Abstract
Objectives
Stress may augment somatic symptoms in central sensitivity syndromes (CSS) such as fibromyalgia, chronic fatigue syndrome, and irritable bowel syndrome. To test this hypothesis, we examined whether the association between COVID-19 stress and somatic symptom severity would be stronger in people with than without CSS and whether psychological flexibility would buffer the impact of this stress on symptom severity.
Methods
In a 2-sample, repeated cross-sectional design, we analysed questionnaire data from Dutch people with and without CSS, collected in two independent surveys: before the COVID-19 pandemic (2018; CSS: n = 194, non-CSS: n = 337) and at the peak of the pandemic (2020; CSS: n = 428, non-CSS: n = 1101). Somatic symptom severity, worry and stress due to the pandemic, and psychological flexibility were examined in regression analyses. Two stress operationalisations were analysed: stress levels during the peak of the pandemic, and a comparison of measurements in 2020 and 2018 (assuming higher stress levels in 2020).
Results
Higher worry and stress during the pandemic (standardized β = 0.14), the presence of a CSS (β = 0.40), and lower psychological flexibility (β = −0.33) were all (p < .0001) associated with more severe somatic symptoms, but the associations of each stress operationalisation with somatic symptoms was not particularly strong in people with CSS (β = −0.026, p = .27; β = −0.037, p = .22), and psychological flexibility (β = −0.025, p = .18; β = 0.076, p = .35) did not buffer this association.
Conclusions
Findings do not support the hypotheses that COVID-19 stress augments somatic symptoms, particularly in CSS, or that psychological flexibility buffers this impact. Rather, COVID-19-related stress appears to have an uncertain impact on somatic symptoms.
Keywords: Chronic fatigue syndrome, COVID-19, Fibromyalgia, Psychological flexibility, SARS-CoV-2 infection, Stress
1. Introduction
Conditions such as fibromyalgia, chronic fatigue syndrome (CFS), and irritable bowel syndrome (IBS) are described with various labels, such as medically unexplained symptoms [1], persistent physical symptoms [2], functional somatic syndromes [3], bodily distress syndromes [4], and central sensitivity syndromes (CSS) [5]. In this paper, we will use the label CSS. The CSS nosology is based on mutual associations among syndromes with overlapping clinical features, and central sensitization as a presumed common pathophysiological mechanism. In this nosology, the term “sensitivity” rather than “sensitization” is used, to emphasize that it is a biopsychological rather than neuropathophysiological phenomenon [5]. Several studies suggest that a sensitive brain may augment pain and other somatic symptoms in response to stress, such as in people with fibromyalgia and widespread pain [6,7], CFS [8] or IBS [9]. The COVID-19 pandemic offers a unique context to study the impact of stress on somatic symptom severity in people with CSS.
The outbreak of the SARS-CoV-2 virus and the measures taken by governments to prevent the spread of COVID-19 have impacted the entire global population [[10], [11], [12]]. Stress during the pandemic may be caused by worry of getting infected, changes in daily routines and caregiving, decreased opportunities for social and leisure activities, the illness or death of family members or friends, loss of work, and financial concerns (e.g., [[13], [14], [15], [16]]). Furthermore, for people with chronic conditions, somatic symptoms may also be enhanced by delayed medical evaluations [17,18], reduced access to health services, and disrupted treatment [7,19,20]. These psychological and health care challenges suggest that pain and other somatic symptoms in people with CSS may be more severe during than before the stressful peak months of the COVID-19 pandemic.
People differ in their ability to deal with stress. Symptom exacerbation may be less likely among people who are able to accept what cannot be changed and find other ways to pursue their goals in life. Psychological flexibility [21] refers to the ability to be open to adapt to new situational demands, while being committed to behaviour that is in line with one's own chosen values [21,22], and is considered key to adapt to challenging circumstances [23,24]. Longitudinal findings suggest that psychological flexibility impacts subsequent mental health, and not the reverse [21]. In people with chronic pain, CFS, or IBS, psychological flexibility is a resilience factor, protecting against and reducing the burden and severity of somatic symptoms [[25], [26], [27], [28], [29], [30]]. If psychological flexibility is also shown to buffer the impact of stress of the COVID-19 pandemic, then enhancing psychological flexibility by acceptance- and mindfulness-based education or interventions, for example, may be of value.
The aim of our study was to determine the impact of stress due to the COVID-19 pandemic on the severity of somatic symptoms in people with CSS, as compared to people without CSS, and as compared to an earlier pandemic-free period. Given that the stress-somatic symptom link may be especially strong in people with CSS, we hypothesized that people with CSS (vs. non-CSS) would show more severe somatic symptoms in response to stress of the pandemic, and that psychological flexibility would buffer the impact of stress on somatic symptoms.
2. Methods
2.1. Participants
Data from two separate online surveys in the general Dutch-speaking population were analysed. The first data collection was from November 2018 to May 2019 (year 2018). The second collection started on March 24, 2020, one day after the Dutch government introduced strict rules and regulations to prevent further spread of COVID-19 and ended on May 2, 2020 (year 2020). This latter period was the first serious pandemic peak period in the Netherlands in terms of number of hospitalizations, patients on the intensive care, and deaths due to COVID-19 (Dutch National Institute for Public Health and the Environment). In the online questionnaires, respondents could indicate with “Yes” or “No” on a list with a variety of diseases, if they had fibromyalgia, chronic fatigue syndrome (CFS), irritable bowel syndrome (IBS), somatoform disorder/somatic symptom disorder, chronic headache (not migraine), or chronic pain elsewhere in the body (not the head). We classified participants reporting any of these syndromes into a CSS group and all other participants into a non-CSS group. Note that someone with, for instance, rheumatoid arthritis or a cardiovascular disorder would be allocated to the CSS group if the person also had fibromyalgia, whereas a person with rheumatoid arthritis or a cardiovascular disorder without any of the CSS disorders was allocated to the non-CSS group. In both samples, all participants with complete assessments on worry, stress, pain, fatigue, and psychological flexibility were retained and analysed. Fig. S1 (supplementary material) shows the flowchart comprising the 2018 and 2020 samples.
2.2. Procedure
For each of the two samples, participants were recruited via social media (e.g., Facebook, Instagram, LinkedIn, local internet sites) and websites of associations including the Dutch national patient associations for fibromyalgia, CFS, and IBS. A hyperlink to the online survey (housed on a secure university website) was provided, where participants were informed about the study and could provide informed consent, after which they were allowed to participate. They were not compensated for their participation. Approval was given by the Ethics Committee of the Faculty of Social and Behavioural Sciences of Utrecht University, the Netherlands for the 2018 (FETC17–120) and 2020 (FETC20–190) data collections.
2.3. Instruments
2.3.1. Somatic symptom severity
In both the 2018 and 2020 samples, the severity of somatic symptoms was measured with the bodily pain and energy/fatigue scales of the Dutch version of the RAND 36-Item Short Form Health Survey (RAND SF-36) [31]. The bodily pain scale consists of two items assessing the level of bodily pain and its interference with daily activities during the past 4 weeks, on 6- and 5-point Likert scales, respectively. The vitality scale consists of two items assessing the level of fatigue and two items on the energy level during the past 4 weeks, all on 6-point Likert scales. After reversing scores, higher scores on the SF-36 reflect more severe pain and fatigue. We used the standardized mean deviation from the norm scores [31] of these pain and energy/fatigue scales as a measure of somatic symptom severity.
2.3.2. Psychological flexibility
Also in both samples, the Flexibility Index Test-60 (FIT-60) was used to measure psychological flexibility [32]. This questionnaire assesses six processes: acceptance, cognitive defusion, contact with the present moment, self as context, values, and committed action [21]. The 60-item questionnaire (10 items for each process) is based on a literature review of psychological flexibility and on four existing questionnaires. Participants rate the extent to which each item applies to them from 0 (‘totally disagree’) to 6 (‘totally agree’). The theoretical range of the total score is from 0 to 360, and higher scores indicate more flexibility. The initial psychometric analyses of the FIT-60 showed that the internal consistency was high, with a Cronbach's alpha of 0.95 for the total scale [32].
2.3.3. COVID-19 stress
The participants of the 2020 sample reported their current level of being worried about getting infected by the virus on a 4-point scale (1 = ‘not worried’, 2 = ‘a little worried’, 3 = ‘worried’, 4 = ‘very worried’) and their current stress compared to their normal stress level, on a 5-point scale (1 = ‘less stressed’, 2 = ‘a little less stressed’, 3 = ‘neither less nor more stressed’, 4 = ‘a little more stressed’ and 5 = ‘more stressed’). The z-scores of each participant on these two items were averaged; the resulting score was labelled “COVID-19 stress”.
2.4. Statistical analyses
The CSS and non-CSS groups were compared using parametric or nonparametric tests, where appropriate. Pearson correlations were calculated to examine associations between independent variables and the dependent variable somatic symptom severity.
Our main analyses consisted of two linear regression, the first in the sample of 2020 only (analysis 1), and the second in the samples of 2018 and 2020 (analysis 2). Two operationalisations of stress were used. In analysis 1, stress was operationalized as the mean of standardized self-reported worry and stress levels during the first peak of the COVID-19 pandemic (‘COVID-19 stress’). In analysis 2, it was assumed that participants during the 2020 pandemic were more stressed than the participants two years earlier. Thus, in this analysis, ‘year’ was the operationalization of stress, with scores in the sample of 2020 representing COVID-19 stress circumstances and scores in the sample of 2018 default circumstances.
In both analyses, linear regressions with bootstrapping (1000 samples) examined the associations of somatic symptom severity (dependent variable) with group (CSS vs. non-CSS), stress (‘COVID-19 stress’ in analysis 1 and ‘year’ in analysis 2), and psychological flexibility (total score of FIT-60) as independent variables (COVID-19 stress and the FIT-60 score were centred in analysis 1). Gender, age, education, and number of (comorbid) diseases were entered as covariates. The 2-way interactions (COVID-19 stress × group, COVID-19 stress × psychological flexibility, and group × psychological flexibility) were included to examine whether belonging to the CSS group was associated with higher somatic symptom levels in response to stress and whether higher levels of psychological flexibility protected against increased stress-related somatic symptom severity. To interpret significant interactions, regression lines for individuals with low (−1 SD) and high (+1 SD) scores on the two interacting variables were plotted [33]. The magnitude of the interaction was indicated with Cohen's d effect sizes, with values of 0.20, 0.50 and 0.80 representing small, medium, and large effects, respectively [34].
To examine, whether findings might be due to the diagnostic overlap of CSS with osteoarthritis or an inflammatory rheumatic disease, we performed ad hoc regression analyses excluding people with osteoarthritis or an inflammatory rheumatic disease. For all analyses, p-values <.05 were considered statistically significant, with all tests being 2-sided. Statistical analyses were done using IBM SPSS statistics version 25.0.
3. Results
3.1. Participants
Table 1 shows the characteristics of the CSS and non-CSS groups in the samples of 2018 (n = 531) and 2020 (n = 1529). The CSS and non-CSS groups did not differ in age in 2018 (F(1, 530) = 3.09, p = .079) and 2020 (F(1, 1528) = 1.71, p = .191). In 2020, groups did not differ on marital status (χ 2(2) = 1.37, p = .505), but in 2018, more people in the CSS than the non-CSS group were in a relationship (χ 2(2) = 8.18, p = .017). In both samples, the CSS groups included more women (2018: χ 2(1) = 36.46; 2020: χ 2(1) = 90.07, p < .0001), people with a lower education level (2018: χ 2(2) = 21.13; 2020: χ 2 (2) = 79.50, p < .0001) and a higher number of (comorbid) diseases (2018: F(1, 530) = 60.78; 2020: F(1,1528) = 94.86, p < .0001). More specifically, the prevalence of osteoarthritis (2018: χ 2(1) = 47.49; 2020: χ 2(1) = 49.06,p < .0001), skin diseases (2018: χ 2(1) = 15.52; 2020: χ 2(1) = 16.95, p < .0001), neurological diseases (2018: χ 2(1) = 11.64, p = .001; 2020: χ 2(1) = 19.32, p < .0001) and obesity (2018: χ 2(1) = 7.85, p = .005, 2020: χ 2(1) = 30.01, p < .0001) was higher in the CSS groups in both samples, whereas in the CSS group pulmonary disease was more prevalent in 2020 (χ 2(1) = 17.09, p < .0001) and cardiovascular disease in 2018 (χ 2(1) = 7.08, p = .008). Table S1 (supplementary material) shows the comorbid conditions for each CSS. Analyses comparing the overall samples from 2018 and 2020, showed a significant age difference (2018: M = 40.3; 2020: M = 47.7; F(1, 2059) = 54.90, p < .0001), whereas gender, education, and marital status did not significantly differ between the two overall samples. Ten out of 1529 people reported having COVID-19 during the first peak: 3 in the CSS group and 7 in the non-CSS group. This may be an underestimate because widespread testing was rare in the Netherlands at that time.
Table 1.
Characteristics of the groups with a central sensitivity syndrome (CSS) and without (non-CSS) before (2018) and during (2020) the first peak of the COVID-19 outbreak in the Netherlands.
| Year |
2018 n = 531 |
2020 n = 1529 |
|||
|---|---|---|---|---|---|
| Group | CSS n = 194 |
non-CSS n = 337 |
CSS n = 428 |
non-CSS n = 1101 |
All n = 2060 |
| Age (years) | |||||
| Mean (SD) | 45.2 (12.1) | 42.8 (16.2) | 48.3 (12.6) | 49.4 (15.2) | 47.7 (14.8) |
| Range | 18–69 | 18–87 | 20–80 | 18–91 | 18–91 |
| Gender, n (%) | |||||
| Women | 186 (95.9) | 254 (75.4) | 406 (94.9) | 802 (72.8) | 1648 (80.0) |
| Education level⁎, n (%) | |||||
| Low | 94 (48.5) | 97 (28.8) | 235 (54.9) | 336 (30.5) | 762 (37.0) |
| High | 98 (50.5) | 238 (70.6) | 190 (44.4) | 761 (69.1) | 1287 (62.5) |
| Missing | 2 (1.0) | 2 (0.6) | 3 (0.7) | 4 (0.4) | 11 (0.5) |
| Marital status, n (%) | |||||
| Single | 42 (21.6) | 112 (33.2) | 139 (32.5) | 325 (29.5) | 618 (30.0) |
| In a relation | 144 (74.2) | 211 (62.6) | 279 (65.2) | 752 (68.3) | 1386 (67.3) |
| Unknown | 8 (4.1) | 14 (4.2) | 10 (2.3) | 24 (2.2) | 56 (2.7) |
| Number of diseases other than a central sensitivity syndrome | |||||
| Mean (SD) | 1.46 (1.41) | 0.69 (0.87) | 1.43 (1.35) | 0.82 (0.98) | 0.98 (1.14) |
| Range | 0–7 | 0–5 | 0–6 | 0–6 | 0–7 |
| Type of other disease, n (%) | |||||
| Inflammatory rheumatic disease† | 27 (13.9) | 47 (13.9) | 52 (12.1) | 113 (10.3) | 239 (11.6) |
| Osteoarthritis | 51 (26.3) | 18 (5.3) | 87 (20.3) | 85 (7.7) | 241 (11.7) |
| Pulmonary | 20 (10.3) | 25 (7.4) | 90 (21.0) | 139 (12.6) | 274 (13.3) |
| Skin | 25 (12.9) | 12 (3.6) | 32 (7.5) | 31 (2.8) | 100 (4.9) |
| Cancer | 4 (2.1) | 4 (1.2) | 7 (4.2) | 29 (2.6) | 46 (2.2) |
| Cardiovascular | 26 (13.4) | 22 (6.5) | 79 (18.5) | 159 (14.4) | 286 (13.9) |
| Psychiatric | 47 (24.2) | 24 (7.1) | 91 (21.3) | 104 (9.4) | 266 (12.9) |
| Neurological | 33 (17.0) | 25 (7.4) | 50 (11.7) | 58 (5.3) | 166 (8.1) |
| Obesity | 26 (13.4) | 21 (6.2) | 71 (16.6) | 80 (7.3) | 198 (9.6) |
| One other non-listed disease | 24 (12.4) | 31 (9.2) | 48 (11.2) | 97 (8.8) | 200 (9.7) |
| Two or three other non-listed diseases | 0 (0.0) | 1 (0.3) | 1 (0.2) | 3 (0.3) | 5 (0.2) |
| Self-report measures, Mean (SD) | |||||
| Somatic symptom severity (RAND SF-36)‡ | 1.56 (0.78) | 0.28 (0.83) | 1.27 (0.79) | 0.08 (0.76) | 0.50 (0.97) |
| Psychological flexibility (FIT-60) § | 213.3 (48.3) | 233.6 (42.9) | 210.3 (53.3) | 236.8 (46.8) | 228.6 (49.1) |
Education level: low: lower general secondary education or lower; high: higher general secondary education or higher.
These participants reported to have a chronic rheumatic disease other than osteoarthritis or fibromyalgia.
This score is the mean of standardized deviation scores from the general adult population norm for pain and fatigue/vitality [31]. Scores were reversed: higher scores reflect more pain and fatigue.
This total score ranges from 0 to 360, with higher scores reflecting more flexibility.
3.2. Stress levels during the first peak of the COVID-19 outbreak in the Netherlands
During this peak in 2020, 80.5% of the people in the non-CSS group and 90.0% in the CSS group reported being “a little” to “very worried” about getting infected by the SARS-CoV-2 virus. When asked about their current stress level compared to their normal stress level, 61.7% of the non-CSS group reported being “a little more stressed” or “more stressed”, versus 71.5% of the CSS-group. The mean standardized worry and stress levels for the CSS and non-CSS groups were 0.21 (SD = 0.86) and − 0.08 (SD = 0.83), respectively. Fig. S2 (supplementary material) shows the distribution of worry and stress levels for the CSS and non-CSS groups. Overall, people indicated that they perceived themselves, on average, to be more worried and stressed than normal during the peak of the pandemic.
3.3. Analysis 1. COVID-19 stress and somatic symptom severity (sample of 2020)
This analysis involved the prediction of the severity of somatic symptoms from all other concurrent variables during the peak of the COVID-19 crisis in 2020 (see Table 2 ). The linear regression model was significant and explained 56% of the variance in somatic symptom severity (F = 199.62, p < .0001, Adjusted R2 = 0.56). Higher levels of COVID-19 stress were significantly associated with more severe somatic symptoms (r = 0.35, p < .0001), also when taking account of all other variables in the model (β = 0.14, p < .0001). All other variables were also significantly and independently associated with higher levels of somatic symptom severity; in order of strength (β): having a central sensitivity syndrome (β = 0.40, p < .0001), a lower level of psychological flexibility (β = −0.33, p < .0001), more (comorbid) diseases (β = 0.23, p < .0001), female gender (β = 0.05, p = .004), lower age (β = −0.05, p = .010), and lower education (β = −0.04, p = .026). The two-way interactions were not significant, indicating that the relationship between COVID-19 stress and symptom severity was statistically not different in people with versus without CSS or in people with lower versus higher psychological flexibility.
Table 2.
COVID-19 stress and other associations with somatic symptom severity in the sample of 2020 (n = 1522)
| r | b | SE | β | t | p | 95% CI | |
|---|---|---|---|---|---|---|---|
| Constant | .103 | .070 | 1.32 | .19 | −.047 to .214 | ||
| Demographics | |||||||
| Gender | .24‡ | .117 | .038 | .051 | 2.85 | .004 | .052 to .202 |
| Age | −.05 | −.003 | .001 | −.047 | −2.57 | .01 | −.005 to −.001 |
| Education⁎ | −.26‡ | −.079 | .038 | −.041 | −2.23 | .03 | −.152 to .001 |
| Number of (comorbid) diseases | .42‡ | .186 | .014 | .226 | 12.13 | <.0001 | .151 to .212 |
| Group† | .57‡ | .836 | .045 | .401 | 21.08 | <.0001 | .743 to .899 |
| COVID-19 stress | .35‡ | .157 | .026 | .142 | 6.48 | <.0001 | .109 to .201 |
| Psychological flexibility | −.53‡ | −.006 | .000 | −.330 | −14.17 | <.0001 | −.007 to −.005 |
| COVID-19 stress × Group | .25‡ | −.052 | .056 | −.026 | −1.10 | .27 | −.144 to .063 |
| COVID-19 stress × Psychological flexibility | −.15‡ | −.001 | .000 | −.025 | −1.34 | .18 | −.001 to .000 |
| Group × Psychological flexibility | −.37‡ | .001 | .001 | .032 | 1.31 | .19 | −.001 to .002 |
Pearson correlations (r) and results of the linear regression analysis with bootstrapping examining the association of somatic symptom severity (SF-36) with gender (0 = men, 1 = women), age, education level, number of (comorbid) diseases, group, COVID-19 stress and psychological flexibility and two-way interactions.
b, unstandardized regression coefficient, SE, Standard Error; β, standardized beta; t, t-test statistic; CI, confidence interval of unstandardized regression coefficient.
Education level: 0 = low: lower general secondary education or lower; 1 = high: higher general secondary education or higher.
Group: 0 = non-CSS; 1 = CSS: people with a central sensitivity syndrome.
Pearson correlation with somatic symptom severity was significant at the 0.01 level (2-tailed).
3.4. Analysis 2. Associations with somatic severity during the peak of the COVID-19 pandemic in 2020 compared to 2018
In this analysis, the severity of somatic symptoms was predicted from year and all other concurrent variables (see Table 3 ); more stress was assumed in 2020 during the peak of the COVID-19 pandemic than before (2018).
Table 3.
Year 2020 (during the first peak of the COVID-19) versus year 2018 and other associations with somatic symptom severity (n = 2049).
| r | b | SE | β | t | p | 95% CI | |
|---|---|---|---|---|---|---|---|
| Constant | 1.901 | .179 | 11.13 | <.0001 | 1.565 to 2.202 | ||
| Demographics | |||||||
| Gender | .26‡ | .165 | .037 | .069 | 4.48 | <.0001 | .095 to .242 |
| Age | −.02 | .000 | .001 | −.004 | −.27 | .79 | −.002 to .001 |
| Education⁎ | −.25‡ | −.081 | .034 | −.040 | −2.58 | .01 | −.167 to .011 |
| Number of (comorbid) diseases | .43‡ | .206 | .013 | .243 | 15.12 | <.0001 | .177 to .236 |
| Group† | .58‡ | .612 | .154 | .291 | 4.04 | <.0001 | .317 to .999 |
| Year | −.15‡ | −.354 | .181 | −.160 | −2.06 | .04 | −.772 to .079 |
| Psychological flexibility | −.51‡ | −.008 | .001 | −.395 | −11.37 | <.0001 | −.010 to −.006 |
| Year × Group | .41‡ | −.087 | .070 | −.037 | −1.24 | .22 | −.246 to .029 |
| Year × Psychological flexibility | −.31‡ | .001 | .001 | .076 | .94 | .35 | −.001 to .002 |
| Group × Psychological flexibility | .50‡ | .001 | .001 | .143 | 2.19 | .03 | .000 to .003 |
Pearson correlations (r) and results of the linear regression analysis with bootstrapping examining the association of somatic symptom severity (SF-36) with gender (0 = men, 1 = women), age, education level, number of (comorbid) diseases, group, year (0 = 2018, 1 = 2020), psychological flexibility and two-way interactions.
b, unstandardized regression coefficient; SE, Standard Error; β, standardized beta; t, t-test statistic; CI, confidence interval of unstandardized regression coefficient.
Education level: 0 = low: lower general secondary education or lower; 1 = high: higher general secondary education or higher.
Group: 0 = non-CSS; 1 = CSS: people with a central sensitivity syndrome.
Pearson correlation with somatic symptom severity was significant at the 0.01 level (2-tailed).
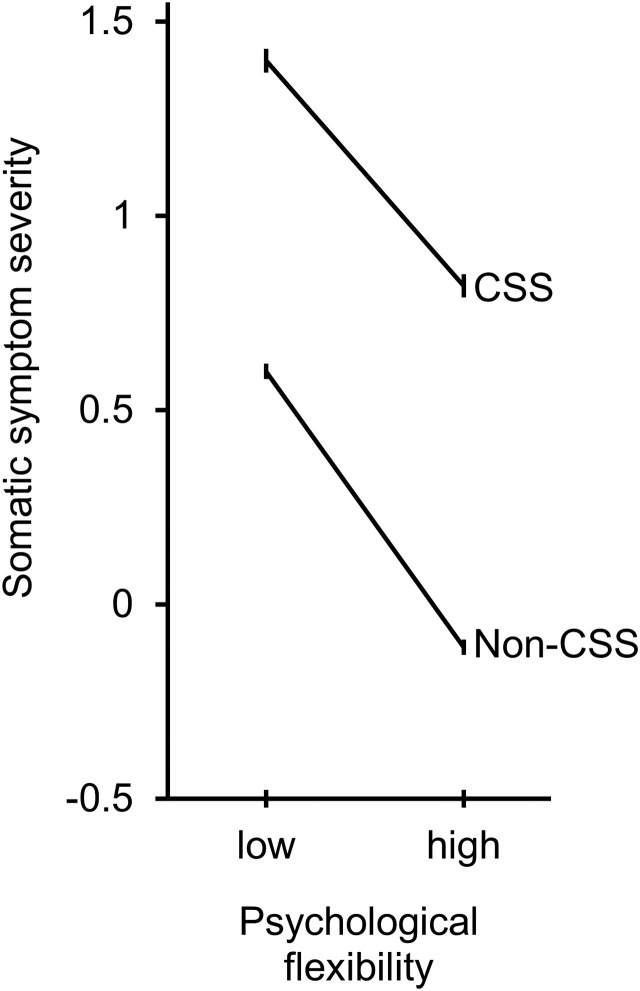
The bootstrap regression model was highly significant and explained 56% of the variance in somatic symptom severity (F = 259.33, p < .0001, Adjusted R 2 = 0.56). Contrary to our hypothesis, the 2018 sample reported more severe somatic symptoms (r = −0.15, p < .0001) than the 2020 sample, and this difference remained significant at including all other variables in the model (β = −0.16, p = .040). All other variables but age were significantly and independently associated with higher levels of somatic symptom severity; in order of strength (β): lower levels of psychological flexibility (β = −0.40, p < .0001), having a CSS (β = 0.29, p < .0001), more (comorbid) diseases (β = 0.24, p < .0001), female gender (β = 0.07, p < .0001), and lower education (β = −0.04, p = .010). Also contrary to expectation, the significant group × psychological flexibility interaction (β = 0.001, p = .029) indicated that in the non-CSS group, higher psychological flexibility buffered somatic symptom severity more, compared to in the CSS group (Fig. 1 ). However, as Fig. 1 shows the regression lines are nearly parallel, indicating a very small interaction; the effect size difference between the two groups for lower (−1 SD) flexibility was 1.01, while it was 1.18 for higher (+1 SD) psychological flexibility; a trivial difference of d = 0.17. All other interactions were not significant, indicating that at the first peak of the COVID-19 outbreak in the Netherlands, the level of symptom severity was not higher for people with CSS or with lower psychological flexibility.
Fig. 1.
Somatic symptom severity (standard deviation from the norm) on y-axis as a function of low (−1 SD) and high (+1 SD) psychological flexibility (x-axis) for having a central sensitivity syndrome disorder (CSS) or not having it (non-CSS), while controlling for gender, age, education level, number of diseases and year (2020 vs. 2018). The error bars show the standard error of measurement.
3.5. Ad hoc analyses
The two hypotheses of our study were also rejected in ad hoc analyses excluding people with osteoarthritis or an inflammatory rheumatic disease; none of the interactions were significant.
4. Discussion
During the first peak of the COVID-19 pandemic in the Netherlands, people perceived themselves to be, on average, more stressed than normal, and these stress levels were associated with more severe somatic symptoms. In contrast, based on another operationalization of stress—comparing the peak period of the pandemic to a previous year—there was no link between stress and more severe somatic symptoms. Also, both moderator hypotheses were rejected: the link between stress and somatic symptom severity was not stronger in people with CSS than those without CSS, and psychological flexibility did not act as a buffer against an increase of somatic symptoms severity in response to stress.
Our cross-sectional analysis during the pandemic peak showed an association between self-reported COVID-19 stress levels and self-reported somatic symptom severity, which is consistent with the larger literature showing correlations between self-reported scores reflecting negative experiences. A 10-day online survey, of people with fibromyalgia during the pandemic, found such an association between intra-individual levels of anxiety and chronic pain [35]. Such concurrent inter-individual and intra-individual associations may reflect mutual influences on a negative affect dimension instead of a specific somatic symptom reaction to stress (e.g., [36]). Guided by the hypothesis that a sensitized brain may augment somatic symptoms in response to stress in people with CSS [[6], [7], [8], [9]], we expected a stronger correlation between COVID-stress and somatic symptoms in people with CSS, compared to people without CSS. Our data did not support this hypothesis. A study that was conducted in parallel to our study showed that longitudinal assessments of pain symptoms measured pre- and post-lockdown did not change significantly on average [37]. Our results showed even a small but statistically significant lower level of symptom severity in the pandemic year (2020), compared to that in the pre-pandemic year (2018). Thus, overall, there is no indication of a COVID-19 stress-somatic symptom link, nor that such a link is stronger in people with CSS.
Several previous studies have examined the effects on people with CSS before and after a major environmental stressor. Pain in people with fibromyalgia was assessed before and after the September 11 attacks [38,39]. Both studies found no increase in symptoms from before to after the attack. Another two studies did not find lower levels of mental well-being during the COVID-19 pandemic in patients with inflammatory rheumatic diseases [40] or patients with systemic lupus erythematosus [41], although both groups were considered at increased risk for acquiring COVID-19 infection and for a more severe course and outcome of this infection. Several other studies compared mental well-being during the pandemic, with data collected before the pandemic. One study in the UK observed a higher prevalence of depressive-, anxiety-, and insomnia symptoms during the pandemic as compared to general population norms [11], whereas two other studies in the Netherlands reported that mental health remained stable as compared to pre-pandemic measurements from one year earlier [42,43]. A prospective study in people with systemic sclerosis from four countries showed that levels of anxiety symptoms increased during the COVID-19 pandemic, whereas the change in depression symptoms was negligible [44]. Together, these studies suggest that somatic symptoms do not reliably increase in response to major environmental stressors. Regarding mental health, the results of all but one study are in agreement by showing that, although increased levels of self-reported worry, anxiety and stress were present during the first peak of the COVID-19 pandemic, there was no clear increase of depressive mood.
There is another possible explanation for the lack of change in somatic symptom severity during the pandemic. The pandemic forced people to focus on external stressors and behaviour changes. This could have had a positive impact in some people with CSS, shifting their focus from internal somatosensory processes and psychological conflicts to environmental issues, which may have positively modulated their pain perception [45]. It is also possible that the impact of the COVID-19 pandemic on people with CSS is, on average, weaker than assumed. Some persons with CSS may have experienced a positive mental impact, for instance, because they felt less pressure from work, more social connectedness, or more recognition for their symptoms and situation during the pandemic.
Studies during the COVID-19 pandemic consistently show that higher scores on psychological flexibility or related constructs such as resilience are associated with mental well-being [14,[46], [47], [48], [49], [50], [51]]. We also found that higher psychological flexibility was associated with less severe somatic symptoms. One study observed that positive personality traits (i.e., optimism, mindfulness, and resilience) served as protective factors in the association between fear of the virus and mental distress [52]. In line with the authors of this study, we hypothesized that psychological flexibility would protect against an increase of somatic symptom severity due to COVID-19 stress, particularly in people with CSS. Our findings do not support this specific hypothesis, although one analysis indicated that psychological flexibility might buffer against somatic symptom severity in people without CSS. Thus, although in both groups higher levels of psychological flexibility were strongly related to lower symptom severity, we did not find evidence that this buffer is particularly strong in people with higher COVID-19 stress or in people with CSS. One possible explanation could be that there was little effect to buffer, because COVID-19 stress did not increase this burden in people with CSS, maybe also because they were already experienced in coping with multiple adversities of life.
A strength of the current study is the time frame in which data were collected. People participated during the first two peak months (March and April of 2020) of the virus outbreak in the Netherlands, when strict safety measurements to limit the spread of the virus were in place, and during which many people got infected and died, and a lot of uncertainty existed on the development of the virus outbreak. Our sample size was large enough to have small margins of error and quite evenly distributed on age and various regions in the Netherlands. However, somatic symptom severity as measured with the RAND SF-36 may be less sensitive to stress as it referred to the past 4 weeks. Another limitation is that CSS conditions were not confirmed by clinical assessment, which may have underestimated CSS in, for instance, rheumatic diseases [53]. A questionnaire for assessing central sensitivity [54,55] would have given insight into the perceived general disability and physical symptoms, central sensitivity features, urological and dermatological problems and emotional distress of our CSS group as compared to the non-CSS group. Our samples were convenience rather than representative, and, importantly, the samples at the two time points were different; obtaining data from the same people at similar periods in the year, rather than from two separate samples, would have yielded a more valid test of intra-individual changes in somatic symptoms between the two sample periods. Results showed an overrepresentation of highly educated women, especially in the non-CSS group, and an association of lower education level with more severe somatic symptoms. Although analyses were adjusted for relevant covariates, including education level and number of comorbid diseases, other uncontrolled variables may be relevant. Finally, our study only targeted the first peak period of the pandemic in the Netherlands, so stress was measured in the acute phase, rather than after a more prolonged experience of stress. A third data collection would give more information about the long-term stress effects of the pandemic.
To our knowledge, this is the only study that has examined the impact of the peak of the COVID-19 crisis on somatic symptom severity in people with CSS. We hypothesized that stress might augment somatic symptoms in people with CSS, but we did not find evidence for this hypothesis; nor did we find a buffering effect of psychological flexibility. Overall, our results suggest that the impact of the COVID-19 pandemic on somatic symptoms in people with CSS is uncertain.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of Competing Interest
The authors have no competing interests to report.
Acknowledgment
The authors thank all participants of the study and all associations that helped in recruiting participants.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychores.2021.110655.
Appendix A. Supplementary data
Supplementary material
References
- 1.Nimnuan C., Hotopf M., Wessely S. Medically unexplained symptoms: an epidemiological study in seven specialities. J. Psychosom. Res. 2001;51:361–367. doi: 10.1016/s0022-3999(01)00223-9. (S0022–3999(01)00223–9 pii) [DOI] [PubMed] [Google Scholar]
- 2.Henningsen P., Gündel H., Kop W.J., Löwe B., Martin A., Rief W., et al. Persistent physical symptoms as perceptual dysregulation: A neuropsychobehavioral model and its clinical implications. Psychosom. Med. 2018;80:422–431. doi: 10.1097/PSY.0000000000000588. [DOI] [PubMed] [Google Scholar]
- 3.Barsky A.J., Borus J.F. Functional somatic syndromes. Ann. Intern. Med. 1999;130:910–921. doi: 10.7326/0003-4819-130-11-199906010-00016. (199906010–00007 pii) [DOI] [PubMed] [Google Scholar]
- 4.Budtz-Lilly A., Schröder A., Rask M.T., Fink P., Vestergaard M., Rosendal M. Bodily distress syndrome: A new diagnosis for functional disorders in primary care? BMC Fam. Pract. 2015;16:180–188. doi: 10.1186/s12875-015-0393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yunus M.B. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin. Arthritis Rheum. 2008;37:339–352. doi: 10.1016/j.semarthrit.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Sluka K.A., Clauw D.J. Neurobiology of fibromyalgia and chronic widespread pain. Neuroscience. 2016;338:114–129. doi: 10.1016/j.neuroscience.2016.06.006. (S0306–4522(16)30236–6 pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serrano-Ibáñez E.R., Esteve R., Ramírez-Maestre C., Ruiz-Párraga G.T., López-Martínez A.E. Chronic pain in the time of COVID-19: Stress aftermath and central sensitization. Br. J. Health Psychol. 2021;26:544–552. doi: 10.1111/bjhp.12483. [DOI] [PubMed] [Google Scholar]
- 8.Meeus M., Nijs J. Central sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clin. Rheumatol. 2007;26:465–473. doi: 10.1007/s10067-006-0433-9. (433 [pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moloney R.D., Johnson A.C., O’Mahony S.M., Dinan T.G., Greenwood-Van Meerveld B., Cryan J.F. Stress and the microbiota-gut-brain axis in visceral pain: Relevance to irritable bowel syndrome. CNS Neurosci Ther. 2016;22:102–117. doi: 10.1111/cns.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torales J., O’Higgins M., Castaldelli-Maia J., Ventriglio A. The outbreak of COVID-19 coronavirus and its impact on global mental health. Int J Soc Psychiatry. 2020;66:317–320. doi: 10.1177/0020764020915212. [DOI] [PubMed] [Google Scholar]
- 11.Pieh C., Budimir S., Delgadillo J., Barkham M., Fontaine J.R.J., Probst T. Mental health during COVID-19 lockdown in the United Kingdom. Psychosom. Med. 2021;83:328–337. doi: 10.1097/PSY.0000000000000871. [DOI] [PubMed] [Google Scholar]
- 12.Holmes E.A., O’Connor R.C., Perry V.H., Tracey I., Wessely S., Arseneault L., et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7:547–560. doi: 10.1016/S2215-0366(20)30168-1. (S2215–0366(20)30168–1 pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Restubog S.L.D., Ocampo A.C.G., Wang L. Taking control amidst the chaos: Emotion regulation during the COVID-19 pandemic. J. Vocat. Behav. 2020;119:103440. doi: 10.1016/j.jvb.2020.103440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barzilay R., Moore T.M., Greenberg D.M., DiDomenico G.E., Brown L.A., White L.K., et al. Resilience, COVID-19-related stress, anxiety and depression during the pandemic in a large population enriched for healthcare providers. Transl. Psychiatry. 2020;10:291–294. doi: 10.1038/s41398-020-00982-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks S.K., Webster R.K., Smith L.E., Woodland L., Wessely S., Greenberg N., et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395:912–920. doi: 10.1016/S0140-6736(20)30460-8. (S0140–6736(20)30460–8 pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park C.L., Russell B.S., Fendrich M., Finkelstein-Fox L., Hutchison M., Becker J. Americans’ COVID-19 stress, coping, and adherence to CDC guidelines. J. Gen. Intern. Med. 2020;35:2296–2303. doi: 10.1007/s11606-020-05898-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choinière M., Dion D., Peng P., Banner R., Barton P.M., Boulanger A., et al. The Canadian STOP-PAIN project - Part 1: Who are the patients on the waitlists of multidisciplinary pain treatment facilities? Can. J. Anaesth. 2010;57:539–548. doi: 10.1007/s12630-010-9305-5. [DOI] [PubMed] [Google Scholar]
- 18.Lynch M.E., Campbell F., Clark A.J., Dunbar M.J., Goldstein D., Peng P., et al. A systematic review of the effect of waiting for treatment for chronic pain. Pain. 2008;136:97–116. doi: 10.1016/j.pain.2007.06.018. (S0304–3959(07)00344–2 pii) [DOI] [PubMed] [Google Scholar]
- 19.Eccleston C., Blyth F.M., Dear B.F., Fisher E.A., Keefe F.J., Lynch M.E., et al. Managing patients with chronic pain during the COVID-19 outbreak: considerations for the rapid introduction of remotely supported (eHealth) pain management services. Pain. 2020;161:889–893. doi: 10.1097/j.pain.0000000000001885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shanthanna H., Strand N.H., Provenzano D.A., Lobo C.A., Eldabe S., Bhatia A., et al. Caring for patients with pain during the COVID-19 pandemic: consensus recommendations from an international expert panel. Anaesthesia. 2020;75:935–944. doi: 10.1111/anae.15076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes S.C., Luoma J.B., Bond F.W., Masuda A., Lillis J. Acceptance and commitment therapy: model, processes and outcomes. Behav. Res. Ther. 2006;44:1–25. doi: 10.1016/j.brat.2005.06.006. (S0005–7967(05)00214–7 pii) [DOI] [PubMed] [Google Scholar]
- 22.Kashdan T.B., Rottenberg J. Psychological flexibility as a fundamental aspect of health. Clin. Psychol. Rev. 2010;30:865–878. doi: 10.1016/j.cpr.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayes S.C., Strosahl K.D., Wilson K.G. Guilford Press; New York City: 2012. Acceptance and Commitment Therapy: The Process and Practice of Mindful Change. [Google Scholar]
- 24.Presti G., McHugh L., Gloster A., Karekla M., Hayes S.C. The dynamics of fear at the time of COVID-19: A contextual behavioral science perspective. Clin. Neuropsychiatry. 2020;17:65–71. doi: 10.36131/CN20200206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gloster A.T., Meyer A.H., Lieb R. Psychological flexibility as a malleable public health target: Evidence from a representative sample. J. Contextual Behav. Sci. 2017;6:166–171. doi: 10.1016/j.jcbs.2017.02.003. [DOI] [Google Scholar]
- 26.Leonidou C., Panayiotou G., Bati A., Karekla M. Coping with psychosomatic symptoms: The buffering role of psychological flexibility and impact on quality of life. J. Health Psychol. 2019;24:175–187. doi: 10.1177/1359105316666657. [DOI] [PubMed] [Google Scholar]
- 27.Merkes M. Mindfulness-based stress reduction for people with chronic diseases. Aust J Prim Health. 2010;16:200–210. doi: 10.1071/PY09063. [DOI] [PubMed] [Google Scholar]
- 28.Densham S., Williams D., Johnson A., Turner-Cobb J. Enhanced psychological flexibility and improved quality of life in chronic fatigue syndrome/myalgic encephalomyelitis. J. Psychosom. Res. 2016;88:42–47. doi: 10.1016/j.jpsychores.2016.07.009. (S0022–3999(16)30350–6 pii) [DOI] [PubMed] [Google Scholar]
- 29.Naliboff B.D., Smith S.R., Serpa J.G., Laird K.T., Stains J., Connolly L.S., et al. Mindfulness-based stress reduction improves irritable bowel syndrome (IBS) symptoms via specific aspects of mindfulness. Neurogastroenterol. Motil. 2020;32:e13828. doi: 10.1111/nmo.13828. [DOI] [PubMed] [Google Scholar]
- 30.Yu L., Scott W., McCracken L.M. Change in fatigue in acceptance and commitment therapy-based treatment for chronic pain and its association with enhanced psychological flexibility. Eur. J. Pain. 2020;24:234–247. doi: 10.1002/ejp.1480. [DOI] [PubMed] [Google Scholar]
- 31.VanderZee K.I., Sanderman R., Heyink J.W., De Haes H. Psychometric qualities of the RAND 36-item health survey 1.0: A multidimensional measure of general health status. Int J Behav Med. 1996;3:104–122. doi: 10.1207/s15327558ijbm0302_2. [DOI] [PubMed] [Google Scholar]
- 32.Batink T., Jansen G., De Mey H. De Flexibiliteits Index Test (FIT-60): Een beknopte beschrijving [The Flexibility Index Test (FIT-60): A concise description] GZ-Psychologie. 2012;4:18–21. doi: 10.1007/s41480-012-0043-x. [DOI] [Google Scholar]
- 33.Aiken L.S., West S.G. Sage Publications; London: 1991. Multiple regression: Testing and interpreting interactions. [Google Scholar]
- 34.Cohen J. A power primer. Psychol. Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- 35.Kharko A.Y., Hansford K.J., Furlong P.L., Hall S.D., Roser M.E. The anxiety and pain of fibromyalgia patients during the COVID-19 pandemic. medRxiv. 2020 doi: 10.1101/2020.11.24.20188011. 2020.11.24.20188011. [DOI] [Google Scholar]
- 36.Crawford J.S. Role of stressors in adult fibromyalgia - a response to Kaleycheva et al. Psychol. Med. 2021:1–2. doi: 10.1017/S003329172100194X. [DOI] [PubMed] [Google Scholar]
- 37.Fallon N., Brown C., Twiddy H., Brian E., Frank B., Nurmikko T., et al. Adverse effects of COVID-19-related lockdown on pain, physical activity and psychological well-being in people with chronic pain. Br. J. Pain. 2021;15:357–368. doi: 10.1177/2049463720973703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raphael K.G., Natelson B.H., Janal M.N., Nayak S. A community-based survey of fibromyalgia-like pain complaints following the World Trade Center terrorist attacks. Pain. 2002;100:131–139. doi: 10.1016/s0304-3959(02)00273-7. (S0304395902002737 [pii) [DOI] [PubMed] [Google Scholar]
- 39.Williams D.A., Brown S.C., Clauw D.J., Gendreau R.M. Self-reported symptoms before and after September 11 in patients with fibromyalgia. JAMA. 2003;289:1637–1638. doi: 10.1001/jama.289.13.1637. (289/13/1637 pii) [DOI] [PubMed] [Google Scholar]
- 40.Koppert T.Y., Jacobs J.W.G., Geenen R. The psychological impact of the COVID-19 pandemic on Dutch people with and without an inflammatory rheumatic disease. Rheumatology (Oxford) 2021;60:3709–3715. doi: 10.1093/rheumatology/keaa842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Quartuccio L., De Marchi G., Azzolina D., Maresio E., Colatutto D., Binutti M., et al. Psychological effects of lockdown measures for the COVID-19 outbreak in patients with systemic lupus erythematosus. J. Multidiscip. Healthc. 2021;14:1475–1488. doi: 10.2147/JMDH.S311325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Tilburg T.G., Steinmetz S., Stolte E., van der Roest H., de Vries D.H. Loneliness and mental health during the COVID-19 pandemic: A study among Dutch older adults. J Gerontol B Psychol Sci Soc Sci. 2021;76:e249–e255. doi: 10.1093/geronb/gbaa111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Velden P.G., Contino C., Das M., van Loon P., MWG Bosmans. Anxiety and depression symptoms, and lack of emotional support among the general population before and during the COVID-19 pandemic. A prospective national study on prevalence and risk factors. J. Affect. Disord. 2020;277:540–548. doi: 10.1016/j.jad.2020.08.026. (S0165–0327(20)32622–7 pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thombs B.D., Kwakkenbos L., Henry R.S., Carrier M.E., Patten S., Harb S., et al. Changes in mental health symptoms from pre-COVID-19 to COVID-19 among participants with systemic sclerosis from four countries: A Scleroderma Patient-centered Intervention Network (SPIN) Cohort study. J. Psychosom. Res. 2020;139:110262. doi: 10.1016/j.jpsychores.2020.110262. (S0022–3999(20)30824–2 pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Villemure C., Bushnell C.M. Cognitive modulation of pain: how do attention and emotion influence pain processing? Pain. 2002;95:195–199. doi: 10.1016/S0304-3959(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 46.Kroska E.B., Roche A.I., Adamowicz J.L., Stegall M.S. Psychological flexibility in the context of COVID-19 adversity: Associations with distress. J. Contextual Behav. Sci. 2020;18:28–33. doi: 10.1016/j.jcbs.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Conversano C., Di Giuseppe M., Miccoli M., Ciacchini R., Gemignani A., Orrù G. Mindfulness, age and gender as protective factors against psychological distress during COVID-19 pandemic. Front. Psychol. 2020;11:1900. doi: 10.3389/fpsyg.2020.01900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fullana M.A., Hidalgo-Mazzei D., Vieta E., Radua J. Coping behaviors associated with decreased anxiety and depressive symptoms during the COVID-19 pandemic and lockdown. J. Affect. Disord. 2020;275:80–81. doi: 10.1016/j.jad.2020.06.027. (S0165–0327(20)32385–5 pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lin L. Longitudinal associations of meaning in life and psychosocial adjustment to the COVID-19 outbreak in China. Br. J. Health Psychol. 2021;26:525–534. doi: 10.1111/bjhp.12492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ran L., Wang W., Ai M., Kong Y., Chen J., Kuang L. Psychological resilience, depression, anxiety, and somatization symptoms in response to COVID-19: A study of the general population in China at the peak of its epidemic. Soc. Sci. Med. 2020;262:113261. doi: 10.1016/j.socscimed.2020.113261. (S0277–9536(20)30480–9 pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dawson D.L., Golijani-Moghaddam N. COVID-19: Psychological flexibility, coping, mental health, and wellbeing in the UK during the pandemic. J. Contextual Behav. Sci. 2020;17:126–134. doi: 10.1016/j.jcbs.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vos L.M.W., Habibović M., Nyklíček I., Smeets T., Mertens G. Optimism, mindfulness, and resilience as potential protective factors for the mental health consequences of fear of the coronavirus. Psychiatry Res. 2021;300:113927. doi: 10.1016/j.psychres.2021.113927. (S0165–1781(21)00224–9 pii) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haliloglu S., Carlioglu A., Akdeniz D., Karaaslan Y., Kosar A. Fibromyalgia in patients with other rheumatic diseases: prevalence and relationship with disease activity. Rheumatol. Int. 2014;34:1275–1280. doi: 10.1007/s00296-014-2972-8. [DOI] [PubMed] [Google Scholar]
- 54.Mayer T.G., Neblett R., Cohen H., Howard K.J., Choi Y.H., Williams M.J., et al. The development and psychometric validation of the central sensitization inventory. Pain Pract. 2012;12:276–285. doi: 10.1111/j.1533-2500.2011.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kregel J., Vuijk P.J., Descheemaeker F., Keizer D., van der Noord R., Nijs J., et al. The Dutch Central Sensitization Inventory (CSI): Factor analysis, discriminative power, and test-retest reliability. Clin. J. Pain. 2016;32:624–630. doi: 10.1097/AJP.0000000000000306. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material