Abstract
Background and Objectives
Convalescent plasma has attracted significant attention as a therapeutic option against infectious agents for more than a century. In March 2020, the use of Convalescent COVID-19 plasma (CCP) as a new research drug for COVID-19 treatment was approved by the FDA. The development of SARS-CoV-2 IgG antibodies following infection or vaccination is likely to be essential to provide adequate immunity for the population to halt the COVID19 pandemic. This study aimed to identify the criteria that would be used to determine the most appropriate CCP donors with the highest effective antiviral antibody titers.
Materials and Methods
In this prospective cohort, univariate analyses and multivariate regression analyses were performed to evaluate the relationship between characteristics of 11949 CCP donors and COVID-19 disease severity prior to donation with antibody titers estimated using ELISA technique and rapid tests.
Results
The antibody titer was measured among 8206 (68.7 %) donors. Elderly male and nulliparous female CCP donors who resided in the areas with high load of virus had positive ELISA and rapid test results as well as high levels of SARS-CoV-2 IgG antibodies titer. Moreover, the long hospital stay and elderly donors were the variables associated with high levels of SARS-CoV-2 IgG antibodies.
Conclusion
This study suggests that nulliparous female and male donors with positive rapid tests who resided in areas with a higher prevalence of SARS-CoV-2, with more than 40 years of age and long hospitalization time can be the preferred donors for CCP donation.
Keywords: COVID-19 serotherapy, SARS-CoV-2, Serologic tests, Donor selection
1. Introduction
The most recent formidable threat to the global health is the proceeding outbreak of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the responsible virus for coronavirus disease-19 (COVID-19), which became epidemic in mid‐December 2019 in Wuhan, China. Three months later than the emergence of COVID-19, the Director General of the World Health Organization, declared the COVID-19 a global pandemic [1]. At the time of writing, COVID-19 pandemic is the gravest current challenge worldwide with approximately 230 million infected patients and over than 4.7 million deaths as of September 26, 2021. By this time, more than 5.4 million people were infected in Iran, and 118,191 people have died. Nowadays, COVID-19 has limited therapeutic options which have mainly been empirical and experimental. There are no specific acknowledged antiviral agents targeting SARS-CoV-2 [2]. Vaccination is the best approach to control rampant epidemic and its destructive effects on global economy and public health [3]. Despite the unprecedented and extensive efforts currently being made to manufacture and distribute enough safe and effective COVID-19 vaccine worldwide, it seems time-consuming to establish active immunity to the SARS-CoV-2 virus in every individual around the world successfully. Therefore, the use of effective therapies to treat severe cases of the disease to reduce mortality rate until equitable global access to the eventual COVID-19 vaccines is achieved, is of substantial importance. Equipping the body with passive immunity through the transfusion of convalescent plasma donated by patients who have recovered from an infection would be effective at least in early stages of outbreaks of such emerging viral pathogens as SARS-CoV-2, for which therapeutic options are limited [4]. Based on the promising results obtained from treating other viral diseases such as influenza, Ebola, SARS, and Middle East respiratory syndrome (MERS), it has been approved that convalescent plasma can also treat COVID-19 [[5], [6], [7]]. Humoral immune response by targeting the immunodominant SARS-CoV-2 Spike (S) protein seems to be highly correlated with virus neutralization through hampering the interaction between S protein and the virus receptor, angiotensin converting enzyme 2 (AEC2) [8]. It has been shown that transfusion of COVID-19 convalescent plasma containing these neutralizing antibodies (nAbs) to patients affected with COVID-19 could effectively reduce viral load in the patients’ body [9]. Convalescent plasma therapy will be successful if plasma be donated by recovered people from COVID-19 who have high concentrations of neutralizing antibodies, without the risk of virus transmission via transfusion to the recipient [10]. Hence, identifying factors associated with the production of anti-SARS-CoV-2 antibodies affects the success rate of convalescent plasma therapy [11]. In this regard, this study aimed to find out how SARS-CoV-2 IgG antibodies are influenced by demographic and clinical characteristics of donors such as age, sex, ABO/Rh blood group, disease severity, ethnicity and the place of residence in terms of virus load. This study could provide a roadmap for selecting individuals possibly with high levels of anti-SARS-CoV-2 IgG antibodies as preferred donors for CCP donation.
2. Materials and methods
A prospective cohort study incorporating a total of 11,949 COVID-19 Convalescent Plasma (CCP) donors in the cohort.
2.1. Donors of convalescent plasma
Iranian Blood Transfusion Organization (IBTO) encouraged Iranian general population via public dissemination channels, including traditional or social media, to donate CCP from May to December 2020. CCP donors were identified as allogeneic and they were considered healthy if a physician examined their clinical history and confirmed so at the time of referral. Inclusion criteria for the CCP donation were considered to be:
-
•
Proven prior COVID-19 infection by real-time PCR,
-
•
Elapse of at least 28 days since being fully recovered
The severity of previous COVID-19 disease in CCP donors was determined in the present study.
Mild illness: donors who had a variety of signs and symptoms at the time of prior COVID-19 (including fever, cough, sore throat, lethargy, headache, muscle aches, nausea, vomiting, diarrhea, loss of taste and smell) but without any shortness of breath, dyspnea or abnormal chest imaging.
Moderate illness: donors who showed lower respiratory disease evidence during clinical evaluation or imaging and had more than 94 % oxygen saturation (SpO2) in room air at sea level.
Severe illness: donors with SpO2 < 94 in room air at sea level, the ratio of partial arterial pressure of oxygen to fraction of oxygen-inspired (PaO2 / FiO2) <300 mm Hg, respiratory rate> 30 breaths per minute, or lung infiltrates > 50 %.
All donors expressed their informed consent to donate CCP via plasmapheresis or whole blood donation. Also, they met the standard criteria for plasma donation, including a negative serological result for hepatitis B, C, HIV, HTLV, and syphilis. CCP donors fully recovered and symptom-free for 28 consecutive days could also return to donate just like allogeneic donors. The study was approved by High Institute for Education and Research in Transfusion Medicine: IR.TMI.REC.1399.018. The maximum plasma volume was 500 cc collected from apheresis (source plasma) or 500 cc whole blood (recovered plasma). Plasma was collected by apheresis equipment using MCS+ (HAEMONETICS, USA) and XJC2000 (NIGALE, China). Therapeutic CCP was collected for injection into patients diagnosed with laboratory-confirmed COVID-19 infection as a therapeutic approach. Therapeutic CCPs included source/recovered COVID-19 FFP from all males and nulliparous females with positive rapid test results. However, nontherapeutic CCP units included source/recovered COVID19- FFP from all primi/multiparous women and men/nulliparous women whose IgG rapid test was negative.
The prepared plasma was frozen in less than one hour after donation at a temperature of ≤-30 °C by blast freezer during 8 h. Three tubes were taken from each donor for the screening tests, blood grouping, and antibody titration. Titration tubes were centrifuged; consequently, the prepared serums were stored in the freezer at −20 °C.
2.2. Serological test
Meeting the inclusion criteria for CCP donation by each donor was followed by performing a rapid antibody test. The COVID-19 IgG / IgM rapid test strip is a qualitative immunoassay for the detection of anti-SARS-CoV-2 IgG and IgM antibodies in blood or plasma samples (Table 1 ). This test was performed based on the test catalog of companies; COVID-19 IgG/IgM Rapid Test Dipstick (Whole blood/serum/plasma) Package insert (China, Hangzhou All Test Biotech Co.), Wondfo (China, Hangzhou Wondfo Biotech Co.), and COVID-19 IgG/IgM Antibody Test Kit (Colloidal Gold) Package insert (China, Biosino Bio-Technology and Science Inc).
Table 1.
Interpretation of serologic test.
| IgG | IgM | Donation status | |
|---|---|---|---|
| 1 | Positive | Positive | *Therapeutic use |
| 2 | Positive | Negative | * Therapeutic use |
| 3 | Negative | Negative | Nontherapeutic use |
| 4 | Negative | Positive | 28-day deferral (donor can apply for donation 28 days later) |
Related to male and nulliparous female donors.
2.3. Determination of IgG level against S1 spike protein and its titration in the donated plasma
Measurement of anti-SARS-CoV-2 IgG antibody levels in CCP units was performed by enzyme-linked immunosorbent assay (ELISA) method via commercial kit (Euroimmun AG, Luebeck, Germany) according to the manufacturer’s instruction. Patient samples were diluted 1:101 in sample buffer to evaluate antibody titers. The ratios for this dilution are linearly interpolated based on the results obtained for 1:80 and 1:160. This assay applies a specific calibrator to report the ratio of the extinction of the control or patient sample over the extinction of the calibrator. A ratio above the cutoff value (≥ 1.1) defines the final interpretation of positivity. A greater ratio represents higher antibody levels. CCPs containing positive ELISA ratio (≥1.1) and titers above 1:40 are considered antibody-containing plasma.
2.4. Data collection tools
Data were collected prospectively using a predetermined case report forms and Negareh organizational dataset from all Iran's blood centers. Preliminary information about donors such as age, gender, blood type, type of donation, place of residence that determines the particular ethnicity, donation history according to the specific code on each bag for each donor was collected from IBTO software (Negareh dataset).
2.5. Statistical analysis
The analysis was done using SPSS software version 25. Descriptive statistics [mean, median, standard deviation (SD) and interquartile range (IQR)] were used appropriately. We assessed the independent factors using logistic and linear regression with antibody titration results by ELISA method and rapid test results. Independent variables that were significantly associated with a dependent variable in univariate analysis (p < 0.1) were included in multivariate binary logistic regression analysis (backward conditional, P for omission <0.1). The results presented as OR with 95 % confidence interval (CI). We used the Chi-Square test to evaluate the relationship between antibody titer and rapid test results. The Mann-Whitney U test was used to execute analyses of continuous variables. The categorized data were appropriately compared using the Chi-Square test or Fisher’s test. Significance was set at p < 0.05.
Role of finding source: The study received no funding and relied on IBTO's internal resources.
3. Results
3.1. Donors of convalescent plasma
CCP was obtained from 11,949 donors with molecular confirmation of previous COVID-19 infection (Fig. 1 , Supplement 1). This population included men (92.5 %) more than women (7.5 %) with a mean age of 39.6: 5113 men out of 5712 (92.7 %) and 402 women out of 464(86.6 %) had positive ELISA test results (≥1.1) (Table 2, Table 3 ). Moreover, comparing males and females regarding positive rapid test results shows the former having 6966 out of 7756 (89.8 %) and the latter 399 out of 606 (65.8 %) (Fig. 1).
Fig. 1.
Flow chart of the study design.
Table 2.
Convalescent plasma donor’s characteristics.
| Independent variable | ELISA IgG positive result (ratio ≥1.1) | ELISA IgG negative result (ratio<0.8) | Total donors | Missing data | P Value |
|---|---|---|---|---|---|
| age (years) - median (IQR) | 40(14) | 39(16) | 40(13) | 1711 (20.8 %) | P<0.0001 |
| Gender – no. (%) | 2030 (24.7 %) | P<0.05 | |||
| male | 5113 (92.7 %) | 599 (90.6 %) | 5712 (92.5 %) | ||
| female | 402 (86.6 %) | 62 (13.4 %) | 464 (7.5 %) | ||
| The location of donors in terms of the prevalence of SARS-CoV-2- no. (%) | 1466 (17.9 %) | P > 0.05 | |||
| Red | 3648 (60.8 %) | 429 (58.1 %) | 4077 (60.5 %) | ||
| Orange | 1497 (24.9 %) | 213 (28.9 %) | 1710 (25.4 %) | ||
| Yellow | 857 (14.3 %) | 96 (13.0 %) | 953 (14.1 %) | ||
| Donation history- no. (%) | 2121 (25.8 %) | P > 0.05 | |||
| First donation | 5062 (93.2 %) | 598 (91.9 %) | 5660 (93.0 %) | ||
| Return | 372 (6.8 %) | 53 (8.1 %) | 425 (7.0 %) | ||
| Ethnicity- no. (%) | 1731 (21.1 %) | P<0.0001 | |||
| Persians | 3521 (61.2 %) | 404 (55.7 %) | 3925 (60.6 %) | ||
| Azeris | 1189 (20.7 %) | 182 (25.1 %) | 1371 (21.2 %) | ||
| Turkmens | 251 (4.4 %) | 5 (0.7 %) | 256 (4.0 %) | ||
| Gilaks | 42 (0.7 %) | 15 (2.1 %) | 57 (0.9 %) | ||
| Arabs | 523 (9.1 %) | 101 (13.9 %) | 624 (9.6 %) | ||
| Baluchs | 224 (3.9 %) | 18 (2.5 %) | 242 (3.7 %) | ||
| Length of hospitalization (days) - median (IQR) | 2.74 – 0(5) | 3.01 – 0(6) | 2.47 – 0(5) | 7206 (87.8 %) | P > 0.05 |
| Chest CT, positive result- no. (%) | 60(11.8 %) | 5(9.1 %) | 65(11.5 %) | 7633 (93.0 %) | P > 0.05 |
| Blood types – no. (%) | 2149 (26.2 %) | P<0.01 | |||
| A | 1837(33.9 %) | 203(31.6 %) | 2040(33.7 %) | ||
| B | 1338(24.7 %) | 147(22.9 %) | 1485(24.5 %) | ||
| AB | 487(9.0 %) | 38(5.9 %) | 57(8.7 %) | ||
| O | 1753(32.4 %) | 254(39.6 %) | 2007(33.1 %) | ||
| Rh- no. (%) | 2149 (26.2 %) | P > 0.05 | |||
| Positive | 4930(91.0 %) | 575(89.6 %) | 5505(90.9 %) | ||
| Negative | 485(87.9 %) | 67(12.1 %) | 552(9.1 %) | ||
| Donation type- no. (%) | 2127 (25.9 %) | NA | |||
| Recovered | 352(6.5 %) | 63(9.6 %) | 415(6.8 %) | ||
| Source | 5071(93.5 %) | 593(90.4 %) | 5664(93.2 %) | ||
| Type of CCP- no. (%) | 2339 (28.5 %) | P<0.0001 | |||
| Therapeutic | 5140 (87.66 %) | ||||
| Nulliparous female with positive rapid test | 187 (4.03 %) | 28 (5.6 %) | 215(4.18 %) | ||
| Men with positive rapid test | 4453 (95.97 %) | 472 (94.4 %) | 4925(95.82 %) | ||
| Nontherapeutic | 723 (12.34 %) | ||||
| ever-parous women/Nulliparous female with negative rapid test | 135 (22.88 %) | 24 (18.04 %) | 159(22.0 %) | ||
| Men with negative rapid test | 455 (77.12 %) | 109 (81.96 %) | 564(78.0 %) |
NA: Not applicable.
Table 3.
Characteristics of non-parous females / males convalescent plasma donors.
| Independent variable | ELISA IgG positive result (ratio ≥1.1) | ELISA IgG negative result (ratio<0.8) | Total donors |
|---|---|---|---|
| age (years) - median (IQR) | 33(39)/40(48) | 28(34)/37(43) | 33(39)/40(47) |
| The location of donors in terms of the prevalence of SARS-CoV-2- no. (%) | |||
| Red | 120(71.0 %)/2337(61.1 %) | 21(77.8 %)/237(58.2 %) | 141(71.9 %)/2574(60.8 %) |
| Orange | 31(18.3 %)/963(25.2 %) | 6(22.2 %)/119(29.2 %) | 37(18.9 %)/1082(25.6 %) |
| Yellow | 18(10.7 %)/525(13.7 %) | 0(0%)/51(12.5 %) | 18(9.2 %)/576(13.6 %) |
| Donation history- no. (%) | |||
| First donation | 181(97.3 %)/4096(92.9 %) | 27(96.4 %)/425(91.4 %) | 208(97.2 %)/4521(92.7 %) |
| Return | 5(2.7 %)/314(7.1 %) | 1(3.6 %)/40(8.6 %) | 6(2.8 %)/354(7.3 %) |
| Ethnicity- no. (%) | |||
| Persians | 97(62.6 %)/2256(62.3 %) | 15(55.6 %)/210(53.3 %) | 112(61.5 %)/2466(61.5 %) |
| Azeris | 33(21.3 %)/819(22.6 %) | 6(22.2 %)/138(35.0 %) | 39(21.4 %)/957(23.8 %) |
| Turkmens | 0(0%)/247(6.8 %) | 0(0%)/5(1.3 %) | 0(0%)/252(6.3 %) |
| Gilaks | 2(1.3 %)/37(1.0 %) | 0(0%)/5(1.3 %) | 2(1.1 %)/42(1.0 %) |
| Arabs | 23(14.8 %)/260(7.2 %) | 6(22.2 %)/36(9.1 %) | 29(15.9 %)/296(7.4 %) |
| Length of hospitalization (days) - median (IQR) | 0 (0) | 0 (0) | 0 (0) |
| Chest CT- no. (%) | |||
| positive result | 1(11.1 %)/60(14.1 %) | 1(100 %)/2(7.7 %) | 2(20.0 %)/62(13.7 %) |
| negative result | 8(88.9 %)/367(85.9 %) | 0(0%)/24(92.3 %) | 8(80 %)/391(86.3 %) |
| Blood types – no. (%) | |||
| A | 66(36.5 %)/1509(34.4 %) | 10(40.0 %)/143(31.1 %) | 76(36.9 %)/1652(34.1 %) |
| B | 42(23.2 %)/1087(24.8 %) | 7(28.0 %)/99(21.5 %) | 49(23.8 %)/1186(24.5 %) |
| AB | 19(10.5 %)/391(8.9 %) | 0(0%)/30(6.5 %) | 19(9.2 %)/421(8.7 %) |
| O | 54(29.8 %)/1403(32.0 %) | 8(32.0 %)/188(40.9 %) | 62(30.1 %)/1591(32.8 %) |
| Rh- no. (%) | |||
| Positive | 165(91.2 %)/3992(90.9 %) | 22(88.0 %)/416(90.4 %) | 187(90.8 %)/4408(90.9 %) |
| Negative | 16(8.8 %)/398(9.1 %) | 3(12.0 %)/44(9.6 %) | 19(9.2 %)/442(9.1 %) |
| Donation type- no. (%) | |||
| Recovered | 3(1.6 %)/238(7.4 %) | 0(0%)/55(11.7 %) | 3(1.4 %)/383(7.8 %) |
| Source | 183(98.4 %)/4113(92.6 %) | 28(100 %)/415(88.3 %) | 211(98.6 %)/4528(92.2 %) |
Most donors had mild to moderate disease severity at the time of infection, 34.8 % had a history of hospitalization, and 17.5 % had a positive CXR/CT scan result.
3.2. Antibody levels in CCP units
ELISA technique had been performed for 8206 donors (68.7 %), of whom 7275 (88.7 %) had positive results. The median SARS-CoV-2 IgG antibody titer was 1:320 (range <1:40 to ≥1:640) among ELISA IgG positive donors. IgG antibody titers were below the threshold of 1:160 in 1508 CCP units (20.8 %) and between 1:160 and 1:640 in 3676 CCP units (50.8 %).
3.3. Correlation between ELISA results, antibody titers, and rapid tests among CCP donors
A significant difference was observed between antibody titration results and rapid results (P < 0.0001). ELISA results were not significantly different from Rapid test (P = 0.14 [95 % CI: 0.87–2.52]) (Supplement 2).
3.4. Logistic regression for variables related to ELISA and Rapid results
We assessed and identified eight variables related to ELISA results (Table 4 ) using univariate analysis:
Table 4.
Univariate and multivariate logistic or linear regression for factors related to ELISA and Rapid outcome and SARS-CoV-2 IgG antibody titers.
| Dependent factor | Independent factor | Univariate P | Multivariate OR (95 % CI) | P value |
|---|---|---|---|---|
| ELISA result | Age | 0.000* | 0.951 (0.921−0.982) | 0.002 |
| Sex | 0.05* | P > 0.05 | ||
| Blood group | 0.001* | P > 0.05 | ||
| Rh | 0.2 | |||
| The location in terms of SARS-CoV-2prevalence and red area as a reference | 0.06* | 2.887 (1.482−5.625) | 0.002 | |
| Ethnicity (Persians as references) | 0.000* | 2.801 (1.015−7.731) | 0.047 | |
| CXR/CT scan result | 0.3 | |||
| Hospitalization history | 0.000* | 2.598 (1.300−5.189) | 0.007 | |
| Number of hospitalization days | 0.9 | |||
| Donation history | 0.2 | |||
| Type of CCP | 0.000* | 18.820 (8.02−44.163) | 0.000 | |
| Antibody titers | Age | 0.000* | 0.106(0.519−4.456) | 0.013 |
| Sex | 0.000* | P > 0.05 | ||
| Blood group | 0.02* | P > 0.05 | ||
| Rh | 0.9 | |||
| The location in terms of SARS-CoV-2prevalence and red area as a reference | 0.000* | 0.547(113.330−157.033) | 0.000 | |
| Ethnicity (Persians as references) | 0.000* | P > 0.05 | ||
| CXR/CT scan result | 0.1 | |||
| Hospitalization history | 0.000* | P > 0.05 | ||
| Number of hospitalization days | 0.06* | 0.122(0.459−8.668) | 0.029 | |
| Donation history | 0.07* | P > 0.05 | ||
| Type of CCP | 0.000* | 0.131(42.084−192.280) | 0.002 | |
| Rapid result | Age | 0.04* | P > 0.05 | |
| Sex | 0.2 | |||
| Blood group | 0.2 | |||
| Rh | 0.7 | |||
| The location in terms of SARS-CoV-2prevalence and red area as a reference | 0.000* | 0.321(0.149−0.690) | 0.004 | |
| Ethnicity (Persians as references) | 0.000* | P > 0.05 | ||
| Donation history | 0.02* | |||
| Type of CCP | 0.06* | 4.52(2.24−9.123) | 0.000 |
The variables entered into the logistic regression are multivariate.
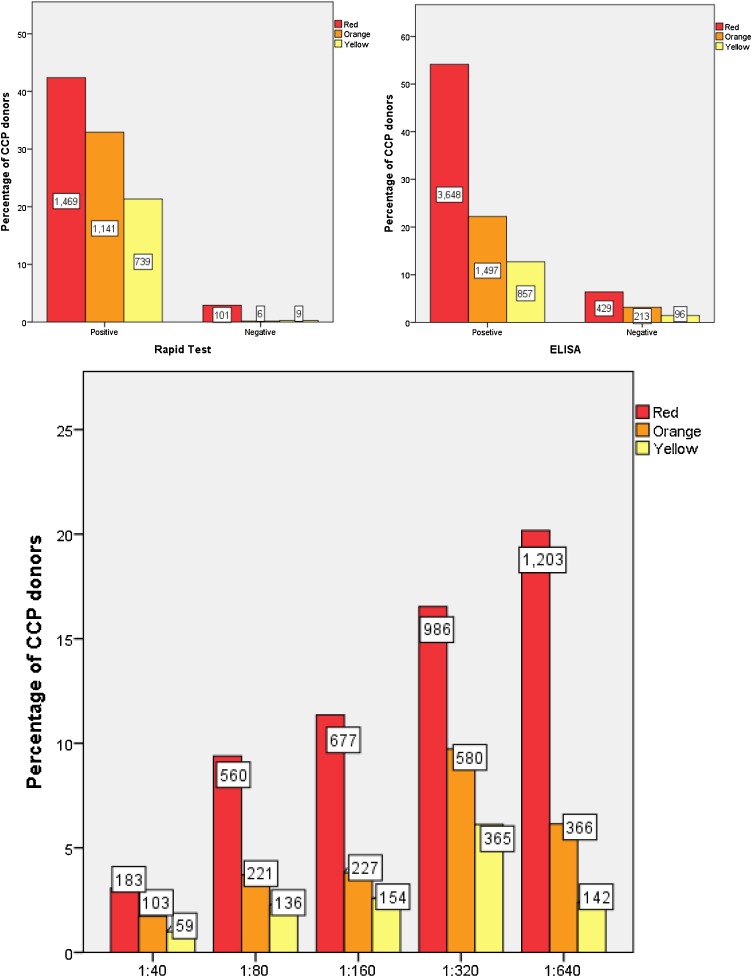
location in terms of SARS-CoV-2 prevalence (Fig. 2 ), gender (Fig. 3 ), blood type (Fig. 4 ), type of CCP (Fig. 5 ), history of hospitalization (Fig. 4), CXR/CT scan results, ethnicity and age.
Fig. 2.
Correlation between ELISA test and rapid test results and antibody titer in different donation centers across the country in terms of SARS-CoV-2 outbreak. The number of donors has been represented by a box in each test.
Yellow: PCR test is seen in one or more cases, and the risk is as expected. The number of definite new cases per 100,000 people is 1–9.
Orange: The number of definite new cases is 10–24 per 100,000 people.
Red: More than 25 cases per day per 100,000 population.
CCP: Convalescent plasma.
Fig. 3.
Correlation between antibody titer results and gender. The number of donors has been represented by a box in each test. CCP: Convalescent plasma.
Fig. 4.
Correlation between ELISA and Rapid test results and antibody titer in positive ELISAs with blood group. The number of donors has been represented by a box in each test. CCP: Convalescent plasma.
Fig. 5.
Antibody titer based on hospitalization history. The number of donors has been represented by a box in each group. CCP: Convalescent plasma.
Moreover, five variables were related to Rapid test results, such as age, location in terms of SARS-CoV-2 prevalence, donation history, ethnicity and type of CCP.
In a multivariate logistic regression analysis of these variables, age, location in red areas in terms of SARS-CoV-2 prevalence, therapeutic CCP, ethnicity, and hospitalization history were associated with ELISA results. Also, we identified two variables, including therapeutic CCP and red areas in terms of SARS-CoV-2 prevalence, associated with Rapid results, using multivariate logistic regression analysis (Table 4).
Furthermore, using linear regression analysis, the variables related to antibody titration were age, location in red areas in terms of SARS-CoV-2 prevalence, number of hospitalization days, donation history, and plasma type (therapeutic CCP).
4. Discussion
This study suggests several factors associated with high antibody titers to understand characteristics for ideal convalescent plasma donation.
There was a correlation between demographic characteristics including age, gender, location with high virus frequency, and antibody levels in CCP units measured both by ELISA and rapid tests and antibody titers assessed in a subset of CCP donors.
Moreover, high antibody titers were also observed in CCP donors with hospitalization history. IgG-antibody titers equal to or above 1: 640 in CCP units of donors with hospitalization history rather than without hospitalization history were more than two-fold. Like our finding, donors recovered from moderate or severe COVID-19 included in another study [12] had high antibody titers. Previous studies demonstrated that many CCP plasma samples have moderate antibody levels, and commercial experiments have varying degrees of accuracy in predicting nAb activity. A central biological question is: What factors cause a large change in the antibody titer (neutralizing or binding) in CCP donors? Some variables, including the dose of SARS-CoV-2 exposure, can cause variation in the extent and spread of SARS-CoV-2. We reported that in areas where the prevalence of COVID-19 virus was high, antibody titer in donors accordingly increased [13].
Donation history is one of the other variables investigated in this study. The antibody titer in the return donors was estimated lower than that in the first donors; though, the difference not being statistically significant. The lack of significance pertains to the fact that the antibody titer did not decrease given the 28-day interval for CCP donation attempts.
When controlling for other variables, such as the use of different Rapid and ELISA kits for donors with various sensitivity and specificity, only age, ethnicity, location with high virus frequency, hospitalization history, male and nulliparous female donors who had a positive rapid test were significantly associated with high antibody titers.
The convalescent plasma therapy in treatment of various infectious diseases is not a novel concept. It has recently been used to treat patients with Ebola. It has subsequently contributed to discovering particular antibodies with high virus-neutralizing activity and the development of synthetic monoclonal antibodies to treat Ebola [14]. Currently, the FDA recommends concentrations of nAb > 160, but a lower titer (1:80) is allowed if no alternative is available. The FDA, however, has not provided a methodology used to obtain these levels, despite potential variables. As already described by others, [15], not all CCP units contain measurable antibodies, and 20 % of donors did not have IgG against S1 above the cutoff of the test employed, while in the current study, this rate was 11.3 % of donors. The median antibody titer in our study was 1:320 for CCP units.
Consistent with our finding, antibody titers in CCP units during the 2009 H1N1 pandemic were correlated with donor age, gender, or location with high virus frequency. Similar to our results, donors included in this study [16] had elevated nAb in younger convalescent donors and those who were older than 55 years. Some small case series suggested that male gender, older age, and hospitalization of patients with COVID-19 were consistently associated with increased antibody responses and worse COVID-19 outcome. They appeared to be factored in identifying individuals most likely to have potent antiviral antibodies [15,17,18]. This study reported a more extensive series of CCP donors with a range of variables that might affect antibody titers' quantity.
Our data indicate that despite the vast epidemiological pattern of blood group O among donors in Iran, [19] it was blood group A in our CCP donors, as being more frequent than the others. This is compatible with two other studies [20,21]. The latter described that anti-A antibody inhibits the binding of SARS-CoV-2 protein-expressing cells explicitly to ACE2-expressing cell lines. Therefore, a lower infection chance of blood type O and a higher in blood type A may exist. In a case-control study on blood types of COVID-19 patients [21], the proportion of blood group A was higher than blood group O among SARS-CoV-2+ patients compared to SARS-CoV-2- cases; although in both cases, the result was significant only in Rh-positive blood groups. Blood types were associated with neither the considered factors (age, sex, hypertension, diabetes, overweight, and chronic cardiovascular and lung disorders) nor intubation or death in COVID-19 patients. Similarly, in the present study, no relationship was observed between blood groups and disease severity in terms of lung involvement (p = 0.5) or hospitalization history (p = 0.1).
This study had some limitations. For example, since CXR/CT scan information and the history and duration of hospitalization were added to this study from October 2020; thus, the amount of data collected is less than other data types, which may be reflected on the results. At the same time, the data such as the duration of previous SARS-CoV-2 infection in donors have not been identified, which may be one factor involved in selecting donors with high antibody titers. The other limitation pertains to antibody titration being performed only by commercial ELISA kits with no cell culture methods or tests to detect neutralizing antibody titer in this study.
Overall, the results of the present study provide a roadmap for selecting individuals possibly with high levels of anti-SARS-CoV-2 IgG antibodies as preferred donors for CCP donation.
CRediT authorship contribution statement
Alieh Fazeli: Methodology, Formal analysis, Investigation, Writing - original draft, Writing - review & editing. Shahin Sharifi: Resources, Supervision. Saeed Mohammadi: Resources, Writing - review & editing. Mehran Bahraini: Methodology, Investigation, Writing - original draft, Writing - review & editing. Ali Arabkhazaeli: Data curation, Writing - review & editing. Nooshin Jelveh: Formal analysis, Investigation. Peyman Eshghi: Conceptualization, Project administration, Funding acquisition.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.transci.2021.103302.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Sohrabi C., Alsafi Z., O’Neill N., Khan M., Kerwan A., Al-Jabir A., et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sarkar C., Mondal M., Torequl Islam M., Martorell M., Docea A.O., Maroyi A., et al. Potential therapeutic options for COVID-19: current status, challenges, and future perspectives. Front Pharmacol. 2020;11:1428. doi: 10.3389/fphar.2020.572870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forni G., Mantovani A. COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ. 2021;28(2):626–639. doi: 10.1038/s41418-020-00720-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14(1):69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- 5.Luke T.C., Casadevall A., Watowich S.J., Hoffman S.L., Beigel J.H., Burgess T.H. Hark back: passive immunotherapy for influenza and other serious infections. Crit Care Med. 2010;38:e66–e73. doi: 10.1097/CCM.0b013e3181d44c1e. [DOI] [PubMed] [Google Scholar]
- 6.Marano G., Vaglio S., Pupella S., Facco G., Catalano L., Liumbruno G.M., et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfusion. 2016;14(2):152. doi: 10.2450/2015.0131-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.-M., Lim W.S., et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211(1):80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zost S.J., Gilchuk P., Case J.B., Binshtein E., Chen R.E., Reidy J.X., et al. Potently neutralizing human antibodies that block SARS-CoV-2 receptor binding and protect animals. bioRxiv. 2020 [Google Scholar]
- 9.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. Jama. 2020;324(5):460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu S.T., Lin H.-M., Baine I., Wajnberg A., Gumprecht J.P., Rahman F., et al. Convalescent plasma treatment of severe COVID-19: a propensity score–matched control study. Nat Med. 2020;26(11):1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 11.The Lancet Haematology The resurgence of convalescent plasma therapy. Lancet Haematol. 2020;7(5):e353. doi: 10.1016/S2352-3026(20)30117-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X., Guo X., Xin Q., Pan Y., Hu Y., Li J., et al. Neutralizing antibodies responses to SARS-CoV-2 in COVID-19 inpatients and convalescent patients. Clin Infect Dis. 2020;71(10):2688–2694. doi: 10.1093/cid/ciaa721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luchsinger L.L., Ransegnola B.P., Jin D.K., Muecksch F., Weisblum Y., Bao W., et al. Serological assays estimate highly variable SARS-CoV-2 neutralizing antibody activity in recovered COVID-19 patients. J Clin Microbiol. 2020;58(12) doi: 10.1128/JCM.02005-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maor Y., Cohen D., Paran N., Israely T., Ezra V., Axelrod O., et al. Compassionate use of convalescent plasma for treatment of moderate and severe pneumonia in COVID-19 patients and association with IgG antibody levels in donated plasma. EClinicalMedicine. 2020;26 doi: 10.1016/j.eclinm.2020.100525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klein S.L., Pekosz A., Park H.-S., Ursin R.L., Shapiro J.R., Benner S.E., et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest. 2020;130(11):6141–6150. doi: 10.1172/JCI142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khalenkov A., He Y., Reed J.L., Kreil T.R., McVey J., Norton M., et al. Characterization of source plasma from self‐identified vaccinated or convalescent donors during the 2009 H 1 N 1 pandemic. Transfusion. 2018;58(5):1108–1116. doi: 10.1111/trf.14530. [DOI] [PubMed] [Google Scholar]
- 17.Scully E.P., Haverfield J., Ursin R.L., Tannenbaum C., Klein S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat Rev Immunol. 2020;11:1–6. doi: 10.1038/s41577-020-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang B., Zhou X., Zhu C., Song Y., Feng F., Qiu Y., et al. Immune phenotyping based on the neutrophil-to-lymphocyte ratio and IgG level predicts disease severity and outcome for patients with COVID-19. Front Mol Biosci. 2020;7:157. doi: 10.3389/fmolb.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pourfathollah A., Oody A., Honarkaran N. Geographical distribution of ABO and Rh (D) blood groups among Iranian blood donors in the year 1361 (1982) as compared with that of the year 1380 (2001) Scientific Journal of Iran Blood Transfus Organ. 2004;1(1):11–17. [Google Scholar]
- 20.Guillon P., Clément M., Sébille V., Rivain J.-G., Chou C.-F., Ruvoën-Clouet N., et al. Inhibition of the interaction between the SARS-CoV spike protein and its cellular receptor by anti-histo-blood group antibodies. Glycobiology. 2008;18(12):1085–1093. doi: 10.1093/glycob/cwn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zietz M., Tatonetti N.P. Testing the association between blood type and COVID-19 infection, intubation, and death. MedRxiv. 2020 doi: 10.1038/s41467-020-19623-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.