Abstract
The current focus for many researchers has turned to the development of therapeutics that have the potential for serving as broad-spectrum inhibitors that can target numerous viruses, both within a particular family, as well as to span across multiple viral families. This will allow us to build an arsenal of therapeutics that could be used for the next outbreak. In that regard, nucleosides have served as the cornerstone for antiviral therapy for many decades. As detailed herein, many nucleosides have been shown to inhibit multiple viruses due to the conserved nature of many viral enzyme binding sites. Thus, it is somewhat surprising that up until very recently, many researchers focused more on “one bug one drug,” rather than trying to target multiple viruses given those similarities. This attitude is now changing due to the realization that we need to be proactive rather than reactive when it comes to combating emerging and reemerging infectious diseases. A brief summary of prominent nucleoside analogues that previously exhibited broad-spectrum activity and are now under renewed interest, as well as new analogues, that are currently under investigation against SARS-CoV-2 and other viruses is discussed herein.
Keywords: Nucleosides, Broad-spectrum, Antiviral, SARS-CoV-2, Pandemic
1. Introduction
Nucleosides have long served as the primary scaffold for treating many diseases, including those caused by cancers, parasites, bacteria, but most notably, against many different families of viruses.1, 2, 3, 4 Because the naturally occurring nucleosides are the building blocks found in our DNA and RNA, and also play critical roles in numerous biological processes, even small modifications to the nucleoside scaffold can have profound therapeutic effects. This observation provides impetus for designing modified nucleoside analogues. Modifications to their structure can be designed in and/or refined, based on the key interactions identified in the binding site of target enzymes. As the field has progressed and new information has become available about nucleoside structure, enzyme recognition, and biological activity, unique and more complex modifications have been pursued, including multiple modifications on the same scaffold.2, 3, 4 As a result of these modifications, there are currently more than 30 nucleoside/tide analogues on the market approved for use in treating viruses, cancers, parasites, as well as bacterial and fungal infections, with many more in preclinical and clinical trials.1, 2, 3, 4
In that regard, we now find ourselves in the middle of a global pandemic caused by the deadly coronavirus, SARS-CoV-2, the zoonotic virus responsible for COVID-19. Never has it been more urgent for researchers to revisit old nucleosides, as well as to explore new and more novel possibilities.5, 6, 7 In addition, given the strong likelihood that this will not be the last pandemic we face, given the number of zoonotic and other diseases circulating globally, it is critical that we find new ways to target future outbreaks.8 As a result, the current focus for many researchers has turned to the development of therapeutics that have the potential for serving as broad-spectrum inhibitors that can target numerous viruses, both within a particular family, as well as to span across multiple viral families so that we can build an arsenal in preparation for the next outbreak.9, 10, 11, 12 This is not as out of reach as it may seem. As detailed below, many nucleosides have been shown to inhibit multiple viruses due to the conserved nature of many viral enzyme binding sites. Thus, it is somewhat surprising that up until very recently, many researchers focused more on “one bug one drug”, rather than trying to target multiple viruses given those similarities.8 This approach is now changing due to the realization that we need to be proactive rather than reactive to combat emerging and reemerging infectious diseases.8
In general, most nucleoside drugs are direct acting antivirals that target the viral polymerases.1, 2, 3, 4, 13 Since they resemble the naturally occurring nucleosides, they are taken up by cells, then sequentially phosphorylated by cellular and/or viral kinases to ultimately form the triphosphate, which is typically the active form of the drug.1, 2, 3, 13 The triphosphate is recognized by the polymerase and subsequently incorporated in the growing nucleic acid chain, but through one of several different possible mechanisms, the drug acts as a chain terminator, thereby stopping the virus from replicating.1, 13, 14 This occurs typically because the nucleoside lacks the necessary 3’-hydroxyl group needed for attachment of the next incoming nucleotide (obligate chain terminator), or if it has a 3’-hydroxyl but also has other substituents that can cause steric issues, the chain either stops immediately (pseudo-obligate chain terminator) or is able to add some additional nucleotides, albeit at an increasingly slower rate until ultimately no more can be added (delayed chain terminator).1, 13, 14 There are several other mechanisms that have seen some promise, and those will be discussed further on in this chapter.
2. Early antiviral nucleosides—Obligate chain terminators
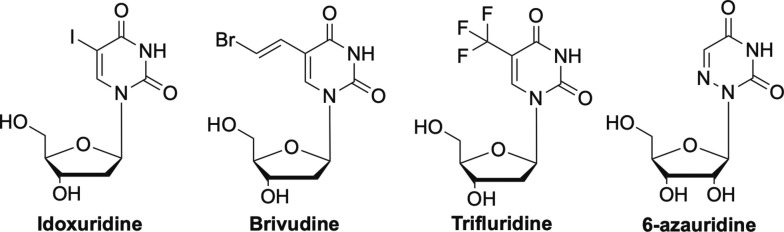
The earliest antiviral nucleoside was idoxuridine (Fig. 1 ), which was approved by the FDA in 1962.15 It was initially developed as an anticancer drug, but was later approved for use against the ocular form of herpes simplex virus (HSV), HSV keratitis.15 It works by mimicking thymidine, and once incorporated into viral DNA, leads to inhibition of thymidylate phosphorylase and the polymerase.15, 16, 17 It is not used much anymore, and has been replaced by another nucleoside analogue, trifluridine (Fig. 1).18 Somewhat related in terms of structure, Brivudine (Fig. 1), another pyrimidine substitute analogue, is widely used in many European countries, but not until recently in the US, for treatment of varicella zoster virus (VZV), which causes herpes zoster, better known as shingles.14, 19, 20 It has also been used to treat HSV-1, but not HSV-2.19 Another older nucleoside that is now being investigated against SARS-CoV-2 is 6-azauridine (Fig. 1). This triazole analogue has shown broad-spectrum activity against a number of different coronaviruses, flaviviruses, including West Nile virus, thus it is not surprising it is now being looked at again.21, 22, 23
Fig. 1.
Early modified nucleoside analogues idoxuridine, brivudine, trifluridine, and 6-azauridine.
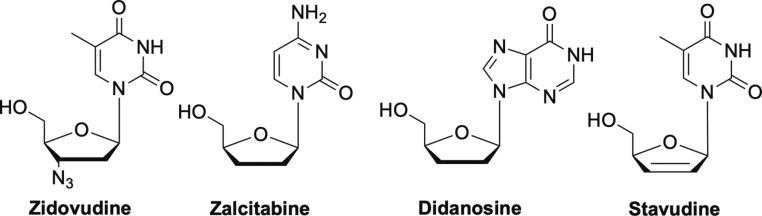
Probably the most well-known of the early FDA-approved antiviral nucleosides is Zidovudine (AZT) shown in Fig. 2 , which was the first nucleoside to be widely used to treat HIV and AIDS.2, 16, 24, 25, 26 Shortly thereafter, Zalcitibine (ddC), Didanosine (ddI) and stavudine (d4T) were developed (Fig. 2), however ddI and ddC were plagued with various toxicity issues and not used extensively after initial excitement.26, 27, 28 All of these are considered obligate chain terminators and work by inhibiting the HIV reverse transcriptase (RT).1, 13
Fig. 2.
Other early modified nucleoside analogues zidovudine, zalcitabine, didanosine, and stavudine.
Other early nucleoside drugs were the arabinose nucleosides vidarabine (Ara-A) and cytarabine (Ara-C) shown in Fig. 3 , both of which are competitive inhibitors of DNA polymerases.1, 13 They are incorporated but then due to the steric configuration of the 2’-hydroxyl on the sugar moiety cause chain termination. Ara-A was used clinically for herpes, but both Ara-A and Ara-C were also shown to inhibit vaccinia (VV) and VZV viruses.15, 29 Ara-C went on to be used primarily as an anticancer drug, due to its toxicity.17, 30
Fig. 3.
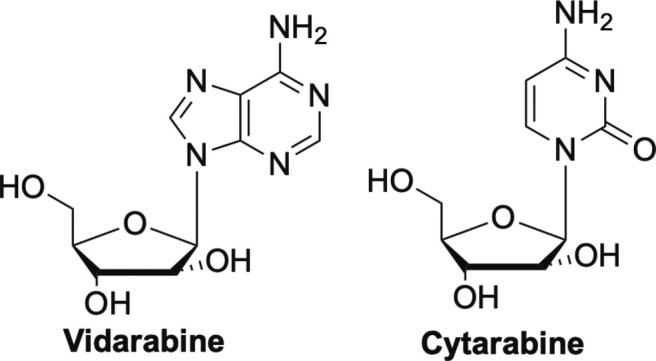
Vidarabine and cytarabine, competitive inhibitors of DNA polymerases.
3. Acyclic obligate chain terminator nucleosides
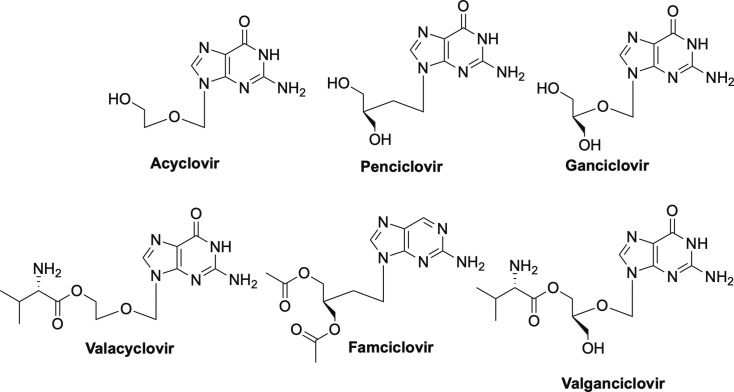
As an extreme approach to obligate chain termination, Professor Antonín Holý developed the acyclic nucleosides and nucleoside phosphonates which lack not only the hydroxyls on the 2’- and 3’-carbons, but also the carbons themselves.16, 31, 32 The acyclic nucleosides that are FDA-approved include acyclovir, penciclovir, and ganciclovir, while the phosphonates include adefovir, cidofovir, and tenofovir.2, 16, 31 In addition, due to issues with poor oral bioavailability, various prodrugs have been subsequently approved including valacyclovir, valganciclovir, famciclovir, tenofovir disoproxil fumarate (TDF), adefovir dipivoxil, and most recently, tenofovir alafenamide (TAF).1, 2, 16 Each will be discussed in more detail below but each work by the same mechanisms – competitive inhibition of the viral polymerases.16
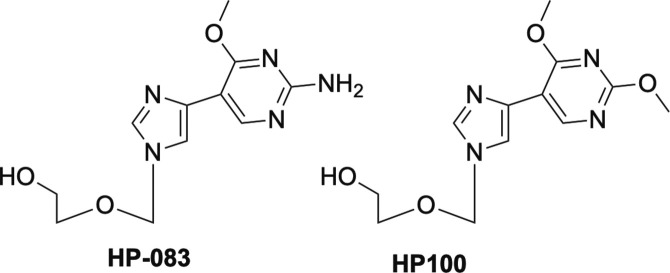
Acyclovir and penciclovir—the carbocyclic version of acyclovir—and their respective prodrugs valacyclovir and famciclovir (Fig. 4) , are used primarily to treat various forms of HSV, while ganciclovir and its prodrug valganciclovir are approved for use against human cytomegalovirus (HCMV).2, 16, 33, 34, 35, 36, 37, 38 Interestingly, HP-083, the fleximer version of acyclovir, and the related fleximer HP-100 (Fig. 5 ) as well as their prodrugs that were developed in our laboratories some years ago, are not active against HSV or HCMV, but rather are potent inhibitors of Ebola (EBOV), Marburg and Sudan viruses of the filovirus class, as well as SARS-CoV-1, MERS and human coronaviruses, in addition to a number of flaviviruses, including dengue (DENV), yellow fever (YFV) and tickborne encephalitis virus (TBEV).39, 40, 41 Notably, acyclovir is not active against any of those at concentrations up to 1000 μM.40 Not surprisingly, these compounds have attracted widespread attention for development as potential broad-spectrum inhibitors and are also currently being investigated for activity against SARS-CoV-2.
Fig. 4.
Approved acyclic nucleosides acyclovir, penciclovir, ganciclovir and their prodrugs valacyclovir, famciclovir, valganciclovir.
Fig. 5.
Fleximer analogues of acyclovir, HP-083 and HP-100.
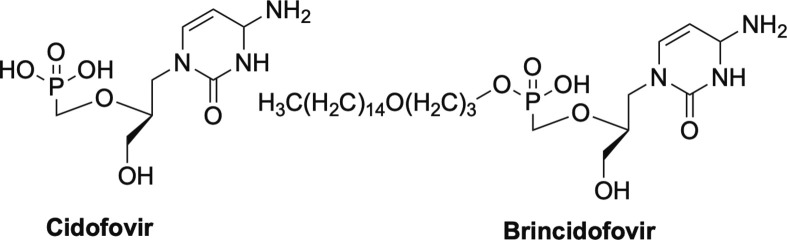
In terms of the acyclic phosphonates, cidofovir (Fig. 6 ) has the broadest spectrum of antiviral activity, with inhibition being noted against both DNA and RNA viruses and numerous different families of viruses.12, 32, 42, 43, 44, 45, 46, 47 Activity exhibited against DNA viruses by cidofovir includes HBV, papillomavirus, polyomavirus, adenovirus, numerous herpes viruses including HSV, HCMV, Epstein Barr virus (EBV) and VZV.12, 32, 42, 43, 44, 45, 46, 47 In addition, cidofovir has been explored extensively against various poxviruses, such as VV, molluscum contagiosum virus (MCV), variola virus, cowpox, monkeypox, and camelpox.42, 44, 45, 47 Related to this, brincidofovir (Fig. 6), the lipid prodrug form of cidofovir, was recently stockpiled by the U.S. government for potential outbreaks or terrorist attacks of smallpox.47, 48, 49, 50 As mentioned earlier, not just limited to DNA viruses, cidofovir also works against retroviruses such as HIV, and the corresponding feline and simian forms of HIV, FIV and SIV respectively.12 As a result, cidofovir and brincidofovir should certainly be revisited for potential use as broad-spectrum antiviral agents.
Fig. 6.
Phosphonate nucleoside cidofovir and its lipid prodrug brincidofovir.
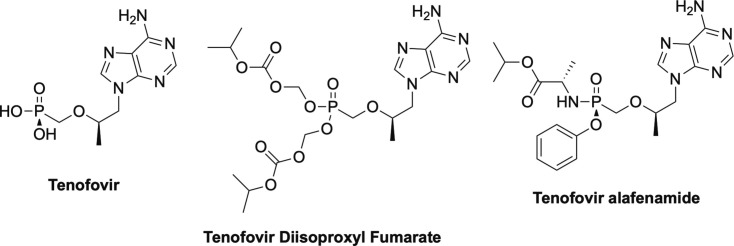
While cidofovir certainly has the widest range of activities, one of the most important nucleosides for the antiviral field is tenofovir (Fig. 7 ).1, 2 Like cidofovir, tenofovir is an acyclic phosphonate and lacks the 3’-OH, thus works as an obligate chain terminator.1, 2 Tenofovir was initially approved as a treatment for HIV, but was later also approved for treatment of HBV, and represents the nucleoside backbone in nearly every combination drug cocktail that has been developed in the past two decades.27, 51, 52, 53 Due to issues with bioavailability, tenofovir was developed as a prodrug, with the first form being tenofovir disoproxil fumarate (TDF, Fig. 7).1, 27, 53 Recently however, a newer form was introduced, which possesses a McGuigan ProTide moiety, tenofovir alafenamide (TAF, Fig. 7).1, 2, 7, 54 This change in prodrug drastically lowered the required dose from 300 mgs/kg to 25 mgs//kg with far less side effects due to more rapid absorption into cells, which lessens the amount of phosphonate in the plasma.55
Fig. 7.
Phosphonate nucleoside tenofovir and its two prodrug forms, tenofovir disoproxil fumarate and tenofovir alafenamide.
Perhaps it is important at this point, to deviate and explain a bit about the impact that the discovery and development of the McGuigan ProTides has had on medicinal chemistry, particularly on nucleoside drugs.56, 57, 58, 59 There are two inherent problems with nucleoside drugs—one, delivery across the cell membrane and two, phosphorylation once inside the cell.56, 57, 58, 59 In terms of delivery, nucleosides as a class are typically polar, so it leads to issues with poor permeability.56, 57, 58, 59 By masking the polar hydroxyl group at the 5’-position with a non-polar moiety, it allows the drug to cross more readily.56, 57, 58, 59 As mentioned previously, typically the active form of the nucleoside is the triphosphate, however you cannot directly administer the triphosphate due to the extreme polarity.56, 57, 58, 59 Moreover, the first phosphorylation step is typically rate limiting due to the acute selectivity of most kinases.56, 58, 59 Once the first phosphorylation has occurred, the second and third tend to be more facile, although not always. As a solution to both problems, a number of years ago the late Professor Chris McGuigan and his team developed what they dubbed the McGuigan ProTide (Fig. 8 ).56, 57, 58, 59
Fig. 8.
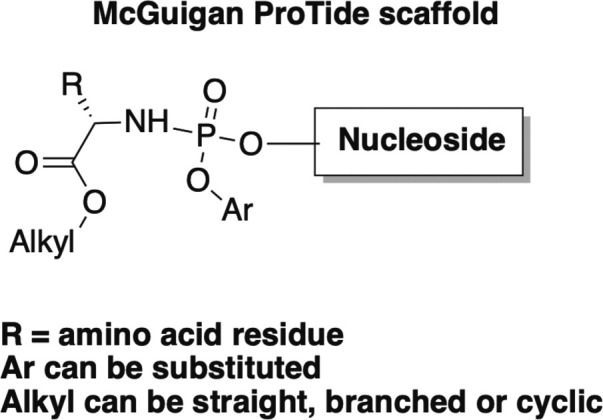
General structure of the McGuigan ProTide.
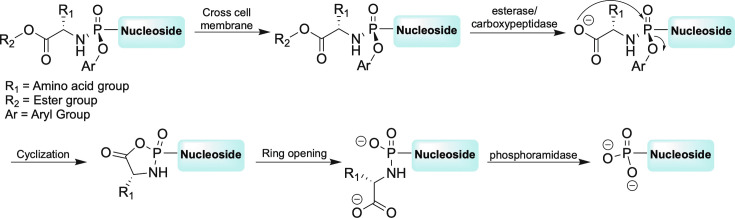
As shown in Fig. 8, there are 3 basic components to the ProTide—the ester, the aromatic moiety, and the amino acid. The ProTide renders the nucleoside less polar, thus it is able to cross the cell membrane.56, 57, 58, 59 Once inside, each piece of the ProTide is sequentially removed by two cellular enzymes as well as spontaneous mechanistic steps (Fig. 9 ). This in turn, reveals the monophosphate form of the nucleoside, thus bypassing that first phosphorylation step and overcoming the rate limiting barrier to activation for the drug.56, 57, 58, 59 After extensive structure activity relationship (SAR) studies on the various components of this clever system, many nucleoside analogues are now being pursued and even marketed, for example, the aforementioned TAF as the McGuigan ProTide form.55, 56
Fig. 9.
Mechanism of McGuigan ProTide metabolism.
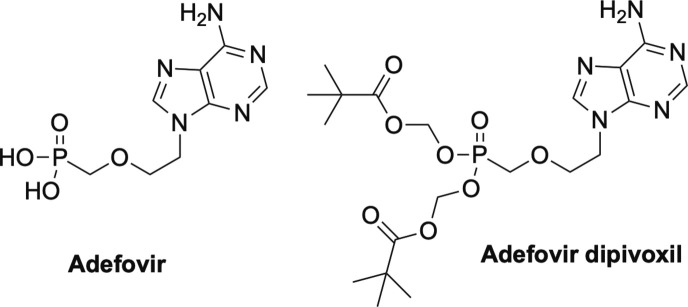
Prior to the development of TDF and TAF, the other FDA approved nucleoside phosphonate was adefovir and its prodrug adefovir dipivoxil (Fig. 10 ), which are used to treat chronic HBV.60, 61, 62 Like tenofovir, adefovir works by competitive inhibition of the viral reverse transcriptase and subsequent chain termination.1, 16 In general, these inhibitors are known as nucleoside reverse transcriptase inhibitors or NRTIs.1, 16, 63
Fig. 10.
Other approved phosphonate nucleosides, adefovir and its prodrug adefovir dipivoxil.
4. Other obligate chain terminator antivirals
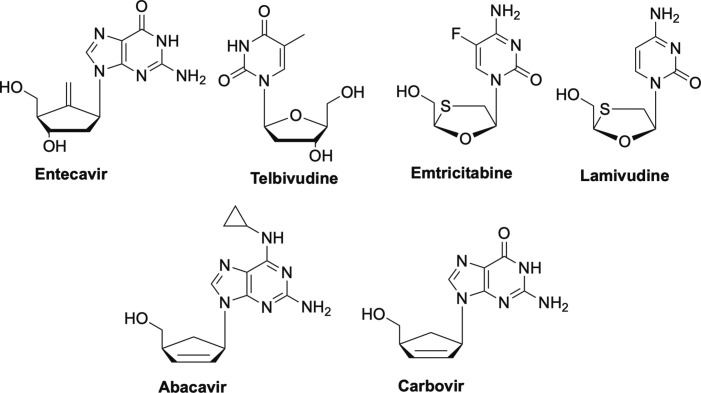
Returning to antivirals possessing a cyclic sugar moiety, other important antiviral NRTIs of note that target HBV are entecavir, and three l-nucleoside analogues—telbivudine, lamivudine (3TC), and its closely related analogue emtricitabine (FTC) (Fig. 11 ).1, 2, 43, 64, 65 Interestingly, entecavir introduced the idea of rigidifying the sugar of the nucleoside with the exocyclic double bond, something that still remains quite novel in terms of scaffold modifications.2 Also of note, the discovery that L-nucleosides could be active was an important finding, as many believed that enzymes would not recognize the unnatural form of the drug.2, 62, 66, 67, 68, 69, 70, 71, 72, 73 Two other FDA-approved NRTIs, abacavir and carbovir (Fig. 11), are carbocyclic nucleosides used to treat HIV.24, 74, 75, 76 Notably, abacavir is one of the few drugs available for use in children with HIV.76
Fig. 11.
Nucleoside reverse transcriptase inhibitors entecavir, telbuvidine, emtricitabine, and lamuvidine (L-nucleosides), as well as abacavir and carbovir.
5. Antivirals that act by lethal mutagenesis
All of the nucleosides discussed to this point have been NRTIs or polymerase inhibitors that act by chain termination, however another mechanism of action has been explored that doesn’t involve chain termination. Lethal mutagenesis is a type of antiviral therapy that essentially increases the viral mutation rate by incorporating nucleosides that act as mutagens, to the point of destroying the viability of the virus.10, 77
One approved nucleoside that has been shown to work by this mechanism is ribavirin, although ribavirin has also been shown to work by other mechanisms and in general, its mechanism of action is not well understood.6 Ribavirin (Fig. 12 ) was originally approved for the treatment of severe respiratory syncytial virus (RSV) infection, it has also been used for treatment of Lassa fever virus infection, as well as influenza A and B.78, 79, 80 Most recently it has been approved for use against HBV and HCV but has been tested against many other viruses including coronaviruses, however without much success in the latter case.6, 81, 82 More recently a prodrug of ribavirin, taribavirin (also known as viramidine) has entered Phase III human trials (Fig. 12). Notably, taribavirin has been reported to target the liver better than ribavirin, which is important because as previously mentioned, both ribavirin and taribavirin have been shown to be highly effective against HCV and HBV.83, 84, 85, 86
Fig. 12.
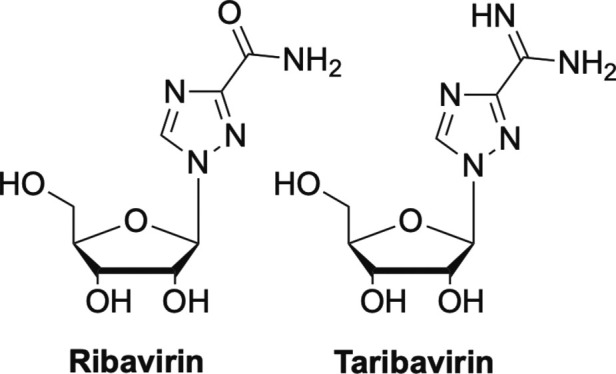
Two nucleosides that work by lethal mutagenesis ribavirin and taribavirin.
Another nucleoside analogue that also works by lethal mutagenesis that has recently gained significant attention against SARS-CoV-2 is molnupiravir (Fig. 13 ).10 Molnupiravir is a prodrug of N-hydroxycytidine (NHC, Fig. 13) and is reported to increase mutations of G to A and C to U in replicating coronaviruses.77 So far it is showing great promise and is currently in Phase II/III clinical trials. Interestingly, the U.S. Department of Health and Human Services has committed to purchasing $1.2B worth of molnupiravir should it ultimately be approved by the FDA.87
Fig. 13.
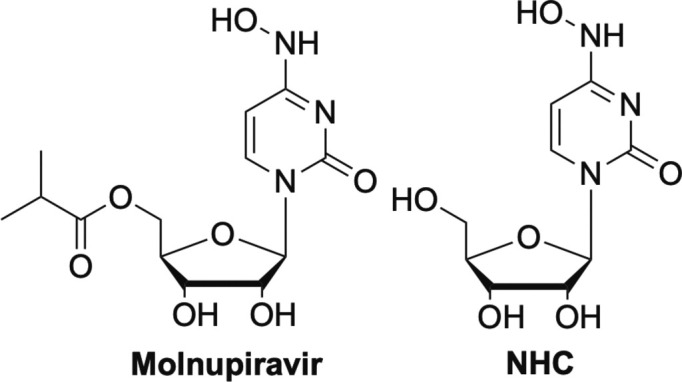
NHC and its prodrug molnupiravir, another nucleoside that works by lethal mutagenesis.
Similarly, the heterocyclic base favipiravir (Fig. 14 ) also works by lethal mutagenesis. Although favipiravir is administered as the base, it is subsequently converted to the nucleoside following ribosylation, and then undergoes normal phosphorylation by the various kinases, thus providing the triphosphate active form.6, 88, 89
Fig. 14.
Favipiravir.
6. Newer antiviral therapeutics—And new mechanisms of action
As more and more information has become known about emerging and reemerging viruses due to increased access to crystal structures and various enzymes involved in viral replication, multiple modifications to the nucleoside scaffold have become quite standard.2, 3 Examples of these complex nucleoside scaffolds include such drugs such as sofosbuvir and the closely related uprifosbuvir, remdesivir and the parent nucleoside GS-441524, EFdA (also known as Islatravir), NITD008, AT-527, and galidesivir, an immunocillin analogue.1, 2, 3
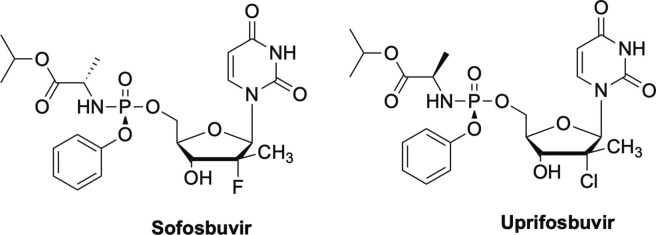
Sofosbuvir is a nucleoside analogue that features an alanine McGuigan ProTide moiety as well as a di-substitution at the C-2 position (Fig. 15 ).90, 91, 92 Sofosbuvir is used to treat HCV, and what makes sofosbuvir stand out among nucleoside analogues is that it is not just a treatment, but rather, a cure.91, 92 For example, HIV patients must take their drug cocktails for their lifetime in order to maintain undetectable levels of viral load, but Sofosbuvir clears the virus completely in 8-12 weeks.91 This was a remarkable breakthrough in antiviral therapy and has set a new standard for researchers to achieve that same effect in other viruses with their nucleoside analogues. Sofosbuvir, and the closely related uprifosbuvir (Fig. 15), are both delayed chain terminators.1, 93 Uprifosbuvir differs from Sofosbuvir at the C-2 position, featuring a chlorine atom instead of the fluorine, as well as the configuration of the methyl group on the ProTide moiety.93, 94 Both have shown broad-spectrum activity against a number of viruses including ZIKV, YFV and chikungunya viruses, as well as more recently, SARS-CoV-2. Uprifosbuvir is currently in Phase III clinical trials.90, 93, 94, 95, 96
Fig. 15.
Delayed chain terminators, sofosbuvir and uprifosbuvir.
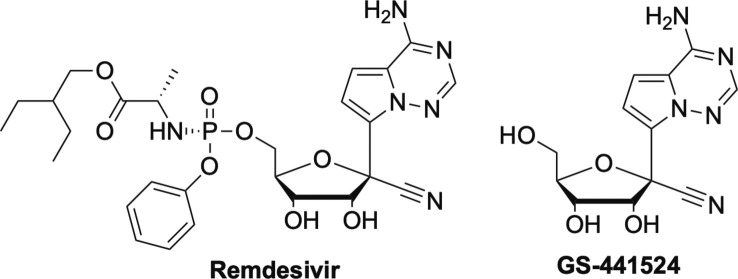
Similarly, remdesivir (RDV, Fig. 16 ), currently the only drug approved for emergency use against SARS-CoV-2 is also a broad-spectrum inhibitor and also contains a McGuigan ProTide moiety like sofosbuvir, uprifosbuvir and TAF, however RDV's ester moiety differs than TAF and sofosbuvir’s.3, 97 RDV was first under development for Ebola, including five compassionate cases, but was later pushed aside due to the development of two monoclonal antibodies that showed significant activity compared to RDV.6, 98, 99 RDV is not ideal as it has to be administered in a hospital setting, which limits its widespread usage and effectiveness. RDV was later shown to have activity against SARS-1 and MERS, thus it wasn’t surprising that it was immediately investigated for SARS-2.6, 98
Fig. 16.
Delayed chain terminators, GS-441524 and its prodrug Remdesivir.
Interestingly, the parent nucleoside of remdesivir, GS-441524 (Fig. 16), has been used for some time for coronaviruses in cats.98 Current efforts involve development a nasal spray as well as an oral form of RDV, both of which would have a major impact on making this drug more accessible globally.6, 98, 100 Regardless, RDV has shown significant broad-spectrum activity against a number of other viruses, therefore certainly has promise for future use in fighting other emerging RNA viruses.9 Remdesivir is also a delayed chain terminator that appears to involve incorporation, followed by pausing and then restarting.6, 9, 98 More recently, RDV has been shown to also work by a second mechanism, as a translocation RT inhibitor, which is currently being studied more in depth.101
A new nucleoside analogue that has recently emerged on the scene due to its excellent activity against SARS-CoV-2 is AT-527 (Fig. 17 ).102 AT-527 is a guanosine analogue being developed by Atea Pharmaceuticals and was recently licensed by Merck to take through clinical trials.103 AT-527 has the C-2’ modification found in sofosbuvir as well as the same McGuigan alanine moiety, but is a double prodrug due to the N-methyl group on the heterocyclic base.102, 104, 105
Fig. 17.
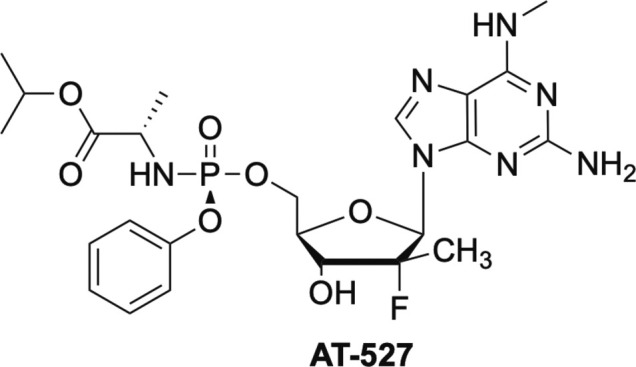
Double prodrug AT-527.
AT-527 has shown significant promise and has numerous advantages over RDV, including being orally bioavailable and safe, thus representing a direct acting antiviral suitable for easy and early administration, as well as for pre- or post-exposure prophylaxis.102, 103, 104, 105 Additionally, AT-527 has exhibited no inhibition of human polymerases, no mitochondrial toxicity in vivo, no toxicity, cardiotoxicity, or neurological effects in rats or monkeys at doses up to 1000 mg/kg, a 10 fold safety profile over RDV for the 550 mg BID dose in humans, a 7 fold higher concentrations in human nasal and lung cells at 72 h, and a much longer half-life.102, 103, 104, 105
Like other nucleoside analogues, AT-527 contains a McGuigan ProTide moiety, which delivers the monophosphate.102, 104, 105 In contrast to other ProTides such as RDV, sofosbuvir and tenofovir, AT-527 exhibits much greater concentrations of the triphosphate, the active form of the drug, in the lungs.102, 105 Most notably however, AT-527 inhibits SARS-CoV-2 in a very unique manner—one molecule of AT-527 is incorporated into the growing strand, while another gets stuck in the active site, both of which lead to chain termination, while a third molecule inhibits the Nidovirus RdRp-Associated Nucleotidyltransferase (NiRAN) binding site, which is adjacent to the RdRp binding site.102 This unique dual mechanism of action is highly synergistic and is likely what is responsible for its superior potency.102
Another nucleoside that is not as well-known as sofosbuvir or RDV, is Islatravir or EFdA (Fig. 18 ). EFdA is an adenosine analogue that has also been shown to work by multiple mechanisms.106, 107 EFdA has shown remarkable activity against HIV, including resistant strains.106, 107, 108 What is even more remarkable, is that unlike other nucleoside analogues that typically work by a single mechanism, EFdA has been shown to work by a number of mechanisms. Similar to RDV, EFdA can function as a delayed chain terminator due to the presence of the 4’-triple bond, which causes steric issues.106, 107 Also like RDV, EFdA can act as a translocation RT inhibitor that essentially slows DNA synthesis, thereby acting as a de facto immediate, or pseudo obligate chain terminator.106, 107 Finally, EFdA-MP can also be misincorporated by RT, leading to mismatched primers that are extremely hard to extend, and are protected from excision by proofreading enzymes.106, 107 This multifaceted mechanism of action is likely to be important for combating the development of viral resistance, something that has plagued medicinal chemists for decades.106, 107
Fig. 18.
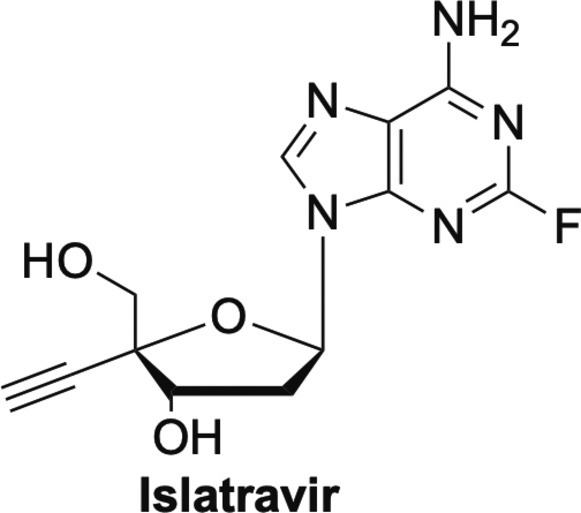
Adenosine analogue islatravir.
Similar to EFdA, another nucleoside analogue that features a triple bond, albeit at the C2′-position, that initially showed promise is NITD008 (Fig. 19 ).109, 110, 111, 112 NITD008 and the closely related MK-608 (Fig. 19), have both exhibit broad-spectrum inhibition against a number of viruses.109, 110, 111, 112, 113, 114, 115 NITD008 has shown potent activity against flaviviruses such as HCV, DENV, TBEV, ZIKV, West Nile virus, and YFV, as well as more exotic viruses such as Powassan virus, Kyasanur forest disease, which is a tickborne virus, and Omsk hemorrhagic fever virus.110, 111, 112, 113, 114, 115 In contrast, MK-608 appears to have only shown activity against DENV, TBEV, ZIKV and poliovirus. Both are delayed chain terminators, both are 7-deaza analogues and both are di-substituted at the C-2’-position, however NITD008 possesses the aforementioned triple bond in the “up” configuration, while MK-608 has a methyl group.3 Unfortunately neither have been pursued extensively after NITD008 proved too toxic in preclinical trials and MK-608 failed in early human clinical trials.116, 117
Fig. 19.
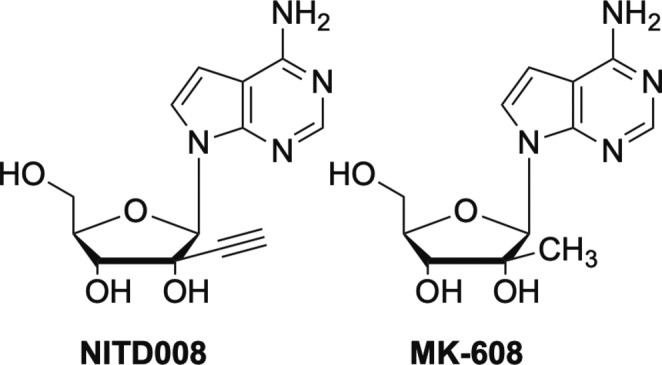
Modified C2’ nucleosides NITD008 and MK-608.
Finally, this brings us to galidesivir, also known as Immucillin A or BX-4430. Galidesivir is interesting structurally due to the presence of a nitrogen in the sugar ring, rather than the typical furanose oxygen found in most nucleoside analogues, or the methylene group found in carbocyclic nucleosides.2 This class of nucleosides are known as immucillins and they have shown potent activity against a number of viruses. Galidesivir is an adenosine analogue that has been investigated for use against numerous viruses from various different families.118, 119, 120, 121, 122 It has shown activity against filoviruses including Ebola and Marburg, against flaviviruses such as YFV and ZIKV, as well as broad spectrum activity against various negative- and positive-sense RNA viruses, including coronaviruses and arenaviruses.11 Because of this broad-spectrum activity, it is now being investigated against SARS-CoV-2, and like many of the nucleosides revisited herein, is an excellent example of how nucleosides can be effective against many viruses123 (Fig. 20 ).
Fig. 20.
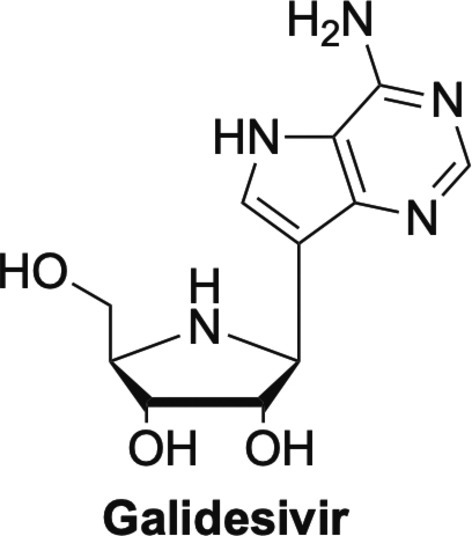
Galidesivir, one of the immucillin nucleosides.
In summary, while this review is by no means comprehensive, rest assured, there are many other nucleoside analogues out there that are being investigated not only as treatments against one virus, but now against multiple viruses. The stark realization brought about by the SARS-CoV-2 pandemic and the over 4.55 M deaths as of September 2021 shows us just how unprepared we were to fight emerging viruses.124 It has never been clear that we must be able to develop and stockpile small molecule broad-spectrum inhibitors to fight emerging and reemerging infectious diseases, particularly zoonotic ones given the vast number of viruses circulating in animals. In that regard, nucleoside inhibitors will indoubtedly remain our best hope just as they have for decades.
Acknowledgments
The authors thank Ms. Christianna Kutz for her excellent editing of the manuscript.
References
- 1.De Clercq E., Li G. Approved Antiviral Drugs Over the Past 50 Years. Clin. Microbiol. Rev. 2016;29:695–747. doi: 10.1128/CMR.00102-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seley-Radtke K.L., Yates M.K. The Evolution of Nucleoside Analogue Antivirals: A Review for Chemists and Non-Chemists. Part 1: Early Structural Modifications to the Nucleoside Scaffold. Antivir. Res. 2018;154:66–86. doi: 10.1016/j.antiviral.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yates M.K., Seley-Radtke K.L. The Evolution of Antiviral Nucleoside Analogues: A Review for Chemists and Non-chemists. Part II: Complex Modifications to the Nucleoside Scaffold. Antivir. Res. 2019;162:5–21. doi: 10.1016/j.antiviral.2018.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seley-Radtke K. Flexibility-Not Just for Yoga Anymore! Antivir. Chem. Chemother. 2018;26 doi: 10.1177/2040206618756788. 2040206618756788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park S.-J., Yu K.-M., Kim Y.-I., Kim S.-M., Kim E.-H., Kim S.-G., Kim E.J., Casel M.A.B., Rollon R., Jang S.-G., Lee M.-H., Chang J.-H., Song M.-S., Jeong H.W., Choi Y., Chen W., Shin W.-J., Jung J.U., Choi Y.K. Antiviral Efficacies of FDA-Approved Drugs Against SARS-CoV-2 Infection in Ferrets. mBio. 2020;11 doi: 10.1128/mBio.01114-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Simonis A., Theobald S.J., Fätkenheuer G., Rybniker J., Malin J.J. A Comparative Analysis of Remdesivir and Other Repurposed Antivirals Against SARS-CoV-2. EMBO Mol. Med. 2021;13 doi: 10.15252/emmm.202013105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanella I., Zizioli D., Castelli F., Quiros-Roldan E. Tenofovir, Another Inexpensive, Well-Known and Widely Available Old Drug Repurposed for SARS-COV-2 Infection. Pharmaceuticals. 2021;14:454. doi: 10.3390/ph14050454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dolgin E. The Race for Antiviral Drugs to Beat COVID—and the Next Pandemic. Nature. 2021;592:340–343. doi: 10.1038/d41586-021-00958-4. [DOI] [PubMed] [Google Scholar]
- 9.Sheahan T.P., Sims A.C., Graham R.L., Menachery V.D., Gralinski L.E., Case J.B., Leist S.R., Pyrc K., Feng J.Y., Trantcheva I., Bannister R., Park Y., Babusis D., Clarke M.O., Mackman R.L., Spahn J.E., Palmiotti C.A., Siegel D., Ray A.S., Cihlar T., Jordan R., Denison M.R., Baric R.S. Broad-Spectrum Antiviral GS-5734 Inhibits Both Epidemic and Zoonotic Coronaviruses. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sheahan T.P., Sims A.C., Zhou S., Graham R.L., Pruijssers A.J., Agostini M.L., Leist S.R., Schäfer A., Dinnon K.H., Stevens L.J., Chappell J.D., Lu X., Hughes T.M., George A.S., Hill C.S., Montgomery S.A., Brown A.J., Bluemling G.R., Natchus M.G., Saindane M., Kolykhalov A.A., Painter G., Harcourt J., Tamin A., Thornburg N.J., Swanstrom R., Denison M.R., Baric R.S. An Orally Bioavailable Broad-Spectrum Antiviral Inhibits SARS-CoV-2 in Human Airway Epithelial Cell Cultures and Multiple Coronaviruses in Mice. Sci. Transl. Med. 2020;12 doi: 10.1126/scitranslmed.abb5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor R., Kotian P., Warren T., Panchal R., Bavari S., Julander J., Dobo S., Rose A., El-Kattan Y., Taubenheim B., Babu Y., Sheridan W.P. BCX4430—A Broad-Spectrum Antiviral Adenosine Nucleoside Analog Under Development for the Treatment of Ebola Virus Disease. J. Infect. Publ. Health. 2016;9:220–226. doi: 10.1016/j.jiph.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Painter W., Robertson A., Trost L.C., Godkin S., Lampert B., Painter G. First Pharmacokinetic and Safety Study in Humans of the Novel Lipid Antiviral Conjugate CMX001, a Broad-Spectrum Oral Drug Active against Double-Stranded DNA Viruses. Antimicrob. Agents Chemother. 2012;56:2726–2734. doi: 10.1128/AAC.05983-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Clercq E., Neyts J. Antiviral Agents Acting as DNA or RNA Chain Terminators. Handb. Exp. Pharmacol. 2009:53–84. doi: 10.1007/978-3-540-79086-0_3. [DOI] [PubMed] [Google Scholar]
- 14.Deval J. Antimicrobial Strategies: Inhibition of Viral Polymerases by 3'-Hydroxyl Nucleosides. Drugs. 2009;69:151–166. doi: 10.2165/00003495-200969020-00002. [DOI] [PubMed] [Google Scholar]
- 15.Wilhelmus K.R. Antiviral Treatment and Other Therapeutic Interventions for Herpes Simplex Virus Epithelial Keratitis. Cochrane Database Systemat. Rev. 2015 doi: 10.1002/14651858.CD002898.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Clercq E., Field H.J. Antiviral Prodrugs—the Development of Successful Prodrug Strategies for Antiviral Chemotherapy. Brit. J. Pharmacol. 2006;147:1–11. doi: 10.1038/sj.bjp.0706446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lauter C.B., Bailey E.J., Lerner A.M. Assessment of Cytosine Arabinoside as an Antiviral Agent in Humans. Antimicrob. Agents Chemother. 1974;6:598–602. doi: 10.1128/aac.6.5.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Brien W.J., Taylor J.L. Therapeutic Response of Herpes Simplex Virus-Induced Corneal Edema to Trifluridine in Combination With Immunosuppressive Agents. Invest. Ophthalmol. Vis. Sci. 1991;32:2455–2461. [PubMed] [Google Scholar]
- 19.De Clercq E. Discovery and Development of BVDU (Brivudin) as a Therapeutic for the Treatment of Herpes Zoster. Biochem. Pharmacol. 2004;68:2301–2315. doi: 10.1016/j.bcp.2004.07.039. [DOI] [PubMed] [Google Scholar]
- 20.Desgranges C., Razaka G., Rabaud M., Bricaud H., Balzarini J., de Clercq E. Phosphorolysis of (E)-5-(2-bromovinyl)-2'-deoxyuridine (BVDU) and Other 5-Substituted-2'-deoxyuridines by Purified Human Thymidine Phosphorylase and Intact Blood Platelets. Biochem. Pharmacol. 1983;32:3583–3590. doi: 10.1016/0006-2952(83)90307-6. [DOI] [PubMed] [Google Scholar]
- 21.Morrey J.D., Smee D.F., Sidwell R.W., Tseng C. Identification of Active Antiviral Compounds Against a New York Isolate of West Nile Virus. Antiviral Res. 2002;55:107–116. doi: 10.1016/s0166-3542(02)00013-x. [DOI] [PubMed] [Google Scholar]
- 22.Pyrc K., Bosch B.J., Berkhout B., Jebbink M.F., Dijkman R., Rottier P., Van Der Hoek L. Inhibition of Human Coronavirus NL63 Infection at Early Stages of the Replication Cycle. Antimicrob. Agents Chemother. 2006;50:2000–2008. doi: 10.1128/AAC.01598-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crance J.M., Scaramozzino N., Jouan A., Garin D. Interferon, Ribavirin, 6-Azauridine and Glycyrrhizin: Antiviral Compounds Active Against Pathogenic Flaviviruses. Antivir. Res. 2003;58:73–79. doi: 10.1016/s0166-3542(02)00185-7. [DOI] [PubMed] [Google Scholar]
- 24.Parker W.B., White E.L., Shaddix S.C., Ross L.J., Buckheit R.W., Jr., Germany J.M., Secrist J.A., 3rd, Vince R., Shannon W.M. Mechanism of Inhibition of Human Immunodeficiency Virus Type 1 Reverse Transcriptase and Human DNA Polymerases Alpha, Beta, and Gamma by the 5'-Triphosphates of Carbovir, 3'-azido-3'-deoxythymidine, 2',3'-Dideoxyguanosine and 3'-Deoxythymidine. A Novel RNA Template for the Evaluation of Antiretroviral Drugs. J. Biol. Chem. 1991;266:1754–1762. [PubMed] [Google Scholar]
- 25.Furman P.A., Fyfe J.A., St Clair M.H., Weinhold K., Rideout J.L., Freeman G.A., Lehrman S.N., Bolognesi D.P., Broder S., Mitsuya H. Phosphorylation of 3'-azido-3'-Deoxythymidine and Selective Interaction of the 5'-Triphosphate With Human Immunodeficiency Virus Reverse Transcriptase. Proc. Natl. Acad. Sci. U. S. A. 1986;83:8333. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yarchoan R., Thomas R., Allain J.-P., McAtee N., Dubinsky R., Mitsuya H., Lawley T., Safai B., Myers C., Perno C., Klecker R., Wills R., Fischl M., McNeely M.C., Pluda J., Leuther M., Collins J., Broder S. Phase I Studies of 2',3'-Dideoxycytidine in Severe Human Immunodeficiency Virus Infection as a Single Agent and Alternating With Zidovudine (AZT) Lancet. 1988;331:76–81. doi: 10.1016/s0140-6736(88)90283-8. [DOI] [PubMed] [Google Scholar]
- 27.Pruvost A., Negredo E., Benech H., Theodoro F., Puig J., Grau E., García E., Moltó J., Grassi J., Clotet B. Measurement of Intracellular Didanosine and Tenofovir Phosphorylated Metabolites and Possible Interaction of the Two Drugs in Human Immunodeficiency Virus-Infected Patients. Antimicrob. Agents Chemother. 2005;49:1907–1914. doi: 10.1128/AAC.49.5.1907-1914.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitsuya H., Broder S. Inhibition of the In Vitro Infectivity and Cytopathic Effect of Human T-Lymphotrophic Virus Type III/Lymphadenopathy-Associated Virus (HTLV-III/LAV) by 2',3'-Dideoxynucleosides. Proc. Natl. Acad. Sci. U. S. A. 1986;83:1911–1915. doi: 10.1073/pnas.83.6.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitley R.J., Tucker B.C., Kinkel A.W., Barton N.H., Pass R.F., Whelchel J.D., Cobbs C.G., Diethelm A.G., Buchanan R.A. Pharmacology, Tolerance, and Antiviral Activity of Vidarabine Monophosphate in Humans. Antimicrob. Agents Chemother. 1980;18:709–715. doi: 10.1128/aac.18.5.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chhikara B.S., Parang K. Development of Cytarabine Prodrugs and Delivery Systems for Leukemia Treatment. Expert Opin. Drug Deliv. 2010;7:1399–1414. doi: 10.1517/17425247.2010.527330. [DOI] [PubMed] [Google Scholar]
- 31.De Clercq E. The Holý Trinity: The Acyclic Nucleoside Phosphonates. Adv. Pharmacol. 2013;67:293–316. doi: 10.1016/B978-0-12-405880-4.00008-1. [DOI] [PubMed] [Google Scholar]
- 32.Tichý T., Andrei G., Dračínský M., Holý A., Balzarini J., Snoeck R., Krečmerová M. New Prodrugs of Adefovir and Cidofovir. Bioorg. Med. Chem. 2011;19:3527–3539. doi: 10.1016/j.bmc.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shaw M., Gurr W., Watts P., Littler E., Field H. Ganciclovir and Penciclovir, but Not Acyclovir, Induce Apoptosis in Herpes Simplex Virus Thymidine Kinasetransformed Baby Hamster Kidney Cells. Antivir. Chem. Chemother. 2001;12:175–186. doi: 10.1177/095632020101200305. [DOI] [PubMed] [Google Scholar]
- 34.Chou T., Hong B. Ganciclovir Ophthalmic Gel 0.15% for the Treatment of Acute Herpetic Keratitis: Background, Effectiveness, Tolerability, Safety, and Future Applications. Ther. Clin. Risk Manage. 2014;665 doi: 10.2147/TCRM.S58242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Worrall G. Acyclovir in Recurrent Herpes labialis. BMJ. 1996;312:6. doi: 10.1136/bmj.312.7022.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spruance S.L., Nett R., Marbury T., Wolff R., Johnson J., Spaulding T. Acyclovir Cream for Treatment of Herpes Simplex Labialis: Results of Two Randomized, Double-Blind, Vehicle-Controlled, Multicenter Clinical Trials. Antimicrob. Agents Chemother. 2002;46:2238–2243. doi: 10.1128/AAC.46.7.2238-2243.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rafailidis P.I., Mavros M.N., Kapaskelis A., Falagas M.E. Antiviral Treatment for Severe EBV Infections in Apparently Immunocompetent Patients. J. Clin. Virol. 2010;49:151–157. doi: 10.1016/j.jcv.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Gershburg E., Pagano J.S. Epstein-Barr Virus Infections: Prospects for Treatment. J. Antimicrob. Chemother. 2005;56:277–281. doi: 10.1093/jac/dki240. [DOI] [PubMed] [Google Scholar]
- 39.Yates M.K., Raje M.R., Chatterjee P., Spiropoulou C.F., Bavari S., Flint M., Soloveva V., Seley-Radtke K.L. Flex-Nucleoside Analogues—Novel Therapeutics Against Filoviruses. Bioorg. Med. Chem. Lett. 2017;27:2800–2802. doi: 10.1016/j.bmcl.2017.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peters H.L., Jochmans D., de Wilde A.H., Posthuma C.C., Snijder E.J., Neyts J., Seley-Radtke K.L. Design, Synthesis and Evaluation of a Series of Acyclic Fleximer Nucleoside Analogues With Anti-Coronavirus Activity. Bioorg. Med. Chem. Lett. 2015;25:2923–2926. doi: 10.1016/j.bmcl.2015.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thames J.E., Waters C.D., Valle C., Bassetto M., Aouadi W., Martin B., Selisko B., Falat A., Coutard B., Brancale A., Canard B., Decroly E., Seley-Radtke K.L. Synthesis and Biological Evaluation of Novel Flexible Nucleoside Analogues That Inhibit Flavivirus Replication In Vitro. Bioorg. Med. Chem. 2020;28:115713. doi: 10.1016/j.bmc.2020.115713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magee W.C., Hostetler K.Y., Evans D.H. Mechanism of Inhibition of Vaccinia Virus DNA Polymerase by Cidofovir Diphosphate. Antimicrob. Agents Chemother. 2005;49:3153–3162. doi: 10.1128/AAC.49.8.3153-3162.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jockusch S., Tao C., Li X., Anderson T.K., Chien M., Kumar S., Russo J.J., Kirchdoerfer R.N., Ju J. A Library of Nucleotide Analogues Terminate RNA Synthesis Catalyzed by Polymerases of Coronaviruses That Cause SARS and COVID-19. Antivir. Res. 2020;180:104857. doi: 10.1016/j.antiviral.2020.104857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Clercq E. Cidofovir in the Treatment of Poxvirus Infections. Antivir. Res. 2002;55:1–13. doi: 10.1016/S0166-3542(02)00008-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Scagliarini A., McInnes C.J., Gallina L., Dal Pozzo F., Scagliarini L., Snoeck R., Prosperi S., Sales J., Gilray J.A., Nettleton P.F. Antiviral Activity of HPMPC (Cidofovir) Against orf Virus Infected Lambs. Antivir. Res. 2007;73:169–174. doi: 10.1016/j.antiviral.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Clercq E. Clinical Potential of the Acyclic Nucleoside Phosphonates Cidofovir, Adefovir, and Tenofovir in Treatment of DNA Virus and Retrovirus Infections. Clin. Microbiol. Rev. 2003;16:569–596. doi: 10.1128/CMR.16.4.569-596.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradbury J. Orally Available Cidofovir Derivative Active Against Smallpox. Lancet. 2002;359:1041. doi: 10.1016/S0140-6736(02)08115-1. [DOI] [PubMed] [Google Scholar]
- 48.Marty F.M., Winston D.J., Chemaly R.F., Mullane K.M., Shore T.B., Papanicolaou G.A., Chittick G., Brundage T.M., Wilson C., Morrison M.E., Foster S.A., Nichols W.G., Boeckh M.J. A Randomized, Double-Blind, Placebo-Controlled Phase 3 Trial of Oral Brincidofovir for Cytomegalovirus Prophylaxis in Allogeneic Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2019;25:369–381. doi: 10.1016/j.bbmt.2018.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lanier R., Trost L., Tippin T., Lampert B., Robertson A., Foster S., Rose M., Painter W., O'Mahony R., Almond M., Painter G. Development of CMX001 for the Treatment of Poxvirus Infections. Viruses. 2010;2:2740–2762. doi: 10.3390/v2122740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Florescu D.F., Keck M.A. Development of CMX001 (Brincidofovir) for the Treatment of Serious Diseases or Conditions Caused by dsDNA Viruses. Expert Rev. Anti Infect. Ther. 2014;12:1171–1178. doi: 10.1586/14787210.2014.948847. [DOI] [PubMed] [Google Scholar]
- 51.Pan C.Q., Duan Z., Dai E., Zhang S., Han G., Wang Y., Zhang H., Zou H., Zhu B., Zhao W., Jiang H. Tenofovir to Prevent Hepatitis B Transmission in Mothers With High Viral Load. New Engl. J. Med. 2016;374:2324–2334. doi: 10.1056/NEJMoa1508660. [DOI] [PubMed] [Google Scholar]
- 52.Charlton M.R., Alam A., Shukla A., Dashtseren B., Lesmana C.R.A., Duger D., Payawal D.A., Duy Cuong D., Jargalsaikhan G., Cua I.H.Y., Sollano J.D., Singh K.R., Madan K., Win K.M., Kyi K.P., Tun K.S., Salih M., Rastogi M., Saraf N., Thuy P.T.T., Hien P.T.D., Gani R.A., Mohamed R., Tanwandee T., Piratvisuth T., Sukeepaisarnjaroen W., Naing W., Hashmi Z.Y. An Expert Review on the Use of Tenofovir Alafenamide for the Treatment of Chronic Hepatitis B Virus Infection in Asia. J. Gastroenterol. 2020;55:811–823. doi: 10.1007/s00535-020-01698-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tao X., Lu Y., Zhou Y., Zhang L., Chen Y. Efficacy and Safety of the Regimens Containing Tenofovir Alafenamide Versus Tenofovir Disoproxil Fumarate in Fixed-Dose Single-Tablet Regimens for Initial Treatment of HIV-1 Infection: A Meta-Analysis of Randomized Controlled Trials. Int. J. Infect. Dis. 2020;93:108–117. doi: 10.1016/j.ijid.2020.01.035. [DOI] [PubMed] [Google Scholar]
- 54.Kutlu Ö. Can Tenofovir Diphosphate be a Candidate Drug for Sars-Cov2? First Clinical Perspective. Int. J. Clin. Pract. 2021;75 doi: 10.1111/ijcp.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ray A.S., Fordyce M.W., Hitchcock M.J.M. Tenofovir Alafenamide: A Novel Prodrug of Tenofovir for the Treatment of Human Immunodeficiency Virus. Antivir. Res. 2016;125:63–70. doi: 10.1016/j.antiviral.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 56.Mehellou Y., Rattan H.S., Balzarini J. The ProTide Prodrug Technology: From the Concept to the Clinic. J. Med. Chem. 2018;61:2211–2226. doi: 10.1021/acs.jmedchem.7b00734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGuigan C., Pathirana R.N., Balzarini J., De Clercq E. Intracellular Delivery of Bioactive AZT Nucleotides by Aryl Phosphate Derivatives of AZT. J. Med. Chem. 1993;36:1048–1052. doi: 10.1021/jm00060a013. [DOI] [PubMed] [Google Scholar]
- 58.McGuigan C., Harris S.A., Daluge S.M., Gudmundsson K.S., McLean E.W., Burnette T.C., Marr H., Hazen R., Condreay L.D., Johnson L., De Clercq E., Balzarini J. Application of Phosphoramidate Pronucleotide Technology to Abacavir Leads to a Significant Enhancement of Antiviral Potency. J. Med. Chem. 2005;48:3504–3515. doi: 10.1021/jm0491400. [DOI] [PubMed] [Google Scholar]
- 59.Cahard D., McGuigan C., Balzarini J. Aryloxy Phosphoramidate Triesters as Pro-Tides. Mini Rev. Med. Chem. 2004;4:371–381. doi: 10.2174/1389557043403936. [DOI] [PubMed] [Google Scholar]
- 60.Marcellin P., Chang T.T., Lim S.G., Tong M.J., Sievert W., Shiffman M.L., Jeffers L., Goodman Z., Wulfsohn M.S., Xiong S., Fry J., Brosgart C.L. Adefovir Dipivoxil for the Treatment of Hepatitis B e Antigen-Positive Chronic Hepatitis B. N. Engl. J. Med. 2003;348:808–816. doi: 10.1056/NEJMoa020681. [DOI] [PubMed] [Google Scholar]
- 61.Manolakopoulos S., Bethanis S., Koutsounas S., Goulis J., Vlachogiannakos J., Christias E., Saveriadis A., Pavlidis C., Triantos C., Christidou A., Papatheodoridis G., Karamanolis D., Tzourmakliotis D. Long-Term Therapy With Adefovir Dipivoxil in Hepatitis B e Antigen-Negative Patients Developing Resistance to Lamivudine. Aliment. Pharmacol. Ther. 2008;27:266–273. doi: 10.1111/j.1365-2036.2007.03567.x. [DOI] [PubMed] [Google Scholar]
- 62.Chan H.L., Heathcote E.J., Marcellin P., Lai C.L., Cho M., Moon Y.M., Chao Y.C., Myers R.P., Minuk G.Y., Jeffers L., Sievert W., Bzowej N., Harb G., Kaiser R., Qiao X.J., Brown N.A. Treatment of Hepatitis B e Antigen Positive Chronic Hepatitis With Telbivudine or Adefovir: A Randomized Trial. Ann. Intern. Med. 2007;147:745–754. doi: 10.7326/0003-4819-147-11-200712040-00183. [DOI] [PubMed] [Google Scholar]
- 63.Fisher E.J., Chaloner K., Cohn D.L., Grant L.B., Alston B., Brosgart C.L., Schmetter B., El-Sadr W.M., Sampson J. The Safety and Efficacy of Adefovir Dipivoxil in Patients With Advanced HIV Disease: A Randomized, Placebo-Controlled Trial. Aids. 2001;15:1695–1700. doi: 10.1097/00002030-200109070-00013. [DOI] [PubMed] [Google Scholar]
- 64.Lai C.L., Shouval D., Lok A.S., Chang T.T., Cheinquer H., Goodman Z., DeHertogh D., Wilber R., Zink R.C., Cross A., Colonno R., Fernandes L. Entecavir Versus Lamivudine for Patients With HBeAg-Negative Chronic Hepatitis B. N. Engl. J. Med. 2006;354:1011–1020. doi: 10.1056/NEJMoa051287. [DOI] [PubMed] [Google Scholar]
- 65.Sims K.A., Woodland A.M. Entecavir: A New Nucleoside Analog for the Treatment of Chronic Hepatitis B Infection. Pharmacotherapy. 2006;26:1745–1757. doi: 10.1592/phco.26.12.1745. [DOI] [PubMed] [Google Scholar]
- 66.Khalighinejad P., Alavian S.M., Fesharaki M.G., Jalilianhasanpour R. Lamivudine's Efficacy and Safety in Preventing Mother-to-Child Transmission of Hepatitis B: A Meta-Analysis. Turk. J. Gastroenterol. 2018 doi: 10.5152/tjg.2018.18148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lai C.L., Leung N., Teo E.K., Tong M., Wong F., Hann H.W., Han S., Poynard T., Myers M., Chao G., Lloyd D., Brown N.A. A 1-Year Trial of Telbivudine, Lamivudine, and the Combination in Patients With Hepatitis B e Antigen-Positive Chronic Hepatitis B. Gastroenterology. 2005;129:528–536. doi: 10.1016/j.gastro.2005.05.053. [DOI] [PubMed] [Google Scholar]
- 68.Lai C.L., Gane E., Liaw Y.F., Hsu C.W., Thongsawat S., Wang Y., Chen Y., Heathcote E.J., Rasenack J., Bzowej N., Naoumov N.V., Di Bisceglie A.M., Zeuzem S., Moon Y.M., Goodman Z., Chao G., Constance B.F., Brown N.A. Telbivudine Versus Lamivudine in Patients With Chronic Hepatitis B. N. Engl. J. Med. 2007;357:2576–2588. doi: 10.1056/NEJMoa066422. [DOI] [PubMed] [Google Scholar]
- 69.Peiris J.S., Chu C.M., Cheng V.C., Chan K.S., Hung I.F., Poon L.L., Law K.I., Tang B.S., Hon T.Y., Chan C.S., Chan K.H., Ng J.S., Zheng B.J., Ng W.L., Lai R.W., Guan Y., Yuen K.Y. Clinical Progression and Viral Load in a Community Outbreak of Coronavirus-Associated SARS Pneumonia: A Prospective Study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Osborn M.K. Safety and Efficacy of Telbivudine for the Treatment of Chronic Hepatitis B. Ther. Clin. Risk Manag. 2009;5:789–798. doi: 10.2147/tcrm.s5318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wohl D., Clarke A., Maggiolo F., Garner W., Laouri M., Martin H., Quirk E. Patient-Reported Symptoms Over 48 Weeks Among Participants in Randomized, Double-Blind, Phase III Non-inferiority Trials of Adults With HIV on Co-formulated Bictegravir, Emtricitabine, and Tenofovir Alafenamide Versus Co-Formulated Abacavir, Dolutegravir. Pat. Pat. Center. Outcomes Res. 2018;11:561–573. doi: 10.1007/s40271-018-0322-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Deeks E.D. Bictegravir/Emtricitabine/Tenofovir Alafenamide: A Review in HIV-1 Infection. Drugs. 2018;78:1817–1828. doi: 10.1007/s40265-018-1010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sculier D., Wandeler G., Yerly S., Marinosci A., Stoeckle M., Bernasconi E., Braun D.L., Vernazza P., Cavassini M., Buzzi M., Metzner K.J., Decosterd L.A., Günthard H.F., Schmid P., Limacher A., Egger M., Calmy A. Efficacy and Safety of Dolutegravir Plus Emtricitabine Versus Standard ART for the Maintenance of HIV-1 Suppression: 48-Week Results of the Factorial, Randomized, Non-Inferiority SIMPL’HIV Trial. PLoS Med. 2020;17 doi: 10.1371/journal.pmed.1003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jackson A., Moyle G., Dickinson L., Back D., Khoo S., Taylor J., Gedela K., Abongomera G., Gazzard B., Boffito M. Pharmacokinetics of Abacavir and Its Anabolite Carbovir Triphosphate Without and With Darunavir/Ritonavir or Raltegravir in HIV-Infected Subjects. Antivir. Ther. 2011;17:19–24. doi: 10.3851/IMP1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yeom Y.H., Remmel R.P., Huang S.H., Hua M., Vince R., Zimmerman C.L. Pharmacokinetics and Bioavailability of Carbovir, a Carbocyclic Nucleoside Active Against Human Immunodeficiency Virus, in Rats. Antimicrob. Agents Chemother. 1989;33:171–175. doi: 10.1128/aac.33.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mega T.A., Usamo F.B., Negera G.Z. Abacavir Versus Zidovudine-Based Regimens for Treatment of HIV-Infected Children in Resource Limited Settings: A Retrospective Cohort Study. BMC Pediat. 2020;20 doi: 10.1186/s12887-020-1995-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kabinger F., Stiller C., Schmitzová J., Dienemann C., Kokic G., Hillen H.S., Höbartner C., Cramer P. Mechanism of Molnupiravir-Induced SARS-CoV-2 Mutagenesis. Nat. Struct. Mol. Biol. 2021 doi: 10.1038/s41594-021-00651-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eberhardt K.A., Mischlinger J., Jordan S., Groger M., Günther S., Ramharter M. Ribavirin for the Treatment of Lassa Fever: A Systematic Review and Meta-Analysis. Int. J. Infect. Dis. 2019;87:15–20. doi: 10.1016/j.ijid.2019.07.015. [DOI] [PubMed] [Google Scholar]
- 79.Pelaez A., Lyon G.M., Force S.D., Ramirez A.M., Neujahr D.C., Foster M., Naik P.M., Gal A.A., Mitchell P.O., Lawrence E.C. Efficacy of Oral Ribavirin in Lung Transplant Patients With Respiratory Syncytial Virus Lower Respiratory Tract Infection. J. Heart Lung Transplant. 2009;28:67–71. doi: 10.1016/j.healun.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smee D.F., Wong M.-H., Bailey K.W., Sidwell R.W. Activities of Oseltamivir and Ribavirin Used Alone and in Combination Against Infections in Mice With Recent Isolates of Influenza a (H1N1) and B Viruses. Antivir. Chem. Chemother. 2006;17:185–192. doi: 10.1177/095632020601700403. [DOI] [PubMed] [Google Scholar]
- 81.Liu C.J., Chen P.J., Lai M.Y., Kao J.H., Jeng Y.M., Chen D.S. Ribavirin and interferon is effective for hepatitis C virus clearance in hepatitis B and C dually infected patients. Hepatology. 2003;37:568–576. doi: 10.1053/jhep.2003.50096. [DOI] [PubMed] [Google Scholar]
- 82.Eslami G., Mousaviasl S., Radmanesh E., Jelvay S., Bitaraf S., Simmons B., Wentzel H., Hill A., Sadeghi A., Freeman J., Salmanzadeh S., Esmaeilian H., Mobarak M., Tabibi R., Jafari Kashi A.H., Lotfi Z., Talebzadeh S.M., Wickramatillake A., Momtazan M., Hajizadeh Farsani M., Marjani S., Mobarak S. The Impact of Sofosbuvir/Daclatasvir or Ribavirin in Patients With Severe COVID-19. J. Antimicrob. Chemother. 2020;75:3366–3372. doi: 10.1093/jac/dkaa331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gish R.G. Treating HCV With Ribavirin Analogues and Ribavirin-Like Molecules. J. Antimicrob. Chemother. 2006;57:8–13. doi: 10.1093/jac/dki405. [DOI] [PubMed] [Google Scholar]
- 84.Poordad F., Lawitz E., Shiffman M.L., Hassanein T., Muir A.J., Bacon B.R., Heise J., Halliman D., Chun E., Hammond J. Virologic Response Rates of Weight-Based Taribavirin Versus Ribavirin in Treatment-Naive Patients With Genotype 1 Chronic Hepatitis C. Hepatology. 2010;52:1208–1215. doi: 10.1002/hep.23827. [DOI] [PubMed] [Google Scholar]
- 85.Lin C.C., Luu K., Lourenco D., Yeh L.T. Pharmacokinetics and Metabolism of [14C]viramidine in Rats and Cynomolgus Monkeys. Antimicrob. Agents Chemother. 2003;47:2458–2463. doi: 10.1128/AAC.47.8.2458-2463.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin C.C., Yeh L.T., Vitarella D., Hong Z. Viramidine, a Prodrug of Ribavirin, Shows Better Liver-Targeting Properties and Safety Profiles Than Ribavirin in Animals. Antivir. Chem. Chemother. 2003;14:145–152. doi: 10.1177/095632020301400304. [DOI] [PubMed] [Google Scholar]
- 87.Zimmer C. The New York Times; 2021. A Pill to Treat Covid-19? The U.S. Is Betting on It. [Google Scholar]
- 88.Ghasemnejad-Berenji M., Pashapour S. Favipiravir and COVID-19: A Simplified Summary. Drug Res. 2021;71:166–170. doi: 10.1055/a-1296-7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Furuta Y., Gowen B.B., Takahashi K., Shiraki K., Smee D.F., Barnard D.L. Favipiravir (T-705), a Novel Viral RNA Polymerase Inhibitor. Antivir. Res. 2013;100:446–454. doi: 10.1016/j.antiviral.2013.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chien M., Anderson T.K., Jockusch S., Tao C., Li X., Kumar S., Russo J.J., Kirchdoerfer R.N., Ju J. Nucleotide Analogues as Inhibitors of SARS-CoV-2 Polymerase, a Key Drug Target for COVID-19. J. Proteome Res. 2020;19:4690–4697. doi: 10.1021/acs.jproteome.0c00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Heo Y.-A., Deeks E.D. Sofosbuvir/Velpatasvir/Voxilaprevir: A Review in Chronic Hepatitis C. Drugs. 2018;78:577–587. doi: 10.1007/s40265-018-0895-5. [DOI] [PubMed] [Google Scholar]
- 92.Mathur P., Kottilil S., Wilson E. Sofosbuvir/Velpatasvir/Voxilaprevir: A Highly Effective Option for Retreatment of Hepatitis C in Difficult-to-Treat Patients. Antivir. Ther. 2019;24:1–10. doi: 10.3851/IMP3264. [DOI] [PubMed] [Google Scholar]
- 93.Lawitz E., Gane E., Feld J.J., Buti M., Foster G.R., Rabinovitz M., Burnevich E., Katchman H., Tomasiewicz K., Lahser F., Jackson B., Shaughnessy M., Klopfer S., Yeh W.W., Robertson M.N., Hanna G.J., Barr E., Platt H.L. Efficacy and Safety of a Two-Drug Direct-Acting Antiviral Agent Regimen Ruzasvir 180 mg and Uprifosbuvir 450 mg for 12 weeks in Adults With Chronic Hepatitis C Virus Genotype 1, 2, 3, 4, 5 or 6. J. Viral Hepat. 2019;26:1127–1138. doi: 10.1111/jvh.13132. [DOI] [PubMed] [Google Scholar]
- 94.Wyles D., Wedemeyer H., Ben-Ari Z., Gane E.J., Hansen J.B., Jacobson I.M., Laursen A.L., Luetkemeyer A., Nahass R., Pianko S., Zeuzem S., Jumes P., Huang H.C., Butterton J., Robertson M., Wahl J., Barr E., Joeng H.K., Martin E., Serfaty L. Grazoprevir, Ruzasvir, and Uprifosbuvir for Hepatitis C Virus After NS5A Treatment Failure. Hepatology. 2017;66:1794–1804. doi: 10.1002/hep.29358. [DOI] [PubMed] [Google Scholar]
- 95.Siqueira-Batista R., De Souza Bayão T., Do Carmo Cupertino M., Alfred Joseph Mayers N., Patrícia Gomes A. Sofosbuvir Use for Yellow Fever: A New Perspective Treatment. Pathog. Glob. Health. 2019;113:207–208. doi: 10.1080/20477724.2019.1679556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mumtaz N., Jimmerson L.C., Bushman L.R., Kiser J.J., Aron G., Reusken C., Koopmans M.P.G., van Kampen J.J.A. Cell-Line Dependent Antiviral Activity of Sofosbuvir Against Zika Virus. Antivir. Res. 2017;146:161–163. doi: 10.1016/j.antiviral.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 97.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H.C., Larson N., Strickley R., Wells J., Stuthman K.S., Van Tongeren S.A., Garza N.L., Donnelly G., Shurtleff A.C., Retterer C.J., Gharaibeh D., Zamani R., Kenny T., Eaton B.P., Grimes E., Welch L.S., Gomba L., Wilhelmsen C.L., Nichols D.K., Nuss J.E., Nagle E.R., Kugelman J.R., Palacios G., Doerffler E., Neville S., Carra E., Clarke M.O., Zhang L., Lew W., Ross B., Wang Q., Chun K., Wolfe L., Babusis D., Park Y., Stray K.M., Trancheva I., Feng J.Y., Barauskas O., Xu Y., Wong P., Braun M.R., Flint M., McMullan L.K., Chen S.S., Fearns R., Swaminathan S., Mayers D.L., Spiropoulou C.F., Lee W.A., Nichol S.T., Cihlar T., Bavari S. Therapeutic Efficacy of the Small Molecule GS-5734 Against Ebola Virus in Rhesus Monkeys. Nature. 2016;531:381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amirian E.S., Levy J.K. Current Knowledge About the Antivirals Remdesivir (GS-5734) and GS-441524 as Therapeutic Options for Coronaviruses. One Health. 2020;9:100128. doi: 10.1016/j.onehlt.2020.100128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agostini M.L., Andres E.L., Sims A.C., Graham R.L., Sheahan T.P., Lu X., Smith E.C., Case J.B., Feng J.Y., Jordan R., Ray A.S., Cihlar T., Siegel D., Mackman R.L., Clarke M.O., Baric R.S., Denison M.R. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio. 2018;9 doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wu K. The New York Times; 2020. Gilead to Test a Version of Remdesivir That Can Be Inhaled. [Google Scholar]
- 101.Bravo J.P.K., Dangerfield T.L., Taylor D.W., Johnson K.A. Remdesivir is a Delayed Translocation Inhibitor of SARS-CoV-2 Replication. Mol. Cell. 2021;81:1548–1552.e4. doi: 10.1016/j.molcel.2021.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Good S.S., Westover J., Jung K.H., Zhou X.J., Moussa A., La Colla P., Collu G., Canard B., Sommadossi J.P. AT-527, a Double Prodrug of a Guanosine Nucleotide Analog, Is a Potent Inhibitor of SARS-CoV-2 In Vitro and a Promising Oral Antiviral for Treatment of COVID-19. Antimicrob. Agents Chemother. 2021;65 doi: 10.1128/AAC.02479-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beasley D. Reuters; 2021. Inside the Race to Find a COVID-19 Treatment Pill. [Google Scholar]
- 104.Good S.S., Moussa A., Zhou X.-J., Pietropaolo K., Sommadossi J.-P. Preclinical Evaluation of AT-527, a Novel Guanosine Nucleotide Prodrug With Potent, Pan-Genotypic Activity Against Hepatitis C Virus. PLoS One. 2020;15 doi: 10.1371/journal.pone.0227104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Berliba E., Bogus M., Vanhoutte F., Berghmans P.-J., Good S.S., Moussa A., Pietropaolo K., Murphy R.L., Zhou X.-J., Sommadossi J.-P. Safety, Pharmacokinetics, and Antiviral Activity of AT-527, a Novel Purine Nucleotide Prodrug, in Hepatitis C Virus-Infected Subjects With or Without Cirrhosis. Antimicrob. Agents Chemother. 2019;63 doi: 10.1128/AAC.01201-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Michailidis E., Ryan E.M., Hachiya A., Kirby K.A., Marchand B., Leslie M.D., Huber A.D., Ong Y.T., Jackson J.C., Singh K., Kodama E.N., Mitsuya H., Parniak M.A., Sarafianos S.G. Hypersusceptibility Mechanism of Tenofovir-Resistant HIV to EFdA. Retrovirology. 2013;10:65. doi: 10.1186/1742-4690-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Michailidis E., Huber A.D., Ryan E.M., Ong Y.T., Leslie M.D., Matzek K.B., Singh K., Marchand B., Hagedorn A.N., Kirby K.A., Rohan L.C., Kodama E.N., Mitsuya H., Parniak M.A., Sarafianos S.G. 4′-Ethynyl-2-fluoro-2′-deoxyadenosine (EFdA) Inhibits HIV-1 Reverse Transcriptase With Multiple Mechanisms. J. Biol. Chem. 2014;289:24533–24548. doi: 10.1074/jbc.M114.562694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Stoddart C.A., Galkina S.A., Joshi P., Kosikova G., Moreno M.E., Rivera J.M., Sloan B., Reeve A.B., Sarafianos S.G., Murphey-Corb M., Parniak M.A. Oral Administration of the Nucleoside EFdA (4′-Ethynyl-2-Fluoro-2′-Deoxyadenosine) Provides Rapid Suppression of HIV Viremia in Humanized Mice and Favorable Pharmacokinetic Properties in Mice and the Rhesus Macaque. Antimicrob. Agents Chemother. 2015;59:4190–4198. doi: 10.1128/AAC.05036-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Deng C.L., Yeo H., Ye H.Q., Liu S.Q., Shang B.D., Gong P., Alonso S., Shi P.Y., Zhang B. Inhibition of Enterovirus 71 by Adenosine Analog NITD008. J. Virol. 2014;88:11915–11923. doi: 10.1128/JVI.01207-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Deng Y.Q., Zhang N.N., Li C.F., Tian M., Hao J.N., Xie X.P., Shi P.Y., Qin C.F. Adenosine Analog NITD008 Is a Potent Inhibitor of Zika Virus. Open Forum Infect. Dis. 2016;3 doi: 10.1093/ofid/ofw175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Enosi Tuipulotu D., Fumian T.M., Netzler N.E., Mackenzie J.M., White P.A. The Adenosine Analogue NITD008 has Potent Antiviral Activity Against Human and Animal Caliciviruses. Viruses. 2019;11 doi: 10.3390/v11060496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lo M.K., Shi P.Y., Chen Y.L., Flint M., Spiropoulou C.F. In Vitro Antiviral Activity of Adenosine Analog NITD008 Against Tick-Borne Flaviviruses. Antivir. Res. 2016;130:46–49. doi: 10.1016/j.antiviral.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schul W., Liu W., Xu H.Y., Flamand M., Vasudevan S.G. A Dengue Fever Viremia Model in Mice Shows Reduction in Viral Replication and Suppression of the Inflammatory Response After Treatment With Antiviral Drugs. J. Infect. Dis. 2007;195:665–674. doi: 10.1086/511310. [DOI] [PubMed] [Google Scholar]
- 114.Wu R., Smidansky E.D., Oh H.S., Takhampunya R., Padmanabhan R., Cameron C.E., Peterson B.R. Synthesis of a 6-Methyl-7-Deaza Analogue of Adenosine That Potently Inhibits Replication of Polio and Dengue Viruses. J. Med. Chem. 2010;53:7958–7966. doi: 10.1021/jm100593s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Eyer L., Valdés J.J., Gil V.A., Nencka R., Hřebabecký H., Šála M., Salát J., Černý J., Palus M., De Clercq E., Růžek D. Nucleoside Inhibitors of Tick-Borne Encephalitis Virus. Antimicrob. Agents Chemother. 2015;59:5483–5493. doi: 10.1128/AAC.00807-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Nelson J., Roe K., Orillo B., Shi P.Y., Verma S. Combined Treatment of Adenosine Nucleoside Inhibitor NITD008 and Histone Deacetylase Inhibitor Vorinostat Represents an Immunotherapy Strategy to Ameliorate West Nile Virus Infection. Antivir. Res. 2015;122:39–45. doi: 10.1016/j.antiviral.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Vittori S., Dal Ben D., Lambertucci C., Marucci G., Volpini R., Cristalli G. Antiviral Properties of Deazaadenine Nucleoside Derivatives. Curr. Med. Chem. 2006;13:3529–3552. doi: 10.2174/092986706779026228. [DOI] [PubMed] [Google Scholar]
- 118.Westover J.B., Mathis A., Taylor R., Wandersee L., Bailey K.W., Sefing E.J., Hickerson B.T., Jung K.H., Sheridan W.P., Gowen B.B. Galidesivir Limits Rift Valley Fever Virus Infection and Disease in Syrian Golden Hamsters. Antivir. Res. 2018;156:38–45. doi: 10.1016/j.antiviral.2018.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Julander J.G., Bantia S., Taubenheim B.R., Minning D.M., Kotian P., Morrey J.D., Smee D.F., Sheridan W.P., Babu Y.S. BCX4430, a Novel Nucleoside Analog, Effectively Treats Yellow Fever in a Hamster Model. Antimicrob. Agents Chemother. 2014;58:6607–6614. doi: 10.1128/AAC.03368-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lim S.-Y., Osuna C.E., Best K., Taylor R., Chen E., Yoon G., Kublin J.L., Schalk D., Schultz-Darken N., Capuano S., Safronetz D., Luo M., Maclennan S., Mathis A., Babu Y.S., Sheridan W.P., Perelson A.S., Whitney J.B. A Direct-Acting Antiviral Drug Abrogates Viremia in Zika Virus–Infected Rhesus macaques. Sci. Transl. Med. 2020;(12) doi: 10.1126/scitranslmed.aau9135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.De Clercq E. New Nucleoside Analogues for the Treatment of Hemorrhagic Fever Virus Infections. Chem. Asian J. 2019;14:3962–3968. doi: 10.1002/asia.201900841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Evans G.B., Tyler P.C., Schramm V.L. Immucillins in Infectious Diseases. ACS Infect. Dis. 2018;4:107–117. doi: 10.1021/acsinfecdis.7b00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Elfiky A., Ribavirin A. Remdesivir, Sofosbuvir, Galidesivir, and Tenofovir Against SARS-CoV-2 RNA Dependent RNA Polymerase (RdRp): A Molecular Docking Study. Life Sci. 2020;253:117592. doi: 10.1016/j.lfs.2020.117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.https://coronavirus.jhu.edu/map.html