Abstract
We review the current data on epidemiology, the clinical significance, the pathophysiologic mechanisms, and the treatment of VAs in the setting of COVID-19. VAs prevail in 0.15% to 8% of hospitalized patients, but only sustained and rapid tachyarrhythmias are purportedly associated with a significant increase in mortality. Multiple factors can elicit VAs, which are ultimately deemed to be a marker of severe systemic disease rather than a distinct cardiac condition. Even though the electrophysiologist plays a determinant role in the secondary prevention of VAs, a multidisciplinary approach is indispensable for primary prophylaxis and acute management.
Keywords: Arrhythmias, COVID-19, Complications, Management, Outcomes, SARS-CoV-2, Ventricular tachycardia
Key points
-
•
Ventricular arrhythmias (VAs) affect a modest proportion of patients with SARS-CoV-2, sometimes representing the only initial symptom.
-
•
The cause of VAs in the setting of acute COVID-19 infection is multifactorial because of direct and indirect myocardial involvement.
-
•
Admission to ICU, use of pressors, pre-existing cardiac disease, but neither QT interval nor left ventricular ejection fraction are consistently associated with such complication.
-
•
Sustained VAs correlate with increased mortality, albeit most of the cardiac arrests originate from not-shockable rhythms.
-
•
Treatment options include correction of metabolic disorder, discontinuation of QT-prolonging agents, antiarrhythmics (especially amiodarone), and ablation in case of a ventricular storm.
Introduction
At the time of writing the present article, SARS-CoV-2, aka COVID-19, has reportedly hit almost 180 million people globally in less than 18 months since its official identification and disclosure as pandemic.1 Initially announced as a severe acute respiratory syndrome due to a novel strain of coronavirus, after the acronym SARS-CoV-2, the first pandemic of the third millennium was unexpectedly far from being an isolated respiratory condition. Instead, a multitude of signs and symptoms, sometimes representing the only atypical manifestation of the disease, have been described with alacrity, making the COVID-19 indeed configuring as a systemic disease requiring a multidisciplinary care.
Pertaining to the cardiovascular complications or manifestations of the SARS-CoV-2, in the present article, we offer an overview of the ventricular arrhythmias related to acute COVID-19 infections, discussing the prevalence, the possible mechanisms due to direct or indirect virus involvement, and the currently proposed therapeutic options.
Epidemiology and clinical outcomes
Prevalence and Nature of VAs
Mitacchione and colleagues described the first case of ventricular storm (12 episodes of sustained ventricular tachycardias [VTs]) with concomitant COVID-19 infection in a patient with defibrillator (ICD) and ischemic cardiomyopathy (ejection fraction = 34%) admitted to an Italian hospital for respiratory distress.2 ICD interrogation showed sustained and nonsustained monomorphic VTs since days before the admission. The authors excluded iatrogenic causes because the systemic use of QT-prolonging immunomodulators and antimicrobials was not recommended at that time, and the VT was probably due to other mechanisms (see paragraph 4). This insightful report suggests that VAs could be the first sign of latent infection in susceptible patients with structural heart disease.
In addition, two descriptions of polymorphic ventricular tachycardia (PMVT) in the settings of prolonged and normal QT, respectively, were also reported.3 , 4 Of interest, two separate cases of newly diagnosed Brugada syndrome were reported, one presenting as an asymptomatic coved-type pattern during febrile peak,5 and the other as PMVT due to fever and drug-induced bradycardia.6
The overall prevalence of VAs in COVID-19 patients ranges between 0.15% and 8% (Table 1 ).7 , 8 Such a discrepancy stems from the definition of VAs and subpopulations analyzed. The first series by Guo showed that malignant arrhythmias were relatively more common in patients with cardiac injury—defined as troponin T elevation—(11% vs 5% over a total of 187 individuals).8 A retrospective study of 700 patients differentiated the prevalence between patients admitted to intensive care unit (ICU) compared with nonintensive care settings.7 In total, only one patient had a life-threatening torsade de pointes (TdP) in ICU (1.4% vs none in the comparison group). Similarly, nonsustained episodes were more common in critical patients (8% vs 0.6%). In line with this analysis, a Scandinavian group observed that incidence of VAs in ICU patients was about 3% (n = 2/155),9 yet, according to a larger cohort of 1053 hospitalized patients, nonsustained episodes are far less prevalent than malignant VAs (0.7% vs ≈ 3%).10
Table 1.
Synopsis of the studies reporting the prevalence of VAs in the setting of acute COVID-19 infection
| Author and Date | Sample | Number and Type of VA | QT/QT-Prolonging Agents | Underlying Cardiac Disease | Primary Cause Hypothesized | Management | Outcome |
|---|---|---|---|---|---|---|---|
| Mitacchione et al,2 2020 | 1 | VT storm |
|
ICM |
|
VT ablation with remote navigation control | Discharged |
| Elsad et al,3 2020 | 1 | Bradycardia-induced TdP; VF |
|
None | Multiorgan failure | Lidocaine Magnesium Dopamine Transcutaneous pacing |
Discharged |
| O'Brien et al,4 2020 | 1 | PMVT |
|
None | Multiorgan failure | Discontinuation of amiodarone Lidocaine Metoprolol |
Demise |
| Chang et al.5 2020 | 1 | NS | NS | Brugada |
|
Observation | Discharged |
| Tsimpoulis et al,6 2020 | 1 | PMVT |
|
Brugada |
|
Supportive care | Demise |
| Bathla et al,7 2020 | 700 |
|
|
|
|
NS |
|
| Guo et al,8 2020 | 187 | 11 VT/VF |
|
NS |
|
NS | NS |
| Wetterslev et al,9 2021 | 155 | 2 NS |
|
NS |
|
NS | NS |
| Peltzer et al,10 2020 | 1053 |
|
|
NS |
|
NS | 59% of patients with VA died |
| Turagam et al,11 2020 | 140 |
|
|
NS |
|
NS |
|
| Mesquita D,12 2021 | 692 | 2 VT | Prolonged in both | NS |
|
NS | Demise in bothb |
| Lanza et al,14 2021 | 324 | 13 PVC |
|
NS | NS | NS | 4 demises |
| Perretto et al,15 2021 | 7 |
|
|
CAD |
|
|
Discharged (ICD in pt with VF and in 1 with NSVT). All alive at 6 months |
| D’Ascenzo et al,30 2021 | 779 | 38 VT/VF |
|
CAD | ACS Multifactorial |
NS | NS |
| Saleh M,39 2020 | 201 |
|
|
No |
|
NS |
|
| Gasparetti A,40 2020 | 649 |
|
|
6 ICM |
|
|
3 demises (VF group) |
Abbreviations: ACS, acute coronary syndrome; AZT, azithromycin; CAD, coronary artery disease; HCQ, hydroxychloroquine; ICM, ischemic cardiomyopathy; NS, nonspecified; NSVT, nonsustained VT; PMVT, polymorphic ventricular tachycardia; pts, patients; PVC, premature ventricular contraction; TdP, torsade de pointes; VF, ventricular fibrillation; VT, ventricular tachycardia.
For atrial fibrillation with rapid ventricular response.
Due to respiratory failure.
Lastly, no data are currently available in regard to VAs incidence in COVID-19 patients with implanted cardiac devices treated conservatively at home. In this regard, it is worth carrying out such analysis, as experienced in different settings.11
Clinical Value and Outcomes
Despite the slight numerical divergence, it is worth noting that the occurrence of VAs consistently clustered with complicated hospitalizations, suggesting that ventricular arrhythmias are a marker of severe systemic disease.7 , 10 Indeed, besides anecdotic cases of VA survivors,2, 3, 4 , 6 the mortality at 1 month is substantially higher in patients experiencing arrhythmias of ventricular nature (VT or ventricular fibrillation [VF], 59% vs 16% in controls; P<.001, respectively).10 On the contrary, nonsustained episodes of VAs do not seem to predict mortality within a year.7
Furthermore, another detailed breakdown of the relationship between VAs and clinical outcomes argues against the role of malignant VTs as the primary/initial cause of death. For instance, as reported by two different groups, a nonshockable rhythm was the predominant cause of cardiac arrest/death (90% in both studies).7 , 10 Correspondingly, in a series of 140 patients admitted on telemetry monitor, fatal VAs were noted in only 12% of deceased patients (6/52).12 All the events were represented by VF, 1 patient survived, and 2 autopsy examinations suggested that the initial cause of death was pulmonary rather than cardiac.
The prevalence and clinical significance of premature ventricular contractions (PVCs) in the setting of acute infection were less investigated; although subjects with SARS-CoV-2 infection can initially complain of palpitations (7%),13 dedicated analysis is scarce. An Italian study showed that 4% (n = 13/324) of COVID-19 positive subjects admitted to the emergency department presented ventricular ectopy,14 compared to 13% of 1053 hospitalized patients (n = 137).10 Despite the evidence that PVCs are seen in cases of novel coronavirus-related myocarditis,15 , 16 the positive predictive value for cardiac involvement is poor,17 and likewise the association with mortality is borderline (HR = 2.79; 95% CI, 1.00–7.79; P = .051).14
Mechanisms and predictors of VAs in the setting of acute COVID-19 infection
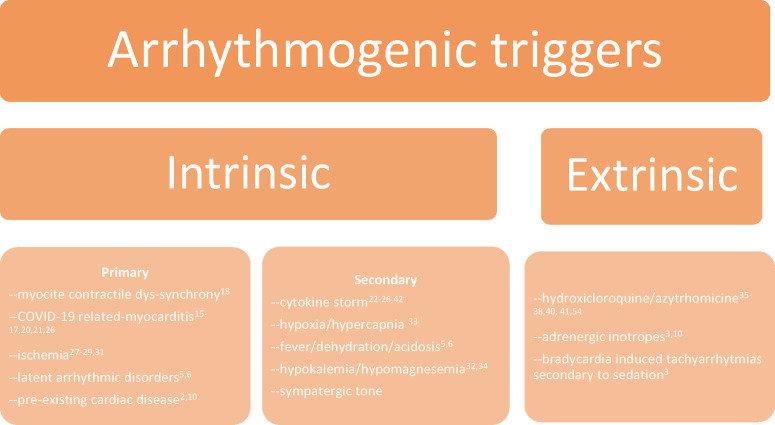
A broad number of articles discuss the possible causes of VAs during acute COVID-19. For the sake of simplification, herein we distinguish between intrinsic and extrinsic causes, depending on the direct or indirect role of the pathogenic agent. The formers can be divided into 2 types: primary intrinsic (directly due to the interaction between the virus and the cardiomyocyte) and secondary intrinsic (after the immune response to the systemic infection; Fig. 1 ). Overall, rarely a single factor can be pointed as the source of VA; instead, plural elements could affect the electrical vulnerability of the ventricles.18
Fig. 1.
Schematic classification of VA causes in COVID-19 patients.
Intrinsic Factors
A brilliant in vitro model showed that infected cardiomyocytes derived from a human pluripotent staminal cell exhibit a significant reduction of the contractile activity measured as beats per minute (4 vs 9 in controls) and a greater extent of contraction dis-synchrony compared with mock SARS-CoV-2 negative cultures.19 Such effect became even more prominent after 48 hours of treatment with interleukin-6. In fact, even though the presence of the virus in cardiac tissue or evidence of myocarditis have been inconsistently detected,20, 21, 22 cytokines surge from the systemic immune response are alone sufficient to dysregulate calcium handling, modulate ion channels expression, increase fibrosis, and exert a negative inotropic effect, ultimately enhancing the susceptibility to VAs.23, 24, 25, 26, 27 For example, late VAs and epicardial fibrosis at the magnetic resonance were documented in one case months after the resolution of the acute illness.28
COVID-19 can provoke cardiac ischemia as well, due to both hypoxia/demanding mechanism,29 but also as it can eminently be thrombogenic and cause coronary microemboli and acute coronary syndromes (ACSs).30 , 31 The relationship with sustained VAs, however, does not differ from negative controls admitted with ACSs (4.9% vs 6.8%, P = ns).32 Beyond the direct involvement of the heart, systemic metabolic derangements such as acidosis, hypercapnia, hypokalemia, dehydration, and catecholamines surge do precipitate VAs.33, 34, 35, 36, 37 Also, fever can incidentally unmask Brugada pattern in predisposed subjects,5 , 6 and obviously, patients with pre-existing cardiomyopathy are more prone to develop VAs in the setting of SARS-CoV-2 infection.2 , 10
Extrinsic Factors and Predictors of VAs
VAs can also be iatrogenic. Based on the evidence that hydroxychloroquine and chloroquine inhibit lysosome turnover, and consequently the antigen presentation initiating the immune response,38 there has been a large empirical use of antimalarial drugs at the beginning of the pandemic with the purpose to damper the adverse events and the hospitalization duration. However, the most concerning side effects of hydroxychloroquine are QT prolongation and TdP, especially if combined with antimicrobial prophylaxis with azithromycin or lopinavir/ritonavir.39 , 40 Indeed, the duration of phase 2 of the action potential is prolonged through hydroxychloroquine inhibition of the hERG channels, slowing the potassium rapid inward currents (iKr), and by means of sodium current enhancement exerted by azithromycin on SC5NA.41, 42, 43 Repolarization prolongation by 10 to 50 ms is relatively frequent (5%–50%),10 , 34 , 35 , 44 and in one series of 201 patients treated with empirical prophylaxis, 18 (11%) developed QTc prolongation greater than 500 ms, albeit only one patient had sustained VT (0.5%), and no TdP was recorded.34 In another series, no difference in VAs incidence was seen between patients treated with antimalarial agents versus controls,10 whereas Gasparetti and colleagues concluded that the 3 VF observed in 28 patients admitted in ICU (10%) were due to ongoing ACS and not hydroxychloroquine-induced QT.37
Therefore, QT prolongation inconsistently predicts VAs in COVID-19 patients,3 , 10 , 35 likewise hydroxychloroquine is rarely associated with malignant arrhythmias (<1%).10 , 35 , 37 ST-T wave changes seemingly are not related to VAs45; however, elevated markers of cardiac injury (troponin, natriuretic peptide) and systemic inflammation (ie, C-reactive protein, interleukin-6) are significantly higher in patients presenting life-threatening arrhythmias,3 , 8 , 9 , 46 , 47 nonetheless it is not univocally acknowledged.10 Differently, a high dose of pressors seems to correlate with VAs incidence, both due to adrenergic stimulation and also for an implication of severe cardiogenic shock, whereas the value of admission left ventricular function is controversial.8, 9, 10, 11 , 15 , 43
Therapeutic options
If supportive management, including correction of electrolyte and acid/base derangement, fluid repletion, blood transfusion, coronary or pulmonary reperfusion, is not adequate, rationale antiarrhythmic strategies should be adopted. Sedatives and anesthetics should be titrated down or discontinued when identified as the cause of bradycardia or QT prolongation,4 and standard dose of beta blockers is certainly useful compatibly with the cardiac output.
Thirty-four percent of the 447 respondents (n = 150) to a global survey (March-April 2020) admitted to using amiodarone for VAs treatment, whereas 15% (n = 64) disclosed having used lidocaine or mexiletine for the same purpose. Other class III agents (sotalol and dofetilide) were rarely reported.48 Pulmonary and hepatic toxicity probably refrained most physicians from adopting amiodarone in secondary prevention in patients with a concomitant viral respiratory syndrome often complicated by liver dysfunction.49
According to some authors, lung toxicity is more likely to occur in ICU patients in which the oxidative damage of high oxygen partial pressure potentiates the free radicals derived from the iodine accumulated in the alveoli.50 However, it is worth highlighting that acute lung toxicity is an adverse event reported anecdotally51 , 52; rather, a cumulative dose of 150 g, equivalent to 400 mg daily for 3 months, or 200 mg for more than 18 months are associated with pulmonary injury.53, 54, 55, 56
On the contrary, liver toxicity requires as high as 300 g of cumulative dose,57 but according to some German scholars, one-third of the SARS-CoV-2 patients admitted in ICU has shock liver, which may compromise amiodarone metabolism.45
In our opinion, the vast experience in daily practice and the existing literature in non-COVID scenarios is more than sufficient to state that amiodarone is efficacious for malignant arrhythmias in COVID-19 patients. Despite isolated warning reports about lung and hepatic toxicity in some subjects with new coronavirus infection,58, 59, 60 the legitimate concern should be the risk of liver injury and of QT prolongation secondary to drug-drug interaction with antimicrobials36 , 40; thus, we think that amiodarone should be cautiously dosed in patients with extreme transaminitis, but still be preferred to other more torsadogenic class III agents. Furthermore, in the case of long QT (>550 ms), the treatment should switch to Ib agents, which can shorten the repolarization.61
Interestingly, amiodarone exhibits pleiotropic effects that can interfere with SARS-CoV-2 infection, by altering the ion channels of the endosomal vesicles.62 Also, it prevents cytokines production supposedly through the same mechanism in lymphocytes in vivo,63 and additionally presents scavenging effects of oxygen free radicals in vitro.64 In light of such anti-inflammatory antioxidant properties, amiodarone has been proposed for the treatment of symptomatic patients; besides a single case report,65 a randomized trial in comparison with verapamil is currently on recruiting (ReCOVery-SIRIO, ClinicalTrials.gov identifier: NCT04351763).66 , 67
ICDs and endocardial ablation should be recommended in agreement with the international guidelines,68, 69, 70 and when available, a remote navigation system should be used for the ablation to minimize the exposure of the medical staff.2
Summary
SARS-CoV-2 is a systemic disease that can also impair the electrical stability of the ventricles. Although a direct cardiac infection by the virus is plausible, the host’s systemic neuroinflammatory response in addition to metabolic disorders are the main triggers of VAs. The physician should be aware that subjects with pre-existing cardiac disease, admitted to ICU, requiring pressor support are more at risk of developing malignant arrhythmias. Therefore, strict monitoring of drugs interactions and precipitating factors (such as hypoxemia, hypokalemia, acidosis) is essential for primary prevention. Amiodarone is generally safe for the secondary prophylaxis of sustained events, and also might show unexplored antiviral/anti-inflammatory effects in human. Intravenous procainamide, lidocaine, or oral mexiletine are alternatives, albeit the evidence is limited. Contrarily, sotalol and dofetilide should be discouraged, because the more significant torsadogenic effect can be detrimental in such a delicate scenario. Lastly, substrate ablation is recommended in case of refractory episodes, possibly by using a remote navigation system to minimize the contact with the providers.
Clinics care points
-
•
Ventricular arrhythmias prevail in less than 10% (<1–8%) of the patients with acute COVID-19 infection, including premature ventricular contractions and life-threatening events.
-
•
The cause is multifactorial and includes direct virus interaction with cardiomyocytes, the effect of interleukins, neurohormonal output, pre-existing cardiac disease, metabolic disorders, iatrogenic toxicity, and latent congenital arrhythmic disorders precipitated by the systemic illness.
-
•
Although VAs are associated with increased mortality, it does not imply that they are the primary cause of death. In contrast, they express the terminal event of a severe systemic metabolic and inflammatory catastrophe.
-
•
In absence of reversible causes (ie, QT-prolonging agents, hypoxemia, hypokalemia), amiodarone is generally safe if other parameters are monitored (ie, liver function) and could theoretically exhibit pleiotropic beneficial effects on the infection itself. Class I agents represent valid alternatives, and ablation should be performed following the international guidelines, possibly with the help of a robotic navigation system to maximize contact isolation.
Acknowledgments
Disclosure
Dr A. Natale has received speaker honoraria from Boston Scientific, Biosense Webster, St. Jude Medical, Biotronik, and Medtronic; and is a consultant for Biosense Webster, St. Jude Medical, and Janssen. Dr L. Di Biase is a consultant for Biosense Webster, RMG, Stereotaxis, Boston Scientific, and Abbott. Dr L. Di Biase received speaker honoraria/travel from Medtronic, Atricure, and Biotronik. All other authors have reported that they have no relationships relevant to the contents of this article to disclose.
Footnotes
Funding: None declared.
References
- 1.Available at: https://coronavirus.jhu.edu/map.html. Accessed on June 12, 2021.
- 2.Mitacchione G., Schiavone M., Gasperetti A., et al. Ventricular tachycardia storm management in a COVID-19 patient: a case report. Eur Heart J Case Rep. 2020;4(FI1):1–6. doi: 10.1093/ehjcr/ytaa217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Elsaid O., McCullough P.A., Tecson K.M., et al. Ventricular fibrillation storm in coronavirus 2019. Am J Cardiol. 2020;135:177–180. doi: 10.1016/j.amjcard.2020.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Brien C., Ning N., McAvoy J., et al. Electrical storm in COVID-19. JACC Case Rep. 2020;2(9):1256–1260. doi: 10.1016/j.jaccas.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang D., Saleh M., Garcia-Bengo Y., et al. COVID-19 infection unmasking brugada syndrome. Heartrhythm Case Rep. 2020;6:237–240. doi: 10.1016/j.hrcr.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsimploulis A., Rashba E.J., Rahman T., et al. Fan R Medication unmasked Brugada syndrome and cardiac arrest in a COVID-19 patient. Heartrhythm Case Rep. 2020;6(9):554–557. doi: 10.1016/j.hrcr.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatla A., Mayer M.M., Adusumalli S., et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020;17(9):1439–1444. doi: 10.1016/j.hrthm.2020.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(7):811–818. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wetterslev M., Jacobsen P.K., Hassager C., et al. Cardiac arrhythmias in critically ill patients with coronavirus disease 2019: a retrospective population-based cohort study. Acta Anaesthesiol Scand. 2021;65(6):770–777. doi: 10.1111/aas.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peltzer B., Manocha K.K., Ying X., et al. Arrhythmic complications of patients hospitalized with COVID-19: incidence, risk factors, and outcomes. Circ Arrhythm Electrophysiol. 2020;13(10):e009121. doi: 10.1161/CIRCEP.120.009121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piro A., Magnocavallo M., Della Rocca D.G., et al. Management of cardiac implantable electronic device follow-up in COVID-19 pandemic: Lessons learned during Italian lockdown. J Cardiovasc Electrophysiol. 2020;31(11):2814–2823. doi: 10.1111/jce.14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turagam M.K., Musikantow D., Goldman M.E., et al. Malignant arrhythmias in patients with COVID-19: incidence, mechanisms, and outcomes. Circ Arrhythm Electrophysiol. 2020;13(11):e008920. doi: 10.1161/CIRCEP.120.008920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu K., Fang Y.Y., Deng Y., et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J (Engl) 2020;133(9):1025–1031. doi: 10.1097/CM9.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lanza G.A., De Vita A., Ravenna S.E., et al. Electrocardiographic findings at presentation and clinical outcome in patients with SARS-CoV-2 infection. Europace. 2021;23(1):123–129. doi: 10.1093/europace/euaa245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peretto G., Villatore A., Rizzo S., et al. The spectrum of COVID-19-associated myocarditis: a patient-tailored multidisciplinary approach. J Clin Med. 2021;10(9):1974. doi: 10.3390/jcm10091974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho J.S., Sia C.H., Chan M.Y., et al. Coronavirus-induced myocarditis: a meta-summary of cases. Heart Lung. 2020;49(6):681–685. doi: 10.1016/j.hrtlng.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siripanthong B., Nazarian S., Muser D., et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17(9):1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Della Rocca D.G., Santini L., Forleo G.B., et al. Novel perspectives on arrhythmia-induced cardiomyopathy: pathophysiology, clinical manifestations and an update on invasive management strategies. Cardiol Rev. 2015;23(3):135–141. doi: 10.1097/CRD.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 19.Siddiq M.M., Chan A.T., Miorin L., et al. Physiology of cardiomyocyte injury in COVID-19. medRxiv. 2020;2020:11. [Google Scholar]
- 20.Bulfamante G.P., Perrucci G.L., Falleni M., et al. Evidence of SARS-CoV-2 transcriptional activity in cardiomyocytes of COVID-19 patients without clinical signs of cardiac involvement. Biomedicines. 2020;8(12):626. doi: 10.3390/biomedicines8120626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wichmann D., Sperhake J.P., Lütgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a Prospective cohort study. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puntmann V.O., Carerj M.L., Wieters I., et al. Outcomes of cardiovascular magnetic resonance Imaging in patients Recently Recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5(11):1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haque R., Kan H., Finkel M.S. Effects of cytokines and nitric oxide on myocardial E-C coupling. Basic Res Cardiol. 1998;93(Suppl 1):86–94. doi: 10.1007/s003950050227. [DOI] [PubMed] [Google Scholar]
- 24.Lazzerini P.E., Laghi-Pasini F., Bertolozzi I., et al. Systemic inflammation as a novel QT-prolonging risk factor in patients with Torsades de Pointes. Heart. 2017;103:1821–1829. doi: 10.1136/heartjnl-2016-311079. [DOI] [PubMed] [Google Scholar]
- 25.Zhabyeyev P., Oudit G.Y. Sickle cell disease, interleukin-18, and arrhythmias. Blood. 2021;137(9):1138–1139. doi: 10.1182/blood.2020009690. [DOI] [PubMed] [Google Scholar]
- 26.Tsai Y.N., Hsiao Y.W., Lin S.F., et al. Proinflammatory cytokine modulates Intracellular calcium handling and Enhances ventricular arrhythmia susceptibility. Front Cardiovasc Med. 2021;8:623510. doi: 10.3389/fcvm.2021.623510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Di Biase L., Romero J., Zado E.S., et al. Variant of ventricular outflow tract ventricular arrhythmias requiring ablation from multiple sites: Intramural origin. Heart Rhythm. 2019;16(5):724–732. doi: 10.1016/j.hrthm.2018.11.028. [DOI] [PubMed] [Google Scholar]
- 28.Solaimanzadeh J., Freilich A., Sood M.R. Ventricular tachycardia with epicardial and pericardial fibrosis 6 months after resolution of subclinical COVID-19: a case report. J Med Case Rep. 2021;15(1):305. doi: 10.1186/s13256-021-02782-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitrani R.D., Dabas N., Goldberger J.J. COVID-19 cardiac injury: implications for long-term surveillance and outcomes in survivors. Heart Rhythm. 2020;17(11):1984–1990. doi: 10.1016/j.hrthm.2020.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Della Rocca D.G., Magnocavallo M., Lavalle C., et al. Evidence of systemic endothelial injury and microthrombosis in hospitalized COVID-19 patients at different stages of the disease. J Thromb Thrombolysis. 2021;51(3):571–576. doi: 10.1007/s11239-020-02330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pellegrini D., Kawakami R., Guagliumi G., et al. Microthrombi as a major cause of cardiac injury in COVID-19: a pathologic study. Circulation. 2021;143(10):1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828. [DOI] [PubMed] [Google Scholar]
- 32.D'Ascenzo F., De Filippo O., Borin A., et al. Impact of COVID-19 pandemic and infection on in hospital survival for patients presenting with acute coronary syndromes: a multicenter registry. Int J Cardiol. 2021;332:227–234. doi: 10.1016/j.ijcard.2021.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luqman N., Sung R.J., Wang C.L., et al. Myocardial ischemia and ventricular fibrillation: pathophysiology and clinical implications. Int J Cardiol. 2007;119(3):283–290. doi: 10.1016/j.ijcard.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 34.Tazmini K., Frisk M., Lewalle A., et al. Hypokalemia Promotes arrhythmia by distinct mechanisms in atrial and ventricular myocytes. Circ Res. 2020;126(7):889–906. doi: 10.1161/CIRCRESAHA.119.315641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sealy W.C., Young W.G., Jr., Harris J.S. Studies on cardiac arrest: the relationship of hypercapnia to ventricular fibrillation. J Thorac Surg. 1954;28(5):447–462. [PubMed] [Google Scholar]
- 36.Gettes L.S. Electrolyte abnormalities underlying lethal and ventricular arrhythmias. Circulation. 1992;85(1 Suppl):I70–I76. [PubMed] [Google Scholar]
- 37.Chen Q., Xu J., Gianni C., et al. Simple electrocardiographic criteria for rapid identification of wide QRS complex tachycardia: the new limb lead algorithm. Heart Rhythm. 2020;17(3):431–438. doi: 10.1016/j.hrthm.2019.09.021. [DOI] [PubMed] [Google Scholar]
- 38.Schrezenmeier E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 39.Saleh M., Gabriels J., Chang D., et al. Effect of chloroquine, hydroxychloroquine, and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ Arrhythm Electrophysiol. 2020;13(6):e008662. doi: 10.1161/CIRCEP.120.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gasperetti A., Biffi M., Duru F., et al. Arrhythmic safety of hydroxychloroquine in COVID-19 patients from different clinical settings. Europace. 2020;22(12):1855–1863. doi: 10.1093/europace/euaa216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang M., Xie M., Li S., et al. Electrophysiologic studies on the risks and potential mechanism underlying the Proarrhythmic nature of azithromycin. Cardiovasc Toxicol. 2017;17(4):434–440. doi: 10.1007/s12012-017-9401-7. [DOI] [PubMed] [Google Scholar]
- 42.Marmolejo-Murillo L.G., Aréchiga-Figueroa I.A., Moreno-Galindo E.G., et al. Chloroquine blocks the Kir4.1 channels by an open-pore blocking mechanism. Eur J Pharmacol. 2017;800:40–47. doi: 10.1016/j.ejphar.2017.02.024. [DOI] [PubMed] [Google Scholar]
- 43.Yang Z., Prinsen J.K., Bersell K.R., et al. Azithromycin causes a novel Proarrhythmic syndrome. Circ Arrhythm Electrophysiol. 2017;10(4):e003560. doi: 10.1161/CIRCEP.115.003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chorin E., Wadhwani L., Magnani S., et al. QT interval prolongation and torsade de pointes in patients with COVID-19 treated with hydroxychloroquine/azithromycin. Heart Rhythm. 2020;17:1425–1433. doi: 10.1016/j.hrthm.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romero J., Alviz I., Parides M., et al. T-wave inversion as a manifestation of COVID-19 infection: a case series. J Interv Card Electrophysiol. 2020;59(3):485–493. doi: 10.1007/s10840-020-00896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lazzerini P.E., Boutjdir M., Capecchi P.L. COVID-19, arrhythmic risk, and inflammation: Mind the Gap. Circulation. 2020;142(1):7–9. doi: 10.1161/CIRCULATIONAHA.120.047293. [DOI] [PubMed] [Google Scholar]
- 47.Zylla M.M., Merle U., Vey J.A., et al. Predictors and Prognostic implications of cardiac arrhythmias in patients hospitalized for COVID-19. J Clin Med. 2021;10(1):133. doi: 10.3390/jcm10010133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gopinathannair R., Merchant F.M., Lakkireddy D.R., et al. COVID-19 and cardiac arrhythmias: a global perspective on arrhythmia characteristics and management strategies. J Interv Card Electrophysiol. 2020;59(2):329–336. doi: 10.1007/s10840-020-00789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roedl K., Jarczak D., Drolz A., et al. Severe liver dysfunction complicating course of COVID-19 in the critically ill: multifactorial cause or direct viral effect? Ann Intensive Care. 2021;11(1):44. doi: 10.1186/s13613-021-00835-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ashrafian H., Davey P. Is amiodarone an underrecognized cause of acute respiratory failure in the ICU? Chest. 2001;120(1):275–282. doi: 10.1378/chest.120.1.275. [DOI] [PubMed] [Google Scholar]
- 51.Argyriou M., Hountis P., Antonopoulos N., et al. Acute fatal post-CABG low dose amiodarone lung toxicity. Asian Cardiovasc Thorac Ann. 2007;15(6):e66–e68. doi: 10.1177/021849230701500626. [DOI] [PubMed] [Google Scholar]
- 52.Baumann H., Fichtenkamm P., Schneider T., et al. Rapid onset of amiodarone induced pulmonary toxicity after lung lobe resection - a case report and review of recent literature. Ann Med Surg (Lond) 2017;21:53–57. doi: 10.1016/j.amsu.2017.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ott M.C., Khoor A., Leventhal J.P., et al. Pulmonary toxicity in patients receiving low-dose amiodarone. Chest. 2003;123:646–651. doi: 10.1378/chest.123.2.646. [DOI] [PubMed] [Google Scholar]
- 54.Terzo F., Ricci A., D'Ascanio M., et al. Amiodarone-induced pulmonary toxicity with an excellent response to treatment: a case report. Respir Med Case Rep. 2019;29:100974. doi: 10.1016/j.rmcr.2019.100974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Camus P., Martin W.J., II, Rosenow E.C., III Amiodarone pulmonary toxicity. Clin Chest Med. 2004;25:65–75. doi: 10.1016/S0272-5231(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 56.Tarantino N., Della Rocca D.G., Faggioni M., et al. Epicardial ablation complications. Card Electrophysiol Clin. 2020;12(3):409–418. doi: 10.1016/j.ccep.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Hussain N., Bhattacharyya A., Prueksaritanond S. Amiodarone-induced cirrhosis of liver: what predicts mortality? ISRN Cardiol. 2013;2013:617943. doi: 10.1155/2013/617943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Azraai M., McMahon M., Dick R. Case report of amiodarone-associated allergic pneumonitis amidst the COVID-19 pandemic. Rev Cardiovasc Med. 2021;22(1):181–184. doi: 10.31083/j.rcm.2021.01.267. [DOI] [PubMed] [Google Scholar]
- 59.Kow C.S., Hasan S.S. Amiodarone in COVID-19: let's not forget its potential for pulmonary toxicity. Eur J Prev Cardiol. 2020 doi: 10.1093/eurjpc/zwaa086. zwaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmit G., Lelotte J., Vanhaebost J., et al. The liver in COVID-19-related death: Protagonist or Innocent Bystander? Pathobiology. 2021;88(1):88–94. doi: 10.1159/000512008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mitra R.L., Greenstein S.A., Epstein L.M. An algorithm for managing QT prolongation in coronavirus disease 2019 (COVID-19) patients treated with either chloroquine or hydroxychloroquine in conjunction with azithromycin: possible benefits of intravenous lidocaine. Heartrhythm Case Rep. 2020;6(5):244–248. doi: 10.1016/j.hrcr.2020.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stadler K., Ha H.R., Ciminale V., et al. Amiodarone alters late endosomes and inhibits SARS coronavirus infection at a post-endosomal level. Am J Respir Cell Mol Biol. 2008;39:142–149. doi: 10.1165/rcmb.2007-0217OC. [DOI] [PubMed] [Google Scholar]
- 63.Ito H., Ono K., Nishio R., et al. Amiodarone inhibits interleukin 6 production and attenuates myocardial injury induced by viral myocarditis in mice. Cytokine. 2002;17:197–202. doi: 10.1006/cyto.2001.0996. [DOI] [PubMed] [Google Scholar]
- 64.Ide T., Tsutsui H., Kinugawa S., et al. Amiodarone protects cardiac myocytes against oxidative injury by its free radical scavenging action. Circulation. 1999;100:690–692. doi: 10.1161/01.cir.100.7.690. [DOI] [PubMed] [Google Scholar]
- 65.Castaldo N., Aimo A., Castiglione V., et al. Safety and efficacy of amiodarone in a patient with COVID-19. JACC Case Rep. 2020;2(9):1307–1310. doi: 10.1016/j.jaccas.2020.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sanchis-Gomar F., Lavie C.J., Morin D.P., et al. Amiodarone in the COVID-19 Era: treatment for symptomatic patients only, or drug to prevent infection? Am J Cardiovasc Drugs. 2020;20(5):413–418. doi: 10.1007/s40256-020-00429-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Available at: https://clinicaltrials.gov/ct2/show/NCT04351763. Accessed on July 5, 2021.
- 68.Cronin E.M., Bogun F.M., Maury P., et al. 2019 HRS/EHRA/APHRS/LAHRS expert consensus statement on catheter ablation of ventricular arrhythmias: Executive summary. J Arrhythm. 2020;36(1):1–58. doi: 10.1002/joa3.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Priori S.G., Blomström-Lundqvist C., Mazzanti A., et al. ESC Scientific Document Group 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task Force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European society of Cardiology (ESC). Endorsed by: association for European Paediatric and congenital Cardiology (AEPC) Eur Heart J. 2015;36(41):2793–2867. [Google Scholar]
- 70.Briceño D.F., Romero J., Villablanca P.A., et al. Long-term outcomes of different ablation strategies for ventricular tachycardia in patients with structural heart disease: systematic review and meta-analysis. Europace. 2018;20(1):104–115. doi: 10.1093/europace/eux109. [DOI] [PubMed] [Google Scholar]