Abstract
Background and aims
The COVID-19 pandemic has turned the world topsy turvy since its emergence and has claimed innumerable lives worldwide. Neurological manifestations of the disease have raised several eyebrows around the world among which Guillain-Barré syndrome (GBS) deserve special mention. Although majority of the cases of the coronavirus disease 2019 (COVID-19) present with respiratory symptoms, extrapulmonary manifestations are being increasingly reported. We conducted this study to analyze detailed clinical presentations and outcome in a series of eight cases (n = 8) with COVID-19 associated GBS.
Methods
An observational prospective study was conducted among patients with post-infectious/para-infectious GBS. 8 patients were subclassified into acute inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy (AMAN) and acute motor and sensory axonal neuropathy (AMSAN) as per electrodiagnostic criteria and were followed up from admission to 6 months post discharge, to obtain a comprehensive clinical profile and outcome in these patients.
Results
The diagnosis of GBS was confirmed as per Asbury criteria, supported by electrodiagnostic features in nerve conduction velocity test. Among the series of 8 patients, 3 were diagnosed as AIDP, 3 had AMAN and the remaining 2 patients had AMSAN. 3 patients of GBS were afebrile and were diagnosed as COVID-19 after a positive assay on routine screening. Cerebro-spinal fluid analysis for SARS-Cov-2 RT-PCR and serum anti-ganglioside antibodies were negative in all the patients.
Conclusion
GBS in patients with COVID-19 should be differentiated from critical illness neuropathy and myopathy. Early diagnosis is important as it is associated with poor outcome and prolonged invasive ventilation.
Keywords: COVID-19, Guillain-barré syndrome, Acute inflammatory demyelinating polyradiculoneuropathy, Acute motor axonal neuropathy, Acute motor and sensory axonal neuropathy
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has wreaked havoc globally and has claimed over 4 million lives as of August 31, 2021; the deaths attributed to its growing range of complications [1]. It was declared a pandemic on March 11, 2020. Apart from the characteristic respiratory illness, COVID-19 has been associated with several extrapulmonary manifestations and complications [2]. In this current pandemic, neurological complications have emerged as a substantial source of morbidity and mortality among patients. Multiple studies have narrated the neurotropic and neuroinvasive potential of this virus. The neurological manifestations vary from non-specific symptoms like headache, dizziness, altered mentation, and ataxia to severe syndromes, like acute cerebrovascular accidents, meningoencephalitis, epilepsy and CNS neuro-immunological disorders [3,4].
Guillain-Barré syndrome (GBS) is an acute-onset, immune mediated polyradiculoneuropathy that may affect sensory, motor and autonomic nerves. The common variants of GBS are: acute inflammatory demyelinating polyradiculoneuropathy (AIDP) which predominantly a demyelinating disorder; acute motor axonal neuropathy (AMAN) which mainly affects axons of motor nerves; and acute motor and sensory axonal neuropathy (AMSAN), which is an axonal disorder involving both sensory and motor nerves [5]. Other uncommon variants include: Miller Fisher Syndrome (MFS), Bickerstaff brainstem encephalitis (BBE), paraparetic GBS, bilateral facial palsy with paresthesia (BFP), polyneuritis cranialis and acute dysautonomia [6].
GBS presents with a wide gamut of neurological manifestations, the most dangerous being rapidly progressive acute flaccid paralysis. Respiratory failure being a common complication of COVID-19, often creates a diagnostic dilemma in co-existent GBS. Therefore, it is critically important for the clinicians to diagnose and treat GBS early in patients with COVID-19 [7].
Infection and re-infection by Campylobacter jejuni, human herpes virus, CMV and EBV account for about 20–30% of the cases. Other viruses like HIV, Zika, H1N1 and Hepatitis-E are also implicated as the causative agents, besides recent immunizations [5,6]. Since the outbreak of the pandemic, the incidence of GBS has increased. There have been many reports describing the association between SARS-CoV-2 infection and GBS.
We planned this study to document the clinical characteristics, responses to treatment and outcome in a series of 8 patients with COVID-19 associated Guillain-Barré syndrome (GBS).
2. Materials and methods
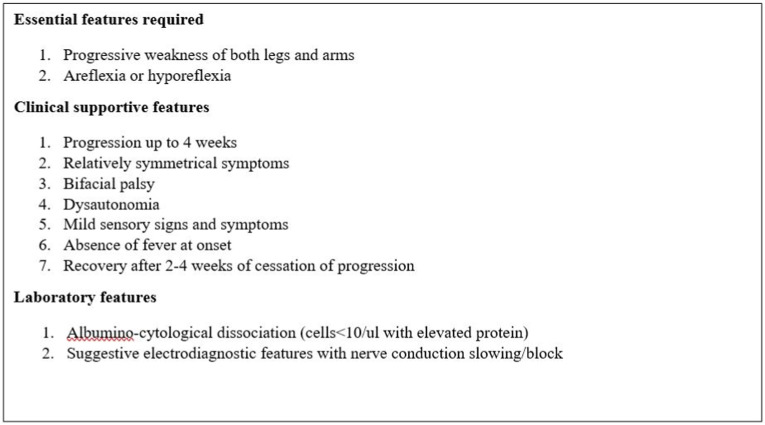
Out of 136 patients, admitted with GBS from July 2020 to June 2021, 8 patients with para-infectious/post-infectious COVID-19 associated GBS were selected after adequate ethical clearance. Patients with pre-existing neurological illness, neurological manifestations following other infections or vaccination were excluded from our study. Detailed neurological history was obtained and examination was done as per the prefixed proforma. Patients were investigated with cerebrospinal fluid analysis (CSF), nerve conduction studies (NCS) and other specialized investigations as per clinical context. The diagnosis of GBS was confirmed as per Asbury criteria [8], supported by electrodiagnostic features in nerve conduction velocity test (Fig. 1 ). The variants of GBS (acute inflammatory demyelinating polyneuropathy, acute motor axonal neuropathy, acute motor and sensory axonal neuropathy) were described on the basis of electrodiagnostic findings. A multi-disciplinary approach was taken and the patients were managed accordingly in consultation with department of Critical Care Medicine and Physical Medicine and Rehabilitation department. They were followed-up for 6 months to assess outcome. As cases are heterogeneous and sample size is also small for statistical analysis, disease groups with special emphasis on a few individual cases are being discussed.
Fig. 1.
Diagnostic criteria for GBS (Asbury criteria).
3. Results
In this series, the mean age of distribution was 47.62 years, with only 1 female patient (12.5%). Among the variants of GBS, 3 cases (37.5%) were diagnosed as AIDP, 3 cases (37.5%) were diagnosed as AMAN and the remaining 2 patients (25%) had AMSAN. 3 patients of GBS were afebrile and were diagnosed with COVID-19 after a positive SARS-Cov-2 reverse transcription-polymerase chain reaction (RT-PCR) assay on routine screening. CSF analysis was done in 7 patients and SARS-Cov-2 RT-PCR in CSF was negative in all of them. Ganglioside antibodies were negative in all 8 patients. The mean Erasmus GBS respiratory insufficiency score (EGRIS) on admission was 3.75 and the mean modified Erasmus GBS Outcome Score (mEGOS) on admission was 6.5. All patients were initiated on intravenous immunoglobulin (IVIG) 400 mg/kg/day for 5 days (Table 1 ).
Table 1.
Comparison of clinical profile among the variants of Covid-19 associated GBS.
| Variant | Case | Age (years) | Latency of weakness from onset of fever (days) | Progression of weakness | Cranial nerve involvement | Bulbar dysfunction | Dysautonomia | EGRIS | CSF findings |
|---|---|---|---|---|---|---|---|---|---|
| AIDP | 1 | 39, Male | 7 | Ascending | Nil | Nil | Present | 3 | Albumino-cytological dissociation |
| 2 | 44, Male | 14 | Ascending | Bilateral facial palsy | Present | Absent | 6 | Not obtained | |
| 3 | 40, Male | 5 | Ascending | Bilateral facial palsy | Nil | Absent | 5 | Albumino-cytological dissociation | |
| AMSAN | 1 | 54, Male | Afebrile | Ascending | Nil | Nil | Present | 5 | Normal |
| 2 | 36, Female | 12 | Ascending | Nil | Nil | Absent | 2 | Albumino-cytological dissociation | |
| AMAN | 1 | 42, Male | Afebrile | Descending | Nil | Nil | Present | 4 | Albumino-cytological dissociation |
| 2 | 75, Male | 18 | Ascending | Unilateral facial palsy | Nil | Present | 2 | Albumino-cytological dissociation | |
| 3 | 51, Male | Afebrile | Ascending | Nil | Nil | Absent | 3 | Albumino-cytological dissociation |
AIDP: Acute inflammatory demyelinating polyradiculoneuropathy, AMSAN: Acute motor and sensory axonal neuropathy, AMAN: Acute motor axonal neuropathy, EGRIS: Erasmus GBS Respiratory Insufficiency Score, CSF: Cerebrospinal fluid.
AIDP: 2 patients of AIDP (Case 1, 3) had a latency period of 7 days and 5 days from onset of weakness and fever were also diagnosed positive for SARS-Cov-2, while 1 patient (Case 2) had history of antecedent infection. 2 patients had bifacial palsy, of which 1 (Case 2) had severe bulbar involvement; dysautonomia was recorded in 1 patient (Case 2) and he required invasive mechanical ventilation, but succumbed within hours of admission despite our best efforts.
AMAN: 2 patients were afebrile and was diagnosed with COVID-19 on routine screening after admission, while 1 patient (Case 2) had history of antecedent COVID-19 infection. The later also had a facial palsy with dysautonomia. 1 patient (Case 1) had a descending type of paresis starting from his proximal upper limbs and progressing downwards with subsequent autonomic dysfunction. 2 patients (Case 1,3) with EGRIS 4 and 3 respectively, required invasive mechanical ventilation.
AMSAN: 1 patient (Case 1) was afebrile and had severe dysautonomia, without albumino-cytological dissociation in CSF analysis; however, he required invasive mechanical ventilation and succumbed to multiple organ dysfunction syndrome (MODS) as a consequence of sepsis.
4. Discussion
GBS is a rare disease of the peripheral nervous system with an annual incidence of 1.11 per 100,000 [9]. Since the outbreak of the COVID-19 pandemic, the incidence of GBS has increased significantly [10]. There have been many reports describing the association between SARS-CoV-2 infection and GBS. GBS and SARS-CoV-2 have been linked in COVID-19 individuals, according to studies.
A number of mechanisms have been proposed to explain the pathogenesis post-COVID-19 GBS, which include systemic inflammation and immune dysregulation [11]. The most accepted mechanism is the formation of antibodies against surface glycoproteins of the pathogen which may damage peripheral nerves due to a similar native protein structure (molecular mimicry). Following infection, SARS-COV-2 attaches to the cell surfaces via the viral spike (S) protein, which then binds to the angiotensin-converting enzyme-2(ACE2) receptor in the capillary endothelium and gangliosides containing sialic acid residue (including the GalNAc residue of GM1). Due to the structural resemblance of the spike glycoproteins or epitopes of SARS-Cov-2 with gangliosides, antibodies generated against the virus may bind the gangliosides found on peripheral neurons via T cell-B cell interactions. These autoantibodies bind to nodes of Ranvier and induce local complement mediated damage leading to formation of membrane attack complex (MAC), resulting in subsequent Wallerian degeneration [12]. Other proposed theories include hyperinflammation as a consequence of cytokine storm in patients with COVID-19 [13].
Antiganglioside autoantibodies of the IgG type are found in 50% of GBS patients, and their presence confirms the diagnosis. Antiganglioside antibodies are more strongly linked to the AMAN subtype than the AIDP subtype. In GBS and its variants, autoantibodies may target ganglioside complexes, with GD1a/GD1b being one of the most common, especially when cranial nerves are affected. Anti-ganglioside antibody testing could also help researchers better understand other, more common neurological symptoms associated with SARS-CoV-2 [14]. However, anti-GM1 antibodies were negative in all our cases.
In AMAN and AMSAN, myelin sheath is relatively intact as the macrophages tend to invade the space between the Schwann cell and axon, leaving the myelin sheath intact. The AMSAN variant has a relatively slower recovery with higher prevalence of dysautonomia, when compared to AMAN [15,16]. Attachment of SARS-CoV-2 virus to cell surfaces, mediated by the spike (S) protein, which binds to gangliosides containing sialic acid residues has been hypothesized as a probable mechanism [17]. Alternatively, T-cell activation followed by release of inflammatory mediators by macrophages maybe another plausible mechanism, supported by a multisystem inflammatory syndrome leading to para-infectious GBS [18].
For analysis, the patients in our study were divided into three groups: AIDP, AMSAN, and AMAN for sub-analysis of specific variables. The incidence of GBS was 0.42% in a large Italian study of 1200 patients admitted with SARS-CoV-2, substantially higher than the normal population [18]. GBS has been identified as a rapidly increasing neurological complication in COVID-19 patients in recentliterature. AIDP and AMAN was the most common GBS variants associated with COVID-19 in our study, which slightly differs from the literature in general [19]. In most of the previous studies, AIDP was found to be the commonest variant among all the subtypes of GBS associated with COVID-19. Among the three variants, both AIDP and AMAN were found in 37.5% of GBS cases. The mean age of distribution in our series was 47.62 years, with only one female patient affected.
In recent articles, a wide variation in the latency interval has been documented between the onset of GBS symptoms and the onset of COVID-19 [18,20]. Caress et al. found an average of 11 days between the onset of COVID symptoms and the appearance of GBS in a previous investigation [21]. The mean time between COVID-19 infection and GBS manifestation among different subtypes in this review was 11–13 days. GBS had also been reported in SARS-COV-2 RT PCR positive people who had no prior symptoms suggestive of Covid-19 [22,23]. Zhao et al. also reported a case where there was no latent period as the diagnosis of GBS-like neurological manifestation preceded the diagnosis of Covid-19, which was detected post-hospitalization [24]. This latent period between the emergence of COVID-19 signs and GBS symptoms provides valuable information regarding the pathogenesis of GBS in COVID-19 infection.
In our series, 2 AIDP patients tested positive for SARS-Cov-2 after a latency period of 7 days and 5 days from onset of weakness and fever, respectively, while 1 patient had a history of antecedent infection. Also, 2 patients were afebrile at the time of admission and were diagnosed with COVID-19 on routine screening, whereas 1 patient had history of antecedent COVID-19 infection.
Surprisingly, none of our patients had a positive PCR for SARS-CoV-2 in their CSF, suggestive of an immune-mediated mechanism in the pathogenesis of GBS in Covid-19. It is unclear whether this immune-mediated process is caused by molecular mimicry in the peripheral immune system or by the release of antigens in the peripheral immune system, thus commencing an autoimmune process [25,26]. Elevated CSF protein is a well-known crucial biomarker that can be used to determine the severity and extent of the disease in addition to the clinical examination [27].
Albumino-cytological dissociation was noted in 6 patients (75%), of which 2 had AIDP (33%), 1 had AMSAN (17%), and all 3 had AMAN (50%). The mean CSF albumin in our study was 106.58 mg/dl.
The EGRIS is a clinical model to predict the probability of respiratory insufficiency within the first week of admission in GBS. In this study, the EGRIS corroborated to an extent with requirement of invasive ventilation in the patients, although a patient with relatively high score (EGRIS-6) did not require mechanical ventilation, compared to a patient with as low as 5% probability of respiratory insufficiency (EGRIS-2).
The modified Erasmus GBS Outcome Score (mEGOS) is a critical prognostic indicator that aids in prediction of long-term outcomes of patients based on their clinical presentation on day 7 of admission. As a result, the higher the score, the more likely the patient will be unable to walk independently at 6 months following admission. Multiple cohort studies in GBS patients have demonstrated that this score has a significant predictive value [28]. In this study, the lowest mEGOS was 4 (Case 2, AIDP) while higher mEGOS was noted in AMAN, AMSAN subtypes. The outcome of ability to walk unaided at 4 weeks, 3 months and 6 months corroborated with mEGOS, suggesting that axonal variants of GBS have a relatively poor recovery compared to the demyelinating variant (Table 2 ).
Table 2.
Comparison of treatment and outcome among the patients with Covid-19 associated GBS.
| Variant | Case | Treatment received | Invasive ventilation | mEGOS (day 7 of admission) | Outcome | mRC Sum score on discharge |
|---|---|---|---|---|---|---|
| AIDP | 1 | IvIG | No | 6 | Discharged | 36 |
| 2 | IvIG | No | 4 | Discharged | 52 | |
| 3 | IvIG | Yes | 8 | Succumbed | N/A | |
| AMSAN | 1 | IvIG | Yes | 7 | Succumbed | N/A |
| 2 | IvIG | No | 6 | Discharged | 28 | |
| AMAN | 1 | IvIG | Yes | 8 | Discharged | 20 |
| 2 | IvIG | No | 6 | Discharged | 42 | |
| 3 | IvIG | Yes | 7 | Discharged | 32 |
AIDP: Acute inflammatory demyelinating polyradiculoneuropathy, AMSAN: Acute motor and sensory axonal neuropathy, AMAN: Acute motor axonal neuropathy, IvIG: intravenous immunoglobulin, mEGOS: modified Erasmus GBS outcome score, mRC: Medical Research Council.
Moreover, critical illness neuropathy and myopathy may mimic GBS in patients with prolonged stay in a critical care unit, thus differentiation between these entities may be difficult at times. Recent reports of GBS after vaccination against COVID-19 have surfaced up in literature [29,30]. However, evidence is limited to anecdotal reports or sparse case scenarios.
5. Conclusion
This study is first of its kind from Eastern India. The limitations include a small sample size, which may not be representative of a broader perspective. All patients were initiated on IVIG and none was initiated on plasmapheresis, hence no comparison could be drawn regarding outcome between two standard therapeutic options. It was not possible to determine whether 2 patients succumbed to COVID-19 and its complications or GBS per se. The patients were followed from admission to 6 months post discharge, thus providing a comprehensive outlook on the clinical profile and outcome of these patients. However, a multicentric study with a larger sample size is required to establish the relationship of COVID-19 and GBS, explore the pathophysiology and a long-term follow-up is required to obtain data regarding outcome in these patients.
Contributorship statement
All the authors contributed to conception, initial drafting of manuscript, critical revision of content and final approval of manuscript. Authors have testified that all persons designated as authors qualify for authorship and have checked the article for plagiarism. All authors had substantial contributions to the conception or design of the work; the acquisition, analysis, or interpretation of the data; drafting the work or revising it critically for important intellectual content, and final approval of the version to be published. All agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not for-profit sectors.
What is known?
Covid-19 has presented with various extrapulmonary manifestations, of which Guillain-Barré syndrome (GBS) has been reported considerably in literature. GBS is known to be associated with infective etiologies, likewise an association with Covid-19 and parainfectious/postinfectious GBS have been in the limelight for a considerable duration.
What is new?
In this study, the authors have provided a comprehensive outlook from clinical presentation to outcome in 8 cases of parainfectious/postinfectious GBS. Among the variants of GBS, the authors found uniform distribution among AIDP, AMAN, AMSAN.
Declaration of competing interest
No, there are no competing interests for any author.
Acknowledgement
Nil.
References
- 1.Lai C.C., Shih T.P., Ko W.C., Tang H.J., Hsueh P.R. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): the epidemic and the challenges. Int J Antimicrob Agents. 2020 Mar;55(3) doi: 10.1016/j.ijantimicag.2020.105924. Epub 2020 Feb 17. PMID: 32081636; PMCID: PMC7127800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai C.C., Ko W.C., Lee P.I., Jean S.S., Hsueh P.R. Extra-respiratory manifestations of COVID-19. Int J Antimicrob Agents. 2020 Aug;56(2) doi: 10.1016/j.ijantimicag.2020.106024. Epub 2020 May 22. PMID: 32450197; PMCID: PMC7243791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montalvan V., Lee J., Bueso T., De Toledo J., Rivas K. Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg. 2020 Jul;194 doi: 10.1016/j.clineuro.2020.105921. Epub 2020 May 15. PMID: 32422545; PMCID: PMC7227498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whittaker A., Anson M., Harky A. Neurological Manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand. 2020 Jul;142(1):14–22. doi: 10.1111/ane.13266. Epub 2020 Jun 2. PMID: 32412088; PMCID: PMC7273036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughes R.A., Cornblath D.R. Guillain-Barré syndrome. Lancet. 2005 Nov 5;366(9497):1653–1666. doi: 10.1016/S0140-6736(05)67665-9. PMID: 16271648. [DOI] [PubMed] [Google Scholar]
- 6.van den Berg B., Walgaard C., Drenthen J., Fokke C., Jacobs B.C., van Doorn P.A. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014 Aug;10(8):469–482. doi: 10.1038/nrneurol.2014.121. Epub 2014 Jul 15. PMID: 25023340. [DOI] [PubMed] [Google Scholar]
- 7.Finsterer J., Scorza F.A. Guillain-Barré syndrome in 220 patients with COVID-19. Egypt J Neurol Psychiatr Neurosurg. 2021;57(1):55. doi: 10.1186/s41983-021-00310-7. Epub 2021 May 4. PMID: 33967575; PMCID: PMC8094972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asbury A.K., Cornblath D.R. Assessment of current diagnostic criteria for Guillain-Barré syndrome. Ann Neurol. 1990;27(Suppl):S21–S24. doi: 10.1002/ana.410270707. PMID: 2194422. [DOI] [PubMed] [Google Scholar]
- 9.Asiri S., Altwaijri W.A., Ba-Armah D., Al Rumayyan A., Alrifai M.T., Salam M., Almutairi A.F. Prevalence and outcomes of Guillain-Barré syndrome among pediatrics in Saudi Arabia: a 10-year retrospective study. Neuropsychiatric Dis Treat. 2019 Mar 1;15:627–635. doi: 10.2147/NDT.S187994. PMID: 30880987; PMCID: PMC6400135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abu-Rumeileh S., Abdelhak A., Foschi M., Tumani H., Otto M. Guillain-Barré syndrome spectrum associated with COVID-19: an up-to-date systematic review of 73 cases. J Neurol. 2021 Apr;268(4):1133–1170. doi: 10.1007/s00415-020-10124-x. Epub 2020 Aug 25. PMID: 32840686; PMCID: PMC7445716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan F., Sharma P., Pandey S., Sharma D., V V, Kumar N., Shukla S., Dandu H., Jain A., Garg R.K., Malhotra H.S. COVID-19-associated Guillain-Barré syndrome: postinfectious alone or neuroinvasive too? J Med Virol. 2021 Oct;93(10):6045–6049. doi: 10.1002/jmv.27159. Epub 2021 Jul 6. PMID: 34170552; PMCID: PMC8426929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kajumba M.M., Kolls B.J., Koltai D.C., Kaddumukasa M., Kaddumukasa M., Laskowitz D.T. COVID-19-Associated guillain-barré syndrome: atypical para-infectious profile, symptom overlap, and increased risk of severe neurological complications. SN Compr Clin Med. 2020 Nov 21:1–13. doi: 10.1007/s42399-020-00646-w. Epub ahead of print. PMID: 33251483; PMCID: PMC7680081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teuwen L.A., Geldhof V., Pasut A., Carmeliet P. COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020 Jul;20(7):389–391. doi: 10.1038/s41577-020-0343-0. Erratum in: Nat Rev Immunol. 2020 Jun 4;: PMID: 32439870; PMCID: PMC7240244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutillo G., Saariaho A.H., Meri S. Physiology of gangliosides and the role of antiganglioside antibodies in human diseases. Cell Mol Immunol. 2020;17:313–322. doi: 10.1038/s41423-020-0388-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vucic S., Kiernan M.C., Cornblath D.R. Guillain-Barré syndrome: an update. J Clin Neurosci. 2009;16:733–741. doi: 10.1016/j.jocn.2008.08.033. [PubMed] [Google Scholar] [DOI] [PubMed] [Google Scholar]
- 16.Yuki N., Kuwabara S., Koga M., Hirata K. Acute motor axonal neuropathy and acute motor-sensory axonal neuropathy share a common immunological profile. J Neurol Sci. 1999;168:121–126. doi: 10.1016/s0022-510x(99)00180-x. [DOI] [PubMed] [Google Scholar]
- 17.Dalakas M.C. Guillain-Barré syndrome: the first documented COVID-19-triggered autoimmune neurologic disease: more to come with myositis in the offing. Neurol Neuroimmunol Neuroinflamm. 2020 Jun 9;7(5) doi: 10.1212/NXI.0000000000000781. PMID: 32518172; PMCID: PMC7309518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toscano G., Palmerini F., Ravaglia S., Ruiz L., Invernizzi P., Cuzzoni M.G., Franciotta D., Baldanti F., Daturi R., Postorino P., Cavallini A., Micieli G. Guillain-barré syndrome associated with SARS-CoV-2. N Engl J Med. 2020 Jun 25;382(26):2574–2576. doi: 10.1056/NEJMc2009191. Epub 2020 Apr 17. PMID: 32302082; PMCID: PMC7182017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sriwastava S., Kataria S., Tandon M., Patel J., Patel R., Jowkar A., Daimee M., Bernitsas E., Jaiswal P., Lisak R.P. Guillain Barré Syndrome and its variants as a manifestation of COVID-19: a systematic review of case reports and case series. J Neurol Sci. 2021 Jan 15;420 doi: 10.1016/j.jns.2020.117263. Epub 2020 Dec 9. PMID: 33321326; PMCID: PMC7725056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sedaghat Z., Karimi N. Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci. 2020 Jun;76:233–235. doi: 10.1016/j.jocn.2020.04.062. Epub 2020 Apr 15. PMID: 32312628; PMCID: PMC7158817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caress J.B., Castoro R.J., Simmons Z., Scelsa S.N., Lewis R.A., Ahlawat A., Narayanaswami P. COVID-19-associated Guillain-Barré syndrome: the early pandemic experience. Muscle Nerve. 2020 Oct;62(4):485–491. doi: 10.1002/mus.27024. Epub 2020 Aug 11. PMID: 32678460; PMCID: PMC7405390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheidl E., Canseco D.D., Hadji-Naumov A., Bereznai B. Guillain-Barré syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. 2020 Jun;25(2):204–207. doi: 10.1111/jns.12382. Epub 2020 May 26. PMID: 32388880; PMCID: PMC7273104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chan J.L., Ebadi H., Sarna J.R. Guillain-barré syndrome with facial diplegia related to SARS-CoV-2 infection. Can J Neurol Sci. 2020 Nov;47(6):852–854. doi: 10.1017/cjn.2020.106. Epub 2020 May 29. PMID: 32468972; PMCID: PMC7308598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao H., Shen D., Zhou H., Liu J., Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020 May;19(5):383–384. doi: 10.1016/S1474-4422(20)30109-5. Epub 2020 Apr 1. PMID: 32246917; PMCID: PMC7176927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ottaviani D., Boso F., Tranquillini E., Gapeni I., Pedrotti G., Cozzio S., Guarrera G.M., Giometto B. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol Sci. 2020 Jun;41(6):1351–1354. doi: 10.1007/s10072-020-04449-8. Epub 2020 May 12. PMID: 32399950; PMCID: PMC7216127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Assini A., Benedetti L., Di Maio S., Schirinzi E., Del Sette M. New clinical manifestation of COVID-19 related Guillain-Barrè syndrome highly responsive to intravenous immunoglobulins: two Italian cases. Neurol Sci. 2020 Jul;41(7):1657–1658. doi: 10.1007/s10072-020-04484-5. Epub 2020 May 28. Erratum in: Neurol Sci. 2020 Jun 8;: PMID: 32468450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brettschneider J., Petzold A., Süssmuth S., Tumani H. Cerebrospinal fluid biomarkers in Guillain-Barré syndrome--where do we stand? J Neurol. 2009 Jan;256(1):3–12. doi: 10.1007/s00415-009-0097-x. Epub 2009 Feb 16. PMID: 19267167. [DOI] [PubMed] [Google Scholar]
- 28.Walgaard C., Lingsma H.F., Ruts L., van Doorn P.A., Steyerberg E.W., Jacobs B.C. Early recognition of poor prognosis in Guillain-Barré syndrome. Neurology. 2011 Mar 15;76(11):968–975. doi: 10.1212/WNL.0b013e3182104407. PMID: 21403108; PMCID: PMC3059137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McKean N., Chircop C. Guillain-Barré syndrome after COVID-19 vaccination. BMJ Case Rep. 2021 Jul 30;14(7) doi: 10.1136/bcr-2021-244125. PMID: 34330729; PMCID: PMC8327820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shapiro Ben David S., Potasman I., Rahamim-Cohen D. Rate of recurrent guillain-barré syndrome after mRNA COVID-19 vaccine BNT162b2. JAMA Neurol. 2021 Sep 1 doi: 10.1001/jamaneurol.2021.3287. Epub ahead of print. PMID: 34468703; PMCID: PMC8411356. [DOI] [PMC free article] [PubMed] [Google Scholar]