In the last few years, Venezuela has been the epicentre for multiple concurrent epidemics (syndemics) [1] of emerging and reemerging infectious and tropical diseases. These events have severe implications for public health control efforts in Latin American and other regions due to the latent threat of case importation [1,2]. At the top of the list, the resurgence of vector-borne diseases (VBD) [3,4], such as malaria and dengue pose substantial challenges for the region [5]. On the other hand, the reemergence of vaccine-preventable diseases (VPD) due to low immunization coverage has led to new and multiple outbreaks of measles, diphtheria and other vaccine-preventable infections that are once again affecting many populations [4,6,7]. The spread of some of these infectious pathogens due to forced migration into neighboring countries or overseas carries an important risk for importation of cases [3,8,9].
The reemergence of human yellow fever (YF) cases in Venezuela is a major public health threat to the region. Yellow fever is a mosquito-borne illness caused by a flavivirus (YF-virus), transmitted by Haemagogus, Sabethes or Aedes mosquitoes, exhibiting urban and sylvatic cycles in endemic regions across the tropical belt. Despite that Aedes aegypti is recognized as the principal vector of YF-virus, the increasing spread of Aedes albopictus through new regions of Venezuela may play a determinant role on enhancing transmissibility of the virus. A highly effective vaccine (17D YF-VAX) has been used safely for decades in areas of transmission, both in Africa and tropical South America. Additionally, YF-VAX is a travel-required vaccine as part of the World Health Organization (WHO) International Health Regulations (IHR) [10].
However, inadequate entomological and animal surveillance, especially in ecotones close to urban areas, constitutes a risk to non-human primates (NHP) and people as has occurred over the past few year in Sao Paulo and Mina Gerais States, Brazil. The low vaccination rate for YF among adults in these affected regions has been one of the determining factors for the expressive epidemic of YF in Brazil. Furthermore, the density and mobility of NHPs along with the intense human mobility during their viremic phase in areas infested by mosquitoes have blended with many other factors contributing to the expansion of the YF virus in Brazil, particularly its dissemination to the Atlantic coast, as observed in the current epidemic [11]. From July 2017-through-June 2018, the State of Minas Gerais was one of the most affected states and the one with the highest number of confirmed cases. Sao Paulo revealed the highest number of reported cases but was the second state when considering the total of confirmed cases. A similar situation occurred with some reported epizootic with Sao Paulo exhibiting the highest number of reported epizootics and Minas Gerais the highest with confirmed epizootics [12]. In Latin America, YF transmission embraces two cycles, sylvatic and urban, both have been reported recently in the region. In Africa, an additional third setting, the intermediate (savannah) cycle, is known to occur, involving transmission of virus from mosquitoes to humans living or working in jungle bordering areas. In this particular setting, the virus can be transmitted from monkey to human or from human to human via mosquitoes (https://www.cdc.gov/yellowfever/transmission/index.html).
The enzootic YF scenery in Latin America is very complex, and many of its ecological and biological aspects are yet not fully understood. Therefore, there is a pressing need to deepen and update many aspects related to the taxonomic knowledge, distribution and bioecology of sylvan vectors capitalizing on previous work [13,14], as well as to study in-depth the dynamics of vertebrate reservoirs, mainly non-human primates, along with other marsupials and rodents populations. The lack of vector competence studies [15] has been another factor affecting our ability to infer and predict potential outbreaks. Different genera of non-human primates have been incriminated as reservoirs of YF in the Americas, as is the case of species at the parvorder Platyrrhini (New World monkeys) (which include 4 families: Aotidae, Atelidae, Cebidae and Pitheciidae) (https://www.ncbi.nlm.nih.gov/Taxonomy/), as well as those from the genus Alouatta sp. (howler monkey), considered the most important reservoir due to its innate high susceptibility to the virus and its ability to move considerable distances. Other species involved in transmission cycles include Aotus sp. (night monkey), Saimiri sp. (squirrel monkey), and Ateles sp. (spider monkey or maquisapa) [16]. Studies assessing the various histopathological changes occurring in wild neotropical primates naturally infected with the YF virus have revealed striking clinical-pathological features including severe necrotizing hepatic lesions, as those observed in Alouatta sp. primates which exhibit a wide distribution in Venezuela [17].
Novel surveillance methods are required in order to assist tracking transmission routes, spatial expansion and viral spillover to humans [18]. Epidemiological and genomic surveillance of human and primate populations at risk are crucial for the early detection and rapid containment of YFV transmission [19]. Knowledge on the ecological interactions between environment, vectors and reservoir hosts can help predict spillover with high accuracy [20], thus paving the way to better understand both sylvatic and interhuman transmission cycles as well as their interfaces and help avoid future YF spillover events. It is imperative to strengthen and establish continuous surveillance programs of non-human primates and mosquitoes [21], in order to design preventive actions focused on reducing potential YF spillover events. In relation to New World non-human primates, one aspect to consider is the differential susceptibility to YF among different primates and how this influences viral load and overall mortality rates amongst these. Such information comes handy when designing strategic surveillance programs of epizootics and YF prevention and control measure [22].
Prevention of YF requires combined vector control strategies and broad immunization to protect individuals in response to outbreaks. The YF-VAX is administered as part of routine childhood immunization programs in some countries, as per recommendation by the WHO (https://www.who.int/es/news-room/fact-sheets/detail/immunization-coverage). However, despite it's proven effectiveness YF vaccination has been facing many hurdles, thus complicating the scenario for disease control in many endemic areas (i.e., Brazil). One such obstacle is the global shortage of vaccine YF-VAX supplies, which has also affected travelers at a domestic and international scale [[23], [24], [25]].
During recent outbreaks in Brazil [11,26,27] and Angola [28], vaccine shortages have had an important impact in disease control efforts. In addition, domestic and international travelers have been affected by the limited availability of the vaccine, with travel agencies not being able to provide adequate information regarding administration of the vaccine in endemic areas [29]. This factor may have influenced the occurrence of imported cases reported in travelers returning from endemic areas to North America, Europe, and China [30]. Also, the impact on travel health within the region has been significant. The occurrence of imported YF cases in South America could lead to potential urban outbreaks in various non-endemic areas due to geographic expansion of the vector, mostly favored by climate change [31]. This represents a challenge particularly for healthcare workers who should be kept up-to-date on the preventive measures and the changes in the levels of risk from tourism areas, which is variable and not always considered by travel medicine practitioners at the right time [10].
Fortunately, YF vaccination every ten years is no longer recommended for immune-competent travelers, based on an assumed life-long immunity conveyed by a single administration of the 17D YF vaccine. Following this paradigm change in yellow fever vaccination policy by the World Health Organization's Strategic Advisory Group of Experts (SAGE) on immunization in May 2013, WHO has now officially changed its YF vaccination policy with an International Health Regulation (IHR) rule which came into place in July 2016 [32]. Due to the shortage of doses necessary to meet the international health demand, some countries have implemented a fractional dose strategy to vaccinate more people as per the Pan American Health Organization (PAHO)/WHO recommendations. However, it is essential to point out that those people vaccinated with fractional doses should receive a full dose to fulfil with IHR when travelling to endemic/epidemic areas or visiting those countries requesting YF vaccination [33].
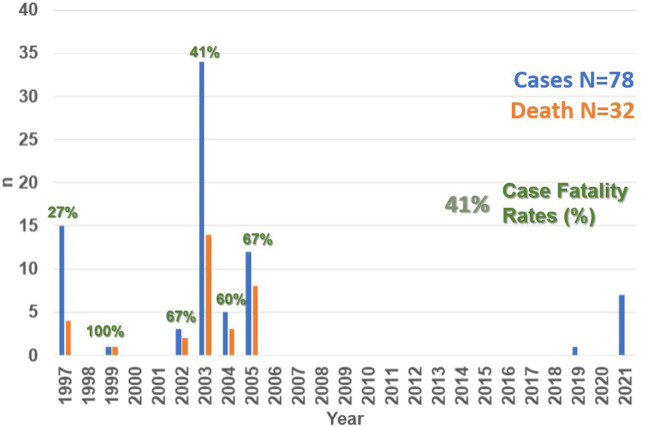
Venezuela is a well-known endemic area for YF, with recent epizootics occurring mainly in three foci, one adjacent to the border with Brazil, and the two other in neighboring areas with Colombia. Phylogenetic analysis of yellow fever virus (YFV) strains isolated from Venezuela strongly supports YFV enzootic maintenance in situ, with evidence of regionally independent evolution within the country. However, there is considerable YFV movement from Brazil to Venezuela as well as between Trinidad and Venezuela [34]. Between 1997 and 2005, 78 cases of YF had been confirmed in Venezuela, with a case fatality rate (CFR%) of approximately 41% (Fig. 1 ). A major outbreak of YF occurred in 2005 (Fig. 1) in rural areas with co-occurrence of Aedes aegypti and Haemagogus casting doubts on whether this was a typical or spurious jungle outbreak linked to Aedes aegypti [[35], [36], [37]]. Between 2002 and 2005, the states of Zulia, Táchira and Apure (bordering with Colombia) as well as Portuguesa, Mérida, Monagas, and Bolivar (bordering with Brazil) reported human cases of YF. Later, in 1999 a fatal case was reported in California in a returning US-traveler who had visited the Canaima National Park. Since 2005, no human cases of YF were documented in Venezuela until to 2019 (Fig. 1).
Fig. 1.
Trends in the number of cases and deaths of YF in Venezuela, 1997–2021.
In November 2019, the Venezuelan Society for Public Health alerted of a YF case on a 46-year-old Pemon Amerindian male from Kamarata, a community whithin the touristic Canaima National Park, in the state of Bolivar (southern Venezuela, border with Brazil), who was admitted to the “Ruiz y Páez” Hospital of Ciudad Bolivar in September 2019, severely ill, with a febrile ictero-haemorrhagic syndrome. The patient had developed acute renal failure, requiring dialysis, recovering and being discharged eight weeks later after resolution of symptoms. YF IgM-ELISA and RT-PCR results, performed by the Venezuelan National Institute of Health Rafael Rangel (NIH) in Caracas, Venezuela, returned positive confirming the diagnosis of YF [38].
Unfortunately, in the shadows of the COVID-19 pandemic [39], a new outbreak with seven confirmed YF cases was recently reported in October 2021 in Venezuela [40]. All of these cases were laboratory confirmed by nucleic acid amplification testing at the Venezuelan NIH in Caracas, Venezuela. Of the total of cases, three were asymptomatic (probably related to symptomatic cases), and four developed signs and symptoms during the epidemiological week (EW) 38 of 2021. Clinically, all of the symptomatic patients presented with fever and one presented with headache and ocular pain, among other symptoms. The probable site of infection for the confirmed cases was traced to the locality of Carapal, a rural parish south of the Maturín Municipality, in the state of Monagas in North Western Venezuela. The first reported case was a 16-year-old pregnant woman who suffered an abortion at the time of diagnosis (most probably related to YF). Of the remaining six cases, five were males with age-range between 24 and 82 years old, and no previous vaccination history [23]. To date, no death has been reported among the seven cases of this current outbreak (Fig. 1).
In this context, it is worth mentioning that between EW 32 and EW 39 of 2021, a total of 10 epizootic events were also recorded among NHP in Venezuela – seven in the state of Monagas and three in Anzoátegui. Two of the epizootic events reported in Monagas State were laboratory confirmed at the National Reference Laboratory, and eight were confirmed by epidemiological data and case definition. In addition, confirmed epizootics were identified at 35 km and 150 km from the major urban area of Maturín [40], the city capital of Monagas. Unfortunately, YF surveillance in populations at risk has been largely impacted by the absence of a robust epidemiological surveillance program and by the limited availability of laboratories to carry out molecular and/or immuno-serological assays for YF diagnostics in the country. This situation has scaled by the profound impact of the COVID-19 pandemic on the Venezuelan health system, which has led to emergence and reemergence of neglected of tropical diseases and a an underdiagnosis of cases shadowing the real impact of these diseases country wide.
This emerging situation followed recently after the Brazil and Peru outbreaks, has generated great concern in the region about the possible scenario of active circulation of YF in the Americas [11,23]. During the current monitoring period (July, 1 to September 27, 2021), a total of 17 suspected human cases of yellow fever were reported in Brazil, of which one was confirmed in the state of Pará, and two remain under investigation. The confirmed case was notified on July 21, in a 21-year-old man resident of Afua, Pará State [40]. Concerns about an YF outbreak in Brazil has regained importance, as new data on COVID-19 cases currently show a stable trend with over 108 million of the Brazilian population being fully vaccinated (2 doses) and around 48 million with a single dose. Nowadays, when internal tourism travel restrictions are being lifted, and with a YF vaccination coverage of <60%, much below the ideal rate for controlling circulation of the YFV, the risk of a new outbreak remains latent. This situation is even more likely to occur in high-risk areas where ecoturism is popular, like for example Santa Catarina were YF cases have already been reported.
In Peru, between EW 1 and EW 37 of 2021, a total of 14 YF cases were reported, ten of which were confirmed, and four catalogued as probable cases that remain under investigation. As recommended by the PAHO/WHO, member states with areas at risk for yellow fever should continue their efforts to immunize the at-risk populations and implement the necessary actions to keep travellers informed and vaccinated before visiting areas where yellow fever is endemic. Epidemiological surveillance at border sites and monitoring the population returning from high-risk area is pivotal. Recommendations for international travellers regarding yellow fever vaccination are available at: http://www.who.int/ith/en/, [40]. Clinicians should maintain a high level of suspicion when assessing returning travelers from high-risk areas exhibiting icterohemorrhagic signs and symptoms and take into consideration that the disease spectrum can vary from nonspecific symptoms to a fatal hemorrhagic disease. From a clinical standpoint, the disease is characterized by fever (lasting three days) that may be accompanied by malaise, conjuctival redness, headache, back pain, generalized myalgias, nausea, and dizziness. Notably, a highly suggestive physical examination finding to consider in YF affected patients is pulse-temperature dissociation and bradycardia. Common laboratory findings include leukopenia with neutropenia; before the onset of jaundice and elevated transaminases Often, fever may be accompanied by general malaise, headache, back pain, generalized myalgias, nausea, and dizziness, a critical sign to consider when examining the patient presents the pulse-temperature dissociation; the conjunctiva may be congested, and when examining the heart rhythm, bradycardia may be found. The fever can last three days; when carrying out a hemogram, we will find leukopenia with neutropenia; before the onset of jaundice, the transaminases are considerably elevated [41].
Yellow Fever along with other febrile icterohemorrhagic diseases like Leptospirosis, Malaria and Arboviruses should be kept top in the list of differential diagnosis [48], and recording of vaccination status within the anamnesis remains a key piece of information to establish diagnosis. In addition, the possibility of coinfection with COVID-19 should also be entertained, given the increasing reports of co-infection with other arboviral diseases, especially dengue [42]. In light of the current humanitarian crisis and forced migration of Venezuelan citizens to neighboring countries like Colombia and Brazil [41], and considering the vast border of Brazil with ten other neighboring countries (Argentina, Bolivia, French Guiana, Guyana, Paraguay, Peru, Suriname, Uruguay, and Venezuela), active surveillance for possible YF cases transiting across disease corridors is of utmost relevance in order to contain the possible spread of this re-emerging infection across the Americas, including the United States (mainly through imported cases), where currently there are no YF vaccination requirements for travelers from these regions.
Moreover, taking into account that co-circulation and co-infection of other arboviruses across the Colombian-Venezuelan border has been previously reported [43,44], areas of Colombia such as Norte de Santander and the Sierra Nevada de Santa Marta should be carefully monitored. Careful monitoring should be observed in cases of epizootics, which often occur first amongst indigenous communities and non-human primates, prompting active preventive measures by regional public health offices on immunizing vulnerable communities.
A call to for action is urged. From a public health perspective, it is unacceptable to observe cases and deaths related to YF in populations that live in places previously recognized as endemic areas. This situation requires urgent action to immunize at-risk populations in a short time frame. Countries such as Venezuela were YF vaccination coverage has been estimated to be 12.02% (only 550,325 administered doses out of 4,577,154 as the goal) require prompt intervention. Before the current outbreak, many states in Venezuela revealed lower vaccination coverage than the national average, particularly Monagas (currently affected state, 11%), Táchira (9%) and Zulia (4%), these two later bordering with Colombia. These scenarios should be translated into disease control actions, which should be intensified in these countries, emphasizing on strict surveillance of epizootics and intensification of vaccination in the region. Another issue we consider key for prevention is health education, by creating educational networking throughout digital sources (social media) targeting at-risk populations.
To date, most of the new threats Latin America has faced have been imported (COVID-19, CHIKV, and ZIKV) or re-emerged as is the case of YF. However, the impending threat of the emergence of a new pathogen in our region requires a different surveillance approach focused on strengthening multi-state surveillance networks and capitalizing on new genomic surveillance approaches [45]. Such approaches should be made accessible to low resource settings using a metagenomic strategies that allow multi-pathogen detection [46]; an example of such approach was revealed by the publication of the first SARS-CoV-2 sequence MN908947 on December 26, 2020 [47] opening a new era for genomic surveillance.
The current outbreak of YF in Venezuela calls for major public health interventions to protect people living in areas at-risk where recent cases were identified. The current situation in Venezuela with a growing number of cases of YF identified amid the COVID-19 pandemic requires urgent attention to improve immunization coverage and optimize surveillance of ictero-haemorrhagic diseases in populations at risk in a short time.
Conflicts of interest
Opinions expressed in this editorial are not the position of GSK, but of A. Lepetic and the rest of the authors. A. J. Rodriguez-Morales, report being medical advisor of Abbott Diagnostics, Amgen, Roche, and Takeda for Latin America, outside the submitted work. The rest of the authors declare no conflict of interest. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Acknowledgements
A. J. Rodriguez-Morales is the Editor in Chief of Travel Medicine and Infectious Diseases, President of the Colombian Association of Infectious Diseases (ACIN) and Vicepresident of the Latin American Society for Travel Medicine (SLAMVI). M. E. Figuera-Esparza is the President of the Venezuelan Society for Infectious Diseases (SVI). Tânia do Socorro Souza Chaves is the President of SLAMVI. We dedicate this article to the memory of Dr. Manuel Velasco Pernia, Professor of Pharmacology of the Universidad Central de Venezuela, Caracas, Venezuela, who died from COVID-19 in 2021. Also, to Dr. Angel Minguez, Infectious Diseases Specialist from Argentina, who also died in 2021.
References
- 1.Rodriguez-Morales A.J., Suarez J.A., Risquez A., Delgado-Noguera L., Paniz-Mondolfi A. The current syndemic in Venezuela: measles, malaria and more co-infections coupled with a breakdown of social and healthcare infrastructure. Quo vadis? Trav Med Infect Dis. 2019;27:5–8. doi: 10.1016/j.tmaid.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Suárez J.A., Carreño L., Paniz-Mondolfi A.E., Marco-Canosa F.J., Freilij H., Riera J.A., et al. Infectious diseases, social, economic and political crises, anthropogenic disasters and beyond: Venezuela 2019 – implications for public health and travel medicine. Rev Panam Enf Inf. 2018;1:73–93. [Google Scholar]
- 3.Grillet M.E., Hernandez-Villena J.V., Llewellyn M.S., Paniz-Mondolfi A.E., Tami A., Vincenti-Gonzalez M.F., et al. Venezuela's humanitarian crisis, resurgence of vector-borne diseases, and implications for spillover in the region. Lancet Infect Dis. 2019 doi: 10.1016/S1473-3099(18)30757-6. [DOI] [PubMed] [Google Scholar]
- 4.Paniz-Mondolfi A.E., Tami A., Grillet M.E., Marquez M., Hernandez-Villena J., Escalona-Rodriguez M.A., et al. Resurgence of vaccine-preventable diseases in Venezuela as a regional public health threat in the Americas. Emerg Infect Dis. 2019;25:625–632. doi: 10.3201/eid2504.181305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabrera M., Taylor G. Modelling spatio-temporal data of dengue fever using generalized additive mixed models. Spatial and Spatio-temporal Epidemiology. 2019;28:1–13. doi: 10.1016/j.sste.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Paniz-Mondolfi A.E., Blohm G., Pinero R., Rondon-Cadenas C., Rodriguez-Morales A.J. Venezuelan equine encephalitis: how likely are we to see the next epidemic? Trav Med Infect Dis. 2017;17:67–68. doi: 10.1016/j.tmaid.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Paniz-Mondolfi A.E., Rodriguez-Morales A.J., Blohm G., Marquez M., Villamil-Gomez W.E. ChikDenMaZika Syndrome: the challenge of diagnosing arboviral infections in the midst of concurrent epidemics. Ann Clin Microbiol Antimicrob. 2016;15:42. doi: 10.1186/s12941-016-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burki T. Re-emergence of neglected tropical diseases in Venezuela. Lancet Infect Dis. 2015;15:641–642. doi: 10.1016/S1473-3099(15)00011-0. [DOI] [PubMed] [Google Scholar]
- 9.Daniels J.P. Increasing malaria in Venezuela threatens regional progress. Lancet Infect Dis. 2018;18:257. doi: 10.1016/S1473-3099(18)30086-0. [DOI] [PubMed] [Google Scholar]
- 10.Reno E., Quan N.G., Franco-Paredes C., Chastain D.B., Chauhan L., Rodriguez-Morales A.J., et al. Prevention of yellow fever in travellers: an update. Lancet Infect Dis. 2020;20:e129–e137. doi: 10.1016/S1473-3099(20)30170-5. [DOI] [PubMed] [Google Scholar]
- 11.Chaves T., Orduna T., Lepetic A., Macchi A., Verbanaz S., Risquez A., et al. Yellow fever in Brazil: epidemiological aspects and implications for travelers. Trav Med Infect Dis. 2018;23:1–3. doi: 10.1016/j.tmaid.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Sacchetto L., Silva N.I.O., Rezende I.M., Arruda M.S., Costa T.A., de Mello E.M., et al. Neighbor danger: yellow fever virus epizootics in urban and urban-rural transition areas of Minas Gerais state, during 2017-2018 yellow fever outbreaks in Brazil. PLoS Neglected Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liria J., Navarro J.-C. Modelo de nicho ecológico en Haemagogus Williston (Diptera: Culicidae), vectores del virus de la fiebre amarilla. Rev Biomed. 2010;21:13. [Google Scholar]
- 14.Liria J., Navarro J.-C. Clave fotográfica para hembras de Haemagogus Williston 1896 (Diptera: Culicidae) de Venezuela, con nuevo registro para el país. Boletín de Malariología y Salud Ambiental. 2009;49:283–292. [Google Scholar]
- 15.Bryant J., Wang H., Cabezas C., Ramirez G., Watts D., Russell K., et al. Enzootic transmission of yellow fever virus in Peru. Emerg Infect Dis. 2003;9:926–933. doi: 10.3201/eid0908.030075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vasconcelos P.F., Sperb A.F., Monteiro H.A., Torres M.A., Sousa M.R., Vasconcelos H.B., et al. Isolations of yellow fever virus from Haemagogus leucocelaenus in Rio Grande do Sul state, Brazil. Trans R Soc Trop Med Hyg. 2003;97:60–62. doi: 10.1016/s0035-9203(03)90023-x. [DOI] [PubMed] [Google Scholar]
- 17.Santos D.O.D., de Oliveira A.R., de Lucena F.P., de Mattos S.A., de Carvalho T.P., Costa F.B., et al. Histopathologic patterns and susceptibility of neotropical primates naturally infected with yellow fever virus. Veterinary pathology. 2020;57:681–686. doi: 10.1177/0300985820941271. [DOI] [PubMed] [Google Scholar]
- 18.Stower H. Monitoring yellow fever. Nat Med. 2018;24 doi: 10.1038/s41591-018-0280-7. 1781-81. [DOI] [PubMed] [Google Scholar]
- 19.Faria N.R., Kraemer M.U.G., Hill S.C., Goes de Jesus J., Aguiar R.S., Iani F.C.M., et al. Genomic and epidemiological monitoring of yellow fever virus transmission potential. Science. 2018;361:894–899. doi: 10.1126/science.aat7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Childs M.L., Nova N., Colvin J., Mordecai E.A. Mosquito and primate ecology predict human risk of yellow fever virus spillover in Brazil. Philos Trans R Soc Lond B Biol Sci. 2019;374:20180335. doi: 10.1098/rstb.2018.0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abreu FVSd, Ferreira-de-Brito A., Azevedo AdS., Linhares J.H.R., de Oliveira Santos V., Hime Miranda E., et al. Survey on non-human primates and mosquitoes does not provide evidences of spillover/spillback between the urban and sylvatic cycles of yellow fever and zika viruses following severe outbreaks in southeast Brazil. Viruses. 2020;12:364. doi: 10.3390/v12040364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.de Azevedo Fernandes N.C.C., Guerra J.M., Diaz-Delgado J., Cunha M.S., Saad L.D., Iglezias S.D., et al. Differential yellow fever susceptibility in new World Nonhuman primates, comparison with humans, and implications for surveillance. Emerg Infect Dis. 2021;27:47–56. doi: 10.3201/eid2701.191220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortiz-Martinez Y., Patino-Barbosa A.M., Rodriguez-Morales A.J. Yellow fever in the Americas: the growing concern about new epidemics. F1000Res. 2017;6:398. doi: 10.12688/f1000research.11280.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elachola H., Ditekemena J., Zhuo J., Gozzer E., Marchesini P., Rahman M., et al. Yellow fever outbreaks, vaccine shortages and the Hajj and Olympics: call for global vigilance. Lancet. 2016;388:1155. doi: 10.1016/S0140-6736(16)31546-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Health O. WHO position on the use of fractional doses - June 2017, addendum to vaccines and vaccination against yellow fever WHO: position paper - June 2013. Vaccine. 2017;35:5751–5752. doi: 10.1016/j.vaccine.2017.06.087. [DOI] [PubMed] [Google Scholar]
- 26.Hamer D.H., Angelo K., Caumes E., van Genderen Pjj, Florescu S.A., Popescu C.P., et al. Fatal yellow fever in travelers to Brazil, 2018. MMWR Morb Mortal Wkly Rep. 2018;67:340–341. doi: 10.15585/mmwr.mm6711e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gossner C.M., Haussig J.M., de Bellegarde de Saint Lary C., Kaasik Aaslav K., Schlagenhauf P., Sudre B. Increased risk of yellow fever infections among unvaccinated European travellers due to ongoing outbreak in Brazil. Euro Surveill. 2018;23 doi: 10.2807/1560-7917.ES.2018.23.11.18-00106. July 2017 to March 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed Q.A., Memish Z.A. Yellow fever from Angola and Congo: a storm gathers. Trop Doct. 2017;47:92–96. doi: 10.1177/0049475517699726. [DOI] [PubMed] [Google Scholar]
- 29.Villanueva-Meyer P.G., Garcia-Jasso C.A., Springer C.A., Lane J.K., Su B.S., Hidalgo I.S., et al. Advice on malaria and yellow fever prevention provided at travel agencies in Cuzco, Peru. J Trav Med. 2015;22:26–30. doi: 10.1111/jtm.12149. [DOI] [PubMed] [Google Scholar]
- 30.Schlagenhauf P., Chen L.H. Yellow Fever importation to China - a failure of pre- and post-travel control systems? Int J Infect Dis. 2017;60:91–92. doi: 10.1016/j.ijid.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Liu-Helmersson J., Brannstrom A., Sewe M.O., Semenza J.C., Rocklov J. Estimating past, present, and future trends in the global distribution and abundance of the arbovirus vector Aedes aegypti under climate change scenarios. Front Public Health. 2019;7:148. doi: 10.3389/fpubh.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grobusch M.P., van Aalst M., Goorhuis A. Yellow fever vaccination - once in a lifetime? Trav Med Infect Dis. 2017;15:1–2. doi: 10.1016/j.tmaid.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 33.Vannice K., Wilder-Smith A., Hombach J. Fractional-dose yellow fever vaccination - advancing the evidence Base. N Engl J Med. 2018;379:603–605. doi: 10.1056/NEJMp1803433. [DOI] [PubMed] [Google Scholar]
- 34.Auguste A.J., Lemey P., Bergren N.A., Giambalvo D., Moncada M., Morón D., et al. Enzootic transmission of yellow fever virus, Venezuela. Emerg Infect Dis. 2015;21:99–102. doi: 10.3201/eid2101.140814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finol E., Berrueta E., Levy A., Añez F., Espina L.M., Maldonado M.B., et al. Retrospective evaluation of jungle yellow fever in Venezuela, during the Years 2003 - 2005. Kasmera. 2008;36:12. [Google Scholar]
- 36.Rifakis P.M., Benitez J.A., De-la-Paz-Pineda J., Rodriguez-Morales A.J. Epizootics of yellow fever in Venezuela (2004-2005): an emerging zoonotic disease. Ann N Y Acad Sci. 2006;1081:57–60. doi: 10.1196/annals.1373.005. [DOI] [PubMed] [Google Scholar]
- 37.Muñoz-Rodríguez M., Arrivillaga J., Navarro J.-C. Casos de Fiebre Amarilla en Portuguesa, Venezuela: ¿un brote selvático espurio? Rev Biomed. 2010;21:15. [Google Scholar]
- 38.Chavero M., Guevara O., Figuera L., Viera M., Sandoval-De Mora M., Carrión-Nessi F.S., et al. Re-emergence of yellow fever in Venezuela: report of the first case after 14 years. Trav Med Infect Dis. 2021;41:102025. doi: 10.1016/j.tmaid.2021.102025. [DOI] [PubMed] [Google Scholar]
- 39.Paniz-Mondolfi A.E., Sordillo E.M., Marquez-Colmenarez M.C., Delgado-Noguera L.A., Rodriguez-Morales A.J. The arrival of SARS-CoV-2 in Venezuela. Lancet. 2020;395:e85–e86. doi: 10.1016/S0140-6736(20)31053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.PAHO https://iris.paho.org/handle/10665.2/54999?locale-attribute=en. 2021 Epidemiological Update Yellow Fever - 6 October 2021.
- 41.Rodriguez-Morales A.J., Villamil-Gómez W.E. Yellow fever: still of concern for travelers of Colombia? Infectio. 2018;22:171–172. [Google Scholar]
- 42.Mejía-Parra J.L., Aguilar-Martinez S., Fernández-Mogollón J.L., Luna C., Bonilla-Aldana D.K., Rodriguez-Morales A.J., et al. Characteristics of patients coinfected with severe acute respiratory syndrome coronavirus 2 and dengue virus, Lambayeque, Peru. Trav Med Infect Dis. 2021;43:102132. doi: 10.1016/j.tmaid.2021.102132. May-August 2020: A retrospective analysis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrillo-Hernández M.Y., Ruiz-Saenz J., Villamizar L.J., Gómez-Rangel S.Y., Martínez-Gutierrez M. Co-circulation and simultaneous co-infection of dengue, chikungunya, and zika viruses in patients with febrile syndrome at the Colombian-Venezuelan border. BMC Infect Dis. 2018;18:1–12. doi: 10.1186/s12879-018-2976-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carrillo-Hernandez M.Y., Ruiz-Saenz J., Jaimes-Villamizar L., Robledo-Restrepo S.M., Martinez-Gutierrez M. Phylogenetic and evolutionary analysis of dengue virus serotypes circulating at the Colombian–Venezuelan border during 2015–2016 and 2018–2019. PLoS One. 2021;16 doi: 10.1371/journal.pone.0252379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gardy J.L., Loman N.J. Towards a genomics-informed, real-time, global pathogen surveillance system. Nat Rev Genet. 2018;19:9–20. doi: 10.1038/nrg.2017.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Govender K.N., Street T.L., Sanderson N.D., Eyre D.W. Metagenomic sequencing as a pathogen-agnostic clinical diagnostic tool for infectious diseases: a systematic review and meta-analysis of diagnostic test accuracy studies. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.02916-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musso D., Rodriguez-Morales A.J., Levi J.E., Cao-Lormeau V.M., Gubler D.J. Unexpected outbreaks of arbovirus infections: lessons learned from the Pacific and tropical America. Lancet Infect Dis. 2018;18(11):e355–e361. doi: 10.1016/S1473-3099(18)30269-X. [DOI] [PubMed] [Google Scholar]