Abstract
Purpose:
Glioblastoma (GBM) is a devastating disease. With the current treatment of surgery followed by chemoradiation, outcomes remain poor with only a median survival of 15 months and a 5-year survival of 6.8%. One challenge to treating GBM is the heterogeneous integrity of the blood-brain barrier (BBB) which limits the bioavailability of systemic therapies to the brain. There is a growing interest in enhancing drug delivery by opening the BBB with the use of focused ultrasound (FUS). We hypothesize that FUS-mediated BBB opening can enhance the delivery of etoposide for a therapeutic benefit in GBM.
Methods and Materials:
A murine glioma cell line (Pdgf+, Pten−/−, P53−/−) was orthotopically injected into B6(Cg)-Tyrc-2J/J mice to establish the syngeneic GBM model for this study. Animals were treated with FUS and microbubbles to open the BBB to enhance the delivery of systemic etoposide. Magnetic resonance (MR) imaging was utilized to evaluate BBB opening and tumor progression. Liquid chromatography tandem mass spectrometry was used to measure etoposide concentrations in the intracranial tumors.
Results:
The murine glioma cell line is sensitive to etoposide in vitro. MR imaging and passive cavitation detection demonstrates the safe and successful BBB opening with FUS. The combined treatment of FUS-mediated BBB opening and etoposide decreased tumor growth by 45% and prolonged median overall survival by 6 days, an approximately 30% increase. FUS-mediated BBB opening increased brain tumor-to-serum ratio of etoposide by 3.5-fold and increased etoposide concentration in brain tumor tissue by 8-fold as compared to without ultrasound.
Conclusion:
The current study demonstrates that BBB opening with FUS increases intratumoral delivery of etoposide in the brain, resulting in local control and overall survival benefits.
Keywords: focused ultrasound, blood-brain barrier, etoposide, glioblastoma
Introduction
Radiation therapy is an integral part of cancer treatment. More than half of cancer patients receive radiotherapy and the treatment paradigm ranges from definitive radiotherapy, post-operative treatment, neoadjuvant treatment, to palliative treatment1. In addition to traditional radiotherapy, acoustic radiation also offers a wide array of treatment options to patients with cancer. Ablative high-intensity focused ultrasound (FUS) has been used in the treatment of metastatic bone disease and prostate cancer2, 3. Recently, there has been a growing interest in using low-intensity FUS to open the blood-brain barrier (BBB) for drug delivery.
The BBB is a physiological barrier that maintains the homeostasis of the brain by protecting it from exogenous and endogenous substances, which can be potentially toxic4. Extensive interest has emerged regarding the development of ways to optimize drug delivery by overcoming the BBB, including intracranial injections, hyperosmotic solutions, convection-enhanced delivery (CED) and FUS mediated BBB opening5. FUS offers a potentially safe and non-invasive method to localize image-guided BBB opening for drug delivery. Optimization of FUS delivery with the combined use of microbubbles (MBs: ultrasound contrast agents) has been shown to achieve local and reversible BBB opening without damaging the brain parenchyma in multiple preclinical models, including rodents and non-human primates6, 7. Several chemotherapeutic drugs for treating brain tumors have shown increased penetrance into the brain parenchyma after FUS and MBs induced BBB disruption, including temozolomide (TMZ), carboplatin, doxorubicin, paclitaxel and cisplatin8–12. Over the past few years, there has been significant clinical advancement of FUS in which multiple FUS devices are being tested in the clinic for BBB opening in diseases, including Alzheimer’s Disease, Amyotrophic Lateral Sclerosis, and brain tumors13, 14. Given that FUS is a new technology, preclinical studies using known systemic therapies with FUS are needed for early clinical trial design for patients with brain tumors.
Glioblastoma (GBM) is a deadly primary brain tumor in which outcomes are poor. In 2005, Stupp and colleagues demonstrated a modest but significant survival benefit for GBM patients, which has become the backbone of the standard-of-care treatment for GBM for the past 15 years. Patients who undergo surgery followed by chemoradiation with TMZ have a median survival of 14.6 months, although almost every patient eventually succumbs to recurrence and their survival rarely exceeds 2 years15. Since then, there has been minimal improvement with systemic therapy. GBM is still an incurable tumor with a median survival of 15 months and a 5-year survival rate of 6.8%16, 17. Despite important advances in our understanding of GBM, current systemic therapies in the clinical treatment of patients with GBM remain largely ineffective. BBB is one of the major limiting factors of systemic treatment in the clinic for GBM. GBM tumors have heterogeneous integrity of the BBB. This includes the invasive mass with destructive BBB and the diffusely infiltrative components hiding in areas of the brain where the BBB remains relatively intact. Microscopic spread is well-documented from biopsy studies, demonstrating that GBM tumor cells infiltrate into adjacent edematous areas of the brain that are impermeable to contrast agents and even up to 1–2 cm beyond visible tumor18. Typically, the destructive mass is surgically resected, while surrounding microscopic disease is targeted using adjuvant chemoradiation-based treatment. Because the penetration of systemic agents into the brain is largely restricted by the BBB, adequate treatment of the microscopic spread, which contains intact BBB, is critical to develop an effective systemic therapy for GBM19.
Etoposide is an anti-cancer chemotherapy drug that inhibits topoisomerase II (TOP2) and induces DNA strand breaks, which is widely used in the treatment of various types of cancers20. Many in vitro and preclinical studies have shown the anti-tumor effects of etoposide against GBM cells21. However, several combination therapy clinical trials with systemically-delivered etoposide have shown limited anti-tumor activity and poor response rates, possibly due to poor BBB penetration and dose-limiting toxicities22–25. Although the molecular size of etoposide is small (588.56 Da), approximately 90% of etoposide found in the body is protein-bound, which may limit its bioavailability in tumors in the brain26. Thus we hypothesize that in the setting of subtherapeutic levels of intratumoral etoposide, the addition of FUS-mediated BBB opening can enhance delivery and efficacy in treating GBM. The aim of this study is to investigate the effects of FUS-enhanced etoposide delivery on local tumor growth and overall survival.
Methods and Materials
Cell culture
The murine glioma cell line is established from the tumor generated by injecting PDGF-IRES-Cre retrovirus into the subcortical white matter of mice with floxed Pten and p5327. The murine glioma cell harboring Pdgf+, Pten−/−, and P53−/− (MGPP3) is cultured in DMEM medium supplemented with 0.5% fetal bovine serum, 100 units/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL amphotericin B, 1% N2 supplement, and 10 ng/mL recombinant human PDGF-AA and FGF-basic in a humidified atmosphere with 5% CO2 at 37°C.
Cell viability assay
MGPP3 cells were seeded at a density of 7000 cells per well in a 96-well plate. After overnight culture, cells were treated with different drugs as indicated. The concentration ranges of each drug are 0.001–30 μM for etoposide, 0.01–100 μM for carboplatin, and 0.01–1000 μM for TMZ. After 72 hours of treatment, later cell viability was determined by the CellTiter-Blue™ Cell Viability Assay (Promega, Madison, WI) according to the manufacturer’s instructions.
Animal studies
All animal studies were approved by the Institutional Animal Care and Use Committee of Columbia University. Four- to six-week-old male B6(Cg)-Tyrc-2J/J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). The mice were housed under pathogen-free conditions and fed autoclaved food and water. All animals were monitored daily for food and water intake, nutritional status (weekly weight), and animal behaviors were assessed routinely and consistently. For etoposide administration, mice were injected intraperitoneally right after FUS application. For survival study, tumor-bearing mice were inspected and weighed daily. The endpoints of the survival curve were weight loss >20%, hunched posture, lethargy, persistent recumbency, coughing, labored breathing, rough hair coats, nasal discharge, jaundice, neurologic signs (circling, head pressing, seizures), bleeding from any orifice, self-induced trauma, any condition interfering with eating or drinking such as difficulty ambulating, and death.
Intracranial injection
Mice were anesthetized by intraperitoneal injection of 100 mg/kg body weight (bw) ketamine and 10mg/kg bw xylazine. Mice were then immobilized in a mouse stereotaxic instrument (Stoelting, Wood Dale, IL) and a 1 cm incision was made in the midline of the scalp to expose the sagittal suture and bregma of the skull. A burr hole of 1mm in diameter was made at a position of 2mm anterior and 2mm lateral right to the bregma. A Hamilton syringe containing MGPP3 cells was inserted 2mm deep from the skull surface. 50,000 cells in 1 μL DMEM medium were injected at 0.2 μL/min into the brains.
Magnetic resonance (MR) imaging
Bruker BioSpec 9.4T Magnetic Resonance Imager and ParaVision 6.0.1 (Bruker, Billerica, MA) were used for the magnetic resonance imaging. Mice were first anesthetized with 1–2% isoflurane and vital signs were monitored throughout the imaging sessions. We then placed the anesthetized mice in a phased-array RF coil. T1-weighted 2D FLASH sequence (TR/TE 230/3.3 ms, flip angle: 70°, number of excitations: 4, field of view: 25.6 mm × 25.6 mm, resolution 100 μm × 100 μm × 400 μm) was performed 10 min after intraperitoneal injection of 0.2 mL gadodiamide (GD-DTPA) (Omniscan®, GE Healthcare, Chicago, IL). The tumor volume of MR images was quantitated using the free open source platform 3D Slicer (www.slicer.org). Briefly, the tumor boundaries of each consecutive slice containing tumor was contoured and then the calculation of whole tumor volumes was conducted. Image processing methods for quantifying BBB opening volume and contrast enhancement were as described previously28.
FUS and passive cavitation detection (PCD)
The experimental setup is shown in Supplementary Figure S1. A single-element, spherical FUS transducer (center frequency: 1.5 MHz, focal length 60 mm; diameter:60 mm, Imasonic, France) was driven by a function generator (33500B Series, Agilent Technologies, Santa Clara, CA) through a 50 dB power amplifier (E&I Inc., Rochester, NY). A single-element, pulse-echo transducer (frequency: 7.5 MHz, focal length 60 mm, diameter 11.2 mm; Olympus NDT, Waltham, MA) utilized for PCD was concentrically aligned with the FUS transducer. The PCD transducer connected to a digitizer (Gage Applied Technologies, Inc., Lachine, QC, Canada) was used to passively acquire acoustic emissions from MBs during the FUS exposure. A cone filled with degassed, distilled water was mounted onto the transducer assembly. The transducers were attached to a computer-controlled 3D positioning system (Velmex Inc., Lachine, QC, Canada). The polydisperse in-house manufactured MBs (concentration: 8×108 bubbles /mL, diameter: 1.37 ± 1.02 μm29, lipid shell composition: DSPC and DSPE-PEG2000 at molar ratio of 9:1, gas core: C4F10, dose: 1 μL/g bw) were diluted in saline to 200 μL and injected intravenously. Prior to administration of MBs, a 2 s sonication was applied in order to get the baseline of acoustic response used in the quantification of cavitation dose. To completely cover the tumor and its surrounding infiltrative region, FUS was applied once at each of four points on a 1.5 mm × 1.5 mm grid. A hundred μl of MBs were slowly injected before the first and third points of sonication. Each point was sonicated for 30 s, with a pulse repetition frequency (PRF) of 5 Hz, a pulse length (PL) of 1 ms, and an estimated derated peak-negative acoustic pressure of 0.7 MPa. Acoustic emissions were recorded passively by the imaging transducer and analyzed as previously described30. The acoustic energy emitted by MBs was measured over the duration of the whole sonication. Moreover, we conducted frequency analysis using a fast Fourier Transform (FFT) in MATLAB (The MathWorks, Natick, MA, USA) to identify MB response based on spectral features. Lastly, we calculated cavitation doses, including SCDh, SCDu, and ICD, as previously described31.
Histology
Ten days after intracranial injection of MGPP3 cells, mice were anesthetized by intraperitoneal injection of 100 mg/kg bw ketamine and 10mg/kg bw xylazine and then transcardially perfused with 0.9% sodium chloride solution for 10 min. After perfusion, brain tissues were collected, fixed in 4 % paraformaldehyde, processed, and embedded in paraffin. H&E staining was performed and the slides were analyzed by the neuropathologists.
Liquid Chromatography with Tandem Mass Spectrometry (LC-MS-MS)
Seven days after intracranial injection of MGPP3 cells, FUS was performed on mice to open the BBB. 5 mg/kg bw of etoposide was intraperitoneally administered right after FUS application. Ninety min after intraperitoneal administration of etoposide, mice were anesthetized by intraperitoneal injection of 100 mg/kg bw ketamine and 10mg/kg bw xylazine. Blood samples were collected by cardiac puncture. We then conducted transcardial perfusion with 0.9% sodium chloride solution for 10 min and collected the brain tissues with the tumor. For serum collection, whole blood samples were allowed to clot for 60 min and then centrifuged at 2000 xg for 20 min to acquire the supernatants (serum). All samples were stored at −80°C until analyzed by the Biomarkers Core Laboratory of the Irving Institute for Clinical and Translational Research (Columbia University, New York, NY).
Animal Numbers and Statistical Analysis
The sizes of the sample groups in histology, the determination of etoposide dose, and LC-MS-MS are n = 6, 5, and 6 respectively. The sizes of the sample groups in the survival study are as indicated (Table 1). All data presented are representative of at least three independent experiments that yielded similar results. Statistical analyses were performed using GraphPad Prism 5.
Table 1.
Summary of Animal Survival Analysis
| Group | Median Survival (d) | Change in Median Survival Time (%) | P-value* | Mean Survival (d)† | Change in Mean Survival Time (%) | P-value‡ |
|---|---|---|---|---|---|---|
| Control (n = 13) | 19 | 100 | 0.0002 | 18.85 ± 3.46 | 100 | 0.0002 |
| Etoposide alone (n = 13) | 19 | 100 | 0.0001 | 19.31 ± 2.59 | 102.44 | 0.0001 |
| Focused ultrasound alone (n = 7) | 19 | 100 | 0.0014 | 19.43 ± 3.1 | 103.08 | 0.0037 |
| Focused ultrasound plus Etoposide (n = 11) | 25 | 131.58 | … | 24.64 ± 2.84 | 130.72 | … |
P-values are relative to Focused ultrasound plus Etoposide in Log-rank Test
Values are means ± standard deviations
P-values are relative to Focused ultrasound plus Etoposide in unpaired t-tests with Welch’s correction
Results
High Sensitivity of Mouse Glioma Cell Line to Etoposide
Initially we examined the drug sensitivity of MGPP3 cells to etoposide, carboplatin and TMZ. TMZ is used as part of standard of care for patients with GBM15. Carboplatin has previously been used in clinical trial with FUS for recurrent GBM. Cells were treated with the chemotherapy drug for 72 hours and CellTiter-Blue Assay was performed to assess for cell viability. The values of half maximal inhibitory concentration (IC50) of etoposide, carboplatin, and TMZ for MGPP3 cells were 0.47, 85.4, and 690.2 μM, respectively, indicating that MGPP3 cells appeared to be most sensitive to etoposide (Figure 1).
Figure 1.
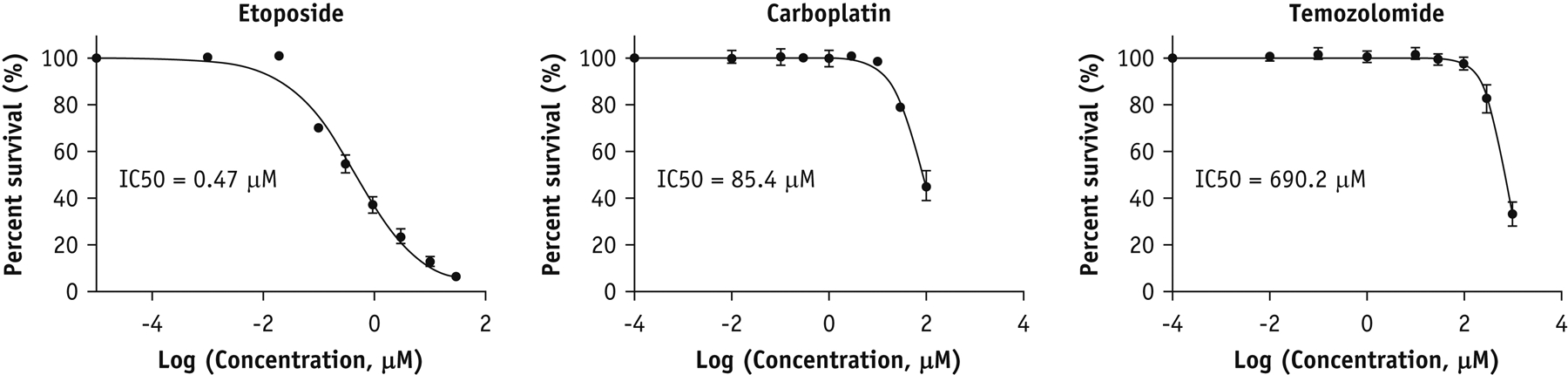
In vitro cytotoxicity of chemotherapy drugs in of mouse glioma cells. Cell viability (mean ± SD) of MGPP3 cells treated with etoposide, carboplatin, and TMZ for 72 hours assessed by MTT assay.
GBM Features of MGPP3-derived Tumor
MGPP3 is a murine glioma cell line that was isolated from mice injected with PDGF-internal ribosomal entry site retrovirus into the cerebral white matter of Pten−/−/p53−/− mice. Intracranial injection of MGPP3 cells into the cerebral white matter demonstrated highly reproducible tumors in mice, recapitulating key features of human GBM. These tumors are heterogeneous with a solid tumor component, as well as microscopic infiltrative disease. Gene expression profile demonstrated a high degree of similarity with proneural GBM27. Typically, in the setting of preclinical murine models for GBM, mice are treated when they have a large destructive tumor in which the BBB is diffusely disrupted. In the clinical setting, patients with GBM undergo maximal safe resection of the primary solid tumor component and radiation and systemic therapy is used to treat the microscopic disease extending beyond the resection margin. To model the microscopic spread where the BBB disruption was minimal, we selected to treat mice implanted with MGPP3 prior to the formation of a destructive tumor mass. Using T1-weighted contrast-enhanced MR imaging, we monitored the tumor progression. At 6 days after tumor implantation, MR images showed a small tumor cavity caused by the injection surrounded by a contrast-enhanced ring with no visible mass (Figure 2A). Mice with this radiographic finding had over 95% tumor formation. Thus we elected to treat our mice one week after tumor implantation to mimic the microscopic diseases with minor BBB disruption. We then further used T1-weighted contrast-enhanced and non-contrast T2 MR imaging to identify the infiltrative characteristic of MGPP3-derived tumor. Mice were scanned at day 10 after tumor implantation once solid tumor was visualized. Correlating to clinical radiographic findings of GBM, T2 hyperintensity (asterisk) was visualized surrounding the region of T1-contrast enhancing mass (arrowhead) (Figure 2B). We also analyzed the histopathological features of the MGPP3-derived tumor. Hematoxylin and eosin (H&E) staining of the tumor at day 10 showed pleomorphic large tumor cells with huge, bizarre nuclei and nuclear hyperchromasia. Moreover, the tumor exerts infiltrative margins, mild vascular proliferation, and moderate mononuclear cell infiltration (Figure 2C, left panel). On high magnification, large tumor cells with multinucleation, prominent nucleoli, increased mitotic figures (count 11), and anisonucleosis (Figure 2C, right panel) were observed. Collectively, the MGPP3-derived tumor exhibited the imaging and histopathological features of GBM with diffusely infiltrative characteristics.
Figure 2.
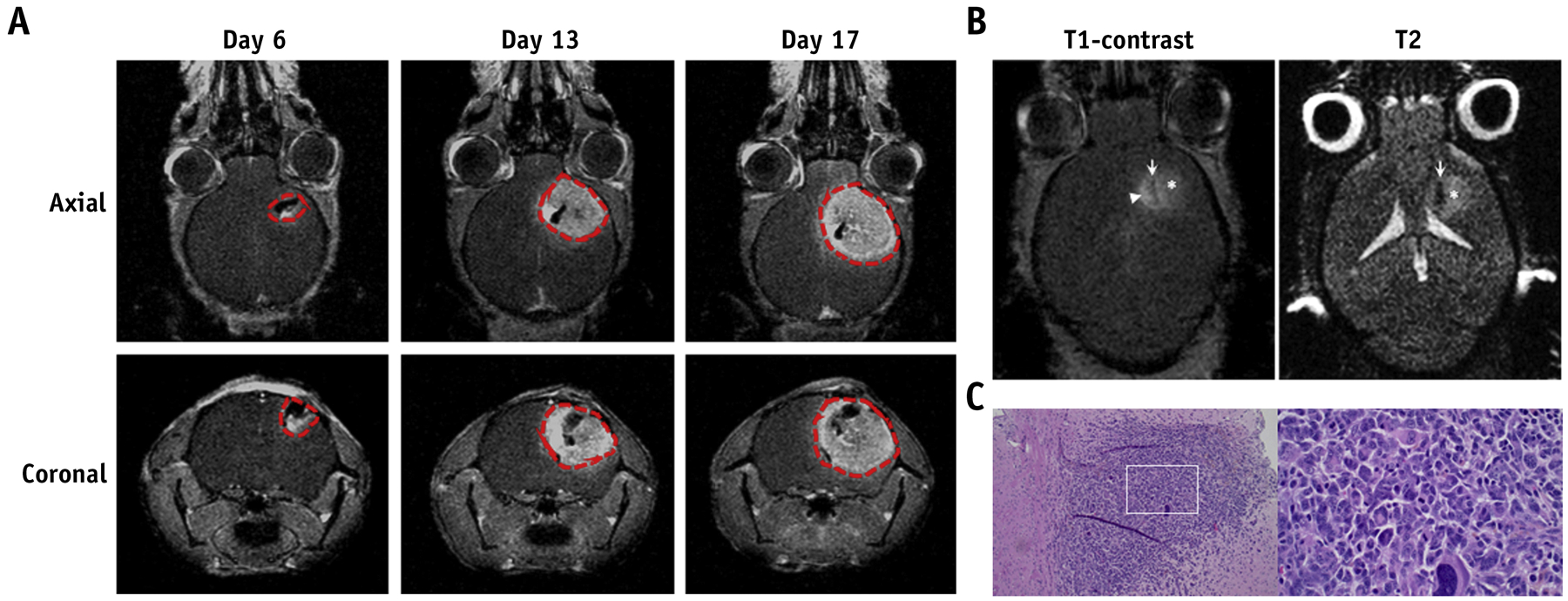
Validation of intracranial GBM model. (A) Representative T1-weighted contrast-enhanced images of mice intracranially injected with MGPP3 cells. Images were taken 6, 13, and 17 days after tumor implantation. Red dotted line: tumor cavity as defined by T1-post contrast enhancement. (B) Representative T1-weighted contrast-enhanced and non-contrast T2 images of mice 10 days after intracranial injection of MGPP3 cells. Left panel: injection tract (arrow), contrast enhancing tumor (arrowhead), and hyperintensity demonstrating peritumoral edema region (asterisk). Right panel: injection tract (arrow) and lightly hyperintense, poorly demarcated, and irregularly tumor lesion (asterisk). (C) Representative H&E staining of brain tumor showing the histological features of MGPP3-derived xenograft. Left panel: original magnification × 100; Right panel: original magnification × 400.
FUS-mediated BBB Opening and Passive Cavitation Detection in Intracranial GBM Model
To cover the tumor cavity and its surrounding infiltrative region, we sonicated four points in a 2×2 square at a distance of 1.5 mm by 1.5 mm apart (Figure 3A). During sonication, PCD was used in real time to detect the acoustic emission of MBs. After sonication, T1-weighted contrast-enhanced MR imaging was used to confirm successful BBB opening. As shown in Figure 3B, the contrast-enhancing region in T1-post contrast MR images confirmed BBB opening in the region of the tumor cavity with additional margins within the mouse brain. Acoustic energy emitted by MBs rose at the beginning due to the first MBs administration (red arrowhead) and maintained the energy level during sonication. In addition to this, the 3 peaks (blue arrowheads) observed in the energy correspond to the motion of the transducer to another spot of the square (Figure 3C). The spectral content of the received signals showed an increase in higher harmonics after MBs administration (Figure 3D). Spectrogram revealed no substantial increase in the broadband floor during the FUS treatment, indicating limited microbubble destruction within the focal volume (Figure 3E). Lastly, stable cavitation doses based on harmonics and ultraharmonics (SCDh, SCDu) and inertial cavitation doses (ICD) were relatively constant throughout the sonication, indicating persistent stable cavitation activity during sonication with minimal inertial cavitation (Figure 3F).
Figure 3.
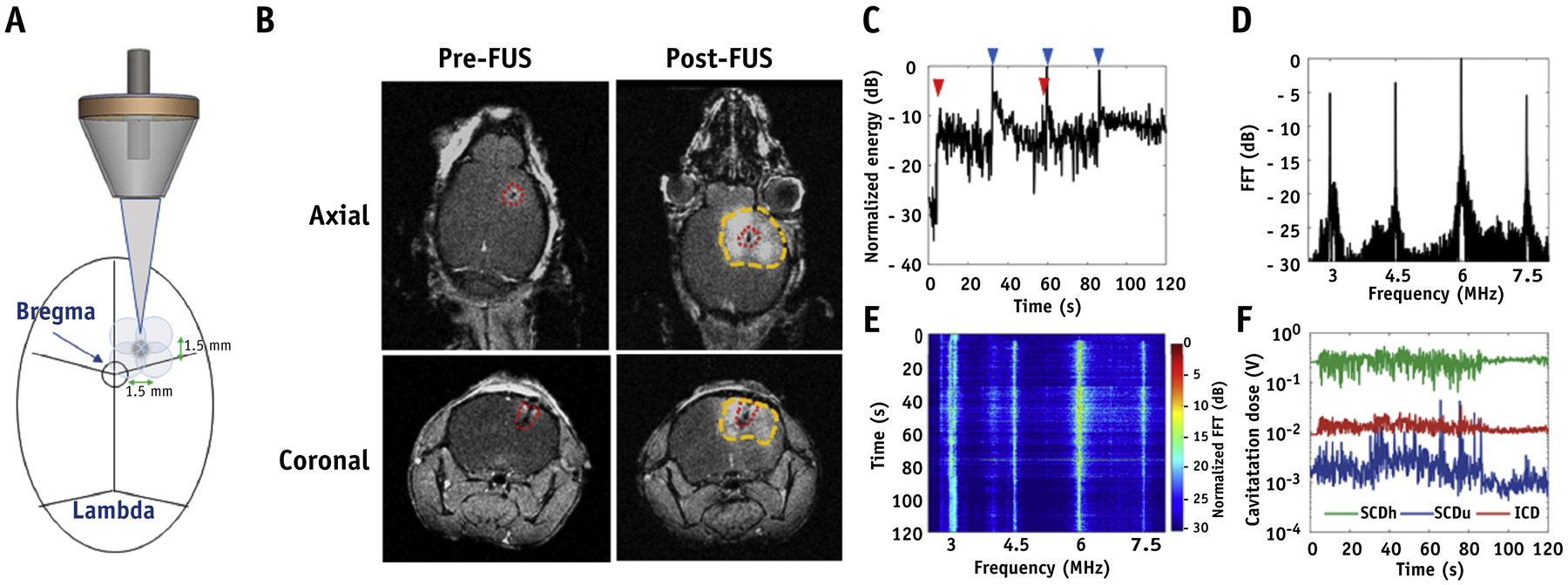
FUS-mediated BBB opening confirmed by MR imaging and acoustic emissions measurement. (A) Schematic diagram of the targeted strategy for BBB opening. (B) Representative T1-weighted contrast-enhanced images of GBM tumor-bearing mice before and after FUS sonication. Red dotted line: tumor; Yellow dotted line: BBB opening. In vivo passive cavitation detection measurements. (C) Acoustic energy, (D) spectral amplitude, (E) spectrogram of MBs cavitation during FUS exposure. (F) The dose of stable harmonic cavitation (SCDh), stable ultraharmonic cavitation (SCDu), and inertial cavitation (ICD) during FUS exposure. Red arrowhead: MBs administration; Blue arrowheads: movement of the transducer.
Treatment Combining FUS and Etoposide Reduces Tumor Growth and Prolongs Survival in Intracranial GBM Model
To establish a subtherapeutic dose of etoposide without FUS in the GBM model, mice with GBM were intraperitoneally treated with 0, 5, 10, and 20 mg/kg bw of etoposide on days 7 and 14 after tumor implantation. We found 5 mg/kg bw of etoposide was subtherapeutic (data not shown). Hence, we elected 5 mg/kg bw of etoposide to combine with FUS to assess whether FUS-mediated BBB opening increased etoposide delivery resulting in an improved therapeutic benefit in GBM tumor-bearing mice. After intracranial tumor implantation, tumor-bearing mice were randomized into four groups: (i) Control, (ii) etoposide alone, (iii) FUS alone, and (iv) FUS plus etoposide. The experimental timeline is shown in Figure 4A. MR imaging T1-post contrast scan was used to assess BBB opening and tumor size. Using MATLAB to quantify the volume of BBB opening and contrast enhancement, there was no significant difference between FUS alone and FUS plus etoposide groups on both days 7 (BBB opening: 35.40 ± 12.59, 29.75 ± 13.46; contrast enhancement: 90.18 ± 31.06, 77.75 ± 32.29) and 14 (BBB opening: 30.55 ± 8.08, 25.67 ± 10.99; contrast enhancement: 81.24 ± 28.36, 56.66 ± 20.20). (Figure 4B,C). Likewise, quantitative results of PCD also showed similar delivery of ultrasound achieving comparable cavitation doses across the two groups (Figure 4D).
Figure 4.
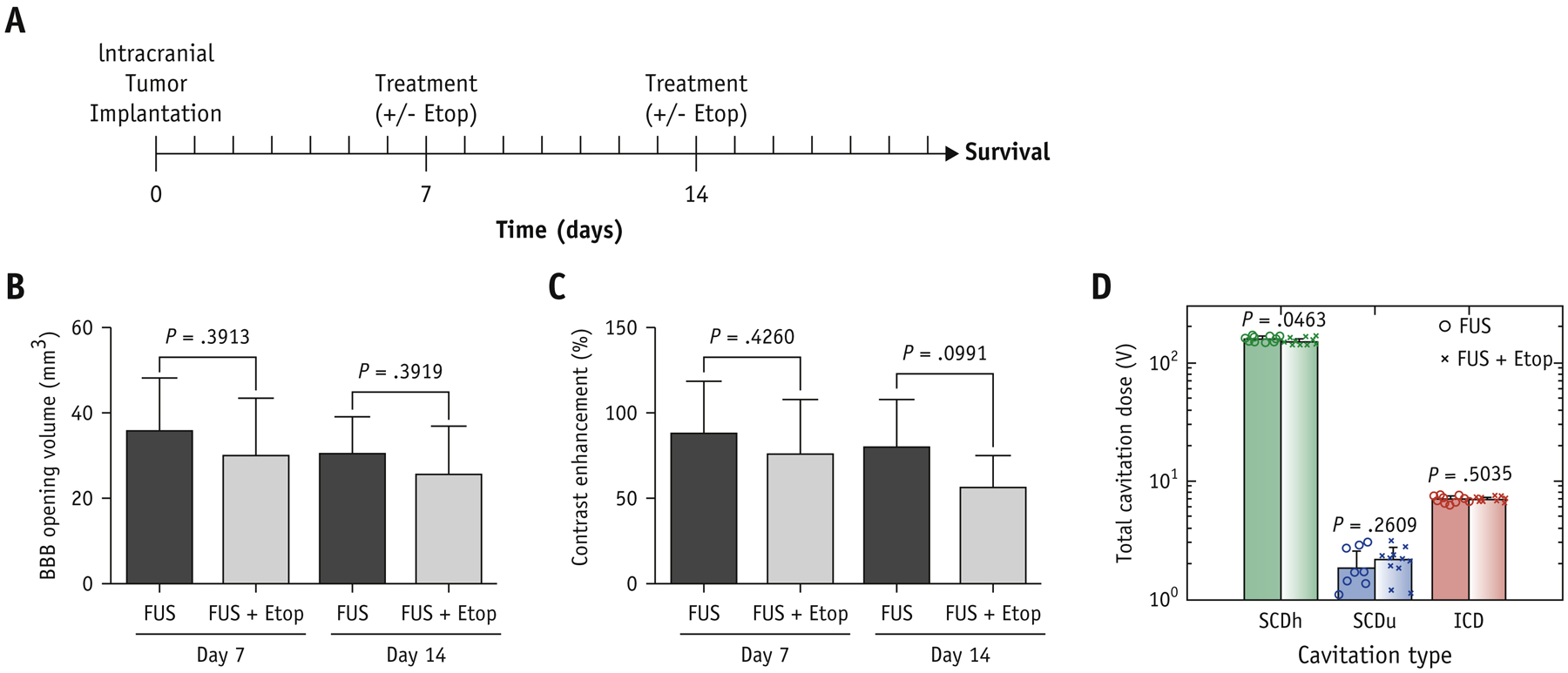
Comparison of BBB opening in vivo. (A) Time course of the experiment. The quantification of MR images for (B) BBB opening volume (mm3) and (C) contrast enhancement (%). (D) Average stable harmonic (SCDh), stable ultraharmonic (SCDu) and inertial (ICD) cavitation dose during FUS sonication. Values are means + SD; P values between FUS and FUS+Etop are indicated by using unpaired t-tests with Welch’s correction.
We also used T1-weighted contrast-enhanced MR imaging to monitor tumor progression by measuring tumor volumes. Mice receiving FUS plus etoposide had increased local control as compared to control mice (Figure 5A). The animals from control, etoposide alone, and FUS alone groups showed progressive tumor growth (tumor volume changes from day 7 to day 14 were 5.39 ± 0.67, 6.04 ± 1.02, and 5.75 ± 0.45 folds, respectively). Treatment with FUS and etoposide inhibited tumor growth (tumor volume changes from day 7 to day 14 were 2.98 ± 0.31 folds) with a 45% reduction in tumor size as compared to control mice (Figure 5B). Kaplan-Meier survival curves showed an increase in survival in the FUS plus etoposide arm (Figure 5C and Table 1). The median survival of control, etoposide alone, and FUS alone groups were all 19 days. The treatment of FUS plus etoposide improved the median survival of mice to 25 days. The Log-rank test revealed that FUS plus etoposide significantly increases 6 days of median survival, approximately 30%, compared to other groups.
Figure 5.
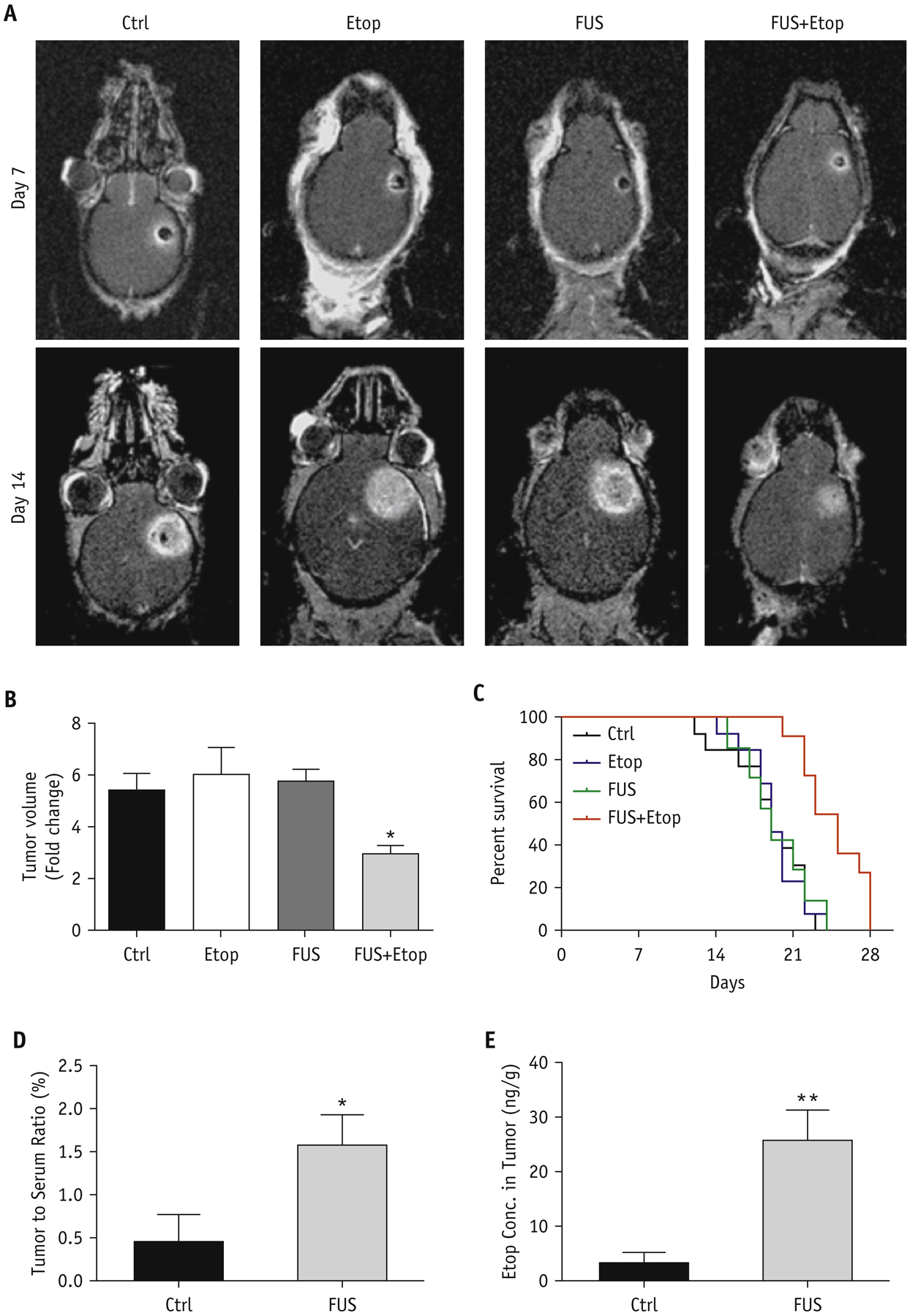
Therapeutic efficacy and delivery of etoposide enhanced by FUS in GBM tumor-bearing mice. (A) Representative T1-weighted contrast-enhanced images of mice intracranially injected with MGPP3 cells with indicated treatment. Images were taken 7 and 14 days after tumor implantation. (B) Quantitative analysis of the relative tumor progression. Fold changes are the tumor volume by MR image on day 14 normalized to the tumor volume on day 7. Values are means + SD; * indicates P<0.05 in unpaired t test with Welch’s correction, compared to the control group. (C) Kaplan-Meier plot of survival of mice intracranially injected with MGPP3 cells with indicated treatment. Ctrl: control group; Etop: etoposide alone group; FUS: FUS alone group; FUS+Etop: the combination of FUS and etoposide group. (D) The tumor-to-serum ratios of etoposide concentration (E) and the concentration of etoposide in brain tumors with or without FUS.
FUS-mediated BBB Opening Enhances Etoposide Delivery to Brain Tumor
The BBB acts as a barrier to limit large molecules from penetrating the brain parenchyma. We examined whether FUS-mediated BBB opening increased intracranial tumor delivery of etoposide. LC-MS-MS was used to measure the concentration of etoposide in tissues and serum of GBM-harboring mice with or without FUS. Seven days after tumor implantation, mice were exposed to FUS to open BBB and intraperitoneally administered with 5 mg/kg bw of etoposide. The LC-MS-MS results showed that mice receiving FUS had a 3.5-fold increased mean brain tumor-to-serum ratio compared to control mice (Figure 5D). The concentration of etoposide in the sonicated tumor tissue was over 8-fold higher than in the nonsonicated tumor tissue (Figure 5E). These results suggest that FUS can increase the accumulation of etoposide in the tumor tissue of mouse brain.
Discussion
GBM is a devastating brain tumor in which there has been minimal clinical systemic therapeutic advancement over the past 15 years. The diffusely infiltrative nature of the disease makes it difficult to eliminate microscopic disease even after gross total resection. GBM is heterogeneous with both a solid component (in which the BBB is disrupted) and an extensively infiltrative microscopic component that extends into regions of the brain where the BBB remains relatively intact. This is demonstrated by MR imaging where the T1-weighted post-contrast images show contrast-enhancement confined to the primary tumor mass with disrupted BBB (which is typically resected), and the area of infiltrating tumor cells, visualized as hyperintense regions on T2-weighted imaging, extends beyond the primary tumor and reveals minimal contrast enhancement32. Radiation treatment has played a critical role in the management of this microscopic disease with ionization radiotherapy; however, the overall survival is dismal. Over 90% of patients with GBM have recurrences within 2–3 cm from the margin of the original resected tumor33, 34. The heterogeneous integrity of the BBB in GBM plays a big role in limiting drug delivery and novel approaches for treatment are necessary.
There is a large disconnect between clinical and preclinical GBM. Despite the triumph of systemic treatments of preclinical mouse models of GBM, there has been very limited success in the translation of these systemic therapies for patients. One potential reason is the heterogeneous nature of the tumor and peritumoral vasculature: the residual, non-enhancing disease that is not targeted with surgery remains protected from systemic therapy by the BBB. As mentioned previously, patients with GBM are treated with maximal safe resection of the bulk tumor while systemic and radiation strategies focus on microscopic disease. This is different from preclinical models of GBM in which many of the studies use mice with destructive solid tumors in which the BBB is completely disrupted. Furthermore, while several preclinical models of GBM have been developed, not all of them have the characteristics to recapitulate genetic, histopathological, and biological features of human GBM35. For example, U87 MG cells used for human orthotopic modeling of GBM often form massive tumors that disrupt the BBB without microscopic infiltration36. This creates a fundamental difference in the therapeutic effect of systemic therapy and its bioavailability.
In this study we elected to use murine syngeneic GBM model using MGPP3. The MGPP3 (Pdgf+, Pten−/−, P53−/−) cell line was established from genetically-engineered mouse models of GBM created by injecting PDGF-IRES-Cre retrovirus into the subcortical white matter of mice that harbor floxed tumor suppressors (PTEN and p53). Tumors from this model underwent genome-wide expression profiling with RNA sequencing and were compared to gene sets of 4 subtypes of GBM established by The Cancer Genome Atlas (TCGA), revealing the highest similarity to the proneural subtype of human GBM. This murine GBM model has been identified with genetic and histological resemblance to human proneural GBM and demonstrates a modest response to radiation therapy27. Moreover, this syngeneic mouse model of GBM uses mice of C57BL/6 origin that do not require a deficient immune system, which may more closely resemble the immune response of human GBM. In order to mimic the microscopic disease after surgical resection seen in humans, we elected to treat the mice that were stereotactically injected with GBM cells at an earlier time point. Through serial imaging, we observed that upon developing a ring enhancement surrounding the injection cavity, over 95% of the mice develop intracranial tumors. MRI imaging of tumors demonstrates T2 hyperintensity consistent with human GBM and pathological analysis of H&E stains demonstrates an infiltrative pattern similar to clinical GBM.
Intracranial therapeutic ultrasound has gained much interest since the first MR imaging-guided FUS device for the brain was FDA-approved for clinical use in 2016 for essential tremor and in 2018 for Parkinson’s disease (PD). The Neuro Exablate, by Insightec, is similar to the Gamma Knife in that the ultrasound transducers are placed in a hemispherical fashion pointing to a single focus. Using MR imaging guidance, ultrasound can be delivered leading to an increase in temperature allowing for thermal thalamotomy for patients with essential tremor and PD37. This same device is capable of focally and transiently opening the BBB using low frequency ultrasound. When low frequency ultrasound is delivered in the presence of ultrasound contrast, or MBs, the bubbles oscillate through repeated expansion and contraction (stable cavitation). This can lead to mechanical and functional alterations of the blood vessels, resulting in temporary and reversible opening of the BBB13, 38. FUS has been shown to be able to transiently, repeatedly, and safely open the BBB in multiple preclinical models, including rodents, rabbits, pigs, and non-human primates by optimizing acoustic parameters and MB dosage6, 7. Safe BBB opening has been demonstrated in multiple phase I and phase II clinical trials in patients with Alzhemier’s disease, amyotrophic lateral sclerosis (ALS), and recurrent glioblastoma39–42. The degree of BBB opening and the duration of BBB opening can be modified by changing the acoustic parameters and MB dosage. With excessively stronger parameters, the MBs continuously grow, resulting in a violent collapse (inertial cavitation) which leads to shock waves and turbulent flow that may result in vascular damage38. The ultrasound parameters used in this study (center frequency: 1.5 MHz, peak-negative pressure: 0.7 MPa, PL: 1 ms, PRF: 5 Hz, in-house manufactured MBs: 1 μL/g bw) have been previously demonstrated to open the BBB without causing neural damage6. Furthermore, real-time assessment of the ultrasound energy delivered along with acoustic monitoring showed evenly maintained energy levels resulting in high levels of stable cavitation with minimal inertial cavitation. This resulted in successful and reproducible BBB opening, without compromising safety.
Several preclinical studies have shown that FUS-mediated BBB opening increased the penetrance of various systemically administered drugs, including chemotherapy drugs8–12 and antibody directed therapies43–46, into the brain parenchyma as well as brain tumors, ranging from metastases to GBM. In order to support rapid translation to clinical trial, we chose a commonly used chemotherapeutic agent to combine with a novel method of drug delivery. Etoposide is a TOP2 inhibitor that is used in both pediatric and adult cancer patients and is well tolerated21. The pharmacokinetics of etoposide is well understood and the drug reaches maximum serum concentration within a couple hours after both IV and oral administration47. Although the molecular weight is small (588.56 Da), the majority of etoposide in the body is protein-bound, limiting its penetrance across the BBB. Previous studies show that local intracranial delivery of etoposide with CED improves therapeutic effects in GBM48. In addition, there is some clinical evidence suggesting a potential response to treatment in patients with high grade glioma49. We elected to compare etoposide with carboplatin and TMZ: TMZ is used as part of standard of care for GBM and carboplatin has been used with ultrasound-mediated BBB opening in patients with recurrent GBM for some response. Interestingly, the IC50 showed higher levels of cell death response with etoposide as compared to either therapy, making it an ideal candidate for rapid translation into clinical trial.
In this study, we observed that combining FUS with etoposide increased the intratumoral delivery of etoposide, which led to a 30% increase in median overall survival. With an increased delivery of chemotherapeutic agent into the brain with FUS, there is some concern whether this enhances toxicities. In a study by Sonabend and colleagues, they examined the use of different formulations of paclitaxel (albumin-bound paclitaxel and paclitaxel dissolved in cremophor) and ultrasound-guided delivery. They observed that paclitaxel dissolved in cremophor induced central nervous system toxicity when combined with FUS, while albumin-bound paclitaxel did not12. The combined usage of FUS and etoposide was well tolerated. We did not observe increased mortality or morbidity with combining FUS and etoposide, thus making etoposide an ideal agent to examine in an early phase clinical trial.
Clinical advancement of FUS technology has progressed rapidly over the past few years with clinical trials showing safety with BBB opening in both patients with brain tumors and with Alzheimer’s disease39–41. Multiple clinical trials are currently ongoing studying the safety and feasibility of BBB opening14. SonoCloud (CarThera, France) is an implantable device fixed to the skull that delivers low frequency (non-focused) ultrasound for BBB opening. In addition, three extracranial devices are currently being tested, including the Exablate Neuro (Insightec Tirat Carmel, Israel), NaviFUS system (NaviFUS, Taiwan), and a single-element neuronavigation guided device developed at Columbia University. The Exablate Neuro is similar to the Gamma Knife in which ultrasound transducers are placed in a hemispherical manner pointing to a single focus. A stereotactic head frame is placed on the patient for radiation planning with a built-in MRI system. In contrast, the NaviFUS system also uses multi-channel hemispherical phased array ultrasound; however, the targeting is based on neuro-navigation. Lastly, a single-element, extracranial FUS system with neuronavigation guidance has been developed at Columbia University. This neuronavigation-guided FUS system consists of a 0.25-MHz single-element transducer coupled with real-time cavitation monitoring31. With advancement of ultrasound technology and the feasibility of clinical applicability, there is an emerging need for research to advance the field. We believe our study establishes preclinical rationale to test the combination of FUS-mediated BBB opening with etoposide in patients with glioblastoma. Currently the treatment of intracranial FUS across the world varies from radiologists, neurologists, neurosurgeons, and radiation oncologists. FUS has the potential to offer a new pathway for non-invasive drug delivery to infiltrative tumors in the brain and researchers in the field of radiation oncology should be aware of the development of this emerging technology.
Supplementary Material
Acknowledgments:
The authors acknowledge Maggie and Jacob Dyson, and the Zuckerman Mind Brain Behavior Institute MRI Platform, a shared resource.
Funding:
This research was funded by the Gary and Yael Fegel Family Foundation, Star and Storm Foundation, Matheson Foundation (UR010590), Herbert Irving Cancer Center Cancer Center Support Grant (P30CA013696), Herbert Irving Cancer Center Cancer Center CAPRI Grant (NIH R38CA231577-01), NIH 5R01EB009041 grant, NIH 5R01AG038961 grant, NIH R01CA204297 grant, and the National Center for Advancing Translational Sciences, NIH (UL1TR001873). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Conflicts of Interest:
Dr. Wang reports personal fees and non-financial support from AbbVie, personal fees from AstraZeneca, personal fees from Cancer Panels, personal fees from Doximity, personal fees and non-financial support from Elekta, personal fees and non-financial support from Merck, personal fees and non-financial support from Novocure, personal fees and non-financial support from RTOG Foundation, personal fees from Rutgers, personal fees from University of Iowa, personal fees from Wolters Kluwer, outside the submitted work. Dr. Konofagou reports a patent US 2013/0046229 issued.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing Statement: All data generated and analyzed during this study are included in this published article and its supplementary information files.
References
- 1.Baskar R, Lee KA, Yeo R, Yeoh KW. Cancer and radiation therapy: current advances and future directions. Int J Med Sci. 2012;9(3):193–9. doi: 10.7150/ijms.3635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Napoli A, Alfieri G, Scipione R, et al. High-intensity focused ultrasound for prostate cancer. Expert Rev Med Devices. May 2020;17(5):427–433. doi: 10.1080/17434440.2020.1755258 [DOI] [PubMed] [Google Scholar]
- 3.Bertrand AS, Iannessi A, Natale R, et al. Focused ultrasound for the treatment of bone metastases: effectiveness and feasibility. J Ther Ultrasound. 2018;6:8. doi: 10.1186/s40349-018-0117-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tajes M, Ramos-Fernandez E, Weng-Jiang X, et al. The blood-brain barrier: structure, function and therapeutic approaches to cross it. Mol Membr Biol. August 2014;31(5):152–67. doi: 10.3109/09687688.2014.937468 [DOI] [PubMed] [Google Scholar]
- 5.Hendricks BK, Cohen-Gadol AA, Miller JC. Novel delivery methods bypassing the blood-brain and blood-tumor barriers. Neurosurg Focus. March 2015;38(3):E10. doi: 10.3171/2015.1.FOCUS14767 [DOI] [PubMed] [Google Scholar]
- 6.Konofagou EE. Optimization of the ultrasound-induced blood-brain barrier opening. Theranostics. 2012;2(12):1223–37. doi: 10.7150/thno.5576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Papademetriou IT, Porter T. Promising approaches to circumvent the blood-brain barrier: progress, pitfalls and clinical prospects in brain cancer. Ther Deliv. 2015;6(8):989–1016. doi: 10.4155/tde.15.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drean A, Lemaire N, Bouchoux G, et al. Temporary blood-brain barrier disruption by low intensity pulsed ultrasound increases carboplatin delivery and efficacy in preclinical models of glioblastoma. J Neurooncol. August 2019;144(1):33–41. doi: 10.1007/s11060-019-03204-0 [DOI] [PubMed] [Google Scholar]
- 9.Liu HL, Huang CY, Chen JY, Wang HY, Chen PY, Wei KC. Pharmacodynamic and therapeutic investigation of focused ultrasound-induced blood-brain barrier opening for enhanced temozolomide delivery in glioma treatment. Plos One. 2014;9(12):e114311. doi: 10.1371/journal.pone.0114311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kovacs Z, Werner B, Rassi A, Sass JO, Martin-Fiori E, Bernasconi M. Prolonged survival upon ultrasound-enhanced doxorubicin delivery in two syngenic glioblastoma mouse models. J Control Release. August 10 2014;187:74–82. doi: 10.1016/j.jconrel.2014.05.033 [DOI] [PubMed] [Google Scholar]
- 11.Coluccia D, Figueiredo CA, Wu MY, et al. Enhancing glioblastoma treatment using cisplatin-gold-nanoparticle conjugates and targeted delivery with magnetic resonance-guided focused ultrasound. Nanomedicine. June 2018;14(4):1137–1148. doi: 10.1016/j.nano.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 12.Zhang DY, Dmello C, Chen L, et al. Ultrasound-mediated Delivery of Paclitaxel for Glioma: A Comparative Study of Distribution, Toxicity, and Efficacy of Albumin-bound Versus Cremophor Formulations. Clin Cancer Res. January 15 2020;26(2):477–486. doi: 10.1158/1078-0432.CCR-19-2182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beccaria K, Canney M, Bouchoux G, et al. Ultrasound-induced blood-brain barrier disruption for the treatment of gliomas and other primary CNS tumors. Cancer Lett. June 1 2020;479:13–22. doi: 10.1016/j.canlet.2020.02.013 [DOI] [PubMed] [Google Scholar]
- 14.Clinicaltrial.gov. Accessed May 3 2020, 2020. https://clinicaltrials.gov/ct2/results?recrs=&cond=&term=Focused+ultrasound%2C+Blood+Brain+Barrier&cntry=&state=&city=&dist=
- 15.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. March 10 2005;352(10):987–96. doi: 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 16.Koshy M, Villano JL, Dolecek TA, et al. Improved survival time trends for glioblastoma using the SEER 17 population-based registries. J Neurooncol. March 2012;107(1):207–12. doi: 10.1007/s11060-011-0738-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2012–2016. Neuro Oncol. November 1 2019;21(Supplement_5):v1–v100. doi: 10.1093/neuonc/noz150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberoi RK, Parrish KE, Sio TT, Mittapalli RK, Elmquist WF, Sarkaria JN. Strategies to improve delivery of anticancer drugs across the blood-brain barrier to treat glioblastoma. Neuro-oncology. January 2016;18(1):27–36. doi: 10.1093/neuonc/nov164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sarkaria JN, Hu LS, Parney IF, et al. Is the blood-brain barrier really disrupted in all glioblastomas? A critical assessment of existing clinical data. Neuro Oncol. January 22 2018;20(2):184–191. doi: 10.1093/neuonc/nox175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hande KR. Etoposide: four decades of development of a topoisomerase II inhibitor. Eur J Cancer. September 1998;34(10):1514–21. doi: 10.1016/s0959-8049(98)00228-7 [DOI] [PubMed] [Google Scholar]
- 21.Mehta A, Awah CU, Sonabend AM. Topoisomerase II Poisons for Glioblastoma; Existing Challenges and Opportunities to Personalize Therapy. Front Neurol. 2018;9:459. doi: 10.3389/fneur.2018.00459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franceschi E, Cavallo G, Scopece L, et al. Phase II trial of carboplatin and etoposide for patients with recurrent high-grade glioma. Br J Cancer. September 13 2004;91(6):1038–44. doi: 10.1038/sj.bjc.6602105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reardon DA, Desjardins A, Vredenburgh JJ, et al. Metronomic chemotherapy with daily, oral etoposide plus bevacizumab for recurrent malignant glioma: a phase II study. Br J Cancer. December 15 2009;101(12):1986–94. doi: 10.1038/sj.bjc.6605412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aoki T, Mizutani T, Nojima K, et al. Phase II study of ifosfamide, carboplatin, and etoposide in patients with a first recurrence of glioblastoma multiforme. J Neurosurg. January 2010;112(1):50–6. doi: 10.3171/2009.5.JNS081738 [DOI] [PubMed] [Google Scholar]
- 25.Kesari S, Schiff D, Doherty L, et al. Phase II study of metronomic chemotherapy for recurrent malignant gliomas in adults. Neuro Oncol. July 2007;9(3):354–63. doi: 10.1215/15228517-2007-006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu B, Earl HM, Poole CJ, Dunn J, Kerr DJ. Etoposide protein binding in cancer patients. Cancer Chemother Pharmacol. 1995;36(6):506–12. doi: 10.1007/BF00685801 [DOI] [PubMed] [Google Scholar]
- 27.Sonabend AM, Yun J, Lei L, et al. Murine cell line model of proneural glioma for evaluation of anti-tumor therapies. J Neurooncol. May 2013;112(3):375–82. doi: 10.1007/s11060-013-1082-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pouliopoulos AN, Jimenez DA, Frank A, et al. Temporal Stability of Lipid-Shelled Microbubbles During Acoustically-Mediated Blood-Brain Barrier Opening. Original Research. Frontiers in Physics. 2020-May-06 2020;8(137)doi: 10.3389/fphy.2020.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pouliopoulos AN, Burgess MT, Konofagou EE. Pulse inversion enhances the passive mapping of microbubble-based ultrasound therapy. Appl Phys Lett. July 23 2018;113(4):044102. doi: 10.1063/1.5036516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pouliopoulos AN, Bonaccorsi S, Choi JJ. Exploiting flow to control the in vitro spatiotemporal distribution of microbubble-seeded acoustic cavitation activity in ultrasound therapy. Phys Med Biol. November 21 2014;59(22):6941–57. doi: 10.1088/0031-9155/59/22/6941 [DOI] [PubMed] [Google Scholar]
- 31.Pouliopoulos AN, Wu SY, Burgess MT, Karakatsani ME, Kamimura HAS, Konofagou EE. A Clinical System for Non-invasive Blood-Brain Barrier Opening Using a Neuronavigation-Guided Single-Element Focused Ultrasound Transducer. Ultrasound Med Biol. January 2020;46(1):73–89. doi: 10.1016/j.ultrasmedbio.2019.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villanueva-Meyer JE, Mabray MC, Cha S. Current Clinical Brain Tumor Imaging. Neurosurgery. September 1 2017;81(3):397–415. doi: 10.1093/neuros/nyx103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gaspar LE, Fisher BJ, Macdonald DR, et al. Supratentorial malignant glioma: patterns of recurrence and implications for external beam local treatment. Int J Radiat Oncol Biol Phys. 1992;24(1):55–7. doi: 10.1016/0360-3016(92)91021-e [DOI] [PubMed] [Google Scholar]
- 34.Lee SW, Fraass BA, Marsh LH, et al. Patterns of failure following high-dose 3-D conformal radiotherapy for high-grade astrocytomas: a quantitative dosimetric study. Int J Radiat Oncol Biol Phys. January 1 1999;43(1):79–88. doi: 10.1016/s0360-3016(98)00266-1 [DOI] [PubMed] [Google Scholar]
- 35.Jacobs VL, Valdes PA, Hickey WF, De Leo JA. Current review of in vivo GBM rodent models: emphasis on the CNS-1 tumour model. ASN Neuro. August 3 2011;3(3):e00063. doi: 10.1042/AN20110014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Radaelli E, Ceruti R, Patton V, et al. Immunohistopathological and neuroimaging characterization of murine orthotopic xenograft models of glioblastoma multiforme recapitulating the most salient features of human disease. Histol Histopathol. July 2009;24(7):879–91. doi: 10.14670/HH-24.879 [DOI] [PubMed] [Google Scholar]
- 37.Bond AE, Shah BB, Huss DS, et al. Safety and Efficacy of Focused Ultrasound Thalamotomy for Patients With Medication-Refractory, Tremor-Dominant Parkinson Disease: A Randomized Clinical Trial. JAMA Neurol. December 1 2017;74(12):1412–1418. doi: 10.1001/jamaneurol.2017.3098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bunevicius A, McDannold NJ, Golby AJ. Focused Ultrasound Strategies for Brain Tumor Therapy. Oper Neurosurg (Hagerstown). December 19 2019;doi: 10.1093/ons/opz374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lipsman N, Meng Y, Bethune AJ, et al. Blood-brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat Commun. July 25 2018;9(1):2336. doi: 10.1038/s41467-018-04529-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Idbaih A, Canney M, Belin L, et al. Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin Cancer Res. March 19 2019;doi: 10.1158/1078-0432.CCR-18-3643 [DOI] [PubMed] [Google Scholar]
- 41.Asquier N, Bouchoux G, Canney M, et al. Blood-brain barrier disruption in humans using an implantable ultrasound device: quantification with MR images and correlation with local acoustic pressure. J Neurosurg. February 1 2019:1–9. doi: 10.3171/2018.9.JNS182001 [DOI] [PubMed] [Google Scholar]
- 42.Abrahao A, Meng Y, Llinas M, et al. First-in-human trial of blood-brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat Commun. September 26 2019;10(1):4373. doi: 10.1038/s41467-019-12426-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinoshita M, McDannold N, Jolesz FA, Hynynen K. Noninvasive localized delivery of Herceptin to the mouse brain by MRI-guided focused ultrasound-induced blood-brain barrier disruption. Proc Natl Acad Sci U S A. August 1 2006;103(31):11719–23. doi: 10.1073/pnas.0604318103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu HL, Hsu PH, Lin CY, et al. Focused Ultrasound Enhances Central Nervous System Delivery of Bevacizumab for Malignant Glioma Treatment. Radiology. October 2016;281(1):99–108. doi: 10.1148/radiol.2016152444 [DOI] [PubMed] [Google Scholar]
- 45.Kobus T, Zervantonakis IK, Zhang Y, McDannold NJ. Growth inhibition in a brain metastasis model by antibody delivery using focused ultrasound-mediated blood-brain barrier disruption. J Control Release. September 28 2016;238:281–288. doi: 10.1016/j.jconrel.2016.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park EJ, Zhang YZ, Vykhodtseva N, McDannold N. Ultrasound-mediated blood-brain/blood-tumor barrier disruption improves outcomes with trastuzumab in a breast cancer brain metastasis model. J Control Release. November 10 2012;163(3):277–84. doi: 10.1016/j.jconrel.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hande K, Messenger M, Wagner J, Krozely M, Kaul S. Inter- and intrapatient variability in etoposide kinetics with oral and intravenous drug administration. Clin Cancer Res. October 1999;5(10):2742–7. [PubMed] [Google Scholar]
- 48.Sonabend AM, Carminucci AS, Amendolara B, et al. Convection-enhanced delivery of etoposide is effective against murine proneural glioblastoma. Neuro Oncol. September 2014;16(9):1210–9. doi: 10.1093/neuonc/nou026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leonard A, Wolff JE. Etoposide improves survival in high-grade glioma: a meta-analysis. Anticancer Res. August 2013;33(8):3307–15. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


