Abstract
Changes in gene expression are a hallmark of learning and memory consolidation. Little is known about how alternative mRNA processing, particularly abundant in neuron-specific genes, contributes to these processes. Prototype RNA binding proteins of the neuronally expressed ELAV/Hu family are candidates for roles in learning and memory, but their capacity to cross-regulate and take over each other’s functions complicate substantiation of such links. Honey bees Apis mellifera have only one elav/Hu family gene elavl2, that has functionally diversified by increasing alternative splicing including an evolutionary conserved microexon. RNAi knockdown demonstrates that ELAVL2 is required for learning and memory in bees. ELAVL2 is dynamically expressed with altered alternative splicing and subcellular localization in mushroom bodies, but not in other brain regions. Expression and alternative splicing of elavl2 change during memory consolidation illustrating an alternative mRNA processing program as part of a local gene expression response underlying memory consolidation.
Subject terms: Alternative splicing, Classical conditioning
Ustaoglu, Gill, et al. investigate the role of the single copy of the elav/Hu family gene in honeybees in learning and memory. RNAi knockdown of elavl2 demonstrates a role in learning and memory, and ELAVL2 is dynamically expressed with altered alternative splicing and changing expression upon learning.
Introduction
Changes in gene expression play pivotal roles in memory consolidation, the process through which memories are stabilized and stored into long-term memory1–4. A common feature of neuronal genes, particularly ion channel and cell adhesion genes, is their often complex pattern of alternative splicing, which alters protein coding and regulatory potential in flanking untranslated regions of the mRNA5–7. Alternative splicing events particularly in cell adhesion and ion channels among other genes have been linked to learning and memory8–12, but little is known how RNA-binding proteins impact on alternative splicing programs that operate in learning and memory. Here, we focused on ELAV (Embryonic Lethal Abnormal Visual system)/Hu family RNA-binding proteins because they are prominently expressed in neurons of all metazoans, regulate alternative splicing and expression of synaptic genes as well as formation of new connections13–17.
Like many RNA-binding proteins (RBPs) ELAV/Hu proteins comprise a family of highly related proteins in humans and many animals. Humans have four ELAV/Hu genes (HuB, HuC, HuD, and HuR), Drosophila have three (elav, fne, and Rbp9) and Hydra have three (ELAV-like 1–3)18,19. Some animals including the lancelet B. floridae, the nematode C. elegans, the honeybee A. mellifera and the cricket G. bimaculatus, however, have only one gene of an ELAV/Hu family orthologue indicating a very dynamic protein family with gains and losses during animal evolution18,19. Of note, the single ELAV/Hu family orthologue found in neurons in crickets has substantially expanded its coding capacity by alternative splicing to encode 24 protein isoforms19.
In mice, all Hu proteins are expressed in largely overlapping patterns in mature neurons20, while in Drosophila pan-neural expression of ELAV and FNE starts with the birth of neurons, and RBP9 is first detected in late larval neurons21–24. Although ELAV family RBPs in Drosophila have distinct neuronal phenotypes based on the analysis of null mutants and genetic interactions among them, they can cross regulate each other’s targets depending on cellular localization and concentrations complicating the analysis of their functions24.
ELAV/Hu proteins are prototype RBPs, which harbor three highly conserved RNA Recognition Motifs (RRMs). The first two RRMs are arranged in tandem and the third RRM is separated by a less-conserved hinge region. ELAV/Hu family RBPs bind to short, uridine-rich motifs, which are ubiquitously found in introns and untranslated regions, but ELAV/Hu proteins are gene-specific and have a complement of dedicated target genes15,17,25–28. Due to the prominent nuclear localization, ELAV in Drosophila has mostly been associated with gene-specific regulation of alternative splicing and polyadenylation, but it can also regulate mRNA stability29–36. Although the three RRMs comprise the evolutionary most conserved parts of ELAV/Hu proteins, individual members are to a large degree functionally interchangeable when adjusting expression levels and subcellular localization24,37,38. Hence, regulation of the activity of ELAV/Hu proteins likely occurs at the level of post-translational modifications and suggest that less conserved and unstructured linker sequences between or within RRMs serve fundamental functional roles, possibly by regulating interactions with other proteins39.
To avoid complications of assigning specific gene functions to individual members of the ELAV/Hu family, we focused on honeybees whose genome encodes only one copy of an elav/Hu family gene18, elavl2, an orthologue of Drosophila fne22. Conveniently, honeybees are a well-established model for the study of learning and memory. Here we show that the single elavl2 gene in honeybees is required for learning, as well as the formation of stable memories by RNAi knockdown. Although bees have only a single elav/Hu family gene elavl2, its coding capacity proliferated by increasing alternative splicing to generate 40 different protein isoforms. The splicing pattern changes during development and between different adult social castes, but also shows variability among the brains of individual adult workers. Likewise, ELAVL2 expression changes in mushroom bodies (brain centers involved in learning and memory), but not in the medulla of the optic system, to generate individual expression patterns reminiscent of experience-dependent neuronal activity that forms the basis of gene expression changes associated with memory consolidation. Consistent with a role in learning and memory consolidation, elavl2 expression and inclusion levels of alternative exons change during the early phases of memory consolidation. In this memory consolidation phase, also transcription is required and hence alternative splicing could be altered then depending on experience40,41.
Results
ELAVL2 is required for learning and memory consolidation in bees after olfactory reward conditioning
To detect bee ELAVL2, we used a polyclonal antiserum raised against Drosophila ELAV42, that cross-reacts with bee ELAVL2 and human HuR, but not with other Drosophila ELAV family members and Drosophila cap methyltransferase CMTr143 as shown by Western blot from bacterially expressed GST-fusion proteins (Supplementary Fig. 1a, b).
The single ELAVL2 in bees is prominently expressed in the brain as determined by Western-blots recognizing the expected 38 kDa protein (Supplementary Fig. 1c). We did not detect ELAVL2 in bee muscle tissue, fat body, or gut (Supplementary Fig. 1c).
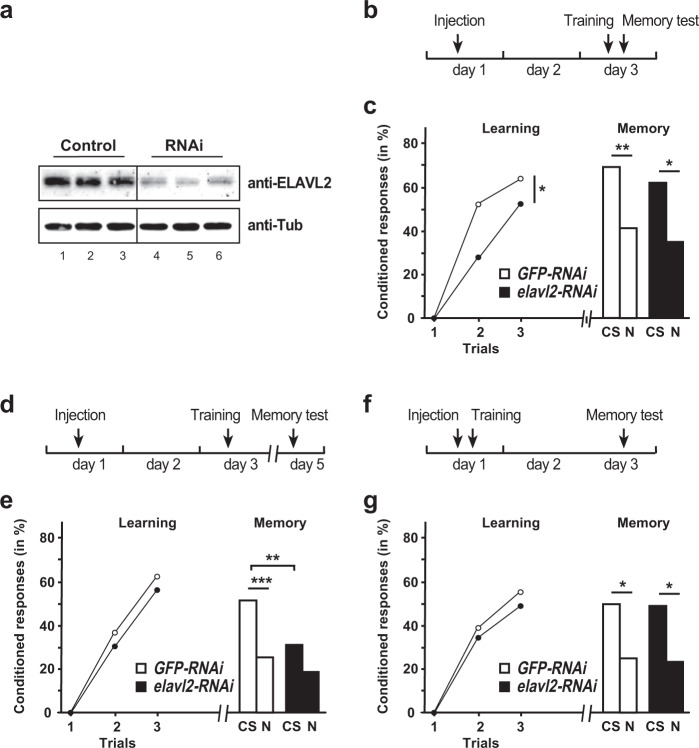
To assess whether ELAVL2 has a role in learning and memory in bees the single bee elavl2 gene was knocked down by RNAi leading to a reduction of 76 ± 5.1% after two days (n = 3, Fig. 1a, Supplementary Fig. 1d, e). Two days after injection of elavl2 or GFP control dsRNA, bees were individually trained and short-term memory was scored 2 h after training (Fig. 1b). Both groups showed significant learning over the successive trials (RM-ANOVA, Trial effect: F = 61.93, p < 0.001), but performance was affected by treatment (Trial × Treatment interaction: F = 4.33, p < 0.05). Indeed, as compared to controls, significantly fewer elavl2 dsRNA-injected bees showed conditioned responses by the end of training (Fischer’s test on 3rd trial: χ2 = 4.22, p < 0.05, Fig. 1c left). However, short-term memory retrieval remained unaffected (χ2 = 0.64, p > 0.05, Fig. 1c right).
Fig. 1. ELAVL2 is required for learning and memory consolidation.
a Western blot detecting ELAVL2 (top) or tubulin (bottom) in bee central brains of control GFP and elavl2 dsRNA-injected workers 50 h after injection. b Schematic of the treatment to test for ELAVL2’s role in learning. c Learning (left) and memory (right) performances of control GFP dsRNA (white, n = 66) and elavl2 dsRNA (black, n = 74) injected worker bees. CS: conditioned odor, N: novel odorant. *p < 0.05; **p < 0.01. d Schematic of the treatment to test for ELAVL2’s s role in memory consolidation. e Learning (left) and memory (right) performances of control GFP dsRNA (white, n = 74) and elavl2 dsRNA (black, n = 77) injected worker bees. CS: conditioned stimulus, N: novel odorant **p < 0.01; ***p < 0.001. f Schematic of the treatment to test for ELAVL2’s role in memory retrieval. g Learning (left) and memory (right) performances of control GFP dsRNA (white, n = 53) and elavl2 dsRNA (black, n = 50) injected worker bees. CS: conditioned stimulus, N: novel odorant *: p < 0.05. The source data underlying this figure are available in Supplementary Data 1.
We then asked whether elavl2 knockdown might impact on the consolidation of long-term memory independently on its effect on acquisition. Therefore, injections and training were performed as before to ensure that elavl2 levels would still be reduced during the hours following training (Fig. 1d), i.e. at a time when crucial transcriptional activity is required for long-term memory consolidation40,41. We then tested for their memory two days after training (a typical delay to assess consolidated long-term memory). In these conditions, learning occurred normally (RM-ANOVA, Trial effect: F = 108.6, p < 0.001; Trial × Treatment interaction: F = 0.50, p > 0.05; Fig. 1e left). Yet, the two groups showed different capacities to recall the memory of the CS-US association (Fischer’s test: χ2 = 10.08, p < 0.01, Fig. 1e right). In addition, only control bees responded significantly more to the CS than to the novel odorant (GFP: χ2 = 11.55, p < 0.001; elavl2: χ2 = 3.77, p > 0.05).
To reject the possibility that loss of ELAVL2 impairs long-term memory retrieval per se due to a prolonged downregulation of elavl2, we performed an additional experiment in which injection was done shortly before training, when RNAi is not yet effective (Fig. 1f). As expected, this treatment did not affect learning (Trial effect: F = 62.93, p < 0.001; Trial × Treatment interaction: F = 0.15, p > 0.05; Fig. 1g left). More importantly, memory retrieval was intact and two days after training both groups responded similarly to the CS (Fischer’s test: χ2 = 0.02, p > 0.05) and responded significantly less to the novel odorant (GFP: χ2 = 6.24, p < 0.05; elavl2: χ2 = 5.66, p < 0.05), thus indicating a preserved memory of the CS-US association.
These results thus argue that elavl2 is required for the early formation of an associative memory over repeated acquisition trials, and for its subsequent consolidation.
The single bee ELAVL2 gene is dynamically alternatively spliced
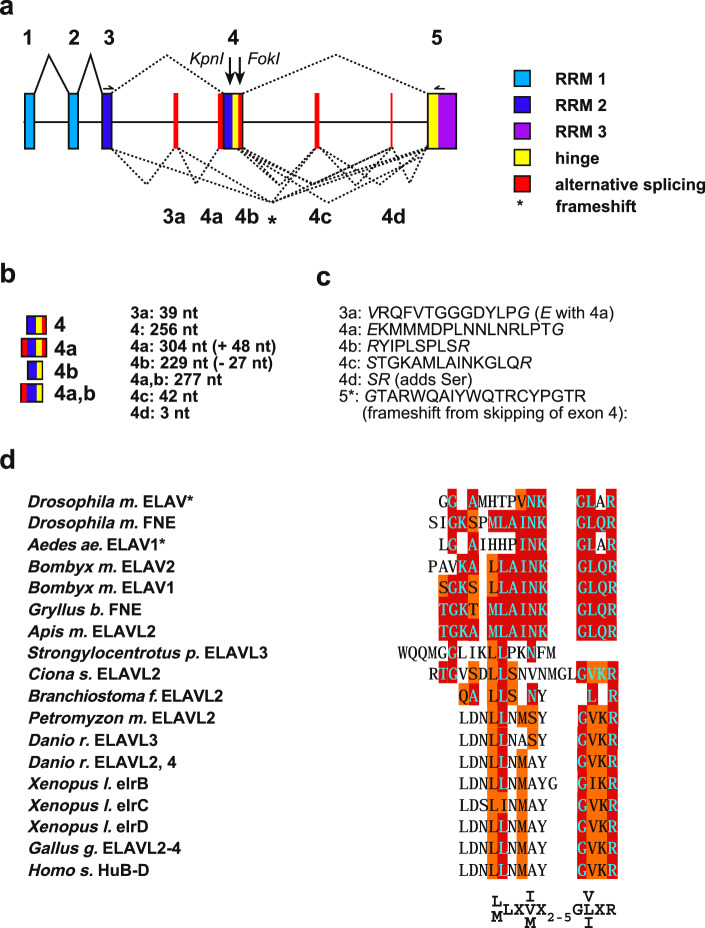
The bee ELAVL2 protein is highly homologous to those of the Drosophila ELAV family (ELAV, FNE, and RBP9) in the three RRM domains, but diverges significantly in the unstructured hinge domain separating RRM2 from RRM3 (Fig. 2a and Supplementary Fig. 2). Given the much more sophisticated tasks associated with the social life of bees compared to Drosophila, it is surprising that bees have only one elav/Hu family gene18,19. However, diversification of gene function can also be achieved by increasing alternative splicing5. This prompted us to investigate whether elavl2 in bees is alternatively spliced. Indeed, cloning of full-length elavl2 from RT-PCR revealed five alternatively spliced exons: exons 3a, exon 4a adding an additional 3′ss, exon 4b adding an additional 5′ss, exon 4c, and exon 4d, (Fig. 2a–c, Supplementary Fig. 2). The 45 nt exon 4c has been described as evolutionary conserved in insects19,37,38. The combination of these exons in addition to skipping of exon 4 variables potentially generates 40 different protein isoforms (Fig. 2a, Supplementary Data 1)44.
Fig. 2. Single bee elavl2 gene is alternatively spliced.
a Gene model of Apis mellifera elavl2 gene depicting exons (boxes) and their splicing. Constant splicing is indicated by solid lines and alternative splicing is indicated by dashed lines. In total, 40 different alternative splice products are possible. Skipping of exon 4 variants results in a frameshift in exon 5 (indicated by an asterisk). b Depiction of exon 4 variants and length of alternative microexons. c Sequence of alternative microexons and frameshift in exon 5* from skipping of exon 4 variants. d Alignment of alternative microexon 4c from bees with a part present in Drosophila ELAV or alternatively spliced microexons in ELAV/Hu family proteins of other species with a consensus motif shown at the bottom. Retroposed genes lacking introns are indicated by an asterisk.
Intriguingly, two of these alternative exons are located in the loop region of RRM2 and the other three are located in the hinge region (Fig. 2a–c, Supplementary Fig. 3a). Exon 4d is only 3 nts long and codes for a serine which can potentially be phosphorylated to impose further control of ELAV function39. Since the sequence of exon 4d is TAG and flanked by AG/GT consensus splice sites it is not a substrate for recursive splicing45–47. Exon 4d is too small to accommodate spliceosomal complexes on both sides and must thus be spliced sequentially5.
Rather unexpectedly, we also detected splice variants, which skip exon 4 and its variants encoding the second half of RRM2 to result in proteins of 19–22 kDa. This results in truncated ELAVL2 proteins by introducing a frameshift removing much of the beta-sheet of RRM2 involved in RNA recognition as well as alpha-helix 2 that makes up the backbone of the RRM structure. Since skipping of variable exons between the exon 3 and 4 deemed unfunctional based on RNA-binding assays48, we employed molecular modeling to explore the capacity of frequently included alternative exons 3a and 4a to build alternative structures that might hold functionality. Inclusion of exon 3a with concomitant exclusion of exon 4, 4a and/or 4b adds an additional beta-sheet potentially increasing the capacity to bind RNA (Supplementary Fig. 3b). The inclusion of exon 4c further adds an additional alpha-helix likely stabilizing this alternative RRM structure (Supplementary Fig. 3c).
Intriguingly, exon 4c from bees has been found conserved in Drosophila FNE and aligns to part of ELAV37,38,44. Human ELAV/Hu family proteins also harbor an alternatively spliced small exon between the second and third RRM at a position similar to that of the hinge region of bee elavl220,49, before a conserved motif involved in nuclear-cytoplasmic shuttling50. Alignment of exon 4c from bees with orthologues in other insects such as the mosquito Aedes aegyptii, the silk moth Bombyx mori and the cricket Gryllus bimaculatus18,19, the sea urchin Strongylocentrotus purpuratus, the sea squirt Ciona savignyi, the lancelet Branchiostoma lanceolatum, the lamprey Petromyzon marinus, the zebrafish Danio rerio, the African clawed frog Xenopus laevis, the Chicken Gallus gallus and human ELAV/Hu proteins revealed a microexon at the same position with a consensus motif L/MLXI/V/MX2–5GV/L/IXR (Fig. 2d), which is consistent with an evolutionary conserved microexon program between vertebrates and invertebrates51,52.
Bees also have a 3 nt microexon (Fig. 2a–c), that adds a serine, which potentially can be phosphorylated39. In vertebrates, this serine is added through an alternative 3’splice site at the same position in Human HuB and HuC, chicken ELAVL2-4, Xenopus elrB, and elrC, and zebrafish ELAVL2 and ELAVL3.
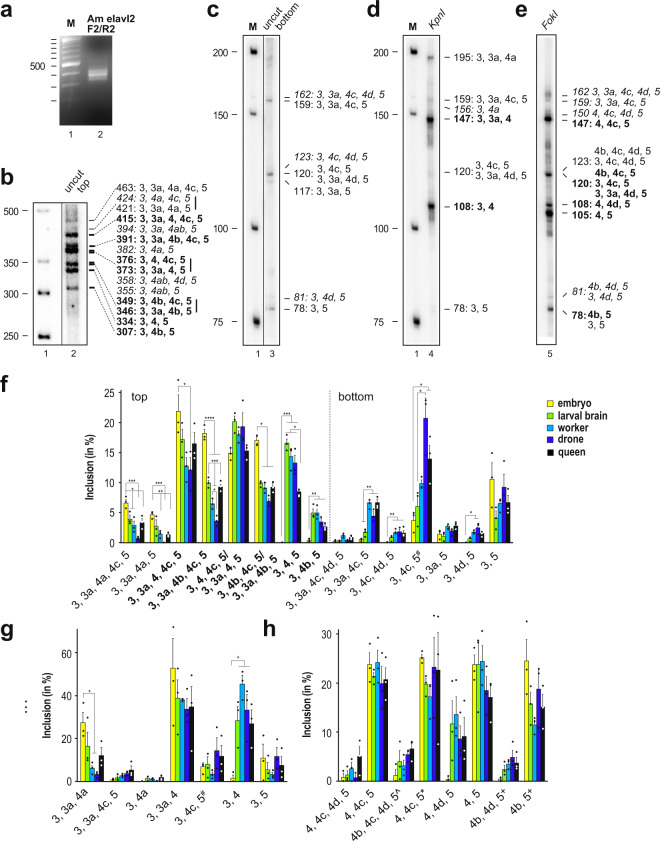
Next, we analyzed alternative splicing in more detail than possible on agarose gels, where multiple alternative splice products, amplified from mRNA of larval brains were detected only as a smear (Fig. 3a). Therefore, we employed a higher resolution separation of 32P-labeled PCR products using denaturing polyacrylamide gels. This analysis revealed 23 distinguishable products with sizes between 78 and 463 nt (Fig. 3b, c). Most frequently found splice variants were 3-4-5, 3-3a-4-4c-5, 3-3a-4-5/3-4-4c-5 and 3-3a-4b-5/3-4b-4c-5 as well as the truncated isoforms 3-4c-5 and 3–5, thus indicating functional relevance for the newly identified alternative splice products.
Fig. 3. elavl2 alternative splicing is dynamic during development.
a Multiple products are detected from RT-PCR of RNA from larval brain in the alternatively spliced part of bee elavl2 on an agarose gel. M: Marker b, c Top and bottom part of a representative 5% denaturing polyacrylamide gel separating 32P-labeled alternative splice products from larval brains. Length of PCR products from splice variants are indicated at the right in bold for prominent products and in italics for very rare products. Vertical lines at the right of indicated splice variants indicate inseparable products. M: Marker. d, e Analysis of alternative splicing from larval brains proximal (KpnI) and distal (FokI) of exon 4 from 32P-labeled labeled forward (d) or return (e) primer after digestion with either KpnI or FokI on a representative 5% denaturing polyacrylamide gels. Length of PCR products from splice variants are indicated at the right in bold for prominent products and in italics for very rare products. M: Marker. f–h Developmental and sex-specific alternative splicing of bee elavl2 quantified from denaturing polyacrylamide gels shown as mean with the standard error from three independent replicates as percent from all splice products from top (f left) and bottom (f right) gel parts and after KpnI or FokI digestion as above from embryos (yellow), larval brains (green), drone brains (light blue), worker brains (dark blue) and queen brains (black). (f and g). #The 120 nt product can be either 3, 4c, 5 or 3, 3a, 4d, 5. (h) ^The 123 nt products are either 4b, 4c, 4d, 5 or 3, 4c, 4d, 5. *The 120 nt products are either 4b, 4c, 5 or 3, 4c, 5 or 3, 3a, 4d, 5. +The 80 nt products are either 4b, 5 or 3, 5 +/− 4d. The source data underlying this figure are available in Supplementary Data 1.
Since some of the splice variants were not separable based on size, we wanted to determine how frequently each alternative exon is included. For this purpose, we digested 5′ 32P-labeled PCR products with KpnI or FokI restriction endonucleases to cleave off their unlabeled 3′ parts (Figs. 2a and 3d, e). For both sides of exon 4, all possible combinations of alternative splice products were detected.
Next, we analyzed the ELAVL2 alternative splicing pattern at different developmental stages and in different tissues (n = 3, Fig. 3f–h and Supplementary Fig. 4). This analysis revealed dynamic inclusion of alternative exons. In particular, splicing from exon 3 to 4 is absent in embryos compared to larval brains and adults (Fig. 3f left, p ≤ 0.001). Skipping of exon 4, 4a or 4b leads to significantly increased abundance of the truncated isoforms 3-4c-5 and 3-3a-4c-5 in adults (Fig. 3f right, p ≤ 0.05).
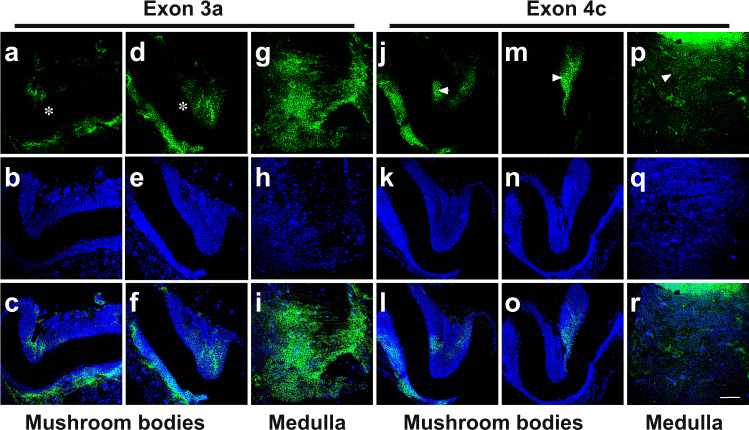
To obtain further insights into the dynamics of elavl2 alternative exon use at a cellular level we performed whole-mount RNA in situ hybridization with antisense probes against alternative exons 3a and 4c in brains of worker bees (Fig. 4a–r, Supplementary Table 1). Most strikingly, both exons 3a and 4c show very dynamic inclusion levels in the mushroom bodies, displaying unique patterns in each individual bee (Fig. 4a, d, j, m, and see Supplementary Fig. 5a for a whole-brain image). In contrast, inclusion levels in the medulla (visual neuropil not involved in the learning process) are uniform for both splice variants (Fig. 4g, p). Control stainings with a probe against Drosophila tango13 alternative exon 7b did not stain and a probe against constant exon 14 of Apis Dscam stained ubiquitously in the mushroom bodies (Supplementary Fig. 5b–g). Likewise, a probe against Drosophila elav only stained Drosophila embryos, but not mushroom bodies of bees and vice versa, a probe against bee elavl2 exon 4c only stained bee mushroom bodies, but not Drosophila embryos (Supplementary Fig. 5h–k).
Fig. 4. Alternative splicing of elavl2 exons 3a and 4c is dynamic in mushroom bodies.
Representative RNA in situ hybridizations in worker bees against elavl2 exon 3a (a, d, and g) and exon 4c (j, m, and p) in mushroom bodies (a, d, j, and m) and the medulla (g and p) counterstained with DAPI to visualize nuclei (b, e, h, k, n, and q) and merged pictures (c, f, i, l, o, and r). Scale bar in R is 30 µm.
ELAVL2 protein levels are dynamic in mushroom bodies of worker bees
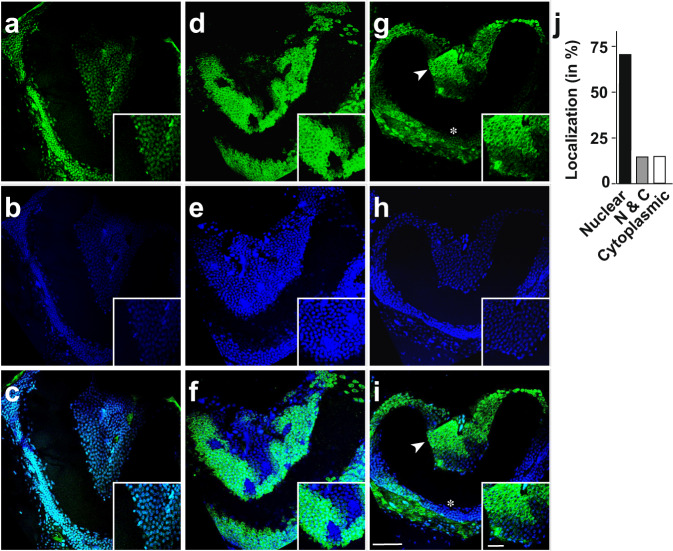
ELAV family proteins are pan-neurally expressed in Drosophila. Their expression seems not to be dynamic as judged from antibody stainings, but changes in nuclear and cytoplasmic distributions have been observed24. When we then analysed ELAVL2 expression in mushroom bodies, we found that expression varied between individual bees (Fig. 5a–i, Supplementary Fig. 5a). In some bees, ELAVL2 localizes to the nucleus (Fig. 5c–e) while in others it was cytoplasmic (Fig. 5f–h), or both nuclear and cytoplasmic (Fig. 5i–k). In addition, we detected areas where ELAVL2 was absent (Fig. 5f–h) or levels were reduced (Fig. 5i–k). Quantification of ELAVL2’s cellular localization revealed that ELAVL2 is nuclear in about 75% of worker bee brains (Fig. 5c–e, l). In the remaining 25%, however, ELAVL2 expression was very dynamic, showing patches of nuclear and cytoplasmic localization, but also small patches of cells with no ELAVL2 expression (Fig. 5f–k, l). Because these analyses were done on animals whose previous experience in the field could not be controlled, we wondered whether such localized changes in ELAVL2 expression might be indicative of experience-dependent plasticity.
Fig. 5. Localization and expression levels of ELAVL2 are dynamic in mushroom bodies.
Representative anti-ELAV antibody stainings in worker bees of mushbodies (a, d, and g) counterstained with DAPI to visualize nuclei (b, e, and h) and merged pictures (c, f, and i). Insets show higher magnifications of ubiquitous expression and nuclear localization (inset in a–c), patchy expression with mostly cytoplasmic localization (inset in d–f) and patchy expression with nuclear and cytoplasmic expression (inset in g–i, arrowhead in g and i). The asterisk in g and i indicates nuclear localization in the lower part of the mushroom body. A summary of ELAVL2 localization in mushroom bodies of worker bees is shown in panel j (n = 20). Scale bars are 30 µm in i and 6 µm in the inset. The source data underlying this figure are available in Supplementary Data 1.
ELAVL2 expression and alternative splicing is altered upon learning
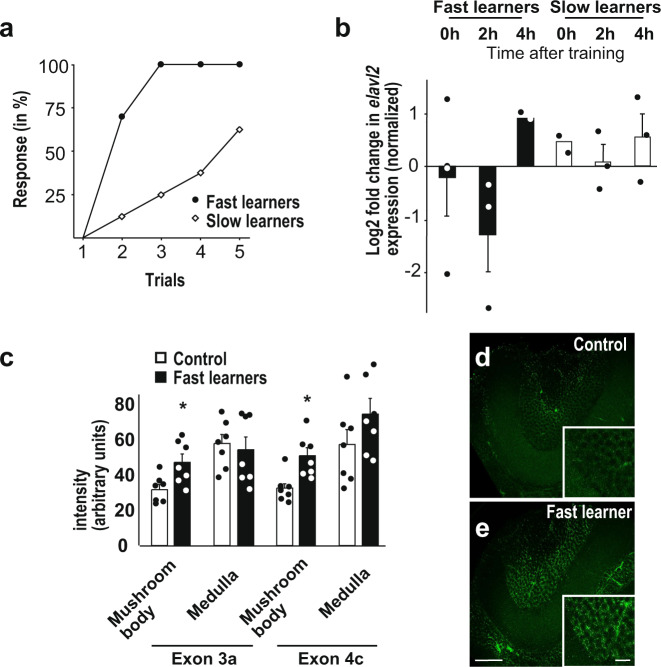
Since bees depend on learning and memory to forage, the pronounced loss of ELAVL2 expression in some of the brains of worker bees might reflect interindividual learning/memory variations. Thus, we thought of testing if such local downregulation might be indicative of a particular individual learning/memory status. To increase the sensitivity of our follow-up molecular analysis we took advantage of the diversity in the speed of learning observed among individuals during a 5-trial training by splitting trained bees into fast and slow learners, e.g., bees that responded in the first two trials and every time after the initial response vs bees with a lack of response in the first two trials or with gaps after the initial response (Fig. 6a). We then monitored elavl2 expression levels from their brains by qPCR at various timepoints after training (Fig. 6b). This analysis indeed revealed that elavl2 steady-state mRNA levels had dropped 50% two hours after training in the fast learners compared to slow learners. We therefore thought to analyze alternative splicing of elavl2 exons 3a and 4c 2 h after training. We detected a significant increase in the inclusion of exons 3a and 4c in the mushroom bodies, but not in the medulla one hour after training in fast learners (Fig. 6c–e). We also analyzed the alternative splicing pattern of elavl2 on denaturing polyacrylamide gels, but no differences were detected after learning in this assay, likely because the observed changes occurred only in relatively few cells (Supplementary Fig. 6).
Fig. 6. elavl2 alternative splicing and expression levels change upon learning.
a Learning response of bees grouped into fast and slow learners (fast learners n = 10 and slow learners n = 8). b elavl2 mRNA expression levels determined by qPCR 0, 2, and 4 h after training shown as mean with the standard error from two to four independent replicates central brains normalized to Appl expression (p = 0.13). c Inclusion levels of elavl2 exons 3a and 4c quantified from RNA in situ hybridizations from control (unpaired) and fast learning (paired) bees in the mushroom body and the medulla (p < 0.05, n = 7). d, e Representative RNA in situ hybridizations against exon 3c in mushroom bodies of control (d) and fast learning (e) bees 2 h after training. Scale bars are 30 µm in E and 6 µm in the inset. The source data underlying this figure are available in Supplementary Data 1.
Discussion
Many RNA-binding proteins including neuronal ELAV/Hu RBPs are comprised of families of highly related proteins15,53. In case of ELAV family RBPs, they have unique individual functions, but depending on cellular localization and concentrations they can cross-regulate targets making the study of their individual functions difficult24,37,38,48. Therefore we took advantage of honeybees due to the presence of only a single elav gene to examine whether ELAVL2 is required for learning and memory.
A role for ELAVL2 in learning and memory
Although neuronal ELAV/Hu family proteins are broadly expressed in the brain, mutants of individual genes in mice and Drosophila revealed only subtle developmental defects thus pointing towards a primary role in regulating neuronal functions as e.g. operating in learning and memory17,24,54,55. A knock-out of HuC in mice revealed a role in the synthesis of the neurotransmitter glutamate resulting in reduced neuronal excitability and impaired motor function17. For HuD, roles in learning and memory have been suggested due to its involvement in regulating GAP43 expression which has established roles in learning and memory56–58. Here, overexpression of HuD, which is cytoplasmic, leads to increased GAP-43 expression by increasing mRNA stability. Since in bees elavl2 steady-state mRNA levels drop for a short period after training early during memory consolidation, this might reflect functional compartmentalization of ELAV/Hu family proteins between nucleus and cytoplasm as bee ELAVL2 is mostly nuclear compared to HuD, which is mostly cytoplasmic in a learning context in the mouse hippocampus57.
The changes in elavl2 expression in the brain occur within two hours following learning consistent with a role in memory consolidation. Indeed, our learning protocol was designed to trigger the formation of stable long-term memories, which can be detected several days later59. Such memories are formed through a consolidation process initiated before the end of training and within a few hours, which depends on gene transcription40,41. It is therefore conceivable, that altered levels of ELAVL2 will impact on newly transcribed genes. In particular, expression of ELAV has been linked to implementing splicing programs governing neuronal characteristics such as changes in cell adhesion. Potentially, reduction of elavl2 levels could reduce cell adhesion for facilitating the creation or pruning of new synaptic connections. Indeed, changes in connectivity, particularly in the mushroom bodies, is an important process underlying long-term memory formation60. Such role is well in agreement with our observations in Drosophila, where reducing alternative splicing of the ELAV target ewg, a transcription factor, results in increased growth of synapses at the NMJ16,61. Likewise, we observed changes in ELAVL2 alternative splicing in bees leading to an increase in exon 3a and 4c inclusion, which is anticipated to have profound effects on target mRNA binding. In addition, skipping of exon 4, 4a, or 4b leads to a frameshift and an altered structure of the third RRM, which will alter target specificity and/or reduce binding affinity.
Local and dynamic expression changes of ELAVL2 as a hallmark for its role in learning and memory
A hallmark of memory formation is altered local gene expression followed by local changes of neuronal properties and establishment of new connections1,3. Activity-induced expression of immediate-early gene transcription factors has been associated with memory40,62–67. Intriguingly, expression of HuD can be induced by neuronal activity68. Stabilization of C/EBP by apELAV1 in Aplysia accompanies long-term memory69, although apELAV1 is mainly nuclear in contrast to apELAV2, which is also cytoplasmic70.
In agreement with a role for ELAVL2 in memory formation we find variable expression patterns for ELAVL2 in the mushroom bodies of worker bees. Even more compelling, the expression pattern of ELAVL2 in the mushroom bodies of worker bees is unique and differs between individuals. Similarly, inclusion levels of alternative exons 3a and 4c also show unique patterns in each individual bee. This can be understood as possible consequences of differences in the previous experience that individuals have had, either within the hive or outdoors (e.g., social interactions, environmental stimuli). The rapid changes of ELAVL2 expression observed after learning occur in the same time-frame as activity-induced expression of immediate-early genes71 suggesting a role for alternative splicing during the early phases of memory consolidation, which requires transcription in addition to protein synthesis from stored mRNA at synapses4,40,41. Notably, elav expression in Drosophila is controlled by miRNAs that can contribute to fast changes in expression, but also restrict the expression of ELAV protein to the nervous system72. Whether ELAV family proteins are expressed in other cells than neurons, as found for human HuR and suggested from fne mRNA expression in crickets, remains to be determined19.
Mushroom body connectivity is shaped by individual experience during a continuous maturation process73,74. Yet, molecular tools available in Drosophila or mice to genetically label individual neurons are currently lacking in the honeybee in order to identify those neurons where elavl2 expression varies and to establish interindividual variations75. In addition, ELAVL2’s cellular localization also varied in individual cells in the mushroom bodies from nuclear to cytoplasmic. Such differences in cellular localization are expected since Drosophila ELAV localizes mostly to the nucleus, RBP9 is cytoplasmic and FNE is found in both compartments. However, ELAV/Hu family proteins also shuttle between nucleus and cytoplasm24,76. Upon removal of ELAV in Drosophila, alternatively spliced microexon 4c is included in FNE leading to nuclear localization and regulation of alternative splicing of genes that are otherwise ELAV targets37,38 suggesting a complex network of interactions among ELAV/Hu proteins.
Alternative splicing could serve as an adaptive mechanism to changes in perception, but also to environmental conditions such as toxic insult77. Although learning and memory is affected by neonicotinoids in insects, we did not find any changes in elavl2 alternative splicing44,78,79. Since we also could not detect any alternative splicing changes after learning in mRNA from central brains, drastic changes in alternative splicing relevant to learning and memory might occur only in few cells.
Alternative splicing of a microexon in ELAV/Hu proteins is evolutionary ancient
Human HuB-D genes contain an alternatively spliced microexon in the hinge region between the second and third RRM20,80,81. We identified an alternatively spliced exon at the same position in the single bee elavl2 gene44 homologous to a previously identified alternative exon in a cricket19. Comparison of the sequence between human and insects of this exon shows a high sequence similarity indicating that this exon is evolutionary ancient. Although we identified a consensus motif in exon 4c, convergent evolution is also possible due to the short sequence51. Previously, exon duplication between humans and Drosophila has been documented in a few ion channel genes leading to alternatively spliced exons at the same position, but without sequence conservation and no longer exons have been found that are evolutionary conserved between invertebrates and vertebrates51,52. Intriguingly, ELAV in Drosophila has lost its introns due to retrotransposition, but retained microexon 4c18. This microexon is involved in regulating nuclear localization of ELAV and FNE in Drosophila37,38,50, and also affects localization of HuD in human cells80,81. Its increased expression shortly after training thus coincides with an initial nuclear role of ELAVL2 at the memory consolidation phase, which requires transcription40,41. The unstructured hinge region between the RRMs 2 and 3 in ELAV/Hu family RBPs has expanded by alternative splicing in humans80, some insects18,19 and in the sea squirt Ciona savignyi with unexpected complexity of eight alternative exons in this species (www.ensembl.org, ENSCSAVG00000003440), but the functional consequences of alternative splicing in this part of ELAV/Hu family proteins have not been determined.
For most neuronally alternative spliced microexons in mice, Srrm4 is required for their inclusion82. Srrm4 contains a novel evolutionary conserved protein domain ‘enhancer of microexons’ (eMIC) that is present in Drosophila Srrm2/3/4 and required for exon inclusion in the Dscam exon 9 cluster83 indicating a conserved neuronal microexon program is present in vertebrates and insects52.
Alternative splicing in bee ELAVL2 is confined to unstructured linker regions, but not RNA recognition domains
A main question arising from the presence of multiple highly related genes is whether they act in an overlapping manner. In case of Drosophila ELAV family members ELAV, FNE, and RBP9, distinct mutant phenotypes and the lack of major genetic interactions among them suggests largely independent functions24. However, cross-regulation between FNE and RBP9 is present in the regulation of synapse numbers. Likewise, expression in non-neuronal cells or swapping of expression and localization regulatory regions can to a large degree substitute for their individual functions and they can cross-regulate. Overlapping functions even extend to more distantly related Sex lethal (Sxl), which is required for neuronal functions in Diptera, but has been recruited in Drosophila for sex determination and dosage compensation84. Here, RBP9 is required for maternal inhibition of dosage compensation, a function that is taken over entirely by Sxl during embryogenesis24. In addition, the ELAV binding site in Drosophila virilis ewg diverged substantially and does not align to the D. melanogaster ELAV binding site, but ELAV regulation is maintained28.
These facts point out that the main distinction among ELAV family members only minimally occurs at the level of RNA recognition. Hence, it is conceivable, that the ELAV family in bees has “merged back” into a single copy gene by incorporating the variable parts between family members by alternative splicing as observed in the honeybee and cricket19. In this respect, it is very interesting that alternative splicing in bee ELAVL2 and cricket FNE occurs in unstructured linker regions between RRMs19. It is conceivable, that these regions mediate protein-protein interactions leading to sub-functionalization. Accordingly, the microexon present in the hinge region likely serves such purpose, but the interacting proteins remain to be identified.
Mis-regulation of microexons has been found as a major cause of autism spectrum disorders revealing essential functions for such microexons in neurons82. Notably, inclusion levels of this microexon in bees is altered upon learning and memory formation. Hence, lack of dynamic inclusion of microexons in ELAV/Hu family proteins might point toward a role in establishing the extensive memories often associated with some autism spectrum disorders85.
Materials and Methods
Honeybees and treatment
Honeybees (Apis mellifera) were collected from flowers or local bee hives in the UK for molecular biology experiments (worker bees were used unless otherwise specified). For behavioral experiments, workers were taken from the experimental apiary on the university campus in Toulouse (France), on the morning of each experiment. Following cold-anesthesia, they were harnessed in metal tubes leaving access to the head, fed with 5 µl of sucrose solution (50% weight/weight in water) and then kept in the dark at room temperature until needed. They were fed in the same way on every morning and evening during the time of each experiment.
Behavioral assays
Learning and memory capacities were assessed using a standard protocol based on the olfactory conditioning of the proboscis extension response (PER)86, which consisted of three learning trials (unless specified otherwise) where animals were trained individually to associate an odorant with a sucrose reward as detailed below. Memory of the association was tested either one hour (short-term memory) or 48 h (long-term memory) after the last learning trial. In all experiments, bees of both treatment groups were trained in parallel. Each learning trial (40 s) started when the restrained bee was placed in front of an odorless airflow. After 15 s, the setup allowed to deliver an odor (conditioned stimulus, CS) for 4 s by partially diverting the flow in a syringe containing a filter paper soaked with 4 µl of pure odorant. (1-hexanol and 1-nonanol were used, alternatively for different bees; data were pooled after checking for any significant effect of the odorant used). Sucrose (unconditioned stimulus, US: same solution as for feeding) was delivered to the antennae using a toothpick, 3 s after CS onset, for 3 s. This triggered the bee’s reflex extension of the proboscis to lick the reward. Whenever the animal already responded to the CS (conditioned response), it was directly allowed to feed upon US onset. Successive learning trials were separated by 10-min intervals to facilitate memory consolidation59. Memory was assessed by placing the animals again in the conditioning setup, and by presenting them the CS without the US86. The presence or absence of a conditioned response was recorded. In case of no response, sucrose was applied to the antennae at the end of the test, to control for the intact motor response. Bees failing to show an intact reflex were discarded. Bees that responded to the training in the first two trials and that responded every time were classified as fast learners. Bees that responded only two times in the four trials were classified as slow learners. The sucrose and odorants were purchased from Sigma-Aldrich (France).
Recombinant DNA technology, RT-PCR, qPCR, and analysis of alternative splicing
The sequence of oligonucleotides used in this study is listed in Supplementary Table 1. Recombinant DNA technology was done according to standard procedures as described29. Bee elavl2 was amplified from oligo dT primed cDNA made from larval brains using primers AM elav F1 and AM elav R1 and cloned into a modified pBS SK+ using NgoMIV and XbaI. Clones (n = 45) were sequenced using primers elav F1 and elav R1.
RNA extraction from whole bees or dissected bee brains and RT-PCR was done as described87. Expression of elavl2 at different timepoints was compared to Appl expression using primers AM elav qF3 and AM elav qR3 to amplify the constant part of elav and normalized to unpaired control animals using qPCR as described33,79.
For high-resolution analysis of elavl2 alternative splicing primers AM elav F2 and AM elav R2 were used to amplify elavl2 from cDNA. One of the primers was labeled using gamma32P-ATP (NEN) and PCR products were separated on sequencing type denaturing polyacrylamide gels. Polyacrylamide gels were dried, exposed to phosphoimager screens (BioRad), and quantified with QuantityOne (BioRad).
RNAi, Western analysis recombinant protein expression, RNA in situ, antibody staining, and imaging
For RNAi knockdown in bees, elavl2 and GFP DNA templates for in vitro transcription were amplified for elavl2 from a cloned cDNA with primers AM ELAV T7 RNAi F1 and AM ELAV T7 RNAi R1 and for GFP a 700 bp fragment was amplified with primers GFP T7 RNAi F1 and GFP T7 RNAi R1. Double-stranded RNA was generated by in vitro transcription with T7 polymerase with the MegaScript kit (Ambion) for 3 h according to the manufacturer’s instructions. After digestion of the template with TurboDNAse (Ambion), dsRNA was phenol/chloroform extracted, ethanol precipitated and taken up in RNAse free water at a concentration of 5 µg/µl. The dsRNA (250 nl) was then injected into the brain through the median ocellus with a Nanoject II microinjector (Drummond).
RNAi efficiency testing for ELAVL2 was done from dissected central brains by Western blotting according to standard protocols as described29 using a polyclonal rat anti-ELAV antibody generated against Drosophila ELAV (1:800)42 and secondary HRP-coupled goat antirat antibody (1:5000, GE Healthcare) by chemiluminescence detection (Pierce) according to the manufacturer’s instructions. Alternatively, infrared dye coupled secondary antibodies (IRDye800CW, LI-COR) were used and detected with an Odyssey infrared imaging system (LI-COR). A polyclonal antiserum raised in rabbits against Drosophila ELAV also cross-reacts with bee ELAVL238. Tubulin was detected with a mouse anti-alpha tubulin antibody (1:10,000, clone DM1A, SIGMA). Quantification of Western blots was done with Quantity ONE 4.6.8 (BioRad) according to the manufacturer’s instructions.
Recombinant bee ELAVL2 was made in E.coli by cloning the cDNA with primers GST AM ELAV F1 and primers GST AM ELAV R1 into a modified pGEX. Drosophila GST ELAV, GST FNE, GST RBP9, and human GST HuR, and GST dCMTr were as described24,43. For protein expression, 1.5 ml of a 3 ml overnight culture was diluted with 2 ml 2YT and IPTG (1 mM final) and proteins were induced for 8 h. For protein gels, 0.5 ml cells were pelleted and taken up in 50 µl 2x SDS loading buffer, boiled and 5 µl loaded onto an 8% SDS gel. For Westerns, proteins were further diluted 1:100.
Brain antibody stainings were done with rat polyclonal anti-ELAV antibody (1:200) and an antirat FITC labeled antibody (1:200, Molecular Probes) for two days each as described16 and counterstained with DAPI (1 µg/ml).
To make probes for RNA in situ hybridizations, a pBS SK+ vector was modified by cloning a U-rich stem loop at the end of the in vitro transcript using EcoRI and KpnI and phosphorylated and annealed oligos RNA IS stem 1A and B, tango13A and B. ELAVL2 alternative exons were then cloned with XhoI and PstI using phosphorylated and annealed oligos AM elav 3a A and B, and AM elav 4c A and B. The sequence of RNA in situ probes used in this study are listed in Supplementary Table 1. Vectors were linearized with Acc56I and DIG-dUTP (Roche) labeled antisense transcripts were generated by in vitro transcription with T3 RNA polymerase (Ambion) for Apis elavl2 exon 3a and 4c, and Drosophila tango13 probes, and with T7 RNA polymerase for Apis Dscam exon 14 in 10 µl from 1 µg template DNA. After digestion of the template with TurboDNAse (Ambion), these transcripts were cleaned by centrifugation through a G50 Microspin column (GE Healthcare) in a final volume of 50 µl.
For in situ hybridizations, whole brains were fixed 30 min in 4% paraformaldehyde in PBT (PBS, 0.1% Tween 20) and then washed in PBT. Hybridizations were then done in 50% formamide buffer88 (50% formamide, 5x SSPE, 50 µg/ml heparin (SIGMA), 0.1% Tween 20, 0.5 mg/ml denatured Salm sperm DNA) using 1:500 diluted probes at 39 °C for 3d as described89. To wash off unhybridized probe, tissues were incubated overnight in hybridization buffer at 39° C. Brains were then washed with PBT and DIG-labeled probes were visualized by incubation with a sheep anti-DIG antibody (1:400, 2 days, Roche), and after washing followed by incubation with an FITC conjugated anti-sheep antibody (1:200, 2 days, SIGMA) and counterstained with DAPI (1 µg/ml). To evaluate that the probe concentration was adequate, bee brains or Drosophila embryos were incubated for two days in anti-DIG Fab fragments coupled to alkaline phosphatase (1:400) after hybridization and washing, and detected with NBT/BCIP (1:50, Roche) in TLMNT (100 mM Tris/HCl pH 9.5, 100 mM NaCl, 50 mM MgCl2, 0.1% Tween 20, 1 mM Levamisole) after washing.
For brain imaging, confocal Z stacks were taken using a Leica SP5/SP2, using a 40x-oil objective. For the quantification of stained Kenyon cells, the cross-section equal to the width of the calyx was scanned and the fluorescence intensity quantification was performed as previously described using ImageJ24. For the imaging of the calyces of the mushroom bodies of honeybee brains, single optical sections were taken in the x-y plane. The image acquisition settings were kept identical for all preparations.
Protein modeling and sequence analysis
Structural modelling of Apis melifera ELAVL2 splice variants was performed in SWISS-MODEL90. For RRM1/2 modelling, the HuR RRM1/2 structure was used as a template (PDB accession: 4ed5.1.A). For RRM3 modelling, HuR RRM3 structure was used as a template (PDB accession: 6gd3.1). The hinge region could not be modelled due to a lack of known structures with a sufficient degree of homology. Structural features in the hinge region between RRMS2 and 3, and the loop consisting of variable exons 3a and 4a inserted between beat sheets 2 and 3 in RRM2 were predicted using the JPRED webserver91. Predicted secondary structure of alpha-helices in alternative exons 3a and 4a were manually added to the model.
The sequence of alternatively spliced exon 4c was retrieved either from annotations for splice variants from humans80, a cricket19, silk moth and a mosquito18, by analysing sequences annotated in the UCSC genome browser (www.genome.ucsc.edu) for a chicken, Xenopus and zebrafish, or from annotations in GenBank (https://www.ncbi.nlm.nih.gov/gene/) for a lancelet (LOC118431358) and lamprey (LOC116951932) and sea urchin (LOC115928867) or for a sea squirt in Ensembl (www.ensembl.org, ENSCSAVG00000003440).
In addition, we validated the annotation of the sea urchin Strongylocentrotus purpuratus exon 4c by retrieving sequence reads from expression data from deeply sequenced transcriptomes92 by realigning RNA sequencing reads using STAR93 (version 2.7.2; parameters: alignSJDBoverhangMin 3 —twopassMode Basic —alignIntronMin 5 —alignMatesGapMax 200000 —alignIntronMax 200000. The genome index has been generated using the S. purpuratus genome version 5 as downloaded 25th of July 2021 from Echinobase94. The index was built without primary gene annotation. The results have been evaluated against the genome annotation using IGV and in-house scripts to assess the expression level of the exon. Of 23 samples, two showed clear expression of the exon.
Statistics and reproducibility
Multiple planned pairwise comparisons of expression levels were done by ANOVA followed by Fisher’s protected least significance difference post hoc test using StatView. To compare proportions of conditioned responses between groups, a repeated-measure analysis of variance (ANOVA) was run for the acquisition data (one factor, treatment, with trial as the repeated measure), and a simple ANOVA for retention data95. Post hoc comparisons of rates of CS-specific responses were done using the Fisher’s exact test.
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
We thank the Winterbourne garden (Birmingham) and Lucie Hotier (Toulouse) for providing bees, Valérie Hilgers for rabbit anti-ELAV antibodies, Karthik Nallasivan for help with imaging, Reinhard Stöger for discussions and comments on the manuscript. For this work we acknowledge funding from the Sukran Sinan Fund, the Genetics Society, the Biochemical Society and BBSRC. JMD acknowledges funding from the CNRS and Université Paul Sabatier.
Author contributions
P.U., I.U.H., T.D. and M.S. performed molecular biology experiments, P.U., J.K.G., and N.D. performed behavioral experiments, and J.K.G., P.U. and T.D. performed antibody stainings and in situ hybridization experiments and imaging. J.M.D. designed behavioral experiments. T.D. analyzed structures and A.R. provided bioinformatics support for sequence analysis. MS conceived the project and wrote the original draft of the manuscript. J.M.D., I.U.H, and all other authors reviewed and edited. M.S. and J.M.D. supervised and acquired funding.
Data availability
All data are available in the main text or the supplementary material (Supplementary Data 1). Reagents are available upon reasonable request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Communications Biology thanks Francis Nunes and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: Caitlin Karniski. Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Pinar Ustaoglu, Jatinder Kaur Gill.
Supplementary information
The online version contains supplementary material available at 10.1038/s42003-021-02763-1.
References
- 1.Alberini CM. Transcription factors in long-term memory and synaptic plasticity. Physiol. Rev. 2009;89:121–145. doi: 10.1152/physrev.00017.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 3.Clayton DF. The genomic action potential. Neurobiol. Learn Mem. 2000;74:185–216. doi: 10.1006/nlme.2000.3967. [DOI] [PubMed] [Google Scholar]
- 4.Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157:163–186. doi: 10.1016/j.cell.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Soller M. Pre-messenger RNA processing and its regulation: a genomic perspective. Cell Mol. Life Sci. 2006;63:796–819. doi: 10.1007/s00018-005-5391-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vuong CK, Black DL, Zheng S. The neurogenetics of alternative splicing. Nat. Rev. Neurosci. 2016;17:265–281. doi: 10.1038/nrn.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ule J, Blencowe BJ. Alternative Splicing Regulatory Networks: Functions, Mechanisms, and Evolution. Mol. Cell. 2019;76:329–345. doi: 10.1016/j.molcel.2019.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Demares F, et al. Differential involvement of glutamate-gated chloride channel splice variants in the olfactory memory processes of the honeybee Apis mellifera. Pharm. Biochem Behav. 2014;124:137–144. doi: 10.1016/j.pbb.2014.05.025. [DOI] [PubMed] [Google Scholar]
- 9.Demares F, Raymond V, Armengaud C. Expression and localization of glutamate-gated chloride channel variants in honeybee brain (Apis mellifera) Insect Biochem Mol. Biol. 2013;43:115–124. doi: 10.1016/j.ibmb.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 10.Beffert U, et al. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor Apoer2. Neuron. 2005;47:567–579. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 11.Poplawski SG, et al. Contextual fear conditioning induces differential alternative splicing. Neurobiol. Learn Mem. 2016;134 Pt B:221–235. doi: 10.1016/j.nlm.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sengar AS, et al. Control of Long-Term Synaptic Potentiation and Learning by Alternative Splicing of the NMDA Receptor Subunit GluN1. Cell Rep. 2019;29:4285–4294 e4285. doi: 10.1016/j.celrep.2019.11.087. [DOI] [PubMed] [Google Scholar]
- 13.Mirisis AA, Carew TJ. The ELAV family of RNA-binding proteins in synaptic plasticity and long-term memory. Neurobiol. Learn Mem. 2019;161:143–148. doi: 10.1016/j.nlm.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perrone-Bizzozero N, Bolognani F. Role of HuD and other RNA-binding proteins in neural development and plasticity. J. Neurosci. Res. 2002;68:121–126. doi: 10.1002/jnr.10175. [DOI] [PubMed] [Google Scholar]
- 15.Soller M, White K. Elav. Curr. Biol. 2004;14:R53. doi: 10.1016/j.cub.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 16.Haussmann IU, White K, Soller M. Erect wing regulates synaptic growth in Drosophila by integration of multiple signaling pathways. Genome Biol. 2008;9:R73. doi: 10.1186/gb-2008-9-4-r73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ince-Dunn G, et al. Neuronal Elav-like (Hu) proteins regulate RNA splicing and abundance to control glutamate levels and neuronal excitability. Neuron. 2012;75:1067–1080. doi: 10.1016/j.neuron.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samson ML. Rapid functional diversification in the structurally conserved ELAV family of neuronal RNA binding proteins. BMC Genomics. 2008;9:392. doi: 10.1186/1471-2164-9-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Watanabe T, Aonuma H. Tissue-specific promoter usage and diverse splicing variants of found in neurons, an ancestral Hu/ELAV-like RNA-binding protein gene of insects, in the direct-developing insect Gryllus bimaculatus. Insect Mol. Biol. 2014;23:26–41. doi: 10.1111/imb.12057. [DOI] [PubMed] [Google Scholar]
- 20.Okano HJ, Darnell RB. A hierarchy of Hu RNA binding proteins in developing and adult neurons. J. Neurosci. 1997;17:3024–3037. doi: 10.1523/JNEUROSCI.17-09-03024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim YJ, Baker BS. The Drosophila gene rbp9 encodes a protein that is a member of a conserved group of putative RNA binding proteins that are nervous system-specific in both flies and humans. J. Neurosci. 1993;13:1045–1056. doi: 10.1523/JNEUROSCI.13-03-01045.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samson ML, Chalvet F. found in neurons, a third member of the Drosophila elav gene family, encodes a neuronal protein and interacts with elav. Mech. Dev. 2003;120:373–383. doi: 10.1016/S0925-4773(02)00444-6. [DOI] [PubMed] [Google Scholar]
- 23.Yao K-M, Samson M-L, Reeves R, White K. Gene elav of Drosophila melanogaster: a prototype for neuronal-specific RNA binding protein gene family that is conserved in flies and humans. J. Neurobiol. 1993;24:723–739. doi: 10.1002/neu.480240604. [DOI] [PubMed] [Google Scholar]
- 24.Zaharieva E, Haussmann IU, Brauer U, Soller M. Concentration and localization of co-expressed ELAV/Hu proteins control specificity of mRNA processing. Mol. Cell Biol. 2015;35:3104–3115. doi: 10.1128/MCB.00473-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukherjee N, et al. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol. Cell. 2012;43:327–339. doi: 10.1016/j.molcel.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uren PJ, et al. Genomic analyses of the RNA-binding protein Hu antigen R (HuR) identify a complex network of target genes and novel characteristics of its binding sites. J. Biol. Chem. 2012;286:37063–37066. doi: 10.1074/jbc.C111.266882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lebedeva S, et al. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol. Cell. 2012;43:340–352. doi: 10.1016/j.molcel.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Haussmann IU, Li M, Soller M. ELAV-mediated 3’-end processing of ewg transcripts is evolutionarily conserved despite sequence degeneration of the ELAV-binding site. Genetics. 2011;189:97–107. doi: 10.1534/genetics.111.131383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Soller M, White K. ELAV multimerizes on conserved AU4-6 motifs important for ewg splicing regulation. Mol. Cell Biol. 2005;25:7580–7591. doi: 10.1128/MCB.25.17.7580-7591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soller M, White K. ELAV inhibits 3’-end processing to promote neural splicing of ewg pre-mRNA. Genes Dev. 2003;17:2526–2538. doi: 10.1101/gad.1106703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toba G, Qui J, Koushika SP, White K. Ectopic expression of Drosophila ELAV and human HuD in Drosophila wing disc cells reveals functional distinctions and similarities. J. Cell Sci. 2002;115:2413–2421. doi: 10.1242/jcs.115.11.2413. [DOI] [PubMed] [Google Scholar]
- 32.Lisbin MJ, Qiu J, White K. The neuron-specific RNA-binding protein ELAV regulates neuroglian alternative splicing in neurons and binds directly to its pre-mRNA. Genes Dev. 2001;15:2546–2561. doi: 10.1101/gad.903101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koushika SP, Soller M, White K. The neuron-enriched splicing pattern of Drosophila erect wing is dependent on the presence of ELAV protein. Mol. Cell Biol. 2000;20:1836–1845. doi: 10.1128/MCB.20.5.1836-1845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koushika SP, Lisbin MJ, White K. ELAV, a Drosophila neuron-specific protein, mediates the generation of an alternatively spliced neural protein isoform. Curr. Biol. 1996;6:1634–1641. doi: 10.1016/S0960-9822(02)70787-2. [DOI] [PubMed] [Google Scholar]
- 35.Simionato E, et al. The Drosophila RNA-binding protein ELAV is required for commissural axon midline crossing via control of commissureless mRNA expression in neurons. Dev. Biol. 2007;301:166–177. doi: 10.1016/j.ydbio.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 36.Rogulja-Ortmann A, et al. The RNA-binding protein ELAV regulates Hox RNA processing, expression and function within the Drosophila nervous system. Development. 2014;141:2046–2056. doi: 10.1242/dev.101519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wei L, et al. Overlapping Activities of ELAV/Hu Family RNA Binding Proteins Specify the Extended Neuronal 3’ UTR Landscape in Drosophila. Mol. Cell. 2020;80:140–155 e146. doi: 10.1016/j.molcel.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carrasco J, et al. ELAV and FNE Determine Neuronal Transcript Signatures through EXon-Activated Rescue. Mol. Cell. 2020;80:156–163 e156. doi: 10.1016/j.molcel.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 39.Brauer U, Zaharieva E, Soller M. Regulation of ELAV/Hu RNA-binding proteins by phosphorylation. Biochem Soc. Trans. 2014;42:1147–1151. doi: 10.1042/BST20140103. [DOI] [PubMed] [Google Scholar]
- 40.Lefer D, Perisse E, Hourcade B, Sandoz J, Devaud JM. Two waves of transcription are required for long-term memory in the honeybee. Learn Mem. 2012;20:29–33. doi: 10.1101/lm.026906.112. [DOI] [PubMed] [Google Scholar]
- 41.Villar ME, Marchal P, Viola H, Giurfa M. Redefining Single-Trial Memories in the Honeybee. Cell Rep. 2020;30:2603–2613 e2603. doi: 10.1016/j.celrep.2020.01.086. [DOI] [PubMed] [Google Scholar]
- 42.Robinow S, White K. Characterization and spatial distribution of the ELAV protein during Drosophila melanogaster development. J. Neurobiol. 1991;22:443–461. doi: 10.1002/neu.480220503. [DOI] [PubMed] [Google Scholar]
- 43.Haussmann, I. U. et al. CMTr cap-adjacent 2‘-O-ribose methyltransferases are required for reward learning and mRNA localization to synapses. bioRxiv, 10.1101/2021.1106.1124.449724 (2021). [DOI] [PMC free article] [PubMed]
- 44.Decio P, et al. Acute thiamethoxam toxicity in honeybees is not enhanced by common fungicide and herbicide and lacks stress-induced changes in mRNA splicing. Sci. Rep. 2019;9:19196. doi: 10.1038/s41598-019-55534-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatton AR, Subramaniam V, Lopez AJ. Generation of alternative Ultrabithorax isoforms and stepwise removal of a large intron by resplicing at exon-exon junctions. Mol. Cell. 1998;2:787–796. doi: 10.1016/S1097-2765(00)80293-2. [DOI] [PubMed] [Google Scholar]
- 46.Duff MO, et al. Genome-wide identification of zero nucleotide recursive splicing in Drosophila. Nature. 2015;521:376–379. doi: 10.1038/nature14475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sibley CR, et al. Recursive splicing in long vertebrate genes. Nature. 2015;521:371–375. doi: 10.1038/nature14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borgeson CD, Samson ML. Shared RNA-binding sites for interacting members of the Drosophila ELAV family of neuronal proteins. Nucleic Acids Res. 2005;33:6372–6383. doi: 10.1093/nar/gki942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inman MV, Levy S, Mock BA, Owens GC. Gene organization and chromosome location of the neural-specific RNA binding protein Elavl4. Gene. 1998;208:139–145. doi: 10.1016/S0378-1119(97)00615-X. [DOI] [PubMed] [Google Scholar]
- 50.Yannoni YM, White K. Domain necessary for Drosophila ELAV nuclear localization: function requires nuclear ELAV. J. Cell Sci. 1999;112:4501–4512. doi: 10.1242/jcs.112.24.4501. [DOI] [PubMed] [Google Scholar]
- 51.Copley RR. Evolutionary convergence of alternative splicing in ion channels. Trends Genet. 2004;20:171–176. doi: 10.1016/j.tig.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Torres-Mendez A, et al. A novel protein domain in an ancestral splicing factor drove the evolution of neural microexons. Nat. Ecol. Evol. 2019;3:691–701. doi: 10.1038/s41559-019-0813-6. [DOI] [PubMed] [Google Scholar]
- 53.Ray D, et al. A compendium of RNA-binding motifs for decoding gene regulation. Nature. 2013;499:172–177. doi: 10.1038/nature12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Akamatsu W, et al. Mammalian ELAV-like neuronal RNA-binding proteins HuB and HuC promote neuronal development in both the central and the peripheral nervous systems. Proc. Natl Acad. Sci. USA. 1999;96:9885–9890. doi: 10.1073/pnas.96.17.9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akamatsu W, et al. The RNA-binding protein HuD regulates neuronal cell identity and maturation. Proc. Natl Acad. Sci. USA. 2005;102:4625–4630. doi: 10.1073/pnas.0407523102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bolognani F, et al. Coordinated expression of HuD and GAP-43 in hippocampal dentate granule cells during developmental and adult plasticity. Neurochem Res. 2007;32:2142–2151. doi: 10.1007/s11064-007-9388-8. [DOI] [PubMed] [Google Scholar]
- 57.Pascale A, et al. Increase of the RNA-binding protein HuD and posttranscriptional up-regulation of the GAP-43 gene during spatial memory. Proc. Natl Acad. Sci. USA. 2004;101:1217–1222. doi: 10.1073/pnas.0307674100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quattrone A, et al. Posttranscriptional regulation of gene expression in learning by the neuronal ELAV-like mRNA-stabilizing proteins. Proc. Natl Acad. Sci. USA. 2001;98:11668–11673. doi: 10.1073/pnas.191388398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Menzel R, Manz G, Menzel R, Greggers U. Massed and spaced learning in honeybees: the role of CS, US, the intertrial interval, and the test interval. Learn Mem. 2001;8:198–208. doi: 10.1101/lm.40001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hourcade B, Muenz TS, Sandoz JC, Rossler W, Devaud JM. Long-term memory leads to synaptic reorganization in the mushroom bodies: a memory trace in the insect brain? J. Neurosci. 2010;30:6461–6465. doi: 10.1523/JNEUROSCI.0841-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haussmann IU, Soller M. Differential activity of EWG transcription factor isoforms identifies a subset of differentially regulated genes important for synaptic growth regulation. Dev. Biol. 2010;348:224–230. doi: 10.1016/j.ydbio.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 62.Sommerlandt FMJ, Brockmann A, Rossler W, Spaethe J. Immediate early genes in social insects: a tool to identify brain regions involved in complex behaviors and molecular processes underlying neuroplasticity. Cell Mol. Life Sci. 2019;76:637–651. doi: 10.1007/s00018-018-2948-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guzowski JF. Insights into immediate-early gene function in hippocampal memory consolidation using antisense oligonucleotide and fluorescent imaging approaches. Hippocampus. 2002;12:86–104. doi: 10.1002/hipo.10010. [DOI] [PubMed] [Google Scholar]
- 64.Minatohara K, Akiyoshi M, Okuno H. Role of Immediate-Early Genes in Synaptic Plasticity and Neuronal Ensembles Underlying the Memory Trace. Front Mol. Neurosci. 2015;8:78. doi: 10.3389/fnmol.2015.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iino S, et al. Neural activity mapping of bumble bee (Bombus ignitus) brains during foraging flight using immediate early genes. Sci. Rep. 2020;10:7887. doi: 10.1038/s41598-020-64701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirano Y, et al. Shifting transcriptional machinery is required for long-term memory maintenance and modification in Drosophila mushroom bodies. Nat. Commun. 2016;7:13471. doi: 10.1038/ncomms13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fujita N, et al. Visualization of neural activity in insect brains using a conserved immediate early gene, Hr38. Curr. Biol. 2013;23:2063–2070. doi: 10.1016/j.cub.2013.08.051. [DOI] [PubMed] [Google Scholar]
- 68.Tiruchinapalli DM, Ehlers MD, Keene JD. Activity-dependent expression of RNA binding protein HuD and its association with mRNAs in neurons. RNA Biol. 2008;5:157–168. doi: 10.4161/rna.5.3.6782. [DOI] [PubMed] [Google Scholar]
- 69.Mirisis, A. A., Kopec, A. M. & Carew, T. J. ELAV proteins bind and stabilize C/EBP mRNA in the induction of long-term memory in Aplysia. J. Neurosci.41, 947–959 (2020). [DOI] [PMC free article] [PubMed]
- 70.Yim SJ, et al. Regulation of ApC/EBP mRNA by the Aplysia AU-rich element-binding protein, ApELAV, and its effects on 5-hydroxytryptamine-induced long-term facilitation. J. Neurochem. 2006;98:420–429. doi: 10.1111/j.1471-4159.2006.03887.x. [DOI] [PubMed] [Google Scholar]
- 71.McNeill MS, Kapheim KM, Brockmann A, McGill TA, Robinson GE. Brain regions and molecular pathways responding to food reward type and value in honey bees. Genes Brain Behav. 2016;15:305–317. doi: 10.1111/gbb.12275. [DOI] [PubMed] [Google Scholar]
- 72.Sanfilippo P, Smibert P, Duan H, Lai EC. Neural specificity of the RNA-binding protein Elav is achieved by post-transcriptional repression in non-neural tissues. Development. 2016;143:4474–4485. doi: 10.1242/dev.141978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cabirol A, Brooks R, Groh C, Barron AB, Devaud JM. Experience during early adulthood shapes the learning capacities and the number of synaptic boutons in the mushroom bodies of honey bees (Apis mellifera) Learn Mem. 2017;24:557–562. doi: 10.1101/lm.045492.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cabirol A, Cope AJ, Barron AB, Devaud JM. Relationship between brain plasticity, learning and foraging performance in honey bees. PLoS One. 2018;13:e0196749. doi: 10.1371/journal.pone.0196749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luo L, Callaway EM, Svoboda K. Genetic Dissection of Neural Circuits: A Decade of Progress. Neuron. 2018;98:256–281. doi: 10.1016/j.neuron.2018.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fan XC, Steitz JA. Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. Embo J. 1998;17:3448–3460. doi: 10.1093/emboj/17.12.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zaharieva E, Chipman JK, Soller M. Alternative splicing interference by xenobiotics. Toxicology. 2012;296:1–12. doi: 10.1016/j.tox.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 78.Tasman K, Hidalgo S, Zhu B, Rands SA, Hodge JJL. Neonicotinoids disrupt memory, circadian behaviour and sleep. Sci. Rep. 2021;11:2061. doi: 10.1038/s41598-021-81548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Decio P, et al. Thiamethoxam exposure deregulates short ORF gene expression in the honey bee and compromises immune response to bacteria. Sci. Rep. 2021;11:1489. doi: 10.1038/s41598-020-80620-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang H, Molfenter J, Zhu H, Lou H. Promotion of exon 6 inclusion in HuD pre-mRNA by Hu protein family members. Nucleic Acids Res. 2010;38:3760–3770. doi: 10.1093/nar/gkq028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hayashi S, Yano M, Igarashi M, Okano HJ, Okano H. Alternative role of HuD splicing variants in neuronal differentiation. J. Neurosci. Res. 2015;93:399–409. doi: 10.1002/jnr.23496. [DOI] [PubMed] [Google Scholar]
- 82.Irimia M, et al. A highly conserved program of neuronal microexons is misregulated in autistic brains. Cell. 2014;159:1511–1523. doi: 10.1016/j.cell.2014.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ustaoglu P, et al. Srrm234, but not canonical SR and hnRNP proteins, drive inclusion of Dscam exon 9 variable exons. RNA. 2019;25:1353–1365. doi: 10.1261/rna.071316.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schutt C, Nothiger R. Structure, function and evolution of sex-determining systems in Dipteran insects. Development. 2000;127:667–677. doi: 10.1242/dev.127.4.667. [DOI] [PubMed] [Google Scholar]
- 85.Gonatopoulos-Pournatzis T, Blencowe BJ. Microexons: at the nexus of nervous system development, behaviour and autism spectrum disorder. Curr. Opin. Genet. Dev. 2020;65:22–33. doi: 10.1016/j.gde.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 86.Matsumoto Y, Menzel R, Sandoz JC, Giurfa M. Revisiting olfactory classical conditioning of the proboscis extension response in honey bees: a step toward standardized procedures. J. Neurosci. Methods. 2012;211:159–167. doi: 10.1016/j.jneumeth.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 87.Koushika SP, Soller M, DeSimone SM, Daub DM, White K. Differential and inefficient splicing of a broadly expressed Drosophila erect wing transcript results in tissue-specific enrichment of the vital EWG protein isoform. Mol. Cell Biol. 1999;19:3998–4007. doi: 10.1128/MCB.19.6.3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sambrook, R. P. & Russell, D. V. Molecular Cloning-A Laboratory Manual. (Cold Spring Harbor Laboratory Press, 2001).
- 89.Saudan P, et al. Ductus ejaculatorius peptide 99B (DUP99B), a novel Drosophila melanogaster sex-peptide pheromone. Eur. J. Biochem. 2002;269:989–997. doi: 10.1046/j.0014-2956.2001.02733.x. [DOI] [PubMed] [Google Scholar]
- 90.Waterhouse A, et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic Acids Res. 2018;46:W296–W303. doi: 10.1093/nar/gky427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Drozdetskiy A, Cole C, Procter J, Barton GJ. JPred4: a protein secondary structure prediction server. Nucleic Acids Res. 2015;43:W389–W394. doi: 10.1093/nar/gkv332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tu Q, Cameron RA, Worley KC, Gibbs RA, Davidson EH. Gene structure in the sea urchin Strongylocentrotus purpuratus based on transcriptome analysis. Genome Res. 2012;22:2079–2087. doi: 10.1101/gr.139170.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cary GA, Cameron RA, Hinman VF. EchinoBase: tools for echinoderm genome analyses. Methods Mol. Biol. 2018;1757:349–369. doi: 10.1007/978-1-4939-7737-6_12. [DOI] [PubMed] [Google Scholar]
- 95.Mota, T. & Giurfa, M. Multiple reversal olfactory learning in honeybees. Front. Behav. Neurosci.4, 48 (2010). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
All data are available in the main text or the supplementary material (Supplementary Data 1). Reagents are available upon reasonable request from the corresponding author.