Abstract
The ability to produce slime and to express a slime-associated antigen was examined in a collection of staphylococcal clinical isolates. Slime-producing strains were found among coagulase-negative staphylococci in percentages comparable to those reported in other studies; surprisingly, a high percentage of Staphylococcus aureus strains also were able to produce this extracellular material. In the latter case, this ability was strongly dependent on the presence of an additional carbohydrate source in the growth medium. Expression of the slime-associated antigen appeared to be species specific and confined to the Staphylococcus epidermidis sensu stricto isolates; its strong association with the ability of these strains to produce thicker biofilms indicated slime-associated antigen as a possible virulence marker for S. epidermidis.
Both Staphylococcus aureus and coagulase-negative staphylococci (CoagNS), particularly Staphylococcus epidermidis, are an important cause of infections associated with indwelling medical devices. Among factors possibly explaining the frequent colonization of indwelling devices by staphylococci, the microbial production of mucoid exopolymeric substances and the presence of receptors to plasma proteins absorbed onto the biomaterial surface have been considered.
As far as CoagNS are concerned, extracellular polysaccharides seem to be the most important factor, and biofilm production (slime) has been investigated (reviewed in reference 8). However, the debate is still open on this point; as a matter of fact, controversial results have been reported in terms of the association between slime production and clinically significant CoagNS infections (6, 11, 12, 18, 19, 24, 28) or differences in pathogenic potential between slime-producing and slime-negative strains (1, 23). No definitive evidence is available on the role of host protein receptors expressed by CoagNS. In fact, while some reports clearly demonstrated the ability of S. epidermidis to adhere to immobilized plasma proteins (4, 22), others have reported such proteins decrease or have a blocking effect on staphylococcal adherence (20). Such contrasting results have been ascribed to a masking action of slime covering protein receptors in in vitro assays (4).
As far as S. aureus is concerned, slime production has never been considered as a virulence factor. The importance of coagulase-positive staphylococci in medical device-associated infections has mostly been ascribed to their ability to express molecules that recognize host matrix proteins (15).
In this study, we wanted to evaluate the occurrence of slime production among clinical isolates of both CoagNS and S. aureus; moreover, we analyzed the expression of a slime-associated antigen (SAA) (7), which preliminary results obtained by our group suggested is strictly correlated with the expression of slime and possibly represents a marker of virulence for staphylococci (3).
MATERIALS AND METHODS
Bacteria.
Two hundred thirteen staphylococcal isolates, specifically S. epidermidis (n = 115), S. aureus (n = 72), Staphylococcus haemolyticus (n = 8), Staphylococcus warneri (n = 4), Staphylococcus xylosus (n = 4), Staphylococcus capitis (n = 4), Staphylococcus intermedius (n = 3), Staphylococcus simulans (n = 2), and Staphylococcus lentus (n = 1), were examined. The majority of strains were from clinically significant infections from humans (Table 1), (kindly provided by V. Monzillo, IRCCS S. Matteo, University of Pavia, Pavia, Italy; and E. Romoli, Ospedale di Orvieto, Orvieto, Italy); nine S. aureus strains were from bovine mastitis, and the three S. intermedius strains were from canine dermatitis (all of the animal strains were kindly provided by S. Hensen, Intervet International bv). Isolates were characterized at the species level by the API-Staph Ident System (Biomerieux). Bacteria were maintained in Trypticase soy broth (TSB) (Oxoid), to which 15% glycerol was added, at −80°C. For the experiments, bacterial cultures were grown in TSB or TSB supplemented with 1% glucose (TSB-G) overnight at 37°C.
TABLE 1.
Slime production of the 201 staphylococcal strains of human origin examined in this study with regard to the source of isolation
| Source | No. (%) of strains
|
||||||
|---|---|---|---|---|---|---|---|
|
S. epidermidis
|
S. aureus
|
Others
|
Total | ||||
| Slime+ | Slime− | Slime+ | Slime− | Slime+ | Slime− | ||
| Catheters | 43 (63.2) | 25 (36.8) | 2 (40) | 3 (60) | 2 (28.6) | 5 (71.4) | 80 (39.8) |
| Blood | 16 (66.7) | 8 (33.3) | 2 (33.3) | 4 (66.7) | 30 (15) | ||
| Orthopedic implants | 12 (75) | 4 (25) | 32 (97) | 1 (3) | 49 (24.4) | ||
| CAPD | 5 (71.4) | 2 (28.6) | 2 (100) | 9 (4.5) | |||
| Urine | 7 (77.8) | 2 (22.3) | 3 (75) | 1 (25) | 13 (6.5) | ||
| Throat and nasal swabs | 12 (100) | 4 (66.7) | 2 (33.3) | 18 (8.9) | |||
| Wounds | 1 (50) | 1 (50) | 2 (1) | ||||
| Total | 76 (66.1) | 39 (33.9) | 56 (88.9) | 7 (11.1) | 11 (47.8) | 12 (52.2) | 201 (100) |
Slime production.
Production of slime was quantitatively determined by a modified version of the plate test (2). Briefly, overnight cultures were diluted 1:100 in 180 μl of TSB or TSB-G in 96-well microtiter plates. After overnight incubation at 37°C, wells were emptied, washed three times with phosphate-buffered solution, and air dried for 1 h at 60°C. Adherent biofilm was stained with Hucker crystal violet, and the excess stain removed by rinsing with tap water. After drying, the optical density (OD) of the biofilm was read by a Novapath microplate reader (BioRad) at a wavelength of 570 nm in the single-wavelength mode.
Evaluation of SAA expression.
To evaluate expression of SAA, 5 ml of overnight cultures in TSB-G was centrifuged at 3,000 rpm (TJ-6 Beckman centrifuge) for 15 min, washed with phosphate-buffered saline, and sonicated in ice for a total of 3 min. Bacterial cells and debris were eliminated by centrifugation, and the supernatant was filtered and concentrated 5 to 10 times. The presence of SAA was detected by double immunodiffusion with a specific antiserum raised against whole cells of the reference slime-producing, SAA-positive strain S. epidermidis ATCC 35984, obtained as previously described (3).
Statistical evaluation.
The relationship between slime and SAA positivity by isolates from a given infectious site in relation to all of the remaining isolates was compared by χ2 analysis with a continuity correction. The significance of ODs of biofilm formed by SAA-expressing strains and SAA-negative strains was analyzed by the Wilcoxon test for related rankable score. All evaluations were done with the Statistica 4.1 software program on a Macintosh computer.
RESULTS
In our strain collection of human isolates, the ability to produce slime (Table 1) was only slightly more frequent among S. epidermidis strains than those of the other CoagNS strains considered in this study (66.1% versus 47.8%), although the difference in the biofilm’s OD was significant (1.08 ± 0.09 versus 0.546 ± 0.18; P < 0.002). On the other hand, a surprisingly high percentage of S. aureus strains (88.9%) were found to produce slime, with a mean biofilm thickness (0.653 ± 0.08) comparable to that of CoagNS other than S. epidermidis.
Almost all of the strong-slime-producing S. aureus strains were those of human origin, while of the nine animal strains, only three produced a detectable biofilm (mean OD, 0.300 ± 0.05). The others were all non-slime producers. The three S. intermedius strains from canine dermatitis were weak slime producers.
The ability of the human strains to produce slime, when considered with regard to the source of isolation, is shown in Table 1. Although the number of isolates was not always comparable among the various species and site of isolation, differences were significant for S. epidermidis strains isolated from catheter-associated infections, for all isolates from orthopedic implants, and for S. aureus from throat and nasal swabs.
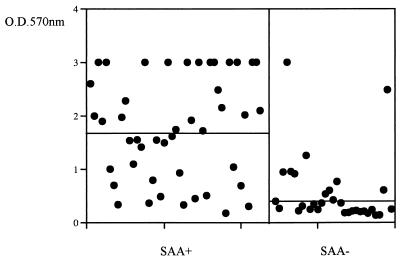
The SAA was identified exclusively within members of the S. epidermidis sensu stricto group, with almost 60% of the slime-producing strains also expressing SAA (Table 2); slime-producing, SAA-negative strains were also detected, while the phenotype slime-negative, SAA-positive strain was never recovered (Table 2). Almost all SAA-expressing strains were strong slime producers (44 of 45); of those, 12 always showed OD values above 3.00. The differences in the OD of the biofilm formed by SAA-expressing strains (mean ± standard error [SE], 1.550 ± 0.99) were statistically significant (P < 0.001) compared to that of SAA-negative strains (0.588 ± 0.06) (Fig. 1). When the slime and SAA data were analyzed by comparing isolates associated with a particular clinical situation with all other clinical isolates, strains from infected orthopedic implants differed significantly (P < 0.002) in their ability to express SAA and to produce slime. Continuous peritoneal ambulatory dialysis (CAPD) isolates were also more likely to express SAA, while blood isolates presented fewer strains elaborating SAA.
TABLE 2.
Combinations of expression of SAA and slime production among the 115 S. epidermidis isolates from different sources
| Phenotype | No. (%) of isolates from:
|
||||
|---|---|---|---|---|---|
| Catheters | Blood | Orthopedic implants | CAPD | All sites | |
| Slime+ SAA+ | 26 (38.2) | 6 (25) | 9 (56.2) | 4 (57) | 45 (39) |
| Slime+ SAA− | 17 (25) | 10 (41.6) | 3 (18.8) | 1 (14) | 31 (27) |
| Slime− SAA+ | 0 | 0 | 0 | 0 | 0 |
| Slime− SAA− | 25 (36.7) | 8 (33.3) | 4 (25) | 2 (28) | 39 (34) |
| Total | 68 | 24 | 16 | 7 | 115 |
FIG. 1.
Slime production of SAA-positive and SAA-negative S. epidermidis clinical isolates. Dots indicate the average OD of triplicate determinations of the biofilm produced by each strain. Bars indicate the medians of the plotted values.
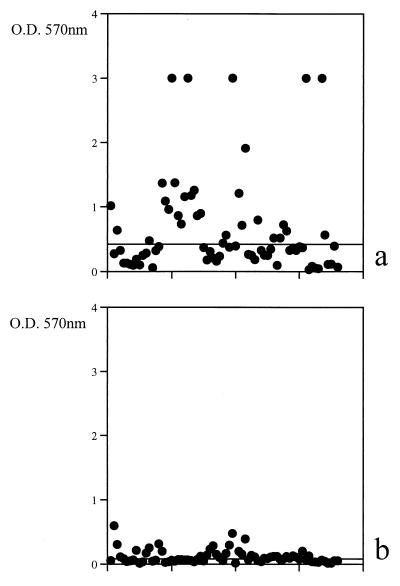
A casual observation that slime production by S. aureus seemed to be affected by the addition of glucose to the medium prompted us to systematically investigate this aspect. The ability of S. aureus, of either human or animal origin, to produce slime was dramatically affected by the presence of an additional carbohydrate source in the medium (Fig. 2). The addition of 1% glucose increased the percentage of slime-producing S. aureus from 34.7% to 83.3%, with a mean OD (±SE) rise from 0.19±0.05 to 0.67±0.09 (P < 0.001); only six of such slime-producing strains did not show a significant difference in biofilm production measured after growth in the presence or absence of glucose. The “carbohydrate effect” was never detected for other staphylococcal species, with the exception of a few nonsignificant value fluctuations for strains with a borderline classification.
FIG. 2.
Slime production of S. aureus strains in the presence of glucose (a) or without glucose addition (b). Dots indicate the average OD of triplicate determinations. Bars indicate the medians of the plotted values.
DISCUSSION
Slime production has been suggested to have therapeutic implications by both Younger and coworkers (28) and by Davenport et al. (10), who observed a link between the production of slime and the persistence of infection. In patients with ventriculoperitoneal shunt infections, Diaz-Mitoma and coworkers (11) also found a significant association between antibiotic failure and slime production. Likewise, in patients with CAPD peritonitis, an association between therapeutic failure, severity of infection, and slime production has been observed (16). All of these authors agree on these properties of slime-producing strains, despite the fact that not all of them were able to demonstrate an epidemiological association between the production of slime and the induction of disease by pathogenic CoagNS isolates (11, 19). In our strain collection, we observed percentages of slime production by CoagNS comparable to those of other studies in which this property was associated with clinically significant infections (10, 28). A more interesting observation is the high frequency of the SAA-positive slime among S. epidermidis strains. The exclusive association of such antigen with slime, with almost 60% of the slime-producing S. epidermidis strains expressing SAA, is indicative of its importance among clinically significant isolates. Also, the thickness of the biofilm produced by SAA-expressing strains, significantly different from that produced by SAA-negative strains, is strongly suggestive of SAA as a virulence marker for S. epidermidis. We did not examine skin isolates to analyze the association between SAA and severity of clinical infections. However, we believe that, as indicated by other authors (21), the majority of clinically important staphylococcal strains are derived from the skin of either patients or hospital personnel involved in their care. The only previous report describing the occurrence of a supposedly slime-associated antigen (PS/A) (26) in a collection of clinical isolates was based on detection of a molecule associated with slime but also expressed by non-slime-producing strains. Different from SAA, PS/A was found to be expressed frequently by S. epidermidis and S. capitis and rarely by other CoagNS species, indicating it was an antigen common among microorganisms belonging to the genus Staphylococcus (21). On the other hand, SAA was definitely slime specific and possibly was confined to S. epidermidis, although the number of other CoagNS studied was limited.
As far as S. aureus is concerned, there are no epidemiological studies available describing slime production in human isolates. A few studies analyzed strains causing bovine mastitis, reporting 12% showed slime production with a strong tendency to phenotypic variation and rapid loss of the slime layer by in vitro subculture (5), compared to 80% of strains reported as slime producing in vivo (27).
The pivotal role of a medium richer in glucose we observed may represent an explanation of why slime production has never been thoroughly investigated before as a possible virulence factor for S. aureus. In fact, the addition of glucose dramatically increased the number of slime-producing strains, suggesting that the gene for slime production is inducible by a suitable metabolic source. It has already been reported that the expression of other characteristics such as the capsule production of serotypes 5 and 8 is greatly influenced by environmental and bacterial growth conditions (9, 17, 25). To verify whether the addition of glucose truly stimulated the production of slime or supported the adherence to plastic through a different mechanism, we examined 10 randomly chosen S. aureus strains by transmission electron microscopy and observed that strains grown in TSB-G presented larger amounts of extracellular polysaccharide material enclosing numerous bacterial cells (data not shown). The observation reported in this study should stimulate investigation into the importance of slime production in S. aureus. Fattom et al. (13) described the use of a vaccine based on polysaccharides with specific antibodies protecting mice against bacterial challenge; this report may suggest the importance of the polysaccharide material, in addition to the capsular one, in eliciting protective antibodies.
As suggested by the data reported by Flock et al. (14), molecules with binding functions may be necessary for colonization when fibronectin-binding proteins and other extracellular matrix-binding proteins are lacking. Because the majority of S. aureus strains examined in this study were from orthopedic implants, a definitive conclusion about the occurrence of slime production among clinical isolates cannot be drawn. However, the large prevalence of slime production among strains isolated from such specific infection sites (infected knee and hip prostheses) may be suggestive of an important role for slime in biomaterial-associated infections supported by S. aureus as is the case for CoagNS biomaterial-centered infections.
Slime production by S. epidermidis and the other staphylococcal strains was only slightly affected by glucose, since the amount of this sugar available in the commercial medium was sufficient for slime production. Only a few strains with borderline values of OD (none to weak or weak to strong) sometimes showed nonsignificant fluctuations (data not shown), suggesting the presence of a gene system for slime production different from that in S. aureus.
The data reported here indicate an important role of SAA as a virulence marker for clinically significant S. epidermidis isolates. Its occurrence among a majority of clinical isolates and its association with the strains’ ability to produce thicker biofilms strongly suggest a role of SAA in pathogenesis. The purified antigen might be considered, together with other antigens suggested to elicit protective antibodies, such as PS/A, for the production of a vaccine to be used in patients at risk for biomaterial-associated infections.
Also, the indication of the predominance of slime-producing S. aureus strains, particularly among prosthesis-centered infections, should stimulate research on this topic, to evaluate the possible role of this factor in biomaterial-associated S. aureus infections.
ACKNOWLEDGMENTS
This work was partially supported by the Italian Ministry of Health, Project 1% “Interactions between opportunistic pathogens and biomaterials in the pathogenesis of prosthesis infections” (ICS 080.1/RS 98.43.).
REFERENCES
- 1.Baddour L M, Christensen G D, Hester M G, Bisno A L. Production of experimental endocarditis by coagulase-negative staphylococci: variability in species virulence. J Infect Dis. 1984;150:721–727. doi: 10.1093/infdis/150.5.721. [DOI] [PubMed] [Google Scholar]
- 2.Baldassarri L, Simpson W A, Donelli G, Christensen G D. Variable fixation of staphylococcal slime by different histochemical fixatives. Eur J Clin Infect Dis. 1993;12:34–37. doi: 10.1007/BF02000411. [DOI] [PubMed] [Google Scholar]
- 3.Baldassarri L, Donelli G, Gelosia A, Voglino M C, Simpson A W, Christensen G D. Purification and characterization of the staphylococcal slime-associated antigen and its occurrence among Staphylococcus epidermidis clinical isolates. Infect Immun. 1996;64:3410–3415. doi: 10.1128/iai.64.8.3410-3415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldassarri L, Donelli G, Gelosia A, Simpson A W, Christensen G D. Expression of slime interferes with in vitro detection of host protein receptors of Staphylococcus epidermidis. Infect Immun. 1997;65:1522–1526. doi: 10.1128/iai.65.4.1522-1526.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baselga R, Albizu I, De La Cruz M, Del Cacho E, Barberan M, Amorena B. Phase variation of slime production in Staphylococcus aureus: implications in colonization and virulence. Infect Immun. 1993;61:4857–4862. doi: 10.1128/iai.61.11.4857-4862.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christensen G D, Simpson W A, Bisno A L, Beachey E H. Adherence of slime-producing strains of Staphylococcus epidermidis to smooth surfaces. Infect Immun. 1982;37:318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christensen G D, Barker L P, Mawhinney T P, Baddour L M, Simpson W A. Identification of an antigenic marker for slime production for Staphylococcus epidermidis. Infect Immun. 1990;58:2906–2911. doi: 10.1128/iai.58.9.2906-2911.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christensen G D, Baldassarri L, Simpson W A. Colonization of medical devices by coagulase-negative staphylococci. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. 2nd ed. Washington, D. C: American Society for Microbiology; 1994. pp. 45–78. [Google Scholar]
- 9.Dassy B, Stringfellow W T, Lieb M, Fournier J M. Production of type 5 capsular polysaccharide by Staphylococcus aureus grown in semisynthetic medium. J Gen Microbiol. 1991;137:1155–1162. doi: 10.1099/00221287-137-5-1155. [DOI] [PubMed] [Google Scholar]
- 10.Davenport D S, Massanari R M, Pfaller M A, Bale M J, Streed S A, Hierholzer W J. Usefulness of a test for slime production as a marker for clinically significant infections with coagulase-negative staphylococci. J Infect Dis. 1986;153:332–339. doi: 10.1093/infdis/153.2.332. [DOI] [PubMed] [Google Scholar]
- 11.Diaz-Mitoma F, Harding G K M, Hoban D J, Roberts R S, Low D E. Clinical significance of a test for slime production in ventriculoperitoneal shunt infections caused by coagulase-negative staphylococci. J Infect Dis. 1987;156:555–560. doi: 10.1093/infdis/156.4.555. [DOI] [PubMed] [Google Scholar]
- 12.Etienne J, Brun Y, Solh N E, Delorme V, Mouren C, Bes M, Fleurette J. Characterization of clinically significant isolates of Staphylococcus epidermidis from patients with endocarditis. J Clin Microbiol. 1988;26:613–617. doi: 10.1128/jcm.26.4.613-617.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fattom A I, Sarwar J, Ortiz A, Naso R. A Staphylococcus aureus capsular polysaccharide (CP) vaccine and CP-specific antibodies protect mice against bacterial challenge. Infect Immun. 1996;64:1659–1665. doi: 10.1128/iai.64.5.1659-1665.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Flock J-I, Hienz S A, Heimdahl A, Schennings T. Reconsideration of the role of fibronectin binding in endocarditis caused by Staphylococcus aureus. Infect Immun. 1996;64:1876–1878. doi: 10.1128/iai.64.5.1876-1878.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foster T J, McDevitt D. Molecular basis of adherence of staphylococci to biomaterials. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C: American Society for Microbiology; 1994. pp. 31–44. [Google Scholar]
- 16.Freeman D J, Falkiner F R. Coagulase-negative staphylococci and continuous peritoneal ambulatory dialysis. Rev Med Microbiol. 1991;2:98–104. [Google Scholar]
- 17.Herbert S, Worlitzsch D, Dassy B, Boutonnier A, Fournier J-M, Bellon G, Dalhoff A, Doring G. Regulation of Staphylococcus aureus capsular polysaccharide type 5: CO2 inhibition in vitro and in vivo. J Infect Dis. 1997;176:431–438. doi: 10.1086/514061. [DOI] [PubMed] [Google Scholar]
- 18.Kotilainen P. Association of coagulase-negative staphylococcal slime production and adherence with the development and outcome of adult septicemias. J Clin Microbiol. 1990;28:2779–2785. doi: 10.1128/jcm.28.12.2779-2785.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristinsson K G, Spencer R C, Brown C B. Clinical importance of production of slime by coagulase-negative staphylococci in chronic ambulatory peritoneal dialysis. J Clin Pathol. 1986;39:117. doi: 10.1136/jcp.39.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muller E, Takeda S, Goldmann D A, Pier G B. Blood proteins do not promote adherence of coagulase-negative staphylococci to biomaterials. Infect Immun. 1991;59:3323–3326. doi: 10.1128/iai.59.9.3323-3326.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muller E, Takeda S, Shiro H, Goldmann D A, Pier G B. Occurrence of capsular polysaccharide/adhesin among clinical isolates of coagulase-negative staphylococci. J Infect Dis. 1993;168:1211–1218. doi: 10.1093/infdis/168.5.1211. [DOI] [PubMed] [Google Scholar]
- 22.Nilsson M, Frykberg L, Flock J-I, Pei L, Lindberg M, Guss B. A fibrinogen-binding protein of Staphylococcus epidermidis. Infect Immun. 1998;66:2666–2673. doi: 10.1128/iai.66.6.2666-2673.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perdreau-Remington F, Sande M A, Peters G, Chambers H F. The abilities of a Staphylococcus epidermidis wild-type strain and its slime-negative mutant to induce endocarditis in rabbits are comparable. Infect Immun. 1998;66:2778–2781. doi: 10.1128/iai.66.6.2778-2781.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steckelberg J M, Osmon D R. Prosthetic joint infections. In: Bisno A L, Waldvogel F A, editors. Infections associated with indwelling medical devices. 2nd ed. Washington, D.C: American Society for Microbiology; 1994. pp. 259–290. [Google Scholar]
- 25.Stringfellow W T, Dassy B, Lieb M, Fournier J-M. Staphylococcus aureus growth and type 5 capsular polysaccharide production in synthetic media. Appl Environ Microbiol. 1991;57:618–621. doi: 10.1128/aem.57.2.618-621.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tojo M, Yamashita N, Goldmann D A, Pier G B. Isolation and characterization of a capsular polysaccharide adhesin from Staphylococcus epidermidis. J Infect Dis. 1988;157:713–722. doi: 10.1093/infdis/157.4.713. [DOI] [PubMed] [Google Scholar]
- 27.Watson D L. Expression of a pseudocapsule by Staphylococcus aureus: influence of cultural conditions and relevance to mastitis. Res Vet Sci. 1989;47:152–157. [PubMed] [Google Scholar]
- 28.Younger J J, Christensen G D, Bartley D L, Simmons J C H, Barrett F F. Coagulase-negative staphylococci isolated from cerebrospinal fluid shunts: importance of slime production, species identification, and shunt removal to clinical outcome. J Infect Dis. 1987;156:548–554. doi: 10.1093/infdis/156.4.548. [DOI] [PubMed] [Google Scholar]