Abstract
The methods commonly used for human brucellosis serological testing are agglutination tests and the complement fixation test (CFT). Among the newer serological tests, primary binding assays were developed to improve sensitivity and specificity. The competitive enzyme immunoassay (CELISA) for the detection of serum antibody to Brucella is a multispecies assay which appears to be capable of differentiating vaccinal and cross-reacting antibodies from antibodies elicited by field infection in cattle. The competing monoclonal antibody used in this assay is specific for a common epitope of smooth lipopolysaccharide (S-LPS). In this study, we compared the CELISA to the classical tests for the diagnosis of human brucellosis. The CELISA cutoff value was determined to calculate its diagnostic specificity and sensitivity. A survey was performed with 911 sera. Of the sera, 341 were from an asymptomatic population that tested negative with conventional serological tests (screening and confirmatory). Based on these samples, the CELISA specificities were determined to be 99.7 and 100% with cutoff values of 28 and 30% inhibition (%I), respectively. In a further study with 393 additional sera from an asymptomatic population found negative by the conventional screening tests, the CELISA specificities were calculated to be 96.5 and 98.8% with cutoff values of 28 and 30%I. The CELISA sensitivities were determined to be 98.3 and 94.8% with cutoff values of 28 and 30%I, respectively, for sera from 116 individuals found positive by the classical tests. For the 51 culture-positive patients, CELISA was positive for 100%, the CFT was positive for 92%, and the standard tube agglutination test (TAT) was positive for 100%. The CELISA specificity was 100% for 31 sera from patients found negative by conventional serological tests but with brucellosis-like symptoms. The CELISA is fairly rapid to perform, somewhat faster than TAT, and cross-reacts less with other antigens (or antibodies) than the conventional tests. Further, the CELISA is simpler to perform that the CFT and may readily be standardized by the use of purified S-LPS antigen and monoclonal antibody for competition.
Although brucellosis has long been recognized as a global problem of animals, it constitutes a significant human health problem. In Argentina, brucellosis in humans is linked to infection in goats in the west and in cattle and swine in the east. In urban centers, the main source of infection is slaughterhouses (7). Since some individuals with brucellosis have been found to be afebrile and asymptomatic, the need for accurate diagnosis is greater, especially in areas of endemicity (16).
The diagnostic methods now commonly used for human serological testing are agglutination tests and the complement fixation test (CFT). Among the newer serological tests, primary binding assays were developed to improve sensitivity and specificity. The indirect enzyme immunoassay (IELISA) appears to be the most sensitive; however, interpretation may be difficult, as false-positive reactions may occur due to exposure to, for instance, Yersinia enterocolitica O:9 (15). Another problem with the IELISA is the standardization of reagents, which should be improved in order to make interlaboratory results easy to interpret (15–17). The competitive enzyme immunoassay (CELISA) for the detection of serum antibody to Brucella is a multispecies assay which appears to be capable of differentiating vaccinal antibodies from antibodies elicited by field infection in cattle (12, 13). The monoclonal antibody used in this assay is specific for a common epitope of smooth lipopolysaccharide (S-LPS). The test is fairly rapid but does require some equipment or manipulation.
In this study, the CELISA was compared to the conventional tests for the diagnosis of human brucellosis. The most appropriate cutoff value for the diagnostic specificity and sensitivity of the CELISA was determined.
MATERIALS AND METHODS
Conventional serological tests.
The plate agglutination test (PAT), Rose Bengal test (RBT), buffered-antigen plate agglutination test (BPAT), and standard tube agglutination test (TAT) were performed as described previously (10). The cold CFT was done as described previously (4), based on the addition of 5 hemolytic units of guinea pig complement, 18 h of fixation at 4°C, the addition of sensitized erythrocytes, incubation for 30 min at 37°C, and photometric reading. All the sera, including positive and negative controls, were inactivated for 30 min at 56°C. Fresh serum usually contains some anticomplement activity, the level of which may be increased by bacterial contamination; the process of inactivation reduces this anticomplement activity. For interpretation, a reaction at a 1:5 serum dilution or higher was considered positive. Sera exhibiting anticomplement activity were not considered suitable for testing.
The antigens used in the tests were prepared at the Administración Nacional de Laboratorios e Institutos de Salud Dr. C. G. Malbrán; antigens supplied by the National Veterinary Services Laboratories, U.S. Department of Agriculture, were used as a reference.
CELISA.
The CELISA involved the adsorption of Brucella S-LPS antigen diluted in 0.06 M sodium carbonate buffer (pH 9.6) to polystyrene plates (Nunc 2-69620; Nunc, Roskilde, Denmark) at 100 μl/well. The plates were incubated overnight (18 h) at 4°C and then washed four times in 0.01 M phosphate-buffered saline containing 0.05% Tween 20 (pH 7.2) (PBS/T). This step was followed by the addition of 95 μl of prediluted ascitic fluid containing mouse monoclonal antibody (MAb), diluted in 0.015 M EDTA–0.015 M EGTA in PBS/T (pH 6.3), specific for an epitope on the O-polysaccharide portion of the immobilized antigen, and the addition of 5 μl of undiluted control or test serum; the mixture was mixed gently for 5 min in a microplate shaker and incubated for 30 min at room temperature. After this incubation period, the plate was washed four times in PBS/T, and goat anti-mouse immunoglobulin G (IgG) antibody conjugated to horseradish peroxidase (diluted in PBS/T) was added; the mixture was mixed gently for 5 min in a shaker and incubated for 30 min at room temperature. The plate was washed four times. The final step was the addition of 100 μl of chromogenic substrate [4.0 mM hydrogen peroxide and 1.0 mM 2,2′-azino-bis(3-ethylbenz-thiazoline-6-sulfonic acid) diammonium salt in 0.05 M citrate buffer (pH 4.5)] per well. After 10 min of continuous shaking, the optical density (OD) was measured photometrically (Labsystems Multiskan EX microplate reader with a 414-nm filter) by use of 100 μl of chromogenic substrate in a plate to serve as a control for the microplate reader. In the absence of anti-Brucella antibody in the test serum, the MAb binds, resulting in color development. If the test is positive, the test serum competes with the MAb for the epitope sites, and inhibition of MAb binding is inversely proportional to subsequent color development (12, 13). Results for control and test sera were expressed as percent inhibition (%I) of MAb activity against the antigen. The %I value for each sample was calculated with the formula %I = [100 − (OD of test sample/OD of conjugate control) × 100].
The antigen (S-LPS from Brucella abortus 413), the MAb (specific for an epitope on the O-polysaccharide portion of the S-LPS), and the control sera (strongly positive and weakly positive bovine anti-Brucella serum and negative bovine serum) were standardized and supplied by the Brucellosis Centre of Expertise and Office International des Epizooties Reference Laboratory, Animal Diseases Research Institute, Nepean, Ontario, Canada.
The conjugate (goat anti-mouse IgG antibody conjugated to horseradish peroxidase) preadsorbed with bovine, equine, and human serum proteins was from Jackson Laboratories Inc. In addition to bovine serum controls, positive and negative reference human sera were included in each CELISA plate.
Bacteriological studies.
Brucella organisms were isolated as previously described (8) from three blood cultures, except in one case, in which cerebrospinal fluid was used. The strains were typed as recommended by the International Committee on Bacterial Nomenclature (ICBN) Subcommittee on Taxonomy of the Genus Brucella (6) at Administración Nacional de Laboratorios e Institutos de Salud Dr. C. G. Malbrán.
Human sera.
The 911 sera included in the study were divided into the following groups.
(i) Asymptomatic population of blood donors.
A total of 341 sera from people with no clinical or epidemiological evidence of brucellosis (18 to 62 years old) and with negative PAT, RBT, BPAT, TAT, and CFT results were used to determine the CELISA cutoff value and diagnostic specificity.
(ii) Asymptomatic population from rural areas.
A total of 393 sera from clinically and epidemiologically healthy people between 10 and 65 years of age and with negative PAT, RBT, and BPAT results were used to determine the CELISA cutoff value and diagnostic specificity.
(iii) Suspected brucellosis patients.
A total of 65 sera were obtained from patients with clinical symptoms compatible with brucellosis and with positive PAT, RBT, BPAT, TAT, and CFT results at any titer.
(iv) Brucella-infected patients.
A total of 51 sera were obtained from patients with positive cultures.
(v) Patients with febrile syndrome.
A total of 31 sera from patients with clinical symptoms compatible with brucellosis but with negative PAT, RBT, BPAT, TAT, and CFT results were used to test CELISA specificity.
(vi) Patients treated with antibiotic therapy.
A total of 30 sera from nine patients receiving appropriate treatment for brucellosis were tested. The treatment consisted basically of doxycycline (200 mg/day)-rifampin (600 to 900 mg/day) or streptomycin (1 g/day) for a minimum of 6 weeks. Sometimes it was combined with trimethoprim (160 mg twice daily) and sulfamethoxazole (800 mg twice daily) in accordance with previous recommendations (3). Tests were done at admission, 1 or 2 months after the initiation of treatment, and in some cases up to 28 months later.
Data analysis.
Diagnostic sensitivity and specificity were determined initially by plotting the data for negative and positive samples in a frequency histogram. The cutoff value was used for calculating initial sensitivity and specificity with 95% confidence limits. The data was subsequently analyzed by receiver-operator characteristics (ROC) analysis (11). This analysis allowed the determination of an optimum cutoff value, estimated by comparing the range of sensitivity and specificity values for a range of cutoff values. The optimum cutoff value results in the maximum sum of the sensitivity and specificity values. If a higher sensitivity is desired, a lower cutoff may be selected, usually at the cost of specificity.
RESULTS
The 341 negative sera yielded a mean %I value of 12.09 and a standard deviation of 6.0 for the CELISA. A frequency distribution showed a characteristic skewing of the results for the serum samples (data not shown). The cutoff value of 28%I resulted in a CELISA specificity of 99.7% and one false-positive (Table 1), while a cutoff value of 30%I resulted in a CELISA specificity of 100% and no false-positives (Table 2).
TABLE 1.
Number of true-positives, false-positives, true-negatives, and false-negatives for the CELISA with a cutoff of 28%I
| CELISA | No. of sera with the following reference method result:
|
|
|---|---|---|
| Positivea | Negativeb | |
| Positive | 114 | 1 |
| Negative | 2 | 340 |
Sera were from patients with suspected brucellosis (65 sera from patients with clinical symptoms compatible with brucellosis and positive PAT, RBT, BPAT, TAT, and CFT results at any titer) and Brucella-infected patients (51 sera from patients with positive cultures).
Sera were from an asymptomatic population of blood donors (341 sera from people with no clinical or epidemiological evidence of brucellosis, 18 to 62 years old, and with negative PAT, RBT, BPAT, TAT, and CFT results).
TABLE 2.
Number of true-positives, false-positives, true-negatives, and false-negatives for the CELISA with a cutoff of 30%I
When 393 sera that were negative in screening tests (PAT, RBT, and BPAT) were added to the original 341 sera, a mean %I value of 12.63 and a standard deviation of 7.15 were obtained for the CELISA. The frequency distribution of the sera again gave a cutoff value of 28%I.
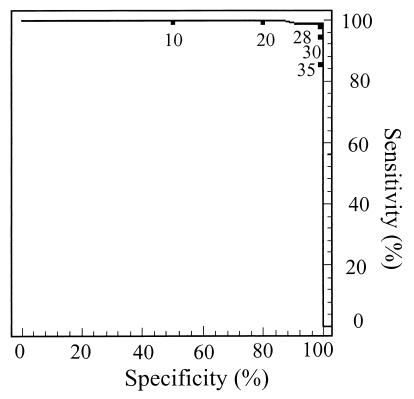
In both cases, the cutoff values were confirmed by ROC analysis (Fig. 1), and in both cases 116 sera were used as positive sera (65 sera positive in PAT, RBT, BPAT, TAT, and CFT at any titer and 51 sera from culture-positive patients) to determine the assay sensitivity (Tables 1 and 2). In the enlarged study (734 sera), when a cutoff value of 28%I was selected, the sensitivity was 98.3% and the specificity was 96.5%.
FIG. 1.
ROC analysis plotting percent sensitivity (y axis) against percent specificity (x axis) for various cutoff values of the CELISA for the detection of antibody to Brucella spp. A value of 28%I yielded the maximum sum of percent sensitivity and percent specificity and was considered to be the optimum cutoff value.
Fifty-one Brucella strains were obtained from patients. Table 3 shows the typing results and serological test results for the sera from these patients. Eighteen strains were B. abortus biovar 1, 1 was B. abortus biovar 1 S19, 6 were B. melitensis biovar 1, 16 were B. suis biovar 1, and 9 were B. suis biovar 1a (atypical strains) (5); 1 strain was not typed at the species level. At a CELISA cutoff value of 28%I, the sensitivity for this group was 100%; however, the CFT gave four negative results, and two sera had anticomplement activity (the sensitivity was 92% when the two sera with anticomplement activity were considered positive). The TAT detected all the positive sera, although three sera had titers of <1:100.
TABLE 3.
Serological results for sera from Brucella sp. culture-positive patients
| Case | Resulta of:
|
Speciesb | |||||
|---|---|---|---|---|---|---|---|
| PAT | RBT | BPAT | TAT | CFT | CELISA | ||
| 1 | >400 | Pos | Pos | 1,600 | 160 | 85 | B. melitensis |
| 2 | 200 | Pos | Pos | 200 | 10 | 84 | B. suis 1a |
| 3 | 200 | Pos | Pos | 100 | 20 | 38 | B. abortus |
| 4 | >400 | Pos | Pos | 1,600 | 160 | 89 | B. abortus 1 S19 |
| 5 | >400 | Pos | Pos | 6,400 | 640 | 77 | B. suis 1a |
| 6 | >400 | Pos | Pos | 400 | 160 | 56 | B. melitensis |
| 7 | 100 | Pos | Pos | 100 | 5 | 47 | B. melitensis |
| 8 | 100 | Pos | Pos | 100 | 40 | 90 | B. suis |
| 9 | 200 | Pos | Pos | 200 | 80 | 47 | B. abortus |
| 10 | 200 | Pos | Pos | 200 | 20 | 79 | B. suis 1a |
| 11 | >400 | Pos | Pos | 1,600 | 320 | 70 | B. abortus |
| 12 | 100 | Pos | Pos | 100 | 40 | 60 | B. abortus |
| 13 | 100 | Pos | Pos | 50 | 20 | 50 | B. suis |
| 14 | 400 | Pos | Pos | 400 | 40 | 88 | B. suis |
| 15 | 400 | Pos | Pos | 400 | 40 | 82 | B. suis 1a |
| 16 | 400 | Pos | Pos | 400 | 40 | 81 | B. abortus |
| 17 | 400 | Pos | Pos | 400 | 40 | 71 | B. suis |
| 18 | 100 | Pos | Pos | 100 | 10 | 35 | B. suis |
| 19 | 100 | Pos | Pos | 50 | Neg | 43 | B. suis |
| 20 | 200 | Pos | Pos | 200 | Neg | 90 | B. abortus |
| 21 | 400 | Pos | Pos | 400 | 40 | 79 | B. suis |
| 22 | 100 | Pos | Pos | 100 | 20 | 71 | B. suis 1a |
| 23 | 100 | Pos | Pos | 100 | Neg | 56 | B. abortus |
| 24 | 400 | Pos | Pos | 400 | 40 | 55 | B. abortus |
| 25 | 400 | Pos | Pos | 400 | 40 | 77 | B. suis 1a |
| 26 | 200 | Pos | Pos | 200 | 40 | 73 | B. abortus |
| 27 | 400 | Pos | Pos | 400 | 40 | 71 | B. suis |
| 28 | 400 | Pos | Pos | 400 | 40 | 66 | B. suis 1a |
| 29 | 400 | Pos | Pos | 400 | 40 | 54 | B. suis |
| 30 | 400 | Pos | Pos | 400 | 40 | 82 | B. abortus |
| 31 | 50 | Pos | Pos | 25 | Neg | 84 | B. suis |
| 32 | 100 | Pos | Pos | 100 | 20 | 47 | B. suis |
| 33 | 400 | Pos | Pos | 400 | 40 | 77 | B. suis |
| 34 | >400 | Pos | Pos | 1,600 | 320 | 39 | B. suis 1a |
| 35 | 200 | Pos | Pos | 200 | 20 | 29 | B. melitensis |
| 36 | >400 | Pos | Pos | 1,600 | 640 | 71 | B. abortus |
| 37 | 100 | Pos | Pos | 100 | 40 | 77 | B. abortus |
| 38 | 200 | Pos | Pos | 200 | PA | 32 | B. suis |
| 39 | >400 | Pos | Pos | 1,600 | PA | 62 | B. suis |
| 40 | 200 | Pos | Pos | 200 | 40 | 35 | B. abortus |
| 41 | 200 | Pos | Pos | 200 | 80 | 58 | B. abortus |
| 42 | >400 | Pos | Pos | 1,600 | 640 | 87 | B. suis |
| 43 | 400 | Pos | Pos | 400 | 160 | 80 | B. melitensis |
| 44 | >400 | Pos | Pos | 3,200 | 640 | 74 | B. suis 1a |
| 45 | >400 | Pos | Pos | 800 | 160 | 86 | Brucella sp. |
| 46 | >400 | Pos | Pos | 3,200 | 640 | 79 | B. abortus |
| 47 | >400 | Pos | Pos | 1,600 | 80 | 68 | B. abortus |
| 48 | >400 | Pos | Pos | 3,200 | 640 | 77 | B. abortus |
| 49 | >400 | Pos | Pos | 3,200 | 320 | 73 | B. abortus |
| 50 | >400 | Pos | Pos | 1,600 | 320 | 77 | B. suis |
| 51 | >400 | Pos | Pos | 400 | 160 | 70 | B. melitensis |
Results are shown as titers for PAT, TAT, CFT, and CELISA. For RBT and BPAT, results are indicated as positive (Pos) or negative (Neg). PA, sera with anticomplement activity.
All species were biovar 1, unless otherwise indicated; 1a, atypical biovar 1.
The %I value for 31 sera from people with clinical symptoms compatible with brucellosis but found negative by PAT, RBT, BPAT, TAT, and CFT was between 7 and 26%, with a mean value of 14.83, for the CELISA.
The serological results for nine patients receiving antibiotic therapy for brucellosis and tested at admission and 1, 2, or more months after treatment were determined. Occupationally exposed workers were monitored for longer periods (data not shown).
DISCUSSION
Human brucellosis is diagnosed on the basis of clinical findings and laboratory studies that include bacteriological and serological tests. Diagnosis is not difficult if the clinical presentation is typical, but the varied manifestations of localized, acute, or chronic infection sometimes lead to misdiagnosis (16).
Little is known about changes in antibody concentrations over time or about the distinction among antibody levels in persons with chronic disease, past infection, and/or occupational exposure.
It has been suggested (9) that complete recovery from infection is normally followed by a sharp reduction in antibody levels beginning 2 months later. A break in this reduction, replaced by a new rise in IgG levels, is highly suggestive of a relapse or an incomplete recovery. Ariza et al. (2) have reported that most patients with persistently high titers of IgG antibody had the highest titers at admission or had focal disease. The persistence of high titers of IgG antibody in patients without a high initial titer may be indicative of chronic disease. Nevertheless, a combination of determinations of IgG and IgM antibody levels allows accurate serological diagnosis at any stage of the disease.
The TAT has the advantage of detecting the combined IgM, IgA, and IgG antibody levels in serum, but its diagnostic specificity is poor, especially when the titers are low. Cross-reactions with other gram-negative bacteria have been observed, and the diagnostic end-point agglutination titer has not been satisfactorily established.
The CFT mostly identifies IgG antibodies predominant in the later stage of the disease or in chronic disease but has several disadvantages, such as the occurrence of anticomplement activity, the need to use a highly labile reagent (such as complement), a failure to detect a CFT response in the early stage of the disease, and technical demands.
It has been stressed that it would be very beneficial to use enzyme immunoassay (ELISA) technology for brucellosis surveillance in human populations in order to improve evaluation of the importance of the disease and to use ELISA technology to assess the efficacy of brucellosis control in animal populations in areas of endemicity (15). The ELISA would also seem to be an appropriate technology for application to individual human diagnoses (1, 2, 9). However, the problem of serologically differentiating infected patients from those who have been exposed to cross-reacting organisms has not been resolved with the use of S-LPS antigens in the IELISA. The O polysaccharide portion of the S-LPS is the immunodominant antigen in response to exposure, and the antigenic epitopes responsible for cross-reactivity also reside in the S-LPS. The CELISA is based on a MAb specific for a common and repeating epitope on the O-polysaccharide portion of the S-LPS, and the MAb competes with antibodies to cross-reacting antigens; specificity can be increased by use of a MAb with a selected affinity for an antigen (13).
The principle objectives of this study were to ascertain the usefulness of the CELISA for the diagnosis of human brucellosis, to determine the cutoff value in relation to infection, and to monitor the course of treatment.
The serological survey was performed with a total of 911 sera. The 341 sera from the asymptomatic population found negative by conventional serological tests showed CELISA specificities of 99.7 and 100%, respectively, when cutoff values of 28 and 30%I were selected. When we tested a total of 734 sera from the asymptomatic population found negative by conventional screening tests, the CELISA specificities were 96.5 and 98.8% with cutoff values of 28 and 30%I, respectively. It is clear that by adjusting the cutoff value upward, specificity may be increased to 100%, although with some loss of sensitivity.
For 116 sera found positive by conventional serological tests or from culture-positive patients, the CELISA sensitivities were 98.3 and 94.8% with cutoff values of 28 and 30%I, respectively. For the 51 culture-positive patients, the CELISA detected 100% of the cases, the CFT detected 92%, and the TAT detected 100%. Three culture-positive cases had TAT titers of <1:100. Similar results were found by another research group (9), who reported that 13 of 46 patients with positive cultures had agglutination titers of <1:80 at admission and that 7 did not have increased TAT titers when monitored sequentially. The three cases with TAT titers of <1:100 in this study were CELISA positive (over 28%I). The CELISA data is summarized in Table 3.
One case of interest (case 8, Table 3) had neurological complications, and Brucella was isolated from cerebrospinal fluid. The agglutination titer at admission was 1:100, the CFT titer was 1:40, and the CELISA titer was 90. A second interesting case (case 35, Table 3) had the lowest CELISA value and involved a patient whose titer had not increased 3 months later. The four CFT-negative cases had CELISA titers over 28. The CELISA specificity was 100% for 31 sera from patients who tested negative in conventional serological tests but had brucellosis-like symptoms.
To focus on the influence of chemotherapy and compliance with medical treatment on test results, we studied nine patients who received classical brucellosis treatment. The serological follow-up performed with serial serum samples showed that the CELISA %I values correlated well with the clinical course. One patient infected with B. melitensis had low but persistent %I values for 3 months after admission.
In the present study, the CELISA with a cutoff value of 28%I had a specificity of 99.7% and a sensitivity of 98.3%. The test detects IgG antibody, which is useful for evaluating treatment effectiveness, for monitoring clinical conditions, and for prognosis. It is relatively easier to perform than the CFT, a technically complicated test that requires continuous titration of reagents, and is somewhat faster than the TAT. Fewer cross-reactions with antigens of other microorganisms (or antibodies) occur (12, 13), and the use of MAb detection reagents enables standardization (14). The CELISA is a suitable test for human brucellosis and could be adopted as the confirmatory test.
ACKNOWLEDGMENTS
This project was funded in part by a grant from the Centro Argentino Brasilero de Biotecnologia (CABBIO).
We are grateful to Jorge Rey, Hospital de Clinicas, Universidad de Buenos Aires, and to Maria Playán, Hospital de Ciudadela, both in Buenos Aires, Argentina, for providing us with sera from blood donors.
REFERENCES
- 1.Araj G F, Lulu A I, Mustafa M Y, Khateeb M I. Evaluation of ELISA in the diagnosis of acute and chronic brucellosis in human beings. J Hyg. 1986;97:457–469. doi: 10.1017/s0022172400063634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ariza J, Pellicer T, Pallarés A, Foz A, Gudiol F. Specific antibody profile in human brucellosis. Clin Infect Dis. 1992;14:131–140. doi: 10.1093/clinids/14.1.131. [DOI] [PubMed] [Google Scholar]
- 3.Ariza J. Antibiotic therapy for human brucellosis. In: Plommet M, editor. Proceedings of the International Seminar Organized by CIHEAM, CEC, MINAG (Malta), FIS (Malta). Wageningen, The Netherlands: Pudoc Scientific Publishers; 1992. pp. 87–92. [Google Scholar]
- 4.Centro Panamericano de Zoonosis/Pan-American Health Organization/World Health Organization. Brucelosis: prueba de fljacion de complemento. Nota técnica no. 24. Buenos Aires, Argentina: Centro Panamericano de Zoonosis; 1981. [Google Scholar]
- 5.Corbel M J, Thomas E L, Garcia Carrillo C. Taxonomic studies on some atypical strains of Brucella suis. Br Vet J. 1984;140:34–43. doi: 10.1016/0007-1935(84)90055-1. [DOI] [PubMed] [Google Scholar]
- 6.Corbel M J, Brinley Morgan W J. Genus Brucella, Meyer and Shaw 1920, 173 AL. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams & Wilkins Co.; 1984. pp. 377–388. [Google Scholar]
- 7.Garcia Carrillo C. Animal and human brucellosis in the Americas. Paris, France: Office International des Epizooties; 1990. Argentina; pp. 4–30. [Google Scholar]
- 8.Garcia Carrillo C, Lucero N E. Diagnóstico bacteriológico. In: De Diego A, editor. Brucelosis bovina. Buenos Aires, Argentina: Hemisferio Sur; 1993. pp. 97–98. [Google Scholar]
- 9.Gazapo E, Gonzalez Lahoz J, Subiza J L, Baquero M, Gil J, de la Concha E. Changes in IgM and IgG antibody concentrations in brucellosis over time—importance for diagnosis and follow up. J Infect Dis. 1989;159:219–225. doi: 10.1093/infdis/159.2.219. [DOI] [PubMed] [Google Scholar]
- 10.Lucero N E, Bolpe J E. The buffered plate antigen as a screening test for diagnosis of human brucellosis. J Clin Microbiol. 1998;36:1425–1427. doi: 10.1128/jcm.36.5.1425-1427.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metz C E. Basic principles of ROC analysis. Semin Nucl Med. 1978;8:283. doi: 10.1016/s0001-2998(78)80014-2. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen K H, Gall D, Kelly W, Vigliocco A, Henning D, Garcia M. Immunoassay development: application to enzyme immunoassay for the diagnosis of brucellosis. Nepean, Ontario, Canada: Agriculture and Agri-Food Canada; 1996. [Google Scholar]
- 13.Nielsen K H, Kelly L, Gall D, Balsevicius S, Nicoletti P, Kelly W. Improved competitive enzyme immunoassay for the diagnosis of bovine brucellosis. Vet Immunol Immunopathol. 1995;46:285–291. doi: 10.1016/0165-2427(94)05361-u. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization. Diagnosis of brucellosis in humans. Report of Working Group Meeting on Brucellosis Diagnosis and Research in Enzyme Immunoassay. WHO/CDS/VPI-1/93.125. Geneva, Switzerland: World Health Organization; 1993. [Google Scholar]
- 15.World Health Organization. Joint FAO/WHO Expert Committee on Brucellosis, 6th report. W H O Tech Rep Ser. 1986;740:62–63. [Google Scholar]
- 16.Young E J. An overview of human brucellosis. Clin Infect Dis. 1995;21:283–290. doi: 10.1093/clinids/21.2.283. [DOI] [PubMed] [Google Scholar]
- 17.Young E J. Utility of the enzyme-linked immunosorbent assay for diagnosing neurobrucellosis. Lett Clin Infect Dis. 1998;26:1481. doi: 10.1086/517653. [DOI] [PubMed] [Google Scholar]