Abstract
Vinculin plays a key role during the first phase of focal adhesion formation and interacts with the plasma membrane through specific binding of its tail domain to the lipid phosphatidylinositol 4,5-bisphosphate (PIP2). Our understanding of the PIP2-vinculin interaction has been hampered by contradictory biochemical and structural data. Here, we used a multiscale molecular dynamics simulation approach, in which unbiased coarse-grained molecular dynamics were used to generate starting structures for subsequent microsecond-long all-atom simulations. This allowed us to map the interaction of the vinculin tail with PIP2-enriched membranes in atomistic detail. In agreement with experimental data, we have shown that membrane binding is sterically incompatible with the intramolecular interaction between vinculin’s head and tail domain. Our simulations further confirmed biochemical and structural results, which identified two positively charged surfaces, the basic collar and the basic ladder, as the main PIP2 interaction sites. By introducing a valency-disaggregated binding network analysis, we were able to map the protein-lipid interactions in unprecedented detail. In contrast to the basic collar, in which PIP2 is specifically recognized by an up to hexavalent binding pocket, the basic ladder forms a series of low-valency binding sites. Importantly, many of these PIP2 binding residues are also involved in maintaining vinculin in a closed, autoinhibited conformation. These findings led us to propose a molecular mechanism for the coupling between vinculin activation and membrane binding. Finally, our refined binding site suggests an allosteric relationship between PIP2 and F-actin binding that disfavors simultaneous interaction with both ligands, despite nonoverlapping binding sites.
Significance
Phosphatidylinositol-4,5-bisphosphate regulates early events in cell adhesion formation by recruiting and coactivating peripheral membrane proteins. Because detailed protein-lipid interactions are difficult to capture experimentally, our understanding of the functional impact of membrane binding is hampered by a lack of high-resolution structural data. Here, we used a multiscale molecular dynamics simulation approach to generate an atomistic model of how vinculin, which mechanically reinforces early adhesions, is recruited to the inner leaflet of the plasma membrane. Our data reveal not only new and refined phosphatidylinositol-4,5-bisphosphate binding sites, they also show that membrane association regulates the access to vinculin’s binding sites by competing with many intra- and intermolecular binding partners. In the course of this study, we have developed a valency-disaggregated binding network analysis that could greatly facilitate the investigation of general protein-ligand systems with molecular dynamics simulations.
Introduction
Focal adhesions are complex and highly dynamic membrane-associated signaling hubs that connect the cytoskeleton with the extracellular matrix (1,2). Their formation requires the recruitment of a myriad of components to the plasma membrane in a defined temporal sequence and stoichiometry (3, 4, 5). The rapid buildup and regulation of adhesion sites is not only achieved through concerted protein-protein interactions but also through direct protein-lipid binding. Phosphatidylinositol phosphates (PIPs) are the most important lipid class for the regulation of adhesions, and phosphatidylinositol 4,5-bisphosphate (PIP2) is its most prominent representative (6, 7, 8, 9). PIP2 is well suited to dynamically regulate protein recruitment because its concentration can be locally increased through phosphorylation or decreased through hydrolysis. The large, highly charged headgroup is a convenient target for strong and specific protein-ligand interactions. The effects of PIP2 binding go beyond the mere local enrichment of target proteins at the membrane. It can orient proteins on the bilayer in positions that favor or hinder association with other binding partners, or it can directly modulate protein function through allosteric or competitive interactions (10, 11, 12). A recent report further suggests that PIP clustering affects the diffusivity of peripheral membrane proteins on the membrane surface, which is likely to play a role in regulating the function of membrane-bound proteins (13).
Vinculin, an essential scaffold and mechanosensitive signaling protein, is one of the many focal adhesion components that are co-regulated by PIP2 binding. Besides PIP2, vinculin has more than 15 confirmed binding partners. It is best known for its ability to reinforce the physical link between the cytoskeleton and the adhesion machinery by forming additional connections between talin and F-actin (14). F-actin and membrane interactions are both mediated by the C-terminal vinculin tail (Vt) domain (15,16). Vinculin’s function is characterized by strong intramolecular interactions. In the cytosol, vinculin assumes a closed conformation in which Vt makes extensive contacts with the N-terminal head domain (17,18). Only when this autoinhibition is disrupted (e.g., through talin binding) can Vt interact strongly with PIP2 or F-actin (19, 20, 21). Although vinculin was already discovered in 1979 (22) and its binding to PIP2 was discovered in 1994 (15), the functional impact of membrane binding remains controversial. Cell studies using mutants deficient in lipid binding produced contradictory results (20,23, 24, 25). Because Vt binds many ligands with often overlapping binding sites, these inconsistencies can probably be attributed to the use of nonspecific mutants.
Vt consists of a five-helix bundle (H1–H5) that is preceded by an unstructured, extended strap region (residues 879–890) and followed by a flexible C-terminus (Fig. 1 A) (17,18,26). The bundle possesses two positively charged surfaces that were shown to be important for the interaction with acidic lipids, particularly PIP2 (26). They are referred to as the basic collar and the basic ladder (Fig. 1 A). Acidic amino acids in the strap partially occlude access to the basic collar (17,18,26). Deletion of these residues leads to an increased affinity for PIP2, but not for other acidic lipids (16). The strap thus provides an additional layer of functional regulation of ligand binding through intramolecular interaction and needs to alter its conformation to allow PIP2 binding (20).
Figure 1.
Computational approach to screen interactions between the vinculin tail (Vt) and PIP2-containing membranes. (A) Vt is a five-helix bundle (H1–H5) with an extended N-terminal unstructured strap and a flexible C-terminus (colored white to black from N- to C-terminus; basic residues are shown in gray and acidic residues are shown in pink). The basic ladder and the basic collar are responsible for PIP2 binding. The top panel shows the basic collar, which consists of residues of H1, H2, and the C-terminus. The basic collar is occluded by the strap, which contains a cluster of acidic residues. The bottom panel shows the basic ladder, which is formed by positively charged residues on H3 and H4. (B) Workflow of our multiscale simulation approach. 32 independent coarse-grained simulations were run for 5 μs. The orientation of Vt on the membrane was recorded as the contact point of the circumscribing sphere around the protein and the membrane in geographical coordinates (y, l). These data were combined into orientational maps. Relevant frames from the coarse-grained screening were selected and transformed into all-atom models. Six atomistic simulations, starting from distinct initial configurations, were run for ∼1 μs each. To increase lipid diffusion, we converted the membrane into a highly mobile membrane mimetic (HMMM) (25). Phosphatidylcholine is shown in beige, PIP2 is shown in different colors, and HMMM solvent is shown in turquois. To see this figure in color, go online.
Chinthalapudi et al. solved the crystal structure of Vt in the presence of short-tailed, soluble PIP2 derivatives (20). Vt crystalized as an asymmetric domain-swapped dimer in which one PIP2 molecule is sandwiched between the basic collar of two monomers and makes contact with S913, K915, and K1061. Additional interactions with PIP2 are established through the basic ladder (K944, R945) of a third Vt molecule. Thus, the PIP2 binding site in the crystal is formed by three Vt molecules that surround one lipid from all sides. It is not clear, however, how this trimer could insert into a planar membrane. This issue is resolved in an alternative model of membrane-bound Vt based on computational docking, hereinafter referred to as the Thompson model (24). Their suggested PIP2 binding site (R910, S913, K915, K924, R925) overlaps with the crystal structure, but results in a position of the PIP2 headgroup that is shifted by several angstroms. The differences between the models might appear small, but any change of the binding pose translates into a change of the orientation Vt assumes on the membrane. As described above, membrane binding can expose or occlude different parts of the protein and thus change the availability of interaction sites for other binding partners. Thus, it is crucial to gain detailed knowledge about the orientation(s) a protein assumes upon membrane association. In the case of vinculin, this affects, for example, Vt dimer formation. In the crystal structure, the dimer is stabilized by domain swapping of the flexible C-terminal region. W1064 of one monomer inserts into a cleft on the H1/H2 surface of the other monomer. In the Thompson model, Vt is oriented such that access to this binding pocket is blocked by the membrane.
Both of the currently available structural models suffer from the same limitation because they do not take into account the spatial constraints imposed by membrane binding. Soluble short-tailed lipids in the crystal lattice, as well as computational docking of a single PIP2 headgroup, can produce binding poses that would not be accessible if the protein was embedded in a membrane. These approaches might produce low free-energy binding modes in isolation that would be prohibited by steric clashes in the context of a full lipid bilayer. On the other hand, additional stabilizing interactions with the membrane could render locally suboptimal binding poses more favorable.
All-atom molecular dynamics (MD) simulations are highly valuable to investigate protein-membrane interfaces at the submolecular level. Accessible simulation times are, however, not sufficient to exhaustively sample lateral diffusion of lipids and rotational diffusion of the protein on the membrane. The choice of initial coordinates will thus bias the simulation outcome. Here, we employed a multiscale approach of the vinculin tail bound to a lipid membrane to overcome this issue. Initially, we performed an unbiased systematic screening with computationally cheap coarse-grained MD that allows for longer simulation times and more replicas. Based on these simulations, we created maps that show the probability of Vt to be bound to the membrane in a given orientation (12,27). Subsequently, we combined the coarse-grained MD data with insights from mutational studies to extract relevant frames from the screening simulations. This allowed us to generate starting coordinates for all-atom MD simulations by using resolution back-transformation. To further enhance lipid diffusion, we replaced the lipid bilayer by a highly mobile membrane mimetic (HMMM) system (25,28). In an HMMM, the ends of the lipids’ hydrocarbon chains (i.e., the core of the membrane) are replaced by a fluid solvent. This allows for faster lateral movement of the resulting short-tailed lipids (see Materials and methods; Fig. 1 B). In the past, similar sequential multiscale approaches have been successfully applied to determine the binding orientations and lipid interaction sites of multiple PIP binding peripheral membrane proteins (12,29, 30, 31). To identify and visualize ligand binding sites, we devised, to our knowledge, a novel network representation that highlights not only how likely a given residue is to bind to a ligand molecule but also how it does so in concert with other residues and thus facilitates quantification as well as the comparison of multiple binding simulations. As the final outcome, we present, to our knowledge, the first structural model of membrane-bound Vt in the presence of multiple PIP2 molecules that is in excellent agreement with published biochemical data.
Materials and methods
Coarse-grained simulations
All coarse-grained simulations were run with GROMACS5.1 (32) with the Martini force field v2.2 (33,34). The coarse-grained membranes were built with the program “insane” (35). In the 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC)/PIP2 membrane, the lower leaflet consists of 100% POPC, the upper leaflet consists of 90% POPC and 10% 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylinositol-4,5-bisphosphate (PIP2). In the complex membrane, the lower leaflet consists of 100% POPC, the upper leaflet of 60% 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylethanolamine, 20% POPC, 10% 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphatidylserine, and 10% PIP2. The membranes were solvated, neutralized with Na+ ions, and the total NaCl concentration was set to 150 mM, minimized for 1000 steps, and equilibrated during 1 μs with a 20 fs time step.
The human vinculin tail domain was taken from the full-length crystal structure (Protein Data Bank: 1TR2) and missing residues were modeled with Modeler (36). Full-length Vt (VtFL) consists of residues 879–1066, and VtΔstrap (VtΔS) consists of residues 891–1066. The structures were equilibrated atomistically for 100 ns (see All-atom simulations) before coarse graining with the martinize.py script (34). An elastic network (37) connecting the backbone beads was applied with a force constant of 400 kJ mol−1 nm−2 and a distance cutoff between 0.5 and 0.9 nm, except for the loops connecting the helices. To better reproduce the flexibility of the strap, a reduced force constant of 200 kJ mol−1 nm−2 was applied to theses residues (residue 879–884). To avoid unphysical side-chain orientations of W1058, we introduced additional bonds between W1058 and I919 as well as between F885 and H906, with a force constant of 5000 kJ mol−1 nm−2. The restraints were applied because the Martini force field underestimated the interaction between these residues compared with all-atom simulations. Overall, these changes lead to a better match between coarse-grained and atomistic simulations.
The screening was set up and analyzed as described previously (12,27). 32 initial orientations were chosen based on an equidistant spacing on a Fibonacci sphere. Each system was minimized for 10 steps in vacuum. The systems were solvated, neutralized with Na+, and the total NaCl concentration set to 150 mM. Subsequently, they were minimized for 5000 steps, then equilibrated for 1 ns using a 20 fs time step with the velocity rescale thermostat (38) and Berendsen pressure coupling scheme (39). For production, pressure was controlled with the Parrinello-Rahman barostat (40). Lennard-Jones and Coulomb forces were cut off at 1.1 nm, with the potentials shifted to zero at the cutoff. Each system was run for 5 μs. To create the orientational maps, we defined a coordinate system relative to a reference structure of Vt. The origin of the coordinate system was set to the center of mass of the protein and the z axis points along the long axis of the helix bundle. For each simulation frame, we fitted the reference structure onto the protein. The geographical coordinates (longitude Ψ, latitude λ) of the contact points between the circumscribing sphere around the protein and the membrane were then recorded. The contact point is defined as the point where the vector from the center of mass of the protein along the negative membrane normal pierces the circumscribing sphere (Fig. S1 A or (27)). Only those frames were considered for which at least one bead made contact with the membrane (distance <6 Å). Because the area of a small surface element on a sphere changes from the equator toward the poles, a normal two-dimensional histogram of the angular directions would introduce a significant bias. To avoid this effect, we performed the binning on the surface of a sphere. 2701 equidistant bins were generated on a unit sphere using the Fibonacci spiral, and the angular data were binned to the respective nearest pinning point (Fig. S1 B). The bins were subsequently projected onto a plane (Fig. S1 C), and a histogram was interpolated on a 60 × 60 grid using the MATLAB (The MathWorks, Natick, MA) function “meshgrid” with the method “nearest.” The resulting matrix was then normalized to the total number of included simulation frames, yielding a probability distribution P, whose logarithm log(P) was plotted as contour lines.
All-atom simulations
All all-atom simulations were run with NAMD2.12 (41) using the CHARMM36 force field (42) with a 2 fs timestep. The pressure was set to 1 atm and controlled with the Nosé-Hoover Langevin piston (43,44). Short-range, nonbonded interactions were cut off at 12 Å, with a shifting function starting at 10 Å. Long-range electrostatic interactions were calculated with the particle-mesh Ewald method (45). The simulations of Vt in solution were prepared with the QwikMD plugin (46) in VMD (47). The systems were solvated with TIP3P water, and the NaCl concentration set to 150 mM. They were minimized for 2000 steps, then stepwise heated to 310 K, and subsequently equilibrated for 1 ns. During these equilibration steps, the backbone atoms were restrained. Production runs were performed without any restraints.
Atomistic models from snapshots of coarse-grained simulations were obtained with the backward.py script (48). The fine-grained systems were minimized for 10,000 steps. All water molecules and ions, except for ions bound to the membrane, were then removed. Although the back-transformation worked well for the lipid molecules, the protein structures were prone to suffer from structural artifacts. This was remediated by replacing the fine-grained protein with a well-equilibrated protein structure. Subsequently, the membrane was transformed into an HMMM with SCSE solvent replacing part of the lipid tails (25,28). The new protein-HMMM system was again minimized for 10,000 steps, heated stepwise to 310 K, and equilibrated during these equilibration steps, whereas the protein backbone and the lipid C21/C31 atoms were restrained. During production runs, we applied restraints on the z position to the C21 and C31 atoms of each lipid to avoid diffusion of short-tailed lipids out of the membrane.
Analysis and visualization
Simulation snapshots and videos were rendered with VMD (47); hydrogen bonds between PIP2 and the proteins were analyzed with PyContact, using the default H-bond parameters (i.e., an acceptor to hydrogen distance cutoff of 2.5 Å and an angel cutoff of 120° (49)). The binding networks were generated with custom-made software available in the Supporting material accompanying this article (and can be visualized with Cytoscape (50)). The workflow of the valency-disaggregated binding network analysis is laid out in Fig. S2. In each simulation frame, we identified all PIP2 molecules forming at least one hydrogen bond with the protein. We then determined, for each of these PIP2 molecules, all protein residues that interact with it. Residues that form simultaneous interactions with the same ligand are marked as connected and entered into a list of binding sites. This is repeated for all simulation frames, and the occurrence of each unique binding site is counted and eventually normalized by the number of frames. Finally, the counts from all simulations are summed up and normalized by the number of simulations. These results can be visualized, as shown in Fig. S3. The more compact representation using conditional probabilities for pairwise interactions, as shown in Fig. 4 and Fig. S3, can be generated by summing all pairwise interactions of a given valency. Mathematically this corresponds to the entry-wise sum of the adjacency matrices of all unique binding sites of a given size. For clearer visualization, thresholds for minimal occurrence of an interaction were applied.
Figure 4.
A novel valency-disaggregated binding network analysis approach to visualize and quantify the simultaneous interactions of Vt residues with membrane-embedded PIP2 molecules. (A) Aggregated binding histogram of the interaction between Vt and PIP2 of all six atomistic simulations (total simulation time =7.1 ms). Although it is possible to determine the PIP2 binding hotspots from the histogram, it is not clear which residues act in concert to form binding sites. The dotted red line at 33% indicates the cut off for residues to be explicitly shown as nodes in (B). (B) PIP2 binding network reconstructed from the time-aggregated binding data of all six all-atom simulations. The node size is proportional to the bin counts of the histogram shown in (A). The edges are color coded by the number of residues that simultaneously bind to the same PIP2 molecule (i.e., a dark blue line means that the connected residues form a pairwise interaction, a light blue line means that the two connected residues bind at the same time with a third one, et cetera) (for a time-resolved representation, see Video S1). The line width of the edges is proportional to the percentage of frames in which the respective interaction occurs. For better visibility, only contributions that occur in at least 5% of the frames and up to pentavalent interactions are colored. The rest is combined into the remaining category. The full network is available as a Cytoscape file in the Supporting material. The binding network separates into two regions. One corresponds to the basic collar, the other one corresponds to the basic ladder. The basic collar network is highly interconnected, with higher-valency binding events dominating. The basic ladder network contains several residues that strongly interact with PIP2, but few higher order binding sites are consistently present across multiple simulations (see also Fig. S3). Binding events in (A) and (B) were defined as hydrogen bonds between Vt and PIP2 (distance cutoff =2.5 Å, angle cutoff =120°) (C) Snapshot of a PIP2 molecule simultaneously bound to six basic collar residues. (D) Visualization of basic ladder residues that interact most strongly with PIP2. In addition, PIP2 bound to the putative novel trivalent binding site formed by K944, R945, and R1008 is shown. To see this figure in color, go online.
Results
Coarse-grained screening reveals the orientational distribution of vinculin tail on the membrane
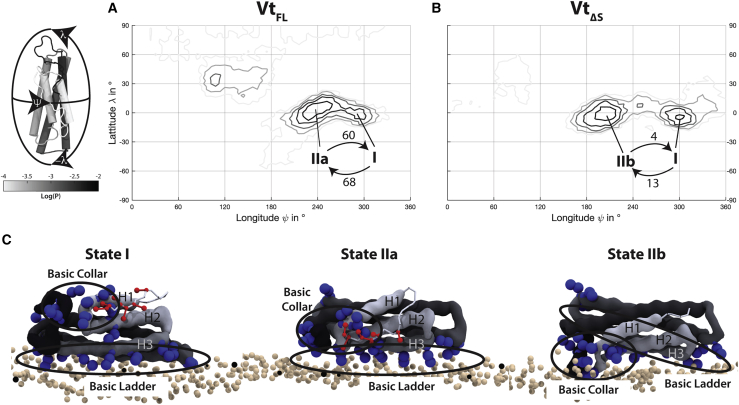
The goal of the coarse-grained simulations was to generate starting coordinates for all-atom MD, with approximately correct binding orientations and membrane configurations that can then be refined on the timescales accessible with atomistic simulations. To this end, we ran 32 independent 5 μs coarse-grained simulations of Vt in the presence of a POPC membrane containing 10% PIP2. To minimize bias from initial conditions, we varied the starting orientation of the protein in each of these systems systematically. We quantified the binding orientations by generating a logarithmic histogram of the contact points between Vt and the membrane in a geographical coordinate system (as described in (27)). The coordinate system was chosen such that a movement along the longitudinal direction (ψ) corresponds to a rotation around the long axis of the Vt helix bundle, whereas changes along the latitudinal angle (λ) correspond to a tilting toward its top or bottom (Fig. 2). Because it has been shown that the disordered strap reduces the accessibility of the basic collar and thus decreases Vt’s affinity for PIP2 (16) (Fig. 1 A), we wanted to investigate the influence of its presence on the binding behavior. Therefore, we performed the coarse-grained screening both with VtFL (residues 879–1066) and VtΔS (residues 891–1066), which is lacking the N-terminal strap. Both constructs show similar orientational distributions. The main peaks are concentrated around the equator, suggesting that the bundle preferentially lies flat on the membrane (Fig. 2). In both cases two peaks are observed. One of them (state I) is in approximately the same position for both constructs, corresponding to an orientation that brings H3 and H4 in contact with the membrane and allows only interactions with the basic ladder (Fig. 2 C). The second peak (state IIa in VtFL and state IIb in VtΔS) is shifted by ∼30° around the long axis (ψ) between the two constructs (Fig. 2, A and B). This means that in VtFL (state IIa) the contact to the membrane is mediated by H2 and H3, whereas for VtΔS (state IIb) the protein is rotated more toward its H1/H2 surface (Fig. 2 C). In both orientations, basic ladder and basic collar residues can simultaneously interact with the membrane. Over the simulation time, a net flux from state I to the respective state II was observed, indicating that states IIa and IIb are energetically more favorable than state I (Fig. 2, A and B). The minor peak observed around λ ≈ 30° in the VtFL simulations was not included in the analysis because it is likely an artifact of the Martini force field. Two out of the 32 simulations were trapped in an orientation stabilized by an insertion of hydrophobic residues of the C-terminus (W1064 and Y1065) into the membrane. However, it was shown experimentally that the last five C-terminal residues (1062–1066) are not involved membrane binding (16).
Figure 2.
(A and B) Orientational maps of Vt on the 90% POPC, 10% PIP2 membrane compiled from 32 simulations of 5 μs each. For each construct two main peaks are observed. State I exists in both cases, whereas the second peak is shifted between (A) VtFL (state IIa) and (D) VtΔS (state IIb). The arrows and numbers indicate the transition between the states. For both constructs, a net flux from state I to state IIa or IIb was observed. (C) The orientation of vinculin tail (Vt) on the membrane is shown for states I, IIa, and IIb in the coarse-grained simulations. The basic collar and the basic ladder are shown in blue, the strap is shown in red. The beige spheres represent phosphate beads of the coarse-grained lipids. In state I, only the basic ladder makes contact with the membrane, whereas in state IIa and IIb, both the basic collar and the basic ladder are engaged. To see this figure in color, go online.
Thompson et al. have suggested that the basic collar is responsible for PIP2 binding, whereas the basic ladder drives membrane insertion by interacting nonspecifically with acidic phospholipids such as phosphatidyl serine (PS) (24). To test this hypothesis, we repeated the screening with a more complex membrane containing both PIP2 and PS. The orientational distribution of Vt was not significantly affected by the change in lipid composition (Fig. S5 A). When both PIP2 and PS were present, PIP2 completely displaced PS from the basic collar and the basic ladder, indicating a strong preference of PIP2 over PS binding (Fig. S5, B and C). This finding led us to proceed with the simpler POPC/PIP2 membrane system.
For the fine graining, we picked frames in which PIP2 interacts with as many residues as possible that have been shown to be important for lipid binding (Table S1). Because we were interested in PIP2 binding to the basic collar, only frames from states IIa and IIb were considered. We chose a total of six frames from the coarse-grained simulations and transformed them into all-atom systems (see Materials and methods). The first three were directly based on VtΔS simulations. In the coarse-grained simulations, the high charge density of the basic collar attracted multiple PIP2 molecules, which made it difficult to find suitable frames with only a single PIP2 in proximity to the basic collar. The binding of several ligands at the same time might hinder PIP2 from finding its preferred binding pose. We observed that the electrostatic shielding effect of the strap reduced the number of PIP2 molecules in the vicinity of the basic collar. Therefore, we ran three all-atom simulations based on initial coordinates derived from coarse-grained simulations of VtFL in which the protein structure was replaced with VtΔS to facilitate binding of a single ligand to the basic collar.
Membrane binding reduces binding site accessibility of multiple interaction partners of the vinculin tail
Because the binding sites for the many intra- and intermolecular binding partners of Vt are distributed all over its surface, membrane association is likely to interfere with some of these interactions or vice versa. Determining the binding orientation of Vt on the bilayer will thus provide new insights into the functional impact of PIP2 binding. Combined, the six all-atom simulations sampled more than 60° around the long axis of the bundle (ψ). However, over time all systems converged to a relatively well-defined region spanning ∼30°, where most of the simulation time was spent (Fig. 3 A). In this pose, membrane contact is mediated by helices H2 and H3, which allows for simultaneous interaction of the basic collar and the basic ladder with the membrane. Higher ψ-values disrupt optimal binding by rotating the basic collar away from the membrane, whereas lower ψ-values reduce the interaction with the basic ladder (Fig. 3 B; Fig. S6). Because, in the Thompson model, Vt binds with a low ψ angle, we started one simulation from such an orientation. Presumably due to suboptimal membrane contact with the basic ladder, the pose was unstable, and Vt reoriented into the 30° region (Fig. 3, A and B; Fig. S6 A). A mapping of the contact frequencies of Vt residues with the membrane reveals a large protein-lipid interface (Fig. 3 C). Most of these interactions are mediated by PIP2 (Fig. S6 B), which binds, as expected, preferably to the basic collar and the basic ladder. However, other regions of Vt preferentially form contacts with the background lipid POPC (Fig. S6 B). The overall orientation of Vt on the membrane will thus be determined by the sum of many distributed interactions.
Figure 3.
Orientations of the vinculin tail (Vt) monomer on the lipid membrane in all-atom simulations. (A) Orientational map based on all six all-atom simulations (total simulation time =7.1 μs) in the same reference coordinate system as in Fig. 1 (light gray contour, log(P) = −3; dark gray contour, log(P) = −2). The green and the red symbols indicate the initial (green) and final (red) orientations of each simulation, respectively. Yellow symbols mark the orientation of selected intermediate frames. The large symbols correspond to the orientations visualized in (B). Although the simulations sample y angles of more than 60°, all final orientations converge to a region spanning ∼30°. (B) Representative snapshots, covering a wide range of sampled y-values. At low y angles, the dimerization pocket is occluded, and membrane contact with the basic ladder is reduced (Fig. S6). At high y-values, contact between the basic collar and the membrane is disrupted. Within the 30° region (210–240°), both dimerization and full engagement of the PIP2 binding sites are possible. Vt is colored from white (N-terminus) to black (C-terminus), the phosphor atoms of the upper membrane leaflet are shown as spheres. (C) Contact frequency of Vt residues with the membrane (both PIP2 and POPC) (5 Å distance cutoff) overlaid with the contact areas of various Vt binding partners. Membrane binding is not mutually permissible with binding to vinculin head, the unstructured and negatively charged strap, or Raver1, whereas the F-actin binding site remains accessible. The same visualization for the individual contact frequencies with PIP2 or POPC is shown in Fig. S6B. To see this figure in color, go online.
The binding poses within the 30° region render both the residue required for homo-dimerization (W1064) and its binding pocket easily accessible and aligned for interaction with a second Vt molecule (Fig. 3, B and C). Rotation toward lower ψ-values, as in the Thompson model, brings helices H1 and H2 in contact with the membrane, which leads to occlusion of the W1064 binding site. To investigate how membrane binding interferes with the interaction between Vt and other binding partners, we determined the regions of Vt that are in contact with the membrane and compared the occluded areas with known binding sites (Fig. 3 C). Our simulations show that two distinct intramolecular interactions compete with membrane binding. As expected, we observed strong contacts between the basic collar and the bilayer, confirming that the strap needs to unfurl for full membrane binding (Fig. 3 C). Highly significant, our data show that the head-tail interactions directly prevent vinculin association with the lipid bilayer or, vice versa, that membrane binding stabilizes the activated conformation of vinculin, thus preventing a rapid transitioning back to the autoinhibited conformation. Some of the regions on Vt that interact most strongly with the membrane overlap with the Vt-vinculin head interface. Furthermore, our simulations show that large parts of the binding site for the RNA binding protein Raver1 are occluded by the membrane (Fig. 3 C; (51)). This is consistent with experimental data showing that Raver1 and membrane binding are mutually exclusive (20). Because their binding sites do not overlap, simultaneous binding to the membrane and F-actin seems permissible. However, this result needs to be interpreted carefully. We have based the binding site on a cryo-EM model of actin-bound Vt (21). This structure shows that F-actin binding induces the release of helix H1 and the C-terminus from Vt, transforming it from a five- to a four-helix bundle. These substantial conformational changes are likely to affect membrane binding. Furthermore, only H2–H5 are resolved in this structure, and it has been recently shown that the rearranged C-terminus is also involved in actin binding (52).
Network representation of PIP2 binding sites reveals high-valency bonding with the basic collar and low-valency binding to the basic ladder
To gain a more detailed understanding how PIP2 binding affects Vt’s functions, we determined its binding sites on the protein surface. As routinely done in MD-based studies, we counted how often each protein residue interacts with a ligand molecule and normalized the tally by the number of simulation frames (Fig. 4 A). This histogram confirms that PIP2 binding is indeed mostly mediated by the basic collar and the basic ladder, although not all members of these regions are actually involved in lipid binding. The two serine residues, S913 and S914, are the only neutral amino acids forming stable, recurrent interactions with PIP2 (Fig. 4 A; Fig. S4). In the crystal structure, S913 forms water-mediated interactions with PIP2, whereas it binds directly to one phosphate group in the Thompson model (20,24). S914 has not been implicated in PIP2 binding in either model.
Simple histograms, however, cannot provide a full description of binding sites because the mapping between binding frequencies and binding poses is ambiguous. This issue is further exacerbated in systems, such as ours, which contain several mobile ligand molecules. For example, a configuration in which multiple protein residues bind one ligand molecule binds simultaneously is indistinguishable from the case in which the same set of amino acids bind multiple ligands. Yet this information is essential, because binding sites acquire their affinity and specificity by forming multiple concurrent contacts with the ligand. The individual interactions are often relatively weak and dynamic; only their combined contributions ensure tight binding. Thus, determining the valency of binding events will improve the detection of bona fide binding sites from MD simulations. The assumption here is that a set of residues that forms recurrent, high-valency interactions with the ligand can be considered a specific binding site.
Binding sites can be visualized more naturally by network graphs, in which the protein residues are represented by nodes. The edges between the nodes encode a measure for the probability of pairwise, simultaneous interaction with the same ligand molecule. However, because only pairwise interactions are considered, the mapping remains ambiguous for valencies greater than two. To reduce this issue, we have developed a method dubbed “valency-disaggregated binding network analysis” that can be easily applied to any protein-ligand system. As the name suggests, the pairwise probabilities are further broken down into conditional probabilities (i.e., we are, for example, asking what the probability is of residue A and B forming a pairwise interaction, given that both are engaged in a trivalent interactions involving an additional residue). This information can then be used to reconstruct the full binding site by finding the residue that has trivalent edges with both A and B. To create these networks, we recorded the residues that interact simultaneously with a given ligand molecule in each simulation frame (Videos S1 and S2). Subsequently, the occurrence of each unique set of binding residues is counted and normalized. The conditional probabilities were estimated by combining these data for all PIP2 molecules in all six simulations, as outlined in Fig. S2 and the Materials and methods (code and data are available in the Supporting material). We then applied thresholding to highlight recurrent binding configurations and to average out less specific interactions. This allowed us to generate binding networks that break down how many and which residues frequently form simultaneous interactions with the same lipid molecule (Fig. 4 B; Fig. S3). The size of the nodes in the graph corresponds to the bin count in the histogram. The width of the edges is proportional to the occurrence of an interaction; the color of the edges refers to the valency of an interaction. Besides the easier detection of binding sites, the advantages of this representation for visualization are evident. Regions that are adjacent in the tertiary fold of the protein, such as the N- and C-terminal portion of the basic collar, can appear far apart in the histogram. In contrast, the network graph reflects the underlying structure of the protein because only residues in close spatially proximity can form shared interactions. An additional benefit is the easier comparability of multiple simulations. Although it is tedious to recognize similarities and differences in the histograms, it is possible to identify them at a glance in the network representation (Fig. S3).
Left: Binding trajectory of a single PIP2molecule (purple C-atoms) to the Basic Collar. Only the Vt residues involved in binding of this lipid molecule are shown explicitly and the rest of the membrane is intermittently omitted for better visibility. Right: Time resolved network representation of the binding process. Each node corresponds to one of the Vt residues involved in binding of the ligand molecule. When the node is shaded in grey, the corresponding protein residue is interacting with the PIP2molecule. Lines between nodes indicate simultaneous binding and their color encodes the valency of the interaction (same color code as in Supplementary Figure 8). Initially the PIP2 molecule forms only few instable, low valency interactions. As the simulation progresses, the ligand moves into the binding pocket formed by R910, S913, S914, K915, K924 and K1061 and the number of simultaneous interactions increases. The binding valency still varies because of thermal fluctuations, but the high number of engaged residues ensures that the ligand remains bound stably. To generate the aggregated binding networks shown in Fig. 4 B and Supplementary Fig. 4 the network for each timestep (as shown on the right) is recorded for each PIP2 molecule. The occurrence of each unique set of binding residues is counted and normalized by the number of simulation frames.
Top: Binding trajectory of a two PIP2 molecules (purple and yellow C-atoms) competing for the Basic Collar. Only the Vt residues involved in binding of this lipid molecule are shown explicitly and the rest of the membrane is intermittently omitted for better visibility. Bottom: Time resolved network representations of the binding process. The color of the frame corresponds to the color of the respective PIP2 molecule shown above. Each node corresponds to one of the Vt residues involved in binding of the ligand molecule. When the node is shaded in grey, the corresponding protein residue is interacting with the PIP2molecule. Lines between nodes indicate simultaneous binding and their color encodes the valency of the interaction (same color code as in Supplementary Figure 9). The prebound PIP2 (yellow) forms initially higher valency interactions but gets partially displaced by another ligand (purple) over time. In the end the two PIP2 molecules share the Basic Collar each donating one phosphate group (see also Supplementary Fig 6 B). To generate the aggregated binding networks shown in Fig. 4 B and Supplementary Fig. 4 the network for each timestep is recorded for each PIP2molecule. The occurrence of each unique set of binding residues is counted and normalized by the number of simulation frames.
The binding network that we obtained separates into two regions. One corresponds to the basic collar, the other one corresponds to the basic ladder (Fig. 4, B–D). The basic collar forms a high-valency binding site with up to six residues (R910, S913, S914, K915, K924, and K1061) interacting simultaneously with the same lipid (see also Fig. S3). This means that binding residues identified in the crystal structure (S913, K915, and K1061) are actually a subset of the binding site determined by us. High-valent binding (n ≥ 4) to this site occurs in all simulations but to a different extent (Fig. S3). A more detailed analysis of the binding site occupancy showed that simultaneous engagement of all six residues occurs only in a small fraction of frames (3.2%). However, because of thermal fluctuations and incomplete sampling of the binding site, smaller subsets of these residues bind to PIP2 more frequently. The accumulated binding time of hexa- down to tetravalent subsets amounts to more than 45% of total simulation time (Fig. S3). When the heptavalent binding event involving R910, S913, S914, K915, K1061, and the backbone of two additional residues that occurred in one simulation (Fig. S3 A) is also considered, this number rises to above 50%. From the network representation, it becomes immediately apparent that the Thompson model does not describe the preferred binding site. In their model, R925 binds PIP2 together with R910, S913, K915, and K924, but our analysis shows that R925 is only weakly coupled to the rest of these residues (Fig. 4 B).
In contrast to the basic collar, the basic ladder forms a series of lower-valency (mostly bi- or trivalent) binding sites that are rather divergent between different simulations (Fig. 3 A; Fig. S3). Among the most strongly interacting basic ladder residues are K944 and R945, which have been implicated in PIP2 binding, both through mutational studies and in the crystal structure (Table S1). These two residues together with R1008 can form a trivalent binding pocket that has not, to our knowledge, been described before (Fig. 4, A and C). It is important to note that this triad forms salt bridges with acidic residues of the vinculin head that contribute significantly to the strength of head-tail interaction in the closed conformation (Video S3) (18,53,54). Similarly, R1049 also interacts regularly with PIP2 and forms a salt bridge in closed vinculin. An interesting observation is the tetravalent binding site formed by K970, K975, R978, and R1049 in one simulation (Fig. S3 F) because the three latter residues bind to an acidic cluster on the vinculin head. Again, mutation of these residues substantially weakens the head-tail interaction and substitutions of K975 and R978 or of their counterparts on vinculin head that are routinely used to produce constitutively open vinculin variants (53). We could show that membrane binding can induce subtle conformational changes in this region that are likely to destabilize the closed conformation (Video S3). Other prominent PIP2 binding residues include K952, K956, and especially R963. Mutation of these residues has been shown to lead to a significant drop in membrane binding activity (Table S1; (23)). The low valency and recurrence of binding sites in some parts of the basic ladder suggests that this region forms rather unspecific interactions with PIP2.
By overlaying the structure of full-length Vinculin onto our membrane bound model, we have identified three regions where PIP2 binding sites overlap with intramolecular contacts, namely at the first and fourth Vinculin Head domains (Vd1 and Vd4) and the Strap. Notably, in the overlay only Vd1 produces severe steric clashes with the membrane, while dissociation of Vd4 and the Strap seems to be no prerequisite to membrane binding (see also Fig. 6 A-C). At all three locations the basic residues responsible for PIP2 binding, bind to acidic residues in full-length Vinculin.
Atomistic insights into the Vt-PIP2 interactions at the basic collar suggest a novel recognition mechanism
Even when a PIP2 molecule is engaged with the same set of residues, it can still assume multiple binding poses. Indeed, inspection of individual MD trajectories revealed that the simulations did not converge to a single pose. However, when we combined the data of all six simulations and calculated the spatial density of PIP2 phosphate atoms, several peaks appeared at the basic collar. High 1′-phosphate densities are present in the vicinity of K915 (Fig. S7 A). This indicates that this residue is responsible for binding to the phosphoglyceride region of the membrane. K915 indeed inserted between the lipid headgroups in four of six simulations. The strongest density peak for the 4′- and 51′-phosphates is located between the side-chains of the serine pair S913/914 and K1061 (Fig. S7, A and B). A high density at this position is not consistent with the Thompson model nor with the crystal structure. The role of the serine pair is especially intriguing. These residues are ideally positioned to simultaneously form H-bonds with one phosphate group, holding onto it like a pincer (Fig. 4 B). We will henceforth refer to them as the serine pincer. Engagement to the serine pincer locks the phosphate group in place and allows the positively charged basic collar residues, especially K1061, to bind PIP2 efficiently. A second large but fuzzier peak appeared toward K924 and within the reach of R910, indicating a second phosphate binding site (Fig. S7 A).
The high local PIP2 concentration around the basic collar leads to the association of multiple ligands with the binding site in the simulations based on VtΔS coordinates. In these cases, we observed binding modes in which one PIP2 molecule binds to the serine pincer, whereas the other donates one of its phosphates to satisfy the second site (Fig. S7 B; Video S2). This issue was resolved by the use of initial coordinates derived from coarse-grained VtFL simulations. In two out of these three simulations, a single PIP2 bound in a very similar binding pose to the basic collar such that both phosphate binding sites were satisfied (Fig. 4 B; Fig. S7 A).
Based on these results, we propose the following mechanism for PIP2 binding and recognition by the basic collar. K915 inserts into the membrane and binds to the 1′-phosphate of PIP2. The serine pincer (S913/S914) in concert with K1061 recognizes the negative charge of either the 4′- or the 5′-phosphate, but the tilted headgroup of PIP2 leads to preferential binding of the 5′ group. R910 and K924 recognize the 4′-phosphate (Fig. 4 B). In this binding mode, PIP2 spatially overlaps with the pose found in the crystal structure but is oriented very differently and forms much more intimate contacts with Vt.
Although it would be interesting to test the role of the serine pincer experimentally, Vt seems to be very sensitive to mutations in this region. The attempt to express a S913A/S914A mutant to test its lipid binding capability failed because of strong protein aggregation (N. Bax, personal communication).
The strap and PIP2 compete for the same binding site at the basic collar
In crystal structures of full-length vinculin in the apo state, the strap adopts different conformations, often forming interactions with few of the basic collar residues that have been implicated in PIP2 binding in all available structural models, including ours (17,18,26). Inspection of these structures revealed that the N-terminal region of the strap is often engaged in crystal contacts and might therefore adopt different conformations than in solution. To investigate the solution behavior of the strap, we performed simulations of VtFL in the absence of the membrane. We found that acidic strap residues interact strongly but dynamically with the basic collar by forming a multitude of changing salt bridges and hydrogen bonds (Fig. 5; Video S4). This allows the strap to sample a wide range of the basic collar without unbinding from it, thus effectively shielding its positive charge. Importantly, all residues that we found to be involved in PIP2 binding were among those that interacted most frequently with the strap, including the serine pincer (Fig. 5). Furthermore, the spatial density of the carboxy-groups of acidic strap residues is very similar to that of the PIP2 phosphates (Fig. S7 C). The presence of density peaks in the same locations corroborates that these regions are indeed well suited to stabilize negative charges and thus strengthens the confidence in our model. These findings refine the structural basis for the reduced affinity of VtFL for PIP2 and offer a detailed mechanistic explanation for how lipid binding can unfurl the strap by direct competition.
Figure 5.
The strap competes for the same binding site at the basic collar as PIP2. (A) Percentage of time that acidic strap residues form H-bonds with the rest of Vt. The inset shows the binding network prepared as described in Fig. 4. The residues that interact the strongest with PIP2 (plus R903) also bind most frequently to the N-terminal, negatively charged strap. (B) Representative snapshot of acidic strap residues engaged in the binding to multiple PIP2 binding residues. To see this figure in color, go online.
The acidic residues of the Strap (pink C-atoms) form changing salt bridges and hydrogen bonds with the Basic Collar PIP2 binding site (grey C-atoms, Van der Waals representation) and other basic residues (R903 and R924) on H1 and H2 (grey C-atoms, stick representation) (see also Fig. 5).
Discussion
Although the force-dependent mechanisms driving the maturation of focal adhesions have been broadly studied (55), relatively little is known about the processes guiding their assembly before the onset of force transmission. Past and recent studies suggest a central role for PIP2-enriched membranes in the recruitment and activation of proteins in nascent adhesions (56,57). However, because protein-lipid interactions are notoriously difficult to capture experimentally, the molecular details of these events often remain unclear. In most cases, the lipid binding sites are only indirectly mapped through mutational studies. For a handful of examples, including Vt, it was possible to co-crystalize PIP2 with the protein (20,58,59). Although these studies are invaluable starting points toward understanding the molecular basis of PIP2 binding, the lack of high-quality structural models of membrane-bound Vt is hampering a mechanistic understanding of the vinculin-lipid interaction. Earlier computational approaches (24) relied on the docking of a single PIP2 headgroup to Vt. Because the orientational distribution of the protein on the membrane is dictated by the sum of specific and unspecific protein-lipid interactions, docking or binding of isolated lipids is not sufficient to generate reliable models. Here, we employed a multiscale MD simulation scheme in which every step was performed in the presence of a membrane. This allowed us to generate a model that is in excellent agreement with biochemical data (Table S1) and provides new insight into the functional impact of membrane binding (Video S5, coordinates available in the Supporting material). To facilitate the detailed analysis and comparison of the protein-lipid interactions we have developed a valency-disaggregated binding network analysis that captures both the interaction frequency and size of a set of protein residues that bind simultaneously to the same PIP2 molecule.
In this simulation frame Vt is simultaneously interacting with multiple PIP2 molecules at both the Basic Collar and the Basic Ladder. In the first rotation only the PIP2 molecules binding tightly to these regions and the Vt residues that interact to them are shown. In the second rotation Vt is shown in a surface representation. In the third rotation the remaining membrane is displayed as well (black: PIP2, grey: POPC).
Our approach showed that the main contact area of Vt with the membrane overlaps significantly with the binding sites of several Vt interaction partners (Fig. 3 C). Depending on the relative strength of the interactions, membrane binding can thus either reduce or, vice versa, it can be reduced by protein-protein interactions. Our findings for Vt provide a complete structural picture for the inverse relationship between vinculin’s autoinhibition and membrane binding. We have identified key residues in the basic collar and in the basic ladder that either bind to acidic residues of the strap or of vinculin head in the autoinhibited conformation or to PIP2 when Vt is bound to the membrane (Figs. 4, 5, and 6). Importantly, these basic ladder residues contribute significantly to the strength of the head-tail interaction (53,54). An overlay of the structure of autoinhibited vinculin onto our membrane-bound model reveals that the PIP2 binding residues fall into two classes, in which simultaneous binding to the membrane and to the vinculin head is sterically prohibited or permissible, respectively (Video S3). The first class comprises K944, R945, and R1008 (i.e., the part of the basic ladder that forms contacts with the first domain of vinculin head (Vd1)). It also includes the neighboring, solvent exposed parts of the basic ladder, especially K952, K956, or R963. In the closed conformation, Vd1 protrudes beyond the membrane binding surface of Vt and thereby sterically prevents its interaction with the bilayer (Fig. 6 A). For the second class, in which the head-tail interactions do not lead to clashes with the membrane, we propose that these interactions can be broken through competition with PIP2, whereas Vt is already associated with the membrane. This includes the interaction between the basic collar and the strap, as well as between K975, R978, R1049, and the fourth vinculin head domain (Fig. 6 C).
Figure 6.
Molecular model of the coactivation of vinculin by PIP2 (A–D) and possible effects of PIP2 binding on the interaction with other binding partners (E–G). (A) PIP2 binding can overcome the autoinhibition of talin but not vinculin. Talin activation exposes a small fraction of vinculin binding sites (VBS) in a force-independent way (57). The colored circles on Vt depict residues that interact with PIP2 in our simulations but form intramolecular salt bridges in autoinhibited vinculin (blue, basic collar; green, K975, R978, and R1049; turquoise, K944, R945, and R1008). The magenta-colored patch indicates what is solvent exposed but sterically prevented from membrane binding in the closed conformation (K952, K956, and R963). (B) VBS binding to vinculin disrupts the autoinhibitory contacts between vinculin head and Vt that enables lipid binding (green arrows). In the absence of a second ligand, vinculin would quickly revert to its autoinhibited state (orange arrows). (C) Once Vt is associated with the membrane, additional PIP2 molecules can attack intramolecular interfaces and disrupt these interactions (green arrows). (D) Full membrane binding prevents rebinding of Vt to vinculin head and thus increases the pool of open vinculin in complex with talin. (E) F-actin binding leads to structural changes that destroy the PIP2 binding site (21,52). Simultaneous binding to both ligands thus seems not permissible. However, it is not clear which interaction prevails under physiological conditions. (F) PIP2 binding releases the strap, which exposes the adjacent polyproline Arp2/3 binding site and increases the affinity between the two proteins. Arp2/3 binding is much stronger when PIP2 binding nucleation factors like WASP contribute a VCA domain to the complex (60). (G) We suggest a symmetric dimer conformation mediated by W1064 alternative to the asymmetric dimer of the crystal structure (20) because the crystal structure is not compatible with our model. To see this figure in color, go online.
Only when the strong intramolecular interactions between its head and its tail domain (Vt) are disrupted, is Vt able to fully engage with its multiple binding partners, such as PIP2. The overlapping interfaces mediating different intra- and intermolecular interactions have made it difficult to isolate the functional impact of membrane binding through mutation studies. Therefore, reliable high-resolution structures of membrane-bound Vt are crucial to fully understand the effects of PIP2 binding. Existing structural models, created through crystallization of soluble lipid derivatives or computational docking, are not able to capture the effects of binding to a full membrane containing multiple, mobile PIP2 molecules. The combinatorial activation model of vinculin states that vinculin activation requires the binding of at least two ligands with intermediate affinity to stably disrupt the subnanomolar interaction between vinculin head and tail (60,61). One of these ligands is a vinculin binding site (VBS), present in adhesion proteins like talin, whereas the second ligand can be either PIP2 or F-actin. In accordance with this model, we propose the following structural mechanism for the coactivation of vinculin by PIP2: in the fully closed conformation, vinculin has no affinity for PIP2 because all binding sites are blocked by intramolecular interactions. Especially the protruding end of Vd1 sterically prevents membrane association (Fig. 6 A). Upon VBS binding, the Vt-Vd1 interface is disrupted, Vd1 gets displaced, and K944, R945, and R1008, as well as adjacent parts of the basic ladder become accessible. In the absence of a second ligand, Vt quickly rebinds to vinculin head, displaces the VBS, and restores the autoinhibited closed state (Fig. 6 B). In the presence of PIP2, however, vinculin is recruited to the membrane through interactions with the basic ladder and thus stabilizes the active conformation. We argue that the central part of the basic ladder is a mostly unspecific, electrostatic membrane anchor, but more specific PIP2 binding to K944, R945, and R1008 could act like a wedge to prevent rebinding of Vd1 (Fig. 6 C; Video S3). Once this initial connection with the membrane is established, PIP2 can compete with the strap and fourth vinculin head domain for its additional binding sites and fully disrupt the head-tail interaction (Fig. 6, C and D).
Significantly, PIP2 binding stabilizes the active conformation of vinculin by engaging Vt residues that normally support the head-tail interaction in the autoinhibited conformation and thereby prevents fast reversion to the autoinhibited state, resulting in a shift of the equilibrium away from the closed toward the open state (Fig. 6, C and D). This interpretation is in line with a recent report by Kelley et al., which suggests that PIP2 co-regulates the joint activation of talin and vinculin (57). The authors showed that PIP2 partially unlocks the autoinhibited conformation of talin but not of vinculin. PIP2-activated talin can in turn coactivate vinculin, most likely through VBS binding (Fig. 6, B and C). Full-length vinculin is only activated if talin is anchored to the membrane, but not in the presence of soluble, constitutively active talin fragments. These findings are consistent with the stabilizing role of membrane binding. Removal of Vt consequently bypasses the need for additional stabilization of the complex; indeed it was found that vinculin head alone stably binds the same talin fragment, even in the absence of a membrane (62). Regarding the physiological significance, these findings tie nicely into a recent experimental study by Han et al. Their findings suggest that nascent adhesions that sequentially recruit first talin, followed by vinculin, quickly disassemble at the onset of force transmission. In contrast, early adhesions formed by talin-vinculin precomplexes have a higher rate of maturation (63). We thus suggest that membrane binding could stabilize and therefore increase the number of precomplexes in very early adhesions, in which no F-actin is present to keep vinculin in the open state (Fig. 6 D). In turn, the number of precomplexes would also be increased when the head-tail interaction is weakened by mutations. This indicates that lipid binding mutants with reduced head-tail affinity are not suitable to investigate the effects of PIP2 binding because these mutations themselves compensate, at least partially, for the loss of affinity toward PIP2 (57).
Our structural model of membrane-bound Vt further suggests a new allosteric connection between PIP2 and F-actin binding; whereas membrane binding does not impede the accessibility of the F-actin binding site (Fig. 3), cryo-EM structures have revealed that Vt undergoes large structural rearrangements upon interaction with F-actin (21,52). These conformational changes displace helix H1, the loop connecting H1 and H2, and the C-terminus. With the exception of K924, all PIP2 binding residues of the basic collar are affected by this reorganization. Because the PIP2 binding residues R1049 and R1008 can also interact with F-actin, filament binding appears to not only destroy the binding site at the basic collar but also to reduce Vt’s affinity for PIP2 overall. Dimerization seems to also be affected because W1064 is displaced as well, and its binding pocket disintegrates upon the actin-induced unfurling of Vt. On the other hand, lipid binding and dimerization might stabilize the five-helix bundle and thereby prevent association with F-actin (Fig. 6 E). Which interaction prevails depends on the relative affinity for either ligand and their respective local concentration. It is thus probable that, at early time points when F-actin content is low, most of the Vt will be bound to the membrane. As the adhesion matures and the actin concentration increases, Vt will shift to the F-actin-bound form. Indeed, super-resolution microscopy studies have shown that the membrane-proximate fraction of vinculin is markedly enhanced in nascent versus mature adhesions (64). Another interesting aspect is that not only the concentration of the ligands but also their relative affinity for Vt could be regulated dynamically. Because Vt forms a catch bond with F-actin (65), mechanical tension arising at the onset of force transmission across talin could also favor filament over membrane binding.
There is a second, more indirect way how Vt-PIP2 interactions can influence F-actin dynamics. The strap is directly preceded by a polyproline motif that mediates interaction with the Arp2/3 complex (60). It was proposed that PIP2 triggers the release of the strap, which leads to better accessibility to the Arp2/3 binding site (17), which is in accordance with our model. Interestingly, the vinculin-Arp2/3 hybrid complex seems to be responsible for the nucleation of new filaments rather than for the branching of existing fibers (66). The induction of new filaments in the immediate vicinity of Vt strongly increases the likelihood for a handover of Vt from the membrane to F-actin. Because Arp2/3 activity also depends on the presence of membrane-associated nucleation-promoting factors, such as WASP or (60), the vinculin-Arp2/3 interaction will be disrupted when Vt is released from the bilayer. Such a negative feedback loop could explain the transient occurrence of the vinculin-Arp2/3 hybrid complex.
Although it has to be noted that the existence of a membrane-bound Vt dimer has not yet been demonstrated, a PIP2-induced release of the strap would be a structural prerequisite for dimerization (20). Contrary to the Thompson model, we could show that membrane binding orients Vt favorable for W1064-mediated dimerization (Fig. 3), suggesting that dimerization is sterically possible. However, an overlay of the dimer as present in the crystal structure with our model leads to severe clashes with the membrane. Even when assuming a somewhat flexible dimer interface, our simulation results are incompatible with the sharing of the PIP2 headgroup between two Vt monomers. An alternative dimer structure that is compatible with our findings would be symmetric and consist of two Vt molecules, each bound to their own PIP2 molecule with their respective basic collars (Fig. 6 F). A similar conformation has been observed for the PIP2-mediated dimer of vinculin’s larger splice variant, metavinculin (58). The potential physiological role of dimer formation remains unclear, but multimerization is a mechanism often used by lipid binding proteins to increase their membrane binding affinity (6).
Membrane binding is a fundamental step in the genesis of early adhesion sites because it effectively increases the local concentration of and thus the probability for productive encounters between proteins of the adhesion signaling hub (6,67). The vinculin-talin binding represents only a small segment of a large interaction network, which also contains other PIP2 binding proteins, such as RIAM and focal adhesion kinase (68,69). One or more membrane-bound proteins can then bind to cytosolic proteins and drive further assembly steps, as exemplified for the Arp2/3 complex (Fig. 6 F). This strategy allows cells to quickly recruit and activate a multitude of adhesion proteins at highly specific locations. High-resolution structural models, such as ours, are crucial for gaining a detailed understanding of this process.
Because we started from a completely unbiased screening, our multiscale approach is a promising tool to not only refine known PIP2 binding sites, but even to determine them de novo for proteins that elude crystallization in the presence of lipids. This study serves as a proof-of-concept for the power of computational methods in this field, illustrated by the convergence of our independent simulations (Fig. 4; Fig. S3) and the excellent agreement with biochemical data (Table S1) of our model. Although biochemical and structural studies are still essential to guide and verify computational approaches, simulations can capture effects that are otherwise inaccessible, such as the orientation of the protein on the membrane, transient interactions, or subtle conformational changes. The broad application of such multiscale simulations can thus substantially increase our knowledge of the peripheral membrane protein in the adhesome and beyond. With rapidly growing computing capacities and lower costs, this approach could become a high-throughput method in the future to efficiently generate atomistic structures of membrane-bound proteins.
Our valency-disaggregated binding network analysis was developed to overcome common challenges in the quantification and visualization of protein-lipid simulations, but it is also directly applicable for the analysis of other protein-ligand interactions. It could thus become an indispensable tool to facilitate the analysis of large data sets by an automation of binding site detection. The method is especially convenient for the analysis of systems containing multiple and/or mobile ligands and, hence, might facilitate the detection of ion binding sites or the determination of key residues in transport events through transmembrane channels. In addition to analyzing time-aggregated data, the network representation can also be used to intuitively visualize the fluctuating interactions during binding processes, as shown in Videos S1 and S2. Although we have developed this network representation independently, we want to acknowledge a related method that has been recently proposed by Barbera et al. (70). Their approach is mathematically very elegant and provides a more rigorous definition of binding sites, whereas our approach provides direct information on the number of residues simultaneously involved in an interaction and thus makes it easier to judge the specificity of a binding event. We suggest that a combination of both approaches with their distinct merits could enable an even more rigorous assessment of protein-ligand interactions from atomistic simulations.
Author contributions
L.B. designed the study with input from V.V. L.B. conceived and designed the analysis. L.B. collected the data. L.B. performed the analysis with input from I.S. L.B. wrote the first draft. All authors edited and commented on the study.
Acknowledgments
The authors thank Alexander Dunn and Nick Bax for fruitful discussions, Nick Bax for his attempt to produce a serine pincer mutant, and Paulina Pacak for her support and input.
We gratefully acknowledge funding from ETH Zurich (V.V.), the Royal College of Surgeons in Ireland (I.S.), and resource grants from the Swiss National Supercomputing Centre under project identifier s791.
Editor: Lucie Delemotte.
Footnotes
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2021.08.018.
Supporting material
The notebook Network_analysis.ipynb contains the code for the network analysis. The ∗hbond.txt files contain example data from our simulations that will you allow to run the code. To visualize our data you can open the file network.cys in cytoscape. It contains the combined binding network for all simulations, as well as the networks for each individual simulation. You can play around with the select option to change the thresholds and other visualization options. Alternatively you can load the files generated by Network_analysis.ipynb into cytoscape. First click “Import network from filesystem” and select the.sif file. Then click “Import table from file” and load the node file. Click “Import table from file” again and select the edge file, make sure you change the “import as” option to Edge Table Columns.
References
- 1.Zamir E., Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J. Cell Sci. 2001;114:3583–3590. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- 2.Kechagia J.Z., Ivaska J., Roca-Cusachs P. Integrins as biomechanical sensors of the microenvironment. Nat. Rev. Mol. Cell Biol. 2019;20:457–473. doi: 10.1038/s41580-019-0134-2. [DOI] [PubMed] [Google Scholar]
- 3.Zaidel-Bar R., Ballestrem C., Geiger B. Early molecular events in the assembly of matrix adhesions at the leading edge of migrating cells. J. Cell Sci. 2003;116:4605–4613. doi: 10.1242/jcs.00792. [DOI] [PubMed] [Google Scholar]
- 4.Zaidel-Bar R., Cohen M., Geiger B. Hierarchical assembly of cell-matrix adhesion complexes. Biochem. Soc. Trans. 2004;32:416–420. doi: 10.1042/BST0320416. [DOI] [PubMed] [Google Scholar]
- 5.Legerstee K., Geverts B., Houtsmuller A.B. Dynamics and distribution of paxillin, vinculin, zyxin and VASP depend on focal adhesion location and orientation. Sci. Rep. 2019;9:10460. doi: 10.1038/s41598-019-46905-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McLaughlin S., Wang J., Murray D. PIP(2) and proteins: interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 2002;31:151–175. doi: 10.1146/annurev.biophys.31.082901.134259. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin S., Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- 8.van den Bout I., Divecha N. PIP5K-driven PtdIns(4,5)P2 synthesis: regulation and cellular functions. J. Cell Sci. 2009;122:3837–3850. doi: 10.1242/jcs.056127. [DOI] [PubMed] [Google Scholar]
- 9.Picas L., Gaits-Iacovoni F., Goud B. The emerging role of phosphoinositide clustering in intracellular trafficking and signal transduction. F1000 Res. 2016;5:422. doi: 10.12688/f1000research.7537.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leslie N.R., Batty I.H., Downes C.P. Understanding PTEN regulation: PIP2, polarity and protein stability. Oncogene. 2008;27:5464–5476. doi: 10.1038/onc.2008.243. [DOI] [PubMed] [Google Scholar]
- 11.Zhou J., Aponte-Santamaría C., Gräter F. Mechanism of focal adhesion kinase mechanosensing. PLoS Comput. Biol. 2015;11:e1004593. doi: 10.1371/journal.pcbi.1004593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herzog F.A., Braun L., Vogel V. Structural insights how PIP2 imposes preferred binding orientations of FAK at lipid membranes. J. Phys. Chem. B. 2017;121:3523–3535. doi: 10.1021/acs.jpcb.6b09349. [DOI] [PubMed] [Google Scholar]
- 13.Yamamoto E., Akimoto T., Sansom M.S.P. Dynamic interactions between a membrane binding protein and lipids induce fluctuating diffusivity. Sci. Adv. 2017;3:e1601871. doi: 10.1126/sciadv.1601871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carisey A., Ballestrem C. Vinculin, an adapter protein in control of cell adhesion signalling. Eur. J. Cell Biol. 2011;90:157–163. doi: 10.1016/j.ejcb.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukami K., Endo T., Takenawa T. alpha-Actinin and vinculin are PIP2-binding proteins involved in signaling by tyrosine kinase. J. Biol. Chem. 1994;269:1518–1522. [PubMed] [Google Scholar]
- 16.Palmer S.M., Playford M.P., Campbell S.L. Lipid binding to the tail domain of vinculin: specificity and the role of the N and C termini. J. Biol. Chem. 2009;284:7223–7231. doi: 10.1074/jbc.M807842200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bakolitsa C., Cohen D.M., Liddington R.C. Structural basis for vinculin activation at sites of cell adhesion. Nature. 2004;430:583–586. doi: 10.1038/nature02610. [DOI] [PubMed] [Google Scholar]
- 18.Borgon R.A., Vonrhein C., Izard T. Crystal structure of human vinculin. Structure. 2004;12:1189–1197. doi: 10.1016/j.str.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Johnson R.P., Craig S.W. F-actin binding site masked by the intramolecular association of vinculin head and tail domains. Nature. 1995;373:261–264. doi: 10.1038/373261a0. [DOI] [PubMed] [Google Scholar]
- 20.Chinthalapudi K., Rangarajan E.S., Izard T. Lipid binding promotes oligomerization and focal adhesion activity of vinculin. J. Cell Biol. 2014;207:643–656. doi: 10.1083/jcb.201404128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim L.Y., Thompson P.M., Alushin G.M. The structural basis of actin organization by vinculin and metavinculin. J. Mol. Biol. 2016;428:10–25. doi: 10.1016/j.jmb.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geiger B. A 130K protein from chicken gizzard: its localization at the termini of microfilament bundles in cultured chicken cells. Cell. 1979;18:193–205. doi: 10.1016/0092-8674(79)90368-4. [DOI] [PubMed] [Google Scholar]
- 23.Chandrasekar I., Stradal T.E.B., Ziegler W.H. Vinculin acts as a sensor in lipid regulation of adhesion-site turnover. J. Cell Sci. 2005;118:1461–1472. doi: 10.1242/jcs.01734. [DOI] [PubMed] [Google Scholar]
- 24.Thompson P.M., Ramachandran S., Campbell S.L. A structural model for vinculin insertion into PIP2-containing membranes and the effect of insertion on vinculin activation and localization. Structure. 2017;25:264–275. doi: 10.1016/j.str.2016.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohkubo Y.Z., Pogorelov T.V., Tajkhorshid E. Accelerating membrane insertion of peripheral proteins with a novel membrane mimetic model. Biophys. J. 2012;102:2130–2139. doi: 10.1016/j.bpj.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bakolitsa C., de Pereda J.M., Liddington R.C. Crystal structure of the vinculin tail suggests a pathway for activation. Cell. 1999;99:603–613. doi: 10.1016/s0092-8674(00)81549-4. [DOI] [PubMed] [Google Scholar]
- 27.Herzog F.A., Braun L., Vogel V. Improved side chain dynamics in MARTINI simulations of protein-lipid interfaces. J. Chem. Theory Comput. 2016;12:2446–2458. doi: 10.1021/acs.jctc.6b00122. [DOI] [PubMed] [Google Scholar]
- 28.Vermaas J.V., Pogorelov T.V., Tajkhorshid E. Extension of the highly mobile membrane mimetic to transmembrane systems through customized in silico solvents. J. Phys. Chem. B. 2017;121:3764–3776. doi: 10.1021/acs.jpcb.6b11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamamoto E., Kalli A.C., Sansom M.S.P. Anomalous dynamics of a lipid recognition protein on a membrane surface. Sci. Rep. 2015;5:18245. doi: 10.1038/srep18245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Buyan A., Kalli A.C., Sansom M.S.P. Multiscale simulations suggest a mechanism for the association of the Dok7 PH domain with PIP-containing membranes. PLoS Comput. Biol. 2016;12:e1005028. doi: 10.1371/journal.pcbi.1005028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amos S.-B.T.A., Kalli A.C., Sansom M.S.P. Membrane recognition and binding by the phosphatidylinositol phosphate kinase PIP5K1A: a multiscale simulation study. Structure. 2019;27:1336–1346.e2. doi: 10.1016/j.str.2019.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Abraham M.J., Murtola T., Lindahl E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. [Google Scholar]
- 33.Monticelli L., Kandasamy S.K., Marrink S.-J. The MARTINI coarse-grained force field: extension to proteins. J. Chem. Theory Comput. 2008;4:819–834. doi: 10.1021/ct700324x. [DOI] [PubMed] [Google Scholar]
- 34.de Jong D.H., Singh G., Marrink S.J. Improved parameters for the martini coarse-grained protein force field. J. Chem. Theory Comput. 2013;9:687–697. doi: 10.1021/ct300646g. [DOI] [PubMed] [Google Scholar]
- 35.Wassenaar T.A., Ingólfsson H.I., Marrink S.J. Computational lipidomics with insane: a versatile tool for generating custom membranes for molecular simulations. J. Chem. Theory Comput. 2015;11:2144–2155. doi: 10.1021/acs.jctc.5b00209. [DOI] [PubMed] [Google Scholar]
- 36.Webb B., Sali A. Comparative protein structure modeling using MODELLER. Curr. Protoc. in Bioinformatics. 2014;47 doi: 10.1002/0471250953.bi0506s47. 5.6.1–5.6.32. [DOI] [PubMed] [Google Scholar]
- 37.Periole X., Cavalli M., Ceruso M.A. Combining an elastic network with a coarse-grained molecular force field: structure, dynamics, and intermolecular recognition. J. Chem. Theory Comput. 2009;5:2531–2543. doi: 10.1021/ct9002114. [DOI] [PubMed] [Google Scholar]
- 38.Bussi G., Donadio D., Parrinello M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007;126:014101. doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- 39.Berendsen H.J.C., Postma J.P.M., Haak J.R. Molecular dynamics with coupling to an external bath. J. Chem. Phys. 1984;81:3684–3690. [Google Scholar]
- 40.Parrinello M., Rahman A. Polymorphic transitions in single crystals: a new molecular dynamics method. J. Appl. Phys. 1981;52:7182–7190. [Google Scholar]
- 41.Phillips J.C., Braun R., Schulten K. Scalable molecular dynamics with NAMD. J. Comput. Chem. 2005;26:1781–1802. doi: 10.1002/jcc.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Best R.B., Zhu X., Mackerell A.D., Jr. Optimization of the additive CHARMM all-atom protein force field targeting improved sampling of the backbone φ, ψ and side-chain χ(1) and χ(2) dihedral angles. J. Chem. Theory Comput. 2012;8:3257–3273. doi: 10.1021/ct300400x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Feller S.E., Zhang Y., Brooks B.R. Constant pressure molecular dynamics simulation: the Langevin piston method. J. Chem. Phys. 1995;103:4613–4621. [Google Scholar]
- 44.Martyna G.J., Tobias D.J., Klein M.L. Constant pressure molecular dynamics algorithms. J. Chem. Phys. 1998;101:4177–4189. [Google Scholar]
- 45.Darden T., York D., Pedersen L. Particle mesh Ewald: an N log(N) method for Ewald sums in large systems. J. Chem. Phys. 1993;98:10089–10092. [Google Scholar]
- 46.Ribeiro J.V., Bernardi R.C., Schulten K. QwikMD - Integrative molecular dynamics toolkit for novices and experts. Sci. Rep. 2016;6:26536. doi: 10.1038/srep26536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38, 27–28.. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 48.Wassenaar T.A., Pluhackova K., Tieleman D.P. Going backward: a flexible geometric approach to reverse transformation from coarse grained to atomistic models. J. Chem. Theory Comput. 2014;10:676–690. doi: 10.1021/ct400617g. [DOI] [PubMed] [Google Scholar]
- 49.Scheurer M., Rodenkirch P., Rudack T. PyContact: rapid, customizable, and visual analysis of noncovalent interactions in MD simulations. Biophys. J. 2018;114:577–583. doi: 10.1016/j.bpj.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shannon P., Markiel A., Ideker T. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee J.H., Rangarajan E.S., Izard T. Raver1 interactions with vinculin and RNA suggest a feed-forward pathway in directing mRNA to focal adhesions. Structure. 2009;17:833–842. doi: 10.1016/j.str.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mei L., Espinosa de Los Reyes S., Alushin G.M. Molecular mechanism for direct actin force-sensing by α-catenin. eLife. 2020;9:e62514. doi: 10.7554/eLife.62514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen D.M., Chen H., Craig S.W. Two distinct head-tail interfaces cooperate to suppress activation of vinculin by talin. J. Biol. Chem. 2005;280:17109–17117. doi: 10.1074/jbc.M414704200. [DOI] [PubMed] [Google Scholar]
- 54.Chorev D.S., Volberg T., Geiger B. Conformational states during vinculin unlocking differentially regulate focal adhesion properties. Sci. Rep. 2018;8:2693. doi: 10.1038/s41598-018-21006-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y., Yan J., Goult B.T. Force-dependent binding constants. Biochemistry. 2019;58:4696–4709. doi: 10.1021/acs.biochem.9b00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Raucher D., Stauffer T., Meyer T. Phosphatidylinositol 4,5-bisphosphate functions as a second messenger that regulates cytoskeleton-plasma membrane adhesion. Cell. 2000;100:221–228. doi: 10.1016/s0092-8674(00)81560-3. [DOI] [PubMed] [Google Scholar]
- 57.Kelley C.F., Litschel T., Mizuno N. Phosphoinositides regulate force-independent interactions between talin, vinculin, and actin. eLife. 2020;9:1597. doi: 10.7554/eLife.56110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chinthalapudi K., Rangarajan E.S., Izard T. Differential lipid binding of vinculin isoforms promotes quasi-equivalent dimerization. Proc. Natl. Acad. Sci. USA. 2016;113:9539–9544. doi: 10.1073/pnas.1600702113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chinthalapudi K., Rangarajan E.S., Izard T. The interaction of talin with the cell membrane is essential for integrin activation and focal adhesion formation. Proc. Natl. Acad. Sci. USA. 2018;115:10339–10344. doi: 10.1073/pnas.1806275115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeMali K.A., Barlow C.A., Burridge K. Recruitment of the Arp2/3 complex to vinculin: coupling membrane protrusion to matrix adhesion. J. Cell Biol. 2002;159:881–891. doi: 10.1083/jcb.200206043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen H., Choudhury D.M., Craig S.W. Coincidence of actin filaments and talin is required to activate vinculin. J. Biol. Chem. 2006;281:40389–40398. doi: 10.1074/jbc.M607324200. [DOI] [PubMed] [Google Scholar]
- 62.Dedden D., Schumacher S., Mizuno N. The architecture of Talin1 reveals an autoinhibition mechanism. Cell. 2019;179:120–131.e13. doi: 10.1016/j.cell.2019.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Han S.J., Azarova E.V., Danuser G. Pre-complexation of talin and vinculin without tension is required for efficient nascent adhesion maturation. eLife. 2021;10:e66151. doi: 10.7554/eLife.66151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Case L.B., Baird M.A., Waterman C.M. Molecular mechanism of vinculin activation and nanoscale spatial organization in focal adhesions. Nat. Cell Biol. 2015;17:880–892. doi: 10.1038/ncb3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang D.L., Bax N.A., Dunn A.R. Vinculin forms a directionally asymmetric catch bond with F-actin. Science. 2017;357:703–706. doi: 10.1126/science.aan2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chorev D.S., Moscovitz O., Sharon M. Regulation of focal adhesion formation by a vinculin-Arp2/3 hybrid complex. Nat. Commun. 2014;5:3758. doi: 10.1038/ncomms4758. [DOI] [PubMed] [Google Scholar]
- 67.Bromberger T., Zhu L., Moser M. Rap1 and membrane lipids cooperatively recruit talin to trigger integrin activation. J. Cell Sci. 2019;132:jcs235531. doi: 10.1242/jcs.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wynne J.P., Wu J., Philips M.R. Rap1-interacting adapter molecule (RIAM) associates with the plasma membrane via a proximity detector. J. Cell Biol. 2012;199:317–330. doi: 10.1083/jcb.201201157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Acebrón I., Righetto R.D., Lietha D. Structural basis of Focal Adhesion Kinase activation on lipid membranes. EMBO J. 2020;39:e104743. doi: 10.15252/embj.2020104743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Barbera N., Ayee M.A.A., Levitan I. Molecular dynamics simulations of Kir2.2 interactions with an ensemble of cholesterol molecules. Biophys. J. 2018;115:1264–1280. doi: 10.1016/j.bpj.2018.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Left: Binding trajectory of a single PIP2molecule (purple C-atoms) to the Basic Collar. Only the Vt residues involved in binding of this lipid molecule are shown explicitly and the rest of the membrane is intermittently omitted for better visibility. Right: Time resolved network representation of the binding process. Each node corresponds to one of the Vt residues involved in binding of the ligand molecule. When the node is shaded in grey, the corresponding protein residue is interacting with the PIP2molecule. Lines between nodes indicate simultaneous binding and their color encodes the valency of the interaction (same color code as in Supplementary Figure 8). Initially the PIP2 molecule forms only few instable, low valency interactions. As the simulation progresses, the ligand moves into the binding pocket formed by R910, S913, S914, K915, K924 and K1061 and the number of simultaneous interactions increases. The binding valency still varies because of thermal fluctuations, but the high number of engaged residues ensures that the ligand remains bound stably. To generate the aggregated binding networks shown in Fig. 4 B and Supplementary Fig. 4 the network for each timestep (as shown on the right) is recorded for each PIP2 molecule. The occurrence of each unique set of binding residues is counted and normalized by the number of simulation frames.
Top: Binding trajectory of a two PIP2 molecules (purple and yellow C-atoms) competing for the Basic Collar. Only the Vt residues involved in binding of this lipid molecule are shown explicitly and the rest of the membrane is intermittently omitted for better visibility. Bottom: Time resolved network representations of the binding process. The color of the frame corresponds to the color of the respective PIP2 molecule shown above. Each node corresponds to one of the Vt residues involved in binding of the ligand molecule. When the node is shaded in grey, the corresponding protein residue is interacting with the PIP2molecule. Lines between nodes indicate simultaneous binding and their color encodes the valency of the interaction (same color code as in Supplementary Figure 9). The prebound PIP2 (yellow) forms initially higher valency interactions but gets partially displaced by another ligand (purple) over time. In the end the two PIP2 molecules share the Basic Collar each donating one phosphate group (see also Supplementary Fig 6 B). To generate the aggregated binding networks shown in Fig. 4 B and Supplementary Fig. 4 the network for each timestep is recorded for each PIP2molecule. The occurrence of each unique set of binding residues is counted and normalized by the number of simulation frames.
By overlaying the structure of full-length Vinculin onto our membrane bound model, we have identified three regions where PIP2 binding sites overlap with intramolecular contacts, namely at the first and fourth Vinculin Head domains (Vd1 and Vd4) and the Strap. Notably, in the overlay only Vd1 produces severe steric clashes with the membrane, while dissociation of Vd4 and the Strap seems to be no prerequisite to membrane binding (see also Fig. 6 A-C). At all three locations the basic residues responsible for PIP2 binding, bind to acidic residues in full-length Vinculin.
The acidic residues of the Strap (pink C-atoms) form changing salt bridges and hydrogen bonds with the Basic Collar PIP2 binding site (grey C-atoms, Van der Waals representation) and other basic residues (R903 and R924) on H1 and H2 (grey C-atoms, stick representation) (see also Fig. 5).
In this simulation frame Vt is simultaneously interacting with multiple PIP2 molecules at both the Basic Collar and the Basic Ladder. In the first rotation only the PIP2 molecules binding tightly to these regions and the Vt residues that interact to them are shown. In the second rotation Vt is shown in a surface representation. In the third rotation the remaining membrane is displayed as well (black: PIP2, grey: POPC).
The notebook Network_analysis.ipynb contains the code for the network analysis. The ∗hbond.txt files contain example data from our simulations that will you allow to run the code. To visualize our data you can open the file network.cys in cytoscape. It contains the combined binding network for all simulations, as well as the networks for each individual simulation. You can play around with the select option to change the thresholds and other visualization options. Alternatively you can load the files generated by Network_analysis.ipynb into cytoscape. First click “Import network from filesystem” and select the.sif file. Then click “Import table from file” and load the node file. Click “Import table from file” again and select the edge file, make sure you change the “import as” option to Edge Table Columns.