Abstract
The central complex is one of the major brain regions that control sleep in Drosophila. However, the circuitry details of sleep regulation have not been elucidated yet. Here, we show a novel sleep-regulating neuronal circuit in the protocerebral bridge (PB) of the central complex. Activation of the PB interneurons labeled by the R59E08-Gal4 and the PB columnar neurons with R52B10-Gal4 promoted sleep and wakefulness, respectively. A targeted GFP reconstitution across synaptic partners (t-GRASP) analysis demonstrated synaptic contact between these two groups of sleep-regulating PB neurons. Furthermore, we found that activation of a pair of dopaminergic (DA) neurons projecting to the PB (T1 DA neurons) decreased sleep. The wake-promoting T1 DA neurons and the sleep-promoting PB interneurons formed close associations. Dopamine 2-like receptor (Dop2R) knockdown in the sleep-promoting PB interneurons increased sleep. These results indicated that the neuronal circuit in the PB, regulated by dopamine signaling, mediates sleep-wakefulness.
Keywords: Drosophila melanogaster, protocerebral bridge, sleep, central complex, dopamine
Introduction
Sleep is a basic physiological state evolutionarily conserved among animal species (Allada and Siegel, 2008; Cirelli and Tononi, 2008). The fruit fly, Drosophila melanogaster, has emerged as a powerful model system for uncovering the molecular and cellular basis of sleep-wakefulness. In Drosophila, sleep is defined by behavioral features, including circadian control of the timing of sleep, homeostasis of the amount of sleep, and increased arousal threshold, similar to other animal species that do not exhibit the characteristic electroencephalogram patterns of sleep (Campbell and Tobler, 1984).
The central complex is a unique midline neuropil structure in the adult insect brain, composed of four substructures: the protocerebral bridge (PB), the fan-shaped body (FB), the ellipsoid body (EB), and the noduli (NO). It serves as a higher-order integrator for sensory information and various motor control (Homberg, 2008). Accumulating evidence from a number of neurogenetic studies has revealed that the central complex plays a crucial role in Drosophila sleep regulation. Sleep-promoting neurons, having extensive presynaptic arborizations, have been identified in the dorsal layer of the FB (dFB) (Donlea et al., 2011; Ueno et al., 2012). Dopamine is a neurotransmitter that is essential for maintaining wakefulness in Drosophila, similar to mammals (Andretic et al., 2005; Kume et al., 2005). The protocerebral posterior medial 3 (PPM3) cluster of dopaminergic (DA) neurons projecting to the dFB neurons controls sleep through Dopamine 1-like receptor 1 (Dop1R1) signaling (Liu et al., 2012; Ueno et al., 2012). Neurons that project to the ventral layer of the FB promote sleep and mediate consolidation of long-term memory (Dag et al., 2019). The homeostatic mechanism of sleep would be associated with changes in neuronal activity of the sleep-promoting dFB neurons and their functional upstream neurons, the R5 neurons in the EB (Donlea et al., 2014; Liu et al., 2016). In addition, the dFB neurons form a recurrent circuit with the EB R5 neurons via helicon cells to regulate sleep homeostasis (Donlea et al., 2018). Regarding circadian regulation of sleep-wakefulness, recent studies have shown that sleep-modulating dorsal neurons 1 (DN1) of circadian clock neurons and EB ring neuron subtypes are functionally connected via sleep-promoting tubercular-bulbar (TuBu) neurons and that this circuit is likely to regulate sleep-wakefulness (Guo et al., 2018; Lamaze et al., 2018). Besides, sleep-promoting lateral posterior neurons (LPNs), another group of clock neurons, appear to form close associations to the sleep-promoting dFB neurons and activate these neurons (Ni et al., 2019). Detailed mechanisms for regulating sleep-wakefulness by the central complex are being clarified. However, because the four substructures of the central complex are interconnected, a population of central complex neurons other than the FB neurons and the EB R5 neurons should also be implicated in the control of sleep-wakefulness.
Here, we begin to explore a novel sleep-regulating neuron type in the central complex and discover that the sleep-promoting PB interneurons have direct neuronal connections to the wake-promoting PB columnar neurons projecting from the PB to the FB and the NO. Moreover, we reveal that the T1 cluster of DA neurons regulates sleep by acting on the sleep-promoting PB interneurons through D2 dopamine receptor signaling.
Materials and Methods
Fly Strains and Rearing Conditions
Fruit flies (Drosophila melanogaster) were raised at 25°C in 50–60% relative humidity on standard medium containing cornmeal, yeast, glucose, wheat germ, and agar. They were maintained under a 12-h light:12-h dark (LD) cycle. Eleven mutant flies with structural defects in the central complex: agnosticX1 (agnX1), central-body-defectKS171 (cbdKS171), cbdKS188, cbd762, central-complex-broadKS145 (ccbKS145), central-complex-derangedKS135 (ccdKS135), central-complexKS181 (cexKS181), ellipsoid-body-openKS263 (eboKS263), ebo678, ebo1041, and no-bridgeKS49 (nobKS49) (Strauss and Heisenberg, 1993), were kindly provided by Roland Strauss. UAS-dTrpA1 (Hamada et al., 2008) lines on the second or third chromosomes were gifts from Paul A. Garrity and were backcrossed over five generations to the control strain (w1118). The teashirt (tsh)-Gal80/CyO (Clyne and Miesenböck, 2008) fly line was a gift from Julie H. Simpson. Cha7.4kb-Gal80 (Cha-Gal80) (Sakai et al., 2009) and MB247-Gal80 (Krashes et al., 2007) were gifts from Takaomi Sakai. The c465-Gal4 (Young and Armstrong, 2010) line was a gift from J. Douglas Armstrong. FLP243 (Alekseyenko et al., 2013) was a gift from Edward A. Kravitz. UAS>stop<mCD8::GFP (Yu et al., 2010) and UAS>stop>dTrpA1 (von Philipsborn et al., 2011) were gifts from Barry J. Dickson. R14F09-Gal4 (stock number: 48652), R15E12-Gal4 (#48608), R16D01-Gal4 (#48722), R37G05-Gal4 (#48133), R38G07-Gal4 (#50019), R40A01-Gal4 (#50072), R44B10-Gal4 (#50202), R45G06-Gal4 (#50244), R52B10-Gal4 (#38820), R52G12-Gal4 (#49581), R52H12-Gal4 (#38856), R55G08-Gal4 (#50422), R59A12-Gal4 (#39206), R59E08-Gal4 (#39219), R67B06-Gal4 (#48294), R74C08-Gal4 (#46711) R83A10-Gal4 (#48371), R83H12-Gal4 (#40374), R91A12-Gal4 (#40573), 10×UAS-IVS–mCD8::GFP (#32187), 10×UAS-IVS-mCD8::GFP (#32188), UAS-DenMark, UAS-syt.eGFP (#33064), R52B10-LexA (#52826), R59E08-LexA (#52832), 13×LexAop2-post-t-GRASP, 20×UAS-pre-t-GRASP (#79040), UAS-CD4-spGFP1-10, lexAop-CD4-spGFP11 (#58755), UAS-Dop2R-RNAi (#50621), and y v; P{CaryP}attP40 (control line for TRiP RNAi lines, #36304) were obtained from the Bloomington Drosophila Stock Center, Indiana University, Indiana, United States. UAS-Dop2R-RNAi (VDRC ID: 1820), UAS-Dicer-2 (60008), and w1118 (the genetic background for VDRC RNAi lines, 60000) were from the Vienna Drosophila RNAi Center (VDRC). P{neoFRT}19A (stock number: 106482) and P{neoFRT}19A, tubP-GAL80, hsFLP; PinYt/CyO (#108064) were obtained from the Kyoto Stock Center, Kyoto Institute of Technology, Kyoto, Japan. Male flies were used in this study unless otherwise noted.
Locomotor Activity and Sleep Analysis
Locomotor activity of individual flies was measured using the Drosophila activity monitoring system (TriKinetics, Waltham, MA, United States) as described previously (Tomita et al., 2015). Flies were placed individually in glass tubes (length, 65 mm; inside diameter, 3 mm) containing either standard medium or sucrose-agar medium (5% sucrose and 1% agar) at one end and were entrained for at least 3 days to LD cycles before they were transferred to constant dark (DD) conditions. Two- to four-day-old male flies were used except for mosaic analysis with a repressible cell marker (MARCM) experiments. Activity data were recorded continuously at 1-min intervals under both LD and DD conditions. Drosophila sleep was defined as continuous immobile periods lasting 5 min or longer, based on previous reports (Hendricks et al., 2000; Shaw et al., 2000; Huber et al., 2004; Kume et al., 2005). Total activity counts, total amount of sleep, and waking activity index were analyzed by Microsoft Excel-based software, as previously described (Kume et al., 2005) and averaged over 3 days. For conditional dTrpA1 activation experiments, control and experimental flies were grown at 22°C. Because daytime sleep under LD conditions is partly due to light-induced suppression of locomotor activity (Kume et al., 2005), sleep measurements were performed in DD following LD cycles. At circadian time (CT) 0 (the beginning of a subjective day), temperature was raised from 22 to 29°C for 24 or 48 h to activate dTrpA1-expressing neurons and then returned to 22°C.
Video Tracking of Locomotion
Offspring from the control (R59E08-Gal4 × tsh-Gal80) and the experimental (R59E08-Gal4 × tsh-Gal80; UAS-dTrpA1) crosses were housed in glass tubes and entrained to an LD cycle at 22°C. After entrainment, these flies were maintained in DD with illumination by near-infrared LEDs. At CT 0 on day 2 in DD, flies were transferred from 22 to 29°C for 12 h to activate dTrpA1. The behavior of flies was recorded at 2 frames/sec using a USB camera (Flea3 USB3.0: FL3-U3-13Y3M-C, Point Gray Research Inc., Richmond, BC, Canada) fitted with a macro zoom lens (MLH-10X, computer, Tokyo, Japan) during late subjective day (CT 8 to CT 12) at 29°C. The captured images were converted into 8-bit grayscale images. A composite background image was subtracted from each video image. The position of each individual fly was calculated for each subtracted image using the Particle Tracker 2D/3D, an ImageJ plugin (Sbalzarini and Koumoutsakos, 2005).
Mechanical Stimulation During Sleep
The control (R59E08-Gal4 × w1118) and the experimental (R59E08-Gal4 × UAS-dTrpA1) flies were placed in glass tubes in the Drosophila Activity Monitor 2 (Trikinetics, Waltham, MA, United States) and maintained in DD followed by 3 days LD cycles at 22°C. At CT 0 on day 3 in DD, flies were transferred from 22 to 29°C for 24 h to activate dTrpA1. Using an Analog MultiTube Vortexer (02215450, Fisher Scientific) controlled by a custom software built on a LabVIEW (National Instruments) platform, mechanical stimuli were provided to flies by shaking the activity monitor for 1 s with a speed setting of 4, 13 times every hour from CT 12 to CT 0 during dTrpA1 activation. Locomotor activity was monitored every 15 s and any activity of sleeping flies during the 1 min interval between 15 and 75 s after a stimulus was scored as a response to the stimulus. Spontaneous arousal was assessed by the percentage of unstimulated sleeping control and experimental flies that started to move within 1 min at the beginning of every hour during subjective night (CT 12 to CT 0) at 29°C. The net responsiveness of flies to mechanical stimuli was obtained by subtracting the percentage of spontaneous arousal from that of flies awakened by mechanical stimuli.
The Mosaic Analysis With a Repressible Cell Marker System
To generate flies for MARCM, female flies carrying FRT19A, tub-GAL80, hs-FLP; UAS-dTrpA1 (X; II) were crossed with males carrying FRT19A; R52B10-Gal4, 10×UAS-IVS-mCD8::GFP (X; III) at 22°C. Following egg-laying for 48 h, embryos or first instar larvae were heat-shocked at 37°C for 1 h to induce FLP-mediated recombination across two FRT sites. The amount of sleep in mosaic female flies was measured at 22 and 29°C under DD conditions. After sleep analysis, GFP co-expression with dTrpA1 in the brains was immunohistochemically determined.
Immunohistochemistry
To detect GFP expression in the MARCM experiments, and reconstituted GFP signals in the GRASP analysis, whole-mount immunofluorescence staining of adult brains was performed as described previously (Wu and Luo, 2006). For determination of GFP expression, a GFP polyclonal antibody (A6455, Thermo Fisher Scientific) was used at 1:250 dilution and goat anti-rabbit IgG, Alexa Fluor 568 (A11036, Thermo Fisher Scientific), was used at 1:200 dilution as a secondary antibody. We used monoclonal anti-GFP, clone GFP-20 (G6539, Sigma-Aldrich) at 1:100 dilution for detection of reconstituted GFP signals in the GRASP experiments. Alexa Fluor 488, goat anti-mouse IgG (A11001, Thermo Fisher Scientific) was used at 1:200 dilution as a secondary antibody.
Confocal Imaging
Immunostained brain tissues were imaged by laser scanning confocal microscopes Zeiss LSM 510 or LSM 800 (Carl Zeiss). Adult brains and ventral nerve cords expressing mCD8::GFP or syt-GFP under the control of Gal4 were dissected and scanned using confocal microscopes, without staining. For the t-GRASP experiments, reconstituted GFP signals were visualized without staining. To examine the inhibitory effect of tsh-Gal80 or Cha-Gal80 on the Gal4 activity of R59E08-Gal4 and R52B10-Gal4, the Gal4-driven mCD8::GFP expression in the brains or the ventral nerve cords in the presence and absence of tsh-Gal80 or Cha-Gal80 was imaged and processed in parallel, using identical settings on a confocal microscope.
Statistical Analysis
Data were analyzed as described in the figure legends using Microsoft Excel and R: a language and environment for statistical computing (R Core Team, 20201).
Results
The Protocerebral Bridge Defective Mutant Flies, nobKS49 Decreased Sleep
In order to examine the role of the central complex in sleep regulation, we tested eleven mutant strains with morphological defects in the central complex. These mutant flies were isolated histologically following ethyl methanesulfonate mutagenesis, except for agnX1, which was isolated from a wild population (Strauss and Heisenberg, 1993). Flies were entrained to 12 h light: 12 h dark (LD) cycles for at least 3 days, then maintained in constant dark (DD) conditions. Locomotor activity was recorded in both LD and DD conditions. Sleep was defined as periods of immobile state lasting 5 min or longer, as previously described (Hendricks et al., 2000; Shaw et al., 2000; Huber et al., 2004; Kume et al., 2005). Because most of these structural mutants are still uncharacterized, their total daily activity and sleep were compared to those of three control strains (Canton-S (CS), y w and w1118) under DD conditions. There were no significant differences in total daily activity and sleep between the three control strains (Figures 1A,B). Among the central complex mutants tested, nobKS49 flies showed significant hyperactivity compared with the two control lines (CS and y w). nobKS49 mutants have a defect in the central part of PB (Strauss et al., 1992). No difference was seen in the waking activity index, defined as the total daily activity divided by the active period length, between nobKS49 and controls (Figure 1C), suggesting that hyperactivity in nobKS49 flies is not attributed to aberrant locomotion. On an average, total daily activity significantly increased by about two-fold in nobKS49 relative to controls (Figure 1A). In contrast, total daily sleep in nobKS49 flies decreased to approximately half of that of the CS and y w controls (Figure 1B). These results suggest that PB is involved in sleep regulation.
FIGURE 1.
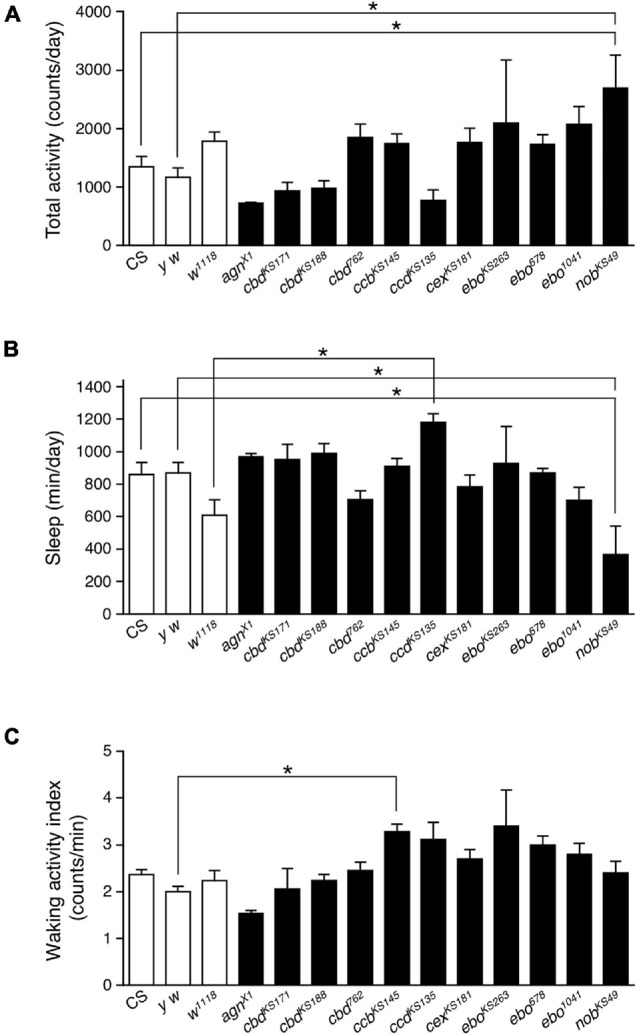
The PB defective nobKS49 decreased sleep. Total daily activity (A), total sleep (B) and waking activity index (C) for control flies (Canton-S, y w and w1118, white bars) and mutant strains with structural defects in the central complex (black bars) in constant dark (DD) conditions. Data are averaged for 3 days and are presented as mean ± standard error of the mean (SEM) (n = 3–11 for each group). Groups with asterisks indicate statistically significant differences from controls (one-way ANOVA followed by Tukey-Kramer post hoc test, p < 0.05).
Activation of Gal4-Expressing Neurons in Gal4 Drivers That Express in the Protocerebral Bridge Affected Sleep
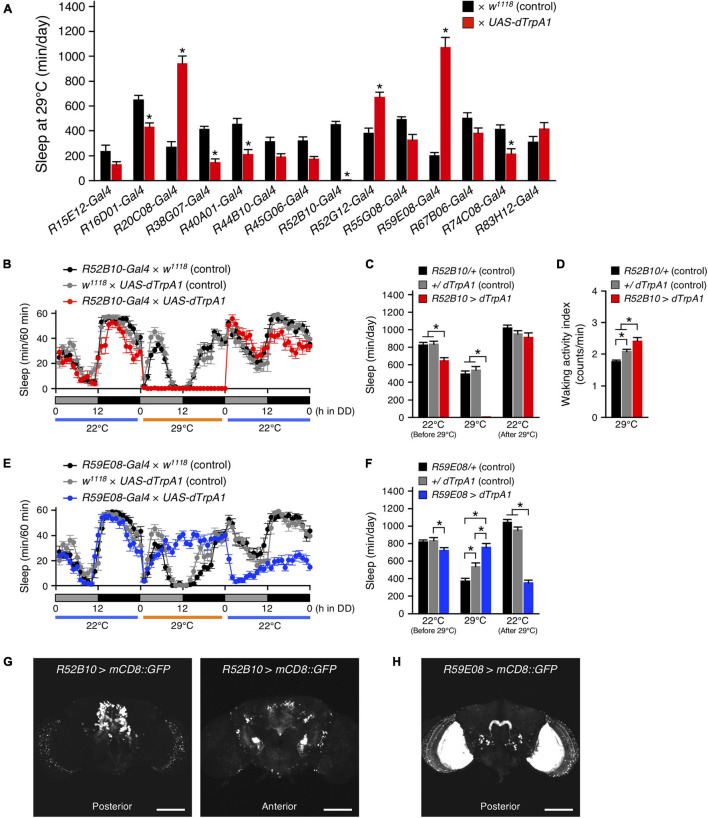
To further explore the involvement of PB neurons in sleep regulation, we examined the effects of transient thermogenetic activation of a subset of PB neurons using the temperature-sensitive cation channel dTrpA1 on the amount of sleep. For this, fourteen Gal4 driver lines that express strongly in PB neurons (PB-Gal4) were selected from the Gal4 image data of the FlyLight Image Database (2 Jenett et al., 2012). Progenies from the control (PB-Gal4 × w1118) and the experimental (PB-Gal4 × UAS-dTrpA1) crosses were grown at 22°C to adulthood. Sleep was measured in adult male progenies in DD following LD cycles. These flies were transferred from 22 to 29°C for 24 h to conditionally activate dTrpA1-expressing neurons and then returned to 22°C. Of the 14 PB-Gal4 drivers tested, the acute activation of neurons with the 5 Gal4 drivers significantly decreased sleep. In particular, the decrease in the sleep amount induced by activation of neurons using the R52B10-Gal4 was most remarkable and flies hardly slept during neuronal activation (Figures 2A–C). This reduced sleep was also observed in male flies under LD conditions (Supplementary Figures 1A,B) and, as shown in a previous report, in female flies in LD (Supplementary Figures 1C,D; Liu et al., 2016). By contrast, the amount of sleep in females under DD conditions was decreased only during subjective night (Supplementary Figures 1E,F). Although sleep loss by the thermogenetic activation using the R52B10-Gal4 caused a clear homeostatic sleep recovery (sleep rebound) during daytime in female flies as has been reported previously (Supplementary Figures 1C,E; Liu et al., 2016), such a clear sleep rebound was not observed in male flies (Figure 2B and Supplementary Figure 1A). The waking activity index was significantly increased during activating R52B10-Gal4 neurons compared to that of controls (Figure 2D), suggesting that R52B10-Gal4-expressing neurons were wake-promoting. On the other hand, the activation of Gal4 expressing neurons in the 3 of 14 PB-Gal4 drivers significantly increased sleep (Figure 2A). The largest effect on sleep induction was caused by the neuronal activation using the R59E08-Gal4. In this Gal4 driver, the amount of sleep in the experimental flies peaked in the middle of the subjective day at 29°C and was maintained at the peak level during neuronal activation (Figure 2E). The increase in sleep amount during activation of R59E08-Gal4-expressing neurons was also observed in female flies in both LD and DD conditions (Supplementary Figures 2C–F), but not in males in LD conditions (Supplementary Figures 2A,B). Sleep amount in the experimental male and female flies was significantly reduced compared with that in the corresponding control flies after stopping the activation (Figures 2E,F and Supplementary Figure 2).
FIGURE 2.
Acute activation of the PB neurons affected sleep. (A) The amount of sleep per day at 29°C in DD conditions in control flies (black bars) and flies expressing dTrpA1 transgene by indicated Gal4 drivers that highly express in PB neurons (red bars). For controls, each Gal4 driver was crossed with w1118 (the genetic background of UAS-dTrpA1 carrying flies). Data are presented as mean ± SEM (n = 6–16 for each group). Asterisks indicate statistically significant differences from control determined by one-way ANOVA followed by Tukey-Kramer post hoc test (p < 0.05). (B–D) Sleep profiles for 60-min intervals (B), total daily sleep (C) or waking activity index (D) for controls (R52B10-Gal4 × w1118, black circles or bars, n = 16; w1118 × UAS-dTrpA1, gray circles or bars, n = 16) or flies expressing dTrpA1 with R52B10-Gal4 (R52B10-Gal4 × UAS-dTrpA1, red circles or bars, n = 16) in DD. Behavior was monitored in DD for 1 day at 22°C, followed by 1 day at 29°C (dTrpA1 activation), and 1 day at 22°C. Gray and black bars under the horizontal axis indicate subjective day and night, respectively. Data are presented as mean ± SEM. *p < 0.05; two-way repeated measures ANOVA with the Holm-Bonferroni method for multiple comparisons (C) and one-way ANOVA followed by Tukey-Kramer post hoc test (D). (E,F) Sleep profiles for 60-min intervals (E) or total daily sleep (F) for controls (R59E08-Gal4 × w1118, black circles or bars, n = 16; w1118 × UAS-dTrpA1, gray circles or bars, n = 16) or flies expressing dTrpA1 with R59E08-Gal4 (R59E08-Gal4 × UAS-dTrpA1, blue circles or bars, n = 10) in DD. Data are presented as mean ± SEM. *p < 0.05; two-way repeated measures ANOVA with the Holm-Bonferroni method for multiple comparisons. (G,H) Maximum-intensity projection of the confocal brain images of R52B10-Gal4 (G) or R59E08-Gal4 (H) crossed to UAS-mCD8::GFP flies. Scale bars represent 100 μm.
The expression patterns of the R52B10-Gal4 or the R59E08-Gal4 drivers in the adult brains were visualized using UAS-mCD8::GFP. The wake-promoting R52B10-Gal4 drove strong expression in several cell types of columnar neurons, named PFN, projecting from the PB to the ventral part of the FB and contralateral NO (Figure 2G, left panel and Supplementary Movie 1), as shown in the FlyLight database (Jenett et al., 2012) and a previous report (Buchanan et al., 2015). In addition to these central complex substructures, some neurons in the anterior ventrolateral protocerebrum (AVLP) were also seen as having intense GFP signals (Figure 2G, right panel). The thermogenetic activation using R52H12-Gal4 or R83A10-Gal4 that express strongly in the AVLP neuropil (the FlyLight database, Jenett et al., 2012) did not result in a marked decrease in the amount of sleep (Supplementary Figure 3). The sleep-promoting R59E08-Gal4 specifically labeled the PB strongly in the central complex (Figure 2H and Supplementary Movie 2). These PB neurons were identified as PB interneurons according to their previously described morphological features (Lin et al., 2013; Wolff et al., 2015). In addition, the ubiquitous strong expression of GFP in the lobula plate cells was also detected in the R59E08-Gal4 driver. Neuronal activation using two Gal4 drivers (R14F09-Gal4 and R59A12-Gal4), whose expression patterns in the lobula plate resemble that of the R59E08-Gal4 (the FlyLight database, Jenett et al., 2012), did not significantly increase the amount of sleep (Supplementary Figure 4).
Activation of Protocerebral Bridge Interneurons With R59E08-Gal4 Increased Sleep
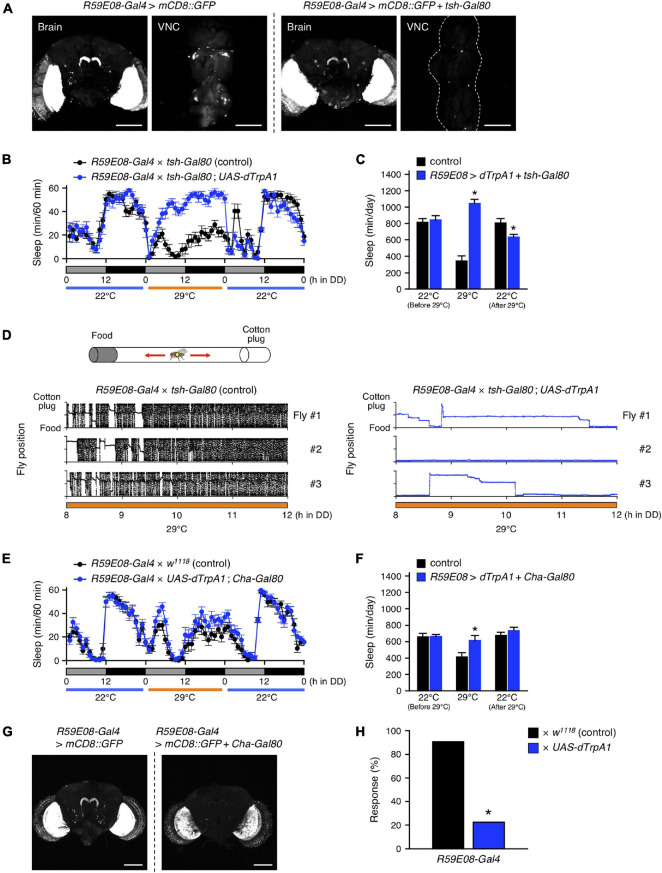
The sleep-promoting R59E08-Gal4 was expressed not only in the adult brain but also in the ventral nerve cord (VNC) (Figure 3A, left panel). To determine which targeted neurons in this driver are responsible for the sleep induction, we employed tsh-Gal80, which inhibits Gal4-mediated transcription in the VNC (Yu et al., 2010). The incorporation of the tsh-Gal80 transgene successfully blocked R59E08-Gal4 induced GFP expression in the VNC without affecting its expression pattern in the brain (Figure 3A). Neuronal activation using R59E08-Gal4 in combination with tsh-Gal80 markedly increased in the amount of sleep, indicating that brain neurons labeled by R59E08-Gal4 contribute dTrpA1-mediated sleep induction (Figures 3B,C, 2E,F). On the other hand, the decrease in sleep caused after dTrpA1 activation was completely restored upon introducing tsh-Gal80, indicating that R59E08-Gal4-expressing VNC neurons are involved in this sleep phenotype. Thus, distinct populations of R59E08-Gal4-expressing neurons contribute to the sleep phenotypes induced during or after dTrpA1 activation.
FIGURE 3.
R59E08-Gal4-expressing PB interneurons promote sleep. (A) Maximum-intensity projection of the confocal brain or ventral nerve cord (VNC) images of flies expressing UAS-mCD8::GFP under the control of R59E08-Gal4 (left two panels) or R59E08-Gal4 with tsh-Gal80 (right two panels). Transgene tsh-Gal80 efficiently suppressed R59E08-Gal4 driven GFP expression in the VNC, while did not in the brains. Scale bars indicate 100 μm. (B,C) Sleep profiles in 60-min intervals (B) or total daily sleep (C) for control flies (R59E08-Gal4 × tsh-Gal80, black circles or bars, n = 12) or flies expressing dTrpA1 in R59E08-Gal4 brain neurons (R59E08-Gal4 × tsh-Gal80; UAS-dTrpA1, blue circles or bars, n = 11) in DD. The behavior was monitored as described in Figure 2. Data are presented as mean ± SEM. *p < 0.05 vs. control; two-way repeated measures ANOVA followed by simple main effect test. (D) Horizontal movements of three representative control flies (R59E08-Gal4 × tsh-Gal80, black circles) or flies expressing dTrpA1 in R59E08-Gal4 brain neurons (R59E08-Gal4 × tsh-Gal80; UAS-dTrpA1, blue circles) in a glass tube at 29°C during the late subjective day (CT 8 to CT 12). Positions of the cotton plug and the food were at the top and the bottom in each plot, respectively. The behavior of flies was recorded at 2 frames/sec using an infrared video camera in DD conditions. The fly position in a glass tube was calculated for each image using an ImageJ plugin. (E,F) Sleep profiles in 60-min intervals (E) or total daily sleep (F) for control flies (R59E08-Gal4 × w1118, black circles or bars, n = 16) or flies expressing dTrpA1 in R59E08-Gal4 except for cholinergic neurons (R59E08-Gal4 × UAS-dTrpA1; Cha-Gal80, blue circles or bars, n = 16) in DD. Data are presented as mean ± SEM. *p < 0.05 vs. control; two-way repeated measures ANOVA followed by simple main effect test. (G) Maximum-intensity projection of the confocal brain images of flies expressing UAS-mCD8::GFP under the control of R59E08-Gal4 (left panel) or R59E08-Gal4 with Cha-Gal80 (right panel). Scale bars indicate 100 μm. (H) Responsiveness to mechanical stimuli of sleeping flies with activation of R59E08-Gal4-expressing neurons. Sleeping control (R59E08-Gal4 × w1118, black bar) or experimental (R59E08-Gal4 × UAS-dTrpA1, blue bar) flies were mechanically stimulated during the subjective night at 29°C. Response rate was calculated by subtracting the percentage of flies spontaneously aroused from that of flies awakened by stimulation (see Materials and Methods for details). Numbers of examined sleeping flies: 65 (R59E08-Gal4 × w1118) and 130 (R59E08-Gal4 × UAS-dTrpA1). *p < 0.05; chi-square test.
Because the DAM system only detects motions of a fly passing through an infrared beam that bisects a glass tube at the center, continuing feeding or grooming away from the beam path are detected as apparent inactivity (continuous bins with the value of zero). To confirm that the flies sleep by activation of R59E08-Gal4-expressing brain neurons, we performed a video analysis of the behavior of these flies in glass tubes during the late subjective-day (CT 8 to CT 12) at 29°C (Figure 3D). The temperature was shifted from 22 to 29°C at CT 0. As expected, based on the sleep patterns observed by the DAM system, the experimental flies (R59E08-Gal4 × tsh-Gal80; UAS-dTrpA1) displayed little locomotor activity compared to controls (R59E08-Gal4 × tsh-Gal80). The immobile flies did not show excessive feeding or grooming upon naked-eye observation.
We also found that the sleep phenotypes induced during or after activation of R59E08-Gal4-expressing neurons were largely rescued by introducing the Cha-Gal80 transgene, which prohibits Gal4 activity in cholinergic neurons (Figures 3E,F). Cha-Gal80 effectively suppressed the GFP expression in all but probably two of the PB interneurons labeled by R59E08-Gal4 (Figure 3G). By contrast, the GFP expression was partially reduced in the lobula plate of the optic lobes.
Furthermore, we examined the response to mechanical stimuli of sleeping flies with activated neurons labeled by R59E08-Gal4 during subjective night. Approximately 20% of the experimental flies (R59E08-Gal4 × UAS-dTrpA1) were awakened by stimuli of an intensity that aroused more than 90% of the control flies (R59E08-Gal4 × w1118) (Figure 3H). Taken together, these results indicate that the PB interneurons labeled by R59E08-Gal4 were sleep-promoting and were primarily cholinergic.
PFN Neurons Were Wake-Promoting Neurons
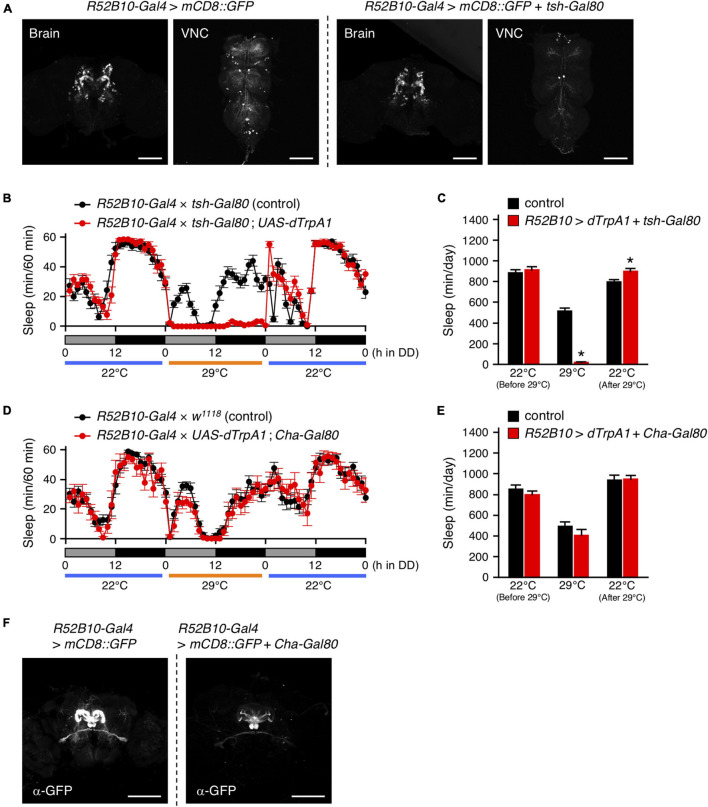
As shown in Figure 4A, tsh-Gal80 effectively attenuated the wake-promoting R52B10-Gal4-driven GFP expression in the VNC without affecting its expression in the brain. Activation of the brain neurons targeted by R52B10-Gal4 resulted in a dramatic reduction in the amount of sleep comparable to that evoked by activating both the brain and the VNC neurons using this Gal4 driver (Figures 4B,C, 2B,C). Cha-Gal80 was able to fully revert the decreased sleep caused by activation of R52B10-Gal4-expressing neurons (Figures 4D,E, 2B,C) and successfully inhibited GFP expression by R52B10-Gal4 in the PB (Figure 4F). These results suggest that cholinergic brain neurons labeled by R52B10-Gal4 are wake-promoting.
FIGURE 4.
Activation of R52B10-Gal4-expressing cholinergic neurons in the brain promotes wakefulness. (A) Maximum-intensity projection of the confocal brain or VNC images of flies expressing UAS-mCD8::GFP under the control of R52B10-Gal4 (left two panels) or R52B10-Gal4 with tsh-Gal80 (right two panels). The tsh-Gal80 efficiently suppressed R52B10-Gal4 driven GFP expression in the VNC, while did not in the brains. Scale bars represent 100 μm. (B,C) Sleep profiles in 60-min intervals (B) or total daily sleep (C) for control flies (R52B10-Gal4 × tsh-Gal80, black circles or bars, n = 16) or flies expressing dTrpA1 in R52B10-Gal4 brain neurons (R52B10-Gal4 × tsh-Gal80; UAS-dTrpA1, red circles or bars, n = 16) in DD. The behavior was monitored as described in Figure 2. Data are presented as mean ± SEM. *p < 0.05 vs. control; two-way repeated measures ANOVA followed by simple main effect test. (D,E) Sleep profiles in 60-min intervals (D) or total daily sleep (E) for control flies (R52B10-Gal4 × w1118, black circles or bars, n = 16) or flies expressing dTrpA1 in R52B10-Gal4 except for cholinergic neurons (R52B10-Gal4 × UAS-dTrpA1; Cha-Gal80, red circles or bars, n = 7) in DD. Data are presented as mean ± SEM. (F) Maximum-intensity projection of the confocal brain images of flies expressing UAS-mCD8::GFP under the control of R52B10-Gal4 (left panel) or R52B10-Gal4 with Cha-Gal80 (right panel). Scale bars represent 100 μm.
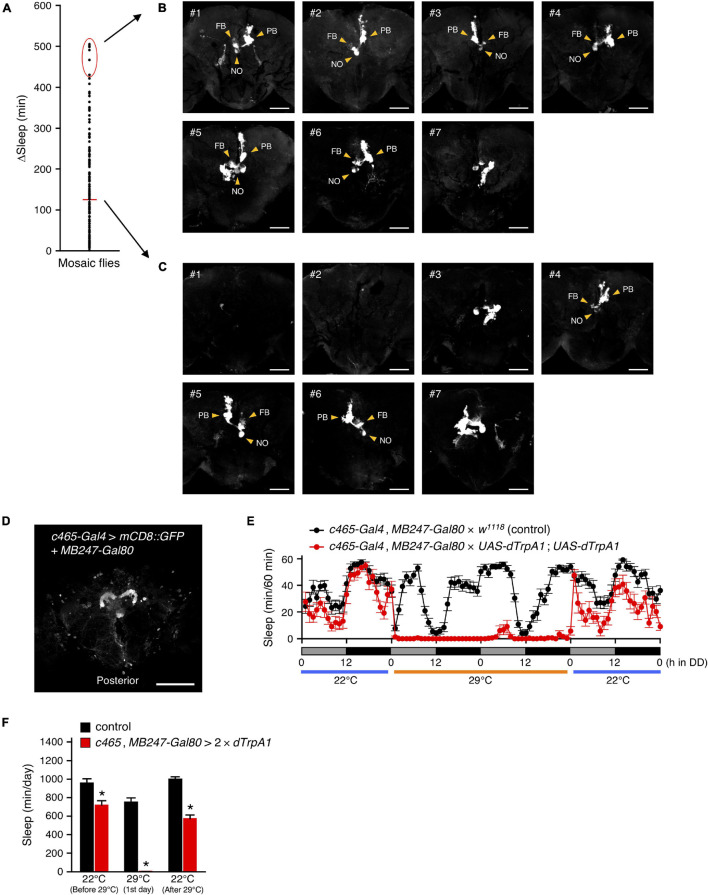
Next, we searched for R52B10-Gal4-expressing brain neurons that contribute to the promotion of wakefulness using a genetic mosaic approach. Using the mosaic analysis with a repressible cell marker (MARCM) system, dTrpA1 and GFP were randomly co-expressed in a subpopulation of neurons using R52B10-Gal4 with FRT/FLP-induced mitotic recombination. We measured the amount of sleep in these genetically mosaic flies at 22°C and then 29°C under DD conditions. The change in sleep amount (ΔSleep) in individual flies was calculated by subtracting the amount of sleep during the 12-h subjective night at 29°C from that at 22°C (Figure 5A). In this experiment, ΔSleep value above the mean plus two times standard deviation (+ 2 SD) was regarded as a significant decrease in subjective-night sleep at 29°C. Of 133 flies examined, 7 flies had a ΔSleep value above the mean + 2 SD (426 min). After measuring the amount of sleep, the brains of these flies were dissected and immunostained to determine GFP expression co-expressed with dTrpA1. GFP expression in the flies with significantly reduced sleep labeled the PFN neurons in all but one of the flies shown in #7 in Figure 5B (see z-stack in Supplementary Movie 3). In contrast, in the 7 individuals having a ΔSleep value near the average, 3 flies were labeled with the PFN neurons (#4, #5 and #6 in Figure 5C), while the remaining flies were either not labeled at all (#2) or labeled with neurons different from the PFN neurons (#1, #3 and #7) (see z-stack in Supplementary Movie 4).
FIGURE 5.
R52B10-Gal4-expressing PFN neurons promote wakefulness. (A) Using the MARCM system, dTrpA1 expression was targeted to a limited number of neurons in R52B10-Gal4. The behavior of mosaic flies was monitored as described in Figure 2. Sleep change (ΔSleep) of a single fly was calculated by subtracting the amount of sleep during the subjective night at 29°C from that at 22°C (before 29°C) (n = 133). The red horizontal bar indicates the mean. (B,C) Maximum-intensity projection of the confocal brain images of the flies whose ΔSleep were > + 2 SD higher than the mean [red oval in (A)] (B) or nearly equal to the mean (C). PB, protocerebral bridge; FB, fan-shaped body; NO, noduli. Scale bars indicate 50 μm. (D) Maximum-intensity projection of the confocal brain images of c465-Gal4, MB247-Gal80 crossed to UAS-mCD8::GFP flies. c465-Gal4 drives expression in the PFN neurons. Scale bars indicate 100 μm. (E,F) Sleep profiles in 60-min intervals (E) or total daily sleep (F) for control (c465-Gal4, MB247-Gal80 × w1118, black circles or bars, n = 15) or flies expressing dTrpA1 in c465-Gal4 except for mushroom body neurons (c465-Gal4, MB247-Gal80 × UAS-dTrpA1; UAS-dTrpA1, red circles or bars, n = 11) in DD conditions. Behavior was monitored as described in Figure 2, except that flies were transferred to 29°C for 2 days to allow dTrpA1 activation. Data are presented as mean ± SEM. *p < 0.05 vs. control; two-way repeated measures ANOVA followed by simple main effect test.
Moreover, to ensure that the PFN neurons labeled by R52B10-Gal4 are wake-promoting neurons, we employed c465-Gal4, which drives expression in these neurons, participating in the tuning of the magnitude of locomotor handedness (Buchanan et al., 2015). Because c465-Gal4 is also strongly expressed in the mushroom bodies, which are another brain neuropil structure involved in Drosophila sleep regulation (Joiner et al., 2006; Pitman et al., 2006; Sitaraman et al., 2015a), we used MB247-Gal80, which blocks the activity of Gal4 in the mushroom bodies (Figure 5D). As expected, activation of c465-Gal4-expressing neurons, except for mushroom body neurons, significantly decreased sleep, as did the activation of R52B10-Gal4-expressing neurons (Figures 5E,F). These results indicate that the PFN neurons labeled by R52B10-Gal4 are wake-promoting.
The Sleep-Promoting Protocerebral Bridge Interneurons Had Synaptic Connections With the Wake-Promoting PFN Neurons
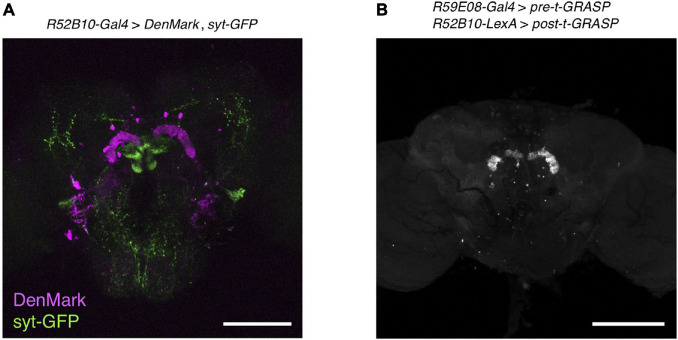
To visualize putative dendritic and axonal terminals of the wake-promoting PFN neurons, both DenMark (Nicolai et al., 2010), a specific somatodendritic marker, and synaptotagmin (syt)-GFP (Zhang et al., 2002), a presynaptic marker localized to synaptic vesicles, were expressed using R52B10-Gal4. These neurons displayed specific dendritic DenMark labeling in the PB and strong labeling of presynaptic syt-GFP in the layer 1 of the FB and in the ventral NO (Figure 6A and Supplementary Movie 5). In addition, weak labeling of presynaptic terminals was also observed near the layers 2 to 3 of the FB and in regions of the NO other than the ventral NO.
FIGURE 6.
The sleep-promoting PB interneurons form synaptic contacts with the wake-promoting PFN neurons. (A) The dendritic arbors and presynaptic terminals of the PFN neurons in R52B10-Gal4 were visualized by expression of the postsynaptic marker DenMark and the presynaptic marker syt-GFP, respectively. (B) The neuronal connection of the sleep-promoting PB interneurons to the wake-promoting PFN neurons was revealed using the t-GRASP method. The pre-t-GRASP and the post-t-GRASP encode the split-GFP fragments, which are targeted to presynaptic endings and dendritic terminals, respectively. Maximum-intensity projection of the confocal images of the brain expressing pre-t-GRASP with R59E08-Gal4 and post-t-GRASP with R52B10-LexA. Scale bars represent 100 μm.
To assess synaptic connectivity between the sleep-promoting PB interneurons and the wake-promoting PFN neurons, we employed a targeted GFP reconstitution across synaptic partners (t-GRASP) technique (Shearin et al., 2018) under the control of two independent binary systems. A part of the cacophony gene tagged with the GFP11 fragment of a split-GFP (pre-t-GRASP), which is targeted to axonal terminals, and a portion of the mouse Icam5 gene tagged with the GFP1-10 (post-t-GRASP), which is targeted to dendritic terminals, were expressed by R59E08-Gal4 and R52B10-LexA, respectively. Reconstituted GFP signals were detected in the PB (Figure 6B and Supplementary Movie 6), suggesting that the sleep-promoting PB interneurons labeled by R59E08-Gal4 have synaptic connections with the wake-promoting PFN neurons marked by R52B10-Gal4.
Dopamine Signaling Acted on the Sleep-Promoting Protocerebral Bridge Interneurons for Sleep Regulation
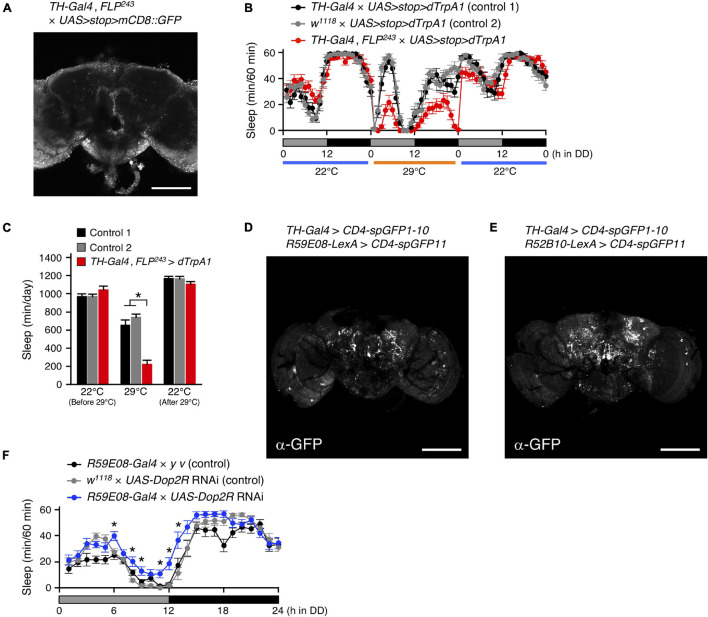
Dopamine has been identified as a key neurotransmitter in the regulation of sleep in Drosophila (Andretic et al., 2005; Kume et al., 2005; Pimentel et al., 2016). In the Drosophila brain, DA neurons are distributed in clusters, and each cluster is involved in various physiological phenomena, including sleep-wake (Liu et al., 2012; Ueno et al., 2012; Sitaraman et al., 2015b). A pair of DA neurons, named T1, has been previously observed with dendrites arborizing in the tritocerebrum and with axons projecting extensively to the PB (Nässel and Elekes, 1992; Alekseyenko et al., 2013). The intersectional method combining the enhancer-trap flippase (FLP) transgenic line, FLP243 with a DA-neuron specific TH-Gal4 driver allows for the selective targeting of the T1 neurons (Figure 7A and Supplementary Movie 7; Alekseyenko et al., 2013). Both activation and inhibition of the T1 DA neurons using this intersectional method have been reported to specifically facilitate inter-male aggression. On the other hand, inactivation of these neurons has no effect on locomotor activity and sleep amount (Alekseyenko et al., 2013). We conditionally activated the T1 neurons with dTrpA1 in male flies under DD conditions and found that the manipulation resulted in a significant decrease in the amount of sleep (Figures 7B,C). Similarly, activation of these neurons in males under LD conditions and in females under LD and DD conditions induced a decrease in the amount of sleep (Supplementary Figure 5). Only female flies whose T1 neurons were activated under LD conditions exhibited a distinct sleep rebound following sleep loss (Supplementary Figure 5C).
FIGURE 7.
Dopamine regulates sleep by acting on the sleep-promoting PB interneurons. (A) Maximum-intensity projection of the confocal brain images of TH-Gal4, FLP243 crossed to UAS>stop>mCD8::GFP flies. TH-Gal4, FLP243 restricts expression to the T1 dopaminergic (DA) neurons that project to the PB. Scale bar indicates 100 μm. (B,C) Sleep profiles in 60-min intervals (B) or total daily sleep (C) for controls [TH-Gal4 × UAS>stop>dTrpA1 (control 1), black circles or bars, n = 16; w1118 × UAS>stop>dTrpA1 (control 2), gray circles or bars, n = 16] or flies expressing dTrpA1 in T1 DA neurons (TH-Gal4, FLP243 × UAS>stop>dTrpA1, red circles or bars, n = 16) in DD conditions. The behavior was monitored as described in Figure 2. Data are presented as mean ± SEM. *p < 0.05; two-way repeated measures ANOVA with the Holm-Bonferroni method for multiple comparisons. (D,E) The anatomical connection of DA neurons in TH-Gal4 to the sleep-promoting PB interneurons (D) or the wake-promoting PFN neurons (E) were examined using the GRASP method. Brains were stained with a reconstituted GFP-specific antibody. Scale bars indicate 100 μm. (F) Sleep profiles in 60-min intervals for controls (R59E08-Gal4 × y v, black circles, n = 16; w1118 × UAS-Dop2R RNAi, gray circles, n = 16) and Dop2R RNAi-expressing flies using the R59E08-Gal4 driver (blue circles, n = 16) in DD conditions. Data are presented as mean ± SEM. *p < 0.05 vs. both controls; two-way repeated measures ANOVA with the Holm-Bonferroni method for multiple comparisons.
Next, we examined whether T1 DA neurons directly connect with sleep-wake regulating PB neurons by GRASP analysis utilizing membrane-tethered two complementary split-GFP fragments (CD4-spGFP1-10 and CD4-spGFP11) (Feinberg et al., 2008). TH-Gal4 was used to express UAS-CD4-spGFP1-10, and R59E08-LexA or R52B10-lexA were employed to drive lexAop-CD4-spGFP11, respectively. Immunohistochemical signals for reconstituted GFP in the PB were detected only in the combination of TH-Gal4 and R59E08-LexA (Figures 7D,E and Supplementary Movies 8, 9). These signals were dot-like and localized to the bilateral bends of the PB.
Alekseyenko and colleagues also demonstrate that Dopamine 2-like receptors (Dop2R also known as D2R or DD2R), a functional counterpart of mammalian D2 receptor, are expressed in the PB but not localized to the presynaptic terminals of T1 neurons. Dop2R knockdown using two independent RNAi lines in the R59E08-Gal4 expressing PB neurons resulted in a significant increase in the amount of sleep compared to controls (Figure 7F and Supplementary Figure 6). These results support the idea that T1 DA neurons regulate sleep by acting on the sleep-promoting PB interneurons via D2 receptor signaling.
Discussion
The data presented provide evidence that Drosophila sleep is controlled by the neuronal circuit in PB, consisting of the wake-promoting PFN neurons and the sleep-promoting PB interneurons, and by the T1-PB dopamine pathway (Figure 8). Previous anatomical, electrophysiological, and genetic studies have revealed the physiological functions of the PB in several insect species. For example, in desert locusts, different types of PB neurons generate a topographical map of the sky polarization pattern underlying sun compass orientation (Homberg et al., 2011). Morphological counterparts of these locust’s PB neurons are found in migratory monarch butterflies and serve similar functions in processing polarized-light information (Heinze and Reppert, 2011). Experiments in fruit flies have demonstrated a role for the PB in locomotor control such as walking speed, leg coordination, maintenance of walking activity, locomotor handedness, and visual targeting in gap-crossing behavior (Strauss et al., 1992; Strauss and Heisenberg, 1993; Martin et al., 1999; Triphan et al., 2010; Buchanan et al., 2015). The locomotor activity of nobKS49 mutants has been reported to be decreased compared to control flies, but was increased in the present study (Martin et al., 1999; Figure 1A). In this study, and in a previous study by Martin et al. (1999) the number of activity counts per day or at a particular time of day (4.5 h), respectively, was assessed as the total locomotor activity in nobKS49 flies. This difference in assessment methods may have caused the phenotypic discrepancy in locomotor activity. nobKS49 flies with a large gap at the sagittal midplane in the PB showed a significant reduction in the amount of sleep (Figure 1B), suggesting a physical impairment of sleep-promoting neurons in this mutant. We discovered the sleep-promoting R59E08-Gal4, which is mainly expressed in the PB interneurons and the lobula plate neurons (Figure 2). Analysis using a combination of R59E08-Gal4 and Cha-Gal80 (Figures 3E–G) and neuronal activation using Gal4 lines that express in the lobula plate (Supplementary Figure 4) showed that the PB interneurons labeled by R59E08-Gal4 should promote sleep. On the other hand, these results did not exclude the possibility that R59E08-Gal4-expressing neurons other than the PB interneurons may also affect sleep. Analysis of single PB neurons labeled by MARCM or multicolor flip-out technique has shown that PB interneurons are classified into three cell types based on their pattern of glomerular innervation in the PB (Lin et al., 2013; Wolff et al., 2015). Two of the three cell types of PB interneurons innervate all glomeruli, including the central part of the PB. Because the sleep-promoting R59E08-Gal4 was expressed throughout the PB, at least one of these two cell types of PB interneurons would have been labeled with this driver. Although the cell type of PB interneurons that promote sleep has been unidentified in our results, the two cell types may be physically damaged in nobKS49 mutants.
FIGURE 8.
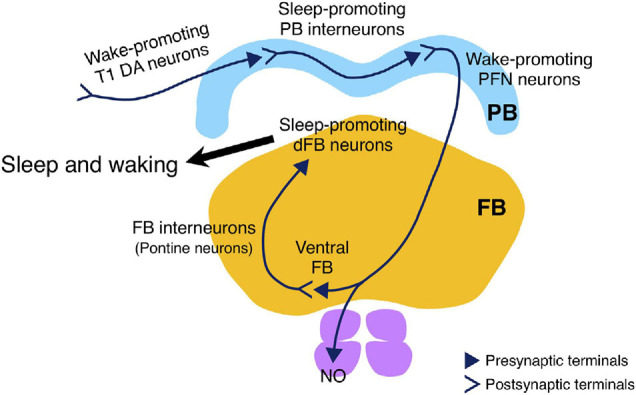
Schematic drawing of the sleep-regulating neuronal circuit in the PB. The circuit consists of the sleep-promoting PB interneurons and the wake-promoting PFN neurons, and is regulated by wake-promoting T1 DA neurons. The wake-promoting PFN neurons indirectly inhibit the sleep-promoting dFB neurons via FB interneurons (Kato YS et al., unpublished results).
Our MARCM experiment using R52B10-Gal4 suggested that distinct types of PFN neurons promote wakefulness (Figures 5A,B). PFN neurons, which are PB output neurons, are classified into five types, according to their patterns of projection to the FB and the NO (Wolff et al., 2015). Each PFN neuron cell type is further classified into subtypes that have different patterns of arborization in the PB and arborize to one of the PB glomeruli. The Gal4 line c465 drives expression in the wake-promoting PFN neurons (Figures 5D–F; Lin et al., 2013; Buchanan et al., 2015). Of the five cell types of PFN neurons, three are commonly labeled by R52B10-Gal4 and c465-Gal4, especially the cell type with axon terminals in layer 2 of the FB and in the ventral NO exhibits high Gal4 expression in these two drivers (Buchanan et al., 2015). On the other hand, neuronal activation with R44B10-Gal4, which is highly expressed in all three cell types of PFN neurons (Buchanan et al., 2015), was not significantly effective in reducing the amount of sleep (Figure 2A). Although R52B10-Gal4, c465-Gal4, and R44B10-Gal4 label the same cell types of PFN neurons, it is possible that the subtypes of PFN neurons arborizing in one specific PB glomerulus, which are co-labeled with R52B10-Gal4 and c465-Gal4, are implicated in promoting arousal.
The t-GRASP experiment using R59E08-Gal4 and R52B10-LexA suggested that the sleep-promoting PB interneurons form synaptic contacts with the wake-promoting PFN neurons (Figure 6B). The symmetrical patterns of the t-GRASP signal intensity in PB glomeruli should indicate that this signal is not non-specific, supporting the idea described above that particular subtypes of PFN neurons promote arousal. Sleep phenotypes of the flies, in which R59E08-Gal4- or R52B10-Gal4-expressing neurons were acutely activated, and axon-dendrite connectivity between these sleep-regulating neurons suggest that the sleep-promoting PB interneurons inhibit the activity of the wake-promoting PFN neurons. Because the sleep-promoting PB interneurons were predominantly cholinergic (Figures 3E–G), the wake-promoting PFN neurons were likely to be regulated by inhibitory acetylcholine receptor signaling (Ren et al., 2015). Functional connectivity analysis of the central complex using Ca2+ imaging combined with optogenetics has demonstrated that activation of one of the cell types of PB interneurons, designated as Δ7, triggers the inhibitory response of one cell type of PFN neurons (Franconville et al., 2018). However, since the Δ7 neurons are either glutamatergic or GABAergic, they must not be the major PB interneurons involved in the promotion of sleep. VT34814-Gal4 has been reported as a driver to label cholinergic PB interneurons (Lin et al., 2013), and these neurons may be involved in the regulation of the wake-promoting PFN neurons. Further anatomical and functional dissection of the sleep-regulating neuronal circuits in the PB, archived with promising split-Gal4 lines, will be required.
Previous studies have elucidated the control of Drosophila sleep by several clusters of DA neurons (Liu et al., 2012; Ueno et al., 2012; Sitaraman et al., 2015b). This study revealed that the PB-projecting T1 DA neurons physically connected to the sleep-promoting PB interneurons promote wakefulness (Figures 7A–D). Because knockdown of Dop2R encoding Gi protein-coupled dopamine receptor in R59E08-Gal4-expressing neurons significantly increased the amount of sleep (Figure 7F and Supplementary Figure 6), the simplest explanation is that dopamine inhibits the sleep-promoting PB interneurons and thus promotes wakefulness. Dop2R null mutants have an increased amount of sleep (Deng et al., 2019). The functioning of Dop2R in the pars intercerebralis (PI) neurons expressing Dilp2 and SIFamide has been shown to contribute to this sleep-increasing phenotype. Our results show that, in addition to the PI, Dop2R also functions in the PB to regulate sleep.
T1 neurons were originally identified as neurons that specifically modulate aggression between pairs of males (Alekseyenko et al., 2013). Regarding the interaction between sleep and aggression, it has been shown that aggressive behaviors are suppressed in sleep-deprived male flies, and the changes are mediated by octopamine signaling (Kayser et al., 2015). Interestingly, Duhart and colleagues have recently reported that T1 neurons act downstream of courtship- and sleep-regulating P1 neurons to modulate nutrition-dependent sleep-courtship balance in male flies (Duhart et al., 2020). Elucidation of the mechanisms controlling the activity of T1 neurons may provide a novel link between sleep, aggression and courtship.
More recently, we have successfully demonstrated that the wake-promoting PFN neurons directly activate the FB interneurons (also known as pontine neurons) that appear to transmit inhibitory acetylcholine signals to the sleep-promoting dFB neurons (Kato YS et al., unpublished results, Figure 8). Further studies are required to determine the cellular and molecular details of how these PB-dFB circuits control sleep-wakefulness.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
JT, GB, YK, and KK: design of experiments. JT, GB, and YK: performing the experiments and analyzing data. JT, YK, and KK: drafting the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Roland Strauss, Paul A. Garrity, Julie H. Simpson, Takaomi Sakai, J. Douglas Armstrong, Edward A. Kravitz, and Barry J. Dickson, the Bloomington Drosophila Stock Center, the Vienna Drosophila RNAi Center and the Kyoto Stock Center for providing the fly lines used in this study. We also thank Taro Ueno (Toho University) and members of the Kume lab for helpful discussions.
Footnotes
Funding
This work was supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Numbers JP18H02481, KK, JP26507008, JP17K08571, JT, and the Takeda Science Foundation, JT.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnins.2021.647117/full#supplementary-material
References
- Alekseyenko O. V., Chan Y.-B., Li R., Kravitz E. A. (2013). Single dopaminergic neurons that modulate aggression in Drosophila. Proc. Natl. Acad. Sci. U. S. A. 110 6151–6156. 10.1073/pnas.1303446110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R., Siegel J. M. (2008). Unearthing the phylogenetic roots of sleep. Curr. Biol. 18 R670–R679. 10.1016/j.cub.2008.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andretic R., van Swinderen B., Greenspan R. J. (2005). Dopaminergic modulation of arousal in Drosophila. Curr. Biol. 15 1165–1175. 10.1016/j.cub.2005.05.025 [DOI] [PubMed] [Google Scholar]
- Buchanan S. M., Kain J. S., de Bivort B. L. (2015). Neuronal control of locomotor handedness in Drosophila. Proc. Natl. Acad. Sci. 112 6700–6705. 10.1073/pnas.1500804112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell S. S., Tobler I. (1984). Animal sleep: a review of sleep duration across phylogeny. Neurosci. Biobehav. Rev. 8 269–300. 10.1016/0149-7634(84)90054-x [DOI] [PubMed] [Google Scholar]
- Cirelli C., Tononi G. (2008). Is sleep essential? PLoS Biol. 6:e216. 10.1371/journal.pbio.0060216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyne J. D., Miesenböck G. (2008). Sex-specific control and tuning of the pattern generator for courtship song in Drosophila. Cell 133 354–363. 10.1016/j.cell.2008.01.050 [DOI] [PubMed] [Google Scholar]
- Dag U., Lei Z., Le J. Q., Wong A., Bushey D., Keleman K. (2019). Neuronal reactivation during post-learning sleep consolidates long-term memory in Drosophila. Elife 8:e42786. 10.7554/eLife.42786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng B., Li Q., Liu X., Cao Y., Li B., Qian Y., et al. (2019). Chemoconnectomics: mapping Chemical Transmission in Drosophila. Neuron 101 876–893.e4. 10.1016/j.neuron.2019.01.045 [DOI] [PubMed] [Google Scholar]
- Donlea J. M., Pimentel D., Miesenböck G. (2014). Neuronal machinery of sleep homeostasis in Drosophila. Neuron 81 860–872. 10.1016/j.neuron.2013.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea J. M., Pimentel D., Talbot C. B., Kempf A., Omoto J. J., Hartenstein V., et al. (2018). Recurrent Circuitry for Balancing Sleep Need and Sleep. Neuron 97 378–389.e4. 10.1016/j.neuron.2017.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donlea J. M., Thimgan M. S., Suzuki Y., Gottschalk L., Shaw P. J. (2011). Inducing sleep by remote control facilitates memory consolidation in Drosophila. Science 332 1571–1576. 10.1126/science.1202249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duhart J. M., Baccini V., Zhang Y., Machado D. R., Koh K. (2020). Modulation of sleep-courtship balance by nutritional status in Drosophila. Elife 9:e60853. 10.7554/eLife.60853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg E. H., Vanhoven M. K., Bendesky A., Wang G., Fetter R. D., Shen K., et al. (2008). GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron 57 353–363. 10.1016/j.neuron.2007.11.030 [DOI] [PubMed] [Google Scholar]
- Franconville R., Beron C., Jayaraman V. (2018). Building a functional connectome of the drosophila central complex. Elife 7:e37017. 10.7554/eLife.37017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F., Holla M., Díaz M. M., Rosbash M. (2018). A Circadian Output Circuit Controls Sleep-Wake Arousal in Drosophila. Neuron 100 624–635.e4. 10.1016/j.neuron.2018.09.002 [DOI] [PubMed] [Google Scholar]
- Hamada F. N., Rosenzweig M., Kang K., Pulver S. R., Ghezzi A., Jegla T. J., et al. (2008). An internal thermal sensor controlling temperature preference in Drosophila. Nature 454 217–220. 10.1038/nature07001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinze S., Reppert S. M. (2011). Sun compass integration of skylight cues in migratory monarch butterflies. Neuron 69 345–358. 10.1016/j.neuron.2010.12.025 [DOI] [PubMed] [Google Scholar]
- Hendricks J. C., Finn S. M., Panckeri K. A., Chavkin J., Williams J. A., Sehgal A., et al. (2000). Rest in Drosophila is a sleep-like state. Neuron 25 129–138. 10.1016/s0896-6273(00)80877-6 [DOI] [PubMed] [Google Scholar]
- Homberg U. (2008). Evolution of the central complex in the arthropod brain with respect to the visual system. Arthropod Struct. Dev. 37 347–362. 10.1016/j.asd.2008.01.008 [DOI] [PubMed] [Google Scholar]
- Homberg U., Heinze S., Pfeiffer K., Kinoshita M., El Jundi B. (2011). Central neural coding of sky polarization in insects. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 366 680–687. 10.1098/rstb.2010.0199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R., Hill S. L., Holladay C., Biesiadecki M., Tononi G., Cirelli C. (2004). Sleep homeostasis in Drosophila melanogaster. Sleep 27 628–639. 10.1093/sleep/27.4.628 [DOI] [PubMed] [Google Scholar]
- Jenett A., Rubin G. M., Ngo T.-T. B., Shepherd D., Murphy C., Dionne H., et al. (2012). A GAL4-Driver Line Resource for Drosophila Neurobiology. Cell Rep. 2 991–1001. 10.1016/j.celrep.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner W. J., Crocker A., White B. H., Sehgal A. (2006). Sleep in Drosophila is regulated by adult mushroom bodies. Nature 441 757–760. 10.1038/nature04811 [DOI] [PubMed] [Google Scholar]
- Kayser M. S., Mainwaring B., Yue Z., Sehgal A. (2015). Sleep deprivation suppresses aggression in Drosophila. Elife 4:e07643. 10.7554/eLife.07643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes M. J., Keene A. C., Leung B., Armstrong J. D., Waddell S. (2007). Sequential use of mushroom body neuron subsets during drosophila odor memory processing. Neuron 53 103–115. 10.1016/j.neuron.2006.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume K., Kume S., Park S. K., Hirsh J., Jackson F. R. (2005). Dopamine is a regulator of arousal in the fruit fly. J. Neurosci. 25 7377–7384. 10.1523/JNEUROSCI.2048-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaze A., Krätschmer P., Chen K.-F., Lowe S., Jepson J. E. C. (2018). A Wake-Promoting Circadian Output Circuit in Drosophila. Curr. Biol. 28 3098–3105.e3. 10.1016/j.cub.2018.07.024 [DOI] [PubMed] [Google Scholar]
- Lin C.-Y., Chuang C.-C., Hua T.-E., Chen C.-C., Dickson B. J., Greenspan R. J., et al. (2013). A comprehensive wiring diagram of the protocerebral bridge for visual information processing in the Drosophila brain. Cell Rep. 3 1739–1753. 10.1016/j.celrep.2013.04.022 [DOI] [PubMed] [Google Scholar]
- Liu Q., Liu S., Kodama L., Driscoll M. R., Wu M. N. (2012). Two dopaminergic neurons signal to the dorsal fan-shaped body to promote wakefulness in Drosophila. Curr. Biol. 22 2114–2123. 10.1016/j.cub.2012.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S., Liu Q., Tabuchi M., Wu M. N. (2016). Sleep Drive Is Encoded by Neural Plastic Changes in a Dedicated Circuit. Cell 165 1347–1360. 10.1016/j.cell.2016.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. R., Raabe T., Heisenberg M. (1999). Central complex substructures are required for the maintenance of locomotor activity in Drosophila melanogaster. J. Comp. Physiol. A 185 277–288. 10.1007/s003590050387 [DOI] [PubMed] [Google Scholar]
- Nässel D. R., Elekes K. (1992). Aminergic neurons in the brain of blowflies and Drosophila: dopamine- and tyrosine hydroxylase-immunoreactive neurons and their relationship with putative histaminergic neurons. Cell Tissue Res. 267 147–67. 10.1007/BF00318701 [DOI] [PubMed] [Google Scholar]
- Ni J. D., Gurav A. S., Liu W., Ogunmowo T. H., Hackbart H., Elsheikh A., et al. (2019). Differential regulation of the Drosophila sleep homeostat by circadian and arousal inputs. Elife 8:e40487. 10.7554/eLife.40487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolai L. J. J., Ramaekers A., Raemaekers T., Drozdzecki A., Mauss A. S., Yan J., et al. (2010). Genetically encoded dendritic marker sheds light on neuronal connectivity in Drosophila. Proc. Natl. Acad. Sci. 107 20553–20558. 10.1073/pnas.1010198107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel D., Donlea J. M., Talbot C. B., Song S. M., Thurston A. J. F., Miesenböck G. (2016). Operation of a homeostatic sleep switch. Nature 536 333–337. 10.1038/nature19055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman J. L., McGill J. J., Keegan K. P., Allada R. (2006). A dynamic role for the mushroom bodies in promoting sleep in Drosophila. Nature 441 753–756. 10.1038/nature04739 [DOI] [PubMed] [Google Scholar]
- R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing. [Google Scholar]
- Ren G. R., Folke J., Hauser F., Li S., Grimmelikhuijzen C. J. P. (2015). The A- and B-type muscarinic acetylcholine receptors from Drosophila melanogaster couple to different second messenger pathways. Biochem. Biophys. Res. Commun. 462 358–364. 10.1016/j.bbrc.2015.04.141 [DOI] [PubMed] [Google Scholar]
- Sakai T., Kasuya J., Kitamoto T., Aigaki T. (2009). The Drosophila TRPA channel, Painless, regulates sexual receptivity in virgin females. Genes. Brain. Behav. 8 546–557. 10.1111/j.1601-183X.2009.00503.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sbalzarini I. F., Koumoutsakos P. (2005). Feature point tracking and trajectory analysis for video imaging in cell biology. J. Struct. Biol. 151 182–195. 10.1016/j.jsb.2005.06.002 [DOI] [PubMed] [Google Scholar]
- Shaw P. J., Cirelli C., Greenspan R. J., Tononi G. (2000). Correlates of sleep and waking in Drosophila melanogaster. Science 287 1834–1837. 10.1126/science.287.5459.1834 [DOI] [PubMed] [Google Scholar]
- Shearin H. K., Quinn C. D., Mackin R. D., Macdonald I. S., Stowers R. S. (2018). t-GRASP, a targeted GRASP for assessing neuronal connectivity. J. Neurosci. Methods 306 94–102. 10.1016/j.jneumeth.2018.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman D., Aso Y., Jin X., Chen N., Felix M., Rubin G. M., et al. (2015a). Propagation of Homeostatic Sleep Signals by Segregated Synaptic Microcircuits of the Drosophila Mushroom Body. Curr. Biol. 25 2915–2927. 10.1016/j.cub.2015.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitaraman D., Aso Y., Rubin G. M., Nitabach M. N. (2015b). Control of Sleep by Dopaminergic Inputs to the Drosophila Mushroom Body. Front. Neural Circuits 9:73. 10.3389/fncir.2015.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R., Hanesch U., Kinkelin M., Wolf R., Heisenberg M. (1992). No-bridge of Drosophila melanogaster: portrait of a structural brain mutant of the central complex. J. Neurogenet. 8 125–155. 10.3109/01677069209083444 [DOI] [PubMed] [Google Scholar]
- Strauss R., Heisenberg M. (1993). A higher control center of locomotor behavior in the Drosophila brain. J. Neurosci. 13 1852–1861. 10.1523/jneurosci.13-05-01852.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita J., Ueno T., Mitsuyoshi M., Kume S., Kume K. (2015). The NMDA Receptor Promotes Sleep in the Fruit Fly, Drosophila melanogaster. PLoS One 10:e0128101. 10.1371/journal.pone.0128101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triphan T., Poeck B., Neuser K., Strauss R. (2010). Visual targeting of motor actions in climbing Drosophila. Curr. Biol. 20 663–668. 10.1016/j.cub.2010.02.055 [DOI] [PubMed] [Google Scholar]
- Ueno T., Tomita J., Tanimoto H., Endo K., Ito K., Kume S., et al. (2012). Identification of a dopamine pathway that regulates sleep and arousal in Drosophila. Nat. Neurosci. 15 1516–1523. 10.1038/nn.3238 [DOI] [PubMed] [Google Scholar]
- von Philipsborn A. C., Liu T., Yu J. Y., Masser C., Bidaye S. S., Dickson B. J. (2011). Neuronal control of Drosophila courtship song. Neuron 69 509–522. 10.1016/j.neuron.2011.01.011 [DOI] [PubMed] [Google Scholar]
- Wolff T., Iyer N. A., Rubin G. M. (2015). Neuroarchitecture and neuroanatomy of the Drosophila central complex: a GAL4-based dissection of protocerebral bridge neurons and circuits. J. Comp. Neurol. 523 997–1037. 10.1002/cne.23705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. S., Luo L. (2006). A protocol for dissecting Drosophila melanogaster brains for live imaging or immunostaining. Nat. Protoc. 1 2110–2115. 10.1038/nprot.2006.336 [DOI] [PubMed] [Google Scholar]
- Young J. M., Armstrong J. D. (2010). Structure of the adult central complex in Drosophila: organization of distinct neuronal subsets. J. Comp. Neurol. 518 1500–1524. 10.1002/cne.22284 [DOI] [PubMed] [Google Scholar]
- Yu J. Y., Kanai M. I., Demir E., Jefferis G. S. X. E., Dickson B. J. (2010). Cellular organization of the neural circuit that drives Drosophila courtship behavior. Curr. Biol. 20 1602–1614. 10.1016/j.cub.2010.08.025 [DOI] [PubMed] [Google Scholar]
- Zhang Y. Q., Rodesch C. K., Broadie K. (2002). Living synaptic vesicle marker: synaptotagmin-GFP. Genesis 34 142–145. 10.1002/gene.10144 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.