Abstract
Severe Coronavirus Disease 2019 (COVID-19) is characterized by numerous complications, complex disease, and high mortality, making its treatment a top priority in the treatment of COVID-19. Integrated traditional Chinese medicine (TCM) and western medicine played an important role in the prevention, treatment, and rehabilitation of COVID-19 during the epidemic. However, currently there are no evidence-based guidelines for the integrated treatment of severe COVID-19 with TCM and western medicine. Therefore, it is important to develop an evidence-based guideline on the treatment of severe COVID-19 with integrated TCM and western medicine, in order to provide clinical guidance and decision basis for healthcare professionals, public health personnel, and scientific researchers involved in the diagnosis, treatment, and care of COVID-19 patients. We developed and completed the guideline by referring to the standardization process of the “WHO handbook for guideline development”, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system, and the Reporting Items for Practice Guidelines in Healthcare (RIGHT).
Abbreviations: AGREE, Appraisal of Guidelines for Research and Evaluation; AMSTAR, Assessing the Methodological Quality of Systematic Reviews; BFJP, Bufei Jianpi; COVID-19, Coronavirus Disease 2019; CPMs, Chinese patent medicines; EEG, the evidence evaluation group; FHN, femoral head necrosis; GDG, the guideline development group; GRADE, Grades of recommendations, assessment, development, and evaluation; HCQ, hydroxychloroquine; HFNC, high flow nasal cannula; HSBD, Huashi Baidu; HXZQ, Huoxiang Zhengqi; IMV, invasive mechanical ventilation; IOM, Institute of Medicine; IVIg, intravenous immunoglobulin; JHQG, Jinhua Qinggan; LHQW, Lianhua Qingwen; Lpv/R, lopinavir/ritonavir; MERS, Middle East Respiratory Syndrome; NHCPRC, National Health Commission of the People's Republic of China; NIV, non-invasive ventilation; PICO, participants, intervention, comparison, outcome; PHEIC, Public Health Emergency of International Concern; QFPD, Qingfei Paidu; RCT, Randomized control trial; RIGHT, Reporting Items for Practice Guidelines in Healthcare; SARS, Severe Acute Respiratory Syndrome; TCM, traditional Chinese medicines; TCZ, tocilizumab; UC, usual care; WHO, World Health Organization; XBJ, Xuebijing; XFBD, Xuanfei Baidu; YQYY, Yiqi Yangyin; YYQR, Yangyin Qingre
Keywords: Guideline, COVID-19, Traditional Chinese medicine, Integrated Chinese and western medicine
Graphical Abstract
1. Introduction
Since the outbreak of Coronavirus Disease 2019 (COVID-19) in December 2019, the epidemic has spread rapidly across several countries and regions worldwide. On January 30, 2020, the World Health Organization (WHO) announced that the outbreak of COVID-19 was classified as a Public Health Emergency of International Concern (PHEIC). Facing the severe global epidemic situation, WHO issued interim guidelines for severe acute respiratory infections caused by suspected novel coronavirus infections [1] on January 12, 2020, and the National Health Commission of the People's Republic of China (NHCPRC) updated the eighth edition of the Diagnosis and treatment of Coronavirus Disease-19 [2] on August 16, 2020. Currently, the international epidemic situation is still not very optimistic. According to data from the official WHO outbreak report [3], by June 13, 2021, the total number of confirmed cases of novel coronavirus pneumonia was 174,919,389 in 215 regions, countries, territories, and areas, with 410,909 new confirmed cases and 3782,619 cumulative deaths.
Severe COVID-19 is characterized by numerous complications and complex conditions, and the mortality rate (12.4%) is about 5 times higher than the total mortality rate of COVID-19 (2.3%) [4]. Therefore, the treatment of severe patients is one of the top priorities in the treatment of COVID-19. Integrated traditional Chinese medicine (TCM) and western medicine played an important role in the prevention and treatment of COVID-19 during the epidemic. To scientifically summarize and evaluate the effect of integrated TCM and western medicine in the prevention and treatment of COVID-19, and to promote the successful experience to the international community, we developed this guideline based on a global perspective and systematic guideline methodology. We hope the guideline might potentially provide guidance to fully utilize the preventive and control role of TCM during the epidemic.
2. Scope
This guideline is intended for patients with COVID-19 who are at risk of severe conversion, have been diagnosed with severe disease, or patients that are recovering from severe COVID-19, including those who are suffering post-acute sequelae of COVID-19 (PASC, or long COVID). The guideline covers the prevention, treatment, rehabilitation, and psychological support of severe COVID-19 with integrated western medicine and TCM. This guideline applies to all healthcare providers, public health workers, and researchers involved in the diagnosis, treatment, and care of patients with COVID-19 in health care facilities and health administrations in all countries and regions.
3. Guideline development process
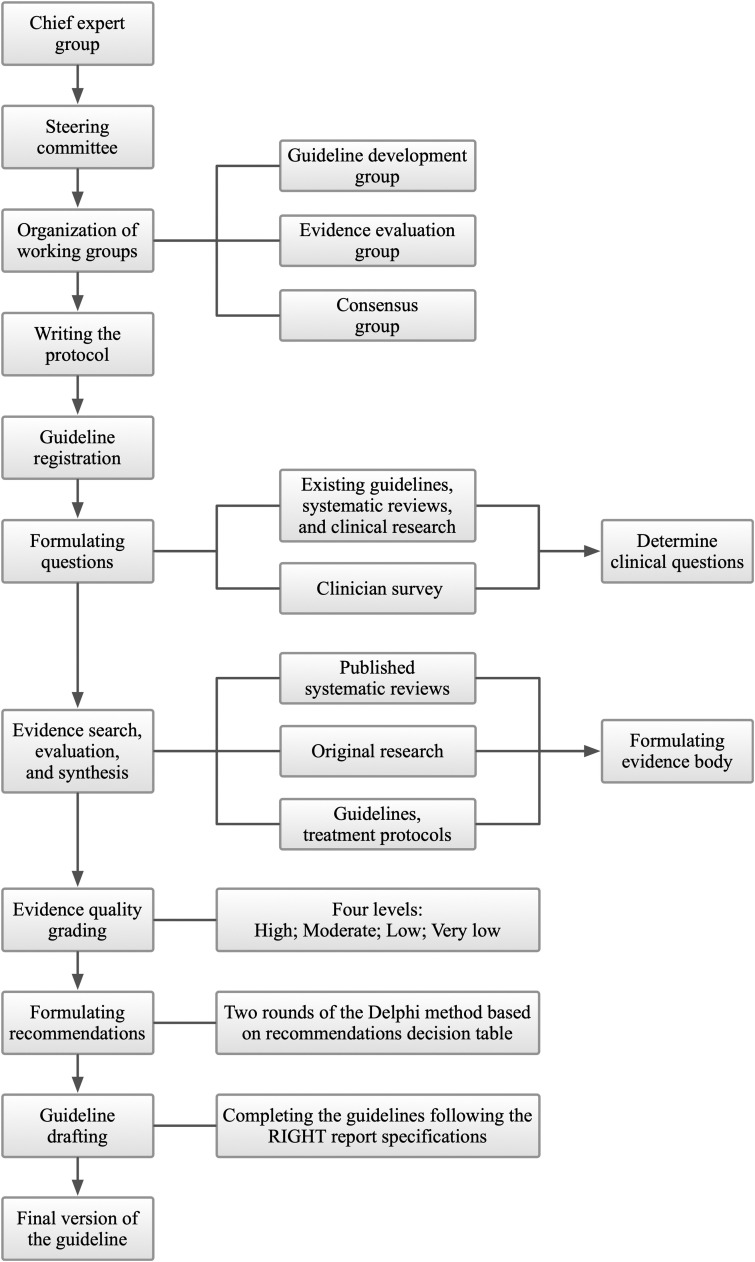
Guideline development was launched on May 4, 2020, and finalized on June 13, 2021. The guideline development followed the Institute of Medicine (IOM) definition of guidelines [5], the specification process recommended by the WHO handbook for guideline development [6], and the Basic methods and procedures for the development/revision of Clinical Practice Guidelines [7]. The guideline used the grading of recommendation evaluation, development and evaluation (GRADE) [8], [9] method to rigorously determine the evidence quality and strength of recommendations. Guidelines were constructed based on the list of entries in the Appraisal of Guidelines for Research and Evaluation (AGREE II) [10], [11] tool. The complete guidelines were written according to the Reporting Items for Practice Guidelines in Healthcare (RIGHT) [12]. The entire guideline development process is shown in Fig. 1.
Fig. 1.
Guideline development process.
3.1. Guideline panel
The Guideline Panel includes the following five groups:
The chief expert group (n = 2) includes one chief clinical expert and one chief methodologist. The chief clinical expert is the overall leader of the guideline, responsible for drafting the scope of the guideline, determining the composition of the guideline team, having decision-making authority at all stages of guideline development, finalizing the draft of the guideline, and being responsible for the clinical applicability of the guideline. The chief methodologist is responsible for the top-level design, guidance, training, and quality control of the guideline methodology and is responsible for the methodological quality.
The steering committee (n = 4) is responsible for managing guideline conflicts of interest, approving guideline protocols, supervising the guideline development process, validating completed guidelines, and providing advice and guidance necessary for the guideline development.
The guideline development group (GDG, n = 5) is responsible for coordinating the work of various working groups, drafting guideline protocols, conducting clinical question investigation, organizing consensus meetings/surveys on recommendations, documenting the entire guideline development process in detail, writing the guidelines draft, and submission of the guidelines.
The evidence evaluation group (EEG, n = 12) is responsible for literature search, screening, inclusion, and quality evaluation; evidence extraction, synthesis, and grading; and creating the evidence profile.
The consensus group (n = 27) is responsible for voting and consensus on clinical questions and recommendations.
3.2. Registration
This guideline has been registered bilingually on the International Practice Guidelines Registry Platform (http://www.guidelines-registry.org/) on May 4, 2020, with a unique registration number: IPGRP-2020CN062.
3.3. Collection and determination of clinical questions
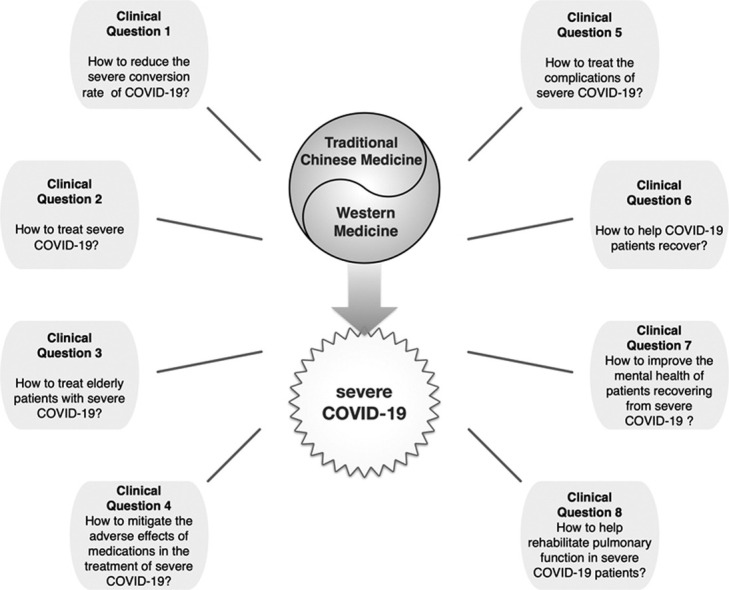
EEG and GDG systematically reviewed and collected clinical questions from existing guidelines, systematic reviews, and clinical studies of COVID-19, severe acute respiratory syndrome (SARS), and Middle East respiratory syndrome (MERS). Together with the clinical questions derived from the steering committee discussions, a total of 264 candidate clinical questions were obtained. After de-duplication, screening, and merging, 24 main clinical questions were determined. These 24 clinical questions were prioritized through an online questionnaire and supplemented with clinical questions that clinicians considered critical. We received questionnaire responses from 21 clinicians (three in TCM, four in respiratory medicine, four in critical care medicine, one in emergency medicine, three in rheumatology, three in nephrology, one in surgery, one in dermatology, and one in orthopedics) with experience in the prevention and treatment of COVID-19 from 17 medical institutions in ten provinces, cities, and autonomous regions in China. The importance of clinical questions was ranked according to the mean importance score (1–7 points) of each question. Finally, eight clinical issues were selected according to the results of the survey and the results of the discussion of the steering committee.
3.4. Evidence collection
Considering the suddenness of outbreaks, the evidence may not be sufficient. Systematic reviews, meta-analyses, randomized controlled trials (RCTs), and observational studies were included. Evidence-based guidelines and official treatment protocols issued by various national and regional health institutions were also included. Considering the characteristics of TCM, we also refer to the consensus opinions of TCM experts (famous and experienced TCM practitioners certified by the National Administration of TCM and have a wealth of experience in the treatment of SARS and influenza).
The EEG deconstructed the final eight included clinical questions according to the PICO (participants, intervention, comparison, outcome) principle and conducted systematic searches according to topic terms combined with free words. We searched MEDLINE (via PubMed), Embase, the Cochrane Library, the China National Knowledge Infrastructure (CNKI), Wanfang, and the China Biology Medicine disc (CBM) for systematic reviews and meta-analyses, network meta-analyses, and clinical studies, supplemented by searches of Google Scholar (https://scholar.google.com/) while tracing reference lists of the included literature; and searched WHO (https://www.who.int/), NICE (http://www.nice.org.uk/), NHCPRC (http://www.nhc.gov.cn/) and the National Administration of TCM (http://www.satcm.gov.cn/) for guidelines, treatment protocol, and consensus of TCM expert. The search period was from the establishment of the database to May 1, 2021, and the language of publication was limited to English and Chinese. Studies, guidelines, and treatment protocols were screened, identified, and checked to meet the inclusion criteria of every specific clinical question by two members of the EEG independently. Disagreements, if any, were resolved by discussion or consultation with a third party.
3.5. Evidence evaluation and grading
EEP applied the AMSTAR scale [13], the Cochrane Collaboration’s tool for assessing risk of bias (ROB) scale [14], the Newcastle-Ottawa Scale (NOS) [15], the National Institutes of Health (NIH) Research Quality Assessment Tool [16], the Canadian Institute of Health Economics (IHE) Quality Assessment of Case Series Studies [17], the Agency for Health Care Research and Quality (AHRQ) Cross-sectional Study Quality Evaluation Tool [18], and AGREE II [10] to evaluate the quality of systematic reviews and meta-analyses (network meta-analysis), RCTs, cohort and case-control studies, before-after studies, case series, cross-sectional studies, and guidelines and consensus, respectively. The GRADE [19], [20] system was used to evaluate the evidence quality and recommendations ( Table 1). Two researchers independently extracted data and assessed the quality of studies.
Table 1.
Evidence quality grades and strength of recommendations.
| Items | Definition |
|---|---|
| Evidence quality | |
| High | Confident enough that the true effect lies close to that of the estimate of the effect. |
| Moderate | Moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. |
| Low | Confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. |
| Very low | Very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. |
| Strength of recommendation | |
| Strong | Advantages of intervention significantly outweigh disadvantages or disadvantages of intervention significantly outweigh advantages. |
| Weak | Advantages of intervention may outweigh disadvantages or disadvantages of intervention may outweigh advantages or the relationship between advantages and disadvantages is not clear. |
3.6. Formulation of recommendations
The GDG initially developed appropriate recommendations for clinical practice based on the evidence included, with considering the preferences and values of patients, the costs of intervention, and the balance between pros and cons. Subsequently, the consensus group voted on every recommendation in Delphi surveys [21] (selected one of "Not sure", "Disagree", or "Agree", reaching a consensus required 80% votes of “agree”). Additionally, the consensus group can propose modification suggestions to those recommendations that have reached a consensus. Two rounds of Delphi surveys were conducted on 8 February 2021 and 19 March 2021, with 22 recommendations reaching consensus and 99 modification suggestions collected. The GDG then completed the guidelines following the RIGHT report specifications.
4. Dissemination and promotion of guidelines
After publication, the guideline will be disseminated and promoted in the following ways: (1) health authorities; (2) publications and official networks; (3) academic conferences and forums in related fields; (4) social media platforms; (5) interpretation of the guideline by invited experts published on common medical websites.
5. Definition of terms
The definition of severe COVID-19 varies slightly around the world, but is primarily based on the presence of signs of pneumonia and severe dyspnea/hypoxemia. The definition of severe COVID-19 in this guideline follows both the WHO definition ( Table 2) and the definition of the Diagnosis and treatment of coronavirus disease-19 (8th trial edition) [2] issued by the NHCPRC ( Table 3).
Table 2.
WHO severe COVID-19 definition.
Adolescent or adult with clinical signs of pneumonia (fever, cough, dyspnoea, fast breathing) plus one of the following:
|
| Child with clinical signs of pneumonia (cough or difficulty breathing) + at least one of the following: |
|
|
| While the diagnosis can be made on clinical grounds; chest imaging (radiograph, CT scan, ultrasound) may assist in diagnosis and identify or exclude pulmonary complications. |
Table 3.
Definition of severe COVID-19 in the Diagnosis and treatment of COVID-19 (8th trial edition).
| Adults meet any of the following criteria: |
| 1. Shortness of breath, respiratory rate (RR) ≥ 30 breaths/min; |
| 2. Oxygen saturation ≤ 93% on air inhalation at rest state; |
| 3. Arterial partial pressure of oxygen (PaO2)/inhaled oxygen concentration (FiO2) ≤ 300 mmHg (1 mmHg = 0.133 kPa); Note: The PaO2/FiO2should be corrected according to the following formula in high altitude (over 1000 m above sea level) areas: PaO2/FiO2× [760/atmosphere (mmHg)]. |
| 4. Progressive worsening of clinical symptoms and lung imaging showing a significant progression of lesions > 50% within 24–48 h. |
| The child meets any of the following criteria: |
| 1. Persistent high fever for more than 3 days; |
| 2. Shortness of breath (<2 months of age, RR ≥ 60 beats/min; 2–12 months of age, RR ≥ 50 beats/min; 1–5 years of age, RR ≥ 40 beats/min; >5 years of age, RR ≥ 30 beats/min), except for the effects of fever and crying; |
| 3. Resting state, oxygen saturation ≤ 93% during air inhalation; |
| 4. Assisted breathing (nasal flapping, trigeminal sign); |
| 5. Drowsiness, convulsions; |
| 6. Refusal of food or feeding difficulties, with signs of dehydration. |
The term "usual care (UC)" (or usual treatment, standard care/treatment, etc.) mentioned in different literature varies slightly depending on the center, region, and time, but mostly includes bed rest, supportive treatment, close monitoring of vital signs and indicators, symptomatic treatment, oxygen therapy, antibiotics (when there is evidence of a secondary bacterial infection) and sometimes empirical medications such as chloroquine phosphate or empirical antivirals (usually one or more of the following: IFN-α, ribavirin, abidol, or lopinavir/ritonavir).
6. Recommendations
Forty-seven articles were finally included in the study, including ten systematic reviews, 15 RCTs, nine cohort studies, two before-after studies, two cross-sectional studies, two case series, three guidelines, and four diagnosis and treatment protocols. A total of 22 recommendations were formed, and the essential points were summarized in Table 4.
Table 4.
Essential points of the recommendations.
| Clinical Question 1 | How to reduce the severe conversion rate of COVID-19 patients with integrated TCM and western medicine? |
| Recommendation 1 | Supportive and symptomatic therapy (consensus recommendation) |
| Triple therapy with IFN β-1b, Lpv/R, and ribavirin (weak recommendation, low evidence quality) | |
| For elderly patients with mild COVID-19: Early treatment with high-titer convalescent plasma (weak recommendation, high evidence quality) | |
| Recommendation 2 | TCM decoctions / CPMs based on syndrome differentiation (strong recommendation, high evidence quality) |
| Clinical Question 2 | How to treat severe COVID-19 with integrated TCM and western medicine? |
| Recommendation 3 | Close monitoring for signs of clinical deterioration, aggressive management of complications and secondary infections, and timely supportive treatment; Immediately respiratory support. (consensus recommendation) |
| For patients HFNC or NIV: Concurrent awake prone ventilation (weak recommendation, low evidence quality) | |
| Recommendation 4 | Corticosteroids (strong recommendation, moderate evidence quality) |
| For adult patients with high inflammatory markers: TCZ (strong recommendation, moderate evidence quality) | |
| For patients receiving oxygen therapy but not on IMV: 5 days of remdesivir treatment (weak recommendation, moderate evidence quality) | |
| For patients who fail to respond to initial therapy: IVIg (strong recommendation, moderate evidence quality) | |
| Recommendation 5 | Severe COVID-19 belongs to the “damp toxin epidemic” in TCM theory, and its main TCM pathogenesis is “epidemic toxin blocking lung” (consensus recommendation) |
| TCM medications based on syndrome differentiation that has the effect of “releasing pulmonary Qi and detoxicating”. (strong recommendation, low evidence quality) | |
| TCM medications that has the effect of “nourishing the spleen and dissipating dampness”. (strong recommendation, moderate evidence quality) | |
| Clinical Question 3 | How to treat elderly patients with severe COVID-19 with integrated TCM and western medicine? |
| Recommendation 6 | Reduce polypharmacy; adjusted drug dose according to the condition, organ function, and drug interactions; prevent adverse events. (consensus recommendation) |
| Recommendation 7 | Potential complications should be monitored and prevented; treatments should focus on supportive, symptomatic treatment and TCM treatment with syndrome differentiation. (consensus recommendation) |
| Recommendation 8 | TCM therapeutic method of “strengthening healthy energy and removing blood stasis” (consensus recommendation) |
| Clinical Question 4 | How to mitigate the adverse effects of medications with integrated TCM and western medicine in the treatment of severe COVID-19? |
| Recommendation 9 | Use the medications that carries the lowest risk of drug-drug interactions with other medications that the patient may be receiving; for medications with dose-dependent negative effects, minimum effective doses should be used for the shortest duration (consensus recommendation) |
| Recommendation 10 | Symptomatic treatment combined with TCM treatment based on syndrome differentiation (consensus recommendation) |
| For liver damage: The combination of symptomatic treatment with TCM decoction based on the therapeutic strategy of “strengthening primordial energy, releasing pulmonary qi, nourishing spleen, and detoxification” (weak recommendation, low evidence quality) | |
| Recommendation 11 | TCM treatment for severe COVID-19 based on syndrome differentiation could reduce adverse events by shortening the application time and total dose of other medications. (strong recommendation, low evidence quality) |
| Clinical Question 5 | How to treat the complications of severe COVID-19 with integrated TCM and western medicine? |
| Recommendation 12 | Active monitoring and evaluation according to the TCM theory of “treating the disease before it occurs (preventive treatment of disease)” (consensus recommendation) |
| Recommendation 13 | For fatigue, chest distress, shortness of breath, loss of appetite, limb pain, and other complications: HXZQ capsule/dropping pill combined with LHQW capsule/granule based on syndrome differentiation (strong recommendation, moderate evidence quality) |
| For digestive system complications, including anorexia, diarrhea, and constipation: TCM decoctions based on syndrome differentiation may be more effective than CPMs (strong recommendation, moderate evidence quality) | |
| For abdominal fullness, anorexia, and nausea: TCM herbs that have the effect of “regulating stomach and dissipating dampness” or “tonifying spleen and appetizing” (consensus recommendation) | |
| For constipation, dry stool, and abdominal distension: Chengqi decoction series based on syndrome differentiation (consensus recommendation) | |
| For pulmonary function damage including pulmonary fibrosis: TCM herbs that have the effects of “dispelling wind, dredging collaterals, and resolving hard lumps” or Guizhi Fuling decoction/pill (for those who do not have high fever) (consensus recommendation) | |
| Clinical Question 6 | How to carry out integrated TCM and western medicine treatment for patients recovering from severe COVID-19? |
| Recommendation 14 | All patients before hospital discharge or those experienced persistent symptoms and/or functional limitations after hospital discharge should be screened for rehabilitation needs in terms of physical, cognitive, and mental disorders, to facilitate onward referral and/or be managed timely (consensus recommendation) |
| Recommendation 15 | Provide education and support for self-management of breathlessness and resumption of activities. (consensus recommendation) |
| Recommendation 16 | The general TCM pathogenesis of patients recovering from severe COVID-19 is “unexhausted evil Qi and unrecovered healthy Qi”, and the specific pathogenesis is mainly characterized by “deficiency, stasis, and dampness”. (consensus recommendation) |
| The basic TCM syndromes are “Qi deficiency of the lung and spleen, and Qi and Yin deficiency”, which are conditionally combined with “uncleared toxin and phlegm-stasis blocking collaterals” (consensus recommendation) | |
| Recommendation 17 | TCM decoctions and CPMs with syndrome differentiation (strong recommendation, low evidence quality) |
| TCM therapy such as acupuncture and moxibustion, manipulation, auricular points therapy, skin-scraping therapy, cupping, foot bath, diet therapy, and emotional therapy; traditional Chinese excises such as Baduanjin and Tai-chi (strong recommendation, moderate evidence quality) | |
| Clinical Question 7 | How to improve the mental health of patients recovering from severe COVID-19 with integrated TCM and western medicine? |
| Recommendation 18 | Establish prompt identification and assessment as well as early warning mechanism; initiate psychosocial support strategies and first-line intervention (consensus recommendation) |
| Recommendation 19 | Provide basic mental health and psychosocial support by asking and addressing their needs and concerns (consensus recommendation) |
| Recommendation 20 | For anxiety, fear, depression, somatization symptoms, and other adverse mental states:Psychological counseling, mental health education, and non-drug treatment such as Tai Chi, breathing relaxation training, mindfulness training, cognitive behavioral therapy, and group intervention (strong recommendation, low evidence quality)Symptomatic treatment combined with TCM decoctions based on syndrome differentiation, which follows the therapeutic method of “soothing the liver and relieve depression, reinforcing earth (spleen) to strengthen metal (lung)” and “eliminating the remaining evil Qi” (weak recommendation, low evidence quality) |
| Clinical Question 8 | How to help rehabilitate pulmonary function in patients with severe COVID-19 with integrated TCM and western medicine? |
| Recommendation 21 | Rehabilitation care should be implemented as soon as possible (could be carried out in parallel with the treatment of the disease according to the correct assessment) (consensus recommendation) |
| Recommendation 22 | For potential impaired pulmonary function and pulmonary fibrosis:Pulmonary rehabilitation training and TCM decoction according to syndrome differentiation, which follows the therapy method of “reinforcing earth (spleen) to strengthen metal (lung), eliminating phlegm to dredging collaterals” and “eliminating the remaining evil Qi (epidemic toxin)” (weak recommendation, low evidence quality)Non-drug treatments such as the Baduanjin, Tai-chi, 6-character breathing exercise, posture management, and breathing exercise management (strong recommendation, low evidence quality) |
IFN: interferon; Lpv/R: lopinavir/ritonavir; TCM: traditional Chinese medicine; CPM: Chinese patent medicine; HFNC: high flow nasal cannula; NIV: noninvasive ventilation; TCZ: tocilizumab; IVIg: intravenous immunoglobulin; HXZQ, Huoxiang Zhengqi; LHQW, Lianhua Qinwen
The following documents are included in the Supplementary Material: 1) Quality assessment of the included studies and guidelines; 2) the details and composition of TCM decoctions and Chinese patent medicines (CPM) mentioned in the recommendations and rationales; 3) the details, effect sizes, and safety data of the outcomes of the included clinical studies; 4) the GRADE evidence profile, which contains detailed information about the quality of evidence assessment and effect sizes of each clinical evidence.
6.1. Clinical Question 1: How to reduce the severe conversion rate of COVID-19 patients with integrated TCM and western medicine?
6.1.1. Recommendation 1
For COVID-19, supportive and symptomatic therapy is recommended (consensus recommendation). Triple therapy with interferon (IFN) β-1b, lopinavir/ritonavir (Lpv/R), and ribavirin is suggested to reduce the severe conversion rate of COVID-19 (weak recommendation, low evidence quality); for elderly patients with mild COVID-19, early treatment with high-titer convalescent plasma can be considered (weak recommendation, high evidence quality).
6.1.2. Rationale
Patients with COVID-19 should rest in bed and receive adequate supportive treatment, such as adequate nutrition and fluid support to ensure water-electrolyte balance and stability of the internal environment; symptomatic treatments such as antipyretic and analgesic treatment should also be given [2], [22]. One RCT [23] showed that, for mild and moderate COVID-19, the viral clearance time of nasopharyngeal swab was significantly reduced with the triple therapy of IFNβ-1b, Lpv/R, and ribavirin compared with Lpv/R alone (effect size and safety data of each study could be found in Supplementary Materials 3, while the effect size and GRADE assessment details of each evidence could be found in Supplementary Materials 4). Another RCT [24] demonstrated that early treatment with high-titer convalescent plasma significantly reduced the severe conversion rate in mild elderly patients with COVID-19 compared to placebo.
Some treatments may have more impairments than benefits in reducing the severe COVID-19 conversion rate. Lpv/R or ribavirin alone may not reduce the rate of severe conversion in patients with COVID-19 or improve important outcomes (moderate evidence quality); Lpv/R may increase the risk of diarrhea and nausea/vomiting [22] (very low evidence quality). An evidence-based guideline [22] and four systematic reviews [25], [26], [27], [28] showed that hydroxychloroquine (HCQ) can postpone pulmonary radiological progression in patients with COVID-19 compared to UC, but there were no significant differences in viral clearance rate, clinical progression, and length of hospital stay, and it may also increase the risk of death and invasive mechanical ventilation (IMV). Furthermore, HCQ may increase the risk of adverse events such as diarrhea and nausea/vomiting (very low to low evidence quality). Two evidence-based guidelines [22], [29] and one RCT [30] indicated that corticosteroid treatment has no significant effect on the rate of clinical deterioration and may prolong the time to viral clearance and increase 28-day mortality rate in patients with mild or moderate COVID-19 (low to moderate evidence quality). Antibiotic therapy or prophylaxis (especially in combination with broad-spectrum antibacterial drugs) is not suggested for patients with mild or moderate COVID-19 unless a bacterial infection is clinically suspected, since few patients with COVID-19 have a secondary bacterial infection and antibiotic misuse may lead to higher rates of bacterial resistance [2], [29], [31] (consensus recommendation).
6.1.3. Recommendation 2
To prevent severe conversion in patients with mild or moderate COVID-19, TCM decoctions or CPM treatment based on syndrome differentiation are recommended in combination with UC (strong recommendation, high evidence quality), such as the medication series “three CPMs and three decoctions” (three CPMs: Jinhua Qinggan [JHQG] granules, Lianhua Qingwen [LHQW] capsules / granules, Xuebijing [XBJ] injection; three TCM decoctions: Qingfei Paidu [QFPD] decoction, Huashi Baidu [HSBD] decoction, Xuanfei Baidu [XFBD] decoction) (strong recommendation, moderate evidence quality); or Hanshiyi decoction (strong recommendation, low evidence quality).
6.1.4. Rationale
For COVID-19 treatment, the Diagnosis and treatment of COVID-19 (8th trial edition) [2] recommended JHQG granules, LHQW capsules/granules, XBJ injection, QFPD decoction, HSBD decoction, and XFBD decoction based on phased syndrome differentiation, which is summarized as “three CPMs and three decoctions”. JHQG granules and LHQW capsules (granules) are used mainly for fatigue with fever during the medical observation phase; XBJ injection is used mainly for the TCM syndrome of “overabundant beat in both Qifen and Yingfen” of severe COVID-19; QFPD decoction can be used for patients at any clinical stage; HSBD decoction is used mainly for the TCM syndrome of “epidemic toxin blocking lung” of severe COVID-19; XFBD decoction is mainly used for the TCM syndrome of “dampness toxin stagnant lung” of moderate COVID-19. Treatment of TCM decoctions or CPMs such as “three CPMs and three decoctions” based on syndrome differentiation in combination with UC (including EM such as Lpv/R, abidol, ribavirin, or interferon) can significantly reduce the severe COVID-19 conversion rate, improve symptoms, reduce complications, improve cardiopulmonary function, increase the cure rate, and reduce mortality compared with UC, with no statistical difference in the incidence of adverse events [32], [33], [34], [35], [36], [37], [38], [39]. A cohort study [40] showed that the Hanshiyi formula can significantly reduce the severe conversion rate of patients with mild and moderate COVID-19 compared with UC (including antivirals such as oseltamivir or arbidol).
6.2. Clinical Question 2: How to treat severe COVID-19 with integrated TCM and western medicine ?
6.2.1. Recommendation 3
For severe COVID-19, close monitoring for signs of clinical deterioration, aggressive management of complications and secondary infections, and timely supportive treatment are recommended; respiratory support such as oxygen therapy, noninvasive ventilation (NIV), IMV, and airway management should be given immediately (consensus recommendation). For patients with COVID-19 who receive high flow nasal cannula (HFNC) or NIV, concurrent awake prone ventilation is recommended (weak recommendation, low evidence quality).
6.2.2. Rationale
Close monitoring of clinical signs of deterioration such as rapid progressive respiratory failure and shock, active management of complications, treatment of underlying diseases, prevention of secondary infections, and timely support of organ function were considered the rules for treating severe COVID-19 [2], [31]. Severe patients with PaO2/FiO2 less than 300 mmHg and those with emergency signs during resuscitation should immediately receive adjuvant oxygen therapy and airway management; after receiving a nasal catheter or mask oxygen inhalation, if respiratory distress or hypoxemia is not improved within 1~2 h, HFNC or NIV should be applied; if the condition is still not improved after 1~2 h, IMV should be performed in time; Extracorporeal membrane oxygenation (ECMO) could be administered if available [2], [31]. One evidence-based guideline [31] and one treatment protocol [2] recommended that patients with severe COVID-19 who require assisted oxygenation (including HFNC) or NIV should be placed in the awake prone position for > 12 h if there is no contraindication.
6.2.3. Recommendation 4
Corticosteroids are recommended for the treatment of severe COVID-19 (strong recommendation, moderate evidence quality). For adult patients with high inflammatory markers, tocilizumab (TCZ) could be considered (strong recommendation, moderate evidence quality). For patients receiving oxygen therapy but not on IMV, 5 days of remdesivir treatment could be considered (weak recommendation, moderate evidence quality). For patients who fail to respond to initial therapy, appropriate treatment with intravenous immunoglobulin (IVIg) could be considered (strong recommendation, moderate evidence quality).
6.2.4. Rationale
Two evidence-based guidelines [22], [29], one systematic review [41], and one RCT [42] indicated that corticosteroid use in patients with severe or critical COVID-19 was associated with lower all-cause mortality and IMV requirements, higher disease improvement rates, and longer survival, with no increase in the incidence of serious adverse events except for a potential increase of hyperglycemia.
One evidence-based guideline [29], two systematic reviews [43], [44], and one RCT [45] showed that TCZ reduced the risk of all-cause mortality and 14-day requirements of NIV and IMV in moderate to critical COVID-19 patients (including those with high interleukin-6 or C-reactive protein [CRP]; on oxygen support; have extensive bilateral lung disease; or need cortisol therapy) compared with UC, and subgroup analyses showed lower mortality at higher CRP levels (≥ 100 mg/L).
An RCT [46] showed that IVIg therapy was associated with a significant reduction in mortality compared with placebo in patients with severe COVID-19 that fail to respond to initial therapy. A cohort study [47] indicated that high-dose IVIg (total dose of 2 g/kg body weight, over 2–5 days) significantly reduced mortality at 28 days in patients with severe COVID-19 compared to UC.
The overall benefit of some treatments for COVID-19 patients is not clear, but their use under specific conditions may be beneficial. One evidence-based guideline [22] indicated that remdesivir could not improve important outcomes in patients with COVID-19 (low certainty evidence). However, another evidence-based guideline [29] suggested 5-days remdesivir treatment for COVID-19 patients (adults and young people aged 12 and over with a weight of 40 kg or more) who are in hospital and on supplemental oxygen but not on IMV, as there is evidence indicated that remdesivir can probably reduce the risk of death slightly (moderate certainty evidence).
Some treatments may have more impairments than benefits for the treatment of severe COVID-19. One evidence-based guideline [22] and one RCT [48] indicated that Lpv/R treatment was not significantly different from UC in terms of mortality, IMV requirements, clinical improvement time, and length of hospital stay of the patients, and was more likely to result in gastrointestinal adverse events (low certainty evidence). One evidence-based guideline [22] and four systematic reviews [25], [26], [27], [28] showed no significant differences in viral clearance rate, clinical progression, or length of hospital stay in patients with treatment of HCQ compared with UC, and it may increase the risk of death, IMV, and adverse effects such as diarrhea and nausea/vomiting.
6.2.5. Recommendation 5
Severe COVID-19 belongs to the “damp toxin epidemic” in TCM theory, and its main TCM pathogenesis is “epidemic toxin blocking lung” (consensus recommendation). TCM medications based on syndrome differentiation, such as “three CPMs and three decoctions” that has the effect of “releasing pulmonary Qi and detoxicating”, are recommended to treat severe COVID-19 in combination with UC; TCM medications such as Ma Huang Liu Jun decoction that has the effect of “nourishing the spleen and dissipating dampness” is also recommended (strong recommendation, low to moderate evidence quality).
6.2.6. Rationale
The Diagnosis and treatment of COVID-19 (8th trial edition) [2] suggested that the main TCM pathogenesis of severe COVID-19 is “epidemic toxin blocking lung”; TCM decoctions including QFPD decoction, HSBD decoction, and Qingwen Baidu decoction, and CPMs such as XBJ injection, are suggested to treat severe COVID-19. Two systematic reviews [37], [38] and nine clinical studies [33], [49], [50], [51], [52] showed that CPMs (LHQW granules, JHQG granule, Tongjie Quwen granules, and XBJ injection) or TCM decoctions (Maxingshigan decoction, QFPD decoction, Qingfei Tongxie Fuzheng decoction, Maxing Xuanfei Jiedu decoction, and other decoctions based on syndrome differentiation) that have the efficacy of “detoxicating and releasing pulmonary Qi” could effectively ameliorate the symptoms of fever, cough, expectoration, shortness of breath, and fatigue; promote CT image improvement and viral clearance rate; shorten the length of hospital stay; and reduce the severe conversion rate and mortality, with no significant differences in safety compared to UC (including IFN-α, Lpv/R, abidol, ribavirin, or chloroquine phosphate).
Severe COVID-19 belongs to the “damp toxin epidemic” in TCM theory [53], [54], therefore, “nourishing the spleen to dissipating dampness” was considered an important treatment method [53], [55], [56]. A cohort study [57] demonstrated that UC (including IFN and ribavirin) combined with Mahuang Liujun decoction based on syndrome differentiation significantly reduced the mortality of patients with severe COVID-19 compared with UC alone. Another cohort study [35] showed that the TCM decoctions based on syndrome differentiation, which has the effect of “detoxicating and releasing pulmonary qi, nourishing spleen, and dissipating dampness”, could significantly improve the CT score of pneumonia, improve symptoms and inflammatory indicators, and reduce the incidence of adverse events in patients with severe COVID-19 compared with UC (including Lpv/R).
6.3. Clinical Question 3: How to treat elderly patients with severe COVID-19 with integrated TCM and western medicine?
6.3.1. Recommendation 6
For elderly patients with COVID-19, polypharmacy should be appropriately reduced; drug dose should be adjusted according to the condition, organ function, and drug interactions; and adverse events should be prevented. (consensus recommendation).
6.3.2. Rationale
Older people are more likely to have deteriorated liver and kidney function, a low drug clearance rate, and a high risk of adverse events and liver/kidney damage. The Pharmacopoeia of the People's Republic of China (2015 edition) [58] stipulates that elderly people (60–80 years old) should be given 3/4–4/5 of the dose as adults (18–60 years old), and the dose for elderly people over 80 years old should be reduced to 1/2 of that for adults. For elderly patients with COVID-19, medication prescriptions should be reviewed to reduce polypharmacy and prevent drug interactions and adverse events; community workers, doctors, nurses, pharmacists, physiotherapists, occupational therapists, mental health and psychosocial care providers, and other health care professionals should facilitate multidisciplinary collaboration in the decision-making process to solve multimorbidity and hypofunction problems in elderly patients [31].
6.3.3. Recommendation 7
For elderly patients with severe COVID-19, potential complications such as secondary infection and disseminated intravascular coagulation should be closely monitored and actively prevented; treatments should focus on supportive, symptomatic treatment and TCM treatment with syndrome differentiation. (consensus recommendation).
6.3.4. Rationale
Elderly COVID-19 patients are characterized by numerous underlying diseases, multiple complications, variable conditions, and limited nutritional intake [59], [60], suggesting that their management should be focused on supportive, symptomatic treatment, and TCM treatment based on syndrome differentiation. The neutrophil ratio in elderly patients with COVID-19 was significantly higher than that in non-elderly patients [60], suggesting that elderly patients may be more susceptible to secondary infections. Therefore, respiratory pathogen monitoring should be actively performed, and targeted anti-infective treatment should be performed timely. D-dimer is significantly elevated in elderly patients [60], suggesting a higher risk of disseminated intravascular coagulation; thus, close monitoring of coagulation indicators and timely implementation of interventions as well as symptomatic, supportive therapies and TCM treatment with syndrome differentiation are recommended. A cohort study on SARS [61] showed that TCM decoction treatment with phased syndrome differentiation could significantly reduce the mortality of elderly patients and increase the improvement rate of clinical symptoms, which may provide a reference for the treatment of elderly COVID-19 patients.
6.3.5. Recommendation 8
Elderly patients with COVID-19 showed more significant signs of the TCM pathogenesis of “deficiency” and “stasis” than non-elderly patients; therefore, the TCM therapeutic method of “strengthening healthy energy and removing blood stasis” is suggested (consensus recommendation).
6.3.6. Rationale
Elderly patients with COVID-19 are characterized by reduced physiological and immune function. Their peripheral blood albumin and prealbumin are significantly lower than those of non-elderly patients, and the number of patients with inappetence is significantly larger than that of non-elderly patients, which might be related to the TCM pathogenesis of “deficiency”. Compared to non-elderly patients, elderly patients with COVID-19 are more likely to have combined cardiovascular disease and significantly higher D-dimer, which might be due to the TCM pathogenesis of “stasis” [60]. Accordingly, the treatment should focus on “strengthening healthy energy and removing blood stasis” based on syndrome differentiation.
6.4. Clinical Question 4: How to mitigate the adverse effects of medications with integrated TCM and western medicine in the treatment of severe COVID-19?
6.4.1. Recommendation 9
Use the medications that carries the lowest risk of drug-drug interactions with other medications that the patient may be receiving; for medications with dose-dependent negative effects, minimum effective doses should be used for the shortest duration (consensus recommendation).
6.4.2. Rationale
Careful consideration should be given to the numerous side effects of medications that may be used in the treatment of COVID-19, as well as drug-drug interactions between medications, both of which may affect COVID-19 symptomatology (including effects on respiratory, cardiac, immune, and mental or neurological function). Both pharmacokinetic and pharmacodynamic effects should be considered [31].
6.4.3. Recommendation 10
Symptomatic treatment combined with TCM treatment based on syndrome differentiation is recommended for reducing potential adverse events in patients with severe COVID-19 (consensus recommendation). For liver damage, the combination of symptomatic treatment with TCM decoction based on the therapeutic strategy of “strengthening primordial energy, releasing pulmonary qi, nourishing spleen, and detoxification” is recommended (weak recommendation, low evidence quality).
6.4.4. Rationale
A cohort study [35] showed that TCM decoctions based on the therapeutic method of “strengthening primordial energy, releasing pulmonary qi, nourishing spleen, and detoxification” in combination with symptomatic treatment could significantly reduce the incidence of adverse events (especially elevated liver enzymes) of severe COVID-19.
6.4.5. Recommendation 11
TCM treatment for severe COVID-19 based on syndrome differentiation could reduce adverse events by shortening the application time and total dose of other medications, such as antivirals or corticosteroids (strong recommendation, low evidence quality).
6.4.6. Rationale
TCM medications based on syndrome differentiation could increase the treatment efficacy for severe COVID-19 with good safety [32], [33], [34], [35], [36], [38], [39], [40], [49], [50], [51], and therefore may reduce the adverse events by shortening the application time and total amount of other medications, such as antivirals or corticosteroids.
6.5. Clinical Question 5: How to treat the complications of severe COVID-19 with integrated TCM and western medicine?
6.5.1. Recommendation 12
For all potential complications of severe COVID-19, active monitoring and evaluation should be carried out according to the TCM theory of “treating the disease before it occurs (preventive treatment of disease)”; if complications have already occurred, it is recommended to immediately follow local treatment protocols for symptomatic treatment (consensus recommendation).
6.5.2. Rationale
Two evidence-based guidelines [29], [31] and one treatment protocol [2] suggested active monitoring of COVID-19 patients for signs or symptoms of thromboembolism, such as stroke, deep vein thrombosis, pulmonary embolism, or acute coronary syndrome; if clinically suspected, an appropriate diagnostic and management pathway needs to be initiated immediately. Adolescent and adult patients with COVID-19 should be evaluated for the risk of hemorrhage immediately after admission. For patients with suspected or confirmed myocardial injury, high-sensitivity troponin measurements and continuous ECG monitoring should be repeated daily, and blood pressure, heart rate, and fluid balance should be measured.
6.5.3. Recommendation 13
For fatigue, chest distress, shortness of breath, loss of appetite, limb pain, and other complications in patients with severe COVID-19, it is recommended to use HXZQ capsule/dropping pill combined with LHQW capsule/granule based on syndrome differentiation (strong recommendation, moderate evidence quality). For digestive system complications, including anorexia, diarrhea, and constipation, TCM decoctions based on syndrome differentiation may be more effective than CPMs (strong recommendation, moderate evidence quality). For abdominal fullness, anorexia, and nausea, TCM herbs, such as Magnolia Bark (Houpu), Cablin patchouli herb (Huoxiang), and Atractylodes Rhizome (Cangzhu), that have the effect of “regulating stomach and dissipating dampness” or TCM herbs, such as roasted Endothelium Corneum Gigeriae Galli (Jineijin), fire-dried Fructus Crataegi (Shanzha), and Fructus Amomi (Sharen), that have the effect of “tonifying spleen and appetizing” could be considered based on syndrome differentiation (consensus recommendation). For constipation, dry stool, and abdominal distension, the Chengqi decoction series based on syndrome differentiation are suggested (consensus recommendation). For pulmonary function damage including pulmonary fibrosis, the TCM herbs, such as Circada Moulting (Chantui), Body of sick Silkworm (Jiangcan), and lumbricus (Dilong), that have the effects of “dispelling wind, dredging collaterals, and resolving hard lumps” could be considered; or Guizhi Fuling decoction (pill) could be considered for those who do not have high fever (consensus recommendation).
6.5.4. Rationale
One RCT [39] indicated that, HXZQ dripping pills combined with LHQW granules can effectively improve nausea, vomiting, limb pain, loss of appetite, limb pain, fatigue, chest tightness, shortness of breath, and other symptoms in patients with COVID-19, and meanwhile reduce the use of antibiotics. With the treatment of UC combining CPMs based on syndrome differentiation, some patients showed improvement in symptoms such as fever, cough, sore throat, and fatigue, while digestive symptoms such as poor appetite, diarrhea, or constipation did not improve significantly; after changing the CPMs to TCM decoctions with syndrome differentiation, the aforementioned symptoms improved [34]. TCM herbs, such as Magnolia Bark (Houpu), Cablin patchouli herb (Huoxiang), Atractylodes Rhizome (Cangzhu), with the effects of “regulating stomach and dissipating dampness” and TCM herbs, such as roasted Endothelium Corneum Gigeriae Galli (Jineijin), fire-dried Fructus Crataegi (Shanzha) and Fructus Amomi (Sharen), with the effects of “tonifying spleen and appetizing” based on syndrome differentiation can improve abdominal fullness, anorexia, nausea, diarrhea, and other gastrointestinal symptoms in patients with severe COVID-19 [34]. Treatment of Chengqi decoction series including Da Chengqi decoction, Xuanbai Chengqi decoction, and Jiedu Chengqi decoction based on syndrome differentiation can improve constipation, dry stool, abdominal distension, and other symptoms in patients with severe COVID-19 [34], [54]. Early use of TCM medications such as Circada Moulting (Chantui), Body of Sick Silkworm (Jiangcan), and lumbricus (Dilong), Red Peony Root (Chishao), Peach Kernel (Taoren), and the Salvia Root (Danshen), with the effects of “dispelling wind, dredging collaterals, and resolving hard lumps” may reduce the severity of pulmonary fibrosis [54]; for those who do not have high fever, Guizhi Fuling decoction (pill) could also be considered for treatment [62].
6.6. 6.6Clinical Question 6: How to carry out integrated TCM and western medicine treatment for patients recovering from severe COVID-19?
6.6.1. Recommendation 14
All patients before hospital discharge or those experienced persistent symptoms and/or functional limitations after hospital discharge should be screened for rehabilitation needs in terms of physical, cognitive, and mental disorders, to facilitate onward referral and/or be managed timely (consensus recommendation).
6.6.2. Rationale
All patients with COVID-19 before hospital discharge should be screened for rehabilitation needs (including physical deconditioning, and respiratory, swallow, cognitive or mental health disorders) to facilitate onward referral; patients who have been discharged from the hospital and experienced persistent symptoms and/or functional limitations should also be screened for rehabilitation needs to be managed in a timely manner [31].
6.6.3. Recommendation 15
All severe COVID-19 patients should be provided with education and support for self-management of breathlessness and resumption of activities. (consensus recommendation).
6.6.4. Rationale
Patients with severe COVID-19 are more likely to have severely impaired pulmonary diffusion capacity and abnormal chest imaging manifestations, and therefore, are the main target population for long-term recovery intervention [63]. All severe COVID-19 patients should be educated and supported. Education on breathing control, such as high side lying, forward lean sitting, pursed lip breathing, and square box breathing, can support patients recovering from respiratory illness. Appropriate walking pace regulation is recommended to reduce breathlessness and prevent oxygen desaturation on exertion. All rehabilitating patients should be educated to resume daily activities conservatively at an appropriate pace that is safe and manageable, and the increase of exercise should be gradual and based on symptoms [31].
6.6.5. Recommendation 16
The general TCM pathogenesis of patients recovering from severe COVID-19 is “unexhausted evil Qi and unrecovered healthy Qi”, and the specific pathogenesis is mainly characterized by “deficiency, stasis, and dampness”. The basic TCM syndromes are “Qi deficiency of the lung and spleen, and Qi and Yin deficiency”, which are conditionally combined with “uncleared toxin and phlegm-stasis blocking collaterals” (consensus recommendation).
6.6.6. Rationale
The general pathogenesis of patients recovering from severe COVID-19 is characterized by “unexhausted evil Qi (epidemic toxin) and unrecovered healthy Qi”. Although the epidemic toxin has been attenuated, the body's Yin and Yang were both damaged, both Qi and Yin were injured, and the epidemic toxin was difficult to be dispelled, which results in the TCM syndrome of “origin deficiency and superficial actuality” or “a mixture of deficiency and actuality”. Specific pathogenesis is mainly “deficiency, stasis, and dampness”. The pathogenesis “Qi deficiency, Yin deficiency, lung and spleen deficiency” belongs to “origin deficiency”, and the pathogenesis “stasis, dampness, and unexhausted epidemic toxin” belongs to “superficial actuality”. The basic TCM syndrome can be summarized as “Qi deficiency of the lung and spleen, and deficiency of both Qi and Yin” combined with “uncleared toxin” or “phlegm-stasis blocking collaterals” [2], [64], [65].
6.6.7. Recommendation 17
For the management of function disorders, pulmonary fibrosis, mental health problems, decreased mobility, glucocorticoids sequelae, and recurrence of positive RT-PCR test results that patients who recovering from severe COVID-19 may experience, TCM decoctions and CPMs with syndrome differentiation are recommended (strong recommendation, low evidence quality). TCM therapy such as acupuncture and moxibustion, manipulation, auricular points therapy, skin-scraping therapy, cupping, foot bath, diet therapy, and emotional therapy, or traditional Chinese excises such as Baduanjin and Tai-chi, are also recommended (strong recommendation, moderate evidence quality).
6.6.8. Rationale
A cohort study [66] showed that, in combining with UC, the TCM decoction series of Yiqi Yangyin (YQYY) decoction, Bufei Jianpi (BFJP) decoction, and Yangyin Qingre (YYQR) decoction, together with Xiaoyao powder, which has the effect of “soothing the liver to relieve depression”, “nourishing spleen and harmonizing Ying”, and “eliminating the remaining evil Qi (epidemic toxin)”, were used with modification according to the patient's specific condition; they significantly improved the life quality of patients recovered from SARS in ten aspects, including asthmatic, cycling, shopping, stairs climbing, chest tightness, melancholy, anger, restricted activity, worrying about the present, and worrying about the future. A treatment protocol [65] recommended that patients recovered from COVID-19 can be treated with acupuncture and moxibustion, manipulation, auricular points therapy, skin-scraping therapy, cupping, and other non-oral drug TCM therapies; diet therapy and emotional therapy; or traditional Chinese excises including the Baduanjin and Tai-chi. A cohort study [67] showed that the comprehensive treatment based on syndrome differentiation, including TCM decoction, CPM, acupuncture, foot bath, and Baduanjin exercise, can significantly reduce the recurrence of positive RT-PCR test results of the viral nucleic acid in patients recovered from COVID-19.
For patients with severe COVID-19 treated with glucocorticoids, sequela may occur after discontinuation, such as femoral head necrosis (FHN), which is the most common and should be prevented early. The main TCM syndrome of FHN is “fire excess from Yin deficiency” and “deficiency of both Yin and Yang” combined with “blood stasis and phlegm-dampness”, which can be treated with Liuwei Dihuang pill, Jinkui Shenqi pill, or Jisheng Shenqi pill based on syndrome differentiation [68].
6.7. Clinical Question 7: How to improve the mental health of patients recovering from severe COVID-19 with integrated TCM and western medicine?
6.7.1. Recommendation 18
For convalescent patients with severe COVID-19, prompt identification and assessment as well as early warning mechanism for possible adverse mental states should be established, and psychosocial support strategies and first-line intervention should be initiated for management. (consensus recommendation).
6.7.2. Rationale
More than 60% of COVID-19 patients may experience adverse mental states such as anxiety and depression [69], [70]. An evidence-based guideline [31] and a treatment protocol [71] suggested establishing a dynamic evaluation and warning mechanism for prompt identification, assessment, and intervention of possible psychological crises. For patients with mild adverse mental states, psychological self-adjustment including breath relaxation training and mindfulness training is recommended. For patients with moderate to severe adverse mental states, intervention by combining medication and psychotherapy is suggested. For patients experiencing anxiety and depression, basic psychological interventions such as psychological first aid, stress management, and brief psychological interventions based on the principles of cognitive behavior therapy should be considered. For severe anxiety, medications, especially those with a short half-life and low risk of drug-drug interactions, at the lowest possible dose and the shortest possible duration as can be considered.
6.7.3. Recommendation 19
It is recommended to provide basic mental health and psychosocial support to all patients with severe COVID-19 by asking and addressing their needs and concerns. (consensus recommendation).
6.7.4. Rationale
Basic mental health and psychosocial support is essential for the treatment of patients and is an indispensable part of medical care for different groups affected by COVID-19, including severe patients. Patients should continue receiving mental health and psychosocial support and be followed up after hospital discharge to ensure that their symptoms continue being improved [31].
6.7.5. Recommendation 20
For anxiety, fear, depression, somatization symptoms, and other adverse mental states that severe COVID-19 patients may experience, psychological counseling, mental health education, and non-drug treatment such as Tai Chi, breathing relaxation training, mindfulness training, cognitive behavioral therapy, and group intervention are recommended (strong recommendation, low evidence quality). Symptomatic treatment combined with TCM decoctions based on syndrome differentiation, which follows the therapeutic method of “soothing the liver and relieve depression, reinforcing earth (spleen) to strengthen metal (lung)” and “eliminating the remaining evil Qi”, is also suggested (weak recommendation, low evidence quality).
6.7.6. Rationale
Two before-after studies [70], [72] showed that psychological counseling, mental health education, cognitive behavioral therapy, mindfulness therapy, group intervention, and Tai Chi can significantly improve the adverse mental status of COVID-19 patients, such as anxiety, fear, depression, and somatization symptoms. Furthermore, two RCTs [66], [73] showed that symptomatic treatment combined with TCM decoctions, including YQYY decoction, BFJP decoction, and YYQR decoction, based on syndrome differentiation, which has the effect of “soothing the liver and relieve depression, reinforcing earth (spleen) to strengthen metal (lung)” and “eliminating the remaining evil Qi”, can significantly improve sadness, depression, anger, worry, and other adverse mental states of COVID-19 patients.
6.8. Clinical Question 8: How to help rehabilitate pulmonary function in patients with severe COVID-19 with integrated TCM and western medicine?
6.8.1. Recommendation 21
Rehabilitation care should be implemented as soon as possible for patients with severe COVID-19, which could be carried out in parallel with the treatment of the disease according to the correct assessment (consensus recommendation).
6.8.2. Rationale
In case of stable oxygenation and hemodynamics, passive and active activities should be carried out as soon as possible to promote sputum drainage and pulmonary rehabilitation [2]. Interventions could be initiated by rehabilitation professionals in the acute phase of the disease to reduce respiratory distress, prevent complications, and support communication. The timing of the start of rehabilitation care should be decided by the multidisciplinary team based on the medical condition of the patient [31].
6.8.3. Recommendation 22
For potential impaired pulmonary function and pulmonary fibrosis in convalescent patients with severe COVID-19, pulmonary rehabilitation training and TCM decoction according to syndrome differentiation, which follows the therapy method of “reinforcing earth (spleen) to strengthen metal (lung), eliminating phlegm to dredging collaterals” and “eliminating the remaining evil Qi (epidemic toxin)”, are suggested (weak recommendation, low evidence quality); Baduanjin, Tai-chi, 6-character breathing exercise, posture management, breathing exercise management, and other Chinese and western non-drug treatments are also suggested (strong recommendation, low evidence quality).
6.8.4. Rationale
A systematic review [74] of interstitial lung disease (including pulmonary fibrosis after severe COVID-19 infection) showed that pulmonary rehabilitation training, including combined exercise (aerobic with strength), combined exercise with specific respiratory exercises, and aerobic exercise with specific respiratory muscle training, significantly improved pulmonary function (forced vital capacity), exercise capacity (6-minute walking distance), and health-related quality of life of convalescent patients with severe COVID-19. Three RCTs [66], [75], [76] showed that, for SARS convalescent patients, TCM decoctions such as YQYY decoction, BFJP decoction, and YYQR decoction, based on syndrome differentiation and following the therapy strategy of “reinforcing earth (spleen) to strengthen metal (lung), eliminating phlegm to dredging collaterals” and “eliminating the remaining evil Qi (epidemic toxin)”, can significantly improve the chest x-ray manifestations of pulmonary fibrosis (blindly scored by the specialized radiologist, in principle based on the density and extent of shadows on chest radiographs), total lung capacity, diffusion capacity for carbon monoxide, and symptoms such as dyspnea and chest distress. Two treatment protocols [65], [71] suggested using posture management, breathing exercise management, or TCM health-preserving exercises such as Baduanjin and Tai-chi, and 6-character breathing exercise for pulmonary function recovery.
7. Conclusion
This is the first guideline for the management of severe COVID-19 by integrated Chinese and western medicine, based on a systematic search of evidence applying the GRADE classification system. As there is still a discrepancy between existing studies and clinical practice due to the suddenness of the epidemic, a large number of high-quality studies are needed in the field of integrated Chinese and western medicine for severe COVID-19, especially for the old patients, as they are the main focus of the severe COVID-19. Because of the inadequacy or lack of clinical evidence, some of the recommendations are of weak recommendation level. Therefore, we suggest physicians make appropriate choices when applying them in clinical practice by considering the actual conditions and values of the patients. Moreover, as the population of patients grows, the rehabilitation of COVID-19, including the management of long-COVID/PACS, has become a facing challenge, while high quality studies remain inadequate. TCM has advantages in long-COVID management for its strengths in rehabilitation, individualized treatment, and self-immunity enhancement, and therefore, will be the focus of the guideline update. In addition, users of this guidelines are welcome to provide valuable suggestions and comments through clinical practice, and we will continue improving them in the next edition.
Funding
This work was supported by the Key Project “Emergency Prevention and Treatment of COVID- 19″ of the Zhejiang Provincial Natural Science Foundation of China (grant number LEZ20H190001).
Declaration
All authors declare that there is no conflict of interest. The opinions covered in this guide should not be used for commercial promotion or publicity.
Acknowledgments
We thank all members of the Guideline Panel for their contribution. The following names are in alphabetical order by Chinese Pinyin of the last name.
Chief Expert Group: Chief Clinical Expert, Chengping Wen; Chief Methodologist, Yaolong Chen.
Steering Committee: Qingquan Liu (Beijing Hospital of Traditional Chinese Medicine, Capital Medical University); Xiaolin Tong (Guang'anmen Hospital, China Academy of Chinese Medical Sciences); Qi Wang (Beijing University of Chinese Medicine); Chengping Wen.
Guideline Development Group (GDG): Zhiyu Li; Haichang Li; Jianjian Wang; Chengping Wen; Zhijun Xie.
Evidence evaluation group: Jiao Chen; Wu Chen; Yunfei Ding; Shan Fang; Yini Gao; Qiangqiang Guo; Yuanfang He; Weidong Lai; Dianming Li; Lin Li; Qiuping Liu; Zhiyu Li; Yu Lou; Wenqing Luo; Yuepeng Jiang; Siyue Song; Jianjian Wang; Keer Wang; Xianghui Wen; Shouyuan Wu; Anran Xi; Xiaofeng Xu; Fengqi Zhang; Juanjuan Zhang; Ming Zhang; Xiafeng Zhang; Mingqian Zhou.
Consensus group: Jianfeng Bao (Hangzhou Xixi Hospital); Erzhen Chen (Ruijin Hospital, Shanghai Jiaotong University School of Medicine); Ming Chen (Beijing Hospital of Traditional Chinese Medicine, Capital Medical University); Sheng Chen (Tuanfeng people's Hospital); Tengfei Chen (Beijing Hospital of Traditional Chinese Medicine, Capital Medical University); Lei Dong (The Second Affiliated Hospital of Zhejiang Chinese Medicine University); Yuanbo Fu (Beijing Hospital of Traditional Chinese Medicine, Capital Medical University); Xiaomin Huang (Zhejiang Provincial Hospital of Traditional Chinese Medicine); Xiangdong Jian (Qilu Hospital, Shandong University); Baoli Liu (Beijing Hospital of Traditional Chinese Medicine, Capital Medical University); Genping Lei (Affiliated Hospital of Shanxi University of Chinese Medicine); Tianhao Li (The Second Affiliated Hospital of Shanxi University of Chinese Medicine); Xi Li (The Second People's Hospital Affiliated to Fujian University of Chinese Medicine); Xiangyang Leng (Changchun University of Chinese Medicine); Jiaju Ma (Beijing Hospital of Traditional Chinese Medicine, Capital Medical University); Denhan Shi (Yueqing People's Hospital); Junling Yang (The Second Hospital of Jilin University); Mingwei Yang (Jingmen Second People's Hospital); Yao Lin (The Sixth Affiliated Hospital of Sun Yat-sen University); Qing Yuan (Beijing Hospital of Traditional Chinese Medicine, Capital Medical University); Rui Yu (Liaoning University of Traditional Chinese Medicine); Xianghong Yang (Zhejiang Provincial People's Hospital); Yongan Ye (Dongzhimen Hospital, Beijing University of Chinese Medicine); Fuzeng Zheng (Henan Provincial Hospital of Traditional Chinese Medicine); Zhang Liming (Beijing Chaoyang Hospital, Capital Medical University); Hongju Zhang (Lishui Hospital of Traditional Chinese Medicine).
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.phrs.2021.105955.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.World Health Organization, Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance, (2020).
- 2.National Health Commission of the People’s Republic of China National Administration of Traditional Chinese Medicine, Diagnosis and treatment of corona virus disease-19 (8th trial edition) China Med. 2020;15(10):1494–1499. doi: 10.3760/j.issn.1673-4777.2020.10.002. [DOI] [Google Scholar]
- 3.World Health Organization, WHO coronavirus (COVID-19) dashboard, 2021. 〈https://covid19.who.int〉. (Accessed June 13 2021).
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Kung J., Miller R.R., Mackowiak P.A. Failure of clinical practice guidelines to meet institute of medicine standards: two more decades of little, if any, progress. Arch. Intern Med. 2012;172(21):1628–1633. doi: 10.1001/2013.jamainternmed.56. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization . WHO Handbook for Guideline Development. 2nd ed. World Health Organization; 2014. [Google Scholar]
- 7.Jiang Z., Zhan S., Jia X., Fang H., Zuo L., Gao R. Basic methods and procedures for the development/revision of Clinical Practice Guidelines [Chinese] Natl. Med. J. China. 2016;96(04):250–253. [Google Scholar]
- 8.Schünemann H.J., Mustafa R., Brozek J., Santesso N., Alonso-Coello P., Guyatt G., Scholten R., Langendam M., Leeflang M.M., Akl E.A., Singh J.A., Meerpohl J., Hultcrantz M., Bossuyt P., Oxman A.D. GRADE Guidelines: 16. GRADE evidence to decision frameworks for tests in clinical practice and public health. J. Clin. Epidemiol. 2016;76:89–98. doi: 10.1016/j.jclinepi.2016.01.032. [DOI] [PubMed] [Google Scholar]
- 9.Canfield S.E., Dahm P. Rating the quality of evidence and the strength of recommendations using GRADE. World J. Urol. 2011;29(3):311–317. doi: 10.1007/s00345-011-0667-2. [DOI] [PubMed] [Google Scholar]
- 10.Brouwers M.C., Kho M.E., Browman G.P., Burgers J.S., Cluzeau F., Feder G., Fervers B., Graham I.D., Grimshaw J., Hanna S.E., Littlejohns P., Makarski J., Zitzelsberger L. AGREE II: advancing guideline development, reporting, and evaluation in health care. Prev. Med. 2010;51(5):421–424. doi: 10.1016/j.ypmed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Schünemann H.J., Wiercioch W., Etxeandia I., Falavigna M., Santesso N., Mustafa R., Ventresca M., Brignardello-Petersen R., Laisaar K.T., Kowalski S., Baldeh T., Zhang Y., Raid U., Neumann I., Norris S.L., Thornton J., Harbour R., Treweek S., Guyatt G., Alonso-Coello P., Reinap M., Brozek J., Oxman A., Akl E.A. Guidelines 2.0: systematic development of a comprehensive checklist for a successful guideline enterprise. CMAJ. 2014;186(3):E123–E142. doi: 10.1503/cmaj.131237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y., Yang K., Marušic A., Qaseem A., Meerpohl J.J., Flottorp S., Akl E.A., Schünemann H.J., Chan E.S., Falck-Ytter Y., Ahmed F., Barber S., Chen C., Zhang M., Xu B., Tian J., Song F., Shang H., Tang K., Wang Q., Norris S.L. A reporting tool for practice guidelines in health care: the RIGHT statement. Ann. Intern Med. 2017;166(2):128–132. doi: 10.7326/m16-1565. [DOI] [PubMed] [Google Scholar]
- 13.Shea B.J., Hamel C., Wells G.A., Bouter L.M., Kristjansson E., Grimshaw J., Henry D.A., Boers M. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J. Clin. Epidemiol. 2009;62(10):1013–1020. doi: 10.1016/j.jclinepi.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 14.Hopp L. Risk of bias reporting in Cochrane systematic reviews. Int J. Nurs. Pract. 2015;21(5):683–686. doi: 10.1111/ijn.12252. [DOI] [PubMed] [Google Scholar]
- 15.Cook D.A., Reed D.A. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale-Education. Acad. Med. 2015;90(8):1067–1076. doi: 10.1097/acm.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 16.The National Institutes of Health (NIH), Quality assessment tool for observational cohort and cross-sectional studies, (2014). 〈https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools〉.
- 17.Institute of Health Economics (IHE) Institute of Health Economics; Edmonton (AB): 2014. Quality Appraisal of Case Series Studies Checklist. [Google Scholar]
- 18.Rostom A., Dubé C., Cranney A., Saloojee N., Sy R., Garritty C., Sampson M., Zhang L., Yazdi F., Mamaladze V. Agency for Healthcare Research and Quality (US); Rockville (MD): 2004. Celiac Disease: Summary, AHRQ Evidence Report Summaries; pp. 1–6. [PMC free article] [PubMed] [Google Scholar]
- 19.Guyatt G., Oxman A.D., Akl E.A., Kunz R., Vist G., Brozek J., Norris S., Falck-Ytter Y., Glasziou P., DeBeer H., Jaeschke R., Rind D., Meerpohl J., Dahm P., Schünemann H.J. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J. Clin. Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Chen Y., Yao L., Norris S., Du L., Chen H., Zeng X., Mao Z., Yang K. Application of GRADE in systematic reviews:necessity, frequently-asked questions and concerns. Chin. J. Evid. Based Med. 2013;13(12):1401–1404. [Google Scholar]
- 21.Jones J., Hunter D. Consensus methods for medical and health services research. BMJ. 1995;311(7001):376–380. doi: 10.1136/bmj.311.7001.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization, Therapeutics and COVID-19: living guideline, (2021). [PubMed]
- 23.Hung I.F.-N., Lung K.-C., Tso E.Y.-K., Liu R., Chung T.W.-H., Chu M.-Y., Ng Y.-Y., Lo J., Chan J., Tam A.R., Shum H.-P., Chan V., Wu A.K.-L., Sin K.-M., Leung W.-S., Law W.-L., Lung D.C., Sin S., Yeung P., Yip C.C.-Y., Zhang R.R., Fung A.Y.-F., Yan E.Y.-W., Leung K.-H., Ip J.D., Chu A.W.-H., Chan W.-M., Ng A.C.-K., Lee R., Fung K., Yeung A., Wu T.-C., Chan J.W.-M., Yan W.-W., Chan W.-M., Chan J.F.-W., Lie A.K.-W., Tsang O.T.-Y., Cheng V.C.-C., Que T.-L., Lau C.-S., Chan K.-H., To K.K.-W., Yuen K.-Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Libster R., Pérez Marc G., Wappner D., Coviello S., Bianchi A., Braem V., Esteban I., Caballero M.T., Wood C., Berrueta M., Rondan A., Lescano G., Cruz P., Ritou Y., Fernández Viña V., Álvarez Paggi D., Esperante S., Ferreti A., Ofman G., Ciganda Á., Rodriguez R., Lantos J., Valentini R., Itcovici N., Hintze A., Oyarvide M.L., Etchegaray C., Neira A., Name I., Alfonso J., López Castelo R., Caruso G., Rapelius S., Alvez F., Etchenique F., Dimase F., Alvarez D., Aranda S.S., Sánchez Yanotti C., De Luca J., Jares Baglivo S., Laudanno S., Nowogrodzki F., Larrea R., Silveyra M., Leberzstein G., Debonis A., Molinos J., González M., Perez E., Kreplak N., Pastor Argüello S., Gibbons L., Althabe F., Bergel E., Polack F.P. Early high-titer plasma therapy to prevent severe Covid-19 in older adults. N. Engl. J. Med. 2021;384(7):610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zang Y., Han X., He M., Shi J., Li Y. Hydroxychloroquine use and progression or prognosis of COVID-19: a systematic review and meta-analysis. Naunyn Schmiede. Arch. Pharm. 2021;394(4):775–782. doi: 10.1007/s00210-020-01964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elsawah H.K., Elsokary M.A., Elrazzaz M.G., Elshafie A.H. Hydroxychloroquine for treatment of nonsevere COVID-19 patients: systematic review and meta-analysis of controlled clinical trials. J. Med. Virol. 2021;93(3):1265–1275. doi: 10.1002/jmv.26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarma P., Kaur H., Kumar H., Mahendru D., Avti P., Bhattacharyya A., Prajapat M., Shekhar N., Kumar S., Singh R., Singh A., Dhibar D.P., Prakash A., Medhi B. Virological and clinical cure in COVID-19 patients treated with hydroxychloroquine: a systematic review and meta-analysis. J. Med. Virol. 2020;92(7):776–785. doi: 10.1002/jmv.25898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayele Mega T., Feyissa T.M., Dessalegn Bosho D., Kumela Goro K., Zeleke Negera G. The outcome of hydroxychloroquine in patients treated for COVID-19: systematic review and meta-analysis. Can. Respir. J. 2020;2020(2020) doi: 10.1155/2020/4312519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The National Institute for Health and Care Excellence (NICE), The COVID-19 rapid guideline: managing COVID-19, (2021). [PubMed]
- 30.Tang X., Feng Y.M., Ni J.X., Zhang J.Y., Liu L.M., Hu K., Wu X.Z., Zhang J.X., Chen J.W., Zhang J.C., Su J., Li Y.L., Zhao Y., Xie J., Ding Z., He X.L., Wang W., Jin R.H., Shi H.Z., Sun B. Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomized control trial. Respiration. 2021;100(2):116–126. doi: 10.1159/000512063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization, The COVID-19 Clinical management: living guidance, (2021).
- 32.Yu P., Li Y., Wan S., Wang Y. Efficacy of Lianhua Qingwen Granules combined with Arbidol in the treatment of mild new coronavirus pneumonia. Chin. Pharm. J. 2020;55(12):1042–1045. [Google Scholar]
- 33.Lian J., Zhang S., Li G., Shang D., Wang Q., Xu L., Shi Y., Liu X. Retrospective analysis of 38 cases of COVID-19 treated by integrated traditional Chinese and western medicine. J. Tradit. Chin. Med. 2020;61(24):2126–2130. [Google Scholar]
- 34.Xia L., Wu H., Liu P., Lu Y., Wang M., Sun Y., Lu H. Analysis on clinical efficacy and liver injury of 100 cases of COVID-19 treated by integrated traditional Chinese and western medicine. Shanghai J. Tradit. Chin. Med. 2020;54(07):23–28. [Google Scholar]
- 35.Xie Z., Bao J., Sun J., Huang L., Chen S., Shi D., Wu S., Zhang Z., Zhou M., Wen C. Curative effects of integrated traditional Chinese and western medicine treatment regimens on COVID-19: a prospective cohort study. Chin. J. Integr. Med. 2021;34:406–416. [Google Scholar]
- 36.Yao K., Liu M., Li X., Huang J., Cai H. Retrospective clinical analysis of traditional Chinese medicine Lianhua Qingwen in the treatment of new coronavirus pneumonia. Chin. J. Exp. Formulas. 2020;26(11):8–12. [Google Scholar]
- 37.Xiong X., Wang P., Su K., Cho W.C., Xing Y. Chinese herbal medicine for coronavirus disease 2019: a systematic review and meta-analysis. Pharm. Res. 2020;160 doi: 10.1016/j.phrs.2020.105056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pang W., Liu Z., Li N., Li Y., Yang F., Pang B., Jin X., Zheng W., Zhang J. Chinese medical drugs for coronavirus disease 2019: a systematic review and meta-analysis. Integr. Med. Res. 2020;9(3) doi: 10.1016/j.imr.2020.100477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao M., Tian J., Zhou Y., Xu X., Min X., Lv Y., Peng M., Zhang Y., Yan D., Lang S., Zhang Q., Fan A., Ke J., Li X., Liu B., Jiang M., Liu Q., Zhu J., Yang L., Zhu Z., Zeng K., Li C., Zheng Y., Wu H., Lin J., Lian F., Li X., Tong X. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharm. Res. 2020;161 doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tian J., Yan S., Wang H., Zhang Y., Zheng Y., Wu H., Li X., Gao Z., Ai Y., Gou X., Zhang L., He L., Lian F., Liu B., Tong X. Hanshiyi Formula, a medicine for Sars-CoV2 infection in China, reduced the proportion of mild and moderate COVID-19 patients turning to severe status: a cohort study. Pharm. Res. 2020;161 doi: 10.1016/j.phrs.2020.105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma S., Xu C., Liu S., Sun X., Li R., Mao M., Feng S., Wang X. Efficacy and safety of systematic corticosteroids among severe COVID-19 patients: a systematic review and meta-analysis of randomized controlled trials. Signal Transduct. Target Ther. 2021;6(1):83. doi: 10.1038/s41392-021-00521-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Edalatifard M., Akhtari M., Salehi M., Naderi Z., Jamshidi A., Mostafaei S., Najafizadeh S.R., Farhadi E., Jalili N., Esfahani M., Rahimi B., Kazemzadeh H., Mahmoodi Aliabadi M., Ghazanfari T., Sattarian M., Ebrahimi Louyeh H., Raeeskarami S.R., Jamalimoghadamsiahkali S., Khajavirad N., Mahmoudi M., Rostamian A. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur. Respir. J. 2020;56(6) doi: 10.1183/13993003.02808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nugroho C.W., Suryantoro S.D., Yuliasih Y., Rosyid A.N., Asmarawati T.P., Andrianto L., Setiawan H.W., Mahdi B.A., Windradi C., Agustin E.D., Fajar J.K. Optimal use of tocilizumab for severe and critical COVID-19: a systematic review and meta-analysis. F1000Research. 2021;10:73. doi: 10.12688/f1000research.45046.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaye A.G., Siegel R. The efficacy of IL-6 inhibitor Tocilizumab in reducing severe COVID-19 mortality: a systematic review. PeerJ. 2020;8 doi: 10.7717/peerj.10322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hermine O., Mariette X., Tharaux P.L., Resche-Rigon M., Porcher R., Ravaud P. Effect of Tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern. Med. 2021;181(1):32–40. doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gharebaghi N., Nejadrahim R., Mousavi S.J., Sadat-Ebrahimi S.R., Hajizadeh R. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect. Dis. 2020;20(1):786. doi: 10.1186/s12879-020-05507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao W., Liu X., Hong K., Ma Z., Zhang Y., Lin L., Han Y., Xiong Y., Liu Z., Ruan L., Li T. High-dose intravenous immunoglobulin in severe coronavirus disease 2019: a multicenter retrospective study in China. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.627844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of Lopinavir-Ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pan G., Du C., Liu Y., Liu Y., Chen Y., Han F., Chen Z., He Z., Xia Z., Yang S. Integrated traditional Chinese and western medicine in the treatment of 40 cases of critical patients with COVID-19. J. Huazhong Univ. Sci. Technol. (Med. Ed.) 2020;49(02):202–207. [Google Scholar]
- 50.Wang J.B., Wang Z.X., Jing J., Zhao P., Dong J.H., Zhou Y.F., Yang G., Niu M., Zhao X., Jiang T.J., Bi J.F., Xu Z., Zhang P., Wu D., Bai Z.F., Guo Y.M., Yu S.M., Sun Y.Q., Zhang Z.T., Zhan X.Y., Li P.Y., Ding J.B., Zhao P.F., Song X.A., Tang J.Y., He D.C., Chen Z., Qin E.Q., Wang R.L., Xiao X.H. Exploring an integrative therapy for treating COVID-19: a randomized controlled trial. Chin. J. Integr. Med. 2020;26(9):648–655. doi: 10.1007/s11655-020-3426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo H., Zheng J., Huang G., Xiang Y., Lang C., Li B., Huang D., Sun Q., Luo Y., Zhang Y., Huang L., Fang W., Zheng Y., Wan S. Xuebijing injection in the treatment of COVID-19: a retrospective case-control study. Ann. Palliat. Med. 2020;9(5):3235–3248. doi: 10.21037/apm-20-1478. [DOI] [PubMed] [Google Scholar]
- 52.Sun Q., An X., Xie P., Jiang B., Tian J., Yang Q., Li X., Luo M., Liu P., Zhao S., Duan L., Lang S., Fan A., Luo P., Lian F., Huang X., Tong X. Traditional Chinese Medicine decoctions significantly reduce the mortality in severe and critically ill patients with COVID-19: a retrospective cohort study. Am. J. Chin. Med. 2021;50 doi: 10.1142/S0192415X21500518. [DOI] [PubMed] [Google Scholar]
- 53.Tong X., Li X., Zhao L., Li Q., Yang Y., Lin Y., Ding Q., Lei Y., Wang Q., Song B., Liu W., Shen S., Zhu X., Huang F., Zhou Y. Discussion on traditional Chinese medicine prevention and treatment strategies of coronavirus disease 2019 (COVID-19) from the Perspective of “Cold-dampness Pestilence”. Chin. Med. J. 2020;61(06):465–470+553. [Google Scholar]
- 54.B. Zhang, Q. Wang, X. Gu, Manual of TCM diagnosis and treatment of COVID-19, (2020).
- 55.Liu J., Huang J., Bao J., Xie Z., Wen C. Differentiation reasoning for treatment of patients with difficulty in expectoration of sticky sputum induced by severe coronavirus disease 2019 and case analysis. Chin. J. Integr. Tradit. Chin. West. Med. Emerg. 2020;27(5):624–626. doi: 10.3969/j.issn.1008-9691.2020.05.027. [DOI] [Google Scholar]
- 56.W.h H.E., DING N., ZHENG C., CAI W., LIU J., LIU Q. Discussion on the medical records of COVID-19 from the perspective of dampness-toxin epidemic disease. J. Nanjing Univ. Tradit. Chin. Med. 2020;36(02):165–167. (+245) [Google Scholar]
- 57.Chen G., Su W., Yang J., Luo D., Xia P., Jia W., Li X., Wang C., Lang S., Meng Q., Zhang Y., Ke Y., Fan A., Yang S., Zheng Y., Fan X., Qiao J., Lian F., Wei L., Tong X. Chinese herbal medicine reduces mortality in patients with severe and critical Coronavirus disease 2019: a retrospective cohort study. Front. Med. 2020;14(6):752–759. doi: 10.1007/s11684-020-0813-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.National Pharmacopoeia Committee, Pharmacopoeia of the People's Republic of China, (2015).
- 59.Special Expert Group for Control of the Epidemic of Novel Coronavirus Pneumonia of the Chinese Preventive Medicine Association An update on the epidemiological characteristics of novel coronavirus pneumonia (COVID-19) Chin. J. Viral Dis. 2020;10(02):86–92. [Google Scholar]
- 60.Zhang X., Wu H., Ling Y., Li W., Li Q., Chen L. Analysis of clinical characteristics of elderly patients with novel coronavirus pneumonia. Geriatr. Health. 2020;26(05):752–756. [Google Scholar]
- 61.Huang X., Li Z., Li B., Sun S., Chen W., Wang M., Liu G. Clinical study on the treatment of elderly patients with SARS by integrated traditional Chinese and western medicine. Chin. J. Gerontol. 2004;03:223–225. [Google Scholar]
- 62.Wang Q., Shi G., Zhang Y., Lu F., Xie D., Wen C., Huang L. Deciphering the potential pharmaceutical mechanism of GUI-ZHI-FU-LING-WAN on systemic sclerosis based on systems biology approaches. Sci. Rep. 2019;9(1):355. doi: 10.1038/s41598-018-36314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang C., Huang L., Wang Y., Li X., Ren L., Gu X., Kang L., Guo L., Liu M., Zhou X., Luo J., Huang Z., Tu S., Zhao Y., Chen L., Xu D., Li Y., Li C., Peng L., Li Y., Xie W., Cui D., Shang L., Fan G., Xu J., Wang G., Wang Y., Zhong J., Wang C., Wang J., Zhang D., Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi: 10.1016/s0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Min X., Xiao M., Lei Y., Li X., Zhou Y., Zhao L., Wang H., Huang F., Lu C., Tong X. Analysis of TCM syndrome types in the recovery period of novel coronavirus pneumonia and key points of treatment. Sichuan Tradit. Chin. Med. 2020;38(07):4–6. [Google Scholar]
- 65.National Administration of Traditional Chinese Medicine, Recommendation on the Rehabilitation Guidance of Traditional Chinese Medicine for Coronavirus Disease in the Recovery Period (Trial), (2020). 〈http://yzs.satcm.gov.cn/zhengcewenjian/2020-02-23/13319.html〉. (Accessed February 26th 2020).
- 66.Zhang Q., Huang J., Liu S., Wang L., Zhang L., Wu M. Clinical study on integrated traditional Chinese and western medicine therapy to improve the quality of life of 31 SARS patients. Beijing Tradit. Chin. Med. 2004;01:22–24. [Google Scholar]
- 67.He S., Tian J., Li X., Zhou Y., Xiao M., Zhang Y., Min X., Li X., Jin D., Zhang Q., Zheng Y., Ke J., Li Q., Tao J., Song P., Wang H., Lv Y., Ding Q., Tang S., Lin J., Jiang Z., Zhang Z., Song J., Lian F., Tong X. Positive RT-PCR test results in 420 patients recovered from COVID-19 in Wuhan: an observational study. Front. Pharm. 2020;11 doi: 10.3389/fphar.2020.549117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Q., Bai M. Concerns and suggestions for management of coronavirus disease 2019 in convalescence with traditional Chinese medicine. J. Tradit. Chin. Med. 2021;62(05):371–374. +380. [Google Scholar]
- 69.Chen J., Liu Y. Investigation of psychological status of patients with COVID-19 and analysis of related influencing factors. Qingdao Med. Health. 2021;53(01):52–54. [Google Scholar]
- 70.Yang D., Chen L., Gu J., Chen L., Ji H., Chen X. Mental health status and psychological intervention of patients with new coronavirus pneumonia in Fangcang shelter hospital. Chin. J. Health Psychol. 2021;29(04):560–564. [Google Scholar]
- 71.The First Affiliated Hospital of the Zhejiang University School of Medicine, Handbook of COVID-19 Prevention and Treatment, (2020).
- 72.Xu X. The influence of Taijiquan on depression and hope level of patients with new coronavirus pneumonia in Fangcang shelter hospital. Gen. Nurs. 2020;18(07):829–830. [Google Scholar]
- 73.Bian Y., Qi W., Song Q., Li G., Fu Y., Tang X., Jiang Z., Wang Y. Effect of integrated traditional Chinese and western medicine treatment on 85 cases of SARS convalescent patients’ quality of life. Chin. J. Integr. Tradit. West. Med. 2003;09:658–660. [PubMed] [Google Scholar]
- 74.Reina-Gutiérrez S., Torres-Costoso A., Martínez-Vizcaíno V., de Arenas-Arroyo S.N., Fernández-Rodríguez R., Pozuelo-Carrascosa D.P. Effectiveness of pulmonary rehabilitation in interstitial lung disease including coronavirus diseases: a systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2021;102:1989–1997. doi: 10.1016/j.apmr.2021.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen X., Lin J., Yu H., Li Y., Zhao X., Su N., Yang M., Wang Z., Jia Y., Nong Y. Effect of traditional Chinese medicine treatment on lung function and quality of life in patients with SARS convalescence. Chin. J. Rehabil. Med. 2006;02:124–126. [Google Scholar]
- 76.Bian Y., Zhang Z., Qi W., Song Q., Tang X., Fu Y., Li G., Jiang Z., Wang Y. Clinical observation on intervention of series of Chinese herbal medicines on pulmonary lesions in the recovery period of SARS. Chin. J. Tradit. Chin. Med. 2003;12:100–102. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material