Abstract
Ovarian cancer is the major cause of gynecologic cancer-related mortality. Regardless of outstanding advances, which have been made for improving the prognosis, diagnosis, and treatment of ovarian cancer, the majority of the patients will die of the disease. Late-stage diagnosis and the occurrence of recurrent cancer after treatment are the most important causes of the high mortality rate observed in ovarian cancer patients. Unraveling the molecular mechanisms involved in the pathogenesis of ovarian cancer may help find new biomarkers and therapeutic targets for ovarian cancer. MicroRNAs (miRNAs) are small noncoding RNAs that regulate gene expression, mostly at the posttranscriptional stage, through binding to mRNA targets and inducing translational repression or degradation of target via the RNA-induced silencing complex. Over the last two decades, the role of miRNAs in the pathogenesis of various human cancers, including ovarian cancer, has been documented in multiple studies. Consequently, these small RNAs could be considered as reliable markers for prognosis and early diagnosis. Furthermore, given the function of miRNAs in various cellular pathways, including cell survival and differentiation, targeting miRNAs could be an interesting approach for the treatment of human cancers. Here, we review our current understanding of the most updated role of the important dysregulation of miRNAs and their roles in the progression and metastasis of ovarian cancer. Furthermore, we meticulously discuss the significance of miRNAs as prognostic and diagnostic markers. Lastly, we mention the opportunities and the efforts made for targeting ovarian cancer through inhibition and/or stimulation of the miRNAs.
1. Introduction
Ovarian cancer is the most frequent type of gynecologic malignancy and it is the fifth leading cause of cancer-related deaths worldwide. According to the estimations, ovarian cancer accounts for 1.3% of all new cancer cases and is the cause of 2.3% of all cancer-related deaths [1]. Despite the administration of standard therapy, which is the combination of debulking surgery and taxane- and platinum-based chemotherapeutic agents, ovarian cancer has witnessed a minimal improvement in the cure rate of the patients. In fact, the average five-year survival of the ovarian cancer patients is less than 50%, highlighting the fact that most of the patients are diagnosed at the advanced stage of the disease [2]. In this regard, studies have indicated that only 15% of the ovarian cancer patients are diagnosed when the tumor is in its early stage and, therefore, most patients are diagnosed after disseminating the tumor to the peritoneal cavity and distant organs. According to the reports, when the tumor is localized to one or both ovaries, ˂10% of the patients succumb to their disease, reflecting the significance of tumor dissemination and metastasis in ovarian cancer mortality [1, 3].
MicroRNAs (miRNAs) are a type of small noncoding RNAs with a length of about 19–25 nucleotides which function in the regulation of gene expression typically through the inhibition of translation and debilitation of messenger RNAs (mRNAs) stability [4]. Therefore, miRNAs control the genes expression correlated with various cellular pathways, including cell cycle regulation, inflammation, cell differentiation, apoptosis, and cell migration. Compelling pieces of evidence have established the role of miRNAs in human cancers. miRNAs dysregulation in human cancers usually occurs through amplification or deletion of miRNA genes, dysregulated epigenetic alterations, defective transcription of miRNAs, and miRNA synthesis machinery [5]. In this regard, the dysregulated miRNAs have been reported as tumor suppressors or oncogenes, augmenting the hallmarks of cancers, including intensified proliferative capacity, resisting cell death signals, and increased cell invasion and angiogenesis [6].
Regarding the high mortality of ovarian cancer which is mostly because of the difficulties in detecting this daunting disease at the primary stage and the paucity of efficacious therapies for patients in the advanced stage or with a recurrent malignancy, the investigation into the role of miRNAs in this cancer pathogenesis and metastasis is highly warranted. Given the fact that an amplitude of mRNAs can be regulated by miRNAs, it is reasonable to expect that a large number of cellular and molecular processes contributing to the formation and dissemination of ovarian cancer are controlled by miRNAs. In this regard, a plethora of studies have explored miRNAs functions in various steps of ovarian cancer, including cancer initiation, progression, and metastasis [7–9]. Understanding the function of microRNAs in the development and metastasis of ovarian cancer will hopefully shed light on finding new diagnostic and prognostic biomarkers for the patients [10–12]. Furthermore, given that miRNAs could act as either tumor suppressor gene or oncogene to regulate gene expression, miRNA-based anticancer therapies are being developed to ameliorate the disease response and cure rate of cancer patients, including ovarian cancer patients, either as a single therapy or with other systemic or targeted treatments [13]. The privilege of miRNA-based therapeutics is in their ability to simultaneously impact various effectors proliferation and tumorigenesis pathways. In this regard, several clinical trials are being recruited to unravel the promising potential of miRNA-based therapies in targeting of ovarian cancer [14].
In this review, we discuss the role of miRNAs in the tumorigenesis and metastasis of ovarian cancer and their use as biomarker for prognostic and diagnostic purposes in this devastating tumor. Furthermore, we illustrate the strategies for the application of miRNA-based therapies in targeting ovarian cancer. We finally present the perspectives, future directions, and the challenges beyond the application of miRNAs in the development of new treatments for targeting ovarian cancer.
2. miRNA Biogenesis and Function
miRNAs are small noncoding RNA fragments with the length of 18–24 nucleotides. Their function is to regulate the gene expression. These pieces were first discovered in Caenorhabditis elegans in 1990s [15]. Thousands of miRNAs have been detected in a variety of species (i.e., single-cell algae to humans) [15, 16]. More studies have suggested that miRNAs are highly conserved in various species [17, 18]. Bioinformatic evaluations have predicted that miRNAs are expected to control more than fifty percent of the human protein-coding genes [19, 20]. Therefore, given the regulatory effects on different mRNAs, miRNAs play crucial role in numerous cellular processes, including differentiation, cell growth, and apoptosis.
The biogenesis of miRNA begins with the simultaneous or subsequent processing of RNA polymerase II/III transcripts [4, 21]. Around half of the known miRNAs are intragenic, most of which are introns originated and somewhat few are created from protein-coding exons. Left over intergenic miRNAs are transcribed and regulated by specific genes and their promoters [4, 22, 23]. miRNAs are considered as a family when they are transcribed as clusters (i.e., one long transcript) that might have analogous seed areas [4, 24].
miRNAs biogenesis is carried out through both canonical and noncanonical pathways. The chief pathway involved in the processing of the miRNA is the canonical biogenesis. In this pathway, RNA polymerase II transcribes pri-miRNAs with more than 200 nucleotides in length from related genes, and then, by microprocessor complex, they can be processed into pre-miRNAs. The microprocessor complex is comprised of an RNA-binding protein called DiGeorge Syndrome Critical Region 8 (DGCR8) and Drosha, a ribonuclease III enzyme [4, 25]. Two steps are required for pri-miRNAs to go through the processing. These two processes can be catalyzed by two enzymes of the RNase III family (i.e., Drosha and Dicer). Initially, N6-methyladenylated GGAC and other motifs within the pri-miRNA should be recognized by the complex of Drosha-DGCR8 recognizes [4, 26]; then Drosha slices the pri-miRNA duplex and generates miRNA precursors (pre-miRNA) which are around 70-nucleotide hairpin intermediates. This causes the formation of a 2-nucleotide 3′ that is overhang on pre-miRNA [27]. When pre-miRNAs are created, through a complex called exportin 5 (XPO5)/RanGTP, they can be transferred to the cytoplasm. The next step is the process of them by the RNase III endonuclease Dicer [25, 28] that slashes the transferred pre-miRNAs into ∼22-nucleotide miRNA/miRNA duplexes in cytoplasm. This process encompasses the deletion of the terminal loop and results in a mature miRNA duplex [4, 29]. The name of the mature miRNA form is determined by the direction of the miRNA strand. The 3p and the 5p strands come from the 3′ end and the 5′end of the pre-miRNA hairpin, respectively. Next, the guide strand is integrated into the RNA-induced silencing complex (RISC). Both 3p and 5p strands which are emanated from the mature miRNA duplex can be overloaded to proteins of Argonaute (AGO) family (in humans called AGO1-4) using an ATP-dependent related manner [4, 30]. For each miRNA, the amount of AGO-loaded 3p or 5p strands varies and it highly depends on the type or environment of the cell. This ranges from almost equal proportions to mainly one cell type and environment or the other [4, 31]. A 5 U at nucleotide position 1 or the thermodynamic stability at the 5 ends of the miRNA duplex has a role in selection of 3p or 5p strands in part [4, 32]. Typically, the guide strand is opted according to stability and Uracil content. Therefore, the strand with lower 5 stability or 5 Uracil is preferred to be loaded into AGO. Through the different mechanisms, the passenger strand (i.e., the unloaded strand) will be separated from the guide strand according to the degree of complementarity. This strand of miRNA which has no mismatches is sliced by AGO2. Then it is degraded by cellular machinery that can generate a strong strand bias. miRNA duplexes with central mismatches or non-AGO2-loaded miRNAs are passively disentangled and degraded [4, 21]. miRNAs can aim at the 3 untranslated regions (UTRs) of mRNAs and also can cause degradation of mRNA or translational inhibition of mRNA. Hence, they can suppress protein synthesis and gene expression (Figure 1) [16–18, 20].
Figure 1.
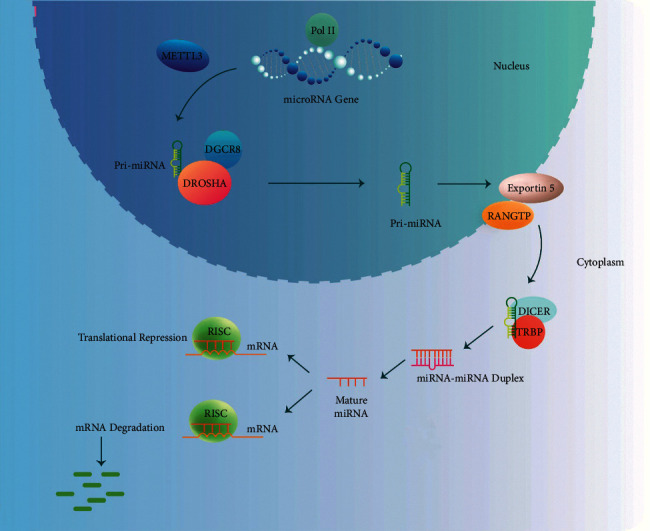
The biogenesis of miRNAs. miRNA genes are usually transcribed by RNA polymerase II, which first produces large transcripts, referred to as pri-miRNAs. The pri-miRNAs are then cleaved by a complex, which is composed of RNA-binding protein DGCR8 and type III RNase, termed Drosha. The resultant ∼85-nucleotide stem-loop structure is pre-miRNA. Following the transportation from nucleus to cytoplasm by Ran/GTP/exportin 5 complex, the pre-miRNAs are cleaved by another RNase III enzyme, which is called Dicer. The miRNA duplexes are then unwound, and the mature miRNA is incorporated into an RISC complex. According to the complementarity between the miRNA and the targeted mRNA, the miRNA-loaded RISC complex mediates gene silencing by cleavage or by a translational repression.
So far, several biogenesis pathways of noncanonical miRNA have been explained. In mentioned pathways, various combinations of the proteins engaged in the canonical pathway are utilized. They are predominantly Drosha, Dicer, exportin 5, and AGO2 (Figure 1). Most of the time, the noncanonical miRNA biogenesis is categorized into Drosha/DGCR8-independent as well as Dicer-independent pathways. Of note, pre-miRNAs that are created by the Drosha/DGCR8-independent pathway seem to be the substrates of Dicer. Mirtrons and 7-methylguanosine- (m7G-) capped are examples of such pre-miRNAs. During splicing, they can be generated from the introns of mRNA [4, 33, 34]. Exportin 1 directly contributes to transporting these emerging RNAs to the cytoplasm without cleavage by Drosha. The m7G cap prevents loading of 5p strands into Argonaute that causes a strong bias in the 3p strand [4, 35]. Drosha, instead, processes the Dicer-independent miRNAs from endogenous short hairpin RNA (shRNA) transcripts [4, 36]. In order to complete the maturation of pre-miRNAs within the cytoplasm, they need AGO2 as their length is not long enough for them to be suitable substrates for Dicer. This process consecutively fosters the loading of the complete pre-miRNA into AGO2 and AGO2-dependent slicing of the 3p strand. The maturation process is completed by the 3–5 trimming of the 5p strand [4, 37].
miRNAs have critical roles in numerous types of biological processes such as cell proliferation, differentiation, development, metabolism, migration, and apoptosis through posttranscriptional regulation mechanisms [16–18, 20]. Also, any change in their expression is associated with several human pathologies [20, 38–40]. Moreover, miRNAs can be found in a stable form in plasma and serum samples [20, 41, 42], saliva [43], urine [44], colostrum, tears, peritoneal fluid, seminal fluid, bronchial lavage [4, 45], milk [46], ovarian follicular fluid [47], and cell culture supernatants [48, 49]. Therefore, miRNAs can be found in two stabilized forms in the extracellular spaces. Also, it has been reported that both extracellular and intracellular miRNAs can regulate physiological and pathological events and, therefore, evaluation of their expression profiles can be utilized as potential beneficial markers for the diagnosis of human diseases [20]. Pritchard et al. evaluated circulating miRNAs in 79 solid tumors and noticed that more than half of them were highly expressed in one or more blood cell type. Furthermore, they indicated that miRNA biomarkers in plasma are highly associated with relevant hemolysis (blood cell counts). This observation suggests that the miRNAs in both plasma and serum are mostly stemmed from blood cells [50]. Researchers [51–54] have detected specific kinds of miRNAs enriched in tissues, such as muscle-enriched miR-133, liver-enriched miR-122, and heart-enriched miR-208 in plasma. There are three pathways for their release into the extracellular space: (1) tissue injury, inflammation, and cell death that cause passive leakage from broken cells, (2) microvesicles (MVs) (i.e., active secretion through membrane-enclosed cell fragments) that consist of discarding vesicles and exosomes (under physiological and pathological conditions, almost all cell types that are involved in releasing MVs [20, 55–59]), and (3) active secretion through a protein-dependent MV-free RNA-binding pathway. It is recommended by some researchers [60–63] that numerous RNA-binding proteins that include AGO2, high-density lipoprotein (HDL), and nucleophosmin 1 (NPM1), are capable of merging with miRNAs and bring them outside places of the cells. miRNAs secretion by MVs and HDL-binding is an active process and requires energy in comparison with passive leakage. Active pathways which secrete extracellular miRNAs can mediate regulation of biological processes.
Lately, it is believed that extracellular/miRNAs and those circulating in the blood can be used as biomarkers of various diseases and also play a significant function in cell-cell communications. Not only do these miRNAs have active functions in recipient cells but also a number of them can interact with receptors in cell surface. Therefore, the miRNAs' activities are like hormones. The existence and stability of extracellular miRNAs in circulation indicate an interesting function of these cell-derived miRNAs, but the detailed functions of many secreted miRNAs, especially MV-free, protein-binding extracellular miRNAs, remain to be elucidated. However, in intercellular communication, the extracellular miRNAs seem to be a unique component. Moreover, a new insight can be given by the crosstalk mediated by these miRNAs to unravel the mechanisms of dysfunctionality. Some studies suggest that, during tumor progression, cancer cells dynamically are capable of packaging the miRNAs into MVs and help them to be transferred to the surroundings in order to change tumor microenvironment. Also, it has been found out that extracellular miRNAs can be considered as diagnosis and prognosis biomarkers in various disorders such as cancers [41–44], diabetes, and viral infections [64, 65]. Furthermore, specific genes can be targeted by miRNAs and siRNAs, and these RNAs can regulate the expression of related proteins. Since these RNA molecules can modulate the genes with abnormal expression in different diseases, they may be considered as the promising potential therapy in near future [66, 67].
3. The Dysregulation of miRNAs in Cancer
Cancer is a multistep procedure, during which genetic changes in normal cells cause progression of a sequence of premalignant conditions (initiation) into a malignant condition (progression). The resulting cellular phenotype that is transformed has many features. This transformation enables the cells to proliferate independently from growth signals and make them irresponsive to inhibitory signals, elude designed cell death pathways (apoptosis), conquer intrinsic cell replication restrictions, stimulate and maintain angiogenesis, and produce new discontinued colonies with the primary tumor [68, 69]. The leading cause of cancer initiation and progression is dysregulation of genes which are involved in biological processes such as cell proliferation, differentiation, and/or apoptosis. Genes that are correlated with development of various cancers are described as oncogenes and tumor suppressors. Based on function, oncogene products can be classified into six groups: growth factors, growth factor receptors, transcription factors, chromatin remodelers, signal transducers, and apoptosis regulators [69, 70]. Overexpression of the products of these genes provides selective growth advantage that causes tumor growth. The genetic changes can mediate the oncogene activation and result in strengthening the gene, alter promoters/enhancers capability to raise gene expression, or permanently modify protein structure to an active state [70–72]. On the other hand, the products of tumor suppressor genes have regulatory functions in biological processes. Loss or reduced function of tumor suppressors causes dysregulation contributing to cancer [73]. Lately, the description of both tumor suppressors and oncogenes has been changed from the classical protein coding genes and, therefore, various types of noncoding RNAs, like miRNAs, are included in this description [74, 75]. miRNAs play a regulatory role in several cellular and metabolic pathways, especially those that control cell proliferation, differentiation, and survival [40, 76–83]. The dysregulated expression of miRNAs has been indicated in most tumors found [74, 84], but the investigation on grouping of miRNA as tumor suppressors or oncogenes has been quite challenging, since expression patterns of miRNAs have been complicated. For various tissues and differentiation states, the expression patterns of miRNAs differ. Thus, there are two difficulties in the miRNA classification [85–89]. It is sometimes unclear that altered miRNA patterns directly or indirectly contribute to cancer in cellular phenotypes. Moreover, several targets can be regulated by a single miRNA [90]. This phenomenon, as well as tissue specific expression, introduces a single miRNA, either oncogene or tumor suppressor gene, in various contexts.
3.1. miRNAs Activity on Cancer and Its Dysregulation
In cancer, the dysregulation of pathway components involved in miRNA biogenesis plus its expression state has been revealed (Figure 2). For example, there is a negative correlation between increase and decrease in the expression of Dicer and Drosha with the advanced stage of tumor and poor clinical outcome. Existing of any flaws in the pathway components of the miRNA biogenesis poses significant change in expression levels of many cellular miRNAs. There are several examples of tumors with global downregulation of miRNA, particularly in differentiated ones. A suggested explanation for this common miRNA downregulation in cancerous cells is that several miRNAs' role is to describe the properties of specific lineages. So, the small abundance of miRNAs raises the undifferentiated state of tumor cells and increases the potential metastasis and invasion of human cancers [88].
Figure 2.
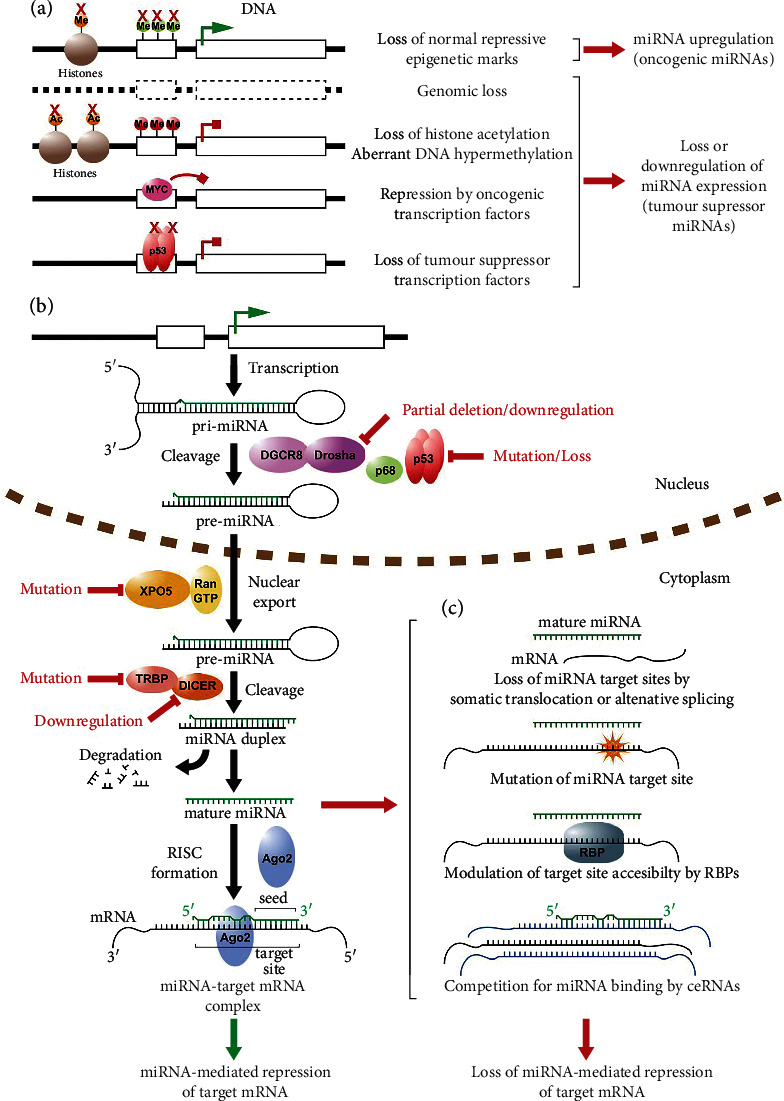
miRNA dysregulation in cancer. A schematic representation depicting the canonical miRNA biogenesis pathway and the general mechanisms whereby normal miRNA expression and function can be dysregulated in cancer. (a) Dysregulation of miRNA gene transcription in cancer through genetic, epigenetic, and transcriptional mechanisms. Active transcription is indicated by green arrow and blocked transcription by red block arrow. Red crosses indicate loss of the gene, epigenetic mechanism, or transcription factor. Me, methylation; Ac, acetylation. (b) Simplified canonical pathway of miRNA biogenesis and processing. Steps commonly dysregulated in cancer are indicated in red. (c) Mechanisms are prevalent in cancer allowing mRNAs to escape regulation by miRNAs. RBP, RNA binding protein. This figure is reprinted with permission from [91] under a Creative Commons Attribution 4.0 International License.
According to conducted studies, haploinsufficiency or knockdown of Dicer 1 in rodents has been reported to promote tumor development and progression. This supports the general “tumor suppressor” action of global miRNAs. Conversely, the levels of the processing enzymes such as Drosha and Dicer have increased in tumor cells even though global upregulation of miRNAs is unusual [92–94]. Some examples of dysregulation of various components of the miRNA biogenesis pathway are as below.
3.2. Drosha Microprocessor Dysregulation
RNA polymerase II transcribes long pri-miRNAs that endure primary processing in the nucleus by the function of a complex, the Drosha microprocessor, which comprises different cofactors including of DGCR8. In various types of cancers, the upregulation of DGCR8 has been observed. Lately, analyses of gene mutation have indicated frequent heterozygous mutations in the Drosha gene in Wilms tumors [45, 95–99]. It is thought that this mutation interferes with metal binding and destructively regulates the processing function related to Drosha in a main fashion. It reveals a consistency with the global downregulation of miRNAs which is shown in Wilms tumors that harbor mutated Drosha [45, 95–98]. Besides E1147K mutation, numerous other splice-site, missense, and nonsense mutations in Drosha gene have been detected in Wilms tumors, but their effect has not been fully elucidated. Other mutations like somatic as well as germline mutations in DGCR8 have also been observed in Wilms tumors [45, 97, 98]. A “hot-spot” in the first double-strand RNA (dsRNA) binding domain (dsRBDe) of DGCR8 is the missense mutation of E518K, which contributes to over 70% of the DGCR8 mutations in Wilms tumors [45, 97, 98]. The E518K mutation decreases vital miRNAs in tumors [45, 97, 98]. This is in accordance with the fact that DGCR8 knockdown stimulates tumor growth [92]. The expression of alternatively spliced variants of Drosha alongside the gene mutations has been stated in some human cancers, including melanoma and teratocarcinoma cells [100]. The variants of splice site can encode a Drosha protein with a truncated carboxyl- (C-) terminal RNase domain. Its dsRBD is not capable of interacting with DGCR8 and thus is functionally cooperated and functions as a detrimental leader [100]. On the other hand, there is a high copy number of the Drosha gene or overexpression of Drosha protein in over half of the advanced cervical squamous cell carcinomas, which in turn cause a global shift in levels of miRNA [101, 102]. Further studies are needed to reveal why mutations in Drosha gene are often observed in Wilms tumors, besides why Drosha levels are regulated in contrary directions reliant on the tumor type.
Drosha acetylation by CGN5, CBP, and P300 inhibits ubiquitin-mediated degradation and leads to Drosha stabilization [103] and DGCR8 deacetylation by histone deacetylase 1 (HDAC1) increases the resemblance between DGCR8 and pri-miRNAs [104], while phosphorylation of DGCR8 by Erk contributes to stabilizing the DGCR8 protein and promotes miRNA production [105]. It is unclear whether alterations in miRNA modification process resulting from the Drosha PTMs (posttranscriptional modifications) are practical in cancer. In contrast, both HDAC1 and GSK3B are regularly dysregulated in cancer; thus it is probable that various Drosha PTMs may be observed in cancer cells in comparison with nontumor cells.
Several nuclear proteins can play a role in regulating the activity of Drosha in a way that it commonly influences the biogenesis of only a small subset of miRNAs. An example of such nuclear protein is adenosine deaminase acting on RNA (ADAR). ADAR is an RNA-editing enzyme that transforms adenosine (A) to inosine (I) in double-stranded RNAs. ADAR1 constructs a complex with DGCR8 and inhibits the activity of Drosha [106]. The level of ADAR1 decreased in metastatic melanoma, and two miRNAs (miR-17 and miR-432) are excessively produced and stimulate tumor development [106]. Also, Smad proteins, which are the signal transducers of the transforming growth factor-β (TGF-β) family of growth factors, mediate the change in Drosha activity in the nucleus. While Smad proteins were predominately cytoplasmic at even state, they are translocated to the nucleus during ligand activation.
Smad proteins are inherent transcription factors with DNA attachment and transcription activating domains [107]. Nevertheless, these proteins are enlisted for the complex of Drosha microprocessor via physical interaction with the RNA helicase p68, also identified as DDX5. Pri-miRNA is escalated here to pre-miRNA processing of approximately 20 miRNAs [108], which include miR-21 and miR-199. The studies have shown that miR-21 as an oncogenic miRNA (onco-miR) is frequently upregulated in roughly all tumor samples and is recognized as an inhibitor of a large number of tumor suppressor genes such as PDCD4, PTEN, SPRY1/2, TPM1, and TP53BP2 [109]. Furthermore, miR-21 is able to lead tumorigenesis by preventing the negative regulators like Ras/MEK/Erk pathway [110].
The regulation of pri-miRNA processing mediated by R-Smad is obtained by direct connection of R-Smads with a sequence component in the stem district of pri-miR-21 [111]. In addition, similar to Smads, p53 can provoke the expression of several suppressor miRNAs (suppressor-miRs) (miR-143, miR-15/16, miR-203, and miR-145) by linking with the complex of Drosha microprocessor through p68 and expediting pri-miRNA processing [112]. Although genotoxic stimuli induce acetylation of K120 in the DNA binding domain of p53, they do not influence the transcription activity of p53. They lead to the correlation of p53 with the complex of Drosha microprocessor and promote miR-203 quantities to increase apoptosis as a replacement for cell cycle arrest [113]. It is not clear yet how p53 can regulate the processing of a specific subset of miRNA and how activation of Smads mediated by TGF-β can influence the process of miRNA regulation mediated by p53.
A new research also detected Yes-associated protein (YAP), a signal transducer of the Hippo pathway, which monitors the size of organ by sensing cell density, as a regulating factor of Drosha microprocessor activity [114]. In normal or cancerous cells at low cell density in which Hippo signaling is not active, YAP is collected in the nucleus and is capable of binding to the RNA helicase p72 (also known as DDX17) and then sequestering it from the Drosha microprocessor, which can inhibit a subset of miRNAs that aim at Myc [114]. There is a possibility that the inhibition of Drosha by YAP/p72 complex may play an important role in the common downregulation of a large group of miRNAs in cancer. The single-strand RNA binding factor KH-type splicing regulatory protein (KSRP, also known as FUBP2) combines to a small subset of pri-miRNAs through their terminal loops. This includes onco-miRs in the let-7 family, miR-21 and miR-125, and stimulates processing by Drosha by an unidentified mechanism [115, 116]. When the PI3K/Akt signaling pathway is activated, KSRP is phosphorylated at the sites of S274 and S670, which triggers enhanced binding to pri-miRNAs and induces their Drosha-dependent processing [115]. Moreover, DNA damage leads to activation of KSRP-enhanced miRNA biogenesis [117]. In comparison with the chronic phase disease, KSRP is exceedingly expressed in chronic myeloid leukemia (CML) acute phase/blast crisis. However, it is not clear whether changed expression or function of KSRP aids in leukemogenesis mediated by dysregulation of miRNA processing [118].
Some other RNA binding proteins, like hnRNP A1, serine/arginine-rich splicing factor 1 (SRSF1), and FUS (also known as TLS), have been discovered to be related to pri-miRNAs and assist Drosha processing, but the direct contribution of them in tumorigenesis has not been evidenced. Modification of pre-miRNA export, dysregulation of the Dicer/TRBP processing complex, and posttranslational modifications of Ago proteins can be other reasons for dysregulation, which are described further in [95].
4. miRNAs and the Pathogenesis and Metastasis of Ovarian Cancer
Ovarian cancer cells could originate from epithelial, stromal, or germline cells, among which epithelial tumors are the most common type [119]. Demographic and lifestyle factors, ethnicity, advanced age, and female reproductive hormones are the risk factors associated with ovarian cancer. However, a positive family history of ovarian, uterine, breast, or colon cancer is linked to mutations in BRCA1, BRCA2, or TP53 genes, the most potent risk factor [120]. At the molecular level, ovarian cancer is considered to be an extremely heterogeneous disease with somatic and germline mutations, in tumor suppressor genes and DNA repair genes, and oncogenes such as BRCA1, BRCA2, KRAS, BARD1, BRAF, PTEN, PIK3CA, BRIP1, MRE11A, MSH6, CHEK2, NBN, RAD50, PALB2, RAD51C, and TP53 have role in proliferation, invasion, and metastasis process of ovarian cancer cells [121]. Alongside genetic mutations, alteration in the expression of these genes and other genes related to carcinogenesis may result in the aberrant proliferation of ovarian cancer cells.
As mentioned earlier, miRNAs are determined to regulate up to half of activities of all protein coding genes [122] and are observed to manage almost all the cellular processes [123]. Several studies discovered that miRNAs' dysregulation leads to different human diseases like cancer [124, 125]. Due to their involvement in oncogenesis, miRNAs function as either tumor suppressors or oncogenes, according to their function and expression pattern [126]. High-throughput technologies indicated that miRNAs have a fundamental effect on cancer-specific targets in all cancers [38, 127]. Broad dysregulation of miRNAs ultimately causes cancer. There are altered mechanisms that are responsible for this circumstance: DNA point mutations, epigenetic mechanisms, translational modulations, chromosomal alterations, and genetic and epigenetic modifications in various stages of transcriptional and posttranscriptional pathways [128]. Low activities of Dicer and Drosha are closely correlated with end-stage ovarian cancer and low suboptimal surgery, respectively [124]. The upregulation and dysregulation of miRNA in ovarian cancer are key features of malignancies regarding tumor suppressor genes or oncogenes [40]. The miRNAs presence in serum is associated with the occurrence of malignancies and tumors and has also been used as a diagnostic tool for detecting cancers [129].
Numerous studies confirmed that approximately 40% of the miRNA genes reveal modified DNA copy numbers [130, 131]. The worldwide miRNA expression of human ovarian cancer and the differential expression in cancer cells and normal ovarian tissue have been investigated by Iorio et al. which revealed the upregulation of miR-200a, miR-141, miR-200b, and miR-200c in carcinomas and the downregulation of miR-125b1, miR-145, miR-140, and miR-199a. The deletion of miR-140 in ovarian cancers leads to the belief that it is related to invasion, involving matrix metalloproteinase-13 (MMP-13), fibroblast growth factor 2, and angiogenic VEGF-A [132]. miR-138 downregulates the expression of SOX4 and HIF-1a and, consequently, suppresses ovarian cancer invasion and metastasis. These are more involved in the regulation of EGFR and Slug pathways, respectively [133]. The overexpression of miR-320 was reported to be tangled in invasion and metastasis in ovarian cancer [134]. Similarly, the overexpression of miR-196a stimulates epithelial ovarian cancer (EOC) to migrate [135].
In ovarian cancer, miRNAs' involvement in various cellular pathways includes cell cycle, tumorigenesis, proliferation, apoptosis, invasion, and metastasis. The expression profiling of miRNAs in various cancer types revealed their pivotal roles in recognizing the miRNAs' function in ovarian cancer. It has been shown that miRNAs are oncogenes and tumor-suppressor genes; hence, they can be considered reliable biomarkers for ovarian cancer. miRNAs are more specific and sensitive than any other biomarkers and their effectiveness is worth to be scrutinized for the diagnosis and prognosis of ovarian cancer [39, 136]. Some significant miRNAs that have a role in the pathogenesis and metastasis of ovarian cancer are listed in Table 1. The following is a concise overview of some important miRNAs associated with ovarian cancer's pathogenesis and metastasis processes.
Table 1.
Targets and functions of important miRNAs associated with ovarian cancer.
| miRNA | Target | Alteration | Cellular function | Ref. |
|---|---|---|---|---|
| miR-214 | Sema 4D (Semaphoring 4D) | Upregulation | Inhibits cell proliferation and promotes apoptosis | [137] |
| LHX6 (LIM Homeobox 6) | Downregulation | Induces apoptosis | [138] | |
| PETN (phosphatase and tensin homolog) | Upregulation | Promotes radioresistance | [139] | |
| miR-182 | BNIP3 (BCL2 interacting protein 3) | Upregulation | Inhibits proliferation and migration | [140] |
| miR-23b | CCNG1 (cyclin G1) | Upregulation | Inhibits ovarian cancer tumorigenesis and progression | [141] |
| miR-381 | YY1 (the ubiquitous transcription factor yin yang 1) | Upregulation | Inhibits ovarian cancer cell proliferation, migration, and invasion | [142] |
| miR-132 | E2F5 (transcription factor) | Upregulation | Inhibits cell migration and invasion of cancer cells | [143] |
| miR-143-3p | TAK1 (transforming growth factor-β-activated kinase 1) | Upregulation | Inhibits the proliferation, migration, and invasion of ovarian cancer cells | [144] |
| CTGF (connective tissue growth factor) | Upregulation | Inhibits ovarian cancer cells proliferation, migration, and invasion | [145] | |
| miR-145 | CCND2 & E2F3 (cyclin D2) | Upregulation | The invasion and proliferation suppressor | [146] |
| miR-34a-5p | NEAT1 (nuclear paraspeckle assembly transcript 1) | Downregulation | Suppresses Cell proliferation and triggers apoptosis | [147] |
| miR-383-5p | TRIM27 (tripartite motif containing 27) | Upregulation | Proliferation, chemosensitivity suppressor and enhancer in ovarian cancer cells | [148] |
| miR-148a-3p | c-Met (tyrosine-protein kinase Met) | Upregulation | Inhibits invasion of ovarian cancer cells and cell migration | [149] |
| miR-141 | KEAP1 (Kelch-like ECH-associated protein 1) | Upregulated | Cisplatin resistance | [150] |
| miR-218 | RUNX2 (runt-related transcription factor 2) | Upregulated | Inhibits proliferation and invasion | [151] |
| miR-199a | HIF-1α & HIF-2α (hypoxia-inducible factor) | Upregulation | Decreases migration and metastatic tumor formation | [152] |
| miR-491-5p | EGFR (epidermal growth factor receptor) and BCL-XL | Upregulation | Induces cancer cell apoptosis by blocking downstream AKT and MAPK signaling pathways | [153] |
| miR-744-5p | HNRNPC (heterogeneous nuclear ribonucleoproteins C1/C2) and NFIX (nuclear factor 1 X-type) | Upregulation | Induces tumor cell apoptosis | [154] |
4.1. miR-182
miR-182-5p is an oncogenic miRNA positioned on 7q32.2 region and its overexpression is prevalent in many solitary malignancies, including melanoma [155], colon cancer [156], breast cancer [157], and ovarian cancer [155, 158]. miR-182 is one of a few miRNAs affected by cellular stress or damage to DNA [19], and, therefore, its overexpression results in tumor development. The oncogenic features of miR-182 in ovarian cancer are noticeably due to impairment in double-strand breaks repair in DNA, negative control of BRCA1, suppression of metastasis suppressor 1 (MTSS1), and overexpression of high-mobility group AT-hook 2 (HMGA2) [155, 159–162]. MTSS1 and HMGA2 may represent a central role in miR-182-mediated tumor metastasis. HMGA2 protein, encoded by the HMGA2 gene, has different effects on a number of biological processes, including responses to DNA damage repair, cell cycle, apoptosis, cell senescence, epithelial-mesenchymal transition (EMT), and telomere restoration, so that its aberrant expression in adult tissues is generally associated with both malignant and benign tumor formation [163]. Recent investigations showed that HMGA2 is significantly overexpressed in most of ovarian cancers [164]. Multiple studies have reported that miR-182 enhanced HMGA2 expression and promoted ovarian cancer invasion via direct binding to the SNAIL1 promoter. SNAIL1 is a zinc-finger transcription factor playing critical roles in tumor progression and EMT [165, 166].
Metastasis suppressor 1 (MTSS1), which is also referred to as missing-in-metastasis (MIM), is a potential metastasis suppressor protein [165], which functions as a cytoskeletal scaffold protein and influences cytoskeletal movements through interacting with Rac, actin, and actin-associated proteins [167]. Stress fiber formation (F-actin) is one of the most critical events of cytoskeleton rearrangement during migration and invasion of cancer cells blocked by MTSS1 (27). MTSS1 is considered as a top target of miR-182 and, therefore, is significantly downregulated in many metastatic types of cancer [162].
Discoidin domain-containing receptor 2 (DDR2) is another miR-182 target gene that mediates cell interactions of extracellular matrix (ECM) so may contribute to cancer cell proliferation and migration [168–170]. DDR2 affects the SNAIL1 function in ovarian cancer and contributes to invasion and cell migration [171]. Ramalho et al. have revealed that the overexpression of DDR2 leads to decreased miR-182 levels and worse progression-free survival. Accordingly, these molecules might be related to cancer progression [172].
DCN (Decorin), AKT3 (AKT Serine/Threonine Kinase 3), and TIMP2 (tissue inhibitor of metalloproteinases 2) are the other novel candidate target genes for miR-182 involved in the regulation of ovarian cancer biological processes [173]. Due to the above statements, blocking the expression of miR-182 will rescue some well-known genes suppressing the tumor and recover the cell's critical biologic functions.
4.2. miR-200 Family
There are five miRNAs (miR-141, miR-200a, b, c, and miR-429) in miR-200 family and they are abundantly expressed in epithelial tissues. miR-200b/a and miR-429 are on chromosome 1p36.33 and miR-200a/141 are located on different clusters of chromosome 12. miR-200c can promote CD44 expression. CD44 is a multifunctional molecule which is involved in cell-to-cell interactions, adhesion, and migration. Consequently, decreased expression of miR-200c could lead to metastatic ovarian cancer [174]. Due to its dual function in various cancer cells, miR-429 is a tumor suppress in bladder cancer [175], gastric cancer [176], and cervical cancer [177] and has an oncogenic role in colorectal and ovarian cancers. Plenty of studies indicate that miR-429 has a fundamental role in the initiation and promotion of EOC through the regulation of EMT. In this regard, it has been identified that promoter hypermethylation of miR-429 suppressed its expression and therefore upregulated its corresponding target genes, including KIAA0101, ZEB1, and ZEB2 [178].
It has been identified that ZEB1, as a mesenchymal marker repressing the transcription of E-cadherin, is an EMT activator [179]. Indeed, inhibiting ZEB1 and ZEB2 upregulates E-cadherin and reduces cell motility [180]. ZEB1 and ZEB2, as targets of miR-429, inhibit this regulatory miRNA and alter the expression of the calcium-dependent adhesion protein E-cadherin [181, 182], inducing EMT in various cell types [183] (Figure 3).
Figure 3.
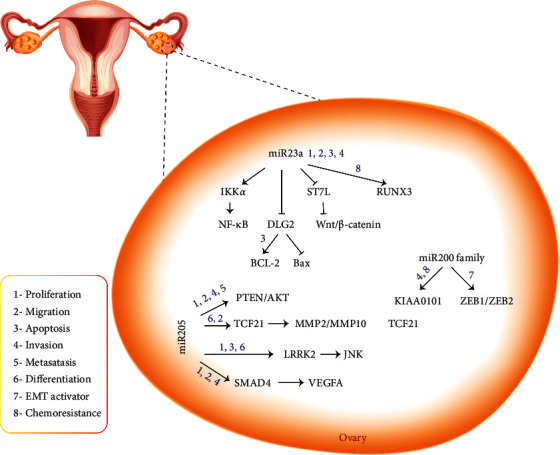
This schematic figure represents the main miRNAs and gene targets involved in the biological processes of ovarian cancer. miR-200 family are abundantly expressed in epithelial tissues. This miRNA induces the expression of CD44, which plays a key role in cell-to-cell interactions, adhesion, and migration. The cluster of the miR-23a is involved in the initiation and progression of ovarian cancer through affecting transcription factors like NF-κB and WNT pathway, regulating the transcription of various genes involved in cytokine production, cell survival, proliferation, and differentiation. miR-205 targets many biological processes like proliferation, differentiation, and migration and facilitates apoptosis in some ovarian cancer cells.
KIAA0101 protein (PCNA-associate factor) is a cell cycle-regulated protein occurring most abundantly during S/G2 phase and acts as a regulator of DNA repair during DNA replication (42). KIAA0101 is required for the EOC cell invasion and chemoresistance (Figure 3) and is the target gene for miR-429. Therefore, decreased expression of miR-429 in the metastatic cells in EOC tissues leads to the upregulation of KIAA0101 and enhances the migratory activity and chemoresistance of EOC cells [184].
4.3. miR-23a
miR-23a belongs to the miR-23∼27∼24 cluster in 19p13, where the miR-23a-27a-24-2 cluster is located [185]. The transcription of this cluster results in the production of a pri-miRNA transcript consisting of miR-23a, miR-27a, and miR-24. miR-23a, as an oncogenic miRNA (onco-miR), has multiple-gene regulatory functions and is significantly increased in different cancer tissues such as bladder cancer, glioblastoma, pancreatic cancer, and gastric cancer [186–188]. Moreover, this miRNA is involved in the initiation and progression of a variety of cancers [189].
Ikappa B kinase-alpha (IKKα) and suppression of tumorigenicity 7 like (ST7L) are two miR-23a target genes that are shown to provoke the oncogenic role of miR-23a and contribution in the malignancy of EOC cells through effects on nuclear factor kappa B (NF-κB) and Wnt pathways [190]. NF-κB transcription factor acts as a protein complex regulating the transcription of various genes involved in cytokine production, cell survival, proliferation, and differentiation [191]. IKKα is one of the NF-κB pathway proteins that activated NF-κB to modulate target gene expression [191]. ST7L is a suppressor of the Wnt/β-catenin signaling pathway and acts as a tumor suppressor in many cancers [192]. For the control of cell proliferation, migration, and apoptosis as well as the promotion of EMT by inducing the expression of EMT transcription factors, the Wnt/β-catenin signaling pathway is essential [193, 194]. Yang et al. reported that, by binding to their 3′-untranslated regions (3′-UTRs) in EOC cells, miR-23a might increase IKKα expression but decrease ST7L expression. Regarding IKKα as an essential factor for the NF-κB pathway and ST7L as a Wnt pathway inhibitor, miR-23a positively regulates these pathways inducing cell growth, migration, and invasion in EOC cells [190] (Figure 3).
Discs large MAGUK scaffold protein 2 (DLG2) is found to be another miR-23a target. DLG2 is a member of the membrane-associated guanylate kinase (MAGUK) family, which has various protein-protein interaction domains having a role in a broad range of cellular pathways [195]. miR-23a can inhibit its expression through binding specifically to the DLG2 gene and inhibit its expression. Zhuang and his colleagues found that miR-23a, via targeting the DLG2 expression, elevates antiapoptotic Bcl-2 expression and suppresses proapoptotic protein Bax expression, blocking ovarian cancer cell apoptosis and inducing tumor stem cells development (Figure 3) [196].
miR-23a is also related to the chemotherapy-resistant ovarian cancer cell lines [197]. The RUNX3 gene is one of the antioncogenes that have a quenching effect on multidrug resistance gene 1 (MDR1) expression. MDR1 acts as a drug pump to excrete the chemotherapeutic agents out of the cells (Figure 3). One of miR-23a targets is the 3′-UTR of RUNX3 gene [198]. Jin and Wei by studying on drug-resistance ovarian cancer A2780 cell lines indicated that, in the presence of a highly expressed level of miR-23a, the silenced gene MDR1 was expressed, and the classic resistance mechanism was initiated [199].
4.4. miR-205
Human miR-205 is a highly conserved miRNA placed on chromosome 1q32.2. miR-205 has dual roles in cell regulation due to its involvement in both physiological and pathological pathways by targeting oncogene or tumor suppressor genes [200]. According to the various evidences, miR-205 is elevated in a broad range of cancers such as lung [201], kidney and bladder cancers or prostate [202], esophageal [203], melanoma [204], and breast [205] cancers. In EOC cells, SMAD4 and PTEN (phosphatase and tensin homolog) genes have been identified as miR-205 targets, and their downregulation contributes to cell proliferation, migration, and cancer cell invasion [206]. Smad4 (SMAD family member 4) is activated through phosphorylation by transmembrane serine-threonine receptor kinases in response to TGF-β signaling and regulates the transcription of target genes related to cellular adhesion, motility, and differentiation. Therefore, the Smad4 reduction correlates the cancer progression [207, 208]. PTEN tumor suppressor gene is located on 10q23.3 and is altered in various cancers at high frequency. Various studies have confirmed its ability in decreasing tumor growth and chemoresistance via blocking various cell signaling pathways [209]. Loss of PTEN expression in several tumor types indicated that low PTEN expression might be an accelerating factor in the development of cancer cells. Shi and his coworkers found that the downregulation of PTEN/AKT pathway via miR-205 is contributed to cisplatin-resistant C13K ovarian cancer cells [210]. Furthermore, findings from the research of He et al. show that exosomal miR-205 significantly accelerated angiogenesis and promoted metastasis in a mouse model and HUVECs human cell lines by targeting PTEN-AKT pathway [211] (Figure 3).
Leucine-rich repeat kinase 2 (LRRK2), as a target of miR-205, has been introduced in another study of Liu et al. They showed that LRRK2 has lower expression in ovarian cancer tissues than in healthy ovarian tissues [212]. LRRK2 is a kinase mediating the c-Jun N-terminal kinase (JNK) phosphorylation [213]. The JNK signaling pathway participates in biological processes of proliferation, differentiation, and apoptosis and facilitates apoptosis in some ovarian cancer cells [214]. Therefore, decreased LRRK2 expression by miR-205 results in the JNK pathway inactivation and the apoptosis inhibition of ovarian cancer cell. VEGFA (vascular endothelial growth factor A) is known as another target gene of miR-205 increasing the ovarian cancer risk by targeting EMT progression factors [215]. Another direct target of miR-205 is transcription factor 21 (TCF21), which is a reduced tumor suppressor in ovarian cancers [216]. TCF21 is an essential factor in epithelial cell differentiation which is located on chromosome 6q23 [217]. MMPs are involved in tissue cell integrity through the regulation of cell-matrix composition and play a pivotal role in tumor progression [218]. TCF21, as a transcription factor, is reported to decrease MMP-2 and MMP-10 expression and lead to the maintenance of cell integrity. In ovarian cancer, miR-205 directly targets TCF21, resulting in high expression of MMP-2, which increases cancer cell migration [216, 219] (Figure 3).
5. miRNAs as a Biomarker for Prognosis and Early Diagnosis of Ovarian Cancer
One of the most common gynecologic malignancies in women is ovarian cancer and the patients show symptoms even at the primary stages of cancer. These symptoms are regularly misunderstood as they are closely associated with other gastro- and gynecological diseases leading to the misdiagnosis of this disease [220, 221]. Around 230,000 new plus 140,000 death cases are reported annually [202]. Generally, ovarian cancer starts from granular-theca and germ cells. Also, more than 90 percent of patients have epithelial histology stemming from surface covering cells or subsurface cyst cells [222]. EOC is the most common cancer type compared to the other stromal and germline tumor types [119, 221]. A family history of cancer, age, and repeated ovulation with mutation in BRCA1 are associated with ovarian cancer [223]. Even though there are advancements in the identification of cases and cytotoxic treatments, only one-third of patients with an advanced stage of cancer live approximately five years after prognosis [224], whereas the mortality rate of the rest of the patients is high because of diagnosis at the late stage [132]. Moreover, about 19% of ovarian cancer patients are diagnosed at the early stages of cancer [132]. Genetic and epigenetic alterations both help in the progression of ovarian cancer which can be created from gene amplification, deletion, and changes in the methylation state [221, 225]. Ovarian cancer is of two types of tumors: low-grade and high-grade types that are based on gene mutations. Low-grade group is less common. These are diagnosed at early stages, and tumor growth is stepwise and encompasses mutations in KRAS, BRAF, PIK3CA, and PTEN. High-grade tumors are more common, which are mostly diagnosed at late stage. They are aggressive in nature and include mutations in TP53, BRCA1, and BRCA2 [221, 226, 227]. TP53 mutations are the most typical in ovarian cancer, whereas inactivation of phosphatase and tensin homolog (PTEN) is less typical. Mutations in DNA repair genes BRCA1 and BRCA2 contribute to heredity to ovarian cancer and accordingly increase the genomic instability [228]. At molecular levels, ovarian cancer has a highly heterogeneous disease etiology [229]. As a consequence of inadequacy of standard diagnostic and pelvic tests, serum cancer antigen 125 (CA125), and transvaginal ultrasonography for the detection of cancer at the early stage, there are more cases of death [124]. The main root of poor prognosis can be due to subtle disease symptoms at early stages, chemotherapy resistance, and lack of precise noninvasive detection methods [132, 220]. Surgery and chemotherapy are preferred treatments for advanced ovarian cancer, and, even with chemotherapy advancements, there is the possibility of chemotherapy resistance or relapses. Ovarian cancer diagnostic methods consist of pelvic examination, transvaginal ultrasound, and CA125 measurements [130]. So, advanced methods for detecting ovarian cancer, especially at early stages, are critical for prescription of the right medications and therapies. Clear cell, endometrioid, serous, and mucinous, with serous as the most frequent type, are the four major histological subtypes of ovarian carcinoma [132]. Analysis of the newest large-scale data of ovarian cancer samples recommends invasiveness at mesenchymal subtype of tumors to be connected with TGF-β. TGF-β, a multifunctional protein, stimulates EMT, resulting in metastasis and chemotherapy resistance in various kinds of cancers [230]. These histological types are related to different morphologic and genetic modifications [231]. Recently, expression profiling technologies have been significantly advanced, which expand the awareness on molecular implications of cancer in order to utilize them for diagnosis, therapy, and cancer drugs development. Therefore, the innovative and superior markers and drugs for diagnosis and treatment of ovarian cancer are figured out. Several researchers have recognized and examined modified expression of miRNAs in ovarian cancer, which results in the establishment of macrometastasis. miRNAs are used as novel diagnostic and prognostic markers and could be considered as novel targets for therapeutic purposes [232] (Table 2).
Table 2.
Significant miRNAs with potential prognostic and diagnostic biomarkers.
| Biomarker | Prognosis | Diagnosis | Tumor stages | Endpoint | Source | Ref. |
|---|---|---|---|---|---|---|
| miR-532-5p | Yes | No | Cell proliferation of ovarian neoplasms | OS | — | [242] |
| miR-200b | Yes | No | EMT and migration | OS | Ascitic fluid | [243, 244] |
| miR-193b | No | Yes | Lymph node metastasis | Poor survival | Tissue | [245] |
| miR-135a-3p | Yes | No | Cell proliferation of ovarian tumors | Decreased PFS | Peritoneal fluid | [246] |
| miR-125b | Yes | Yes | Lymph node involvement and distant metastasis | Decreased OS | Serum | [247] |
| miR-613 | Yes | No | Lymph node involvement | Short PFS and OS | Tissue | [248] |
| miR-506 | Yes | No | EMT | Decreased OS and PFS | Tissue | [249] |
| miR-205 | Yes | Yes | Advanced stages III/IV | — | Plasma | [250] |
| miR-200c-3p, miR-346, miR-127-3p | No | Yes | Distant metastasis | — | Serum | [251] |
| miR-200c | No | Yes | Stage I | OS and PFS | Tissue | [252] |
| miR-27a and miR-23a | Yes | No | Stages I-IV | OS, RFS, and short PFS (miR-27a) | Serum | [253] |
| miR-let-7 | Yes | No | Early tumor progression | Poor survival | Tissue | [241] |
| miR-221 | Yes | Yes | Stages I–IV | Decreased OS | Serum | [254] |
| miR-21 | Yes | Yes | Stages I–IV | OS | Serum | [255] |
| miR-335 | Yes | No | Distant metastasis (stages III/IV) | OS and RFS | Tissue | [256] |
| miR-429 | Yes | Yes | Stages III-IV | OS | Serum | [168] |
| miR-199a | Yes | Yes | Stages III-IV | Poor OS | [257] | |
| miR-1246 | No | Yes | Stages III-IV | — | Serum | [258] |
PFS: progression-free survival, OS: overall survival, RFS: recurrence-free survival.
5.1. The Patterns of miRNA Expression in Ovarian Cancer
Numerous experiments of miRNA and cDNA microarrays in ovarian cancer have shown large transcriptional variations [233]. Various miRNAs are indistinguishable to be downregulated in miRNA expression profiles of ovarian cancer, validating miRNAs' role in tumorigenesis [124]. Iorio et al. compared the expression profiles of miRNAs between ovarian cancer tissues or cell lines with normal tissues and showed that only 29 miRNAs, miR-14, miR-200a, miR-200b, and miR-200c were found to be upregulated in cancer cells, and there is a downregulation in the rest of all the 25 miRNAs comprising miR-140, miR-145, miR-199a, and miR-125b-1 [132]. They also mentioned that there is a distinction between the miRNA signatures in different histotypes of ovarian cancer. In another study by Vang et al., miRNA expression patterns of primary serous ovarian cancer and their omental metastasis had significant differences, measured by qPCR. miR-146a and miR-150 were related to omental metastases, cisplatin resistance, and spheroid formation [234]. Also, Calura et al. showed the profile of miRNA expression of each stage I EOC histotype and revealed the overexpression of miR-30a-5p and miR-30a-3p on clear cell type and the higher level of miR-192 and miR-194 on mucinous histotype [235]. Furthermore, Nam et al. showed the upregulation of 11 miRNAs (miR-16, miR-20a, miR-21, miR-23a, miR-23b, miR-27a, miR-93, miR-141, miR-200a, miR-200b, and miR-200c) and downregulation of 12 (miR-10b, miR-226a, miR-29a, miR-99a, miR-100, miR-125a, miR-125b, miR-143, miR-145, miR-199a, miR-214, and let-7b) through the miRNA microarray of 20 serous ovarian carcinomas and their comparison with normal samples [236].
The Cancer Genome Atlas (TCGA) project has revealed the mRNA and miRNA expression, promoter methylation, and DNA copy number in 489 patients with high-grade serous ovarian adenocarcinomas [237] and it has been stated that TP53 mutation has been detected in nearly 96% of tumors plus recurring somatic mutations of NF1, RB1, CDK12, BRCA1, and BRCA2 [124]. Eight key miRNAs (miR-141, miR-182, miR-200a, miR-506, miR-25, miR-29c, miR-101, and miR-128) were found and thought to target up to 90% of its network [124]. A combination of genomic methods, including array-based comparative genomic hybridization (aCGH), miR microarray, cDNA microarray, and tissue array, was utilized to evaluate alterations in the miRs' expression in EOC [233]. It was discovered that genomic losses along with epigenetic variations might cause the downregulation of miRNA. From 35 dysregulated miRs in ovarian cancer (immortalized ovarian surface epithelial cells), 31 (88.6%) had almost lost their expression in comparison with normal cells, including the miRs that are tumor suppressors, like let-7d [238] and miR-127 [239].
Experiments also disclosed that miR-199a, miR-214, miR-200, miR-100, and let-7 family are the most extremely expressed candidates in normal ovarian cells and EOC in different ways [130, 202, 240]. The expression of miR-200 family detected in epithelial tissues includes five members (miR-200a, miR-200b, miR-200c, miR-141, and miR-429). These miRNAs are divided into two groups in the human genome. miR-200a, miR-200b, and miR-429 are grouped on chromosome 1, while miR-200c and miR-141 are found on chromosome 12 [241]. Numerous researches have indicated variations in the expressions of distinct components of the miR-200 family and exhibited their essential functions in ovarian carcinoma development. These experiments showed the miR-200 family in three categories of ovarian cancer: serous, endometrioid, and clear cell carcinomas. However, they revealed the upregulation of miR-200b and miR-141 merely in endometrioid and serous categories. This recommends a more intricate and distinct role of the miR-200 family in ovarian cancer than it was believed primarily [132]. Therefore, different miRNAs have been identified as vital prognostic and diagnostic biomarkers that can be served as a potential utility in ovarian cancer.
5.2. miRNAs Alterations in Ovarian Cancer
The copy number variations of genes that are near cancer-associated genomic regions (CAGRs) influence miRNA expression [259]. A comprehensive study of cancer modifications was presented with aCGH to recognize miRNA loci that are gained or lost in some human cancers, including ovarian cancer, breast cancer, and melanoma [131]. Zhang et al. studied 283 miRNA loci and discovered that 37.1% of these miRNAs had significant alterations in their copy number [131]. Similarly, substantial copy variations were noticed in 72.8% of breast cancer samples and 85.9% of melanomas samples. The miR-15a/16-1 locus is lost in up to 24% of ovarian cancer samples and miR-17-92 in all tumor tissues [123]. A copy number increase in Dicer1 and Ago2 loci has also been reported in, respectively, 24.8% and 51.5% of ovarian tumor regions [131]. As stated earlier, an effectual miRNA processing and operation requires Dicer and Ago2 proteins. Short-hairpin-RNA- (shRNA-) mediated knockout of Dicer1 and Ago2 boosts colony development in both in vivo and in vitro environments [92]. Modifications in Dicer1/Ago2 expression result in huge alterations of miRNA expression, which commonly occur in cancer through either germline deletions, mutations, or promoter methylation [239, 260]. Mutation of p53 is also one of the most widespread changes noticed in EOC, especially in high-grade serous tumors [261].
5.3. Dysregulation of miRNA in Ovarian Cancer
Many experiments on the expression of miRNAs in ovarian cancer and normal epithelial ovarian cells as well as immortalized ovarian surface epithelial cells [262–264] have reported the dysregulation of 310 miRNAs in ovarian cancers, among which 34 miRNAs were constantly dysregulated in carcinoma tissues in at least three separate studies. Numerous miRNAs discovered to control growth of ovarian cancers are miR-31, miR-34abc, miR-125b, miR-127, and let-7a/b/d/f miR-31 [265]. According to studies, oncogenic miR-182 expression is upregulated in high-grade ovarian tumors, resulting in proliferation and progression of cancer cells by enhancing the BRCA1/HMGA2 dysregulation [266]. miR-221-3p plays a tumor suppressor role by affecting ARF4 to repress the proliferation and migration of EOC cells. Those having the high miR-221-3p expression can survive better as compared to others [267]. miRNAs are the regulators of chemosensitivity, since dysregulated miRNA expressions contribute to therapeutic chemoresistance. The upregulation of miR-106a and downregulation of miR-591 cause chemoresistance to Paclitaxel, so the chemosensitivity of Paclitaxel can be achieved by downregulation of miR-106a and upregulation of miR-591 [268]. miR-145 also sensitizes the resistant ovarian cell to Paclitaxel by regulating the Sp1 and Cdk6 [185, 269].
5.4. miRNAs as Prognostic Markers in Ovarian Cancer
For ovarian cancer diagnosis and detection, the miRNAs can be utilized as an essential tool. Downregulation of miR-9 has been shown in ovarian cancer cases [270]. Upregulation of miR-92, miR-21, and miR-15a has been proposed to be a signature of ovarian cancer. The underexpression of miR-31 indicates the initial phase of ovarian cancer growth [265]. On the other hand, the downregulation of miR-34 a/b/c/miR-449b, miR-503, and miR-507 has been noticed in patients of last phases [233, 271, 272]. Research has also shown the overexpression of miR-200 family and underexpression of let-7 family in EOC cases. In this regard, it has been reported that serum miRNAs, miR-21, miR-155, and miR-210, were significantly higher in cancer patients compared with normal participants [273]. Chung et al. showed let-7b, miR-26a, miR-132, and miR-145 as diagnostic markers in EOC patients [274]. Zheng et al. also indicated let-7f and miR-205 as biomarkers for the EOC diagnosis [275]. Since miRNAs with tissue-specific expressions are often stated for being dysregulated in cancer cells, they appear to be tumor indicators [276]. Several reports have revealed an unusual miRNA expression pattern as a prognostic indicator showing the disease result during the treatment. For example, in ovarian cancer cases, loss of let-7 expression along with the overexpression of HMGA2 is an indicator of a bad prognosis. Hence, the ratio of HMGA2 to let-7 is used to predict treatment outcomes; it means that cases with a higher ratio of HMGA2/let-7 have poor survival (<10%) in comparison with patients with a low ratio (40%) [241]. Furthermore, let-7 is underexpressed in chemotherapy-resistant patients and is associated with shorter survival; hence, it might be a potential biomarker for observing treatment outcomes and patients survival [240]. So, the miR-200 family was found to be a prognostic marker for ovarian cancer. Hu et al. studied 55 ovarian cancer patients with stages III and IV [277]. They found that expression of miR-200 family group, comprising miR-200a/b/c and miR-429, has been extremely reduced in patients with a recurrent cancer related to patients without recurrence. Eitan et al. in their research revealed the same conclusions [278]. They studied 57 patients with either serous or endometrioid histology who were at stage I or stage III of the disease and revealed downregulation of miR-200a, miR-34, and miR-449b miRNAs in advanced ovarian cancer (stage III) and high expression of miR-200a in initial stages of disease (stage I) with a more satisfactory conclusion. Also, studying 107 other patients from all types and stages disclosed higher survival in high-grade ovarian cancer patients mixed with high miR-200a levels in comparison to patients with lower expression of miR-200a [279]. miR-9 and miR-223 might be applicable as prognostic markers since they are correlated with the recurrent type of ovarian cancer [270]. Recently, the overexpression of miR-30d has been detected in patients that are sensitive to platinum-based chemotherapy compared to in chemoresistant patients. Moreover, the downregulation of miR-30d in patients with recurrent ovarian cancer has been noticed [280]. Lee et al. who were investigating miRNA expression in patients with ovarian cancer, benign ovarian cancers, and borderline malignancy discovered a higher expression of miR-30c, miR-30d, miR30e-3p, and miR-181d as survival prognostic markers [280]. Others have identified low expression of miR-34c and miR-422b in patients with a dropped survival of high-grade ovarian cancer [272]. Corney et al. indicated that downregulation of miR-34b/c, which is detected in the late stage of ovarian cancer, is related to mesenchymal-to-epithelial transition (MET) [271]. Kim et al. discovered a link between notable upregulation of miR-519a and poor survival [281]. miR-22 downregulated in ovarian cancer is correlated with overall survival and progression-free survival of patients; hence, it might be used as an efficient prognostic factor [282].
5.5. miRNAs as Novel Tools for Ovarian Cancer Diagnosis
miRNAs play a role as a perfect diagnostic and monitoring factor for ovarian cancer. Patients with a minimum level of miR-200 genes expression have poor survival with cancer growth [277]. Also, patients with low let 7a-3 methylation have minimal survival rate compared to patients with high methylation [202]. Precursor miR-146a causes G to C variants in patients with ovarian and breast cancer [283]. Ratio of HMGA2 to let-7 has been discovered as a prognosis factor in ovarian carcinoma patients [241]. The serum miRNAs are useful for recognition of cancer [273, 284]. Tumor-derived exosomes (small lipid vesicles) encompass diagnostic miRNAs [285]. Early detection of life-threatening malignancies, including ovarian cancer, has long been the key to successful therapy among the different considered preventive measurements [130]. The abnormal expression pattern of miRNAs expression can be useful for identifying an early-stage ovarian cancer. miR-200a and miR-141 were identified as exceedingly upregulated miRNAs in ovarian cancer, whereas miR-199a, miR-140, miR-145, and miR-125b1 were most astonishingly downregulated. In addition, to detect the histological types of ovarian tumors, dysregulation of a particular miRNA was also applied. For instance, there is an overexpression of miR-200a and miR-200c in all types (mucinous, endometrioid, and clear cells), miR-200b and miR-141 were overexpressed in serous as well as endometrioid carcinomas, and miR-21, miR-203, and miR-205 were overexpressed in endometrioid tumors. In fact, miR-145 is underexpressed in serous and clear-cell tumors, while miR-222 is underexpressed in endometrioid and clear-cell malignancies.
A different research has proven outstanding downregulation of serum miR-145 in malignant and benign ovarian cancers compared to healthy cases, and outcomes suggested that miR-145 can forcefully be used to discover ovarian and other types of human cancers [286]. Nam et al. determined 23 haphazardly expressed miRNAs in at least 60% of specimens of ovarian cancer with miR-21 as the most overexpressed miRNA (85% samples) and miR-125b (95% samples) as the most downregulated miRNA [236]. Yang et al. finalized 36 miRNAs differently expressed in normal and ovarian cancer cells, comprising miR-199a, miR-214, and miR-200a, respectively, overexpressed in 53, 56, and 43% of tumors of high-grade cancers [240]. miR-100 instead is downregulated in 76% of tumors. In comparison with previously mentioned data, Eitan et al. exhibited downregulation of miR-200a, miR-34a, and miR-449b in the evolved (stage III) ovarian tumors with miR-200a relation to the initial-stage cancer and enhanced overall survival [278]. Furthermore, miR-200a was discovered as a satisfactory outcome predictor in another research of 55 patients [277]. In addition, Marchini et al. recognized the correlation of miR-200c with progression-free survival or overall survival or both in phase I ovarian cancers [252]. The root cause for upregulation of miR-21, miR-203, and miR-205 in ovarian cancer tissues is hypomethylation of miRNA genes as the most essential epigenetic mechanism [132]. A different research has reported the overexpression of miRNAs as a consequence of miRNA genes amplification in tumors [233]. Among 35 miRNAs studied, there have been upregulation in four miRNAs (miR-26, miR-182, miR-103, and miR-26a) and downregulation in two miRNAs (let-7d and miR-127). These findings proposed alterations in copy number and epigenetic mediators as two reasons for unusual expression of miRNAs in ovarian cancer [233]. Thus, miR-30c, miR-30d, and miR-30e are frequently upregulated, whereas miR-493 is downregulated in ovarian tumors compared to in normal HOSE cell lines.
6. Implications of miRNAs in Targeting Ovarian Cancer
If ovarian cancer cells are confined to the ovaries, they can be effectively treated by debulking surgery. However, most of tumors are not diagnosed but are at more advanced metastatic stages of the disease, when chemotherapy treatment is required [287]. The majority of ovarian cancer cells respond effectively to neoadjuvant platinum-based chemotherapies. On the other hand, mesenchymal-like metastasis initiating cancer stem cells are usually resistant to these chemotherapies, resulting in cancer recurrence in many ovarian cancer patients [288]. Targeted therapy is another strategy in which crucial pathways for ovarian cancer cells growth or survival are blocked, while normal cells are not affected [289]. A possible targeted therapy for the treatment of ovarian cancer metastasis is based on targeting miRNAs. As described above, many miRNAs act as tumor promoters or suppressors in various cancers, and they can be given more attention as new therapeutic targets.
Thanks to the enhancement of cancer studies, patient-specific treatments soon would be developed. Since the expressions of a plethora of various genes are regulated by miRNAs, they can control and coordinate multiple cellular pathways [109, 130]. Therefore, miRNAs have been recommended as potential therapies against cancer. Correction of the mRNA expressions is an appealing strategy to target cancer cells, which can be done through the application of miRNA mimics (miRNA replacement therapy) to inhibit the upregulated onco-miRs by antisense miRs (miRNA inhibition therapy) [256]. miRNA drug MRX34, which is a miR-34 mimic, is the first and the only anticancer miRNA that has entered clinical trials in cancer patients. This miRNA mimic is being evaluated in clinical trials of phase I in hepatocellular carcinoma patients, which may finally open new avenues toward the application of miRNAs in cancer treatments.
Chen et al. suggested that the targeted delivery of miR-429 to carcinoma cells may have therapeutic effect for reducing cancer cell metastasis and tumor recurrence in advance-staged cancer patients [290]. miR-492 plays a significant role in controlling EMT and MET pathways; therefore, the overexpression of miR-429 may reduce metastasis by inducing epithelial phenotype in mesenchymal stem cell-like metastasizing cells reducing their tumorigenicity and metastatic potential [291]. Also, miR-492 is shown to be negatively correlated with resistance to Paclitaxel and cisplatin in ovarian adenocarcinoma cell lines [292]. Chen et al. have investigated the EOC cell lines, SKOV3 and COV644, and revealed the inhibition of metastasis and chemoresistance of EOC cells by miR-429 via KIAA0101-mediated Wnt/β-catenin signaling [184]. As a consequence, delivery of miR-429 inhibits the recurrence and improves survival rates of ovarian cancer.
The X-linked inhibitor of apoptosis protein (XIAP) acts as an apoptosis blockage and directly inhibits the effector caspases (caspases 3, 6, 7, and 9) [293] and promotes tumor formation and metastasis [294]. XIAP plays a crucial role in chemotherapy resistance in ovarian cancer and various tumors [295]. It is also highly expressed in ovarian cancer cells, which indicates the chemosensitivity of cancer cells [296]. XIAP is a direct target gene of miR-149 and miR-137. miR-137 [297] and miR-149 [298] are usually downregulated in ovarian cancer. The results obtained from several studies suggest that ectopic expression of miR-137 and miR-149 can suppress the XIAP 3′UTR function and decrease the levels of XIAP protein in ovarian cancer cells [297–299]. miR-150 is considered as an EMT process regulator but is downregulated in primary EOC tissues [234], and ectopic expression of miR-150 blocks proliferation, invasion, and metastasis of EOC cells [300].
miR-320, which is a tumor suppressor, is markedly downregulated in EOC cells and cell lines. MAPK1 [301] and TWIST [302] are two direct target genes of miR-320 in EOC, which are significantly upregulated in EOC tissues, and their downregulation represses the proliferation and invasive ability of EOC cells. MAPK1 plays a crucial role in the MAPK/ERK pathway and mediates various biological processes, including cell growth, cell survival, and differentiation through the regulation of transcription, translation, and cytoskeletal rearrangements [303, 304]. Therefore, decreased expression of MAPK1 suppresses SKOV3 cells growth and invasion in ovarian cancer [305]. TWIST1 is one member of the basic helix-loop-helix transcription factor twist family that plays important roles in the mesenchymal phenotype, serves as one potent oncogene [306], and is overexpressed in various cancers. Overexpression of TWIST1 promoted tumor cell migration, proliferation, and invasion [307]. Besides, miR-186 is another miRNA that inhibits EMT in ovarian cancer cells and stimulates G1 cell-cycle arrest and promotes apoptosis by regulating TWIST1 [308].
miR-34 family is thought to be underexpressed in EOC, especially those with mutations in TP53, and was linked to tumor stage [271]. It is also demonstrated that replacement therapy with miR-34 in SKOV3 cell line has drastically reduced proliferation, migration, and invasion. In most of ovarian cancers, miR-200 family members (miR-141, miR-200a, miR-200b, miR-200c, and miR-429) are underexpressed correlating with poor survival [132, 236]. The miR-200 family inhibit EMT, cell migration, and metastasis through impacting on ZEB1 (zinc-finger E-box-binding homeobox 1) and ZEB2 [279, 309]. Chen et al. reported that IKK expression is regulated by miR-199a, which changes the inflammatory microenvironment in ovarian cancer [310]. Epigenetic silencing of miR-199b-5p is linked to chemoresistance in ovarian cancer, since it activates JAG1/Notch1 signaling [311]. Recently, meta-analysis of the transcriptome of high-grade serous ovarian carcinoma (HGSOC) has revealed that let-7b, as a weak prognostic marker, helps predict molecular and clinical classes of HG-SOC [312].
Studies have reported that miR-200 affects interleukin-8 (IL-8) and CXCL1 in epithelial cancer cells, thereby inhibiting angiogenesis, demonstrating the therapeutic role of miR-200 in ovarian cancer treatment [313]. It is also found that loss of miR-31 results in TP53 pathway deficiency, highlighting the potential therapeutic benefits of this miRNA in patients with TP53 deficient activity [124, 262]. Taken together, since a variety of human cancer cells, including ovarian cancer cells, have been shown to have altered expression of different miRNAs, the development of a targeted therapeutic agent that would suppress or provoke oncogenic/tumor suppressor miRNAs would be a promising antitumor therapy against cancer.
7. Conclusions
Regardless of the advancements achieved for prognosis, diagnosis, and monitoring of ovarian cancer, most of the patients succumb to this devastating malignancy, mostly because of the late-stage diagnosis and recurrent disease. Therefore, the need for identifying reliable markers for early detection and EOC patients monitoring, which are both sensitive and specific, remains a long-awaited priority. EOC management could be well supported by the application of the biomarkers for discriminating malignant tumors from benign pelvic masses, early diagnosis, estimating prognosis, monitoring the treatment efficacy, and predicting response to individual drugs. Current screening and monitoring indicators for ovarian cancer do not meet the requirements for fulfilling cancer diagnosis, tumor subtype classification, chemoresistance monitoring, and outcome prognosis. For instance, carcinoembryonic antigen (CEA) is a broad-spectrum biomarker for diagnosis of various types of cancers and lacks sensitivity. CA125, which is the most well-known biomarker for EOC screening, lacks sensitivity, especially for the diagnosis of early-stage patients and could not discriminate the malignant tumors from benign pelvic cysts. Human epididymis protein 4 (HE4), which is another marker usually applied for EOC detection, lacks acceptable specificity and could not monitor the tumor burden during the treatment course. Therefore, incorporating other types of biomarkers, either alone or in combination with the traditional biomarkers, may help ameliorate the diagnosis and monitoring of the patients. Considering the miRNAs as biomarkers for prognostication and screening of cancer patients is promising, because they usually exhibit aberrant expression under different pathological conditions, including ovarian malignancy. miRNAs are involved in various cellular processes. Therefore, a plethora of these markers are available, making it possible to trace any aspect of cellular and molecular mechanisms involved in cancer progression. In addition, miRNAs benefit from structural stability, making them promising for being considered as screening and monitoring biomarkers (Table 2). Furthermore, since each miRNA usually targets a vast number of mRNAs, targeting or the delivery of miRNAs may be an interesting approach for efficiently targeting ovarian cancer cells. Further studies are, however, needed to suffice the role of miRNAs as biomarkers in clinical settings for ovarian cancer management.
Acknowledgments
This study was supported by the Department of Medical Genetics and Molecular Medicine, Faculty of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
Contributor Information
Arash Salmaninejad, Email: arash.salmany@yahoo.com.
Amirhossein Sahebkar, Email: amir_saheb2000@yahoo.com.
Data Availability
There are no raw data associated with this review article.
Conflicts of Interest
The authors declare no conflicts of interest.
Authors' Contributions
A.S. and Y.M. contributed to conception, design, statistical analysis, and drafting of the manuscript. M.F., R.N., M.M.S., F.T., M.Y., Z.N., and A.S. contributed to data collection and manuscript drafting. All authors approved the final version for submission.
References
- 1.Treiber T., Treiber N., Meister G. Regulation of microRNA biogenesis and its crosstalk with other cellular pathways. Nature Reviews Molecular Cell Biology . 2019;20(1):5–20. doi: 10.1038/s41580-018-0059-1. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R. L., Miller K. D., Jemal A. Cancer statistics, 2017. CA: A Cancer Journal for Clinicians . 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Yeung T.-L., Leung C. S., Yip K.-P., Au Yeung C. L., Wong S. T. C., Mok S. C. Cellular and molecular processes in ovarian cancer metastasis. A review in the theme: cell and molecular processes in cancer metastasis. American Journal of Physiology-Cell Physiology . 2015;309(7):C444–C456. doi: 10.1152/ajpcell.00188.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Brien J., Hayder H., Zayed Y., Peng C. Overview of microRNA biogenesis, mechanisms of actions, and circulation. Frontiers in Endocrinology . 2018;9:p. 402. doi: 10.3389/fendo.2018.00402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peng Y., Croce C. M. The role of MicroRNAs in human cancer. Signal Transduction and Targeted Therapy . 2016;1(1):p. 15004. doi: 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van Roosbroeck K., Calin G. A. Cancer hallmarks and MicroRNAs: the therapeutic connection. Advances in Cancer Research . 2017;135:119–149. doi: 10.1016/bs.acr.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Katz B., Tropé C. G., Reich R., Davidson B. MicroRNAs in ovarian cancer. Human Pathology . 2015;46(9):1245–1256. doi: 10.1016/j.humpath.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Khan S., Ayub H., Khan T., Wahid F. MicroRNA biogenesis, gene silencing mechanisms and role in breast, ovarian and prostate cancer. Biochimie . 2019;167:12–24. doi: 10.1016/j.biochi.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Yousefi M., Dehghani S., Nosrati R., Ghanei M., Salmaninejad A., Rajaie S. Current insights into the metastasis of epithelial ovarian cancer-hopes and hurdles. Cellular Oncology . 2020;43(4):515–538. doi: 10.1007/s13402-020-00513-9. [DOI] [PubMed] [Google Scholar]
- 10.Pal M. K., Jaiswar S. P., Dwivedi V. N., Tripathi A. K., Dwivedi A., Sankhwar P. MicroRNA: a new and promising potential biomarker for diagnosis and prognosis of ovarian cancer. Cancer Biology & Medicine . 2015;12(4):328–341. doi: 10.7497/j.issn.2095-3941.2015.0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Staicu C. E., Predescu D. V., Rusu C. M, et al. Role of microRNAs as clinical cancer biomarkers for ovarian cancer: a short overview. Cells . 2020;9(1) doi: 10.3390/cells9010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z.-H., Xu C.-J. Research progress of MicroRNA in early detection of ovarian cancer. Chinese Medical Journal . 2015;128(24):3363–3370. doi: 10.4103/0366-6999.171459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garzon R., Marcucci G., Croce C. M. Targeting microRNAs in cancer: rationale, strategies and challenges. Nature Reviews Drug Discovery . 2010;9(10):775–789. doi: 10.1038/nrd3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. U.S. National Library of Medicine, 2020, https://clinicaltrials.gov/ct2/results?term=microrna&cond=Ovarian+Cancer. [DOI] [PubMed]
- 15.Lee R. C., Feinbaum R. L., Ambros V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell . 1993;75(5):843–854. doi: 10.1016/0092-8674(93)90529-y. [DOI] [PubMed] [Google Scholar]
- 16.Kim V. N., Han J., Siomi M. C. Biogenesis of small RNAs in animals. Nature Reviews Molecular Cell Biology . 2009;10(2):126–139. doi: 10.1038/nrm2632. [DOI] [PubMed] [Google Scholar]
- 17.Pasquinelli A. E. MicroRNAs and their targets: recognition, regulation and an emerging reciprocal relationship. Nature Reviews Genetics . 2012;13(4):271–282. doi: 10.1038/nrg3162. [DOI] [PubMed] [Google Scholar]
- 18.Peláez N., Carthew R. W. MicroRNAs in Development: Current Topics in Developmental Biology . Vol. 99. Amsterdam, Netherlands: Elsevier; 2012. Biological robustness and the role of MicroRNAs; pp. 237–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman R. C., Farh K. K., Burge C. B., Bartel D. P. Most mammalian mRNAs are conserved targets of microRNAs. Genome Research . 2009;19(1):92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao C., Sun X., Li L. Biogenesis and function of extracellular miRNAs. ExRNA . 2019;1(1):p. 38. doi: 10.1186/s41544-019-0039-4. [DOI] [Google Scholar]
- 21.Ha M., Kim V. N. Regulation of microRNA biogenesis. Nature Reviews Molecular Cell Biology . 2014;15(8):509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 22.De Rie D., Abugessaisa I., Abugessaisa I., et al. An integrated expression atlas of miRNAs and their promoters in human and mouse. Nature Biotechnology . 2017;35(9):872–878. doi: 10.1038/nbt.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim Y.-K., Kim V. N. Processing of intronic microRNAs. The EMBO Journal . 2007;26(3):775–783. doi: 10.1038/sj.emboj.7601512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tanzer A., Stadler P. F. Molecular evolution of a microRNA cluster. Journal of Molecular Biology . 2004;339(2):327–335. doi: 10.1016/j.jmb.2004.03.065. [DOI] [PubMed] [Google Scholar]
- 25.Denli A. M., Tops B. B. J., Plasterk R. H. A., Ketting R. F., Hannon G. J. Processing of primary microRNAs by the microprocessor complex. Nature . 2004;432(7014):231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- 26.Alarcón C. R., Lee H., Goodarzi H., Halberg N., Tavazoie S. F. N6-methyladenosine marks primary microRNAs for processing. Nature . 2015;519(7544):482–485. doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han J., Lee Y., Yeom K.-H., Kim Y.-K., Jin H., Kim V. N. The Drosha-DGCR8 complex in primary microRNA processing. Genes & Development . 2004;18(24):3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okada C., Yamashita E., Lee S. J., et al. A high-resolution structure of the pre-microRNA nuclear export machinery. Science . 2009;326(5957):1275–1279. doi: 10.1126/science.1178705. [DOI] [PubMed] [Google Scholar]
- 29.Zhang H., Kolb F. A., Jaskiewicz L., Westhof E., Filipowicz W. Single processing center models for human dicer and bacterial RNase III. Cell . 2004;118(1):57–68. doi: 10.1016/j.cell.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 30.Yoda M., Kawamata T., Paroo Z. ATP-dependent human RISC assembly pathways. Nature Structural & Molecular Biology . 2010;17(1):17–23. doi: 10.1038/nsmb.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meijer H. A., Smith E. M., Bushell M. Regulation of miRNA Strand Selection: Follow the Leader? London, UK: Portland Press Ltd.; 2014. [DOI] [PubMed] [Google Scholar]
- 32.Khvorova A., Reynolds A., Jayasena S. D. Functional siRNAs and miRNAs exhibit strand bias. Cell . 2003;115(2):209–216. doi: 10.1016/s0092-8674(03)00801-8. [DOI] [PubMed] [Google Scholar]
- 33.Babiarz J. E., Ruby J. G., Wang Y., Bartel D. P., Blelloch R. Mouse ES cells express endogenous shRNAs, siRNAs, and other microprocessor-independent, dicer-dependent small RNAs. Genes & Development . 2008;22(20):2773–2785. doi: 10.1101/gad.1705308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruby J. G., Jan C. H., Bartel D. P. Intronic microRNA precursors that bypass Drosha processing. Nature . 2007;448(7149):83–86. doi: 10.1038/nature05983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie M., Li M., Vilborg A., et al. Mammalian 5′-capped MicroRNA precursors that generate a single microRNA. Cell . 2013;155(7):1568–1580. doi: 10.1016/j.cell.2013.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang J.-S., Maurin T., Robine N., et al. Conserved vertebrate mir-451 provides a platform for dicer-independent, Ago2-mediated microRNA biogenesis. Proceedings of the National Academy of Sciences . 2010;107(34):15163–15168. doi: 10.1073/pnas.1006432107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheloufi S., Dos Santos C. O., Chong M. M. W., Hannon G. J. A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature . 2010;465(7298):584–589. doi: 10.1038/nature09092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Calin G. A., Croce C. M. MicroRNA signatures in human cancers. Nature Reviews Cancer . 2006;6(11):857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 39.Cho W. C. S. MicroRNAs: potential biomarkers for cancer diagnosis, prognosis and targets for therapy. The International Journal of Biochemistry & Cell Biology . 2010;42(8):1273–1281. doi: 10.1016/j.biocel.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 40.Esquela-Kerscher A., Slack F. J. Oncomirs–microRNAs with a role in cancer. Nature Reviews Cancer . 2006;6(4):259–269. doi: 10.1038/nrc1840. [DOI] [PubMed] [Google Scholar]
- 41.Chen X., Ba Y., Ma L., et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Research . 2008;18(10):997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell P. S., Parkin R. K., Kroh E. M., et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proceedings of the National Academy of Sciences . 2008;105(30):10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Park N. J., Zhou H., Elashoff D., et al. Salivary microRNA: discovery, characterization, and clinical utility for oral cancer detection. Clinical Cancer Research . 2009;15(17):5473–5477. doi: 10.1158/1078-0432.ccr-09-0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanke M., Hoefig K., Merz H., Feller A. C., Kausch I., Jocham D. A robust methodology to study urine microRNA as tumor marker: microRNA-126 and microRNA-182 are related to urinary bladder cancer. Urologic Oncology: Seminars and Original Investigations . 2010;28(6):655–661. doi: 10.1016/j.urolonc.2009.01.027. [DOI] [PubMed] [Google Scholar]
- 45.Weber J. A., Baxter D. H., Zhang S., et al. The microRNA spectrum in 12 body fluids. Clinical Chemistry . 2010;56(11):1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kosaka N., Izumi H., Sekine K., Ochiya T. microRNA as a new immune-regulatory agent in breast milk. Silence . 2010;1(1):p. 7. doi: 10.1186/1758-907x-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.da Silveira J. C., Veeramachaneni D. R., Winger Q. A., Carnevale E. M., Bouma G. J. Cell-secreted vesicles in equine ovarian follicular fluid contain miRNAs and proteins: a possible new form of cell communication within the ovarian follicle. Biology of Reproduction . 2012;86(3):1–10. doi: 10.1095/biolreprod.111.093252. [DOI] [PubMed] [Google Scholar]
- 48.Skog J., Würdinger T., Van Rijn S., et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature Cell Biology . 2008;10(12):1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J. J., Lötvall J. O. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biology . 2007;9(6):654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 50.Pritchard C. C., Kroh E., Wood B., et al. Blood cell origin of circulating microRNAs: a cautionary note for cancer biomarker studies. Cancer Prevention Research . 2012;5(3):492–497. doi: 10.1158/1940-6207.capr-11-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Corsten M. F., Dennert R., Jochems S., et al. Circulating MicroRNA-208b and MicroRNA-499 reflect myocardial damage in cardiovascular disease. Circulation: Cardiovascular Genetics . 2010;3(6):499–506. doi: 10.1161/circgenetics.110.957415. [DOI] [PubMed] [Google Scholar]
- 52.Laterza O. F., Lim L., Garrett-Engele P. W., et al. Plasma MicroRNAs as sensitive and specific biomarkers of tissue injury. Clinical Chemistry . 2009;55(11):1977–1983. doi: 10.1373/clinchem.2009.131797. [DOI] [PubMed] [Google Scholar]
- 53.Lewis A. P., Jopling C. L. Regulation and biological function of the liver-specific miR-122. Biochemical Society Transactions . 2010;38(6):1553–1557. doi: 10.1042/bst0381553. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Y., Jia Y., Zheng R., et al. Plasma microRNA-122 as a biomarker for viral-, alcohol-, and chemical-related hepatic diseases. Clinical Chemistry . 2010;56(12):1830–1838. doi: 10.1373/clinchem.2010.147850. [DOI] [PubMed] [Google Scholar]
- 55.Cocucci E., Racchetti G., Meldolesi J. Shedding microvesicles: artefacts no more. Trends in Cell Biology . 2009;19(2):43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Mathivanan S., Ji H., Simpson R. J. Exosomes: extracellular organelles important in intercellular communication. Journal of Proteomics . 2010;73(10):1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Ratajczak J., Wysoczynski M., Hayek F., Janowska-Wieczorek A., Ratajczak M. Z. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia . 2006;20(9):1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 58.Simons M., Raposo G. Exosomes-vesicular carriers for intercellular communication. Current Opinion in Cell Biology . 2009;21(4):575–581. doi: 10.1016/j.ceb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 59.Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nature Reviews Immunology . 2002;2(8):569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 60.Arroyo J. D., Chevillet J. R., Kroh E. M., et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proceedings of the National Academy of Sciences . 2011;108(12):5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turchinovich A., Weiz L., Langheinz A., Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic Acids Research . 2011;39(16):7223–7233. doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vickers K. C., Palmisano B. T., Shoucri B. M., Shamburek R. D., Remaley A. T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nature Cell Biology . 2011;13(4):423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang K., Zhang S., Weber J., Baxter D., Galas D. J. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Research . 2010;38(20):7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Y., Li L., Zhou Z., Wang N., Zhang C.-Y., Zen K. A pilot study of serum microRNA signatures as a novel biomarker for occult hepatitis B virus infection. Medical Microbiology and Immunology . 2012;201(3):389–395. doi: 10.1007/s00430-011-0223-0. [DOI] [PubMed] [Google Scholar]
- 65.Li L.-M., Hu Z.-B., Zhou Z.-X., et al. Serum microRNA profiles serve as novel biomarkers for HBV infection and diagnosis of HBV-positive hepatocarcinoma. Cancer Research . 2010;70(23):9798–9807. doi: 10.1158/0008-5472.can-10-1001. [DOI] [PubMed] [Google Scholar]
- 66.Ryther R. C. C., Flynt A. S., Phillips, Patton J. G. siRNA therapeutics: big potential from small RNAs. Gene Therapy . 2005;12(1):5–11. doi: 10.1038/sj.gt.3302356. [DOI] [PubMed] [Google Scholar]
- 67.Weiler J., Hunziker J., Hall J. Anti-miRNA oligonucleotides (AMOs): ammunition to target miRNAs implicated in human disease? Gene Therapy . 2006;13(6):496–502. doi: 10.1038/sj.gt.3302654. [DOI] [PubMed] [Google Scholar]
- 68.Hanahan D., Weinberg R. A. The hallmarks of cancer. Cell . 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 69.MacFarlane L.-A., Murphy P. R. MicroRNA: biogenesis, function and role in cancer. Current Genomics . 2010;11(7):537–561. doi: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Croce C. M. Oncogenes and cancer. New England Journal of Medicine . 2008;358(5):502–511. doi: 10.1056/nejmra072367. [DOI] [PubMed] [Google Scholar]
- 71.Konopka J. B., Watanabe S. M., Singer J. W., Collins S. J., Witte O. N. Cell lines and clinical isolates derived from Ph1-positive chronic myelogenous leukemia patients express c-abl proteins with a common structural alteration. Proceedings of the National Academy of Sciences . 1985;82(6):1810–1814. doi: 10.1073/pnas.82.6.1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsujimoto Y., Jaffe E., Cossman J., Gorham J., Nowell P. C., Croce C. M. Clustering of breakpoints on chromosome 11 in human B-cell neoplasms with the t(11; 14) chromosome translocation. Nature . 1985;315(6017):340–343. doi: 10.1038/315340a0. [DOI] [PubMed] [Google Scholar]
- 73.Sherr C. J. Principles of tumor suppression. Cell . 2004;116(2):235–246. doi: 10.1016/s0092-8674(03)01075-4. [DOI] [PubMed] [Google Scholar]
- 74.Garzon R., Fabbri M., Cimmino A., Calin G. A., Croce C. M. MicroRNA expression and function in cancer. Trends in Molecular Medicine . 2006;12(12):580–587. doi: 10.1016/j.molmed.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 75.Wu W., Sun M., Zou G. M., Chen J. MicroRNA and cancer: current status and prospective. International Journal of Cancer . 2007;120(5):953–960. doi: 10.1002/ijc.22454. [DOI] [PubMed] [Google Scholar]
- 76.Dalmay T., Edwards D. R. MicroRNAs and the hallmarks of cancer. Oncogene . 2006;25(46):6170–6175. doi: 10.1038/sj.onc.1209911. [DOI] [PubMed] [Google Scholar]
- 77.Esau C., Davis S., Murray S. F., et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metabolism . 2006;3(2):87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 78.Garzon R., Pichiorri F., Palumbo T., et al. MicroRNA fingerprints during human megakaryocytopoiesis. Proceedings of the National Academy of Sciences . 2006;103(13):5078–5083. doi: 10.1073/pnas.0600587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hwang H., Mendell J. MicroRNAs in cell proliferation, cell death, and tumorigenesis. British Journal of Cancer . 2007;96:R40–R44. [PubMed] [Google Scholar]
- 80.Krützfeldt J., Rajewsky N., Braich R., et al. Silencing of microRNAs in vivo with ‘antagomirs’. Nature . 2005;438(7068):685–689. doi: 10.1038/nature04303. [DOI] [PubMed] [Google Scholar]
- 81.Monticelli S., Ansel K. M., Xiao C., et al. MicroRNA profiling of the murine hematopoietic system. Genome Biology . 2005;6(8):p. R71. doi: 10.1186/gb-2005-6-8-r71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Naguibneva I., Ameyar-Zazoua M., Polesskaya A., et al. The microRNA miR-181 targets the homeobox protein Hox-A11 during mammalian myoblast differentiation. Nature Cell Biology . 2006;8(3):278–284. doi: 10.1038/ncb1373. [DOI] [PubMed] [Google Scholar]
- 83.Zhao Y., Samal E., Srivastava D. Serum response factor regulates a muscle-specific microRNA that targets Hand2 during cardiogenesis. Nature . 2005;436(7048):214–220. doi: 10.1038/nature03817. [DOI] [PubMed] [Google Scholar]
- 84.Volinia S., Calin G. A., Liu C.-G., et al. A microRNA expression signature of human solid tumors defines cancer gene targets. Proceedings of the National Academy of Sciences . 2006;103(7):2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Calin G. A., Liu C.-G., Sevignani C., et al. MicroRNA profiling reveals distinct signatures in B cell chronic lymphocytic leukemias. Proceedings of the National Academy of Sciences . 2004;101(32):11755–11760. doi: 10.1073/pnas.0404432101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lagos-Quintana M., Rauhut R., Yalcin A., Meyer J., Lendeckel W., Tuschl T. Identification of tissue-specific microRNAs from mouse. Current Biology . 2002;12(9):735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 87.Lee Y. S., Dutta A. The tumor suppressor microRNA let-7 represses the HMGA2 oncogene. Genes & Development . 2007;21(9):1025–1030. doi: 10.1101/gad.1540407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lu J., Getz G., Miska E. A., et al. MicroRNA expression profiles classify human cancers. Nature . 2005;435(7043):834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 89.Mayr C., Hemann M. T., Bartel D. P. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science . 2007;315(5818):1576–1579. doi: 10.1126/science.1137999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Krek A., Grün D., Poy M. N., et al. Combinatorial microRNA target predictions. Nature Genetics . 2005;37(5):495–500. doi: 10.1038/ng1536. [DOI] [PubMed] [Google Scholar]
- 91.Jansson M. D., Lund A. H. MicroRNA and cancer. Molecular Oncology . 2012;6(6):590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kumar M. S., Lu J., Mercer K. L., Golub T. R., Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nature Genetics . 2007;39(5):673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- 93.Kumar M. S., Pester R. E., Chen C. Y., et al. Dicer1 functions as a haploinsufficient tumor suppressor. Genes & Development . 2009;23(23):2700–2704. doi: 10.1101/gad.1848209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lambertz I., Nittner D., Mestdagh P., et al. Monoallelic but not biallelic loss of Dicer1 promotes tumorigenesis in vivo. Cell Death & Differentiation . 2010;17(4):633–641. doi: 10.1038/cdd.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hata A., Kashima R. Dysregulation of microRNA biogenesis machinery in cancer. Critical Reviews in Biochemistry and Molecular Biology . 2016;51(3):121–134. doi: 10.3109/10409238.2015.1117054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rakheja D., Chen K. S., Liu Y., et al. Somatic mutations in DROSHA and DICER1 impair microRNA biogenesis through distinct mechanisms in Wilms tumours. Nature Communications . 2014;5(1):p. 4802. doi: 10.1038/ncomms5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Torrezan G. T., Ferreira E. N., Nakahata A. M., et al. Recurrent somatic mutation in DROSHA induces microRNA profile changes in Wilms tumour. Nature Communications . 2014;5(1):p. 4039. doi: 10.1038/ncomms5039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Walz A. L., Ooms A., Gadd S., et al. Recurrent DGCR8, DROSHA, and SIX homeodomain mutations in favorable histology Wilms tumors. Cancer Cell . 2015;27(2):286–297. doi: 10.1016/j.ccell.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wegert J., Ishaque N., Vardapour R., et al. Mutations in the SIX1/2 pathway and the DROSHA/DGCR8 miRNA microprocessor complex underlie high-risk blastemal type Wilms tumors. Cancer Cell . 2015;27(2):298–311. doi: 10.1016/j.ccell.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 100.Grund S. E., Polycarpou-Schwarz M., Luo C., Eichmüller S. B., Diederichs S. Rare Drosha splice variants are deficient in microRNA processing but do not affect general microRNA expression in cancer cells. Neoplasia . 2012;14(3):238–IN26. doi: 10.1593/neo.111586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muralidhar B., Goldstein L., Ng G., et al. Global microRNA profiles in cervical squamous cell carcinoma depend on Drosha expression levels. The Journal of Pathology . 2007;212(4):368–377. doi: 10.1002/path.2179. [DOI] [PubMed] [Google Scholar]
- 102.Muralidhar B., Winder D., Murray M., et al. Functional evidence that Drosha overexpression in cervical squamous cell carcinoma affects cell phenotype and microRNA profiles. The Journal of Pathology . 2011;224(4):496–507. doi: 10.1002/path.2898. [DOI] [PubMed] [Google Scholar]
- 103.Tang X., Wen S., Zheng D., et al. Acetylation of Drosha on the N-terminus inhibits its degradation by ubiquitination. PLoS One . 2013;8(8) doi: 10.1371/journal.pone.0072503.e72503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wada T., Kikuchi J., Furukawa Y. Histone deacetylase 1 enhances microRNA processing via deacetylation of DGCR8. EMBO Reports . 2012;13(2):142–149. doi: 10.1038/embor.2011.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Herbert K. M., Pimienta G., DeGregorio S. J., Alexandrov A., Steitz J. A. Phosphorylation of DGCR8 increases its intracellular stability and induces a progrowth miRNA profile. Cell Reports . 2013;5(4):1070–1081. doi: 10.1016/j.celrep.2013.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Nemlich Y., Greenberg E., Ortenberg R., et al. MicroRNA-mediated loss of ADAR1 in metastatic melanoma promotes tumor growth. Journal of Clinical Investigation . 2013;123(6):2703–2718. doi: 10.1172/jci62980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Massagué J. TGFβ signalling in context. Nature Reviews Molecular Cell Biology . 2012;13(10):616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davis B. N., Hilyard A. C., Lagna G., Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature . 2008;454(7200):56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Di Leva G., Garofalo M., Croce C. M. MicroRNAs in cancer. Annual Review of Pathology: Mechanisms of Disease . 2014;9(1):287–314. doi: 10.1146/annurev-pathol-012513-104715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hatley M. E., Patrick D. M., Garcia M. R., et al. Modulation of K-Ras-dependent lung tumorigenesis by MicroRNA-21. Cancer Cell . 2010;18(3):282–293. doi: 10.1016/j.ccr.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Davis B. N., Hilyard A. C., Nguyen P. H., Lagna G., Hata A. Smad proteins bind a conserved RNA sequence to promote microRNA maturation by Drosha. Molecular Cell . 2010;39(3):373–384. doi: 10.1016/j.molcel.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suzuki H. I., Yamagata K., Sugimoto K., Iwamoto T., Kato S., Miyazono K. Modulation of microRNA processing by p53. Nature . 2009;460(7254):529–533. doi: 10.1038/nature08199. [DOI] [PubMed] [Google Scholar]
- 113.Chang J., Davis-Dusenbery B. N., Kashima R., et al. Acetylation of p53 stimulates miRNA processing and determines cell survival following genotoxic stress. The EMBO Journal . 2013;32(24):3192–3205. doi: 10.1038/emboj.2013.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Mori M., Triboulet R., Mohseni M., et al. Hippo signaling regulates microprocessor and links cell-density-dependent miRNA biogenesis to cancer. Cell . 2014;156(5):893–906. doi: 10.1016/j.cell.2013.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Briata P., Lin W.-J., Giovarelli M., et al. PI3K/AKT signaling determines a dynamic switch between distinct KSRP functions favoring skeletal myogenesis. Cell Death & Differentiation . 2012;19(3):478–487. doi: 10.1038/cdd.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Trabucchi M., Briata P., Garcia-Mayoral M., et al. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature . 2009;459(7249):1010–1014. doi: 10.1038/nature08025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang X., Wan G., Berger F. G., He X., Lu X. The ATM kinase induces microRNA biogenesis in the DNA damage response. Molecular Cell . 2011;41(4):371–383. doi: 10.1016/j.molcel.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Radich J. P., Dai H., Mao M., et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proceedings of the National Academy of Sciences . 2006;103(8):2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Romero I., Bast R. C., Jr. Minireview: human ovarian cancer: biology, current management, and paths to personalizing therapy. Endocrinology . 2012;153(4):1593–1602. doi: 10.1210/en.2011-2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pothuri B., Leitao M. M., Levine D. A., et al. Genetic analysis of the early natural history of epithelial ovarian carcinoma. PLoS One . 2010;5(4) doi: 10.1371/journal.pone.0010358.e10358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Walsh T., Casadei S., Lee M. K., et al. Mutations in 12 genes for inherited ovarian, fallopian tube, and peritoneal carcinoma identified by massively parallel sequencing. Proceedings of the National Academy of Sciences . 2011;108(44):18032–18037. doi: 10.1073/pnas.1115052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krol J., Loedige I., Filipowicz W. The widespread regulation of microRNA biogenesis, function and decay. Nature Reviews Genetics . 2010;11(9):597–610. doi: 10.1038/nrg2843. [DOI] [PubMed] [Google Scholar]
- 123.Huang Y., Shen X. J., Zou Q., Wang S. P., Tang S. M., Zhang G. Z. Biological functions of microRNAs: a review. Journal of Physiology and Biochemistry . 2011;67(1):129–139. doi: 10.1007/s13105-010-0050-6. [DOI] [PubMed] [Google Scholar]
- 124.Kinose Y., Sawada K., Nakamura K., Kimura T. The role of microRNAs in ovarian cancer. BioMed Research International . 2014;2014:11. doi: 10.1155/2014/249393.249393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Perera R. J., Ray A. MicroRNAs in the search for understanding human diseases. BioDrugs . 2007;21(2):97–104. doi: 10.2165/00063030-200721020-00004. [DOI] [PubMed] [Google Scholar]
- 126.Calin G. A., Croce C. M. MicroRNA-cancer connection: the beginning of a new tale. Cancer Research . 2006;66(15):7390–7394. doi: 10.1158/0008-5472.can-06-0800. [DOI] [PubMed] [Google Scholar]
- 127.Croce C. M. Causes and consequences of microRNA dysregulation in cancer. Nature Reviews Genetics . 2009;10(10):704–714. doi: 10.1038/nrg2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Di Leva G., Croce C. M. Roles of small RNAs in tumor formation. Trends in Molecular Medicine . 2010;16(6):257–267. doi: 10.1016/j.molmed.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Boeri M., Verri C., Conte D., et al. MicroRNA signatures in tissues and plasma predict development and prognosis of computed tomography detected lung cancer. Proceedings of the National Academy of Sciences . 2011;108(9):3713–3718. doi: 10.1073/pnas.1100048108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mahdian-shakib A., Dorostkar R., Tat M., Hashemzadeh M. S., Saidi N. Differential role of microRNAs in prognosis, diagnosis, and therapy of ovarian cancer. Biomedicine & Pharmacotherapy . 2016;84:592–600. doi: 10.1016/j.biopha.2016.09.087. [DOI] [PubMed] [Google Scholar]
- 131.Zhang L., Huang J., Yang N., et al. microRNAs exhibit high frequency genomic alterations in human cancer. Proceedings of the National Academy of Sciences . 2006;103(24):9136–9141. doi: 10.1073/pnas.0508889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Iorio M. V., Visone R., Di Leva G., et al. MicroRNA signatures in human ovarian cancer. Cancer Research . 2007;67(18):8699–8707. doi: 10.1158/0008-5472.can-07-1936. [DOI] [PubMed] [Google Scholar]
- 133.Yeh Y.-M., Chuang C.-M., Chao K.-C., Wang L.-H. MicroRNA-138 suppresses ovarian cancer cell invasion and metastasis by targeting SOX4 and HIF-1α. International Journal of Cancer . 2013;133(4):867–878. doi: 10.1002/ijc.28086. [DOI] [PubMed] [Google Scholar]
- 134.Wang W., Yang J., Xiang Y. Y., Pi J., Bian J. Overexpression of hsa-miR-320 is associated with invasion and metastasis of ovarian cancer. Journal of Cellular Biochemistry . 2017;118(11):3654–3661. doi: 10.1002/jcb.26009. [DOI] [PubMed] [Google Scholar]
- 135.Yang B., Li S.-Z., Ma L., Liu H.-L., Liu J., Shao J.-J. Expression and mechanism of action of miR-196a in epithelial ovarian cancer. Asian Pacific Journal of Tropical Medicine . 2016;9(11):1105–1110. doi: 10.1016/j.apjtm.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 136.Cho W. C. Great potential of miRNAs as predictive and prognostic markers for cancer. Expert Review of Molecular Diagnostics . 2012;12(4):315–318. doi: 10.1586/erm.12.21. [DOI] [PubMed] [Google Scholar]
- 137.Liu Y., Zhou H., Ma L., et al. MiR-214 suppressed ovarian cancer and negatively regulated semaphorin 4D. Tumor Biology . 2016;37(6):8239–8248. doi: 10.1007/s13277-015-4708-0. [DOI] [PubMed] [Google Scholar]
- 138.Yang C., Kim H. S., Park S. J., et al. Inhibition of miR-214-3p aids in preventing epithelial ovarian cancer malignancy by increasing the expression of LHX6. Cancers . 2019;11(12) doi: 10.3390/cancers11121917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang Q., Zhang S. miR-214 promotes radioresistance in human ovarian cancer cells by targeting PETN. Bioscience Reports . 2017;37(4) doi: 10.1042/BSR20170327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jia X. N., Yin S. D., Wei Y., Chen L. MiR-182-5p inhibited proliferation and migration of ovarian cancer cells by targeting BNIP3. European Review for Medical and Pharmacological Sciences . 2019;23(8):3270–3276. doi: 10.26355/eurrev_201904_17688. [DOI] [PubMed] [Google Scholar]
- 141.Yan J., Jiang J.-Y., Meng X.-N., Xiu Y.-L., Zong Z.-H. MiR-23b targets cyclin G1 and suppresses ovarian cancer tumorigenesis and progression. Journal of Experimental & Clinical Cancer Research . 2016;35(1):p. 31. doi: 10.1186/s13046-016-0307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xia B., Li H., Yang S., Liu T., Lou G. MiR-381 inhibits epithelial ovarian cancer malignancy via YY1 suppression. Tumor Biology . 2016;37(7):9157–9167. doi: 10.1007/s13277-016-4805-8. [DOI] [PubMed] [Google Scholar]
- 143.Tian H., Hou L., Xiong Y. M., et al. miR-132 targeting E2F5 suppresses cell proliferation, invasion, migration in ovarian cancer cells. American Journal of Translational Research . 2016;8(3):1492–1501. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 144.Shi H., Shen H., Xu J., Zhao S., Yao S., Jiang N. MiR-143-3p suppresses the progression of ovarian cancer. American Journal of Translational Research . 2018;10(3):866–874. [PMC free article] [PubMed] [Google Scholar]
- 145.Wang L., He J., Xu H., Xu L., Li N. MiR-143 targets CTGF and exerts tumor-suppressing functions in epithelial ovarian cancer. American Journal of Translational Research . 2016;8(6):2716–2726. [PMC free article] [PubMed] [Google Scholar]
- 146.Hua M., Qin Y., Sheng M., Cui X., Chen W., Zhong J. miR-145 suppresses ovarian cancer progression via modulation of cell growth and invasion by targeting CCND2 and E2F3. Molecular Medicine Reports . 2019;19(5):p. 3575. doi: 10.3892/mmr.2019.10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ding N., Wu H., Tao T., Peng E. NEAT1 regulates cell proliferation and apoptosis of ovarian cancer by miR-34a-5p/BCL2. OncoTargets and Therapy . 2017;10:4905–4915. doi: 10.2147/ott.s142446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jiang J., Xie C., Liu Y., Shi Q., Chen Y. Up-regulation of miR-383-5p suppresses proliferation and enhances chemosensitivity in ovarian cancer cells by targeting TRIM27. Biomedicine & Pharmacotherapy . 2019;109:595–601. doi: 10.1016/j.biopha.2018.10.148. [DOI] [PubMed] [Google Scholar]
- 149.Wang W., Dong J., Wang M., et al. miR-148a-3p suppresses epithelial ovarian cancer progression primarily by targeting c-Met. Oncology Letters . 2018;15(5):6131–6136. doi: 10.3892/ol.2018.8110. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 150.van Jaarsveld M. T. M., Helleman J., Boersma A. W. M., et al. miR-141 regulates KEAP1 and modulates cisplatin sensitivity in ovarian cancer cells. Oncogene . 2013;32(36):4284–4293. doi: 10.1038/onc.2012.433. [DOI] [PubMed] [Google Scholar]
- 151.Li N., Wang L., Tan G., et al. MicroRNA-218 inhibits proliferation and invasion in ovarian cancer by targeting Runx2. Oncotarget . 2017;8(53):91530–91541. doi: 10.18632/oncotarget.21069. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 152.Joshi H. P., Subramanian I. V., Schnettler E. K., et al. Dynamin 2 along with microRNA-199a reciprocally regulate hypoxia-inducible factors and ovarian cancer metastasis. Proceedings of the National Academy of Sciences . 2014;111(14):5331–5336. doi: 10.1073/pnas.1317242111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Denoyelle C., Lambert B., Meryet-Figuière M., et al. miR-491-5p-induced apoptosis in ovarian carcinoma depends on the direct inhibition of both BCL-XL and EGFR leading to BIM activation. Cell Death & Disease . 2014;5(10):p. e1445. doi: 10.1038/cddis.2014.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Kleemann M., Schneider H., Unger K., et al. MiR-744-5p inducing cell death by directly targeting HNRNPC and NFIX in ovarian cancer cells. Scientific Reports . 2018;8(1):p. 9020. doi: 10.1038/s41598-018-27438-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Segura M. F., Hanniford D., Menendez S., et al. Aberrant miR-182 expression promotes melanoma metastasis by repressing FOXO3 and microphthalmia-associated transcription factor. Proceedings of the National Academy of Sciences . 2009;106(6):1814–1819. doi: 10.1073/pnas.0808263106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Liu H., Du L., Wen Z., et al. Up-regulation of miR-182 expression in colorectal cancer tissues and its prognostic value. International Journal of Colorectal Disease . 2013;28(5):697–703. doi: 10.1007/s00384-013-1674-0. [DOI] [PubMed] [Google Scholar]
- 157.Lei R., Tang J., Zhuang X., et al. Suppression of MIM by microRNA-182 activates RhoA and promotes breast cancer metastasis. Oncogene . 2014;33(10):1287–1296. doi: 10.1038/onc.2013.65. [DOI] [PubMed] [Google Scholar]
- 158.Yemelyanova A., Vang R., Kshirsagar M., et al. Immunohistochemical staining patterns of p53 can serve as a surrogate marker for TP53 mutations in ovarian carcinoma: an immunohistochemical and nucleotide sequencing analysis. Modern Pathology . 2011;24(9):1248–1253. doi: 10.1038/modpathol.2011.85. [DOI] [PubMed] [Google Scholar]
- 159.Moskwa P., Buffa F. M., Pan Y., et al. miR-182-mediated downregulation of BRCA1 impacts DNA repair and sensitivity to PARP inhibitors. Molecular Cell . 2011;41(2):210–220. doi: 10.1016/j.molcel.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li A. Y. J., Boo L. M., Wang S.-Y., et al. Suppression of nonhomologous end joining repair by overexpression of HMGA2. Cancer Research . 2009;69(14):5699–5706. doi: 10.1158/0008-5472.can-08-4833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tran H., Brunet A., Grenier J., Datta S., Fornace A, Jr., DiStefano P. DNA repair pathway stimulated by the forkhead transcription factor FOXO3a through the Gadd45 protein. Science . 2002;296(5567):530–534. doi: 10.1126/science.1068712. [DOI] [PubMed] [Google Scholar]
- 162.Wei J.-J., Wu J., Luan C., et al. HMGA2: a potential biomarker complement to P53 for detection of early-stage high-grade papillary serous carcinoma in fallopian tubes. American Journal of Surgical Pathology . 2010;34(1):18–26. doi: 10.1097/pas.0b013e3181be5d72. [DOI] [PubMed] [Google Scholar]
- 163.Zhang S., Mo Q., Wang X. Oncological role of HMGA2. International Journal of Oncology . 2019;55(4):p. 775. doi: 10.3892/ijo.2019.4856. [DOI] [PubMed] [Google Scholar]
- 164.Wu J., Liu Z., Shao C., et al. HMGA2 overexpression-induced ovarian surface epithelial transformation is mediated through regulation of EMT genes. Cancer Research . 2011;71(2):349–359. doi: 10.1158/0008-5472.can-10-2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Parr C., Jiang W. Metastasis suppressor 1 (MTSS1) demonstrates prognostic value and anti-metastatic properties in breast cancer. European Journal of Cancer . 2009;45(9):p. 1673. doi: 10.1016/j.ejca.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 166.Thuault S., Tan E.-J., Peinado H., Cano A., Heldin C.-H., Moustakas A. HMGA2 and Smads co-regulate SNAIL1 expression during induction of epithelial-to-mesenchymal transition. Journal of Biological Chemistry . 2008;283(48):33437–33446. doi: 10.1074/jbc.m802016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Woodings J. A., Sharp S. J., Machesky L. M. MIM-B, a putative metastasis suppressor protein, binds to actin and to protein tyrosine phosphatase delta. The Biochemical Journal . 2003;371(2):463–71. doi: 10.1042/BJ20021962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Meng X., Joosse S. A., Müller V., et al. Diagnostic and prognostic potential of serum miR-7, miR-16, miR-25, miR-93, miR-182, miR-376a and miR-429 in ovarian cancer patients. British Journal of Cancer . 2015;113(9):1358–1366. doi: 10.1038/bjc.2015.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Walsh L. A., Nawshad A., Medici D. Discoidin domain receptor 2 is a critical regulator of epithelial-mesenchymal transition. Matrix Biology . 2011;30(4):243–247. doi: 10.1016/j.matbio.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Corsa C. A. S., Brenot A., Grither W. R., et al. The action of discoidin domain receptor 2 in basal tumor cells and stromal cancer-associated fibroblasts is critical for breast cancer metastasis. Cell Reports . 2016;15(11):2510–2523. doi: 10.1016/j.celrep.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Grither W. R., Divine L. M., Meller E. H., et al. TWIST1 induces expression of discoidin domain receptor 2 to promote ovarian cancer metastasis. Oncogene . 2018;37(13):1714–1729. doi: 10.1038/s41388-017-0043-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ramalho S., Andrade L. A. A., Filho C. C., et al. Role of discoidin domain receptor 2 (DDR2) and microRNA-182 in survival of women with high-grade serous ovarian cancer. Tumour Biology: The Journal of the International Society for Oncodevelopmental Biology and Medicine . 2019;41(1) doi: 10.1177/1010428318823988.1010428318823988 [DOI] [PubMed] [Google Scholar]
- 173.Li Y., Li L. Prognostic values and prospective pathway signaling of MicroRNA-182 in ovarian cancer: a study based on gene expression omnibus (GEO) and bioinformatics analysis. Journal of Ovarian Research . 2019;12(1):p. 106. doi: 10.1186/s13048-019-0580-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Fang Z., Li T., Chen W., et al. Gab2 promotes cancer stem cell like properties and metastatic growth of ovarian cancer via downregulation of miR-200c. Experimental Cell Research . 2019;382(1) doi: 10.1016/j.yexcr.2019.06.007.111462 [DOI] [PubMed] [Google Scholar]
- 175.Wu C.-L., Ho J.-Y., Chou S.-C., Yu D.-S. MiR-429 reverses epithelial-mesenchymal transition by restoring E-cadherin expression in bladder cancer. Oncotarget . 2016;7(18):26593–26603. doi: 10.18632/oncotarget.8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zhang M., Dong B. B., Lu M., et al. miR-429 functions as a tumor suppressor by targeting FSCN1 in gastric cancer cells. OncoTargets and Therapy . 2016;9:1123–1133. doi: 10.2147/OTT.S91879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Fan J.-Y., Fan Y.-J., Wang X.-L., et al. miR-429 is involved in regulation of NF-κBactivity by targeting IKKβ and suppresses oncogenic activity in cervical cancer cells. FEBS Letters . 2017;591(1):118–128. doi: 10.1002/1873-3468.12502. [DOI] [PubMed] [Google Scholar]
- 178.Mao Z., Li T., Zhao H., Qin Y., Kang Y. Identification of epigenetic interactions between MicroRNA and DNA methylation associated with polycystic ovarian syndrome. 2019. [DOI] [PMC free article] [PubMed]
- 179.Spaderna S., Schmalhofer O., Wahlbuhl M., et al. The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Research . 2008;68(2):537–544. doi: 10.1158/0008-5472.can-07-5682. [DOI] [PubMed] [Google Scholar]
- 180.Wiklund E. D., Bramsen J. B., Hulf T., et al. Coordinated epigenetic repression of the miR-200 family and miR-205 in invasive bladder cancer. International Journal of Cancer . 2011;128(6):1327–1334. doi: 10.1002/ijc.25461. [DOI] [PubMed] [Google Scholar]
- 181.Zou J., Liu L., Wang Q., et al. Downregulation of miR-429 contributes to the development of drug resistance in epithelial ovarian cancer by targeting ZEB1. American Journal of Translational Research . 2017;9(3):1357–1368. [PMC free article] [PubMed] [Google Scholar]
- 182.Park S.-M., Gaur A. B., Lengyel E., Peter M. E. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes & Development . 2008;22(7):894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Gregory P. A., Bert A. G., Paterson E. L., et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nature Cell Biology . 2008;10(5):593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 184.Chen H., Xia B., Liu T., Lin M., Lou G. KIAA0101, a target gene of miR-429, enhances migration and chemoresistance of epithelial ovarian cancer cells. Cancer Cell International . 2016;16(1):p. 74. doi: 10.1186/s12935-016-0353-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Donadelli M., Dando I., Fiorini C., Palmieri M. Regulation of miR-23b expression and its dual role on ROS production and tumour development. Cancer Letters . 2014;349(2):107–113. doi: 10.1016/j.canlet.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 186.Cai S., Chen R., Li X., et al. Downregulation of microRNA-23a suppresses prostate cancer metastasis by targeting the PAK6-LIMK1 signaling pathway. Oncotarget . 2015;6(6):3904–3917. doi: 10.18632/oncotarget.2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Liu P., Wang C., Ma C., Wu Q., Zhang W., Lao G. MicroRNA-23a regulates epithelial-to-mesenchymal transition in endometrial endometrioid adenocarcinoma by targeting SMAD3. Cancer Cell International . 2016;16(1):p. 67. doi: 10.1186/s12935-016-0342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Hu X., Wang Y., Liang H., et al. miR-23a/b promote tumor growth and suppress apoptosis by targeting PDCD4 in gastric cancer. Cell Death & Disease . 2017;8(10):p. e3059. doi: 10.1038/cddis.2017.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wang N., Tan H.-Y., Feng Y.-G., Zhang C., Chen F., Feng Y. microRNA-23a in human cancer: its roles, mechanisms and therapeutic relevance. Cancers . 2018;11(1):p. 7. doi: 10.3390/cancers11010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Yang Z., Wang X.-L., Bai R., et al. miR-23a promotes IKKα expression but suppresses ST7L expression to contribute to the malignancy of epithelial ovarian cancer cells. British Journal of Cancer . 2016;115(6):731–740. doi: 10.1038/bjc.2016.244. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 191.Albensi B. C. What is nuclear factor kappa B (NF-κB) doing in and to the mitochondrion? Frontiers in Cell and Developmental Biology . 2019;7(154) doi: 10.3389/fcell.2019.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kirikoshi H., Katoh M. Expression of ST7R (ST7-like, ST7L) in normal tissues and cancer. International Journal of Oncology . 2002;21(1):p. 193. doi: 10.3892/ijo.21.1.193. [DOI] [PubMed] [Google Scholar]
- 193.Zhuang L., Wang X., Wang Z., et al. MicroRNA-23b functions as an oncogene and activates AKT/GSK3β/β-catenin signaling by targeting ST7L in hepatocellular carcinoma. Cell Death & Disease . 2017;8(5):e2804. doi: 10.1038/cddis.2017.216.e2804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sebio A., Kahn M., Lenz H.-J. The potential of targeting Wnt/β-catenin in colon cancer. Expert Opinion on Therapeutic Targets . 2014;18(6):611–615. doi: 10.1517/14728222.2014.906580. [DOI] [PubMed] [Google Scholar]
- 195.Zhu J., Shang Y., Xia Y., Zhang R., Zhang M. An atypical MAGUK GK target recognition mode revealed by the interaction between DLG and KIF13B. Structure (London, England: 1993) . 2016;24(11):p. 1876. doi: 10.1016/j.str.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 196.Zhuang R.-J., Bai X.-X., Liu W. MicroRNA-23a depletion promotes apoptosis of ovarian cancer stem cell and inhibits cell migration by targeting DLG2. Cancer Biology & Therapy . 2019;20(6):897–911. doi: 10.1080/15384047.2019.1579960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Chhabra R., Dubey R., Saini N. Cooperative and individualistic functions of the microRNAs in the miR-23a∼27a∼24-2 cluster and its implication in human diseases. Molecular Cancer . 2010;9(1):p. 232. doi: 10.1186/1476-4598-9-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.He R., Xie L., Zhao Q., Wang J., Wen L., Wang Y. Expressions of miR-23a and RUNX1 in gastric cancer and their clinical significances. Chinese Journal of General Surgery . 2012;21:563–567. [Google Scholar]
- 199.Jin A. H., Wei Z. L. Molecular mechanism of increased sensitivity of cisplatin to ovarian cancer by inhibition of microRNA-23a expression. International Journal of Clinical and Experimental Medicine . 2015;8(8):13329–13334. [PMC free article] [PubMed] [Google Scholar]
- 200.Vosgha H., Salajegheh A., Smith R., Lam A. The important roles of miR-205 in normal physiology, cancers and as a potential therapeutic target. Current Cancer Drug Targets . 2014;14(7):621–637. doi: 10.2174/156800961407140926105634. [DOI] [PubMed] [Google Scholar]
- 201.Cai J., Fang L., Huang Y., et al. miR-205 targets PTEN and PHLPP2 to augment AKT signaling and drive malignant phenotypes in non-small cell lung cancer. Cancer Research . 2013;73(17):5402–5415. doi: 10.1158/0008-5472.can-13-0297. [DOI] [PubMed] [Google Scholar]
- 202.Majid S., Dar A. A., Saini S., et al. MicroRNA-205-directed transcriptional activation of tumor suppressor genes in prostate cancer. Cancer . 2010;116(24):5637–5649. doi: 10.1002/cncr.25488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Feber A., Xi L., Luketich J. D., et al. MicroRNA expression profiles of esophageal cancer. The Journal of Thoracic and Cardiovascular Surgery . 2008;135(2):255–260. doi: 10.1016/j.jtcvs.2007.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Dar A. A., Majid S., de Semir D., Nosrati M., Bezrookove V., Kashani-Sabet M. miRNA-205 suppresses melanoma cell proliferation and induces senescence via regulation of E2F1 protein. Journal of Biological Chemistry . 2011;286(19):16606–16614. doi: 10.1074/jbc.m111.227611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sempere L. F., Christensen M., Silahtaroglu A., et al. Altered MicroRNA expression confined to specific epithelial cell subpopulations in breast cancer. Cancer Research . 2007;67(24):11612–11620. doi: 10.1158/0008-5472.can-07-5019. [DOI] [PubMed] [Google Scholar]
- 206.Li J., Hu K., Gong G., et al. Upregulation of MiR-205 transcriptionally suppresses SMAD4 and PTEN and contributes to human ovarian cancer progression. Scientific Reports . 2017;7(1):p. 41330. doi: 10.1038/srep41330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Sunde J. S., Donninger H., Wu K., et al. Expression profiling identifies altered expression of genes that contribute to the inhibition of transforming growth factor-β signaling in ovarian cancer. Cancer Research . 2006;66(17):8404–8412. doi: 10.1158/0008-5472.can-06-0683. [DOI] [PubMed] [Google Scholar]
- 208.Moustakas A., Souchelnytskyi S., Heldin C. H. Smad regulation in TGF-beta signal transduction. Journal of Cell Science . 2001;114(24):4359–69. doi: 10.1242/jcs.114.24.4359. [DOI] [PubMed] [Google Scholar]
- 209.Kohnoh T., Hashimoto N., Ando A., et al. Hypoxia-induced modulation of PTEN activity and EMT phenotypes in lung cancers. Cancer Cell International . 2016;16:p. 33. doi: 10.1186/s12935-016-0308-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Shi X., Xiao L., Mao X., et al. miR-205-5p mediated downregulation of PTEN contributes to cisplatin resistance in C13K human ovarian cancer cells. Frontiers in Genetics . 2018;9(555):p. 555. doi: 10.3389/fgene.2018.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.He L., Zhu W., Chen Q., et al. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics . 2019;9(26):8206–8220. doi: 10.7150/thno.37455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Liu M., Shen C., Wang C. Long noncoding RNA LINC01133 confers tumor-suppressive functions in ovarian cancer by regulating leucine-rich repeat kinase 2 as an miR-205 sponge. The American Journal of Pathology . 2019;189(11):2323–2339. doi: 10.1016/j.ajpath.2019.07.020. [DOI] [PubMed] [Google Scholar]
- 213.Trancikova A., Mamais A., Webber P. J., et al. Phosphorylation of 4E-BP1 in the mammalian brain is not altered by LRRK2 expression or pathogenic mutations. PLoS One . 2012;7(10) doi: 10.1371/journal.pone.0047784.e47784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kim M. K., Choi H. S., Cho S.-G., Shin Y. C., Ko S.-G. Rubus coreanus Miquel extract causes apoptosis of doxorubicin-resistant NCI/ADR-RES ovarian cancer cells via JNK phosphorylation. Molecular Medicine Reports . 2016;13(5):4065–4072. doi: 10.3892/mmr.2016.4996. [DOI] [PubMed] [Google Scholar]
- 215.Wang L., Zhao F., Xiao Z., Yao L. Exosomal microRNA-205 is involved in proliferation, migration, invasion, and apoptosis of ovarian cancer cells via regulating VEGFA. Cancer Cell International . 2019;19(1):p. 281. doi: 10.1186/s12935-019-0990-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chen W., Liu Y., Chen H., Ning H., Ding K. Loss of miR-449a-caused PrLZ overexpression promotes prostate cancer metastasis. International Journal of Oncology . 2017;51(2):435–444. doi: 10.3892/ijo.2017.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Smith L. T., Lin M., Brena R. M., et al. Epigenetic regulation of the tumor suppressor gene TCF21 on 6q23-q24 in lung and head and neck cancer. Proceedings of the National Academy of Sciences . 2006;103(4):982–987. doi: 10.1073/pnas.0510171102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.John A., Tuszynski G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathology & Oncology Research . 2001;7(1):14–23. doi: 10.1007/bf03032599. [DOI] [PubMed] [Google Scholar]
- 219.Cai K. Q., Yang W.-L., Capo-Chichi C. D., et al. Prominent expression of metalloproteinases in early stages of ovarian tumorigenesis. Molecular Carcinogenesis . 2007;46(2):130–143. doi: 10.1002/mc.20273. [DOI] [PubMed] [Google Scholar]
- 220.Deb B., Uddin A., Chakraborty S. miRNAs and ovarian cancer: an overview. Journal of Cellular Physiology . 2018;233(5):3846–3854. doi: 10.1002/jcp.26095. [DOI] [PubMed] [Google Scholar]
- 221.Khan S., Ayub H., Khan T., Wahid F. MicroRNA biogenesis, gene silencing mechanisms and role in breast, ovarian and prostate cancer. Biochimie . 2019;167:12–24. doi: 10.1016/j.biochi.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 222.Feeley K. M., Wells M. Precursor lesions of ovarian epithelial malignancy. Histopathology . 2001;38(2):87–95. doi: 10.1046/j.1365-2559.2001.01042.x. [DOI] [PubMed] [Google Scholar]
- 223.Corney D. C., Nikitin A. Y. MicroRNA and ovarian cancer. Histology and Histopathology . 2008;23(9):1161–9. doi: 10.14670/HH-23.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Greenlee R. T., Hill-Harmon M. B., Murray T., Thun M. Cancer statistics, 2001. CA: A Cancer Journal for Clinicians . 2001;51(1):15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 225.Bast R. C., Hennessy B., Mills G. B. The biology of ovarian cancer: new opportunities for translation. Nature Reviews Cancer . 2009;9(6):415–428. doi: 10.1038/nrc2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lim D., Oliva E. Precursors and pathogenesis of ovarian carcinoma. Pathology . 2013;45(3):229–242. doi: 10.1097/pat.0b013e32835f2264. [DOI] [PubMed] [Google Scholar]
- 227.Wentzensen N., Poole E. M., Trabert B., et al. Ovarian cancer risk factors by histologic subtype: an analysis from the ovarian cancer cohort consortium. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology . 2016;34(24):2888–98. doi: 10.1200/JCO.2016.66.8178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Andrews L., Mutch D. G. Hereditary ovarian cancer and risk reduction. Best Practice & Research Clinical Obstetrics & Gynaecology . 2017;41:31–48. doi: 10.1016/j.bpobgyn.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 229.Kroeger P. T., Jr., Drapkin R. Pathogenesis and heterogeneity of ovarian cancer. Current Opinion in Obstetrics & Gynecology . 2017;29(1):26–34. doi: 10.1097/gco.0000000000000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Parikh A., Lee C., Joseph P., et al. microRNA-181a has a critical role in ovarian cancer progression through the regulation of the epithelial-mesenchymal transition. Nature Communications . 2014;5(1):p. 2977. doi: 10.1038/ncomms3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Bell D. A. Origins and molecular pathology of ovarian cancer. Modern Pathology . 2005;18(2):S19–S32. doi: 10.1038/modpathol.3800306. [DOI] [PubMed] [Google Scholar]
- 232.Bartels C. L., Tsongalis G. J. MicroRNAs: novel biomarkers for human cancer. Clinical Chemistry . 2009;55(4):623–631. doi: 10.1373/clinchem.2008.112805. [DOI] [PubMed] [Google Scholar]
- 233.Zhang L., Volinia S., Bonome T., et al. Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proceedings of the National Academy of Sciences . 2008;105(19):7004–7009. doi: 10.1073/pnas.0801615105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Vang S., Wu H. T., Fischer A., et al. Identification of ovarian cancer metastatic miRNAs. PLoS One . 2013;8(3) doi: 10.1371/journal.pone.0058226.e58226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Calura E., Fruscio R., Paracchini L., et al. MiRNA landscape in stage I epithelial ovarian cancer defines the histotype specificities. Clinical Cancer Research . 2013;19(15):4114–4123. doi: 10.1158/1078-0432.ccr-13-0360. [DOI] [PubMed] [Google Scholar]
- 236.Nam E. J., Yoon H., Kim S. W., et al. MicroRNA expression profiles in serous ovarian carcinoma. Clinical Cancer Research . 2008;14(9):2690–2695. doi: 10.1158/1078-0432.ccr-07-1731. [DOI] [PubMed] [Google Scholar]
- 237.Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature . 2011;474(7353):p. 609. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Johnson S. M., Grosshans H., Shingara J., et al. RAS is regulated by the let-7 microRNA family. Cell . 2005;120(5):635–647. doi: 10.1016/j.cell.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 239.Saito Y., Liang G., Egger G., et al. Specific activation of microRNA-127 with downregulation of the proto-oncogene BCL6 by chromatin-modifying drugs in human cancer cells. Cancer Cell . 2006;9(6):435–443. doi: 10.1016/j.ccr.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 240.Yang H., Kong W., He L., et al. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Research . 2008;68(2):425–433. doi: 10.1158/0008-5472.can-07-2488. [DOI] [PubMed] [Google Scholar]
- 241.Shell S., Park S.-M., Radjabi A. R., et al. Let-7 expression defines two differentiation stages of cancer. Proceedings of the National Academy of Sciences . 2007;104(27):11400–11405. doi: 10.1073/pnas.0704372104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Wang F., Chang J. T.-H., Kao C. J., Huang R. S. High expression of miR-532-5p, a tumor suppressor, leads to better prognosis in ovarian cancer both in vivo and in vitro. Molecular Cancer Therapeutics . 2016;15(5):1123–1131. doi: 10.1158/1535-7163.mct-15-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Záveský L., Jandáková E., Weinberger V., et al. Ascites-derived extracellular microRNAs as potential biomarkers for ovarian cancer. Reproductive Sciences . 2019;26(4):510–522. doi: 10.1177/1933719118776808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Cui Y., Hong S., Zhu X. The accuracy of single MicroRNAs in peripheral blood to diagnose ovarian cancer: an updated meta-analysis. Disease Markers . 2020;2020:7. doi: 10.1155/2020/1075942.1075942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Li H., Xu Y., Qiu W., Zhao D., Zhang Y. Tissue miR-193b as a novel biomarker for patients with ovarian cancer. Medical Science Monitor . 2015;21:3929–3934. doi: 10.12659/msm.895407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Fukagawa S., Miyata K., Yotsumoto F., et al. MicroRNA-135a-3p as a promising biomarker and nucleic acid therapeutic agent for ovarian cancer. Cancer Science . 2017;108(5):886–896. doi: 10.1111/cas.13210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zuberi M., Khan I., Mir R., Gandhi G., Ray P. C., Saxena A. Utility of serum miR-125b as a diagnostic and prognostic indicator and its alliance with a panel of tumor suppressor genes in epithelial ovarian cancer. PLoS One . 2016;11(4) doi: 10.1371/journal.pone.0153902.e0153902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Zhang X., Zhang H. Diminished miR-613 expression as a novel prognostic biomarker for human ovarian cancer. European Review for Medical and Pharmacological Sciences . 2016;20(5):837–841. [PubMed] [Google Scholar]
- 249.Sun Y., Hu L., Zheng H., et al. MiR-506 inhibits multiple targets in the epithelial-to-mesenchymal transition network and is associated with good prognosis in epithelial ovarian cancer. The Journal of Pathology . 2015;235(1):25–36. doi: 10.1002/path.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kusch M. W., Chapman J. A., Weiner C. P., Roby K. F., Khabele D., Zhou H. H. Abstract 2235: circulating microRNA-205 as a potential prognostic biomarker for recurrent ovarian cancer. Cancer Research . 2019;79(13):p. 2235. doi: 10.1158/1538-7445.am2019-2235. [DOI] [Google Scholar]
- 251.Wang W., Wu L.-R., Li C., et al. Five serum microRNAs for detection and predicting of ovarian cancer. European Journal of Obstetrics & Gynecology and Reproductive Biology: X . 2019;3 doi: 10.1016/j.eurox.2019.100017.100017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Marchini S., Cavalieri D., Fruscio R., et al. Association between miR-200c and the survival of patients with stage I epithelial ovarian cancer: a retrospective study of two independent tumour tissue collections. The Lancet Oncology . 2011;12(3):273–285. doi: 10.1016/s1470-2045(11)70012-2. [DOI] [PubMed] [Google Scholar]
- 253.Ghafouri-Fard S., Shoorei H., Taheri M. miRNA profile in ovarian cancer. Experimental and Molecular Pathology . 2020;113:p. 104381. doi: 10.1016/j.yexmp.2020.104381. [DOI] [PubMed] [Google Scholar]
- 254.Hong F., Li Y., Xu Y., Zhu L. Prognostic significance of serum microRNA-221 expression in human epithelial ovarian cancer. Journal of International Medical Research . 2013;41(1):64–71. doi: 10.1177/0300060513475759. [DOI] [PubMed] [Google Scholar]
- 255.Xu Y.-Z., Xi Q.-H., Ge W.-L., Zhang X.-Q. Identification of serum microRNA-21 as a biomarker for early detection and prognosis in human epithelial ovarian cancer. Asian Pacific Journal of Cancer Prevention . 2013;14(2):1057–1060. doi: 10.7314/apjcp.2013.14.2.1057. [DOI] [PubMed] [Google Scholar]
- 256.Cao J., Cai J., Huang D., et al. miR-335 represents an independent prognostic marker in epithelial ovarian cancer. American Journal of Clinical Pathology . 2014;141(3):437–442. doi: 10.1309/ajcplytzgb54iszc. [DOI] [PubMed] [Google Scholar]
- 257.Zuberi M., Khan I., Gandhi G., Ray P. C., Saxena A. The conglomeration of diagnostic, prognostic and therapeutic potential of serum miR-199a and its association with clinicopathological features in epithelial ovarian cancer. Tumor Biology . 2016;37(8):11259–11266. doi: 10.1007/s13277-016-4993-2. [DOI] [PubMed] [Google Scholar]
- 258.Todeschini P., Salviato E., Paracchini L., et al. Circulating miRNA landscape identifies miR-1246 as promising diagnostic biomarker in high-grade serous ovarian carcinoma: a validation across two independent cohorts. Cancer Letters . 2017;388:320–327. doi: 10.1016/j.canlet.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 259.Rossi S., Sevignani C., Nnadi S. C., Siracusa L. D., Calin G. A. Cancer-associated genomic regions (CAGRs) and noncoding RNAs: bioinformatics and therapeutic implications. Mammalian Genome: Official Journal of the International Mammalian Genome Society . 2008;19(7-8):526–40. doi: 10.1007/s00335-008-9119-8. [DOI] [PubMed] [Google Scholar]
- 260.Calin G. A., Ferracin M., Cimmino A., et al. A MicroRNA signature associated with prognosis and progression in chronic lymphocytic leukemia. New England Journal of Medicine . 2005;353(17):1793–1801. doi: 10.1056/nejmoa050995. [DOI] [PubMed] [Google Scholar]
- 261.Corney D. C., Flesken-Nikitin A., Choi J., Nikitin A. Y. Ovarian Cancer . Berlin, Germany: Springer; 2008. Role of p53 and Rb in ovarian cancer; pp. 99–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Creighton C. J., Fountain M. D., Yu Z., et al. Molecular profiling uncovers a p53-associated role for microRNA-31 in inhibiting the proliferation of serous ovarian carcinomas and other cancers. Cancer Research . 2010;70(5):1906–1915. doi: 10.1158/0008-5472.can-09-3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Nagaraja A. K., Creighton C. J., Yu Z., et al. A link between mir-100 and FRAP1/mTOR in clear cell ovarian cancer. Molecular Endocrinology . 2010;24(2):447–463. doi: 10.1210/me.2009-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Vaksman O., Stavnes H. T., Kaern J., Trope C. G., Davidson B., Reich R. miRNA profiling along tumour progression in ovarian carcinoma. Journal of Cellular and Molecular Medicine . 2011;15(7):1593–1602. doi: 10.1111/j.1582-4934.2010.01148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Zhang S., Lu Z., Unruh A. K., et al. Clinically relevant microRNAs in ovarian cancer. Molecular Cancer Research . 2015;13(3):393–401. doi: 10.1158/1541-7786.mcr-14-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Liu Z., Liu J., Segura M. F., et al. MiR-182 overexpression in tumourigenesis of high-grade serous ovarian carcinoma. The Journal of Pathology . 2012;228(2):204–215. doi: 10.1002/path.4000. [DOI] [PubMed] [Google Scholar]
- 267.Wu Q., Ren X., Zhang Y., et al. MiR-221-3p targets ARF4 and inhibits the proliferation and migration of epithelial ovarian cancer cells. Biochemical and Biophysical Research Communications . 2018;497(4):1162–1170. doi: 10.1016/j.bbrc.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 268.Huh J. H., Kim T. H., Kim K., et al. Dysregulation of miR-106a and miR-591 confers paclitaxel resistance to ovarian cancer. British Journal of Cancer . 2013;109(2):452–461. doi: 10.1038/bjc.2013.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zhu X., Li Y., Xie C., et al. miR-145 sensitizes ovarian cancer cells to paclitaxel by targeting Sp1 and Cdk6. International Journal of Cancer . 2014;135(6):1286–1296. doi: 10.1002/ijc.28774. [DOI] [PubMed] [Google Scholar]
- 270.Laios A., O’Toole S., Flavin R., et al. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Molecular Cancer . 2008;7(1):p. 35. doi: 10.1186/1476-4598-7-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Corney D. C., Hwang C.-I., Matoso A., et al. Frequent downregulation of miR-34 family in human ovarian cancers. Clinical Cancer Research . 2010;16(4):1119–1128. doi: 10.1158/1078-0432.ccr-09-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lee C. H., Subramanian S., Beck A. H., et al. MicroRNA profiling of BRCA1/2 mutation-carrying and non-mutation-carrying high-grade serous carcinomas of ovary. PLoS One . 2009;4(10) doi: 10.1371/journal.pone.0007314.e7314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Lawrie C. H., Gal S., Dunlop H. M., et al. Detection of elevated levels of tumour-associated microRNAs in serum of patients with diffuse large B-cell lymphoma. British Journal of Haematology . 2008;141(5):672–675. doi: 10.1111/j.1365-2141.2008.07077.x. [DOI] [PubMed] [Google Scholar]
- 274.Chung Y.-W., Bae H.-S., Song J.-Y., et al. Detection of microRNA as novel biomarkers of epithelial ovarian cancer from the serum of ovarian cancer patient. International Journal of Gynecological Cancer . 2013;23(4):673–679. doi: 10.1097/igc.0b013e31828c166d. [DOI] [PubMed] [Google Scholar]
- 275.Zheng H., Zhang L., Zhao Y., et al. Plasma miRNAs as diagnostic and prognostic biomarkers for ovarian cancer. PLoS One . 2013;8(11) doi: 10.1371/journal.pone.0077853.e77853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Prahm K. P., Karlsen M. A., Høgdall E., et al. The prognostic value of dividing epithelial ovarian cancer into type I and type II tumors based on pathologic characteristics. Gynecologic Oncology . 2015;136(2):205–211. doi: 10.1016/j.ygyno.2014.12.029. [DOI] [PubMed] [Google Scholar]
- 277.Hu X., Macdonald D. M., Huettner P. C., et al. A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecologic Oncology . 2009;114(3):457–464. doi: 10.1016/j.ygyno.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 278.Eitan R., Kushnir M., Lithwick-Yanai G., et al. Tumor microRNA expression patterns associated with resistance to platinum based chemotherapy and survival in ovarian cancer patients. Gynecologic Oncology . 2009;114(2):253–259. doi: 10.1016/j.ygyno.2009.04.024. [DOI] [PubMed] [Google Scholar]
- 279.Mateescu B., Batista L., Cardon M., et al. miR-141 and miR-200a act on ovarian tumorigenesis by controlling oxidative stress response. Nature Medicine . 2011;17(12):1627–1635. doi: 10.1038/nm.2512. [DOI] [PubMed] [Google Scholar]
- 280.Lee H., Park C. S., Deftereos G., et al. MicroRNA expression in ovarian carcinoma and its correlation with clinicopathological features. World Journal of Surgical Oncology . 2012;10(1):p. 174. doi: 10.1186/1477-7819-10-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Kim T. H., Kim Y. K., Kwon Y., et al. Deregulation of miR-519a, 153, and 485-5p and its clinicopathological relevance in ovarian epithelial tumours. Histopathology . 2010;57(5):734–743. doi: 10.1111/j.1365-2559.2010.03686.x. [DOI] [PubMed] [Google Scholar]
- 282.Wan W.-N., Zhang Y.-X., Wang X.-M., et al. ATAD2 is highly expressed in ovarian carcinomas and indicates poor prognosis. Asian Pacific Journal of Cancer Prevention . 2014;15(6):2777–2783. doi: 10.7314/apjcp.2014.15.6.2777. [DOI] [PubMed] [Google Scholar]
- 283.Shen J., Ambrosone C. B., DiCioccio R. A., Odunsi K., Lele S. B., Zhao H. A functional polymorphism in the miR-146a gene and age of familial breast/ovarian cancer diagnosis. Carcinogenesis . 2008;29(10):1963–1966. doi: 10.1093/carcin/bgn172. [DOI] [PubMed] [Google Scholar]
- 284.Feng G., Li G., Gentil-Perret A., Tostain J., Genin C. Elevated serum-circulating RNA in patients with conventional renal cell cancer. Anticancer Research . 2008;28(1A):321–326. [PubMed] [Google Scholar]
- 285.Taylor D. D., Gercel-Taylor C. MicroRNA signatures of tumor-derived exosomes as diagnostic biomarkers of ovarian cancer. Gynecologic Oncology . 2008;110(1):13–21. doi: 10.1016/j.ygyno.2008.04.033. [DOI] [PubMed] [Google Scholar]
- 286.Liang H., Jiang Z., Xie G., Lu Y. Serum microRNA-145 as a novel biomarker in human ovarian cancer. Tumor Biology . 2015;36(7):5305–5313. doi: 10.1007/s13277-015-3191-y. [DOI] [PubMed] [Google Scholar]
- 287.Lheureux S., Gourley C., Vergote I., Oza A. M. Epithelial ovarian cancer. The Lancet . 2019;393(10177):1240–1253. doi: 10.1016/s0140-6736(18)32552-2. [DOI] [PubMed] [Google Scholar]
- 288.Mullen M. M., Kuroki L. M., Thaker P. H. Novel treatment options in platinum-sensitive recurrent ovarian cancer: a review. Gynecologic Oncology . 2019;152(2):416–425. doi: 10.1016/j.ygyno.2018.10.023. [DOI] [PubMed] [Google Scholar]
- 289.Jelovac D., Armstrong D. K. Recent progress in the diagnosis and treatment of ovarian cancer. CA: A Cancer Journal for Clinicians . 2011;61(3):183–203. doi: 10.3322/caac.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Chen J., Wang L., Matyunina L. V., Hill C. G., McDonald J. F. Overexpression of miR-429 induces mesenchymal-to-epithelial transition (MET) in metastatic ovarian cancer cells. Gynecologic Oncology . 2011;121(1):200–205. doi: 10.1016/j.ygyno.2010.12.339. [DOI] [PubMed] [Google Scholar]
- 291.Wang L., Mezencev R., Švajdler M., Benigno B. B., McDonald J. F. Ectopic over-expression of miR-429 induces mesenchymal-to-epithelial transition (MET) and increased drug sensitivity in metastasizing ovarian cancer cells. Gynecologic Oncology . 2014;134(1):96–103. doi: 10.1016/j.ygyno.2014.04.055. [DOI] [PubMed] [Google Scholar]
- 292.Leskelä S., Leandro-García L. J., Mendiola M., et al. The miR-200 family controls -tubulin III expression and is associated with paclitaxel-based treatment response and progression-free survival in ovarian cancer patients. Endocrine Related Cancer . 2010;18(1):85–95. doi: 10.1677/erc-10-0148. [DOI] [PubMed] [Google Scholar]
- 293.Lin Y.-F., Lai T.-C., Chang C.-K., et al. Targeting the XIAP/caspase-7 complex selectively kills caspase-3-deficient malignancies. Journal of Clinical Investigation . 2013;123(9):3861–3875. doi: 10.1172/jci67951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Mehrotra S., Languino L. R., Raskett C. M., Mercurio A. M., Dohi T., Altieri D. C. IAP regulation of metastasis. Cancer Cell . 2010;17(1):53–64. doi: 10.1016/j.ccr.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Zhao W.-J., Deng B.-Y., Wang X.-M., Miao Y., Wang J.-N. XIAP associated factor 1 (XAF1) represses expression of X-linked inhibitor of apoptosis protein (XIAP) and regulates invasion, cell cycle, apoptosis, and cisplatin sensitivity of ovarian carcinoma cells. Asian Pacific Journal of Cancer Prevention . 2015;16(6):2453–2458. doi: 10.7314/apjcp.2015.16.6.2453. [DOI] [PubMed] [Google Scholar]
- 296.Sasaki H., Sheng Y., Kotsuji F., Tsang B. K. Down-regulation of X-linked inhibitor of apoptosis protein induces apoptosis in chemoresistant human ovarian cancer cells. Cancer Research . 2000;60(20):5659–66. [PubMed] [Google Scholar]
- 297.Li X., Chen W., Zeng W., Wan C., Duan S., Jiang S. microRNA-137 promotes apoptosis in ovarian cancer cells via the regulation of XIAP. British Journal of Cancer . 2017;116(1):66–76. doi: 10.1038/bjc.2016.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Sun L., Zhai R., Zhang L., Zhao S. MicroRNA-149 suppresses the proliferation and increases the sensitivity of ovarian cancer cells to cisplatin by targeting X-linked inhibitor of apoptosis. Oncology Letters . 2018;15(5):7328–7334. doi: 10.3892/ol.2018.8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Zhang Y., Huang F., Luo Q., et al. Inhibition of XIAP increases carboplatin sensitivity in ovarian cancer. OncoTargets and Therapy . 2018;11:8751–8759. doi: 10.2147/ott.s171053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Jin M., Yang Z., Ye W., Xu H., Hua X. MicroRNA-150 predicts a favorable prognosis in patients with epithelial ovarian cancer, and inhibits cell invasion and metastasis by suppressing transcriptional repressor ZEB1. PLOS ONE . 2014;9(8) doi: 10.1371/journal.pone.0103965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Xu Y., Hu J., Zhang C., Liu Y. MicroRNA-320 targets mitogen-activated protein kinase 1 to inhibit cell proliferation and invasion in epithelial ovarian cancer. Molecular Medicine Reports . 2017;16(6):8530–8536. doi: 10.3892/mmr.2017.7664. [DOI] [PubMed] [Google Scholar]
- 302.Li C., Duan P., Wang J., Lu X., Cheng J. miR-320 inhibited ovarian cancer oncogenicity via targeting TWIST1 expression. American Journal of Translational Research . 2017;9(8):3705–3713. [PMC free article] [PubMed] [Google Scholar]
- 303.Cronise K. E., Hernandez B. G., Gustafson D. L., Duval D. L. Identifying the ErbB/MAPK signaling cascade as a therapeutic target in canine bladder cancer. Molecular Pharmacology . 2019;96(1):36–46. doi: 10.1124/mol.119.115808. [DOI] [PubMed] [Google Scholar]
- 304.Rahman M., Nakayama K., Rahman M., et al. KRAS and MAPK1 gene amplification in type II ovarian carcinomas. International Journal of Molecular Sciences . 2013;14(7):13748–13762. doi: 10.3390/ijms140713748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Yiwei T., Hua H., Hui G., Mao M., Xiang L. HOTAIR interacting with MAPK1 regulates ovarian cancer skov3 cell proliferation, migration, and invasion. Medical Science Monitor . 2015;21:1856–1863. doi: 10.12659/msm.893528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zhao H., Wang J., Kong X., et al. CD47 promotes tumor invasion and metastasis in non-small cell lung cancer. Scientific Reports . 2016;6(1):p. 29719. doi: 10.1038/srep29719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Zhu Q.-Q., Ma C., Wang Q., Song Y., Lv T. The role of TWIST1 in epithelial-mesenchymal transition and cancers. Tumor Biology . 2016;37(1):185–197. doi: 10.1007/s13277-015-4450-7. [DOI] [PubMed] [Google Scholar]
- 308.Zhu X., Shen H., Yin X., et al. miR-186 regulation of Twist1 and ovarian cancer sensitivity to cisplatin. Oncogene . 2016;35(3):323–332. doi: 10.1038/onc.2015.84. [DOI] [PubMed] [Google Scholar]
- 309.Wu Q., Guo R., Lin M., Zhou B., Wang Y. MicroRNA-200a inhibits CD133/1+ ovarian cancer stem cells migration and invasion by targeting E-cadherin repressor ZEB2. Gynecologic Oncology . 2011;122(1):149–154. doi: 10.1016/j.ygyno.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 310.Chen R., Alvero A. B., Silasi D. A., et al. Regulation of IKKβ by miR-199a affects NF-κB activity in ovarian cancer cells. Oncogene . 2008;27(34):4712–4723. doi: 10.1038/onc.2008.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Liu M. X., Siu M. K., Liu S. S., Yam J. W., Ngan H. Y., Chan D. W. Epigenetic silencing of microRNA-199b-5p is associated with acquired chemoresistance via activation of JAG1-Notch1 signaling in ovarian cancer. Oncotarget . 2014;5(4):944–958. doi: 10.18632/oncotarget.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Tang Z., Ow G. S., Thiery J. P., Ivshina A. V., Kuznetsov V. A. Meta-analysis of transcriptome reveals let-7b as an unfavorable prognostic biomarker and predicts molecular and clinical subclasses in high-grade serous ovarian carcinoma. International Journal of Cancer . 2014;134(2):306–318. doi: 10.1002/ijc.28371. [DOI] [PubMed] [Google Scholar]
- 313.Pecot C. V., Rupaimoole R., Yang D., et al. Tumour angiogenesis regulation by the miR-200 family. Nature Communications . 2013;4(1):p. 2427. doi: 10.1038/ncomms3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There are no raw data associated with this review article.


