Abstract
Introduction:
Mother’s own milk improves health outcomes in infants of all gestational ages. Although, pump-dependent mothers of extremely premature infants are at risk of insufficient milk production, whether mother’s milk production is impacted by gestational age and pump dependency in mothers of more mature critically ill infants is unknown.
Purpose:
To determine if there is a relationship between gestational age, milk production, and time to secretory activation in mothers delivering a critically ill infant.
Methods:
A convenience sample of 136 pump-dependent mothers whose infants were admitted to the neonatal intensive care unit were enrolled between 2013 and 2016 as part of a quality improvement project. Group I (early preterm) delivered infants at 30–33 weeks gestation (n = 41), Group 2 (late preterm) 34–36 weeks (n = 48), and Group 3 (term) ≥37 weeks (n = 47). Milk volume on days 1–7 was measured by weighing each vial of expressed milk and compared using general linear mixed model analysis. Time to the onset of secretory activation was compared using censored regression analysis.
Results:
Main effect for gestational age controlling for day was statistically significant (p = .0234). The early preterm group produced more milk over the 7 day study than the term (p = .01) and late preterm (p = .02) groups. The early preterm group achieved secretory activation earlier than the late preterm group (adjusted p = .039).
Implications for Practice:
Pump-dependent mothers of all infants admitted to the NICU may be at risk of inadequate milk production and delayed secretory activation and may therefore benefit from early milk production monitoring and lactation support.
Implications for Research:
Further studies should examine targeted interventions to increase milk production in pump-dependent mothers.
Keywords: human milk, lactation, pump-dependent mothers, neonatal intensive care, infant, breast milk expression, gestational age, secretory activation, mother’s own milk
Introduction
Mother’s own milk (MOM) is the optimal source of nutritional support and improves health outcomes including feeding tolerance, neurodevelopmental, lower rates of sepsis,1 and mortality.2 The World Health Organization (WHO)3 and the American Academy of Pediatrics (AAP)4 recommend infants receive exclusive MOM feedings for at least the first six months of life. The AAP further recommends preterm infants receive donor human milk (DHM), when MOM is unavailable.4 Infants admitted to the neonatal intensive care unit (NICU) are often unable to breastfeed due to illness, prematurity,5 and mother/infant separation.6 Mothers must therefore express their milk via a breast pump (pump dependency), which may limit milk production.7 Furthermore, mothers delivering critically ill and premature infants have a high rate of comorbidities8 known to negatively affect milk production,9 and delay the onset of secretory activation (SA, copious milk production). A delayed (>72hours) onset of SA has been associated with lower sustained milk production10,11 and early cessation of lactation.12 Therefore, mothers delivering an infant requiring admission to the NICU8 may experience decreased milk production7,13 resulting in supplementation14 with formula and/or DHM15 which are far less protective than MOM.16,17,18,19
It is well known that pump-dependent mothers of very low birth weight infants (VLBW, less than 1500 grams) are at risk of insufficient milk production.8,13,20 However, it is unknown whether milk production is also affected by gestational age (GA) and pump dependency in mothers of more mature critically ill infants. Therefore, the primary aim of this observational study was to determine an association between GA and milk production in pump-dependent mothers of critically ill infants. We hypothesized that milk production in pump-dependent mothers of critically ill infants is positively associated with GA. A secondary objective was to test for a relationship between the time from birth to onset of secretory activation (SA) and GA. We expected a shorter time to onset of secretory activation with increased GA.
Methodology
In this observational cohort study, a convenience sample of 136 pump-dependent mothers whose infants were admitted to the NICU were enrolled between 2013 and 2016. This retrospective study was part of an over-reaching quality improvement project in the NICU whose goal was to determine barriers to provision of MOM and increase consumption of MOM by critically ill infants. Infants were admitted to a 72-bed level IV NICU located within a Level III tertiary care hospital, which receives patients from rural and semi-rural areas in the Southeastern United States. Mothers were included if they delivered an infant weighing >1500 grams and at least 30 weeks GA admitted to the NICU and who supplied MOM for the first seven days after birth and excluded if their infant died or was discharged prior to 7 days of age. Mothers were placed into one of three groups based upon their infant’s GA at birth. Group 1 delivered an infant 30–33 6/7 weeks gestation (early preterm); Group 2 delivered an infant between 34–36 6/7 weeks (late preterm); and Group 3 delivered an infant at least 37 weeks gestation (term). The project was approved by the Quality Improvement Committee at UFHealth Shands Children’s Hospital.
Demographics:
Maternal and infant demographics including maternal age, race, ethnicity, parity, mode of delivery, body mass index (BMI), as well as history of hypertension, diabetes, and antenatal steroid administration were obtained from the electronic medical record (EMR). Socioeconomic status was determined by maternal enrollment in Medicaid.
Measurement of MOM volume:
As per standard practice in this NICU, all mothers, including those who delivered outside the study hospital, were instructed to date and time each vial of expressed MOM and bring all vials to the NICU. Volume of milk produced on Days 1–7 was determined by weighing each vial of expressed milk using an electronic digital scale (Scout Balance, Florham Park, NJ, USA) to the nearest 0.1 gram (weight conversion of 1gm = 1mL) and then recorded in a designated mother’s milk log book by trained human milk technicians blinded to the infant’s GA. If the mother did not bring in vial of expressed milk to the NICU, a value of zero was recorded for that day.
Time to the first expression session following delivery was defined as number of hours from birth to the first expression that yielded milk and data was collected from the mother’s milk log. Number of times mothers expressed per day was determined by the vials of milk which were dated and timed by the mother. Breastfeeding episodes were measured by those recorded by nurses in the EMR as excellent or good indicating nutritive sucking and likely milk transfer to avoid including breastfeeding attempts associated with negligible milk transfer.21 Additionally, whether or not mothers engaged in skin to skin care (STS) during the seven days was charted by bedside nurses and data collected from the EMR.
Determination of secretory activation:
Time to the onset of SA was measured as hours from birth to the second consecutive expression session producing at least 20mL of MOM.22 If SA was not achieved within seven days after delivery, time to the onset of SA was recorded as 168 hours (the study period) and marked as right censored.
Statistical Analysis:
Maternal and infant demographics were compared between groups using contingency tables with chi square for categorical variables, and one-way analysis of variance (ANOVA) for continuous variables. Number of breastfeeding episodes were described using mean and standard deviation. Demographic variables found to differ between groups at p < .05 were included as covariates in the models and Bonferroni adjustments were made for multiple comparisons. Inferential and descriptive analyses were conducted using SAS® (v 9.4, SAS Institute, Cary, NC).
General linear mixed model (GLMM) for repeated measures was used to analyze the primary outcomes of daily (Day 1–7) and total MOM volume over the first 7 days following delivery between groups. Autoregressive (AR) covariance structure, unstructured, compound symmetry and variance components models were tested to determine the best fit for the data with minimal data loss. The model with the lowest AIC value (3478.2) was the unstructured covariance matrix and was thus selected for analysis. A natural log transformation of milk volume was applied to correct the non-linear trend of residuals (large standard deviation in milk supply) to meet assumptions of repeated measures GLMM testing. Twin delivery, birthweight, and antenatal steroid administration were not included as covariates in the model due to known collinearity with GA. The full model contained main effects, which included the covariates GA, day (time), maternal age, socioeconomic status, and mode of delivery, as well as the covariate interaction effect based on day or GA. Interactions and covariates with p > .05 were removed from the model resulting in a final model containing main effects for GA and Day, as well as the GA by Day interaction.
Time from delivery to onset of SA was examined with SAS PROC LIFEREG23 using a censored regression analysis with right-censored values and controlling for covariates. SAS PROC LIFEREG fits parametric models of data that can be uncensored, right censored, left censored, or interval censored. Parameter values are estimated by maximum likelihood with a Newton-Raphson algorithm.23 Data from seven mothers who did not achieve SA within the first seven days were marked as right-censored values, then included in the analysis. Seven mothers were excluded from analysis due to missing data regarding time to onset of SA. To determine the best model fit, we assessed the exponential, gamma, llogistic, lnormal, logistic, normal and Weibull models concluding a log-logistic distribution model was the best fit based on the lowest AIC (152.738) and BIC (164.177). We began with a full model controlling for maternal age, socioeconomic status, and mode of delivery. Covariates with a p > .05 were removed from the model leaving only GA effect on time to onset of SA in the final model.
Results
The study cohort consisted of 136 primarily White (67%), non-Hispanic (89%) mothers with an average age of 27.29 years and 32.55 body mass index. The majority of mothers were multiparous (73%), delivered a single infant (90%), underwent a cesarean section delivery (54%), and without a documented history of hypertension (68%) or diabetes (86%). Sixty-five percent of participants were enrolled in Medicaid. Differences between groups included administration of antenatal steroids, frequency of multiple births, maternal age, socioeconomic status, and mode of delivery (Table 1). Participants in the term group were older and less likely to be Medicaid recipients. Mothers delivering late preterm infants were more likely to deliver via cesarean section, than those delivering early preterm or term infants. Time from delivery to the first expression, expression frequency, and proportion of mothers with STS episodes was statistically similar between groups. While not statistically significant, mothers of late preterm infants breastfed more often during the seven day study, followed by mothers of term infants and finally those of early preterm infants [total episodes (range) 36 (1–8); 29 (1–9); 13 (1–4)]. As expected, more mothers in the early preterm group (21%) received antenatal steroids (p < .0001).
Table 1.
Sample Characteristics by Gestational Age Group.
| Characteristic | Group 1 (n = 41) | Group 2 (n = 48) | Group 3 (n= 47) | P Value |
|---|---|---|---|---|
| Gestational age | 31.9 [1.09] | 34.8 [0.83] | 38.4 [1.25] | |
| Birthweight (grams) | 1778.3 [301] | 2210.9 [470] | 3191.6 [713] | |
| Maternal age | 25.5 [4.97] | 27.75 [5.01] | 28.38 [5.63] | .03 |
| BMI (kg/m2) | 32.2 [8.56] | 32.9 [8.3] | 32.7 [12.7] | .97 |
| Maternal race | .08 | |||
| White | 25 (61%) | 35(73%) | 31(66%) | |
| African American | 13 (32%) | 10 (21%) | 9 (19%) | |
| Asian | 0 | 1 (2%) | 5 (11%) | |
| Other | 3 (7%) | 2 (4%) | 2 (4%) | |
| Ethnicity | ||||
| non-Hispanic/ Latino | 37 (90%) | 40 (82%) | 46 (96%) | .09 |
| SES (Medicaid) | ||||
| Yes | 31 (76%) | 37 (77%) | 20 (43%) | <.001 |
| History hypertension | ||||
| Yes | 13 (32%) | 20 (42%) | 11 (23%) | .15 |
| History diabetes | ||||
| Yes | 2 (5%) | 8 (17%) | 9 (19%) | .09 |
| Antenatal steroids | ||||
| Yes | 28 (68%) | 19 (40%) | 2 (4%) | <.001 |
| Primigravida | ||||
| Yes | 11 (27%) | 9 (19%) | 17 (36%) | .17 |
| Delivery type | ||||
| Caesarean | 20 (49%) | 33 (69%) | 21 (45%) | .04 |
| Multiple births | ||||
| Yes | 8 (20%) | 4 (8%) | 1 (2%) | .02 |
| Achievement of SA | ||||
| Yes | 41 (100%) | 44 (92%) | 44 (94%) | .18 |
| Skin to skin care episodes | ||||
| Yes | 11 (26%) | 12 (25%) | 9 (19%) | .67 |
| Time from delivery to first expression (hours) | 25.2 [24.82] | 37.1 [41.56] | 38.3 [34.24] | .17 |
| Breastfeeding attempts | 1.77 [1.09] | 3.14 [2.11] | 2.34 [1.99] | .06 |
| Expression frequency | 3.19 [2.5] | 2.84 [2.5] | 2.76 [2.5] | .08 |
Note. Continuous variables reported with mean [standard deviation], ANOVA p value. Categorical variables reported with number (percent), Chi square Exact p-value.
Abbreviations: Body mass index (BMI), Socioeconomic status (SES), Secretory activation (SA).
Milk volume:
The cumulative loge-transformed volume of milk produced over the first 7 days differed based on GA (p = .023). Subsequent t-test paired comparisons (Table 2) using a Bonferroni-adjusted p-value resulted in a statistically significant difference in volume of milk produced between the early preterm and term groups (.154, adjusted p = .039). However, no statistically significant differences were found between the late preterm groups and either the early preterm or term groups. Milk production across all three groups increased from birth to 7 days (p < .0001), and the slope of the increase was similar between groups (Table 2; Figure 1). Mothers in the early preterm group produced more milk over the first seven days (1409.64 mL) than mothers in the late preterm (908.87 mL) and term (1028.59 mL) groups (Table 3).
Table 2.
General Linear Mixed Model Results for Final Reduced Model, Loge Transformed MOM Volume.
| Effect | F (DF) | P Value | Least Square Mean (95% CI) |
|---|---|---|---|
| GA Group | 3.86 (2, 133) | .02 | |
| Early preterm | 3.80 (3.44, 4.17) | ||
| Late preterm | 3.21 (2.87, 5.55) | ||
| Term | 3.17 (2.82, 3.51) | ||
| GA Group | |||
| Early vs Late preterm | .06 | 0.60 (0.10, 1.10) | |
| Early preterm vs Term | .03 | 0.64 (0.14, 1.14) | |
| Late preterm vs Term | 1.00 | 0.04 (−0.44, 0.53) | |
| Day | 200.9 (6, 133) | <.001 | |
| Day 1 | 0.67 (0.47, 0.87) | ||
| Day 2 | 1.41 (1.16, 1.66) | ||
| Day 3 | 2.62 (2.28, 2.96) | ||
| Day 4 | 3.95 (3.59, 4.31) | ||
| Day 5 | 4.88 (4.62, 5.14) | ||
| Day 6 | 5.01 (4.84, 5.37) | ||
| Day 7 | 5.10 (4.82, 5.39) | ||
| GA Group * Day | 0.75 (12, 133) | .70 |
Note. Abbreviations: Mother’s own milk (MOM), degrees of freedom (DF), gestational age (GA).
Figure 1:
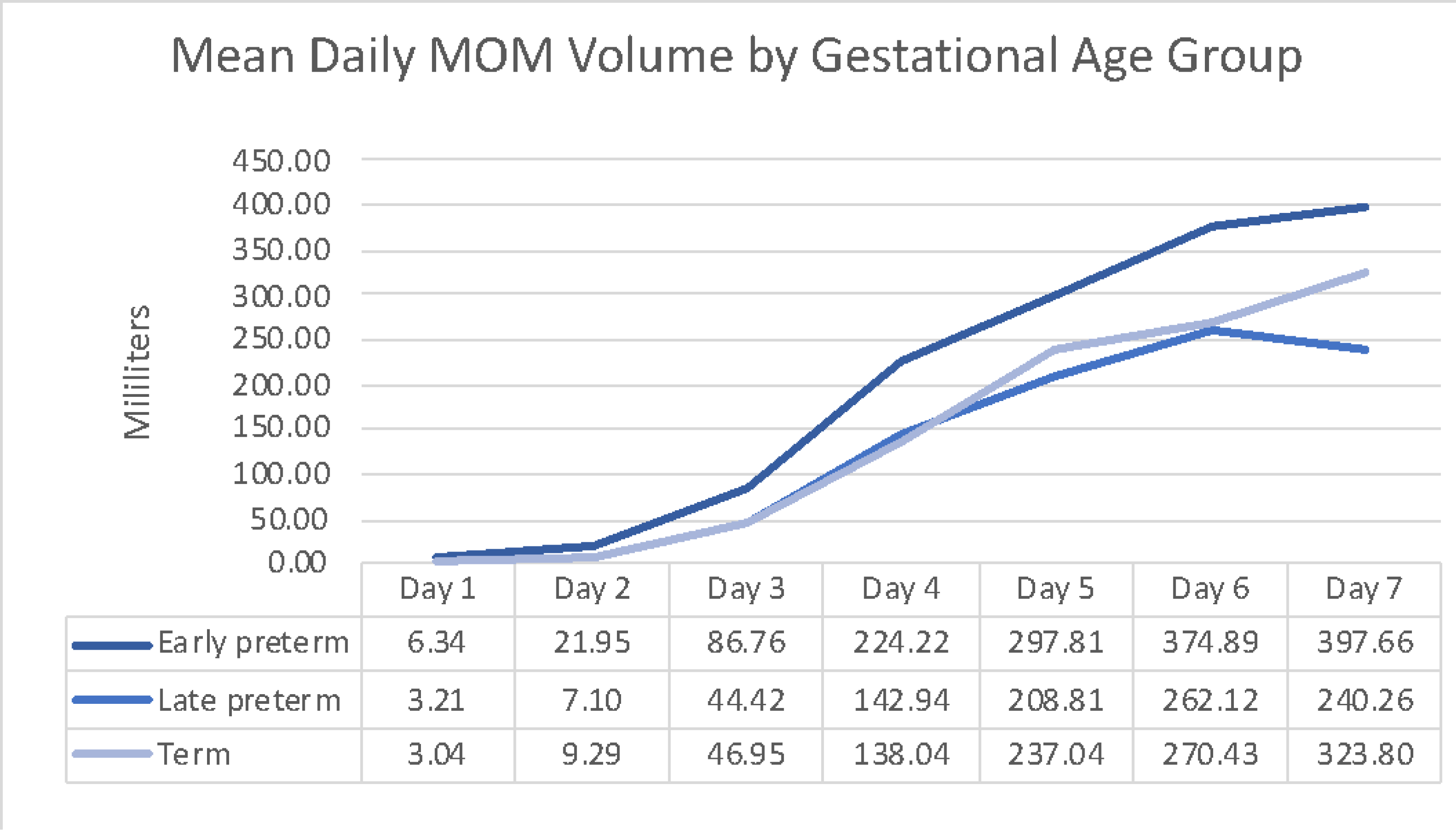
Mean daily MOM volume by gestational age group. Daily MOM volume by gestational age group using untransformed values for clinical reference.
Table 3.
Non-transformed MOM Volume (mL) Values Provided for Clinical Reference.
| Effect | Group | Mean +SD | Median [IQR] |
|---|---|---|---|
| Day = 1 | Early preterm | 6.35 ± 16.52 | 0 [0,2.10] |
| Late preterm | 3.21 ± 7.26 | 0 [0,2.37] | |
| Term | 3.04 ± 9.34 | 0 [0,1.70] | |
| Day = 2 | Early preterm | 21.95 ± 35.60 | 8.0 [0, 26.87] |
| Late preterm | 7.11 ± 13.40 | 1.25 [0, 8.85] | |
| Term | 9.29 ± 20.41 | 0.54 [0, 8.43] | |
| Day = 3 | Early preterm | 86.76 ± 123.72 | 29.87 [4.40, 110.50] |
| Late preterm | 44.42 ± 54.54 | 18.64 [0, 83.09] | |
| Term | 46.95 ± 81.16 | 8.63 [0, 47.74] | |
| Day = 4 | Early preterm | 224.22 ± 199.07 | 212.18 [43.85, 356.42] |
| Late preterm | 142.94 ± 156.13 | 99.66 [11.35, 218.13] | |
| Term | 138.04 ± 165.48 | 108.52 [4.80, 193.59] | |
| Day = 5 | Early preterm | 297.81 ± 218.56 | 258.25 [112.22, 381.65] |
| Late preterm | 208.81 ± 210.95 | 151.59 [71.19, 327.53] | |
| Term | 237.04 ± 214.23 | 215.80 [56.07, 371.70] | |
| Day = 6 | Early preterm | 374.89 ± 266.43 | 347.58 [188.75, 499.34] |
| Late preterm | 262.12 ± 284.11 | 182.89 [83.75, 336.70] | |
| Term | 270.43 ± 228.32 | 242.00 [86.90, 424.80] | |
| Day = 7 | Early preterm | 397.66 ± 287.53 | 364.05 [181.61, 599.20] |
| Late preterm | 240.26 ± 249.38 | 206.45 [75.28, 336.44] | |
| Term | 323.80 ± 269.17 | 250.20 [122.30, 479.50] |
Note. Abbreviations: Milliliter (mL), mother’s own milk (MOM), standard deviation (SD), inter quartile range [IQR].
Time to Onset of Secretory Activation:
Ninety-five percent of mothers achieved SA during the seven day study. Although the proportion of mothers that achieved SA were similar between groups (Table 1), the time to SA (loge hours) was different (Table 4). Mothers in the early preterm group achieved SA sooner than mothers in the late preterm group (Bonferroni-adjusted p = .042, Table 4). However, there were no differences between the early preterm group and term group or the late preterm and term group. Median time to SA was delayed (greater than 72 hours) in all three groups (Table 4).
Table 4.
Time from Birth to Secretory Activation Censored Regression Results Reported as Censored Right Data Between Gestational Age Groups.
| Effect Censored (n=129) | Z Value | DF | Least Square Mean (95% CI) | P Value | Median [IQR]a (hours) |
|---|---|---|---|---|---|
| GA group | 6.05* | 2 | .049 | ||
| Early preterm (<34 weeks) | 4.26 (4.12, 4.39) | 73.55 [54.53, 86.87] | |||
| Late preterm (34-<37 weeks) | 4.48 (4.36, 4.60) | 85.52 [68.03, 121.07] | |||
| Term (≥37 weeks) | 4.38 (4.26, 4.51) | 76.03 [60.61, 108.66] | |||
| Post hoc tests | |||||
| Early vs Late preterm | −2.46 | −0.228 (−0.41; −0.05) | .042 | ||
| Early preterm vs Term | −1.36 | −0.128 (−0.31; 0.06) | .51 | ||
| Late preterm vs Term | 1.10 | 0.099 (−0.08; 0.28) | .81 |
Note.
Wald chi-square.
Median [inter quartile range] provided for clinical reference only.
Abbreviations: Gestational age (GA), degrees of freedom (DF).
Discussion
This study examined the relationship between GA and MOM production and time to the onset of SA in a cohort of racially and ethnically diverse pump-dependent mothers of critically ill infants. We found mothers of early preterm infants (30–33 6/7 weeks GA) produced more MOM over the first seven days following delivery than mothers of term infants (≥37 weeks). While a single small study24 of 48 mothers of infants less than 31 weeks gestation found a similar inverse relationship between GA and milk production at 8 weeks postpartum, several studies report that mothers delivering less mature infants are at greater risk of decreased milk production.6,11,20,25,26,27,28 In fact, an observational study of 193 mothers found those delivering infants less than 32 weeks gestation produced 2.8 times less milk over the first six weeks compared to breastfeeding mothers of healthy term infants (relative risk = 3.0; 95% confidence interval = 1.9–4.8).20 Other research has reported no difference in milk production based on GA in mothers of infants born at 23–26 weeks GA compared to 26–28 weeks at 3 weeks postpartum26 or over the first week in mothers delivering infants less than 32 weeks gestation.27 However, previous research generally included mothers who were not in the first week postpartum or compared milk production in pump-dependent mothers of preterm infants with that of breastfeeding mothers of term healthy infants, which may not accurately describe milk production in pump-dependent mothers of critically ill late preterm and term infants.20,26
Furthermore, all mothers in this study regardless of GA produced less milk than previously reported.20,29 Compared to Hill et al,20 who reported an average milk production on days 6 and 7 following delivery of 433.4 mL in mothers of infants less than 32 weeks gestation and 494.6 mL in mothers of term infants, we found lower milk production during the same time period [averages over days 6 and 7; 386.28 mL (early preterm), 251.19 mL (late preterm), 297.12 mL (term)]. However, compared to Lussier et al.30 who found mothers of infants less than 32 weeks GA produced 1,371 mL over the first week, we found mothers of early preterm infants produced a similar cumulative milk volume of 1,410 mL. Conversely, mothers of both late preterm (909 mL) and term (1029 mL) infants produced less milk than reported in Lussier et al.30 over the first seven days.
Similar to previous sentinel studies in mothers delivering term breastfeeding infants,31,32 we found a temporal trend in milk production over the first 7 days. Furthermore, milk production during the first 1 to 2 weeks 30,33 may be predictive of milk production at 6 weeks,20,25,28 as well as long term breastfeeding success.20 Mothers of infants born at less than 32 weeks gestation who produce less than 140 mL/day at 4 days postpartum are over 9 times more likely to have an inadequate milk supply at 6 weeks.25 Our findings that both mothers of term and late preterm infants produced less than 140 mL/d on day 4, is concerning, and suggests they may be at risk for continued inadequate milk production.
Mothers of early preterm infants achieved SA significantly earlier than mothers of late preterm infants. An earlier onset of SA is associated with increased milk production31,34,35,36,37 and with future lactation success,12 which may be clinically important to increase the provision of MOM for infant feeds. In an observational study10 of 100 mothers of preterm infants (mean 33.92 weeks GA), delayed SA was associated with decreased milk production over the first 14 days following delivery.
This study had several limitations. Because we only examined milk production during the first 7 days after delivery, any differences in production after the first week are unknown. As commonly reported in human milk research,20,38 there was large variability in milk production with individual mothers across the study groups which required statistical correction during analysis. While the number of breastfeeding attempts and expression sessions between groups was not statistically significant, the early preterm group had fewer breastfeeding attempts and more expression sessions as well as the shortest time to the first expression which may have affected results. Although test-weighing measures milk consumption during breastfeeding, this practice was not standard care in this institution and thus the amount of milk consumed during breastfeeding was not measured. Finally, because this was a retrospective study, we were unable to include analysis of factors which may affect milk production including use of hand expression24 and although all mothers intended to provide milk to their infants, we did not collect information on intended length of lactation.39 In addition, this is an exploratory study of retrospective data collected four years ago and thus additional prospective studies are needed to conclusively determine potential differences in milk production among mothers of different gestational ages.
Conclusions
Providing MOM to NICU hospitalized infants is critical to improve health outcomes. Results of this exploratory study suggest pump-dependent mothers may be at significant risk of inadequate milk production and delayed SA, which may decrease MOM availability, thereby potentially increasing formula and DHM supplementation. It is possible that our findings of increased milk production in mothers of more premature infants could be attributed to the intense research and quality improvement projects focused on this vulnerable population of women. Therefore, it is essential to recognize that all pump-dependent mothers of critically ill infants may be at risk for inadequate milk production regardless of GA. Early close monitoring of milk production and lactation support should be provided for all mothers of infants admitted to the NICU. Future research is needed to further understand milk production and SA in women delivering infants greater than 1500 grams and to examine the effect of targeted interventions to improve milk production.
Acknowledgements:
The authors thank Clara Englemann, MHA and Molly McCoy for their assistance with data collection, as well as the healthcare workers and mothers in our NICU. The authors also thank Charlene Krueger, PhD, ARNP for her help in editing the manuscript. No one received compensation outside of their usual salary.
Funding Source: Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under University of Florida Clinical and Translational Science Awards TL1TR001428 and UL1TR001427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acronyms and Abbreviations:
- AIC
Akaike information criterion
- AAP
American Academy of Pediatrics
- ANOVA
analysis of variance
- AR
autoregressive
- BIC
Bayesian information criterion
- DHM
donor human milk
- EMR
electronic medical records
- GLMM
general linear mixed model
- GA
gestational age
- MOM
mother’s own milk
- NICU
neonatal intensive care unit
- SA
secretory activation
- STS
skin to skin
- VLBW
very low birth weight
- WHO
World Health Organization
Footnotes
Potential Conflicts of Interest: Dr. Parker, who is a Section Editor for Advances in Neonatal Care and the coauthor and mentor to the primary author, was not involved in the editorial review or decision to publish this article. The entire process from submission, referee assignment, and editorial decisions was handled by other members of the editorial team for the journal. The other authors have no conflicts of interest.
Contributor Information
Marion M. Bendixen, PO Box 100197, College of Nursing, University of Florida, Gainesville, FL 32610.
Michael T. Weaver, University of Florida, College of Nursing, Gainesville, Florida.
Leslie A. Parker, University of Florida, College of Nursing, Gainesville, Florida.
References
- 1.Cortez J, Makker K, Kraemer DF, Neu J, Sharma R, Hudak ML. Maternal milk feedings reduce sepsis, necrotizing enterocolitis and improve outcomes of premature infants. J Perinatol. Jan 2018;38(1):71–74. [DOI] [PubMed] [Google Scholar]
- 2.Patel A, Engstrom J, Goldman J, Fogg L, Meier P. Dose Response Benefits of Human Milk in Extremely Low Birth Weight Premature Infants. Pediatric Academic Socities; 2008. [Google Scholar]
- 3.Guideline: Protecting, Promoting and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services. 2017. [PubMed]
- 4.Eidelman AK, Schanler RJ. Breastfeeding and the use of human milk. Pediatrics. Mar 2012;129(3):e827–841. [DOI] [PubMed] [Google Scholar]
- 5.Furman L, Minich N, Hack M. Correlates of lactation in mothers of very low birth weight infants. Pediatrics. Apr 2002;109(4):e57. [DOI] [PubMed] [Google Scholar]
- 6.Acuna-Muga J, Ureta-Velasco N, de la Cruz-Bertolo J, et al. Volume of milk obtained in relation to location and circumstances of expression in mothers of very low birth weight infants. J Hum Lact. Feb 2014;30(1):41–46. [DOI] [PubMed] [Google Scholar]
- 7.Hill PD, Aldag JC, Zinaman M, Chatterton RT. Predictors of preterm infant feeding methods and perceived insufficient milk supply at week 12 postpartum. J Hum Lact. Feb 2007;23(1):32–38; quiz 39–43. [DOI] [PubMed] [Google Scholar]
- 8.Craighead DV, Elswick RK. The influence of early-term birth on NICU admission, length of stay, and breastfeeding initiation and duration. J Obstet Gynecol Neonatal Nurs. 2014 Jul-Aug 2014;43(4):409–421. [DOI] [PubMed] [Google Scholar]
- 9.Murase M, Nommsen-Rivers L, Morrow AL, et al. Predictors of low milk volume among mothers who delivered preterm. J Hum Lact. Nov 2014;30(4):425–435. [DOI] [PubMed] [Google Scholar]
- 10.Yu X, Li J, Lin X, Luan D. Association between Delayed Lactogenesis Ⅱ and Early Milk Volume among Mothers of Preterm Infants. Asian Nurs Res (Korean Soc Nurs Sci). May 2019;13(2):93–98. [DOI] [PubMed] [Google Scholar]
- 11.Henderson JJ, Hartmann PE, Newnham JP, Simmer K. Effect of preterm birth and antenatal corticosteroid treatment on lactogenesis II in women. Pediatrics. Jan 2008;121(1):e92–100. [DOI] [PubMed] [Google Scholar]
- 12.Brownell E, Howard CR, Lawrence RA, Dozier AM. Delayed onset lactogenesis II predicts the cessation of any or exclusive breastfeeding. J Pediatr. Oct 2012;161(4):608–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hill PD, Humenick SS. Insufficient milk supply. Image J Nurs Sch. Fall 1989;21(3):145–148. [DOI] [PubMed] [Google Scholar]
- 14.Chiang KV, Sharma AJ, Nelson JM, Olson CK, Perrine CG. Receipt of Breast Milk by Gestational Age - United States, 2017. MMWR Morb Mortal Wkly Rep. Jun 2019;68(22):489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoban R, Bigger H, Patel AL, Rossman B, Fogg LF, Meier P. Goals for Human Milk Feeding in Mothers of Very Low Birth Weight Infants: How Do Goals Change and Are They Achieved During the NICU Hospitalization? Breastfeed Med. 2015 Jul-Aug 2015;10(6):305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cacho NT, Lawrence RM. Innate Immunity and Breast Milk. Front Immunol. 2017;8:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cong X, Judge M, Xu W, et al. Influence of Feeding Type on Gut Microbiome Development in Hospitalized Preterm Infants. Nurs Res. Mar/Apr 2017;66(2):123–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meier PP, Bode L. Health, nutrition, and cost outcomes of human milk feedings for very low birthweight infants. Adv Nutr. Nov 2013;4(6):670–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schanler RJ, Lau C, Hurst NM, Smith EO. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics. Aug 2005;116(2):400–406. [DOI] [PubMed] [Google Scholar]
- 20.Hill PD, Aldag JC, Chatterton RT, Zinaman M. Comparison of milk output between mothers of preterm and term infants: the first 6 weeks after birth. Journal of Human Lactation. Feb 2005. (a);21(1):22–30. [DOI] [PubMed] [Google Scholar]
- 21.Sakalidis VS, Kent JC, Garbin CP, Hepworth AR, Hartmann PE, Geddes DT. Longitudinal changes in suck-swallow-breathe, oxygen saturation, and heart rate patterns in term breastfeeding infants. J Hum Lact. May 2013;29(2):236–245. [DOI] [PubMed] [Google Scholar]
- 22.Meier PP, Engstrom JL, Janes JE, Jegier BJ, Loera F. Breast pump suction patterns that mimic the human infant during breastfeeding: greater milk output in less time spent pumping for breast pump-dependent mothers with premature infants. J Perinatol. Feb 2012;32(2):103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.SAS Institute Inc. SAS/STAT 15.1 User’s Guide. Cary, NC: SAS Institute Inc.; 2018. [Google Scholar]
- 24.Morton J, Hall JY, Wong RJ, Thairu L, Benitz WE, Rhine WD. Combining hand techniques with electric pumping increases milk production in mothers of preterm infants. J Perinatol. Nov 2009;29(11):757–764. [DOI] [PubMed] [Google Scholar]
- 25.Hill PD, Aldag JC. Milk volume on day 4 and income predictive of lactation adequacy at 6 weeks of mothers of nonnursing preterm infants. J Perinat Neonatal Nurs. 2005 Jul-Sep 2005;19(3):273–282. [DOI] [PubMed] [Google Scholar]
- 26.Bishara R, Dunn MS, Merko SE, Darling P. Volume of foremilk, hindmilk, and total milk produced by mothers of very preterm infants born at less than 28 weeks of gestation. Journal of Human Lactation. Aug 2009;25(3):272–279. [DOI] [PubMed] [Google Scholar]
- 27.Chatterton RT Jr., Hill PD, Aldag JC, Hodges KR, Belknap SM, Zinaman MJ. Relation of plasma oxytocin and prolactin concentrations to milk production in mothers of preterm infants: influence of stress. J Clin Endocrinol Metab. Oct 2000;85(10):3661–3668. [DOI] [PubMed] [Google Scholar]
- 28.Ru X, Huang X, Feng Q. Successful Full Lactation Achieved by Mothers of Preterm Infants Using Exclusive Pumping. Frontiers in Pediatrics. 2020;8(191). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kent JC, Gardner H, Geddes DT. Breastmilk Production in the First 4 Weeks after Birth of Term Infants. Nutrients Nov 25 2016;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lussier MM, Brownell EA, Proulx TA, et al. Daily Breastmilk Volume in Mothers of Very Low Birth Weight Neonates: A Repeated-Measures Randomized Trial of Hand Expression Versus Electric Breast Pump Expression. Breastfeed Med. 2015 Jul-Aug 2015;10(6):312–317. [DOI] [PubMed] [Google Scholar]
- 31.Casey CE, Neifert MR, Seacat JM, Neville MC. Nutrient intake by breast-fed infants during the first five days after birth. Am J Dis Child. Sep 1986;140(9):933–936. [DOI] [PubMed] [Google Scholar]
- 32.Neville MC, Morton J. Physiology and endocrine changes underlying human lactogenesis II. J Nutr. Nov 2001;131(11):3005S–3008S. [DOI] [PubMed] [Google Scholar]
- 33.Hoban R, Bigger H, Schoeny M, Engstrom J, Meier P, Patel AL. Milk Volume at 2 Weeks Predicts Mother’s Own Milk Feeding at Neonatal Intensive Care Unit Discharge for Very Low Birthweight Infants. Breastfeed Med. March 2018;13(2):135–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cregan MD, De Mello TR, Kershaw D, McDougall K, Hartmann PE. Initiation of lactation in women after preterm delivery. Acta Obstetricia et Gynecologica Scandinavica. Sep 2002;81(9):870–877. [DOI] [PubMed] [Google Scholar]
- 35.Kent JC, Prime DK, Garbin CP. Principles for maintaining or increasing breast milk production. J Obstet Gynecol Neonatal Nurs. 2012 Jan-Feb 2012;41(1):114–121. [DOI] [PubMed] [Google Scholar]
- 36.Neville MC, Keller R, Seacat J, et al. Studies in human lactation: milk volumes in lactating women during the onset of lactation and full lactation. Am J Clin Nutr. Dec 1988;48(6):1375–1386. [DOI] [PubMed] [Google Scholar]
- 37.Parker LA, Sullivan S, Krueger C, Mueller M. Association of timing of initiation of breastmilk expression on milk volume and timing of lactogenesis stage II among mothers of very low-birth-weight infants. Breastfeed Med. Mar 2015;10(2):84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parker LA, Sullivan S, Krueger C, Kelechi T, Mueller M. Effect of early breast milk expression on milk volume and timing of lactogenesis stage II among mothers of very low birth weight infants: a pilot study. J Perinatol. Mar 2012;32(3):205–209. [DOI] [PubMed] [Google Scholar]
- 39.Guo JL, Wang TF, Liao JY, Huang CM. Efficacy of the theory of planned behavior in predicting breastfeeding: Meta-analysis and structural equation modeling. Appl Nurs Res. Feb 2016;29:37–42. [DOI] [PubMed] [Google Scholar]


