID-19
Patients with moderate and severe COVID-19 had CTP Class C predominantly at presentation. Of the 136 patients with mild COVID-19, the CTP Class A, B, and C at presentation was seen in 29 (21.3%), 66 (48.5%), and 41 (30.1%), respectively. Of the 21 patients with moderate COVID-19, the CTP class A, B, and C at presentation was seen in 1 (4.8%), 5 (23.8%), and 15 (71.4%), respectively. Of the 64 patients with severe COVID-19, the CTP class A, B, and C at presentation was seen in 4 (6.3%), 13 (20.3%), and 47 (73.4%), respectively (P < 0.001).
COVID-19 Specific Therapy
Specific therapy for COVID-19 included HCQ in 55 (24.8%), ivermectin 30 (13.5%) and Methylprednisolone/dexamethasone in 23 (10.4%).
Predictors of Death
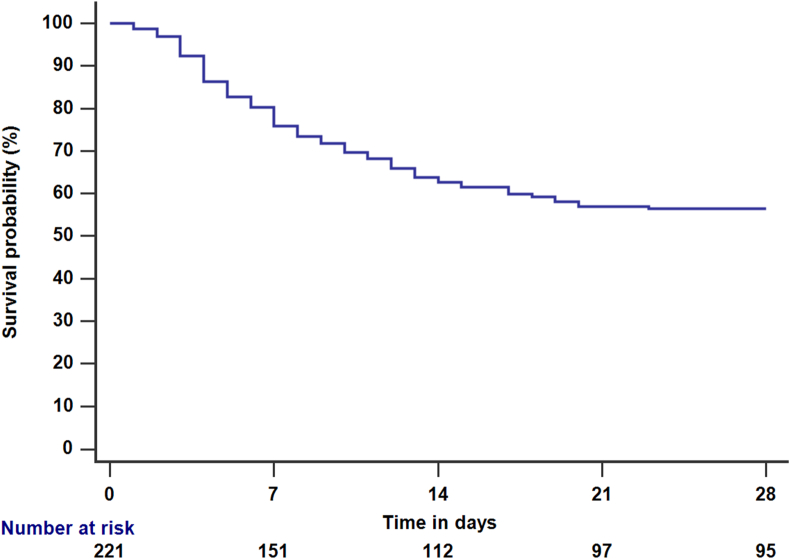
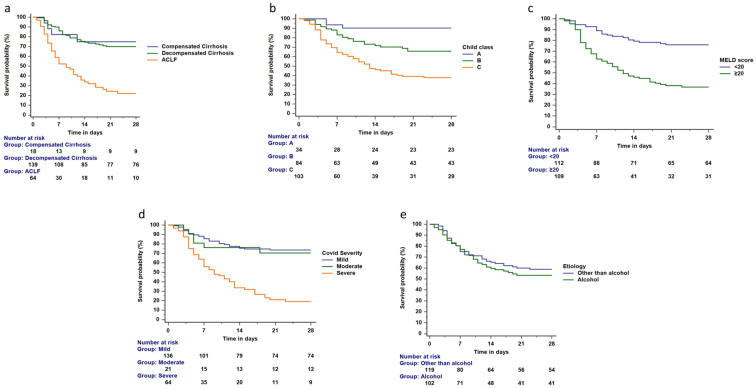
The overall in-hospital mortality was 90/221 (40.7%) (Figure 2). The mortality was highest in the ACLF subgroup, compared to compensated and decompensated cirrhosis (71.9% vs. 22.2% and 28.8%; P < 0.001) (Figure 3a).
Figure 2.
Survival probability among patients with cirrhosis and COVID-19.
Figure 3.
a-e. Survival probability among patients with cirrhosis and COVID-19. Figure 3a. The 28-day mortality among patients with ACLF was higher than decompensated and compensated cirrhosis (log-rank test, P < 0.001). Figure 3b. The 28-day mortality among patients with CTP-C was higher than CTP B and A class (log-rank test, P < 0.001). Figure 3c. The 28-day mortality among patients with MELD scores ≥20 than <20 (log-rank test, P < 0.001). Figure 3d. The 28-day mortality among patients with severe COVID-19 was higher than those with mild and moderate COVID-19 (log-rank test, P < 0.001). Figure 3e. The 28-day mortality among patients with alcohol and other etiologies was similar (log-rank test, P = 0.600).
On univariate Cox regression analysis, the predictors of mortality included the presence of diabetes, severe COVID-19, Child C, ACLF, higher total leucocyte count, INR, bilirubin, creatinine, MELD score, aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and low albumin (Table 2). Alcohol as an etiology was not a predictor of outcome.
Table 2.
Univariate Analysis of Predictors of Outcome in Patients With COVID-19 and Cirrhosis.
| Parameter | Alive (n = 131) | Died (n = 90) | Univariate HR | P-value |
|---|---|---|---|---|
| Mean Age (years) | 46 (37–54) | 46 (37–57) | 0.999 (0.982–1.016) | 0.896 |
| Sex, male n (%) | 106 (80.9%) | 65 (72.2%) | 0.745 (0.466–1.191) | 0.219 |
| Diabetes | 32 (24.4%) | 16 (17.8%) | 0.631 (0.366–1.086) | 0.097 |
| Etiology of cirrhosis | ||||
| Alcohol | 59 (45.0%) | 43 (47.8%) | ||
| HBV | 11 (8.4%) | 13 (14.4%) | ||
| HCV | 12 (9.2%) | 3 (3.3%) | ||
| AIH | 9 (6.9%) | 3 (3.3%) | ||
| NAFLD | 13 (9.9%) | 9 (10.0%) | ||
| Cryptogenic | 19 (14.5%) | 17 (18.9%) | ||
| Others | 8 (6.1%) | 2 (2.2%) | ||
| Etiology Alcohol: Others | 59 (45.0%): 72 (55.0%) | 43 (47.8%): 47 (52.2%) | 1.075 (0.709–1.630) | 0.734 |
| Hemoglobin | 8.5 (7.1–10.4) | 8.2 (7.1–10.0) | 0.942 (0.865–1.026) | 0.172 |
| TLC | 5950 (3675–8925) | 9400 (5935–17105) | 1.084 (1.058–1.111) | <0.001 |
| Platelet count | 70.5 (47.7–105.2) | 75 (48.5–126) | 1.003 (0.999–1.006) | 0.109 |
| INR | 1.5 (1.3–2.0) | 1.9 (1.6–2.7) | 1.415 (1.206–1.661) | <0.001 |
| Total bilirubin | 2.1 (0.9–4.1) | 4.4 (2.3–13.6) | 1.060 (1.036–1.084) | <0.001 |
| Creatinine | 0.9 (0.7–1.3) | 1.5 (0.8–2.9) | 1.231 (1.122–1.351) | <0.001 |
| AST | 46 (36–70) | 64 (46–114) | 1.000 (1.000–1.001) | 0.015 |
| ALT | 32 (26–46) | 43 (29–64) | 1.000 (1.000–1.001) | 0.014 |
| Alk P | 123 (90–138) | 132 (94–168) | 1.003 (1.001–1.005) | 0.004 |
| Albumin | 2.8 (2.4–3.1) | 2.5 (2.3–2.8) | 0.697 (0.478–1.015) | 0.059 |
| Child class | ||||
| A | 31 (23.7%) | 3 (3.3%) | 1 | |
| B | 58 (44.3%) | 26 (28.9%) | 2.633 (0.794–8.735) | 0.114 |
| C | 42 (32.1%) | 61 (67.8%) | 5.749 (1.801–18.356) | 0.003 |
| MELD | 15.6 (11.3–22.5) | 25.0 (19.4–35.1) | 1.068 (1.045–1.091) | <0.001 |
| Underlying liver disease | ||||
| Compensated cirrhosis (n = 18) | 14 (10.7%) | 4 (4.4%) | 1 | |
| Decompensated cirrhosis (n = 139) | 99 (75.6%) | 40 (44.4%) | 1.196 (0.427–3.349) | 0.733 |
| ACLF (n = 64) | 18 (13.7%) | 46 (51.1%) | 3.402 (1.224–9.456) | 0.019 |
| COVID severity | ||||
| Mild | 104 (79.4%) | 32 (35.6%) | 1 | |
| Moderate | 14 (10.7%) | 7 (7.8%) | 0.729 (0.303–1.754) | 0.481 |
| Severity | 13 (9.9%) | 51 (56.7%) | 3.151 (2.021–4.913) | <0.001 |
| Hospital stay | 8 (6–12) | 7 (5–12) | ||
Note: all data are presented as median (IQR) unless specified. Albumin (G/dl), AST/ALT (iu/L), Alk P (iu/L), Bilirubin (mg/dl), creatinine (mg/dl), Hemoglobin: (g/dl), Platelets (x109/L), TLC (cells per Cmm).
Abbreviations: AIH, autoimmune hepatitis; ALT, alanine aminotransferase; Alk P, alkaline phosphatase; AST, aspartate aminotransferase; CTP, Child-Turcotte-Pugh; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; MELD, model for end-stage liver disease; NAFLD, nonalcoholic fatty liver disease; TLC, Total leukocyte count.
On multivariate analysis, independent predictors of mortality included higher total leucocyte count, creatinine, MELD score, alkaline phosphatase, severe COVID-19, and ACLF. We created multiple models based on the above data to predict mortality in patients with cirrhosis and COVID-19 (Table 3).
Table 3.
Multivariate Analysis of Predictors of Outcomes in COVID-19 and Cirrhosis.
| Parameter | Model 1 HR (95% CI) | P-value | Model 2 HR (95% CI) | P-value | Model 3 HR (95% CI) | P-value | Model 4 | P-value |
|---|---|---|---|---|---|---|---|---|
| Diabetes | 0.930 (0.491–1.760) | 0.823 | 1.035 (0.555–1.930) | 0.915 | 0.859 (0.461–1.599) | 0.631 | 0.711 (0.410–1.232) | 0.224 |
| TLC | 1.054 (1.017–1.093) | 0.004 | 1.053 (1.020–1.088) | <0.001 | 1.052 (1.019–1.087) | 0.002 | ||
| INR | 1.141 (0.915–1.424) | 0.240 | ||||||
| Total bilirubin | 1.005 (0.974–1.037) | 0.772 | ||||||
| Creatinine | 1.184 (1.032–1.358) | 0.016 | 1.186 (1.037–1.357) | 0.013 | ||||
| AST | ||||||||
| ALT | 1.000 (0.999–1.002) | 0.642 | 1.000 (0.999–1.002) | 0.854 | 1.000 (0.999–1.002) | 0.768 | ||
| Alk P | 1.003 (1.001–1.005) | 0.010 | 1.003 (1.001–1.005) | 0.011 | 1.002 (1.000–1.004) | 0.031 | ||
| Albumin | 0.926 (0.597–1.438) | 0.733 | 0.935 (0.604–1.445) | 0.761 | ||||
| Child class | ||||||||
| A | 1 | |||||||
| B | 2.827 (0.660–12.110) | 0.161 | ||||||
| C | 3.884 (0.902–16.728) | 0.069 | ||||||
| MELD | 1.038 (1.010–1.066) | 0.007 | ||||||
| Underlying liver disease | ||||||||
| Compensated cirrhosis | 1 | |||||||
| Decompensated cirrhosis | 0.742 (0.309–1.783) | 0.505 | ||||||
| ACLF | 2.573 (1.617–4.095) | <0.001 | ||||||
| COVID severity | ||||||||
| Mild | 1 | 1 | 1 | 1 | ||||
| Moderate | 0.678 (0.276–1.663) | 0.396 | 0.631 (0.259–1.536) | 0.311 | 0.544 (0.215–1.373) | 0.197 | 0.742 (0.309–1.783) | 0.505 |
| severity | 2.109 (1.222–3.640) | 0.007 | 1.933 (1.155–3.236) | 0.012 | 1.954 (1.128–3.385) | 0.017 | 2.573 (1.617–4.095) | <0.001 |
Abbreviations: ACLF, acute-on-chronic liver failure; ALT, alanine aminotransferase; Alk P, alkaline phosphatase; AST, aspartate aminotransferase; INR, international normalized ratio; MELD, model for end-stage liver disease; TLC, Total leukocyte count.
Survival in Various Groups Based on the Severity of Presentation
The 28-day mortality was higher in patients with Child C than those with Child A and B (log-rank P < 0.001)- Figure 3b. The 28-day mortality was higher in patients with MELD score ≥20 (log-rank P < 0.001)- Figure 3c, and those with severe COVID-19 (log-rank P < 0.001)- Figure 3d. There was no difference in the 28-day outcome between alcohol and other etiologies (log-rank P = 0.600)- Figure 3e. There were differences in mortality across the various grades of ACLF. The mortality was highest in patients with grade 3 ACLF. Among the 64 patients with ACLF, grades 1, 2, and 3 ACLF were seen in 16, 18, and 30 patients, respectively. The mortality in grades 1, 2, and 3 ACLF were 9/16 (56.3%), 9/18 (50%), and 28/30 (93.3%), respectively (P = 0.001).
Cause of Death
The most common cause of death was multiorgan dysfunction syndrome (MODS) in 50 (55.6%) patients, followed by acute hypoxemic respiratory failure in 18 (20.0%), complications of cirrhosis in 13 (14.4%), sudden cardiac arrest in 6 (6.7%) and GI bleeding in 3 (3.3%).
Comparison Between the First and Second Waves
There were no differences in the two groups with respect to age, gender or comorbidities, laboratory parameters, and etiology of cirrhosis (Table 4). Clinical presentation with severe COVID-19 infection was more frequent in the second wave than the first wave of infections (51.5% vs. 25.0%; P = 0.006). However, both the subgroups had similar duration of hospital stay and mortality.
Table 4.
Comparison of Patients Admitted in the First Wave and Second Wave of COVID-19.
| Characteristics | First wave (n = 188) | Second wave (n = 33) | P-value |
|---|---|---|---|
| Mean Age (years) | 46 (37–55) | 47 (39–55) | 0.540 |
| Sex, male n (%) | 145 (77.1%) | 26 (78.8%) | 1.000 |
| Diabetes | 40 (21.3%) | 8 (24.2%) | 0.655 |
| Etiology of cirrhosis | 0.726 | ||
| Alcohol | 90 (47.9%) | 12 (36.4%) | |
| HBV | 20 (10.6%) | 4 (12.1%) | |
| HCV | 12 (6.4%) | 3 (9.1%) | |
| AIH | 11 (5.9%) | 1 (3.0%) | |
| NAFLD | 18 (9.6%) | 4 (12.1%) | |
| Cryptogenic | 30 (16.0%) | 6 (18.2%) | |
| Others | 7 (3.7%) | 3 (9.1%) | |
| Etiology Alcohol: Others | 90 (47.9%): 98 (52.1%) | 12 (36.4%): 21 (63.6%) | 0.259 |
| Hemoglobin | 8.5 (7.2–10.2) | 8.0 (6.7–11.3) | 0.572 |
| TLC | 7000 (4400–10725) | 6540 (3600–12700) | 0.656 |
| Platelet count | 70.5 (47.2–110) | 78 (52–131) | 0.537 |
| INR | 1.6 (1.4–2.3) | 1.6 (1.3–2.3) | 0.757 |
| Total bilirubin | 2.8 (1.2–5.9) | 2.5 (0.9–6.0) | 0.380 |
| Creatinine | 1.0 (0.7–1.9) | 1.2 (0.7–2.7) | 0.313 |
| AST | 54 (38–83) | 58 (37–86) | 0.756 |
| ALT | 37 (26–53) | 41 (28–55) | 0.466 |
| Alk P | 130 (98–162) | 96 (78–136) | 0.024 |
| Albumin | 2.6 (2.3–3.0) | 2.5 (2.3–2.8) | 0.249 |
| CTP | 9 (7–11) | 9 (7–12) | 0.575 |
| Child class | 0.520 | ||
| A | 27 (14.4%) | 7 (21.2%) | |
| B | 71 (37.8%) | 13 (39.4%) | |
| C | 90 (47.9%) | 13 (39.4%) | |
| MELD | 19.4 (12.5–26.2) | 22.4 (11.8–31.2) | 0.640 |
| COVID severity | 0.006 | ||
| Mild | 121 (64.4%) | 15 (45.5%) | |
| Moderate | 20 (10.6%) | 1 (3.0%) | |
| Severity | 47 (25.0%) | 17 (51.5%) | |
| Compensated Cirrhosis | 11 (5.9%) | 7 (21.2%) | 0.009 |
| Decompensated Cirrhosis | 123 (65.4%) | 16 (48.5%) | |
| ACLF | 54 (28.7%) | 10 (30.3%) | |
| Hospital stay | 8 (6–11) | 8 (5–16) | 0.464 |
| Died | 77 (41.0%) | 13 (39.4%) | 1.000 |
Note: all data are presented as median (IQR) unless specified. Albumin (G/dl), AST/ALT (iu/L), Alk P (iu/L), Bilirubin (mg/dl), creatinine (mg/dl), Hemoglobin: (g/dl), Platelets (x109/L), TLC (cells per Cmm).
Abbreviations: ACLF, acute-on-chronic liver failure; AIH, autoimmune hepatitis; ALT, alanine aminotransferase; Alk P, alkaline phosphatase; AST, aspartate aminotransferase; CTP, Child-Turcotte-Pugh; HBV, hepatitis B virus; HCV, hepatitis C virus; INR, international normalized ratio; MELD, model for end-stage liver disease; NAFLD, nonalcoholic fatty liver disease; TLC, total leukocyte count.
Discussion
The present study includes a large number of patients with cirrhosis and COVID-19 evaluated at a tertiary care facility and provides an insight into the outcomes of such patients and predictors of mortality. The in-hospital mortality of our patients was 40%, with higher mortality among those with advanced liver disease as suggested by Child class C, higher MELD score, and associated ACLF. Mortality was higher in those with elevated total leukocyte count, creatinine, and severe COVID-19 infection.
A multicenter international registry reported outcomes of 386 patients with cirrhosis and COVID-19. The overall mortality was 32%, which was highest in the CTP-C class. A multicenter study from Latin America that included 96 patients with cirrhosis reported an overall mortality of 47%.15 Our results are in accordance with these published results of outcomes of cirrhosis with COVID-19 infection. During the hospital stay, 20 AD patients developed ACLF. It is possible that SARS-CoV2 may have caused/accelerated the development of ACLF. Our data suggest that patients with higher MELD scores and those with ACLF have increased mortality. In addition, patients with severe COVID-19 have poor outcomes. As per guidelines, COVID-19 severity is defined based on oxygen saturation,8 and those with severe COVID-19 would likely have respiratory failure as defined by EASL-CLIF.16 An increasing number of organ failures are associated with high mortality in patients with cirrhosis.16,17 Our results suggest that, in cirrhosis patients with COVID-19, organ failures-respiratory and renal failure-independently predict the outcome.
In contrast to the results from the international registry, we did not find age or alcohol as etiology to be independent predictors of outcome. We have previously reported that alcohol as an acute precipitant of ACLF is associated with a worse outcome compared to other etiologies like the hepatitis E virus.18 Similar results were not observed in the present cohort of patients with cirrhosis and COVID-19. Our patients were young, with a median age of 46 years, in contrast to the international registry cohort, where the median age was 59.3
In this analysis, we included the subgroup of patients we previously presented as preliminary analysis in the initial part of the pandemic.5,7 Due to the small sample size at the time of the initial study, we could not assess the predictors of outcomes. Therefore, in this large sample study, which to our knowledge is the largest to date from India, we assessed the independent predictors of outcomes and created multiple models. In times of scarcity of available medical resources for the management of such sick patients, our results can help triage patients and identify those who will need urgent medical care. In addition, our results reconfirm the higher rates of mortality in patients with cirrhosis and COVID-19 infection. This subgroup of patients at greater risk needs to be vaccinated on priority, and we hope that our data will help create awareness among policymakers, as well as the lay public.
Though most patients succumbed to MODS (55.6%), we acknowledge that in patients with severe COVID-19 and ACLF, it is difficult to point out whether these organ failures resulted from hyperinflammatory responses due to COVID-19 or ACLF per se. Although our results differ in this regard from the international registry, in which the major cause of death was COVID related lung injury (71%), we hypothesize that this may be due to the fact that COVID-19 related lung injury and liver dysfunction are not mutually exclusive and are well known to augment each other. Respiratory failure is a well-known complication of cirrhosis and ACLF arising as a result of tense ascites, pleural effusion, portopulmonary hypertension, and hepatopulmonary syndrome; and the presence of cirrhosis is well known to impart poor prognosis in patients with pneumonia.19
We compared the clinical presentations of cirrhosis and COVID-19 patients admitted during the first and second waves of the pandemic in India. With the faster transmission and a higher risk of severe disease, VOC and VOI pose new challenges even as countries worldwide engage in the vaccination of their citizens.20 According to a recent report by Indian SARS- CoV2 Genomic Consortia (INSACOG), VOCs-particularly B.1.1.7 (alpha) and B.1.617.2 (delta) variants-constituted nearly 50% of the disease burden during the second wave of the pandemic in India.21 Although the exact distinction between the first and second waves is arbitrary, the number of COVID-19 cases started increasing significantly after March 15, 2021, which we took as the start of the second wave. Overall, we did not find any significant differences between the two cohorts, except that a greater proportion of patients in the second wave had severe COVID-19. Despite the severe presentation, there was no difference in the overall mortality. There were no differences in the MELD and CTP scores among the two cohorts, which may influence the outcomes in patients with cirrhosis.7,22
Our study has a few limitations. We did not collect data on markers of inflammation such as C-reactive protein, procalcitonin, erythrocyte sedimentation rate, and interleukin-6, which are associated with severe COVID-19. The drug therapies for the management of COVID-19 varied as per the evolving guidelines. None of the therapies used has been approved for the treatment of COVID-19. It is doubtful that the therapy given for COVID-19 affected the outcome. The proportion of patients admitted with advanced liver disease was higher than the compensated cirrhosis patients due to the tertiary center referral bias. We did not assess the VOC and VOI based on sequencing; instead, we analyzed the data according to time-frames for the first and second waves. The number of available vacant beds limited the hospital admissions during the second wave. We did not record the weight and height data as many patients were sick and exact weight could not be assessed. Although the previous data for outcomes of COVID-19 in patients with cirrhosis on immunosuppressants is reassuring, we did not assess outcomes in patients with autoimmune hepatitis (AIH) and post LT patients due to a small representation of these patients in our database.23,24 The data regarding the previous vaccination status of the patients and their immunization record was not collected, which could have helped in analyzing the efficacy of the vaccine in this subgroup of patients.
In conclusion, patients with cirrhosis and COVID-19 have high in-hospital mortality. The factors determining mortality include advanced liver disease, elevated total leukocyte count, and severity of COVID-19 infection. Although the second wave of the pandemic had more cases with severe COVID-19, the overall mortality was similar between the two waves.
Credit authorship contribution statement
Anshuman Elhence: data collection and writing. Manas Vaishnav: data collection and writing. Sagnik Biswas: data collection and writing. Abhinav Anand: data collection and writing. Deepak Gunjan: patient management. Saurabh Kedia: patient management. Soumya J Mahapatra: patient management. Baibaswata Nayak: draft writing. Sabreena Sheikh: data collection. Kapil Dev Soni: patient management. Anjan Trikha: patient management. Amit Goel: critical revision and inputs. Shalimar: concept, draft writing, patient management, and critical revision.
Conflicts of interest
The authors have none to declare.
FUNDING INFORMATION
The study was supported by a grant from the All India Institute of Medical Sciences, New Delhi. Grant number A-COVID-28; 02.06.2020. Mr Amar, Hemant and Dilshad for coordination and data maintenance.
Ethics clearance
Not applicable.
References
- 1.WHO Coronavirus (COVID-19) Dashboard. Accessed June 29, 2021. https://covid19.who.int.
- 2.Elhence A., Vaishnav M., Biswas S., Chauhan A., Anand A., Shalimar Coronavirus disease-2019 (COVID-19) and the liver. J Clin Transl Hepatol. 2021;9:247–255. doi: 10.14218/JCTH.2021.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marjot T., Moon A.M., Cook J.A., et al. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: an international registry study. J Hepatol. 2021;74:567–577. doi: 10.1016/j.jhep.2020.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mallet V, Beeker N, Bouam S, Sogni P, Pol S, Demosthenes research group. Prognosis of French COVID-19 patients with chronic liver disease: a national retrospective cohort study for 2020. J Hepatol. Published online May 13, 2021:S0168-827800329-9. 10.1016/j.jhep.2021.04.052. [DOI] [PMC free article] [PubMed]
- 5.Shalimar, Elhence A., Vaishnav M., et al. Poor outcomes in patients with cirrhosis and Corona virus disease-19. Indian J Gastroenterol. 2020;39:285–291. doi: 10.1007/s12664-020-01074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin J., Bao B., Khurram N.A., et al. Chronic liver disease not a significant comorbid condition for COVID-19. Sci Rep. 2021;11:11734. doi: 10.1038/s41598-021-91238-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shalimar, Vaishnav M., Elhence A., et al. Outcome of conservative therapy in coronavirus disease-2019 patients presenting with gastrointestinal bleeding. J Clin Exp Hepatol. 2021;11:327–333. doi: 10.1016/j.jceh.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.UpdatedDetailedClinicalManagementProtocolforCOVID19adultsdated24052021.pdf. 2021. https://www.mohfw.gov.in/pdf/UpdatedDetailedClinicalManagementProtocolforCOVID19adultsdated24052021.pdf Accessed June 29. [Google Scholar]
- 9.Clinical guidance for management OF adult COVID-19 patients | AIIMS covid information portal. 2021. https://covid.aiims.edu/clinical-guidance-for-management-of-adult-covid-19-patients/ Accessed June 29. [Google Scholar]
- 10.Tsoris A., Marlar C.A. StatPearls. StatPearls Publishing; 2021. Use of the child pugh score in liver disease.http://www.ncbi.nlm.nih.gov/books/NBK542308/ Accessed June 30, 2021. [PubMed] [Google Scholar]
- 11.Kamath P.S., Kim W.R., Advanced Liver Disease Study Group The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 12.Jalan R., Yurdaydin C., Bajaj J.S., et al. World Gastroenterology Organization Working Party. Toward an improved definition of acute-on-chronic liver failure. Gastroenterology. 2014;147:4–10. doi: 10.1053/j.gastro.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 13.Angeli P. EASL Clinical Practice Guidelines for the management of patients with decompensated cirrhosis. J Hepatol. 2018:55. doi: 10.1016/j.jhep.2018.03.024. Published online. [DOI] [PubMed] [Google Scholar]
- 14.Kar S.K., Ransing R., Arafat S.M.Y., Menon V. Second wave of COVID-19 pandemic in India: barriers to effective governmental response. EClinicalMedicine. 2021;36:100915. doi: 10.1016/j.eclinm.2021.100915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendizabal M., Ridruejo E., Piñero F., et al. Comparison of different prognostic scores for patients with cirrhosis hospitalized with SARS-CoV-2 infection. Ann Hepatol. 2021;25:100350. doi: 10.1016/j.aohep.2021.100350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jalan R., Saliba F., Pavesi M., et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J Hepatol. 2014;61:1038–1047. doi: 10.1016/j.jhep.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Shalimar, Kumar D., Vadiraja P.K., et al. Acute on chronic liver failure because of acute hepatic insults: etiologies, course, extrahepatic organ failure and predictors of mortality. J Gastroenterol Hepatol. 2016;31:856–864. doi: 10.1111/jgh.13213. [DOI] [PubMed] [Google Scholar]
- 18.Shalimar, kedia S., Mahapatra S.J., et al. Severity and outcome of acute-on-chronic liver failure is dependent on the etiology of acute hepatic insults: analysis of 368 patients. J Clin Gastroenterol. 2017;51:734–741. doi: 10.1097/MCG.0000000000000823. [DOI] [PubMed] [Google Scholar]
- 19.Fine M.J., Auble T.E., Yealy D.M., et al. A prediction rule to identify low-risk patients with community-acquired pneumonia. N Engl J Med. 1997;336:243–250. doi: 10.1056/NEJM199701233360402. [DOI] [PubMed] [Google Scholar]
- 20.Tracking SARS-CoV-2 variants. Accessed June 29, 2021. https://www.who.int/activities/tracking-SARS-CoV-2-variants.
- 21.INSACOG Dashboard. Accessed June 30, 2021. http://clingen.igib.res.in/insacog/.
- 22.Rout G., Sharma S., Gunjan D., et al. Development and validation of a novel model for outcomes in patients with cirrhosis and acute variceal bleeding. Dig Dis Sci. 2019;64:2327–2337. doi: 10.1007/s10620-019-05557-y. [DOI] [PubMed] [Google Scholar]
- 23.Marjot T, Buescher G, Sebode M, et al. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. Published online January 26, 2021. 10.1016/j.jhep.2021.01.021. [DOI] [PMC free article] [PubMed]
- 24.Colmenero J., Rodríguez-Perálvarez M., Salcedo M., et al. Epidemiological pattern, incidence, and outcomes of COVID-19 in liver transplant patients. J Hepatol. 2021;74:148–155. doi: 10.1016/j.jhep.2020.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]