Abstract
Objective
In the 19 years since the Korean College of Neuropsychopharmacology and the Korean Society for Affective Disorders developed the Korean Medication Algorithm Project for Depressive Disorder (KMAP-DD) in 2002, four revisions have been conducted.
Methods
To increase survey efficiency in this revision, to cover the general clinical practice, and to compare the results with previous KMAP-DD series, the overall structure of the questionnaire was maintained. The six sections of the questionnaire were as follows 1) pharmacological treatment strategies for major depressive disorder (MDD) with/without psychotic features; 2) pharmacological treatment strategies for persistent depressive disorder and other depressive disorder subtypes; 3) consensus for treatment-resistant depression; 4) the choice of an antidepressant in the context of safety, adverse effects, and comorbid physical illnesses; 5) treatment strategies for special populations (children/adolescents, elderly, and women); and 6) non-pharmacological biological therapies. Recommended first-, second-, and third-line strategies were derived statistically.
Results
There has been little change in the four years since KMAP-DD 2017 due to the lack of newly introduced drug or treatment strategies. However, shortened waiting time between the initial and subsequent treatments, increased preference for atypical antipsychotics (AAPs), especially aripiprazole, and combination strategies with AAPs yield an active and somewhat aggressive treatment trend in Korea.
Conclusion
We expect KMAP-DD to provide clinicians with useful information about the specific strategies and medications appropriate for treating patients with MDD by bridging the gap between clinical real practice and the evidence-based world.
Keywords: Algorithm, Depressive disorder, Guideline, Pharmacotherapy
INTRODUCTION
Nineteen years have passed since the first Korean Medication Algorithm Project for Depressive Disorder (KMAP-DD), the expert’s consensus guideline with clinical evidence on the treatment of depressive disorder, was developed in 2002 [1]. Since then, three revisions have been conducted by the KMAP-DD executive committee within the Korean Society for Affective Disorders (KSAD) and the Korean College of Neuropsychopharmacology (KCNP): KMAP-DD 2006 [2], KMAP-DD 2012 [3], and KMAP-DD 2017 [4].
Because depressive disorder is a heterogeneous disorder that has various symptoms, clinical courses, and outcomes, the KMAP-DD executive committee conducted a fourth revision in 2021 to assist clinicians with decisions on proper treatment strategies, to standardize the quality of pharmacological treatments as the definitive clinical guideline [5], and to reflect changes that have been made for several years in pharmacological practice in Korea.
We summarized the results of the fourth revision of Korean experts’ opinions on the pharmacological treatment of patients with depressive disorder and compared the 4th revision results to those of the previous KMAP-DD, from 2017, 2012, and 2006; KMAP-DD 2002 was excluded due to questionnaire format and differences in the lists of antidepressants (ADs) and atypical antipsychotics (AAPs).
METHODS
The overall study design and method used in previous revisions were maintained in this revision for the comparison across the KMAP-DD series. To obtain the experts’ consensus, the executive committee composed a review committee, and the review committee completed the questionnaire (Table 1).
Table 1.
Comparison among the first (2006), second (2012), third (2017), and fourth (2021) revisions of the KMAP-DD
| First revision in 2006 | Second revision in 2012 | Third revision in 2017 | Fourth revision in 2021 | |
|---|---|---|---|---|
| Depressive episode | Mild Moderate Non-psychotic severe Psychotic severe |
Mild to moderate Non-psychotic severe Psychotic severe |
Same as 2012 | Same as 2017 |
| AD dosage and duration of treatment | Present | Deletion | Change: duration of initial treatment and number of choosing AD as initial treatment | Same as 2017 |
| Subtype | Dysthymia Minor depressive disorder Atypical features Melancholic features. |
Dysthymia Minor depressive disorder Atypical features Melancholic features Seasonal pattern |
Dysthymia Minor depressive disorder Atypical features Melancholic features Seasonal pattern Mixed features Anxious distress |
PDD (Dysthymia) Atypical features Melancholic features Seasonal pattern Mixed features Anxious distress |
| Comorbid physical illness | Absent | Newly added | DM Thyroid disease Liver disease Renal disease |
DM Thyroid disease Liver disease Renal disease Hypertension Seizure disorder Cardiovascular disease Parkinson’s disease Arrythmia Chronic pain (fibromyalgia, etc.) |
| Special population | Child only | Child and adolescent Elderly Women |
Same as 2012 | Child (up to primary school) Adolescent (up to high school) Elderly Women |
| Non-pharmacological biological therapy | ECT only | Including TMS Light therapy nutritional therapy, sleep deprivation, VNS, DBS as well as ECT |
Same as 2012 | ECT rTMS VNS DBS Light therapy Nutritional therapy tDCS |
| Response rate of review committee | 66.3% (67/101) | 54.5% (67/123) | 54.9% (79/144) | 68.5% (98/143) (adult 67.0% [65/97], child/adolescent 71.7%, [33/46]) |
KMAP-DD, Korean Medication Algorithm for Depressive Disorder; AD, antidepressant; DBS, deep brain stimulation; DM, diabetes mellitus; ECT, electroconvulsive therapy; PDD, persistent depressive disorder; rTMS, repetitive transcranial magnetic stimulation; tDCS, transcranial direct current stimulation; VNS, vagal nerve stimulation.
Review Committee
The composition criteria and qualifications of the review committee were similar to those of KMAP-DD 2012 and 2017. We recruited 143 Korean psychiatrists (97 adult psychiatrists and 46 child/adolescent psychiatrists) who were lifelong members of KCNP and KSAD, had more than 15 years of clinical experience in the field of psychiatry, and who had each published at least one paper related to mood disorders during the previous year or who have been running a mood clinic in their hospital. Psychiatrists for adult members worked in various clinical settings (university hospitals, n = 68; general and mental hospitals, n = 22; private psychiatric clinics, n = 7), and the child/adolescent psychiatrists worked in university hospitals (n = 29), general and mental hospitals (n = 6), and private psychiatric clinics (n = 11). All members of the review committee provided written informed consent for their participation in this survey. Of the 143 psychiatrists, 98 responded to our survey (response rate = 68.5%; adult = 67.0% [65/97], child/adolescent = 71.7% [33/46]). Respondents received a predetermined fee for their participation.
Questionnaire
The questionnaire included six sections and thirty three general questions, including 118 sub-items and 764 options. The six sections of the questionnaire were as follows: 1) pharmacological treatment strategies for mild-to-moderate, severe without psychotic features (non-psychotic severe episode), and severe episode with psychotic features (psychotic severe episode) (first to third step); 2) pharmacological treatment strategies for persistent depressive disorder (PDD) and depressive disorder subtypes; 3) consensus for treatment-resistant depression; 4) the choice of an antidepressant in the context of safety, adverse effects, and comorbid physical illnesses; 5) treatment strategies for special populations (children/adolescents, elderly, and women); and 6) non-pharmacological biological therapies (electroconvulsive therapy, ECT; repetitive transcranial magnetic stimulation, rTMS; etc.). The executive committee decided to exclude fluvoxamine due to lack of usage in Korea and include esketamine (nasal spray), a newer antidepressant approved for treatment-resistant depression (TRD) (Table 2). In this revision, the list of AAPs is the same as in 2017, including amisulpride, aripiprazole, blonanserin, clozapine, olanzapine, paliperidone, quetiapine, risperidone, and ziprasidone.
Table 2.
Lists of drugs used in KMAP-DD 2021
| Antidepressant | Agomelatine |
| Bupropion | |
| Esketamine (nasal spray)a | |
| Mirtazapine | |
| SNRI (desvenlafaxine, duloxetine, milnacipran, venlafaxine) | |
| SSRI (escitalopram, fluoxetine, paroxetine, sertraline)b | |
| Tianeptine | |
| TCA (amitriptyline, clomipramine, imipramine, etc.) | |
| Vortioxetinea | |
| Mood stabilizer | Carbamazepine, lamotrigine, lithium, valproate |
| Antipsychotics | Amisulpride, aripiprazole, blonanserin, clozapine, olanzapine, paliperidone, quetiapine, risperidone, ziprasidone Typical antipsychotics |
| Augmentation drugs | Buspirone, mood stabilizer, psychostimulant, thyroid hormone, etc. |
KMAP-DD, Korean Medication Algorithm for Depressive Disorder; SNRI, serotonin and norepinephrine reuptake inhibitor; SSRI, selective serotonin reuptake inhibitor; TCA, tricyclic antidepressant.
aNewly included in AD lists in this survey. bFluvoxamine was deleted in AD lists.
Unlike previous surveys, children (primary school students and younger) and adolescents (middle and high school students) were investigated separately within the children/adolescents with depression group.
Rating Scale
The overall rating method was the same as before. Each treatment option was scored on a nine-point scale. Nine indicates extremely appropriate, 7−8 indicates usually appropriate, 4−6 indicates ambivalence about its appropriateness, 2−3 indicates usually inappropriate (a treatment the clinician would rarely use), and 1 indicates extremely inappropriate (a treatment the clinician would never use). Some questions had a numeric response instead of a rating. For example, “How long do you wait before switching ongoing AD or AAP?”
Data Analysis and Decision of Preference and Categories
Means and 95% confidence intervals (CI) of each question or option were calculated. We divided them into three categories according to the lowest score of 95% CI: first- line/preferred treatment, ≥ 6.5; second-line/reasonable treatment, < 6.5 and ≥ 3.5; and third-line/inappropriate treatment, < 3.5. Treatment of choice (TOC) was defined as an option that was rated at 9 points by 50% or more of the experts.
A chi-square test was used to confirm the presence or absence of consensus on each option/question. No significant difference between categories indicated lack of consensus.
The SPSS 15.0 software package (SPSS Inc., Chicago, IL, USA) was used for the analyses of preference rankings and multiple responses.
Development of Treatment Guidelines and Algorithms
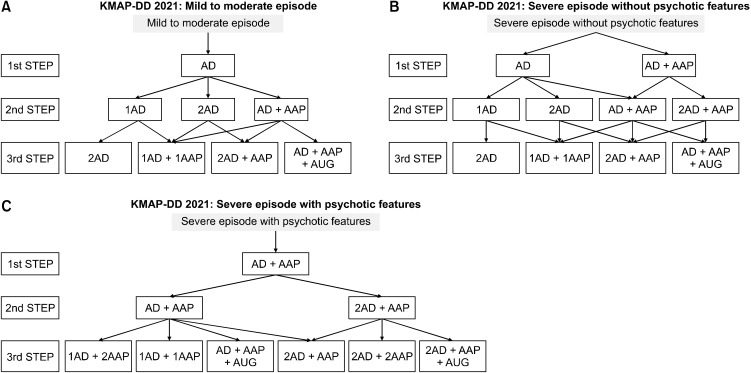
After the advisory committee and the executive committee discussed these results and reviewed the clinical evidence, considering Korean clinical situations, the executive committee drew up the fourth revised KMAP-DD algorithms (Fig. 1) and distributed KMAP-DD 2021 to psychiatrists and related experts in Korea.
Fig. 1.
The Korean Medication Algorithm for Depressive Disorder 2021. KMAP-DD, Korean Medication Algorithm for Depressive Disorder; AD, antidepressants; AAP, atypical antipsychotics; AUG, augmenting agents.
Ethics
The present study was conducted according to the Declaration of Helsinki. The study protocol was approved by the Institutional Review or Ethics Committee at Wonkwang University (approved number: WKUH 2020-12-012). The revision process was funded entirely by KCNP and KSAD without external financial support.
RESULTS
Treatment Strategy for Acute Depression with or Without Psychotic Features (Table 3)
Table 3.
Initial and next step strategies for depressive disorder between the KMAP-DD 2021, 2017, 2012, and 2006
| KMAP-DD 2021 | KMAP-DD 2017 | KMAP-DD 2012 | KMAP-DD 2006 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| 1st line | 2nd line | 1st line | 2nd line | 1st line | 2nd line | 1st line | 2nd line | ||||||
| Initial step treatment strategy | |||||||||||||
| Depressive episode | |||||||||||||
| Mild to moderate | AD monotherapya | AD + AD AD + AAPb |
AD monotherapya | AD + AD AD + AAP AD + MS |
AD monotherapya | AD + AD AD + AAP |
AD monotherapya | AD + AD AD + AUG |
|||||
| Non-psychotic severe | AD monotherapy AD + AAP |
AD + AD AD + MS AAP monotherapy |
AD monotherapy AD + AAP |
AAP monotherapy AD + AD AD + MS ECT |
AD monotherapy | AD + AAP AAP monotherapy AD + AD ECT |
AD monotherapy | AD + AD AD + AAP AD + AUG |
|||||
| Psychotic severe | AD + AAPa | AAP monotherapy AD + MS AD + ADb AD monotherapyb |
AD + AAPa | ECT AAP monotherapy AD + TAP AD + MS AD monotherapy AD + AD |
AD + AAPa | AD + TAP AAP monotherapy ECT AD + AD AD monotherapy |
AD + AAPa | AD + TAP ECT AD + AD AD + AUG AD monotherapy AAP monotherapy |
|||||
| 2nd step treatment strategies | |||||||||||||
| Mild to moderate | No response | Adding AAP Switching AD Adding AD |
Adding AUG | Switching AD Adding AD Adding AAP |
Adding AUG | Switching AD Adding other AD |
Adding AAP AUG |
Switching AD Adding other AD |
Adding AUG Adding AAP |
||||
| Non-psychotic severe | Partial response | Adding AD Switching AAP |
Adding AAP Switching AD Adding AUG |
Adding AD Adding AAP |
Switching AD AUG |
Adding other AD Adding AAP |
AUG Switching AD |
Adding other AD AUG |
Switching AD Adding AAP |
||||
| Psychotic severe | Inadequate response | Adding AD Switching AAP |
Adding AAP Switching AD Adding AUG |
Switching AAP Adding AD Switching AD |
Adding AAP AUG Adding TAP |
Adding other AD Switching AAP Switching AD |
Adding other AAP AUG Adding TAP |
Adding AAP Switching AD Adding other AD |
AUG | ||||
KMAP-DD, Korean Medication Algorithm for Depressive Disorder; AAP, atypical antipsychotics; AD, antidepressant; ECT, electroconvulsive therapy; MS, mood stabilizer; TAP, typical anti-psychotics. AUG, augmenting drug such as buspirone, mood stabilizer, psychostimulant, thyroid hormone, etc.
aTreatment of choice (TOC), defined as an option that was rated at 9 points by 50% or more of the experts. bNo consensus.
Initial step strategies for depressive episode
For mild-to-moderate depressive episodes, AD monotherapy (95% CI 8.47−8.84) was recommended as the TOC. For non-psychotic severe episodes, AD monotherapy and AD + AAP were the preferred first-line strategies (Table 4). For psychotic severe episodes, the AD + AAP combination was the TOC in all four KMAP-DD revisions.
Table 4.
Comparison of preference of antipsychotics in the Korean Medication Algorithm for Depressive Disorder
| Preference of atypical antipsychotics | Fourth revision in 2021, severe episode | Third revision in 2017, severe episode | Second revision in 2012, severe episode | First revision in 2006 | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Without psychotic features | With psychotic features | Without psychotic features | With psychotic features | Without psychotic features | With psychotic features | ||||
| Amisulpride | 4.2 (3.7−4.7) | 5.7 (5.3−6.2) | 5.0 (4.6−5.5) | 6.0 (5.6−6.3) | 5.5 (5.0−5.9) | 6.6 (6.1−7.0) | 5.8 (5.3−6.2) | ||
| Aripiprazole | 7.6 (7.2−8.0)a | 8.4 (8.2−8.6)a,b | 8.3 (8.2−8.5)a,b | 8.3 (8.1−8.5)a | 7.9 (7.6−8.2)a | 7.9 (7.6−8.2)a | 6.3 (5.8−6.7) | ||
| Blonanserin | 3.9 (3.5−4.4) | 5.8 (5.3−6.2) | 4.6 (4.2−5.0) | 6.1 (5.7−6.5) | 4.4 (3.7−5.1) | 5.8 (5.1−6.4) | - | ||
| Clozapine | 2.6 (2.2−3.1) | 4.3 (3.8−4.8) | 2.7 (2.3−3.1) | 3.9 (3.4−4.3) | 2.9 (2.4−3.4) | 4.1 (3.6−4.6) | 3.5 (3.0−4.0) | ||
| Olanzapine | 5.2 (4.6−5.7)c | 7.3 (6.9−7.7)a | 6.0 (5.6−6.4) | 7.3 (7.0−7.7)a | 6.6 (6.2−7.0) | 7.6 (7.3−7.9)a | 7.1 (6.7−7.5)a | ||
| Paliperidone | 4.0 (3.5−4.6) | 6.1 (5.6−6.6) | 4.5 (4.1−5.0) | 6.9 (5.6−6.5) | - | - | - | ||
| Quetiapine | 6.9 (6.4−7.8) | 8.0 (7.7−8.2)a | 7.8 (7.6−8.0)a | 7.9 (7.7−8.1)a | 7.7 (7.4−8.0)a | 8.1 (7.8−8.3)a | 7.3 (6.9−7.7)a | ||
| Risperidone | 4.7 (4.2−5.2) | 6.8 (6.4−7.2) | 5.3 (4.8−5.7) | 6.7 (6.3−7.1) | 6.0 (5.5−6.4) | 7.3 (6.9−7.6)a | 7.3 (6.9−7.7)a | ||
| Ziprasidone | 4.3 (3.9−4.8) | 5.7 (5.3−6.1) | 5.1 (4.6−5.6) | 5.9 (5.6−6.3) | 5.7 (5.2−6.3) | 6.5 (6.1−6.9) | 6.5 (6.0−6.9) | ||
| Typical antipsychotics | 2.3 (1.9−2.7) | 3.4 (2.9−3.9) | 2.9 (2.5−3.3) | 4.0 (3.4−4.3) | 3.2 (2.8−3.6) | 4.5 (4.0−5.0) | 4.8 (4.3−5.3) | ||
Values are presented as mean (95% confidence interval).
aFirst-line drug:score of preference is 9 points. bTreatment of choice (TOC), defined as an option that was rated at 9 points by 50% or more of the experts. cNo consensus.
Second-step strategies when initial strategies yield no or partial response
When the patient is unresponsive to the initial AD monotherapy, adding AD or AAP and switching AD were pre-ferred. With a partial response rather than ‘no response’ to AD monotherapy, adding AD or AAP was preferred. When unresponsive to the initial AD + AAP combination, switching AAP or AD and adding AD were preferred. With partial response to the initial AD + AAP, adding AD and switching AAP were preferred.
In general, changing ADs occurred among SSRIs, SNRIs, and mirtazapine, and adding ADs occurred among SSRIs, SNRIs, mirtazapine, bupropion, agomelatine, and vortioxetine, depending on the drug being used. Changing or adding AAPs occurred among aripiprazole, quetiapine, and olanzapine in the same way.
Third-step strategies when second-step strategies have no or partial response
When there is inadequate response to the second-step treatment, adding another AAP or AD or changing AAP or AD were recommended. Adding augmentation drugs, such as buspirone, mood stabilizer, psychostimulant, and thyroid hormone, was preferred when there was an inadequate response to adding AAP to AD monotherapy for non-psychotic depression and when there was an inadequate response to either adding AD to an AD + AAP combination (2ADs + AAP) or switching or adding AAP to ongoing AD + AAP for psychotic depression.
AD Choices
Preferred AD for initial treatment
For mild-to-moderate depressive episodes, escitalopram (95% CI 8.3−8.7) was the TOC, and sertraline, desvenlafaxine, fluoxetine, venlafaxine, vortioxetine, duloxetine, mirtazapine, and paroxetine were recommended as first-line ADs. For non-psychotic severe episodes, escitalopram (95% CI 8.1−8.5) was the TOC, and desvenlafaxine, venlafaxine, sertraline, mirtazapine, fluoxetine, duloxetine, paroxetine, and vortioxetine were the first-line ADs. For psychotic severe episodes, escitalopram (95% CI 8.1−8.5) was the TOC, and venlafaxine, desvenlafaxine, sertraline, mirtazapine, fluoxetine, paroxetine, and duloxetine were recommended as the first-line ADs.
AD choice considering adverse effects, safety, or comorbid physical illness
We asked the experts to choose three ADs when considering adverse effects, drug safety, and comorbid physical illness, respectively. Considering adverse effects, bupropion, mirtazapine, and vortioxetine were preferred in terms of sexual dysfunction. For sedation and somnolence, bupropion, fluoxetine, and tianeptine; for weight gain, bupropion, fluoxetine, and vortioxetine; for insomnia, mirtazapine, paroxetine, and tricyclic antidepressants (TCAs); for gastrointestinal trouble, mirtazapine, tianeptine and bupropion; and for anticholinergic side effects, escitalopram, agomelatine, and vortioxetine were selected.
Regarding safety issues, for safety accidents such as falling or traffic accidents, bupropion, escitalopram, and fluoxetine; for serotonin syndrome, bupropion, tianeptine, and agomelatine; for orthostatic hypotension, bupropion, escitalopram, and mirtazapine; and for suicidal ideation, mirtazapine, bupropion, and agomelatine were chosen.
Regarding comorbid conditions, for diabetes mellitus (DM), escitalopram, sertraline and bupropion; for thyroid disease, escitalopram, sertraline and fluoxetine; for liver disease, escitalopram, sertraline, and tianeptine; for renal disease, escitalopram, sertraline, and tianeptine; for hypertension, escitalopram, sertraline, and tianeptine; for cardiovascular illness, escitalopram, sertraline, and tianeptine; for seizure disorder, escitalopram, sertraline, and tianeptine; for parkinsonism, escitalopram, sertraline, and bupropion; for arrhythmia, sertraline, escitalopram, and fluoxetine; and for chronic pain, duloxetine, milnacipran, and venlafaxine were preferred.
AAP Choices
For non-psychotic severe episodes, aripiprazole was only first-line AAP, and quetiapine, olanzapine with no consensus, risperidone, ziprasidone, and amisulpride were recommended as second-line. However, for psychotic severe episodes, aripiprazole was the TOC, and quetiapine and olanzapine were recommended as first-line AAPs (Table 4).
Treatment Duration with Initial AD before Next Strategy
The reviewers were asked, “How long do you keep using the initial drug until the next strategic change, such as switching or adding, due to lack of efficacy?” With AD monotherapy for mild-to-moderate depressive episodes, their answer was a minimum 2.2 ± 0.9−maximum 6.1 ± 2.3 weeks (no response: 2.2−4.3 weeks; partial response: 3.3−6.1 weeks). With AD monotherapy for non-psychotic severe episodes, the answer was 1.9 ± 0.8−5.2 ± 2.2 weeks (no response: 2.2−4.3 weeks; partial response: 2.9−5.2 weeks). With AD monotherapy for psychotic severe episodes, their answer was a minimum 1.7 ± 0.8−maximum 4.8 ± 2.3 weeks (no response: 1.7−3.3 weeks; partial response: 2.6−4.8 weeks).
Treatment Strategies for Persistent Depressive Disorder (Dysthymia) and Subtypes of Depression
Treatment strategies for PDD
AD monotherapy with escitalopram was the TOC for PDD. AD + AAP, AD + AD, AD + mood stabilizer (MS), and AAP monotherapy were recommended as second-line strategies.
AD choice according to subtype of depressive episode
For patients with melancholic features, escitalopram was the TOC and desvenlafaxine, venlafaxine, sertraline, fluoxetine, duloxetine, mirtazapine, paroxetine, vortioxetine, and milnacipran were the first-line ADs. Agomelatine, bupropion, tianeptine, TCA, and esketamine (with no consensus) were second-line ADs.
For atypical features, escitalopram, desvenlafaxine, fluoxetine, sertraline, venlafaxine, duloxetine, vortioxetine, bupropion, paroxetine, milnacipran, and agomelatine were the first-line ADs. Mirtazapine, tianeptine, esketamine, and TCA were second-line ADs.
For seasonal patterns, escitalopram, sertraline, fluoxetine, desvenlafaxine, venlafaxine, duloxetine, paroxetine, vortioxetine, bupropion, and mirtazapine were the first-line ADs. Agomelatine, milnacipran, tianeptine, TCA, and esketamine (with no consensus) were second-line ADs.
Treatment strategies for anxious distress specifiers and mixed features (Table 5)
Table 5.
Initial treatment strategies and drugs of choice for anxious distress or mixed features in KMAP-DD 2017 and 2021
| Subtype of depressive disorder | 2021 | 2017 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||
| Initial treatment strategies | AD | AAP or MS | Initial treatment strategies | AD | AAP or MS | ||||||||
|
|
|
|
|
|
|
|
|||||||
| 1st line | 2nd line | 1st line | 1st line | 1st line | 2nd line | 1st line | 1st line | ||||||
| Anxious distress |
AD + AAP AD monotherapy |
AD + AD AD + MS AAP monotherapy |
Escitalopram Fluoxetine Paroxetine Sertraline Duloxetine Venlafaxine Desvenlafaxine Mirtazapine Vortioxetine |
Aripiprazole Olanzapine Quetiapine |
AD + AAP AD monotherapy |
MS monotherapy AD + AD AD + MS AAP monotherapy AD + TAP |
Escitalopram Fluoxetine Paroxetine Sertraline Duloxetine Venlafaxine Desvenlafaxine Mirtazapine |
Quetiapine |
|||||
| Mixed features | AD + AAP AD + MS |
AAP monotherapy MS monotherapy AD monotherapy AD + AD AD + TAP |
Escitalopram Sertraline Fluoxetine Bupropion Mirtazapine Desvenlafaxine Venlafaxine Paroxetine |
Aripiprazole Quetiapine Olanzapine Valproate Lithium |
AD+AAP AD+MS |
AAP monotherapy MS monotherapy AD monotherapy AD + TAP AD + AD ECT |
Escitalopram Fluoxetine Sertraline Venlafaxine Bupropion Mirtazapine |
Aripiprazole Quetiapine valproate Olanzapine Lithium |
|||||
KMAP-DD, Korean Medication Algorithm for Depressive Disorder; AAP, atypical antipsychotics; AD, antidepressant; MS, mood stabilizer; TAP, typical antipsychotics; ECT, electroconvulsive therapy.
For anxious distress, an AD + AAP combination or AD monotherapy were the initial treatment strategies. AD + AD, AD + MS, and AAP monotherapy were recommended as the second-line strategies. As an initial AD, escitalopram, fluoxetine, paroxetine, sertraline, duloxetine, venlafaxine, desvenlafaxine, mirtazapine, and vortioxetine were preferred. Aripiprazole, olanzapine, and quetiapine were the first-line AAPs for anxious distress.
For mixed features, AD + AAP or AD + MS were the first-line strategies, and AAP, MS, or AD monotherapy were recommended as second-line strategies. As preferred ADs, escitalopram, sertraline, fluoxetine, bupropion, mirtazapine, desvenlafaxine, venlafaxine, and paroxetine were recommended, and as AAPs and MSs, aripiprazole, quetiapine, olanzapine, valproate, and lithium were recommended.
Criteria for TRD Chosen by Experts
Experts were asked about the proper criteria for TRD. The largest number of experts (44.4%) chose the criteria for TRD as “Failure to respond to two ADs + one AAP combination treatment”. “Failure to respond to two AD treatments of separate pharmacological AD classes” was the second most answered (20.6%). “Failure to respond to two ADs + two AAPs combination treatment (19.0%)” and “Failure to respond to three AD treatments of separate pharmacological AD classes (9.5%)” were in the third and fourth place, respectively. As fifth place, “ECT should be considered for no longer responsive to medications” (3.2%), and surprisingly, “Failure to response to a two-AD combination treatment” (1.6%) was the least preferred (Table 6).
Table 6.
Consensus of clinical definition for treatment resistant depression in KMAP-DD 2021
| Definition for treatment resistant depression | Respondent |
|---|---|
| Failure to respond to two AD treatments of separate pharmacological AD class | 13 (20.6) |
| Failure to respond to three AD treatments of separate pharmacological AD class | 6 (9.5) |
| Failure to respond to two AD combination treatment | 1 (1.6) |
| Failure to respond to two AD + one AAP combina-tion treatment of pharmacological AD classes | 28 (44.4) |
| Failure to respond to two AD + two AAP combination treatment | 12 (19.0) |
| ECT should be considered for no longer responsive to medications. | 2 (3.2) |
| Others | 1 (1.6) |
| Total | 62 (100) |
Values are presented as number (%).
KMAP-DD, Korean Medication Algorithm for Depressive Disorder; AAP, atypical antipsychotic; AD, antidepressant; ECT, electroconvulsive therapy.
Treatment Strategies for Special Populations (Table 7)
Table 7.
Treatment strategies for major depressive disorder in special populations
| Special populations | Disorder | Severity of episode | Initial treatment strategies | AD | AAP or MS | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||
| 1st line | 2nd line | 1st line | 2nd line | 1st line | 2nd line | |||||
| Child and adolescent | Disruptive mood dysregulation disorder |
- | AAP monotherapy AD + AAP AD monotherapy MS + AAPb MS monotherapy MS + ADb |
Escitalopram Fluoxetine |
Sertraline Bupropion Duloxetine |
Aripiprazolea
Risperidone Valproate |
Quetiapine Paliperidone Amisulpride Olanzapineb |
|||
| MDD | Mild-to-moderate episode, child | AD monotherapya | AD + AAP AAP monotherapy AD + AD AD + MSb |
Escitalopram Fluoxetine |
Sertraline Bupropion Duloxetine Paroxetineb |
|||||
| Mild to moderate episode, adolescent | AD monotherapy |
AD + AAP AAP monotherapy AD + MS AD + ADb |
Escitaloprama
Fluoxetine Sertraline |
Venlafaxine Duloxetineb Bupropionb Paroxetineb Desvenlafaxineb Agomelatineb Vortioxetineb Mirtazapineb |
||||||
| Non-psychotic severe episode, child | AD monotherapy AD + AAP |
AD + AD AD + MS AAP monotherapyb |
Escitaloprama
Fluoxetine Sertraline |
Bupropion Paroxetineb Duloxetineb Venlafaxine Desvenlafaxineb |
Aripiprazole Quetiapineb Risperidoneb |
|||||
| Non-psychotic severe episode, adolescent | AD monotherapy AD + AAP |
AAP monotherapy AD + MS AD + ADb |
Fluoxetinea Escitaloprama Sertraline | Bupropion Paroxetineb Venlafaxine Duloxetineb Desvenlafaxineb Agomelatineb Vortioxetineb |
Aripiprazole Quetiapineb Risperidoneb |
|||||
| Psychotic severe episode, child | AD + AAPa | AAP monotherapy AD + MS AD monotherapyb AD + ADb |
Escitalopram fluoxetine Sertraline |
Bupropion Mirtazapine Paroxetineb Venlafaxineb Desvenlafaxineb Duloxetineb |
Aripiprazolea
Risperidone Quetiapine |
Amisulpride Olanzapineb Paliperidoneb |
||||
| Psychotic severe episode, adolescent | AD + AAPa | AAP monotherapy AD + MS AD monotherapyb AD + ADb |
Escitaloprama
Fluoxetine Sertraline |
Paroxetineb
Venlafaxine Duloxetineb Bupropionb Desvenlafaxineb Vortioxetineb Mirtazapineb |
Aripiprazolea
Risperidone |
Quetiapine Amisulpride Olanzapineb Paliperidoneb |
||||
| Elderly | MDD | Mild to moderate episode | AD monotherapya | AD + AAP AD + AD AAP monotherapy AD + MS AD + STM |
Escitaloprama
Sertraline Desvenlafaxine Vortioxetine Duloxetine Venlafaxine Fluoxetine Mirtazapine Milnacipran |
Paroxetine Agomelatine Bupropion Tianeptine |
||||
| Non-psychotic severe episode | AD monotherapy AD+AAP |
AD + AD AD + MS AAP monotherapy AD + STM |
Escitaloprama
Sertraline Desvenlafaxine Duloxetine Mirtazapine Vortioxetine Venlafaxine Fluoxetine Milnacipran Paroxetine |
Agomelatine Bupropion Tianeptine Esketamine (nasal spray)b TCAs |
||||||
| Psychotic severe episode | AD + AAPa | AD monotherapy AD + MS AD + AD AD + STM AAP monotherapyb |
Escitaloprama
Sertraline Desvenlafaxine Duloxetine Mirtazapine Venlafaxine Vortioxetine Fluoxetine Milnacipran Paroxetine |
Agomelatine Bupropion Tianeptine Esketamine (nasal spray)b TCAs |
Aripiprazolea Quetiapine |
Olanzapine Risperidone Paliperidone Blonanserin Amisulpride Ziprasidone |
||||
| Women | Premenstrual dysphoric disorder | AD monotherapya | Fluoxetine Escitalopram Sertraline Paroxetine Desvenlafaxine Venlafaxine |
Duloxetine Vortioxetine Milnacipran Mirtazapine Agomelatine Bupropion Tianeptine TCA |
||||||
| MDD in pregnancy | Mild to moderate episode | AD monotherapy | AAP monotherapy AD + AAPb ECTb |
|||||||
| Non-psychotic severe episode | AD monotherapy |
AD + AAP AAP monotherapy ECT |
||||||||
| Psychotic severe episode | ECT AD + AAP |
AAP monotherapy AD monotherapy AAP + MS |
||||||||
| Postpartum depression | Mild to moderate episode | AD monotherapy AD + AAP |
AAP monotherapy AD + MS MS monotherapy MS + AAPb ECTb |
|||||||
| Non-psychotic severe episode | AD + AAP | AD monotherapy AAP monotherapy AD + MS MS monotherapy AAP + MS ECT |
||||||||
| Psychotic severe episode | AD + AAPa | AD monotherapy AAP monotherapy AD + MS MS monotherapy AAP + MS ECT |
||||||||
AAP, atypical antipsychotics; AD, antidepressant; ECT, electroconvulsive therapy; MDD, major depressive disorder; MS, mood stabilizer; STM, psychostimulant; TAP, typical antipsychotics; TCA, tricyclic antidepressant.
aTreatment of choice (TOC), defined as an option that was rated at 9 points by 50% or more of the experts. bNo consensus.
Depressive disorder in children or adolescents
For more detailed results, the fourth revision separately surveyed the children (elementary school students) and adolescents (middle and high school students). There is no first-line treatment for disruptive mood dysregulation disorder (DMDD). AAP, or AD monotherapy, and AD + AAP were recommended as second-line treatment. Escitalopram and fluoxetine were the first-line ADs, aripiprazole was the TOC, and risperidone and valproate were the first-line AAP and MS, respectively. AD monotherapy was the TOC for mild-to-moderate episodes in children and the first-line treatment strategy for mild-to-moderate episodes in adolescents. AD monotherapy and AD + AAP were the first-line strategies for children and adolescents with non-psychotic severe episodes. AD + AAP were the TOC for children and adolescents with psychotic severe episodes.
As first-line AD, escitalopram and fluoxetine for children, and escitalopram, fluoxetine, and sertraline for adolescents with mild-to-moderate depressive episodes were preferred. For children and adolescents with psychotic or non-psychotic severe episodes, escitalopram, fluoxetine, and sertraline were preferred. Among AAPs, aripiprazole was the TOC for children and adolescents with psychotic severe episodes, and risperidone and quetiapine were the first-line treatment preferences for children with psychotic severe episodes.
Elderly patients with MDD
AD monotherapy was the TOC for elderly patients with mild-to-moderate depressive episodes. AD monotherapy and AD + AAP were the first-line strategies for non-psychotic severe episodes, whereas AD + AAP were the TOC for psychotic severe episodes. Moreover, escitalopram was the TOC for all three types of episodes. Aripiprazole as the TOC and quetiapine as the first-line AAP were recommended for psychotic severe episodes.
Women with depressive disorder
AD monotherapy was the first-line treatment strategy for premenstrual dysphoric disorder (PMDD). Fluoxetine, escitalopram, sertraline, paroxetine, desvenlafaxine, and venlafaxine were first-line ADs for PMDD. For MDD in pregnant women, AD monotherapy was recommended as the first-line treatment for mild-to-moderate and non- psychotic severe depression. However, AD + AAP and ECT were recommended for psychotic severe depression. For postpartum depression, AD monotherapy and AD + AAP were the first-line strategies for mild-to-moderate episodes, and AD + AAP were recommended as the first-line treatment for non-psychotic severe episodes. For psychotic severe episodes, AD + AAP were the recommended TOC.
Non-pharmacological Biological Treatment
Electroconvulsive therapy (ECT)
Ninety-two percent of experts considered ECT to be an MDD treatment modality, and 43.8% of experts were applying it for MDD in clinical practice. On average, one expert conducts ECT with 7.0 persons per year, with 2.8 sessions per patient per week, totaling 10.5 sessions per patient during one treatment plan. The first-line indications for ECT were urgent suicidal risks in patients regardless of psychotic features and nonresponsiveness on pharmacotherapy with moderate episodes or severe episodes in pregnant patients.
Indications for repetitive transcranial magnetic stimulation (rTMS)
Eighty-nine percent of experts considered rTMS an MDD treatment option, but only 40.6% apply it in clinical practice for MDD. On average, one expert conducts rTMS with 12.6 persons per year, with 3.4 sessions per patient per week, totaling 12.6 sessions per patient during one treatment plan. In Korea, MDD in pregnant patients was the first-line indication for rTMS.
Choice of complementary or novel agents
Transcranial direct current stimulation (tDCS), vagus nerve stimulation (VNS), deep brain stimulation (BDS), light therapy, and omega-3 were considered as second-line treatment options for MDD.
DISCUSSION
Compared to KMAP-DD 2017, there are no significant changes in KMAP-DD 2021. However, we confirmed the increased preference of AAPs in various depressive conditions and the criteria for TRD that was first investigated in this revision and compared these results with previous versions of KMAP and foreign guidelines for depressive disorder [5-7].
Treatment Strategies for Non-psychotic or Psychotic Depression
Initial step
As a first step, the preferred initial treatment strategy for non-psychotic depressive episodes was AD monotherapy regardless of the severity of the depressive episodes, as recommended in KMAP-DD 2017, 2012, and 2006. Also, AAP + AD combination as the first-line strategy for non-psychotic or psychotic severe depressive episodes was the same recommendation as in KMAP-DD 2017, while AD monotherapy was the only first-line strategy for non-psychotic severe episodes in KMAP-DD 2012, which implicated the increased preference of AAPs in Korea.
Foreign clinical guidelines recommended AD monotherapy instead of AD + AAP combination as the first-line strategy for non-psychotic severe episodes on the bases of a benefit-harm comparison about initially using AAP for depression [5-8] and lack of evidence that AAP as an initial treatment strategy for depression is superior to AD monotherapy [5].
However, given that the response rate of initial AD monotherapy was 40−60% [9,10], the remission rate of initial AD monotherapy was 20−30% [11,12], and that a recent meta-analysis showed that the AD + AAP combination is superior to AD monotherapy (response rate, odds ratio [OR] = 1.69, 95% CI = 1.46−1.95, p < 0.00001; remission rates, OR = 2.00, 95% CI = 1.69−2.37, p < 0.00001) [13], AD + AAP combination could also be a good choice as the first-line strategy for non-psychotic or psychotic severe depressive episodes.
The AD + AAP combination was recommended as the TOC for psychotic depression in KMAP-DD 2006, 2012, 2017, and 2021. Canadian Network for Mood and Anxiety Treatments (CANMAT) recommended AD + AAP for psychotic depression based on the finding that an AD + AAP combination was superior to placebo 2 randomized controlled trials (RCTs), AD monotherapy (3 RCTs), and AAP monotherapy (4 RCTs) [5].
Next step: Second and third steps
As the next step after inadequate response to initial treatment, we should consider adding (combination or augmentation) or switching, after reevaluating the factors for the lack of response, such as inadequate dose, inadequate duration, and adherence. As the second step after an inadequate response to initial treatment, adding AD or AAP with a partial response and switching ongoing AD or AAP with no response to initial treatment is the same recommendation as in KMAP previous series and CANMAT 2016 [5]. Based on the most consistent evidence for efficacy of AAP in TRD, CANMAT recommended aripiprazole, quetiapine, and risperidone as adjunctive therapy with level I evidence [5].
AD Choice
Preferred AD as initial treatment
For mild-to-moderate episodes, escitalopram was the only TOC in 2021, while escitalopram and sertraline were the TOCs in 2017. Escitalopram was the TOC for psychotic and non-psychotic severe depressive episodes in 2021 as well as in 2017 in contrast with the KMAP-DD 2012, in which there was no TOC among the ADs.
Although no single AD has proven to be more effective than others [6], Cipriani and colleagues, who conducted network analyses among 21 ADs, suggested that in head-to-head studies, agomelatine, amitriptyline, escitalopram, mirtazapine, paroxetine, venlafaxine, and vortioxetine were more effective than other antidepressants (range of ORs 1.19−1.96) [14]. Like these results, the preference of escitalopram for all severities of depressive episodes is the highest in Korea for more than 9 years.
The preference of vortioxetine, newly included in KMAP-DD 2021, for psychotic depression was as a second-line treatment in 2021. CANMAT 2016 recommended vortioxetine and agomelatine as well as SSRIs, SNRIs, bupropion, and mirtazapine as first-line [5]. But the preference of vortioxetine for psychotic depression was second-line in 2021. Also, the preference of agomelatine in Korea was second-line in KMAP-DD 2021 following 2017. This result reflects the Korean situation that agomelatine was first withdrawn in Korea in 2017 due to medical insurance issues with Korean government and then re-marketed in 2019. The preference of agomelatine has recently been increasing.
Esketamine (nasal spray), new and not yet widely used in Korea, was recommended as the second-line for non- psychotic or psychotic severe episodes, which suggests higher expectations for this drug as an emergent drug as well as an AD by Korean experts. The US Food and Drug Administration (FDA) approved esketamine (nasal spray) for TRD [15,16]. A recent meta-analysis including 8 double-blinded, randomized controlled trials and 1,488 patients showed that esketamine significantly improved the Montgomery Asberg depression rating scale (MADRS) total score compared to placebo starting from 2−4 hours after the first administration (standardized mean difference, −0.41 [95% CI = −0.58 to −0.25], p < 0.00001) [17], and this superiority was maintained until the end of the double-blinded period (28 days). Esketamine (nasal spray) has indication for suicidal ideation as well as TRD in Korea.
AD choice considering adverse effects, safety, and comorbid physical illness
Due to a lack of evidence regarding this section, we mainly compare these results with previous KMAP-DD series. These results are almost the same in 2021, 2017, and 2012. When considering adverse effects, the following ADs were recommended with higher preference: 1) bupropion for sexual dysfunction, sleepiness or sedation, weight gain, safety accidents, serotonin syndrome, and orthostatic hypotension and 2) mirtazapine for insomnia, GI trouble, and suicidal ideation.
A recent systemic review regarding AD and weight gain showed that SSRIs including fluoxetine increase mean weight while bupropion decreases the mean weight [18]. Most experts in Korea would have the same clinical experience.
The clear mechanism for orthostatic hypotension in the elderly is unknown but due to its commonality [19], it can be assumed that bupropion was recommended as the first-line to take into consideration both orthostatic hypotension and anticholinergic side effects. However, CANMAT 2016 found patients taking bupropion-XL had more headache and dry mouth, by as much as 28% and 34%, respectively, compared to other ADs. Therefore, careful prescription of bupropion is needed due to headache and dry mouth [5].
For suicidality, SSRIs are related to nearly twice the risk (OR 1.92) of suicide and suicidal attempts among adolescents in observational studies [20]. The US FDA warned that all antidepressants are related with an increase in suicidality among children and adolescents, including young adults (18−24 years), during initial treatment [21].
During the investigation period of KMAP-DD 2021, esketamine had only been approved for TRD in Korea. As a result, Korean experts recommended mirtazapine rather than esketamine when considering suicidality. However, a recent meta-analysis as described above showed that esketamine had superiority over placebo in TRD and suicidal ideation (OR = 2.04, 95% CI = 1.37−3.05), but the groups did not statistically differ at 24 hours and day 28 [17]. With careful monitoring and assessment for suicidality at the beginning of AD treatment, particularly in children, adolescents, and young adults, esketamine is promising to protect against suicide.
When considering comorbid physical illness, escitalopram and sertraline were recommended as first- or second-line drugs for comorbidities of DM, thyroid disease, liver disease, renal disease, hypertension, seizure disorder, cardiovascular disease, cerebrovascular disease, parkinsonism, and arrhythmia. When choosing the initial AD, after all, it is reasonable to select drugs by considering various factors including socioeconomic condition, safety issues, clinical experience, and comorbid physical conditions, as well as efficacy at the same time.
AAP Choices
For 15 years, there was a high preference of aripiprazole for non-psychotic severe episodes and aripiprazole, quetiapine, and olanzapine for psychotic severe episodes (Table 4). With level I evidence, foreign guidelines recommended aripiprazole, quetiapine, olanzapine, brexpiprazole, and lurasidone as adjunctive, not as AAP monotherapy [5,6]. That is, Korean experts recommend AAP as a first step, but foreign guidelines recommend AAP as the second step. Brexpiprazole and lurasidone are currently not available in Korea.
Treatment Duration with the Initial AD before the Next Strategy (Switching to or Adding Another AD, etc.)
We observed a shorter waiting duration between the initial and next-step treatment strategies, such as augmentation, switching, or combination. With no response to the initial treatment for mild-to-moderate depressive episodes, waiting durations were 3.3−6.1 weeks (2006), 3.2−7.5 weeks (2012), 2.9−6.4 weeks (2017), and 2.2−4.3 weeks (2021). With no response to the initial treatment for severe psychotic episodes, waiting durations were 2.4−4.7 weeks (2012), 2.3−4.7 weeks (2017), and 1.7−3.3 weeks (2021). With a partial response to initial treatment for psychotic severe episodes, waiting durations were 3.4−6.9 weeks (2012), 3.4−6.5 weeks (2017), and 2.6−4.8 weeks (2021).
We observed a trend of shorter durations when there was no response than when there was a partial response to initial drugs, as was seen in previous KMAP series. This trend can also be seen in foreign guidelines. World Federation of Societies of Biological Psychiatry (WSFBP) 2017 [6] recommended that optimization be considered with an inadequate response to AD therapy for 2 weeks. In addition, CANMAT 2016 introduced ‘early improvement’, defined as > 20−30% reduction from baseline on a depression rating scale after 2−4 weeks, as a predictor for later outcomes and prognoses and recommended increasing AD dosage or switching AD when intolerable at 2−4 weeks [5].
Treatment Strategies for Persistent Depressive Disorder (dysthymia) and Strategies Specific to Subtype or Specifiers such as Mixed or Anxious Distress
Treatment strategies for PDD
The recommendation for AD monotherapy as the initial strategy was the same as that of KMAP-DD 2021, 2017, 2012, and 2006. The preference of escitalopram as initial AD was the TOC in 2021 while a first-line treatment in 2017.
Antidepressant choice according to subtypes
Melancholia
Little information about the most effective agents for the melancholic and atypical subtypes is available [22]. Compared to the result of escitalopram and venlafaxine being TOCs in 2017, only escitalopram was the TOC in 2021. The preference of desvenlafaxine increased in 2021 compared to 2017. Recent Australian and New Zealand guidelines for major depression recommended venlafaxine and amitriptyline for melancholic types [22].
Atypical features and seasonal patterns
Mirtazapine was downgraded to a second-line treatment in 2021 after being a first-line treatment in 2017, which may reflect the selection of less sedative ADs considering atypical symptoms, such as hypersomnia and psychomotor retardation. Agomelatine was recommended as second-line in 2017 but was promoted to first-line in 2021, which seems to have considered the stimulation effect via blocking 5-HT2C of agomelatine and effect of improving hypersomnia through adjusting the sleep cycle [23].
For seasonal patterns, the first-line ADs in 2021 were the same as those recommended in 2017. These results were presumed to be a choice considering the (hypo)manic switching of seasonal patterns that can be related to bipolarity [24]; bupropion XR was approved for depressive patients with seasonal patterns in the US [25].
Treatment strategies for anxious distress specifiers and mixed feature (Table 5)
Preferred initial strategies for “with anxious distress” were AD + AAP or AD monotherapy both in 2017 and 2021. First-line ADs in 2017 were the same in 2021, except escitalopram was the TOC and vortioxetine was newly included as a first-line AD in 2021. Quetiapine was the only recommended first-line AAP for ‘with anxious distress’ in 2017, but aripiprazole and olanzapine as well as quetiapine were the first-line AAPs in 2021. This showed that AAPs are not limited to psychosis or bipolar disorder but are also used for comorbid anxiety or agitation.
For mixed features, both KMAP-DD 2017 and 2021 had the same first-line strategies (AAP + AD and AD + MS). Aripiprazole, quetiapine, olanzapine, valproate, and lithium were recommended as the first-line augmenting medications for mixed features. These recommended medications were consistent with previous trials [26] and similar to those for mixed state of bipolar disorder [27]. Despite the controversies about the criteria for mixed features in the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) [28, 29], it is reasonable that mixed features in depressive episodes itself can be interpreted as bipolarity.
The Consensus on the Definition of TRD
There is no universal criteria or definition of TRD, but it is most commonly defined as at least two failures of AD monotherapy with adequate dose, for 6-9 weeks during a major depressive episode [30]. We expected a similar answer from Korean experts; however, contrary to our expectation, among 6 questions, 44% of experts chose the criteria of TRD as “Failure to respond to two ADs + one AAP combination treatment”. “Failure to respond to a two-AD combination treatment” was chosen the least, by 1.6% of experts.
“Treatment-resistance” can be defined when the treatment response is outside the boundaries of standard therapy. Of course, AAP is not literally an AD; however, a few AAPs demonstrated antidepressant efficacy in clinical trials with TRD [5,6], and CANMAT 2016 recommended quetiapine as one of the second-line ADs. Therefore, it is necessary to extend the criteria of TRD to include AAPs [5]. These results also demonstrated the Korean trend of using AAPs in an earlier step in treating depression, with increased preference for AAPs.
Treatment Strategies for Special Populations (Table 7)
Because it is ethically difficult to conduct RCTs with special populations, such as children, pregnant subjects, and elderly subjects with depression, experts’ consensus could be useful. However, experts’ experience is not always right, as evidence is not always right. Special populations with depression should be carefully treated according to an advantage-disadvantage evaluation.
Treatment strategy for children and adolescents
Disruptive mood dysregulation disorder (DMDD)
This section has been included since KMAP-2012. In this revision, more detailed results could be obtained by distinguishing between children (5 to 12 years old) and adolescents (13 to 17 years old) in MDD, except for disruptive mood dysregulation disorder (DMDD).
The prevalence of DMDD, newly introduced in DSM-5, among children and adolescents has been as much as 2−5% [31,32]; however, there was no first-line strategy for DMDD in KMAP-DD 2021. Korean experts cautiously recommended AAP monotherapy, AD + AAP, and AD monotherapy as a second-line treatment. Given that two key symptoms are severe recurrent temper outbursts and persistent irritability observable by others, a higher preference for AAPs rather than ADs was reasonable, although DMDD is a type of depressive disorder.
Among AAPs, aripiprazole was the TOC for DMDD. Preference for risperidone was increased to a first-line drug in 2021 after being a second-line drug in 2017. These changes are consistent with results on tic disorder in Korea: aripiprazole was preferred over risperidone in terms of side effects [33], and results of the effects of AAP on DMDD demonstrated the efficacy of risperidone [31,32].
Considering the risks associated with valproate for pregnant women [34, 35], experts were asked to consider their choice of mood stabilizer differently between male and female patients. Valproate was the first-line treatment for DMDD in men but a second-line treatment for DMDD in women.
Children and adolescents with major depression
Similar to the recommendations of KMAP-DD 2017 and 2012, AD monotherapy was recommended as the TOC for mild-to-moderate episodes in children and the first-line strategy for mild-to-moderate episodes in adole-scents. AD monotherapy and AD + AAP combination were the first-line strategies for non-psychotic severe depression in children and adolescents, and AD + AAP was the TOC for psychotic severe depression in children and adolescents, which was the same as in KMAP-DD 2017.
Aripiprazole was the TOC and risperidone and quetiapine were the first-line treatments for psychotic severe depression in children and adolescents in 2021, while aripiprazole and risperidone were the first-line treatments in 2017. However, AAPs were not included in the treatment strategy of CANMAT 2016 for child/adolescent depression [36].
Caution should be paid to AD use in children and adolescents, because ADs may be associated with increased risk of suicide in adolescents [36] and with (hypo)manic switching in young patients with bipolarity [37]. As the first-line AD for children and adolescents with MDD, escitalopram, fluoxetine, and sertraline were recommended. A recent Cochrane review of 19 trials with subjects aged 6−18 years (n = 3,335) showed that fluoxetine was significantly more effective than placebo, and sertraline was also significantly effective with a small effect size [38]. CANMAT 2016 recommended fluoxetine rather than escitalopram as the second step after initial cognitive-behavioral therapy (CBT) or interpersonal therapy (IPT) and internet-based psychotherapy [36], while the preference for escitalopram seems to be higher than that of fluoxetine in Korea. It can be seen as a result of considering the superior efficacy of escitalopram on improving children and adolescents’ function and symptoms compared to placebo [38-40], its effect during maintenance treatment [41], and favorable safety issues including drug-drug interactions via CYP450 2D6, 3A4 [42].
By risk-benefit evaluation, psychosocial intervention was recommended as the first step for mild depressive episodes in children/adolescents, but for more severe episodes or when initial psychosocial intervention for mild depressive episodes failed in children and adolescent, pharmacotherapy could be recommended as the first step [36].
Treatment strategy for elderly adults
According to big data analysis from the Korea Health Insurance Review and Assessment Service (HIRA), the number of patients treated for depression increased from 588,000 in 2014 to 681,000 in 2017, and among the 684,690 patients treated in 2018, 40% (n = 275,684) of them were over the age of 60 [43]. Unlike typical middle-aged depression, memory loss, fatigue, loss of appetite, insomnia, and pain, rather than depressed mood, are more common in elderly depression [44].
The recommendations for the first-line treatment strategies for each severity of episode in 2021 were the same as those in 2017: AD monotherapy for mild-to-moderate depressive episodes as TOC, AD + AAP and AD monotherapy for non-psychotic severe depression, and an AD + AAP combination for psychotic severe episodes.
Compared to KMAP-DD 2017, the preference of aripiprazole increased from first-line to the TOC in 2021. Adjunctive aripiprazole with various ADs [45] and aripiprazole augmentation for treatment-resistant depression was found to be effective for elderly depression [46]. Considering diabetes, hypertension, metabolic syndrome, the anticholinergic effect, and somnolence, aripiprazole was more highly preferred than other AAPs [47].
Due to lack of evidence for escitalopram, CANMAT [36] recommended duloxetine, mirtazapine, and nortriptyline instead of escitalopram as first-line ADs with level I evidence, while escitalopram was considered to be the preferred AD by many clinicians including the Korean experts [48,49].
Despite clinical limitations in treating elderly depression, it is clear that certain factors, such as comorbid physical illnesses, drug-drug interaction, and decreased metabolism due to the aging effect, should be considered in treating depression in the elderly.
Treatment strategy for women with premenstrual dysphoric disorder or postpartum depression
According to HIRA data survey, among the 681,000 depressed patients in Korea in 2017, there were 450,000 female patients, twice as many as the males [43]. As in KMAP-DD 2012 and 2017, AD monotherapy was recommended as the TOC for PMDD; in particular, escitalopram was the TOC for PMDD in 2017, while fluoxetine, escitalopram, sertraline, paroxetine, desvenlafaxine, and venlafaxine were the recommended first-line drugs. That is, the preference for SSRIs is higher than for other ADs in treating PMDD, which is consistent with foreign research [50 -52].
In treating pregnant women, as with older people and children and adolescents, we should evaluate the benefit-risk ratio. CANMAT 2016 recommends escitalopram and sertraline after initially applying CBT and IPT for mild-to-moderate major depressive disorder during pregnancy with level I evidence, but recommends pharmacotherapy alone or combined with CBT or IPT for severe depression during pregnancy [36]. The fact that AD monotherapy was recommended for mild-to-moderate and non-psychotic severe episodes while ECT or AD + AAP was recommended for psychotic severe episodes, in KMAP-DD 2021 reflects a careful choice of treatment strategies as in CANMAT 2016.
Changes in the recommendations for postpartum depression were that AD monotherapy was the TOC for mild-to-moderate episodes in 2017, while AD + AAP as well as AD monotherapy were the first-line strategies in 2021. In addition, an AD + AAP combination was recommended as first-line for severe episodes with/without psychotic features as in the previous version. For postpartum depression, CANMAT 2016 also recommends CBT and IPT as first-line and escitalopram and sertraline as second-line [36].
Non-pharmacological Biological Therapy
ECT, rTMS
ECT for non-psychotic severe MDD with self-harm or suicidal risk regardless of psychotic features and TMS for pregnant women were recommended as first-line. Most Korean experts consider ECT (92% in 2021 vs. 92.4% in 2017) and rTMS (89% in 2021 vs. 86.0% in 2017) to be good treatment strategies in line with recent evidence [53,54]. Compared to 44.3% in 2017, 43.8% of experts conduct ECT in 2021, while compared to 31.6% in 2017, 40.6% in 2021 have used rTMS in real practice.
For the treatment of MDD, CANMAT 2016 recommended ECT as a second-line treatment, and rTMS for patients who have had failed treatment, based on efficacy, tolerability, and safety, with at least 1 AD as a first-line treatment [55]. As with previous revisions, the executive committee recommended that ECT could be applied whenever depressed patients have potential suicidality or attempt at self-harm.
Alternative biological therapies
In Korea, these alternative therapies are less popular than ECT or rTMS. As second-line treatment strategies, the frequencies of use in 2021 of light therapy, omega-3 nutritional therapy, and tDCS combined with initial pharmacotherapy, which are complementary or novel agents, were 17.2% (vs. 27.8% in 2017), 18.8% (vs. 22.8% in 2017), and 7.8%, respectively, which currently indicates a low utilization rate. CANMAT 2016 recommended light therapy alone as a first-line treatment for seasonal MDD and as a second-line treatment for non-seasonal, mild-to- moderate MDD [51]. Although tDCS is less effective for TRD, the onset of its effect was faster than AD monotherapy with equal efficacy [56], and when adjuvant with AD, its efficacy was superior to AD monotherapy [57], and it is a relatively safe, non-invasive modality that can improve cognition in MDD patients [58,59].
Summary: Advantages and Limitations of KMAP-DD 2021
A main limitation is the characteristics of the experts’ consensus guidelines. As stated in another paper [3], experts’ consensus and evidence-based guidelines are not contradictory, but complementary. To do this, the executive committee published final guidelines through the process of drawing opinions from experts and reviewing the results with clinical evidence.
Second, the review committee may have been too small (n = 94) to reach a valid consensus and to select a TOC. However, given that there are only 3,800 psychiatrists in Korea and given that the total lifelong membership of the KSAD is only 258, a sample of 94 psychiatrists may be not insufficient. Finally, we did not explore psychosocial approaches, which should be addressed in a future study.
In summary, a shortened waiting time between the initial and subsequent treatments, increased preference for AAPs, especially aripiprazole, and combination strategies with AAPs yield an active and somewhat aggressive treatment trend in Korea. To our knowledge, the KMAP-DD series is the only experts’ consensus guideline in the world that has been updated and revised at regular intervals since 2002. Reminding one of the principles of KMAP development that this guideline cannot go beyond the physicians’ clinical decision, we expect KMAP-DD to provide clinicians with useful information about the specific strategies and medications appropriate for treating patients with MDD by bridging the gap between clinical real practice and evidence-based world.
Acknowledgements
The present manuscript is a secondary publication of our group’s papers, which were already published in the Korean language.
Though we have already published the papers in Korea, we decided to present and share the results with English- speaking experts according to the conditions for acceptable secondary publications as stated in Uniform Require-ments for Manuscripts Submitted to Biomedical Journals by the International Committee of Medical Journal Editors.
This study was supported by the Korean Society for Affective Disorders and the Korean College of Neuropsychopharmacology. This research did not receive any specific grant from funding agencies in the commercial sector.
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Author Contributions
Conceptualization: Jeong Seok Seo, Won-Myong Bahk. Study design and data collection: Jeong Seok Seo, Won-Myong Bahk, Young Sup Woo, Young-Min Park, Won Kim, Jong-Hyun Jeong, Se-Hoon Shim, Jung Goo Lee, Seung-Ho Jang, Chan-Mo Yang, Sheng-Min Wang, Myung Hun Jung, Hyung Mo Sung, IL Han Choo, Bo-Hyun Yoon, Sang-Yeol Lee, Duk-In Jon, Kyung Joon Min. Data analysis and interpretation: Jeong Seok Seo, Won-Myong Bahk, Young Sup Woo, Young-Min Park, Won Kim, Jong-Hyun Jeong, Se-Hoon Shim, Jung Goo Lee. Funding acquisition: Won-Myong Bahk, Jeong Seok Seo. Draft manuscript preparation: Jeong Seok Seo. All authors reviewed the results and approved the final version of the manuscript.
References
- 1.Lee MS, Lim SW, Cha JH, Chung SK, Kim KS Kasper S; The Executive Committee for the Korean Medication Algorithm Project for Major Depressive Disorder, author. The Korean Medica-tion Algorithm for Major Depressive Disorder (KMA-MDD): report of the Korean Society of Depressive and Bipolar Disorders. Int J Psychiatry Clin Pract. 2006;10:186–194. doi: 10.1080/13651500600633584. [DOI] [PubMed] [Google Scholar]
- 2.Seo JS, Min KJ, Kim W, Seok JH, Bahk WM, Song HC, et al. Korean Medication Algorithm for Depressive Disorder 2006 (I) J Korean Neuropsychiatr Assoc. 2007;46:453–460. doi: 10.1016/S0924-977X(07)70448-0. [DOI] [Google Scholar]
- 3.Seo JS, Song HR, Lee HB, Park YM, Hong JW, Kim W, et al. The Korean Medication Algorithm for Depressive Disorder: second revision. J Affect Disord. 2014;167:312–321. doi: 10.1016/j.jad.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 4.Seo JS, Bahk WM, Wang HR, Woo YS, Park YM, Jeong JH, et al. Korean Medication Algorithm for Depressive Disorders 2017: third revision. Clin Psychopharmacol Neurosci. 2018;16:67–87. doi: 10.9758/cpn.2018.16.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kennedy SH, Lam RW, McIntyre RS, Tourjman SV, Bhat V, Blier P, et al. Canadian Network for Mood and Anxiety Treat-ments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 3. Pharmacological treatments. Can J Psychiatry. 2016;61:540–560. doi: 10.1177/0706743716659417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer M, Severus E, Möller HJ Young AH; WFSBP Task Force on Unipolar Depressive Disorders, author. Pharmacological treatment of unipolar depressive disorders: summary of WFSBP guidelines. Int J Psychiatry Clin Pract. 2017;21:166–176. doi: 10.1080/13651501.2017.1306082. [DOI] [PubMed] [Google Scholar]
- 7.Cleare A, Pariante CM, Young AH, Anderson IM, Christmas D, Cowen PJ, et al. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29:459–525. doi: 10.1177/0269881115581093. [DOI] [PubMed] [Google Scholar]
- 8.Spielmans GI, Berman MI, Linardatos E, Rosenlicht NZ, Perry A, Tsai AC. Adjunctive atypical antipsychotic treatment for major depressive disorder: a meta-analysis of depression, quality of life, and safety outcomes. PLoS Med. 2013;10:e1001403. doi: 10.1371/journal.pmed.1001403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulberg HC, Katon W, Simon GE, Rush AJ. Treating major depression in primary care practice: an update of the Agency for Health Care Policy and Research Practice Guidelines. Arch Gen Psychiatry. 1998;55:1121–1127. doi: 10.1001/archpsyc.55.12.1121. [DOI] [PubMed] [Google Scholar]
- 10.Williams JW, Jr, Mulrow CD, Chiquette E, Noël PH, Aguilar C, Cornell J. A systematic review of newer pharmacotherapies for depression in adults: evidence report summary. Ann Intern Med. 2000;132:743–756. doi: 10.7326/0003-4819-132-9-200005020-00011. [DOI] [PubMed] [Google Scholar]
- 11.Fava M, Rush AJ, Trivedi MH, Nierenberg AA, Thase ME, Sackeim HA, et al. Background and rationale for the sequenced treatment alternatives to relieve depression (STAR*D) study. Psychiatr Clin North Am. 2003;26:457–494. x. doi: 10.1016/s0193-953x(02)00107-7. [DOI] [PubMed] [Google Scholar]
- 12.Fava M, Davidson KG. Definition and epidemiology of treatment-resistant depression. Psychiatr Clin North Am. 1996;19:179–200. doi: 10.1016/s0193-953x(05)70283-5. [DOI] [PubMed] [Google Scholar]
- 13.Nelson JC, Papakostas GI. Atypical antipsychotic augmentation in major depressive disorder: a meta-analysis of placebo- controlled randomized trials. Am J Psychiatry. 2009;166:980–991. doi: 10.1176/appi.ajp.2009.09030312. [DOI] [PubMed] [Google Scholar]
- 14.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.U.S. Food and Drug Administration, author. Drugs@FDA: FDA-approved drugs [Internet] U.S. Food and Drug Administration; Silver Spring: 2019. [cited at 2019 May 9]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/211243s001lbl.pdf . [Google Scholar]
- 16.U.S. Food and Drug Administration, author. FDA approves new nasal spray medication for treatment-resistant depression; available only at a certified doctor’s office or clinic [Internet] U.S. Food and Drug Administration; Silver Spring: 2019. [cited at 2019 May 3]. https://www.fda.gov/news-events/press-announcements/fda-approves-new-nasal-spray-medication-treatment-resistant-depression-available-only-certified . [Google Scholar]
- 17.Wang SM, Kim NY, Na HR, Lim HK, Woo YS, Pae CU, et al. Rapid onset of intranasal esketamine in patients with treatment resistant depression and major depression with suicide ideation: a meta-analysis. Clin Psychopharmacol Neurosci. 2021;19:341–354. doi: 10.9758/cpn.2021.19.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alonso-Pedrero L, Bes-Rastrollo M, Marti A. Effects of antidepressant and antipsychotic use on weight gain: a systematic review. Obes Rev. 2019;20:1680–1690. doi: 10.1111/obr.12934. [DOI] [PubMed] [Google Scholar]
- 19.Pollock BG. Adverse reactions of antidepressants in elderly patients. J Clin Psychiatry. 1999;60 Suppl 20:4–8. [PubMed] [Google Scholar]
- 20.Barbui C, Esposito E, Cipriani A. Selective serotonin reuptake inhibitors and risk of suicide: a systematic review of observational studies. CMAJ. 2009;180:291–297. doi: 10.1503/cmaj.081514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman RA. Antidepressants’ black-box warning - 10 years later. N Engl J Med. 2014;371:1666–1668. doi: 10.1056/NEJMp1408480. [DOI] [PubMed] [Google Scholar]
- 22.Malhi GS, Bell E, Singh AB, Bassett D, Berk M, Boyce P, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders: major depression summary. Bipolar Disord. 2020;22:788–804. doi: 10.1111/bdi.13035. [DOI] [PubMed] [Google Scholar]
- 23.Norman TR, Olver JS. Agomelatine for depression: expanding the horizons? Expert Opin Pharmacother. 2019;20:647–656. doi: 10.1080/14656566.2019.1574747. [DOI] [PubMed] [Google Scholar]
- 24.Sohn CH, Lam RW. Treatment of seasonal affective disorder: unipolar versus bipolar differences. Curr Psychiatry Rep. 2004;6:478–485. doi: 10.1007/s11920-004-0014-z. [DOI] [PubMed] [Google Scholar]
- 25.Galima SV, Vogel SR, Kowalski AW. Seasonal affective disorder: common questions and answers. Am Fam Physician. 2020;102:668–672. [PubMed] [Google Scholar]
- 26.Han C, Wang SM, Bahk WM, Lee SJ, Patkar AA, Masand PS, et al. The potential utility of aripiprazole augmentation for major depressive disorder with mixed features specifier: a retrospective study. Clin Psychopharmacol Neurosci. 2019;17:495–502. doi: 10.9758/cpn.2019.17.4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeong JH, Bahk WM, Woo YS, Lee JG, Kim MD, Sohn I, et al. Korean Medication Algorithm for Bipolar Disorder 2018: comparisons with other treatment guidelines. Clin Psycho-pharmacol Neurosci. 2019;17:155–169. doi: 10.9758/cpn.2019.17.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park YM. The mixed-features specifier of major depressive disorder in DSM-5: is it practical? Psychiatry Investig. 2018;15:1009–1010. doi: 10.30773/pi.2018.11.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koukopoulos A, Sani G, Ghaemi SN. Mixed features of depression: why DSM-5 is wrong (and so was DSM-IV) Br J Psychiatry. 2013;203:3–5. doi: 10.1192/bjp.bp.112.124404. [DOI] [PubMed] [Google Scholar]
- 30.Ng CH, Kato T, Han C, Wang G, Trivedi M, Ramesh V, et al. Definition of treatment-resistant depression - Asia Pacific perspectives. J Affect Disord. 2019;245:626–636. doi: 10.1016/j.jad.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 31.Baweja R, Mayes SD, Hameed U, Waxmonsky JG. Disruptive mood dysregulation disorder: current insights. Neuropsychiatr Dis Treat. 2016;12:2115–2124. doi: 10.2147/NDT.S100312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.American Psychiatric Association, author. Diagnostic and statistical manual of mental disorders. 5th ed. American Psychiatric Association; Virginia: 2013. [Google Scholar]
- 33.Yoo HK, Lee JS, Paik KW, Choi SH, Yoon SJ, Kim JE, et al. Open-label study comparing the efficacy and tolerability of aripiprazole and haloperidol in the treatment of pediatric tic disorders. Eur Child Adolesc Psychiatry. 2011;20:127–135. doi: 10.1007/s00787-010-0154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomson T, Battino D, Bonizzoni E, Craig J, Lindhout D, Sabers A, et al. Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol. 2011;10:609–617. doi: 10.1016/S1474-4422(11)70107-7. [DOI] [PubMed] [Google Scholar]
- 35.Meador K, Reynolds MW, Crean S, Fahrbach K, Probst C. Pregnancy outcomes in women with epilepsy: a systematic review and meta-analysis of published pregnancy registries and cohorts. Epilepsy Res. 2008;81:1–13. doi: 10.1016/j.eplepsyres.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.MacQueen GM, Frey BN, Ismail Z, Jaworska N, Steiner M, Lieshout RJ, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 6. Special populations: youth, women, and the elderly. Can J Psychiatry. 2016;61:588–603. doi: 10.1177/0706743716659276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghaemi SN, Ko JY, Goodwin FK. “Cade’s disease” and beyond: misdiagnosis, antidepressant use, and a proposed definition for bipolar spectrum disorder. Can J Psychiatry. 2002;47:125–134. doi: 10.1177/070674370204700202. [DOI] [PubMed] [Google Scholar]
- 38.Hetrick SE, McKenzie JE, Cox GR, Simmons MB, Merry SN. Newer generation antidepressants for depressive disorders in children and adolescents. Cochrane Database Syst Rev. 2012;11:CD004851. doi: 10.1002/14651858.CD004851.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wagner KD, Jonas J, Findling RL, Ventura D, Saikali K. A double-blind, randomized, placebo-controlled trial of escitalopram in the treatment of pediatric depression. J Am Acad Child Adolesc Psychiatry. 2006;45:280–288. doi: 10.1097/01.chi.0000192250.38400.9e. [DOI] [PubMed] [Google Scholar]
- 40.Cipriani A, Zhou X, Del Giovane C, Hetrick SE, Qin B, Whittington C, et al. Comparative efficacy and tolerability of antidepressants for major depressive disorder in children and adolescents: a network meta-analysis. Lancet. 2016;388:881–890. doi: 10.1016/S0140-6736(16)30385-3. [DOI] [PubMed] [Google Scholar]
- 41.Findling RL, Robb A, Bose A. Escitalopram in the treatment of adolescent depression: a randomized, double-blind, placebo- controlled extension trial. J Child Adolesc Psychopharmacol. 2013;23:468–480. doi: 10.1089/cap.2012.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical application. 4th ed. Cambridge University Press; Cambridge: 2013. pp. 48–51. [Google Scholar]
- 43.Korea Social Security Information Service, author. Last year, 450,000 women suffered from depression… twice that of men [Internet] Korea Social Security Information Service; Seoul: Available from: https://www.bokjiro.go.kr/nwel/welfareinfo/livwelnews/news/retireveIssueDetail.do?dataSid=6634143 . [Google Scholar]
- 44.Jung YE, Kim MD, Bahk WM, Woo YS, Nam B, Seo JS, et al. Validation of the Korean version of the depression in old age scale and comparison with other depression screening questionnaires used in elderly patients in medical settings. Clin Psychopharmacol Neurosci. 2019;17:369–376. doi: 10.9758/cpn.2019.17.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Steffens DC, Nelson JC, Eudicone JM, Andersson C, Yang H, Tran QV, et al. Efficacy and safety of adjunctive aripiprazole in major depressive disorder in older patients: a pooled subpopulation analysis. Int J Geriatr Psychiatry. 2011;26:564–572. doi: 10.1002/gps.2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lenze EJ, Mulsant BH, Blumberger DM, Karp JF, Newcomer JW, Anderson SJ, et al. Efficacy, safety, and tolerability of augmentation pharmacotherapy with aripiprazole for treatment-resistant depression in late life: a randomised, double-blind, placebo-controlled trial. Lancet. 2015;386:2404–2412. doi: 10.1016/S0140-6736(15)00308-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cakir S, Senkal Z. Atypical antipsychotics as add-on treatment in late-life depression. Clin Interv Aging. 2016;11:1193–1198. doi: 10.2147/CIA.S114244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mulsant BH, Blumberger DM, Ismail Z, Rabheru K, Rapoport MJ. A systematic approach to pharmacotherapy for geriatric major depression. Clin Geriatr Med. 2014;30:517–534. doi: 10.1016/j.cger.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Canadian Coalition for Seniors’ Mental Health, author. National guide-lines for seniors’ mental health-the assessment and treatment of depression [Internet] Canadian Coalition for Seniors’ Mental Health; Toronto: 2006. [cited at 2006 May]. https://ccsmh.ca/wp-content/uploads/2016/03/NatlGuideline_LTC.pdf . [Google Scholar]
- 50.Sachs GS, Printz DJ, Kahn DA, Carpenter D, Docherty JP. The expert consensus guideline series: medication treatment of bipolar disorder 2000. Postgrad Med. 2000;Spec No:1–104. [PubMed] [Google Scholar]
- 51.Hofmeister S, Bodden S. Premenstrual syndrome and premenstrual dysphoric disorder. Am Fam Physician. 2016;94:236–240. [PubMed] [Google Scholar]
- 52.Lanza di Scalea T, Pearlstein T. Premenstrual dysphoric disorder. Med Clin North Am. 2019;103:613–628. doi: 10.1016/j.mcna.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 53.Kar SK. Predictors of response to repetitive transcranial magnetic stimulation in depression: a review of recent updates. Clin Psychopharmacol Neurosci. 2019;17:25–33. doi: 10.9758/cpn.2019.17.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chou PH, Lin YF, Lu MK, Chang HA, Chu CS, Chang WH, et al. Personalization of repetitive transcranial magnetic stimulation for the treatment of major depressive disorder according to the existing psychiatric comorbidity. Clin Psychopharmacol Neurosci. 2021;19:190–205. doi: 10.9758/cpn.2021.19.2.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Milev RV, Giacobbe P, Kennedy SH, Blumberger DM, Daskalakis ZJ, Downar J, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 4. Neurostimulation treatments. Can J Psychiatry. 2016;61:561–575. doi: 10.1177/0706743716660033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rigonatti SP, Boggio PS, Myczkowski ML, Otta E, Fiquer JT, Ribeiro RB, et al. Transcranial direct stimulation and fluoxetine for the treatment of depression. Eur Psychiatry. 2008;23:74–76. doi: 10.1016/j.eurpsy.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Brunoni AR, Valiengo L, Baccaro A, Zanão TA, de Oliveira JF, Goulart A, et al. The sertraline vs. electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry. 2013;70:383–391. doi: 10.1001/2013.jamapsychiatry.32. [DOI] [PubMed] [Google Scholar]
- 58.Oliveira JF, Zanão TA, Valiengo L, Lotufo PA, Benseñor IM, Fregni F, et al. Acute working memory improvement after tDCS in antidepressant-free patients with major depressive disorder. Neurosci Lett. 2013;537:60–64. doi: 10.1016/j.neulet.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 59.Brunoni AR, Zanao TA, Vanderhasselt MA, Valiengo L, de Oliveira JF, Boggio PS, et al. Enhancement of affective processing induced by bifrontal transcranial direct current stimulation in patients with major depression. Neuromodulation. 2014;17:138–142. doi: 10.1111/ner.12080. [DOI] [PubMed] [Google Scholar]