Abstract
Coronavirus disease 2019 (COVID-19) has been associated with Acute Ischemic Stroke (AIS). Here, we characterize our institutional experience with management of COVID-19 and AIS. Baseline demographics, clinical, imaging, and outcomes data were determined in patients with COVID-19 and AIS presenting within March 2020 to October 2020, and November 2020 to August 2021, based on institutional COVID-19 hospitalization volume. Of 2512 COVID-19 patients, 35 (1.39%, mean age 63.3 years, 54% women) had AIS. AIS recognition was frequently delayed after COVID-19 symptoms (median 19.5 days). Four patients (11%) were on therapeutic anticoagulation at AIS recognition. AIS mechanism was undetermined or due to multiple etiologies in most cases (n = 20, 57%). Three patients underwent IV TPA, and three underwent mechanical thrombectomy, of which two suffered re-occlusion. Three patients had incomplete mRNA vaccination course. Fourteen (40%) died, with 26 (74%) having poor outcomes. Critical COVID-19 severity was associated with worsened mortality (p = 0.02). More patients (12/16; 75%) had either worsened or similar 3-month functional outcomes, than those with improvement, indicating the devastating impact of co-existing AIS and COVID-19. Comparative analysis showed that patients in the later cohort had earlier AIS presentation, fewer stroke risk factors, more comprehensive workup, more defined stroke mechanisms, less instance of critical COVID-19 severity, more utilization of IV TPA, and a trend towards worse outcomes for the sub-group of mild-to-moderate COVID-19 severity. AIS incidence, NIHSS, and overall outcomes were similar. Further studies should investigate outcomes beyond 3 months and their predictive factors, impact of completed vaccination course, and access to neurologic care.
Keywords: COVID-19, SARS-CoV-2, Acute ischemic stroke, Large vessel occlusion, Encephalopathy
1. Background
Complex neurologic manifestations of COVID-19 have been identified, including Acute Ischemic Stroke (AIS), whose pathophysiology is thought to be hypercoagulability from COVID-19 [1]. Stroke care during the pandemic has been complicated due to delayed presentation and recognition, especially during the early surge of COVID-19 cases in Michigan. Here, we describe our institutional experience in the treatment of patients with COVID-19 presenting with AIS during the initial and subsequent COVID-19 infection surges, including reporting long-term outcomes. We highlight challenges encountered in identification and investigative workup of these patients, treatment concerns, how AIS characteristics and management evolved from 2020 to 2021, the effect of COVID-19 vaccination, and considerations for future investigations.
2. Methods
All consecutive patients with COVID-19 and AIS treated at our institution during the various COVID-19 outbreaks, between March 1, 2020 and August 31st, 2021, were included. All patients had either positive nasal swab samples for SARS-CoV-2 using Reverse-Transcriptase Polymerase Chain Reaction or chest radiography findings consistent with COVID-19. Due to consideration for differences in patient management early and later in the COVID-19 pandemic, patients were categorized into two separate cohorts: 1) patients diagnosed with AIS during the initial peak of COVID-19 (March 2020 through October 2020) and 2) subsequent surges in infection (November 2020 through August 2021). These cut-offs were based on changes in COVID-19 hospitalization volumes at our institution. Baseline demographic and clinical characteristics were retrospectively determined. COVID-19 severity was categorized into asymptomatic or mild, moderate, and severe or critical as per published criteria [2]. We defined large vascular territory strokes as stroke involving at least 1/3rd of the vascular territory of a large intracranial artery. Stroke mechanisms were defined by the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) criteria and recent review and clinical update of embolic stroke of undetermined source [3], [4]. ModifiedRankin Scale (mRS) was recorded at discharge and then at 3 months. Poor functional outcome was defined as mRS of 4–6. Cohort comparisons amongst various variables were made using Student’s t-test or Mann-Whitney U-test for continuous variables, and chi-squared tests for categorical variables. The study was approved by local Institutional Review Board.
3. Results
3.1. Demographics, risk factors, and clinical features
The study identified 35 patients (females [19/35, 54%]; mean age 63.3 [SD 13.4, range 30–89 years]) with AIS and COVID-19, out of a total of 2512 COVID-19 patients (1.39%) (Table 1, Table 2 ). A total of 19 AIS cases were identified from the first cohort of 1400 COVID-19 patients (1.36%), and 16 from the second cohort of 1112 patients (1.44%). Thirty patients (86%) presented from home, whereas the remaining presented from a nursing facility. Twenty-nine (83%) had ≥ 2 vascular risk factors, most commonly hypertension, prior AIS, and diabetes. Cardioembolic risk factors including atrial fibrillation, mitral prosthetic valve, patent foramen ovale, EF <45% or hypokinetic/akinetic left ventricular segments were noted in nine patients (26%). Other factors included underlying hypercoagulable states in four (11%), and Moyamoya vasculopathy in only one (3%). The most common nonvascular risk factor was chronic kidney disease (6/35; 17%). Prior to identification of stroke, most patients (21/35; 60%) had respiratory symptoms. COVID-19 severity was severe or critical in 12 (34%), moderate in 11 (31%), and mild or asymptomatic in the remaining patients, prior to or concomitant with identification of stroke. Within 48 hours prior to stroke onset, only five patients (14%) were treated with vasopressors. Clinical symptoms of stroke included either focal neurologic signs (24/35; 69%) or persistent/worsening encephalopathy (11/35; 31%). Three patients (9%) from the second cohort each received one dose of a mRNA COVID-19 vaccine.
Table 1.
Table showing differences of various variables between the two different AIS and COVID-19 cohorts.
| Characteristics |
P-value |
Total |
||
|---|---|---|---|---|
| Acute Ischemic Stroke |
(n = 35) |
|||
| Cohort 1 (n = 19; Mar 2020 – Oct 2020) | Cohort 2 (n = 16; Nov 2020 – August 2021) | |||
| Stroke Incidence | 19/1400 (1.36%) | 16/1112 (1.44%) | 0.86 | 35/2512 (1.39%) |
| Demographics | ||||
| Age (mean, SD) | 64.4 (12.2) | 62 (15) | 0.61 | 63.3 (13) |
| Male | 8 (42%) | 8 (50%) | 0.64 | 16 (46%) |
| Two or more stroke risk factors | 18 (95%) | 11(69%) | 0.04 | 29 (83%) |
| Time from initial COVID-19 symptoms to stroke diagnosis, d (median, IQR) | 31.5 (49) | 12.5 (25) | 0.19 | 19.5 (43) |
| Stroke as the initial presentation on COVID-19 diagnosis | 6 (32%) | 9 (56%) | 0.14 | 15 (43%) |
| Stroke presentation >30 days after COVID-19 diagnosis | 10 (53%) | 3 (19%) | 0.04 | 13 (37%) |
| Encephalopathy as the main presenting symptom | 6 (32%) | 5 (31%) | 0.98 | 11 (31%) |
| Critical COVID-19 severity | 10 (53%) | 2 (13%) | 0.01 | 12 (34%) |
| Coagulation factor, n (%) | ||||
| On therapeutic anticoagulation at time of stroke | 3 (16%) | 1 (6%) | 0.38 | 4 (11%) |
| C-reactive protein (mg/L, mean, SD) | 142 (105) | 115 (89) | 0.42 | 132 (98) |
| D-dimer (fibrinogen-equivalent units μg/mL, mean, SD) | 6.9 (8.8) | 14 (15) | 0.09 | 9.5 (11.8) |
| Fibrinogen (mg/dL, mean, SD) | 569 (188) | 527 (215) | 0.54 | 551 (196) |
| Stroke characteristics | ||||
| NIHSS (median, IQR) | 15 (21) | 11 (21) | 0.65 | 12 (21) |
| Large vascular territory | 13 (68%) | 10 (63%) | 0.71 | 23 (66%) |
| Two or more vascular territories | 10 (53%) | 5 (31%) | 0.20 | 15 (43%) |
| Bilateral vascular territories | 8 (42%) | 4 (25%) | 0.29 | 12 (34%) |
| Cortical involvement on imaging | 13 (68%) | 12 (75%) | 0.67 | 25 (71%) |
| Subcortical involvement on imaging | 2 (11%) | 4 (25%) | 0.85 | 6 (17%) |
| Computer tomography negative for acute stroke | 5 (26%) | 1 (6%) | 0.12 | 6 (17%) |
| Number of patients with intracranial vessel imaging | 12 (63%) | 15 (94%) | 0.03 | 27 (77%) |
| Symptomatic vascular stenosis/reduced flow | 5/12 (42%) | 7/15 (47%) | 0.80 | 12/27 (44%) |
| Treatment with IV TPA | 0 (0%) | 3 (19%) | N/A | 3 (9%) |
| Treatment with mechanical thrombectomy | 2 (11%) | 1 (6%) | 0.65 | 3 (9%) |
| Stroke mechanism, n (%) | ||||
| Cardioembolism | 3 (16%) | 2 (13%) | 0.78 | 5 (14%) |
| Large artery atherosclerosis (LAA) | 0 (0%) | 5 (31%) | N/A | 5 (14%) |
| Small artery occlusion (SAO) | 0 (0%) | 2 (13%) | N/A | 2 (6%) |
| Other determined mechanism | 1 (5%) | 2 (13%) | 0.45 | 3 (9%) |
| Undetermined or more than one potential cause | 15 (79%) | 5 (31%) | 0.004 | 20 (57%) |
| Outcomes, n (%) | ||||
| In-hospital death | 6 (32%) | 3 (19%) | 0.39 | 9 (26%) |
| 3-month mortality | 8 (42%) | 6 (38%) | 0.78 | 14 (40%) |
| 3-month Modified Rankin Scale of 0–3 | 5 (26%) | (%) | 0.93 | 9 (26%) |
| Discharge to hospice, LTAC, or nursing facility | 9 (47%) | 8 (50%) | 0.88 | 17 (49%) |
Foot Notes. COVID-19, coronavirus 2019; d, day; IQR, interquartile range; LTAC, long term acute care facility; NIHSS, NIH stroke scale; SD, standard deviation.
Table 2.
Characteristic of COVID-19 severity, stroke mechanism and patient outcome.
| Cohort 1 (Mar 2020 – Oct 2020) |
Cohort 2 (Nov 2020 – August 2021) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | COVID-19 severity prior to stroke onset | Stroke Mechanism | NIHSS | MRS (m) |
Patient | COVID-19 severity prior to stroke onset | Stroke Mechanism | NIHSS | MRS (m) |
||
| 0 | 3 | 0 | 3 | ||||||||
| 1 | Mild | Other determined etiology | 15 | 6 | 6 | 1 | Mild | Cardioembolism | 6 | 1 | – |
| 2 | Critical | SAO + Cardioembolism | 32 | 6 | 6 | 2 | Moderate | Cardioembolism | 12 | 4 | – |
| 3 | Critical | Incomplete evaluation | 26 | 6 | 6 | 3 | Mild | Other determined etiology | 3 | 1 | 0 |
| 4 | Critical | Incomplete evaluation | 8 | 5 | 4 | 4 | Mild | LAA | 27 | 5 | – |
| 5 | Moderate | Incomplete evaluation | 4 | 4 | – | 5 | Moderate | Incomplete evaluation | 26 | 6 | 6 |
| 6 | Critical | Incomplete evaluation | 29 | 5 | 6 | 6 | Mild | SAO | 7 | 5 | 6 |
| 7 | Critical | Incomplete evaluation | 30 | 6 | 6 | 7 | Critical | Other determined etiology | 31 | 5 | – |
| 8 | Critical | Incomplete evaluation | 29 | 6 | 6 | 8 | Moderate | Negative evaluation | 5 | 5 | 6 |
| 9 | Critical | ESUS | 23 | 5 | 5 | 9 | Moderate | LAA | 6 | 3 | – |
| 10 | Moderate | Incomplete evaluation | 8 | 3 | 3 | 10 | Moderate | Negative evaluation | 19 | 6 | 6 |
| 11 | Critical | Cardioembolism | 12 | 5 | 6 | 11 | Mild | SAO | 10 | 4 | 6 |
| 12 | Critical | Incomplete evaluation | 26 | 5 | – | 12 | Moderate | LAA | 31 | 5 | – |
| 13 | Mild | Cardioembolism | 18 | 5 | 5 | 13 | Mild | Negative evaluation | 3 | 0 | – |
| 14 | Moderate | Cardioembolism | 12 | 5 | 5 | 14 | Mild | LAA | 20 | 4 | 5 |
| 15 | Mild | Incomplete evaluation | 4 | 0 | – | 15 | Mild | LAA | 2 | 4 | 4 |
| 16 | Moderate | Incomplete evaluation | 4 | 0 | 0 | 16 | Critical | Negative evaluation | 35 | 6 | 6 |
| 17 | Critical | Incomplete evaluation | 27 | 6 | 6 | ||||||
| 18 | Mild | Negative evaluation | 4 | 1 | 0 | ||||||
| 19 | Moderate | Negative evaluation | 6 | 4 | 2 | ||||||
Foot Notes. COVID-19, coronavirus 2019; ESUS, Embolic stroke of undetermined source; LAA, large artery atherosclerosis; m, month; MRS; Modified Rankin Scale; NIHSS, NIH stroke scale; SAO, small-artery occlusion.
3.2. Stroke characteristics and workup in patients with COVID-19
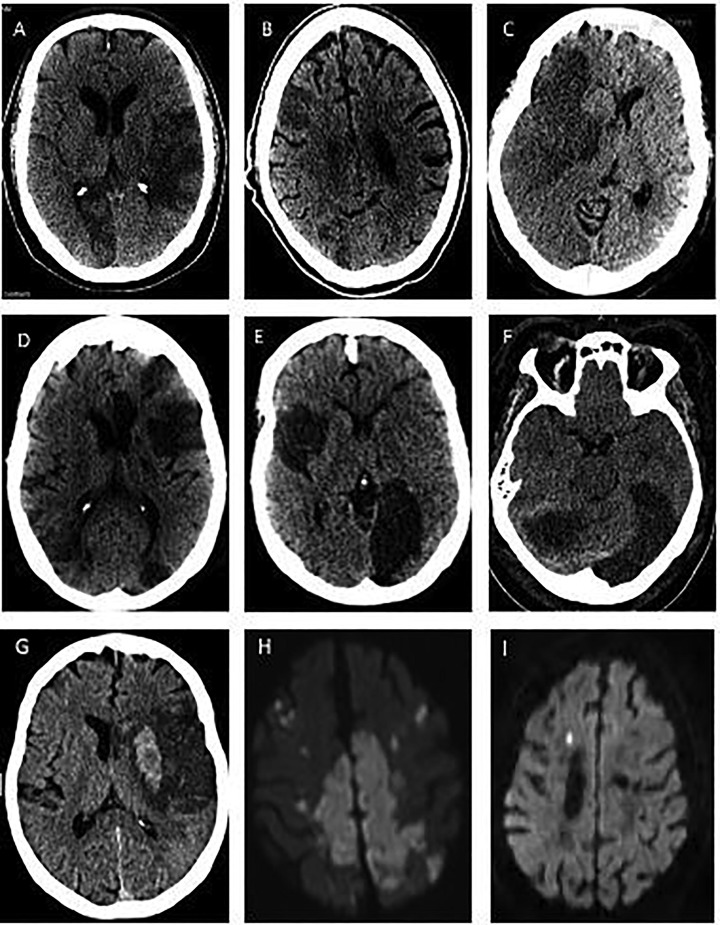
Mechanism of stroke (Table 1, Table 2) was cardioembolic in five patients (14%), large artery atherosclerosis in five (14%), small vessel disease in two (6%), other determined etiology in three (9%), and undetermined or more than one etiology in 20 (57%). Of the latter, 12 (60%) were due to incomplete evaluation, seven (35%) were cryptogenic with complete evaluation, of which one (5%) had embolic stroke of undetermined source (ESUS), and one (5%) had multiple etiologies (small vessel occlusion and cardioembolism). Amongst the 29/35 (83%) patients with radiographically proven new stroke (Fig. 1 ), large vessel territory infarcts occurred in 23 (80%) patients, two or more vascular territories in 15 (52%) patients, and bilateral vascular territories in 12 (41%) patients. Of the 27 patients with neurovascular imaging, symptomatic focal intravascular occlusion and/or flow attenuation were seen in 12 patients (44%).
Fig. 1.
Intracranial images of selected study patients. Representative images demonstrate large vascular, small vascular and multi-vascular territory infarcts. Non-contrast head computed tomography scan is shown in A-G, whereas Diffusion-weighted Magnetic Resonance Imaging of the brain is shown in H-I.
Stroke accounted for hospital presentation with or without respiratory symptoms in 15/35 (43%) cases. Median time from onset of COVID-19 symptoms to identification of stroke was 19.5 (IQR 42.5) days. Thirteen patients (37%) presented with stroke >30 days from COVID-19 diagnosis and 11 (31%) had radiographic stroke associated with persistent encephalopathy. Median National Institutes of Health Stroke Scale (NIHSS) upon stroke recognition was 12 [IQR 21]. Three patients (9%) received IV TPA and three patients (9%) underwent thrombectomy. Two patients (6%) demonstrated re-stenosis of the same large vessel within 24 hours of successful thrombectomy (TICI 2b revascularization). Significant laboratory markers (mean, [SD]) at stroke onset were elevated CRP (132 mg/L [98]), D-dimer (9.5 μg/mL [11.8]), and fibrinogen (551 mg/dL [196]). Positive cardiolipin antibody was observed in three patients. Eight patients (23%) received anti-platelet therapy, four (11%) received therapeutic anticoagulation, and 11 (31%) received prophylactic anticoagulation prior to stroke onset.
3.3. Comparison of AIS patients with COVID-19 presenting in early and late cohorts
Patients in the later cohort had a lower proportion of multiple stroke risk factors (p = 0.04) and delayed stroke presentation of >30 days from initial COVID-19 diagnosis (p = 0.04) compared to the cohort prior to November 2020 (Table 1). Three patients in the later cohort received IV TPA compared to none in the earlier cohort, and there was a higher proportion of patients with completed stroke work up with identified stroke mechanisms. Patients in the later cohort had a lower instance of undetermined mechanisms (p = 0.004), and a higher completion rate of neurovascular imaging (0.032). More patients had critical COVID-19 severity in the earlier cohort (p = 0.01). Three patients had an incomplete course of a COVID-19 mRNA vaccine in the second cohort. AIS incidence, NIHSS, and outcomes between the cohorts were similar. Despite a higher instance of mild-to-moderate COVID-19 severity in the later cohort, there was a trend towards higher mortality and poor outcomes within the mild-to-moderate COVID-19 severity subgroup (mRS of 4–6). However, the findings were statistically non-significant (Table 3A ).
Table 3A.
Table showing distribution of COVID-19 severity along-with long-term outcomes across the two separate patient cohorts.
| Mild to moderate COVID-19 severity | |||
|---|---|---|---|
| Deaths | Poor outcome* | Total patients | |
| Cohort 1 | 1 (11.1%) | 4 (44.4%) | 9 |
| Cohort 2 | 5 (35.7%) | 10 (71.4%) | 14 |
| p-value | 0.190 | 0.196 | 23 |
| Critical COVID-19 severity | |||
| Cohort 1 | 7 (70%) | 10 (100.0%) | 10 |
| Cohort 2 | 1 (50%) | 2 (100.0%) | 2 |
| p-value | 0.584 | N/A | 12 |
Foot Notes. COVID-19, coronavirus 2019.
Poor outcome is defined as Modified Rankin Scale 4–6.
3.4. Complications and overall outcomes
Five patients (14%) developed venous thromboembolism during hospitalization, including limb ischemia, pulmonary emboli, lower extremity venous thrombosis, and splenic infarct. One out of 18 (6%) patients with blood cultures obtained developed MRSA bacteremia following stroke. Five patients (14%) required vasopressor support 48 hours prior to stroke onset. Overall, the outcome of the patients was poor with 14 (40%) expiring and 17 (49%) discharged to nursing facility or hospice. Only nine patients (26%) had a 3-month mRS of 0–3. Of the 26 (74%) patients who survived, 3-month mRS was available in 16. Five patients (31%) had a 3-month mRS of 0–3. Modified Rankin Scale was improved in only four patients (25%), worsened in six (38%) of which five had died, and remained the same in the rest (Table 2). COVID-19 severity significantly impacted outcomes. There was higher mortality in patients with critical COVID-19 severity compared to mild-to-moderate (p = 0.02) (Table 3B ).
Table 3B.
Table showing distribution of COVID-19 severity along-with long-term outcomes across both patient cohorts combined.
| COVID-19 severity | Deaths | Poor outcome* | Total patients |
|---|---|---|---|
| Mild-to-moderate | 6 (26.1%) | 14 (60.1%) | 23 |
| Critical | 8 (75.0%) | 12 (100.0%) | 12 |
| p-value | 0.020 | N/A | 35 |
Foot Notes. COVID-19, coronavirus 2019.
Poor outcome is defined as Modified Rankin Scale 4–6.
4. Discussion
We present our experience with managing AIS patients with concomitant COVID-19 infection. Our region was particularly affected at the early stages of the pandemic and harbors a population with multiple stroke risk factors. We noticed that the overall incidence of AIS in COVID-19 has not significant changed during the course of pandemic. There were selected differences in stroke characteristics, treatments and outcomes within the later cohort, including more utilization of IV TPA, lower incidence of critical COVID-19 severity, more acute AIS presentation, and a tendency for more complete workup and identification of the stroke mechanism. Although vaccines became available in the later cohort, majority of patients were still unvaccinated, potentially due to slow vaccine roll-out and lower-than-average vaccination rates of 37% in the city of Detroit, as of September 2021 [5]. Three patients each received a single dose of an mRNA COVID-19 vaccine prior to stroke onset, but no fully vaccinated patient presented with AIS. Large scale observational studies investigating the effect of vaccination on stroke incidence in COVID-19 would be of particular interest, as although stroke incidence in COVID-19 is low, outcomes are devastating. This was particularly highlighted in our data where a non-significant trend was discovered for mortality and poor outcomes even with mild-to-moderate COVID-19 severity in the later cohort.
Due to critical limitations in medical personnel and testing availability at the start of the pandemic, stroke workups were often incomplete for patients diagnosed early in the pandemic (11/19 patients had limited evaluation in the early cohort), but most patients had full evaluations in the later period (15/16). Thus, identified stroke mechanisms became evident in patients presenting later in the pandemic with fewer undetermined mechanisms, with one factor being a higher completion rate of neurovascular imaging. Due to this, large artery atherosclerosis was identified in five patients in the later cohort and small-vessel occlusion in two, compared to none in the earlier cohort. It is likely that the mechanisms have not necessarily changed, as other stroke features such as the NIHSS, and large, multiple and bilateral vascular territorial involvement remained the same. Overall, strokes were severe, evidenced by an abundance of large vascular territory involvement and poor outcomes.
It is likely that with time, access to neurologic care has improved with greater utilization of TPA and earlier presentation, as we noted in our institution. Other studies also show similarly poor access to neurologic care within the time-frame of our early cohort, with significant decreases in the number of patients with critical neurologic conditions such as AIS presenting to the emergency room and subsequent admission, and reduced treatment utilization such as IV TPA and thrombectomy [6], [7]. Another factor determining this compromise was a time-lag between onset of COVID-19 infection and stroke recognition, potentially due to a delayed stroke inducing mechanism, and an inability to conduct neurologic exams due to sedation required for mechanical ventilation. This lag significantly shrank in the later cohort, likely from earlier recognition of and familiarity with stroke in COVID-19, full availability of neuroimaging, and more patients seeking care for neurologic conditions in the emergency departments. Despite these factors, outcomes have not improved with more patients experiencing worsening or static functional 3-month outcomes compared to those with improvement. There is a need for further studies investigating whether measures taken to improve access to neurologic care has had a meaningful benefit on earlier patient presentation, more treatment utilization, and better outcomes.
The severity of COVID-19-related symptoms evolved with early patients experiencing more instance of critical respiratory severity. There was a trend noted for patients with even mild-to-moderate COVID-19 severity in the later cohort suffering poor outcomes indicating a potential independent impact of strokes on poor outcomes, with co-existing COVID-19. However, critical COVID-19 severity for the whole cohort was significantly associated with higher mortality, indicating the major driving force of poor outcomes was critical COVID-19 rather than severe stroke. Other published reports also echo similar findings [8], [9], [10], [11], [12].
The exact mechanism of stroke in COVID-19 remains unknown, but is likely secondary to the neurotropic potential of COVID-19. Autopsy findings suggest that stroke could be related to underlying prothrombotic state secondary to endothelial invasion via ACE-2 leading to platelet deposition and microthrombi formation within vasculature [13]. However, presence of traditional vascular risk factors, systemic inflammation and sepsis might increase predisposition to thrombotic events. We noticed this phenemon in two of three patients who underwent successful thrombectomy, but later developed re-occlusion of the same vessel within 24 hours. Older patients with multiple risk factors are particularly vulnerable, although severe strokes from large vessel occlusions have been reported in young patients also [8], [9], [10], [11], [12]. Additional thrombotic risk may be imparted by D-dimer and fibrinogen levels, which are elevated in patients of COVID-19 with stroke, with positive correlation to disease severity and prognosis. These factors have been used as rationale for therapeutic anticoagulation in COVID-19[1], however it is not known if it would prevent stroke. Three patients (9%) suffered severe multi-vascular territory strokes despite being on adequate anticoagulation. Current evidence lacks sufficient evidence to necessitate the use of therapeutic anticoagulation in COVID-19 [14], [15].
Our study is a retrospective review with a small sample size, and the weaknesses are inherent to any retrospective single-institutional studies. Nevertheless, it does lay a foundation to generate further hypotheses to investigate in AIS and COVID-19 using large multi-center observational cohorts. These include a comprehensive investigation of the effect of a completed COVID-19 vaccination course on AIS incidence and outcomes with either co-existing COVID-19, or a history of COVID-19. Effect of both the vaccination and measures taken to improve access to neurologic care should also be diligently investigated for effectiveness and the impact on outcomes. Improved utilization of IV TPA and thrombectomy, as well as reduction in delay to these treatments as experienced during the COVID-19 pandemic, are also of research interest. There is a paucity of data regarding long-term outcomes in patients with coexisting AIS and COVID-19, and it is imperative that access to both acute rehabilitation services and ongoing neurologic care are not compromised. These items should also be investigated. Finally, the exact role of therapeutic anticoagulation in AIS that is strongly attributed to the hypercoagulability of a severe COVID-19 infection remains to be elucidated further.
5. Conclusions
Although AIS in COVID-19 is a rare phenomenon, we recommend vigilance in anticipating this issue, particularly in patients with prolonged unexplained encephalopathy, abnormal pro-thrombotic markers, and history of prior stroke. Although AIS characteristics and incidence in COVID-19 have not changed over the course of pandemic, there has been earlier presentation, more comprehensive evaluation including neurovascular imaging, increased utilization of IV TPA, and lower instance of concomitant critical COVID-19 severity in the later cohort. Outcomes are particularly worse with AIS and COVID-19 and worsen further with critical COVID-19 severity. Long-term outcomes also tend to worsen more often in survivors of AIS and COVID-19. Despite similar overall outcomes between the two cohorts, there was a possible trend towards worse outcomes within the mild-to-moderate COVID-19 severity subgroup in the later cohort, indicating the devastating impact of co-existing AIS and COVID-19. Future studies should investigate outcomes longer than 3 months and factors leading to worsening long-term outcomes, the effect of a completed vaccination course, access to neurologic care, and the role of therapeutic anticoagulation in AIS with COVID-19.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved with a waiver from consent by the DMC Institutional Review Board without the need for informed consent due to the use solely of retrospective, existing data from medical records. The research involved no more than minimal risk to the participants. The research could not practicably be carried out without the requested waiver or alteration. The waiver or alteration did not adversely affect the rights and welfare of the participants. Whenever appropriate, the participants or legally authorized representative were provided with additional pertinent information after participation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Zhang S., Zhang J., Wang C., Chen X., Zhao X., Jing H., et al. COVID–19 and ischemic stroke: Mechanisms of hypercoagulability (Review) Int J Mol Med. 2021 Mar;47(3) doi: 10.3892/ijmm10.3892/ijmm.2021.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at https://www.covid19treatmentguidelines.nih.gov/. Accessed [09/19/2021]. [PubMed]
- 3.Adams H.P., Bendixen B.H., Kappelle L.J., Biller J., Love B.B., Gordon D.L., et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35–41. doi: 10.1161/01.STR.24.1.35. [DOI] [PubMed] [Google Scholar]
- 4.Hart R.G., Catanese L., Perera K.S., Ntaios G., Connolly S.J. Embolic Stroke of Undetermined Source: A Systematic Review and Clinical Update. Stroke. 2017;48(4):867–872. doi: 10.1161/STROKEAHA.116.016414. [DOI] [PubMed] [Google Scholar]
- 5.COVID-19 vaccine dashboard. Available at https://www.michigan.gov/coronavirus/0,9753,7-406-98178_103214-547150--,00.html. Accessed [09/22/2021].
- 6.Jansen R., Lee J.-I., Turowski B., Kaschner M., Caspers J., Bernhard M., et al. Consequences of COVID-19 pandemic lockdown on emergency and stroke care in a German tertiary stroke center. Neurol Res Pract. 2021;3(1) doi: 10.1186/s42466-021-00118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegler J.E., Zha A.M., Czap A.L., et al. Influence of the COVID-19 Pandemic on Treatment Times for Acute Ischemic Stroke: The Society of Vascular and Interventional Neurology Multicenter Collaboration. Stroke. 2021 Jan;52(1):40–47. doi: 10.1161/STROKEAHA.120.032789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siow I., Lee K.S., Zhang J.J.Y., Saffari S.E., Ng A., Young B. Stroke as a Neurological Complication of COVID-19: A Systematic Review and Meta-Analysis of Incidence, Outcomes and Predictors. J Stroke Cerebrovasc Dis. 2021;30(3):105549. doi: 10.1016/j.jstrokecerebrovasdis.2020.105549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin C., Zhou L., Hu Z., Yang S., Zhang S., Chen M., et al. Clinical Characteristics and Outcomes of COVID-19 Patients With a History of Stroke in Wuhan. China. Stroke. 2020;51(7):2219–2223. doi: 10.1161/STROKEAHA.120.030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oxley T.J., Mocco J., Majidi S., Kellner C.P., Shoirah H., Singh I.P., et al. Large-Vessel Stroke as a Presenting Feature of Covid-19 in the Young. N Engl J Med. 2020;382(20):e60. doi: 10.1056/NEJMc2009787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaghi S, Ishida K, Torres J et al. SARS-CoV-2 and Stroke in a New York Healthcare System [published correction appears in Stroke. 2020 Aug;51(8):e179]. Stroke. 2020;51(7):2002-2011. doi:10.1161/STROKEAHA.120.030335. [DOI] [PMC free article] [PubMed]
- 12.Li Y., Li M., Wang M., Zhou Y., Chang J., Xian Y., et al. Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Stroke Vasc Neurol. 2020;5(3):279–284. doi: 10.1136/svn-2020-000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bryce C, Grimes Z, Pujadas E et al. Pathophysiology of SARS-CoV-2: targeting of endothelial cells renders a complex disease with thrombotic microangiopathy and aberrant immune response. The Mount Sinai COVID-19 autopsy experience. [Published online May22,2020.]MedRxiv.2020.
- 14.Cuker A, Tseng EK, Nieuwlaat R et al. American Society of Hematology 2021 guidelines on the use of anticoagulation for thromboprophylaxis in patients with COVID-19. Blood Adv. 2021 Feb 9;5(3):872-888. 10.1182/bloodadvances.2020003763. [DOI] [PMC free article] [PubMed]
- 15.Chandra A, Chakraborty U, Ghosh S et al. Anticoagulation in COVID-19: current concepts and controversies. Postgrad Med J. 2021 Apr 13:postgradmedj-2021-139923. 10.1136/postgradmedj-2021-139923. [DOI] [PubMed]