Abstract
Cardiomyocytes generate force for the contraction of the heart to pump blood into the lungs and body. At the same time, they are exquisitely tuned to the mechanical environment and react to e.g. changes in cell and extracellular matrix stiffness or altered stretching due to reduced ejection fraction in heart disease, by adapting their cytoskeleton, force generation and cell mechanics. Both mechanical sensing and cell mechanical adaptations are multiscale processes. Receptor interactions with the extracellular matrix at the nanoscale will lead to clustering of receptors and modification of the cytoskeleton. This in turn alters mechanosensing, force generation, cell and nuclear stiffness and viscoelasticity at the microscale. Further, this affects cell shape, orientation, maturation and tissue integration at the microscale to macroscale. A variety of tools have been developed and adapted to measure cardiomyocyte receptor-ligand interactions and forces or mechanics at the different ranges, resulting in a wealth of new information about cardiomyocyte mechanobiology. Here, we take stock at the different tools for exploring cardiomyocyte mechanosensing and cell mechanics at the different scales from the nanoscale to microscale and macroscale.
Keywords: Mechanobiology, Force, Cardiomyocytes, Cardiac tissue, Scale, Tools
Introduction
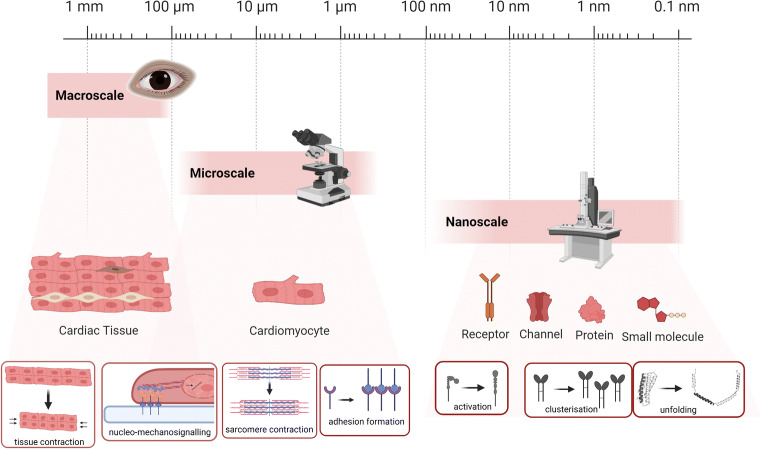
It is now clear that mechano-regulation is an integral part of cells and tissues in both physiological and pathological conditions (Beedle et al. 2017; Rivas-Pardo et al. 2016). Along with the recognition of mechanobiology as an indispensable partner of biological samples studies, new techniques emerge to address this topic. The advent of new tools advanced our fundamental knowledge of mechano-regulation in a range of cell and tissue types. Likewise, understanding mechanosensing and transduction processes is pivotal for explaining cardiovascular physiology and pathology (Sit et al. 2019; Ward and Iskratsch 2020). The heart is experiencing different types of forces such as shear stress and tensile force (Lu and Kassab 2011; Lunkenheimer et al. 2004). Structural and mechanical hierarchies span from nanoscale to macroscale. The interconnection through the extracellular matrix leads to sensing of macroscale forces at specific mechanosensor molecules, which again leads to mechanical control of cell fate switching and tissue development (Ingber 2008). Most (mechanobiology) studies focus on a single scale, and mechanical measurements of single myofibrils, cardiomyocytes, trabeculae or cardiac tissue individually shed more light on the overall cardiac mechanobiology (Brady et al. 1979; Carson et al. 2016; Kim et al. 2006; Saleem et al. 2020). Here, we allocated various approaches that map cardiac mechanical alterations based on the scale of operation, nano, micro and macro (Fig. 1). We will progressively introduce tools in their respective range of action, subsequently illustrating the obtained outcome. This review will disseminate the different techniques and newest data that have been gained from these studies. Work carried out at different scales and by different techniques resulted in confounding results every so often (examples are the different mechanical measurements of the cardiac stiffness, different forces necessary to unfold monomers or higher ordered structures, such as filaments or fibrils, or different types of forces measured with nano vs micropillars) (Ghassemi et al. 2012; Meacci et al. 2016; Roca-Cusachs et al. 2012; Ward and Iskratsch 2020). Therefore, it is becoming ever clearer that approaches, models and theories are needed that bridge the scales in cardiovascular mechanobiology and mechanobiology in general (Regazzoni et al. 2020). In the last paragraph, we will discuss the challenges and future perspectives for the cardiac mechanobiology techniques, including attempts to integrate the data from the different scales for an improved understanding of cardiomyocyte mechanical sensing.
Fig. 1.
Studying cardiac biology across the scales. Nanoscale platforms enable the study of forces and mechanosensitive dynamics at single molecule, single adhesion or single adhesion cluster level that effectively cause protein activation, cluster formation or protein (domain) unfolding. Microscale and macroscale tools allow studying intracellular changes, the cell–cell or cell–ECM cross-talk
Cardiovascular mechanosensing at the nanoscale: from single molecules to single adhesions
In recent years, a surge in new nanoscale approaches can be observed that aim to study forces and mechanosensitive dynamics at single molecule, single adhesion or single adhesion cluster level (Fig. 2). Despite the dimensions, the final effect can be likewise observed at a bigger scale (Sanchez-Alonso et al. 2020). These approaches include studies applying forces at single molecules using magnetic tweezers, laser traps or atomic force microscopes; or measurement of forces and protein dynamics at the single adhesion level using nanofabricated tools such as nanopillars or nanopatterns. Other nanofabrication techniques are being employed to study the effect of topography or ligand presentation on cell behaviour (nanopatterns, nanofibers, nanotubes or nanowires).
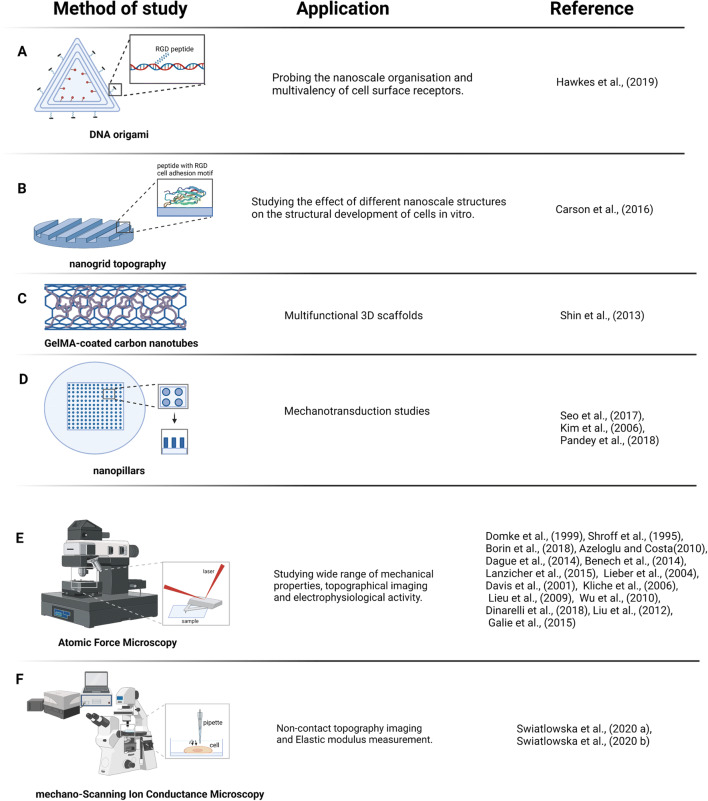
Fig. 2.
Selected nanoscale tools that have been employed for the investigation of cardiovascular mechanobiology (first column), as well as their applications (second column) and respective references (third column). (A) DNA origami, (B) nanogrid topography, (C) gelatin methacryloyl (GelMa)-coated carbon nanotubes, (D) nanopillars, (E) atomic force microscopy and (F) mechano-scanning ion conductance microscopy. The increasing number of available tools, as well as the number of studies using these tools, reflects the importance of the processes happening at the nanoscale
Single-molecule studies — magnetic tweezers, optical tweezers and atomic force microscopy (AFM)
Applying forces onto single molecules gives insights into nanoscale mechanics, including force-dependent domain folding and unfolding events, opening of catalytic domains or cryptic binding sites that change protein–protein interactions and ultimately determine mechanosensitive responses at the cellular scale. The main techniques in general and especially for cardiovascular mechanobiology are AFM, magnetic and optical tweezers. The details of the techniques have been discussed elsewhere (e.g. by Neuman and Nagy 2008) and are out of scope for this short review, but briefly, all techniques measure force-dependent change in length of a single molecule that is attached to either the tip of the cantilever (AFM) or a bead (magnetic tweezer, optical tweezer) on one side and a solid surface or another bead on the other end. Depending on the technique, forces are applied through a piezo controlled cantilever (AFM), a magnetic field on paramagnetic beads (magnetic tweezers), or a focused laser beam onto a dielectric bead (optical tweezers) (Neuman and Nagy 2008).
Notable studies that are pertinent for the cardiovascular field are especially experiments exploring the mechanics of titin, which is a major contributor to cardiomyocyte mechanics. Here, recent studies investigated the unfolding and the refolding of titin domains that could contribute to the force generation in muscle cells (Mártonfalvi et al. 2017; Rivas-Pardo et al. 2016), although the relevance for cardiac muscle might be limited due to much smaller amount of force generated through this mechanism, compared to muscle myosin contractions, as well as a 60-fold slower shortening velocity (Bianco et al. 2016). Other single-molecule studies on titin observed the modulation of titin stiffness through oxidative folding (Beedle et al. 2017) or mechanical activation of ATP binding to the titin kinase domain (Puchner et al. 2008), albeit this has been since identified as a pseudokinase, which nonetheless is involved in scaffolding processes of regulatory significance (Bogomolovas et al. 2014; Lange et al. 2005).
Looking at the cell-matrix interface, especially two molecules of relevance have been studied using such single-molecule approaches: talin and dystrophin (del Rio et al. 2009; Haining et al. 2018; Le et al. 2018; Yao et al. 2016). Talin was first identified to unfold cryptic binding sites to enable vinculin binding (del Rio et al. 2009) and later also to regulate the interaction with the Rho GTPase activating protein DLC1 (Haining et al. 2018) and potentially additional binding partners (Yao et al. 2016). Recently, magnetic tweezer studies suggested dystrophin, which is the central component of the dystrophin–glycoprotein complex that links the extracellular matrix with the actin cytoskeleton, to act as molecular shock adsorber through force-dependent unfolding and refolding of its spectrin domains (Le et al. 2018). Overall, these studies reinforce the idea that cells such as cardiomyocytes are finely tuned to a specific mechanical environment and use mechanosensitive proteins to react to small changes in the force landscape.
Measuring cellular forces at the nanoscale — nanopillars
Nanopillars, typically fabricated by using e-beam lithography to make a negative master, followed by PDMS soft lithography, have been shown to be a very practical tool in the mechanical characterization of different cell types, including cardiomyocytes. These can at the same time mimic substrate stiffness — typically modulated by adjusting the pillar aspect ratio — and be used to measure the cellular forces. Together, this allows obtaining detailed information of cell–ECM interactions and cell mechanics. Compared to micropillars, nanopillars are recognised by cells as uniform surfaces and fibroblast adhesions spread over multiple pillars instead of forming around individual posts. Therefore, nanopillars can be used to pick up forces that are generated e.g. during early adhesion formation (Iskratsch et al. 2013; Wolfenson et al. 2014; Haguy Wolfenson et al. 2015). Applied to a study of cardiovascular mechanosensing, our previous work could demonstrate cardiomyocyte rigidity sensing depending on slow non-muscle myosin and fast muscle myosin contractions. This results in oscillating stretching of talin protein on physiological substrates but continuous stretching on fibrotic stiffness (Pandey et al. 2018). Other groups used nanopillars to further study the effect on cardiomyocyte differentiation and behaviour. Nanoscaled gradient pillar patterned plates used by Seo et al. increased cardiomyocyte differentiation, showing highly organised sarcomere formation and mature cardiac gene expression on 200–280 nm-sized pillars. This process was associated with phospho-cofilin mediated actin cytoskeleton reorganisation (Seo et al. 2017). Nanopillar platforms were also applied to neonatal rat cardiomyocytes cultures. Poly(ethylene glycol) (PEG) hydrogel pillars have shown lower cell adhesion but higher action potential amplitude (Kim et al. 2006).
Contact points between the cell and extracellular matrix serve as anchoring points and mechanotransduction nodes activating further signalling pathways. The cell mechanosensing is influenced not only by stiffness but also by the type and organisation of the extracellular matrix molecules or respective receptors and e.g. different integrins show different mechanosensing behaviours (Elosegui-Artola et al. 2014; Schvartzman et al. 2011; Ward and Iskratsch 2020). To study the organisation of receptors, for instance integrins, DNA origami nanoarrays functionalized with peptides have been employed to examine cardiomyocyte–ECM interaction at the single receptor (cluster) level. Studies on neonatal rat cardiomyocytes demonstrated that both the distance between individual integrin ligands and the density of the ligands influence cardiomyocyte adhesion — in contrast to fibroblasts which only respond to the inter-ligand distance (Hawkes et al. 2019). Since costameres connect the cytoskeleton to the ECM not only through integrins and associated proteins, but also through the dystrophin–glycoprotein complex (DGC), it will be intriguing to expand these studies onto different adhesion systems or to study the cross-talk between the receptors as done e.g. between EGF and integrins in cancer cells (Huang et al. 2018).
Measuring cardiomyocyte mechanics at the nanoscale — AFM and scanning ion conductance microscopy (SICM)
AFM
Application of atomic force microscopy (AFM) for biological sample studies, including cardiomyocytes, started in the 90s. First reports showed a link between regional cell stiffness to changes in the cytoskeleton. Moreover, AFM was employed to measure cell contractile activity by following the cantilever deflection over time with nanoscale resolution (Domke et al. 1999; Shroff et al. 1995). These first studies sparked interest in this method and inspired researchers to use it for a range of different research questions. To date, AFM has been employed to study cardiomyocyte properties, such as topographical changes, contraction activity and mechanics (Borin et al. 2018). The elastic modulus was assessed in different states (diastole and systole) to assess chemically induced cellular changes, effects of knockouts or disease-causing mutations. These studies pointed out key proteins that regulate the elastic modulus, such as actin and vinculin, or whole membrane microdomains (Azeloglu and Costa 2010; Benech et al. 2014; Dague et al. 2014; Lanzicher et al. 2015). Using AFM, age was also shown to be an important determinant of the elastic modulus, whereby cardiomyocytes isolated from 30-month-old rats were stiffer than cells from 4-month-old animals, suggesting that change in single cardiomyocyte mechanical properties can also contribute to left ventricular diastolic dysfunction observed in elder patients (Lieber et al. 2004).
AFM as an imaging tool was applied to resolve high-resolution sarcolemma features in adult guinea pig cardiomyocytes (Davis et al. 2001), aldosterone-mediated sarcolemma changes in mouse neonatal cardiomyocytes (Kliche et al. 2006) and used to demonstrate lack of t-tubular cardiomyocyte membrane invaginations in mouse and human embryonic stem cell-derived cardiomyocytes (ESC-CMs) (Lieu et al. 2009). Moreover, since the cardiomyocyte membrane presents a rich repertoire of different receptors that play a major role in the overall cell physiology, coating the AFM tip with specific protein allowed to measure adhesion forces that were shown to be disrupted in mutant cardiomyocytes (Lanzicher et al. 2015; Wu et al. 2010).
When AFM was used to measure contraction dynamics of hiPSC-derived cardiomyocytes from control and myotonic dystrophy type 1 patients, a higher mechanical resistance was observed based on altered beating impulse or beat duration (Dinarelli et al. 2018). Similarly, a multi-parameter AFM-based study measured contraction force, rate and duration as well as elastic modulus in hESC-CM and iPSC-CM, allowing to map spatial heterogeneity of height, elastic modulus and contraction force, together demonstrating aberrant contractility and mechanical properties in iPSC–cardiomyocytes from dilated cardiomyopathy patients (DCM) patients (Liu et al. 2012). Also, AFM was also used to stimulate cell contraction in a cardiac microtissue in vitro (Galie et al. 2015), by applying oscillating indentations to train the cells. Further modifications to the system present the possibility to study depolarization and repolarization wavefronts.
SICM
A non-contact, high-resolution mechano-scanning ion conductance microscopy (mechano-SICM) technique, measuring the transverse Young’s modulus (tYM) by a constant pressure application through a nanopipette was recently used to investigate specific membrane subdomains, enabled by the excellent resolution in all dimensions (Swiatlowska et al. 2020a; Swiatlowska et al. 2020b). When applying pressure, the probe’s vertical position is recorded, and a deformation map is generated from which the tYM can be calculated and corrected for the uneven geometry. Both tYM and topography maps are recorded simultaneously and non-invasively, leaving the cell intact in non-contact mode. This work demonstrated increased tYM in cardiomyocytes from a myocardial infarction (MI) rat model, where the mechanical load is high compared to control animals. On the other hand, in cardiomyocytes from a load-deficient MI model, the tYM was reduced. Observed changes were due to an altered microtubular network that has also been shown to regulate the modified tYM in the Angiotensin II-treated adult rat cardiomyocytes (Swiatlowska et al. 2020a; Swiatlowska et al. 2020b). The high resolution and non-invasive nature of the contactless scanning mode are major benefits of this platform, suggesting great potential for expanding the use to other mechano-regulatory cell measurements.
Nanotopography sensing and tools for controlling cardiomyocyte differentiation and behaviour
Different tools have been employed to study topography sensing at the nanoscale. Fundamental knowledge of the topography sensing mechanisms has further influenced the design of nanotopographies that mimic the native tissue in vitro and thus improve the maturity and function of cardiomyocytes. These include nanofibers or nanowires.
Nanofibers
Obtaining induced pluripotent stem cell-derived cardiomyocytes (iPSC-CM) functionally and structurally similar to adult cardiomyocytes is still very challenging. Moreover, one of the overlooked aspects in graft implantation is the maintenance of cell directionality. If poorly performed, region-specific alterations are observed, and myofiber orientation differences between the transplant and diseased tissue occur. However, carefully designed topographies can be harnessed for improved maturation. This was shown for instance when iPSC-CM were plated on nanogrooved topographies, which were mimicking ECM fibre orientation and were functionalised with RGD as cell adhesive peptides (Carson et al. 2016). Morphological analysis of cell alignment and area, as well as sarcomere length, suggested that cells plated on 800-nm diameter grooves improved maturation of the cardiomyocytes and showed the strongest resemblance to adult-like phenotypes among all nanotopographic patterns that were studied (Carson et al. 2016). In order to recapitulate the extracellular matrix (ECM) organisational structure, Lin et al. (2014) produced aligned and randomly oriented electrospun patches. Cardiomyocytes plated on aligned substrates demonstrated improved beating capabilities and, after patching onto infarcted hearts, showed significantly enhanced performance in vivo, as measured by improved hemodynamics, electrocardiography, optical mapping or reduced infarct size, as well as demonstrated by cell morphology with aligned anisotropic cardiomyocytes present after implantation; all are important features for therapeutic application (Lin et al. 2014). Similarly, when aligned nanofiber scaffolds were used to mimic ECM organisation, iPSC-cardiac progenitor cells showed improved cardiac maturation as shown by increased cTnt-positive cells, elongated nuclei and synchronised Ca2+ fluctuations (Ding et al. 2020).
Nanotubes
The advent of carbon nanotubes (CNTs) sparked a lot of interest in the biological field, opening doors for new cross-discipline studies. These 1–100 nm diameter graphene, cylindrically shaped structures offer good mechanical, electrical and thermal properties that enabled to use them in scaffolds in different forms. Although typically not used to modify the topography but rather for electrical excitation or sensing, it is noted in several studies that cardiomyocytes interact specifically with the nanotubes, forming a nanofibrous network (Martinelli et al. 2013b; Shin et al. 2013), and hence, this is discussed here as well. Studies on neonatal cardiomyocytes show that CNT substrates had a positive outcome on cell physiology, presenting higher cell viability, proliferation, tighter cell–cell contacts and improved electrophysiological parameters (Martinelli et al. 2013b). In a different study from the same group, CNTs also showed a protective effect from pathological hypertrophy by demonstrating no change in the gene expression profiles following phenylephrine stimulation. Together, this work illustrates the higher maturity of cardiomyocytes and disease preventive properties of CNTs (Gerwig et al. 2012; Martinelli et al. 2013a; Martinelli et al. 2013b). Also, an improvement towards cardiomyocyte lineage differentiation from mesenchymal stem cells (MSCs) was observed using CNT platforms. Here, by taking the advantage of the electrical properties, stimulated MSCs re-oriented and showed elongated morphology. Additionally, an increase in GATA-4, Nkx2.5, connexin43 and cardiac troponin T was observed (Mooney et al. 2012). To improve biocompatibility, biodegradability, electrical and mechanical properties, a CNT platform was developed, in which the nanostructures were homogenously embedded in gelatin methacrylate (GelMA) hydrogels with highly porous structures for tissue development. This new platform demonstrated improved cell adhesion, organisation, cell–cell coupling, mechanical integrity and electrophysiological properties in neonatal rat cardiomyocytes when compared to GelMA only arrays (Shin et al. 2013). Also, a protective effect from doxorubicin and heptanol was detected, together demonstrating the potential of CNT containing scaffolds for cardiovascular applications.
Nanowires
By looking at the specific nature of myocardial tissue architecture, it is noticeable that cellular orientation in the tissue is not random. Different techniques have been employed to address cardiomyocyte alignment in vitro cultures. Laser-patterned linear nanowires resulted in human cardiomyocyte alignment (Kiefer et al. 2014). In another study, fabricated gold nanowires (AuNWs) were incorporated in hydrogels, such as alginate or GelMA. Both studies showed that neonatal rat cardiomyocytes had a more mature state when cultured on NW hydrogels versus hydrogels alone (Dvir et al. 2011; Li et al. 2020).
Translational approaches based on nanofabrication
Nanoscale techniques have been increasingly applied for drug testing purposes. Hart et al. generated a new in vitro platform that employed nanostructured interdigitated electrodes (nIDEs) patterned on polyacrylonitrile. The long-term culture of iPSC cardiomyocytes demonstrated that these nanopatterned arrays are highly sensitive and suitable for cardiotoxicity testing (Hart et al. 2018). On the other hand, inotropic and chronotropic drug effects were tested using AFM contractility measurements (Chang et al. 2013; Liu et al. 2012). The same technique was used to demonstrate an increase in membrane roughness in neonatal rat cardiomyocytes, following a hypertrophy protective drug uptake (Yang et al. 2013).
Microscale tools
At the microscale, cardiomyocytes apply forces onto (and sense forces from) neighbouring cardiomyocytes and the extracellular matrix. Notably, the maturity and function of cardiomyocytes are affected at this scale by cell shape and extracellular matrix elasticity; hence, tools have been developed and applied to mimic and correlate between these healthy and diseased hearts. Early studies by the Discher lab established a clear correlation between cell differentiation and function with the substrate elasticity of polyacrylamide gels, whereby myogenic differentiation of MSCs and optimal work of quail cardiomyocytes were observed at a stiffness found in the native heart (~10 kPa) (Engler et al. 2004, 2008). Subsequently, similar results were obtained with a range of other materials and fabrication techniques, including PDMS, poly-e-caprolactone or PEG (Forte et al. 2012; Pandey et al. 2018; Wan et al. 2019), whereby the latter was also used to pattern the surface at the same time and found that patterning and stiffness together affected gene expression. Similarly, micropatterning was used to look at both healthy and fibrotic elastic moduli in combination with cardiomyocyte shape (i.e. aspect ratios of cardiomyocytes in healthy hearts, ~ 7:1; hypertrophic hearts, ~ 5:1; or dilated hearts, ~ 11:1), which suggests that the ECM elastic modulus regulates cardiomyocyte shape in order to obtain the most efficient contractile properties (McCain et al. 2014). Physiological shape and stiffness (10 kPa) were also shown to improve the cellular function of hPSC-CM. Defects in the myofibrils accumulated when increasing the substrates Young’s modulus, while keeping the aspect ratio constant at 7:1 (Ribeiro et al. 2015). Consequently, using patterned surfaces that impose biophysical cues such as geometrical constraint in 2D or 3D (eventually regulating cell shape) and substrate stiffness demonstrates a suitable approach to increase cardiomyocyte maturation and improve electrophysiological properties (Guo and Pu 2020).
Compared to nanopillars (see previous text), micropillars have been more widely used to study the mechanobiology of cardiomyocytes at different developmental stages. Micropost arrays coated with different ECM proteins showed no difference in contractile properties of hiPSC-CMs between laminin, fibronectin and collagen coatings. However, higher contractile properties were observed after thyroid hormone T3 treatment (Beussman et al. 2016; Rodriguez et al. 2014). HiPSC-CM cultured on micropillars exhibited different beating frequencies, elastic modulus, calcium signalling and sarcomere and integrin organisation compared to planar substrates (Palankar et al. 2016). Also, a more mature state of cultured neonatal rat myocytes was observed when these were cultured on microposts, inferred from higher twitch force, correlating with improved sarcomere organisation and intracellular calcium (Rodriguez et al. 2011).
Equally important to cell–ECM interaction is the cell–cell communication that includes a mechano-electrical activity. Using controlled substrate deformation similar to those exerted by neighbouring cardiomyocytes, microenvironment mechanical properties influenced cell mechanical coupling and hence led to synchronisation of the electrical functionality (Nitsan et al. 2016). Similarly, neonatal cardiomyocyte pairs on micropatterned surfaces demonstrated regularly repeating Ca2+ transients. These homogeneous cardiomyocyte pairs formed high traction stresses that were distributed at the lateral ends of the islands. In contrast, heterogeneous cell pairs of neonatal cardiomyocytes and stem cell-derived cardiomyocytes resulted in a tension variability with region-specific differences, with additional high stresses at the cell–cell junction (Aratyn-Schaus et al. 2016).
Micropatterned fibronectin islands on hydrogel supports were further used for the formation of multicellular ‘mini-tissues’, which showed stiffness dependent differences (Pasqualini et al. 2018). Cells on soft hydrogels (1 kPa) demonstrated shorter sarcomeres length and lower myofibrillar packing density and consequentially generated reduced contractile stresses in traction force microscopy (TFM) measurements when compared to tissues on normal (13 kPa) and stiff surfaces (90 kPa). Similar to previous observations (Engler et al. 2008), stresses increased with stiffness, while maximal contractile work was observed on normal gel stiffness (Pasqualini et al. 2018). Interestingly, metabolic measurements of basal respiration and ATP production suggested an inverse correlation with stiffness, while the spare respiratory capacity was independent of stiffness, suggesting that the additional available ATP was used for non-contractile purposes, such as cytoskeletal maturation.
In addition to TFM, intracellular and extracellular Förster resonance energy transfer (FRET) tension sensors have gained popularity, including cardiovascular research (Pandey et al. 2018). These are included here as microscale tools, due to optical limitations to the resolution for the detection of the forces, although super-resolution microscopy has been recently employed to surpass these limitations (Schlichthaerle et al. 2021). FRET tension sensors build on DNA double strands, DNA hairpins (both for extracellular application) or elastic peptides (for intracellular sensors). These force-sensitive elements are flanked by fluorescent molecules or quenchers that can be used to quantify the energy transfer as a function of the distance and after calibration with e.g. magnetic tweezers, as function of force (recently reviewed together with TFM and other tools in Lavrenyuk et al. 2021). Especially the intracellular tension sensors are promising tools to study poorly understood cardiomyocyte Z-disc, M-band or intercalated discs mechanobiology.
Micro to macro
Employing microscale and nanoscale tools greatly improved our knowledge of cardiomyocyte mechanobiology. However, macroscale tools enabling tissue level experiments are needed for a comprehensive understanding at higher organisational levels (Fig. 3). Engineered heart tissues (EHTs) have emerged as a physiological in vitro platform for heart research that is now widely in use. EHTs are 3D cardiac tissue-like structures able to generate contractile force. These commonly used platforms are produced in different shapes from different cells, such as neonatal cardiomyocytes or stem cell-derived cardiomyocytes, which are undergoing either general or chamber specific differentiation programmes (Breckwoldt et al. 2017; Eschenhagen et al. 2002; Goldfracht et al. 2020; Lemme et al. 2018; Schaaf et al. 2011). Mixed with extracellular matrices, for instance, collagen or fibrinogen, heart tissues mature and allow for the analysis of contractile force in dependence of mutations (e.g. ANKRD1 or alpha-actinin 2) (Crocini et al. 2013; Prondzynski et al. 2019), drug treatments (Mannhardt et al. 2016) or presence of additional cell types, such as fibroblasts (Liau et al. 2011), or epicardial cells. In this way, they are used to mimic heart disease or study potential therapies (Bargehr et al. 2019; Hawkes et al. 2019). EHT platforms allow for a range of molecular biology, electrophysiology and force measurement experiments (Goldfracht et al. 2020; Mannhardt et al. 2016; Saleem et al. 2020; Schaaf et al. 2011). Continuous contractile work and tissue spanning between the silicone posts is a probable reason for a good cell alignment and sarcomere organisation based on histological experiments, indicating structural maturation (Schaaf et al. 2011). EHTs have been studies not only as a potential tool for cardiac repair but also for toxicology studies and disease modelling (El-Armouche et al. 2007; Mosqueira et al. 2018; Mühlhäuser et al. 2006; Zimmermann et al. 2006). Work from Xu et al. (2015) presented magnetic actuation as a mechanical stimulator for the microtissue assembled around micropillars, with a potential application to different cell types. In order to study mechano-electrical coupling, Galie et al. (2015) designed a 3D in vitro model of neonatal cardiomyocyte-fibroblast suspended in fibrin-collagen gel (called μTUGs) with incorporated AFM to mechanically control the stimulation. This system allows for direct quantification of contraction velocity and force magnitude, defining properties of striated muscle. Further, Boudou et al. modified previously generated microfabricated tissue gauges (μTUG) for cardiac microtissues (CMTs) that are formed by mixing neonatal cardiomyocytes with collagen-fibrin matrices. This platform allowed to investigate electrical stimulation, mechanical load, matrix stiffness and chemical stimulation on structural and functional properties of CMTs with a potential for further drug screening application (Boudou et al. 2012).
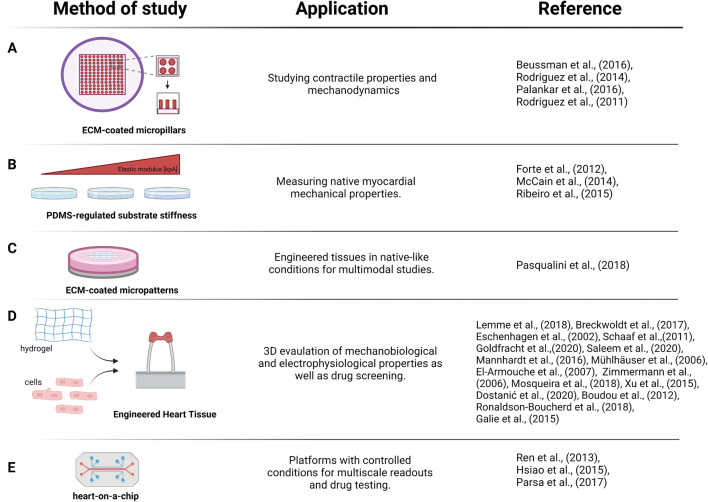
Fig. 3.
Selected micro and macro tools that have been employed for the investigation of cardiovascular mechanobiology (first column), as well as their applications (second column) and respective references (third column). (A) ECM-coated micropillars, (B) PDMS substrates, (C) ECM-coated micropatterns, (D) engineered heart tissues and (E) heart on a chip. Microscale and macroscale models have allowed the investigation of complex multicellular behaviours, mechanics and mechanobiology
Recent developments are aimed at reducing the size and maturity of the tissues. A mini-EHT model was recently developed, where a reduced cell number (down 16,000 cells compared to ~500,000 cells in normal EHTs) was needed for contractile measurement (Dostanić et al. 2020). To improve maturity, Ronaldson-Bouchard et al. (2018) varied the stimulation frequency during the differentiation, resulting in an adult-like gene expression profile, electrophysiology and tissue ultrastructure. Nunes et al. used both hESC- and hiPSC-derived cardiomyocytes, combining structural and electrical stimulators to generate biowire platforms. Cell suspension in collagen was seeded into the main channel around a suture with progressively increasing electrical stimulation. Biowires exhibited improved organisation, electrophysiological and Ca2+ handling properties when compared to a non-stimulated platform (Nunes et al. 2013).
In addition to EHTs, microfluidic heart-on-a-chip platforms have been developed by different groups to study multicellular microtissues. Ren et al. investigated the H9c2 embryonic cardiomyocyte-like cell line under hypoxic conditions, which showed changes in cell size, mitochondria and suggested more cellular apoptotic events (Ren et al. 2013). The same cell line was also used by Hsiao et al. to investigate hydraulic pressure application, which resulted in bigger cell size and higher levels of natriuretic peptide. This effect could be reversed by a focal adhesion kinase blocker (Hsiao et al. 2015). A microfluidic high-throughput platform incorporating microtissues from small cell numbers (~5000 cells per tissue) and pneumatic mechanical stimulation was developed as a model for cardiac hypertrophy with volume overload and recapitulated the upregulation of the foetal gene programme, associated with this pathology (Parsa et al. 2017).
Finally, cardiac slices emerged as a good model to bridge the gap between in vitro and in vivo models (Pitoulis et al. 2021; Watson et al. 2019). These ultrathin 100–400 μm slices of living myocardium largely maintain tissue architecture, multicellular structure, physiology and load-induced remodelling and hence are an especially useful tool for cardiovascular research, at least until a similarly high degree of maturity can be achieved with in vitro models.
Conclusions
Cardiovascular diseases (CVD) are still the leading cause of death globally, estimated to reach 17.9 million deaths per year (World Health Organization 2021). Heart failure (HF) is most prevalent among all CVD diseases, whereby around 50% of the cases are identified as HF with reduced ejection fraction and the other half as HF with preserved ejection fraction (HFpEF). To date, there is no effective treatment for HFpEF. With the number of cases rising, this heart disease is posing a significant health burden. However, HF is just at the top of a long list of other heart diseases that need extensive research (Virani et al. 2020). Therefore, studying CVD is of utmost importance. A better understanding of the behaviour of cardiac pathologies is needed but, due to the complexity, requires a multiscale approach to get to the heart of the matter.
The collection of newly developed techniques and tools led to an increasing understanding of cardiomyocyte behaviour and function — temporally and spatially, at different scales. Applying these techniques led to new insights into cardiomyocyte structural properties, electrophysiology and mechanobiology and showed potential to be used as future drug screening platforms. Importantly, they also further established mechanobiology as an integral and crucial part of cardiomyocyte biology. Further interdisciplinary approaches and studies, spanning the different scales, will help to address open questions in cardiovascular research, including the identification of novel therapies for heart failure or how the mechanical environment affects pluripotent stem cells maturation for improved patient-specific models and personalized medicine approaches.
Acknowledgements
We would like to thank BBSRC (BB/S001123/1) and BHF (PG/20/6/34835) for their generous support. Figures were created with BioRender.com software.
Code availability
Not applicable.
Author contribution
The authors contributed equally to the manuscript preparation.
Funding
BBSRC new investigator award: BB/S001123/1, BHF project grant: PG/20/6/34835.
Data availability
Not applicable.
Declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Publication and licensing rights were granted for the use of Biorender.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aratyn-Schaus Y, Pasqualini FS, Yuan H, McCain ML, Ye GJC, Sheehy SP, Campbell PH, Parker KK. Coupling primary and stem cell-derived cardiomyocytes in an in vitro model of cardiac cell therapy. J. Cell Biol. 2016;212(4):389–397. doi: 10.1083/jcb.201508026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azeloglu EU, Costa KD (2010) Cross-bridge cycling gives rise to spatiotemporal heterogeneity of dynamic subcellular mechanics in cardiac myocytes probed with atomic force microscopy. Am. J. Physiol. Heart Circ. Physiol. 298(3). 10.1152/ajpheart.00427.2009 [DOI] [PubMed]
- Bargehr J, Ong LP, Colzani M, Davaapil H, Hofsteen P, Bhandari S, Gambardella L, Le Novère N, Iyer D, Sampaziotis F, Weinberger F, Bertero A, Leonard A, Bernard WG, Martinson A, Figg N, Regnier M, Bennett MR, Murry CE, Sinha S. Epicardial cells derived from human embryonic stem cells augment cardiomyocyte-driven heart regeneration. Nature Biotechnol. 2019;37(8):895–906. doi: 10.1038/s41587-019-0197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beedle AEM, Mora M, Lynham S, Stirnemann G, Garcia-Manyes S. Tailoring protein nanomechanics with chemical reactivity. Nat Comm. 2017;8(1):1–11. doi: 10.1038/ncomms15658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benech JC, Benech N, Zambrana AI, Rauschert I, Bervejillo V, Oddone N, Damián JP. Diabetes increases stiffness of live cardiomyocytes measured by atomic force microscopy nanoindentation. Am. J. Physiol. Cell Physiol. 2014;307(10):C910–C919. doi: 10.1152/ajpcell.00192.2013. [DOI] [PubMed] [Google Scholar]
- Beussman KM, Rodriguez ML, Leonard A, Taparia N, Thompson CR, Sniadecki NJ. Micropost arrays for measuring stem cell-derived cardiomyocyte contractility. Methods. 2016;94:43–50. doi: 10.1016/j.ymeth.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P, Reconditi M, Piazzesi G, Lombardi V. Is muscle powered by springs or motors? J. Muscle Res. Cell Motil. 2016;37(4–5):165–167. doi: 10.1007/S10974-016-9454-4. [DOI] [PubMed] [Google Scholar]
- Bogomolovas, J., Gasch, A., Simkovic, F., Rigden, D. J., Labeit, S., & Mayans, O. (2014). Titin kinase is an inactive pseudokinase scaffold that supports MuRF1 recruitment to the sarcomeric M-line. Open Biol. 4(MAY). 10.1098/RSOB.140041 [DOI] [PMC free article] [PubMed]
- Borin, D., Pecorari, I., Pena, B., & Sbaizero, O. (2018). Novel insights into cardiomyocytes provided by atomic force microscopy. In Seminars in cell and developmental biology (Vol. 73, pp. 4–12). Elsevier Ltd. 10.1016/j.semcdb.2017.07.003 [DOI] [PubMed]
- Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB, Chen CS. A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues. Tissue Engineering - Part A. 2012;18(9–10):910–919. doi: 10.1089/ten.tea.2011.0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady, A. J., Tan, S. T., & Ricchiuti, N. V. (1979). Contractile force measured in unskinned isolated adult rat heart fibres [22]. In Nature (Vol. 282, Issue 5740, pp. 728–729). Nature. 10.1038/282728a0 [DOI] [PubMed]
- Breckwoldt K, Letuffe-Brenière D, Mannhardt I, Schulze T, Ulmer B, Werner T, Benzin A, Klampe B, Reinsch MC, Laufer S, Shibamiya A, Prondzynski M, Mearini G, Schade D, Fuchs S, Neuber C, Krämer E, Saleem U, Schulze ML, et al. Differentiation of cardiomyocytes and generation of human engineered heart tissue. Nat. Protoc. 2017;12(6):1177–1197. doi: 10.1038/nprot.2017.033. [DOI] [PubMed] [Google Scholar]
- Carson, D., Hnilova, M., Yang, X., Nemeth, C. L., Tsui, J. H., Smith, A. S. T., Jiao, A., Regnier, M., Murry, C. E., Tamerler, C., & Kim, D. H. (2016). Nanotopography-induced structural anisotropy and sarcomere development in human cardiomyocytes derived from induced pluripotent stem cells. In ACS applied materials and interfaces (Vol. 8, Issue 34, pp. 21923–21932). American Chemical Society. 10.1021/acsami.5b11671 [DOI] [PMC free article] [PubMed]
- Chang WT, Yu D, Lai YC, Lin KY, Liau I. Characterization of the mechanodynamic response of cardiomyocytes with atomic force microscopy. Anal. Chem. 2013;85(3):1395–1400. doi: 10.1021/ac3022532. [DOI] [PubMed] [Google Scholar]
- Crocini C, Arimura T, Reischmann S, Eder A, Braren I, Hansen A, Eschenhagen T, Kimura A, Carrier L (2013) Impact of ANKRD1 mutations associated with hypertrophic cardiomyopathy on contraction parameters of engineered heart tissue. Basic Res. Cardiol. 108(3). 10.1007/S00395-013-0349-X [DOI] [PubMed]
- Dague E, Genet G, Lachaize V, Guilbeau-Frugier C, Fauconnier J, Mias C, Payré B, Chopinet L, Alsteens D, Kasas S, Severac C, Thireau JÔ, Heymes C, Honton B, Lacampagne A, Pathak A, Sénard JM, Galés C. Atomic force and electron microscopic-based study of sarcolemmal surface of living cardiomyocytes unveils unexpected mitochondrial shift in heart failure. J. Mol. Cell. Cardiol. 2014;74:162–172. doi: 10.1016/j.yjmcc.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Davis JJ, Hill HAO, Powell T. High resolution scanning force microscopy of cardiac myocytes. Cell Biol. Int. 2001;25(12):1271–1277. doi: 10.1006/cbir.2001.0813. [DOI] [PubMed] [Google Scholar]
- del Rio A, Perez-Jimenez R, Liu R, Roca-Cusachs P, Fernandez J, Sheetz M. Stretching single talin rod molecules activates vinculin binding. Science. 2009;323(5914):638–641. doi: 10.1126/SCIENCE.1162912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarelli S, Girasole M, Spitalieri P, Talarico RV, Murdocca M, Botta A, Novelli G, Mango R, Sangiuolo F, Longo G (2018) AFM nano-mechanical study of the beating profile of hiPSC-derived cardiomyocytes beating bodies WT and DM1. J. Mol. Recognit. 31(10). 10.1002/jmr.2725 [DOI] [PubMed]
- Ding M, Andersson H, Martinsson S, Sabirsh A, Jonebring A, Wang QD, Plowright AT, Drowley L. Aligned nanofiber scaffolds improve functionality of cardiomyocytes differentiated from human induced pluripotent stem cell-derived cardiac progenitor cells. Sci. Rep. 2020;10(1):13575. doi: 10.1038/s41598-020-70547-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domke J, Parak WJ, George M, Gaub HE, Radmacher M. Mapping the mechanical pulse of single cardiomyocytes with the atomic force microscope. Eur. Biophys. J. 1999;28(3):179–186. doi: 10.1007/s002490050198. [DOI] [PubMed] [Google Scholar]
- Dostanić M, Windt LM, Stein JM, van Meer BJ, Bellin M, Orlova V, Mastrangeli M, Mummery CL, Sarro PM, Sarro are, P. M. (2020) A miniaturized EHT platform for accurate measurements of tissue contractile properties. J. Microelectromech. Syst. 29(5). 10.1109/JMEMS.2020.3011196
- Dvir T, Timko BP, Brigham MD, Naik SR, Karajanagi SS, Levy O, Jin H, Parker KK, Langer R, Kohane DS. Nanowired three-dimensional cardiac patches. Nat. Nanotechnol. 2011;6(11):720–725. doi: 10.1038/nnano.2011.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Armouche A, Singh J, Naito H, Wittköpper K, Didié M, Laatsch A, Zimmermann WH, Eschenhagen T. Adenovirus-delivered short hairpin RNA targeting PKCα improves contractile function in reconstituted heart tissue. J. Mol. Cell. Cardiol. 2007;43(3):371–376. doi: 10.1016/j.yjmcc.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Elosegui-Artola A, Bazellières E, Allen MD, Andreu I, Oria R, Sunyer R, Gomm JJ, Marshall JF, Jones JL, Trepat X, Roca-Cusachs P. Rigidity sensing and adaptation through regulation of integrin types. Nat Mat. 2014;13(6):631–637. doi: 10.1038/nmat3960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A, Carag-Krieger C, Johnson C, Raab M, Tang H, Speicher D, Sanger J, Sanger J, Discher D. Embryonic cardiomyocytes beat best on a matrix with heart-like elasticity: scar-like rigidity inhibits beating. J. Cell Sci. 2008;121(Pt 22):3794–3802. doi: 10.1242/JCS.029678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A, Griffin M, Sen S, Bönnemann C, Sweeney H, Discher D. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004;166(6):877–887. doi: 10.1083/JCB.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eschenhagen T, Didié M, Münzel F, Schubert P, Schneiderbanger K, Zimmermann W-H. 3D engineered heart tissue for replacement therapy. Basic Res. Cardiol. 2002;97(7):1–1. doi: 10.1007/s003950200043. [DOI] [PubMed] [Google Scholar]
- Forte G, Pagliari S, Ebara M, Uto K, Van Tam JK, Romanazzo S, Escobedo-Lucea C, Romano E, Di Nardo P, Traversa E, Aoyagi T. Substrate stiffness modulates gene expression and phenotype in neonatal cardiomyocytes in vitro. Tissue Engineering - Part A. 2012;18(17–18):1837–1848. doi: 10.1089/ten.tea.2011.0707. [DOI] [PubMed] [Google Scholar]
- Galie PA, Byfield FJ, Chen CS, Kresh JY, Janmey PA. Mechanically stimulated contraction of engineered cardiac constructs using a microcantilever. IEEE Trans. Biomed. Eng. 2015;62(2):438–442. doi: 10.1109/TBME.2014.2357778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwig R, Fuchsberger K, Schroeppel B, Link GS, Heusel G, Kraushaar U, Schuhmann W, Stett A, Stelzle M. PEDOT-CNT composite microelectrodes for recording and electrostimulation applications: fabrication, morphology, and electrical properties. Front Neuroeng. 2012;5(MAY):8. doi: 10.3389/fneng.2012.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghassemi S, Meacci G, Liu S, Gondarenko AA, Mathur A, Roca-Cusachs P, Sheetz MP, Hone J. Cells test substrate rigidity by local contractions on submicrometer pillars. Proc. Natl. Acad. Sci. 2012;109(14):5328–5333. doi: 10.1073/PNAS.1119886109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfracht I, Protze S, Shiti A, Setter N, Gruber A, Shaheen N, Nartiss Y, Keller G, Gepstein L. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat. Commun. 2020;11(1):1–15. doi: 10.1038/s41467-019-13868-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Pu WT. Cardiomyocyte maturation: new phase in development. Circ. Res. 2020;126(8):1086–1106. doi: 10.1161/CIRCRESAHA.119.315862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haining AWM, Rahikainen R, Cortes E, Lachowski D, Rice A, von Essen M, Hytönen VP, del Hernández AR. Mechanotransduction in talin through the interaction of the R8 domain with DLC1. PLoS Biol. 2018;16(7):e2005599. doi: 10.1371/JOURNAL.PBIO.2005599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C, Kundu A, Kumar K, Varma SJ, Thomas J, Rajaraman S (2018) Rapid nanofabrication of nanostructured interdigitated electrodes (NIDES) for long-term in vitro analysis of human induced pluripotent stem cell differentiated cardiomyocytes. Biosensors 8(4). 10.3390/bios8040088 [DOI] [PMC free article] [PubMed]
- Hawkes W, Huang D, Reynolds P, Hammond L, Ward M, Gadegaard N, Marshall JF, Iskratsch T, Palma M. Probing the nanoscale organisation and multivalency of cell surface receptors: DNA origami nanoarrays for cellular studies with single-molecule control. Faraday Discuss. 2019;219(0):203–219. doi: 10.1039/c9fd00023b. [DOI] [PubMed] [Google Scholar]
- Hsiao YF, Pan HJ, Tung YC, Chen CC, Lee CH (2015) Effects of hydraulic pressure on cardiomyoblasts in a microfluidic device. Biomicrofluidics 9(2). 10.1063/1.4917080 [DOI] [PMC free article] [PubMed]
- Huang D, Patel K, Perez-Garrido S, Marshall JF, Palma M. DNA origami nanoarrays for multivalent investigations of cancer cell spreading with nanoscale spatial resolution and single-molecule control. ACS Nano. 2018;13(1):728–736. doi: 10.1021/ACSNANO.8B08010. [DOI] [PubMed] [Google Scholar]
- Ingber DE. Tensegrity-based mechanosensing from macro to micro. Prog. Biophys. Mol. Biol. 2008;97(2–3):163–179. doi: 10.1016/J.PBIOMOLBIO.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iskratsch T, Yu C, Mathur A, Liu S, Stévenin V, Dwyer J, Hone J, Ehler E, Sheetz M. FHOD1 is needed for directed forces and adhesion maturation during cell spreading and migration. Dev. Cell. 2013;27(5):545–559. doi: 10.1016/J.DEVCEL.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer K, Lee J, Haidar A, Miró MM, Akkan CK, Veith M, Aktas OC, Abdul-Khaliq H (2014) Alignment of human cardiomyocytes on laser patterned biphasic core/shell nanowire assemblies. Nanotechnology 25(49). 10.1088/0957-4484/25/49/495101 [DOI] [PubMed]
- Kim DH, Kim P, Song I, Cha JM, Lee SH, Kim B, Suh KY. Guided three-dimensional growth of functional cardiomyocytes on polyethylene glycol nanostructures. Langmuir. 2006;22(12):5419–5426. doi: 10.1021/la060283u. [DOI] [PubMed] [Google Scholar]
- Kliche K, Kuhn M, Hillebrand U, Ludwig Y, Stock C, Oberleithner H. Direct aldosterone action on mouse cardiomyocytes detected with atomic force microscopy. Cell. Physiol. Biochem. 2006;18(4–5):265–274. doi: 10.1159/000097673. [DOI] [PubMed] [Google Scholar]
- Lange S, Xiang F, Yakovenko A, Vihola A, Hackman P, Rostkova E, Kristensen J, Brandmeier B, Franzen G, Hedberg B, Gunnarsson L, Hughes S, Marchand S, Sejersen T, Richard I, Edström L, Ehler E, Udd B, Gautel M. The kinase domain of titin controls muscle gene expression and protein turnover. Science. 2005;308(5728):1599–1603. doi: 10.1126/SCIENCE.1110463. [DOI] [PubMed] [Google Scholar]
- Lanzicher T, Martinelli V, Puzzi L, Del Favero G, Codan B, Long CS, Mestroni L, Taylor MRG, Sbaizero O. The cardiomyopathy lamin A/C D192G mutation disrupts whole-cell biomechanics in cardiomyocytes as measured by atomic force microscopy loading-unloading curve analysis. Sci. Rep. 2015;5(1):1–14. doi: 10.1038/srep13388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrenyuk K, Conway D, Dahl KN. Imaging methods in mechanosensing: a historical perspective and visions for the future. Mol. Biol. Cell. 2021;32(9):842–854. doi: 10.1091/MBC.E20-10-0671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le S, Yu M, Hovan L, Zhao Z, Ervasti J, Yan J. Dystrophin as a molecular shock absorber. ACS Nano. 2018;12(12):12140–12148. doi: 10.1021/ACSNANO.8B05721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemme M, Ulmer BM, Lemoine MD, Zech ATL, Flenner F, Ravens U, Reichenspurner H, Rol-Garcia M, Smith G, Hansen A, Christ T, Eschenhagen T. Atrial-like engineered heart tissue: an in vitro model of the human atrium. Stem Cell Reports. 2018;11(6):1378–1390. doi: 10.1016/j.stemcr.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XP, Qu KY, Zhang F, Jiang HN, Zhang N, Nihad C, Liu CM, Wu KH, Wang XW, Huang NP. High-aspect-ratio water-dispersed gold nanowires incorporated within gelatin methacrylate hydrogels for constructing cardiac tissues: in vitro. J. Mater. Chem. B. 2020;8(32):7213–7224. doi: 10.1039/d0tb00768d. [DOI] [PubMed] [Google Scholar]
- Liau B, Christoforou N, Leong K, Bursac N. Pluripotent stem cell-derived cardiac tissue patch with advanced structure and function. Biomaterials. 2011;32(35):9180–9187. doi: 10.1016/J.BIOMATERIALS.2011.08.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieber, S. C., Aubry, N., Pain, J., Diaz, G., Kim, S. J., & Vatner, S. F. (2004). Aging increases stiffness of cardiac myocytes measured by atomic force microscopy nanoindentation. Am J Physiol - Heart Circ Physiol, 287(2 56-2). 10.1152/ajpheart.00564.2003 [DOI] [PubMed]
- Lieu DK, Liu J, Siu CW, McNerney GP, Tse HF, Abu-Khalil A, Huser T, Li RA. Absence of transverse tubules contributes to non-uniform Ca2+ wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes. Stem Cells Dev. 2009;18(10):1493–1500. doi: 10.1089/scd.2009.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YD, Ko MC, Wu ST, Li SF, Hu JF, Lai YJ, Harn HIC, Laio IC, Yeh ML, Yeh HI, Tang MJ, Chang KC, Su FC, Wei EIH, Lee ST, Chen JH, Hoffman AS, Wu WT, Hsieh PCH. A nanopatterned cell-seeded cardiac patch prevents electro-uncoupling and improves the therapeutic efficacy of cardiac repair. Biomater. Sci. 2014;2(4):567–580. doi: 10.1039/c3bm60289c. [DOI] [PubMed] [Google Scholar]
- Liu J, Sun N, Bruce MA, Wu JC, Butte MJ (2012) Atomic force mechanobiology of pluripotent stem cell-derived cardiomyocytes. PLoS One 7(5). 10.1371/journal.pone.0037559 [DOI] [PMC free article] [PubMed]
- Lu, D., & Kassab, G. S. (2011). Role of shear stress and stretch in vascular mechanobiology. In Journal of the Royal Society Interface (Vol. 8, Issue 63, pp. 1379–1385). Royal Society. 10.1098/rsif.2011.0177 [DOI] [PMC free article] [PubMed]
- Lunkenheimer PP, Redmann K, Florek J, Fassnacht U, Cryer CW, Wübbeling F, Niederer P, Anderson RH. The forces generated within the musculature of the left ventricular wall. Heart. 2004;90(2):200–207. doi: 10.1136/hrt.2003.011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannhardt I, Breckwoldt K, Letuffe-Brenière D, Schaaf S, Schulz H, Neuber C, Benzin A, Werner T, Eder A, Schulze T, Klampe B, Christ T, Hirt MN, Huebner N, Moretti A, Eschenhagen T, Hansen A. Human engineered heart tissue: analysis of contractile force. Stem Cell Reports. 2016;7(1):29–42. doi: 10.1016/j.stemcr.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinelli VGC, Toma F, Long C, Caldwell J, Zentilin L, Giacca M, Turco A, Prato M, Ballerini L, Mestroni L. Carbon nanotubes instruct physiological growth and functionally mature syncytia: nongenetic engineering of cardiac myocytes. ACS Nano. 2013;7(7):5746–5756. doi: 10.1021/NN4002193. [DOI] [PubMed] [Google Scholar]
- Martinelli, Valentina, Cellot, G., Fabbro, A., Bosi, S., Mestroni, L., & Ballerini, L. (2013b). Improving cardiac myocytes performance by carbon nanotubes platforms. In Frontiers in Physiology: Vol. 4 SEP (p. 239). Frontiers 10.3389/fphys.2013.00239 [DOI] [PMC free article] [PubMed]
- Mártonfalvi Z, Bianco P, Naftz K, Ferenczy G, Kellermayer M. Force generation by titin folding. Prot Sci: A Publ Protein Soc. 2017;26(7):1380–1390. doi: 10.1002/PRO.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain ML, Yuan H, Pasqualini FS, Campbell PH, Parker KK (2014) Matrix elasticity regulates the optimal cardiac myocyte shape for contractility. Am. J. Physiol. Heart Circ. Physiol. 306(11). 10.1152/ajpheart.00799.2013 [DOI] [PMC free article] [PubMed]
- Meacci G, Wolfenson H, Liu S, Stachowiak M, Iskratsch T, Mathur A, Ghassemi S, Gauthier N, Tabdanov E, Lohner J, Gondarenko A, Chander A, Roca-Cusachs P, O’Shaughnessy B, Hone J, Sheetz M. α-Actinin links extracellular matrix rigidity-sensing contractile units with periodic cell-edge retractions. Mol. Biol. Cell. 2016;27(22):3471–3479. doi: 10.1091/MBC.E16-02-0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney E, Mackle JN, Blond DJP, O’Cearbhaill E, Shaw G, Blau WJ, Barry FP, Barron V, Murphy JM. The electrical stimulation of carbon nanotubes to provide a cardiomimetic cue to MSCs. Biomaterials. 2012;33(26):6132–6139. doi: 10.1016/j.biomaterials.2012.05.032. [DOI] [PubMed] [Google Scholar]
- Mosqueira D, Mannhardt I, Bhagwan JR, Lis-Slimak K, Katili P, Scott E, Hassan M, Prondzynski M, Harmer SC, Tinker A, Smith JGW, Carrier L, Williams PM, Gaffney D, Eschenhagen T, Hansen A, Denning C. CRISPR/Cas9 editing in human pluripotent stemcell-cardiomyocytes highlights arrhythmias, hypocontractility, and energy depletion as potential therapeutic targets for hypertrophic cardiomyopathy. Eur. Heart J. 2018;39(43):3879–3892. doi: 10.1093/eurheartj/ehy249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mühlhäuser U, Zolk O, Rau T, Münzel F, Wieland T, Eschenhagen T. Atorvastatin desensitizes β-adrenergic signaling in cardiac myocytes via reduced isoprenylation of G-protein γ-subunits. FASEB J. 2006;20(6):785–787. doi: 10.1096/fj.05-5067fje. [DOI] [PubMed] [Google Scholar]
- Neuman KC, Nagy A. Single-molecule force spectroscopy: optical tweezers, magnetic tweezers and atomic force microscopy. Nature Methods. 2008;5(6):491–505. doi: 10.1038/nmeth.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsan I, Drori S, Lewis YE, Cohen S, Tzlil S. Mechanical communication in cardiac cell synchronized beating. Nat. Phys. 2016;12(5):472–477. doi: 10.1038/nphys3619. [DOI] [Google Scholar]
- Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Massé S, Gagliardi M, Hsieh A, Thavandiran N, Laflamme MA, Nanthakumar K, Gross GJ, Backx PH, Keller G, Radisic M. Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes. Nat. Methods. 2013;10(8):781–787. doi: 10.1038/nmeth.2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palankar R, Glaubitz M, Martens U, Medvedev N, von der Ehe M, Felix SB, Münzenberg M, Delcea M. 3D micropillars guide the mechanobiology of human induced pluripotent stem cell-derived cardiomyocytes. Adv. Healthc. Mater. 2016;5(3):335–341. doi: 10.1002/adhm.201500740. [DOI] [PubMed] [Google Scholar]
- Pandey P, Hawkes W, Hu J, Megone WV, Gautrot J, Anilkumar N, Zhang M, Hirvonen L, Cox S, Ehler E, Hone J, Sheetz M, Iskratsch T. Cardiomyocytes sense matrix rigidity through a combination of muscle and non-muscle myosin contractions. Dev Cell. 2018;44(3):326–336.e3. doi: 10.1016/j.devcel.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsa H, Wang BZ, Vunjak-Novakovic G. A microfluidic platform for the high-throughput study of pathological cardiac hypertrophy. Lab Chip. 2017;17(19):3264–3271. doi: 10.1039/c7lc00415j. [DOI] [PubMed] [Google Scholar]
- Pasqualini FS, Agarwal A, O’Connor BB, Liu Q, Sheehy SP, Parker KK. Traction force microscopy of engineered cardiac tissues. PLoS One. 2018;13(3):e0194706. doi: 10.1371/journal.pone.0194706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitoulis F, Nunez-Toldra R, Xiao K, Kit-Anan W, Mitzka S, Jabbour R, Harding S, Perbellini F, Thum T, de Tombe P, Terracciano C (2021) Remodelling of adult cardiac tissue subjected to physiological and pathological mechanical load in vitro. Cardiovasc. Res.10.1093/CVR/CVAB084 [DOI] [PMC free article] [PubMed]
- Prondzynski M, Lemoine M, Zech A, Horváth A, Di Mauro V, Koivumäki J, Kresin N, Busch J, Krause T, Krämer E, Schlossarek S, Spohn M, Friedrich F, Münch J, Laufer S, Redwood C, Volk A, Hansen A, Mearini G et al (2019) Disease modeling of a mutation in α-actinin 2 guides clinical therapy in hypertrophic cardiomyopathy. EMBO Mole Med 11(12). 10.15252/EMMM.201911115
- Puchner EM, Alexandrovich A, Ay LK, Hensen U, Schäfer LV, Brandmeier B, Gräter F, Grubmüller H, Gaub HE, Gautel M. Correction for Puchner et al., Mechanoenzymatics of titin kinase. Proc. Natl. Acad. Sci. 2008;105(52):21045–21045. doi: 10.1073/PNAS.0810209105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regazzoni F, Dedè L, Quarteroni A. Biophysically detailed mathematical models of multiscale cardiac active mechanics. PLoS Comput. Biol. 2020;16(10):e1008294. doi: 10.1371/JOURNAL.PCBI.1008294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren L, Liu W, Wang Y, Wang JC, Tu Q, Xu J, Liu R, Shen SF, Wang J. Investigation of hypoxia-induced myocardial injury dynamics in a tissue interface mimicking microfluidic device. Anal. Chem. 2013;85(1):235–244. doi: 10.1021/ac3025812. [DOI] [PubMed] [Google Scholar]
- Ribeiro AJS, Ang YS, Fu JD, Rivas RN, Mohamed TMA, Higgs GC, Srivastava D, Pruitt BL. Contractility of Single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness. Proc. Natl. Acad. Sci. U. S. A. 2015;112(41):12705–12710. doi: 10.1073/pnas.1508073112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Pardo JA, Eckels EC, Popa I, Kosuri P, Linke WA, Fernández JM. Work done by titin protein folding assists muscle contraction. Cell Rep. 2016;14(6):1339–1347. doi: 10.1016/j.celrep.2016.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca-Cusachs P, Iskratsch T, Sheetz MP. Finding the weakest link – exploring integrin-mediated mechanical molecular pathways. J. Cell Sci. 2012;125(13):3025–3038. doi: 10.1242/JCS.095794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AG, Han SJ, Regnier M, Sniadecki NJ. Substrate stiffness increases twitch power of neonatal cardiomyocytes in correlation with changes in myofibril structure and intracellular calcium. Biophys. J. 2011;101(10):2455–2464. doi: 10.1016/j.bpj.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez ML, Graham BT, Pabon LM, Han SJ, Murry CE, Sniadecki NJ (2014) Measuring the contractile forces of human induced pluripotent stem cell-derived cardiomyocytes with arrays of microposts. J. Biomech. Eng. 136(5). 10.1115/1.4027145 [DOI] [PMC free article] [PubMed]
- Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song LJ, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature. 2018;556(7700):239–243. doi: 10.1038/s41586-018-0016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem U, Mannhardt I, Braren I, Denning C, Eschenhagen T, Hansen A. Force and calcium transients analysis in human engineered heart tissues reveals positive force-frequency relation at physiological frequency. Stem Cell Reports. 2020;14(2):312–324. doi: 10.1016/j.stemcr.2019.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Alonso JL, Loucks A, Schobesberger S, van Cromvoirt AM, Poulet C, Chowdhury RA, Trayanova N, Gorelik J. Nanoscale regulation of L-type calcium channels differentiates between ischemic and dilated cardiomyopathies. EBioMed. 2020;57:102845. doi: 10.1016/j.ebiom.2020.102845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaf S, Shibamiya A, Mewe M, Eder A, Stöhr A, Hirt MN, Rau T, Zimmermann WH, Conradi L, Eschenhagen T, Hansen A. Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology. PLoS One. 2011;6(10):26397. doi: 10.1371/journal.pone.0026397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlichthaerle T, Lindner C, Jungmann R. Super-resolved visualization of single DNA-based tension sensors in cell adhesion. Nature Comm. 2021;12(1):1–8. doi: 10.1038/s41467-021-22606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schvartzman M, Palma M, Sable J, Abramson J, Hu X, Sheetz MP, Wind SJ. Nanolithographic control of the spatial organization of cellular adhesion receptors at the single-molecule level. Nano Lett. 2011;11(3):1306–1312. doi: 10.1021/NL104378F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo HR, Joo HJ, Kim DH, Cui LH, Choi SC, Kim JH, Cho SW, Lee KB, Lim DS. Nanopillar surface topology promotes cardiomyocyte differentiation through cofilin-mediated cytoskeleton rearrangement. ACS Appl. Mater. Interfaces. 2017;9(20):16803–16812. doi: 10.1021/acsami.7b01555. [DOI] [PubMed] [Google Scholar]
- Shin SR, Jung SM, Zalabany M, Kim K, Zorlutuna P, Kim SB, Nikkhah M, Khabiry M, Azize M, Kong J, Wan KT, Palacios T, Dokmeci MR, Bae H, Tang X, Khademhosseini A. Carbon-nanotube-embedded hydrogel sheets for engineering cardiac constructs and bioactuators. ACS Nano. 2013;7(3):2369–2380. doi: 10.1021/nn305559j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shroff, S. G., Saner, D. R., & Lal, R. (1995). Dynamic micromechanical properties of cultured rat atrial myocytes measured by atomic force microscopy. Am J Physiol - Cell Physiol, 269(1 38-1). 10.1152/ajpcell.1995.269.1.c286 [DOI] [PubMed]
- Sit B, Gutmann D, Iskratsch T. Costameres, dense plaques and podosomes: the cell matrix adhesions in cardiovascular mechanosensing. J Muscle Res Cell Motil. 2019;40(2):197–209. doi: 10.1007/S10974-019-09529-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiatlowska P, Sanchez-Alonso JL, Mansfield C, Scaini D, Korchev Y, Novak P, Gorelik J. Short-term angiotensin II treatment regulates cardiac nanomechanics via microtubule modifications. Nanoscale. 2020;12(30):16315–16329. doi: 10.1039/D0NR02474K. [DOI] [PubMed] [Google Scholar]
- Swiatlowska P, Sanchez-Alonso JL, Wright PT, Novak P, Gorelik J. Microtubules regulate cardiomyocyte transversal Young’s modulus. Proc. Natl. Acad. Sci. 2020;117(6):2764–2766. doi: 10.1073/PNAS.1917171117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virani, S. S., Alonso, A., Benjamin, E. J., Bittencourt, M. S., Callaway, C. W., Carson, A. P., Chamberlain, A. M., Chang, A. R., Cheng, S., Delling, F. N., Djousse, L., Elkind, M. S. V., Ferguson, J. F., Fornage, M., Khan, S. S., Kissela, B. M., Knutson, K. L., Kwan, T. W., Lackland, D. T., … Heard, D. G. (2020). Heart disease and stroke statistics—2020 update: a report from the American Heart Association. In Circulation (Vol. 141, pp. E139–E596). Lippincott Williams and Wilkins. 10.1161/CIR.0000000000000757 [DOI] [PubMed]
- Wan W, Bjorkman KK, Choi ES, Panepento AL, Anseth KS, Leinwand LA (2019) Cardiac myocytes respond differentially and synergistically to matrix stiffness and topography. BioRxiv 682930. 10.1101/682930
- Ward M, Iskratsch T (2020) Mix and (mis-)match — the mechanosensing machinery in the changing environment of the developing, healthy adult and diseased heart. Biochimica et Biophysica Acta (BBA) - Mole Cell Res 2020(3):118436. 10.1016/J.BBAMCR.2019.01.017 [DOI] [PMC free article] [PubMed]
- Watson SA, Duff J, Bardi I, Zabielska M, Atanur SS, Jabbour RJ, Simon A, Tomas A, Smolenski RT, Harding SE, Perbellini F, Terracciano CM. Biomimetic electromechanical stimulation to maintain adult myocardial slices in vitro. Nat Comm. 2019;10(1):1–15. doi: 10.1038/s41467-019-10175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfenson H, Iskratsch T, Sheetz M. Early events in cell spreading as a model for quantitative analysis of biomechanical events. Biophys. J. 2014;107(11):2508–2514. doi: 10.1016/J.BPJ.2014.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfenson H, Meacci G, Liu S, Stachowiak MR, Iskratsch T, Ghassemi S, Roca-Cusachs P, O’Shaughnessy B, Hone J, Sheetz MP. Tropomyosin controls sarcomere-like contractions for rigidity sensing and suppressing growth on soft matrices. Nat Cell Biol. 2015;18(1):33–42. doi: 10.1038/ncb3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2021). Cardiovascular diseases (CVDs). https://www.who.int/en/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds)
- Wu X, Sun Z, Foskett A, Trzeciakowski JP, Meininger GA, Muthuchamy M (2010) Cardiomyocyte contractile status is associated with differences in fibronectin and integrin interactions. Am. J. Physiol. Heart Circ. Physiol. 298(6). 10.1152/ajpheart.01156.2009 [DOI] [PMC free article] [PubMed]
- Xu F, Zhao R, Liu AS, Metz T, Shi Y, Bose P, Reich DH. A microfabricated magnetic actuation device for mechanical conditioning of arrays of 3D microtissues. Lab Chip. 2015;15(11):2496–2503. doi: 10.1039/c4lc01395f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Liu Y, Lu XL, Li XH, Zhang HG. Transmembrane transport of the Gαq protein carboxyl terminus imitation polypeptide GCIP-27. Eur. J. Pharm. Sci. 2013;49(5):791–799. doi: 10.1016/j.ejps.2013.05.028. [DOI] [PubMed] [Google Scholar]
- Yao M, Goult BT, Klapholz B, Hu X, Toseland CP, Guo Y, Cong P, Sheetz MP, Yan J. The mechanical response of talin. Nat Comm. 2016;7(1):1–11. doi: 10.1038/ncomms11966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann WH, Melnychenko I, Wasmeier G, Didié M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat. Med. 2006;12(4):452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.