Abstract
Objective
To evaluate the effectiveness of the Pfizer BNT162b2 vaccine against the SARS-Cov-2 Beta variant.
Study Design and Setting
Israel's mass vaccination program, using two doses of the Pfizer BNT162b2 vaccine, successfully curtailed the Alpha variant outbreak during winter 2020–2021, However, the virus may mutate and partially evade the immune system. To monitor this, sequencing of selected positive swab samples of interest was initiated. Comparing vaccinated with unvaccinated PCR positive persons, we estimated the odds ratio for a vaccinated case to have the Beta vs. the Alpha variant, using logistic regression, controlling for important confounders.
Results
There were 19 cases of Beta variant (3.2%) among those vaccinated more than 14 days before the positive sample and 79 (3.4%) among the unvaccinated. The estimated odds ratio was 1.26 (95% CI: 0.65–2.46). Assuming the effectiveness against the Alpha variant to be 95%, the estimated effectiveness against the Beta variant was 94% (95% CI: 88%–98%).
Conclusion
Despite concerns over the Beta variant, the BNT162b2 vaccine seemed to provide substantial immunity against both the Beta and the Alpha variants. From 14 days following the second vaccine dose, the effectiveness of BNT162b2 vaccine was at most marginally affected by the Beta variant.
Keywords: Alpha variant, COVID-19, Israel, Logistic regression, Odds ratio, Variants of concern, Beta variant
What is new.
-
•
Estimating the vaccine effectiveness against variants of concern is vital for planning and modifying current vaccination strategies.
-
•
We compared the proportion of the Beta variant (versus the Alpha variant) among SARS-CoV-2 cases fully vaccinated with the BNT162b2 vaccine with the proportion of the Beta variant among unvaccinated cases.
-
•
We estimated a modest, non-statistically significant reduction in vaccine effectiveness against the Beta variant compared to the Alpha variant.
-
•
Assuming that the vaccine effectiveness against the Alpha variant is 95%, the effectiveness against the Beta variant is estimated to be 94%, with 95% confidence limits between 88% and 98%.
1. Introduction
The impressive success of mass vaccination in curtailing the coronavirus 2 (SARS-CoV-2) by halting transmission has paved the road for returning to “pre-pandemic life.” However, several open questions challenge the triumph of controlling the pandemic by vaccination. One major concern is the ability of the virus to mutate and evolve; this potentially can cause SARS-CoV-2 to partially evade the immune system, reducing vaccine effectiveness (VE) in preventing disease and viral transmission. Determining VE against variants of concern (VOC) [1] is vital for planning and modifying vaccination strategies.
On December 19, 2020, Israel launched a massive COVID vaccination campaign based on the Pfizer BNT162b2 vaccine, and by end of May 2021, had administered over 10,500,000 doses, to approximately 5,400,000 individuals, more than 80% of the population over 16y receiving two doses. Both in clinical trials and real-world studies, the vaccine has proven highly effective in both averting infections and preventing severe disease and death [2], [3], [4], [5].
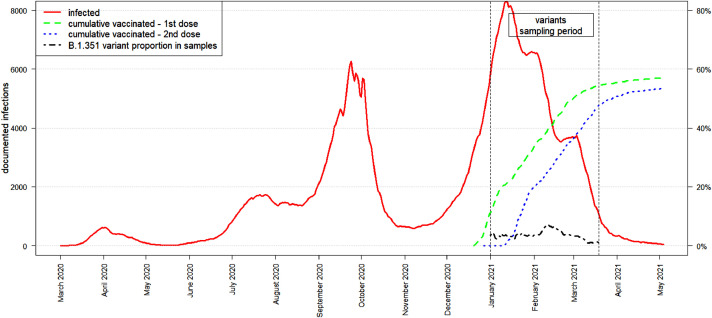
The Israeli vaccination campaign took place during the third and largest wave of the pandemic (see Fig. 1). During this wave, the Alpha variant became the dominant strain in Israel, reaching over 95% dominance [6]. Since detection of the Alpha variant in November 2020 in the United Kingdom, it spread rapidly and became the dominant strain in many countries. There is also evidence that it caused higher rates of morbidity and mortality [7]. Nevertheless, the BNT162b2 vaccine, which was developed based on the original Wuhan strain sequence, has been found very effective against the Alpha variant, both in blocking transmission and reducing morbidity and mortality following infection [2], [3], [4], [5]. The Beta variant, first documented in South Africa, is also considered a VOC mainly because in vitro experiments demonstrated its ability to overcome previous immunity to SARS-CoV2. Specifically, experimental work demonstrated significant decrease in neutralization capacity of the Beta variant [8]. However other research found that neutralizing antibodies remained sufficiently high against this variant [9]. Humoral protection measured by antibody responses and neutralization studies do not assess the role of cellular immunity mediated by T-cell responses. A recent study showed that cellular protection established following previous infection or vaccination remains high against both Alpha and Beta variants [10]. However, two real world studies have raised concern that the BNT162b2 vaccine has reduced effectiveness against the Beta variant. A study from Qatar showed that the effectiveness of BNT162b2 against the Beta variant was ∼75% compared to ∼90% against the Alpha variant [11]. A second study from Israel estimated that the odds ratio (OR), in a matched study of SARS-Cov-2 cases occurring in unvaccinated persons vs. persons who had received their second dose of vaccine at least one week previous to sample collection, was 1/8, implying considerably lower VE against the Beta variant [6]. Thus, our goal was to further quantify the risk of the Beta variant causing a significant breakthrough in a real-world environment.
Fig. 1.
Number of documented COVID19 infections in Israel from March 2020 until May 2021 indicated by the solid line. The dashed line and dotted line represent the percent of the population vaccinated with the first and second doses respectively. The dot-dashed line indicates the proportion of the Beta variant (vs. the Alpha variant) in the dataset. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2. Methods
2.1. Collection of samples
With the start of the vaccination campaign in Israel, the Central Virology Laboratory (CVL) of the Ministry of Health, initiated collection and sequencing of selected swab samples that had tested positive on polymerase chain reaction (PCR). Samples were selected for sequencing to i) monitor the circulating and imported variants in Israel, ii) characterize viral variants among cases occurring after vaccination and matching cases in unvaccinated persons iii) monitor local outbreaks, severe clinical cases and transmission among specific population groups, and iv) follow-up those coming into contact with persons infected with the Beta variant. Samples identified as being of interest were retrieved from the 48 laboratories that perform SARS-CoV-2 PCR tests in Israel, and sent to CVL, where they were assessed by whole genome sequencing.
2.2. Laboratory methods
RNA extracted from SARS-CoV-2 positive samples was sequenced with COVID-Seq library preparation on NovaSeq (Sp 300 cycles, Illumina, CA, USA). Resulting fastq files were processed, including quality filtering, mapping to the reference genome (NC_045512.2), construction of consensus fasta sequences, alignment to the reference genome and mutation analyses via a custom python-based pipeline. Specific variants were determined based on identification of relevant mutations for each variant (Alpha [doi: 10.1016/j.jinf.2020.12.024], Beta [doi: https://doi.org/10.1101/2020.12.21.20248640]) in the genome sequence and identification of the relevant lineage by Pangolin (doi:10.1038/s41564-020-0770-5). Additional details are provided in the Supplementary Information.
2.3. Creation of the database
Information on all cases sent for sequencing was entered in a database, containing: socio-demographical variables (e.g. age, city/town/village of residence, subpopulation - Arab, Ultra-Orthodox Jewish, other), date of collection of first positive sample, date of recovery, vaccination dates, symptoms and hospitalizations, and the infecting variant as determined by whole genome sequencing. All information was retrieved from the Israeli Ministry of Health's databases.
2.4. Statistical methods
Our analyses were restricted to vaccinated and unvaccinated cases that were positive either for the Alpha variant or the Beta variant, using whole genome sequencing. Vaccinated cases were defined as those where the first positive sample was taken at least 14 days after the second dose. Those with unknown dates of vaccination, or who received only one vaccine dose, or for whom the sample was taken between the first dose and second dose, were excluded. Cases based on samples taken before January 1, 2021 were also excluded, because no persons were fully vaccinated before that date. Those for whom the sample was taken less than 14 days after their second dose were excluded from the main analysis, but were included in a secondary analysis. Individuals who had acquired the infection outside Israel, those aged less than 16y (not eligible for vaccination) and individuals without information regarding their place of residence, were all excluded.
The main principle of the statistical analysis was to estimate the odds ratio, OR, for a vaccinated case to have the Beta variant, within vaccinated and unvaccinated persons who tested positive. One can show (see Supplementary Information) that:
where Vβ is the VE against the Beta variant, and Vα the VE against the Alpha variant. Thus, assuming Vα is estimated well from observational studies of VE conducted in Israel (e.g., 95%), we estimated the VE against the Beta variant from:
From this equation, when OR equals 1, then Vβ = Vα. Thus, a test of the hypothesis that OR = 1, also tests whether the vaccine is equally effective against each variant.
We estimated the odds ratio by logistic regression [12], with variant type as the dependent variable and vaccination status as the main explanatory variable. The following variables were entered as potential confounding covariates: city/town/village of residence (as a random effect), date of taking the swab sample (in four categories: 1–31 Jan, 2021; 1–14 Feb 2021; 15–28 Feb 2021; 1–31 Mar 2021), subpopulation (Arab, Jewish ultra-orthodox, other), and age group (16–44, 45–64, 65–79, ≥80 y). The analysis was implemented using the glmer procedure in the lme4 package of the R software [13]. This approach also provided a p-value for the test that OR = 1 and a 95% confidence interval for the OR.
A secondary analysis was conducted to examine the influence of time of infection following full vaccination, using the same methods as described above and comparing unvaccinated cases vs. cases where the sample from the vaccinated case was taken within the first 14 days after the second dose of vaccination.
Alternative analyses were conducted using matching to control for confounding variables, and are reported in the Supplementary Information. Unlike the main analyses presented here, such matching entails exclusion of approximately 60% of the cases. We regard their results as providing information supportive to the main analyses.
3. Results
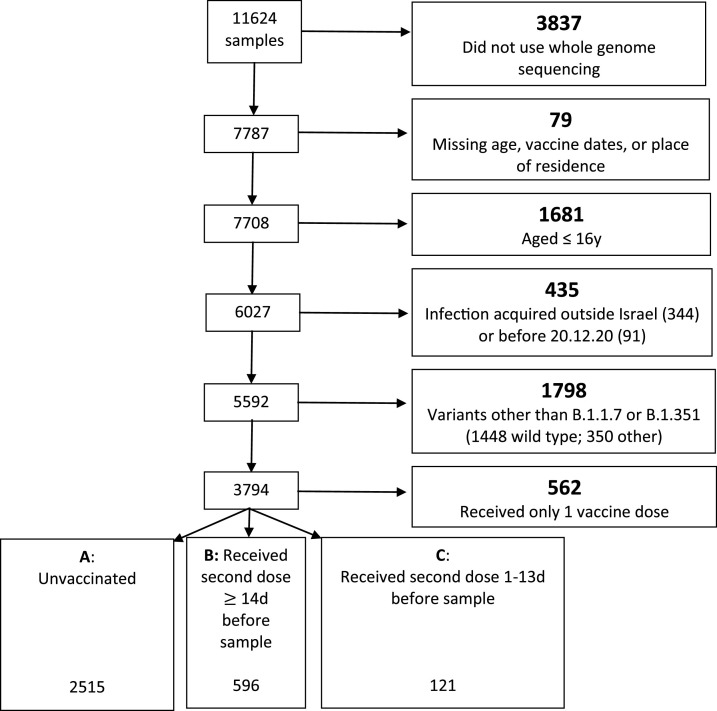
The database contained the sequencing results of 11,624 samples obtained from distinct individuals. After the exclusions described in the Methods section, 596 vaccinated and 2,333 unvaccinated cases were left eligible for analysis ( Fig. 2). Characteristics of these vaccinated and unvaccinated individuals are shown in Table 1 , and according to variant in Supplemental Table A1. The vaccinated groups were on average older and had a smaller proportion of ultra-orthodox Jews. Variant Beta appeared more in the Arab sector and less among Ultra-orthodox Jews. These differences emphasize the need to control for these potential confounders. The proportion of the Beta variant (vs. the Alpha) in the dataset over the period of our study is shown in the dot-dashed line in Fig. 1.
Fig. 2.
Flow chart showing number of exclusions (in bold) from the analysis, with the reasons for exclusion. Numbers included in the analyses are shown in the A, B, C panel at the bottom. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Table 1.
Characteristics of those included in the analysis
| Characteristic | Unvaccinated | 2nd vaccine dose ≥14d before | 2nd vaccine dose1–13 d before |
|---|---|---|---|
| No. persons | 2333 | 596 | 121 |
| Mean age (SD), y | 36.0 (16.5) | 55.8 (18.3) | 61.1 (19.8) |
| Gender (% male) | 43.5 | 48.4 | 49.6 |
| Arab (%) | 15.0 | 11.1 | 8.3 |
| Ultra-orthodox Jewish (%) | 23.0 | 3.4 | 13.2 |
| Rest of population (%) | 62.0 | 85.6 | 78.5 |
| No. villages, town or cities | 296 | 148 | 56 |
The distribution of variants (Alpha and Beta) by vaccination status is shown in Table 2 . There were 19 cases of Beta variant (3.2%) among those vaccinated more than 14d before the positive sample and 79 (3.4%) among the unvaccinated. The estimated OR (Table 3 ) was 1.26 (P = 0.50; 95% CI: 0.65–2.46). Note that the estimated OR was larger than one, even though the crude proportion of Beta was slightly lower (3.2% vs. 3.4%) among the vaccinated cases. This was due to the regression adjustment for the confounders age and subpopulation – younger age and ultra-orthodox Jews both had a negative association with the Beta variant. Using the estimated OR, assuming that the VE against the Alpha variant is 95% [2], [3], [4], [5], the estimated effectiveness against the Beta variant was estimated to be 94% (95% CI: 88%–98%). Results from the supportive analysis when matching was employed gave an estimated OR of 0.90 (95%CI: 0.40–2.01), and VE against Beta of 95% (95%CI: 90%–98%) (see Supplementary Tables A2-A5 for details).
Table 2.
Distribution of variants among vaccinated and unvaccinated cases
| Variant | Unvaccinated | 2nd vaccine dose ≥14d before | 2nd vaccine dose1–13 d before |
|---|---|---|---|
| Beta | 79 (3.4%) | 19 (3.2%) | 14 (11.6%) |
| Alpha | 2254 | 577 | 107 |
| Total | 2333 | 596 | 121 |
Table 3.
Estimated odds ratio and vaccine effectiveness against beta variant
| Estimated odds ratio | 95% CI | P value | Vaccine effectiveness against beta variant (95% CI)a |
|
|---|---|---|---|---|
| = 90% | = 95% | |||
| 1.26 | 0.65–2.46 | 0.50 | 87% (75–94) | 94% (88–98) |
Calculated from 1 - (Odds Ratio × ).
The same methods as above were applied to persons who received their second dose between 1 and 13 days before collection of the sample. In the 121 cases, there were 14 (11.1%) Beta variants; the estimated OR was 2.62 (P = 0.016, 95%CI = 1.19–5.76). A similar estimate was obtained from the supportive analysis using matching, but with a wider confidence interval (estimated OR = 2.12, 95%CI = 0.49–9.05).
4. Discussion
The success of the Israeli vaccination campaign against SARS-CoV-2 can be appreciated by the fact that, as of June 1st, Israel lifted all COVID emergency restrictions except the requirement to wear masks indoors and regulations governing international travel.
Variants can affect a vaccine's impact on the transmission of an infection by two factors. First, VOC may have a higher rate of transmission that will require a higher vaccination coverage or a greater VE to curtail the spread of infection [14]. In this regard, the Alpha variant is about 50% more infectious than the original Wuhan strain [7], leading to the subsequent global dominance of Alpha. This higher transmission rate requires higher vaccine coverage to allow relaxation of social distancing and other non-pharmaceutical interventions while still curtailing new outbreaks. The second factor is vaccine breakthrough by VOC. Initially, evidence regarding the evolutionary dynamics governed by the relatively slow mutation rate bolstered the hope that VOC would not endanger the effectiveness of vaccination. However, concerns over reduced effectiveness of the Pfizer BNT162b2 vaccine arose after laboratory experiments estimated a large drop in sensitivity of the Alpha variant to sera collected from vaccinated individuals and an even larger reduction in sensitivity to convalescent sera [8]. The Beta variant has caused further anxiety regarding the potential vaccine breakthrough due to the co-occurrence of supplementary mutations in the receptor-binding domain, which were proven to have a significantly increased resistance to vaccine-induced and convalescent sera [15]. Two real-world studies which examined the protection of the BNT162b2 vaccine against Alpha and Beta variants found a substantial reduction in VE against Beta compared to Alpha [6,11].
Results from a previous study in Israel based on a matched pairs case-case design pointed to a significant vaccine breakthrough of the Beta variant compared to the Alpha. The study had a modest sample size and period-length, and found eight Beta variant breakthroughs compared to a single case of an Alpha breakthrough in the subgroup of nine discordant matched pairs (i.e., those for which the vaccinated and unvaccinated cases differed in the variant-type) of vaccinated persons receiving two vaccine doses and unvaccinated persons [6]; all these cases were PCR confirmed in a short time window, on days 7–13 after receiving the second dose. No positive individuals infected by the Beta variant were found in vaccinated persons more than two weeks after the second dose even though about half of the vaccinated cases were from that later time frame. The investigators speculated that “this cohort may have been infected before the immunity from the boost was fully established, and it is thus possible that enhanced immunity from the boost, which develops over time, may more effectively prevent infection with the Beta variant.”
In our study, a much larger number of vaccinated and unvaccinated cases was included. In addition, our main analysis focused on vaccinated cases infected with Alpha or Beta variants occurring more than two weeks after their second dose and did not find a statistically significant reduction in protection of the vaccine. However, a sub-analysis, which examined vaccinated cases occurring only 1–13 days after the second dose found an increased proportion of Beta variant compared to unvaccinated cases, with an estimated OR of 2.62. Although both studies were conducted in Israel, there is no overlap of data between the two studies. Integrating the information and findings from both studies, supports the hypothesis that a higher level of immunity is required for protection against the Beta variant, thus inducing different levels of effectiveness against the Alpha and Beta variants during the first two weeks after the boost from the second dose [16]. The fact that the Beta variant, first diagnosed in Israel in January 2021 concomitantly with the start of the vaccination program, has not caused significant community transmission in Israel is further encouraging evidence supporting the ability of two doses of the BNT162b2 vaccine combined with high coverage to halt transmission.
A second study used a test-negative design to examine the effectiveness of the BNT162b2 vaccine in Qatar [11], and found that the VE was 89.5% (95% CI:[85.9–92.3]) against the Alpha variant but only 75% (95% CI:[70.5–78.9]) against the Beta variant, at least 14 days after the second dose. In contrast, several large studies from Israel have shown that the efficacy of the BNT162b2 vaccine is close to 95% against infection during a time when the Alpha variant was predominant [3], [4], [5]. The difference in VE to the Alpha variant between the Qatar and Israeli studies could be due to different methodologies used to estimate VE, or different social, cultural, racial or environmental conditions; the latter potentially can affect the transmission dynamics and thus alter VE. The above differences could also explain the difference in vaccine breakthrough of the Beta variant between the two countries. The current reduction in transmission seen in Qatar after the vaccination campaign gives further support to the ability of the BNT162b2 vaccine to control the spread of the Beta variant.
Our results are further supported by the combined adaptive humoral and cellular immune responses documented following BNT162b2 vaccination [17] and by the efficient in-vitro responses against both Alpha and Beta variants observed in blood samples derived from BNT162b2 vaccinated individuals [18], [19], [20].
Limitations of our study include the relatively low number of Beta variant cases, owing to its low prevalence in Israel, and also the fact that the sequencing was not done on a sample selected randomly from the total population of SARS-Cov2 positive cases in Israel. In particular, samples were selected for sequencing based on a variety of concerns, but partly to check on the variants among cases occurring after vaccination and partly to follow-up on those coming into contact with persons found to have the Beta variant. Thus, the database has over-representation of vaccinated cases, and also over-representation of the Beta variant among cases in Israel. In order to test the robustness of our results, we used two different statistical methods, unconditional logistic regression and conditional logistic regression with matching to estimate the OR. Both analyses revealed no statistically significant difference, and indicated that any reduction in VE against the Beta variant relative to Alpha is at most marginal with an OR not exceeding 2.5. Also, we have shown in computer simulations that this type of selection should not introduce bias when estimating the odds ratio of a vaccinated case having the Beta variant (see Supplemental Information for details). The criterion for validly using such a database for estimating the OR is that the selection of vaccinated cases or Beta cases should be independent of each other; when selecting a vaccinated case this should be without regard to whether the case was Alpha or Beta, and when selecting a case suspected to be Beta this should be without regard to whether the case was vaccinated or unvaccinated. As far as we can ascertain, such conditions applied to the selection of cases for sequencing.
Despite the concerns caused by the Beta variant, the BNT162b2 vaccine seems to provide substantial immunity against both that variant and the Alpha. Our results suggest that from 14 days following the second vaccine dose the effectiveness of BNT162b2 vaccine is at most marginally affected by the Beta variant. The next generation of vaccines might include modifications to better deal with different VOC; nevertheless, the current vaccines may still provide substantial immunity against both current and future VOC.
Footnotes
Conflict of interest: None declared.
Ethics approval: The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board of the Sheba Medical Center (7045-20-SMC). Patient consent was waived because the study used remains of clinical samples and the analysis used anonymous clinical data.
Authors’ contributions: LF, AH and OM were responsible for study design and writing the manuscript. LF, and RF analyzed the data. IH, NG, SA, NA, EM, OM and NZ were responsible for collecting data and for data management. LF, AH and OM did the literature survey. LF and RF developed the statistical analysis. All authors interpreted the data and reviewed the draft and final versions of the manuscript.
Funding: None.
Data availability: The data underlying this article cannot be shared publicly due to the privacy of individuals that participated in the study.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jclinepi.2021.10.011.
Appendix. Supplementary materials
References
- 1.CDC Centers for Disease Control and Prevention. (Accessed June 2021) Available at: https://www.cdc.gov/coronavirus/2019-ncov/variants/variant-info.html.
- 2.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. Epub 2020 Dec 10. PMID: 33301246; PMCID: PMC7745181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dagan N, Barda N, Kepten E, Miron O, Perchik S, Katz MA, et al. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384(15):1412–1423. doi: 10.1056/NEJMoa2101765. Epub 2021 Feb 24. PMID: 33626250; PMCID: PMC7944975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haas JE, Angulo FJ, McLaughlin JM, Anis E, Singer SR, Khan F, et al. Impact and effectiveness of mRNA BNT162b2 vaccine against SARS-CoV-2 infections and COVID-19 cases, hospitalizations, and deaths following a nationwide vaccination campaign in Israel: an observational study using national surveillance data. Lancet. 2021 doi: 10.1016/S0140-6736(21)00947-8. published online May 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg Y, Mandel M, Woodbridge Y, Fluss R, Novikov I, Yaari R, et al. Protection of previous SARS-CoV-2 infection is similar to that of BNT162b2 vaccine protection: a three-month nationwide experience from Israel. medRxiv. 2021 doi: 10.1093/aje/kwac060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kustin T, Harel N, Finkel U, Perchik S, Harari S, Tahor M, et al. Evidence for increased breakthrough rates of SARS-CoV-2 variants of concern in BNT162b2 mRNA vaccinated individuals. medRxiv. 2021 doi: 10.1038/s41591-021-01413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies NG, Jarvis CI, Working Group, Edmunds WJ, Jewell NP. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021;593:270–274. doi: 10.1038/s41586-021-03426-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Collier DA, De Marco A, Ferreira I, Meng B, Datir RP, Walls AC, et al. Sensitivity of SARS- CoV-2 B.1.1.7 to mRNA. vaccine- elicited antibodies. Nature. 2021 doi: 10.1038/s41586-021-03412-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Lin J, Xia H, Zhang X, Fontes-Garfias CR, Swanson KA, et al. Neutralizing activity of BNT162b2-elicited serum — preliminary report. N Engl J Med. 2021 doi: 10.1056/NEJMc2102017. Published online 2021 Mar 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tarke A, Sidney J, Methot N, Zhang Y, Dan JM, Goodwin B, et al. Negligible impact of SARS-CoV-2 variants on CD4 + and CD8 + T cell reactivity in COVID-19 exposed donors and vaccines. bioRxiv. 2021 doi: 10.1101/2021.02.27.433180. Preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abu-Raddad LJ, Chemaitelly H, Butt AA. Effectiveness of the BNT162b2 Covid-19 vaccine against the B. 1.1. 7 and B. 1.351 variants. N Engl J Med. 2021 doi: 10.1056/NEJMc2104974. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosmer D, Lemeshow S. Wiley; New York: 1989. Applied logistic regression. [Google Scholar]
- 13.Bates D, Maechler M, Bolker B, Walker S, Christensen RHB, Singmann H, et al. Linear mixed effects model using ’Eigen’ and S4. 2021. Available at: https://cran.r-project.org/web/packages/lme4/index.html. Accessed-Jun 22, 2021.
- 14.Keeling M, Rohani P. Princeton University Press; New Jersey: 2007. Modeling infectious diseases in humans and animals. [Google Scholar]
- 15.Cele S, Gazy I, Jackson L, Hwa SH, Tegally H, Lustig G, et al. Escape of SARS-CoV-2 501Y.V2 from neutralization by convalescent plasma. Nature. 2021;593:142–146. doi: 10.1038/s41586-021-03471-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bar On Y, Noor E, Gottlieb N, Sigal A, Milo R. The importance of time post-vaccination in determining the decrease in vaccine efficacy against SARS-CoV-2 variants of concern. Medrxiv. 2021 doi: 10.1101/2021.06.06.21258429. [DOI] [Google Scholar]
- 17.Sahin U, Muik A, Derhovanessian E, Vogler I, Kranz LM, Vormehr M, et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 18.Barros-Martins J, Hammerschmidt SI, Cossmann A, Odak I, Stankov MV, Ramos GM, et al. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat Med. 2021 doi: 10.1038/s41591-021-01449-9. (published online July 14.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lustig Y, Zuckerman N, Nemet I, Atari N, Kliker L, Regev-Yochay G, et al. Neutralising capacity against Delta (B.1.617.2) and other variants of concern following Comirnaty (BNT162b2, BioNTech/Pfizer) vaccination in health care workers, Israel. Eurosurveillance. 2021;26(26) doi: 10.2807/1560-7917.ES.2021.26.26.2100557. pii= [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lustig Y, Nemet I, Kliker L, Zuckerman N, Yishai R, Alroy-Preis S, et al. Neutralizing response against variants after SARS-CoV-2 infection and one dose of BNT162b2. N Engl J Med. 2021;384:2453–2454. doi: 10.1056/NEJMc2104036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.